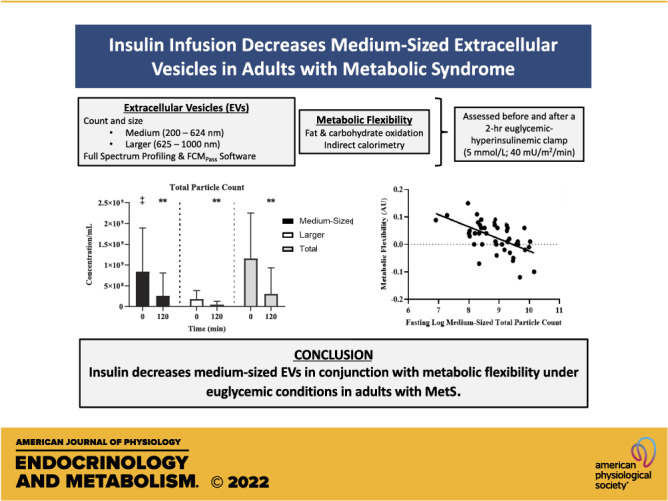
Keywords: extracellular vesicles, inflammation, insulin sensitivity, substrate oxidation
Abstract
Elevated extracellular vesicles (EVs) are associated with glucose dysmetabolism. However, the effects of insulin on EVs and subsequent relationships with insulin sensitivity, substrate oxidation, and inflammation are unknown. We tested the hypothesis that insulin would lower EVs and relate to insulin action. Fifty-one sedentary adults (54.8 ± 1.0 yr; V̇o2peak : 22.1 ± 0.6 mL/kg/min) with metabolic syndrome (MetS) and obesity (36.4 ± 0.65 kg/m2) underwent a 2-h euglycemic-hyperinsulinemic clamp (5 mmol/L; 40 mU/m2/min). Count and size (medium: 200–624 nm; larger: 625–1,000 nm) for total particle count, endothelial- (CD105+), leukocyte- (CD45+), platelet- (CD41+), and tetraspanin- (TX+: CD9/CD81/CD63), as well as platelet endothelial cell adhesion molecule- (CD31+) derived EVs were determined before and following the clamp using Full Spectrum Profiling (FSPM). Size and MESF (molecules of equivalent soluble fluorochrome) data were generated using FCMPASS Software. Fat and carbohydrate oxidation, in addition to high-sensitivity c-reactive protein (hsCRP), were measured to understand insulin effects and associations between EVs, metabolic flexibility, and inflammation. Despite low metabolic insulin sensitivity (M-Value = 2.56 ± 0.17 mg/kg/min), insulin increased carbohydrate (P = 0.015) and decreased fat oxidation (P = 0.048) and hsCRP (P = 0.016) compared with fasting. Insulin also decreased total particle count (P < 0.001), attributable to decreased medium-sized CD105+ (P = 0.052) and CD45+ EVs (P < 0.001). Elevated fasting insulin was associated with reduced insulin-stimulated changes in all EVs phenotypes (P < 0.001). Interestingly, fasting EVs were associated with increased fasting carbohydrate oxidation (all P < 0.05). These findings suggest that insulin decreases medium-sized EVs in conjunction with metabolic flexibility under euglycemic conditions in adults with MetS. More research is needed to determine how therapies alter EV phenotype/size and consequent cardiometabolic risk.
NEW & NOTEWORTHY This study is one of the first to investigate the effects of insulin on medium and larger extracellular vesicles (EVs) in relation to metabolic insulin sensitivity and fuel use in adults with metabolic syndrome. Our data suggest that insulin infusion decreases the concentration of total particle counts, mainly due to reductions in medium-sized EVs. Furthermore, EVs, predominantly medium-sized, are inversely associated with metabolic flexibility.
INTRODUCTION
It is estimated that 37% of all adults in the United States are classified as having metabolic syndrome (MetS) (1), which increases the risk of developing type 2 diabetes (T2D) and cardiovascular disease (CVD) (2). Although decreased insulin sensitivity is a key underlying factor regarding MetS pathogenesis, it, along with other traditional factors, only predicts around half of future CVD risk (3). Extracellular vesicles (EVs) have recently been identified in intercellular communication and as potential biomarkers in T2D and CVD (4). Once described as inactive cellular debris (5), EVs are now recognized as microstructures that can modify both the function and phenotype of target cells (6, 7). Subsequently, EVs appear to have potential roles in disease development and progression.
EVs contain cargo that includes bioactive materials and genetic information (8) and are classified into three different groups based upon their biogenesis and size: exosomes, microvesicles, and apoptotic bodies (9). Exosomes form by inward budding and are generally a smaller subtype of EV (30–150 nm), whereas microvesicles develop via direct outward budding and can range from 100 nm (or lower) to 1 µm in diameter. Apoptotic bodies are released by dying cells and although they can vary in size (50 nm–5 µm), they are typically larger (10). Interestingly, both EV size (11) and cargo are influenced by physiological states (12). In fact, fasting EV concentrations, specifically small particles (∼ <250 nm), as measured by nanoparticle tracking analysis, are elevated in states of obesity (13) and T2D (14), suggesting that EV size may differentially impact cardiometabolic health. Furthermore, obesity, as a metabolic state reflected by energy surplus and/or hormonal alterations, is thought to alter EV cargo and promote chronic inflammation (15, 16). This is clinically important as chronic inflammation has been linked to decreased insulin sensitivity and blunted metabolic flexibility (17, 18), which is traditionally the inability to switch between fuel sources in fasted versus insulin-stimulated states.
Although insulin has overall anti-inflammatory effects (19) and induces a shift in fuel usage from fat to carbohydrate oxidation in lean but not in people with obesity (20), less is known about the impact of insulin on EV phenotype and cargo. Interestingly, elevated insulin levels may affect EVs, as in vitro work has highlighted that hyperinsulinemia increases EV secretion, which subsequently impairs insulin signaling (14). However, there is limited research regarding postprandial EV responses in humans (21–23). We recently demonstrated that a 75-g oral glucose load induced a reduction in several EV types among adults with normal glucose tolerance and prediabetes in relation to postprandial insulin (21). Although this suggests that insulin may modify EVs, these in vivo data are confounded by elevated glucose concentrations, which are associated with concentrations (11, 24). Together, these observations highlight that no study in humans has determined and/or isolated the effect of insulin on EVs under euglycemic conditions. Furthermore, it is unknown if metabolic flexibility (i.e., fasted vs. insulin-stimulated fuel use) and inflammation relate to EVs during insulin stimulation. Therefore, the overall purpose of this study was to test the hypothesis that insulin would reduce EV count using the euglycemic-hyperinsulinemic clamp in people with MetS. We secondarily sought to assess EV sizes ranging from medium to large EVs. We then related EV characterization to insulin sensitivity, substrate oxidation, and inflammation to enhance our understanding of EVs for clinical relevance.
METHODS
Study Population
Adults (Table 1) were recruited from the Charlottesville, VA, area via flyers and social media. Eligibility criteria included age between 40 and 70, sedentary (<60 min/wk physical activity), nonsmoking, no medication usage that affected glucose metabolism (e.g., biguanides, insulin, etc.), no history of cardiovascular, renal, or hepatic disease, and characterized as having MetS via ATP III criteria (25). Individuals were classified as MetS at the screening visit via assessment of fasting blood measurements (plasma glucose, triglycerides, and high-density lipoprotein), waist circumference, and blood pressure, and/or medication usage (hypertensives and/or statins). All individuals provided written and verbal informed consent as approved by the University of Virginia Institutional Review Board (IRB-HSR: #19364 and 21244). This study conformed to the standards set by the Declaration of Helsinki, except for registration in a database.
Table 1.
