Optogenetics, which uses light to control genetically modified neurons, is revolutionizing neuroscience research by allowing for the precise deconstruction of neural circuits with neuron-type specificity (1, 2). However, due to limited tissue penetration of visible light, invasive craniotomy and intracranial implantation of obtrusive optical fibers or light-emitting diodes (LEDs) are usually required for in-vivo optogenetic neural modulation in the brain of rodents and other mammalian subjects (3). Perturbation to the endogenous neural and glial activity has been reported as a consequence of chronic gliosis at the implant/neural interface, as has permanent damage to neural tissue (4–7).
The challenge of invasive optogenetics
The need for invasive delivery of a light source represents a long-standing challenge for in-vivo optogenetics. This is because visible photons can only achieve limited penetration in brain tissue (8). The conventional optogenetic toolbox is comprised of opsins with activation spectra in the range of 430–610 nm that have limited tissue penetration due to scattering and absorption of photons by the brain tissue (9). Several strategies have been employed to increase penetration, including employing opsins with red-shifted activation spectra (10, 11), two-photon stimulation of opsins (12), and converting brain-penetrant near-infrared light into visible photons via intracortically injected upconversion nanoparticles (13, 14).
Despite these advances, such approaches are still invasive, requiring either partial removal of the scalp and skull (10, 12), or intracranial delivery of nanomaterials into deep brain tissue (13, 14).
Convert sound into light
Our approach (15) to address this challenge has been to replace direct light illumination with focused ultrasound (FUS), the latter of which affords much deeper penetration (~5 cm for FUS vs. 100 μm for visible photons) in biological tissues including the brain (16–19). However, ultrasound does not activate opsins directly. Thus, a local transducer that converts sound into light in the brain is required.
I am a materials scientist with extensive training in neurobiology (7), and am therefore aware of the types of materials that can absorb sound waves and emit light in return. Such substances are called “mechanoluminescent materials,” and have previously been used primarily in dynamic stress mapping of structures (20), displays (21, 22) and photonic skins (23). But if these materials could be delivered to a deep brain region, they could become a light source that can be turned on and off by FUS from outside the brain.
