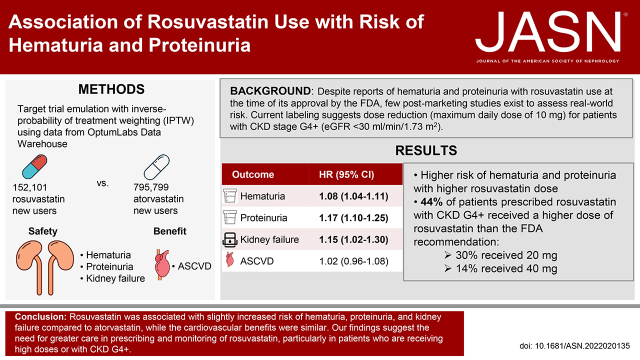
Significance Statement
Despite reports of hematuria and proteinuria with rosuvastatin use at the time of its approval by the US Food and Drug Administration (FDA), current labeling mentions dose reduction (maximum daily dose of 10 mg) only for patients with severe CKD. In this real-world study, 44% of patients with severe CKD were prescribed a higher dose of rosuvastatin than recommended by the FDA. Compared with atorvastatin, rosuvastatin use was associated with slightly increased risk of hematuria and proteinuria in a dose-dependent manner and slightly increased risk of kidney failure with replacement therapy; the cardiovascular benefits were similar. These findings suggest the need for greater care in prescribing and monitoring rosuvastatin, particularly in patients who receive high doses or who have severe CKD.
Keywords: statins, drug nephrotoxicity, clinical epidemiology, chronic kidney disease, rosuvastatin calcium, hematuria, proteinuria
Visual Abstract
Abstract
Background
Despite reports of hematuria and proteinuria with rosuvastatin use at the time of its approval by the US Food and Drug Association (FDA), little postmarketing surveillance exists to assess real-world risk. Current labeling suggests dose reduction (maximum daily dose of 10 mg) for patients with severe CKD.
Methods
Using deidentified electronic health record data, we analyzed 152,101 and 795,799 new users of rosuvastatin and atorvastatin, respectively, from 2011 to 2019. We estimated inverse probability of treatment–weighted hazard ratios (HRs) of hematuria, proteinuria, and kidney failure with replacement therapy (KFRT) associated with rosuvastatin. We reported the initial rosuvastatin dose across eGFR categories and evaluated for a dose effect on hematuria and proteinuria.
Results
Overall, we identified 2.9% of patients with hematuria and 1.0% with proteinuria during a median follow-up of 3.1 years. Compared with atorvastatin, rosuvastatin was associated with increased risk of hematuria (HR, 1.08; 95% confidence interval [95% CI], 1.04 to 1.11), proteinuria (HR, 1.17; 95% CI, 1.10 to 1.25), and KFRT (HR, 1.15; 95% CI, 1.02 to 1.30). A substantial share (44%) of patients with eGFR <30 ml/min per 1.73 m2 was prescribed high-dose rosuvastatin (20 or 40 mg daily). Risk was higher with higher rosuvastatin dose.
Conclusions
Compared with atorvastatin, rosuvastatin was associated with increased risk of hematuria, proteinuria, and KFRT. Among patients with eGFR <30 ml/min per 1.73 m2, 44% were prescribed a rosuvastatin daily dose exceeding the FDA’s recommended 10 mg daily dose. Our findings suggest the need for greater care in prescribing and monitoring rosuvastatin, particularly in patients who receive high doses or who have severe CKD.
There was considerable controversy over rosuvastatin—the most potent of the currently available hydroxymethylglutaryl–coenzyme A reductase inhibitors1—at the time of drug approval.2–6 In preapproval clinical trials evaluating the safety and efficacy of rosuvastatin, patients with hematuria and proteinuria were reported.7 Most of these patients were on high-dose rosuvastatin (80 mg), which was subsequently discontinued from development. However, 10% and 5% of patients on 40 mg also developed dipstick hematuria ≥+ and proteinuria ≥++, respectively, compared with 2%–4% and 0.4%–2% of patients on any dose of atorvastatin.7 Furthermore, there have also been several case reports suggesting rosuvastatin causes hematuria and proteinuria through direct renal tubular toxicity since the US Food and Drug Administration (FDA) approval of rosuvastatin in 2003.8,9 Despite these safety signals,10 very little postmarketing surveillance exists on rosuvastatin’s potential nephrotoxicity.
The FDA approved rosuvastatin at doses <40 mg, with a conclusion that the risks of adverse events at lower doses appear to be comparable with other marketed statins. However, the FDA also recognized that these risks may increase in special populations, such as those with CKD, where patients may be exposed to higher systemic drug concentrations.7 Thus, the FDA label suggests a starting dose for rosuvastatin of 5 mg and a maximum dose of 10 mg in patients with severe CKD (i.e., creatinine clearance <30 ml/min).11 Adherence to this dosing recommendation in real-world practice is unknown.
Using a large, geographically diverse electronic health record (EHR) database covering >80 million patients in the United States, we aimed to assess the associations of rosuvastatin use versus atorvastatin use with the risk of hematuria and proteinuria across the range of kidney function, and rosuvastatin-dosing practice patterns in relation to kidney function.
Methods
Data Source
We used deidentified EHR data from 40 health care organizations (“cohorts”) participating in Optum Labs Data Warehouse to conduct a multicenter observational cohort study. The database contains longitudinal administrative claims and EHR data on enrollees and patients, representing a mixture of ages and geographical regions across the United States.12 The Optum Labs Data Warehouse includes a subset of EHR data that has been normalized and standardized into a single database. Data structure for included cohorts did not change during the study period.
Emulating a Target Trial
Eligibility Criteria and Study Population
We explicitly emulated a target trial to examine the risk of proteinuria and hematuria with rosuvastatin use (Supplemental Table 1).13 The eligible study population included patients aged ≥18 years between 2011 and 2019, who had ≥1 year of prior engagement with the health system, were free of kidney failure with replacement therapy (KFRT), did not have history of any study outcome (i.e., hematuria, proteinuria), did not have any statin prescriptions within the year before study medication initiation (baseline, T0), and had at least one outpatient value for serum creatinine, systolic blood pressure, serum potassium, and body mass index within the year before T0 (Supplemental Figure 1). Probability of exclusion due to missing data was similar between rosuvastatin and atorvastatin group. History of hematuria and proteinuria were ascertained by the presence of relevant diagnostic codes before T0 (Supplemental Table 2). We also excluded those with outpatient urine albumin-creatinine ratio or converted urine albumin-creatinine ratio (from urine protein-creatinine ratio)14 ≥30 mg/g, dipstick proteinuria ≥+, dipstick hematuria ≥+, or microscopic hematuria. Because false-positive urine dipstick hematuria could be due to subclinical rhabdomyolysis, we also excluded individuals with history of rhabdomyolysis or serum creatinine kinase >200 U/L to avoid misclassification of hematuria outcome. This study was approved by the Johns Hopkins University Institutional Review Board.
