Abstract
Objective:
To evaluate a transition from standard-of-care (SC) management of type 1 diabetes (any insulin delivery method including hybrid closed-loop systems plus real-time continuous glucose monitoring [CGM]) to use of the insulin-only configuration of the iLet® bionic pancreas (BP) in 90 adults and children (age 6–71 years).
Research Design and Methods:
After the SC group completed the randomized controlled trial (RCT) portion of the Insulin-Only BP Pivotal Trial, 90 of the 107 participants participated in a 13-week study using the BP. The key outcomes were change from baseline in HbA1c and CGM metrics after 13 weeks on the BP.
Results:
Using the BP, mean HbA1c decreased from 7.7% ± 1.0% (61 ± 10.9 mmol/mol) at baseline to 7.1% ± 0.6% (54 ± 6.6 mmol/mol) at 13 weeks (mean change −0.55% ± 0.72% [−6 ± 7.9 mmol/mol], P < 0.001), time in range 70–180 mg/dL increased by 12.0% ± 12.5% (from 53% ± 17% to 65% ± 9%, P < 0.001), and mean glucose decreased by −18 ± 23 mg/dL (from 182 ± 32 to 164 ± 15 mg/dL, P < 0.001). The higher the baseline HbA1c level, the greater the change in HbA1c. Results were similar in the adult (N = 42) and pediatric (N = 48) cohorts. Time <70 mg/dL decreased from baseline over the 13 weeks by −0.50% ± 1.86% (P = 0.02), and time <54 mg/dL was similar (change from baseline −0.08% ± 0.59%, P = 0.24). Two severe hypoglycemia events (in same participant) and one diabetic ketoacidosis event occurred.
Conclusions:
Glycemic control improved after adult and pediatric participants in the SC arm in the Insulin-Only BP Pivotal Trial transitioned to use of the BP. Improvement using the BP was of similar magnitude to that observed during the RCT.
ClinicalTrials.gov number, NCT04200313.
Keywords: bionic pancreas, type 1 diabetes, adults, pediatrics, artificial pancreas, evaluation, automated insulin delivery
Introduction
Automated insulin delivery systems hold great promise for transforming the management of type 1 diabetes. The systems that have become available commercially over the past 5 years (Medtronic Minimed™ 670G and 780G,1–4 Tandem t:slim X2 with Control-IQ® Technology [Control-IQ],5,6 and Insulet Omnipod® 57) are referred to as hybrid closed loop (HCL) systems since they partially automate insulin delivery but still require insulin titration and dosing decisions on the part of the health care provider and user.8
This includes requiring the user to enter an estimate of the grams of carbohydrate in a meal and then initiate a meal bolus and to treat hyperglycemia as needed or desired with correction doses of insulin. These systems also require determination and programming of multiple settings before they are used, which may include insulin basal rates, insulin- to-carbohydrates ratios, insulin sensitivity factors, glucose targets, active insulin time, and/or total daily dose (TDD) of insulin.
In contrast, the iLet® bionic pancreas (BP; Beta Bionics) is an automated insulin delivery system initialized only with body weight and without requiring the input of any information about previous insulin dosing. All insulin titration, including for meals, is determined autonomously by the BP insulin-dosing algorithms, and it cannot be modified by the user or health care provider. These algorithms continually adapt basal insulin doses, correction insulin doses, and meal-announcement doses to meet the individual's insulin needs in response to the continuous glucose monitoring (CGM) input signal to the BP.
Meals are announced by the user without carbohydrate counting as “Usual For Me,” “More” (around 50% more than usual), or “Less” (around 50% less than usual) than other meals of the same type (i.e., “Breakfast,” “Lunch,” “Dinner”). In response to these qualitative meal announcements, the system delivers ∼75% of the autonomously estimated insulin need immediately, and then it will autonomously add or refrain from additional basal or correction insulin dosing postprandially, as necessary.
When CGM data are not available, the BP continues to make all insulin-dosing decisions autonomously, based on a basal insulin profile determined, continually updated, and stored by the BP when CGM data were available, and in response to any entered blood glucose values obtained from a capillary glucometer and any meal announcements without carbohydrate counting. Insulin dosing can be maintained, increased, or temporarily suspended autonomously by the BP in response to the entered blood-glucose values. The BP has been developed both as an insulin-only system and as a bihormonal system that doses both insulin and glucagon.
The pivotal randomized controlled trial (RCT) evaluating the insulin-only configuration of the BP demonstrated it to be effective and safe in both adults and youth ≥6 years old with type 1 diabetes in a 13-week trial that included 440 participants using either insulin aspart, insulin lispro, or fast-acting insulin aspart in the BP compared with a standard-of-care (SC) control group using their standard insulin delivery (which included HCL systems) plus real-time CGM.9–12
After completion of the RCT, participants in the SC control group were provided the opportunity to participate in an extension study in which they used the BP for 13 weeks. The results from the extension study expand on the RCT results and are reported herein.
Methods
The study was conducted at 16 diabetes centers in the United States. The protocol was approved by a central institutional review board. Informed consent, or parental consent and assent for children, was obtained. An investigational device exemption for the conduct of the trial was approved by the U.S. Food and Drug Administration (FDA). The protocol is available at https://s3.amazonaws.com/publicfiles.jaeb.org/FinalIOBP_PROTOCOL.pdf, and key aspects are summarized herein.12 The eligibility criteria for the RCT are available at ClinicalTrials.gov NCT04200313.
As part of the SC group in the RCT, participants continued use of their personal insulin delivery method, which could be multiple daily injections (MDI), an insulin pump without automation, an insulin pump with a low glucose suspend feature, or an insulin pump with an HCL system. Participants were provided with education and supplies to use real-time CGM (Dexcom G6) for the duration of the 13 weeks of the RCT; they could continue to use their own CGM if different. To be eligible for the Extension Study, participants needed to complete all visits during the RCT, at least 10 of 13 weekly surveys regarding hypoglycemia, and have used the Dexcom G6 CGM for at least 80% of the time.
Participants who transitioned to the extension study were provided with the iLet device, which is part of the BP system, a Contour®Next One Blood Glucose Monitoring System (Ascensia Diabetes Care, Basel, CH), a Precision Xtra ketone meter (Abbott Diabetes Care), and an additional supply of Dexcom G6 sensors and transmitters. Insulin was delivered subcutaneously using a commercially available Inset I infusion set (Unomedical), which was the only type of infusion set used in the trial. Pediatric participants used prefilled fast-acting insulin aspart (Fiasp®) in 1.6-mL glass cartridges to provide data on the use of this insulin in the iLet in the pediatric population.
Adult participants used their personal insulin aspart or insulin lispro vials to fill 1.6-mL glass, ready-to-fill cartridges (an arm in which adults used fast-acting insulin aspart was included in the RCT).9 If they used pens or a different insulin for the SC arm, the study provided them with insulin aspart or lispro in 10-mL vials.
