Significance Statement
Biomarker studies in the setting of CKD have increased considerably within the past 15 years, but vary significantly by design and clinical context. The authors conducted a systematic review and meta-analysis to summarize the prognostic value of preclinical plasma and urine biomarkers for CKD outcomes (incident CKD, CKD progression, or incident ESKD), including 129 studies in the meta-analysis. Pooled risk ratios (RRs) and 95% confidence intervals (CIs) among some of the most studied CKD biomarkers were 2.17 (95% CI, 1.91 to 2.47) for plasma TNFR1, 1.21 (95% CI, 1.15 to 1.28) for plasma FGF23, 2.07 (95% CI, 1.82 to 2.34) for plasma TNFR2, 1.10 (95% CI, 1.05 to 1.16) for urine KIM-1, and 1.12 (95% CI, 1.06 to 1.19) for urine NGAL. The study’s findings suggest these biomarkers merit assessment of their performance in clinical practice.
Keywords: chronic kidney disease, chronic allograft failure
Abstract
Background
Sensitive and specific biomarkers are needed to provide better biologic insight into the risk of incident and progressive CKD. However, studies have been limited by sample size and design heterogeneity.
Methods
In this assessment of the prognostic value of preclinical plasma and urine biomarkers for CKD outcomes, we searched Embase (Ovid), MEDLINE ALL (Ovid), and Scopus up to November 30, 2020, for studies exploring the association between baseline kidney biomarkers and CKD outcomes (incident CKD, CKD progression, or incident ESKD). We used random-effects meta-analysis.
Results
After screening 26,456 abstracts and 352 full-text articles, we included 129 studies in the meta-analysis for the most frequently studied plasma biomarkers (TNFR1, FGF23, TNFR2, KIM-1, suPAR, and others) and urine biomarkers (KIM-1, NGAL, and others). For the most frequently studied plasma biomarkers, pooled RRs for CKD outcomes were 2.17 (95% confidence interval [95% CI], 1.91 to 2.47) for TNFR1 (31 studies); 1.21 (95% CI, 1.15 to 1.28) for FGF-23 (30 studies); 2.07 (95% CI, 1.82 to 2.34) for TNFR2 (23 studies); 1.51 (95% CI, 1.38 to 1.66) for KIM-1 (18 studies); and 1.42 (95% CI, 1.30 to 1.55) for suPAR (12 studies). For the most frequently studied urine biomarkers, pooled RRs were 1.10 (95% CI, 1.05 to 1.16) for KIM-1 (19 studies) and 1.12 (95% CI, 1.06 to 1.19) for NGAL (19 studies).
Conclusions
Studies of preclinical biomarkers for CKD outcomes have considerable heterogeneity across study cohorts and designs, limiting comparisons of prognostic performance across studies. Plasma TNFR1, FGF23, TNFR2, KIM-1, and suPAR were among the most frequently investigated in the setting of CKD outcomes.
CKD is one of the leading causes of death worldwide. In 2017, the prevalence of CKD was estimated to be 15% in the United States and 9% globally, corresponding to over 38 million and 850 million patients with CKD, respectively.1,2 Given the significant burden of disease and increased risk for cardiovascular events and death, accurate prediction tools are needed to anticipate the onset of CKD and identify patients at increased risk for developing ESKD.3 The Kidney Disease: Improving Global Outcomes 2012 guidelines recommend the use of serum creatinine and albuminuria for assessment and risk-stratification of patients with CKD.4 However, intrinsic limitations to these metrics such as delayed onset, prognostic heterogeneity, and high intra- and interindividual variability pose major challenges to prediction of adverse events.5
Over the last 15 years, there has been an explosion in the discovery of preclinical biomarkers of kidney disease reflecting different biologic pathway disturbances implicated in CKD development.6,7 These biomarkers expand the evaluation of kidney health beyond glomerular dysfunction as measured by creatinine-based metrics and glomerular injury (albuminuria), thereby exploring tubulointerstitial damage, general inflammation, and repair. However, when these preclinical biomarkers are assessed in clinical studies, they exhibit varying strengths or attenuated associations with CKD outcomes after adjusting for confounders.8–15 These results may in part be due to heterogeneous study designs, lack of adequate follow-up intervals, or different biomarker platforms used across studies. Hence, the reliability of preclinical biomarkers to independently prognosticate kidney events across CKD groups and provide meaningful clinical insights is unclear.
In view of the marked heterogeneity of available studies and the need to define the clinical utility of preclinical biomarkers in the prognosis of CKD outcomes (CKD incidence, progression, or incident ESKD), we conducted a systematic review and meta-analysis of the literature. We comprehensively assessed the data on the most frequent plasma and urine biomarkers of kidney inflammation, injury, and health and their association with CKD outcomes in diverse clinical scenarios. We hypothesize that top candidate plasma and urine biomarkers would exhibit significant and independent associations with CKD outcomes.
Methods
Search Strategy and Selection Criteria
We conducted a literature search in the databases Embase (Ovid), MEDLINE ALL (Ovid), and Scopus (Elsevier) for articles published from database inception through November 30, 2020.16 The results are reported in accordance to Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.17 The search captured studies evaluating the association(s) between single or multiple kidney biomarkers and any of the following CKD outcomes: incident CKD, CKD progression, or incident ESKD (e.g., initiation of chronic hemodialysis, peritoneal dialysis requirement, or transplant). We used outcome definitions as defined by investigators in their studies. The full search strategy can be seen in Supplemental Appendix I, Supplemental Table 1. No restrictions were placed on publication date or language. Additional manuscripts included for final review were obtained by examination of references from index studies, an environmental search of the literature, or personal communications with investigators.
Two investigators (CL and ND) screened search results using the software Covidence.18 We included the following study designs: prospective cohort studies, retrospective cohort studies, nested case-control studies, and post hoc analyses of randomized clinical trials. We further screened for studies with adjudicated or defined CKD outcomes over a span of ≥1 year of follow-up, had a sample size of ≥100 participants, and examined baseline kidney biomarker(s) at least once during the study period. Studies were excluded if they included nonhuman subjects, were cross-sectional in design, or were exclusive to biomarkers that are widely available in clinical practice. Studies with sample sizes of <100 participants were excluded due to the potential for selection bias and the inherent inability to provide a reasonably precise adjusted effect size due to the limited power to adjust for key covariates. Studies without results in the form of odds ratios (ORs), relative risks (RRs), rate ratios, or hazards ratios (HRs) were excluded from the meta-analysis. Any screening discrepancies were resolved by both reviewers. Full-text screening for eligibility was performed by CL, ND, and GM. All data were entered into a standardized collection form and checked for accuracy by at least two reviewers. All results were presented either as HRs, ORs, incidence rate ratios, RR, or beta coefficients in regressions for eGFR decline with corresponding 95% confidence intervals (95% CI). No gray literature sources were assessed.
Statistical Methods
Results were reported by study cohort for manuscripts that reported on separate associations in multiple cohorts within the same publication. Publication bias was assessed through funnel plots. We used the DerSimonian and Laird method with inverse-variance random-effect models for estimates of heterogeneity.19 The I2 test statistic was used to quantify the magnitude of heterogeneity. I2 values >50% were considered as having significant heterogeneity per the Cochrane Handbook for Systematic Review of Interventions.20 In this study, we focused on the 12 most frequently studied biomarkers in plasma and 12 most frequently studied biomarkers in urine on the basis of the number of available studies in individual cohorts for systematic review.
Among the most frequently studied plasma and urine biomarkers, we assessed between-group heterogeneity (τ2) by predetermined subgroups: study design (randomized clinical trial versus outpatient cohort versus inpatient cohort), diabetes mellitus status (all type 1 diabetes, all type 2 diabetes, no diabetes, mixed), cohort age (adult versus pediatric cohorts), cohort race/ethnicity (all Black, all Native American, all Asian or mixed), method of assessing the association with outcome per biomarker increment (per log2, natural log, per SD, or quantiles only), biomarker measurement platform, and measure of association. Given the heterogeneity of outcomes, we reported pooled RRs for both composite outcomes and individual outcome types (incident CKD versus CKD progression versus incident ESKD) when available. All analyses were performed in Stata (version 14; StataCorp LLC, College Station, TX). All tests of statistical significance were two-sided, with P<0.05 considered significant.
