Abstract
Background:
Testosterone is one of the strategies that transmasculine persons can elect in order to align physical traits to their gender identity. Previous studies have shown morphologic changes in the genital tract associated with testosterone. Here, we aim to evaluate cervicovaginal cytology specimens (Pap tests) and high-risk HPV (HR-HPV) testing from transmasculine individuals receiving testosterone.
Methods:
This is a retrospective cohort of 61 transmasculine individuals receiving testosterone from 2013 to 2021. Cytologic diagnoses from 65 Pap tests were correlated with HPV status and histologic follow-up and compared to the institutional data and a cohort of cisgender women with atrophic changes.
Results:
The median age was 28 years and median time of testosterone use was 3 years. Transmasculine persons showed significantly higher rates of HSIL (2%) and unsatisfactory (16%) when compared to the institutional data and atrophic cohort of cisgender women. After reviewing slides of 46 cases, additional findings were noted: atrophy was present in 87%, glycogenated cells were seen in 30% and Lactobacilli were substantially decreased in 89%. Among 32 available HPV tests, 19% were positive for HR-HPV and 81% were negative. On histologic follow-up, all HR-HPV-positive cases with abnormal cytology showed HSIL, while none of the HPV-negative cases revealed HSIL.
Conclusion:
Our study cohort demonstrated a high percentage of abnormal Pap tests in transmasculine persons receiving testosterone. Testosterone seems to induce changes in squamous cells and shifts in vaginal flora. HR-HPV testing can be a useful adjunct in the workup of abnormal Pap tests from transmasculine individuals.
Keywords: Transgender Persons, Testosterone, Papanicolaou Test, Cervical Cancer, HPV
Introduction
Transmasculine individuals is a broad term that refers to persons who were assigned female at birth, but identify themselves as male or within the masculine spectrum. The majority of transmasculine individuals do not undergo gender-affirming hysterectomies according to the 2015 US transgender survey1; therefore, those persons maintain their cervix and are at risk of developing cervical cancer.
After the implementation of screening programs based on cervicovaginal cytology and high-risk HPV testing, cervical cancer incidence has substantially decreased. However, there are notable barriers for the access of appropriate screening in transmasculine individuals with evidence in the literature showing that they do not receive the same level of care in cervical cancer screening when compared to cisgender women 2–5. Reasons for this disparity include high rate of negative experiences reported by transgender individuals seeking healthcare, insurance coverage denial, physical discomfort and emotional distress during cervicovaginal sample collection and traditional gender representations of screening programs initiatives, which primarily focus on cisgender women 2.
In addition to barriers in screening, morphologic changes in the genital tract following testosterone may affect screening results 6–9. Testosterone is one of the strategies that transmasculine individuals can elect to align physical traits to their gender identity. A few reports have shown that cytopathologic findings in cervicovaginal cytology specimens (Pap tests) from transmasculine individuals receiving testosterone are more likely to be unsatisfactory and show atrophic changes 10–13. In addition to cancer screening, Pap tests can also reveal important information regarding vaginal infections and vaginal flora. To the best of our knowledge only one study in the literature evaluated the vaginal flora in transmasculine individuals receiving testosterone 14. Here, we aim to evaluate Pap tests, HPV testing and vaginal flora in transmasculine persons receiving testosterone.
Materials and Methods
This is an institutional review board-approved retrospective cohort of transgender persons receiving testosterone with available Pap tests performed at NYU Langone Medical Center from December 2013 to December 2021. Available cytology slides were reviewed to confirm diagnoses according to the Bethesda System of Cervicovaginal Reporting (TBS) and to evaluate for additional findings not included in the original reports.
HPV testing and histologic follow-up were retrieved from institutional laboratory information systems. HPV testing was performed on samples collected in PreservCyt Solution (Thinprep) by using the Roche Cobas 4800 HPV Test System Roche. Clinical and demographic data were extracted from electronic health records, including age, race, ethnicity, preferred pronouns, marital status, residence setting (urban or rural area), sexual activity, type and duration of testosterone use, comorbidities, smoking history, and body mass index. Rural areas were considered those with fewer than 2,500 residents as of March 20th, 2021.