Participant demographics
| n | 51 (42 F/9 M) |
| Age, yr | 54.8 ± 1.0 |
| V̇o2max, L/min | 2.33 ± 0.1 |
| V̇o2max, mL/kg/min | 22.2 ± 0.6 |
| Body Composition | |
| Weight, kg | 105 ± 2.4 |
| BMI, kg/m2 | 36.4 ± 0.7 |
| ^Fat, % | 44.1 ± 0.8 |
| ^Estimated VAT, cm2 | 198 ± 8.4 |
| Metabolic Syndrome Criteria | |
| ATP III Score | 3.47 ± 0.1 |
| Glucose, mmol/L | 5.60 ± 0.1 |
| HDL, mmol/L | 1.20 ± 0.0 |
| Triglycerides, mmol/L | 1.55 (0.76, 4.73) |
| Systolic BP, mmHg | 133 ± 1.5 |
| Diastolic BP, mmHg | 77.7 ± 1.3 |
| WC, cm | 115 ± 1.5 |
| Medications | |
| Blood Pressure (ACE, ARB) | 14 (27%) |
| Statins | 4 (8%) |
| Ethnicity and Race | |
| White, non-Hispanic | 47 (92%) |
| Black, non-Hispanic | 3 (6%) |
| Hispanic, regardless of race | 1 (2%) |
^n = 41 (35 F/6 M). Data are means ± SE, median (min, max), and frequency (percentage). ACE, angiotensin-converting enzyme inhibitors; ARB, angiotensin II receptor blockers; BMI, body mass index; BP, blood pressure; HDL, high-density lipoprotein; VAT, visceral adipose tissue; WC, waist circumference.
Aerobic Fitness and Body Composition
Maximal oxygen consumption (V̇o2max) was assessed using a modified Astrand incremental treadmill protocol with indirect calorimetry (Carefusion, Vmax Encore, Yorba Linda, CA). A test was considered maximal if at least 3 out of 4 criteria were met: 1) heart rate within 10 beats of age-predicted max, 2) volitional fatigue (≥17 ration of perceived exertion), 3) V̇o2 change ≤150 mL/min, and 4) RER ≥1.1. Fat and estimated visceral adipose tissue were determined by dual-energy X-ray absorptiometry (Horizon DXA System; Hologic, Marlborough, MA). Body mass was measured on a digital scale while height was recorded without shoes using a wall-mounted stadiometer (Hamburg, Germany) to calculate body mass index (BMI).
Metabolic Control
Participants were provided food and snacks the day before testing to minimize the impact of nutrient intake on respective outcomes, as previously described (26). Briefly, caloric needs were determined using resting metabolic rate obtained from indirect calorimetry and multiplied by a factor of 1.2. Participants were also instructed to drink only water and abstain from caffeine, medication, and alcohol 24 h before testing procedures.
Euglycemic-Hyperinsulinemic Clamp
Individuals arrived at the Clinical Research Unit following an overnight fast. Two intravenous lines were placed for infusion (antecubital vein) and blood collection (wrist), as described previously (26). Briefly, insulin (Humulin R U-100; Eli Lilly and company, Indianapolis, IN) was diluted in saline containing 4% (vol/vol) of the participant’s own blood. A primed (250 mU/m2/min) constant infusion (40 mU/m2/min) of insulin and variable glucose was administered for 120 min to maintain a blood glucose of 5 mmol/L. Indirect calorimetry with a canopy was used to estimate CHO oxidation (CHOox = 4.55 × V̇co2 – 3.21 × V̇o2), fat oxidation (Fox = 1.67 × V̇o2 – 1.67 × V̇co2), and respiratory exchange ratio (RER) at baseline and during the steady-state (i.e., final 30 min) of the clamp to determine metabolic flexibility (120 – 0 min RER) (27). Glucose infusion rate metabolized (M-Value) during the last 30 min of the clamp was used to define insulin sensitivity.
Blood Biochemistry
Glucose was measured every 5 min during the clamp and immediately analyzed upon collection using the glucose oxidase method (YSI Instruments 2300, Yellow Springs, OH). Insulin was collected at 0, 90, 105, and 120 min of the clamp while high-sensitivity c-reactive protein (hsCRP) was measured only at 0 and 120 min. Both insulin and hsCRP were stored at −80°C until later for duplicate-batched analysis via radioimmunoassay and chemiluminescent assay, respectively.
EV Isolation
Blood was collected at 0 and 120 min of the clamp in citrate vacutainers and underwent differential centrifugation at 5,000 g for 15 min (Sorvall RC 5B Plus Centrifuge: Rotor SS-34 Fixed Angle Rotor). The platelet-poor plasma was then transferred to 1.5-mL microcentrifuge tubes (Axygen) and frozen at −80°C. Prior to analysis, blood was thawed and centrifuged at 17,000 g for 20 min (called P17 pellet) and fluorescently labeled with antibodies for EV phenotyping by spectral flow cytometry. Six microliter of Ab mix and 4 µL of HEPES buffer was added to the pEV solution for a total final incubation volume of 30 µL. An antibody titer was performed for each antibody used and the following amount was optimized in the cocktail: 0.25 µL (CD9/CD81/CD63 cocktail; BioLegend, San Diego, CA); 7 µL (CD45); and 5 µL (CD105/CD31/CD41; BioLegend, San Diego, CA). The EV sample (30 µL) was then stained with 20 µL of buffer plus reagents for 1 h in the dark at room temperature (Fig. 1C). After staining, each sample was washed in 0.1 µM HEPES PBS at 21,000 g for 1 h and resuspended in 50 µL (28). Before flow acquisition, each sample was diluted in different volumes (D:500; D:1,000; D:2,000) according to MISEV 2018 guidelines (Minimal information for studies of EVs; Supplemental Table; see https://doi.org/10.6084/m9.figshare.20045723.v1) to verify single particle definition (29, 30). Isotype controls were used to compare unstained controls and stained samples to identify confounding effects related to antibody specificity. In addition, the gate strategy was adjusted for the positive staining and detergent-treated EV samples (Ripa Detergent) were performed to lyse membrane-enclosed vesicles to reduce their number and signals.
Figure 1.
