Abstract
Beyond the palliative reach of today’s medicines, medical therapies of tomorrow aim to treat the root cause of age-related diseases by targeting fundamental aging mechanisms. Pillars of aging include, among others, genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, dysregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intercellular communication. The unitary theory of fundamental aging processes posits that by targeting one fundamental aging process, it may be feasible to impact several or all others given its interdependence. Indeed, pathologic accumulation of senescent cells is implicated in chronic diseases and age-associated morbidities, suggesting that senescent cells are a good target for whole-body aging intervention. Preclinical studies using senolytics, agents that selectively eliminate senescent cells, and senomorphics, agents that inhibit production or release of senescence-associated secretory phenotype (SASP) factors, show promise in several aging and disease preclinical models. Early clinical trials using a senolytic combination (dasatinib and quercetin), and other senolytics including flavonoid, fisetin, and BCL-xL inhibitors, illustrate the potential of senolytics to alleviate age-related dysfunction and diseases including wound healing. Translation into clinical applications requires parallel clinical trials across institutions to validate senotherapeutics as a vanguard for delaying, preventing, or treating age-related disorders and aesthetic aging.
Keywords: senolytics, senomorphics, dasatinib, quercetin, fisetin, cellular senescence, unitary theory of fundamental aging processes
Aging and the geroscience framework
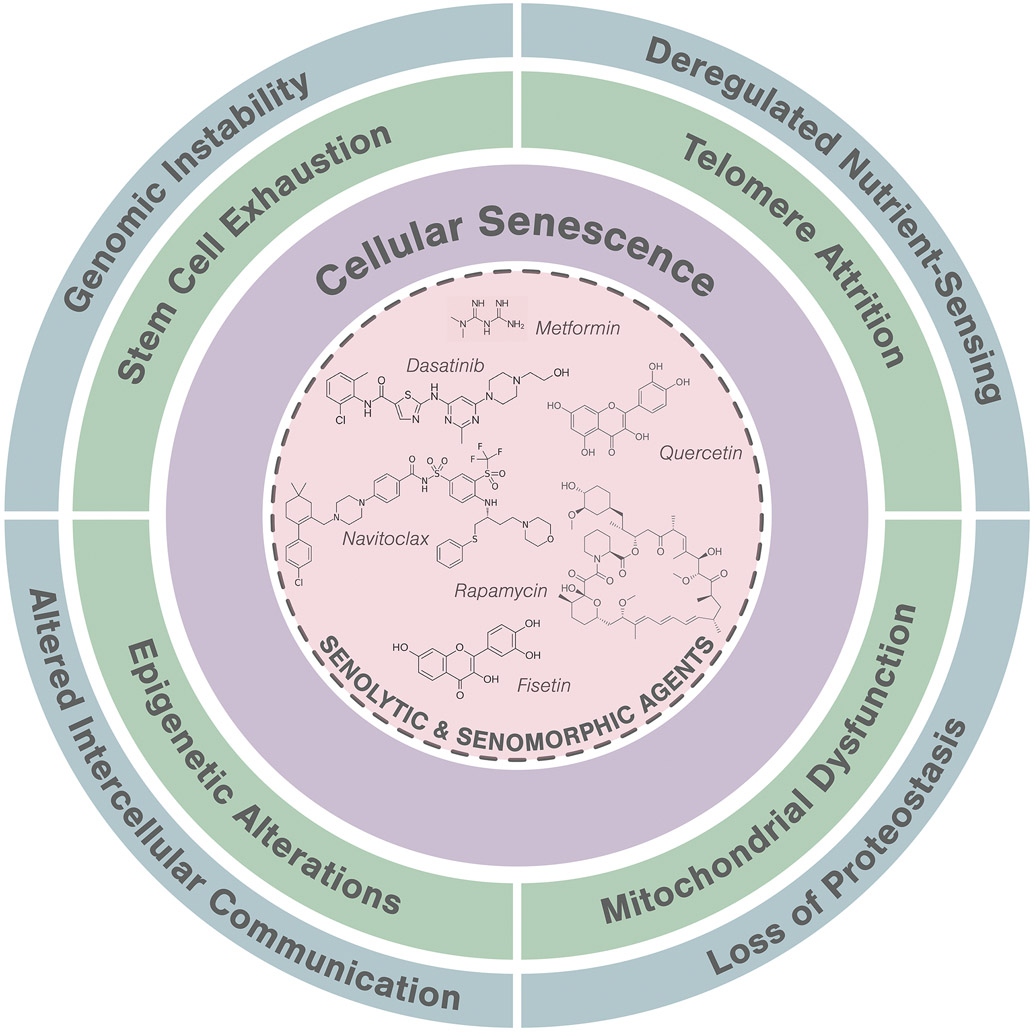
The elderly population continues to rapidly rise. According to the World Health Organization (WHO), the number of persons aged 80 years or older is expected to triple between 2020 and 2050 to reach 426 million.1 Consequently, the global epidemic of age-related chronic diseases represents an increasing burden on all healthcare systems.2,3 The scope of age-related diseases ranges from congestive heart failure, myocardial infarction, dementias, strokes, most cancers, diabetes and metabolic diseases, renal dysfunction, chronic lung disease, osteoporosis, arthritis, blindness, and many more.4-6 In the context of skin aging, which is induced by chronological aging (intrinsic aging) and environmental factors such as ultraviolet (UV) light (extrinsic aging), a compromised skin barrier coupled with geriatric syndromes (including frailty, sarcopenia, immobility, falling, depression, mild cognitive impairment, incontinence, weight loss, and metabolic and immunological dysfunction),7 results in heightened risk for skin diseases and poor wound healing.8 Such examples of compounding multimorbidity in elderly individuals offsets potential gains from devising a treatment for any single age-related disorder.9,10 The geroscience hypothesis postulates that fundamental aging processes may be root cause contributors to all of these disorders, suggesting a new therapeutic avenue to target whole-body aging (Figure 1).11
Figure 1.
