Abstract
Aging of the skin is evidenced by increased wrinkles, age spots, dryness and thinning with decreased elasticity. Extrinsic and intrinsic factors including UV, pollution and inflammation lead to an increase in senescent cells (SnCs) in skin with age that contribute to these observed pathological changes. Cellular senescence is induced by multiple types of damage and stress and is characterized by the irreversible exit from the cell cycle with upregulation of cell cycle-dependent kinase inhibitors p16INK4a and p21CIP1. Most SnCs also developed an inflammatory senescence-associated secretory phenotype (SASP) that drives further pathology through paracrine effects on neighboring cells and endocrine effects on cells at a distance. Recently, compounds able to kill senescent cells specifically, termed senolytics, or suppress the SASP, termed senomorphics, have been developed that have the potential to improve skin aging as well as systemic aging in general. Here we provide a summary of the evidence for a key role in cellular senescence in driving skin aging. In addition, the evidence for the potential application of senotherapeutics for skin treatments is presented. Overall, topical, and possibly oral senotherapeutic treatments, have tremendous potential to eventually become a standard of care for skin aging and related skin disorders.
Introduction
Skin is under continuous environmental assault and its ability to protect the body from these assaults declines with age. The aging of skin is manifested by increased wrinkles, age spots, dryness and thinning with decreased elasticity. As we age, each layer of the skin accrues alterations that contribute to these physical signs of aging and reduce the protective function of skin. The epidermis thins with age, and the distribution of keratinocytes and melanocytes becomes altered. The outer surface of the epidermis, or stratum corneum, maintains the same thickness with aging but has increased permeability and stiffness and is slower to recover following injury. Additionally, the epidermal-dermal junction flattens with the loss of rete ridges resulting in decreased resistance to shearing force (25,26). The dermis of an aged individual has fewer collagen bundles and there is loss of the upper or papillary dermis containing papillary fibroblast and capillaries (27). Dermal fibroblasts that are responsible for producing collagen and other extracellular matrix (ECM) proteins become misshapen and large with decreased collagen production with age. The decline of ECM collagen and elastin results in wrinkling and sagging of skin with reduced resiliency and strength (28). Furthermore, the depletion of skin stem cells with aging hinders wound healing and contributes to atrophy and frailty (29). The symptoms of aging skin subsequently make chronic wounds and ulcers more common in the elderly and these unhealing wounds can precipitate the development of cancer.
Cellular senescence is a damage and stress response that results in an irreversible cell cycle arrest distinct from quiescence or differentiation into post-mitotic cells. Senescent cells (SnCs) cannot be stimulated to proliferate like quiescent cells and the mitotic arrest is due to cellular stress and damage instead of terminal differentiation. SnCs are characterized by multiple markers, but due to senescent cell heterogeneity, no single marker is sufficient to confirm senescence. Some of these senescence-associated markers include elevated levels of senescence-associated β-galactosidase activity (SA-β-Gal), increased cell cycle inhibitors p16INK4A and p21CIP1, and a proinflammatory secretome called the senescence-associated secretory phenotype (SASP) comprised of cytokine, chemokines, metalloproteinases, growth factors, reactive metabolites and even extracellular vesicles. In addition, the nuclear scaffold protein lamin B1 is reduced in senescence cells, affecting the nuclear architecture (7–10). Cellular senescence is thought to play a key role in tumor suppression with oncogene-induced senescence (OIS) preventing expansion of pre-malignant cells and the inflammatory SASP stimulating immune clearance of the SnCs. However, with aging, SnCs accumulate due, in part, to the reduced function of the immune system and directly contribute to driving aging and diseases.
Senescence has both cell-autonomous and non-cell-autonomous effects on tissue homeostasis. The cell-autonomous effects of senescence can hinder tissue regeneration through reduced proliferation, especially senescence in progenitor cells, leading to loss of tissue homeostasis. The non-cell-autonomous effects are mediated by the SASP that can alter the tissue microenvironment and promote aging phenotypes through paracrine effects on neighboring cells and endocrine effects on cells at a distance (11). In particular, SnCs can reduce stem cell function indirectly through the inflammatory SASP. It is important to note that the transient accumulation of cells with markers of senescence and SASP has a beneficial role in skin for development, parturition and wound healing (9,12–14). However, when these transient and other types of SnCs are not cleared by the immune system, they instead can create a proinflammatory microenvironment that contributes to chronic wounds and cancer (15–17).
Intrinsic as well as extrinsic factors lead to an increase in SnCs in skin with age that contribute to an observable functional decline (1–6). Intrinsic factors that induce senescence include oxidative stress and telomere shortening (18–20). Interestingly, telomeres appear to be highly sensitive to oxidate stress, resulting in the accumulation of telomere-associated foci (TAFs) comprised of DNA repair components. In addition, increases in senescence and SASP in tissues other than skin can drive senescence in the skin through indirect mechanisms. Other intrinsic factors include genetic defects that result in hereditary progeria syndromes with increased DNA damage, premature aging and increased cellular senescence (21). Extrinsic factors that increase skin cellular senescence and precipitate aging phenotypes include cellular damage caused by UV radiation (UVR) or pollution (9,14,22). In addition, OIS driven by oncogenic mutations in genes such as RAS and BRAF are common in skin cancers (23,24). Thus, it appears as if many of the same factors that drive cellular senescence also drive skin aging. Several skin cell types including keratinocytes, fibroblast, and melanocytes have evidence of cellular senescence that contributes to aging pathologies (30,31).
Keratinocytes
The continuous differentiation and turnover of keratinocytes may limit the effects of senescent keratinocytes on skin homeostasis. However, with exposure to chronic UV, environmental factors and aging there is an increase in epidermal keratinocytes with senescence markers including increased SA-β-Gal, p16INK4a, p21CIP1 and p53 and decreased lamin B1 (1,14). UV-induced senescence in keratinocytes also increases expression of SASPs including MMPs and proinflammatory cytokines such as IL-6, IL-1β, IL-1α, and TNFα (14,32). Additionally, senescent keratinocytes have reduced ECM production and cell adhesion (33), which could contribute to the increased permeability of skin with aging. Importantly, senescence can also be induced in keratinocytes by pollution (34). Therefore, despite the high turnover rate in keratinocytes, aging and the chronic exposure to environmental hazards both contribute to the increased senescent burden in keratinocytes, affecting tissue function.
