Abstract
Metastatic castration resistant prostate cancer (CRPC) is an inevitably fatal disease. However, in recent years, several treatments have been shown to improve the outcome of CRPC patients both in the non-metastatic (nmCRPC) as well as the metastatic setting (mCRPC). In nmCRPC patients with a PSA doubling time <10 months, the addition of enzalutamide, apalutamide and darolutamide to androgen deprivation therapy (ADT) compared to ADT alone resulted in improved metastases free (MFS) and overall survival (OS). For mCRPC patients, several treatment options have been shown to be effective: two taxane based chemotherapies (docetaxel and cabazitaxel), two androgen-receptor pathway inhibitors (ARPI) (abiraterone and enzalutamide), two radiopharmaceutical agents (radium 223 and 177Lutetium-PSMA-617), one immunotherapy treatment (sipuleucel-T) and two poly ADP-ribose polymerase (PARP) inhibitors (olaparib and rucaparib). Pembrolizumab is US Food and Drug Administration (FDA) approved in all MSI high solid tumors, although a very small proportion of prostate cancer patients harboring this characteristic will benefit. Despite having a broad variety of treatments available, there are still several unmet clinical needs for CRPC. The objective of this review was to describe the therapeutic landscape in CRPC patients, to identify criteria for selecting patients for specific treatments currently available, and to address the current challenges in this setting.
Keywords: prostate cancer, castration resistant prostate cancer, non-metastatic castration resistant prostate cancer, metastatic castration resistant prostate cancer
Introduction
With an estimated almost 1.4 million new cases and 375,000 deaths worldwide, prostate cancer (PC) was the second most frequent cancer and the leading cause of cancer death among men in 2020 in 48 countries.1 Androgen deprivation therapy (ADT) has been the standard of care in metastatic disease for more than 80 years.2 Although ADT is able to initially induce a response in more than 90% of patients, progression occurs after a median of 12–14 months despite suppressed testosterone levels, and patients are thereafter diagnosed as castration resistant (CRPC).3–8 Thus, as reported from a large retrospective patient series, there is wide prognostic variability between patients being de-novo metastatic or metachronous with prior treatment to the primary and further also depending on volume of disease.9 Over the past 20 years various therapies have been shown to improve outcome in castration resistant disease in both the non-metastatic (nmCRPC) and metastatic (mCRPC) setting.10–20 Despite the improved prognosis of patients with CRPC, there are still several unmet clinical needs concerning the optimal therapeutic management of these patients. The aim of this review was to describe treatments currently available for patients with nmCRPC and mCRPC, to discuss criteria for patient selection for different treatments, and to address the current challenges in these settings.
Treatment Landscape for Patients with nmCRPC
Without additional treatment to ADT, most patients with nmCRPC will progress over time to a metastatic disease stage.21 Since metastatic castration resistant disease is associated with decreased overall survival (OS) and decreased quality of life, delaying the time to metastasis is a clinically relevant endpoint in the nmCRPC setting. Xie et al showed that MFS is a strong surrogate for overall survival (OS) for localized PC.22 The median MFS among nmCRPC patients ranges from 25 to 30 months and the risk of metastases is associated with an increasing prostate-specific antigen (PSA) level and a short PSA doubling time (PSA-DT of 10 months or less).23,24 However, until 2018 there was no standard of care in the nmCRPC setting until progression to metastatic disease regardless of the PSA value or the PSA-DT. Since, three randomized, double-blind, placebo-controlled, phase 3 trials with a similar design demonstrated that nmCRPC patients with no detectable metastases on conventional imaging (computed tomography and bone scan or magnetic resonance imaging) and a PSA-DT <10 months on continuous ADT had a significant improvement of MFS with the addition of an ARPI compared to placebo.10–12 More specifically, the PROSPER trial with a median follow-up of 18.5 showed a median MFS of 36.6 months in the enzalutamide group versus 14.7 months in the placebo group [hazard ratio (HR) 0.29; 95% confidence interval (CI), 0.24–0.35; p <0.001].10 In the SPARTAN trial, apalutamide prolonged MFS compared to placebo (40.5 vs 16.2 months, HR 0.28, 95% CI, 0.23–0.35; p<0.001; median follow up 20.3 months).11 Finally, in the ARAMIS trial, darolutamide was superior to placebo in the nmCRPC setting: darolutamide was found to reduce the risk for metastases by 59% compared to placebo (40.4 vs 18.4 months; HR 0.41, 95% CI 0.34–0.51; p <0.001; median follow up 17.9 months).12 Subsequent analyses with longer follow-up showed that the addition of enzalutamide, apalutamide or darolutamide to ADT also resulted in improved OS by reducing the risk of death compared to placebo by 22–31% (Table 1).25–27 Based on these results, international guidelines recommend the use of enzalutamide, apalutamide or darolutamide in addition to ADT in nmCRPC with a PSA-DT < 10 months.28,29 The recommendation and approval in nmCRPC patients with a PSA-DT < 10 months is based on the inclusion criteria in the trials, but about 70% of patients actually had a PSA-DT < 6 months and therefore more aggressive disease. In nmCRPC patients with PSA-DT > 10 months, we have no evidence for relevant efficacy of adding an ARPI or other therapies to ADT. Therefore, in these patients observation plus continuous ADT, to date still represents the standard of care.28,29
Table 1.
Phase III Trials in Non-Metastatic Castration Resistant Prostate Cancer (nmCRPC)
| Study Design | Clinical Trial | n | Median Follow-Up (Months) | Median MFS (Months) | Median OS (Months) | |
|---|---|---|---|---|---|---|
| Enzalutamide | ENZA vs placebo | PROSPER [10,25] | 1401 | 48 | 36.6 vs 14.7 (HR 0.29, 95% CI 0.24–0.35, p<0.001 | 67 vs 56.3 (HR 0.73, 95% CI 0.73, 95% CI 0.61–0.89, p=0.001 |
| Apalutamide | APA vs placebo | SPARTAN [11,26] | 1207 | 52 | 40.5 vs 16.2 (HR 0.28, 95% CI 0.23–0.35, p<0.001) | 73.9 vs 59.9 (HR 0.78, 95% CI 0.78, 95% CI 0.64–0.96, p=0.016 |
| Darolutamide | DARO vs placebo | ARAMIS [12,27] | 1509 | 29 | 40.4 vs 18.4 (HR 0.41, 95% CI 0.34–0.5, p<0.001) | 3 years OS: 83 vs 77% (HR 0.69, 95% CI 0.53–0.88, p=0.003 |
Abbreviations: ENZA, enzalutamide; APA, apalutamide; DARO, darolutamide; MFS, metastasis free survival; OS, overall survival; HR, hazard ratio; CI, confidence interval; n, number of patients.
