Abstract
Background
Endoscopic submucosal dissection (ESD) has gained increasing popularity in the management of complicated colorectal polyps. However, clinical outcomes for ESD have remained highly inconsistent worldwide. This study investigated and analysed factors that significantly affect ESD outcomes.
Methods
We conducted a single-centred retrospective study on 220 colorectal polyps removed by ESD from 1st January 2016 to 31st December 2020. Data were collected and retrieved from clinical records. Variables studied included patient demographics, ESD technicalities and polyp characteristics. The primary outcome was completeness of resection based on en bloc and R0 resection rates. The secondary outcomes were recurrence, complications and hospital stay. Further analysis was performed for significant outcome determining factors.
Results
The en bloc resection and R0 resection rates were 97.3% and 65% respectively. Intraprocedural and delayed perforation rates were 3.2% and 0.5% respectively. Intraprocedural and delayed bleeding rates were both 1.8%. Post-polypectomy syndrome rate was 2.7%. The median hospital stay was 4 days. Submucosal fibrosis was a significant determining factor for lower en bloc resection (p = 0.004), lower R0 resection (p = 0.002), intraprocedural perforation (p = 0.001), intraprocedural bleeding (p = 0.025) and post-polypectomy syndrome (p = 0.039). Hybrid snaring was associated with lower en bloc resection (p < 0.001), while longer ESD time was associated with lower R0 resection (p = 0.003) and post-polypectomy syndrome (p = 0.025). Other significant factors for post-polypectomy syndrome included young age (p = 0.021) and large polyp size (p = 0.018). Secondary analysis showed that submucosal fibrosis was significantly associated with non-granular lesions (p < 0.001) and prior biopsy (p = 0.003).
Conclusion
Submucosal fibrosis, hybrid snaring, ESD time, age and polyp size were significant outcome determining factors for ESD. By identifying these factors, strategies may be formulated to improve ESD outcomes.
Keywords: Endoscopic submucosal dissection, Colorectal polyps, Submucosal fibrosis, En bloc resection, R0 resection
Over the past decade, endoscopic submucosal dissection (ESD) has emerged as the mainstay of management for large and sessile colorectal polyps, especially lateral spreading tumours. ESD was first introduced in the 1990s in Japan as a treatment modality for early gastric cancers. Since then, its indications have extended to the colorectal sector. Compared to traditional surgery and endoscopic mucosal resection (EMR), colorectal ESD has the advantage of being less invasive with lower overall operative risk [1] and a higher en bloc resection rate respectively. However, ESD is known for being technically demanding and challenging with substantial perforation and bleeding risks. While studies in Japan showed high en bloc resection rates of > 90% with low complication rates of 1–5% [2], such results have not been replicated globally. In order to seek ways and formulate strategies to improve colorectal ESD outcomes, we conducted a study to identify important determining factors.
Materials and methods
Patients
Our study was a single-centred retrospective study on colorectal ESDs performed over a 5-year period. The study was conducted in Queen Elizabeth Hospital, a local tertiary hospital with around 50 colorectal ESDs performed every year. As this retrospective study was a review of clinical records, IRB approval or written consent was not required. We included all colorectal polyps removed by ESD from 1st January 2016 to 31st December 2020. The indications for ESD were lesions that cannot be removed en bloc with EMR. This was in accordance to the Colorectal ESD Standardization Implementation Working Group [3]. The feasibility of ESD was assessed prior to ESD and during ESD. Lesions that showed non-lifting sign or muscle invasion were deemed not suitable for ESD and excluded from analysis. The endoscopists in our study were colorectal specialists who had performed more than 1000 colonoscopies and were equipped with advanced skills. All of them had no prior experience in gastric ESD and received ESD training in simulators and animal models in Japan. The indications, risks and benefits of ESD were explained to the patients and all patients provided written informed consents. Patients were given mechanical bowel preparation and antibiotics prior to the procedures. The procedural details of ESD were similar to that described by Saito [4]. All procedures were done under conscious sedation with midazolam and fentanyl. Water-jet endoscopes with short-type ST hoods were used. Air was used for colonic insufflation. All polyps were assessed endoscopically under white line and Narrow Band Imaging (NBI). Hyaluronic acid mixed with indigo carmine was used for submucosal elevation. Lesions were marked with electrocautery prior to dissection. Scissors-type and/or needle-type ESD knives were used for dissection. Hybrid snaring was used for selected lesions to facilitate resection. Hybrid snaring is a technique that combines ESD and snaring, in which a circumferential mucosal incision was made followed by partial submucosal dissection and snaring [5]. Haemostatic graspers were used for haemostasis or control of non-bleeding visible vessels. Endoclips were used to manage small perforations or appose mucosal defects whenever possible. All resected specimens were retrieved and sent for pathological assessment. All patients were hospitalised to observe for any complications. Surveillance colonoscopies were arranged 6 months later for lesions with piecemeal resection or positive margins. For lesions with complete en bloc resection and negative margins, surveillance colonoscopies were arranged 12 months later.
Data collection
Data were collected retrospectively from clinical notes, endoscopic records and photos. Data recorded included patient demographics such as age and sex; operating endoscopists; procedural technicalities such as procedural time, type of ESD knife used, hybrid snaring, use of traction aids, use of haemostatic grasper and use of endoclip; polyp characteristics such as size, location, morphology, granularity, capillary pattern, pit pattern, submucosal fibrosis, previous tattoo injection, previous biopsy and previous submucosal elevation; pathological assessment such as histology, degree of dysplasia and margin; complications such as perforation, bleeding, post-polypectomy syndrome and outcomes such as en bloc resection rate, R0 resection rate, recurrence rate, reintervention rate, 30-day readmission rate and length of hospital stay. Morphology was based on Paris classification. Pit pattern was based on Kudo’s classification, and Capillary pattern was based on Sano’s classification. Submucosal fibrosis was defined as the endoscopic appearance of white fibres in the transparent submucosal layer. En bloc resection was defined as complete gross resection of the lesion in a single specimen. R0 resection was defined as microscopic absence of dysplastic or neoplastic cells at the circumferential and deep margins. Intraprocedural perforation was defined as full-thickness defect with visualisation of visceral fat during ESD. Intraprocedural bleeding was defined as the presence of oozing or spurting during ESD. Delayed perforation was defined as perforation occurring after completion of the procedure [6] evidenced by abdominal pain, peritoneal sign and the presence of pneumoperitoneum on imaging. Delayed bleeding was defined as clinically significant bleeding manifesting as per-rectal bleeding or melena, or a drop in the haemoglobin by more than 2 g/dL after ESD [7]. Post-polypectomy syndrome was defined as the presence of abdominal pain, fever and leukocytosis after ESD in the absence of perforation [8]. Recurrence was defined as histologically proven lesion at the ESD site during follow-up colonoscopy 6 or 12 months later. Missing data were recorded and excluded from analysis.