Participants were trained on the use of the BP system and given specific written instructions for identifying and managing possible infusion set failures. There were no restrictions on diet or exercise during the trial period. All participants had phone contacts after 1–2 days and 1 week and had follow-up visits at 2, 6, 10, and 13 weeks. Some visits were completed remotely via video conference due to the COVID pandemic.
A blood sample from venipuncture or fingerstick13 was collected after 13 weeks for the measurement of HbA1c by a central laboratory at the University of Minnesota Advanced Research and Diagnostic Laboratory (measured with a Tosoh BioScience instrument). Patient-reported outcome surveys were completed at baseline and during follow-up and will be reported separately.
Reporting of adverse events was solicited throughout the trial. Severe hypoglycemia was defined as hypoglycemia requiring assistance because of altered consciousness. Diabetic ketoacidosis was defined by the criteria established by the Diabetes Control and Complications Trial.14
Statistical methods
Analyses were conducted overall and separately for the adult and pediatric cohorts based on participant age at the time of initiation of the randomized trial (≥18 and <18 years—one participant in the pediatric cohort turned 18 during the RCT). The 13-week HbA1c measurement in the RCT and the 13 weeks of CGM data collected during the RCT were used as baseline metrics for the analyses.
Descriptive statistics include means with standard deviations and medians with interquartile ranges (IQR), depending on the distribution of data. For continuous outcomes, a paired t-test was used to evaluate the mean change from baseline to 13 weeks. In addition, a likelihood ratio test was used to compare the standard deviation of HbA1c, mean CGM glucose, and time in range (TIR) 70–180 mg/dL between baseline and 13 weeks. Binary outcomes were compared between baseline and 13 weeks using McNemar's test. Missing data were handled using multiple imputation. P-values are two-sided and have been adjusted for multiple comparisons to control the false discovery rate using the Benjamini-Hochberg method adapted using the two-stage test.15 Analyses were performed with SAS software, version 9.4 (SAS Institute).
Results
The SC group in the RCT included 107 participants, of whom 103 were eligible for the Extension Study, and 90 consented to participate in this study. Reasons for not participating included: fear of hypoglycemia (3), changes in work schedule (2), planned travel (2), life stresses (2), moved out of state (1), COVID-19 (1), not wanting to switch from MDI to a pump (1), and skin reaction using a Dexcom sensor (1).
The 90 participants ranged in age from 6 to 71 years: 42 were in the adult cohort and 48 in the pediatric cohort. Twenty-three percent were of a minority race/ethnicity overall: 14% of adults and 31% of youth. For insulin delivery in the SC arm in the preceding RCT, 39% used MDI, 28% used a pump without automation, 4% used a pump with a predictive low glucose suspend, and 29% used an HCL system (11% Medtronic 670G/770G system and 18% Tandem Control-IQ). The baseline HbA1c at the start of the Extension Study was 7.7% ± 1.0% (61 ± 10.9 mmol/mol). Other characteristics are shown in Table S1 in the Supplementary Appendix.
The 13 weeks of the study were completed by 84 (93%) of the 90 participants. The scheduled visit and contact completion rate was 99% for those who completed the study. Two pediatric participants used insulin aspart in the BP instead of fast-acting insulin aspart (protocol deviation). The three adult participants who withdrew all used an HCL system before the study and expressed dissatisfaction with either glucose control (N = 2) or lack of ability to self-adjust insulin delivery (N = 1).
Among the three pediatric participants who discontinued, one was an investigator decision after a diabetic ketoacidosis (DKA) event, one was related to hyperglycemia, and one was for personal reasons. Over the 13 weeks of the trial (or until the time of study discontinuation), median autonomous insulin dosing by the BP was 96% (IQR 92%–98%), with CGM data available a median of 88% (IQR 82%–92%) of the time. When the BP was being used, median autonomous dosing was 96% (IQR 94%–98%), with CGM data available for 89% (IQR 84%–93%) of the time (Table S2 in the Supplementary Appendix).
HbA1c and CGM outcomes
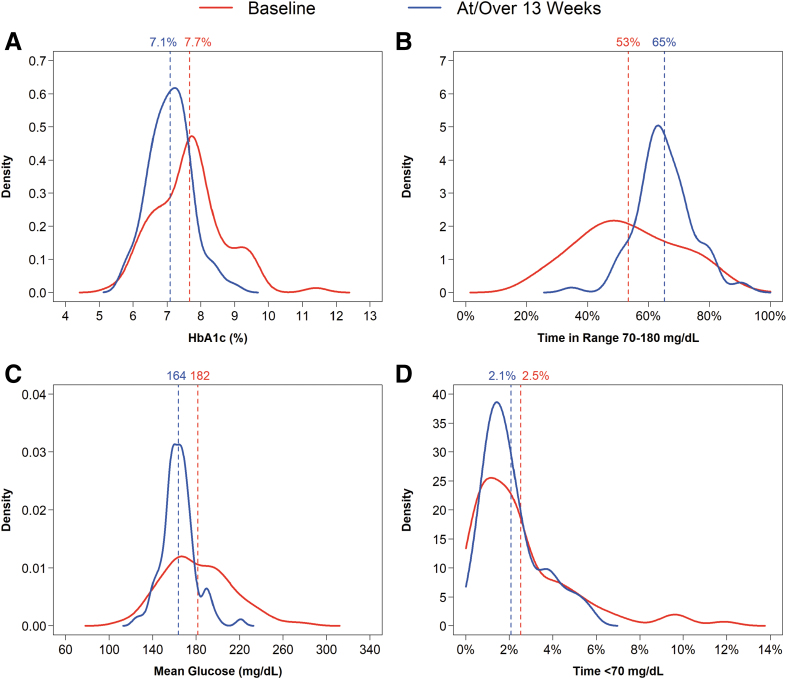
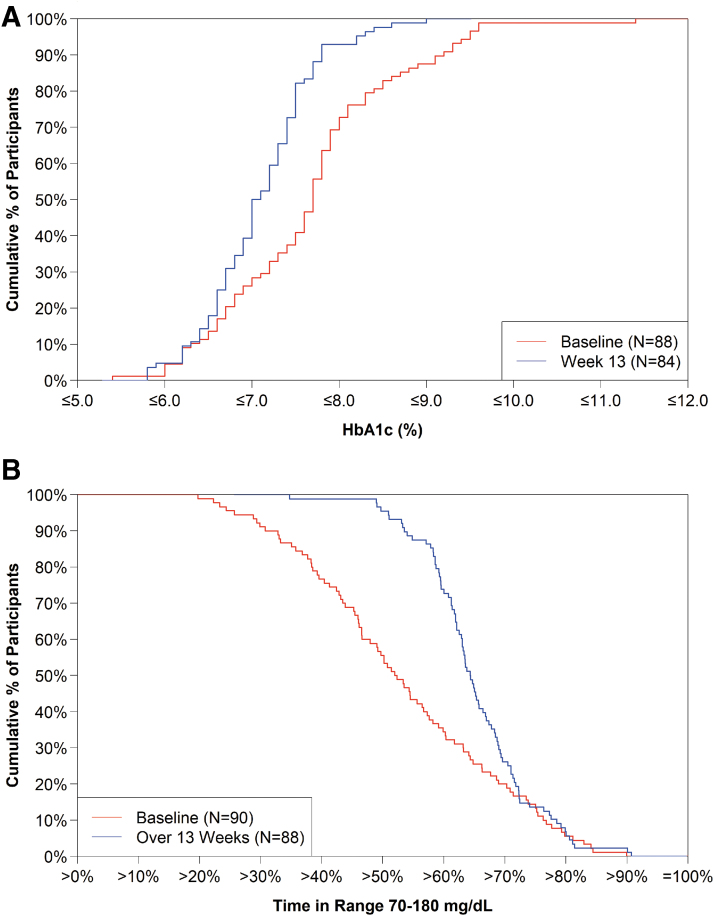
Overall, HbA1c decreased from 7.7% ± 1.0% (61 ± 10.9 mmol/mol) at baseline to 7.1% ± 0.6% (54 ± 6.6 mmol/mol) at 13 weeks (mean change −0.55% ± 0.72% [−6.0 ± 7.9 mmol/mol], P < 0.001). A substantial shift and narrowing in the distributions of HbA1c values, mean CGM glucose levels, and TIR 70–180 mg/dL from baseline to 13 weeks is evident in Figures 1 and 2. The change from baseline in HbA1c was similar in the adult and pediatric cohorts: −0.56% ± 0.78% (−6.1 ± 8.5 mmol/mol) and −0.55% ± 0.68% (−6.0 ± 7.4 mmol/mol), respectively (Table 1 and Figs. S1 and S2 in the Supplementary Appendix).