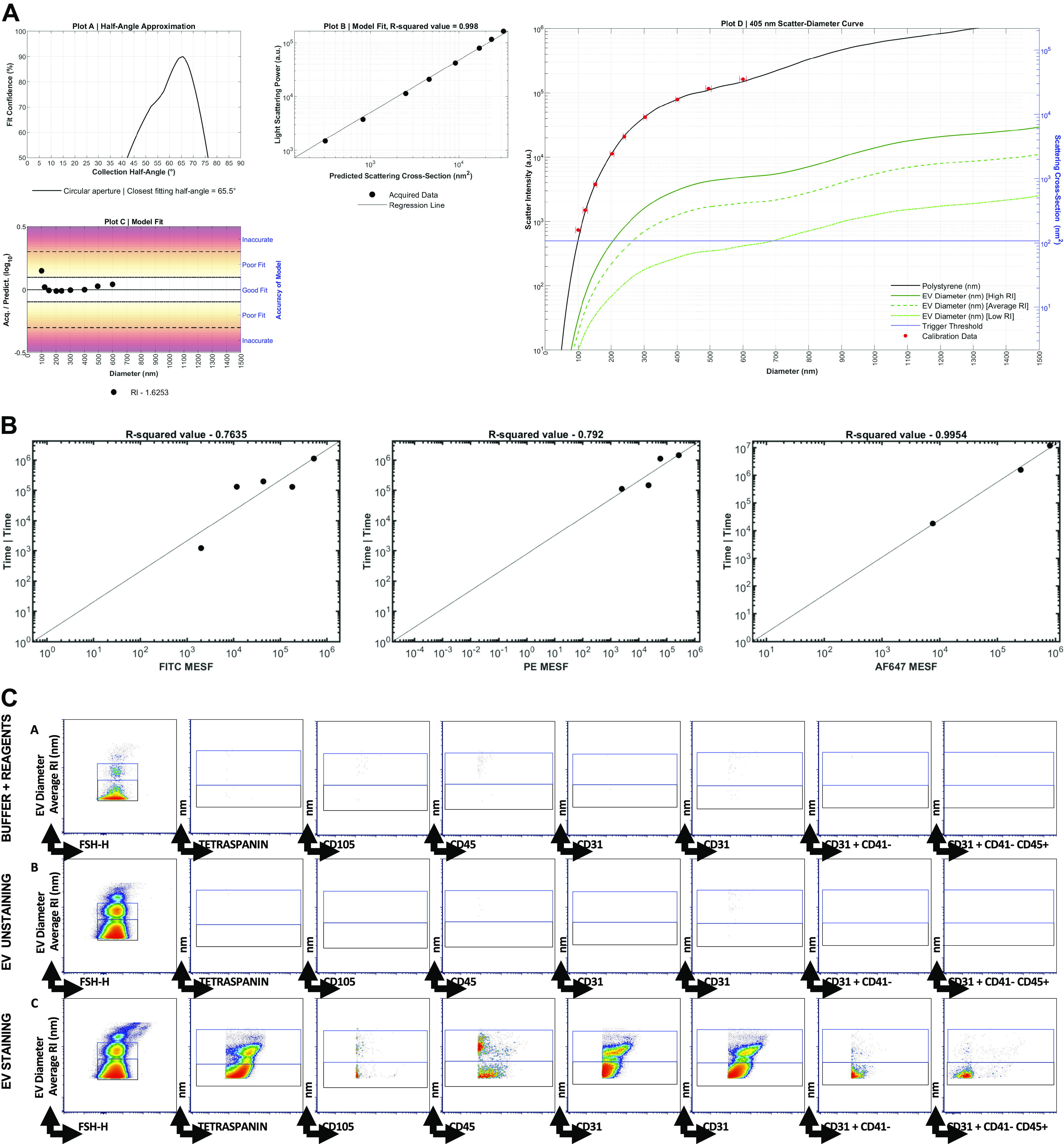
Representative data from one participant. A: light scatter calibration. Scatter-diameter curve (Plot D) report represents the collection angle of the spectrum flow acquiring accurately beads of known diameter and refractive index. The good model fit (Plot B) refers to adjusting the parameters in the model to improve accuracy. Plot C shows the accuracy of the setting to be able to acquire particles of 100 nm in size. B: fluorescence calibration plot. Fluorescence regression from The FCMPASS software is well reported for the following fluorochromes: FITC, PE, and AF647. C: manual gating strategy. Calibration beads were used to set EV particle count. Medium (black gate) and large (blue gate) extracellular vesicles (EVs) were accurately characterized by size. Arrows are used to visualize x, y across plots, and letters A, B, and C are used to call attention to the gating strategy. A: Buffer plus Reagents; B: EV unstaining; C: EV staining. EV phenotyping was performed for the main EV subsets.
EV Morphology and Phenotype
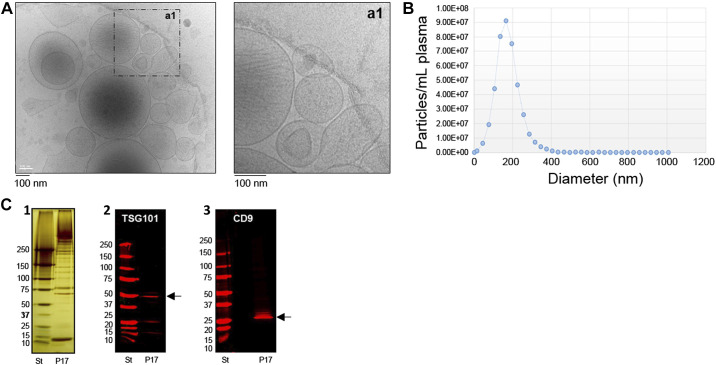
Basic EV characterization was performed using cryo-electron microscopy (cryo-EM) for EV morphology analysis, NTA for EV count and sizing, and Western blotting (WB) for detection of EV and non-EV-related proteins (Fig. 2), as described previously (28, 31, 57). EV-targeted phenotyping using Full Spectrum Profiling (FSP) (Aurora from Cytek) was performed to detect, count, and size total particle count endothelial- (CD105+), leukocyte- (CD45+), platelet- (CD41+), and tetraspanin- (TX+, CD9/CD81/CD63 cocktail), as well as platelet endothelial cell adhesion molecule- (CD31+) derived EVs. EV concentrations were calculated using a volumetric method provided by the full spectrum flow cytometer (FSFC) utilized herein. Of note, information about total particle counts (PC) <1,000 nm in size, which can contain other EV particles, was also collected. Scatter intensity data were calibrated in standardized units of scattering cross-section (nm2) and diameter (nm), which allowed for specific phenotype analysis of two ranges of EV size (medium-sized: 200–624 nm; larger EVs: 625–1,000 nm). Size and molecules of soluble fluorescence (MESF beads, Bangs Laboratories, Inc) data were generated using FCMPASS Software (32). This software performed a particle diameter approximation using Mie Theory and NIST traceable polystyrene particles of known size and refraction index (RI). Following the input of scatter intensity values of the calibration particles, size distribution curves were generated. Diameters were then extrapolated using raw SSC data and particle diameter distributions were produced. Likewise, calibrated units of MESF were extrapolated from the arbitrary units (a.u.) of fluorescence intensity reported by the FSFC using intensity of calibrated fluorescent particles.
Figure 2.
Basic EV characterization. This figure summarizes the basic characterization of extracellular vesicles (EVs) enriched for analysis in this study from platelet poor plasma with differential centrifugation. A: provides cryo-electron microscopy images in low and high resolutions. EVs of various sizes and densities are present. The higher magnification shows also the bilipid layers of EVs studied here. B: shows an example of a particle detection with Nanosight tracking analysis (NTA) using “Zetaview by Particle matrix.” The mean size of EVs in this example is 174.2 nm (median 161.8 nm and mode 171.8 nm) and the majority of EVs is less than 200 nm in size. For all other samples size and phenotype of single EVs are detected with flow cytometry (see Fig. 1C for gating strategy and also information about fluorescence and light scatter calibration for sizing which is provided in the Supplemental information (FCM pass report, MiFlowcyt check list; available: https://doi.org/10.6084/m9.figshare.20045723.v1). C: summarize results of Western blotting analysis of enriched EV pellets with differential centrifugation. The left image (C1) shows a silverstain of protein content of the EV pellet [here enriched with differential centrifugation (P17 pellet)] and a standard (ST). EV antigens such as TSG101 (C2) and CD9 (C3) are present in this P17 pellet.
Statistical Analysis
Data were analyzed using the Statistical Package for Social Sciences (SPSS Software v. 27) and the statistical software R (v. 4.0.2). Shapiro–Wilk was used to assess normality and all nonnormally distributed data (EVs, Fox, hsCRP) were log-transformed for analysis. Outliers were determined using the interquartile rule (IQR × 1.5) and subsequently removed if noted to be influential based on sensitivity analyses. Paired two-tailed t tests were used to compare fasting EVs as well as determine the effects of insulin on EVs and substrate oxidation (0 vs. 120 min). Effect sizes were calculated using Cohen’s d, whereas associations were examined using Pearson correlations. Statistical significance was accepted as P ≤ 0.05. Normally distributed and log-transformed data are mean ± standard error of the mean (SEM), whereas nonnormally distributed data are presented as medians (min, max).
