Keywords: chemotherapy, macrophages, monocytes, muscle repair, skeletal muscle
Abstract
5-Fluorouracil (5FU) remains a first-line chemotherapeutic for several cancers despite its established adverse side effects. Reduced blood counts with cytotoxic chemotherapies not only expose patients to infection and fatigue, but can disrupt tissue repair and remodeling, leading to lasting functional deficits. We sought to characterize the impact of 5FU-induced leukopenia on skeletal muscle in the context of remodeling. First, C57BL/6 mice were subjected to multiple dosing cycles of 5FU and skeletal muscle immune cells were assessed. Second, mice given 1 cycle of 5FU were subjected to 1.2% BaCl2 intramuscularly to induce muscle damage. One cycle of 5FU induced significant body weight loss, but only three dosing cycles of 5FU induced skeletal muscle mass loss. One cycle of 5FU reduced skeletal muscle CD45+ immune cells with a particular loss of infiltrating CD11b+Ly6cHi monocytes. Although CD45+ cells returned following three cycles, CD11b+CD68+ macrophages were reduced with three cycles and remained suppressed at 1 mo following 5FU administration. One cycle of 5FU blocked the increase in CD45+ immune cells 4 days following BaCl2; however, there was a dramatic increase in CD11b+Ly6g+ neutrophils and a loss of CD11b+Ly6cHi monocytes in damaged muscle with 5FU compared with PBS. These perturbations resulted in increased collagen production 14 and 28 days following BaCl2 and a reduction in centralized nuclei and myofibrillar cross-sectional area compared with PBS. Together, these results demonstrate that cytotoxic 5FU impairs muscle damage repair and remodeling concomitant with a loss of immune cells that persists beyond the cessation of treatment.
NEW & NOTEWORTHY We examined the common chemotherapeutic 5-fluorouracil’s (5FU) impact on skeletal muscle immune cells and skeletal muscle repair. 5FU monotherapy decreased body weight and muscle mass, and perturbed skeletal muscle immune cells. In addition, 5FU decreased skeletal muscle immune cells and impaired infiltration following damage contributing to disrupted muscle repair. Our results demonstrate 5FU’s impact on skeletal muscle and provide a potential explanation for why some patients may be unable to properly repair damaged tissue.
INTRODUCTION
It is estimated that over 275,000 patients with cancer receive 5-fluorouracil (5FU) each year with some patients experiencing debilitating or even fatal toxicities (1). 5FU either alone or in combination treatment, is a first-line chemotherapy classically used to treat colon, breast, head and neck, and pancreatic cancers (2). Although cardiotoxicity is the primary cause of death, other striated muscles, and nonmalignant cells, are susceptible to 5FU’s toxicities contributing to poor treatment outcomes, reduced dose intensity (RDI), and lower life quality (3–6). Moreover, 5FU is an antimetabolite that, in addition to its anticancer properties, can induce severe cytopenia that can exacerbate fatigue, increase risk for infection, and impair tissue repair/recovery (7–10). Regimented exercise has emerged as a potential therapy to offset a number of chemotherapy-induced side effects (11, 12); however, there are significant barriers that remain as clinical trials have failed to show consistent benefits (13, 14). This is likely due, at least in part, to a lack of understanding of the impact of chemotherapy on skeletal muscle’s ability to respond to an exercise stimuli. Developing a better understanding of the off-target effects of chemotherapies and their impact on physiology continues to be an important public health need.
Skeletal muscle immune cells play a pivotal role in skeletal muscle repair and remodeling observed typically following damage, regardless of the insult type (e.g., exercise or injury) (15). Predominately myeloid-derived, each cell type (e.g., neutrophil, monocyte/macrophage) plays a unique role in repair and remodeling based on the type and extent of the injury as well as the duration. Although CD11b+Ly6g+ neutrophils are typically the first responders to damage, they are in low abundance in healthy muscle given their inflammatory and catabolic properties (15). Resident skeletal muscle monocytes are more common and are classically characterized as CD11b+Ly6cLo monocytes and/or CD68+CD11c−CD206− (quiescent—M0) macrophages (16, 17). These cells help maintain the inflammatory microenvironment and are susceptible to intrinsic and external stimuli—upon which they can become polarized. In addition to the resident cells, CD11b+Ly6CHi monocytes are recruited by chemokines/myokines from the bone marrow to the muscle (18, 19). Once extravasated from circulation, these cells can either remain CD11b+Ly6CHi or differentiate into CD11b+CD68+CD11c+CD206− proinflammatory, prophagocytic (M1-like) macrophages to aid in the removal of damaged tissue (20, 21). These M1-like macrophages will then downregulate CD11c expression and increase CD206 to reflect a more anti-inflammatory, profibrotic (M2-like), and resolving macrophage that helps remodel the extracellular matrix (ECM) and downregulate the proinflammatory environment (22–24). The introduction of infiltrating monocytes/macrophages will serve to transition away from the nonspecific innate neutrophil activity to coordinate the later stages of repair (17).
Proper balance and maintenance of these immune cell phenotypes is required for appropriate skeletal muscle remodeling and repair. We have previously demonstrated that 5FU-induced cytopenia extends beyond circulation to impact skeletal muscle immune cells (4); however, the duration of these effects and physiological significance was not examined. Skeletal muscle immune cell depletion has been demonstrated to delay recovery, disrupt ECM remodeling, increase fibrosis, and promote metabolic homeostatic imbalance (25–28). Although 5FU monotherapy’s ability to induce cachexia has not been demonstrated (5, 29), its cytopenic effects have been well established (30–35). In addition, chemotherapy (doxorubicin) has been shown to blunt the inflammatory response following muscle damage (36). The overall purpose of the current study was to examine the impact of 5FU on immune cells and skeletal muscle repair and remodeling. We hypothesized that 5FU would deplete skeletal muscle monocytes sufficient to disrupt skeletal muscle repair leading to aberrant skeletal muscle remodeling. Our results demonstrate that three cycles of 5FU monotherapy was sufficient to induce muscle mass loss that was not fully returned 1 mo following recovery. In addition, we show that skeletal muscle immune cells are differentially lost following one or three cycles of 5FU and one cycle of 5FU is sufficient to disrupt skeletal muscle repair leading to myofibrillar cross sectional area (mCSA) loss and increased collagen formation. Together, these results demonstrate that chemotherapy disrupts the physiological response to damage and may provide a mechanistic explanation for why some patients with cancer may not properly respond to certain exercise stimuli.
METHODS
Animals
Male C57BL/6 mice were purchased from Jackson Laboratories at 4 wk of age and housed in the Department of Laboratory Animal Resources at the University of South Carolina. Mice were group housed (n = 3–5/cage) and maintained on a 12:12-h light-dark cycle at 22°C. Animals were placed on a purified AIN-76A (BioServ, Frenchtown, NJ; Catalog No. F1515) diet for 5 wk before any experimental procedures. Body weights were measured daily, and animals were monitored for signs of distress. Animals were given food and water ad libitum throughout the duration of the study. Food weights were measured daily and used to assess daily food intake for each cage. All animals were fasted for 5 h before tissue collection. Mice were anesthetized using 2% isoflurane with 2 L/min O2 and hindlimb muscles and select organs were carefully dissected, weighed, and either snap-frozen in liquid nitrogen or placed in the appropriate buffers for flow cytometry analysis. Hindlimb muscles included the soleus (Sol), plantaris (Plan), gastrocnemius (Gas), extensor digitorum longus (EDL), tibialis anterior (TA), and the rectus femoris (RF). The RF was teased away from the vastus medialis, lateralis, and intermedius from the excised quadriceps to obtain a reliable weight. Organs included spleen, liver, kidney, heart, and the visceral gonadal fat pad. All animal experiments were approved by the University of South Carolina’s Institutional Animal Care and Use Committee.
Experimental Design
Experiment 1.
Male mice (n = 36) were purchased from Jackson laboratories at 4 wk of age, acclimated to the new facilities for 5 wk, and given a purified AIN-76A diet for 5 wk before the start of the study. At 14 wk of age mice were randomized into four groups, Control (n = 12), one cycle 5FU (n = 8), three cycles 5FU (n = 8), and three cycles 5FU with 1 mo recovery (n = 8). 5FU was solubilized in warmed PBS at 3.5 mg/mL and administered to the mice at 35 mg/kg ip once daily for 5 days (1 cycle). This dosing regimen has been previously shown to be comparable with clinical doses and recapitulates one cycle of chemotherapy (37, 38). Mice receiving multiple cycles were given 9 days rest between cycles (3 cycles). Control mice received PBS injections. Tissue was collected and the animals were euthanized 48 h following the final 5FU/PBS injection (1 cycle/3 cycles) or 1 mo following (Recovery). PBS mice were randomized and taken with each time point (n = 4/group—12 total). No differences were observed in any measured outcome across PBS time points and therefore collapsed into one group (PBS).
Experiment 2.