A hallmark of aging, cellular senescence, as a key target for intervention.
Fundamental aging mechanisms can be grouped into hallmarks or pillars of aging. One such grouping includes nine hallmarks that contribute to age-related disease and dysfunction: genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, dysregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, progenitor cell exhaustion, and altered intercellular communication.12 The unitary theory of fundamental aging processes suggests that targeting one fundamental aging process (e.g., cellular senescence) could impact several or all other aspects of aging (Figure 1).13,14 Given its known and well-documented interactions with other aging hallmarks, cellular senescence is an ideal target for pharmacological intervention.15 This recent awareness that age-related disorders can be driven by one or more fundamental aging processes has inspired efforts to identify and develop root-cause drug development strategies that target cellular senescence for improving health span and decreasing multi-morbidity.16
Cellular senescence
Description of cellular senescence dates to the 1960s when Hayflick and Moorhead observed that cells cultures in vitro ceased proliferation after limited number of replication cycles.17 This phenomenon, now referred to as replicative senescence, is associated with telomere and dyssfunction.18 Cellular senescence is a cell fate that occurs as a defense mechanism in response to damaging stimuli, leading to cell cycle arrest, apoptosis resistance, and acquisition of a senescence-associated secretory phenotype.19,20 Both external and internal age-associated signals contribute to driving a cell into the senescence state.16,19 These include DNA alterations bioactive lipids, mitochondrial dysfunction), protein alterations (aggregates, misfolding, failed autophagy), inflammatory signals, and other damage/danger signals.21
Select features of senescent cells include morphologic changes (increased size, increased granularity),22 cell cycle blockade signals (p21-p53, p16-RB),23 mitochondrial changes (increased size, ROS production, decreased membrane integrity), lysosomal changes (increased SA-β-gal activity),24 nuclear changes (telomere shorting, telomere-associated foci, ϒ-H2AX),25 and others.26 Senescent cells typically have high p16INK4A and/or p21CIP1 and exhibit DNA damage foci, particularly in telomeres, senescence-associated distention of pericentromeric satellite DNA, and increased β-galactosidase activity.5,27 Given that these markers vary in sensitivity and specificity, multimodal senescent marker evaluation is needed for diagnosis and predicting senescence-associated disease states and ascertaining target engagement with senolytics and other geroscience-based therapies.
Senescent cells naturally resist apoptosis but can be cleared by the immune system until reaching its limit.28,29 The threshold theory of senescent cell burden posits that there is a tipping point beyond which senescent cell accumulation exceeds immune system capacity to clear senescent cells, leading to decline in tissue functionality and increase in age-related dysfunction.10 Indeed, senescent cells appear at pathogenic sites of major diseases including Alzheimer’s, cardiovascular diseases, osteoporosis, diabetes, renal disease, cirrhosis, and others.4,6 In preclinical experiments, transplanting relatively small numbers of senescent cells around knee joints or intraperitoneally into younger mice caused persistent physical dysfunction and spread of cellular senescence to host tissues.30 Transplanting even fewer senescent cells had the same effects in older mice leading to increased frailty and reduced survival, suggesting the potency of senescent cells in shortening health- and lifespan.30 These studies further support that senescent cell accumulation can be at the nidus of many chronic age-related diseases and as a key pharmacological target.31
In the context of plastic, reconstructive, and aesthetic surgery, deciphering cellular and molecular processes of skin evolution through aging is an active scientific domain.32 Aged skin is generally characterized by skin atrophy resulting from reduced proliferative capacity of skin cells and degradation of extracellular matrix (ECM) proteins such as collagen, elastin, and glycosaminoglycans.33 In vivo, early senescence plays a protective role to prevent cancer cell proliferation,34 and has an important physiological role in acute wound healing.35 Yet, late senescence, triggered by aging and chronic inflammation, is often detrimental.36 Indeed, senescent fibroblasts and keratinocytes accumulate in aged human skin with an estimated 20 to 50% senescent cells present in elderly skin37-39. Functionally, senescent dermal fibroblasts express increased matrix metalloproteinase and decreased tissue inhibitor of matrix metalloproteinases (TIMP-1 and TIMP-3), switching from a matrix-producing to a matrix-degrading phenotype.40 Targeting the emergence of senescent cells in the dermis and hypodermis may portend a new era for the aesthetic domain and skin aging physiology.