RESULTS
Participant Characteristics
Fifty-one adults (54.8 ± 1.0 yr) completed the current study, with a majority being female (82%) and Caucasian (92%). All individuals were classified as having MetS (ATP III: 3.47 ± 0.1) and obesity (BMI: 36.4 ± 0.7 kg/m2; Table 1).
Euglycemic-Hyperinsulinemic Clamp
Insulin infusion decreased glucose (P < 0.001, d = 0.87) and increased insulin concentration (P < 0.001, d = 2.1) by design (Table 2). Despite individuals having low metabolic insulin sensitivity (2.56 ± 0.17 mg/kg/min) (33, 34) compared with literature in healthy controls, insulin decreased hsCRP (P = 0.016, d = 0.36), Fox (P = 0.048, d = 0.30) as well as increased CHOox (P = 0.015, d = 0.37), suggesting metabolic flexibility (0.02 ± 0.01 AU, Table 2).
Table 2.
Euglycemic-hyperinsulinemic clamp
| 0 Min | 120 Min | P Value | Cohen’s d | |
|---|---|---|---|---|
| Blood Biochemistries | ||||
| Glucose, mmol/L | 5.72 ± 0.09 | 4.96 ± 0.08 | <0.001 | 0.87 |
| ^Insulin, pmol/mL | 109 ± 9.60 | 495 ± 22.0 | <0.001 | 2.10 |
| hsCRP, nmol/L | 56.2 (1.81, 248) | 50.0 (1.33, 257) | 0.016 | 0.36 |
| †Substrate Oxidation | ||||
| CHOox, mg/kg/min | 2.17 ± 0.47 | 2.53 ± 0.43 | 0.015 | 0.37 |
| Fox, mg/kg/min | 0.54 (0.01, 1.17) | 0.44 (0.01, 0.74) | 0.048 | 0.30 |
| RER, AU | 0.83 ± 0.03 | 0.85 ± 0.04 | 0.003 | 0.46 |
^n = 46 (38 F/8 M). †n = 47 (39 F/8 M). Data are means ± SE and median (min, max). AU, arbitrary unit; CHOox, carbohydrate oxidation; Fox, fat oxidation; hsCRP, high-sensitivity c-reactive protein; RER, respiratory exchange ratio.
EV Size and Phenotype
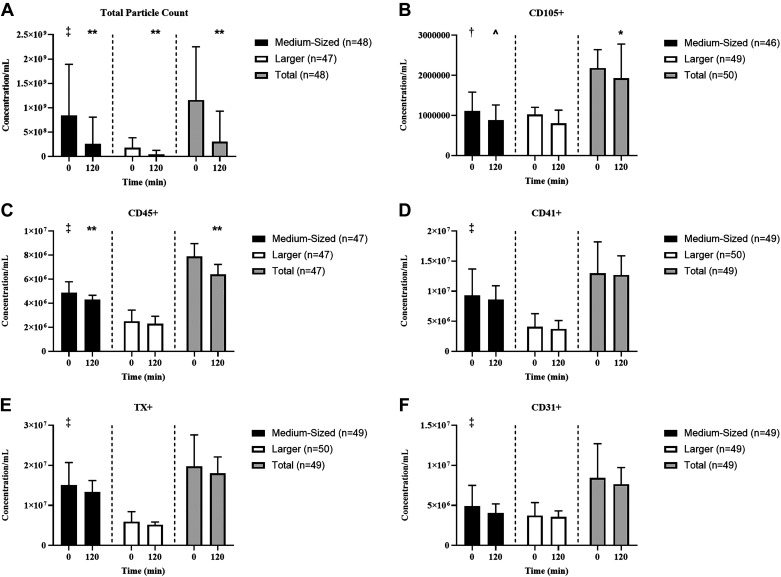
At rest, there were increased concentrations of total PC (P < 0.001, d = 1.70), medium-sized CD105+ (endothelial; P = 0.006, d = 0.42), CD45+ (P < 0.001, d = 1.18), CD41+ (leukocyte; P < 0.001, d = 1.22), TX+ (P < 0.001, d = 1.59), and CD31+ EVs (platelet endothelial cell adhesion molecule; P < 0.001, d = 0.71) compared with larger EVs (Fig. 3). Insulin infusion decreased total PC (P < 0.001, d = 0.96), including both medium- (P < 0.001, d = 1.09) and larger-sized EVs (P < 0.001, d = 1.08). This decrease in total PC was mainly attributed to decreases in total CD105+ (P = 0.020, d = 0.34) and CD45+ (P = 0.001, d = 0.48) EVs. Interestingly, medium-sized (CD105+: P = 0.052, d = 0.28; CD45+: P < 0.001, d = 0.67) but not larger EVs (CD105+: P = 0.29, d = 0.15; CD45+: P = 0.074, d = 0.27) were decreased by insulin infusion. However, insulin did not alter CD31+/41- (medium: P = 0.065, d = 0.27), CD41+ (medium: P = 0.37, d = 0.13; larger: P = 0.63, d = 0.07; total PC: P = 0.43, d = 0.12), TX+ (medium: P = 0.16, d = 0.20; larger: P = 0.28, d = 0.16; total PC: P = 0.17, d = 0.20), or CD31+ EVs (medium: P = 0.15, d = 0.21; larger: P = 0.30, d = 0.15; total OC: P = 0.220, d = 0.18; Fig. 3).
Figure 3.
Effect of insulin infusion on medium-sized, larger, and total extracellular vesicles (EVs). Data are presented as medians with 95% confidence intervals for total particle count (A), CD105+ (B), CD45+ (C), CD41+ (D), TX+ (E), and CD31+ (F). Medium-sized (black bar, 200–624 nm), larger (white bar, 625–1,000 nm), and total EVs (gray bar). Fasting size distribution: †P = 0.006, ‡P < 0.001. Effect of insulin: ^P = 0.052, *P < 0.05, **P < 0.001. TX, tetraspanin.