Statistical analysis
Statistical analysis was carried out using IBM SPSS statistics 20. Univariate analysis was performed for continuous and categorical variables. Mean with standard deviation and median with interquartile range were calculated for continuous variables. Multivariate analysis was performed to examine the association and relationship between variables and outcome. Point biserial correlation was used for continuous variables, while chi-squared test and fisher’s exact test were used for categorical variables. Odds ratio for statistically significant associating factors were generated using logistic regression. Statistical significance was two-tailed and defined as p ≤ 0.05. For significant outcome determining factors, multiple logistic regression was carried out to identify significant independent predictors (Table 1).
Table 1.
Patient demographics, distribution of endoscopists, characteristics of polyps and technicalities of ESD
| Gender | |
| Male (n, %) | 142 (64.5) |
| Female (n, %) | 78 (35.5) |
| Age (y, median ± IQR) | 69 (46–91) |
| Endoscopist (n, %) | |
| A | 26 (11.8) |
| B | 112 (50.9) |
| C | 69 (31.4) |
| D | 13 (5.9) |
| Location (n, %) | |
| Right colon | 86 (39.1) |
| Transverse colon | 34 (15.5) |
| Left colon | 62 (28.2) |
| Rectum | 38 (17.3) |
| Morphology (n, %) | |
| Ip | 6 (2.7) |
| Is | 35 (15.9) |
| LST | 179 (81.4) |
| Granularity (n, %) | |
| Granular | 113 (51.4) |
| Non-granular | 107 (48.6) |
| Capillary pattern (Sano) (n, %) | |
| 1 | 13 (5.9) |
| 2 | 147 (66.8) |
| 3a | 59 (26.8) |
| 3b | 0 (0) |
| Missing | 1 (0.5) |
| Pit pattern (Kudo) (n, %) | |
| I | 1 (0.5) |
| II | 20 (9.1) |
| III | 74 (33.6) |
| IV | 118 (53.6) |
| V | 6 (2.7) |
| Missing | 1 (0.5) |
| Prior tattoo injection (n, %) | 33 (15) |
| Prior biopsy (n, %) | 182 (82.7) |
| Multiple biopsy | 46 (20.9) |
| Prior submucosal injection (n, %) | 6 (2.7) |
| Submucosal fibrosis (n, %) | 88 (40) |
| Size (cm, mean + / − SD) | 2.58 + / − 0.081 |
| Histology (n, %) | |
| Adenocarcinoma | 21 (9.5) |
| Villous adenoma | 5 (2.3) |
| Tubulovillous adenoma | 71 (32.3) |
| Tubular adenoma | 98 (44.5) |
| Serrated adenoma | 19 (8.6) |
| Hyperplastic | 3 (1.4) |
| Others | 3 (1.4) |
| Degree of dysplasia (n, %) | |
| None | 10 (4.5) |
| Low grade | 118 (53.6) |
| High grade | 71 (32.3) |
| Not applicable | 21 (9.5) |
| Type of knife (n, %) | |
| Forceps type | 186 (84.5) |
| Needle type | 14 (6.4) |
| Both | 20 (9.1) |
| Use of traction aids (n, %) | 3 (1.4) |
| Use of haemostatic grasper (n, %) | 87 (39.5) |
| Clipping of defect (n, %) | 148 (67.3) |
| Hybrid snaring (n, %) | 28 (12.7) |
| ESD time (mins, mean + / − SD) | 78.37 + / − 4.51 |
| Surveillance colonoscopy (n, %) | 130 (59.1) |
Results
Flowchart and basic characteristics
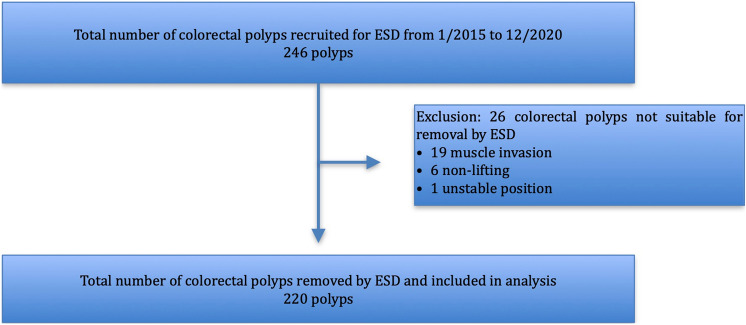
From 1st January 2015 to 31th December 2020, 246 colorectal polyps were recruited for ESD. 220 polyps were successfully removed with ESD and included for analysis, while 26 polyps were deemed not suitable for resection during ESD and excluded. Figure 1 shows the study flowchart. Table 2 summarises the patient demographics, distribution of endoscopists, polyp characteristics and technicalities. In our sample, there were more male patients (64.5%) and the median age was 69. Majority of the polyps were granular (51.4%) lateral spreading tumours (81.4%) located in the right colon (39.1%), with Sano II capillary pattern (66.8%) and Kudo IV pit pattern (53.6%). Most of the polyps had biopsy prior to ESD (82.7%). The mean polyp size was 2.58 cm. For pathological examination, polyps were mainly tubular adenomas (44.5%) and low-grade dysplastic lesions (53.6%). For technicalities, the majority of ESDs were performed with forceps-type knives (84.5%) with no use of traction aids (98.6%) or haemostatic graspers (60.5). Most of the defects were clipped (67.3%). The mean ESD time was 78.37 min. Majority of patients had follow-up surveillance colonoscopy (59.1%).
