Abstract
Purpose
To determine the change in Humphrey visual field and clinical parameters after minimally invasive glaucoma surgery combined with cataract surgery.
Patients and Methods
Patients undergoing minimally invasive glaucoma surgery combined with cataract surgery in a multicenter retrospective case series between 2013 and 2021 with reliable preoperative and 12 to 18 month postoperative visual field measurements were included. Devices included iStent, XEN, and Hydrus. Clinical parameters were compared with a generalized linear model with generalized estimating equations between preoperative and postoperative visits including best corrected visual acuity, intraocular pressure, number of glaucoma medications and visual fields. Visual field metrics included mean deviation (MD), pattern standard deviation (PSD), visual field index (VFI), and Collaborative Initial Glaucoma Treatment Study (CIGTS) score of total deviation probability and pattern deviation probability.
Results
Forty-four eyes from 39 patients were included. During the follow up period, visual acuity improved from 0.23±0.17 to 0.10±0.14 logMAR (mean ± standard deviation, p<0.001), number of glaucoma medications was reduced from 2.68±1.06 to 1.46±1.32 (p<0.001), and intraocular pressure decreased from 17.08±4.23 mmHg to 14.92±3.13 mmHg (p=0.003). Differences across devices were negligible. The only significant difference was a greater reduction in number of glaucoma medications in the XEN group (p<0.001). There were no significant changes in the global parameters of VFI, MD, PSD, or CIGTS.
Conclusion
Overall, minimally invasive glaucoma surgery combined with cataract surgery appears to be effective at stabilizing visual field function, reducing intraocular pressure, reducing number of glaucoma medications, and improving visual acuity over a 12 to 18 month follow-up period across MIGS devices.
Keywords: glaucoma, MIGS, visual field, cataract
Introduction
Glaucoma is rising in prevalence and intraocular pressure (IOP) is the only known modifiable risk factor.1,2 Ocular hypotensive medications can be effective, but their effect is limited by adherence, self-administration is often sub-optimal, and they can cause ocular surface toxicity and systemic adverse effects.3–7, In recent years, minimally invasive glaucoma surgery (MIGS) combined with cataract surgery has risen in popularity as an alternative to reduce eye drop burden or replace more invasive and complicated glaucoma surgeries.8–11 MIGS is aptly named given the micro incisional, conjunctival sparing, ab interno approach typically employed.12 Several different types of MIGS interventions have been developed and target various outflow mechanisms. These mechanisms include increasing trabecular outflow via trabecular meshwork bypass, subconjunctival filtration, increasing outflow via suprachoroidal shunts, and reducing aqueous production.12–14
Many studies have previously shown MIGS combined with cataract surgery to be safe and to have significant IOP reducing and medication lowering effects.10,13,15–17 Visual acuity (VA) of MIGS combined with cataract surgery is similar compared to cataract surgery alone.15 Current evidence assessing postoperative visual fields (VF) following MIGS has been limited, yet preliminary findings indicate MIGS better stabilizes VF compared to cataract surgery alone.18 The goal of this study is to evaluate clinical and VF outcomes following cataract surgery combined with iStent, XEN, and Hydrus.
Materials and Methods
This was a retrospective review of patients who underwent cataract surgery with concurrent implantation at two clinical sites: the University of California, San Francisco (UCSF) and Prism Eye Institute in Brampton, Ontario, Canada. This study was compliant with the Health Insurance Portability and Accountability Act and conformed to the tenets of the Declaration of Helsinki for research involving human participants. Institutional Review Board approval was obtained from UCSF and from the Trillium Health Partners Research Ethics Board.
Inclusion and Exclusion Criteria
This was a retrospective review of all patients who underwent cataract surgery with concurrent implantation of an iStent Trabecular Micro-Bypass device (Glaukos, Laguna Hills, California) or a Hydrus Microstent (Alcon, Fort Worth, Texas) at UCSF between January 2017 and March 2021, or a XEN Gel Stent (Allergan Inc., Dublin, Ireland) at the Prism Eye Institute between March 2013 and August 2018. The inclusion criteria were patients 18 years of age or older who had at least one preoperative VF, at least one postoperative VF, and follow-up data greater than 12 months. For follow-up period, the range was limited to 12–18 months. Patients were excluded if they had best corrected visual acuity worse than 20/200 in the operative eye, had prior trabeculectomy or glaucoma drainage device implantation, had an ocular or neurological comorbidity affecting their VF, or unreliable VF parameters with fixation losses or false negatives >33% or false positives >15%. Baseline demographics were collected including age, sex, lens status, and type of glaucoma. Number of glaucoma medications, VF metrics, IOP, and cup to disc ratio were recorded preoperatively and postoperatively. Preoperative central corneal thickness was recorded.