Results
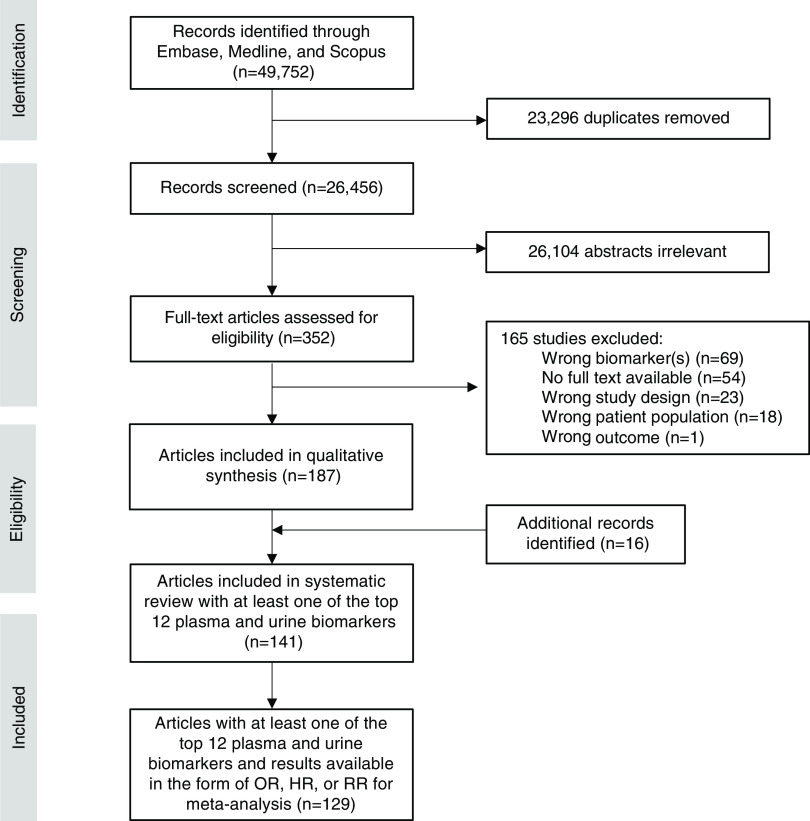
The search algorithm identified 16,523 articles from Embase (Ovid), 16,894 from Scopus, and 16,335 from Medline (Ovid), for a total of 49,752 studies (Figure 1). After removal of duplicates (n=23,296) in Covidence, 26,456 studies were available for abstract screening. After abstract screening and full-text screening, 187 total manuscripts were available for systematic review (141 manuscripts for the top 12 plasma and top urine biomarkers) and 129 manuscripts were included in meta-analyses for the top 12 most frequently studied plasma and top 12 most frequently studied urine biomarkers (Figure 2). All analysis and results focused on the top 12 most frequently studied plasma and urine biomarkers with the most qualifying studies for meta-analysis.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-analyses flow chart of study selection for systematic review and meta-analysis.
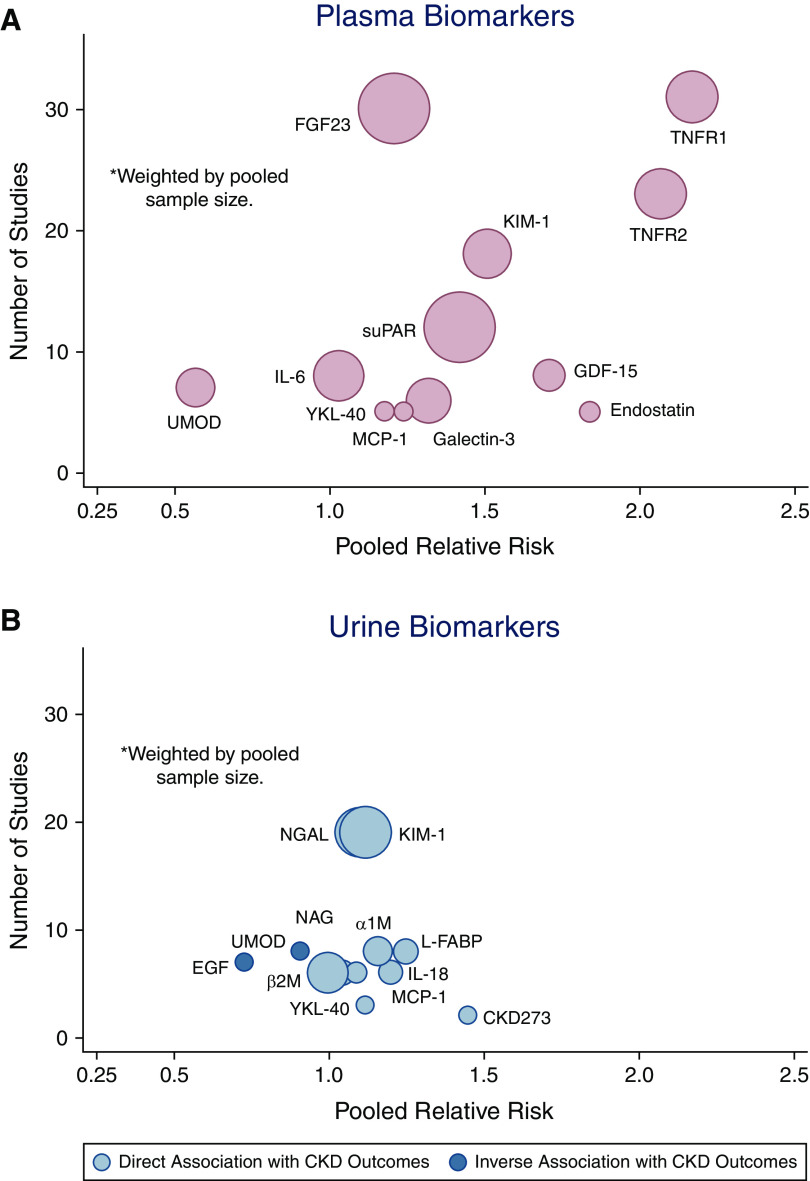
Figure 2.
Number of studies and pooled relative risks for 12 most frequently studied biomarkers for CKD outcomes. (A) plasma; (B) urine.
Plasma Biomarkers
The 12 most studied plasma biomarkers were as follows: TNFR1 (n=31), FGF23 (n=30), TNFR2 (n=23), KIM-1 (n=18), suPAR (n=12), GDF-15 (n=8), IL-6 (n=8), UMOD (n=7), Endostatin (n=5), MCP-1 (n=5), Galectin-3 (n=6), and YKL-40 (n=5). Funnel plots assessing variability can be seen in Supplemental Appendix I, Supplemental Figure 1, A–L. Details of all studies, with and without quantitative results for meta-analysis, can be seen in Supplemental Appendix II, Supplemental Table 1, A–L.
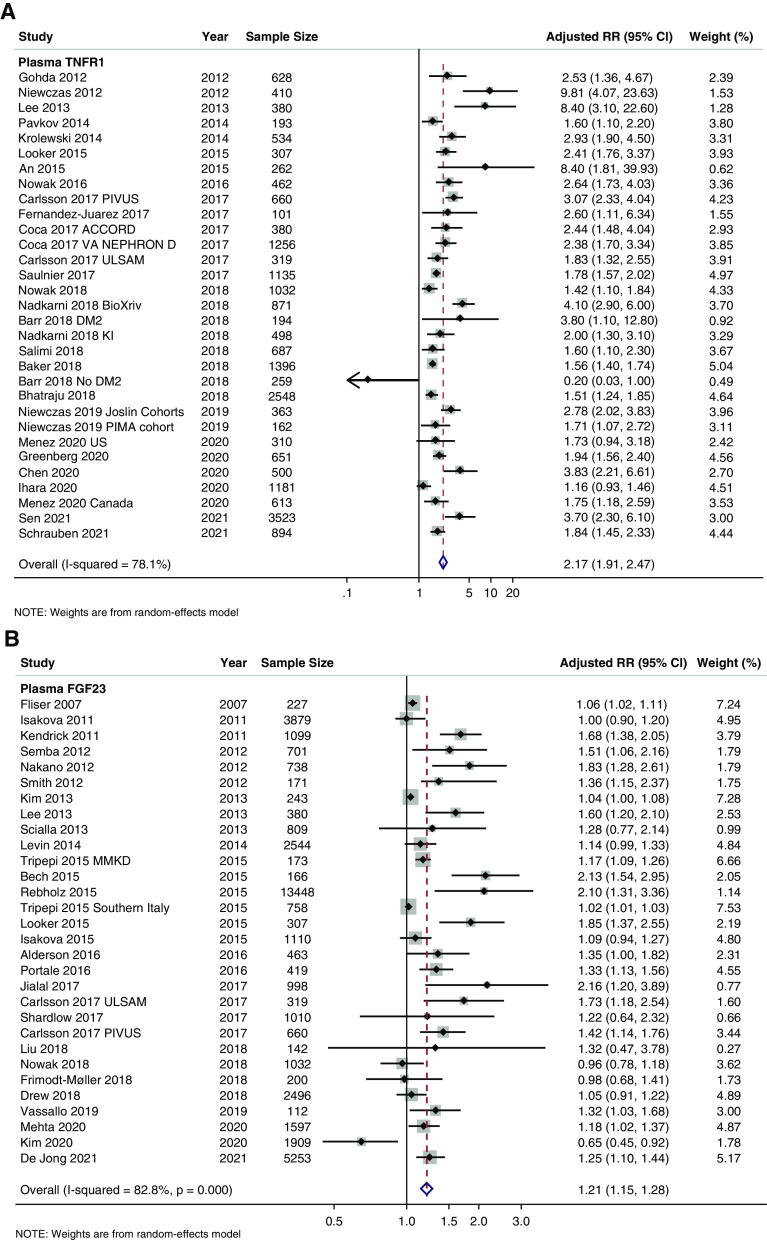
Plasma TNFR1 was the most studied plasma biomarker in the setting of our CKD outcomes with 33 total studies, 31 studies available for meta-analysis, and 22,709 total pooled study participants (Table 1, Supplemental Appendix II, Supplemental Table 1A). Before pooling, 27 of the 31 available studies had statistically significant positive associations between plasma TNFR1 and CKD outcomes (Figure 3A). The overall pooled RR for TNFR1 was 2.17 (95% CI, 1.91 to 2.47) with RRs of 2.11 (95% CI, 1.60 to 2.78), 1.56 (95% CI, 1.27 to 1.91), 3.20 (95% CI, 1.99 to 5.16), and 2.29 (95% CI, 1.91 to 2.73) when further stratified by incident CKD, CKD progression, incident ESKD, and composite outcomes, respectively (Supplemental Appendix I, Supplemental Figure 2A). There was statistically significant heterogeneity across all TNFR1 studies overall (I2=78.1%, P<0.001) and significant between-group heterogeneity by cohort race/ethnicity, biomarker transformation, and biomarker measurement platform (Supplemental Appendix I, Supplemental Figure 2, B–H).