Fig. 1.
Flowchart of analysis
Table 2.
Clinical and pathological outcomes
| En bloc resection (n, %) | 214 (97.3) |
| R0 resection (n, %) | 143 (65) |
| R1 resection (n, %) | 70 (31.8) |
| – Circumferential margin | 51 (72.9) |
| – Deep margin | 0 (0) |
| – Circumferential and deep margins | 2 (2.9) |
| – Not specified | 24 (24.2) |
| Undetermined resection (n, %) | 7 (3.2) |
| Perforation (n, %) | |
| – Intraprocedural | 7 (3.2) |
| – Delayed | 1 (0.5) |
| Bleeding (n, %) | |
| – Intraprocedural | 4 (1.8) |
| – Delayed | 4 (1.8) |
| Post-polypectomy syndrome | 6 (2.7) |
| Length of hospital stay (days, median, IQR) | 4 (1–23) |
| Reintervention (n, %) | 4 (1.8) |
| 30 day readmission (n, %) | 5 (2.3) |
| Local recurrence (n, %) | 5 (2.3) |
Outcomes
Table 2 summarises the ESD outcomes. The en bloc resection rate was 97.3%. The R0 resection rate was 65% and 3.2% were undetermined. Out of those with positive margins, 72.9% were circumferential, 2.9% were circumferential and deep and 24.2% were nonspecific. The intraprocedural and delayed perforation rates were 3.2% and 0.5%, respectively. All intraprocedural perforations were managed with endoscopic clipping. There was one case of delayed perforation, which required emergency operation and colectomy. The intraprocedural and delayed bleeding rates were both 1.8%. 3 out of 4 cases of intraprocedural bleeding required the use of haemostatic graspers. 2 out of 4 cases of delayed bleeding were managed with endoscopic clipping. The other 2 were managed conservatively with medical treatment. The post-polypectomy syndrome rate was 2.7%. The median hospital stay was 4 days. 30-day readmission and reintervention rates were 2.3% and 1.8%, respectively. 2 readmissions were due to delayed bleeding, while the other 3 were not related to ESD. There were 4 reintervention cases, 1 for delayed perforation, 2 for delayed bleeding and 1 for re-clipping of mucosal defect. Local recurrence rate was 2.3%. In our series, there were 21 malignant polyps (9.5%), in which 6 had subsequent colectomies (28.6%) due to high-risk histological features, close or involved margins and deep submucosal invasion. There was 1 local recurrence (4.8%) that occurred in a patient who refused colectomy for close margin.
Completeness of resection and recurrence
En bloc resection was significantly lower in the presence of submucosal fibrosis (p = 0.004, OR 0.0478) and with the use of hybrid snaring (p < 0.001; OR 0.024). R0 resection was significantly lower in the presence of submucosal fibrosis (p = 0.002; OR 0.353) and with longer duration of ESD (p = 0.025; OR 0.993). Recurrence was significantly higher with lower en bloc resection (p = 0.006; OR 0.028). Tables 3, 4 and 5 show the relationship between the determining factors and resection completeness and recurrence.
Table 3.
Determining factors for en bloc resection
| P value | Odds ratio | 95% Confidence Interval | |
|---|---|---|---|
| Gender | 0.668 | ||
| Age | 0.948 | ||
| Endoscopist | 0.07 | ||
| Location | 0.649 | ||
| Morphology | 1 | ||
| Granularity | 1 | ||
| Prior tattoo injection | 0.222 | ||
| Prior biopsy | 1 | ||
| Prior submucosal injection | 0.155 | ||
| Submucosal fibrosis | 0.004 | 0.0478 | 0.00267–0.754 |
| Size | 0.591 | ||
| Histology | 0.702 | ||
| Degree of dysplasia | 0.761 | ||
| Type of knife | 1 | ||
| Use of traction aids | 1 | ||
| Hybrid snaring | < 0.001 | 0.024 | 0.003–0.215 |
| ESD time | 0.287 |
Table 4.
Determining factors for R0 resection
| P value | Odds ratio | 95% Confidence Interval | |
|---|---|---|---|
| Gender | 0.647 | ||
| Age | 0.079 | ||
| Endoscopist | 0.258 | ||
| Location | 0.824 | ||
| Morphology | 0.230 | ||
| Granularity | 0.47 | ||
| Prior tattoo injection | 0.836 | ||
| Prior biopsy | 0.126 | ||
| Prior submucosal injection | 0.334 | ||
| Submucosal fibrosis | 0.002 | 0.353 | 0.196–0.638 |
| Size | 0.8 | ||
| Histology | 0.107 | ||
| Degree of dysplasia | 0.104 | ||
| Type of knife | 0.214 | ||
| Use of traction aids | 1 | ||
| Hybrid snaring | 0.112 | ||
| ESD time | 0.025 | 0.993 | 0.988–0.998 |
Table 5.
Determining factors for recurrence
| P value | Odds Ratio | 95% Confidence Interval | |
|---|---|---|---|
| Gender | 0.658 | ||
| Age | 0.561 | ||
| Endoscopist | 0.672 | ||
| Location | 0.929 | ||
| Morphology | 1 | ||
| Granularity | 1 | ||
| Submucosal fibrosis | 0.085 | ||
| Size | 0.616 | ||
| Histology | 0.471 | ||
| Degree of dysplasia | 0.45 | ||
| Hybrid snaring | 0.123 | ||
| ESD time | 0.325 | ||
| En bloc resection | 0.006 | 0.028 | 0.004–0.22 |
| R0 resection | 0.599 |
Complications
Intraprocedural perforation and bleeding were both significantly higher in the presence of submucosal fibrosis (p = 0.001, OR 24.386; p = 0.025, OR 14.126, CI 1.477 to 1879.95). Delayed perforation and delayed bleeding were not significantly associated with any factors. Post-polypectomy syndrome was significantly higher for younger age (p = 0.021, OR 0.905), submucosal fibrosis (p = 0.039, OR 5.766), larger polyp size (p = 0.018, OR 1.505) and longer duration of ESD (p = 0.029, OR 1.008). Tables 6, 7 and 8 show the relationship between the determining factors and complications.