Cervicovaginal cytology results from transmasculine individuals were compared to institutional data from 2015 to 2021 and a cohort of cisgender women with atrophy on cervicovaginal cytology in 2021. The institutional data included all patients with a Pap test and served as a general population control group. The cohort of atrophic patients was selected by searching for the word “atrophy” in the pathology electronic medical record and included only cisgender women as a control group in which atrophy would not be a confounding factor. Only cisgender women with a cervix and within the same age range as transmasculine individuals were included in the atrophic control group. To determine the percentage of unsatisfactory Pap tests in cases with atrophic change, all unsatisfactory Pap tests in 2021 were reviewed to determine the number of those cases with atrophy. Statistical analysis was performed using Fisher’s exact test and Mann-Whitney test.
To better determine the accuracy of HPV testing to detect HSIL in transmasculine individuals receiving testosterone, a review of the literature was performed and cases from the current cohort were combined with those from the literature. Sensitivity, specificity, positive predictive value, and negative predictive value were calculated based on histologic outcome as the gold standard for the detection of HSIL. Cases with NILM Pap results were also included in the analysis as negative cases for HSIL because histopathology is not routinely obtained.
Results
A total of 61 transmasculine persons receiving testosterone, with a median age of 28 years old (range: 19–56), were included. A summary of clinical and demographic data is presented in Table 1. Twenty-seven individuals self-identified as white (46%), 16 as black (28%), and 15 as other race (26%). A total of 13 patients self-identified as Hispanic (22%). The majority of patients were single (69%), resided in an urban area (98%), and were sexually active (89%). The median time of testosterone use was 3 years (range: 0.2–25) and included multiple different preparations, such as injectable testosterone cypionate (48/61), and testosterone gel (11/61), 1 transdermic testosterone patch (1/61), and injectable testosterone enanthate (1/61). Comorbidities included anxiety and/or depression in 24 patients, asthma in 12, endocrine disorders (e.g., diabetes, polycystic ovary syndrome) in 6, and conditions associated with immunosuppression (e.g., HIV infection, autoimmune disease) in 3.
Table 1:
Clinical and demographic data of transmasculine individuals
| Age, years, mean (range) | 28 (19–56) |
| Age groups (%) | |
| <30 years | 38/61 (62) |
| 30–49 years | 20/61 (33) |
| >49 years | 3/61 (5) |
| Race (%) | |
| White | 27/58 (46) |
| Black | 16/58 (28) |
| Other | 15/58 (26) |
| Ethnicity (%) | |
| Hispanic | 13/58 (22) |
| Non-Hispanic | 45/58 (78) |
| Preferred pronoun (%) | |
| He/him/his | 48/56 (86) |
| They/them/their | 7/56 (12) |
| He/him/his or they/them/their | 1/56 (2) |
| Marital status (%) | |
| Single | 41/59 (69) |
| Married/partnered | 17/59 (29) |
| Separated | 1/59 (2) |
| Residence (%) | |
| Urban | 60/61 (98) |
| Rural | 1/61 (2) |
| Sexually active (%) | |
| Yes | 53/60 (89) |
| Not currently | 5/60 (8) |
| Never | 2/60 (3) |
| Receptive penile-vaginal intercourse (%) | |
| Yes | 15/57 (26) |
| No | 42/57 (74) |
| Immunosuppression/HIV (%) | |