Patient Selection and Unmet Clinical Needs in nmCRPC Setting
As mentioned, in the nmCRPC setting only patients with PSA-DT <10 months should be evaluated for the addition of enzalutamide, apalutamide or darolutamide.28,29 There is no head-to-head comparison between the three different ARPIs. Some meta-analyses however indirectly compared the efficacy and safety in the nmCRPC setting.30–33 These results should be interpreted with caution because there are differences between the studies with regards to design, patient characteristics, and follow-up duration. Therefore, to compare the efficacy of these treatments in meta-analyses is very controversial. Kumar et al showed that apalutamide and enzalutamide had similar but higher MFS rates compared to darolutamide, with no difference in OS.30 In the Mori et al network meta-analysis, apalutamide resulted as the most effective treatment option regarding MFS and PSA progression-free survival.31 Similar results were obtained from the network meta-analysis published by Hird et al where apalutamide seemed to show the strongest benefit in terms of OS as well.32 In contrast, Wenzel et al in their network meta-analysis suggested that darolutamide could have the highest OS benefit.33 Although these meta-analyses are discordant in determining the most effective ARPI in this setting, they all agree in suggesting darolutamide as the best tolerated agent. The favorable toxicity profile of darolutamide can be explained by its unique molecular structure representing a lower penetration of the blood-brain barrier compared to enzalutamide and apalutamide.34 While enzalutamide and apalutamide are known to induce seizures at higher doses probably due to their off-target activity of inducing an inhibition of GABA-A receptors, especially in predisposed patients, darolutamide penetrates less through the blood-brain barrier and is hence thought to have a lower risk for seizures as underlined by clinical data.12,34,35
Prostate cancer patients are often elderly men with concomitant comorbidities that require additional drug treatment. For this reason, the drug interactions of ARPIs should be carefully assessed when selecting treatment. For example, enzalutamide and apalutamide are known to cause interactions with drugs commonly used in the elderly population such as proton pump inhibitors, lipid-lowering drugs, anticoagulants or benzodiazepines.36,37 Conversely, it would appear that darolutamide has fewer drug-drug interactions. In fact, in a pre-specified post hoc analysis of ARAMIS trial, darolutamide demonstrated a lower risk of clinically relevant drug interactions with commonly used drugs in these patients.38
In summary, in the absence of direct comparisons between these three ARPIs, the factors that can help us to best choose treatment in the nmCRPC setting are represented by patient comorbidities, treatment toxicity, drug interactions, and access meaning not only approval but also reimbursement of treatments.
Another challenge in nmCRPC is represented by the increasing use of modern imaging techniques, specifically positron emission tomography (PET) with labeled prostate specific membrane antigen (PSMA) (PSMAPET). In fact, in all outlined phase 3 studies in the nmCRPC setting, conventional imaging techniques were used for disease staging.10–12 A retrospective analysis of PSMA-PET in 200 high-risk nmCRPC patients who had already been evaluated with conventional imaging demonstrated that PSMA-PET was positive in 98% of the patients, showing pelvic disease and distant metastases in 44% and 55% of cases, respectively.39 Consequently, the majority of patients categorized as nmCRPC on conventional imaging would be classified as mCRPC on next-generation imaging. However, it remains unclear if the use of PSMAPET in this setting improves outcome. To date, in asymptomatic patients, classified as nmCRPC, using conventional imaging, performing additional next-generation imaging would not relevantly change the therapeutic strategy since regardless of the PSMAPET result, the patient would likely receive treatment with an ARPI (enzalutamide, apalutamide or darolutamide if PSMAPET was negative or enzalutamide or abiraterone if PSMAPET detected metastatic sites).
Finally, another unmet clinical need in this setting is what treatment to give patients who progress to metastatic disease on an ARPI in nmCRPC. Several studies demonstrated that the sequential use of ARPI is not very effective due to cross-resistance mechanisms.40,41 Based on these results, it would be intuitive to use docetaxel as the best choice of first-line therapy in the mCRPC setting in these patients.
Treatment Landscape for Patients with mCRPC
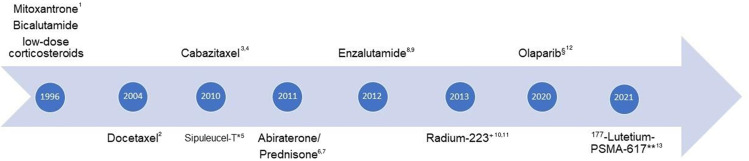
Over the past twenty years, several treatments demonstrated efficacy in the mCRPC setting (Figure 1). To date, the treatment landscape for mCRPC patients includes chemotherapy (docetaxel and cabazitaxel), ARPI (enzalutamide and abiraterone), radiopharmaceutical therapy (radium-223 and 177Lutetium-PSMA-617) and poly ADP-ribose polymerase (PARP) inhibitors (olaparib and rucaparib).13–20 In 2004 docetaxel led to improved OS compared to mitoxantrone (18.9 vs 16.5 months, HR 0.76; 95% CI 0.62–0.94, p=0.009) as well as when combined with estramustine compared to mitoxantrone (17.5 months vs 15.6 months, HR 0.8; 95% CI 067–0.97, p=0.02) and became the standard of care as first-line therapy in mCRPC patients.13,42 Subsequently, cabazitaxel was demonstrated to be superior to mitoxantrone in terms of OS in mCRPC patients progressing on or after docetaxel (15.1 vs 12.7 months, HR 0.7; 95% CI 0.59–0.83, p <0.0001).14 The phase 3 trials with abiraterone and enzalutamide demonstrated improved OS in mCRPC patients post docetaxel and subsequently also in docetaxel naive patients.15,16,43,44 In the ALSYMPCA trial, radium-223 reduced the risk of death by 30% compared to placebo in symptomatic mCRPC patients with predominant bone metastases who had received, were ineligible to or refused to receive docetaxel (HR 0.7; 95% CI 0.58–0.83; p<0.001).17 This trial was however done in an era where only docetaxel was available as life-prolonging treatment and the interpretation of this study is therefore difficult. Most recently in the VISION trial 177Lutetium-PSMA-617 (Lu-PSMA) plus protocol-permitted standard care was shown to improve OS in PSMA-positive mCRPC patients previously treated with at least one ARPI and one or two taxane regimens compared to the protocol permitted standard care alone (15.3 vs 11.3 months, HR 0.62; 95% CI 0.52–0.74; p <0.001).18 The protocol permitted standard of care consisting of bisphosphonates, radiotherapy, denosumab, corticosteroid or ARPI.18 In the Phase III PROFOUND study the PARP inhibitor olaparib was found to reduce the risk of death by 31% in mCRPC patients who had an alteration in BRCA1, BRCA2, or ATM and whose disease had progressed during previous treatment with enzalutamide or abiraterone (HR 0.69; 95% CI 0.50 to 0.97, p = 0.02).19 Another PARP inhibitor approved by US Food and Drug Administration (FDA) but not by European Medicines Agency (EMA) is rucaparib which was evaluated in the Phase II, single arm study (TRITON 2).20 In this trial, rucaparib showed an objective response rate of 43.5% and a PSA response rate of 54.8% in 115 mCRPC patients with BRCA1 or BRCA2 mutations who progressed after at least one ARPI and one taxane-based chemotherapy.20
Figure 1.
