Abstract
Society and our healthcare system are facing unprecedented challenges due to the expansion of the aged population. As plastic surgeons, we can improve care of our aged patients through understanding the mechanisms of aging that inevitably impact their outcomes and wellbeing. One of the major hallmarks of aging, cellular senescence, has recently become the focus of vigorous research in academia and industry. Senescent cells, which are metabolically active but in a state of stable cell cycle arrest, are implicated in causing aging and numerous age-related diseases. Further characterization of the biology of senescence revealed that it can be both detrimental and beneficial to organisms depending on tissue context and senescence chronicity. Here, we review the role of cellular senescence in aging, wound healing, tissue regeneration, and other domains relevant to plastic surgery. We also review the current state of research on therapeutics that modulate senescence to improve conditions of aging.
Keywords: senescence, aging, wound healing, regeneration, fibrosis, senolytics
Introduction
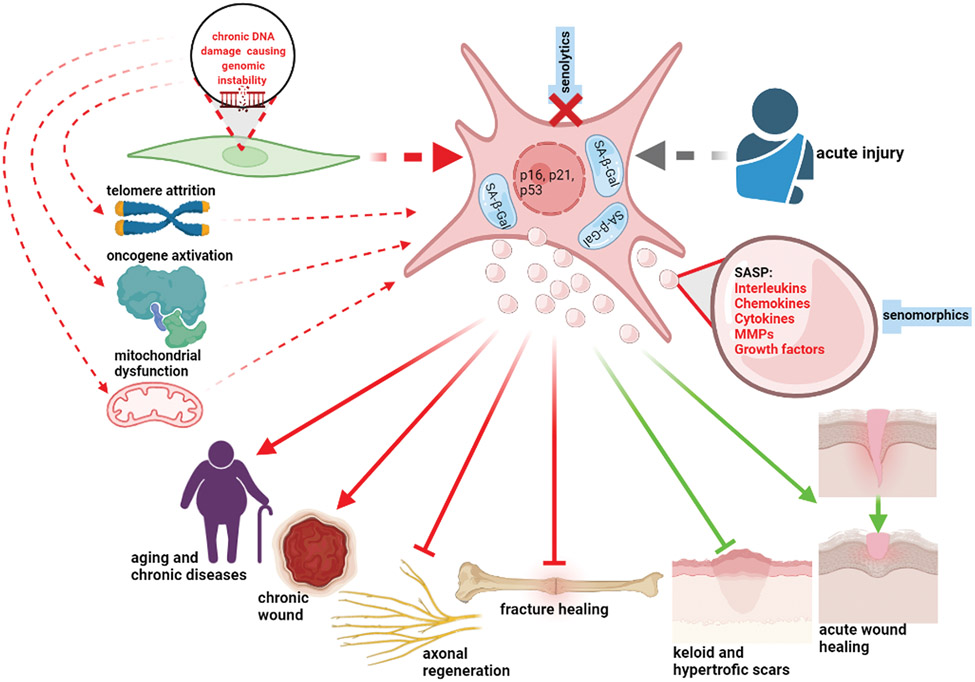
Globally, the number of aged individuals is expanding dramatically due to improved management of chronic diseases. In 2004, 461 million people were older than 65 years-of-age, and it is projected that this will reach 2 billion by 2050, leading to unprecedented challenges in planning and delivery of healthcare1. As aging is recognized as a major contributor to almost all chronic conditions2, the increasing socio-economic pressure associated with the growing aged population prompted the expansion of aging research to increase efforts aimed at extending human healthspan3. This brought attention to the hallmarks of aging: genomic instability, telomere shortening, epigenetic changes, cellular senescence (CS), stem cell exhaustion, loss of proteostasis, deregulated nutrient-sensing, mitochondrial dysfunction, and altered intercellular communication4. Here we focus on CS and its role in aging, tissue repair, and regeneration, relevant to plastic surgery and discuss potential therapeutic strategies targeting senescence (Figure-1).
Figure 1.
Senescent cells (enlarged, hypersecretory (SASP-producing), expressing cell cycle arrest markers, with expanded lysosomal compartment containing senescence-associated β-galactosidase) accumulate with aging largely as a result of genomic stress, telomere attrition, oncogene activation, and mitochondrial dysfunction, and other external and internal stress stimuli. Upregulation of senescence also arises after acute tissue injury. The SASP influences surrounding cells affecting their function and drives chronic sterile inflammation. Chronic senescence contributes to aging, many chronic diseases, delayed wound healing and chronic wounds. Senescent cells also negatively affect axonal and bone regeneration. Sells, expressing senescence markers, however, play a positive role in wound healing after acute injury, and prevent hypertrophic and keloid scar formation. Senolytics act by eliminating senescent cells; senomorphics modulate the SASP (created in BioRender.com)
Cellular senescence
The link between senescent cell (SnC) accumulation and numerous aging-related disorders is firmly established5,6. CS is characterized by stable cell cycle arrest triggered by exposure to a damage or stress stimuli, while remaining metabolically active and unresponsive to mitogenic and apoptotic signals7,8. CS can be triggered by oxidative, mechanical, or genotoxic stress, telomere shortening, mitochondrial dysfunction, and oncogene activation7. DNA damage caused by oxidative stress appears to be a pivotal factor driving the accumulation of SnCs with aging and impacting other causes of senescence and hallmarks of aging9,10. As DNA damage increases with age, mutations accumulate resulting in genomic instability11. Genomic instability causes a decrease in the efficiency of DNA repair machinery, which, in turn, contributes to more DNA damage, forming a vicious cycle of cellular decline occurring with aging11-14. Endogenous oxidative DNA damage can drive mitochondrial dysfunction, increased abundance of reactive oxygen species, and further damage to many cellular macromolecules and organelles15.