| Yes | 3/61 (5) |
| No | 58/61 (95) |
| Other comorbidities (%) | |
| Cardiovascular disorder (Hypertension, hyperlipidemia) | 6/61 (10) |
| Endocrine disorders (PCOS, diabetes, thyroid disease) | 6/61 (10) |
| Asthma | 12/61 (20) |
| Anxiety/depression | 24/61 (39) |
| Other psychiatric disorders | 7/61 (11) |
| Body mass index (kg/m2) | 25.9 (18.7 – 42.9) |
| Smoking (%) | |
| Never smoker | 40/60 (67) |
| Former smoker | 14/60 (23) |
| Current smoker | 6/60 (10) |
| Testosterone use (years)* | 3 (0.2 – 25) |
| Testosterone type (%) | |
| Intramuscular | 49/61 (80) |
| Gel | 11/61 (18) |
| Transdermal patch | 1/61 (2) |
information not available in 1 patient; PCOS: polycystic ovary syndrome
A total of 65 Pap tests results were available with a trend of increase of cases in the last years (39 cases from 2020–21, 24 cases from 2018–19, and 2 cases from before 2017). The final diagnoses included 48 negative for intraepithelial lesion or malignancy (NILM) (74%), 10 unsatisfactory (16%), 4 atypical squamous cells of undetermined significance (ASC-US) (6%), 1 low-grade squamous intraepithelial lesion (LSIL) (1%) and 2 high-grade squamous intraepithelial lesions (HSIL) (3%) (Table 2). The main reason for unsatisfactory cytology was scant squamous cell cellularity (9/10), while 1 case had obscuring inflammation. An endocervical component was seen in 72% of cases (47/65) and no cases of glandular cell abnormalities were identified.
Table 2:
Cytologic diagnosis in cervicovaginal smears from transmasculine individuals receiving testosterone, institutional cytologic data and atrophic cohort of cisgender women
| Transmasculine | Institutional cytologic data | Atrophic cohort cisgender women | |||
|---|---|---|---|---|---|
|
|
|||||
| Group | cohort (n=65) | Cases (n=80,385) | p-value* | Cases (n=439) | p-value* |
|
| |||||
| NILM | 48 (74%) | 67460 (83.9%) | 0.04** | 403 (92%) | <0.0001** |
| UNSAT | 10 (16%) | 1648 (2%) | <0.0001** | 9 (2%) | <0.0001** |
| ASC-US | 4 (6%) | 7386 (9.2) | 0.53 | 27 (6%) | >0.99 |
| ASC-H | 0 (0%) | 226 (0.3%) | >0.99 | 0 | >0.99 |
| LSIL | 1 (1%) | 3184 (4%) | 0.52 | 0 | 0.129 |
| HSIL | 2 (3%) | 243 (0.3%) | 0.02** | 0 | 0.02** |
| AGC | 0 (0%) | 204 (0.3%) | >0.99 | 0 | >0.99 |
| Cancer | 0 (0%) | 34 (0.0%) | >0.99 | 0 | >0.99 |
p-value for comparison with transmasculine cohort;
statistically significant;
NILM: negative for intraepithelial lesion or malignancy; FU: follow-up; ASCUS: atypical squamous cells of undetermined significance; HSIL: high-grade squamous intraepithelial lesion; LSIL: low-grade squamous intraepithelial lesion; ASC-H: atypical squamous cells of undetermined significance, cannot rule out HSIL; AGC: atypical glandular cells; UNSAT: unsatisfactory
When compared to the institutional cytologic data (n=80,385), transmasculine persons showed significantly lower rates of NILM (74% vs 83.9%; p=0.04) and higher rates of HSIL (3% vs 0.3%; p=0.02) and unsatisfactory (16% vs 2%; p<0.0001). Similarly, compared to the atrophic cohort of cisgender women (n=439), transmasculine individuals showed significantly lower rates of NILM (74% vs 92%; p<0.0001) and higher rates of HSIL (3% vs 0%; p=0.02) and unsatisfactory (16% vs 2%; p<0.0001). The median age in the atrophic cohort of cisgender women was 54 years (range: 21–56), which is significantly higher than in transmasculine individuals in this study (p<0.0001).