Timeline of treatments for mCRPC (year of reported positive pivotal trial). 1,2Tannock, IF et al. N Engl J Med 2004; 3De Bono, J et al. Lancet 2010, 4Oudard, S et al. J Clin Oncol 2017, 5Kantoff, P.W. et al. N Engl J Med. 2010; 6De Bono, J et al. N Engl J Med. 2011, 7Ryan, CJ et al. N Engl J Med. 2013, 8Scher, HI et al. N Engl J Med 2012; 9Beer, TM et al. N Engl J Med 2014, 10Parker, C et al. N Engl J Med. 2013, 11Nilsson S et al. Ann Oncol. 2016; 12De Bono, J et al. N Engl J Med 2020;13Sartor O et al. N Engl J Med. 2021. *Approval EMA withdrawn, not available in Europe; +symptomatic, bone only, LN<3cm, visceral mets. excluded; approval §EMA: BRCA1,2 FDA: HRD panel PROfound, Rucaparib (FDA only); **FDA only approval, not EMA.
In addition to the previously mentioned treatments, two immunotherapy treatments (sipuleucel-T and pembrolizumab) are available for treatment in the mCRPC setting, thus sipuleucel-T is only approved by FDA and pembrolizumab has a site-agnostic approval for microsatellite instability (MSI)-high solid tumors and tumor mutational burden (TMB) high ≥10 mutations/megabase by the FDA but not EMA.28,29 Sipuleucel-T improved OS compared to placebo in the phase 3 IMPACT study (25.8 vs 21.7 months; HR 0.78; 95% CI 0.61–0.98; p = 0.03).45 After initial approval by the EMA, the company restrained approval because of logistics with production, therefore this treatment is not currently available in Europe. Pembrolizumab has very limited anti-tumor activity for unselected patients albeit with an acceptable safety profile (Table 2).46 Treatment with pembrolizumab is not recommended outside of clinical trials for unselected patients.
Table 2.
Phase III Trials Reporting Overall Survival Benefit in Metastatic Castration Resistant Prostate Cancer (mCRPC) Setting
| Study Design | Clinical Trial | n | Median OS (Months) | Endpoints | |
|---|---|---|---|---|---|
| Docetaxel | DOCE 60 mg/m2 + Estramustine/PRED vs MTX/PRED | SWOG 99–16 [42] | 770 | 17.5 vs 15.6 (HR 0.8, 95% CI 0.67–0.97, p=0.02) | DOCE/Estramustine/PRED improves OS |
| DOCE 75 mg/m2 vs DOCE 30 mg/m2 weekly vs MTX | TAX327 [13] | 1006 | 18.9 vs.17.4 vs 16.5. HR (DOCE 75 mg/m2 vs DOCE 30 mg/m2 weekly vs MTX) 0.83, 95% CI 0.7–0.99, p=0.04 | DOCE improves OS | |
| Cabazitaxel | CBZ vs MTX | TROPIC [14] | 755 | 15.1 vs 12.7 (HR 0.7, 95% CI 0.59–0.83, p<0.0001) | CBZ improves OS after DOCE-based therapy |
| CBZ vs ENZA or AB+PRED | CARD [50] | 255 | 13.6 vs 11 (HR 0.64, 95% CI 0.46–0.89, p=0.008) | CBZ improves OS over second ARTA | |
| Abiraterone (2nd-line after DOCE) | AB+PRED vs Placebo + PRD | COU-301 [15] | 1195 | 14.8 vs 10.9 (HR 0.65, 95% CI 0.54–0.77, p<0.001 | AB+PRED improves OS after DOCE treatment |
| Abiraterone (TX naive mCRPC) asymptomatic, mildly symptomatic, no visceral mets | AB+PRED vs Placebo + PRED | COU-302 [43] | 1088 | 34.7 vs 30.3 (HR 0.75, 95% CI 0.61–0.93, P=0.01) | AB+PRED improves OS in CHT naive patients |
| Enzalutamide | ENZA vs Placebo | AFFIRM [16] | 1199 | 18.4 vs 13.6 (HR 0.63, 95% CI 0.53–0.75, p<0.001) | ENZA improves OS after CHT |
| Enzalutamide (TX naive mCRPC) asymptomatic or mildly symptomatic | ENZA vs Placebo | PREVAIL [44] | 1717 | 32.4 vs 30.2 (HR 0.71, 95% CI 0.6–0.84, p<0.001 | ENZA improves OS in CHT naive patients |
| Radium-223 | Ra-223 vs Placebo | ALSYMPCA [17] | 921 | 14.9 vs 11.3 (HR 0.7, 95% CI 0.55–0.88, p=0.002 | Ra-223 improves OS |
| Olaparib | Olaparib vs ENZA or AB + PRED | PROFOUND [19] | 387 Cohort A (BRCA1, BRCA2, ATM alteration= 245 Cohort B (all other 12 prespecified HRD defects) = 142 |
Cohort A: 19.1 vs 14.7 (HR 0.69, 95% CI 0.5–0.97, p=0.02) Cohort B: 14.1 vs 11.5 (HR 0.96, 95% CI 0.63–1.49) |
Olaparib improves OS over second ARTA in patients with BRCA1, BRCA2 and ATM mutation Olaparib does not improve OS over second ARPI in patients with HRD defects other than BRCA1, BRCA2, ATM |
| 177-Lutetium-PSMA-617 | 177-Lutetium-PSMA-617 plus protocol-permitted SOC vs protocol-permitted SOC | VISION [18] | 617 | 15.3 vs 11.3 (HR 0.62, 95% CI 0.52–0.74; P<0.001) | 177-Lutetium-PSMA-617 improves OS when added to systemic treatment according to VISION protocol |
| Sipuleucel-T | Sipuleucel-T vs placebo | IMPACT [45] | 512 | 25.8 vs 21.7 (HR 0.78, 95% CI 0.61–0.98; p=0.03 | Sipuleucel-T improves OS |
Abbreviations: DOCE, docetaxel; PRED, prednisone; MTX, mitoxantrone; OS, overall survival; CBZ, cabazitaxel; AB, abiraterone; mCRPC, metastatic castration-resistant prostate cancer; CHT, chemotherapy; Ra-223, radium-223; GCSF, granulocyte stimulating factor; ARPI, androgen-receptor pathway inhibitor; HRD, homologous repair-deficiency; SOC, standard of care; HR=hazard ratio; CI, confidence interval; n, number of patients; PSMA, prostate-specific membrane antigen; BRCA, breast cancer gene; ATM, ataxia telangiectasia mutated; ENZA, enzalutamide; TX, treatment.