Fibroblasts
Senescence in dermal fibroblast is well documented and thought to be a key contributor to skin aging pathologies (1,4,35). Fibroblasts residing in the dermis are slow growing and maintain their position in unwounded skin. During aging, the number of fibroblasts is depleted and they become extended to compensate for the reduced cell density (36). Senescent fibroblasts upregulate SASP factors including MMP2, MMP9, IL-6 and IL-8 (9,37,38). Additionally, UV exposure induces fibroblasts to upregulate p16INK4a, p21CIP1 and p53 taking on a senescence phenotype (39). Furthermore, senescent fibroblasts can induce apoptosis in neighboring cells, contributing to cell number loss with aging (40). The pigmentation changes associated with aging have also been attributed to fibroblast senescence through decreased stromal cell-derived factor −1 (SDF-1) expression (41). Senescent fibroblasts have reduced secretion of insulin-like growth factor-1 (IGF-1) due to mitochondrial dysfunction and superoxide anion production (42). This decrease in IGF-1 production by fibroblasts can subsequently reduce keratinocyte proliferation and differentiation, decrease collagen production and increase DNA damage in epidermal cells (35). Thus, senescent dermal fibroblasts can drive adverse changes in skin function and precipitate aging phenotypes.
Melanocytes
Melanocytes are responsible for distributing melanin to keratinocytes and are found in the basal layer of the epidermis. The number of melanocytes decreases with aging in non-sun exposed skin areas which causes skin to lighten. However, sun exposed areas express hypo and hyperpigmentation areas with abnormal melanocyte distribution and function with aging (43,44). Additionally, increasing p16INK4a positive melanocytes correlate with an aging skin phenotype and wrinkle grading (45,46). Decreased lamin B1 and increased p16INK4a are senescence-associated markers found in melanocytic nevi and could be used to identify senescence melanocytes in the epidermis and track clearance of senescence cells (2,8,47,48). Telomere dysfunction can be induced in fibroblasts in a paracrine manner using conditioned media from senescent melanocytes (49). In addition, co-culture with senescent melanocytes results in reduced keratinocyte proliferation (49). Taken together, these results indicate that senescent melanocytes contribute to skin aging and have the ability to reduce proliferation and increase cellular dysfunction in melanocytes and other skin cell types.
Wound healing
Senescent cells can play both positive and negative roles in tissue and wound repair. Senescent fibroblasts have a positive role in limiting excessive collagen deposition that leads to fibrosis. The SASP contains several MMPs capable of degrading collagen, and senescent fibroblasts can restrict fibrosis in wound healing (11,50). Conversely, the elimination of at least certain types of senescent cells can reduce the efficiency of wound healing (12). While this transient, acute senescence contributes to wound healing, the clearance of senescent cells is necessary for wound closure and their continued presence can result in chronic wounds (16,17). Fibroblasts from chronic wounds when compared to normal fibroblasts were found to express several markers of senescent cells including increased SA-ßGal, reduced proliferation, oxidative stress and pro-inflammatory cytokines (16,51). Interestingly, premature senescence observed in chronic wound fibroblasts was telomere-independent and characterized by reduced wound repopulation as evaluated by the in vitro scratch assay (51).
Cancer
Senescence is generally considered to have evolved as a suppressor of tumorigenesis by preventing premalignant cell growth (15). However, when the immune system does not clear senescent cells, the resulting pro-inflammatory environment can drive aging pathologies and cancer (15,52). This pro-inflammatory can lead to a pro-tumor microenvironment that can help drive malignancy and drug resistance (53,54). The senescence cell burden and the risk of cancer both increase with age. This correlation between senescence and cancer has been confirmed using a genetic mouse model where the continuous elimination of p16INK4 positive senescent cells resulted in delayed development of age-associated cancer (55). Likewise, using a mouse model of squamous cell carcinoma, the SASP was found to promote tumor growth and malignant progression through upregulation of p38MAPK and MAPK/ERK signaling (56). Furthermore, overriding senescence can lead to a more aggressive cancer phenotype and possibly even be required for malignancy to develop (57–61). In fact, the CDNK2A locus for p16INK4a is a commonly mutated in cancer, especially skin cancer (62,63). Additionally, OIS is often a precursor to skin cancer development with over 60% of malignant melanoma having BRAF activating mutations. These same BRAF mutations are typically found in growth arrested nevi (64,65) which have a senescent phenotype with increased expression of p16INK4a and SA-β-gal activity. OIS is a result of the persistent DNA damage response from hyper-replication (2,66,67). Similarly, many cutaneous cancers have RAS mutations that can also cause OIS (11,23,68). Thus, although OIS is a tumor suppressor mechanism, cancer can be initiated by OIS cells by the accumulation of additional mutations, and subsequently further activated and transformed by the senescent cells in the microenvironment to become malignant.
Senotherapeutics
The accumulation of senescent cells is a druggable hallmark of aging and a key cause of age-related decline of function in skin. Many labs, including ours, have demonstrated the efficacy of utilizing small molecules that specifically target senescent cells, termed senotherapeutics, for the improvement of age-related tissue dysfunction mediated by reduced inflammation, restoration of tissue homeostasis, and increased health- and lifespan in many model organisms and human clinical trials (Figure 1). Senotherapeutics are classified as either being senolytic or senomorphic and function to selectively clear senescent cells (senolysis) or attenuate their deleterious SASP (senostasis) respectively. Senotherapeutics target upregulated senescent cell anti-apoptotic pathways (SCAPs) and other senescence-associated pathways that give rise to their pro-inflammatory secretome. To date, many senotherapeutic compounds have been identified (see Table, Supplemental Digital Content 1, which shows senotherapeutics reported to have positive effects on skin and skin-derived cell types, INSERT HYPERLINK HERE) and have been extensively reviewed elsewhere (69,70). Here, below we summarize the therapeutic benefit of senotherapeutic treatments on age-related skin dysfunction, which provides further evidence of a key role of SnCs in driving skin aging.