Correlations
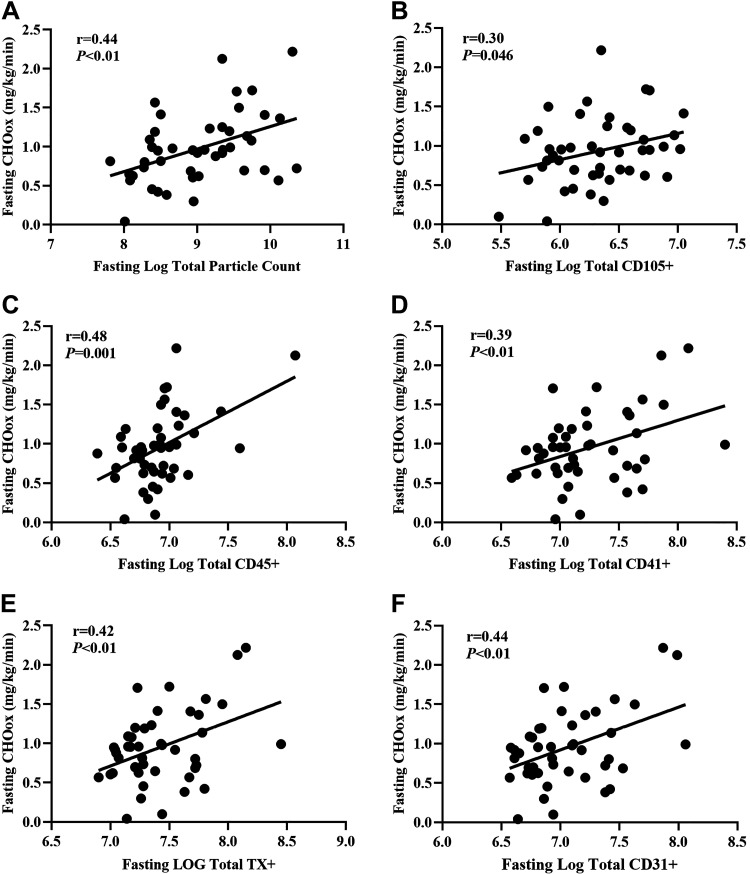
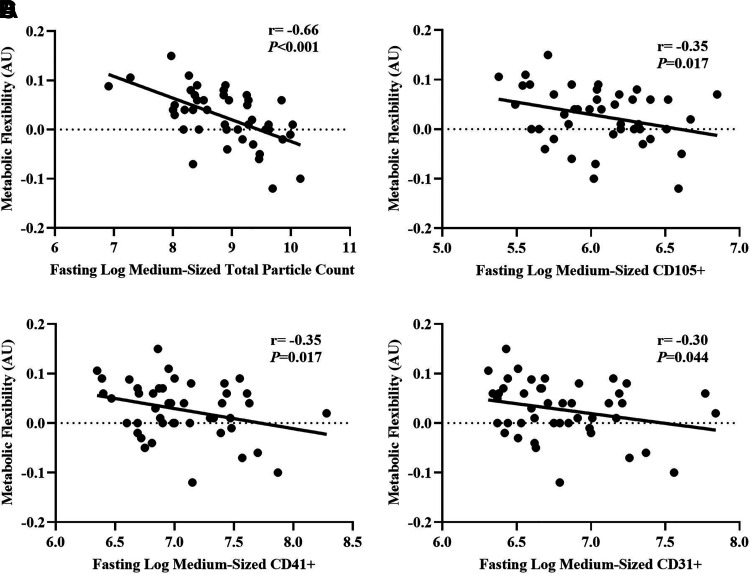
Fasting total PC positively correlated with fasting insulin (n = 43; r = 0.34, P = 0.02), triglycerides (n = 48; r = 0.33, P = 0.02) and negatively with metabolic insulin sensitivity (n = 48; r = −0.29, P = 0.049), but not fasting glucose concentrations (n = 48; r = 0.04, P = 0.79). Likewise, higher fasting insulin was linked to lower insulin-stimulated changes (delta: 120 − 0 min) in total PC (n = 43; r = 0.69, P < 0.001), CD105+ (n = 44; r = 0.63, P < 0.001), CD45+ (n = 44; r = 0.64, P < 0.001), CD41+ (n = 44; r = 0.66, P < 0.001), CD31+ (n = 44; r = 0.67, P < 0.001), and TX+ EV counts (n = 44; r = 0.67, P < 0.001, Table 3). Although there were no correlations between metabolic insulin sensitivity and total or medium-sized EVs, higher metabolic insulin sensitivity correlated with greater insulin-stimulated changes in larger CD45+ EVs (n = 47; r = −0.34, P = 0.02). Despite associations between fasting hsCRP and insulin (n = 44; r = 0.32, P = 0.036) as well as insulin sensitivity (n = 48; r = −0.41, P = 0.004), there were no correlations between hsCRP and EVs, whether at fasting or following insulin stimulation (Table 3). Interestingly, elevated fasting total EV concentrations were associated with higher fasting CHOox (Fig. 4). Subsequently, total PC (r = −0.55, P < 0.001), CD105+ (r = −0.39, P = 0.017), CD31+ (r = −0.36, P = 0.016), and CD41+ EV counts (r = −0.33, P = 0.025) were inversely associated with metabolic flexibility. In addition, this relationship appeared to be driven mainly by medium-sized EVs (Fig. 5).
Table 3.
Correlations between fasting blood biochemistries, insulin sensitivity, and EVs
| Glucose | hsCRP | Insulin | Triglyceride | M-Value | |
|---|---|---|---|---|---|
| Fasting | |||||
| Total PC | 0.04 | 0.10 | 0.34 | 0.33 | −0.29 |
| CD105+ | −0.25 | 0.24 | −0.17 | 0.22 | −0.09 |
| CD45+ | 0.01 | 0.12 | −0.16 | 0.19 | −0.10 |
| CD41+ | 0.06 | 0.06 | −0.07 | 0.06 | 0.02 |
| TX+ | 0.1 | 0.05 | −0.05 | 0.08 | −0.03 |
| CD31+ | 0.06 | −0.04 | −0.13 | 0.07 | 0.03 |
| Δ(120−0 min) | |||||
| Total PC | 0.02 | −0.02 | 0.69 | −0.10 | −0.15 |
| CD105+ | −0.04 | 0.01 | 0.63 | −0.15 | −0.12 |
| CD45+ | −0.03 | −0.01 | 0.64 | −0.13 | −0.12 |
| CD41+ | −0.03 | 0.01 | 0.66 | −0.15 | −0.13 |
| TX+ | −0.03 | <0.01 | 0.67 | −0.14 | −0.13 |
| CD31+ | −0.01 | 0.02 | 0.67 | −0.11 | −0.13 |
Bolded values indicate significant associations (P < 0.05). hsCRP, high-sensitivity c-reactive protein; M-Value, metabolic insulin sensitivity; PC, particle count.
Figure 4.
Positive associations between fasting carbohydrate oxidation (CHOox) and fasting total particle count (A), CD105+ (B), CD45+ (C), CD41+ (D), TX+ (E), and CD31+ (F). EV data are log-transformed. Total particle count, CD45+, CD41+, TX+, CD31+ is n = 45 (37 F/8 M). CD105+ is n = 46 (38 F/8 M). TX, tetraspanin.
Figure 5.
Negative associations between metabolic flexibility and medium-sized total particle count (A), CD105+ (B), CD41+ (C), and CD31+ (D). EV data are log transformed. Total particle count, CD41+, and CD31+ are n = 45 (37 F/8 M). CD105+ is n = 43 (35 F/8 M). Metabolic flexibility reflects 120 − 0 min RER. RER, respiratory exchange ratio.
DISCUSSION
The major finding of this study is that insulin infusion reduced total particle counts in adults with MetS, which was primarily attributed to decreases in total CD105+ and CD45+ EV concentrations. This observation was also mainly due to decreased medium-sized (200–624 nm) compared with larger EVs (625–1,000 nm). The EV characterization used herein offers a novel calibrated approach to size individual EVs and their specific phenotype with flow cytometry. Indeed, to our knowledge, this is the first clinical study to provide information on calibrated EV size and their specific phenotype. Research thus far has only described the overall size range of EVs, mostly via NTA or tunable resistive pulse sensing. However, those tools are particle analyzers and can detect non-EV particles such as albumin and other protein droplets which are coisolated by various EV enrichment methods, including differential centrifugation, as utilized here. In addition, more flow cytometers only measure larger EVs (>400 nm), unless small particle analyzers are used (35). Thus, a large number of published studies are biased toward larger EVs (36).
Although prior work observed that small fasting EVs and particles, as characterized by tunable resistive pulse sensing and NTA, were elevated in individuals with T2D (14) and swine with MetS (37), this is the first study in humans to corroborate these findings with and without insulin in adults with MetS. Interestingly, the reduction in EVs does contrast with prior work by Freeman et al (14), who observed that hyperinsulinemic conditions (i.e., 48 h with 200 nmol/L insulin) increased EV secretion. However, this was done in vitro in isolated rat neurons and therefore may not be indicative of in vivo responses in humans. Furthermore, these cells were cultured in insulin concentrations around 1,000 times what was utilized herein, suggesting dose-response relations may occur. In addition, EV concentrations were measured with particle analyzers and might not reflect true EV counts. Alternatively, the increases observed by Freeman et al. may be related to differences in the limit of detections (LODs) of the instruments between the two technologies used (NTA and FSFC) and/or a shift toward EVs below the 200 nm LOD. Nonetheless, insulin may play an important role in EV concentrations, as we noted herein that elevated clinical fasting insulin was associated with increased EV concentrations as well as lower insulin-stimulated changes. This was also corroborated by the negative association between total particle concentrations in the fasted state and metabolic insulin sensitivity as measured by the clamp. Although further investigation is needed to identify how insulin modifies small and/or large EVs, EVs have insulin receptors as well as Akt proteins, suggesting that insulin can act directly on EVs (14, 38). Alternatively, we acknowledge that it is possible insulin acted on respective tissues to dampen the release of EVs into circulation and/or that insulin directly influenced EV synthesis/clearance/size. Indeed, it is also possible that the hyperinsulinemic conditions utilized herein promoted uptake by recipient cells. In either case, additional work is required to understand how insulin acts to influence EV physiology. In fact, prior work noted that EVs often contain unique cargo, including microRNAs that likely contribute to the development of insulin resistance (39), potentially via cleavage of insulin receptors (40). Thus, the reduction of EVs seen with insulin infusion in our study suggests that EVs are not only under the influence of insulin but also may be involved in insulin sensitivity.