Clearance of senescent cells can reduce inflammation, decrease macromolecular dysregulation, and enhance function of stem and progenitor cells.14,41 This fundamental aging process presents a unique target for intervention through two mechanisms: 1) eliminating senescent cells (senolytics) and 2) inhibiting of senescent-associated secretory phenotype (SASP) production (senomorphics).10 SASP factors, which are secreted by senescent cells, can include a variety of cytokines, chemokines, proteases, micro-RNAs, cell-free mitochondrial DNA, and other factors42 that can cause stem cell and progenitor cell damage as well as tissue dysfunction.43 In other types of senescent cells, the SASP can include growth factors and be much less pro-apoptotic and inflammatory).44 Senotherapeutics include agents that target both of these processes: senolytics (selectively targeting those senescent cells with a pro-apoptotic, inflammatory SASP) and senomorphics (targeting SASP either by controlling the regulatory SASP network or a specific SASP component).45 Cellular senescence therefore serves an ideal target for clinical intervention given its fundamental and forefront mechanistic contribution to age-related phenotypes and disease states.
Discovery of senotherapeutic agents
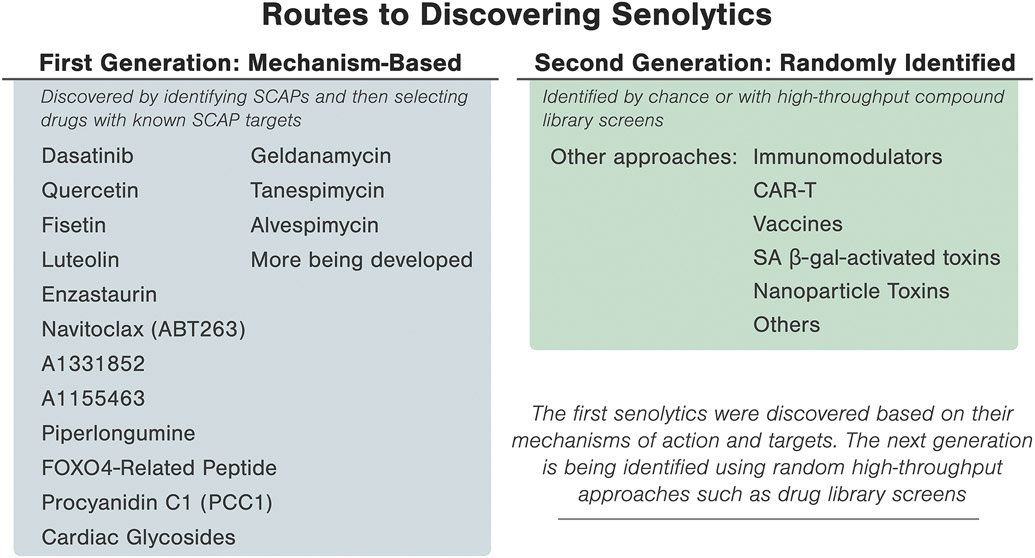
The road to discovering senotherapeutic interventions was catapulted by the finding that cellular senescence is linked to a decline in longevity.46 To identify senotherapeutic agents, a hypothesis-based, mechanism-driven drug discovery methodology was utilized (Figure 2). Guided by the principle that those senescent cells with a pro-apoptotic, tissue-destructive SASP resist self-induced clearance through a network of senescent cell anti-apoptotic pathways (SCAPs), various SCAPs were targeted by RNA interference to identify key nodes in the SCAP network.21 Bioinformatics approaches were then used to identify compounds that target these nodes, effectively inducing apoptosis in those senescent cells with a pro-apoptotic SASP.47 The first-generation cadre of senolytics included Dasatinib (D), an anti-cancer agent and senolytic against human senescent adipocyte progenitors, and Quercetin (Q), a natural flavonoid and senolytic against umbilical vein endothelial cells. The combination, D+Q, provided a synergistic and potent senolytic agent against broader senescent cell types.48 Fisetin, a natural flavonoid and relative to Q, was also found to be senolytic.49,50 Other flavonoids that have been tested as senolytic agents include resveratrol, luteolin, and curcumin.50 Piperlongumine, which is also related to Q, was noted to be senolytic in certain senescent cell types (e.g., fibroblasts).51
Figure 2.