Although CS is a tumor-suppressive mechanism, preventing duplication of damaged cells, SnCs instigate deleterious effects on their tissue microenvironment16,17. SnCs influence surrounding cells via their senescence-associated secretory phenotype (SASP) characterized by secretion of proinflammatory interleukins, chemokines, cytokines, matrix metalloproteinases, and growth factors16-18. CS also plays a beneficial role in embryogenesis, development, and tissue regeneration. For example, transient senescence is detected during mammalian embryonic development at multiple sites, including the mesonephros, the endolymphatic sac of the inner ear19, the apical ectodermal ridge, and the neural roof plate20. Embryonic senescence is dependent on p21CIP1, but independent of DNA damage19. Senescence during embryogenesis is followed by clearance of SnCs by macrophages, and tissue remodeling19, and represents a physiological programmed mechanism that plays instructive roles in development and limb patterning20. Fin amputation in zebrafish leads to the transient accumulation of SnCs at the site of wounding, and their removal impairs tissue regeneration21. In these contexts, CS is a tightly regulated and a transient process5,22 distinct from persistent CS in aging.
The heterogeneity of senescent phenotypes
CS encompasses diverse phenotypes varying based on the stressor inducing CS, the cell type, and physiological contexts7,23. Biomarkers of senescence are neither absolutely sensitive nor specific, and a combination of biomarkers is required to identify SnCs 7,24. The most commonly used CS markers include upregulation of cell cycle inhibitors (in particular, p16INK4a, p21CIP1, and p53) and SASP, morphological changes such as cell enlargement and flattened morphology, expanded lysosomal compartment with increased senescence-associated β-galactosidase (SA-β-Gal) activity, and characteristic chromatin and epigenetic alterations7,8.
Multiple pathological conditions are characterized by increased abundance of SnCs. Examples include idiopathic pulmonary fibrosis25, osteoarthritis26, posttransplant state, inflammatory bowel disease, hepatocellular carcinoma, obesity27, and viral infections28. Whether accumulation of SnCs is related to the etiology in each of these conditions or is a compensatory mechanism is yet to be elucidated. Whether elimination of SnCs in humans with these conditions could bring substantial long-term benefits also remains to be defined.
The heterogeneity of SnCs prompted the idea of classifying SnCs into “Deleterious” and “Helper” types23. “Helper”-SnCs promote stem cell function, wound healing, and tissue regeneration, while deleterious cells promote chronic sterile inflammation and impaired wound healing and regeneration in aging23.
Interestingly, cells can express senescence markers temporarily. A recent study demonstrated that expression of p16Ink4a and SA-β-Gal in macrophages is induced as part of the physiological response to immune stimuli, consistent with data that p16Ink4a plays a role in macrophage polarization and response to stimuli29. These results prompt the need to discriminate helper and deleterious SnCs in health and disease, and to elucidate whether cells are truly senescent or express senescent markers transiently as a part of a physiological response.
The relevance of cellular senescence to plastic surgery
SnCs impact aging and tissue repair, areas relevant to plastic surgery. Although SnCs accumulate with aging, there is increasing evidence that senescence signaling also upregulates during tissue repair and regeneration5,22. Indeed, senescence signaling plays an important role in cutaneous wound healing, musculoskeletal regeneration, as well as regeneration of liver, heart, and lung, and the response of the kidney and spinal cord to injury30,31. These critical physiological roles of CS were established by selective genetic or pharmacological elimination of SnCs or disruption of immune clearance of SnCs leading to impaired tissue regeneration21,30.
Facial and skin aging
Skin from older individuals has significant cellular, biomechanical, biochemical, and physiological differences compared to skin from young individuals32. The number of p16INK4a positive cells in sun-protected human skin significantly correlates with age-associated loss of elastic fibers, increase in facial wrinkles, and higher perceived age33. Independent of chronological age, individuals in the lowest tertile of epidermal p16INK4a cell counts appear three years younger than those in the highest tertile33. In particular, senescent fibroblasts are implicated in skin aging by expressing SASP containing ECM-degrading matrix metalloproteinases (MMP-2 and MMP-9) and proinflammatory interleukins (IL-6 and IL-8)34-37. Additionally, senescent fibroblasts produce decreased insulin-like growth factor-1 what decreases epidermal cell proliferation and collagen expression34,38, all features of skin aging.
Acute wounds and delayed wound healing
Transient upregulation of CS plays an important role in wound healing39,40. Cell cycle arrest proteins (in particular p16, p21, and p53) and SASP are transiently upregulated after cutaneous wounding in both mice and humans41,42. This upregulation appears to be essential for the healing process, as selective elimination of SnCs impairs wound healing in mice41. Wound-associated senescent fibroblast and endothelial cells express platelet derived growth factor-AA (PDGF-AA) as part of the transient SASP, and in mice, topical administration of PDGF-AA alleviates delayed wound healing in the absence of SnCs41. Activated macrophages, which are critical for infection control and elimination of dead cells, can transiently express CS markers p16INK4a and SA-β-Gal29,43.