Insulin typically alters substrate metabolism by decreasing fat and increasing carbohydrate oxidation (41). Prior research has suggested that individuals with insulin resistance have lower levels of metabolic flexibility (42), in part, via impaired mitochondria capacity and dysregulated fatty acid metabolism (43). Herein, we observed that insulin infusion decreased Fox and increased CHOox, even though individuals had MetS. In addition, our data highlight for the first time that EVs may contribute to metabolic flexibility and fuel usage. During the fasted state, fat is the preferred fuel source. However, high fasting CHOox has been noted in people with T2D (42). This altered substrate use, in turn, has been linked to mitochondrial disturbances reflected by excessive oxidative stress/inflammation that impairs insulin signaling (44). Our work suggests that EVs may play a role in fuel source utilizations. In particular, EVs have been noted to increase reactive oxygen species, which decrease mitochondrial energy flux (45) and fat oxidation. Furthermore, studies in cancer report that exosomes, and thus small EVs, contained more glycolytic enzymes (46) and impaired mitochondrial oxidative phosphorylation (47). This, in combination with our data, suggest that size and phenotype can influence metabolic function.
The change in EV concentration may be physiologically meaningful for several reasons. Increased EVs, specifically endothelial EVs, have been associated with reduced vasorelaxation (48). In the present work, insulin infusion decreased medium-sized CD105+, an endothelial-derived EV. Although we did not determine vascular function within this analysis, recent work by our group noted that insulin reduced augmentation index, a measure of arterial stiffness and/or aortic waveform in a cohort of individuals with MetS (26). Furthermore, work by Eichner et al (21) reported that total EVs decreased in parallel with augmentation index in response to an oral glucose load. Thus, it is plausible that insulin increased blood flow and/or perfusion in the current study, related to EV-physiology. In addition to altered vasorelaxation, EVs have been linked to increased reactive oxygen species production as well as stimulation by increased inflammatory cytokines (48). In contrast, insulin is thought to decrease inflammation via regulation of the NLRP3 inflammasome (19). Indeed, we saw that insulin infusion reduced medium-sized CD45+, a leukocyte-derived EV that is associated with immune responses and inflammation (49). Furthermore, hsCRP, a conventional biomarker used as a clinical diagnostic tool to understand inflammation and CVD risk, was assessed in the present study (50). Although all individuals enrolled in the current study were classified as having MetS without diagnosed vascular disease, hsCRP levels were indicative of being at high risk of CVD (>28.6 mmol/L) (51). Prior work too has noted associations between hsCRP and EVs (52). Interestingly, hsCRP and CD45+ were reduced with insulin. This may be relevant as work in vitro and in vivo found that the EV concentration was reduced when inflammation was inhibited by the TNF-α pathway (53), and TNF-α induces IL-6 synthesis via the JAK/STAT3 pathway (54). In turn, IL-6 is the primary cytokine that leads to hepatic CRP production (55). Because inflammation also alters metabolic fuel selection (56), the collective work highlights a potential complex relationship among EVs, insulin, inflammation, and substrate oxidation.
There are limitations in the current study that warrant discussion. We did not include a lean healthy control group, and it is possible that EVs could differ by race and sex (52). Thus, it is likely that these results are not generalizable, as the study was comprised predominantly of White, non-Hispanic females with MetS. Furthermore, although our data support relationships among EVs, insulin, and substrate oxidation, these correlations do not equate to causation. We did not measure EV cargo of EVs <200 nm, which likely play a role in insulin signaling and insulin resistance (14), and limits our knowledge on the mechanism(s) by which insulin impacts EV phenotype and function overall. Finally, additional work is warranted to understand how different doses of insulin and/or hyperglycemia impact EV physiology. Notable strengths, however, include use of the euglycemic clamp with indirect calorimetry to directly test insulin on EV concentration as well as characterization (including size) of specific EV phenotypes using both FSFC and MESF in a clinically relevant population.
Conclusions
Insulin infusion decreases the concentration of plasma-derived nanoparticles, including medium-sized endothelial- (CD105+) and leukocyte-derived EVs (CD45+), under euglycemic conditions in adults with MetS. In addition, total EV concentrations correlated with alterations in fasting carbohydrate oxidation. This suggests that EVs may be a novel mechanism connected to insulin resistance and future disease development and that EV size may have differential impacts. Further work is needed to explore how different treatments, such as lifestyle and/or pharmacological, affect EV characteristics, including smaller EVs, to impact T2D and CVD risk.
DATA AVAILABILITY
Some or all datasets generated and analyzed during the current study are not publicly available but are available from the corresponding author (S.K.M.) on reasonable request.
SUPPLEMENTAL DATA
Supplemental Table: https://doi.org/10.6084/m9.figshare.20045723.v1.
GRANTS
This study was supported by Grants NIH R01-HL130296 (to S.K.M.), UVA LaunchPad (to S.K.M. and U.E.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.K.M. conceived and designed research; E.M.H., A.B., N.R.S., S.L.S., L.M., J.L., and S.K.M. performed experiments; E.M.H., A.B., S.L.S., L.M., J.L., U.E., and S.K.M. analyzed data; E.M.H., A.B., N.R.S., S.L.S., L.M., J.L., U.E., and S.K.M. interpreted results of experiments; E.M.H., S.L.S., and L.M. prepared figures; E.M.H., S.L.S., U.E., and S.K.M. drafted manuscript; E.M.H., A.B., N.R.S., S.L.S., L.M., J.L., U.E., and S.K.M. edited and revised manuscript; E.M.H., A.B., N.R.S., S.L.S., L.M., J.L., U.E., and S.K.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the individuals for participating in the current study as well as the staff of the Exercise Physiology Core Lab for testing aid, the Clinical Research Unit for technical assistance, the Ligand Assay and Analysis Core at the University of Virginia for hsCRP analysis, and Vanderbilt University for insulin analysis.