Identification of senotherapeutic agents using mechanism-based versus random or high-throughput library screening.
Drugs that target BCL-2-related pathways such as navitoclax (ABT263) were identified as potential senolytics, yet off-target effects on non-senescent cells is a limiting factor.49,52,53 The more specific BCL-XL inhibitors, A1331852 and A1155463,54 were found to be senolytic in human lung fibroblasts and umbilical vein endothelial cells.49 Navitoclax and other BCL-2 family inhibitors that act on only a single SCAP can have substantial off-target apoptotic effects on non-senescent cell types, such as platelets and immune cells in the case of navitoclax.55,56 Arguably, navitoclax (ABT263), A1155463, and possibly A1331852 may have more side effects since they can be ‘panolytic,’ causing apoptosis or dysfunction of a number of cell types other than senescent cells, compared to agents with a broader range of molecular targets, such as dasatinib, quercetin, piperlongumine, or fisetin, which hit multiple targets “lightly”, potentially flattening side effect profiles. The target of senolytics is senescent cells, not a single molecule.
Additional senolytic drugs are currently being developed. Many first generation senolytic agents were developed based on the mechanism-based approach of targeting nodes in the SCAP network that confers resistance to apoptosis in tissue-destructive senescent cells.21 Using this method, FOXO4-related peptide was recently noted to be senolytic, perturbing p53 interaction in human fibroblast-like culture-habituated cell strains.57 In aged preclinical models, FOXO4-related peptide restored fitness, fur density, and renal function.57 Unlike small molecule senolytic agents, such peptides are not usually orally-active.
Screening an autophagy regulator library resulted in the discovery of HSP90 inhibitors (geldanamycin, tanespimycin, and alvespimycin) as a new class of senolytics in human cultured cells and in a progeroid mouse model of accelerated aging.58 Studies have also identified natural senotherapeutic agents by screening a natural medicinal product library and detecting procyanidin C1 (PCC1), a polyphenolic flavonoid component of grape seed extract (GSE), as a senolytic agent in human lung fibroblasts, umbilical vein endothelial cells and mesenchymal cells.59
A second generation cadre of senotherapeutic agents is being developed using random, high-throughput-based drug library screening.60 New characterization of senescent cells has revealed senescence-associated self-antigens, which can be co-opted for immune system-mediated senolytic clearance. This mechanism has been recently utilized with chimeric antigen receptor (CAR) T cells targeted against urokinase-type plasminogen activator receptor (uPAR) in a preclinical senescence model.61 Cytotoxic CAR T cells selectively cleared uPAR-expressing senescent cells in vitro and in vivo.61 Limitations for CAR T cell approaches in humans include initial immune system suppression, expense, and risk for acute to subacute adverse reactions, requiring further optimizing prior to clinical deployment. Other strategies for senescent cell clearance have included development of galacto-oligosaccharide-coated nanoparticles with toxic cargos that are engulfed by senescent cells and opened by SA-β-gal, vaccines, and immunomodulators.60,62,63
Based on the unitary theory of fundamental aging processes, senotherapeutic agents could act as the gateway to alleviate adverse effects from other fundamental aging mechanisms. Moreover, senescent cells are non-dividing and therefore unlikely to develop drug resistance, a problem encountered with compounds targeting dividing cancer cells or microbes. The most promising senolytic agents are already being moved through preclinical studies towards early phase clinical trials for multiple disorders and diseases.
Translating senotherapeutic agents to clinical practice
The NIH Geroscience Network, a consortium of aging centers engaged in translating senotherapeutic interventions from bench-to-bedside, have delivered the premises for possible clinical trial scenarios.4,64 Suggested guidelines for clinical trials include simultaneous alleviation of co-morbidities (i.e. 3 or more chronic conditions), delaying accelerated aging-like conditions (i.e. frailty and endurance measures in bone marrow transplant or childhood cancer survivors), conditions with localized senescence (i.e. osteoarthritis, fracture non-union, glaucoma, and macular degeneration), intervening for otherwise fatal cell senescence-driven conditions (i.e. idiopathic pulmonary fibrosis), increasing resilience or clinical stresses in pre-frail subjects (i.e. single course of senolytic agent prior to vaccination),65 and frailty.21
The first senolytic clinical trial was a pilot, open-label study (ClinicalTrials.gov identifier NCT02874989) using dasatinib plus quercetin (D+Q; D: 100 mg/day, Q:1250 mg/day, three-days/week over three-weeks) in a small cohort of patients (n=14) with idiopathic pulmonary fibrosis, a cellular senescence-associated disease.66 Participants reported statistically significant improvement in physical function within one week evaluated as 6-min walk distance, 4-m gait speed, chair-stands time, and the short physical performance battery. Interestingly, correlations were observed between change in function and change in SASP-related matrix-remodeling proteins, microRNAs, and pro-inflammatory cytokines.66 Another pilot open-label clinical trial of D+Q (D:100 mg/day, Q: 1000 mg/day, three consecutive days total) was reported in patients (n=9) with obesity and diabetic kidney disease (NCT02848131). D+Q reduced adipose tissue senescent cell burden, SASP factors, and p16INK4A- and p21CIP1-expression.67,68 These initial results have given way to additional placebo-controlled senotherapeutic clinical trials, which are currently underway or being planned.