Delayed wound healing in aging, although multifactorial44, is also associated with an altered senescence response. Subcutaneous irradiation-induced senescent fibroblast transfer to young mice causes delayed wound healing similar to that registered in aged mice45. In a recent human study a punch biopsy was used to induce a cutaneous wound, and p21 and p53 were upregulated during wound healing in younger, but not older subjects42. Interestingly, local and transient inhibition of p21 expression by in vivo-delivery of a p21-targeting siRNA ameliorated the delayed wound healing in aged mice46. In summary, transient senescence is beneficial for wound healing and without it, delayed healing can occur. However, the persistence of SnCs can contribute to chronic wounds47,48.
Chronic wounds
Chronic wounds are usually stalled at the inflammatory stage49. Senescent fibroblasts are found in venous ulcers, diabetic foot ulcers, and pressure ulcers, and their incidence decreases after healing49,50. Exposure to chronic wound fluid causes dermal fibroblasts to switch their secretion profile from extracellular matrix deposition to a matrix-degrading phenotype50, analogous to SnC SASP. A fraction of SnCs greater than 15% in cells isolated from venous ulcers negatively correlates with healing rate51. There is also a correlation between decreased collagen area and the presence of SnCs in human venous leg ulcers, diabetic foot ulcers, and pressure ulcers52, and collagen imaging has been proposed as a non-invasive method to predict CS and wound healing trajectory52.
SnCs are implicated in impaired wound healing in diabetics, which has multiple pathological components analogous to delayed wound healing in aging53. Hyperglycemia, oxidative stress, and mitochondrial and nuclear DNA damage may act as major drivers of SnC accumulation in diabetic ulcers, and it negatively impacts wound healing54. One of the mechanisms through which chronic CS contributes to impaired diabetic wound healing is via the CXCR2 receptor, which when blocked promotes repair 48,55.
Pathological scarring and fibrosis
Senescent fibroblasts appear at the site of wound repair presumably as the result of oxidative stress56. CS controls fibrotic response during wound healing inhibiting fibroblast proliferation and extracellular matrix synthesis56. This process is modulated by the matricellular protein CCN1, which triggers the DNA damage response and fibroblast senescence with concomitant expression of antifibrotic genes at the site of wound repair in wild type mice. Mice with mutant CCN1, that cannot bind integrin α6β1 and cell surface heparan sulfate proteoglycans, are unable to induce CS in granulation tissue and display exacerbated wound fibrosis57. Therefore, CS inhibits fibroblast proliferation and extracellular matrix synthesis dampening the fibrotic response in wound healing56.
Based on the evidence that CS could limit fibrosis, it was hypothesized that insufficient number of SnCs could result in uncontrolled proliferation in keloid formation56. Therefore, “pro-senescence” therapeutic approaches were proposed as a mean to control keloid onset and recurrence58. For example, ionizing irradiation may prevent the recurrence of keloids by decreasing fibroblast proliferation and inducing CS59.
Conversely, there is evidence that CS promotes fibrosis and scar formation. Interestingly, both hypertrophic and keloid scars demonstrate a unique CD34−/α-SMA+/p16+ scar phenotype, with p16 positivity being greater in keloid60.
In addition, fibrotic capsules surrounding surgically excised human breast implants contain increased numbers of interleukin 17 (IL-17)–producing T cells and senescent stromal cells. In mice, the IL-6 produced by SnCs in response to implanted synthetic material is sufficient to induce IL-17 expression in T cells. When SnCs were eliminated, IL-17 expression and fibrosis in the murine implant model were reduced61, suggesting that SnCs contribute to capsular contracture formation. Furthermore, species capable of scar-free healing are more resistant to oxidative stress and downregulate cellular senescence62,63.
Interestingly, fetal and newborn wounds are characterized by rapid and scarless healing22. As transient senescence during embryonic development is distinct from the chronic senescence associated with aging, and contributes to the tissue remodeling19,20, deeper investigation of the developmentally programmed senescence may give cues to novel approaches to improving wound healing and cosmetic outcomes in adult population.
Nerve repair and regeneration
Schwann cell senescence is involved in decreasing axonal regeneration in acellular nerve allografts in rats 64. Compared to short acellular nerve allografts that heal faster, longer allografts are repopulated with greater percentage of p16+ Schwann cells and stromal cells, suggesting a role of SnCs in poor axonal regeneration of long acellular nerve allografts65. Similar data were obtained in a rat sciatic nerve defect model where long isografts had significantly higher expression of SA-β-Gal, p21, and p16, and distinct chromatin changes in Schwann cells compared to short isografts66.
SnC and SASP were also discovered in the carpal tunnel synovium and subsynovial connective tissue67.
Fracture healing
In silico analysis of public mRNAseq data reveal that senescence and SASP markers are increased during fracture healing68. Consistent with that, in a murine fracture model, a significant increase in the expression of senescence markers occurs in the fracture callus during bone healing68. Using mice containing a Cdkn2aInk4a-driven luciferase reporter, a transient in vivo accumulation of SnCs is detected during callus formation68. Treatment of young adult mice after bone fracture intermittently with the senolytics Dasatinib and quercetin, decreased senescence and SASP markers in the fracture callus and accelerated fracture healing68. Furthermore, senescent osteocytes are implicated in age-associated bone loss, and senolytic treatment can improve bone mass, strength, and microarchitecture69,70 to protect from fractures and potentially improve healing.