Table 1.
Study characteristics of most frequently studied plasma biomarkers for CKD outcomes
| Plasma Biomarker | N Studies Available for Pooling | N Studies by Outcome Typea | Pooled N of Participants | Clinical Settings Studied | Diabetes Status of Studies Available | Pooled Relative Risk (95% CI) | I2b | Qb |
|---|---|---|---|---|---|---|---|---|
| TNFR1 | 31 | Incident CKD (5); CKD progression (5); incident ESKD (6); composite outcomes (16) | 22,709 | 6 clinical trials, 3 inpatient cohorts, 22 outpatient cohorts | 13 cohorts with T2DM only, 3 cohorts with T1DM only, 4 cohorts without DM, 11 cohorts of mixed DM status | Overall: 2.17 (1.91 to 2.47); incident CKD: 2.11 (1.60 to 2.78); CKD progression: 1.56 (1.27 to 1.91); incident ESKD 3.20 (1.99 to 5.16); composite: 2.29 (1.91 to 2.73) | 78.06% | 136.73 |
| FGF23 | 30 | Incident CKD (7); CKD progression (5); incident ESKD (10); composite (10) | 43,363 | 4 clinical trials, 26 outpatient cohorts | 5 cohorts with T2DM only, 2 cohorts without DM, 20 cohorts of mixed DM status | Overall: 1.21 (1.15 to 1.28); incident CKD 1.21 (1.09 to 1.35); CKD progression 1.23 (0.93 to 1.63); incident ESKD 1.37 (1.19 to 1.58); composite 1.12 (1.05 to 1.20) | 82.8% | 168.94 |
| TNFR2 | 23 | Incident CKD (7); CKD progression (2); incident ESKD (5); composite (10) | 22,503 | 5 clinical trials, 1 inpatient cohort, 17 outpatient cohorts | 8 cohorts with T2DM only, 2 cohorts with T1DM, 4 cohorts without DM, 9 cohorts of mixed DM status | Overall: 2.07 (1.82 to 2.34); incident CKD 1.87 (1.44 to 2.42); CKD progression 3.93 (0.75 to 20.59); incident ESKD 2.33 (1.69 to 3.21); composite 2.07 (1.80 to to 2.37) |
70.72% | 75.14 |
| KIM-1 | 18 | Incident CKD (4); incident ESKD (2); composite (13) | 20,219 | 3 clinical trials, 4 inpatient cohorts, 11 outpatient cohorts | 7 cohorts with T2DM, 2 cohorts with T1DM, 1 cohort without DM, 8 cohorts of mixed DM status | Overall: 1.51 (1.38 to 1.66); incident CKD 1.33 (1.16 to 1.53); incident ESKD 1.52 (0.85 to 2.72); composite 1.59 (1.43 to 1.76) | 75.43% | 69.19 |
| suPAR | 12 | Incident CKD (5); incident ESKD (2); composite (5) | 42,662 | 1 clinical trial, 1 inpatient cohort, 1 cohort of mixed setting, 9 outpatient cohorts | 1 cohort with T1DM, 5 cohorts without DM, 6 cohorts of mixed DM status | Overall 1.42 (1.30 to 1.55); incident CKD 1.46 (1.28 to 1.66); incident ESKD 1.44 (1.21 to 1.71); composite 1.37 (1.12 to 1.69) | 53.49% | 23.65 |
| GDF-15 | 8 | Incident CKD (3); CKD progression (2); composite (4) | 8285 | All outpatient cohorts | 2 cohorts with T2DM only, remaining 6 cohorts of mixed DM status | Overall 1.71 (1.55 to 1.90); incident CKD 1.79 (1.58 to 2.04); CKD progression 2.02 (1.49 to 2.75); composite 1.55 (1.42 to 1.69) | 25.68% | 9.42 |
| IL-6 | 8 | Incident CKD (5); incident ESKD (1); composite (2) | 21,568 | 1 clinical trial, 7 outpatient cohorts | 1 cohort with T1DM only, 1 cohort without DM, 6 cohorts of mixed DM status | Overall 1.03 (0.96 to 1.10); incident CKD 1.10 (0.94 to 1.28); incident ESKD 0.92 (0.77 to 1.09); composite 1.02 (0.97 to 1.07) | 38.21% | 11.33 |
| UMOD | 7 | Incident CKD (3); incident ESKD (2); composite (4) | 13,151 | All outpatient cohorts | 2 cohorts with T1DM only, 5 cohorts of mixed diabetes status | Overall 0.57 (0.43 to 0.75); incident CKD 0.41 (0.27 to 0.62); incident ESKD 0.61 (0.37 to 1.00); composite 0.70 (0.54 to 0.91) | 75.89% | 24.89 |
| Galectin-3 | 6 | Incident CKD (3); incident ESKD (2); composite (1) | 16,822 | All outpatient cohorts | 2 cohorts with T2DM only, 4 cohorts of mixed DM status | Overall 1.32 (1.11 to 1.58); incident CKD 1.38 (0.95 to 2.00); incident ESKD 1.40 (1.18 to 1.67); composite 1.19 (1.14 to 1.24) | 89.25% | 46.51 |
| Endostatin | 5 | Incident CKD (2); composite (3) | 3439 | 4 outpatient cohorts, 1 clinical trial | 3 cohorts with T2DM only, 2 cohorts of mixed DM status | Overall 1.84 (1.47 to 2.30); incident CKD 1.59 (1.33 to 1.89); composite 2.24 (1.72 to 2.91) | 50.1% | 8.02 |
| MCP-1 | 5 | Incident CKD (2); incident ESKD (1); composite (2) | 2889 | All outpatient cohorts | 1 cohort with T1DM only, 1 cohort without DM, and 3 cohorts of mixed DM status | Overall 1.24 (0.95 to 1.62); incident CKD 1.19 (0.82 to 1.74); incident ESKD 1.44 (1.17 to 1.77); composite 1.53 (0.48 to 4.92) | 81.75% | 21.92 |
| YKL-40c | 5 | Incident CKD (2); composite (3) | 3137 | 1 inpatient cohort, 4 outpatient cohorts | 1 cohort without DM, 4 cohorts of mixed DM status | Overall 1.18 (1.07 to 1.31); incident CKD; 1.11 (0.91 to 1.34) composite 1.21 (1.07 to 1.37) | 25.20% | 5.35 |
T2DM, type 2 diabetes mellitus; FGF23, fibroblast growth factor-23; KIM-1, kidney injury molecule-1; suPAR, soluble urokinase plasminogen activator receptor; GDF-15, growth differentiation factor 15; UMOD, uromodulin; MCP-1, monocyte chemoattractant protein-1.
Number of studies not additive as studies may report multiple outcomes of interest.
Results in reference to overall pooled RR.
Also known as Chitinase 3-like protein 1.