Figure 1: Schematic of skin aging and senotherapeutic treatment.
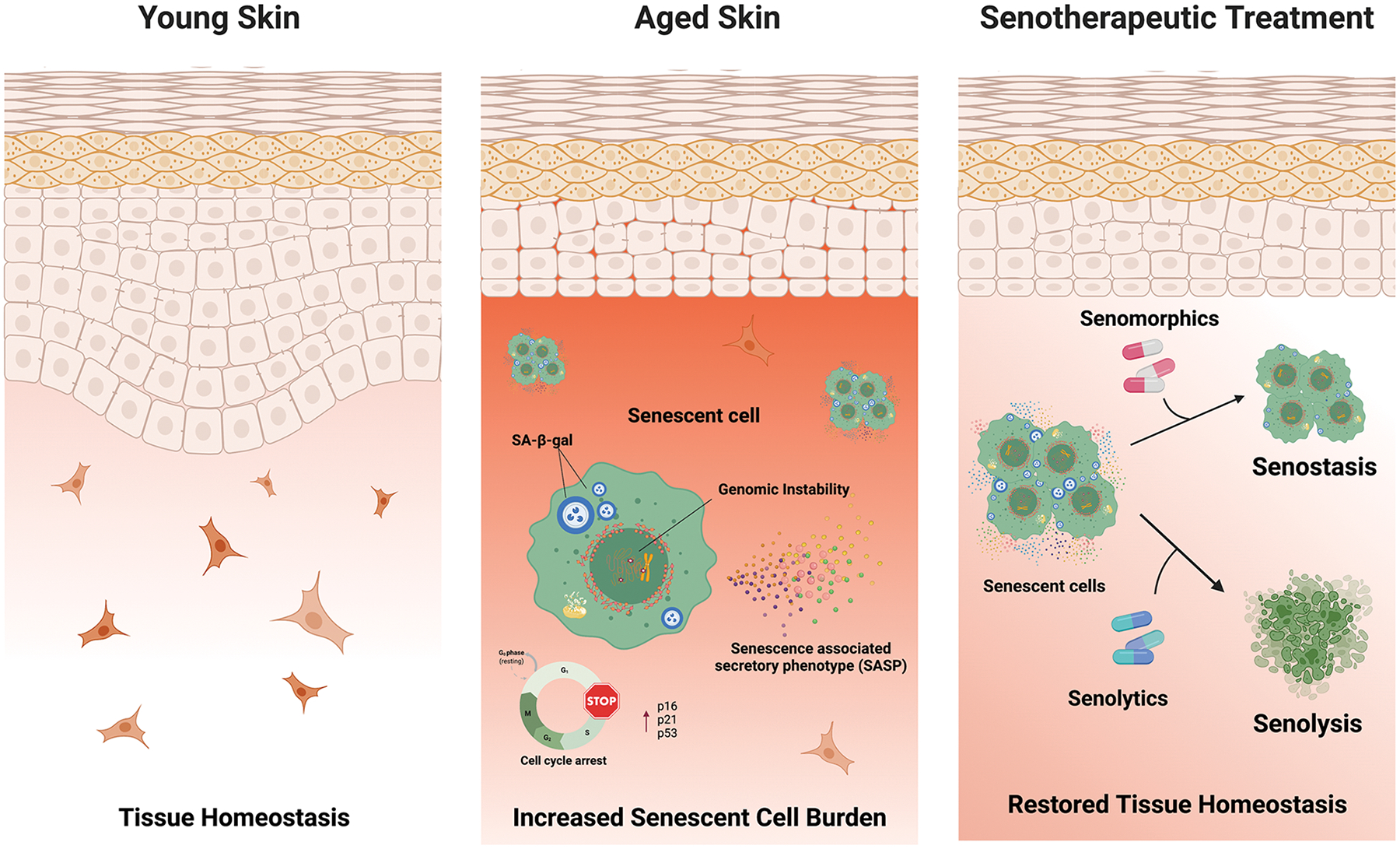
Skin aging is associated with many phenotypic alterations including increased permeability and stiffness of the stratum corneum, flattening and loss of rete ridges in the epidermal-derma junction, alterations in cellular composition, increased senescence cell burden and other aging phenotypes that reduces the protective function and wound healing of the skin. However, senotherapeutic treatments that selectively target senescent cells promoting their clearance (senolysis) or suppression of their SASP (senostasis) decreases inflammation, attenuates tissue dysfunction, and improves regenerative capacity in skin.
The majority of the senolytics identified promote senolysis by targeting critical enzymes involved in pro-survival and anti-apoptotic mechanisms, such as p53, p21, Bcl-2 family proteins, Akt, PI3K, FOXO4, and others (69,70). The first identified senolytic, the combination of Dasatinib and quercetin (D+Q), is one of the most utilized senolytic treatments demonstrating induction of senolysis in many senescent cell types, despite incredible senescent cell heterogeneity, likely through targeting multiple SCAPs (71,72). Indeed, D+Q treatment has been shown to promote senolysis of radiation-induced senescent human oral keratinocytes and skin fibroblasts in vitro and was shown to downregulate p16Ink4a and SASP, upregulate proliferation marker Ki67 and mitigate radiation induced ulcers in both mouse oral ulcer and rat skin ulcer models (73). Further, in one of the first senolytic clinical trials examining the effect of D+Q-mediated senescent cell clearance in patients with diabetic kidney disease, D+Q treatment reduced p16INK4A+ and p21CIP1+ skin epidermal cells as well as circulating SASP factors (NCT02848131) (74). Another senolytic, ABT-737, which targets Bcl-2 and Bcl-xL, was able to clear senescent cells, reduce mitochondrial ROS and rescue epidermal atrophy in 3D living epidermal equivalents (melanoderms) induced to senesce via repeated UVA+B exposure (49). ABT-737 was also found to eliminate senescent cells in the lung and epidermis of double-transgenic K5-rtTA/tet-p14 mice, resulting in increased hair-follicle stem cell proliferation (75). The senolytic FOXO4-D-retoro-inverso (FOXO4-DRI) peptide targets the interaction of p53 and FOXO4. Treatment of the XpdTTD/TTD mouse model of accelerated aging and naturally aged mice with the FOXO4-DRi peptide alleviated aging skin phenotypes as well as hair loss and discoloration (76). Fisetin, a natural flavonoid with diverse pharmacological effects including senolytic activity (77), was shown to protect UVB irradiated hairless mice from photodamage, photo induced inflammation and reduced collagen degradation, wrinkling, and transepidermal water loss through inhibition of mitogen-activated protein kinase (MAPK), activator protein-1 (AP-1), and matrix metalloproteinases (MMPs) when applied topologically for 10 weeks (78). Further, the ATP-competitive inhibitor of spleen tyrosine kinase (Syk), R406, demonstrated senolytic activity in replicatively-induced senescent human diploid dermal fibroblasts by targeting SCAPs that include focal adhesion kinase (FAK) and p38 mitogen-activated protein kinase (MAPK) pathways (79). Finally, Nav-Gal, a senolytic prodrug consisting of the senolytic navitoclax containing an acetylated galactose moiety for selective activation in SA-ßgal expression SnCs, exhibited potent senolytic activity in chemotherapy-induced senescent SK-Mel-103 melanoma cell lines, greater than what is observed with navitoclax alone (80). Thus, there are many senotherapeutic compounds effective against senescence skin cells that could be used for the improvement of age-related alteration in skin structure, inflammation, tissues damage and dysfunction, as well as regenerative capacity. It is important to note that it is still unclear if these senotherapeutic compounds also target the transient senescent cells that appear at sites of wound healing, which may have different SCAPs that those in SnCs that accumulate chronically. It may be possible to improve wound healing by senotherapeutic treatment to reduce the burden of SnCs without affecting the function of the beneficial SnCs.