New and ongoing clinical trials for D+Q senolytic therapies target a wide-spectrum of age-related indications including diabetic kidney disease (NCT02848131), Alzheimer’s disease (NCT04785300, NCT04585590), accelerated age-like state post bone marrow transplantation (NCT02652052), accelerated age-like state in childhood cancer survivors (NCT04733534), and age-related osteoporosis (NCT04313634). Flavonoid-based oral fisetin senolytic clinical studies have also launched for frailty in older women (NCT03430037), diabetic and chronic kidney disease (NCT03325322), age-related osteoporosis (NCT04313634), and COVID-19 in hospitalized patients (NCT04476953), among others.26 UBX1325, a Bcl-xL inhibitor, is another senolytic agent being tested as a single intravitreal injection for patients with diabetic macular edema. These studies could serve as critical evaluators of potential benefits, adverse side effects, and off-target events that may arise from senotherapeutic agents. To date, there have been no serious adverse events related to senolytic therapies in placebo-controlled trials, yet longitudinal monitoring is needed. Thus, over the counter or routine clinical practice use of senotherapeutic agents should be discouraged at this point; only in closely monitored clinical studies. To accelerate progress, multicenter alliances have been established, including the NIH-funded Translational Geroscience Network in hopes to conduct multiple parallel trials instead of doing trials in serially over time for safety, tolerability, target engagement, and efficacy.
Conclusions
The aging global population has exposed an unmet gap in addressing the need to enhance healthspan: current disease-focused treatments often decrease mortality without reversing decline in overall health. Recent awareness that age-related disorders can be driven by one or more fundamental aging processes has inspired a paradigm shift to develop pharmacological intervention strategies that target aging mechanisms. Indeed, effective introduction of senotherapeutic agents that target fundamental aging processes could transform medical and surgical practice. Preclinical and early clinical studies are demonstrating feasibility for senolytics and senomorphics to alleviate age-related dysfunction, yet caution must still be emphasized until clinical trials and post-marketing surveillance for potential adverse effects are done. Current senotherapeutic clinical trials are carefully conducted in accordance to national and intuitional regulatory oversight. It is conceivable that the repertoire of senotherapeutic agents may soon transform our clinical practice by reducing morbidity and increasing healthspan. Nevertheless, more extensive clinical studies are required to better elucidate the role of cellular senescence in whole-body aging.
Acknowledgements
This work was supported by Robert and Arlene Kogod Center on Aging (S.P.W). This work was also supported by National Institute of Health grants AG013925 (J.L.K.), AG062413 (J.L.K), and the Translational Geroscience Network (AG061456: J.L.K), the Connor Group (J.L.K.), Robert J. and Theresa W. Ryan (J.L.K.), and and Noaber Foundations (J.L.K.).
Footnotes
Disclosures: Patents on senolytic drugs are held by Mayo Clinic. This research has been reviewed by the Mayo Clinic Conflict of Interest Review Board and was conducted in compliance with Mayo Clinic Conflict of Interest policies.