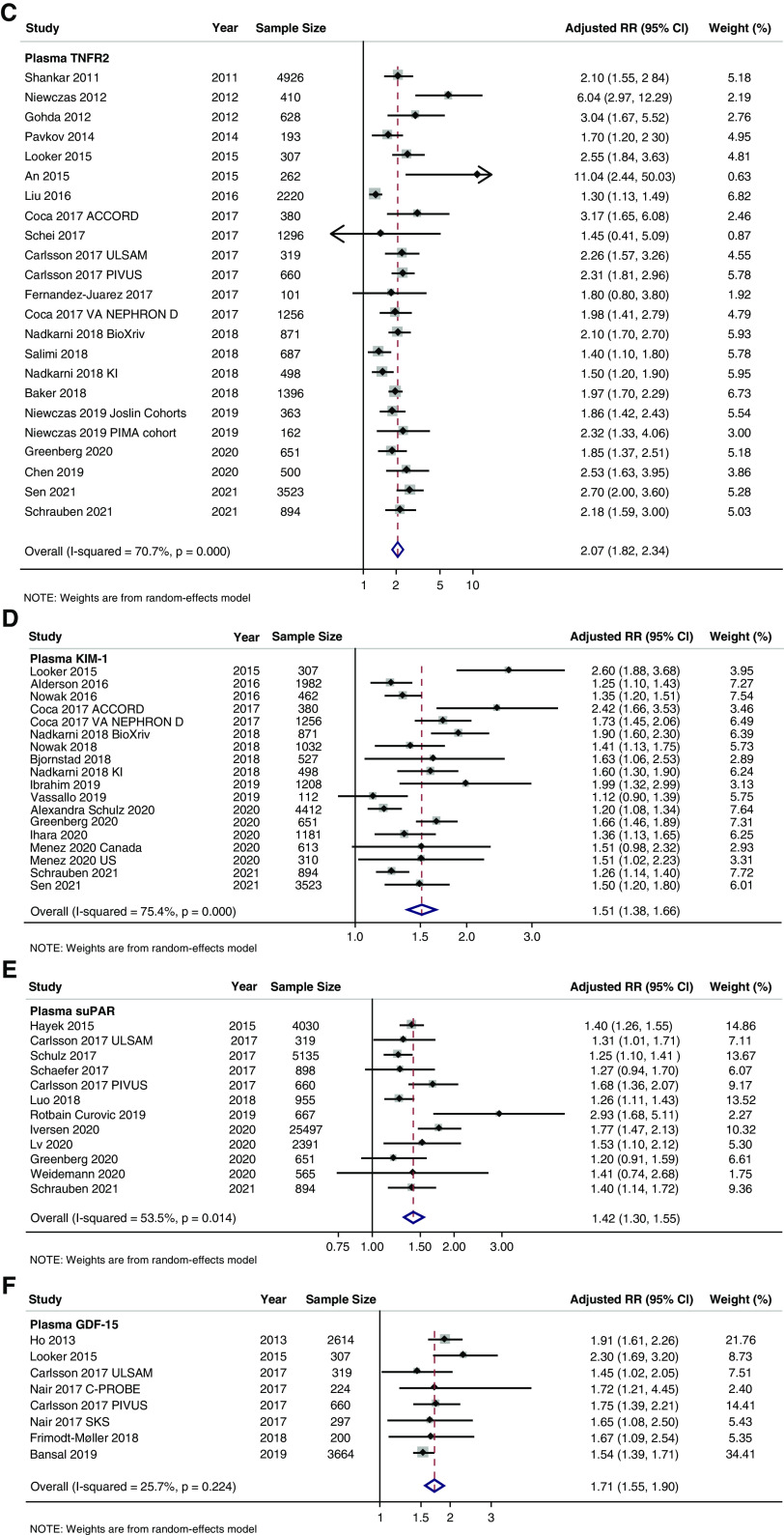
Figure 3.
Forest plots of plasma for CKD outcomes. (A) TNFR1, (B) FGF23, (C) TNFR2, (D) KIM-1, (E) suPAR, and (F) GDF-15.
Plasma FGF23 was the second most studied plasma biomarker with 32 total studies, 30 studies available for meta-analysis, and 43,190 total participants with the majority coming from the Atherosclerosis Risk in Communities cohort (Table 1, Supplemental Appendix II, Supplemental Table 1B). The pooled RR for plasma FGF23 was 1.21 (95% CI, 1.15 to 1.28) overall (Figure 3B), 1.21 (95% CI, 1.09 to 1.35) for incident CKD, 1.23 (95% CI, 0.93 to 1.63) for CKD progression, 1.27 (95% CI, 1.19 to 1.58) for incident ESKD, and 1.12 (95% CI, 1.05 to 1.20) for composite CKD outcomes (Supplemental Appendix I, Supplemental Figure 3A). There was statistically significant heterogeneity between the identified plasma FGF23 studies (I2=82.8%, P<0.001) with significant between-group heterogeneity when grouped by study design, cohort age, diabetes status, race/ethnicity, biomarker transformation, and biomarker measurement platform (Supplemental Appendix I, Supplemental Figure 3, B–H).
Plasma TNFR2 had a total of 25 studies, 23 studies available for meta-analysis, and 22,503 total pooled study participants (Table 1, Supplemental Appendix II, Supplemental Table 1C, Figure 3C). The pooled RR was 2.07 (95% CI, 1.82 to 2.34) with high heterogeneity (I2=70.7%, P<0.001). The RR by outcome type were as follows: 1.87 (95% CI, 1.44 to 2.42) for incident CKD, 3.93 (95% CI, 0.75 to 20.59) for CKD progression, 2.33 (95% CI, 1.69 to 3.21) for incident ESKD, and 2.07 (95% CI, 1.80 to 2.37) for composite CKD outcomes (Supplemental Appendix I, Supplemental Figure 4A). We identified significant between-group heterogeneity by study design, cohort diabetes status, race/ethnicity, biomarker transformation, biomarker, and measurement platform (Supplemental Appendix I, Supplemental Figure 4, B–H).
We identified a total of 20 studies, 18 studies with results available for meta-analysis, and 20,905 pooled study participants for plasma KIM-1 (Table 1, Supplemental Appendix II, Supplemental Table 1D). The pooled RR was 1.51 (95% CI, 1.38 to 1.66) (Figure 3D) with statistically significant heterogeneity (I2=75.4%, P<0.001). By outcome type, the RRs were 1.33 (95% CI, 1.16 to 1.53) for incident CKD, 1.52 (95% CI, 0.85 to 2.72) for incident ESKD, and 1.59 (95% CI, 1.43 to 1.76) for composite CKD outcomes (Supplemental Appendix I, Supplemental Figure 5A). We identified statistically significant between-group heterogeneity by study design, biomarker transformation, cohort age, diabetes status, race/ethnicity, biomarker transformation, and biomarker measurement platform shown in Supplemental Appendix I, Supplemental Figure 5, B–H.
Plasma suPAR was the fifth most studied plasma biomarker with 16 total studies, 12 studies available for meta-analysis, and 42,662 total pooled study participants (Table 1, Supplemental Appendix II, Supplemental Table 2E, Figure 3E). The pooled RR was 1.42 (95% CI, 1.30 to 1.55) with statistically significant heterogeneity (I2=53.5%, P=0.01). When stratified by outcome type, the pooled RRs were 1.46 (95% CI, 1.28 to 1.66) for incident CKD, 1.44 (95% CI, 1.21 to 1.71) for incident ESKD, and 1.37 (95% CI, 1.12 to 1.69) for composite CKD outcomes (Supplemental Appendix I, Supplemental Figure 6A). Statistically significant between-group heterogeneity was found when stratified by study design, cohort diabetes status, and biomarker transformation (Supplemental Appendix I, Supplemental Figure 6, B–H).
We identified eight studies for plasma GDF-15 with 8285 pooled study participants (Table 1, Supplemental Appendix II, Supplemental Table 1H). All studies had statistically significant, positive associations between plasma GDF-15 and CKD outcomes before pooling and were conducted among adult, outpatient cohorts (Figure 3F). The pooled RR was 1.71 (95% CI, 1.55 to 1.90) with statistically insignificant heterogeneity (I2=25.7%, P=0.22). By outcome type, pooled RR were as follows for plasma GDF-15: 1.79 (95% CI, 1.58 to 2.04) for incident CKD, 2.02 (95% CI, 1.49 to 2.75) for CKD progression, and 1.55 (95% CI, 1.42 to 1.69) for composite outcomes (Supplemental Appendix I, Supplemental Figure 7A). Statistically significant between-group heterogeneity was found when stratified by race/ethnicity and measure of association (Supplemental Appendix I, Supplemental Figure 7, B–F).
Fewer studies were available for the remaining top 12 plasma biomarkers (IL-6, UMOD, Endostatin, MCP-1, Galectin-3, and YKL-40)—details regarding these studies can be seen in Supplemental Appendix II, Supplemental Table 1, F, G, I–L. Forest plots for these plasma biomarkers are shown in Supplemental Appendix I, Figure 8, A–F). The following had statistically significant pooled RRs for all CKD outcomes: plasma UMOD (0.57; 95% CI, 0.43 to 0.75), Endostatin (1.84; 95% CI, 1.47 to 2.30), Galectin-3 (1.32; 95% CI, 1.11 to 1.58), YKL-40 (1.18; 95% CI, 1.07 to 1.31). Most studies of the remaining plasma biomarkers had substantial heterogeneity. Only plasma IL-6 and YKL-40 had lower heterogeneity (I2=38.2%, P=0.13 and I2=25.2%, P=0.25, respectively).
Urine Biomarkers
The 12 most studied urine biomarkers in the setting of CKD outcomes were as follows: KIM-1 (n=19 studies), NGAL (n=19), L-FABP (n=8), α1M (n=8), UMOD (n=8), EGF (n=7), IL-18 (n=6), β2M (n=6), NAG (n=6), MCP-1 (n=5), YKL-40 (n=3), and CKD273 (n=2). Funnel plots assessing variability can be seen in Supplemental Appendix I, Supplemental Figure 9, A–L. Details of all studies with and without quantitative results for meta-analysis can be seen in Supplemental Appendix II, Supplemental Table 2, A–L.