FIG. 1.
Distribution of HbA1c, TIR 70–180 mg/dL, mean glucose, and time <70 mg/dL. (A–D) Show the HbA1c, TIR 70–180 mg/dL, mean glucose, and time <70 mg/dL data, respectively, at baseline (red line) and 13 weeks (blue line). The solid curves represent the distribution of values at baseline and 13 weeks, with higher density values representing a greater proportion of individuals. The vertical dashed lines represent the mean values that are indicated numerically at the top of each line. TIR, time in range.
FIG. 2.
Cumulative distribution of HbA1c and TIR 70–180 mg/dL. (A, B) Show the HbA1c and TIR 70–180 mg/dL data, respectively, at baseline (red line) and 13 weeks (blue line).
Table 1.
Key Glycemic Outcomes
| Baseline, N = 90 | At or over 13 weeks, N = 88 | Change from baseline, N = 88 | P a | |
|---|---|---|---|---|
| Overall | ||||
| HbA1c %, mmol/mol | 7.7 (1.0) | 7.1 (0.6) | −0.55 (0.72) | <0.001 |
| [61 (10.9)] | [54 (6.6)] | [−6.0 (7.9)] | ||
| % Time in range 70–180 mg/dL | 53% (17%) | 65% (9%) | 12.0% (12.5%) | <0.001 |
| Mean glucose, mg/dL | 182 (32) | 164 (15) | −18 (23) | <0.001 |
| % Time >180 mg/dL | 44% (17%) | 33% (9%) | −11.5% (12.9%) | <0.001 |
| % Time >250 mg/dL | 18.4% (13.8%) | 9.5% (5.8%) | −9.1% (10.4%) | <0.001 |
| Hyperglycemic event rate per week (≥90 min >300 mg/dL in 120 min)b | 2.7 (2.3) | 1.3 (1.3) | −1.4 (1.7) | <0.001 |
| % Time <70 mg/dL | 2.53% (2.35%) | 2.08% (1.32%) | −0.50% (1.86%) | 0.02 |
| % Time <54 mg/dL | 0.56% (0.75%) | 0.49% (0.49%) | −0.08% (0.59%) | 0.24 |
| Hypoglycemic event rate per weekc | 1.00 (1.30) | 0.99 (1.04) | −0.03 (0.92) | 0.83 |
| Glucose SD, mg/dL | 68 (16) | 60 (11) | −8.7 (10.0) | <0.001 |
| Glucose coefficient of variation | 37% (5%) | 36% (4%) | −1.2% (3.9%) | 0.009 |
| N = 42 | N = 41 | N = 41 | ||
|---|---|---|---|---|
| Adult | ||||
| HbA1c %, mmol/mol | 7.5 (0.9) | 7.0 (0.6) | −0.56 (0.78) | <0.001 |
| [58 (9.8)] | [53 (6.6)] | [−6.1 (8.5)] | ||
| % Time in range 70–180 mg/dL | 57% (17%) | 68% (8%) | 11.6% (13.6%) | <0.001 |
| Mean glucose, mg/dL | 176 (30) | 159 (12) | −17 (24) | <0.001 |
| % Time >180 mg/dL | 41% (18%) | 30% (8%) | −11.5% (13.9%) | <0.001 |
| % Time >250 mg/dL | 15.0% (12.3%) | 7.2% (4.1%) | −8.1% (10.4%) | <0.001 |
| Hyperglycemic event rate per week (≥90 min >300 mg/dL in 120 min)b | 2.0 (1.9) | 0.9 (0.9) | −1.1 (1.6) | <0.001 |
| % Time <70 mg/dL | 2.12% (1.96%) | 2.03% (1.20%) | −0.14% (1.52%) | 0.64 |
| % Time <54 mg/dL | 0.43% (0.54%) | 0.43% (0.48%) | −0.01% (0.47%) | 0.96 |
| Hypoglycemic event rate per weekc | 0.76 (0.93) | 0.85 (0.97) | 0.08 (0.78) | 0.49 |
| Glucose SD, mg/dL | 63 (15) | 56 (9) | −7.4 (11.1) | <0.001 |
| Glucose coefficient of variation | 35% (4%) | 35% (4%) | −0.7% (3.5%) | 0.35 |
| N = 48 | N = 47 | N = 47 | ||
|---|---|---|---|---|
| Pediatric | ||||
| HbA1c %, mmol/mol | 7.8 (1.1) | 7.2 (0.6) | −0.55 (0.68) | <0.001 |
| [62 (12)] | [55 (6.6)] | [−6.0 (7.4)] | ||
| % Time in range 70–180 mg/dL | 50% (16%) | 63% (9%) | 12.3% (11.6%) | <0.001 |
| Mean glucose, mg/dL | 187 (34) | 168 (16) | −19 (24) | <0.001 |
| % Time >180 mg/dL | 47% (17%) | 35% (9%) | −11.5% (12.1%) | <0.001 |
| % Time >250 mg/dL | 21.4% (14.4%) | 11.6% (6.3%) | −10.0% (10.5%) | <0.001 |
| Hyperglycemic event rate per week (≥90 min >300 mg/dL in 120 min)b | 3.2 (2.5) | 1.7 (1.4) | −1.6 (1.8) | <0.001 |
| % Time <70 mg/dL | 2.88% (2.61%) | 2.12% (1.43%) | −0.81% (2.08%) | 0.01 |
| % Time <54 mg/dL | 0.67% (0.88%) | 0.54% (0.50%) | −0.14% (0.68%) | 0.17 |
| Hypoglycemic event rate per weekc | 1.22 (1.53) | 1.12 (1.10) | −0.12 (1.02) | 0.45 |
| Glucose SD, mg/dL | 73 (15) | 63 (11) | −9.8 (9.0) | <0.001 |
| Glucose coefficient of variation | 39% (5%) | 37% (5%) | −1.7% (4.2%) | 0.01 |
Data are reported as mean (SD). Ns for HbA1c are Overall: Baseline 88, week 13 84, and Change 82. Adult: 41, 39, and 38, respectively. Pediatric: 47, 45, 44, respectively. Amount of CGM data (mean [SD] hours) is Overall: Baseline 2095 (130) and Over 13 weeks: 1899 (294). Adult: 2155 (98) and 1928 (326), respectively. Pediatric: 2042 (132) and 1874 (265), respectively.