Humphrey Visual Field Testing
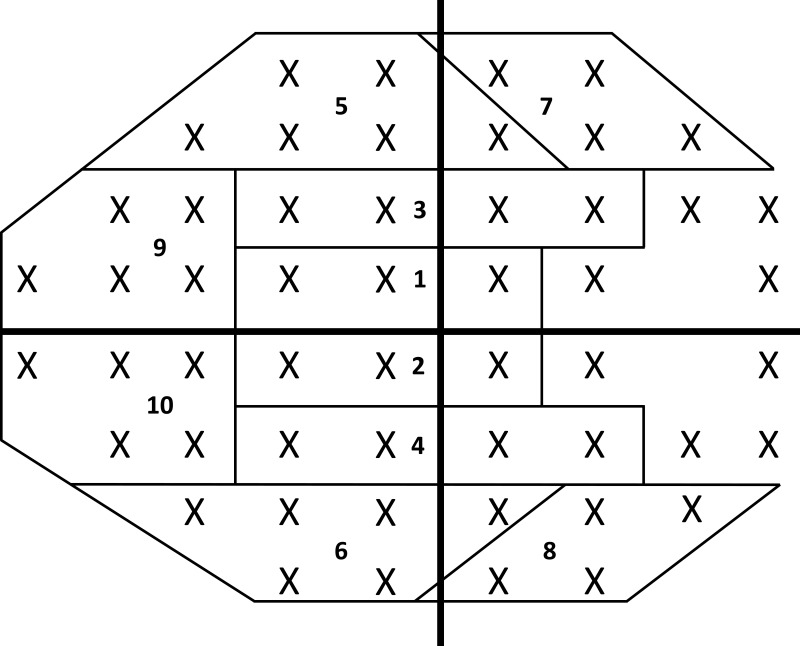
All visual field testing was performed using the Humphrey VF analyzer (Carl Zeiss Ophthalmic Systems, Dublin, California) using a size III white stimulus, with a Swedish Interactive Threshold Algorithm (SITA) standard strategy to complete 24–2 or 30–2 protocols. Tape was used to elevate the upper eyelid for patients with symptomatic ptosis. Global indices including mean deviation (MD), pattern standard deviation (PSD), and visual field index (VFI) were obtained. For specific sector analyses, pointwise total deviation (TD), pattern deviation (PD), TD probability (TDP), and PD probability (PDP) values were extracted using a validated, open-sourced script (https://pypi.org/project/hvf-extraction-script/).19 The script was used to extract pointwise data from the Ophthalmic Perimetry Values (OPV) DICOM files obtained directly from the Humphrey Visual Field device or extracted JPEG files. The sector wise values were then calculated by averaging the pointwise values across each sector based on prior established sectorial mapping.20 Each VF hemifield was divided into five subfields: central, paracentral, nasal, arcuate 1, and arcuate 2 (Figure 1), as previously described.20 For 30–2 VF test, only data for points falling within the 24–2 domain were collected. Analysis of VF defects was carried out based on the classification system proposed by the Ocular Hypertension Treatment study.21,22
Figure 1.
Humphrey VF 24–2 regional pattern. The VF pattern layout was divided into 10 subfields (five per hemifield) based on the characteristics of glaucomatous VF defect. Regions were outlined as superior central (1) or inferior central (2); superior paracentral (3) or inferior paracentral (4); superior arcuate 1 (5) or inferior arcuate 1 (6); superior arcuate 2 (7) or inferior arcuate 2 (8); and superior nasal (9) or inferior nasal (10). Regional analyses of the subfields were performed using total deviation (TD), total deviation probability (TDP), pattern deviation (PD), and pattern deviation probability (PDP).
Surgical Procedure
The type of MIGS performed was determined based on surgeon preference. Cataract surgery was performed prior to MIGS by phacoemulsification through a clear corneal incision. For iStent insertion, acetylcholine (Miochol-E, Bausch & Lomb, Rochester, NY) was given intracamerally, the nasal angle was visualized using a gonioprism, and the device was inserted using the manufacturer-provided handpiece, as previously described.23 Similarly, for Hydrus implantation, the nasal anterior chamber angle was visualized and the device was inserted into Schlemm’s canal, as previously described.24,25 For Xen stent placement, 0.1 mL mitomycin C 0.01% was injected in the intended quadrant of device insertion through the subconjunctiva. An ab interno approach was used where the anterior chamber angle was visualized and the device was inserted into the subconjunctival space using previously described technique.26 Bleb development was observed to confirm correct device placement. All patients were prescribed postoperative antibiotics for one week and a steroid taper over three to four weeks. Glaucoma drops were continued or discontinued at surgeon discretion.
Calculation of CIGTS Score
Calculation of Collaborative Initial Glaucoma Treatment Study (CIGTS) score was originally designed to measure the extent of VF defects longitudinally.27 In our study, CIGTS score was calculated for both the total deviation probability (TDP) and the pattern deviation probability (PDP) to calculate the global metrics CIGTS_TDP and CIGTS_PDP.
Statistical Analysis
Global VF analysis was performed for VFI, MD, and PSD. Analysis of each metric to compare preoperative to postoperative visits was performed using a generalized linear regression model with a generalized estimating equation, which accounted for the inter-eye correlation as well as longitudinal correlation among repeated measurements of the same eye. Each visit was treated as a categorical variable to avoid assuming a linear relationship between VF measurements and time. Comparisons across types of MIGS were analyzed using one-way analysis of variance (ANOVA). Change scores between visits for stent comparisons were calculated by subtracting postoperative values from preoperative values. Statistical analyses were performed using SAS version 9.4 (Cary, North Carolina). P-values <0.05 were considered statistically significant for all analyses. Bonferroni correction was applied for calculations with a large number of tests. Specifically, as there were 10 tests for each regional index, a corrected p-value threshold for statistical significance resulted in an alpha value of alpha/n, effectively necessitating a p<0.005 for significance, that was applied to the correspondingly resultant p-values.