Table 6.
Determining factors for perforation
| Intraprocedural | Delayed | |||||
|---|---|---|---|---|---|---|
| P value | Odds ratio | 95% Confidence Interval | P value | Odds ratio | 95% Confidence Interval | |
| Gender | 0.248 | 0.355 | ||||
| Age | 0.634 | 0.455 | ||||
| Endoscopist | 0.769 | 1 | ||||
| Location | 0.296 | 1 | ||||
| Morphology | 0.673 | 1 | ||||
| Granularity | 1 | 0.486 | ||||
| Prior tattoo injection | 0.071 | 1 | ||||
| Prior biopsy | 0.607 | 1 | ||||
| Prior submucosal injection | 1 | 1 | ||||
| Submucosal fibrosis | 0.001 | 24.386 | 2.907–3181.15 | 1 | ||
| Size | 0.461 | 0.216 | ||||
| Histology | 0.669 | 1 | ||||
| Degree of dysplasia | 0.761 | 1 | ||||
| Type of knife | 1 | 0.064 | ||||
| Traction aids | 1 | 1 | ||||
| Haemostatic grasper | 0.249 | 0.395 | ||||
| Clipping of defect | N/A | 0.327 | ||||
| Hybrid snaring | 0.219 | 1 | ||||
| ESD time | 0.309 | 0.557 | ||||
Table 7.
Determining factors for bleeding
| Intraprocedural | Delayed | |||||
|---|---|---|---|---|---|---|
| P value | Odds ratio | 95% Confidence Interval | P value | Odds ratio | 95% Confidence Interval | |
| Gender | 1 | 1 | ||||
| Age | 0.644 | 0.479 | ||||
| Endoscopist | 0.068 | 0.204 | ||||
| Location | 0.7551 | 0.123 | ||||
| Morphology | 1 | 1 | ||||
| Granularity | 0.358 | 0.358 | ||||
| Prior tattoo injection | 1 | 1 | ||||
| Prior biopsy | 1 | 0.534 | ||||
| Prior submucosal injection | 1 | 1 | ||||
| Submucosal fibrosis | 0.025 | 14.126 | 1.477–1879.95 | 0.651 | ||
| Size | 0.086 | 0.754 | ||||
| Histology | 0.248 | 1 | ||||
| Degree of dysplasia | 1 | 1 | ||||
| Type of knife | 1 | 1 | ||||
| Traction aids | 0.054 | 1 | ||||
| Haemostatic grasper | 0.303 | 0.303 | ||||
| Clipping of defect | N/A | 0.599 | ||||
| Hybrid snaring | 1 | 1 | ||||
| ESD time | 0.113 | 0.543 | ||||
Table 8.
Determining factors for post-polypectomy syndrome
| Post-polypectomy syndrome | |||
|---|---|---|---|
| P value | Odds ratio | 95% Confidence Interval | |
| Gender | 0.189 | ||
| Age | 0.021 | 0.909 | 0.822–0.996 |
| Endoscopist | 0.901 | ||
| Location | 0.275 | ||
| Morphology | 0.655 | ||
| Granularity | 0.435 | ||
| Prior tattoo injection | 1 | ||
| Prior biopsy | 1 | ||
| Prior submucosal injection | 1 | ||
| Submucosal fibrosis | 0.039 | 5.766 | 1.126–56.997 |
| Size | 0.018 | 1.517 | 1.017–2.177 |
| Histology | 0.357 | ||
| Degree of dysplasia | 0.571 | ||
| Type of knife | 0.639 | ||
| Use of traction aids | 1 | ||
| Use of haemostatic grasper | 0.683 | ||
| Clipping of defect | 0.181 | ||
| Hybrid snaring | 0.563 | ||
| ESD time | 0.029 | 1.008 | 1.001–1.016 |
Hospital stay
Hospital stay was significantly longer for elder patients (p = 0.002), females (p < 0.001), longer duration of ESD (p < 0.001), intraprocedural perforation (p < 0.001), delayed perforation (p = 0.012) and post-polypectomy syndrome (p < 0.001). Table 9 shows the relationship between different factors and hospital stay.
Table 9.
Determining factors for length of hospital stay
| P value | |
|---|---|
| Gender | < 0.001 |
| Age | 0.002 |
| ESD time | < 0.001 |
| Perforation | |
| Intraprocedural | < 0.001 |
| Delayed | 0.012 |
| Bleeding | |
| Intraprocedural | 0.237 |
| Delayed | 0.257 |
| Post-polypectomy syndrome | < 0.001 |
Outcome determining factor
Submucosal fibrosis was found to be a significant outcome determining factor for completeness of resection and complications. Further analysis was performed to identify independent predictors of submucosal fibrosis. Table 10 summarises the results. Submucosal fibrosis was significantly higher for non-granular lesions (p < 0.001, OR 3.539) and prior biopsy (p = 0.003, OR 4.813).
Table 10.