Co-testing for high-risk HPV was performed on 32 cases in this cohort which included 6 positive and 26 negative results (Table 3). The corresponding cytologic diagnoses for the HPV-positive cases included 2 NILM, 2 ASC-US, and 2 HSIL, while the HPV-negative cases included 22 NILM, 1 ASC-US, and 3 unsatisfactory results. Histologic follow-up was available in 20 cases; histologic HSIL was diagnosed in all 4 cases with abnormal cytology and HPV-positive results, while all HPV-negative cases had benign histologic follow-up. The 4 cases of histologic HSIL were diagnosed in 3 patients; 2 of them had a history of persistent HSIL for several years and systemic autoimmune diseases, while 1 received a first diagnosis after a NILM result 2 years prior and had no associated comorbidities.
Table 3:
HPV testing and histologic outcomes of Pap tests from transmasculine individuals receiving testosterone from this cohort and the literature
| Study | Current study | Adkins et al (2018) | Williams et al (2020) | Plummer et al (2021) | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Cytology | Histology | Cytology | Histology | Cytology* | Cytology | Histology | |
|
| |||||||
| HPV-positive cases | 2 NILM | 1 benign/1 no FU | 1 NILM | 1 benign | 1 NILM | 4 NILM | 1 benign/3 no FU |
| 2 ASC-US | 2 HSIL | 1 ASC-US/1 ASC-H | 1 no FU/1 LSIL | 1 ASCUS | 1 LSIL | ||
| 2 HSIL | 2 HSIL | 1 HSIL | 1 HSIL | ||||
|
| |||||||
| HPV-negative cases | 22 NILM | 12 benign/10 no FU | 6 NILM | 6 benign | 3 NILM | 16 NILM | 6 benign/10 no FU |
| 1 ASC-US | 1 benign | 2 ASC-US | 2 benign | 1 ASC-US | 2 ASC-US/1 AGC | 2 benign/1 LSIL | |
| 3 UNSAT | 1 benign/2 no FU | 4 UNSAT | 4 benign | ||||
|
| |||||||
| HPV-positive rate | 6/32 (19%) | 4/12 (33%) | 1/5 (20%) | 5/27 (18.5%) | |||
no histologic outcome available according to HPV testing;
NILM: negative for intraepithelial lesion or malignancy; FU: follow-up; ASCUS: atypical squamous cells of undetermined significance; HSIL: high-grade squamous intraepithelial lesion; LSIL: low-grade squamous intraepithelial lesion; ASC-H: atypical squamous cells of undetermined significance, cannot rule out HSIL; AGC: atypical glandular cells; UNSAT: unsatisfactory
Due to the limited number of cases with HPV co-testing in this cohort, we combined our cases with those from studies in the literature 11–13 for statistical analysis to better determine the accuracy of HPV testing in the detection of HSIL in transmasculine individuals receiving testosterone. A total of 72 HPV tests with either concurrent negative cytology or histologic follow-up were included (Table 3). HPV testing sensitivity in the combined data was 100%, specificity 87%, positive predictive value 36%, and negative predictive value 100% for the detection of HSIL.
Slides were reviewed for the 46 cases available and additional findings not included in the pathology reports were noted. Atrophy (shown in Fig. 1a), a known mimicker of HSIL, was present in 87% (40/46), including those with ASCUS (shown in Fig. 2a), LSIL (shown in Fig.2b), and HSIL (shown in Fig.2c). Even cases with unsatisfactory Pap tests showed atrophic changes in the scant cells available for evaluation. There was no significant difference in the duration of testosterone exposure between patients with and without atrophic changes (mean time of testosterone use: 2.4 ± 2.0 years in the group without atrophy vs 6.0 ± 5.4 years in the group with atrophy; p= 0.07). Glycogenated cells (shown in Fig. 1b), which can be mistaken for koilocytes, were seen in 30% (14/46).
Fig. 1.