References
- 1.World Health Organization (WHO): Aging and Health Fact Sheet. World Health Organization. https://www.who.int/news-room/fact-sheets/detail/ageing-and-health [Google Scholar]
- 2.Cortese DA. A vision of individualized medicine in the context of global health. Clin Pharmacol Ther. Nov 2007;82(5):491–3. doi: 10.1038/sj.clpt.6100390 [DOI] [PubMed] [Google Scholar]
- 3.Waldman SA, Terzic A. Individualized medicine and the imperative of global health. Clin Pharmacol Ther. Nov 2007;82(5):479–83. doi: 10.1038/sj.clpt.6100398 [DOI] [PubMed] [Google Scholar]
- 4.Kirkland JL, Tchkonia T. Cellular Senescence: A Translational Perspective. EBioMedicine. Jul 2017;21:21–28. doi: 10.1016/j.ebiom.2017.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kirkland JL, Tchkonia T, Zhu Y, Niedernhofer LJ, Robbins PD. The Clinical Potential of Senolytic Drugs. J Am Geriatr Soc. Oct 2017;65(10):2297–2301. doi: 10.1111/jgs.14969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tchkonia T, Kirkland JL. Aging, Cell Senescence, and Chronic Disease: Emerging Therapeutic Strategies. JAMA. Oct 2 2018;320(13):1319–1320. doi: 10.1001/jama.2018.12440 [DOI] [PubMed] [Google Scholar]
- 7.Inouye SK, Studenski S, Tinetti ME, Kuchel GA. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. May 2007;55(5):780–91. doi: 10.1111/j.1532-5415.2007.01156.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Norman RA. Geriatric dermatology. Dermatol Ther. 2003;16(3):260–8. doi: 10.1046/j.1529-8019.2003.01636.x [DOI] [PubMed] [Google Scholar]
- 9.Kirkland JL. Translating advances from the basic biology of aging into clinical application. Exp Gerontol. Jan 2013;48(1):1–5. doi: 10.1016/j.exger.2012.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirkland JL, Tchkonia T. Senolytic drugs: from discovery to translation. J Intern Med. Nov 2020;288(5):518–536. doi: 10.1111/joim.13141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kennedy BK, Berger SL, Brunet A, et al. Geroscience: linking aging to chronic disease. Cell. Nov 6 2014;159(4):709–13. doi: 10.1016/j.cell.2014.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. Jun 6 2013;153(6):1194–217. doi: 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prata L, Ovsyannikova IG, Tchkonia T, Kirkland JL. Senescent cell clearance by the immune system: Emerging therapeutic opportunities. Semin Immunol. Dec 2018;40:101275. doi: 10.1016/j.smim.2019.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu M, Palmer AK, Ding H, et al. Targeting senescent cells enhances adipogenesis and metabolic function in old age. Elife. Dec 19 2015;4:e12997. doi: 10.7554/eLife.12997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wissler Gerdes EO, Misra A, Netto JME, Tchkonia T, Kirkland JL. Strategies for late phase preclinical and early clinical trials of senolytics. Mech Ageing Dev. Dec 2021;200:111591. doi: 10.1016/j.mad.2021.111591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wissler Gerdes EO, Zhu Y, Tchkonia T, Kirkland JL. Discovery, development, and future application of senolytics: theories and predictions. FEBS J. Jun 2020;287(12):2418–2427. doi: 10.1111/febs.15264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayflick L. The Limited in Vitro Lifetime of Human Diploid Cell Strains. Exp Cell Res. Mar 1965;37:614–36. doi: 10.1016/0014-4827(65)90211-9 [DOI] [PubMed] [Google Scholar]
- 18.Liu J, Wang L, Wang Z, Liu JP. Roles of Telomere Biology in Cell Senescence, Replicative and Chronological Ageing. Cells. Jan 15 2019;8(1)doi: 10.3390/cells8010054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest. Mar 2013;123(3):966–72. doi: 10.1172/JCI64098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LeBrasseur NK, Tchkonia T, Kirkland JL. Cellular Senescence and the Biology of Aging, Disease, and Frailty. Nestle Nutr Inst Workshop Ser. 2015;83:11–8. doi: 10.1159/000382054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu Y, Tchkonia T, Pirtskhalava T, et al. The Achilles' heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. Aug 2015;14(4):644–58. doi: 10.1111/acel.12344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biran A, Zada L, Abou Karam P, et al. Quantitative identification of senescent cells in aging and disease. Aging Cell. Aug 2017;16(4):661–671. doi: 10.1111/acel.12592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Summer R, Shaghaghi H, Schriner D, et al. Activation of the mTORC1/PGC-1 axis promotes mitochondrial biogenesis and induces cellular senescence in the lung epithelium. Am J Physiol Lung Cell Mol Physiol. Jun 1 2019;316(6):L1049–L1060. doi: 10.1152/ajplung.00244.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Debacq-Chainiaux F, Erusalimsky JD, Campisi J, Toussaint O. Protocols to detect senescence-associated beta-galactosidase (SA-betagal) activity, a biomarker of senescent cells in culture and in vivo. Nat Protoc. 2009;4(12):1798–806. doi: 10.1038/nprot.2009.191 [DOI] [PubMed] [Google Scholar]