Patient Selection and Unmet Clinical Need in the mCRPC Setting
Docetaxel, abiraterone, and enzalutamide have all shown a significant survival benefit as first-line therapy for mCRPC patients and are considered standard options in this setting.13,42–44 No randomized comparison between chemotherapy and ARPI and between enzalutamide and abiraterone has been done so far. In clinical practice, docetaxel can be a valuable option in the first-line mCRPC setting in case of symptomatic disease, short duration of response to previous ADT in the metastatic hormone-sensitive PC (mHSPC) setting, and in general in the presence of adverse disease features (short PSA-DT, high tumor burden and presence of visceral metastases) but there is no high level evidence data to prove a superiority of chemotherapy in this situations. Abiraterone and enzalutamide are instead preferred not only in asymptomatic or mildly symptomatic patients and with a long response to previous treatment with ADT in the mHSPC setting, but also in elderly patients, with impaired performance status and with significant comorbidities due to their generally better tolerability compared to chemotherapy. The choice between abiraterone and enzalutamide is mainly based on their toxicity profile, on the patient’s comorbidities and the concomitant medications as mentioned earlier. Enzalutamide should be avoided in patients with a predisposition to develop seizures due to its known ability to interact with GABA receptors by lowering the seizure threshold.47 Abiraterone, on the other hand, is not the preferred treatment in patients with cardiovascular comorbidities due to its increased risk of causing cardiac events and in patients with uncompensated diabetes mellitus or in general, with any other comorbidities that could be worsened by concomitant use of corticosteroids.48 It is crucial to perform a baseline cardiac ultrasound to ensure a preserved ejection fraction (EF), since in New York Heart Association (NYHA) III and IV patients abiraterone is contraindicated as well as in patients with impaired liver function (Child-Pugh class C). In most abiraterone trials the inclusion criteria were even more rigid, requiring an EF of > 50% at baseline and patients with cardiac events in the past 6 months as well as atrial fibrillation and other arrhythmias requiring therapy were excluded.5,6
Enzalutamide and abiraterone have also been shown to be effective in mCRPC patients previously treated with docetaxel while there are no prospective trials demonstrating the efficacy of docetaxel in mCRPC patients previously treated with ARPI.15,16 However, in daily clinical practice the majority of chemotherapy-fit patients who received abiraterone or enzalutamide as first-line treatment for mCRPC will receive docetaxel subsequently. Cabazitaxel was shown to be effective in patients progressing on or after docetaxel14 while it was not superior to docetaxel in the first-line setting.14,49 If patients progress on first line docetaxel there are no data that guide us regarding which therapy to choose between cabazitaxel or an ARPI. Also in this case, clinical criteria can help in treatment selection. Cabazitaxel is the preferred option in case of adverse clinical features (multimetastatic disease progression, short PSA-DT, presence of symptoms, poor response to docetaxel) while ARPI can be the treatment choice in case of mild disease progression, in asymptomatic or mildly symptomatic patients and in case of poor performance status or older age. In the CARD trial cabazitaxel was shown to improve OS in patients previously treated with docetaxel and who progressed within 12 months to abiraterone or enzalutamide compared to the other ARPI not previously used.50 As previously mentioned, there are data in the mCRPC setting that the sequential use of enzalutamide and abiraterone and vice versa is not very effective due to the presence of common cross-resistance mechanisms.40,41 Therefore, in current clinical practice the use of enzalutamide after abiraterone or the reverse sequence in mCRPC patients is generally discouraged and should only be offered in selected cases.28,29
Regarding the other treatments available in the mCRPC setting, radium-223 use is restricted by EMA for patients who have previously received docetaxel and an ARPI.51 This restriction is based on the results of the ERA-223 trial in which the combination of radium-223 and abiraterone showed an increased incidence of fractures (29% versus 11%) and a numerical, although not statistically significant, reduction in median OS (30.7 versus 33.3 months; p = 0.13) compared to abiraterone alone.52 To date we can only use Ra-223 from third line onwards in symptomatic patients who have predominantly bone metastases.
According to the results of the pivotal phase VISION trial, 177Lu-PSMA-617 has shown a benefit in OS in mCRPC patients with PSMA positive lesions on 68Ga-PSMA-11 PET previously treated with docetaxel and one ARPI.18 PSMA-PET positivity and eligibility for the study was defined by at least one 68Ga-PSMA-PET positive lesion and no PSMA-PET negative lesions with the following dimensions: > 2.5 cm (short axis) in lymph nodes, > 1 cm bone metastasis with soft-tissue component and > 1 cm solid organ metastases. Moreover, olaparib is indicated for mCRPC patients harboring mutations in DNA damage repair (DDR) genes who progressed to prior ARPI.19 FDA approved olaparib for mCRPC patients with deleterious or suspected deleterious germline or somatic homologous recombination repair (HRR) gene-mutated metastatic castration-resistant prostate cancer (mCRPC) while EMA restricted the approval to BRCA1 and BRCA2 mutated tumors.28,29
Current evidence available in the mCRPC setting is derived mostly from studies in which patients received ADT alone in the hormone-sensitive setting, although some trials have allowed to having received docetaxel in the mHSPC setting. For example in the CARD study 16% of patients have received prior docetaxel in the mHSPC setting.50 However, with the knowledge of the CHAARTED data a revolution of the therapeutic landscape of mHSPC patients began in 2015 and currently ADT monotherapy no longer represents the standard of care in this setting.3,28,29 First docetaxel and subsequently three ARPIs (abiraterone, enzalutamide and apalutamide) in addition to ADT were shown to improve OS compared to ADT monotherapy in mHSPC patients.3–8,53 Current guidelines recommend upfront docetaxel use preferably in high-volume mHSPC according to the CHAARTED criteria while abiraterone, enzalutamide and apalutamide can be used in both low- and high-volume patients.28,29 We currently have no high level evidence regarding which therapy to recommend in the mCRPC setting in patients who received prior combination therapy in the mHSPC setting. The significant OS benefit of abiraterone or enzalutamide in patients progressing after docetaxel in mCRPC 15,16 supports the hypothesis that an ARPI is reasonable treatment in patients progressing after docetaxel upfront.15,16 Cabazitaxel may alternatively be an option in patients with negative clinical prognostic factors. In patients treated with an ARPI in the mHSPC setting, docetaxel is a reasonable option for first-line mCRPC treatment with the idea to switch the mode of action once resistant to an ARPI. Due to the well-known cross resistance between ARPIs, a second ARPI as first line treatment in mCRPC setting is generally not recommended.40,41 However this evidence comes from studies including mCRPC patients and is limited to the sequence of enzalutamide and abiraterone and vice versa.40,41 In the TITAN trial, the first life-prolonging subsequent therapy in patients randomized to apalutamide arm was abiraterone or enzalutamide in 31% and taxane chemotherapy in 36% of patients. In this post hoc analysis of TITAN, apalutamide was shown to improve PFS2 regardless of the first subsequent treatment chosen.54 These results, are thus advised to be interpreted with caution due to its post-hoc statistic.