Atrophic pattern. (a) Syncytial-like sheet of parabasal and basal cells (Papanicolaou, 400X). (b) Glycogenated cell with perinuclear halo and yellow pigment (Papanicolaou, 600X).
Fig. 2.
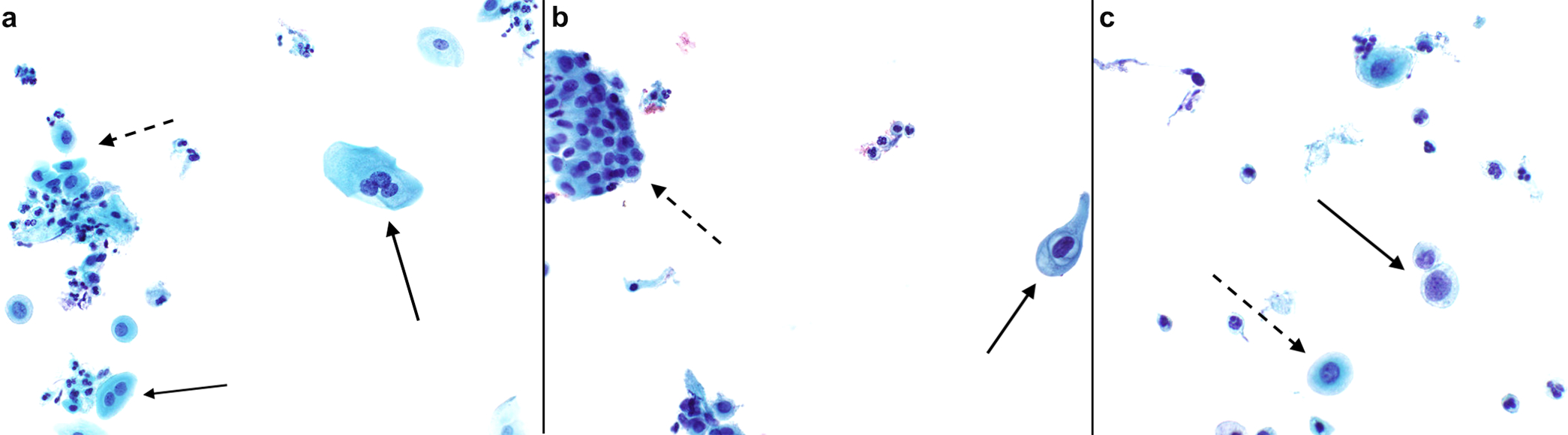
Abnormal squamous cells in atrophic background. (a) ASC-US with binucleation and trinucleation (solid arrow) and parabasal cells (dashed arrow). (b) LSIL with mild nuclear size increase, irregular nuclear membranes and perinuclear halo (solid arrow) and atrophy (dashed arrow) (Papanicolaou, 400X). (c) HSIL with discohesive cells with increased nuclear to cytoplasmic ratio and irregular nuclear contours (solid arrow) and parabasal cells (dashed arrow) (Papanicolaou, 600X).
Lactobacilli were substantially decreased in 89% (41/46) (shown in Fig. 3a) and no evidence of cervicovaginal infection was detected. All cases with atrophic changes showed near absence of Lactobacilli. Of the 6 cases without significant atrophic changes, 2 cases showed predominantly intermediate squamous cells and overall decrease in Lactobacilli (shown in Fig. 3b), while the remaining 4 cases revealed numerous superficial squamous cells with abundant Lactobacilli (shown in Fig. 3c) in 3 cases and absence of Lactobacilli in 1 case. Patients with near absence in Lactobacilli were receiving testosterone for significantly longer time when compared to those with presence of Lactobacilli (mean time of testosterone use: 5.9 ± 5.3 years in group with near absence of Lactobacilli vs 1.8 ± 1.6 years in group with Lactobacilli; p= 0.017).