Treatment Strategies
Rosuvastatin initiation was ascertained from outpatient prescription records, and was compared with atorvastatin initiation because atorvastatin is the only other statin that is classified as high intensity (atorvastatin 40 and 80 mg; rosuvastatin 20 and 40 mg) by the American Heart Association guideline (new user, active comparator design).15
Treatment Assignment: Emulation of Randomization by Inverse-Probability of Treatment Weighting
To achieve balance in baseline characteristics between the two treatment groups and estimate average treatment effect of rosuvastatin, we used inverse probability of treatment weighting (IPTW) methods. Because we used 40 cohorts (“study sites”), we emulated a trial that stratified randomization by study site, deriving IPTW within each cohort.16 Specifically, we fit a logistic regression model in each cohort including the covariates to estimate the conditional probability of an individual receiving rosuvastatin over atorvastatin (i.e., propensity score). Then, we derived stabilized IPTW in each cohort using the marginal probability of treatment instead of “1” in the weight numerator. Within each cohort, we evaluated covariate balance between weighted rosuvastatin versus atorvastatin users using Cohen’s d, which estimates the standardized mean difference, and considered an absolute standardized mean difference <10% to demonstrate good balance across treatment groups.
We included variables that potentially affect the risk of hematuria and proteinuria as covariates in the propensity score model.17 Demographic characteristics and smoking history were abstracted from the EHR. We used systolic BP, serum creatinine, serum potassium, and body mass index from the most recent outpatient measurement within 1 year before T0. We eGFR on the basis of on serum creatinine concentration using the CKD Epidemiology Collaboration equation.18 Baseline comorbidities including diabetes, hypertension, coronary artery disease, cerebrovascular disease, heart failure, and hypothyroidism were ascertained by the presence of relevant diagnostic codes before T0: (1) at least one code in the inpatient setting or problem list or (2) at least two codes within 2 years in other encounters. Concurrent prescription of other medications (either affect the risk of hematuria or proteinuria or interact with rosuvastatin) included angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, aldosterone receptor antagonists, other antihypertensive medications, sodium-glucose cotransporter 2 inhibitors, dipeptidyl peptidase 4 inhibitors, glucagon-like peptide 1 receptor agonists, other oral antidiabetes medications, insulin, direct oral anticoagulants, warfarin, antiplatelets, cyclosporine, itraconazole, clarithromycin, protease inhibitors, fibrates, niacin, ezetimibe, and proprotein convertase subtilisin/kexin type 9 inhibitors. Baseline calendar year was also included as a covariate.
Outcomes, Follow-Up, and Causal Contrasts
Outcomes included hematuria and proteinuria, ascertained exclusively by outpatient laboratory measurements. We required at least two separate timepoints with abnormal values to ascertain persistent hematuria and proteinuria. Hematuria was defined as dipstick hematuria ≥+ or presence of at least three red blood cells in urine microscopy; proteinuria as dipstick proteinuria ≥++ or urine albumin-creatinine ratio (measured or converted from urine protein-creatinine ratio) ≥300 mg/g. Patients who did not have laboratory measurements for the outcomes during the follow-up were considered as not developing the outcomes. We verified the testing rates for the outcomes were similar between the two treatment groups, overall and across eGFR categories (≥60, 30–59, and <30 ml/min per 1.73 m2) according to the Kidney Disease: Improving Global Outcomes criteria19 (Supplemental Table 3). Time at risk began at medication initiation (baseline, T0), and ended at the first occurrence of a study outcome, KFRT, or end of study follow-up (i.e., death or last encounter), whichever came first. Our causal contrast of interest was the intention-to-treat effect, so patients were categorized by their initial treatment strategy.
Statistical Analysis
Baseline characteristics of the entire study population before applying IPTW were reported as mean (SD), or number (percentage), as appropriate. We showed covariate balance within each cohort before and after applying IPTW.
We plotted Kaplan–Meier curves by the combination of treatment group and eGFR category (≥60, 30–59, and <30 ml/min per 1.73 m2). We fit stratified Cox proportional hazards regression using a cohort indicator as a stratifying variable to estimate a single IPTW–hazard ratio (HR) for the entire study population. We assessed whether the associations differed by baseline eGFR using the same analytic approach with interaction terms between rosuvastatin use and eGFR category. We used Poisson regression to estimate the incidence rate difference. The heterogeneity of incidence rate difference across eGFR subgroups was examined using fixed effects meta-analysis.20
To evaluate for potential residual confounding, we assessed the risk of urinary tract infection, ascertained by diagnostic codes, as a negative control outcome thought to be unaffected by statin type. In addition to the laboratory-based kidney safety outcomes, we examined the risk of KFRT, ascertained by diagnostic codes and procedure codes (Supplemental Table 2). We also examined a benefit outcome, comparing the risk of atherosclerotic cardiovascular disease (ASCVD; defined as myocardial infarction or stroke) among those without prevalent ASCVD at baseline.
Analysis with Rosuvastatin Dose
We reported the distribution of initial rosuvastatin prescription dose (5, 10, 20, and 40 mg) across eGFR categories among all rosuvastatin initiators who had baseline eGFR measurements. To examine whether risk varied across dose, we first fit multinomial logistic regression with an outcome of rosuvastatin dose to estimate propensity score of receiving each dose (10, 20, and 40 mg) versus 5 mg (reference), including the aforementioned covariates and a cohort indicator. Then, we derived stabilized IPTW and compared the risk of the outcomes (hematuria, proteinuria, and urinary tract infection) across doses among rosuvastatin users using stratified Cox proportional hazards regression with IPTW.
Sensitivity Analysis
We performed a sensitivity analysis limited to patients with at least two prescriptions to address potential misclassification of treatment strategy. We performed as-treated analysis with additional censoring at medication discontinuation or switch, defining discontinuation as >60-day gap between consecutive prescriptions. We repeated the analysis with rosuvastatin dose after excluding individuals with a recent (<30 days) heart failure or myocardial infarction, or very high cholesterol level (total cholesterol ≥240 mg/dl [6.21 mmol/L] or LDL cholesterol ≥160 mg/dl [4.14 mmol/L]) to address potential confounding by acute cardiovascular events or high-risk status. Because the FDA’s rosuvastatin dosing recommendation is on the basis of creatinine clearance, we also examined the initial rosuvastatin dose across creatinine clearance levels. Creatinine clearance was estimated using Cockcroft–Gault Formula.21 We conducted all analyses using Stata/MP 16.1 (StataCorp, College Station, TX).
Results
Baseline Characteristics of the Study Population
There were 152,101 rosuvastatin and 795,799 atorvastatin new users in 40 cohorts from Optum Labs Data Warehouse (Table 1). The mean age (SD) of the study population was 60 (12) years and approximately half were women. Even before applying IPTW, most of patient characteristics were similar between rosuvastatin and atorvastatin users, including eGFR, body mass index, systolic BP, total cholesterol, comorbidities, and concomitant medication use. Those included in the study population were slightly younger, more likely to have hypertension and hypothyroidism, and less likely to have coronary artery disease than those excluded from the study due to missing data (Supplemental Table 4). After applying IPTW, covariate balance was achieved for each covariate in all 40 cohorts included in the analyses (Supplemental Figure 2).
Table 1.