Therapeutic strategies targeting senescence and aging
Given the growing evidence that CS impacts aging and disease, multiple efforts have been focused on manipulation of SnCs to treat age-related pathology. Senotherapeutic approaches aimed at targeting SnCs include substances that selectively kill SnCs (senolytics) and suppress the SASP (senomorphics)71-73 (Table-1). Some of these drugs, however, have severe side effects limiting their translational potential. For example, senolytic Navitoclax causes life-threatening thrombocytopenia74.
Table 1.
The list of most studied senotherapeutic molecules.
| Substance | Mechanism of action | References |
|---|---|---|
| Senolytics – eliminate senescent cells | ||
| Dasatinib | Tyrosine kinase inhibitor | 84 |
| Quercetin | Targets BCL-2 family Regulates NF-kß and P13K/AKT/mTOR Induces HIF-1a |
85 |
| Navitoclax (ABT-263) and ABT-737 | Targets BCL-2, BCL-x(L), BCL-w | 86 |
| Fisetin | Inhibits PI3K/Akt/mTOR, MAPK, NF-κB, and ERK-MYC Targets BCL-2 |
87-90 |
| FOXO4-DRI peptide | Disrupts the p53-FOXO4 interaction | 91,92 |
| Senomorphics - inhibit SASP | ||
| Metformin | Targets NF-κB and Dicer | 93,94 |
| Rapamycin | mTOR inhibitor | 95 |
| Ruxolitinib | Targets JAK (Janus kinase) pathway | 96 |
Removal of SnCs using senolytics dasatinib plus quercetin, which cause apoptosis of SnCs, increases lifespan and alleviates diabetes, osteoporosis, neurodegeneration, pulmonary fibrosis, and cardiovascular disease in mice23,75,76. Because of these promising findings, there are already multiple clinical trials targeting senescence in humans77. Furthermore, a recent explosion of biotechnology companies entering the field of senotherapeutics has occurred (reviewed in 78). Chimeric antigen receptor (CAR) T-cells, that target SnCs, is another approach being used to target SnCs in vivo79.
While senolytic decrease SnCs in humans77, longitudinal studies of longer duration are needed to understand the clinical significance of these findings and long-term efficiency, safety, and side-effects. At this time, it remains unclear whether senescence modification can decrease frailty and delay aging, or if this approach should be restricted to particular pathologies or topical application only. The results demonstrating transient expression of senescence markers by macrophages suggested that improved health outcomes in animal models following eradication of p16Ink4a-positive cells could involve elimination of p16Ink4a/SA-β-Gal-positive macrophages. The importance of exercising careful approach when attempting to eradicate SnCs is emphasized, while acknowledging the importance of senolytic exploration29. Continuous or acute elimination of p16High SnCs in transgenic mice, leads to disruption of blood-tissue barriers resulting in liver and perivascular tissue fibrosis80. It has also been postulated that elimination of SnCs may accelerate aging-related decline by driving remaining cells into senescence81. Currently, senotherapeutics are not selective for pathologic SnCs and are anticipated to impact beneficial SnCs as well. To summarize, while senotherapeutics deserve thorough investigation, many questions remain: Will systemic elimination of SnCs bring a long-lasting improvement without side effects? Whether it is beneficial to use senolytics in very old individuals (as many cells are potentially damaged and/or senescent)? When is it optimal to initiate senolytic therapy?
In addition to systemic administration, topical approaches are being developed to target localized senescence-associated conditions. Topical approaches might alleviate conditions such as chronic wounds or fibrotic processes and are presumed to be safer than systemic administration. A pilot study in humans yielded improved wound healing in diabetic ulcers treated with nano-hydrogel embedded with the senolytic quercetin and oleic acid82. Topical senolytics are also being tested in pre-clinical models for treatment of carpal tunnel syndrome67. A proprietary topically-delivered senotherapeutic peptide reduces skin biological age and improves skin health markers in ex vivo human skin models83.
Conclusion
CS elicits diverse and potent effects on aging, numerous age-related diseases, as well as tissue repair and regeneration. Thus, targeting SnCs can have a potentially profound impact on the restorative aspects of plastic surgery. The therapeutic potential of CS modification is evident in preclinical studies and is currently being pursued in clinical trials, however, with exercised caution. The development of targeted and localized delivery technologies to modulate CS may be a promising area to influence outcomes in plastic surgery. The dichotomous ability of SnCs to both facilitate and hinder tissue repair processes, means continuing research on SnC biology is essential. Efforts to both harness the benefits of transient senescence and reduce the detrimental effects of chronic senescence may have a transformative impact on wound healing and tissue repair in general. Notably, many senotherapeutics identified to date are natural products that are available over the counter (OTC). Until further clinical studies are completed, it may be prudent for surgeons to be vigilant regarding patient OTC supplementation that could influence tissue regeneration and wound healing.
The heterogeneity of SnCs is now being revealed using single-cell and spacialomics approaches7 with the hopes of creating an atlas that fully characterizes SnC subtypes in health and disease and discover biomarkers that can distinguish between beneficial versus detrimental SnCs. This is the focus of the NIH Common Fund Initiative: Cellular Senescence Network that was initiated in 2021 to create Tissue Mapping Centers, develop novel analytics and technologies for SnC identification, and create a Consortium Organization and Data Coordinate Center (https://sennetconsortium.org/).