Fig. 3:

Vaginal flora. (a) Absence of Lactobacilli in an atrophic background (Papanicolaou, 600X). (b) Absence of Lactobacilli in test composed predominantly by intermediate squamous cells (Papanicolaou, 600X). (c) Abundant Lactobacilli and superficial squamous cell (Papanicolaou, 600X).
Discussion/Conclusion
Our study cohort demonstrated a high percentage of abnormal cervicovaginal cytology tests in transmasculine persons receiving testosterone, including previous reported high rates of unsatisfactory Pap tests, mainly due to scant squamous cell cellularity. In contrast to previous studies 10–13,15, we found a higher percentage of HSIL in our population, when compared to our institutional data and cohort of cisgender women with atrophic changes. The increased rate of HSIL could be due to the relatively small sample size, but other factors may play a role. One possible explanation is the fact that transmasculine individuals are under-screened when compared to cisgender women which could potentially contribute to the progression of cervical lesions. The vast majority of our transmasculine patients do not receive preventive care in our institution and are referred for gender-affirming care; therefore, this cohort is composed of a selected group and may not represent the general transmasculine population. Another consideration that might influence higher rates of HSIL in this cohort compared to others in the literature is sexual behavior in large urban centers16–18.
The diagnosis of HSIL in transmasculine persons receiving testosterone can be challenging due to a high percentage of atrophic changes, showing hyperchromatic crowded groups of cells with increased nuclear to cytoplasmic ratio. These findings are not commonly seen in young individuals and can represent a diagnostic pitfall for HSIL not only in cytology, but also in histologic specimens 6. Due to frequent atrophic changes and unsatisfactory Pap tests, HPV testing can aid in the screening of transmasculine individuals. HPV testing has a high sensitivity for the detection of HSIL and has been shown to be useful in follow-up of unsatisfactory Pap tests 19,20. In the analysis of cases compiled from our cohort and studies in the literature, HPV testing showed an elevated sensitivity for the detection of HSIL in transmasculine individuals receiving testosterone. One of the barriers to cervical cancer screening in transmasculine individuals is the procedure of Pap test sample collection by the provider, which can lead to physical discomfort, especially in those receiving testosterone, and significant psychological distress, increasing negative experiences towards healthcare providers 2. An alternative for patients who decline screening due to discomfort with pelvic examination is the use of self-collected vaginal swabs, which were shown to have moderate concordance with provider-collected HPV testing and a high acceptability by transmasculine individuals 21,22.
There was a prominent change in vaginal flora in transmasculine individuals with significant decrease in Lactobacilli associated with longer exposure time to testosterone. To our knowledge, only one study evaluated the vaginal microbiome of transmasculine persons using molecular techniques and showed very little relative abundance of Lactobacilli and high microbial diversity in the majority of transmasculine individuals 14. Transitions in vaginal flora and microbiota with predominance of non-Lactobacillus species in cisgender women have been associated with HPV infection23. Changes in vaginal flora are usually associated with bacterial vaginosis 24, which is frequently seen in cisgender women 25,26. However, in this cohort, none of the transmasculine patients showed clinical or cytologic evidence of bacterial vaginosis, suggesting that changes in vaginal flora secondary to testosterone might have different implications when compared to cisgender women of reproductive age.
One of the limitations of our study is its relatively small sample size, therefore definite conclusions cannot be reached. However, it still represents one of the largest cohorts of transmasculine individuals in which cytology slides were reviewed with available high-risk HPV testing and histologic follow-up. Other limitations include the retrospective nature of the study and the fact that our institution is a large tertiary care center in which the majority of patients do not receive their primary care, contributing to incomplete information on HPV vaccination and history of cervical cancer screening.
In conclusion, this study demonstrated a high percentage of abnormal Pap tests in our cohort of transmasculine persons receiving testosterone with changes in squamous cells that can represent diagnostic pitfalls. High-risk HPV testing can aid in the detection of precursor lesions due to its high sensitivity for HSIL detection. The vaginal flora of transmasculine individuals show a shift with a decrease in Lactobacilli following the use of testosterone, which might have implications for vaginal infections. A better understanding of the pathophysiologic impact of long-term testosterone therapy can improve healthcare and contribute to developing healthcare strategies for transmasculine persons.