Mice were acquired and housed similar to experiment 1. Mice were then randomized into PBS or one cycle of 5FU. On day 5, 4 h after the last PBS/5FU injection, mice were anesthetized with 2% isoflurane with 2 L/min O2, hindlimb shaved, and given a ∼50 μL of 1.2% BaCl2 intramuscularly into the RF. Mice were maintained on a heating pad until ambulatory. Mice were euthanized 18 h (n = 4/group), 96 h (n = 4 PBS, n = 6 5FU), 14 days (n = 7/group), and 28 days (n = 3/group) following BaCl2-induced muscle damage. The damaged quadriceps and contralateral undamaged quadriceps were excised, weighed, and either snap-frozen (14 and 28 day) for collagen gene analysis (col3a1 and col1a1) and histology (hematoxylin and eosin, H&E and immunofluorescence, IF) or placed in the appropriate solutions for flow cytometry (18 and 96 h) and electron microscopy (96 h and 14 days). Using contralateral controls for each outcome increased our power and reduced the total animal needed for each outcome.
Blood Analysis
Blood was collected at euthanasia via the inferior vena cava, placed in an EDTA-coated vacutainer (VWR, Suwanee, GA; Catalog No. 454428) and stored briefly on ice until analysis. A complete blood count was performed using the VetScan HMT (Abaxis, Union City, CA) for determination of white blood cells (WBCs), lymphocytes (LYM), monocytes (MON), neutrophils (NEU), red blood cells (RBCs), hemoglobin (HGB), hematocrit (HCT), and platelets (PLTs) (Supplemental Fig. S1).
Flow Cytometry
The quadriceps were excised, minced in Dulbecco’s modified Eagle medium (DMEM), and cells were extracted using the skeletal muscle dissociation kit (Miltenyi Biotec, Auburn, CA; Cat. No. 130-098-305) following the manufacturer’s instruction as previously described (4). Using the entire quadriceps (vastus lateralis, medialis, intermedius, and rectus femoris—∼200 mg) provides a sufficient number of cells for each analysis without pooling animals. After isolation, live/dead cell staining was completed using ZombieGreen (BioLegend, San Diego, CA) according to the manufacturer’s instruction. Cells were then suspended in flow buffer (5% FBS, 100 mM HEPES, 2 mM EDTA in PBS), washed with PBS, resuspended in flow buffer, and blocked with Fc-block against CD16 and CD32 (Cat. No. 101302). Cells were then incubated with fluorescently labeled antibodies (BioLegend; 1:100) against CD45 (PE/Cy7; Cat. No. 103114), CD11b (APC; Cat. No. 101212), Ly6c (PerCP/Cy5.5; Cat. No. 128012), CD68 (APC/Cy7; Cat. No. 137024), Ly6g (PE; Cat. No. 127607), CD11c (PerCP/Cy5.5; Cat. No. 117328), or CD206 (PE; Cat. No. 141706). Cells were measured using a FACS Aria II and analyzed using FlowJo V10.6.2 (BD Biosciences, Ashland, OR). Prior to cellular analysis, all colors were compensated using Invitrogen UltraComp eBeads Compensation Beads (Life technologies, Carlsbad CA). A total of 106 cells were analyzed for each muscle.
Skeletal Muscle Morphology
The rectus femoris was isolated 14 and 28 days following BaCl2. The muscle was cut ∼1 mm proximal to the midbelly and immediately snap-frozen in liquid nitrogen with Optimum Cutting Temperature (OCT) embedding medium placed on the most distal portion of the RF. Muscles were then placed in −80°C overnight before being acclimated to the cryostat maintained at −24°C. Transverse sections (10 μm) were then cut up to the midbelly at which cryosections were placed on Fisherbrand Superfrost Plus Microscope Slides and then stored at −80°C again until staining. After confirming proper fiber orientation, no more than four slides were taken with two to three serial sections per slide.
Slides used for morphology were then fixed in ice-cold acetone and hematoxylin and eosin (H&E) staining was completed as previously described (39). Images of H&E stains were taken at ×20 and ×40 magnification using a Keyence BZX800 microscope. Presence of noncontractile tissue was observed and centralized nuclei were quantified. Nuclei with no margins in contact with the extracellular matrix were considered centralized. mCSA was measured using ImageJ by measuring the circumference of 500–700 myofibers/mouse (n = 3/group).
Slides used for immunofluorescence (IF) were air-dried and sections were circled with hydrophobic pen. Whole slides were then fixed in ice-cold acetone for 3 min, washed three times with PBS at room temperature, and blocked against nonspecific binding with 10% goat serum in 5%BSA/PBS for 1 h. Primary FITC conjugated F4/80 (macrophages) antibody (Bio-Rad, Hercules CA; Cat. No. MCA497F) was diluted 1:75 in PBS and dropped onto sections within hydrophobic pen and sections were incubated overnight (>12 h) in a dark humid chamber at 4°C. Slides were then washed with PBS three times before being incubated with primary Texas Red-X conjugated Wheat Germ Agglutinin (WGA; lectins/ECM) diluted 1:200 in PBS (Invitrogen, Cat. No. W21405). Again, slides were washed with PBS three times before counterstaining with DAPI (nuclei) for 5 min. Slides were then washed a final three times before mounting with a glycerol mounting solution. Pictures were taken at ×40 using a Leica DM2500 fluorescent microscope. Slides from each condition were completed in tandem.
RNA Isolation and RT-PCR
The RF was isolated 14 and 28 days following BaCl2 and snap-frozen in liquid nitrogen. RNA isolation was then completed as previously described (40). Briefly, RNA was extracted from the RF using the TRIzol/isopropanol/chloroform procedure (Life Technologies, Gibco-BRL, Carlsbad, CA). RNA sample quality and quantities were verified using a Nanodrop One Microvolume UV-Vis Spectrophotometer (Thermo Fisher Scientific, Waltham, MA) and determined to be of good quality based on A260/A280 values (>1.8) before cDNA synthesis using High-capacity Reverse Transcriptase kit (Applied Biosystems, Foster City, CA). Probes for collagen genes, col3a1 (Mm00802300_m1) and col1a1 (Mm0080166_g1), and housekeeping gene 18s (Mm04277571_s1) were purchased from Applied Biosystems. 18s was not different across groups and 18s CT value standard deviation was 0.25. Data were analyzed using the 2−ΔΔCT method.
Electron Microscopy
As previously stated, the RF was isolated 96 h and 14 days following BaCl2 and the muscle was cut ∼1 mm from the midbelly. The distal portion was used for morphology and IF, whereas the remaining proximal portion was placed in 2.5% glutaraldehyde at room temperature for either transmission (96 h) or scanning (14 days) electron microscopy. For transmission electron microscopy (TEM), the tissue was excised and initially fixed in 2.5% EM-grade glutaraldehyde followed by a secondary fixation in 1% osmium tetroxide and 1.5% K+ ferricyanide. After dehydration with increasing concentrations of ethanol, the samples were transferred to acetonitrile and embedded in Polybed 812. Ultrathin sections were cut with a Leica Reichert Ultracut R ultramicrotome (Leica Microsystems GmbH, Wetzlar, Germany), collected on copper grids and stained with uranyl acetate and lead citrate. A JEOL 1400+ transmission electron microscope (JEOL USA, Peabody, MA) was used to view sections, and digital images were obtained with an Advanced Microscopy Techniques XR 81 Camera (Advanced Microscopy Techniques, Woburn, MA). For scanning electron microscopy (SEM), the tissue was excised and initially fixed in 2.5% EM-grade glutaraldehyde followed by a secondary fixation in 1% osmium tetroxide, and then dehydrated with increasing concentrations of ethanol. Tissue was then critical point dried using the Samdri-PVT-3B (Tousimis, Rockville, MD). Tissue was grossly broken up and Au sputter coated (Cressington Sputter Coater 108auto). Images at ×1,000 and ×5,000 were then collected using JEOL JSM-IT100 scanning electron microscope. Images were obtained through nonspecific searching for adjacent parallel fibers to examine the ECM and collagen abundance.
Statistics
Values are presented as means ± standard error of the mean (SEM). Quantifiable data were graphed using Prism GraphPad (San Diego, CA) and statistical analysis was run with IBM SPSS (Armonk, NY). Power analyses were performed using G*Power (Düsseldorf, Germany). One-way ANOVAs were performed to determine the differences between PBS and multiple cycles of 5FU. A two-way ANOVA was used to determine differences between 5FU and PBS when given BaCl2 or sham. Post hoc analyses were performed with a Tukey multiple-comparisons test. A Bartlett’s test was used to assess the normality of the data and determine significantly different standard deviations. Significance was set at P ≤ 0.05.