Funding information:
This work was supported by the grant from the NIA [R03AG067983]; Laszlo N. Tauber Professorship in Surgery; Boston Claude D. Pepper Older Americans Independence Center (NIA) Research Education Core Award 5P30AG031679-10: Subaward 115900 to Daniel S. Roh. R01 AG063543, U19 AG056278, P01 AG062413 grants to Laura Niedernhofer.
Footnotes
Disclosure: The authors declare no conflicts of interest. None of the authors has a financial interest in any of the products, devices, or drugs mentioned in this manuscript.
Reference list
- 1.Brivio P, Paladini MS, Racagni G, et al. From Healthy Aging to Frailty: In Search of the Underlying Mechanisms. Curr Med Chem 2019; 26: 3685–3701. [DOI] [PubMed] [Google Scholar]
- 2.Marengoni A, Angleman S, Melis R, et al. Aging with multimorbidity: A systematic review of the literature. Ageing Res Rev 2011; 10: 430–439. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy BK, Berger SL, Brunet A, et al. Aging: a common driver of chronic diseases and a target for novel interventions. Cell 2014; 159: 709.25417146 [Google Scholar]
- 4.López-Otín C, Blasco MA, Partridge L, et al. The hallmarks of aging. Cell 2013; 153: 1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yun MH. Cellular senescence in tissue repair: every cloud has a silver lining. Int J Dev Biol 2018; 62: 591–604. [DOI] [PubMed] [Google Scholar]
- 6.Childs BG, Gluscevic M, Baker DJ, et al. Senescent cells: an emerging target for diseases of ageing. Nat Rev Drug Discov 2017; 16: 718–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.González-Gualda E, Baker AG, Fruk L, et al. A guide to assessing cellular senescence in vitro and in vivo. FEBS J 2021; 288: 56–80. [DOI] [PubMed] [Google Scholar]
- 8.Gorgoulis V, Adams PD, Alimonti A, et al. Cellular Senescence: Defining a Path Forward. Cell 2019; 179: 813–827. [DOI] [PubMed] [Google Scholar]
- 9.Yu Y, Cui Y, Niedernhofer LJ, et al. Occurrence, Biological Consequences, and Human Health Relevance of Oxidative Stress-Induced DNA Damage. Chem Res Toxicol 2016; 29: 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kruk PA, Rampino NJ, Bohr VA. DNA damage and repair in telomeres: relation to aging. Proc Natl Acad Sci U S A 1995; 92: 258–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niedernhofer LJ, Gurkar AU, Wang Y, et al. Nuclear Genomic Instability and Aging. https://doi.org/101146/annurev-biochem-062917-012239 2018; 87: 295–322. [DOI] [PubMed] [Google Scholar]
- 12.Goyns MH. Genes, telomeres and mammalian ageing. Mech Ageing Dev 2002; 123: 791–799. [DOI] [PubMed] [Google Scholar]
- 13.Yousefzadeh M, Henpita C, Vyas R, et al. DNA damage—how and why we age? Elife 2021; 10: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitchell JR, Hoeijmakers JHJ, Niedernhofer LJ. Divide and conquer: nucleotide excision repair battles cancer and ageing. Curr Opin Cell Biol 2003; 15: 232–240. [DOI] [PubMed] [Google Scholar]
- 15.Robinson AR, Yousefzadeh MJ, Rozgaja TA, et al. Spontaneous DNA damage to the nuclear genome promotes senescence, redox imbalance and aging. Redox Biol 2018; 17: 259–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coppé J-P, Desprez P-Y, Krtolica A, et al. The Senescence-Associated Secretory Phenotype: The Dark Side of Tumor Suppression. Annu Rev Pathol 2010; 5: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirkland JL, Tchkonia T, Zhu Y, et al. The Clinical Potential of Senolytic Drugs. J Am Geriatr Soc 2017; 65: 2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chambers CR, Ritchie S, Pereira BA, et al. Overcoming the senescence-associated secretory phenotype (SASP): a complex mechanism of resistance in the treatment of cancer. Mol Oncol 2021; 15: 3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muñoz-Espín D, Cañamero M, Maraver A, et al. Programmed cell senescence during mammalian embryonic development. Cell 2013; 155: 1104. [DOI] [PubMed] [Google Scholar]
- 20.Storer M, Mas A, Robert-Moreno A, et al. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell 2013; 155: 1119. [DOI] [PubMed] [Google Scholar]
- 21.Da Silva-Álvarez S, Guerra-Varela J, Sobrido-Cameán D, et al. Cell senescence contributes to tissue regeneration in zebrafish. Aging Cell 2020; 19: e13052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pratsinis H, Mavrogonatou E, Kletsas D. Scarless wound healing: From development to senescence. Adv Drug Deliv Rev 2019; 146: 325–343. [DOI] [PubMed] [Google Scholar]
- 23.Tripathi U, Misra A, Tchkonia T, et al. Impact of Senescent Cell Subtypes on Tissue Dysfunction and Repair: Importance and Research Questions. Mech Ageing Dev; 198. Epub ahead of print 1 September 2021. DOI: 10.1016/J.MAD.2021.111548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kohli J, Wang B, Brandenburg SM, et al. Algorithmic assessment of cellular senescence in experimental and clinical specimens. Nat Protoc 2021; 16: 2471–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meiners S, Lehmann M. Senescent Cells in IPF: Locked in Repair? Front Med; 7. Epub ahead of print 18 December 2020. DOI: 10.3389/FMED.2020.606330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeon OH, David N, Campisi J, et al. Senescent cells and osteoarthritis: a painful connection. J Clin Invest 2018; 128: 1229–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paez-Ribes M, González-Gualda E, Doherty GJ, et al. Targeting senescent cells in translational medicine. EMBO Mol Med; 11. Epub ahead of print December 2019. DOI: 10.15252/EMMM.201810234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barathan M, Mohamed R, Saeidi A, et al. Increased frequency of late-senescent T cells lacking CD127 in chronic hepatitis C disease. Eur J Clin Invest 2015; 45: 466–474. [DOI] [PubMed] [Google Scholar]