REFERENCES
- 1. Hirode G, Wong RJ. Trends in the prevalence of metabolic syndrome in the United States, 2011–2016. JAMA 323: 2526–2528, 2020. doi: 10.1001/jama.2020.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Klein BEK, Klein R, Lee KE. Components of the metabolic syndrome and risk of cardiovascular disease and diabetes in Beaver Dam. Diabetes Care 25: 1790–1794, 2002. doi: 10.2337/diacare.25.10.1790. [DOI] [PubMed] [Google Scholar]
- 3. Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA 297: 611–619, 2007. [Erratum in JAMA 297: 1433, 2007]. doi: 10.1001/jama.297.6.611. [DOI] [PubMed] [Google Scholar]
- 4. Théry C. Exosomes: secreted vesicles and intercellular communications. F1000 Biol Rep 3: 15, 2011. doi: 10.3410/B3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol 13: 269–288, 1967. doi: 10.1111/j.1365-2141.1967.tb08741.x. [DOI] [PubMed] [Google Scholar]
- 6. Tetta C, Ghigo E, Silengo L, Deregibus MC, Camussi G. Extracellular vesicles as an emerging mechanism of cell-to-cell communication. Endocrine 44: 11–19, 2013. doi: 10.1007/s12020-012-9839-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vechetti IJ Jr, Valentino T, Mobley CB, McCarthy JJ. The role of extracellular vesicles in skeletal muscle and systematic adaptation to exercise. J Physiol 599: 845–861, 2021. doi: 10.1113/JP278929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yáñez-Mó M, Siljander PR-M, Andreu Z, Zavec AB, Borràs FE, Buzas EI, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles 4: 27066, 2015. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. EL Andaloussi S, Mäger I, Breakefield XO, Wood MJA. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov 12: 347–357, 2013. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 10. Doyle LM, Wang MZ. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells 8: 727, 2019. doi: 10.3390/cells8070727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Burger D, Turner M, Xiao F, Munkonda MN, Akbari S, Burns KD. High glucose increases the formation and pro-oxidative activity of endothelial microparticles. Diabetologia 60: 1791–1800, 2017. doi: 10.1007/s00125-017-4331-2. [DOI] [PubMed] [Google Scholar]
- 12. Hutcheson JD, Aikawa E. Extracellular vesicles in cardiovascular homeostasis and disease. Curr Opin Cardiol 33: 290–297, 2018. doi: 10.1097/HCO.0000000000000510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stepanian A, Bourguignat L, Hennou S, Coupaye M, Hajage D, Salomon L, Alessi M-C, Msika S, de Prost D. Microparticle increase in severe obesity: not related to metabolic syndrome and unchanged after massive weight loss. Obesity (Silver Spring) 21: 2236–2243, 2013. doi: 10.1002/oby.20365. [DOI] [PubMed] [Google Scholar]
- 14. Freeman DW, Hooten NN, Eitan E, Green J, Mode NA, Bodogai M, Zhang Y, Lehrmann E, Zonderman AB, Biragyn A, Egan J, Becker KG, Mattson MP, Ejiogu N, Evans MK. Altered extracellular vesicle concentration, cargo, and function in diabetes. Diabetes 67: 2377–2388, 2018. doi: 10.2337/db17-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Renovato-Martins M, Matheus ME, de Andrade IR, Moraes JA, da Silva SV, Citelli dos Reis M, de Souza AAP, da Silva CC, Bouskela E, Barja-Fidalgo C. Microparticles derived from obese adipose tissue elicit a pro-inflammatory phenotype of CD16+, CCR5+ and TLR8+ monocytes. Biochim Biophys Acta Mol Basis Dis 1863: 139–151, 2017. doi: 10.1016/j.bbadis.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 16. Liu J, Sun X, Zhang F-L, Hang J, Yan X-L, Huang S, Guo Z-N, Yang Y. Clinical potential of extracellular vesicles in type 2 diabetes. Front Endocrinol (Lausanne) 11: 596811, 2021. doi: 10.3389/fendo.2020.596811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Corpeleijn E, Saris WHM, Blaak EE. Metabolic flexibility in the development of insulin resistance and type 2 diabetes: effects of lifestyle. Obes Rev 10: 178–193, 2009. doi: 10.1111/j.1467-789X.2008.00544.x. [DOI] [PubMed] [Google Scholar]
- 18. Smith RL, Soeters MR, Wüst RCI, Houtkooper RH. Metabolic flexibility as an adaptation to energy resources and requirements in health and disease. Endocr Rev 39: 489–517, 2018. doi: 10.1210/er.2017-00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chang Y-W, Hung L-C, Chen Y-C, Wang W-H, Lin C-Y, Tzeng H-H, Suen J-L, Chen Y-H. Insulin reduces inflammation by regulating the activation of the NLRP3 inflammasome. Front Immunol 11: 587229, 2020. doi: 10.3389/fimmu.2020.587229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sidossis LS, Wolfe RR. Glucose and insulin-induced inhibition of fatty acid oxidation: the glucose-fatty acid cycle reversed. Am J Physiol Endocrinol Metab 270: E733–E738, 1996. doi: 10.1152/ajpendo.1996.270.4.E733. [DOI] [PubMed] [Google Scholar]
- 21. Eichner NZM, Gilbertson NM, Musante L, La Salvia S, Weltman A, Erdbrügger U, Malin SK. An oral glucose load decreases postprandial extracellular vesicles in obese adults with and without prediabetes. Nutrients 11: 580, 2019. doi: 10.3390/nu11030580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tushuizen ME, Nieuwland R, Rustemeijer C, Hensgens BE, Sturk A, Heine RJ, Diamant M. Endothelial microparticles following consecutive meals are associated with vascular endothelial dysfunction in type 2 diabetes. Diabetes Care 30: 728–730, 2007. doi: 10.2337/dc06-1473. [DOI] [PubMed] [Google Scholar]
- 23. Jenkins NT, Landers RQ, Thakkar SR, Fan X, Brown MD, Prior SJ, Spangenburg EE, Hagberg JM. Prior endurance exercise prevents postprandial lipaemia-induced increases in reactive oxygen species in circulating CD31+ cells. J Physiol 589: 5539–5553, 2011. doi: 10.1113/jphysiol.2011.215277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kobayashi Y, Eguchi A, Tempaku M, Honda T, Togashi K, Iwasa M, Hasegawa H, Takei Y, Sumida Y, Taguchi O. Circulating extracellular vesicles are associated with lipid and insulin metabolism. Am J Physiol Endocrinol Metab 315: E574–E582, 2018. doi: 10.1152/ajpendo.00160.2018. [DOI] [PubMed] [Google Scholar]
- 25.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 106: 3143–3421, 2002. [PubMed] [Google Scholar]
- 26. Dotson BL, Heiston EM, Miller SL, Malin SK. Insulin stimulation reduces aortic wave reflection in adults with metabolic syndrome. Am J Physiol Heart Circ Physiol 320: H2305–H2312, 2021. doi: 10.1152/ajpheart.00975.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol Respir Environ Exerc Physiol 55: 628–634, 1983. doi: 10.1152/jappl.1983.55.2.628. [DOI] [PubMed] [Google Scholar]