P-values for the change in means were calculated from a paired t-test comparing the week 13 extension phase value with the extension baseline value. P-values were adjusted to control the false discovery rate.
A CGM-measured hyperglycemic event is defined as ≥90 cumulative minutes with a CGM sensor value >300 mg/dL within a 120-min period. The event ends when there is ≥15 consecutive minutes with a CGM sensor value ≤180 mg/dL, at which point the participant becomes eligible for another hyperglycemic event.
A CGM-measured hypoglycemic event is defined as ≥15 consecutive minutes with a CGM sensor value <54 mg/dL. The event ends when there is ≥15 consecutive minutes with a CGM sensor value ≥70 mg/dL, at which point the participant becomes eligible for another sensor-measured hypoglycemic event.
CGM, continuous glucose monitoring; SD, standard deviation.
HbA1c improvement of >0.5% (>5.5 mmol/mol) was achieved by 46% of participants overall (45% of the adult cohort and in 48% of the pediatric cohort) (Table S3 in the Supplementary Appendix). The percentage achieving an HbA1c level <7% (<53 mmol/mol) increased from 26% at baseline to 39% at 13 weeks (P = 0.02), and the percentage with HbA1c <7.5% (<58 mmol/mol) increased from 38% at baseline to 73% at 13 weeks (P < 0.001, Table 2).
Table 2.
Binary Glycemic Metrics Measured at Baseline and 13 Weeks
| Overall |
Adult |
Pediatric |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline, N = 88 | At or over 13 weeks, N = 84 | P a | Baseline, N = 41 | At or over 13 weeks, N = 39 | P a | Baseline, N = 47 | At or over 13 weeks, N = 45 | P a | |
| HbA1c | |||||||||
| HbA1c <7.0% (53 mmol/mol) | 23 (26) | 33 (39) | 0.02 | 12 (29) | 18 (46) | 0.04 | 11 (23) | 15 (33) | 0.25 |
| HbA1c <7.5% (58 mmol/mol) | 33 (38) | 61 (73) | <0.001 | 17 (41) | 30 (77) | <0.001 | 16 (34) | 31 (69) | <0.001 |
| HbA1c <8.0% (64 mmol/mol) | 61 (69) | 78 (93) | <0.001 | 32 (78) | 37 (95) | 0.04 | 29 (62) | 41 (91) | <0.001 |
| HbA1c >9.0% (75 mmol/mol) | 11 (13) | 0 (0) | <0.001 | 4 (10) | 0 (0) | 0.05 | 7 (15) | 0 (0) | 0.008 |
| N = 90 | N = 88 | N = 42 | N = 41 | N = 48 | N = 47 | ||||
|---|---|---|---|---|---|---|---|---|---|
| CGM-measured | |||||||||
| Time in range 70–180 mg/dL >70% | 18 (20) | 23 (26) | 0.19 | 13 (31) | 13 (32) | 0.84 | 5 (10) | 10 (21) | 0.05 |
| Time <70 mg/dL <4% | 71 (79) | 79 (90) | 0.008 | 34 (81) | 37 (90) | 0.10 | 37 (77) | 42 (89) | 0.04 |
| Time <54 mg/dL <1% | 74 (82) | 77 (88) | 0.10 | 34 (81) | 36 (88) | 0.19 | 40 (83) | 41 (87) | 0.34 |
| Mean glucose <154 mg/dL and time <54 mg/dL <1% | 12 (13) | 14 (16) | 0.51 | 8 (19) | 9 (22) | 0.65 | 4 (8) | 5 (11) | 0.62 |
| Time in range 70–180 mg/dL >70% and time <54 mg/dL <1% | 12 (13) | 22 (25) | 0.01 | 9 (21) | 12 (29) | 0.29 | 3 (6) | 10 (21) | 0.007 |
Data are N (%) unless otherwise indicated.
P-values were calculated from McNemar's test. Missing data were handled using multiple imputation. P-values were adjusted to control the false discovery rate.
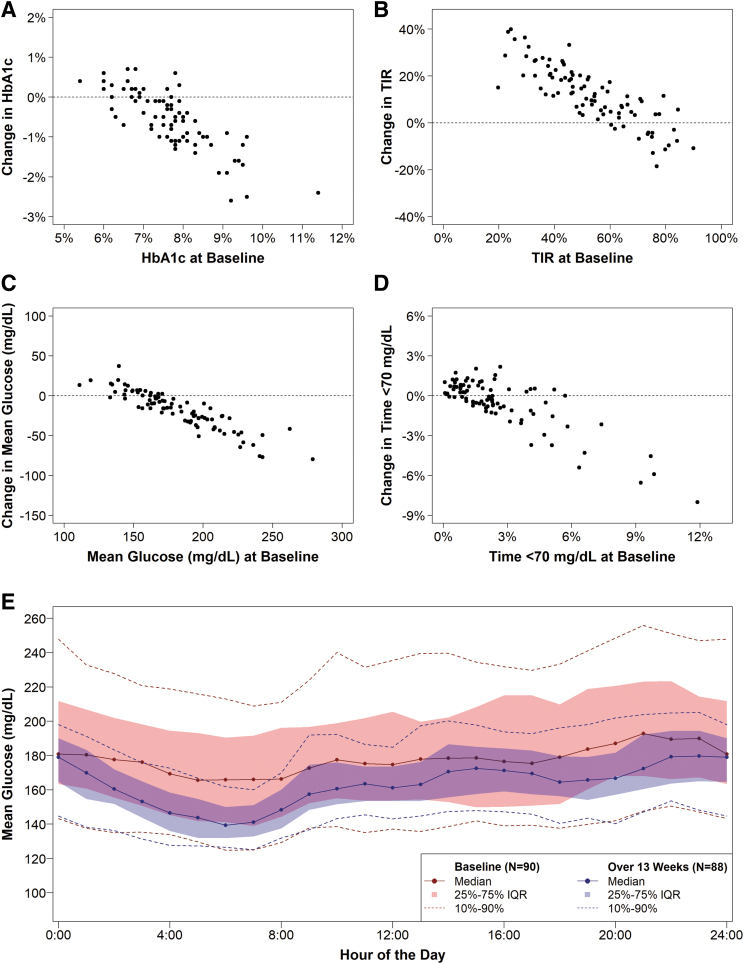
As can be seen in Figure 3 and Figure S3 in the Supplementary Appendix, the degree of improvement in HbA1c was strongly associated with the baseline HbA1c level: The higher the baseline HbA1c level, the greater the change in HbA1c. Participants with baseline HbA1c ≥8.0% (≥64 mmol/mol) (n = 25) had a mean HbA1c reduction at 13 weeks of 1.28% ± 0.61% (14 ± 6.7 mmol/mol), participants with baseline HbA1c 7.5%–7.9% (58–63 mmol/mol) (n = 27) had a mean reduction of 0.47% ± 0.48% (5.1 ± 5.2 mmol/mol), and participants with baseline HbA1c 7.0%–7.4% (53–57 mmol/mol) (n = 9) had a mean reduction of 0.39% ± 0.40% (4.3 ± 4.4 mmol/mol). There was a nonsignificant increase of 0.13% ± 0.36% (1.4 ± 3.9 mmol/mol) in the 21 participants with baseline HbA1c <7.0% (<53 mmol/mol).