Another therapeutic option in patients treated with ADT + ARPI in mHSPC harboring DDR mutations is olaparib, since in the pivotal study olaparib was shown to prolong OS in patients pre-treated with an ARPI.19
The choice of first-line treatment in the mCRPC setting will be influenced in the near future by the implementation of triplet therapy into clinical practice in mHSPC patients. In 2022, two phase 3 randomized clinical trials showed that treatment intensification by adding an ARPI to ADT + docetaxel (triplet therapy) in mHSPC patients prolongs OS compared to ADT + docetaxel.55,56 Particularly in the ARASENS study the combination of ADT + docetaxel + darolutamide was shown to reduce the risk of death of 32% (HR 0.68; 95% CI 0.57–0.80, p<0.001) in 1306 mHSPC patients compared to ADT + docetaxel.55 Similarly in the PEACE-1 trial ADT + docetaxel + abiraterone were shown to improve OS in 1173 mHSPC patients compared to ADT + docetaxel (HR 0.82; 95% CI 0.69–0.98, p=0·030) especially in high-volume patients according to CHAARTED criteria.56 To date, we do not yet know which mHSPC patients should be offered triplet therapy; however, with the introduction of this new therapeutic option in this setting it will become even more complex to choose a first-line treatment in the mCRPC disease stage.
Furthermore, the results of some more recent studies could introduce a new standard of care as a first-line therapy in the mCRPC setting, as summarized in Table 3. Preliminary data from two phase 3 studies were presented at ASCO GU 2022 which showed interesting results for the combination of a PARP inhibitor and an ARPI as first-line therapy in the mCRPC setting.57,58 In the MAGNITUDE trial the combination of niraparib and abiraterone versus abiraterone alone was investigated in patients with and without DDR genes alterations. In patients without alterations there was no benefit from the experimental arm while in patients with DDR mutations niraparib + abiraterone resulted in a statistically significant improvement in radiological progression-free survival (rPFS) (16.5 months vs 13.7 months HR 0.73, 95% CI 0.56–0.96, p = 0.0217).57 In the PROPEL study, the combination of olaparib + abiraterone improved rPFS compared to abiraterone alone (27.6 vs 16.4 months, HR 0.61, 95% CI 0.49–0.74, p <0.0001) irrespective of homologous recombination repair status.58 If these encouraging results are also confirmed in the OS analyses, the PARP inhibitor + abiraterone combination could be considered a new standard of care as a first-line therapy for mCRPC patients. Of note however, in both of these trials the majority of patients had only received ADT monotherapy in the mHSPC setting and therefore it is unclear to date how these results can be implemented daily in clinic now where mostly the combination of ADT plus an ARTA is given in mHSPC patients.
Table 3.
Recently Reported Trials in Metastatic Castration Resistant Prostate Cancer (mCRPC)
| NCT Number (Trial Name) | Phase | Setting | Treatment Arms | Primary Endpoints | Results |
|---|---|---|---|---|---|
|
NCT03072238 (IPATential150) |
3 | mCRPC 1st-line |
ADT+Abiraterone+Ipatasertib vs ADT+Abiraterone+Placebo | rPFS investigator-assessed in the PTEN-loss-by-IHC population and in the intention-to-treat population | rPFS PTEN-loss by IHC: 18.5 months vs 16.5 months, HR 0.77, 95% CI 0.61–0.98; p=0.034 rPFS intention-to-treat population: 19.2 months vs 16.6 months, HR 0.84, 95% CI 0.71–0.99, p=0.043 |
|
NCT02257736 (ACIS) |
3 | mCRPC 1st-line |
ADT+Abiraterone+Apalutamide vs ADT+Abiraterone+Placebo | rPFS intention-to-treat population | rPFS (median follow-up 25.7 months): 22.6 months vs 16.6 months, HR 0.69, 95% CI 0.58–0.83; p<0.0001 rPFS updated analysis (median follow-up 54.8 months): 24.0 months vs 16.6 months, HR 0.70, 95% CI 0.60–0.83; p<0.0001 |
|
NCT03016312 (IMbassador250) |
3 | mCRPC 2nd-line after docetaxel |
ADT+Enzalutamide+Atezolizumab vs ADT+Enzalutamide | OS | OS: 15.2 months vs 16.6 months, HR 1.12, (95% CI 0.91, 1.37); p = 0.28 |
|
NCT03732820 (PROPEL) |
3 | mCRPC 1st-line Prior Docetaxel in mHSPC allowed Prior ARTA in mHSPC or nmCRPC allowed if stopped ≥ 12 months prior |
ADT+Abiraterone+Olaparib vs ADT+Abiraterone+Placebo | rPFS investigator-assessed | rPFS investigator assessed: 24.8 months vs 16.6 months, HR 0.66; (95% CI 0.54–0.81), p<0.0001 rPFS blinded independent central review: 27.6 months vs 16.4 months, HR 0.61, 95% CI 0.49–0.74, p<0.0001 OS: immature data (23% events), HR 0.86, 95% CI 0.66–1.12 ORR: 58% vs 48% |
|
NCT03748641 (MAGNITUDE) |
3 | mCRPC 1st-line HRR± vs HRR- prospectively assessed Prior Docetaxel in mHSPC allowed Prior ARTA in mHSPC or nmCRPC allowed if stopped ≥ 12 months; prior abiraterone allowed if ≤ 4 months |
ADT+Abiraterone+Niraparib vs ADT+Abiraterone+Placebo | rPFS central independent blinded review | rPFS: central independent blinded review HRR-: stopped enrollment after futility analysis (defined HR > 1.0) after 233 patients, HR 1.09 HRR±: 16.5 months vs 13.7 months, HR 0.75, 95% CI 0.57–0.97, p<0.0001 OS: immature data (27% events), RR: 2.13 ORR: 60% vs 28% |
Abbreviations: mCRPC, metastatic castration-resistant prostate cancer; CHT, chemotherapy; ARPI, androgen-receptor pathway inhibitor; ADT, androgen-deprivation therapy; PTEN, phosphatase and tensin homolog; IHC, immunohistochemistry; rPFS, radiographic progression-free survival; CI, confidence interval; HR, hazard ratio; mHSPC, metastatic hormone-sensitive prostate cancer; nmCRPC, non-metastatic castration-resistant prostate cancer; OS, overall survival; ORR, overall response rate; HRR, homologous recombination repair; RR, relative risk.