Results
A total of 175 eyes of 153 patients were screened, of which 44 eyes of 39 patients were included in this study. Of the 131 eyes excluded, 65 eyes were due to not having adequate VF testing, 37 eyes due to history of prior glaucoma surgery, 22 eyes having preoperative BCVA worse than 20/200, and four eyes having ocular or neurological comorbidity affecting the visual field, and three eyes with visual field not typical for glaucomatous defects. Baseline demographic and clinical characteristics are shown in Table 1. Five patients (12.8%) underwent MIGS implantation in both eyes. The most common diagnosis was primary open-angle glaucoma (68.2%), and the most common type of MIGS was iStent (52.3%). The average patient age was 72.4 years at time of surgery, with 51.3% of patients being female. The average IOP was 17.1 mmHg and the average number of glaucoma drops was 2.7. Postoperatively, IOP, logarithm of the minimum angle of resolution (logMAR) visual acuity, and number of glaucoma medications were all significantly reduced from baseline (Table 2). Specific preoperative to postoperative changes included: IOP reduced from 17.1±4.2 mmHg to 14.9±3.1 mmHg (p=0.003), LogMAR decreased from 0.23±0.17 to 0.10±0.14 (p<0.001), and number of glaucoma drops decreased from 2.7±1.1 to 1.5±1.3 (p<0.001), a 44% reduction.
Table 1.
Baseline Patient and Ocular Characteristics
| Total (39 Patients and 44 Eyes) | |
|---|---|
| Age at time of surgery in years (mean ±SD)a | 72.4 (7.2) |
| Gender | |
| Male | 48.7% (19) |
| Female | 51.3% (20) |
| Number of eyes eligible for study | |
| 1 | 87.2% (34) |
| 2 | 12.8% (5) |
| Laterality | |
| Right | 45.4% (20) |
| Left | 54.5% (24) |
| Diagnosis | |
| Primary open angle glaucoma | 68.2% (30) |
| Primary angle closure glaucoma | 9.1% (4) |
| Combined mechanism glaucoma | 8.1% (4) |
| Normal-tension glaucoma | 4.5% (2) |
| Pigmentary glaucoma | 4.5% (2) |
| Pre-perimetric glaucoma | 2.3% (1) |
| Pseudoexfoliative glaucoma | 2.3% (1) |
| Procedure type | |
| iStent | 52.3% (23) |
| XEN | 31.8% (14) |
| Hydrus | 15.9% (7) |
| Central corneal thickness (microns) | |
| Mean ±SD (22 eyes) | 532.9 (30.6) |
| Cup to disc ratio | |
| Mean ±SD (41 eyes) | 0.73 (0.15) |
Notes: aFor the five patients with both eyes included in the study, age at time of surgery differed by 0–12 months.
Abbreviation: SD, standard deviation.
Table 2.
IOP, Visual Acuity, and Number of Glaucoma Medications Over Time
| Baseline | Follow-up | p-valuea | |
|---|---|---|---|
| IOP (mmHg) | |||
| Number of eyes | 44 | 35 | 0.003 |
| Mean (SD) | 17.1 (4.2) | 14.9 (3.1) | |
| BCVA (LogMAR) | |||
| Number of eyes | 44 | 35 | <0.001 |
| Mean (SD) | 0.23 (0.17) | 0.10 (0.14) | |
| Glaucoma drops (n) | |||
| Number of eyes | 44 | 35 | <0.001 |
| Mean (SD) | 2.7 (1.1) | 1.5 (1.3) |
Notes: aGeneralized linear model with generalized estimating equation (GEE) method was used for comparison of difference between baseline and follow-up.
Abbreviations: BCVA, best corrected visual acuity; IOP, intraocular pressure; LogMAR, logarithm of the minimum angle of resolution; SD, standard deviation.
The number of eyes available for analysis for the 12 to 18 month follow-up examination was 35, with nine eyes having follow-up greater than 18 months. Over the course of the study period, there was no significant change in the global parameters of VFI, MD, PSD, CIGTS_TDP, and CIGTS_PDP (Table 3).
Table 3.
Global Visual Field Change Over Time
| Baseline | Follow-up | p-valuea | |
|---|---|---|---|
| VFI (%) | |||
| Count (eyes) | 44 | 32 | 0.74 |
| Mean (SD) | 80 (20) | 80 (20) | |
| MD (dB) | |||
| Count (eyes) | 44 | 32 | 0.34 |
| Mean (SD) | −8.5 (6.7) | −7.8 (7.1) | |
| PSD (dB) | |||
| Count (eyes) | 44 | 32 | 0.78 |
| Mean (SD) | 6.5 (4.4) | 6.6 (4.7) | |
| CIGTS_TDP | |||
| Count (eyes) | 44 | 32 | 0.07 |
| Mean (SD) | 9.7 (6.3) | 8.3 (6.6) | |
| CIGTS_PDP | |||
| Count (eyes) | 44 | 32 | 0.31 |
| Mean (SD) | 4.9 (4.3) | 5.6 (4.8) |
Notes: aGeneralized linear model with generalized estimating equation (GEE) method was used for comparison of difference between baseline and follow-up.
Abbreviations: CIGTS_PDP, Collaborative Initial Glaucoma Treatment Study score on pattern deviation probability; CIGTS_TDP, Collaborative Initial Glaucoma Treatment Study score on total deviation probability; dB, decibel; MD, mean deviation; PSD, pattern standard deviation; VFI, visual field index.
Across MIGS device groups, XEN, iStent, and Hydrus, there were significant differences in preoperative BCVA, VFI, MD, and PSD. Baseline BCVA values were significantly different across stents (p=0.02), as were baseline VFI values (p=0.002), and baseline MD (p=0.001) and PSD (p<0.001) values (Tables 4 and 5). Also, significant differences were observed between groups for postoperative measurements. BCVA (p=0.006) values at postoperative measurements were significantly different between stent groups, as were the number of glaucoma drops (p<0.001), VFI (p=0.01), MD (p=0.003), and PSD (p=0.009) (Tables 4 and 5).
Table 4.