Predictors of submucosal fibrosis
| P value | Odds ratio | 95% Confidence Interval | |
|---|---|---|---|
| Right colon | 0.33 | ||
| Transverse colon | 0.493 | ||
| Left colon | 0.095 | ||
| Rectum | 0.403 | ||
| LST | 0.173 | ||
| Ip | 0.417 | ||
| Is | 0.078 | ||
| Granularity | < 0.001 | 3.539 | 1.789–6.999 |
| Capillary pattern | 0.659 | ||
| Pit pattern | 0.629 | ||
| Prior tattoo injection | 0.221 | ||
| Prior biopsy | 0.003 | 4.813 | 1.733–13.369 |
| Prior submucosal injection | 0.130 | ||
| Size | 0.190 | ||
| Hyperplastic | 0.771 | ||
| Serrated Adenoma | 0.453 | ||
| Tubular Adenoma | 0.434 | ||
| Tubulovillous Adenoma | 0.386 | ||
| Villous adenoma | 0.516 | ||
| Adenocarcinoma | 0.479 | ||
| Others | 0.956 | ||
| Degree of dysplasia | 0.425 |
Discussion
Our study was the largest local series on colorectal ESD by far. Overall, we achieved a high en bloc resection rate and a low complication rate. The en bloc resection rate was 97.3%, which was consistent with reports worldwide (79.4–99.5%) [9]. The largest retrospective case series of 1259 colorectal lesions by the Hiroshima group showed an en bloc resection rate of 92.6% [10], while a meta-analysis of 97 studies showed an en bloc resection rate of 91% for colorectal ESD [11]. Although our en bloc resection rate was high, there were considerable differences in case selection compared to studies at expert centres. Our sample consisted of smaller lesions and fewer malignant lesions. Also, we included non-lifting sign as an exclusion criterion for ESD, which could have potentially excluded more difficult lesions. In our study, submucosal fibrosis and hybrid snaring were significant determining factors for low en bloc resection rate. These findings were demonstrated in previous studies, which also revealed other significant factors including poor submucosal lifting and long duration of ESD [12, 13]. Our R0 resection rate was 65%, which was lower than that from the literature (69–97%) [9]. In our study, the majority of involved margins were circumferential rather than deep. This may be due to inadequate lateral margin during dissection. Prior to dissection, lesions were assessed under white light and NBI only. Sometimes, the borders of subtle lesions, especially non-granular lesions, may be difficult to delineate. The combination of chromoendoscopy with 0.4% indo-carmine dye spray may facilitate the demarcation of lesions [4]. Furthermore, taking a wider margin during dissection may also enhance the R0 resection rate. In our study, submucosal fibrosis and long duration of ESD were significant factors associated with low R0 resection rate. This coheres with previous studies, which additionally also showed polyp location and polyp size as significant factors [14–16]. Despite a low R0 resection rate, our recurrence rate was only 2.3%, which was similar to other case series and meta-analyses (0–5.8%) [11, 17–22]. However, we had a suboptimal rate of surveillance colonoscopy (59.1%) and the interval of follow-up was short, which may underestimate the true recurrence rate. On the contrary, there may be an underestimation of the true R0 resection rate due to the cytological artefacts such as hyperchromasia and elongation of the nuclei created during electrocautery [23], which can affect the evaluation of the margin. In fact, multiple studies have shown that recurrence was not correlated with positive lateral margin following complete en bloc resection [24, 25]. In our study, recurrence rate was significantly correlated with en bloc resection rate, but not R0 resection rate. Therefore, en bloc resection rate might be a more clinically relevant outcome compared to R0 resection rate. Overall, our study showed that ESD is effective in treating large colorectal polys with a high success rate.
Our overall complication rate was 0.5–3.2%, which was at the lower end compared with international studies (0–9.3%) [9]. Our intraprocedural and delayed perforation rates were 3.2% and 0.5%, respectively, comparable to a recent meta-analysis that reported rates of 4.2% and 0.22%, respectively [26]. In our study, we found that submucosal fibrosis was significantly associated with intraprocedural perforation. This echoes with findings from reports worldwide, which also revealed other risk factors such as large polyp size, polyp location and lack of operator experience [27]. All our intraprocedural perforations were successfully managed endoscopically with clipping, which is the recommended first-line treatment for intraprocedural perforations [28, 29]. There was one delayed perforation in our study that required surgery. Delayed perforation is caused by thermal injury to the bowel wall during dissection. It is associated with poor prognosis and often requires surgery [22]. Our intraprocedural and delayed bleeding rates were both 1.8%, comparable to results from a meta-analysis (0.75% and 2.1%, respectively) [21]. Submucosal fibrosis was the only significant factor associated with intraprocedural bleeding, while no factors were significantly associated with delayed bleeding. Previous studies have shown that the use of anticoagulation, large polyp size, rectal polyps and intraprocedural bleeding were significant risks factors for delayed bleeding [30, 31]. In our study, most cases of intraprocedural and delayed bleeding required the use of haemostatic graspers and endoclips. In fact, studies have recommended their use in tackling significant bleeding from major vessels and preventing delayed bleeding by coagulating or clipping major exposed vessels [23]. Our post-polypectomy syndrome rate was 2.7%, lower than that in the literature (4.8–14.2%) [32]. Our study showed that younger age, larger polyp size and longer ESD duration were significantly correlated with post-polypectomy syndrome. In addition to polyp size and ESD duration, reports have also shown that females, right-sided colonic lesions and submucosal fibrosis were associated with post-polypectomy syndrome. Although age was not reported previously as a significant factor, we postulate younger patients may mount a more florid inflammatory reaction and result in a more obvious clinical presentation. With low complication rates, our study showed that ESD is safe in treating large colorectal polyps with minimal morbidity.