Senomorphics function by suppressing secretion of deleterious pro-inflammatory SASP components without inducing senolysis by targeting NF-κB, mTOR, IL-1α, p38 MAPK, and other signaling pathways (81–88). Suppression of SASP also prevents the spread of paracrine senescence, thus reducing the SnC burden. Rapamycin, an mTOR inhibitor, is one the most well-established senomorphics demonstrated to extend health and lifespan in aged mice even when used as a late-life intervention. Rapamycin treatment in a UVA-induced model of photoaging resulted in a significant reduction in senescence and SASP markers as well as oxidative and genotoxic stress and increases autophagy and type I collagen expression levels in human dermal fibroblasts (89–91). Nanoparticle delivery of rapamycin in early and late passage, as well as doxorubicin induced-senescent human dermal fibroblasts, reduced senescence phenotypes, SASP, and ROS while improving migration ability and cell proliferation in vitro (92). Finally, an interventional trial examining the effects of topological rapamycin treatment in participants greater than 40 years of age displaying evidence of photoaging found that treatment improved clinical appearance of the skin, increased levels of collagen VII, and reduced expression of p16Ink4a and other histological markers of aging and senescence (93).
Metformin, a compound used clinically for type II diabetes, also has been extensively used for the treatment of a variety of age-related disorders in humans including hidradenitis suppurativa, acanthosis nigricans, hormonal acne, psoriasis, cutaneous malignancies, hirsutism and hyper pigmentary disorders when administered both orally and topologically (94). The therapeutic benefit of metformin can be attributed, in part, to its pleiotropic senomorphic abilities to influence many signaling pathways associated with SASP secretion. Accordingly, chronic low dose treatment in senescent human dermal fibroblasts and mesenchymal stem cells reduced SA-β-gal activity, reduced expression of SASP and other markers of senescence and increased the percentage of Ki67+ cells (95,96). Many natural products, particularly apigenin and kaempferol, were demonstrated to significantly inhibit SASP production in bleomycin-induced senescent BJ fibroblasts (97) while other natural polyphenols oleuropein aglycone and hydroxytyrosol also reduce SA-β-gal-positive cells and p16INK4a protein expression and attenuate a myriad of SASP factors in pre-senescent neonatal human dermal fibroblasts(98). Thus, treatment with senomorphics also can alleviate age-related skin dysfunction and stem cell exhaustion through both antioxidant and anti-inflammatory mechanisms by means of targeting a variety of signaling pathways demonstrated to attenuate senescence phenotypes.
Conclusion
Targeting fundamental hallmarks of skin aging has the potential to alleviate many cutaneous disorders simultaneously. Cellular senescence is a fundament hallmark and well-established driver of aging and age-related chronic diseases and thus serves as an important therapeutic target. Although the discovery of senotherapeutics occurred relatively recently, their beneficial effects are well documented in an increasing number of preclinical and clinical studies. However, although extensive pre-clinical and still limited clinical data has demonstrated the potential of senotherapeutic, there is still much to be understood in the context of skin aging and senotherapeutic treatment. Studies investigating which cells undergo senescence in the skin as well as single-cell multi-OMIC and spatial-OMIC characterization of senescent cell heterogeneity will be required to identify the key SnC types and their SCAPs as well as determine the effects of different senescent cell populations on the skin microenvironment. These and other approaches are also needed for the identification, optimization and application of novel senotherapeutics. Moreover, more work is needed to determine the optimal senotherapeutic treatment regimens and determine the effect of long-term use regarding off-target effects and the impact on other senescence-associated processes such as wound healing. However, there have been incredible efforts in improving senotherapeutic specificity including increasing their specificity for senescent cells and improving their delivery to certain cells or tissues. Also, it is likely that certain oral senotherapeutic treatment regimens will not only increase healthspan, but slow aging of skin. Finally, unlike many drugs that need continuous administration to be effective, in theory, senolytic treatments can be administered topically or orally intermittently, reducing the chance of possible side effects. In summary, senotherapeutic treatments have tremendous potential to eventually become a standard of care for skin aging and related skin disorders and revolutionize how we treat aging in general.
Supplementary Material
Table, Supplemental Digital Content 1: Senotherapeutics reported to have positive effects on skin and skin-derived cell types.
Acknowledgements
Elizabeth L. Thompson is partly funded by NIA Training Grant T32-AG029796.
Footnotes
Financial Disclosure statement
Dr. Niedernhofer and Dr. Robbins have a financial interest related to this research with patents issued or filed on senotherapeutic drugs. The other authors have no financial interest in any of the drugs mentioned in this manuscript.