RESULTS
Animal Characteristics
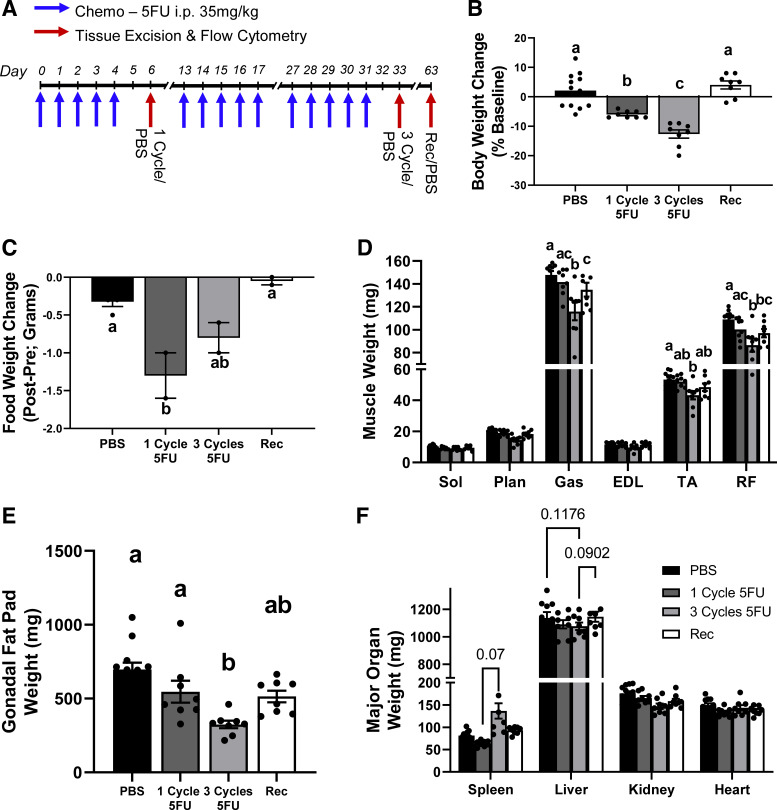
In experiment 1, mice underwent either one cycle of 5FU, three cycles of 5FU, or three cycles of 5FU and were allowed 30 days to recover (Fig. 1A). Mice exhibited significant body weight loss after one cycle (P = 0.0015), and even greater body weight loss after three cycles (P < 0.0001), which was returned following a 1 mo recovery (Fig. 1B). This was accompanied with a loss of food intake following one cycle (P = 0.01) but was not statistically different from PBS following three cycles or recovery (Fig. 1C). Although one cycle of 5FU did not demonstrate differences in any skeletal muscle weights, mice that underwent three cycles of 5FU had lower gastrocnemius (P < 0.0001; Gas), tibialis anterior (P = 0.04; TA), and rectus femoris (P < 0.0001; RF) weights compared with PBS controls (Fig. 1D). Recovery mice had greater Gas weight compared with three cycles (P < 0.0001) but was still smaller compared with PBS control (P = 0.004). Recovery mice did not have significantly different TA weights compared with all groups. Interestingly, Recovery mice had lower RF weights compared with PBS (P = 0.0097) and were not different from one or three cycle (Fig. 1D). There was an observed loss of gonadal fat weight only after three cycles of 5FU compared with PBS (P < 0.0001) and one cycle (P = 0.028), whereas Recovery mice were not statistically different from any group (Fig. 1E). No statistical differences were observed in major organ weights (Fig. 1F).
Figure 1.
Experimental design and animal characteristics. A: 5-fluorouracil (5FU) was solubilized in phosphate-buffered saline at 3.5 mg/mL and administered to the mice at 35 mg/kg via intraperitoneal injection once daily for 5 days (1 cycle), 9 day rest and five additional daily injections, another 9 day rest, with a final five injections (3 cycles) and allowed to recover for 30 days (Recovery). PBS controls were taken with each time point (n = 4/group—12). B: relative body weight change shown as the % change from day 0 to the end of each treatment period. C: daily food intake change shown as the % change from day 0 to the end of each treatment period. D: select hindlimb muscle weights given in milligrams (mg) at euthanasia from each group. E: gonadal fat pad weight given in mg at euthanasia from each group. F: select organ weights in mg at euthanasia from each group. PBS n = 12, one cycle n = 8, three cycles n = 8, and Recovery n = 8; n represents number of animals. Significance was set at P < 0.05. Different letter indicates significant different groups when an interaction was observed. One-way ANOVA.
The Effect of Multiple Dosing Cycles of 5FU on Skeletal Muscle Immune Cells
Cells isolated from the quadriceps were gated to obtain live (ZombieGreen−/Lo) single cells (SSC-A and FSC-A singlets). Mice that received one cycle of 5FU had a lower number of CD45+ immune cells (gated from live single cells) compared with PBS (P = 0.036) controls as previously observed (Fig. 2A) (24). Interestingly, immune cells were not different following three cycles of 5FU or in the Recovery mice compared with PBS controls (Fig. 2A); however, the total number of CD45+ immune cells were less following three cycles compared with PBS (P < 0.0001) and one cycle (P = 0.007; Table 1). Immune cells were then gated with CD11b (myeloid cells) and Ly6c (resident/infiltrating monocytes) (Fig. 2B). A dramatic loss of CD11b+Ly6cHi infiltrating monocytes was observed following one cycle (P < 0.0001) and three cycles (P = 0.0085) of 5FU compared with PBS; however, Recovery mice were not different from PBS controls (Fig. 2B). One cycle of 5FU had less CD11b+Ly6cInt cells compared with PBS controls (P = 0.0066); however, three cycles and recovery were not different from all groups. As expected then, one cycle of 5FU had more CD11b+Ly6cLo cells compared with PBS controls (P = 0.021). Three cycles of 5FU had a lower abundance of CD11b+Ly6cLo compared with one cycle (P < 0.0001) and recovery (P = 0.016) but was not different from PBS (Fig. 2B).
Figure 2.
The effects of 5-fluorouracil (5FU) on skeletal muscle immune cells. Cells were gated for nondebris (SSC-A × FSC-A), FSC singlets (FSC-W × FSC-H), SSC singlets (SSC-W × SSC-H), and Live ZombieGreen−/Low. A: live single cells were then gated with SSC-A and CD45 for immune cells and the relative abundance of CD45+ cells as % of live cells were graphed. B: CD45+ immune cells were then gated with CD11b and Ly6c and the relative abundance of CD11b+Ly6cLo/Int/Hi as a % of CD45+ immune cells were graphed. C: CD45+ immune cells were then gated with CD11b and CD68 for total macrophages and the relative abundance of CD11b+CD68+ as a % of CD45+ immune cells were graphed. D: CD11b+CD68+ macrophages were gated with CD11c and CD206. CD11c−CD206− cells were considered M0-like macrophages, CD11c+CD206− cells were considered M1-like macrophages, CD11c−CD206+ cells were considered M2-like macrophages, and CD11c+CD206+ cells were considered M1-M2-like transitional macrophages. The relative abundance of each cell subset as a % of CD11b+CD68+ macrophages were graphed. PBS n = 12, one cycle n = 8, three cycles n = 8, and Recovery n = 8; n represents number of animals. Significance was set at P < 0.05. Different letter indicates significant different groups when an interaction was observed. Two-way ANOVA.
Table 1.
Total immune cells counts
| PBS | One Cycle 5FU | Three Cycles 5FU | Recovery | ||
|---|---|---|---|---|---|
| CD45+ Immune Cells | 3720 ± 315a | 2745 ± 275b | 1346 ± 154c | 2342 ± 181cb | |
| > | CD11b+CD68+ Macrophages | 1912 ± 198a | 1422 ± 166a | 469 ± 145b | 580 ± 87b |
| >> | M1-Like CD206−CD11c+ | 127 ± 18a | 46 ± 10b | 32 ± 4b | 44 ± 6b |
| >> | M1-2 CD206+CD11c+ | 280 ± 50a | 174 ± 25ab | 44 ± 18b | 64 ± 9b |
| >> | M2-Like CD206+CD11c− | 1291 ± 151a | 1067 ± 145a | 360 ± 118b | 380 ± 66b |
| >> | M0 Naïve CD206−CD11c− | 214 ± 25a | 135 ± 12b | 33 ± 8c | 92 ± 12bc |
| > | CD11b+Ly6cHi | 223 ± 51a | 3 ± 1b | 59 ± 11b | 86 ± 17b |
| > | CD11b+Ly6cInt | 567 ± 88a | 207 ± 83b | 234 ± 26b | 392 ± 40ab |
| > | CD11b+Ly6cLo | 1227 ± 195a | 608 ± 178bc | 221 ± 81c | 1136 ± 114ab |
Values are represented as means ± SE. 1 × 106 cells isolated from the quadriceps from PBS, one cycle, three cycles, and Recovery mice. Single cells were gated for ZombieGreen−/Lo and total cell counts for each immune cell population are shown. Arrows indicate gating depths. One-way ANOVA with a Tukey post hoc multiple comparisons. Different letters indicate statistically different groups. Significance was set at P < 0.05.
Immune cells were also gated with CD11b and CD68 to examine relative abundance of macrophages (Fig. 2C). Although 1 cycle of 5FU did not change the relative abundance of CD11b+CD68+ macrophages, both three cycles (P = 0.0052) and Recovery (P < 0.0001) mice had less macrophages than one cycle and PBS mice (Fig. 2C). A similar pattern was observed when examining total number of CD11b+CD68+ macrophages (Table 1). CD11b+CD68+ macrophages were then gated with CD11c and CD206 to examine macrophage polarization (Fig. 2D). One cycle (P = 0.028) and three cycles (P = 0.008) of 5FU had a lower relative abundance of M1-like CD11c+CD206− macrophages compared with PBS (Fig. 2D). The relative abundance of M1-like CD11c+CD206− macrophages were not different in Recovery mice compared with PBS but were greater when compared with one (P = 0.0007) and three (P = 0.0002) cycles; however, the total number of cells was less in one cycle (P = 0.0005), three cycles (P < 0.0001), and Recovery (P = 0.0004) compared with PBS (Table 1). There were no observed changes in relative abundance of M0 CD11c−CD206− Naïve or M2-like CD11c−CD206+ macrophages (Fig. 2D); however, total M0 macrophages were lower in one cycle (P = 0.023) and Recovery (P = 0.0002) mice compared with PBS and further lessened in three cycles compared with one cycle (P = 0.0054) and PBS (P < 0.0001; Table 1). Total M2-like macrophage counts were less in three cycles (P < 0.0001) and Recovery (P = 0.0001) mice compared with PBS and one cycle (Table 1). The relative abundance of M1-2 CD11c+CD206+ transitional macrophages was less in three cycles (P = 0.0014) and Recovery (P = 0.003) mice compared with PBS and three cycles (P = 0.03) had less M1-2 macrophages when compared with one cycle (Fig. 2D). The total M1-2 macrophages were lower in three cycles (P = 0.0002) and Recovery (P = 0.0008) mice compared with PBS (Table 1).