Submucosal fibrosis was found to be a significant factor for outcomes of colorectal ESD. The presence of it resulted in lower en bloc and R0 resection rates, as well as higher perforation and bleeding rates. Similar findings were reported in the literature [12–16, 27]. Submucosal fibrosis is often induced by inflammation, tumour invasion or desmoplastic reaction [33]. In the presence of fibrosis, the submucosal plane becomes less well defined and obliterated at times. As a result, lifting and dissecting the tumour from the muscle layer becomes difficult, causing more incomplete resection and complications. The degree of submucosal fibrosis can be assessed endoscopically or histologically into no fibrosis (F0), mild fibrosis (F1) and severe fibrosis (F2). Endoscopically, it is based on the appearance after submucosal injection of indigo carmine solution [34]. Histologically, it is based on the extent and intensity of fibrosis [33]. Our study showed that non-granular LSTs and prior biopsy were significant independent predictors of submucosal fibrosis. While multiple studies have demonstrated more submucosal fibrosis in non-granular LSTs [33, 35–37], the effects of prior biopsy were more controversial [37–39]. A recent study showed specifically that prior biopsy of non-granular LSTs increased the risk of submucosal fibrosis [37]. This is because in flat lesions, biopsy has a higher possibility of reaching the deeper layers, thereby generating subsequent inflammatory reaction. Previous studies also revealed other risk factors of submucosal fibrosis including large polyp size and submucosal cancers [14, 33, 35]. In order to reduce submucosal fibrosis, our findings suggest the importance of avoiding biopsies of colorectal polyps prior to ESD, especially non-granular LSTs. While biopsy has the benefit of distinguishing adenoma from adenocarcinoma, the accuracy was only 54% based on a previous study [37]. In fact, the Japan Gastroenterological Endoscopy Society (JGES) guideline recommends against biopsy prior to ESD [29]. This reinforces the importance of magnifying endoscopy, chromoendoscopy and NBI, which evaluate the morphology, surface pit and capillary patterns of colorectal polyps. This valuable information can help predict the risk of malignancy and the invasiveness of colorectal polyps. Other adjuncts such as endoscopic ultrasound and endocytoscopy have also been advocated [40]. However, these diagnostic adjuncts require training, expertise and experience. In our series, there was poor correlation between surface patterns and the risk of malignancy and submucosal invasion. Furthermore, there was a high rate of pre-ESD biopsy. This was because obtaining tissue biopsy still remains a common practice in our locality and most of the polyps referred for ESD were performed elsewhere.
Our study had several strengths. Firstly, it is the largest ESD case series in Hong Kong. Secondly, it was conducted in a high-volume centre. Thirdly, we examined a large and comprehensive list of variables that were well defined. Lastly, there were very few missing data. However, there were also a few limitations. Firstly, our study was retrospective. Secondly, it was conducted in a single centre, which would limit its generalisability. Thirdly, there were multiple endoscopists, which could introduce observation and performance biases. Lastly, the surveillance colonoscopy rate was low, which could have underestimated the true recurrence. In fact, all patients were offered surveillance colonoscopy. However, the low rate could be explained by a combination of patient refusal due to advanced age and postponement of elective colonoscopies due to the COVID-19 pandemic.
Submucosal fibrosis, large polyp size, hybrid snaring, long duration of ESD and young age were significant factors associated with poor ESD outcomes. Non-granular LSTs and polyps with prior biopsy were more likely to harbour or develop submucosal fibrosis. These factors can help identify difficult lesions that should be managed by more experienced operators and help formulate strategies to improve ESD outcomes.
Acknowledgements
The authors would like to acknowledge the endoscopy and ward staffs for their assistance.
Declarations
Disclosures
Chow Chi Woo Samuel, Fung Tak Lit Derek, Chan Pak Tat and Kwok Kam Hung have no conflicts of interest or financial ties to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fung TLD, Chan PT, Lee HM, Kwok KH. Case-matched analysis comparing endoscopic submucosal dissection and surgical removal of difficult colorectal polyps. J Laparoendosc Adv Surg Tech. 2018;28:1188–1191. doi: 10.1089/lap.2018.0112. [DOI] [PubMed] [Google Scholar]
- 2.Fukuzawa M, Gotoda T. History of endoscopic submucosal dissection and role for colorectal endoscopic submucosal dissection: a Japanese perspective. Gastrointest Interv. 2012;1:30–35. doi: 10.1016/j.gii.2012.09.001. [DOI] [Google Scholar]
- 3.Colorectal ESD Standardization Implementation Working Group, Ed . Colorectal ESD guidebook. Tokyo: Nihon Medical Center; 2009. [Google Scholar]
- 4.Saito Y, Otake Y, Sakamoto T, Nakajima T, Yamada M, Haruyama S, So E, Abe S, Matsuda T. Indications for and technical aspects of colorectal endoscopic submucosal dissection. Gut and liver. 2013;7:263–269. doi: 10.5009/gnl.2013.7.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toyonaga T, Man-I M, Morita Y, Sanuki T, Yoshida M, Kutsumi H, Inokuchi H, Azuma T. The new resources of treatment for early stage colorectal tumors: EMR with small incision and simplified endoscopic submucosal dissection. Dig Endosc. 2009;21:31–37. doi: 10.1111/j.1443-1661.2009.00872.x. [DOI] [PubMed] [Google Scholar]