FIG. 3.
Change in HbA1c, TIR 70–180 mg/dL, mean glucose, and time <70 mg/dL from baseline. (A–D) Show a scatter plot of the change in HbA1c, TIR 70–180 mg/dL, mean glucose, and time <70 mg/dL, respectively, from baseline to 13 weeks versus baseline value, with the horizontal line representing zero change. (E) Shows the mean glucose by hour of the day at baseline (blue lines) and over 13 weeks (red lines). Dots represent the median mean glucose, the shaded area represents the interquartile range, and the dashed lines represent the 10th and 90th percentiles over each hour of the day.
The mean CGM glucose level and mean TIR also showed significant improvements from baseline to the 13 weeks (Table 1 and Figs. 1 and 2 and Fig. S3 in the Supplementary Appendix). The mean TIR increased from 53% ± 17% to 65% ± 9% (mean change 12.0% ± 12.5%, P < 0.001), and the mean CGM glucose level decreased from 182 ± 32 to 164 ± 15 mg/dL (mean change −18 ± 23 mg/dL, P < 0.001). Time >300 mg/dL decreased from 8.4% to 3.5% (P < 0.001, Table S4 in the Supplementary Appendix). Similar results were observed for the adult and pediatric cohorts. The mean CGM glucose level was lower throughout the 24 h of the day (Fig. 3).
In addition to improvements in the mean of the key metrics, the between-participant variances for HbA1c, mean CGM glucose level, and mean TIR substantially decreased at or over 13 weeks relative to baseline (P < 0.001, Table S5 in the Supplementary Appendix), which is evident in Figure 1 and Figure S4 in the Supplementary Appendix.
Improvement in HbA1c, mean CGM glucose level, and TIR occurred in both MDI users and pump users, including those who were using an HCL system at baseline (Table 3). The amount of improvement among the different insulin delivery types reflected the baseline level of the metrics, with MDI users, who had higher baseline HbA1c and mean glucose and lower baseline TIR, showing the greatest amount of improvement.
Table 3.
Efficacy Outcomes by Insulin Modality Used During Baseline Period
| Baseline |
At or over 13 weeks |
Change from baseline |
||||
|---|---|---|---|---|---|---|
| N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | |
| HbA1c %, mmol/mol | ||||||
| MDI | 35 | 8.0 (1.1) | 33 | 7.2 (0.7) | 33 | −0.72 (0.79) |
| [64 (12)] | [55 (7.7)] | [−7.9 (8.6)] | ||||
| Pump without automation | 23 | 7.7 (1.1) | 25 | 7.0 (0.6) | 23 | −0.62 (0.67) |
| [61 (12)] | [53 (6.6)] | [−6.8 (7.3)] | ||||
| Pump with PLGS | 4 | 7.7 (1.2) | 4 | 7.1 (0.4) | 4 | −0.55 (0.93) |
| [61 (13.1)] | [54 (4.4)] | [−6.0 (10.2)] | ||||
| Pump with HCL | 26 | 7.3 (0.8) | 22 | 7.0 (0.6) | 22 | −0.24 (0.55) |
| [56 (8.7)] | [53 (6.6)] | [−2.6 (6.0)] | ||||
| Mean glucose, mg/dL | ||||||
| MDI | 35 | 192 (33) | 34 | 166 (13) | 34 | −26 (27) |
| Pump without automation | 25 | 180 (34) | 25 | 162 (18) | 25 | −18 (20) |
| Pump with PLGS | 4 | 177 (40) | 4 | 163 (18) | 4 | −14 (26) |
| Pump with HCL | 26 | 170 (26) | 25 | 162 (14) | 25 | −8 (18) |
| % Time in range 70–180 mg/dL | ||||||
| MDI | 35 | 48% (16%) | 34 | 64% (8%) | 34 | 16.7% (13.2%) |
| Pump without automation | 25 | 52% (17%) | 25 | 66% (12%) | 25 | 13.9% (8.6%) |
| Pump with PLGS | 4 | 56% (23%) | 4 | 65% (10%) | 4 | 8.5% (14.4%) |
| Pump with HCL | 26 | 62% (14%) | 25 | 66% (8%) | 25 | 4.3% (11.2%) |
| % Time <54 mg/dL | ||||||
| MDI | 35 | 0.58% (0.88%) | 34 | 0.37% (0.33%) | 34 | −0.22% (0.68%) |
| Pump without PLGS and HCL | 25 | 0.72% (0.79%) | 25 | 0.52% (0.56%) | 25 | −0.20% (0.60%) |
| Pump with PLGS | 4 | 0.56% (0.80%) | 4 | 0.49% (0.42%) | 4 | −0.06% (0.41%) |
| Pump with HCL | 26 | 0.38% (0.46%) | 25 | 0.62% (0.59%) | 25 | 0.23% (0.32%) |
HCL, hybrid closed loop; MDI, multiple daily injections; PLGS, predictive low glucose suspend.
A beneficial treatment effect on HbA1c, mean CGM glucose level, and TIR was evident in subgroups based on sex, race/ethnicity, body mass index (BMI), and diabetes duration. Benefit was not observed when baseline HbA1c was <7.0% (<53 mmol/mol), TIR was ≥70%, or time >180 mg/dL was <25% (Tables S6 and S7 in the Supplementary Appendix). Other CGM outcomes showing significant improvements included TIR of 70–140 mg/dL and improved BG Risk index, as shown in Table S8 in the Supplementary Appendix.