5FU and Infiltrating Skeletal Muscle Immune Cells following Damage
To examine the potential physiological relevance of 5FU-induced skeletal muscle leukopenia, we subjected mice that received 5FU or PBS to 1.2% BaCl2 intramuscularly into the RF to examine the infiltration of immune cells and morphology changes (Fig. 3A) (41). Sagittal sections of the damaged and undamaged RF 4 days following 1.2% BaCl2 exhibit damaged myofibrils, mild to severe sarcomeric disruption, and disorganized z-disk alignment as seen with other damage models (Fig. 3B) (42). Cells isolated from the quadriceps were gated to obtain live (ZombieGreen−/Lo) single cells (SSC-A and FSC-A singlets) similar to experiment 1 (Fig. 2). CD45+ immune cell abundance was greater in BaCl2-induced damaged muscle only in PBS controls at 96 h (P < 0.0001; Fig. 3, C and F). Both PBS- and 5FU-treated mice had a greater abundance of CD11b+CD68+ macrophages at 18 h and 96 h following BaCl2 (Main effect: P < 0.0001; Fig. 3, D and G). Both PBS and 5FU had less M1 (PBS: P = 0.05; 5FU: P = 0.01), M2 (PBS: P < 0.0001; 5FU: P < 0.0001), and M1-2 (PBS: P = 0.017; 5FU: P = 0.0177) macrophages 18 h following BaCl2 (Fig. 3, E, H, I, and J) with 95%–100% of cells being M0 at this time point in both groups (Fig. 3K). Interestingly, M1-like macrophage abundance was greater in damaged muscle 96 h following BaCl2 only in PBS (P = 0.002) mice (Fig. 3H). M1-like macrophage abundance was greater in 5FU at 96 h compared with 18 h (P = 0.014) but were not different compared with undamaged controls and were lower than PBS at 96 h (P = 0.0003; Fig. 3H). M1-2 transitional macrophage abundance was greater in damaged muscle 96 h following BaCl2 only in PBS (P = 0.024; Fig. 3I). M1-2 macrophage abundance was less in 96 h following BaCl2 in 5FU (P = 0.015) compared with undamaged controls and were not different from 5FU at 18 h (Fig. 3I). M2-like macrophage abundance was greater in damaged muscle 96 h following BaCl2 in PBS controls compared with PBS at 18 h (P < 0.0001) and 5FU at 96 h (P < 0.0001; Fig. 3J). M2-like macrophage abundance was less in 96 h following BaCl2 in 5FU compared with undamaged controls (P < 0.0001) but were not different from 5FU at 18 h (Fig. 3J). M0 naïve macrophage abundance was less in damaged muscle 96 h following BaCl2 in PBS controls compared with 5FU at 96 h (P < 0.0001) and PBS at 18 h (P < 0.0001; Fig. 3K). M0 naïve macrophage abundance was greater in 96 h following BaCl2 in 5FU compared with undamaged controls (P < 0.0001) but was not different from 5FU at 18 h (Fig. 3K).
Figure 3.
The impact of 5-fluorouracil (5FU) on skeletal muscle macrophages following BaCl2-induced muscle damage. A: experiment 2 experimental design. 5FU was solubilized in phosphate-buffered saline at 3.5 mg/mL and administered to the mice at 35 mg/kg via intraperitoneal injection once daily for 5 days. Mice were then subjected to intramuscular injection of 1.2% barium chloride (BaCl2) and tissue was collected at 18 h, 96 h, 14 days, and 28 days following BaCl2. B: transmitted electron microscopy (TEM) images taken at 96 h following BaCl2 in mice given PBS or 5FU to verify muscle damage. Scale bar = 600 nm. C: single live cells isolated from undamaged (und) 18 hours (h) post BaCl2 and 96 h post BaCl2 were gated with SSC-A and CD45 for immune cells. D: CD45+ immune cells were then gated with CD11b and CD68 for total macrophages. E: CD11b+CD68+ macrophages were gated with CD11c and CD206 to examine macrophage polarization. CD11c−CD206− cells were considered M0-like macrophages, CD11c+CD206− cells were considered M1-like macrophages, CD11c−CD206+ cells were considered M2-like macrophages, and CD11c+CD206+ cells were considered M1-M2-like transitional macrophages. F: the relative abundance of CD45+ cells as % of live cells. G: the relative abundance of CD11b+CD68+ as a % of CD45+ immune cells. H–K: the relative abundance of each macrophages cell subset as a % of CD11b+CD68+ macrophages. PBS undamaged n = 4, 5FU undamaged n = 6, PBS at 18 h n = 4, 5FU at 18 h n = 4, PBS at 96 h n = 4, 5FU at 96 h n = 6. Significance was set at P < 0.05. Different letter indicates significant different groups when an interaction was observed. $Significant main effect of damage. Two-way ANOVA.
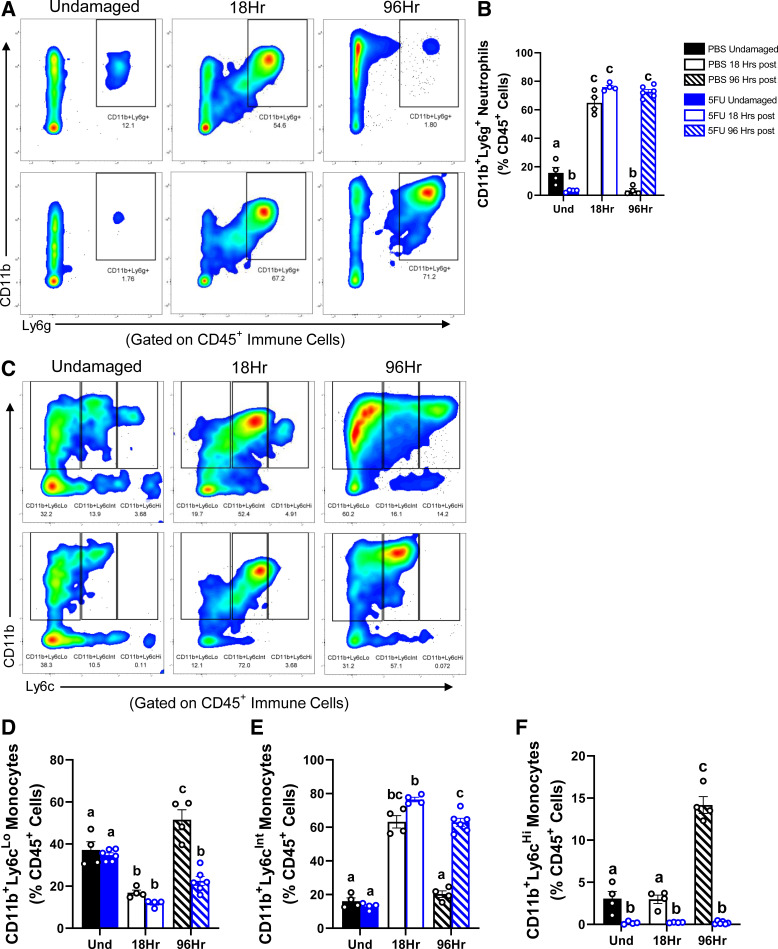
We then sought to examine other myeloid-derived immune cells, Ly6g+ neutrophils (Fig. 4A) and Ly6c monocytes (Fig. 4C). Although CD11b+Ly6g+ neutrophils abundance was less in the 5FU undamaged muscle compared with PBS undamaged muscle (P = 0.02), there was a greater abundance of neutrophils at 18 h following BaCl2 in both PBS (P < 0.0001) and 5FU (P < 0.0001). Interestingly, although neutrophil abundance was less at 96 h following BaCl2 in PBS compared with undamaged (P = 0.02) and PBS at 18 h (P < 0.0001), neutrophil abundance was greater at 96 h following BaCl2 in 5FU compared with to PBS at 96 h (P < 0.0001) and undamaged but were not different from at 18 h (Fig. 4, A and B). Interestingly, there was a shift in Ly6c expression at 18 h following BaCl2 to have greater CD11b+Ly6cInt abundance (PBS: P < 0.0001; 5FU: P < 0.0001) and lower CD11b+Ly6cLo abundance (PBS: P = 0.0004; 5FU: P = 0.0006) in both PBS and 5FU (Fig. 4, C–E). CD11b+Ly6cLo monocyte abundance was then greater at 96 h following BaCl2 in PBS mice compared with all groups and time points (P < 0.01 to P < 0.0001), whereas 5FU at 96 h were not different from 5FU at 18 h and were less when compared with undamaged controls (P = 0.009; Fig. 4D). CD11b+Ly6cInt monocyte abundance was less at 96 h following BaCl2 in PBS mice compared with at 18 h (P < 0.0001) but not different from undamaged, whereas 5FU at 96 h was less when compared with 5FU at 18 h (P = 0.0042), but still greater than undamaged controls (P < 0.0001) and PBS at 96 h (P < 0.0001; Fig. 4E). Most strikingly, however, CD11b+Ly6cHi monocyte abundance was greater in PBS at 96 h compared with all groups and time points (P < 0.0001), where 5FU-treated mice had dramatic losses across all time points (P = 0.014, P = 0.02, P < 0.0001; Fig. 4F).