Since the arrival of 177Lu-PSMA-617 in mCRPC setting, treatment sequence got even more challenging. According to the pivotal VISION trial, Lu-PSMA can be used in patients previously treated with an ARPI and docetaxel in a setting currently predominantly represented by cabazitaxel following the results of the CARD study.18,50 A direct comparison between Lu-PSMA and cabazitaxel was performed in the phase 2 Thera-P study.59 In this trial 177Lu-PSMA-617 led to a higher PSA response (65 vs 37%, p<0·0001) and fewer grade 3 or 4 adverse events (33 vs 53%).59 Based on these promising results, the Lu-PSMA-cabazitaxel sequence could reasonably be preferred to the reverse sequence. However, the updated TheraP study results recently presented at the ASCO 2022 annual meeting showed that there was no difference in OS between 177Lu-PSMA-617 and cabazitaxel (HR 0.97, 95% 0.7–1.4, p=0.99).60 177Lu-PSMA-617 treatment is also characterized by very high costs and difficulties in logistics, and lack of sufficient nuclear medicine departments could lead to difficulties to access this substance.
Therefore, it is most important to select patients very carefully for treatment with 177Lu-PSMA-617 and recent data have underlined that a critical and careful validation of the PSMA uptake with certain SUV cut-offs, as highlighted in an abstract of a sub-study of the VISION and the TheraP trial at ASCO 2022, might help us to select patients that will benefit the most.60,61 A mean SUV of > 10 of all lesions has shown higher odds of PSA-50 response rate to 177-Lu-PSMA-617 in the TheraP trial and patients with the highest quartile of SUV (which was also around > 10) had the longest rPFS and OS in the VISION trial.60,61
Finally, one of the most important challenges in the management of PC patients in all disease settings is represented by the search for biomarkers predicting response to different treatments. Until a few years ago, the androgen receptor splice variant 7 (AR-V7) was the only biomarker recommended by the National Comprehensive Cancer Network (NCCN) guideline, but not by the European guidelines, potentially predicting a poor response to an ARPI.28,29 With the recent implementation of PARP inhibitors and pembrolizumab as therapeutic options in patients with DDR gene mutations or with MSI-high, respectively, the era of precision medicine has finally also arrived in metastatic prostate cancer.19,20,46 However, other future studies are needed to identify new predictive biomarkers that can help us select treatment based on the biomolecular characteristics of each individual patient.
Conclusion
In the last two decades, treatment of CRPC was revolutionized by the implementation of various therapeutic agents that were demonstrated to prolong OS in both the nmCRPC and mCRPC settings.10–20 This has certainly created undeniable benefits for the patients and for oncologists for the management of this disease but on the other hand, it has generated new challenges. The few direct comparison studies between the various treatments available and the few predictive biomarkers do not allow us to optimally select the therapeutic sequence for each individual patient. Furthermore, the therapeutic management of mCRPC patients has become even more complex after the developments of treatment intensification in the mHSPC setting.3–8,53 Among other things, the revolution in the mHSPC setting continues: the recent introduction of a triplet therapy approach in this setting is destined to further modify the therapeutic choice in mCRPC patients.55,56 Therefore, the results of recent studies could further change the therapeutic sequence of CRPC patients. Recently, treatment intensification has even arrived in locally advanced PC: based on the results of the STAMPEDE trial, international guidelines have recently included abiraterone in the treatment of PC patients with clinically node positive or with localized disease and > 2 high-risk factors (cT3–4, Gleason score > 8 or PSA > 40 ng/mL).28,29,62 Furthermore, if the promising results of the PROPEL and MAGNITUDE trial are confirmed and the OS results are positive as well, the combination of a PARP inhibitor (olaparib or niraparib) plus abiraterone could become a possible first-line therapeutic option in mCRPC patients.57,58 Along with these studies that are evaluating new therapeutic options in different disease settings, further future studies are needed to identify new biomarkers that can help us tailor treatment for each individual PC patient.
Funding Statement
No funding was received for this article.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Fabio Turco: travel grant: Bayer. Silke Gillessen: received personal honoraria for participation in advisory boards for Sanofi, Orion, Roche, Amgen, AstraZeneca, Novartis, Myriad Genetics, MSD; other honoraria from RSI (Televisione Svizzera Italiana); invited speaker for ESMO, Swiss group for Clinical Cancer Research (SAKK), Swiss Academy of Multidisciplinary oncology (SAMO), Orikata academy research group, China Anti-Cancer Association Genitourinary Oncology Committee (CACA-GU), S. Grasso Consulting, Beijing United Family Hospital and Clinics, Deso St Gallen; Speaker’s bureau for Janssen Cilag; travel grant from ProteoMEdiX; institutional honoraria for advisory boards for Bayer, Janssen Cilag, Roche, AAA International including Independent Data Monitoring Committee and IDMC; Steering Committee member for Amgen, Menarini Silicon Biosystems, Astellas Pharma, Tolero Pharmaceuticals, MSD, Pfizer, Telixpharma, BMS, and Orion; fees for the institute for faculty activity ASCO GU from WedMed-Medscape; patent royalties and other intellectual property for a research method for biomarker WO2009138392. Richard Cathomas: Advisory board (institutional): Astellas, Astra Zeneca, BMS, Merck, MSD, Pfizer, Ipsen, Roche, Debiopharm, Novartis, Bayer, Janssen (personal), Sanofi. Honoraria (institutional): Janssen, Astellas. Ursula Maria Vogl: Honorary for Advisory Board and Speaker fee (institutional): Pfizer, Bayer, MSD, BMS, Eisei, Astellas, Janssen, Sanofi, Novartis AAA, Merck, Ipsen; Honorary for Speaker fee, travel grant (private): SAMO, Inselspital Bern, Kantonsspital St. Gallen, Kantonsspital Chur, Healthbook, Merck, Ipsen. The authors report no other conflicts of interest in this work.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1:293–297. [DOI] [PubMed] [Google Scholar]
- 3.Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373(8):737–746. doi: 10.1056/NEJMoa1503747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387(10024):1163–1177. doi: 10.1016/S0140-6736(15)01037-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fizazi K, Tran N, Fein L, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377(4):352–360. doi: 10.1056/NEJMoa1704174 [DOI] [PubMed] [Google Scholar]