References
- 1.Dimri G, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano E, Linskens M, Rubelj I and Pereira-Smith O (1995) A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proceedings of the National Academy of Sciences of the United States of America, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michaloglou C, Vredeveld L, Soengas M, Denoyelle C, Kuilman T, van der Horst C, Majoor D, Shay J, Mooi W and Peeper D (2005) BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature, 436. [DOI] [PubMed] [Google Scholar]
- 3.Krishnamurthy J, Torrice C, Ramsey M, Kovalev G, Al-Regaiey K, Su L and Sharpless N (2004) Ink4a/Arf expression is a biomarker of aging. The Journal of clinical investigation, 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ressler S, Bartkova J, Niederegger H, Bartek J, Scharffetter-Kochanek K, Jansen-Dürr P and Wlaschek M (2006) p16INK4A is a robust in vivo biomarker of cellular aging in human skin. Aging cell, 5. [DOI] [PubMed] [Google Scholar]
- 5.Coppé J, Rodier F, Patil C, Freund A, Desprez P and Campisi J (2011) Tumor suppressor and aging biomarker p16(INK4a) induces cellular senescence without the associated inflammatory secretory phenotype. The Journal of biological chemistry, 286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gruber F, Kremslehner C, Eckhart L and Tschachler E (2020) Cell aging and cellular senescence in skin aging - Recent advances in fibroblast and keratinocyte biology. Experimental gerontology, 130. [DOI] [PubMed] [Google Scholar]
- 7.Dreesen O, Chojnowski A, Ong P, Zhao T, Common J, Lunny D, Lane E, Lee S, Vardy L, Stewart C et al. (2013) Lamin B1 fluctuations have differential effects on cellular proliferation and senescence. The Journal of cell biology, 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ivanov A, Pawlikowski J, Manoharan I, van Tuyn J, Nelson D, Rai T, Shah P, Hewitt G, Korolchuk V, Passos J et al. (2013) Lysosome-mediated processing of chromatin in senescence. The Journal of cell biology, 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang AS and Dreesen O (2018) Biomarkers of Cellular Senescence and Skin Aging. Front Genet, 9, 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah P, Donahue G, Otte G, Capell B, Nelson D, Cao K, Aggarwala V, Cruickshanks H, Rai T, McBryan T et al. (2013) Lamin B1 depletion in senescent cells triggers large-scale changes in gene expression and the chromatin landscape. Genes & development, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coppé J, Patil C, Rodier F, Sun Y, Muñoz D, Goldstein J, Nelson P, Desprez P and Campisi J (2008) Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS biology, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR, Laberge RM, Vijg J, Van Steeg H, Dollé ME et al. (2014) An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell, 31, 722–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drosten M, Lechuga C and Barbacid M (2014) Ras signaling is essential for skin development. Oncogene, 33. [DOI] [PubMed] [Google Scholar]
- 14.Fitsiou E, Pulido T, Campisi J, Alimirah F and Demaria M (2021) Cellular Senescence and the Senescence-Associated Secretory Phenotype as Drivers of Skin Photoaging. J Invest Dermatol, 141, 1119–1126. [DOI] [PubMed] [Google Scholar]
- 15.Campisi J (2005) Suppressing cancer: the importance of being senescent. Science (New York, N.Y.), 309. [DOI] [PubMed] [Google Scholar]
- 16.Vande Berg J, Rose M, Haywood-Reid P, Rudolph R, Payne W and Robson M (2005) Cultured pressure ulcer fibroblasts show replicative senescence with elevated production of plasmin, plasminogen activator inhibitor-1, and transforming growth factor-beta1. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society, 13. [DOI] [PubMed] [Google Scholar]
- 17.Wilkinson H, Clowes C, Banyard K, Matteuci P, Mace K and Hardman M (2019) Elevated Local Senescence in Diabetic Wound Healing Is Linked to Pathological Repair via CXCR2. The Journal of investigative dermatology, 139. [DOI] [PubMed] [Google Scholar]
- 18.Passos J, Saretzki G, Ahmed S, Nelson G, Richter T, Peters H, Wappler I, Birket M, Harold G, Schaeuble K et al. (2007) Mitochondrial dysfunction accounts for the stochastic heterogeneity in telomere-dependent senescence. PLoS biology, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Velarde MC, Flynn JM, Day NU, Melov S and Campisi J (2012) Mitochondrial oxidative stress caused by Sod2 deficiency promotes cellular senescence and aging phenotypes in the skin. Aging (Albany NY), 4, 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cristofalo V, Lorenzini A, Allen R, Torres C and Tresini M (2004) Replicative senescence: a critical review. Mechanisms of ageing and development, 125. [DOI] [PubMed] [Google Scholar]
- 21.Foo M, Ong P and Dreesen O (2019) Premature aging syndromes: From patients to mechanism. Journal of dermatological science, 96. [DOI] [PubMed] [Google Scholar]
- 22.Shin J, Kim JH and Kim EK (2012) Repeated exposure of human fibroblasts to UVR induces secretion of stem cell factor and senescence. J Eur Acad Dermatol Venereol, 26, 1577–1580. [DOI] [PubMed] [Google Scholar]
- 23.Pierceall W, Goldberg L, Tainsky M, Mukhopadhyay T and Ananthaswamy H (1991) Ras gene mutation and amplification in human nonmelanoma skin cancers. Molecular carcinogenesis, 4. [DOI] [PubMed] [Google Scholar]
- 24.Dhomen N, Reis-Filho J, da Rocha Dias S, Hayward R, Savage K, Delmas V, Larue L, Pritchard C and Marais R (2009) Oncogenic Braf induces melanocyte senescence and melanoma in mice. Cancer cell, 15. [DOI] [PubMed] [Google Scholar]
- 25.Branchet MC, Boisnic S, Frances C and Robert AM (1990) Skin thickness changes in normal aging skin. Gerontology, 36, 28–35. [DOI] [PubMed] [Google Scholar]