Figure 4.
The impact of 5-fluorouracil (5FU) on skeletal muscle monocyte and neutrophil infiltration following BaCl2-induced muscle damage. A: single live CD45+ immune cells isolated from undamaged (und) 18 hours (h) post BaCl2 and 96 h post BaCl2 were gated with CD11b and Ly6g for neutrophils. B: the relative abundance of CD11b+Ly6g+ neutrophils as a % of CD45+ immune cells were graphed. C: similarly live CD45+ immune cells were gated with CD11b and Ly6c for infiltrating monocytes. D–F: the relative abundance of CD11b+Ly6cLo/Int/Hi as a % of CD45+ immune cells were graphed. PBS undamaged n = 4, 5FU undamaged n = 6, PBS at 18 h n = 4, 5FU at 18 h n = 4, PBS at 96 h n = 4, 5FU at 96 h n = 6; n represents number of animals. Significance was set at P < 0.05. Different letter indicates significant different groups when an interaction was observed. Two-way ANOVA.
5FU and Skeletal Muscle Repair/Remodeling following Damage
We then examined the morphological consequences of 5FU-induced aberrant immune cell infiltration. In PBS mice, skeletal muscle demonstrated more centralized nuclei but a return of myofibrillar structure 14 days following BaCl2-induced injury (P < 0.0001; Fig. 5, A and B). Interestingly, damaged muscle from 5FU mice had less centralized nuclei compared with damaged muscle from PBS mice (P = 0.0003; Fig. 5, A and B), with greater evidence of increased noncontractile tissue when compared with PBS BaCl2. We then examined collagen gene transcription that demonstrated greater col3a1 and col1a1 in PBS and 5FU 14 days following BaCl2 with even higher expression in 5FU compared with PBS in col3a1 transcription (P = 0.001; Fig. 5, C and D). This was further demonstrated by more wheat germ agglutinin (WGA) staining in 5FU BaCl2 without any observable F4/80+DAPI+ overlap via IF that was present in PBS BaCl2 (Fig. 5E).
Figure 5.
The impact of 5-fluorouracil (5FU) on skeletal muscle regeneration and remodeling 14 days following BaCl2-induced muscle damage. A: representative hematoxylin and eosin (H&E) stain rectus femoris muscles from control and damaged skeletal muscle in mice given 5FU or PBS 14 days after insult. Scale bars = 50 µm. B: number of centralized nuclei per 100 fibers measured. col3a1 (C) and col1a1 (D) gene expression in the rectus femoris 14 days following BaCl2. Data were normalized to PBS undamaged. E: representative immunofluorescent ×40 images of wheat germ agglutinin (WGA), macrophages (F4/80), and nuclei (DAPI) in rectus femoris muscle from control and damaged skeletal muscle in mice given 5FU or PBS 14 days after insult. Scale bar = 50 µm. PBS undamaged n = 7, 5FU undamaged n = 7, PBS 14 at days n = 7, 5FU at 14 days n = 7. n = 3/group histology and n = 4/group gene expression; n represents number of animals. Significance was set at P < 0.05. Different letter indicates significant different groups when an interaction was observed. $Significant main effect of damage. Two-way ANOVA.
We then sought to examine morphology 28 days following BaCl2 at which point morphology should resemble an undamaged muscle with centralized nuclei (41). As expected, PBS mice had more centralized nuclei compared with undamaged with a return to normal morphological structure (P < 0.0001; Fig. 6, A and B). Similar to 14 days, 5FU treated mice had a less centralized nuclei compared with PBS BaCl2 (P = 0.0001) with evidence of increased noncontractile tissue and smaller fibers (Fig. 6, A and B). To follow-up with this observation, fiber size and distribution was examined and although PBS mice return to their mean mCSA 28 days following BaCl2, 5FU-treated mice had dramatically smaller mCSA (P = 0.0003) with a leftward shift in fiber distribution (Fig. 6, C and D). In addition, given the visible evidence of increased noncontractile tissue 28 days following BaCl2 in 5FU-treated mice, we examined gene expression of abundant muscle collagens col3a1 and col1a1. There was an increase in both col3a1 and col1a1 28 days following BaCl2 in both PBS and 5FU, with greater increases occurring in 5FU treatment mice (Col3a1: p = 0.009, Col1a1: P = 0.035; Fig. 6, E and F).
Figure 6.
The impact of 5-fluorouracil (5FU) on skeletal muscle regeneration and remodeling 28 days following BaCl2-induced muscle damage. A: representative hematoxylin and eosin (H&E) stain rectus femoris muscles from control and damaged skeletal muscle in mice given 5FU or PBS 28 days after insult. ×20 scale bar = 100 µm, ×40 scale bar = 50 µm. B: number of centralized nuclei per 100 fibers measured. C: fiber size (µm2) distribution in all groups. D: mean myofibrillar cross-sectional area (mCSA) 28 days following BaCl2. col3a1 (E) and col1a1 (F) gene expression in the rectus femoris 28 days following BaCl2. Data were normalized to PBS undamaged. PBS undamaged n = 3, 5FU undamaged n = 3, PBS at 28 days n = 3, 5FU at 28 days n = 3; n represents number of animals. Significance was set at P < 0.05. Different letter indicates significant different groups when an interaction was observed. Two-way ANOVA.
In addition, scanning electron microscopy images demonstrated an increase in the size of collagen bundles with a lack of proper coordination with 5FU BaCl2 compared with PBS BaCl2 (Figs. 7 and 8). Together these results demonstrate impaired regeneration and increased noncontractile tissue deposition following BaCl2-induced skeletal muscle injury in 5FU-treated mice.
Figure 7.
Representative scanning electron micrographs at ×1,000 magnification 14 days following BaCl2-induced muscle damage. A proximal portion of the rectus femoris was subjected to scanning electron microscopy (SEM). Muscles were nonspecifically searched for adjacent parallel fibers to examine the extracellular matrix and collagen fibers. Images were collected at ×1.000. Scale bar = 10 μm. 5FU, 5-fluorouracil; Und, undamaged.
Figure 8.
Representative scanning electron microscopy images at ×5,000 magnification 14 days following BaCl2-induced muscle damage. A proximal portion of the rectus femoris was subjected to SEM. Muscles were nonspecifically searched for adjacent parallel fibers to examine the extracellular matrix and collagen fibers. Images were collected at ×5,000. Scale bar = 5 μm. 5FU, 5-fluorouracil; SEM, scanning electron microscopy; Und, undamaged.
DISCUSSION
Skeletal muscle immune cells have an important role in the maintenance of the skeletal muscle microenvironment during physiological and pathological conditions (17, 43–45). We have previously demonstrated that 5FU acutely reduced skeletal muscle immune cells (4), and chemotherapy has been previously shown to disrupt the physiological response to damage (36). Regimented exercise continues to show efficacy toward improving functional status and life quality of patients with cancer (11, 12); however, barriers remain as clinical trials fail to show consistent benefits (13, 14). In addition, whether skeletal muscle maintains the ability to properly respond to an exercise stimulus during chemotherapy or with significant wasting is still largely unknown. The purpose of this study was to examine the lasting impact of 5FU on skeletal muscle, particularly its effects on immune cells and skeletal muscle repair. Our results demonstrate that multiple cycles of 5FU are sufficient to induce body weight and muscle mass loss. We also demonstrate that one cycle of 5FU induced skeletal muscle immune cell loss and impaired immune cell infiltration following damage, contributing to disrupted remodeling and aberrant deposition of the ECM.
Monocytes, particularly macrophages, are the predominate immune cell within the skeletal muscle microenvironment and serve to coordinate the local inflammatory environment, the removal of damaged materials, and the break down and deposition of collagens in the ECM (15, 17, 43, 46, 47). These functions rely on the macrophage’s phenotype, which while dichotomously described (M1 vs. M2), is flexible and responsive to a variety of cues (48). Although disrupted macrophage polarization has been demonstrated with aged muscle (24, 44, 49, 50), the changes that occur with cancer and cancer treatments are just beginning to be unearthed (4, 51, 52). We previously reported that 5FU induced bone marrow cell cycle arrest, and acutely decreased circulating and skeletal muscle immune cells, with a particular loss of inflammatory or infiltrating monocytes/macrophages (4). The results from the current study extend these findings to demonstrate that the loss of CD45+ total immune cells with one cycle of 5FU is not present after three cycles; however, it is apparent that macrophages are not replenished in the skeletal muscle as a reduction in the relative abundance of CD11b+CD68+ cells remain. This can be explained by the reduction in circulating leukocytes and the reduction in CD11b+Ly6cHi monocytes that is not returned until 1 mo following 5FU treatments. In addition, while we see a return in several relative immune cell populations, it can be appreciated that the absolute number of CD45+ immune cells, CD11b+CD68+ total macrophages, M1-like macrophages, M0 naïve macrophages, CD11b+Ly6cHi monocytes, CD11b+Ly6cInt monocytes, and CD11b+Ly6cLo monocytes were reduced after three cycles and following recovery.