IOP, Visual Acuity, and Number of Glaucoma Medications Changes Across Stents and Over Time
| Baseline | Follow-up | Change from Baseline at Follow-up | p-valuea | |
|---|---|---|---|---|
| IOP (mmHg) | ||||
| XEN | ||||
| Count (eyes) | 14 | 12 | 12 | |
| Mean (SD) | 16.9 (4.5) | 14.4 (3.1) | −2.5 (4.6) | 0.09 |
| iStent | ||||
| Count (eyes) | 23 | 16 | 16 | |
| Mean (SD) | 17.1 (4.6) | 15.5 (3.6) | −1.6 (3.4) | 0.07 |
| Hydrus | ||||
| Count (eyes) | 7 | 7 | 7 | |
| Mean (SD) | 17.4 (3.3) | 14.9 (2.4) | −2.5 (2.6) | 0.07 |
| p-value | 0.97 | 0.79 | 0.97 | |
| BCVA (LogMAR) | ||||
| XEN | ||||
| Count (eyes) | 14 | 12 | 12 | |
| Mean (SD) | 0.29 (0.17) | 0.20 (0.15) | −0.09 (0.2) | 0.13 |
| iStent | ||||
| Count (eyes) | 23 | 16 | 16 | |
| Mean (SD) | 0.24 (0.17) | 0.06 (0.10) | −0.18 (0.1) | 0.0008 |
| Hydrus | ||||
| Count (eyes) | 7 | 7 | 7 | |
| Mean (SD) | 0.07 (0.12) | 0.02 (0.11) | −0.05 (0.1) | 0.44 |
| p-value | 0.02 | 0.006 | 0.18 | |
| Glaucoma drops (#) | ||||
| XEN | ||||
| Count (eyes) | 14 | 12 | 12 | |
| Mean (SD) | 3.1 (1.0) | 0.3 (0.7) | −2.8 (1.3) | 0.0010 |
| iStent | ||||
| Count (eyes) | 23 | 16 | 16 | |
| Mean (SD) | 2.7 (1.1) | 2.3 (1.3) | −0.4 (0.8) | 0.08 |
| Hydrus | ||||
| Count (eyes) | 7 | 7 | 7 | |
| Mean (SD) | 2.0 (1.0) | 1.6 (1.0) | −0.4 (1.1) | 0.47 |
| p-value | 0.09 | <0.001 | <0.001 |
Notes: aGeneralized linear model with generalized estimating equation (GEE) method was used for comparison of difference between baseline and follow-up.
Abbreviations: BCVA, best corrected visual acuity; IOP, intraocular pressure; LogMAR, logarithm of the minimum angle of resolution.
Table 5.
Visual Field Changes Across Stents and Over Time
| Baseline | Follow-up | Change from Baseline at Follow-up | p-valuea | |
|---|---|---|---|---|
| VFI (%) | ||||
| XEN | ||||
| Count (eyes) | 14 | 10 | 10 | |
| Mean (SD) | 70 (20) | 70 (10) | 0.0 (0.1) | 0.67 |
| iStent | ||||
| Count (eyes) | 23 | 15 | 15 | |
| Mean (SD) | 80 (20) | 80 (20) | 0.0 (0.1) | 0.60 |
| Hydrus | ||||
| Count (eyes) | 7 | 7 | 7 | |
| Mean (SD) | 90 (10) | 90 (10) | 0.0 (0.1) | 0.37 |
| p-value | 0.002 | 0.01 | 0.57 | |
| MD (dB) | ||||
| XEN | ||||
| Count (eyes) | 14 | 10 | 10 | |
| Mean (SD) | −13.3 (5.9) | −12.8 (5.4) | 0.5 (2.0) | 0.73 |
| iStent | ||||
| Count (eyes) | 23 | 15 | 15 | |
| Mean (SD) | −7.1 (6.3) | −7.2 (7.1) | −0.1 (2.4) | 0.98 |
| Hydrus | ||||
| Count (eyes) | 7 | 7 | 7 | |
| Mean (SD) | −3.5 (3.5) | −1.9 (3.8) | 1.6 (1.3) | 0.04 |
| p-value | 0.001 | 0.003 | 0.16 | |
| PSD (dB) | ||||
| XEN | ||||
| Count (eyes) | 14 | 10 | 10 | |
| Mean (SD) | 9.8 (3.7) | 9.9 (3.6) | 0.1 (1.4) | 0.97 |
| iStent | ||||
| Count (eyes) | 23 | 15 | 15 | |
| Mean (SD) | 5.5 (4.1) | 6.0 (4.9) | 0.5 (1.3) | 0.50 |
| Hydrus | ||||
| Count (eyes) | 7 | 7 | 7 | |
| Mean (SD) | 3.0 (2.3) | 3.4 (2.5) | 0.4 (0.6) | 0.20 |
| p-value | <0.001 | 0.009 | 0.86 |
Notes: aGeneralized linear model with generalized estimating equation (GEE) method was used for comparison of difference between baseline and follow-up.
Abbreviations: BCVA, best corrected visual acuity; dB, decibel; IOP, intraocular pressure; LogMAR, logarithm of the minimum angle of resolution; MD, mean deviation; PSD, pattern standard deviation; SD, standard deviation; VFI, visual field index.
Change from baseline to follow up in the different MIGS devices demonstrated significantly greater improvement for BCVA in the iStent group (p=0.0008), significantly greater decrease for number of glaucoma drops in the XEN group (p=0.001), and significantly greater improvement for MD in the Hydrus group (p=0.04), as compared to other groups. However, when comparing the baseline to follow up change values across stents, only the number of glaucoma drops change value was statistically significant compared to other stent change values for a given variable (p<0.001) (Tables 4 and 5).
We identified and compared local regions to determine which region had the worst VF function for each of the 10 indices at baseline and follow-up in addition to whether the pattern of VF defect remained the same or changed between baseline and follow-up (Table 6). No subfield was significantly different than others pre- or postoperatively.