Urine KIM-1 was the most studied urine biomarker with a total of 22 studies, 19 of which had results available for meta-analysis corresponding to a total of 22,569 pooled participants (Table 2, Supplemental Appendix II, Supplemental Table 2A, Figure 4A). The pooled RR was statistically significant (1.10; 95% CI, 1.05 to 1.16) with statistically insignificant heterogeneity (I2 = 26.0%, P=0.14). By outcome type, the pooled RR was 1.09 (95% CI, 1.03 to 1.15) for incident CKD, 1.19 (95% CI, 1.07 to 1.32) for incident ESKD, and 1.07 (95% CI, 1.00 to 1.15) for composite outcomes (Supplemental Appendix I, Supplemental Figure 10A). No significant between-group heterogeneity was identified in subgroup analyses (Supplemental Appendix I, Supplemental Figure 10, B–G).
Table 2.
Study characteristics of most frequently studied urine biomarkers for CKD outcomes
| Urine Biomarker | N Studies Available for Pooling | N Studies by Outcome Typea | Pooled N of Participants | Clinical Settings Studied | Diabetes Status of Studies Available | Pooled Relative Risk (95% CI) | I2b | Qb |
|---|---|---|---|---|---|---|---|---|
| KIM-1 | 19 | Incident CKD (5); incident ESKD (4); composite (12) | 22,569 | 4 clinical trials, 15 outpatient cohorts | 5 cohorts with T2DM only, 2 cohorts with T1DM only, 2 cohorts without DM, 10 cohorts of mixed DM status | Overall 1.10 (1.05 to 1.16); incident CKD 1.09 (1.03 to 1.15); incident ESKD 1.19 (1.07 to 1.32); composite 1.07 (1.00 to 1.15) | 26.02% | 24.33 |
| NGAL | 19 | Incident CKD (3); incident ESKD (6); composite (10) | 25,101 | 3 clinical trials, 2 inpatient cohorts, 14 outpatient cohorts | 4 cohorts with T2DM only, 3 cohorts with DM, 12 cohorts of mixed DM status | Overall 1.12 (1.06 to 1.19); incident CKD 1.03 (0.98 to 1.08); incident ESKD 1.39 (1.10 to 1.74); composite 1.10 (1.01 to 1.19) | 71.93% | 64.13 |
| L-FABP | 8 | Incident ESKD (4); composite (4) | 5372 | 2 inpatient cohorts, 6 outpatient cohorts | 3 cohorts with T2DM, 5 cohorts of mixed DM status | Overall 1.25 (1.01 to 1.54); incident ESKD 1.16 (0.66 to 1.55); composite 1.44 (0.94 to 2.21) | 75.38% | 28.43 |
| α1M | 8 | Incident CKD (2); composite (6) | 7966 | 3 clinical trials, 5 outpatient cohorts | 3 cohorts without DM, 5 cohorts of mixed DM status | Overall 1.16 (1.02 to 1.31); incident CKD 1.04 (0.89 to 1.22); composite 1.22 (1.02 to 1.46) | 35.71% | 10.89 |
| UMOD | 8 | Incident CKD (3); Iincident ESKD (1); composite (5) | 7361 | 3 clinical trials, 5 outpatient cohorts | 1 cohort with T2DM only, 3 cohorts without DM, 4 cohorts of mixed DM status | Overall 0.91 (0.74 to 1.11); incident CKD 1.13 (0.87 to 1.45); incident ESKD 0.84 (0.31 to 2.27); composite 0.78 (0.60 to 1.02) | 76.44% | 29.71 |
| EGF | 8 | Incident CKD (1); composite (7) | 7362 | All outpatient cohorts | 1 cohort with T2DM only, 3 cohorts without DM, 1 cohort of mixed DM status | Overall 0.73 (0.53 to 0.99) incident CKD 1.95 (0.92 to 4.13); composite 0.73 (0.53 to 0.99) | 91.43% | 70.00 |
| IL-18 | 6 | Composite outcomes only | 5189 | 3 clinical trials, 3 outpatient cohorts | 1 cohort with T2DM only, 2 cohorts without DM, 3 cohorts of mixed DM status | Overall/composite outcomes only 1.20 (1.07 to 1.36) | 0.00% | 3.86 |
| β2M | 6 | Incident CKD (1); incident ESKD (1); composite (4) | 15,852 | 3 clinical trials, 3 outpatient cohorts | 3 cohorts without DM, 3 cohorts of mixed DM status | Overall 1.23 (0.94 to 1.63) incident CKD 1.70 (0.57 to 5.10); incident ESKD 0.97 (0.83 to 1.13); composite 1.01 (0.93 to 1.09) | 0.00% | 1.36 |
| NAG | 6 | Incident ESKD (2); composite (4) | 3941 | 1 inpatient cohort, 5 outpatient cohorts | 2 cohorts with T2DM only, 4 cohorts of mixed DM status | Overall 1.09 (0.94 to 1.28) incident ESKD 1.05 (0.84 to 1.32); composite 1.13 (0.91 to 1.39) | 63.58% | 13.73 |
| MCP-1 | 6 | Composite outcomes only | 5553 | 2 clinical trials, 4 outpatient cohorts | 3 cohort swith T2DM only, 2 cohorts without DM, 1 cohort of mixed DM status | Overall/composite outcomes only 1.05 (0.71 to 1.53) | 91.1% | 56.33 |
| YKL-40c | 3 | Composite outcomes only | 3132 | 2 clinical trials, 1 outpatient cohort | 1 cohort with T2DM only, 2 cohorts without DM | Overall/composite outcomes only 1.12 (0.98 to 1.29) | 0.00% | 0.46 |
| CKD273 | 2 | Incident CKD only | 3101 | 2 outpatient cohorts | 2 cohorts of mixed DM status | Overall/incident CKD only 1.45 (0.92 to 2.31) | 81.43% | 5.39 |
KIM-1, kidney injury molecule-1; T2DM, type 2 diabetes mellitus; L-FABP, liver-type fatty acid binding protein; α1M, alpha-1-microglobulin; UMOD, uromodulin; EGF, epidermal growth factor; β2M, beta 2 microglobulin NAG, N-acetyl-β-D-glucosaminidase; ; MCP-1, monocyte chemoattractant protein-1.
Number of studies not additive as studies may report multiple outcomes of interest.
Results in reference to overall pooled RR.
Also known as Chitinase 3-like protein 1.
Figure 4.
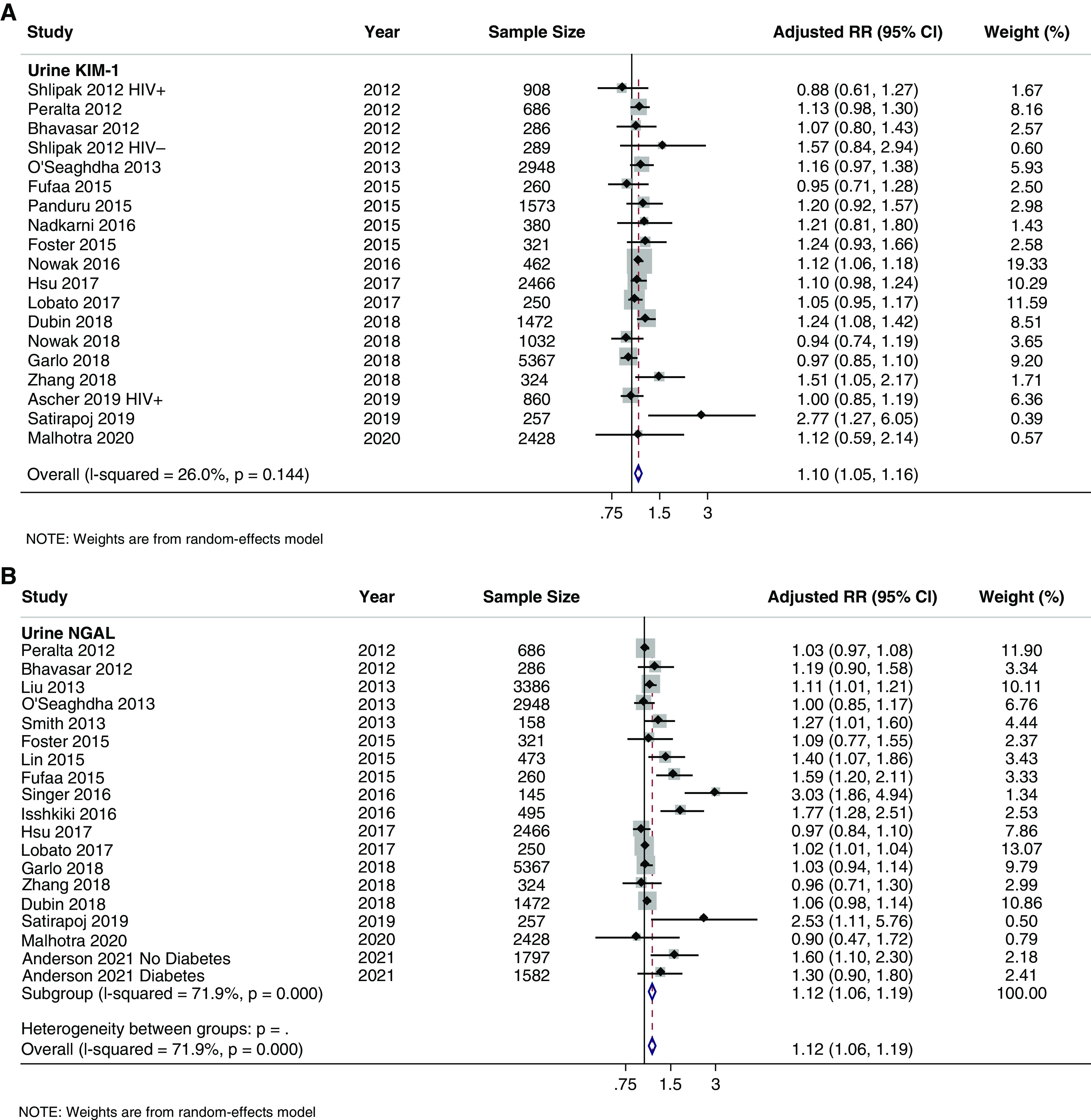
Forest plots of urine for CKD outcomes. (A) KIM-1; (B) NGAL.