- 6.Nakajima T, Saito Y, Tanaka S, Iishi H, Kudo SE, Ikematsu H, Igarashi M, Saitoh Y, Inoue Y, Kobayashi K, Hisasbe T, Matsuda T, Ishikawa H, Suhijara KI. Current status of endoscopic resection strategy for large, early colorectal neoplasia in Japan. Surg Endosc. 2013;27:3263–3270. doi: 10.1007/s00464-013-2903-x. [DOI] [PubMed] [Google Scholar]
- 7.Tajiri H, Kitano S. Complication associated with endoscopic mucosal resection: definition of bleeding that can be viewed as accidental. Dig Endosc. 2004;16:134–136. doi: 10.1111/j.1443-1661.2004.00377.x. [DOI] [Google Scholar]
- 8.Benson BC, Myers JJ, Laczek JT. Postpolypectomy electrocoagulation syndrome: a mimicker of colonic perforation. Case Rep Emerg Med. 2013;2013:687931. doi: 10.1155/2013/687931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mann R, Gajendran M, Umapathy C, Perisetti A, Goyal H, Saligram S, Echavarria J. Endoscopic management of complex colorectal polyps: current insights and future trends. Front Med (Lausanne) 2022;8:728704. doi: 10.3389/fmed.2021.728704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boda K, Oka S, Tanaka S, Nagata S, Kunihiro M, Kuwai T, Hiraga Y, Furudoi A, Terasaki M, Nakadoi K, Higashiyama M, Okanobu H, Akagi M, Chayama K. Clinical outcomes of endoscopic submucosal dissection for colorectal tumors: a large multicenter retrospective study from the Hiroshima GI endoscopy research group. Gastrointest Endosc. 2018;87:714–722. doi: 10.1016/j.gie.2017.05.051. [DOI] [PubMed] [Google Scholar]
- 11.Fuccio L, Hassan C, Ponchon T, Mandolesi D, Farioli A, Cucchetti A, Frazzoni L, Bhandari P, Bellisario C, Bazzoli F, Repici A. Clinical outcomes after endoscopic submucosal dissection for colorectal neoplasia: a systematic review and meta-analysis. Gastrointest Endosc. 2017;86:74–86. doi: 10.1016/j.gie.2017.02.024. [DOI] [PubMed] [Google Scholar]
- 12.Seo M, Yang DH, Kim J, Song EM, Kim GU, Hwang SW, Park SH, Kim KJ, Ye BD, Byeon JS, Myung SJ, Yang SK. Clinical outcomes of colorectal endoscopic submucosal dissection and risk factors associated with piecemeal resection. Turkish J Gastroenterol. 2018;29:473–480. doi: 10.5152/tjg.2018.17400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takeuchi Y, Iishi H, Tanaka S, Saito Y, Ikematsu H, Kudo SE, Sano Y, Hisabe T, Yahagi N, Saitoh Y, Igarashi M, Kobayashi K, Yamano H, Shimizu S, Tsuruta O, Inoue Y, Watanabe T, Nakamura H, Fujii T, Uedo N, Shimokawa T, Ishikawa H, Sugihara K. Factors associated with technical difficulties and adverse events of colorectal endoscopic submucosal dissection: retrospective exploratory factor analysis of a multicenter prospective cohort. Int J Colorectal Dis. 2014;29:1275–1284. doi: 10.1007/s00384-014-1947-2. [DOI] [PubMed] [Google Scholar]
- 14.Sato K, Ito S, Kitagawa T, Kato M, Tominaga K, Suzuki T, Maetani I. Factors affecting the technical difficulty and clinical outcome of endoscopic submucosal dissection for colorectal tumors. Surg Endosc. 2014;28:2959–2965. doi: 10.1007/s00464-014-3558-y. [DOI] [PubMed] [Google Scholar]
- 15.Shiga H, Ohba R, Matsuhashi T, Jin M, Kuroha M, Endo K, Moroi R, Kayaba S, Iijima K. Feasibility of colorectal endoscopic submucosal dissection (ESD) carried out by endoscopists with no or little experience in gastric ESD. Dig Endosc. 2017;29:58–65. doi: 10.1111/den.12814. [DOI] [PubMed] [Google Scholar]
- 16.Isomoto H, Nishiyama H, Yamaguchi N, Fukuda E, Ishii H, Ikeda K, Ohnita K, Nakao K, Kohno S, Shikuwa S. Clinicopathological factors associated with clinical outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms. Endoscopy. 2009;41:679–683. doi: 10.1055/s-0029-1214979. [DOI] [PubMed] [Google Scholar]
- 17.Niimi K, Fujishiro M, Kodashima S, Goto O, Ono S, Hirano K, Minatsuki C, Yamamichi N, Koike K. Long-term outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms. Endoscopy. 2010;42:723–729. doi: 10.1055/s-0030-1255675. [DOI] [PubMed] [Google Scholar]
- 18.Saito Y, Uraoka T, Yamaguchi Y, Hotta K, Sakamoto N, Ikematsu H, Fukuzawa M, Kobayashi N, Nasu J, Michida T, Yoshida S, Ikehara H, Otake Y, Nakajima T, Matsuda T, Saito D. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video) Gastrointest Endosc. 2010;72:1217–1225. doi: 10.1016/j.gie.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Lee EJ, Lee JB, Lee SH, Kim DS, Lee DH, Lee DS, Youk EG. Endoscopic submucosal dissection for colorectal tumors−1,000 colorectal ESD cases: one specialized institute's experiences. Surg Endosc. 2013;27:31–39. doi: 10.1007/s00464-012-2403-4. [DOI] [PubMed] [Google Scholar]
- 20.Yamada M, Saito Y, Takamaru H, Sasaki H, Yokota T, Matsuyama Y, Sato Y, Sakamoto T, Nakajima T, Taniguchi H, Sekine S, Matsuda T. Long-term clinical outcomes of endoscopic submucosal dissection for colorectal neoplasms in 423 cases: a retrospective study. Endoscopy. 2017;49:233–242. doi: 10.1055/s-0042-124366. [DOI] [PubMed] [Google Scholar]
- 21.Shigita K, Oka S, Tanaka S, Sumimoto K, Hirano D, Tamaru Y, Ninomiya Y, Asayama N, Hayashi N, Shimamoto F, Arihiro K, Chayama K. Long-term outcomes after endoscopic submucosal dissection for superficial colorectal tumors. Gastrointest Endosc. 2017;85:546–553. doi: 10.1016/j.gie.2016.07.044. [DOI] [PubMed] [Google Scholar]
- 22.Draganov PV, Aihara H, Karasik MS, Ngamruengphong S, Aadam AA, Othman MO, Sharma N, Grimm IS, Rostom A, Elmunzer BJ, Jawaid SA, Westerveld D, Perbtani YB, Hoffman BJ, Schlachterman A, Siegel A, Coman RM, Wang AY, Yang D. Endoscopic submucosal dissection in North America: a large prospective multicenter study. Gastroenterology. 2021;160:2317–2327. doi: 10.1053/j.gastro.2021.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldstein NS, Watts JC, Neill JS, Vogel LM, Barkel D, Kadro O, Priest S, Klein S. The effect of electrothermal cautery-assisted resection of diminutive colonic polyps on histopathologic diagnosis. Am J Clin Pathol. 2001;115:356–361. doi: 10.1309/0KPE-1RG6-KA78-R49Y. [DOI] [PubMed] [Google Scholar]