Baseline characteristics of the participants initiating rosuvastatin or atorvastatin before applying IPTW
| Characteristic | Rosuvastatin Initiators | Atorvastatin Initiators | Standardized Mean Differences (%) |
|---|---|---|---|
| No. of cohorts | 40 | 40 | − |
| No. of participants | 152,101 | 795,799 | − |
| Age, mean (SD), yr | 60.6 (11.7) | 60.0 (12.2) | 5.37 |
| Women, n (%) | 77,230 (50.8) | 373,794 (47.0) | 7.62 |
| Race/ethnicity, n (%) | |||
| Black | 12,099 (8.0) | 80,224 (10.1) | −7.17 |
| Hispanic | 6006 (3.9) | 31,287 (3.9) | 0.09 |
| White | 125,853 (82.7) | 641,237 (80.6) | 5.51 |
| Othera | 8143 (5.4) | 43,051 (5.4) | −0.25 |
| eGFR, mean (SD), ml/min per 1.73 m2 | 80.5 (18.9) | 81.7 (19.4) | −6.17 |
| eGFR category, n (%) | |||
| ≥60 ml/min per 1.73 m2 | 130,506 (85.8) | 687,461 (86.4) | −1.70 |
| 30–59 ml/min per 1.73 m2 | 20,427 (13.4) | 102,392 (12.9) | 1.68 |
| <30 ml/min per 1.73 m2 | 1168 (0.8) | 5946 (0.7) | 0.24 |
| BMI, mean (SD), kg/m2 | 30.9 (6.5) | 31.3 (6.9) | −5.45 |
| Smoking, n (%) | |||
| Current | 9620 (6.3) | 63,557 (8.0) | −6.23 |
| Former | 25,142 (16.5) | 137,723 (17.3) | −2.06 |
| Never | 117,339 (77.1) | 594,519 (74.7) | 5.64 |
| SBP, mean (SD), mm Hg | 128.1 (16.0) | 129.1 (16.5) | −6.08 |
| Serum K, mean (SD), mmol/L | 4.31 (0.41) | 4.28 (0.41) | 6.92 |
| Comorbidities, n (%) | |||
| Diabetes | 42,904 (28.2) | 236,744 (29.7) | −3.38 |
| Hypertension | 100,712 (66.2) | 535,710 (67.3) | −2.35 |
| Coronary artery disease | 39,031 (25.7) | 185,365 (23.3) | 5.57 |
| Cerebrovascular disease | 14,506 (9.5) | 84,096 (10.6) | −3.38 |
| Heart failure | 8156 (5.4) | 51,274 (6.4) | −4.46 |
| Hypothyroidism | 26,445 (17.4) | 120,861 (15.2) | 6.07 |
| Concomitant medications, n (%) | |||
| ACE inhibitors | 28,348 (18.6) | 182,927 (23.0) | −10.46 |
| ARBs | 18,941 (12.5) | 81,497 (10.2) | 7.19 |
| Aldosterone antagonists | 2022 (1.3) | 11,702 (1.5) | −1.18 |
| Other HTN medications | 49,370 (32.5) | 278,790 (35.0) | −5.41 |
| SGLT2 inhibitors | 1594 (1.0) | 6259 (0.8) | 2.88 |
| DPP4 inhibitors | 3189 (2.1) | 13,004 (1.6) | 3.57 |
| GLP1RAs | 1731 (1.1) | 6883 (0.9) | 2.88 |
| Other oral DM medications | 19,859 (13.1) | 123,491 (15.5) | −6.87 |
| Insulin | 6236 (4.1) | 35,886 (4.5) | −1.99 |
| DOACs | 1715 (1.1) | 11,651 (1.5) | −2.85 |
| Warfarin | 2481 (1.6) | 15,881 (2.0) | −2.64 |
| Antiplatelets | 7879 (5.2) | 50,054 (6.3) | −4.63 |
| Cyclosporine | 143 (0.1) | 474 (0.1) | 1.35 |
| Itraconazole | 28 (0.0) | 152 (0.0) | −0.05 |
| Clarithromycin | 105 (0.1) | 435 (0.1) | 0.60 |
| Protease inhibitors | 76 (0.0) | 323 (0.0) | 0.46 |
| Fibrates | 5605 (3.7) | 18,874 (2.4) | 8.28 |
| Niacin | 1422 (0.9) | 3241 (0.4) | 7.54 |
| PCSK9 inhibitor | 100 (0.1%) | 59 (0.0) | 4.50 |
| Ezetimibe | 3850 (2.5) | 7251 (0.9) | 15.08 |
| Baseline year, n (%) | |||
| 2011 | 12,993 (8.5) | 23,568 (3.0) | 29.15 |
| 2012 | 14,013 (9.2) | 47,819 (6.0) | 12.99 |
| 2013 | 12,756 (8.4) | 65,200 (8.2) | 0.70 |
| 2014 | 15,270 (10.0) | 94,762 (11.9) | −5.83 |
| 2015 | 14,744 (9.7) | 116,337 (14.6) | −14.29 |
| 2016 | 14,646 (9.6) | 120,628 (15.2) | −15.83 |
| 2017 | 18,307 (12.0) | 115,576 (14.5) | −7.14 |
| 2018 | 21,633 (14.2) | 105,943 (13.3) | 2.67 |
| 2019 | 27,739 (18.2) | 105,966 (13.3) | 14.16 |
| Total cholesterolb | |||
| Missing, n (%) | 42,028 (27.6) | 219,096 (27.5) | |
| Mean (SD), mmol/L | 4.9 (1.0) | 5.0 (1.0) | −7.16 |
| LDL cholesterolb | |||
| Missing | 32,169 (21.1) | 164,774 (20.7) | |
| Mean (SD), mmol/L | 3.5 (1.0) | 3.5 (1.0) | 2.61 |
BMI, body mass index; SBP, systolic BP; ACE inhibitors, angiotensin converting enzyme inhibitors; ARBs, angiotensin II receptor blockers; SGLT2 inhibitors, sodium-glucose cotransporter 2 inhibitors; DPP4 inhibitors, dipeptidyl peptidase-4 inhibitor; GLP1RAs, glucagon-like peptide 1 receptor agonists; DOACs, direct oral anticoagulants; PCSK9 inhibitors, proprotein convertase subtilisin/kexin type 9 inhibitors.
Other includes Asian patients and patients with unknown race/ethnicity.
Only among patients with values available. Cholesterol level was not included in the propensity score model.
Hematuria
Overall, we identified hematuria in 2.9% of patients (5178 [3.4%] for rosuvastatin versus 22,604 [2.8%] atorvastatin) during a median follow-up of 3.0 years. In the IPTW analysis, incidence rates (95% confidence interval [95% CI]) of hematuria were 9.2 (8.9 to 9.5) and 8.6 (8.4 to 8.7) events per 1000 person-years in the rosuvastatin and atorvastatin groups, respectively. Incidence rates among those with eGFR <30 ml/min per 1.73 m2 (23.1 events for rosuvastatin versus 18.8 events for atorvastatin, per 1000 person-years) were approximately two-fold higher than those with eGFR ≥60 ml/min per 1.73 m2 (8.4 events for rosuvastatin versus 7.9 events for atorvastatin, per 1000 person-years). In survival analysis, rosuvastatin (versus atorvastatin) use was associated with slightly higher risk of hematuria (IPTW-HR, 1.08; 95% CI, 1.04 to 1.11), consistently across eGFR levels (P for heterogeneity=0.40, Figure 1A and Table 2). The results were similar in sensitivity analyses of patients with at least two prescriptions of each study medication (Supplemental Table 5) and using as-treated approach (Supplemental Table 6).
Figure 1.