- 6.James ND, de Bono JS, Spears MR, et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377(4):338–351. doi: 10.1056/NEJMoa1702900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armstrong AJ, Azad AA, Iguchi T, et al. Improved survival with enzalutamide in patients with metastatic hormone-sensitive prostate cancer. Am J Clin Oncol. 2022;40(15):1616–1622. doi: 10.1200/JCO.22.00193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chi KN, Agarwal N, Bjartell A, et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med. 2019;381(1):13–24. doi: 10.1056/NEJMoa1903307 [DOI] [PubMed] [Google Scholar]
- 9.Francini E, Gray KP, Xie W, et al. Time of metastatic disease presentation and volume of disease are prognostic for metastatic hormone sensitive prostate cancer (mHSPC). Prostate. 2018;78(12):889–895. doi: 10.1002/pros.23645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hussain M, Fizazi K, Saad F, et al. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2018;378(26):2465–2474. doi: 10.1056/NEJMoa1800536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith MR, Saad F, Chowdhury S, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378(15):1408–1418. doi: 10.1056/NEJMoa1715546 [DOI] [PubMed] [Google Scholar]
- 12.Fizazi K, Shore N, Tammela TL, et al. Darolutamide in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2019;380(13):1235–1246. doi: 10.1056/NEJMoa1815671 [DOI] [PubMed] [Google Scholar]
- 13.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502–1512. doi: 10.1056/NEJMoa040720 [DOI] [PubMed] [Google Scholar]
- 14.de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376(9747):1147–1154. doi: 10.1016/S0140-6736(10)61389-X [DOI] [PubMed] [Google Scholar]
- 15.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995–2005. doi: 10.1056/NEJMoa1014618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187–1197. doi: 10.1056/NEJMoa1207506 [DOI] [PubMed] [Google Scholar]
- 17.Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213–223. doi: 10.1056/NEJMoa1213755 [DOI] [PubMed] [Google Scholar]
- 18.Sartor O, de Bono J, Chi KN, et al. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N Engl J Med. 2021;385(12):1091–1103. doi: 10.1056/NEJMoa2107322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hussain M, Mateo J, Fizazi K, et al. Survival with olaparib in metastatic castration-resistant prostate cancer. N Engl J Med. 2020;383(24):2345–2357. doi: 10.1056/NEJMoa2022485 [DOI] [PubMed] [Google Scholar]
- 20.Abida W, Patnaik A, Campbell D, et al. Rucaparib in men with metastatic castration-resistant prostate cancer harboring a BRCA1 or BRCA2 Gene alteration. Am J Clin Oncol. 2020;38(32):3763–3772. doi: 10.1200/JCO.20.01035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karantanos T, Corn PG, Thompson TC. Prostate cancer progression after androgen deprivation therapy: mechanisms of castrate resistance and novel therapeutic approaches. Oncogene. 2013;32(49):5501–5511. doi: 10.1038/onc.2013.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie W, Regan MM, Buyse M, et al. Metastasis-free survival is a strong surrogate of overall survival in localized prostate cancer. Am J Clin Oncol. 2017;35(27):3097–3104. doi: 10.1200/JCO.2017.73.9987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith MR, Cook R, Lee KA, et al. Disease and host characteristics as predictors of time to first bone metastasis and death in men with progressive castration-resistant nonmetastatic prostate cancer. Cancer. 2011;117(10):2077–2085. doi: 10.1002/cncr.25762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith MR, Saad F, Oudard S, et al. Denosumab and bone metastasis-free survival in men with nonmetastatic castration-resistant prostate cancer: exploratory analyses by baseline prostate-specific antigen doubling time. Am J Clin Oncol. 2013;31(30):3800–3806. doi: 10.1200/JCO.2012.44.6716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sternberg CN, Fizazi K, Saad F, et al. Enzalutamide and survival in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2020;382(23):2197–2206. doi: 10.1056/NEJMoa2003892 [DOI] [PubMed] [Google Scholar]
- 26.Smith MR, Saad F, Chowdhury S, et al. Apalutamide and overall survival in prostate cancer. Eur Urol. 2021;79(1):150–158. doi: 10.1016/j.eururo.2020.08.011 [DOI] [PubMed] [Google Scholar]
- 27.Fizazi K, Shore N, Tammela TL, et al. Nonmetastatic, castration-resistant prostate cancer and survival with darolutamide. N Engl J Med. 2020;383(11):1040–1049. doi: 10.1056/NEJMoa2001342 [DOI] [PubMed] [Google Scholar]
- 28.Mottet N, Cornford P, van den Bergh RCN, et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG guidelines on prostate cancer update. Eur Urol. 2022;79:243–262. [DOI] [PubMed] [Google Scholar]
- 29.Schaeffer E, Srinivas S, Antonarakis ES, et al. NCCN guidelines insights: prostate cancer, version 1.2021. J Natl Compr Cancer Netw. 2021;19(2):134–143. doi: 10.6004/jnccn.2021.0008 [DOI] [PubMed] [Google Scholar]
- 30.Kumar J, Jazayeri SB, Gautam S, et al. Comparative efficacy of apalutamide darolutamide and enzalutamide for treatment of non-metastatic castrate-resistant prostate cancer: a systematic review and network meta-analysis. Urol Oncol. 2020;38(11):826–834. doi: 10.1016/j.urolonc.2020.03.022 [DOI] [PubMed] [Google Scholar]
- 31.Mori K, Mostafaei H, Pradere B, et al. Apalutamide, enzalutamide, and darolutamide for non-metastatic castration-resistant prostate cancer: a systematic review and network meta-analysis. Int J Clin Oncol. 2020;25(11):1892–1900. doi: 10.1007/s10147-020-01777-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hird AE, Magee DE, Bhindi B, et al. A systematic review and network meta-analysis of novel androgen receptor inhibitors in non-metastatic castration-resistant prostate cancer. Clin Genitourin Cancer. 2020;18(5):343–350. doi: 10.1016/j.clgc.2020.02.005 [DOI] [PubMed] [Google Scholar]
- 33.Wenzel M, Nocera L, Collà Ruvolo C, et al. Overall survival and adverse events after treatment with darolutamide vs. apalutamide vs. enzalutamide for high-risk non-metastatic castration-resistant prostate cancer: a systematic review and network meta-analysis. Prostate Cancer Prostatic Dis. 2021;25:139–148. doi: 10.1038/s41391-021-00395-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moilanen AM, Riikonen R, Oksala R, et al. Discovery of ODM-201, a new-generation androgen receptor inhibitor targeting resistance mechanisms to androgen signaling-directed prostate cancer therapies. Sci Rep. 2015;5:12007. doi: 10.1038/srep12007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fizazi K, Massard C, Bono P, et al. Activity and safety of ODM-201 in patients with progressive metastatic castration-resistant prostate cancer (ARADES): an open-label Phase 1 dose-escalation and randomised phase 2 dose expansion trial. Lancet Oncol. 2014;15(9):975–985. doi: 10.1016/S1470-2045(14)70240-2 [DOI] [PubMed] [Google Scholar]