- 25.Bonner WM, Redon CE, Dickey JS, et al. GammaH2AX and cancer. Nat Rev Cancer. Dec 2008;8(12):957–67. doi: 10.1038/nrc2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gasek NS, Kuchel GA, Kirkland JL, Xu M. Strategies for Targeting Senescent Cells in Human Disease. Nat Aging. Oct 2021;1(10):870–879. doi: 10.1038/s43587-021-00121-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hernandez-Segura A, Nehme J, Demaria M. Hallmarks of Cellular Senescence. Trends Cell Biol. Jun 2018;28(6):436–453. doi: 10.1016/j.tcb.2018.02.001 [DOI] [PubMed] [Google Scholar]
- 28.Calcinotto A, Kohli J, Zagato E, Pellegrini L, Demaria M, Alimonti A. Cellular Senescence: Aging, Cancer, and Injury. Physiol Rev. Apr 1 2019;99(2):1047–1078. doi: 10.1152/physrev.00020.2018 [DOI] [PubMed] [Google Scholar]
- 29.Wang E. Senescent human fibroblasts resist programmed cell death, and failure to suppress bcl2 is involved. Cancer Res. Jun 1 1995;55(11):2284–92. [PubMed] [Google Scholar]
- 30.Xu M, Pirtskhalava T, Farr JN, et al. Senolytics improve physical function and increase lifespan in old age. Nat Med. Aug 2018;24(8):1246–1256. doi: 10.1038/s41591-018-0092-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirkland JL. Translating the Science of Aging into Therapeutic Interventions. Cold Spring Harb Perspect Med. Mar 1 2016;6(3):a025908. doi: 10.1101/cshperspect.a025908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Contet-Audonneau JL, Jeanmaire C, Pauly G. A histological study of human wrinkle structures: comparison between sun-exposed areas of the face, with or without wrinkles, and sun-protected areas. Br J Dermatol. Jun 1999;140(6):1038–47. doi: 10.1046/j.1365-2133.1999.02901.x [DOI] [PubMed] [Google Scholar]
- 33.Haydont V, Bernard BA, Fortunel NO. Age-related evolutions of the dermis: Clinical signs, fibroblast and extracellular matrix dynamics. Mech Ageing Dev. Jan 2019;177:150–156. doi: 10.1016/j.mad.2018.03.006 [DOI] [PubMed] [Google Scholar]
- 34.Baker DJ, Childs BG, Durik M, et al. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature. Feb 11 2016;530(7589):184–9. doi: 10.1038/nature16932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Demaria M, Ohtani N, Youssef SA, et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell. Dec 22 2014;31(6):722–33. doi: 10.1016/j.devcel.2014.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herranz N, Gil J. Mechanisms and functions of cellular senescence. J Clin Invest. Apr 2 2018;128(4):1238–1246. doi: 10.1172/JCI95148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dimri GP, Lee X, Basile G, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. Sep 26 1995;92(20):9363–7. doi: 10.1073/pnas.92.20.9363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lewis DA, Travers JB, Machado C, Somani AK, Spandau DF. Reversing the aging stromal phenotype prevents carcinoma initiation. Aging (Albany NY). Apr 2011;3(4):407–16. doi: 10.18632/aging.100318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weinmullner R, Zbiral B, Becirovic A, et al. Organotypic human skin culture models constructed with senescent fibroblasts show hallmarks of skin aging. NPJ Aging Mech Dis. 2020;6:4. doi: 10.1038/s41514-020-0042-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Campisi J. The role of cellular senescence in skin aging. J Investig Dermatol Symp Proc. Aug 1998;3(1):1–5. [PubMed] [Google Scholar]
- 41.Zhu Y, Armstrong JL, Tchkonia T, Kirkland JL. Cellular senescence and the senescent secretory phenotype in age-related chronic diseases. Curr Opin Clin Nutr Metab Care. Jul 2014;17(4):324–8. doi: 10.1097/MCO.0000000000000065 [DOI] [PubMed] [Google Scholar]
- 42.Iske J, Seyda M, Heinbokel T, et al. Senolytics prevent mt-DNA-induced inflammation and promote the survival of aged organs following transplantation. Nat Commun. Aug 27 2020;11(1):4289. doi: 10.1038/s41467-020-18039-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lewis-McDougall FC, Ruchaya PJ, Domenjo-Vila E, et al. Aged-senescent cells contribute to impaired heart regeneration. Aging Cell. Jun 2019;18(3):e12931. doi: 10.1111/acel.12931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tripathi U, Misra A, Tchkonia T, Kirkland JL. Impact of Senescent Cell Subtypes on Tissue Dysfunction and Repair: Importance and Research Questions. Mech Ageing Dev. Sep 2021;198:111548. doi: 10.1016/j.mad.2021.111548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Birch J, Gil J. Senescence and the SASP: many therapeutic avenues. Genes Dev. Dec 1 2020;34(23–24):1565–1576. doi: 10.1101/gad.343129.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krishnamurthy J, Torrice C, Ramsey MR, et al. Ink4a/Arf expression is a biomarker of aging. J Clin Invest. Nov 2004;114(9):1299–307. doi: 10.1172/JCI22475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Subramanian A, Kuehn H, Gould J, Tamayo P, Mesirov JP. GSEA-P: a desktop application for Gene Set Enrichment Analysis. Bioinformatics. Dec 1 2007;23(23):3251–3. doi: 10.1093/bioinformatics/btm369 [DOI] [PubMed] [Google Scholar]