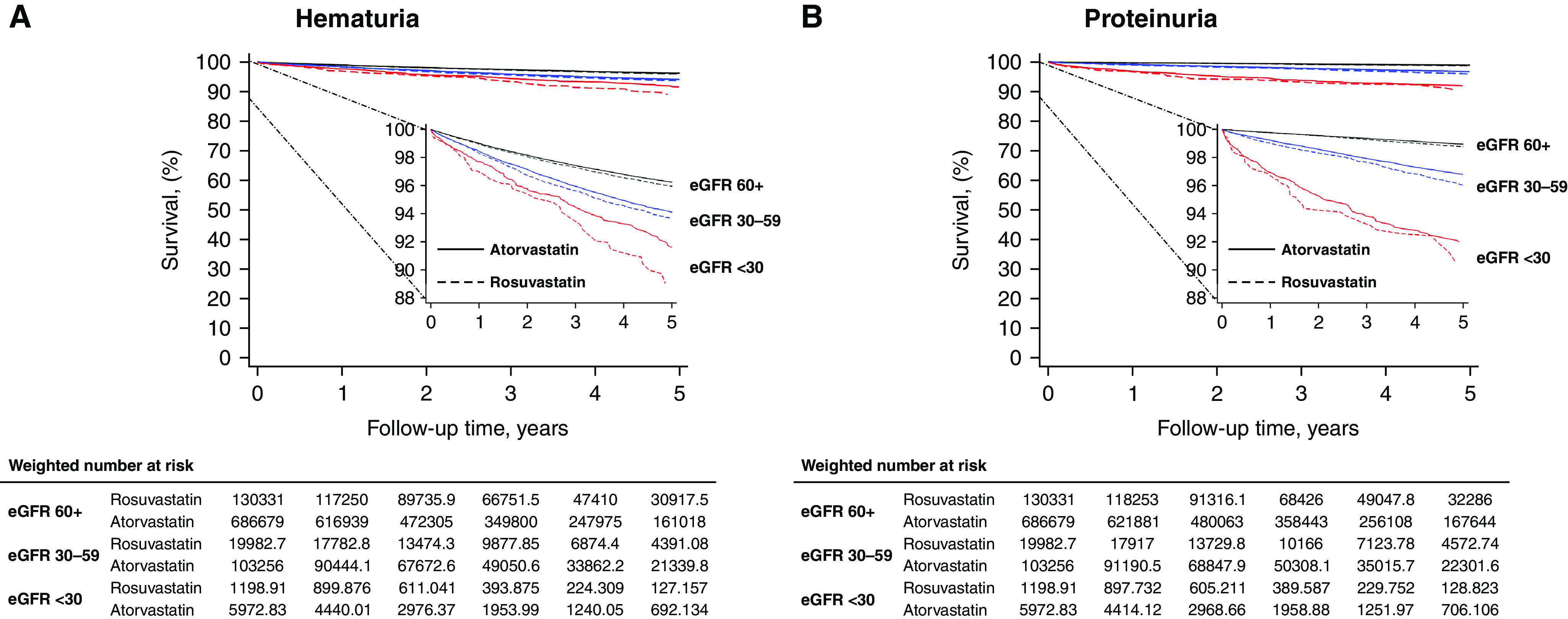
Kaplan–Meier curves by eGFR levels and treatment group in the weighted study population.
Table 2.
Risk of outcomes comparing rosuvastatin use versus atorvastatin use, overall and across eGFR levels
| Outcome | Unweighted No. of Events/N | IPTW-IR (95% CI), per 1000 PYs | IPTW-IRD (95% CI), per 1000 PYs | P for Heterogeneitya | IPTW-HR (95% CI) | P for Heterogeneitya | ||
|---|---|---|---|---|---|---|---|---|
| Rosuvastatin | Atorvastatin | Rosuvastatin | Atorvastatin | |||||
| Hematuria | ||||||||
| Overall | 5178/152,101 | 22,604/795,799 | 9.2 (8.9 to 9.5) | 8.6 (8.4 to 8.7) | 0.63 (0.31 to 0.95) | 1.08 (1.04 to 1.11) | ||
| eGFR (ml/min per 1.73 m2) | ||||||||
| ≥60 | 4138/130,506 | 18,215/687,461 | 8.4 (8.1 to 8.7) | 7.9 (7.8 to 8.0) | 0.53 (0.20 to 0.86) | 0.42 | 1.07 (1.03 to 1.11) | 0.40 |
| 30–59 | 971/20,427 | 4115/102,392 | 13.7 (12.7 to 14.7) | 12.7 (12.3 to 13.1) | 0.98 (−0.09 to 2.05) | 1.09 (1.01 to 1.18) | ||
| <30 | 69/1168 | 274/5946 | 23.1 (17.6 to 30.9) | 18.8 (16.7 to 21.3) | 4.25 (−2.58 to 11.07) | 1.22 (0.90 to 1.64) | ||
| Proteinuria | ||||||||
| Overall | 1776/152,101 | 7495/795,799 | 3.2 (3.1 to 3.4) | 2.8 (2.7 to 2.8) | 0.47 (0.28 to 0.66) | 1.17 (1.10 to 1.25) | ||
| eGFR (ml/min per 1.73 m2) | ||||||||
| ≥60 | 1155/130,506 | 4971/687,461 | 2.4 (2.3 to 2.6) | 2.1 (2.0 to 2.2) | 0.33 (0.15 to 0.52) | 0.16 | 1.16 (1.07 to 1.25) | 0.74 |
| 30–59 | 552/20,427 | 2225/102,392 | 7.8 (7.1 to 8.6) | 6.7 (6.4 to 7.0) | 1.12 (0.32 to 1.93) | 1.18 (1.06 to 1.31) | ||
| <30 | 69/1168 | 299/5946 | 22.6 (17.1 to 30.6) | 20.5 (18.3 to 23.1) | 2.10 (−4.83 to 9.03) | 1.10 (0.81 to 1.50) | ||
| Urinary tract infection (negative control outcome) | ||||||||
| Overall | 11,829/152,101 | 55,213/795,799 | 21.9 (21.4 to 22.4) | 21.5 (21.3 to 21.7) | 0.42 (−0.08 to 0.93) | 1.02 (1.00 to 1.04) | ||
| eGFR (ml/min per 1.73 m2) | ||||||||
| ≥60 | 8962/130,506 | 41,667/687,461 | 19.0 (18.6 to 19.5) | 18.5 (18.3 to 18.7) | 0.50 (−0.001 to 1.0) | 0.32 | 1.03 (1.00 to 1.05) | 0.31 |
| 30–59 | 2676/20,427 | 12,579/102,392 | 39.9 (38.2 to 41.8) | 40.8 (40.1 to 41.5) | −0.86 (−2.8 to 1.1) | 0.99 (0.94 to 1.03) | ||
| <30 | 191/1168 | 967/5946 | 68.2 (57.7 to 81.0) | 72.0 (67.4 to 76.9) | −3.8 (−16.2 to 8.7) | 0.94 (0.78 to 1.12) | ||
IPTW-HRs were from stratified Cox proportional hazards regression models by cohort. IPTW, inverse-probability of treatment weight; IR, incidence rate; PYs, person-years; IRD, incidence rate difference.
P for heterogeneity in IRD across eGFR subgroups was estimated using fixed effects meta-analysis and P for heterogeneity in HR was estimated using stratified Cox models with interaction term between rosuvastatin use and eGFR category.
Proteinuria
Overall, we identified proteinuria in 1.0% of patients (1776 [1.2%] for rosuvastatin versus 7495 [0.9%] for atorvastatin) during a median follow-up of 3.1 years. In the IPTW analysis, incidence rates (95% CI) of proteinuria were 3.2 (3.1 to 3.4) and 2.8 (2.7 to 2.8) events per 1000 person-years in the rosuvastatin and atorvastatin groups, respectively. Incidence rates among those with eGFR <30 ml/min per 1.73 m2 (22.6 events for rosuvastatin versus 20.5 events for atorvastatin, per 1000 person-years) were approximately 9 times higher than those with eGFR ≥60 ml/min per 1.73 m2 (2.4 events for rosuvastatin versus 2.1 events for atorvastatin, per 1000 person-years). In survival analysis, rosuvastatin (versus atorvastatin) use was associated with slightly higher risk of proteinuria (IPTW-HR, 1.17; 95% CI, 1.10 to 1.25, consistently across eGFR levels (P for heterogeneity=0.74, Figure 1B and Table 2). The results were similar when the analysis was limited to patients with at least two prescriptions of each study medication (Supplemental Table 5) and in as-treated analysis (Supplemental Table 6).