Table 6.
Cross-sectional Analysis of Local Visual Field Metrics
| Inferior Arcuate 1 | Inferior Arcuate 2 | Inferior Central | Inferior Nasal | Inferior Paracentral | Superior Arcuate 1 | Superior Arcuate 2 | Superior Central | Superior Nasal | Superior Paracentral | p-valuea | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | |||||||||||
| TD | |||||||||||
| n | 44 | 44 | 44 | 44 | 44 | 44 | 44 | 44 | 44 | 44 | 0.18 |
| Mean (SD) | −8.59 (8.79) | −7.63 (8.36) | −7.66 (8.55) | −10.41 (10.08) | −9.49 (10.30) | −8.58 (8.96) | −6.63 (7.28) | −8.72 (8.60) | −10.33 (10.27) | −9.58 (9.35) | |
| PD | |||||||||||
| n | 42 | 42 | 42 | 42 | 42 | 42 | 42 | 42 | 42 | 42 | 0.21 |
| Mean (SD) | −5.62 (6.52) | −4.73 (6.81) | −5.02 (7.40) | −7.43 (7.91) | −6.33 (8.20) | −6.10 (7.09) | −4.29 (5.57) | −5.67 (6.89) | −7.54 (8.55) | −6.56 (7.24) | |
| TDP | |||||||||||
| n | 44 | 44 | 44 | 44 | 44 | 44 | 44 | 44 | 44 | 44 | 0.01 |
| Mean (SD) | 2.10 (1.55) | 1.79 (1.53) | 2.08 (1.59) | 2.21 (1.58) | 2.41 (1.64) | 1.83 (1.64) | 1.38 (1.49) | 2.32 (1.61) | 2.06 (1.69) | 2.40 (1.64) | |
| PDP | |||||||||||
| n | 44 | 44 | 44 | 44 | 44 | 44 | 44 | 44 | 44 | 44 | 0.09 |
| Mean (SD) | 1.05 (1.38) | 0.77 (1.28) | 0.98 (1.38) | 1.32 (1.63) | 1.22 (1.42) | 1.06 (1.53) | 0.63 (1.20) | 1.13 (1.37) | 1.32 (1.64) | 1.27 (1.48) | |
| Follow-up | |||||||||||
| TD | |||||||||||
| n | 32 | 32 | 32 | 32 | 32 | 32 | 32 | 32 | 32 | 32 | 0.34 |
| Mean (SD) | −8.22 (9.77) | −6.27 (8.57) | −6.47 (9.99) | −8.81 (10.03) | −9.09 (11.42) | −8.10 (9.14) | −7.23 (7.78) | −7.28 (8.04) | −9.94 (10.85) | −8.98 (9.77) | |
| PD | |||||||||||
| n | 32 | 32 | 32 | 32 | 32 | 32 | 32 | 32 | 32 | 32 | 0.36 |
| Mean (SD) | −6.89 (8.11) | −4.91 (7.02) | −5.17 (8.98) | −7.50 (8.34) | −7.84 (9.95) | −7.22 (8.03) | −5.92 (6.16) | −6.09 (7.24) | −8.66 (9.53) | −7.62 (8.37) | |
| TDP | |||||||||||
| n | 32 | 32 | 32 | 32 | 32 | 32 | 32 | 32 | 32 | 32 | 0.04 |
| Mean (SD) | 1.91 (1.55) | 1.41 (1.54) | 1.44 (1.60) | 1.74 (1.61) | 2.14 (1.64) | 1.69 (1.67) | 1.45 (1.55) | 1.77 (1.66) | 1.88 (1.75) | 2.03 (1.72) | |
| PDP | |||||||||||
| n | 32 | 32 | 32 | 32 | 32 | 32 | 32 | 32 | 32 | 32 | 0.054 |
| Mean (SD) | 1.32 (1.55) | 0.90 (1.43) | 0.81 (1.48) | 1.18 (1.61) | 1.51 (1.63) | 1.33 (1.60) | 1.03 (1.39) | 1.16 (1.39) | 1.38 (1.73) | 1.44 (1.66) |
Notes: aGeneralized linear model with generalized estimating equation (GEE) method was used for comparison of difference between baseline and follow-up.
Abbreviations: n, number; PD, pattern deviation; PDP, pattern deviation probability; SD, standard deviation; TD, total deviation; TDP, total deviation probability.
Changes in regional TD, PD, TPD, and PDP VF index from baseline to follow-up are shown in Supplemental Table 1. None of the changes were statistically significant.
Discussion
As MIGS devices gain more popularity, it will be important to better characterize visual field changes after surgery. Our study found that MIGS combined with cataract surgery yielded an IOP reduction of 2.2 mmHg, a 1.2 reduction of number of glaucoma medications, a 0.13 logMAR reduction of BCVA, and stable VF function on regional and global analysis over a 12 to 18 month follow-up period. Furthermore, when comparing change values from baseline to follow-up across stent groups, the only significant difference was found in the change in number of glaucoma drops, with XEN exhibiting the largest decrease at follow-up.