- 48.Zhu Y, Tchkonia T, Fuhrmann-Stroissnigg H, et al. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell. Jun 2016;15(3):428–35. doi: 10.1111/acel.12445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu Y, Doornebal EJ, Pirtskhalava T, et al. New agents that target senescent cells: the flavone, fisetin, and the BCL-XL inhibitors, A1331852 and A1155463. Aging (Albany NY). Mar 8 2017;9(3):955–963. doi: 10.18632/aging.101202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yousefzadeh MJ, Zhu Y, McGowan SJ, et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine. Oct 2018;36:18–28. doi: 10.1016/j.ebiom.2018.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Y, Chang J, Liu X, et al. Discovery of piperlongumine as a potential novel lead for the development of senolytic agents. Aging (Albany NY). Nov 19 2016;8(11):2915–2926. doi: 10.18632/aging.101100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.He Y, Zhang X, Chang J, et al. Using proteolysis-targeting chimera technology to reduce navitoclax platelet toxicity and improve its senolytic activity. Nat Commun. Apr 24 2020;11(1):1996. doi: 10.1038/s41467-020-15838-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chang J, Wang Y, Shao L, et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med. Jan 2016;22(1):78–83. doi: 10.1038/nm.4010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leverson JD, Phillips DC, Mitten MJ, et al. Exploiting selective BCL-2 family inhibitors to dissect cell survival dependencies and define improved strategies for cancer therapy. Sci Transl Med. Mar 18 2015;7(279):279ra40. doi: 10.1126/scitranslmed.aaa4642 [DOI] [PubMed] [Google Scholar]
- 55.Wilson WH, O'Connor OA, Czuczman MS, et al. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: a phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol. Dec 2010;11(12):1149–59. doi: 10.1016/S1470-2045(10)70261-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mahfoudhi E, Lordier L, Marty C, et al. P53 activation inhibits all types of hematopoietic progenitors and all stages of megakaryopoiesis. Oncotarget. May 31 2016;7(22):31980–92. doi: 10.18632/oncotarget.7881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baar MP, Brandt RMC, Putavet DA, et al. Targeted Apoptosis of Senescent Cells Restores Tissue Homeostasis in Response to Chemotoxicity and Aging. Cell. Mar 23 2017;169(1):132–147 e16. doi: 10.1016/j.cell.2017.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fuhrmann-Stroissnigg H, Ling YY, Zhao J, et al. Identification of HSP90 inhibitors as a novel class of senolytics. Nat Commun. Sep 4 2017;8(1):422. doi: 10.1038/s41467-017-00314-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu Q, Fu Q, Li Z, et al. The flavonoid procyanidin C1 has senotherapeutic activity and increases lifespan in mice. Nat Metab. Dec 2021;3(12):1706–1726. doi: 10.1038/s42255-021-00491-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Munoz-Espin D, Rovira M, Galiana I, et al. A versatile drug delivery system targeting senescent cells. EMBO Mol Med. Sep 2018;10(9)doi: 10.15252/emmm.201809355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Amor C, Feucht J, Leibold J, et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature. Jul 2020;583(7814):127–132. doi: 10.1038/s41586-020-2403-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen Z, Hu K, Feng L, et al. Senescent cells re-engineered to express soluble programmed death receptor-1 for inhibiting programmed death receptor-1/programmed death ligand-1 as a vaccination approach against breast cancer. Cancer Sci. Jun 2018;109(6):1753–1763. doi: 10.1111/cas.13618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nakagami H. Cellular senescence and senescence-associated T cells as a potential therapeutic target. Geriatr Gerontol Int. Feb 2020;20(2):97–100. doi: 10.1111/ggi.13851 [DOI] [PubMed] [Google Scholar]
- 64.Burd CE, Gill MS, Niedernhofer LJ, et al. Barriers to the Preclinical Development of Therapeutics that Target Aging Mechanisms. J Gerontol A Biol Sci Med Sci. Nov 2016;71(11):1388–1394. doi: 10.1093/gerona/glw112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mannick JB, Del Giudice G, Lattanzi M, et al. mTOR inhibition improves immune function in the elderly. Sci Transl Med. Dec 24 2014;6(268):268ra179. doi: 10.1126/scitranslmed.3009892 [DOI] [PubMed] [Google Scholar]
- 66.Justice JN, Nambiar AM, Tchkonia T, et al. Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study. EBioMedicine. Feb 2019;40:554–563. doi: 10.1016/j.ebiom.2018.12.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hickson LJ, Langhi Prata LGP, Bobart SA, et al. Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine. Sep 2019;47:446–456. doi: 10.1016/j.ebiom.2019.08.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hickson LJ, Langhi Prata LGP, Bobart SA, et al. Corrigendum to 'Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease' EBioMedicine 47 (2019) 446-456. EBioMedicine. Feb 2020;52:102595. doi: 10.1016/j.ebiom.2019.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]