In our study, we made zinc sulfide (ZnS) nanoparticles co-doped with trace amounts of silver (Ag) and cobalt (Co) to afford the mechanoluminescence property. Unlike pristine, dopant-free ZnS, which is a photoluminescent material with a sub-microsecond lifetime after 400-nm photoexcitation, Co dopant ions trap the excited electrons and store the photoexcitation energy until being triggered by FUS. Ag dopant ions tune the emission color to 470-nm blue light for activation of channelrhodopsin-2 (ChR2) (Fig. 1A).
Figure 1. Sono-optogenetics facilitated by mechanoluminescent materials and endogenous circulatory system.
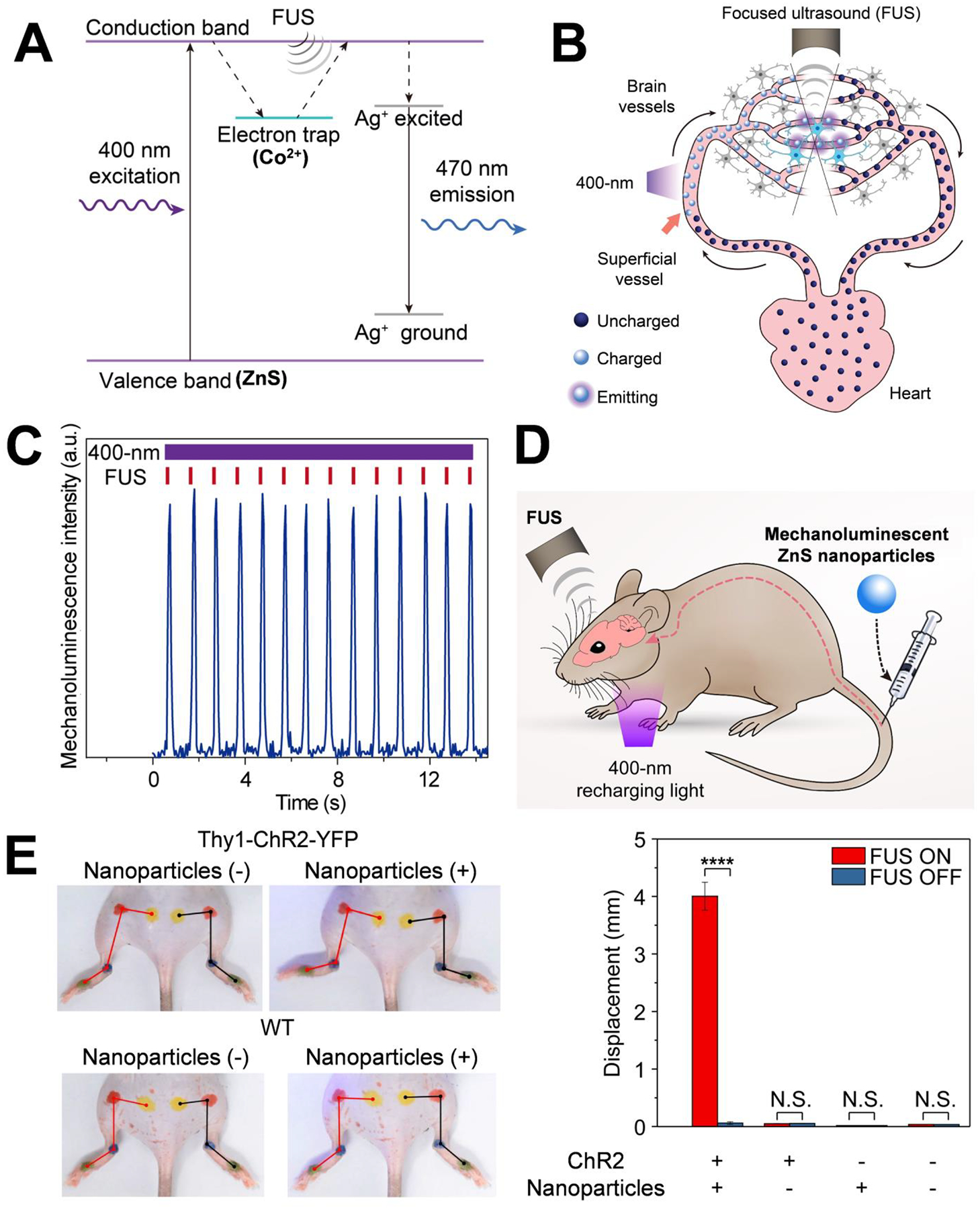
(A) Mechanism of ultrasound-triggered light emission from Ag and Co co-doped ZnS nanoparticles. In this drawing, the electron trap is created in the host material of ZnS by Co2+ dopant ions, causing the photoexcited electrons to be trapped after absorption of 400 nm excitation light. FUS allows the trapped electrons to transfer energy into the luminescent centers created by Ag+ dopant ions, resulting in 470-nm light emission. (B) Schematic showing blood circulation of mechanoluminescent ZnS nanoparticles, transporting the continuous 400-nm photoexcitation energy at superficial vessels into FUS-gated 470-nm emission in deep-brain regions for optogenetic stimulation. (C) Intensity of the 470-nm emission from mechanoluminescent ZnS nanoparticles in an artificial circulatory system under repetitive FUS stimulation (red ticks) and continuous 400-nm recharging light (violet bar). (D) Schematic of in vivo sono-optogenetic stimulation. Mechanoluminescent ZnS nanoparticles are injected intravenously via the tail vein, the 400-nm recharging light is placed near the neck skin, and the FUS transducer is placed on the intact mouse head. (E) Left: Photographs of a Thy1-ChR2-YFP mouse (top) and a wild-type (WT) mouse (bottom) during sono-optogenetic stimulation through intact scalp and skull, before and after injection of mechanoluminescent ZnS nanoparticles. Right: Statistics of left hindlimb displacement in different groups of subjects (n = 3 per group) in response to FUS excitation. The bar heights indicate the mean, and the error bars indicate SEM. ****P < 0.0001; N.S., not significant.
An endogenous mechanism to deliver and recharge the nanoscopic light source
This method, however, has a significant drawback: since 400-nm photoexcitation is required to “pre-charge” the nanoparticles and FUS simply controls when light should emit by releasing the trapped energy (Fig. 1A), the material must be charged with 400-nm irradiation outside the brain before delivery into deep tissue and can only be used once after FUS.