- 26.Russell-Goldman E and Murphy GF (2020) The Pathobiology of Skin Aging: New Insights into an Old Dilemma. Am J Pathol, 190, 1356–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mine S, Fortunel N, Pageon H and Asselineau D (2008) Aging alters functionally human dermal papillary fibroblasts but not reticular fibroblasts: a new view of skin morphogenesis and aging. PloS one, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rittié L and Fisher G (2015) Natural and sun-induced aging of human skin. Cold Spring Harbor perspectives in medicine, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmed A, Sheng M, Wasnik S, Baylink D and KW L (2017) Effect of aging on stem cells. World journal of experimental medicine, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee YI, Choi S, Roh WS, Lee JH and Kim TG (2021) Cellular Senescence and Inflammaging in the Skin Microenvironment. Int J Mol Sci, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pils V, Ring N, Valdivieso K, Lämmermann I, Gruber F, Schosserer M, Grillari J and Ogrodnik M (2021) Promises and challenges of senolytics in skin regeneration, pathology and ageing. Mech Ageing Dev, 200, 111588. [DOI] [PubMed] [Google Scholar]
- 32.Quan T, Little E, Quan H, Qin Z, Voorhees JJ and Fisher GJ (2013) Elevated matrix metalloproteinases and collagen fragmentation in photodamaged human skin: impact of altered extracellular matrix microenvironment on dermal fibroblast function. J Invest Dermatol, 133, 1362–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sprenger A, Weber S, Zarai M, Engelke R, Nascimento JM, Gretzmeier C, Hilpert M, Boerries M, Has C, Busch H et al. (2013) Consistency of the proteome in primary human keratinocytes with respect to gender, age, and skin localization. Mol Cell Proteomics, 12, 2509–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ryu YS, Kang KA, Piao MJ, Ahn MJ, Yi JM, Bossis G, Hyun YM, Park CO and Hyun JW (2019) Particulate matter-induced senescence of skin keratinocytes involves oxidative stress-dependent epigenetic modifications. Exp Mol Med, 51, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wlaschek M, Maity P, Makrantonaki E and Scharffetter-Kochanek K (2021) Connective Tissue and Fibroblast Senescence in Skin Aging. J Invest Dermatol, 141, 985–992. [DOI] [PubMed] [Google Scholar]
- 36.Marsh E, Gonzalez DG, Lathrop EA, Boucher J and Greco V (2018) Positional Stability and Membrane Occupancy Define Skin Fibroblast Homeostasis In Vivo. Cell, 175, 1620–1633.e1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Acosta JC, O’Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, Fumagalli M, Da Costa M, Brown C, Popov N et al. (2008) Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell, 133, 1006–1018. [DOI] [PubMed] [Google Scholar]
- 38.Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ, Aarden LA, Mooi WJ and Peeper DS (2008) Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell, 133, 1019–1031. [DOI] [PubMed] [Google Scholar]
- 39.Chen W, Kang J, Xia J, Li Y, Yang B, Chen B, Sun W, Song X, Xiang W, Wang X et al. (2008) p53-related apoptosis resistance and tumor suppression activity in UVB-induced premature senescent human skin fibroblasts. Int J Mol Med, 21, 645–653. [PubMed] [Google Scholar]
- 40.Kim DE, Dollé MET, Vermeij WP, Gyenis A, Vogel K, Hoeijmakers JHJ, Wiley CD, Davalos AR, Hasty P, Desprez PY et al. (2020) Deficiency in the DNA repair protein ERCC1 triggers a link between senescence and apoptosis in human fibroblasts and mouse skin. Aging Cell, 19, e13072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoon JE, Kim Y, Kwon S, Kim M, Kim YH, Kim JH, Park TJ and Kang HY (2018) Senescent fibroblasts drive ageing pigmentation: A potential therapeutic target for senile lentigo. Theranostics, 8, 4620–4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singh K, Maity P, Krug L, Meyer P, Treiber N, Lucas T, Basu A, Kochanek S, Wlaschek M, Geiger H et al. (2015) Superoxide anion radicals induce IGF-1 resistance through concomitant activation of PTP1B and PTEN. EMBO Mol Med, 7, 59–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gilchrest B, Blog F and Szabo G (1979) Effects of aging and chronic sun exposure on melanocytes in human skin. The Journal of investigative dermatology, 73. [DOI] [PubMed] [Google Scholar]
- 44.Ortonne JP (1990) Pigmentary changes of the ageing skin. Br J Dermatol, 122 Suppl 35, 21–28. [DOI] [PubMed] [Google Scholar]
- 45.Waaijer MEC, Gunn DA, van Heemst D, Slagboom PE, Sedivy JM, Dirks RW, Tanke HJ, Westendorp RGJ and Maier AB (2018) Do senescence markers correlate in vitro and in situ within individual human donors? Aging (Albany NY), 10, 278–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Waaijer M, Gunn D, Adams P, Pawlikowski J, Griffiths C, van Heemst D, Slagboom P, Westendorp R and Maier A (2016) P16INK4a Positive Cells in Human Skin Are Indicative of Local Elastic Fiber Morphology, Facial Wrinkling, and Perceived Age. The journals of gerontology. Series A, Biological sciences and medical sciences, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang AS, Ong PF, Chojnowski A, Clavel C and Dreesen O (2017) Loss of lamin B1 is a biomarker to quantify cellular senescence in photoaged skin. Sci Rep, 7, 15678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gray-Schopfer V, Cheong S, Chong H, Chow J, Moss T, Abdel-Malek Z, Marais R, Wynford-Thomas D and Bennett D (2006) Cellular senescence in naevi and immortalisation in melanoma: a role for p16? British journal of cancer, 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Victorelli S, Lagnado A, Halim J, Moore W, Talbot D, Barrett K, Chapman J, Birch J, Ogrodnik M, Meves A et al. (2019) Senescent human melanocytes drive skin ageing via paracrine telomere dysfunction. EMBO J, 38, e101982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jun JI and Lau LF (2010) The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat Cell Biol, 12, 676–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wall IB, Moseley R, Baird DM, Kipling D, Giles P, Laffafian I, Price PE, Thomas DW and Stephens P (2008) Fibroblast dysfunction is a key factor in the non-healing of chronic venous leg ulcers. J Invest Dermatol, 128, 2526–2540. [DOI] [PubMed] [Google Scholar]