The amount of sensor-measured hypoglycemia was low at baseline (mean of 2.53% of time <70 mg/dL and 0.56% of time <54 mg/dL), and changes over the 13 weeks were small (Table 1 and Table S9 in the Supplementary Appendix). Time <70 mg/dL decreased over the 13 weeks by −0.50% ± 1.86% (P = 0.02), with the reduction predominately seen in the pediatric cohort, and time <54 mg/dL was similar to baseline over 13 weeks (change from baseline −0.08% ± 0.59%, P = 0.24). As seen in Figure 1 and Figure S3 in the Supplementary Appendix, use of the BP had a large impact in reducing the number of participants with large amounts of sensor-measured hypoglycemia.
The overall TDD of insulin was 0.82 ± 0.34 units/kg at baseline and 0.85 ± 0.31 units/kg over 13 weeks (change from baseline = 0.02 ± 0.25, P = 0.35). Participants with baseline HbA1c >9.0% (>75 mmol/mol) (N = 11) had a nominal, nonsignificant increase in TDD on average, whereas those with HbA1c ≤9.0% (≤75 mmol/mol) did not (Table S10 in the Supplementary Appendix). The TDD of insulin measured in units/kg increased by 8% ± 21% in the last 2 weeks compared with the first 2 weeks of BP use (P = 0.003) (Table S11 in the Supplementary Appendix).
Body weight increased over the 13 weeks by a mean of 1.9 ± 3.2 kg (P < 0.001), with the increase being 1.6 ± 3.2 kg (P = 0.003) in adult participants and 2.2 ± 3.1 kg (P < 0.001) in pediatric participants. Commensurate increases in BMI were observed (Table S12 in the Supplementary Appendix).
Safety outcomes
Two severe hypoglycemia events occurred in one adult participant who also experienced two such events during the RCT while using MDI for insulin delivery. Neither event was related to a device malfunction. No other participants experienced a severe hypoglycemia event either during this study or during the preceding RCT. One pediatric participant developed DKA associated with an infusion set failure. Most other reported adverse events involved hyperglycemia related to infusion set failures (Table S13 in the Supplementary Appendix). Device issues are summarized in Table S14 in the Supplementary Appendix.
Discussion
In this multi-center single-arm trial, the insulin-only configuration of the BP was shown to be effective in reducing HbA1c and improving CGM metrics of mean glucose, hyperglycemia, and TIR compared with prospectively collected data for the study participants who participated in the SC control group of an RCT during the immediately preceding 13-week period. The percentage of time that the BP was autonomously dosing insulin was very high (median 96%). Comparable glycemic benefits were observed in pediatric and adult participants, with the benefit being the greatest in those with the worst baseline glycemic levels.
Benefit of the BP on HbA1c or TIR was not observed in participants with baseline HbA1c <7.0% (<53 mmol/mol) or baseline TIR >70%. The amount of hypoglycemia was low at baseline, particularly for time <54 mg/dL, and showed little change over the 13 weeks.
During the preceding RCT, the study participants in the current study used their standard insulin delivery method, which could be an HCL system, and all used real-time CGM. It is notable that improvement in glycemic metrics occurred in both MDI users and nonautomated pump users after transitioning to the BP, even though there was no run-in or training period for the BP.
Although studies of many HCL systems have included such a run-in period, the BP does not have an open-loop mode of operation, and all insulin dose decisions are fully automated as soon as the BP is initiated using only the user's body weight. In participants who previously had been using an HCL system, there were smaller improvements in HbA1c, mean glucose, and TIR after switching to the BP than in the participants not previously using some form of automation.
The results in this study are very similar to the results in the preceding RCT where the mean reduction in HbA1c in the participants in the BP group (using insulin aspart/lispro) compared with baseline was 0.57% and mean increase in TIR was 13.5%.12 Results for the pediatric and adult participants were also similar to the preceding RCT results.10,11 It is difficult to compare the study results with the results from studies of other systems that automate insulin delivery due to differences in study design and participant characteristics.8 However, the improvement in glycemic measures observed with use of the BP is at least as great, if not in some cases greater, than the improvement reported with other systems.1–7
With respect to safety, there was one participant who experienced two severe hypoglycemic events, and this participant also experienced two severe hypoglycemic events in the SC group in the preceding RCT. Infusion set failures were frequent but may be no higher than what would be expected with the particular infusion set used in this study. Assuming that infusion sets were changed on average every 3 days per participant instructions, the 51 hyperglycemia adverse events associated with infusion set failures in the BP group represent a failure rate of 1.9% for 2657 infusion sets.
A recent analysis of infusion set changes associated with prolonged hyperglycemia from a different system suggests that this rate is likely no higher than it is with other systems.16 There was an increase in body weight in both adult and pediatric participants without an increase in total daily insulin. In the preceding RCT, which was the same duration as this study, an increase in body weight was observed in both the BP and control groups, suggesting that the weight gain was not related to BP use.
A novel aspect of the study was the use of fast-acting insulin aspart in the BP by the pediatric cohort. Fast-acting insulin aspart was developed to increase the speed of insulin absorption and reduce its duration of action.17,18 The active pharmaceutical ingredients in fast-acting insulin aspart and insulin aspart are identical and, therefore, once systemically absorbed, fast-acting insulin aspart has the same biological action at the insulin receptor as that of insulin aspart.
No differential information was provided to the BP about the absorption kinetics of fast-acting insulin aspart versus insulin apart or insulin lispro. In the preceding RCT, a group of adult participants used fast-acting insulin aspart in the BP. Although substantial benefit was observed compared with the SC control group in the RCT, there was little or no benefit compared with a group of adults in the same RCT using insulin aspart or insulin lispro in the BP.9
In particular, the change in HbA1c in the RCT was virtually identical with use of either fast-acting insulin aspart or insulin aspart/lispro in the BP; however, there was a 2% greater increase in TIR with fast-acting insulin aspart. In the current study, reduction in HbA1c and increase in TIR in the pediatric cohort using fast-acting insulin aspart in the BP was of similar magnitude to that observed in the pediatric cohort in the RCT using insulin aspart/lispro.
A strength of this trial was the inclusion of participants who, before the study, were using either an HCL system, a pump without automation, or MDI for insulin delivery, and had a wide range of levels of glycemic control, with baseline HbA1c values ranging from 5.4% to 11.4% (36–101 mmol/mol). Further, the cohort was racially and socioeconomically diverse, with 23% being of minority race/ethnicity. Although the study design did not include a concurrent control group, the baseline data were prospectively and systematically collected over a 13-week period before the study, which strengthens the analyses comparing outcomes with baseline.
The low amount of baseline CGM-measured hypoglycemia precluded an evaluation as to whether the insulin-only BP system can reduce hypoglycemia, but it was clear from the results that it does not increase hypoglycemia as measured with CGM despite a substantial decrease in HbA1c.
In conclusion, this study demonstrated that the BP safely improves HbA1c and CGM metrics of TIR, mean glucose, and hyperglycemia without increasing CGM-measured hypoglycemia. These findings are consistent with those of the preceding RCT comparing BP use with an SC control group using CGM and any method of insulin delivery. The use of fast-acting insulin aspart in the BP was found to be safe for pediatric participants, with an efficacy that appears comparable to that of insulin aspart/lispro in the BP.