Urine NGAL was the second most studied urine biomarker with 22 total studies, 19 studies with quantitative analyses for meta-analysis, and 25,101 pooled study participants (Table 2, Supplemental Appendix II, Supplemental Table 2B). The pooled RR was 1.12 (95% CI, 1.06 to 1.18) with statistically significant heterogeneity (I2=71.9%, P<0.001), Figure 4B. By outcome type, the pooled RRs were as follows: 1.03 (95% CI, 0.98 to 1.08) for incident CKD, 1.39 (95% CI, 1.10 to 1.74) for incident ESKD, and 1.10 (95% CI, 1.01 to 1.19) for composite outcomes (Supplemental Appendix I, Supplemental Figure 11A). Subgroup analyses identified statistically significant (P<0.05) between-group heterogeneity by study design, cohort race/ethnicity, biomarker transformation, biomarker measurement platform, and measure of association (Supplemental Appendix I, Supplemental Figure 11, B–G).
Fewer studies were identified among the remaining urine biomarkers (L-FABP, α1M, UMOD, EGF, IL-18, β2M, NAG, MCP-1, YKL-40, and CKD273) as shown in Supplemental Appendix II, Supplemental Table 2, C–L. Forest plots for these urine biomarkers can be seen in Supplemental Appendix I, Supplemental Figure 12, A–J. Urine KIM-1 (1.15; 95% CI, 1.07 to 1.22), NGAL (1.12; 95% CI, 1.06 to 1.19), L-FABP (1.25; 95% CI, 1.01 to 1.54), α1M (1.16; 95% CI, 1.02 to 1.31), and IL-18 (1.20; 95% CI, 1.07 to 1.36) had statistically significant pooled RR with positive associations between the urine biomarkers and CKD outcomes.
Discussion
The last two decades have seen a marked expansion into the investigation of biomarkers to prognosticate future CKD outcomes (incident CKD, CKD progression, or incident ESKD). Our systematic review and meta-analyses of the literature demonstrate that the data density of some of the key plasma and urine biomarkers has reached sufficient accumulation to garner meaningful insights into their individual performances. Plasma biomarkers were more frequently studied than their urine biomarker counterparts. Plasma TNFR1, FGF23, TNFR2, KIM-1, and suPAR were among the most frequently studied plasma biomarkers, whereas urine KIM-1, NGAL, L-FABP, UMOD, and α1M were among the most frequently studied urine biomarkers.
Key pathologic pathways such as kidney/endothelial inflammation (TNFR1, TNFR2, suPAR), tubular injury (KIM-1), parenchymal integrity (UMOD), and fibrosis (FGF23) seemed to predominate in our analyses.6,7,21 Plasma TNFR1 and TNFR2 in particular are key mediators of the inflammatory milieu in diverse tissues including the kidney. TNFRs are found in the endothelial cells, podocytes, and renal tubular epithelial cells, and are responsible for the upregulation of proapoptotic signals.22 Plasma suPAR has been implicated in the development of FSGS and diabetic nephropathy by impairing podocyte migration and apoptosis.23,24 KIM-1 expression is upregulated in cases of ischemia, hypoxia, and cellular tubular injury and has been implicated in biologic mechanisms of CKD in the setting of diabetes mellitus (activation of phagocytic cells, autophagy, and immune cellular activation).25 FGF23 is a regulator of bone homeostasis that is overexpressed during inflammation, thereby promoting profibrotic signals in the kidney via transforming growth factor beta.26 In contrast, plasma UMOD levels reflect repair function and decrease with structural derangement of the thick ascending limb, indicating lower repair function.27,28 Future studies could consider evaluating their performance in alternative clinical contexts such as nondiabetic CKD and post-AKI CKD.29
Stratified analyses identified biomarkers and settings for further investigation. In particular, few studies of plasma TNFRs, KIM-1, and FGF23 took place in inpatient settings, despite associations with acute, inpatient outcomes. Urinary biomarkers were relatively understudied compared with their plasma counterparts, with lower effect sizes. The lower effect sizes may be explained in part due to the relative paucity of urine biomarker studies and the effect of tonicity and urinary flow on biomarker collection. Although the heterogeneity across studies cannot be underestimated, as established by the predetermined subgroups set in our analysis and variables unaccounted for in our analysis, there is considerable concordance and stability across pooled estimates.
Prognostic biomarkers with reliable, reproducible, and robust findings should be assessed for their possible clinical utilities including, but not limited to risk-stratification of patients in clinical practice (inpatient versus outpatient), discriminative performance against eGFR and albuminuria, and identification of patients with evidence of tubule-interstitial damage to detect CKD development in a timely and noninvasive manner.30,31 Incorporation of such biomarkers could provide greater insight into the investigation of biologic pathways and their responses to therapy, as in the case of sodium-glucose transporter type 2 inhibitors, which have been shown to attenuate TNFR1 and TNFR2 levels and reduce KIM-1 levels among patients who are diabetic.32,33 Moreover, identification of high-risk individuals for CKD outcomes can serve to enrich clinical trials by reducing sample size and the length of follow-up without compromising statistical power. This approach has been successfully used in heart failure and oncology trials.34,35 In nephrology, models using data from the Chronic Renal Insufficiency Cohort demonstrated that using TNFR2 levels ≥75th percentile to identify high-risk individuals could reduce the sample size needed to detect a 20% reduction in diabetic kidney disease progression over a 5-year period by 50% with substantial, associated cost reductions.13 Further studies are needed to explore the prognostic role of biomarkers of other biologic pathways that showed promising signs in our analysis, such as UMOD, suPAR, galectin-3, and endostatin.
Our study has several strengths. We used broad search algorithms in three large databases to capture the maximum number of applicable studies. Our inclusion criteria and evaluation of the literature were such that most moderate-to-high quality studies were included in the meta-analysis. To the best of our knowledge, our study provides the most comprehensive systematic review and meta-analysis of biomarker literature accounting for CKD outcomes (incident CKD, CKD progression, incident ESKD, and composite CKD outcomes) to maximize the likelihood of strong findings.
However, our study is not without limitations. First, there was significant publication bias for most biomarkers studied, the implications of which cannot be downplayed. Significant publication bias is concerning for over-representation of strong associations, impairing the ability to draw definite conclusions from our results. Caution should be applied in using the results of our study to directly compare pooled biomarker associations and inform the design of future studies and application of these biomarkers in clinical settings. Second, due to the broad scope of our project, there was marked heterogeneity across studies determined by study-level characteristics such as sample size, race/ethnicity, baseline CKD staging, pre-existing comorbidities, biomarker transformation (i.e., log2, quartiles, per SD increase), and biomarker measurement platform. With the latter, biomarker measurement platforms are diverse, limiting the crossvalidation of results among studies and development of clinically meaningful cutoffs. Future efforts are needed to amass biomarker data across cohorts with standardized assays for the development of further discriminatory metrics such as exposure-risk curves with thresholds and cutoffs for levels of risk. However, even with imprecision in pooled point estimates, the wide-ranging swaths of data allow the reader and the scientific community at large to get a broad sense of the totality of data published to date. Another limitation is the variation in follow-up periods across individual cohorts, which could compromise the adjudication or detection of CKD outcomes. This limitation is expected to be at least partially mitigated by the proportional hazards assumption for the studies that assessed the kidney outcome as a “time to event” outcome (the vast majority). We explored the heterogeneity by conducting various subgroup analysis of the study characteristics (race/ethnicity, inpatient versus outpatient clinical setting, pediatric versus adult cohort, diabetes status, biomarker transformation, and biomarker measurement platform), but were limited by the number of available publications and the reporting quality of these study characteristics. Finally, despite broad search algorithms in Embase, Medline, and Scopus, the inherent time restriction of conducting a systematic review and meta-analysis excludes any new studies published after November 30, 2020.
In conclusion, out of a diverse panel of plasma and urine biomarkers, plasma TNFR1, and TNFR2, plasma FGF23, plasma suPAR, and plasma KIM-1 were among the most frequently studied biomarkers in the setting of CKD outcomes, with marked heterogeneity across study cohorts and study design. These biomarkers may provide meaningful information on the risk of incident and progressive CKD and ESKD and should be assessed for their performance in clinical practice.