MIGS has been shown to reduce IOP significantly, with a stronger effect than glaucoma medications alone and over a longer period of time.28 Furthermore, the efficacy of MIGS in IOP and medication reduction has been demonstrated with or without the combination of cataract surgery.29–31 Arriola-Villabobos et al demonstrated a 17.5% drop in IOP with a follow-up of five years in an iStent only cohort.31 In addition, Fea et al demonstrated that phacoemulsification combined with iStent produced a 3.1 mmHg decrease in IOP over a 15 month period, which was significantly greater than the decrease with phacoemulsification alone, which produced a 1.6 mmHg decrease in IOP.32 It has been shown that multiple iStent implantations can produce an even greater reduction in IOP, however, we only used data from single iStent implantation in our study.33 A two-year study on Hydrus devices also reported significant decrease in IOP compared to phacoemulsification alone, with a reduction of greater than or equal to 20% of the IOP in 77% of eyes with Hydrus use.34 Lastly, a systematic review of XEN gel stent found an overall IOP reduction of 35% across their meta-analysis.35 We found that patients who underwent either of the three stent implantations had an average of 2.2 mmHg drop in IOP, or a 12.9% decrease, within 12 to 18 months. Notably, with a baseline IOP of 17.1 mmHg and a final average number of glaucoma drops of 1.5, it is unsurprising there was a modest IOP reduction. Furthermore, we found the change in IOP over our follow-up period to be similar across all three devices. While the decrease in IOP in the entire MIGS cohort was significant, individual analysis of stents yielded partial significance, which is most likely attributable to the small sample sizes of the individual groups.
MIGS has also been shown to reduce number of glaucoma medications significantly. A systematic review of XEN stent and cataract surgery revealed an average reduction of 1.86 drops with a range of 1.4 to 3.1.35 In addition, a meta-analysis of iStent implants revealed an average medication reduction of 1.2 for a follow-up time between 36 and 60 months.36 Our study demonstrated an average number of glaucoma medication reduction of 1.2, with XEN being significantly different than the other two stent groups with a reduction of 2.8 number of medications compared to 0.4 in the iStent group and the Hydrus group (Table 4). One explanation for this result could be that XEN stents are inherently superior in terms of lowering IOP compared to iStent and Hydrus. However, the change in number of glaucoma medications is based on clinical decision making and is a value that is dependent on and balanced with IOP target goal, which varies across glaucoma diagnoses and patient and physician clinical decision making. Notably, the XEN group in our study was located at a different care center than the Hydrus and iStent groups, which could contribute to the differences in balance of medication reduction and IOP with stent placement. Altogether, similarly to IOP reduction, number of glaucoma medication reduction across the literature post iStent, Hydrus, and XEN exhibited similar results to our findings, with an average of one-to-three medications reduced after implantation.31–35
Research on VF stability after MIGS is forthcoming. A retrospective study of XEN implants found MD to be stable across various types of glaucoma 12 months after surgery.37 Another study on first-generation iStent showed MD decline over five years to be insignificant, in accordance with the noted stability of our findings.38 Of note, studies with newer versions of the stents employed in this study, namely XEN 45 and iStent inject, demonstrated significant reduction in visual field loss compared to control.39,40 Moreover, Liu et al demonstrated that the superior hemifield was most affected over a three-year follow-up period in glaucoma patients, although this analysis was after GDD implantation.20 Our study cohort demonstrated no significant global or regional change in VF metrics over the follow-up period, which indicates a preservation of visual function after MIGS. These findings are contrasted against the regular rate of VF change in glaucomatous patients, which typically range from −0.5 to −0.4 dB per year in mild and moderate glaucoma.41 Our patient population had an average MD at baseline of −8.5 dB, which is representative of moderate stage glaucoma. While we did find that our global visual field outcome metrics were different at baseline between MIGS groups, when comparing change over follow-up period across MIGS groups, XEN, iStent, and Hydrus exhibited no significant change in VFI, MD, or PSD with the exception of mean Hydrus MD change (p=0.04). This change can most likely be attributed to the small sample size of our Hydrus group (n=7), which also displayed significant overlap in mean plus or minus standard deviation between baseline and follow-up. Furthermore, comparison of change from baseline to follow up across MIGS groups did not find the Hydrus change to be statistically different from the other groups (p=0.16). The iStent group was found to have significantly improved BCVA compared to XEN and Hydrus. The mechanism for this finding is unknown, and a comparison of change values across stents did not indicate this change to be significant (p=0.18). Lastly, we did not find any subfield to be significantly worse than others at either baseline or at follow up in our study after applying Bonferroni correction.
This study has several limitations. The study’s retrospective nature introduces the possibility of selection bias and problems related to incomplete data and attrition. Expected changes in visual acuity following cataract surgery may confound glaucoma-related visual field changes, and future studies could consider a post-cataract surgery visual field as baseline. In addition, the 12 to 18 month postoperative follow-up period is short. However, this study provides strong preliminary data that could serve as the foundation of other prospective clinical trials, which are currently lacking for MIGS. Future studies looking at longer-term outcomes, especially with VF analysis, are needed to continue to validate treatment effect and visual function changes in MIGS implantation.
Conclusion
We present the first study to describe detailed VF and clinical metrics over a longitudinal postoperative period following MIGS with cataract surgery. In our cohort utilizing three different MIGS devices, the number of glaucoma medications decreased, the average IOP decreased, and the global and sector-based VF metrics largely remained stable Additional studies are needed to better understand the long-term clinical and VF outcomes following MIGS.