KFRT
Rosuvastatin (versus atorvastatin) use was associated with slightly higher risk of KFRT (IPTW-HR, 1.15; 95% CI, 1.02 to 1.30), consistently across eGFR levels (P for heterogeneity=0.71, Table 3).
Table 3.
Risk of KFRT comparing rosuvastatin use versus atorvastatin use, overall and across eGFR levels
| Group | Unweighted No. of Events/N | IPTW-IR (95% CI), per 1000 PYs | IPTW-IRD (95% CI), per 1000 PYs | P for Heterogeneitya | IPTW-HR (95% CI) | P for Heterogeneitya | ||
|---|---|---|---|---|---|---|---|---|
| Rosuvastatin | Atorvastatin | Rosuvastatin | Atorvastatin | |||||
| Overall eGFR (ml/min per 1.73 m2) |
464/152,101 | 2190/795,799 | 0.92 (0.82 to 1.03) | 0.80 (0.76 to 0.83) | 0.12 (0.02 to 0.23) | 1.15 (1.02 to 1.30) | ||
| ≥60 | 125/130,506 | 568/687,461 | 0.27 (0.22 to 0.34) | 0.24 (0.22 to 0.26) | 0.034 (−0.03 to 0.10) | 0.31 | 1.14 (0.90 to 1.44) | 0.71 |
| 30–59 | 171/20,427 | 827/102,392 | 2.54 (2.14 to 3.03) | 2.40 (2.24 to 2.57) | 0.14 (−0.33 to 0.61) | 1.06 (0.88 to 1.28) | ||
| <30 | 168/1168 | 795/5946 | 60.9 (50.9 to 73.5) | 52.1 (48.5 to 56.0) | 8.8 (−2.9 to 20.5) | 1.21 (0.99 to 1.47) | ||
IPTW-HRs were from stratified Cox proportional hazards regression models by cohort. IPTW, inverse-probability of treatment weight; IR, incidence rate; PYs, person-years; IRD, incidence rate difference.
P for heterogeneity in IRD across eGFR subgroups was estimated using fixed-effects meta-analysis and P for heterogeneity in HR was estimated using stratified Cox models with interaction term between rosuvastatin use and eGFR category.
Negative Control and ASCVD Benefit Outcome
Rosuvastatin users had a similar risk of urinary tract infection compared with atorvastatin users in all eGFR categories (IPTW-HR, 1.02; 95% CI, 1.00 to 1.04, Table 2). The risk of ASCVD was similar between the two groups (IPTW-HR, 1.02; 95% CI, 0.96 to 1.08) consistently across eGFR categories (P for heterogeneity=0.24, Supplemental Table 7).
Rosuvastatin Dosing Patterns and Dose-Risk Gradient
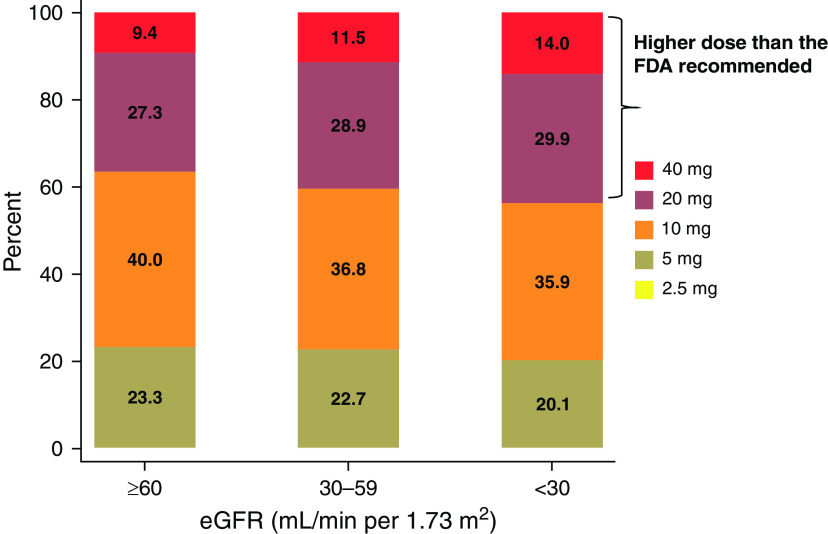
Among patients with eGFR <30 ml/min per 1.73 m2, 80% started rosuvastatin with a higher dose (10, 20, or 40 mg) than the FDA-recommended starting dose of 5 mg. In total, 44% received an initial rosuvastatin dose that exceeded the maximal recommended dose of 10 mg (20 mg, 29.9%; 40 mg, 14.0%, Figure 2). In the adjusted analysis, there was an increasing risk gradient for higher rosuvastatin dose for hematuria and proteinuria, but not for urinary tract infection (negative control outcome) (Figure 3). The results were consistent in the sensitivity analysis, after excluding individuals with a recent (<30 days) heart failure or myocardial infarction or very high cholesterol value (Supplemental Figure 3 and Supplemental Figure 4). When creatinine clearance was used instead of eGFR categories, 36.3% were prescribed rosuvastatin dose higher than FDA recommendation (20 mg, 23.3%; 40 mg, 13.0%, Supplemental Figure 5).
Figure 2.
Prescribed rosuvastatin dose by eGFR category. Rosuvastatin initiators between 2011 and 2019 who had eGFR measurements within 1 year before medication initiation (eGFR ≥60 ml/min per 1.73 m2, n=150,591; eGFR 30–59 ml/min per 1.73 m2, n=24,278; eGFR <30 ml/min per 1.73 m2, n=1504).
Figure 3.