- 29.Hall BM, Balan V, Gleiberman AS, et al. p16(Ink4a) and senescence-associated β-galactosidase can be induced in macrophages as part of a reversible response to physiological stimuli. Aging (Albany NY) 2017; 9: 1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Antelo-Iglesias L, Picallos-Rabina P, Estévez-Souto V, et al. The role of cellular senescence in tissue repair and regeneration. Mech Ageing Dev; 198. Epub ahead of print 1 September 2021. DOI: 10.1016/J.MAD.2021.111528. [DOI] [PubMed] [Google Scholar]
- 31.Majewska J, Krizhanovsky V. Breathe it in - Spotlight on senescence and regeneration in the lung. Mech Ageing Dev; 199. Epub ahead of print 1 October 2021. DOI: 10.1016/J.MAD.2021.111550. [DOI] [PubMed] [Google Scholar]
- 32.Sgonc R, Gruber J. Age-related aspects of cutaneous wound healing: a mini-review. Gerontology 2013; 59: 159–164. [DOI] [PubMed] [Google Scholar]
- 33.Waaijer MEC, Gunn DA, Adams PD, et al. P16INK4a Positive Cells in Human Skin Are Indicative of Local Elastic Fiber Morphology, Facial Wrinkling, and Perceived Age. J Gerontol A Biol Sci Med Sci 2016; 71: 1022–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wlaschek M, Maity P, Makrantonaki E, et al. Connective Tissue and Fibroblast Senescence in Skin Aging. J Invest Dermatol 2021; 141: 985–992. [DOI] [PubMed] [Google Scholar]
- 35.Wang AS, Dreesen O. Biomarkers of Cellular Senescence and Skin Aging. Front Genet; 9. Epub ahead of print 23 August 2018. DOI: 10.3389/FGENE.2018.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuilman T, Michaloglou C, Vredeveld LCW, et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 2008; 133: 1019–1031. [DOI] [PubMed] [Google Scholar]
- 37.Coppé JP, Rodier F, Patil CK, et al. Tumor suppressor and aging biomarker p16(INK4a) induces cellular senescence without the associated inflammatory secretory phenotype. J Biol Chem 2011; 286: 36396–36403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh K, Maity P, Krug L, et al. Superoxide anion radicals induce IGF-1 resistance through concomitant activation of PTP1B and PTEN. EMBO Mol Med 2015; 7: 59–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thanapaul RJRS, Shvedova M, Shin GH, et al. An Insight into Aging, Senescence, and Their Impacts on Wound Healing. Adv Geriatr Med Res; 3. Epub ahead of print 2021. DOI: 10.20900/AGMR20210017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Resnik SR, Egger A, Abdo Abujamra B, et al. Clinical Implications of Cellular Senescence on Wound Healing. Curr Dermatology Reports 2020 94 2020; 9: 286–297. [Google Scholar]
- 41.Demaria M, Ohtani N, Youssef SA, et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell 2014; 31: 722–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chia CW, Sherman-Baust CA, Larson SA, et al. Age-associated expression of p21and p53 during human wound healing. Aging Cell; 20. Epub ahead of print 1 May 2021. DOI: 10.1111/acel.13354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hall BM, Balan V, Gleiberman AS, et al. Aging of mice is associated with p16(Ink4a)- and β-galactosidase-positive macrophage accumulation that can be induced in young mice by senescent cells. Aging (Albany NY) 2016; 8: 1294–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ding X, Kakanj P, Leptin M, et al. Regulation of the Wound Healing Response during Aging. J Invest Dermatol 2021; 141: 1063–1070. [DOI] [PubMed] [Google Scholar]
- 45.Samdavid Thanapaul RJR, Shvedova M, Shin GH, et al. Elevated skin senescence in young mice causes delayed wound healing. GeroScience. Epub ahead of print 11 April 2022. DOI: 10.1007/S11357-022-00551-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang D, de Vries JC, Muschhammer J, et al. Local and transient inhibition of p21 expression ameliorates age-related delayed wound healing. Wound Repair Regen 2020; 28: 49–60. [DOI] [PubMed] [Google Scholar]
- 47.Vande Berg JS, Rose MA, Haywood-Reid PL, et al. Cultured pressure ulcer fibroblasts show replicative senescence with elevated production of plasmin, plasminogen activator inhibitor-1, and transforming growth factor-beta1. Wound Repair Regen 2005; 13: 76–83. [DOI] [PubMed] [Google Scholar]
- 48.Wilkinson HN, Clowes C, Banyard KL, et al. Elevated Local Senescence in Diabetic Wound Healing Is Linked to Pathological Repair via CXCR2. J Invest Dermatol 2019; 139: 1171–1181.e6. [DOI] [PubMed] [Google Scholar]