Disclosures
C. R. Parikh reports serving as a consultant for Genfit and Novartis; reports receiving reports research funding from the National Heart, Lung and Blood Institute and the National Institute of Diabetes and Digestive and Kidney Diseases; and reports having an advisory or leadership role with Genfit Biopharmaceutical Company. C. R. Parikh and S. G. Coca are members of the scientific advisory board of, and own equity in, Renalytix; and report receiving consulting fees from 3ive, Axon Therapies, Bayer, Boehringer-Ingelheim, ProKidney, Reprieve Cardiovascular, Renalytix, Nuwellis, and Vifor in the past 3 years. S. Coca reports having consultancy: agreements with CHF Solutions and Takeda; reports having an ownership interest in pulseData; reports receiving research funding from ProKidney, Renalytix, Renal Research Institute, and XORTX; reports patents or royalties with Renalytix; reports having an advisory or leadership role with Reprieve Cardiovascular; and reports having other interests or relationships as Associate Editor for Kidney360, Editorial Boards of JASN, CJASN, and Kidney International. S. Menez reports receiving research funding from RenalytixAI. All remaining authors have nothing to disclose.
Funding
None.
Supplementary Material
Acknowledgments
The analyses, conclusions, opinions, and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred. Furthermore, these funding organizations had no role in the study design; collection, analysis, and interpretation of data; writing the report; or the decision to submit the report for publication.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Author Contributions
S. Coca conceptualized the study; K. Chauhan, N. Debnath, C. Liu, G. Mosoyan, and C. Soudant were responsible for the data curat ion; C. Liu was responsible the formal analysis and investigation; S. Coca was responsible the funding acquisition; N. Debnath, C. Liu, and C. Soudant were responsible for the methodology; C. Liu was responsible the software; S. Coca provided supervision; S. Coca and C. Liu wrote the original draft; and K. Chauhan, S. Coca, N. Debnath, C. Liu, S. Menez, G. Mosoyan, C. Parikh, C. Soudant, and G. Vasquez-Rios reviewed and edited the manuscript.
Data Sharing Statement
All data used in this study are available in this article.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2022010098/-/DCSupplemental.
Supplemental Table 1. Search terms.
Supplemental Figure 1. Funnel plots of top 12 plasma biomarkers. (A) TNFr1, (B) FGF23, (C) TNFr2, (D) KIM-1, (E) suPAR, (F) UMOD, (G) IL-6, (H) GDF-15, (I) Endostatin, (J) MCP-1, (K) Galectin-3, (L) YKL-40.
Supplemental Figure 2A. Plasma TNFR1 forest plots by outcome type (incident CKD, CKD progression, incident ESKD, or composite outcome).
Supplemental Figure 2B. Plasma TNFR1 forest plot by study design.
Supplemental Figure 2C. Plasma TNFR1 forest plot by cohort age.
Supplemental Figure 2D. Plasma TNFR1 forest plot by cohort diabetes status.
Supplemental Figure 2E. Plasma TNFR1 forest plot by race/ethnicity.
Supplemental Figure 2F. Plasma TNFR1 forest plot by biomarker transformation.
Supplemental Figure 2G. Plasma TNFR1 forest plot by biomarker measurement platform.
Supplemental Figure 2H. Plasma TNFR1 forest plot by measure of association.
Supplemental Figure 3A. Plasma FGF23 forest plots by outcome type (incident CKD, CKD progression, incident ESKD, or composite outcome).
Supplemental Figure 3B. Plasma FGF23 forest plot by study design.
Supplemental Figure 3C. Plasma FGF23 forest plot by cohort age.
Supplemental Figure 3D. Plasma FGF23 forest plot by cohort diabetes status.
Supplemental Figure 3E. Plasma FGF23 forest plot by cohort race/ethnicity.
Supplemental Figure 3F. Plasma FGF23 forest plot by biomarker transformation.
Supplemental Figure 3G. Plasma FGF23 forest plot by biomarker measurement platform.
Supplemental Figure 3H. Plasma FGF23 forest plot by measure of association.
Supplemental Figure 4A. Plasma TNFR2 forest plot by outcome type (incident CKD, CKD Progression, incident ESKD, or composite outcome).
Supplemental Figure 4B. Plasma TNFR2 forest plot by study design.
Supplemental Figure 4C. Plasma TNFR2 forest plot by cohort age.
Supplemental Figure 4D. Plasma TNFR2 forest plot by cohort diabetes status.
Supplemental Figure 4E. Plasma TNFR2 forest plot by cohort race/ethnicity.
Supplemental Figure 4F. Plasma TNFR2 forest plot by biomarker transformation.
Supplemental Figure 4G. Plasma TNFR2 forest plot by biomarker measurement platform.
Supplemental Figure 4H. Plasma TNFR2 forest plot by measure of association.
Supplemental Figure 5A. Plasma KIM-1 forest plot by outcome type (incident CKD, CKD progression, incident ESKD, or composite outcome).
Supplemental Figure 5B. Plasma KIM-1 forest plot by study design.
Supplemental Figure 5C. Plasma KIM-1 forest plot by cohort age.
Supplemental Figure 5D. Plasma KIM-1 forest plot by cohort diabetes status.
Supplemental Figure 5E. Plasma KIM-1 forest plot by cohort race/ethnicity.
Supplemental Figure 5F. Plasma KIM-1 forest plot by biomarker transformation.
Supplemental Figure 5G. Plasma KIM-1 forest plot by biomarker measurement platform.
Supplemental Figure 5H. Plasma KIM-1 forest plot by measure of association.
Supplemental Figure 6A. Plasma suPAR forest plot by outcome type (incident CKD, CKD progression, incident ESKD, or composite outcome).
Supplemental Figure 6B. Plasma suPAR forest plot by study design.
Supplemental Figure 6C. Plasma suPAR forest plot by cohort age.
Supplemental Figure 6D. Plasma suPAR forest plot by cohort diabetes status.
Supplemental Figure 6E. Plasma suPAR forest plot by cohort race/ethnicity.
Supplemental Figure 6F. Plasma suPAR forest plot by biomarker transformation.
Supplemental Figure 6G. Plasma suPAR forest plot by biomarker measurement platform.
Supplemental Figure 6H. Plasma suPAR forest plot by measure of association.
Supplemental Figure 7A. Plasma GDF-15 forest plot by outcome type (incident CKD, CKD progression, incident ESKD, or composite outcome).
Supplemental Figure 7B. Plasma GDF-15 forest plot by cohort diabetes status.
Supplemental Figure 7C. Plasma GDF-15 forest plot by cohort race/ethnicity.
Supplemental Figure 7D. Plasma GDF-15 forest plot by biomarker transformation.
Supplemental Figure 7E. Plasma GDF-15 forest plot by biomarker measurement platform.
Supplemental Figure 7F. Plasma GDF-15 forest plot by measure of association.
Supplemental Figure 8. Forest plots of plasma biomarkers. (A) IL-6, (B) UMOD, (C) Endostatin, (D) MCP-1, (E) Galectin-3, (F) YKL-40 for CKD outcomes.
Supplemental Figure 9. Funnel plots of top 12 urine biomarkers. (A) KIM-1, (B) NGAL, (C) L-FABP, (D) α1M, (E) UMOD, (F) EGF, (G) IL-18, (H) β2M, (I) NAG, (J) MCP-1, (K) YKL-40, (L) CKD273.
Supplemental Figure 10A. Urine KIM-1 forest plots by outcome type (incident CKD, CKD progression, incident ESKD, or composite outcome).
Supplemental Figure 10B. Urine KIM-1 forest plot by study design.
Supplemental Figure 10C. Urine KIM-1 forest plot by cohort diabetes status.
Supplemental Figure 10D. Urine KIM-1 forest plot by cohort race/ethnicity.
Supplemental Figure 10E. Urine KIM-1 forest plot by biomarker transformation.
Supplemental Figure 10F. Urine KIM-1 forest plot by biomarker measurement platform.
Supplemental Figure 10G. Urine KIM-1 forest plot by measure of association.
Supplemental Figure 11A. Urine NGAL forest plots by outcome type (incident CKD, CKD progression, incident ESKD, or composite outcome).
Supplemental Figure 11B. Urine NGAL forest plot by cohort study design.
Supplemental Figure 11C. Urine NGAL forest plot by cohort diabetes.
Supplemental Figure 11D. Urine NGAL forest plot by cohort race/ethnicity.
Supplemental Figure 11E. Urine NGAL forest plot by biomarker transformation.
Supplemental Figure 11F. Urine NGAL forest plot by biomarker measurement platform.
Supplemental Figure 11G. Urine NGAL forest plot by measure of association.
Supplemental Figure 12. Forest plots for urine. (A) L-FABP, (B) α1M, (C) UMOD, (D) EGF, (E) IL-18, (F) β2M, (G) NAG, (H) MCP-1, (I) YKL-40, (J) CKD273 and CKD outcomes.
Supplemental Table 1A. Study characteristics of plasma TNFR1 studies.
Supplemental Table 1B. Study characteristics of plasma FGF23 studies.
Supplemental Table 1C. Study characteristics of plasma TNFR2 studies.