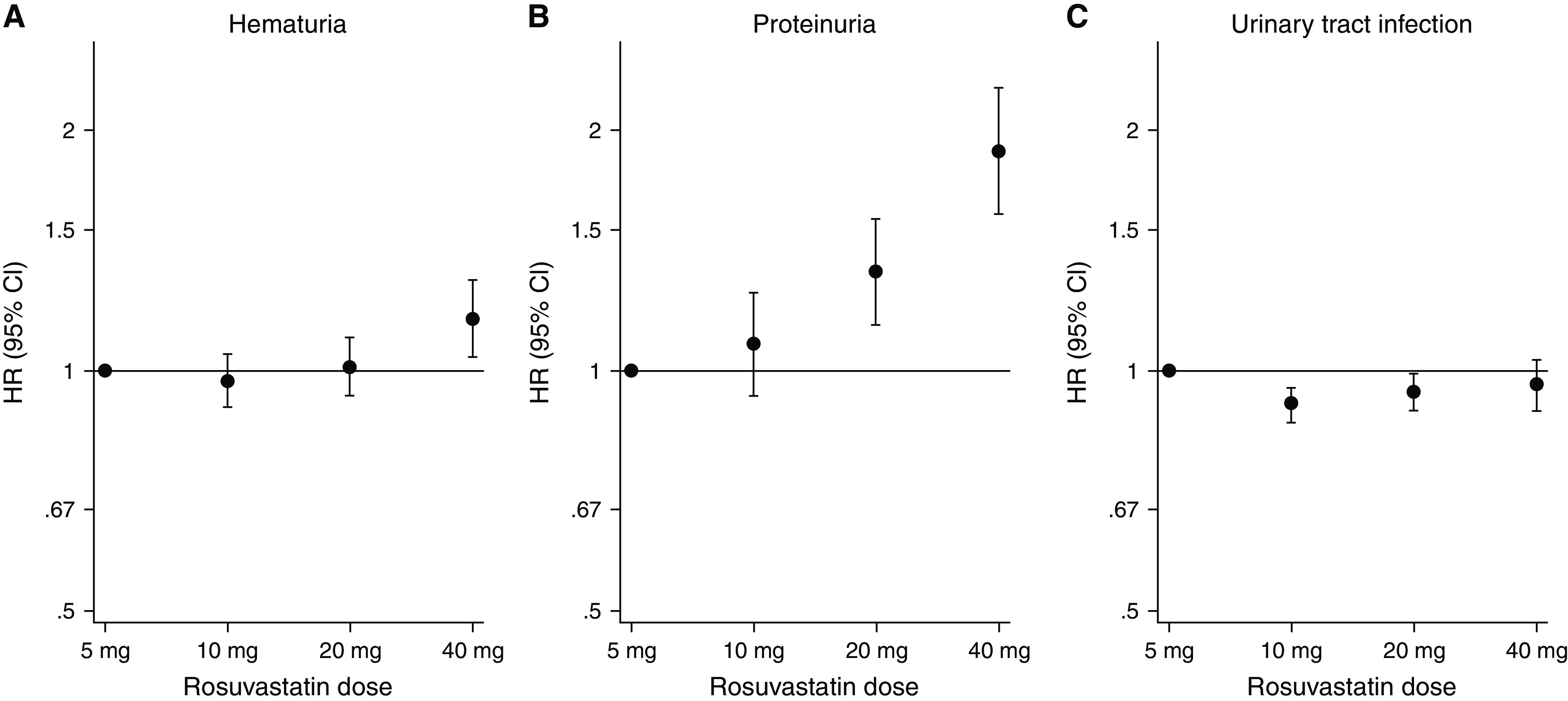
Risks of outcomes comparing different doses of rosuvastatin among rosuvastatin users. Reference dose 5 mg.
Discussion
In this large observational cohort study spanning 40 health systems across the United States and nearly 1 million patients initiating statin therapy, patients treated with rosuvastatin had a slightly higher risk of hematuria, proteinuria, and KFRT than those treated with atorvastatin, whereas the benefit of ASCVD was similar. Nearly 45% of patients with severe CKD were on higher dose of rosuvastatin than that recommended by the FDA, and a rosuvastatin dose-risk gradient was consistently observed for both hematuria and proteinuria. Taken together, these findings suggest the need for closer attention and monitoring to rosuvastatin use, particularly in patients who are receiving high doses or with severe CKD.
The mechanism of proteinuria and hematuria with statin use has not been fully elucidated. In vitro studies of animal and human kidney cells demonstrated a class effect, whereby high-dose statin therapy inhibited protein uptake by the proximal nephron as a result of hydroxymethylglutaryl–coenzyme A reductase inhibition in the proximal tubule cells.22,23 Another potential mechanism is statin-induced mitochondrial dysfunction and oxidation injury due to depletion of mevalonate-derived endproduct ubiquinone.8,24 The extent of renal excretion across the different statins may be important in this pathologic process. Approximately 10% of rosuvastatin, but only 1% of atorvastatin, is renally excreted.25,26 Therefore, systemic exposure of rosuvastatin may be more likely to increase as kidney function declines, particularly in patients with severe CKD.27 Indeed, the FDA’s rosuvastatin dosing guidance for patients with severe CKD (i.e., creatinine clearance <30 ml/min) was on the basis of the data that there was 3.16-fold increased rosuvastatin exposure in individuals with creatinine clearance <30 ml/min compared with those with normal kidney function.28
This study is one of the first and largest real-world studies examining rosuvastatin versus atorvastatin on the risk of hematuria and proteinuria and KFRT across the range of eGFR in a heterogeneous population. Shortly after the FDA approval of rosuvastatin, data from the FDA adverse events reporting system showed higher 1-year rates of proteinuria with rosuvastatin use than other statins.29 This study provided important safety signals of rosuvastatin in a timely manner; however, it was limited by a small sample size with short follow-up times, potential reporting bias (i.e., preferential reporting of adverse events with rosuvastatin [a newly marketed drug] than other statins), and lack of information on rosuvastatin dose or kidney function. Other observational studies reporting no increased risk of adverse events with rosuvastatin use (versus other statin use) were limited by small number of adverse events, incomplete adjustment for confounding, or lack of proteinuria and hematuria outcomes.30–32 More recent safety data on rosuvastatin are mostly from case reports.8,9,33,34
There have been small clinical trials evaluating rosuvastatin versus atorvastatin in patients with CKD, albeit with different research questions than our study. In an analysis of 353 patients with diabetes and proteinuria (urine protein creatinine ratio 500–5000 mg/g) enrolled in the PLANET I clinical trial, atorvastatin 80 mg reduced proteinuria with a stable eGFR compared with rosuvastatin 40 or 10 mg, which had no effect on proteinuria and decreased eGFR over the 1 year of follow-up.35 A follow-up post-hoc analysis combining PLANET I (n=353, diabetes) and II (n=220, no diabetes) also suggested atorvastatin may have a safer kidney profile than rosuvastatin, finding decreased proteinuria and less decline in eGFR in the atorvastatin arm.35
Our study suggests rosuvastatin confers a dose-related risk of adverse outcomes. The dose-related risk of statin-induced rhabdomyolysis is well established36; however, previous data were insufficient to determine the risk of statin-related hematuria and proteinuria across different doses. Among the patients with hematuria and proteinuria in the preapproval clinical trials of rosuvastatin, there appeared to be a dose-related risk, beginning at 40 mg and greater at 80 mg (the latter of which was subsequently not approved).7,37 In the analysis of >2 million statin users, the use of high-potency statins (including rosuvastatin >10 mg) was associated with higher risk of AKI compared with the use of low-potency statins.38
Although the overall absolute and relative risk of hematuria and proteinuria with rosuvastatin use was low, patients with eGFR <30 ml/min per 1.73 m2 were at the highest risk, with approximately two-fold risk of hematuria and nine-fold risk of proteinuria than those with eGFR ≥60 ml/min per 1.73 m2. We observed a higher risk of KFRT with rosuvastatin use and similar cardiovascular benefits between rosuvastatin and atorvastatin group, and evidence that rosuvastatin may cause proteinuria and hematuria, especially with high dose. Thus, high-dose rosuvastatin may not merit the risk, even if small, particularly in low eGFR. Future studies are warranted to shed light on the discrepancy between real-world practice and FDA dosing recommendations.