The BP differs from the current FDA-approved/cleared HCL systems in not requiring any information about the previous insulin regimen, and not requiring carbohydrate counting at mealtimes or user-initiated correction boluses to treat hyperglycemia. This reduced user interaction compared with current HCL systems may facilitate the adoption of automated insulin delivery by a wider spectrum of people with type 1 diabetes and a broad spectrum of health care providers.
Supplementary Material
Acknowledgments
A complete listing of the Bionic Pancreas Research Group appears in the Supplementary Appendix. A listing of authors and nonauthor contributors is given next.
Authors:
Massachusetts General Hospital, Boston, MA: Steven J. Russell, Jordan S. Sherwood, Luz E. Castellanos, Mallory A. Hillard, Marwa Tuffaha, Melissa S. Putman, Mollie Y. Sands, Courtney A. Balliro. Barbara Davis Center for Diabetes, University of Colorado, Aurora, CO: R. Paul Wadwa, Gregory Forlenza, Robert Slover, Laurel H. Messer, Erin Cobry, Viral N. Shah, Sarit Polsky. Department of Pediatrics, Division of Pediatric Endocrinology and Diabetes, Stanford University School of Medicine, Palo Alto, CA: Bruce Buckingham, Rayhan Lal, Laya Ekhlaspour, Michael S. Hughes, Marina Basina. Cleveland Clinic, Cleveland, OH: Betul Hatipoglu, MD, Keren Zhou, MD, Leann Olansky. Children's Hospital of Orange County: Mark Daniels, MD, Amrit Bhangoo, Nikta Forghani, Himala Kashmiri, Francoise Sutton. University of Texas Southwestern (Adults), Dallas, TX: Philip Raskin. University of Texas Southwestern (Pediatrics), Dallas, TX: Perrin White, Abha Choudhary, Jimmy Penn. University of Texas Health Science Center, San Antonio, San Antonio, TX: Jane Lynch, Rabab Jafri, Maria Rayas, Elia Escaname, Catherine Kerr, Ruby Favela-Prezas. University of California, San Diego, CA: Jeremy Pettus, Schafer Boeder. University of Washington, Seattle, WA: Irl B. Hirsch, Subbulaxmi Trikudanathan. Naomi Berrie Diabetes Center, Columbia University, New York City, NY: Robin Goland, Kristen Williams, Natasha Leibel. University of North Carolina, Chapel Hill, NC: John B. Buse, M. Sue Kirkman, Kate Bergamo, Klara R. Klein, Jean M. Dostou, Sriram Machineni, Laura A. Young, Jamie C. Diner. Henry Ford Health System, Detroit, MI: Davida Kruger, Arti Bhan, J. Kimberly Jones. Nemours Children's Health Jacksonville, Jacksonville, FL: Nelly Mauras, Matthew Benson, Keisha Bird, Kimberly Englert, Joe Permuy. Emory University, Atlanta GA: Andrew Muir, MD, Kristina Cossen, Eric Felner. Washington University, St. Louis, MO: Janet B. McGill, Maamoun Salam, Julie M. Silverstein, Samantha Adamson, Andrea Cedeno. Children's National Hospital, Washington, D.C.: Fran Cogen, Seema Meighan, Andrew Dauber. Ann and Robert Lurie Children's Hospital, Pritzker Department of Psychiatry and Behavioral Health, Chicago, IL: Jill Weissberg-Benchell. Boston University, Boston, MA and Beta Bionics, Concord, MA: Edward R. Damiano, Firas H. El-Khatib. Jaeb Center for Health Research, Tampa, FL: Katrina Ruedy, Roy Beck, Katrina Ruedy, Zoey Li, Peter Calhoun.
Nonauthor contributors:
Massachusetts General Hospital, Boston, MA: Evelyn Greaux, Barbara Steiner, Sarah Gaston, Rachel Bartholomew, Kim Martin. Barbara Davis Center for Diabetes, University of Colorado, Aurora, CO: Emily Jost, Cari Berget, Lindsey Towers, Samantha Lange, Estella Escobar, Christie Beatson, Sonya Walker, Angela Karami, Emily Boranian. Department of Pediatrics, Division of Pediatric Endocrinology and Diabetes, Stanford University School of Medicine, Palo Alto, CA: Liana Hsu. Cleveland Clinic, Cleveland, OH: Ana Surckla, Laura Lomeli, Diana Isaacs, Shannon Knapp, Andrea Debs, Tracy Tomaro, Julia Blanchette. Children's Hospital of Orange County: Heather Speer, Marissa Erickson, Samantha Thompson, Allyson McDaniel. University of Texas Southwestern (Adults), Dallas, TX: Suzanne Strowig, Lin Jordan. University of Texas Southwestern (Pediatrics), Dallas, TX: Michael Henson, Yasmin Molina, Chantal Nwosu, Vandana Kumar, Angie Burris, Kim Jernigan. University of Texas Health Science Center, San Antonio, San Antonio, TX: Sara Olivarri. University of California, San Diego, CA: Todd May, Adrienne Armstrong, Erin Giovanetti. University of Washington, Seattle, WA: Nancy Sanborn, Xenia Averkiou. Naomi Berrie Diabetes Center, Columbia University, New York City, NY: Jamie Hyatt, Sarah Pollak, Elizabeth Robinson, Emily Casciano, Analia Alvarez, Eleanor Zagoren, Jaclynn Johnson, Silpa Sharma. University of North Carolina, Chapel Hill, NC: Alex Kass, Virginia Purrington, Rachel Fraser, Julie Uehling. Henry Ford Health System, Detroit, MI: Terra Cushman, Heather Hunter, Natalie Corker, Shereen Mukhashen. Nemours Children's Health Jacksonville, Jacksonville, FL: Kimberly Ponthieux, Albina Tarko. Emory University, Atlanta GA: Amber Antich, Wanda Sanchez, Mone Anzai, Kathryn Lucas, Catherine Simpson. Washington University, St. Louis, MO: Mary Jane Clifton, Toni Schweiger, Traci Bell. Children's National Hospital, Washington, D.C.: Meryll Castro, Tara McCarthy, Kimberly Boucher. Jaeb Center for Health Research: Sarah Borgman, Sydnee Bradshaw, Paige Miller, Rosa Pritchard, Elizaveta Dolzhenko. University of Minnesota Advanced Research and Diagnostic Laboratory: Deanna Gabrielson, Julie Idzorek, Anne Elstrom-Park
Contributor Information
Collaborators: Bionic Pancreas Research Group
Authors' Contributions
J.L. researched and interpreted the data and wrote the article; L.G.K. performed statistical analysis and contributed to writing, reviewed the article; S.J.R., E.R.D., F.H.E.-K., K.J.R., C.B., P.C., and R.W.B. researched data, contributed to the discussion, and reviewed the article.