Supplemental Table 1D. Study characteristics of plasma KIM-1 studies.
Supplemental Table 1E. Study characteristics of plasma suPAR studies.
Supplemental Table 1F. Study characteristics of plasma UMOD studies.
Supplemental Table 1G. Study characteristics of plasma IL-6 studies.
Supplemental Table 1H. Study characteristics of plasma GDF-15 studies.
Supplemental Table 1I. Study characteristics of plasma Endostatin studies.
Supplemental Table 1J. Study characteristics of plasma MCP-1 studies.
Supplemental Table 1K. Study characteristics of plasma Galectin-3 studies
Supplemental Table 1L. Study characteristics of plasma YKL-40 studies.
Supplemental Table 2A. Study characteristics of urine KIM-1 studies.
Supplemental Table 2B. Study characteristics of urine NGAL studies.
Supplemental Table 2C. Study characteristics of urine L-FABP studies.
Supplemental Table 2D. Study characteristics of urine α1M studies.
Supplemental Table 2E. Study characteristics of urine UMOD studies.
Supplemental Table 2F. Study characteristics of urine EGF studies.
Supplemental Table 2G. Study characteristics of urine IL-18 studies.
Supplemental Table 2H. Study characteristics of urine β2M studies.
Supplemental Table 2I. Study characteristics of urine NAG studies.
Supplemental Table 2J. Study characteristics of urine MCP-1 studies.
Supplemental Table 2K. Study characteristics of urine YKL-40 studies.
Supplemental Table 2L. Study characteristics of urine CKD273 studies.
References
- 1.Bikbov B, Purcell CA, Levey AS, Smith M, Abdoli A, Abebe M, et al. ; GBD Chronic Kidney Disease Collaboration : Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 395: 709–733, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.USRDS . 2017. USRDS Annual Data Report: Executive Summary. Available at: https://www.usrds.org/media/1652/v1_00_execsummary_17.pdf. Accessed December 2, 2021
- 3.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Levin A, Stevens PE: Summary of KDIGO 2012 CKD Guideline: Behind the scenes, need for guidance, and a framework for moving forward. Kidney Int 85: 49–61, 2014. Available at: https://www.sciencedirect.com/science/article/pii/S0085253815538933?via%3Dihub. Accessed December 2, 2021. 10.1038/KI.2013.444 [DOI] [PubMed] [Google Scholar]
- 5.Waikar SS, Rebholz CM, Zheng Z, Hurwitz S, Hsu CY, Feldman HI, et al. ; Chronic Kidney Disease Biomarkers Consortium Investigators : Biological variability of estimated GFR and albuminuria in CKD. Am J Kidney Dis 72: 538–546, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang WR, Parikh CR: Biomarkers of acute and chronic kidney disease. Annu Rev Physiol 81: 309–333, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wen Y, Parikh CR. Current concepts and advances in biomarkers of acute kidney injury. Rev Clin Lab Sci 58: 354–368, 2021 10.1080/10408363.2021.1879000 [DOI] [PubMed] [Google Scholar]
- 8.Gohda T, Niewczas MA, Ficociello LH, Walker WH, Skupien J, Rosetti F, et al. : Circulating TNF receptors 1 and 2 predict stage 3 CKD in type 1 diabetes. J Am Soc Nephrol 23: 516–524, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nadkarni GN, Rao V, Ismail-Beigi F, Fonseca VA, Shah SV, Simonson MS, et al. : Association of urinary biomarkers of inflammation, injury, and fibrosis with renal function decline: The ACCORD trial. Clin J Am Soc Nephrol 11: 1343–1352, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foster MC, Coresh J, Bonventre JV, Sabbisetti VS, Waikar SS, Mifflin TE, et al. ; CKD Biomarkers Consortium : Urinary biomarkers and risk of ESRD in the atherosclerosis risk in communities study. Clin J Am Soc Nephrol 10: 1956–1963, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan CH, Xie D, Waikar SS, Bonventre JV, Zhang X, Sabbisette V, et al. : Urine biomarkers of tubular injury do not improve on the clinical model predicting chronic kidney disease progression. Kidney Int 91: 196–203, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coca SG, Nadkarni GN, Huang Y, Moledina DG, Rao V, Zhang J, et al. : Plasma biomarkers and kidney function decline in early and established diabetic kidney disease. J Am Soc Nephrol 28: 2786–2793, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schrauben SJ, Shou H, Zhang X, Anderson AH, Bonventre JV, Chen J, et al. ; CKD Biomarkers Consortium and the Chronic Renal Insufficiency Cohort (CRIC) Study Investigators : Association of multiple plasma biomarker concentrations with progression of prevalent diabetic kidney disease: Findings from the Chronic Renal Insufficiency Cohort (CRIC) study. J Am Soc Nephrol 32: 115–126, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niewczas MA, Gohda T, Skupien J, Smiles AM, Walker WH, Rosetti F, et al. : Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. J Am Soc Nephrol 23: 507–515, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Panduru NM, Sandholm N, Forsblom C, Saraheimo M, Dahlström EH, Thorn LM, et al. ; FinnDiane Study Group : Kidney injury molecule-1 and the loss of kidney function in diabetic nephropathy: A likely causal link in patients with type 1 diabetes. Diabetes Care 38: 1130–1137, 2015 [DOI] [PubMed] [Google Scholar]
- 16.Bramer W, Bain P: Updating search strategies for systematic reviews using EndNote. J Med Libr Assoc 105: 285–289, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. : The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 372: n71, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia. Available at: https://www.covidence.org/. Accessed December 13, 2021.
- 19.DerSimonian R, Laird N: Meta-analysis in clinical trials. Control Clin Trials 7: 177–188, 1986 [DOI] [PubMed] [Google Scholar]
- 20.Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Training. Available at: https://training.cochrane.org/handbook/current. Accessed December 14, 2020.
- 21.Zabetian A, Coca SG: Plasma and urine biomarkers in chronic kidney disease: Closer to clinical application. Curr Opin Nephrol Hypertens 30: 531–537, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Lamki RS, Mayadas TN: TNF receptors: Signaling pathways and contribution to renal dysfunction. Kidney Int 87: 281–296, 2015 [DOI] [PubMed] [Google Scholar]
- 23.Wei C, Möller CC, Altintas MM, Li J, Schwarz K, Zacchigna S, et al. : Modification of kidney barrier function by the urokinase receptor. Nat Med 14: 55–63, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Wei C, El Hindi S, Li J, Fornoni A, Goes N, Sageshima J, et al. : Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med 17: 952–960, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonventre JV. Kidney injury molecule-1: A translational journey. Trans Am Clin Climatol Assoc. 125: 293–299, 2014 [PMC free article] [PubMed] [Google Scholar]
- 26.Smith ER, Holt SG, Hewitson TD: FGF23 activates injury-primed renal fibroblasts via FGFR4-dependent signalling and enhancement of TGF-β autoinduction. Int J Biochem Cell Biol 92: 63–78, 2017 [DOI] [PubMed] [Google Scholar]
- 27.Puthumana J, Thiessen-Philbrook H, Xu L, Coca SG, Garg AX, Himmelfarb J, et al. : Biomarkers of inflammation and repair in kidney disease progression. J Clin Invest 131: 139927, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scherberich JE, Gruber R, Nockher WA, Christensen EI, Schmitt H, Herbst V, et al. : Serum uromodulin-a marker of kidney function and renal parenchymal integrity. Nephrol Dial Transplant 33: 284–295, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menez S, Moledina DG, Garg AX, Thiessen-Philbrook H, McArthur E, Jia Y, et al. : Results from the TRIBE-AKI Study found associations between post-operative blood biomarkers and risk of chronic kidney disease after cardiac surgery. Kidney Int 99: 716–724, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Menn-Josephy H, Lee CS, Nolin A, Christov M, Rybin DV, Weinberg JM, et al. : Renal interstitial fibrosis: An imperfect predictor of kidney disease progression in some patient cohorts. Am J Nephrol 44: 289–299, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rule AD, Amer H, Cornell LD, Taler SJ, Cosio FG, Kremers WK, et al. : The association between age and nephrosclerosis on renal biopsy among healthy adults. Ann Intern Med 152: 561–567, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heerspink HJL, Perco P, Mulder S, Leierer J, Hansen MK, Heinzel A, et al. : Canagliflozin reduces inflammation and fibrosis biomarkers: A potential mechanism of action for beneficial effects of SGLT2 inhibitors in diabetic kidney disease. Diabetologia 62: 1154–1166, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sen T, Li J, Neuen BL, Neal B, Arnott C, Parikh CR, et al. : Effects of the SGLT2 inhibitor canagliflozin on plasma biomarkers TNFR-1, TNFR-2, and KIM-1 in the CANVAS trial. Diabetologia 64: 2147–2158, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu C, Dignam JJ. Biomarker-driven oncology clinical trials: Key design elements, types, features, and practical considerations. JCO Precis Oncol 3: 1–12, 2019. 10.1200/PO.19.00086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ibrahim NE, Gaggin HK, Konstam MA, Januzzi JL Jr: Established and emerging roles of biomarkers in heart failure clinical trials. Circ Heart Fail 9: e002528, 2016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.