Acknowledgement
We would like to thank Marie-Ange Exilhomme, Dana Warfield and Gary Vazquez for providing the institutional cytology data. We also would like to thank the NYULH Center for Biospecimen Research and Development, Histology and Immunohistochemistry Laboratory (RRID:SCR_018304) for the timely slide retrieval.
Funding Sources
NYULH Center for Biospecimen Research and Development, Histology and Immunohistochemistry Laboratory (RRID:SCR_018304) is supported in part by the Laura and Isaac Perlmutter Cancer Center Support Grant; NIH/NCI P30CA016087. The funding agencies had no direct role in the generation of the data or the manuscript.
Footnotes
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Statement of Ethics
This study protocol was reviewed and approved by New York University Institutional Review Board, approval number i20–00345. Due to the nature of this study, informed consent was not required per New York University Institutional Review Board.
This work’s preliminary results were previously presented as a poster at the American Society of Cytopathology’s 69th Annual Scientific meeting, Las Vegas, Nevada, November 12, 2021.
Data Availability Statement
The data are not publicly available due to privacy and ethical restriction.
References
- 1.James SE, Herman JL, Rankin S, Keisling M, Mottet L, Anafi M The Report of the 2015 U.S. Transgender Survey. Washington, DC: National Center for Transgender Equality; 2016. [Google Scholar]
- 2.Dhillon N, Oliffe JL, Kelly MT, Krist J. Bridging barriers to cervical cancer screening in transgender men: A scoping review. Am J Mens Health. 2020;14(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Connolly D, Hughes X, Berner A. Barriers and facilitators to cervical cancer screening among transgender men and non-binary people with a cervix: A systematic narrative review. Prev Med (Baltim). 2020;135:106071. [DOI] [PubMed] [Google Scholar]
- 4.Tabaac AR, Sutter ME, Wall CSJ, Baker KE. Gender identity disparities in cancer screening behaviors. Am J Prev Med. 2018;54(3):385–393. [DOI] [PubMed] [Google Scholar]
- 5.Peitzmeier SM, Khullar K, Reisner SL, Potter J. Pap test use is lower among female-to-male patients than non-transgender women. Am J Prev Med. 2014;47(6):808–812. [DOI] [PubMed] [Google Scholar]
- 6.Lin LH, Hernandez A, Marcus A, Deng F-M, Adler E. Histologic findings in gynecologic tissue from transmasculine individuals undergoing gender-affirming surgery. Arch Pathol Lab Med. 2021. Sep 30. doi: 10.5858/arpa.2021-0199-OA. September. [DOI] [PubMed] [Google Scholar]
- 7.Grimstad FW, Fowler KG, New EP, et al. Uterine pathology in transmasculine persons on testosterone: a retrospective multicenter case series. Am J Obstet Gynecol. 2019;220(3):257.e1–257.e7. [DOI] [PubMed] [Google Scholar]
- 8.Grimstad FW, Fowler KG, New EP, et al. Ovarian histopathology in transmasculine persons on testosterone: A multicenter case series. J Sex Med. 2020;17(9):1807–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khalifa MA, Toyama A, Klein ME, Santiago V. Histologic features of hysterectomy specimens from female-male transgender individuals. Int J Gynecol Pathol. 2019;38(6):520–527. [DOI] [PubMed] [Google Scholar]
- 10.Peitzmeier SM, Reisner SL, Harigopal P, Potter J. Female-to-male patients have high prevalence of unsatisfactory paps compared to non-transgender females: Implications for cervical cancer screening. J Gen Intern Med. 2014;29(5):778–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adkins BD, Barlow AB, Jack A, et al. Characteristic findings of cervical Papanicolaou tests from transgender patients on androgen therapy: Challenges in detecting dysplasia. Cytopathology. 2018;29(3):281–287. [DOI] [PubMed] [Google Scholar]