Author Disclosure Statement
J.L. has nothing to disclose. L.G.K., K.J.R., and P.C. have no personal financial disclosures but report that their employer has received grant support from Beta Bionics, Dexcom, and Tandem Diabetes Care. S.J.R. has a patent and patents pending on aspects of the bionic pancreas that are assigned to Massachusetts General Hospital and are licensed to Beta Bionics, has received honoraria and/or travel expenses for lectures from Novo Nordisk, Roche, and Ascensia, serves on the scientific advisory boards of Unomedical and Companion Medical, has received consulting fees from Beta Bionics, Novo Nordisk, Senseonics, and Flexion Therapeutics, has received grant support from Zealand Pharma, Novo Nordisk, and Beta Bionics, and has received in-kind support in the form of technical support and/or donation of materials from Zealand Pharma, Ascencia, Senseonics, Adocia, and Tandem Diabetes. ERD has issued patents and pending patents on aspects of the bionic pancreas, and is an employee, the Executive Chair of the Board of Directors, and shareholder of Beta Bionics. F.H.E.-K. has issued patents and pending patents on aspects of the bionic pancreas and is an employee and shareholder of Beta Bionics. C.B. reports receiving consulting payments from Beta Bionics, Novo Nordisk, and Zealand Pharma. R.W.B. reports no personal financial disclosures but reports that his institution has received funding on his behalf as follows: grant funding and study supplies from Tandem Diabetes Care, Beta Bionics, and Dexcom; study supplies from Medtronic, Ascencia, and Roche; consulting fees and study supplies from Eli Lilly and Novo Nordisk; and consulting fees from Insulet, Bigfoot Biomedical, vTv Therapeutics, and Diasome.
Funding Information
Study funding has been provided by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (Grant #1UC4DK108612–01), by an Investigator-Initiated Study award from Novo Nordisk, and by Beta Bionics, Inc., which also provided the experimental bionic pancreas devices used in the study. Fast-acting insulin aspart and insulin aspart were provided by Novo Nordisk, and insulin lispro was provided by Eli Lilly. Blood glucose meters and test strips (Contour®Next One Blood Glucose Monitoring System) were provided by Ascensia Diabetes Care, Basel, CH. Continuous glucose monitor sensors and transmitters were purchased from Dexcom, Inc. at a discounted price.
Supplementary Material
References
- 1. Bergenstal RM, Garg S, Weinzimer SA, et al. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA 2016;316(13):1407–1408; doi: 10.1001/jama.2016.11708 [DOI] [PubMed] [Google Scholar]
- 2. Carlson AL, Sherr JL, Shulman DI, et al. Safety and glycemic outcomes during the minimed advanced hybrid closed-loop system pivotal trial in adolescents and adults with type 1 diabetes. Diabetes Technol Ther 2022;24(3):178–189; doi: 10.1089/dia.2021.0319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Forlenza GP, Pinhas-Hamiel O, Liljenquist DR, et al. Safety evaluation of the MiniMed 670G system in children 7–13 years of age with type 1 diabetes. Diabetes Technol Ther 2019;21(1):11–19; doi: 10.1089/dia.2018.0264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Garg SK, Weinzimer SA, Tamborlane WV, et al. Glucose outcomes with the in-home use of a hybrid closed-loop insulin delivery system in adolescents and adults with type 1 diabetes. Diabetes Technol Ther 2017;19(3):155–163; doi: 10.1089/dia.2016.0421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Breton MD, Kanapka LG, Beck RW, et al. A randomized trial of closed-loop control in children with type 1 diabetes. N Engl J Med 2020;383:836–845; doi: 10.1056/NEJMoa2004736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brown SA, Kovatchev BP, Raghinaru D, et al. Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med 2019;381(18):1707–1717; doi: 10.1056/NEJMoa1907863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brown SA, Forlenza GP, Bode BW, et al. Multicenter trial of a tubeless, on-body automated insulin delivery system with customizable glycemic targets in pediatric and adult participants with type 1 diabetes. Diabetes Care 2021;44(7):1630–1640; doi: 10.2337/dc21-0172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Forlenza GP, Lal RA. Current status and emerging options for automated insulin delivery systems. Diabetes Technol Ther 2022;24(5):362–371; doi: 10.1089/dia.2021.0514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bionic Pancreas Research Group; Beck RW, Russell SJ, Damiano E, et al. A multicenter randomized trial evaluating fast-acting insulin aspart in the bionic pancreas in adults with type 1 diabetes. Diabetes Technol Ther 2022;24(10):681–696; doi: 10.1089/dia.2022.0167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bionic Pancreas Research Group; Kruger D, Kass A, Lonier J, et al. A multicenter randomized trial evaluating the insulin-only configuration of the bionic pancreas in adults with type 1 diabetes. Diabetes Technol Ther 2022;24(10):697–711; doi: 10.1089/dia.2022.0200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bionic Pancreas Research Group; Messer LH, Buckingham B, Cogen F, et al. Positive impact of the bionic pancreas on diabetes control in youth 6–17 years old with type 1 diabetes: A multicenter randomized trial. Diabetes Technol Ther 2022;24(10):712–725; doi: 10.1089/dia.2022.0201.pub [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bionic Pancreas Research Group; Russell SJ, Beck RW, Damiano ER, et al. Multicenter, randomized trial of a bionic pancreas in type 1 diabetes. N Engl J Med 2022;387(31):1161–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beck RW, Bocchino LE, Lum JW, et al. An evaluation of two capillary sample collection kits for laboratory measurement of HbA1c. Diabetes Technol Ther 2021;23(8):537–545; doi: 10.1089/dia.2021.0023 [DOI] [PubMed] [Google Scholar]
- 14. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329(14):977–986; doi: 10.1056/nejm199309303291401 [DOI] [PubMed] [Google Scholar]
- 15. Benjamini Y, Krieger AM, Yekutieli D. Adaptive linear step-up procedures that control the false discovery rate. Biometrika 2006;93(3):491–507; doi: 10.1093/biomet/93.3.491 [DOI] [Google Scholar]
- 16. Kanapka LG, Lum JW, Beck RW. Insulin pump infusion set failures associated with prolonged hyperglycemia: Frequency and relationship to age and type of infusion set during 22,741 infusion set wears. Diabetes Technol Ther 2022;24(6):396–402; doi: 10.1089/dia.2021.0548 [DOI] [PubMed] [Google Scholar]
- 17. Heise T, Pieber TR, Danne T, et al. A pooled analysis of clinical pharmacology trials investigating the pharmacokinetic and pharmacodynamic characteristics of fast-acting insulin aspart in adults with type 1 diabetes. Clin Pharmacokinet 2017;56(5):551–559; doi: 10.1007/s40262-017-0514-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Heise T, Stender-Petersen K, Hovelmann U, et al. Pharmacokinetic and pharmacodynamic properties of faster-acting insulin aspart versus insulin aspart across a clinically relevant dose range in subjects with type 1 diabetes mellitus. Clin Pharmacokinet 2017;56(6):649–660; doi: 10.1007/s40262-016-0473-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.