Our solution to this problem is made possible by the pervasive nature of the blood circulation inside the body. Not only do blood vessels penetrate the entire brain, allowing delivery of ZnS nanoparticles to any region, but blood also passes through superficial vessels near the skin, providing accessible locations shallow enough for 400-nm light to penetrate and recharge these nanoscopic light sources as they circulate in the body. After the ZnS nanoparticles are injected intravenously, they can be charged by 400-nm light when passing through superficial vessels, pumped by the heart into cerebral vessels, and then gated by FUS to release the stored energy locally, all without exiting the blood circulation (Fig. 1B).
Sono-optogenetics: a rechargeable light source inside the body
We investigated the feasibility of such an approach first in an artificial circulatory system, where we were able to confirm that the nanoparticles light up, recharge, and light up again without losing the intensity (Fig. 1C). The average intensity of FUS-triggered light emission was above 1 mW/mm2; an amount sufficient to activate ChR2 (24).
We then carried out in vivo experiments, injecting the nanoparticles into the tail vein of mice that expressed ChR2 in the motor cortex. We positioned the shaved mouse head underneath a transducer that aimed ultrasound at the motor cortex in the brain (Fig. 1D). Then— without any surgery to the scalp, skull or the brain, and without any implantation of optical fiber or LED devices— we were able to evoke hindlimb motion in the animal, which was synchronized with the FUS pulses. The same animals did not exhibit any hindlimb motion before nanoparticle injection under the same stimulation protocol, nor did the wild-type mice demonstrate any hindlimb motion, regardless of nanoparticle injection (Fig. 1E).
In our approach, which we refer to as “sono-optogenetics,” the focal size of FUS in the mouse brain is ~500 μm. The localized light source thus has a similar cross-sectional diameter in the brain. Additionally, FUS can be turned on and off with sub-millisecond precision with a latency time of 10 ms before light emission. These features have made the virtual light source in our work similar in spatial and temporal resolutions to conventional fiber optics (3).
Sono-optogenetics provides a unique tool for rapidly screening different target regions in the brain for optogenetic neural modulation, because one can easily change the location of ultrasound focus. We envisage that this approach will also be able to be used in other organs that are usually refractory to fiber implantation (e.g., spinal cord, heart and lungs), and for any application in which a light source is needed deep in the body.
REFERENCES
- 1.Deisseroth K, Nat. Neurosci 18, 1213 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Airan RD, Thompson KR, Fenno LE, Bernstein H, Deisseroth K, Nature 458, 1025 (2009). [DOI] [PubMed] [Google Scholar]
- 3.Zhang F et al. , Nat. Protoc 5, 439 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salatino JW, Ludwig KA, Kozai TDY, Purcell EK, Nat. Biomed. Eng 1, 862 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hong GS, Lieber CM, Nat. Rev. Neurosci 20, 330 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim TI et al. , Science 340, 211 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hong GS et al. , Science 360, 1447 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hong GS, Antaris AL, Dai HJ, Nat. Biomed. Eng 1, 0010 (2017). [Google Scholar]
- 9.Tye KM, Deisseroth K, Nat. Rev. Neurosci 13, 251 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marshel JH et al. , Science 365, eaaw5202 (2019).31320556 [Google Scholar]
- 11.Lin JY, Knutsen PM, Muller A, Kleinfeld D, Tsien RY, Nat. Neurosci 16, 1499 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prakash R et al. , Nat. Methods 9, 1171 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen S et al. , Science 359, 679 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Chen S, Science 365, 456 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Wu X et al. , Proc. Natl. Acad. Sci. USA 116, 26332 (2019). [Google Scholar]
- 16.Wang JB, Aryal M, Zhong Q, Vyas DB, Airan RD, Neuron 100, 728 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Legon W et al. , Nat. Neurosci 17, 322 (2014). [DOI] [PubMed] [Google Scholar]
- 18.Airan R, Science 357, 6350 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Zagzebski JA, Essentials of ultrasound physics. (Mosby, 1996). [Google Scholar]
- 20.Liu LS et al. , Adv. Mater. Technol 4, 1800336 (2019). [Google Scholar]
- 21.Jeong SM, Song S, Lee SK, Ha NY, Adv. Mater 25, 6194 (2013). [DOI] [PubMed] [Google Scholar]
- 22.Wang XD et al. , Adv. Mater 27, 2324 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Qian X et al. , Adv. Mater 30, 1800291 (2018). [Google Scholar]
- 24.Klapoetke NC et al. , Nat. Methods 11, 338 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]