Acknowledgments
All authors attest that they meet the current ICMJE criteria for authorship. A subset of the data contained in this manuscript was presented at the Association for Research in Vision and Ophthalmology 2022 Annual Meeting as a poster presentation with interim findings. The poster’s abstract was published in ”Poster Abstracts” in Investigative Ophthalmology & Visual Science: https://iovs.arvojournals.org/article.aspx?articleid=2781501
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Ederer F, VanVeldhuisen P. The advanced glaucoma intervention study (AGIS): 7. the relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130(4):429–440. doi: 10.1016/S0002-9394(00)00538-9 [DOI] [PubMed] [Google Scholar]
- 2.Allison K, Patel D, Alabi O. Epidemiology of glaucoma: the past, present, and predictions for the future. Cureus. 2020. doi: 10.7759/cureus.11686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zanden JAV, Valuck RJ, Bunch CL, Perlman JI, Anderson C, Wortman GI. Systemic adverse effects of ophthalmic β-Blockers. Ann Pharmacother. 2001;35(12):1633–1637. doi: 10.1345/aph.18464 [DOI] [PubMed] [Google Scholar]
- 4.Costagliola C, Sbordone M, Gandolfi S, Cesari L, Furneri G, Fea AM. Minimally invasive surgery in mild-to-moderate glaucoma patients in Italy: is it time to change? Clin Ophthalmol. 2020;14:2639–2655. doi: 10.2147/OPTH.S264839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nordstrom BL, Friedman DS, Mozaffari E, Quigley HA, Walker AM. Persistence and adherence with topical glaucoma therapy. Am J Ophthalmol. 2005;140(4):598.e1–598.e11. doi: 10.1016/j.ajo.2005.04.051 [DOI] [PubMed] [Google Scholar]
- 6.Belhassen M, Laforest L, Licaj I, Van Ganse É. Étude observationnelle sur l’adhésion précoce aux traitements hypotonisants anti-glaucomateux. [Observational study on early adherence to anti-glaucoma treatments]. Therapies. 2016;71(5):491–499. doi: 10.1016/j.therap.2016.02.033 [DOI] [PubMed] [Google Scholar]
- 7.Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311(18):1901. doi: 10.1001/jama.2014.3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agrawal P, Bradshaw SE. Systematic literature review of clinical and economic outcomes of micro-invasive glaucoma surgery (MIGS) in primary open-angle glaucoma. Ophthalmol Ther. 2018;7(1):49–73. doi: 10.1007/s40123-018-0131-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang SA, Mitchell W, Hall N, et al. Trends and usage patterns of minimally invasive glaucoma surgery in the United States. Ophthalmol Glaucoma. 2021;4(6):558–568. doi: 10.1016/j.ogla.2021.03.012 [DOI] [PubMed] [Google Scholar]
- 10.Clement CI, Howes F, Ioannidis AS, Shiu M, Manning D. One-year outcomes following implantation of second-generation trabecular micro-bypass stents in conjunction with cataract surgery for various types of glaucoma or ocular hypertension: multicenter, multi-surgeon study. Clin Ophthalmol. 2019;13:491–499. doi: 10.2147/OPTH.S187272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gedde SJ, Herndon LW, Brandt JD, Budenz DL, Feuer WJ, Schiffman JC. Postoperative complications in the Tube Versus Trabeculectomy (TVT) study during five years of follow-up. Am J Ophthalmol. 2012;153(5):804–814.e1. doi: 10.1016/j.ajo.2011.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ansari E. An Update on Implants for Minimally Invasive Glaucoma Surgery (MIGS). Ophthalmol Ther. 2017;6(2):233–241. doi: 10.1007/s40123-017-0098-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.SooHoo JR, Seibold LK, Radcliffe NM, Kahook MY. Minimally invasive glaucoma surgery: current implants and future innovations. Can J Ophthalmol. 2014;49(6):528–533. doi: 10.1016/j.jcjo.2014.09.002 [DOI] [PubMed] [Google Scholar]
- 14.Schehlein EM, Kaleem MA, Swamy R, Saeedi OJ. Microinvasive glaucoma surgery: an evidence-based assessment. Expert Rev Ophthalmol. 2017;12(4):331–343. doi: 10.1080/17469899.2017.1335597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarkisian SR, Radcliffe N, Harasymowycz P, et al. Visual outcomes of combined cataract surgery and minimally invasive glaucoma surgery. J Cataract Refract Surg. 2020;46(10):1422–1432. doi: 10.1097/j.jcrs.0000000000000317 [DOI] [PubMed] [Google Scholar]
- 16.Samuelson TW, Sarkisian SR, Lubeck DM, et al. Prospective, randomized, controlled pivotal trial of an ab interno implanted trabecular micro-bypass in primary open-angle glaucoma and cataract. Ophthalmology. 2019;126(6):811–821. doi: 10.1016/j.ophtha.2019.03.006 [DOI] [PubMed] [Google Scholar]
- 17.Pyfer MF, Gallardo M, Campbell A, et al. Suppression of Diurnal (9AM–4PM) IOP fluctuations with minimally invasive glaucoma surgery: an analysis of data from the prospective, multicenter, single-arm GEMINI study. Clin Ophthalmol. 2021;15:3931–3938. doi: 10.2147/OPTH.S335486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montesano G, Ometto G, Crabb DP, Gazzard G. Five-year visual field outcomes of the HORIZON trial. Invest Ophthalmol Vis Sci. 2022;63(7):4383. [Google Scholar]
- 19.Saifee M, Wu J, Liu Y, et al. Development and validation of automated visual field report extraction platform using computer vision tools. Front Med. 2021;8:625487. doi: 10.3389/fmed.2021.625487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Q, Saifee M, Yu Y, et al. Evaluation of long-term visual field function in patients undergoing glaucoma drainage device implantation. Am J Ophthalmol. 2020;216:44–54. doi: 10.1016/j.ajo.2020.03.025 [DOI] [PubMed] [Google Scholar]