- 52.Campisi J (2013) Aging, cellular senescence, and cancer. Annu Rev Physiol, 75, 685–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thompson EL, Hu JJ and Niedernhofer LJ (2021) The Role of Senescent Cells in Acquired Drug Resistance and Secondary Cancer in BRAFi-Treated Melanoma. Cancers (Basel), 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Prasanna P, Citrin D, Hildesheim J, Ahmed M, Venkatachalam S, Riscuta G, Xi D, Zheng G, van Deursen J, Goronzy J et al. (2021) Therapy-Induced Senescence: Opportunities to Improve Anti-Cancer Therapy. Journal of the National Cancer Institute. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baker D, Childs B, Durik M, Wijers M, Sieben C, Zhong J, Saltness R, Jeganathan K, Verzosa G, Pezeshki A et al. (2016) Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature, 530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alimirah F, Pulido T, Valdovinos A, Alptekin S, Chang E, Jones E, Diaz D, Flores J, Velarde M, Demaria M et al. (2020) Cellular Senescence Promotes Skin Carcinogenesis through p38MAPK and p44/42MAPK Signaling. Cancer research, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Krayem M, Najem A, Journe F, Morandini R, Sales F, Awada A and Ghanem G (2018) Acquired resistance to BRAFi reverses senescence-like phenotype in mutant BRAF melanoma. Oncotarget, 9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 58.Collado M, Gil J, Efeyan A, Guerra C, Schuhmacher A, Barradas M, Benguría A, Zaballos A, Flores J, Barbacid M et al. (2005) Tumour biology: senescence in premalignant tumours. Nature, 436. [DOI] [PubMed] [Google Scholar]
- 59.Vernot J (2020) Senescence-Associated Pro-inflammatory Cytokines and Tumor Cell Plasticity. Frontiers in molecular biosciences, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Milanovic M, Fan D, Belenki D, Däbritz J, Zhao Z, Yu Y, Dörr J, Dimitrova L, Lenze D, Monteiro Barbosa I et al. (2018) Senescence-associated reprogramming promotes cancer stemness. Nature, 553. [DOI] [PubMed] [Google Scholar]
- 61.Milanovic M, Yu Y and Schmitt C (2018) The Senescence-Stemness Alliance - A Cancer-Hijacked Regeneration Principle. Trends in cell biology, 28. [DOI] [PubMed] [Google Scholar]
- 62.Soufir N, Molès J, Vilmer C, Moch C, Verola O, Rivet J, Tesniere A, Dubertret L and Basset-Seguin N (1999) P16 UV mutations in human skin epithelial tumors. Oncogene, 18. [DOI] [PubMed] [Google Scholar]
- 63.Foulkes W, Flanders T, Pollock P and Hayward N (1997) The CDKN2A (p16) gene and human cancer. Molecular medicine (Cambridge, Mass.), 3. [PMC free article] [PubMed] [Google Scholar]
- 64.Liu Y and Sheikh MS (2014) Melanoma: Molecular Pathogenesis and Therapeutic Management. Mol Cell Pharmacol, 6, 228. [PMC free article] [PubMed] [Google Scholar]
- 65.Pollock P, Harper U, Hansen K, Yudt L, Stark M, Robbins C, Moses T, Hostetter G, Wagner U, Kakareka J et al. (2003) High frequency of BRAF mutations in nevi. Nature genetics, 33. [DOI] [PubMed] [Google Scholar]
- 66.Bennett D (2003) Human melanocyte senescence and melanoma susceptibility genes. Oncogene, 22. [DOI] [PubMed] [Google Scholar]
- 67.Di Micco R, Fumagalli M, Cicalese A, Piccinin S, Gasparini P, Luise C, Schurra C, Garre’ M, Nuciforo PG, Bensimon A et al. (2006) Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature, 444, 638–642. [DOI] [PubMed] [Google Scholar]
- 68.Oberholzer PA, Kee D, Dziunycz P, Sucker A, Kamsukom N, Jones R, Roden C, Chalk CJ, Ardlie K, Palescandolo E et al. (2011) RAS Mutations Are Associated With the Development of Cutaneous Squamous Cell Tumors in Patients Treated With RAF Inhibitors. 10.1200/JCO.2011.36.7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang L, Pitcher LE, Prahalad V, Niedernhofer LJ and Robbins PD (2021) Recent advances in the discovery of senolytics. Mechanisms of Ageing and Development, 200, 111587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang L, Pitcher LE, Prahalad V, Niedernhofer LJ and Robbins PD (2022) Targeting Cellular Senescence with Senotherapeutics: Senolytics and Senomorphics. The FEBS journal. [DOI] [PubMed] [Google Scholar]
- 71.Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, Palmer AK, Ikeno Y, Hubbard GB, Lenburg M et al. (2015) The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell, 14, 644–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xu M, Pirtskhalava T, Farr JN, Weigand BM, Palmer AK, Weivoda MM, Inman CL, Ogrodnik MB, Hachfeld CM, Fraser DG et al. (2018) Senolytics improve physical function and increase lifespan in old age. Nat Med, 24, 1246–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang H, Wang Z, Huang Y, Zhou Y, Sheng X, Jiang Q, Wang Y, Luo P, Luo M and Shi C (2020) Senolytics (DQ) Mitigates Radiation Ulcers by Removing Senescent Cells. Frontiers in Oncology, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hickson LJ, Langhi Prata LGP, Bobart SA, Evans TK, Giorgadze N, Hashmi SK, Herrmann SM, Jensen MD, Jia Q, Jordan KL et al. (2019) Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine, 47, 446–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yosef R, Pilpel N, Tokarsky-Amiel R, Biran A, Ovadya Y, Cohen S, Vadai E, Dassa L, Shahar E, Condiotti R et al. (2016) Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nature Communications, 7, 11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang C, Xie Y, Chen H, Lv L, Yao J, Zhang M, Xia K, Feng X, Li Y, Liang X et al. (2020) FOXO4-DRI alleviates age-related testosterone