The role of immune cells in skeletal muscle homeostasis is most appreciated with respect to repair, regeneration, and remodeling. Although macrophages coordinate repair, neutrophils (injury specific) (21) are recruited within the first few hours of damage and can induce or exacerbate myofibrillar breakdown to aid in the removal of damaged tissue (17, 53). We demonstrate that 5FU blocks the increase in CD45+ immune cells with a particular loss of CD11b+Ly6cHi monocytes. Unexpectedly, we saw a dramatic increase in CD11b+Ly6g+ neutrophils 4 days following BaCl2 only in 5FU-treated mice demonstrating a prolonged catabolic and atrophic environment. This was further supported by a decrease in the relative number of centralized nuclei 14 days following BaCl2 and an increase in noncontractile tissue and collagen production without an increase in F4/80 positive staining. In addition, these perturbations were not resolved following 28 days as we observed a dramatic loss of mCSA in damaged muscle with mice receiving 5FU and greater increases in collagen production compared with PBS. Together, these results demonstrate that 5FU delays skeletal muscle damage recovery, contributing to increased fibrosis and delayed repair. Future studies should also include time course recovery with a particular emphasis on skeletal muscle function as well as further examine other cell types that contribute to increased fibrosis.
The current study has certain limitations that require discussion. One limitation is the impact that 5FU may have on skeletal muscle satellite cells that was not investigated. It is conceivable that 5FU may block the activation and proliferation of satellite cells leading to impaired recovery (54, 55). In addition, immune cells play a role in satellite cell maintenance that may be perturbed when immune cells are depleted. Investigations delineating the role of satellite cells in these disruptions are an important area of future inquiry that requires specific attention. Another limitation is the translatability of chemical injury (1.2% BaCl2) to induce skeletal muscle damage and the timing chosen to induce damage following 5FU. Although this is an established model of muscle damage to investigate skeletal muscle repair (41), examination of more physiologically translatable damage models should be investigated. Indeed, inflammation induced by downhill running (eccentric contraction-induced muscle damage) was blunted by doxorubicin administration in rats (36). Our results also suggest that some metrics of immune cells and physiology are different between one and three cycles and may return after 1 mo. Examination into the impact of one cycle on chemotherapy on skeletal muscle repair was the first step in understanding the impact of 5FU-induced cytopenia on remodeling. Given the observed altered response to damage following one cycle, follow-up experiments examining damage following these additional time points are needed. It is also important to note that while we observed changes following one cycle of 5FU in experiment 1, these same changes were not consistent in the undamaged leg in experiment 2; however, the damaged muscle reflected the hypothesis gleaned from experiment 1. There are several potential explanations for these discrepancies including not being powered to observe baseline changes and that compensatory changes in the undamaged leg may have occurred following BaCl2. The final limitation is that these experiments were completed in tumor-free mice. Tumor presence may introduce distinct inflammatory changes that may alter the muscle’s physiological response to cytotoxic chemotherapy and damage (56). In addition, certain tumor types elicit different effects on skeletal muscle and importantly require different anticancer therapies. There is an increasing need to understand the differential off-target effects of cancer therapies spanning the cancer and therapy spectrums.
The results from the current study add to the limited body of information regarding the impact of 5FU and 5FU-containing therapies on skeletal muscle. Folfiri, but not Folfox, induce cachexia through mitogen-activated protein kinase (MAPK) activation and disruption of mitochondrial content and quality (29). In contrast, 5FU monotherapy did not induce cachexia; however, 5FU did disrupt skeletal muscle anabolic and inflammatory signaling pathways (5). In addition, few studies have examined cachexia with 5FU in tumor-bearing mice demonstrating that while 5FU and oxaliplatin reduced C26 tumor size this treatment exaggerated muscle weakness (57) and increased neuromuscular junction (NMJ) degeneration (58). Finally, our results demonstrated 5FU monotherapy can induce body weight and muscle mass loss given sustained treatment. Although body weight is returned following recovery, there were signs of sustained perturbations as the Gas and RF did not return to control levels. It is unclear where the increase in body weight following recovery comes from which highlights a need for additional studies and examination into bone loss, adipose distribution, and water volume as these are all potentially impacted by chemotherapy. Independent of cachexia, however, we demonstrate unique disruptions to immune cells within the muscle microenvironment that contribute to impaired repair and remodeling. Again, future inquiry should pay close attention to the remaining aspects of the muscle’s microenvironment (i.e., fibroblasts and satellite cells).
Although more sophisticated anticancer strategies continue to emerge in attempt to provide relief from the toxicities of traditional chemotherapies (59), 5FU has remained a first-line therapy for many patients with cancer. In addition, cancer survivors that have already received 5FU may suffer the lasting off-target effects of this cytotoxic chemotherapy. Therefore, it is of critical importance that we understand the impact of these therapies on host tissues and physiology. In addition to halting tumor cell proliferation, 5FU reduces blood counts, has established cardiotoxicities, promotes oral, and intestinal mucositis, and recently has been demonstrated to perturb skeletal muscles (6, 60, 61). The results from the current study add to the current literature demonstrating 5FU’s impact on skeletal muscle and provide a potential contributor to anabolic resistance often observed with cancer and cachexia (62, 63). Although increasing muscle use has been demonstrated to improve muscle mass maintenance and function with cancer and chemotherapy (64, 65), careful attention should be given to whether the patient has the machinery to properly adapt to any given stimuli.
ETHICS APPROVAL
All animal experiments were approved by the University of South Carolina’s Institutional Animal Care and Use Committee.
SUPPLEMENTAL DATA
Supplemental Fig. S1: https://doi.org/10.6084/m9.figshare.20006174.v1.
GRANTS
This work was supported by the National Institutes of Health, National Center for Complementary and Integrative Health R43AT011171 (to B. N. VanderVeen and K. T. Velazquez), the University of South Carolina’s Office of the Vice President for Research Advanced Support for Innovative Research Excellence (ASPIRE) ASPIRE-I Track IIb (to B. N. VanderVeen) and ASPIRE-II (to E. A. Murphy).
DISCLAIMERS
The funding agency played no part in this projects data collection, analysis, or interpretation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.N.V. and E.A.M. conceived and designed research; B.N.V., T.D.C., S.S.M., S.J.M., and B.M.B. performed experiments; B.N.V., S.S.M., and R.L.P. analyzed data; B.N.V., T.D.C., S.S.M., S.J.M., R.L.P., J.A.C., D.F., and E.A.M. interpreted results of experiments; B.N.V. and T.D.C. prepared figures; B.N.V. drafted manuscript; B.N.V., T.D.C., S.J.M., B.M.B., R.L.P., J.A.C., D.F., and E.A.M. edited and revised manuscript; B.N.V., T.D.C., S.S.M., S.J.M., B.M.B., R.L.P., J.A.C., D.F., and E.A.M. approved final version of manuscript.