- 21.Atalay E, Nongpiur ME, Yap SC, et al. Pattern of visual field loss in primary angle-closure glaucoma across different severity levels. Ophthalmology. 2016;123(9):1957–1964. doi: 10.1016/j.ophtha.2016.05.026 [DOI] [PubMed] [Google Scholar]
- 22.Keltner JL. Classification of visual field abnormalities in the ocular hypertension treatment study. Arch Ophthalmol. 2003;121(5):643. doi: 10.1001/archopht.121.5.643 [DOI] [PubMed] [Google Scholar]
- 23.Nichamin LD. Glaukos iStent trabecular micro-bypass. Middle East Afr J Ophthalmol. 2009;16(3):138–140. doi: 10.4103/0974-9233.56227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pfeiffer N, Garcia-Feijoo J, Martinez-de-la-Casa JM, et al. A randomized trial of a schlemm’s canal microstent with phacoemulsification for reducing intraocular pressure in open-angle glaucoma. Ophthalmology. 2015;122(7):1283–1293. doi: 10.1016/j.ophtha.2015.03.031 [DOI] [PubMed] [Google Scholar]
- 25.Ahmed IIK, Rhee DJ, Jones J, et al. Three-Year Findings of the HORIZON Trial. Ophthalmology. 2021;128(6):857–865. doi: 10.1016/j.ophtha.2020.11.004 [DOI] [PubMed] [Google Scholar]
- 26.Bilgic A, Eltanamly R, Osman A. XEN glaucoma implant with mitomycin c 1-year follow-up: result and complications. J Ophthalmol. 2017;2017:1–5. doi: 10.1155/2017/5457246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Musch DC, Gillespie BW, Lichter PR, Niziol LM, Janz NK. Visual field progression in the collaborative initial glaucoma treatment study. Ophthalmology. 2009;116(2):200–207.e1. doi: 10.1016/j.ophtha.2008.08.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giamporcaro B, Sanchis JI, Chang L, et al. Prospective unmasked randomized evaluation of the iStent inject® versus two ocular hypotensive agents in patients with primary open-angle glaucoma. Clin Ophthalmol. 2014:875. doi: 10.2147/OPTH.S59932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hengerer FH, Auffarth GU, Riffel C, Conrad-Hengerer I. Prospective, non-randomized, 36-month study of second-generation trabecular micro-bypass stents with phacoemulsification in eyes with various types of glaucoma. Ophthalmol Ther. 2018;7(2):405–415. doi: 10.1007/s40123-018-0152-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Samuelson TW, Katz LJ, Wells JM, Duh YJ, Giamporcaro JE. Randomized evaluation of the trabecular micro-bypass stent with phacoemulsification in patients with glaucoma and cataract. Ophthalmology. 2011;118(3):459–467. doi: 10.1016/j.ophtha.2010.07.007 [DOI] [PubMed] [Google Scholar]
- 31.Arriola-Villalobos P, Martinez-de-la-Casa JM, Diaz-Valle D, Morales-Fernandez L, Fernandez-Perez C, Garcia-Feijoo J. Glaukos iStent inject® trabecular micro-bypass implantation associated with cataract surgery in patients with coexisting cataract and open-angle glaucoma or ocular hypertension: a long-term study. J Ophthalmol. 2016;2016:1–7. doi: 10.1155/2016/1056573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fea AM. Phacoemulsification versus phacoemulsification with micro-bypass stent implantation in primary open-angle glaucoma: randomized double-masked clinical trial. J Cataract Refract Surg. 2010;36(3):407–412. doi: 10.1016/j.jcrs.2009.10.031 [DOI] [PubMed] [Google Scholar]
- 33.Belovay GW, Naqi A, Chan BJ, Rateb M, Ahmed IIK. Using multiple trabecular micro-bypass stents in cataract patients to treat open-angle glaucoma. J Cataract Refract Surg. 2012;38(11):1911–1917. doi: 10.1016/j.jcrs.2012.07.017 [DOI] [PubMed] [Google Scholar]
- 34.Samuelson TW, Chang DF, Marquis R, et al. A schlemm canal microstent for intraocular pressure reduction in primary open-angle glaucoma and cataract. Ophthalmology. 2019;126(1):29–37. doi: 10.1016/j.ophtha.2018.05.012 [DOI] [PubMed] [Google Scholar]
- 35.Chen XZ, Liang ZQ, Yang KY, et al. The Outcomes of XEN gel stent implantation: a systematic review and meta-analysis. Front Med. 2022;9:804847. doi: 10.3389/fmed.2022.804847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Healey PR, Clement CI, Kerr NM, Tilden D, Aghajanian L. Standalone iStent trabecular micro-bypass glaucoma surgery: a systematic review and meta-analysis. J Glaucoma. 2021. doi: 10.1097/IJG.0000000000001805 [DOI] [PubMed] [Google Scholar]
- 37.Schargus M, Theilig T, Rehak M, Busch C, Bormann C, Unterlauft JD. Outcome of a single XEN microstent implant for glaucoma patients with different types of glaucoma. BMC Ophthalmol. 2020;20(1):490. doi: 10.1186/s12886-020-01764-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ansari E. 5-year outcomes of single iStent (G1) trabecular microbypass implantation with phacoemulsification in moderately advanced primary open angle glaucoma. PLoS One. 2021;16(9):e0257015. doi: 10.1371/journal.pone.0257015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hengerer FH, Auffarth GU, Conrad-Hengerer I. iStent inject trabecular micro-bypass with or without cataract surgery yields sustained 5-year glaucoma control. Adv Ther. 2022;39(3):1417–1431. doi: 10.1007/s12325-021-02039-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schlatter A, Rauchegger T, Schmid E, Teuchner B. Effects of glaucoma surgery on visual field progression in open‐angle glaucoma considering the floor effect. Acta Ophthalmol. 2021;15048. doi: 10.1111/aos.15048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Forchheimer I, de Moraes CG, Teng CC, et al. Baseline mean deviation and rates of visual field change in treated glaucoma patients. Eye. 2011;25(5):626–632. doi: 10.1038/eye.2011.33 [DOI] [PMC free article] [PubMed] [Google Scholar]