- 36.Del Re M, Fogli S, Derosa L, et al. The role of drug-drug interactions in prostate cancer treatment: focus on Abiraterone acetate/prednisone and enzalutamide. Cancer Treat Rev. 2017;55:71–82. doi: 10.1016/j.ctrv.2017.03.001 [DOI] [PubMed] [Google Scholar]
- 37.Duran I, Carles J, Bulat I, et al. Pharmacokinetic drug-drug interaction of apalutamide, part 1: clinical studies in healthy men and patients with castration-resistant prostate cancer. Clin Pharmacokinet. 2020;59(9):1135–1148. doi: 10.1007/s40262-020-00882-2 [DOI] [PubMed] [Google Scholar]
- 38.Shore N, Zurth C, Fricke R, et al. Evaluation of clinically relevant drug-drug interactions and population pharmacokinetics of darolutamide in patients with nonmetastatic castration-resistant prostate cancer: results of pre-specified and post hoc analyses of the phase III ARAMIS trial. Target Oncol. 2019;14(5):527–539. doi: 10.1007/s11523-019-00674-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fendler WP, Weber M, Iravani A, et al. Prostate-specific membrane antigen ligand positron emission tomography in men with nonmetastatic castration-resistant prostate cancer. Clin Cancer Res. 2019;25(24):7448–7454. doi: 10.1158/1078-0432.CCR-19-1050 [DOI] [PubMed] [Google Scholar]
- 40.Noonan KL, North S, Bitting RL, et al. Clinical activity of Abiraterone acetate in patients with metastatic castration-resistant prostate cancer progressing after enzalutamide. Ann Oncol. 2013;24(7):1802–1807. doi: 10.1093/annonc/mdt138 [DOI] [PubMed] [Google Scholar]
- 41.Antonarakis ES, Lu C, Wang H, et al. AR-V7 and resistance to enzalutamide and Abiraterone in prostate cancer. N Engl J Med. 2014;371(11):1028–1038. doi: 10.1056/NEJMoa1315815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351(15):1513–1520. doi: 10.1056/NEJMoa041318 [DOI] [PubMed] [Google Scholar]
- 43.Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368(2):138–148. doi: 10.1056/NEJMoa1209096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(5):424–433. doi: 10.1056/NEJMoa1405095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422. doi: 10.1056/NEJMoa1001294 [DOI] [PubMed] [Google Scholar]
- 46.Antonarakis ES, Piulats JM, Gross-Goupil M, et al. Pembrolizumab for treatment-refractory metastatic castration-resistant prostate cancer: multicohort, open-label Phase II KEYNOTE-199 study. Am J Clin Oncol. 2020;38(5):395–405. doi: 10.1200/JCO.19.01638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Golshayan AR, Antonarakis ES. Enzalutamide: an evidence-based review of its use in the treatment of prostate cancer. Core Evid. 2013;8:27–35. doi: 10.2147/CE.S34747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cone EB, Reese S, Marchese M, et al. Cardiovascular toxicities associated with Abiraterone compared to enzalutamide-A pharmacovigilance study. EClinicalMedicine. 2021;36:100887. doi: 10.1016/j.eclinm.2021.100887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oudard S, Fizazi K, Sengeløv L, et al. Cabazitaxel versus docetaxel as first-line therapy for patients with metastatic castration-resistant prostate cancer: a randomized phase III Trial-FIRSTANA. Am J Clin Oncol. 2017;35(28):3189–3197. doi: 10.1200/JCO.2016.72.1068 [DOI] [PubMed] [Google Scholar]
- 50.de Wit R, de Bono J, Sternberg CN, et al. Cabazitaxel versus abiraterone or enzalutamide in metastatic prostate cancer. N Engl J Med. 2019;381(26):2506–2518. doi: 10.1056/NEJMoa1911206 [DOI] [PubMed] [Google Scholar]
- 51.EMA. EMA restricts use of prostate cancer medicine XOFIGO. Available from: https://www.ema.europa.eu/en/news/ema-restricts-use-prostate-cancer-medicine-xofigo. Accessed September 14, 2022.
- 52.Smith M, Parker C, Saad F, et al. Addition of radium-223 to Abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(3):408–419. doi: 10.1016/S1470-2045(18)30860-X [DOI] [PubMed] [Google Scholar]
- 53.Davis ID, Martin AJ, Stockler MR, et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. 2019;381(2):121–131. doi: 10.1056/NEJMoa1903835 [DOI] [PubMed] [Google Scholar]
- 54.Agarwal N, Chowdhury S, Bjartell A, et al. Time to second progression (PFS2) in patients (pts) from TITAN with metastatic castration-sensitive prostate cancer (mCSPC) by first subsequent therapy (hormonal vs. taxane). J Clin Oncol. 2020;38:82. doi: 10.1200/JCO.2020.38.6_suppl.82 [DOI] [Google Scholar]
- 55.Smith MR, Hussain M, Saad F, et al. Darolutamide and survival in metastatic, hormone-sensitive prostate cancer. N Engl J Med. 2022;386(12):1132–1142. doi: 10.1056/NEJMoa2119115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fizazi K, Foulon S, Carles J, et al. Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): a multicentre, open-label, randomised, phase 3 study with a 2 × 2 factorial design. Lancet. 2022;399(10336):1695–1707. doi: 10.1016/S0140-6736(22)00367-1 [DOI] [PubMed] [Google Scholar]
- 57.Chi NK, Rathkopf DE, Smith MR, et al. Phase 3 MAGNITUDE study: first results of niraparib (NIRA) with Abiraterone acetate and prednisone (AAP) as first-line therapy in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) with and without homologous recombination repair (HRR) gene alterations. J Clin Oncol. 2022;40:12. doi: 10.1200/JCO.21.01891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saad FA, Armstrong AJ, Thiery-Vuillemin A, et al. PROpel: phase III trial of olaparib (ola) and Abiraterone (abi) versus placebo (pbo) and abi as first-line (1L) therapy for patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol. 2022;40:11. doi: 10.1200/JCO.2022.40.6_suppl.011 [DOI] [Google Scholar]
- 59.Hofman MS, Emmett L, Sandhu S, et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet. 2021;397(10276):797–804. doi: 10.1016/S0140-6736(21)00237-3 [DOI] [PubMed] [Google Scholar]
- 60.Hofman MS, Emmett L, Sandhu S, et al. TheraP: 177 Lu-PSMA-617 (LuPSMA) versus cabazitaxel in metastatic castration-resistant prostate cancer (mCRPC) progressing after docetaxel—Overall survival after median follow-up of 3 years (ANZUP 1603). J Clin Oncol. 2022;40:5000. doi: 10.1200/JCO.2022.40.16_suppl.5000 [DOI] [Google Scholar]
- 61.Kuo P, Hsu H-C, Lin T-M, et al. H1-antihistamines reduce the risk of hepatocellular carcinoma in patients with hepatitis B virus, hepatitis C virus, or dual hepatitis B virus-hepatitis C virus infection. J Clin Oncol. 2022;40:1206–1219. doi: 10.1200/JCO.21.01802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Attard G, Murphy L, Clarke NW, et al. Abiraterone acetate and prednisolone with or without enzalutamide for high-risk non-metastatic prostate cancer: a meta-analysis of primary results from two randomised controlled phase 3 trials of the STAMPEDE platform protocol. Lancet. 2022;399(10323):447–460. doi: 10.1016/S0140-6736(21)02437-5 [DOI] [PMC free article] [PubMed] [Google Scholar]