- 24.Lee S, Kim J, Soh J, Bae J, Hwang SW, Park SH, Ye B, Leong R, Myung S-J, Yang S-K, Yang D-H. Recurrence rate of lateral margin-positive cases after en bloc endoscopic submucosal dissection of colorectal neoplasia. Int J Colorectal Dis. 2018;33:11. doi: 10.1007/s00384-018-3012-z. [DOI] [PubMed] [Google Scholar]
- 25.Park EY, Baek DH, Song G, Kim GH, Lee B, Park DY. Long-term outcomes of endoscopically resected laterally spreading tumors with a positive histological lateral margin. Surg Endosc. 2020;34:9. doi: 10.1007/s00464-019-07187-x. [DOI] [PubMed] [Google Scholar]
- 26.Akintoye E, Kumar N, Aihara H, Nas H, Thompson CC. Colorectal endoscopic submucosal dissection: a systematic review and meta-analysis. Endosc Int Open. 2016;10:1030–1044. doi: 10.1055/s-0042-114774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim ES, Cho KB, Park KS, Lee KI, Jang BK, Chung WJ, Hwang JS. Factors predictive of perforation during endoscopic submucosal dissection for the treatment of colorectal tumors. Endoscopy. 2011;43:573–578. doi: 10.1055/s-0030-1256339. [DOI] [PubMed] [Google Scholar]
- 28.Kim ER, Chang DK. Management of complications of colorectal submucosal dissection clinical. Endoscopy. 2019;52:114–119. doi: 10.5946/ce.2019.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanaka S, Kashida H, Saito Y, Yahagi N, Yamano H, Saito S, Hisabe T, Yao T, Watanabe M, Yoshida M, Kudo SE, Tsuruta O, Sugihara KI, Watanabe T, Saitoh Y, Igarashi M, Toyonaga T, Ajioka Y, Ichinose M, Matsui T, Sugita A, Sugano K, Fujimoto K, Tajiri H. JGES guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc. 2015;27:417–434. doi: 10.1111/den.12456. [DOI] [PubMed] [Google Scholar]
- 30.Kataoka Y, Tsuji Y, Sakaguchi Y, Minatsuki C, Asada-Hirayama I, Niimi K, Ono S, Kodashima S, Yamamichi N, Fujishiro M, Koike K. Bleeding after endoscopic submucosal dissection: risk factors and preventive methods. World J Gastroenterol. 2016;22:5927–5935. doi: 10.3748/wjg.v22.i26.5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ogasawara N, Yoshimine T, Noda H, Kondo Y, Izawa S, Shinmura T, Ebi M, Funaki Y, Sasaki M, Kasugai K. Clinical risk factors for delayed bleeding after endoscopic submucosal dissection for colorectal tumors in Japanese patients. Eur J Gastroenterol Hepatol. 2016;28:1407–1414. doi: 10.1097/MEG.0000000000000723. [DOI] [PubMed] [Google Scholar]
- 32.Cha JM, Lim KS, Lee SH, Joo YE, Hong SP, Kim TI, Kim HG, Park DI, Kim SE, Yang DH, Shin JE. Clinical outcomes and risk factors of post-polypectomy coagulation syndrome: a multicenter, retrospective, case-control study. Endoscopy. 2013;45:202–207. doi: 10.1055/s-0032-1326104. [DOI] [PubMed] [Google Scholar]
- 33.Lee SP, Kim JH, Sung I-K, Lee S-Y, Park HS, Shim CS, Han HS. Effect of submucosal fibrosis on endoscopic submucosal dissection of colorectal tumors: pathologic review of 173 cases. J Gastroenterol Hepatol. 2015;30:872–878. doi: 10.1111/jgh.12886. [DOI] [PubMed] [Google Scholar]
- 34.Matsumoto A, Tanaka S, Oba S, Kanao H, Oka S, Yoshihara M, Chayama K. Outcome of endoscopic submucosal dissection for colorectal tumors accompanied by fibrosis. Scand J Gastroenterol. 2010;45:1329–1337. doi: 10.3109/00365521.2010.495416. [DOI] [PubMed] [Google Scholar]
- 35.Inada Y, Yoshida N, Kugai M, Kamada K, Katada K, Uchiyama K, Handa O, Takagi T, Konishi H, Yagi N, Naito Y, Wakabayashi N, Yanagisawa A, Itoh Y. Prediction and treatment of difficult cases in colorectal endoscopic submucosal dissection. Gastroenterol Resident Practice. 2013;2013:523084. doi: 10.1155/2013/523084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goto SP, Sakamoto N, Mitomi H, Murakami T, Ritsuno H, Ueyama H, Matsumoto K, Shibuya T, Osada T, Nagahara A, Ogihara T, Yao T, Watanabe S. Histological distinction between the granular and nongranular types of laterally spreading tumors of the colorectum. Gastroenterol Resident Practice. 2014;2014:153935. doi: 10.1155/2014/153935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuroha M, Shiga H, Kanazawa Y, Nagai H, Handa T, Ichikawa R, Onodera M, Naito T, Moroi R, Kimura T, Endo K, Kakuta Y, Kinouchi Y, Shimosegawa T, Masamune A. Factors associated with fibrosis during colorectal endoscopic submucosal dissection: does pretreatment biopsy potentially elicit submucosal fibrosis and affect endoscopic submucosal dissection outcomes? Digestion. 2021;102:590–598. doi: 10.1159/000510145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim EK, Han DS, Ro Y, Eun CS, Yoo KS, Oh YH. The submucosal fibrosis: what does it mean for colorectal endoscopic submucosal dissection? Intest Res. 2016;14:358–364. doi: 10.5217/ir.2016.14.4.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fukunaga S, Nagami Y, Shiba M, Sakai T, Maruyama H, Ominami M, Otani K, Hosomi S, Tanaka F, Taira K, Tanigawa T, Yamagami H, Watanabe T, Fujiwara Y. Impact of preoperative biopsy sampling on severe submucosal fibrosis on endoscopic submucosal dissection for colorectal laterally spreading tumors: a propensity score analysis. Gastrointest Endosc. 2019;89:470–478. doi: 10.1016/j.gie.2018.08.051. [DOI] [PubMed] [Google Scholar]
- 40.Lee BI, Matsuda T. Estimation of invasion depth: the first key to successful colorectal ESD. Clin Endosc. 2019;52:100–106. doi: 10.5946/ce.2019.012. [DOI] [PMC free article] [PubMed] [Google Scholar]