- 49.Pulido T, Velarde MC, Alimirah F. The senescence-associated secretory phenotype: Fueling a wound that never heals. Mech Ageing Dev; 199. Epub ahead of print 1 October 2021. DOI: 10.1016/J.MAD.2021.111561. [DOI] [PubMed] [Google Scholar]
- 50.Ho CY, Dreesen O. Faces of cellular senescence in skin aging. Mech Ageing Dev; 198. Epub ahead of print 1 September 2021. DOI: 10.1016/J.MAD.2021.111525. [DOI] [PubMed] [Google Scholar]
- 51.Stanley A, Osler T. Senescence and the healing rates of venous ulcers. J Vasc Surg 2001; 33: 1206–1211. [DOI] [PubMed] [Google Scholar]
- 52.Lim DXE, Richards T, Kanapathy M, et al. Extracellular matrix and cellular senescence in venous leg ulcers. Sci Rep; 11. Epub ahead of print 1 December 2021. DOI: 10.1038/S41598-021-99643-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilkinson HN, Hardman MJ. Senescence in Wound Repair: Emerging Strategies to Target Chronic Healing Wounds. Front cell Dev Biol; 8. Epub ahead of print 11 August 2020. DOI: 10.3389/FCELL.2020.00773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berlanga-Acosta JA, Guillén-Nieto GE, Rodríguez-Rodríguez N, et al. Cellular Senescence as the Pathogenic Hub of Diabetes-Related Wound Chronicity. Front Endocrinol (Lausanne) 2020; 11: 573032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tomic-Canic M, DiPietro LA. Cellular Senescence in Diabetic Wounds: When Too Many Retirees Stress the System. J Invest Dermatol 2019; 139: 997–999. [DOI] [PubMed] [Google Scholar]
- 56.Blažic TM, Blažic B, Brajac I. Defective induction of senescence during wound healing is a possible mechanism of keloid formation. DOI: 10.1016/j.mehy.2005.09.033. [DOI] [PubMed] [Google Scholar]
- 57.J JI, L LF. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat Cell Biol 2010; 12: 676–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Varmeh S, Egia A, McGrouther D, et al. Cellular senescence as a possible mechanism for halting progression of keloid lesions. Genes Cancer 2011; 2: 1061–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ji J, Tian Y, Zhu YQ, et al. Ionizing irradiation inhibits keloid fibroblast cell proliferation and induces premature cellular senescence. J Dermatol 2015; 42: 56–63. [DOI] [PubMed] [Google Scholar]
- 60.Limandjaja GC, Belien JM, Scheper RJ, et al. Hypertrophic and keloid scars fail to progress from the CD34−/α-smooth muscle actin (α-SMA)+ immature scar phenotype and show gradient differences in α-SMA and p16 expression. Br J Dermatol 2020; 182: 974–986. [DOI] [PubMed] [Google Scholar]
- 61.Chung L, Maestas DR, Lebid A, et al. Interleukin 17 and senescent cells regulate the foreign body response to synthetic material implants in mice and humans. Sci Transl Med 2020; 12: 3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saxena S, Vekaria H, Sullivan PG, et al. Connective tissue fibroblasts from highly regenerative mammals are refractory to ROS-induced cellular senescence. Nat Commun; 10. Epub ahead of print 1 December 2019. DOI: 10.1038/S41467-019-12398-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Durant F, Whited JL. Finding Solutions for Fibrosis: Understanding the Innate Mechanisms Used by Super-Regenerator Vertebrates to Combat Scarring. Adv Sci (Weinheim, Baden-Wurttemberg, Ger; 8. Epub ahead of print 1 August 2021. DOI: 10.1002/ADVS.202100407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saheb-Al-Zamani M, Yan Y, Farber SJ, et al. Limited regeneration in long acellular nerve allografts is associated with increased Schwann cell senescence. Exp Neurol 2013; 247: 165–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Poppler LH, Ee X, Schellhardt L, et al. Axonal Growth Arrests After an Increased Accumulation of Schwann Cells Expressing Senescence Markers and Stromal Cells in Acellular Nerve Allografts. Tissue Eng Part A 2016; 22: 949–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hoben GM, Ee X, Schellhardt L, et al. Increasing Nerve Autograft Length Increases Senescence and Reduces Regeneration. Plast Reconstr Surg 2018; 142: 952–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.CARPAL TUNNEL SYNDROME: A DISEASE OF CELLULAR SENESCENCE? ∣ Orthopaedic Proceedings, https://online.boneandjoint.org.uk/doi/abs/10.1302/1358-992X.2021.13.032?ai=z4&mi=3fqos0&af=R (accessed 5 January 2022). [Google Scholar]
- 68.Saul D, Monroe DG, Rowsey JL, et al. Modulation of fracture healing by the transient accumulation of senescent cells. Elife; 10. Epub ahead of print 1 October 2021. DOI: 10.7554/ELIFE.69958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Farr JN, Fraser DG, Wang H, et al. Identification of Senescent Cells in the Bone Microenvironment. J Bone Miner Res 2016; 31: 1920–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Farr JN, Xu M, Weivoda MM, et al. Targeting cellular senescence prevents age-related bone loss in mice. Nat Med 2017; 23: 1072–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boccardi V, Mecocci P. Senotherapeutics: Targeting senescent cells for the main age-related diseases. Mech Ageing Dev; 197. Epub ahead of print 1 July 2021. DOI: 10.1016/J.MAD.2021.111526. [DOI] [PubMed] [Google Scholar]