- 12.Williams MPA, Kukkar V, Stemmer MN, Khurana KK. Cytomorphologic findings of cervical Pap smears from female-to-male transgender patients on testosterone therapy. Cancer Cytopathol. 2020;128(7):491–498. [DOI] [PubMed] [Google Scholar]
- 13.Plummer RM, Kelting S, Madan R, O’Neil M, Dennis K, Fan F. Cervical Papanicolaou tests in the female-to-male transgender population: should the adequacy criteria be revised in this population? An Institutional Experience. J Am Soc Cytopathol. 2021;10(3):255–260. [DOI] [PubMed] [Google Scholar]
- 14.McPherson GW, Long T, Salipante SJ, et al. The vaginal microbiome of transgender men. Clin Chem. 2019;65(1):199–207. [DOI] [PubMed] [Google Scholar]
- 15.Cao CD, Amero MA, Marcinkowski KA, Rosenblum NG, Chan JSY, Richard SD. Clinical Characteristics and Histologic Features of Hysterectomy Specimens From Transmasculine Individuals. Obstet Gynecol. 2021;138(1):51–57. [DOI] [PubMed] [Google Scholar]
- 16.Rotola A, Costa S, Monini P, et al. Impact of sexual habits on the clinical evaluation of male HPV infection. Eur J Epidemiol. 1994;10(4):373–380. [DOI] [PubMed] [Google Scholar]
- 17.Azocar J, Abad SM, Acosta H, et al. Prevalence of cervical dysplasia and HPV infection according to sexual behavior. Int J cancer. 1990;45(4):622–625. [DOI] [PubMed] [Google Scholar]
- 18.Reisner SL, White JM, Mayer KH, Mimiaga MJ. Sexual risk behaviors and psychosocial health concerns of female-to-male transgender men screening for STDs at an urban community health center. AIDS Care. 2014;26(7):857–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen F, Hindi I, Sun W, et al. HPV co-testing of unsatisfactory papanicolaou tests: Implications for follow-up intervals. Mod Pathol. 2021;34:205. [DOI] [PubMed] [Google Scholar]
- 20.Perkins RB, Guido RS, Castle PE, et al. 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J Low Genit Tract Dis. 2020;24(2):102–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reisner SL, Deutsch MB, Peitzmeier SM, et al. Test performance and acceptability of self-versus provider-collected swabs for high-risk HPV DNA testing in female-to-male trans masculine patients. PLoS One. 2018;13(3):1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McDowell M, Pardee DJ, Peitzmeier S, et al. Cervical cancer screening preferences among trans-masculine individuals: Patient-collected human papillomavirus vaginal swabs versus provider-administered pap tests. LGBT Heal. 2017;4(4):252–259. [DOI] [PubMed] [Google Scholar]
- 23.Berggrund M, Gustavsson I, Aarnio R, et al. Temporal changes in the vaginal microbiota in self-samples and its association with persistent HPV16 infection and CIN2. Virol J. 2020;17(1):147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ling Z, Kong J, Liu F, et al. Molecular analysis of the diversity of vaginal microbiota associated with bacterial vaginosis. BMC Genomics. 2010;11:488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lamont RF, Morgan DJ, Wilden SD, Taylor-Robinson D. Prevalence of bacterial vaginosis in women attending one of three general practices for routine cervical cytology. Int J STD AIDS. 2000;11(8):495–498. [DOI] [PubMed] [Google Scholar]
- 26.Yen S, Shafer M-A, Moncada J, Campbell CJ, Flinn SD, Boyer CB. Bacterial vaginosis in sexually experienced and non-sexually experienced young women entering the military. Obstet Gynecol. 2003;102(5 Pt 1):927–933. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are not publicly available due to privacy and ethical restriction.