There are several limitations to this study. Despite the use of IPTW to control for confounding, there might still exist residual or unmeasured confounding. For example, we applied IPTW within each cohort to adjust for heterogeneity across cohorts,16 but there is likely heterogeneity within each cohort (e.g., different practices within a health care organization). However, the null results with a negative control outcome are reassuring that residual confounding is less likely. There is a potential for selection bias, although the probability of exclusion due to missing data were similar between the treatment groups. Despite the large sample size in our study, subgroups of eGFR <30 ml/min per 1.73 m2 were relatively small, with <1% of the study population. Medication information is from prescription data, and we cannot verify whether the prescription was filled. The exposure to medication is on the basis of initial prescription after the intention-to-treat principle, and thus adherence to the prescribed dosing regimen was not taken into account. However, a previous study suggests medication adherence is similar between rosuvastatin and atorvastatin users.39 Indeed, our results were consistent in the as-treated analysis. We could not capture patient outcomes occurring outside the health care organizations. Moreover, we may have underestimated incidence rates of hematuria and proteinuria because urine was not routinely or universally monitored in this real-world data. However, our estimates of absolute risk with atorvastatin were similar to safety data from previous trials with scheduled monitoring. For example, in 9656 atorvastatin users in the TNT (Treating to New Target) trial, 3.7% and 1.6% of patients experienced hematuria and proteinuria during a median follow-up of 5 years,40 respectively, whereas we identified hematuria and proteinuria in 2.8% and 0.9% of our study population during a median follow-up of 3 years. Given we defined hematuria on the basis of a dipstick, we could not distinguish hematuria from that of nonrenal origin, such as subclinical rhabdomyolysis. To address this concern, we excluded individuals with history of rhabdomyolysis and adjusted for risk factors of rhabdomyolysis in our analysis. We counted only severe albuminuria (urine albumin-creatinine ratio >300 mg/g) in our study. Lastly, our findings may have limited generalizability to uninsured patients because our study included mostly insured patients with active engagement with the health systems.
In summary, we found that, compared with atorvastatin, rosuvastatin use was associated with slightly higher risks of hematuria and proteinuria in a dose-dependent manner. Correspondingly, rosuvastatin use was associated with higher risk of KFRT, whereas the cardiovascular benefits were similar. Almost 45% of patients with eGFR <30 ml/min per 1.73 m2 were prescribed higher doses of rosuvastatin than the dose recommended by the FDA. Thus, our findings emphasize the need for greater care in prescribing and monitoring of rosuvastatin, particularly in patients who are receiving high doses, or with severe CKD.
Disclosures
A. Chang reports having consultancy agreements with Amgen, Novartis, and Reata; reports receiving research funding from a Novo Nordisk Investigator Sponsored Study; reports having an advisory or leadership role with Reata, Relypsa; and reports having other interests or relationships with National Kidney Foundation grant support and the National Kidney Foundation Patient Network. D. Fine reports having consultancy agreements with Fresenius Kidney Care Medical Advisory Board and GlaxoSmithKline Data and Safety Monitoring Board; and reports having an advisory or leadership role with the Fresenius Medical Corporation Medical Advisory Board. J. Shin reports receiving research funding to the institute for research from Merck and the National Institutes of Health. L. Inker reports having consultancy agreements with Diamtrix; reports receiving research funding to the institute for research and contracts with the National Institutes of Health, National Kidney Foundation, Omeros, and Reata Pharmaceuticals; reports having consulting agreements to her institution with Omeros and Tricida Inc.; reports having an advisory or leadership role with the Alport Syndrome Foundation; and reports having other interests or relationships as a member of the American Society of Nephrology, the National Kidney Disease Education Program, and the National Kidney Foundation. M. Grams reports having an advisory or leadership role with American Journal of Kidney Disease, Clinical Journal of the American Soceity of Nephrology, Journal of the American Society of Nephrology Editorial Board, Kidney Disease Improving Global Outcomes Executive Committee, National Kidney Foundation Scientific Advisory Board, and the United States Renal Data System Scientific Advisory Board; and reports having other interests or relationships with grant funding from National Kidney Foundation, which receives funding from multiple pharmaceutical companies, and grant funding from the National Institutes of Health. S. Dunning reports employment with and having an ownership interest in Outset Medical, Inc. T. Nolin reports having consultancy agreements with CytoSorbents and MediBeacon; reports having an ownership interest with Healthmap Solutions; and reports having an advisory or leadership role with the American College of Clinical Pharmacology Board of Regents, Clinical Journal of the American Society of Nephrology Editorial Board, Healthmap Solutions Scientific Advisory Board, Kidney Health Initiative Board of Directors, and McGraw-Hill Editor. All remaining authors have nothing to disclose.
Funding
This work was supported by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases grants R01DK115534 (Principal Investigators M. Grams and L. Inker) and K01DK121825 (Principal Investigator J. Shin).
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Author Contributions
D. Fine, M. Grams, and J. Shin conceptualized the study; Y. Sang was responsible for the data curation and the formal analysis; M. Grams, L. Inker, and J. Shin were responsible for the funding acquisition; M. Grams and J. Shin were responsible for the investigation and the methodology; S. Dunning was responsible for the resources; Y. Sang was responsible for the software; M. Grams and J. Shin provided supervision; J. Shin wrote the original draft; and A. Chang, S. Dunning, D. Fine, M. Grams, L. Inker, T. Nolin, Y. Sang, J. Shin, and A. Surapaneni reviewed and edited the manuscript.
Data Sharing Statement
Data are not available per Optum Labs data use agreement; verification analysis requests will be run.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2022020135/-/DCSupplemental.
Supplemental Table 1. Specification and emulation of a target trial of rosuvastatin therapy (versus atorvastatin therapy) and risk of hematuria and proteinuria using observation data.
Supplemental Table 2. International Classification of Diseases (ICD) codes.
Supplemental Table 3. Inverse probability-of-treatment–weighted urine testing rates.
Supplemental Table 4. Comparison of patient characteristics between those included and excluded due to missing data.
Supplemental Table 5. Results in the users of rosuvastatin or atorvastatin with at least two prescriptions.
Supplemental Table 6. As-treated analysis: comparing rosuvastatin use versus atorvastatin use, overall and across eGFR levels.
Supplemental Table 7. Risks of atherosclerotic cardiovascular disease associated with rosuvastatin versus atorvastatin across eGFR levels.
Supplemental Figure 1. Derivation of study population in 40 health care organizations (cohorts) in Optum Labs Data Warehouse.
Supplemental Figure 2. Standardized mean differences (SMD) (%) across covariates in all individual cohorts.
Supplemental Figure 3. Prescribed rosuvastatin dose by eGFR category after excluding individuals with recent heart failure or myocardial infarction or very high cholesterol value.
Supplemental Figure 4. Risks of outcomes comparing different doses of rosuvastatin among rosuvastatin users after excluding individuals with recent heart failure or myocardial infarction or very high cholesterol value.
Supplemental Figure 5. Prescribed rosuvastatin dose by creatinine clearance category.
References
- 1.Jones PH, Davidson MH, Stein EA, Bays HE, McKenney JM, Miller E, et al. ; STELLAR Study Group : Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR* Trial). Am J Cardiol 92: 152–160, 2003 [DOI] [PubMed] [Google Scholar]
- 2.The statin wars: Why AstraZeneca must retreat. Lancet 362: 1341, 2003 [PubMed] [Google Scholar]
- 3.Cohen JS: Should rosuvastatin be withdrawn from the market? Lancet 364: 1579, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Florentinus SR, Heerdink ER, Klungel OH, de Boer A: Should rosuvastatin be withdrawn from the market? Lancet 364: 1577, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Kastelein JJ: Should rosuvastatin be withdrawn from the market? Lancet 364: 1577–1578, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Olsson GO: Safety and efficacy of rosuvastatin. Lancet 364: 135, 2004 [DOI] [PubMed] [Google Scholar]
- 7.FDA : Center for drug evaluation and research. Approval package for: Application number 21-366. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2003/21-366_Crestor_Medr_P1.pdf. Accessed April 27, 2021.