REFERENCES
- 1. Ma WW, Saif MW, El-Rayes BF, Fakih MG, Cartwright TH, Posey JA, King TR, von Borstel RW, Bamat MK. Emergency use of uridine triacetate for the prevention and treatment of life-threatening 5-fluorouracil and capecitabine toxicity. Cancer 123: 345–356, 2017. doi: 10.1002/cncr.30321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen Y-J, Ciombor KK, Cohen S, Cooper HS, Deming D, Farkas L, Garrido-Laguna I, Grem JL, Gunn A, Hecht JR, Hoffe S, Hubbard J, Hunt S, Johung KL, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen K, Saltz L. Colon cancer, Version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 19: 329–359, 2021. doi: 10.6004/jnccn.2021.0012. [DOI] [PubMed] [Google Scholar]
- 3. Latchman J, Guastella A, Tofthagen C. 5-Fluorouracil toxicity and dihydropyrimidine dehydrogenase enzyme: implications for practice. Clin J Oncol Nurs 18: 581–585, 2014. doi: 10.1188/14.CJON.581-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. VanderVeen BN, Sougiannis AT, Velazquez KT, Carson JA, Fan D, Murphy EA. The acute effects of 5 fluorouracil on skeletal muscle resident and infiltrating immune cells in mice. Front Physiol 11: 593468, 2020. doi: 10.3389/fphys.2020.593468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Campelj DG, Timpani CA, Cree T, Petersen AC, Hayes A, Goodman CA, Rybalka E. Metronomic 5-fluorouracil delivery primes skeletal muscle for myopathy but does not cause cachexia. Pharmaceuticals (Basel) 14: 478, 2021. doi: 10.3390/ph14050478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sougiannis AT, VanderVeen BN, Davis JM, Fan D, Murphy EA. Understanding chemotherapy-induced intestinal mucositis and strategies to improve gut resilience. Am J Physiol Gastrointest Liver Physiol 320: G712–G719, 2021. doi: 10.1152/ajpgi.00380.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Deptuła M, Zieliński J, Wardowska A, Pikuła M. Wound healing complications in oncological patients: perspectives for cellular therapy. Postepy Dermatol Alergol 36: 139–146, 2019. doi: 10.5114/ada.2018.72585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Malik I, Hussain M, Yousuf H. Clinical characteristics and therapeutic outcome of patients with febrile neutropenia who present in shock: need for better strategies. J Infect 42: 120–125, 2001. doi: 10.1053/jinf.2001.0798. [DOI] [PubMed] [Google Scholar]
- 9. Vardy JL, Dhillon HM, Pond GR, Renton C, Dodd A, Zhang H, Clarke SJ, Tannock IF. Fatigue in people with localized colorectal cancer who do and do not receive chemotherapy: a longitudinal prospective study. Ann Oncol 27: 1761–1767, 2016. doi: 10.1093/annonc/mdw252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mahoney SE, Davis JM, Murphy EA, McClellan JL, Gordon B, Pena MM. Effects of 5-fluorouracil chemotherapy on fatigue: role of MCP-1. Brain Behav Immun 27: 155–161, 2013. doi: 10.1016/j.bbi.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Waart H, Stuiver MM, van Harten WH, Geleijn E, Kieffer JM, Buffart LM, de Maaker-Berkhof M, Boven E, Schrama J, Geenen MM, Meerum Terwogt JM, van Bochove A, Lustig V, van den Heiligenberg SM, Smorenburg CH, Hellendoorn-van Vreeswijk JAJH, Sonke GS, Aaronson NK. Effect of low-intensity physical activity and moderate- to high-intensity physical exercise during adjuvant chemotherapy on physical fitness, fatigue, and chemotherapy completion rates: results of the paces randomized clinical trial. J Clin Oncol 33: 1918–1927, 2015. doi: 10.1200/JCO.2014.59.1081. [DOI] [PubMed] [Google Scholar]
- 12. Mustian KM, Alfano CM, Heckler C, Kleckner AS, Kleckner IR, Leach CR, Mohr D, Palesh OG, Peppone LJ, Piper BF, Scarpato J, Smith T, Sprod LK, Miller SM. Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: a meta-analysis. JAMA Oncol 3: 961–968, 2017. doi: 10.1001/jamaoncol.2016.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Courneya KS, Segal RJ, Mackey JR, Gelmon K, Reid RD, Friedenreich CM, Ladha AB, Proulx C, Vallance JKH, Lane K, Yasui Y, McKenzie DC. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J Clin Oncol 25: 4396–4404, 2007. doi: 10.1200/JCO.2006.08.2024. [DOI] [PubMed] [Google Scholar]
- 14. Fairman CM, Lønbro S, Cardaci TD, VanderVeen BN, Nilsen TS, Murphy AE. Muscle wasting in cancer: opportunities and challenges for exercise in clinical cancer trials. JCSM Rapid Commun 5: 52–67, 2022. doi: 10.1002/rco2.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tidball JG. Inflammatory processes in muscle injury and repair. Am J Physiol Regul Integr Comp Physiol 288: R345–R353, 2005. doi: 10.1152/ajpregu.00454.2004. [DOI] [PubMed] [Google Scholar]
- 16. Krippendorf BB, Riley DA. Distinguishing unloading- versus reloading-induced changes in rat soleus muscle. Muscle Nerve 16: 99–108, 1993. doi: 10.1002/mus.880160116. [DOI] [PubMed] [Google Scholar]
- 17. Tidball JG. Regulation of muscle growth and regeneration by the immune system. Nat Rev Immunol 17: 165–178, 2017. doi: 10.1038/nri.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liao X, Shen Y, Zhang R, Sugi K, Vasudevan NT, Alaiti MA, Sweet DR, Zhou L, Qing Y, Gerson SL, Fu C, Wynshaw-Boris A, Hu R, Schwartz MA, Fujioka H, Richardson B, Cameron MJ, Hayashi H, Stamler JS, Jain MK. Distinct roles of resident and nonresident macrophages in nonischemic cardiomyopathy. Proc Natl Acad Sci USA 115: E4661–E4669, 2018. doi: 10.1073/pnas.1720065115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res 29: 313–326, 2009. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guilliams M, Ginhoux F, Jakubzick C, Naik SH, Onai N, Schraml BU, Segura E, Tussiwand R, Yona S. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol 14: 571–578, 2014. doi: 10.1038/nri3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Frenette J, Chbinou N, Godbout C, Marsolais D, Frenette PS. Macrophages, not neutrophils, infiltrate skeletal muscle in mice deficient in P/E selectins after mechanical reloading. Am J Physiol Regul Integr Comp Physiol 285: R727–R732, 2003. doi: 10.1152/ajpregu.00175.2003. [DOI] [PubMed] [Google Scholar]
- 22. Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med 204: 1057–1069, 2007. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol 5: 953–964, 2005. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 24. Reidy PT, McKenzie AI, Mahmassani ZS, Petrocelli JJ, Nelson DB, Lindsay CC, Gardner JE, Morrow VR, Keefe AC, Huffaker TB, Stoddard GJ, Kardon G, O'Connell RM, Drummond MJ. Aging impairs mouse skeletal muscle macrophage polarization and muscle-specific abundance during recovery from disuse. Am J Physiol Endocrinol Metab 317: E85–E98, 2019. doi: 10.1152/ajpendo.00422.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu X, Liu Y, Zhao L, Zeng Z, Xiao W, Chen P. Macrophage depletion impairs skeletal muscle regeneration: the roles of regulatory factors for muscle regeneration. Cell Biol Int 41: 228–238, 2017. doi: 10.1002/cbin.10705. [DOI] [PubMed] [Google Scholar]
- 26. Farini A, Meregalli M, Belicchi M, Battistelli M, Parolini D, D'Antona G, Gavina M, Ottoboni L, Constantin G, Bottinelli R, Torrente Y. T and B lymphocyte depletion has a marked effect on the fibrosis of dystrophic skeletal muscles in the scid/mdx mouse. J Pathol 213: 229–238, 2007. doi: 10.1002/path.2213. [DOI] [PubMed] [Google Scholar]
- 27. Xiao W, Liu Y, Chen P. Macrophage depletion impairs skeletal muscle regeneration: the roles of pro-fibrotic factors, inflammation, and oxidative stress. Inflammation 39: 2016–2028, 2016. doi: 10.1007/s10753-016-0438-8. [DOI] [PubMed] [Google Scholar]
- 28. Zhao W, Lu H, Wang X, Ransohoff RM, Zhou L. CX3CR1 deficiency delays acute skeletal muscle injury repair by impairing macrophage functions. FASEB J 30: 380–393, 2016. doi: 10.1096/fj.14-270090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Barreto R, Waning DL, Gao H, Liu Y, Zimmers TA, Bonetto A. Chemotherapy-related cachexia is associated with mitochondrial depletion and the activation of ERK1/2 and p38 MAPKs. Oncotarget 7: 43442–43460, 2016. doi: 10.18632/oncotarget.9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Abraham JE, Hiller L, Dorling L, Vallier A-L, Dunn J, Bowden S, Ingle S, Jones L, Hardy R, Twelves C, Poole CJ, Pharoah PDP, Caldas C, Earl HM. A nested cohort study of 6,248 early breast cancer patients treated in neoadjuvant and adjuvant chemotherapy trials investigating the prognostic value of chemotherapy-related toxicities. BMC Med 13: 306, 2015. doi: 10.1186/s12916-015-0547-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Han Y, Yu Z, Wen S, Zhang B, Cao X, Wang X. Prognostic value of chemotherapy-induced neutropenia in early-stage breast cancer. Breast Cancer Res Treat 131: 483–490, 2012. doi: 10.1007/s10549-011-1799-1. [DOI] [PubMed] [Google Scholar]