- 72.Niedernhofer LJ, Robbins PD. Senotherapeutics for healthy ageing. Nat Rev Drug Discov 2018 175 2018; 17: 377–377. [DOI] [PubMed] [Google Scholar]
- 73.Kirkland JL, Tchkonia T. Senolytic drugs: from discovery to translation. J Intern Med 2020; 288: 518–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Afreen S, Bohler S, Müller A, et al. BCL-XL expression is essential for human erythropoiesis and engraftment of hematopoietic stem cells. Cell Death Dis; 11. Epub ahead of print 1 January 2020. DOI: 10.1038/S41419-019-2203-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu M, Pirtskhalava T, Farr JN, et al. Senolytics improve physical function and increase lifespan in old age. Nat Med 2018 248 2018; 24: 1246–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhu Y, Tchkonia T, Pirtskhalava T, et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 2015; 14: 644–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Robbins PD, Jurk D, Khosla S, et al. Senolytic Drugs: Reducing Senescent Cell Viability to Extend Health Span. Annu Rev Pharmacol Toxicol 2021; 61: 779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dolgin E Send in the senolytics. Nat Biotechnol 2020; 38: 1371–1377. [DOI] [PubMed] [Google Scholar]
- 79.Amor C, Feucht J, Leibold J, et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature 2020; 583: 127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Grosse L, Wagner N, Emelyanov A, et al. Defined p16 High Senescent Cell Types Are Indispensable for Mouse Healthspan. Cell Metab 2020; 32: 87–99.e6. [DOI] [PubMed] [Google Scholar]
- 81.Michael F. Cell Senescence, Telomerase, and Senolytic Therapy. OBM Geriatr 2019, Vol 3, Page 1 2019; 3: 1–1. [Google Scholar]
- 82.Gallelli G, Cione E, Serra R, et al. Nano-hydrogel embedded with quercetin and oleic acid as a new formulation in the treatment of diabetic foot ulcer: A pilot study. Int Wound J 2020; 17: 485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zonari A, Brace LE, Al-Katib KZ, et al. Senotherapeutic peptide reduces skin biological age and improves skin health markers. bioRxiv 2020; 2020.10.30.362822. [Google Scholar]
- 84.Miura M. Therapeutic drug monitoring of imatinib, nilotinib, and dasatinib for patients with chronic myeloid leukemia. Biol Pharm Bull 2015; 38: 645–654. [DOI] [PubMed] [Google Scholar]
- 85.Protocol K, Kaufmann S, Cerny-Garcia J. The Kaufmann Anti-Aging Institute The Kaufmann Anti-Aging Institute Senescent Cells. [Google Scholar]
- 86.Mérino D, Khaw SL, Glaser SP, et al. Bcl-2, Bcl-x(L), and Bcl-w are not equivalent targets of ABT-737 and navitoclax (ABT-263) in lymphoid and leukemic cells. Blood 2012; 119: 5807–5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chamcheu JC, Esnault S, Adhami VM, et al. Fisetin, a 3,7,3’,4’-Tetrahydroxyflavone Inhibits the PI3K/Akt/mTOR and MAPK Pathways and Ameliorates Psoriasis Pathology in 2D and 3D Organotypic Human Inflammatory Skin Models. Cells; 8. Epub ahead of print 15 September 2019. DOI: 10.3390/CELLS8091089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ren Q, Guo F, Tao S, et al. Flavonoid fisetin alleviates kidney inflammation and apoptosis via inhibiting Src-mediated NF-κB p65 and MAPK signaling pathways in septic AKI mice. Biomed Pharmacother; 122. Epub ahead of print 1 February 2020. DOI: 10.1016/J.BIOPHA.2019.109772. [DOI] [PubMed] [Google Scholar]
- 89.Kim N, Kang MJ, Lee SH, et al. Fisetin Enhances the Cytotoxicity of Gemcitabine by Down-regulating ERK-MYC in MiaPaca-2 Human Pancreatic Cancer Cells. Anticancer Res 2018; 38: 3527–3533. [DOI] [PubMed] [Google Scholar]
- 90.Ghani MFA, Othman R, Nordin N. Molecular Docking Study of Naturally Derived Flavonoids with Antiapoptotic BCL-2 and BCL-XL Proteins toward Ovarian Cancer Treatment. J Pharm Bioallied Sci 2020; 12: S676–S680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huang Y, He Y, Makarcyzk MJ, et al. Senolytic Peptide FOXO4-DRI Selectively Removes Senescent Cells From in vitro Expanded Human Chondrocytes. Front Bioeng Biotechnol; 9. Epub ahead of print 29 April 2021. DOI: 10.3389/FBIOE.2021.677576/FULL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang C, Xie Y, Chen H, et al. FOXO4-DRI alleviates age-related testosterone secretion insufficiency by targeting senescent Leydig cells in aged mice. Aging (Albany NY) 2020; 12: 1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Noren Hooten N, Martin-Montalvo A, Dluzen DF, et al. Metformin-mediated increase in DICER1 regulates microRNA expression and cellular senescence. Aging Cell 2016; 15: 572–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chiang CF, Chao TT, Su YF, et al. Metformin-treated cancer cells modulate macrophage polarization through AMPK-NF-κB signaling. Oncotarget 2017; 8: 20706–20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Laberge RM, Sun Y, Orjalo AV., et al. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat Cell Biol 2015; 17: 1049–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xu M, Tchkonia T, Ding H, et al. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc Natl Acad Sci U S A 2015; 112: E6301–E6310. [DOI] [PMC free article] [PubMed] [Google Scholar]