- 28. La Salvia S, Musante L, Lannigan J, Gigliotti JC, Le TH, Erdbrügger U. T cell-derived extracellular vesicles are elevated in essential HTN. Am J Physiol Renal Physiol 319: F868–F875, 2020. doi: 10.1152/ajprenal.00433.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R. , et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 7: 1535750, 2018. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Welsh JA, Van Der Pol E, Arkesteijn GJA, Bremer M, Brisson A, Coumans F, Dignat-George F, Duggan E, Ghiran I, Giebel B, Görgens A, Hendrix A, Lacroix R, Lannigan J, Libregts SFWM, Lozano-Andrés E, Morales-Kastresana A, Robert S, De Rond L, Tertel T, Tigges J, De Wever O, Yan X, Nieuwland R, Wauben MHM, Nolan JP, Jones JC. MIFlowCyt-EV: a framework for standardized reporting of extracellular vesicle flow cytometry experiments. J Extracell Vesicles 9: 1713526, 2020. doi: 10.1080/20013078.2020.1713526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Eichner NZM, Gilbertson NM, Gaitan JM, Heiston EM, Musante L, LaSalvia S, Weltman A, Erdbrügger U, Malin SK. Low cardiorespiratory fitness is associated with higher extracellular vesicle counts in obese adults. Physiol Rep 6: e13701, 2018. doi: 10.14814/phy2.13701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Welsh JA, Horak P, Wilkinson JS, Ford VJ, Jones JC, Smith D, Holloway JA, Englyst NA. FCMPASS Software aids extracellular vesicle light scatter standardization. Cytometry A 97: 569–581, 2020. doi: 10.1002/cyto.a.23782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tam CS, Xie W, Johnson WD, Cefalu WT, Redman LM, Ravussin E. Defining insulin resistance from hyperinsulinemic-euglycemic clamps. Diabetes Care 35: 1605–1610, 2012. doi: 10.2337/dc11-2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22: 1462–1470, 1999. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 35. van der Pol E, Sturk A, van Leeuwen T, Nieuwland R, Coumans F; ISTH-SSC-VB Working group. Standardization of extracellular vesicle measurements by flow cytometry through vesicle diameter approximation. J Thromb Haemost 16: 1236–1245, 2018. doi: 10.1111/jth.14009. [DOI] [PubMed] [Google Scholar]
- 36. Eichner NZM, Erdbrügger U, Malin SK. Extracellular vesicles: a novel target for exercise-mediated reductions in type 2 diabetes and cardiovascular disease risk. J Diabetes Res 2018: 7807245, 2018. doi: 10.1155/2018/7807245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Conley SM, Shook JE, Zhu X-Y, Eirin A, Jordan KL, Woollard JR, Isik B, Hickson LJ, Puranik AS, Lerman LO. Metabolic syndrome induces release of smaller extracellular vesicles from porcine mesenchymal stem cells. Cell Transplant 28: 1271–1278, 2019. doi: 10.1177/0963689719860840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. van der Mijn JC, Sol N, Mellema W, Jimenez CR, Piersma SR, Dekker H, Schutte LM, Smit EF, Broxterman HJ, Skog J, Tannous BA, Wurdinger T, Verheul HMW. Analysis of AKT and ERK1/2 protein kinases in extracellular vesicles isolated from blood of patients with cancer. J Extracell Vesicles 3: 25657, 2014. doi: 10.3402/jev.v3.25657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim Y, Kim O-K. Potential roles of adipocyte extracellular vesicle-derived miRNAs in obesity-mediated insulin resistance. Adv Nutr 12: 566–574, 2021. doi: 10.1093/advances/nmaa105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yuasa T, Amo-Shiinoki K, Ishikura S, Takahara M, Matsuoka T, Kaneto H, Kuroda A, Matsuhisa M, Hashida S. Sequential cleavage of insulin receptor by calpain 2 and γ-secretase impairs insulin signalling. Diabetologia 59: 2711–2721, 2016. doi: 10.1007/s00125-016-4102-5. [DOI] [PubMed] [Google Scholar]
- 41. Yki-Järvinen H, Bogardus C, Howard BV. Hyperglycemia stimulates carbohydrate oxidation in humans. Am J Physiol Endocrinol Physiol 253: E376–E382, 1987. doi: 10.1152/ajpendo.1987.253.4.E376. [DOI] [PubMed] [Google Scholar]
- 42. Kelley DE, Simoneau JA. Impaired free fatty acid utilization by skeletal muscle in non-insulin-dependent diabetes mellitus. J Clin Invest 94: 2349–2356, 1994. doi: 10.1172/JCI117600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Turner N, Kowalski GM, Leslie SJ, Risis S, Yang C, Lee-Young RS, Babb JR, Meikle PJ, Lancaster GI, Henstridge DC, White PJ, Kraegen EW, Marette A, Cooney GJ, Febbraio MA, Bruce CR. Distinct patterns of tissue-specific lipid accumulation during the induction of insulin resistance in mice by high-fat feeding. Diabetologia 56: 1638–1648, 2013. doi: 10.1007/s00125-013-2913-1. [DOI] [PubMed] [Google Scholar]
- 44. Kelley DE, Mandarino LJ. Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes 49: 677–683, 2000. doi: 10.2337/diabetes.49.5.677. [DOI] [PubMed] [Google Scholar]
- 45. Forrester SJ, Kikuchi DS, Hernandes MS, Xu Q, Griendling KK. Reactive oxygen species in metabolic and inflammatory signaling. Circ Res 122: 877–902, 2018. doi: 10.1161/CIRCRESAHA.117.311401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang J, Lu S, Zhou Y, Meng K, Chen Z, Cui Y, Shi Y, Wang T, He Q-Y. Motile hepatocellular carcinoma cells preferentially secret sugar metabolism regulatory proteins via exosomes. Proteomics 17, 2017. doi: 10.1002/pmic.201700103. [DOI] [PubMed] [Google Scholar]
- 47. Zhao H, Yang L, Baddour J, Achreja A, Bernard V, Moss T, Marini JC, Tudawe T, Seviour EG, San Lucas FA, Alvarez H, Gupta S, Maiti SN, Cooper L, Peehl D, Ram PT, Maitra A, Nagrath D. Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. eLife 5: e10250, 2016. doi: 10.7554/eLife.10250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jansen F, Li Q, Pfeifer A, Werner N. Endothelial- and immune cell-derived extracellular vesicles in the regulation of cardiovascular health and disease. JACC Basic Transl Sci 2: 790–807, 2017. doi: 10.1016/j.jacbts.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cross JL, Kott K, Miletic T, Johnson P. CD45 regulates TLR-induced proinflammatory cytokine and IFN-beta secretion in dendritic cells. J Immunol 180: 8020–8029, 2008. doi: 10.4049/jimmunol.180.12.8020. [DOI] [PubMed] [Google Scholar]
- 50. Ridker PM. Clinician’s guide to reducing inflammation to reduce atherothrombotic risk: JACC review topic of the week. J Am Coll Cardiol 72: 3320–3331, 2018. doi: 10.1016/j.jacc.2018.06.082. [DOI] [PubMed] [Google Scholar]
- 51. Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Taubert K, Tracy RP, Vinicor F; American Centers for Disease Control and Prevention; American Heart Association. Markers of Inflammation and Cardiovascular Disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers of Disease Control and Prevention and the American Heart Association. Circulation 107: 499–511, 2003. doi: 10.1161/01.CIR.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 52. Noren Hooten N, McFarland MH, Freeman DW, Mode NA, Ezike N, Zonderman AB, Evans MK. Association of extracellular vesicle protein cargo with race and clinical markers of mortality. Sci Rep 9: 17582, 2019. doi: 10.1038/s41598-019-53640-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yang Y, Boza-Serrano A, Dunning CJR, Clausen BH, Lambertsen KL, Deierborg T. Inflammation leads to distinct populations of extracellular vesicles from microglia. J Neuroinflammation 15: 168, 2018. doi: 10.1186/s12974-018-1204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tanabe K, Matsushima-Nishiwaki R, Yamaguchi S, Iida H, Dohi S, Kozawa O. Mechanisms of tumor necrosis factor-α-induced interleukin-6 synthesis in glioma cells. J Neuroinflammation 7: 16, 2010. doi: 10.1186/1742-2094-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bataille R, Klein B. C-reactive protein levels as a direct indicator of interleukin-6 levels in humans in vivo. Arthritis Rheum 35: 982–983, 1992. doi: 10.1002/art.1780350824. [DOI] [PubMed] [Google Scholar]
- 56. Hotamisligil GS, Erbay E. Nutrient sensing and inflammation in metabolic diseases. Nat Rev Immunol 8: 923–934, 2008. doi: 10.1038/nri2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Heiston EM, Ballantyne A, La Salvia S, Musantee L, Erdbrugger U, Malin SK. Acute exercise decreases insulin-stimulated extracellular vesicles in conjunction with augmentation index in adults with obesity. J Physiol 26, 2022. doi: 10.1113/JP282274. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table: https://doi.org/10.6084/m9.figshare.20045723.v1.
Data Availability Statement
Some or all datasets generated and analyzed during the current study are not publicly available but are available from the corresponding author (S.K.M.) on reasonable request.