- 32. Yamanaka T, Matsumoto S, Teramukai S, Ishiwata R, Nagai Y, Fukushima M. Predictive value of chemotherapy-induced neutropenia for the efficacy of oral fluoropyrimidine S-1 in advanced gastric carcinoma. Br J Cancer 97: 37–42, 2007. doi: 10.1038/sj.bjc.6603831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shitara K, Matsuo K, Takahari D, Yokota T, Inaba Y, Yamaura H, Sato Y, Najima M, Ura T, Muro K. Neutropaenia as a prognostic factor in metastatic colorectal cancer patients undergoing chemotherapy with first-line FOLFOX. Eur J Cancer 45: 1757–1763, 2009. doi: 10.1016/j.ejca.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 34. Baechler S, Hobbs RF, Jacene HA, Bochud FO, Wahl RL, Sgouros G. Predicting hematologic toxicity in patients undergoing radioimmunotherapy with 90Y-ibritumomab tiuxetan or 131I-tositumomab. J Nucl Med 51: 1878–1884, 2010. doi: 10.2967/jnumed.110.079947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kvinnsland S. The leucocyte nadir, a predictor of chemotherapy efficacy? Br J Cancer 80: 1681, 1999. doi: 10.1038/sj.bjc.6690583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huang S-C, Wu J-F, Saovieng S, Chien W-H, Hsu M-F, Li X-F, Lee S-D, Huang C-Y, Huang C-Y, Kuo C-H. Doxorubicin inhibits muscle inflammation after eccentric exercise. J Cachexia Sarcopenia Muscle 8: 277–284, 2017. doi: 10.1002/jcsm.12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Phillips E, France A, Thatvihane G, Nnaemeka U, Zaidi S. Mucositis and cardiotoxicity due to 5-fluorouracil. Am J Ther 25: e712–e714, 2018. doi: 10.1097/MJT.0000000000000725. [DOI] [PubMed] [Google Scholar]
- 38. Sougiannis AT, VanderVeen BN, Enos RT, Velazquez KT, Bader JE, Carson M, Chatzistamou I, Walla M, Pena MM, Kubinak JL, Nagarkatti M, Carson JA, Murphy EA. Impact of 5 fluorouracil chemotherapy on gut inflammation, functional parameters, and gut microbiota. Brain Behav Immun 80: 44–55, 2019. doi: 10.1016/j.bbi.2019.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hardee JP, Mangum JE, Gao S, Sato S, Hetzler KL, Puppa MJ, Fix DK, Carson JA. Eccentric contraction-induced myofiber growth in tumor-bearing mice. J Appl Physiol (1985) 120: 29–37, 2016. doi: 10.1152/japplphysiol.00416.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. VanderVeen BN, Hardee JP, Fix DK, Carson JA. Skeletal muscle function during the progression of cancer cachexia in the male ApcMin/+ mouse. J Appl Physiol (1985) 124: 684–695, 2018. doi: 10.1152/japplphysiol.00897.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hardy D, Besnard A, Latil M, Jouvion G, Briand D, Thépenier C, Pascal Q, Guguin A, Gayraud-Morel B, Cavaillon J-M, Tajbakhsh S, Rocheteau P, Chrétien F. Comparative study of injury models for studying muscle regeneration in mice. PLoS One 11: e0147198, 2016. doi: 10.1371/journal.pone.0147198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mahdy MA, Warita K, Hosaka YZ. Early ultrastructural events of skeletal muscle damage following cardiotoxin-induced injury and glycerol-induced injury. Micron 91: 29–40, 2016. doi: 10.1016/j.micron.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 43. VanderVeen BN, Murphy EA, Carson JA. The impact of immune cells on the skeletal muscle microenvironment during cancer cachexia. Front Physiol 11: 1037, 2020. doi: 10.3389/fphys.2020.01037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Reidy PT, Dupont-Versteegden EE, Drummond MJ. Macrophage regulation of muscle regrowth from disuse in aging. Exerc Sport Sci Rev 47: 246–250, 2019. doi: 10.1249/JES.0000000000000201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Long DE, Peck BD, Lavin KM, Dungan CM, Kosmac K, Tuggle SC. Skeletal muscle properties show collagen organization and immune cell content are associated with resistance exercise response heterogeneity in older persons. J Appl Physiol (1985) 132: 1432–1447, 2022. doi: 10.1152/japplphysiol.00025.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Novak ML, Weinheimer-Haus EM, Koh TJ. Macrophage activation and skeletal muscle healing following traumatic injury. J Pathol 232: 344–355, 2014. [Erratum in J Pathol 233: 319, 2014]. doi: 10.1002/path.4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mochizuki Y, Ojima K, Uezumi A, Masuda S, Yoshimura K, Takeda S. Participation of bone marrow-derived cells in fibrotic changes in denervated skeletal muscle. Am J Pathol 166: 1721–1732, 2005. doi: 10.1016/S0002-9440(10)62482-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Orecchioni M, Ghosheh Y, Pramod AB, Ley K. Macrophage Polarization: Different Gene Signatures In M1(LPS+) vs. classically and M2(LPS-) vs. alternatively activated macrophages. Front Immunol 10: 1084, 2019. [Erratum in Front Immunol 11: 234, 2020]. doi: 10.3389/fimmu.2019.01084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fix DK, Ekiz HA, Petrocelli JJ, Mckenzie AM, Mahmassani ZS, O'Connell RM, Drummond MJ. Disrupted macrophage metabolic reprogramming in aged soleus muscle during early recovery following disuse atrophy. Aging Cell 20: e13448, 2021. doi: 10.1111/acel.13448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang Y, Wehling-Henricks M, Samengo G, Tidball JG. Increases of M2a macrophages and fibrosis in aging muscle are influenced by bone marrow aging and negatively regulated by muscle-derived nitric oxide. Aging Cell 14: 678–688, 2015. doi: 10.1111/acel.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Costamagna D, Duelen R, Penna F, Neumann D, Costelli P, Sampaolesi M. Interleukin-4 administration improves muscle function, adult myogenesis, and lifespan of colon carcinoma-bearing mice. J Cachexia Sarcopenia Muscle 11: 783–801, 2020. doi: 10.1002/jcsm.12539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Duong L, Radley-Crabb HG, Gardner JK, Tomay F, Dye DE, Grounds MD, Pixley FJ, Nelson DJ, Jackaman C. Macrophage depletion in elderly mice improves response to tumor immunotherapy, increases anti-tumor T cell activity and reduces treatment-induced cachexia. Front Genet 9: 526, 2018. doi: 10.3389/fgene.2018.00526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dumont N, Bouchard P, Frenette J. Neutrophil-induced skeletal muscle damage: a calculated and controlled response following hindlimb unloading and reloading. Am J Physiol Regul Integr Comp Physiol 295: R1831–R1838, 2008. doi: 10.1152/ajpregu.90318.2008. [DOI] [PubMed] [Google Scholar]
- 54. McCarthy JJ, Mula J, Miyazaki M, Erfani R, Garrison K, Farooqui AB, Srikuea R, Lawson BA, Grimes B, Keller C, Van Zant G, Campbell KS, Esser KA, Dupont-Versteegden EE, Peterson CA. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development 138: 3657–3666, 2011. doi: 10.1242/dev.068858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development 138: 3625–3637, 2011. doi: 10.1242/dev.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Barreto R, Mandili G, Witzmann FA, Novelli F, Zimmers TA, Bonetto A. Cancer and chemotherapy contribute to muscle loss by activating common signaling pathways. Front Physiol 7: 472, 2016. doi: 10.3389/fphys.2016.00472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wijler LA, Raats DAE, Elias SG, Dijk FJ, Quirindongo H, May AM, Furber MJW, Dorresteijn B, Dijk M, Kranenburg O. Specialized nutrition improves muscle function and physical activity without affecting chemotherapy efficacy in C26 tumour-bearing mice. J Cachexia Sarcopenia Muscle 12: 796–810, 2021. doi: 10.1002/jcsm.12703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Huot JR, Pin F, Bonetto A. Muscle weakness caused by cancer and chemotherapy is associated with loss of motor unit connectivity. Am J Cancer Res 11: 2990–3001, 2021. [Erratum in Am J Cancer Res 12: 1435, 2022]. [PMC free article] [PubMed] [Google Scholar]
- 59. Golshani G, Zhang Y. Advances in immunotherapy for colorectal cancer: a review. Therap Adv Gastroenterol 13: 1756284820917527, 2020. doi: 10.1177/1756284820917527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Campelj DG, Goodman CA, Rybalka E. Chemotherapy-induced myopathy: the dark side of the cachexia sphere. Cancers (Basel) 13: 3615, 2021. doi: 10.3390/cancers13143615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Abdel-Rahman O. Effect of body mass index on 5-fu-based chemotherapy toxicity and efficacy among patients with metastatic colorectal cancer; a pooled analysis of 5 randomized trials. Clin Colorectal Cancer 18: e385–e393, 2019. doi: 10.1016/j.clcc.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 62. Liva SG, Tseng Y-C, Dauki AM, Sovic MG, Vu T, Henderson SE, Kuo Y-C, Benedict JA, Zhang X, Remaily BC, Kulp SK, Campbell M, Bekaii-Saab T, Phelps MA, Chen C-S, Coss CC. Overcoming resistance to anabolic SARM therapy in experimental cancer cachexia with an HDAC inhibitor. EMBO Mol Med 12: e9910, 2020. doi: 10.15252/emmm.201809910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hardee JP, Montalvo RN, Carson JA. Linking cancer cachexia-induced anabolic resistance to skeletal muscle oxidative metabolism. Oxid Med Cell Longev 2017: 8018197, 2017. doi: 10.1155/2017/8018197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Halle JL, Counts BR, Zhang Q, Carson JA. Short duration treadmill exercise improves physical function and skeletal muscle mitochondria protein expression after recovery from FOLFOX chemotherapy in male mice. FASEB J 36: e22437, 2022. doi: 10.1096/fj.202200460R. [DOI] [PubMed] [Google Scholar]
- 65. Vanderveen BN, Fix DK, Counts BR, Carson JA. The effect of wheel exercise on functional indices of cachexia in tumor-bearing mice. Med Sci Sports Exerc 52: 2320–2330, 2020. doi: 10.1249/MSS.0000000000002393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. S1: https://doi.org/10.6084/m9.figshare.20006174.v1.