secretion insufficiency by targeting senescent Leydig cells in aged mice. Aging (Albany NY), 12, 1272–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yousefzadeh M, Zhu Y, McGowan S, Angelini L, Fuhrmann-Stroissnigg H, Xu M, Ling Y, Melos K, Pirtskhalava T, Inman C et al. (2018) Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu P-Y, Lyu J-L, Liu Y-J, Chien T-Y, Hsu H-C, Wen K-C and Chiang H-M (2017) Fisetin Regulates Nrf2 Expression and the Inflammation-Related Signaling Pathway to Prevent UVB-Induced Skin Damage in Hairless Mice. International journal of molecular sciences, 18, 2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cho H-J, Yang EJ, Park JT, Kim J-R, Kim E-C, Jung K-J, Park SC and Lee Y-S (2020) Identification of SYK inhibitor, R406 as a novel senolytic agent. Aging, 12, 8221–8240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.González-Gualda E, Pàez-Ribes M, Lozano-Torres B, Macias D, Wilson JR 3rd, González-López C, Ou H-L, Mirón-Barroso S, Zhang Z, Lérida-Viso A et al. (2020) Galacto-conjugation of Navitoclax as an efficient strategy to increase senolytic specificity and reduce platelet toxicity. Aging cell, 19, e13142–e13142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fryer LG, Parbu-Patel A and Carling D (2002) The Anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J Biol Chem, 277, 25226–25232. [DOI] [PubMed] [Google Scholar]
- 82.Moiseeva O, Deschênes-Simard X, St-Germain E, Igelmann S, Huot G, Cadar AE, Bourdeau V, Pollak MN and Ferbeyre G (2013) Metformin inhibits the senescence-associated secretory phenotype by interfering with IKK/NF-κB activation. Aging Cell, 12, 489–498. [DOI] [PubMed] [Google Scholar]
- 83.Kulkarni SS and Cantó C (2015) The molecular targets of resveratrol. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, 1852, 1114–1123. [DOI] [PubMed] [Google Scholar]
- 84.Holmes-McNary M and Baldwin AS Jr. (2000) Chemopreventive properties of trans-resveratrol are associated with inhibition of activation of the IkappaB kinase. Cancer Res, 60, 3477–3483. [PubMed] [Google Scholar]
- 85.Pitozzi V, Mocali A, Laurenzana A, Giannoni E, Cifola I, Battaglia C, Chiarugi P, Dolara P and Giovannelli L (2012) Chronic Resveratrol Treatment Ameliorates Cell Adhesion and Mitigates the Inflammatory Phenotype in Senescent Human Fibroblasts. The Journals of Gerontology: Series A, 68, 371–381. [DOI] [PubMed] [Google Scholar]
- 86.Alimbetov D, Davis T, Brook AJ, Cox LS, Faragher RG, Nurgozhin T, Zhumadilov Z and Kipling D (2016) Suppression of the senescence-associated secretory phenotype (SASP) in human fibroblasts using small molecule inhibitors of p38 MAP kinase and MK2. Biogerontology, 17, 305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Goldstein DM, Kuglstatter A, Lou Y and Soth MJ (2010) Selective p38alpha inhibitors clinically evaluated for the treatment of chronic inflammatory disorders. J Med Chem, 53, 2345–2353. [DOI] [PubMed] [Google Scholar]
- 88.Velarde MC and Demaria M (2016) Targeting Senescent Cells: Possible Implications for Delaying Skin Aging: A Mini-Review. Gerontology, 62, 513–518. [DOI] [PubMed] [Google Scholar]
- 89.Bai GL, Wang P, Huang X, Wang ZY, Cao D, Liu C, Liu YY, Li RL and Chen AJ (2021) Rapamycin Protects Skin Fibroblasts From UVA-Induced Photoaging by Inhibition of p53 and Phosphorylated HSP27. Front Cell Dev Biol, 9, 633331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Walters HE, Deneka-Hannemann S and Cox LS (2016) Reversal of phenotypes of cellular senescence by pan-mTOR inhibition. Aging (Albany NY), 8, 231–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thapa RK, Nguyen HT, Jeong JH, Kim JR, Choi HG, Yong CS and Kim JO (2017) Progressive slowdown/prevention of cellular senescence by CD9-targeted delivery of rapamycin using lactose-wrapped calcium carbonate nanoparticles. Sci Rep, 7, 43299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Thapa RK, Nguyen HT, Jeong J-H, Kim JR, Choi H-G, Yong CS and Kim JO (2017) Progressive slowdown/prevention of cellular senescence by CD9-targeted delivery of rapamycin using lactose-wrapped calcium carbonate nanoparticles. Scientific Reports, 7, 43299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chung CL, Lawrence I, Hoffman M, Elgindi D, Nadhan K, Potnis M, Jin A, Sershon C, Binnebose R, Lorenzini A et al. (2019) Topical rapamycin reduces markers of senescence and aging in human skin: an exploratory, prospective, randomized trial. GeroScience, 41, 861–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bubna AK (2016) Metformin - For the dermatologist. Indian J Pharmacol, 48, 4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Moiseeva O, Deschenes-Simard X, St-Germain E, Igelmann S, Huot G, Cadar AE, Bourdeau V, Pollak MN and Ferbeyre G (2013) Metformin inhibits the senescence-associated secretory phenotype by interfering with IKK/NF-kappaB activation. Aging Cell, 12, 489–498. [DOI] [PubMed] [Google Scholar]
- 96.Fang J, Yang J, Wu X, Zhang G, Li T, Wang X, Zhang H, Wang CC, Liu GH and Wang L (2018) Metformin alleviates human cellular aging by upregulating the endoplasmic reticulum glutathione peroxidase 7. Aging Cell, 17, e12765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lim H, Park H and Kim HP (2015) Effects of flavonoids on senescence-associated secretory phenotype formation from bleomycin-induced senescence in BJ fibroblasts. Biochem Pharmacol, 96, 337–348. [DOI] [PubMed] [Google Scholar]
- 98.Menicacci B, Cipriani C, Margheri F, Mocali A and Giovannelli L (2017) Modulation of the Senescence-Associated Inflammatory Phenotype in Human Fibroblasts by Olive Phenols. Int J Mol Sci, 18, 2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table, Supplemental Digital Content 1: Senotherapeutics reported to have positive effects on skin and skin-derived cell types.