- 8.van Zyl-Smit R, Firth JC, Duffield M, Marais AD: Renal tubular toxicity of HMG-CoA reductase inhibitors. Nephrol Dial Transplant 19: 3176–3179, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Ward FL, John R, Bargman JM, McQuillan RF: Renal tubular toxicity associated with rosuvastatin therapy. Am J Kidney Dis 69: 473–476, 2017 [DOI] [PubMed] [Google Scholar]
- 10.Wolfe S: Rosuvastatin: Winner in the statin wars, patients’ health notwithstanding. BMJ 350: h1388, 2015 [DOI] [PubMed] [Google Scholar]
- 11.The US Food and Drug Administration : Highlights of prescribing information for Crestor (rosuvastatin calcium) tablets. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021366s016lbl.pdf. Accessed April 27, 2021.
- 12.Optum Labs [website] . Available at: https://www.optumlabs.com/. Accessed April 27, 2021.
- 13.Hernán MA, Robins JM: Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol 183: 758–764, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sumida K, Nadkarni GN, Grams ME, Sang Y, Ballew SH, Coresh J, et al. ; Chronic Kidney Disease Prognosis Consortium : Conversion of urine protein-creatinine ratio or urine dipstick protein to urine albumin-creatinine ratio for use in chronic kidney disease screening and prognosis: An individual participant-based meta-analysis. Ann Intern Med 173: 426–435, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. : 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 139: e1082–e1143, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arpino B, Cannas M: Propensity score matching with clustered data. An application to the estimation of the impact of caesarean section on the Apgar score. Stat Med 35: 2074–2091, 2016 [DOI] [PubMed] [Google Scholar]
- 17.Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Stürmer T: Variable selection for propensity score models. Am J Epidemiol 163: 1149–1156, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int 3[Suppl]: 1–150, 2013. Available at https://kdigo.org/wp-content/uploads/2017/02/KDIGO_2012_CKD_GL.pdf. Accessed June 14, 2022 [DOI] [PubMed] [Google Scholar]
- 20.Jardine MJ, Zhou Z, Mahaffey KW, Oshima M, Agarwal R, Bakris G, et al. ; CREDENCE Study Investigators : Renal, cardiovascular, and safety outcomes of canagliflozin by baseline kidney function: A secondary analysis of the CREDENCE randomized trial. J Am Soc Nephrol 31: 1128–1139, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cockcroft DW, Gault MH: Prediction of creatinine clearance from serum creatinine. Nephron 16: 31–41, 1976 [DOI] [PubMed] [Google Scholar]
- 22.Verhulst A, D’Haese PC, De Broe ME: Inhibitors of HMG-CoA reductase reduce receptor-mediated endocytosis in human kidney proximal tubular cells. J Am Soc Nephrol 15: 2249–2257, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Sidaway JE, Davidson RG, McTaggart F, Orton TC, Scott RC, Smith GJ, et al. : Inhibitors of 3-hydroxy-3-methylglutaryl-CoA reductase reduce receptor-mediated endocytosis in opossum kidney cells. J Am Soc Nephrol 15: 2258–2265, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Bargossi AM, Grossi G, Fiorella PL, Gaddi A, Di Giulio R, Battino M: Exogenous CoQ10 supplementation prevents plasma ubiquinone reduction induced by HMG-CoA reductase inhibitors. Mol Aspects Med 15[Suppl]: s187–s193, 1994 [DOI] [PubMed] [Google Scholar]
- 25.Stern RH, Yang BB, Horton M, Moore S, Abel RB, Olson SC: Renal dysfunction does not alter the pharmacokinetics or LDL-cholesterol reduction of atorvastatin. J Clin Pharmacol 37: 816–819, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Martin PD, Warwick MJ, Dane AL, Hill SJ, Giles PB, Phillips PJ, et al. : Metabolism, excretion, and pharmacokinetics of rosuvastatin in healthy adult male volunteers. Clin Ther 25: 2822–2835, 2003 [DOI] [PubMed] [Google Scholar]
- 27.McKenney JM: Efficacy and safety of rosuvastatin in treatment of dyslipidemia. Am J Health Syst Pharm 62: 1033–1047, 2005 [DOI] [PubMed] [Google Scholar]
- 28.FDA : Center for Drug Evaluation and Research. Approval package for: Application number 21-366. Clinical pharmacology and biophamaceutics review. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2003/21-366_Crestor_BioPharmr.pdf. Accessed April 22, 2022.
- 29.Alsheikh-Ali AA, Ambrose MS, Kuvin JT, Karas RH: The safety of rosuvastatin as used in common clinical practice: A postmarketing analysis. Circulation 111: 3051–3057, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Goettsch WG, Heintjes EM, Kastelein JJ, Rabelink TJ, Johansson S, Herings RM: Results from a rosuvastatin historical cohort study in more than 45,000 Dutch statin users, a PHARMO study. Pharmacoepidemiol Drug Saf 15: 435–443, 2006 [DOI] [PubMed] [Google Scholar]
- 31.García Rodríguez LA, Herings R, Johansson S: Use of multiple international healthcare databases for the detection of rare drug-associated outcomes: A pharmacoepidemiological programme comparing rosuvastatin with other marketed statins. Pharmacoepidemiol Drug Saf 19: 1218–1224, 2010 [DOI] [PubMed] [Google Scholar]
- 32.García-Rodríguez LA, Massó-González EL, Wallander MA, Johansson S: The safety of rosuvastatin in comparison with other statins in over 100,000 statin users in UK primary care. Pharmacoepidemiol Drug Saf 17: 943–952, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Chitralli D, Raheja R, Br K: Clinical rhabdomyolysis with acute kidney injury secondary to high-intensity rosuvastatin use: A case report. Cureus 12: e10932, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hussain K, Xavier A: Rosuvastatin-related rhabdomyolysis causing severe proximal paraparesis and acute kidney injury. BMJ Case Rep 12: e229244, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Zeeuw D, Anzalone DA, Cain VA, Cressman MD, Heerspink HJ, Molitoris BA, et al. : Renal effects of atorvastatin and rosuvastatin in patients with diabetes who have progressive renal disease (PLANET I): A randomised clinical trial. Lancet Diabetes Endocrinol 3: 181–190, 2015 [DOI] [PubMed] [Google Scholar]
- 36.Holbrook A, Wright M, Sung M, Ribic C, Baker S: Statin-associated rhabdomyolysis: Is there a dose-response relationship? Can J Cardiol 27: 146–151, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Wolfe SM: Dangers of rosuvastatin identified before and after FDA approval. Lancet 363: 2189–2190, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Dormuth CR, Hemmelgarn BR, Paterson JM, James MT, Teare GF, Raymond CB, et al. ; Canadian Network for Observational Drug Effect Studies : Use of high potency statins and rates of admission for acute kidney injury: Multicenter, retrospective observational analysis of administrative databases. BMJ 346: f880, 2013 [DOI] [PubMed] [Google Scholar]
- 39.Naci H, Brugts J, Ades T: Comparative tolerability and harms of individual statins: A study-level network meta-analysis of 246 955 participants from 135 randomized, controlled trials. Circ Cardiovasc Qual Outcomes 6: 390–399, 2013 [DOI] [PubMed] [Google Scholar]
- 40.Shepherd J, Kastelein JJ, Bittner V, Deedwania P, Breazna A, Dobson S, et al. ; TNT (Treating to New Targets) Investigators : Intensive lipid lowering with atorvastatin in patients with coronary heart disease and chronic kidney disease: The TNT (Treating to New Targets) study. J Am Coll Cardiol 51: 1448–1454, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.