Abstract
Selenium is an indispensable trace element for all living organisms. It is an essential structural component of several selenium-dependent enzymes, which support the human body’s defense mechanism. Recently, the significance of selenium in preventing/treating COVID-19 has been documented in the literature. This review highlights the clinical studies, compositions, and patent literature on selenium to prevent/treat COVID-19. Selenium exerts its anti-COVID-19 action by reducing oxidative stress, declining the expression of the ACE-2 receptor, lowering the discharge of pro-inflammatory substances, and inhibiting the 3CLPro (main protease) and PLpro enzyme of SARS-CoV-2. The data of clinical studies, inventive compositions, and patent literature revealed that selenium monotherapy and its compositions with other nutritional supplements/drugs (vitamin, iron, zinc, copper, ferulic acid, resveratrol, spirulina, N-acetylcysteine, fish oil, many herbs, doxycycline, azithromycin, curcumin, quercetin, etc.,) might be practical to prevent/treat COVID-19. The studies have also suggested a correlation between COVID-19 and selenium deficiency. This indicates that adequate selenium supplementation may provide promising treatment outcomes in COVID-19 patients. The authors foresee the development and commercialization of Selenium-based compositions and dosage forms (spray, inhalers, control release dosage forms, etc.) to battle COVID-19. We also trust that numerous selenium-based compositions are yet to be explored. Accordingly, there is good scope for scientists to work on developing novel and inventive selenium-based compositions to fight against COVID-19. However, there is also a need to consider the narrow therapeutic window and chemical interaction of selenium before developing selenium-based compositions.
Keywords: Selenium, COVID-19, SARS-CoV-2, Clinical trials, Patent
1. Introduction
The infection with SARS-CoV-2 (a RNA virus) causes contagious COVID-19, which has disastrously impacted the world’s demography [1]. The first case of COVID-19 emerged in December 2019, whereas the World Health Organization (WHO) announced it as a pandemic in March 2020 [1], [2], [3]. SARS-CoV-2 is transmitted through the respiratory droplets of the infected person and virus-contaminated surfaces/items [1]. The general symptoms of COVID-19 include fever, cough, sore throat, nasal congestion, headache, fatigue, pain in muscles/joints, loss of smell/taste, diarrhea, and breathlessness [1], [2], [3], [4]. Most of the COVID-19 patients (about 80%) recover without hospitalization, about 15% get serious symptoms, and about 5% require intensive care [1], [2]. The death-causing complications of COVID-19 comprise respiratory failure, septic shock, thromboembolism, and multiorgan failure [1], [2]. As of June 27, 2022, the World Health Organization (WHO) reported 539,893,858 confirmed cases of COVID-19, 6324,112 deaths due to COVID-19, and administration of 11,912,594,538 COVID-19 vaccine doses to the people around the globe [5]. COVID-19 has impacted the quality of life of ordinary people, caused severe complications among the immunocompromised population, and affected the economy/healthcare systems of almost all countries [1], [2]. The scientific fraternity has developed some novel antivirals (remdesivir, molnupiravir, and paxlovid), vaccines (Pfizer BioNTech COVID-19 Vaccine, Moderna COVID-19 Vaccine, and Comirnaty) for COVID-19 [6], [7], [8], [9], [10], [11]. Other possible treatments have also been explored, including lopinavir/ritonavir combination, ivermectin, convalescent plasma, REGN-COV2, sotrovimab, tocilizumab, baricitinib, ruxolitinib, tofacitinib, and ibrutinib [1], [6], [7], [8], [9], [10], [11]. However, the efficacy of the available/explored COVID-19 treatment and vaccines against the emerging and highly transmittable variants of SARS-CoV-2 like Omicron (B.1.1.529), Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), Delta (B.1.617.2), Mu (B.1.621), Lambda (C.37), Kappa (B.1.617.1), Iota (B.1.526), Theta (P.3), Eta (B.1.525), Zeta (P.2), and Epsilon (B.1.429 & B.1.427) is not fully established [1], [3], [12]. Many countries have also reported the emergence of second and third waves of COVID-19 despite implementing vaccination programs [1]. Some existing COVID-19 treatments/prophylactics are not patient-compliant (injectable remdesivir and vaccines) and may also cause serious side effects (teratogenic effect of molnupiravir) [1], [3], [12]. Further, the available vaccines are not 100% effective for all COVID-19 patients [1]. These issues forced scientists to develop new treatments and supportive therapy for COVID-19.
The COVID-19 treatment with medicines is accompanied by supportive therapy, which encompasses the administration of minerals (zinc, copper, selenium, etc.), vitamins (C and D), and a diet rich in immunity stimulants along with medicines [2], [3], [4], [13], [14], [15]. This type of supportive therapy reduces the recovery time of the COVID-19 patient. Selenium is a recognized trace element and an essential nutrient for human health [13], [14], [15], [16]. Recently, the importance of selenium in preventing/treating COVID-19 has been documented in the literature [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27]. When writing this review, the authors believe that no publication covers the clinical studies, inventive composition, and patent literature of selenium to prevent/treat COVID-19 in a single document. This review fills this literature gap and will be valuable to scientists developing Selenium-based COVID-19 treatment.
2. Selenium
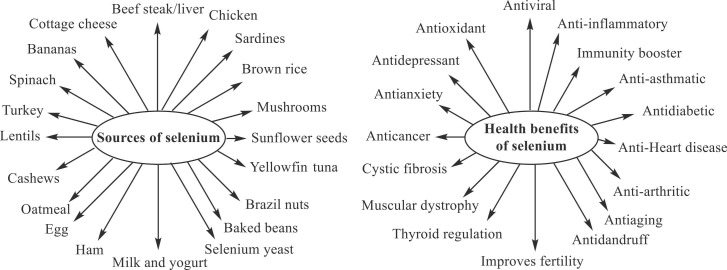
Selenium, designated as Se (atomic number 34), is known as a chemical element since 1818 [28]. This trace or essential micronutrient is one of the critical structural components of several selenium-dependent enzymes, including glutathione peroxidase 1 and 2 (control intracellular oxidative stress), glutathione peroxidase 4 (lowers lipid hydroperoxides), iodothyronine deiodinases (help in immunity building), methionine-R-sulfoxide reductase (regulate oxidative stress), thioredoxin reductase, selenoprotein H and T (regulates redox homeostasis), selenoprotein I (controls phospholipid biosynthesis), selenoprotein K (regulates lymphocyte activity and calcium signaling), selenoprotein P (relates to selenium status and its supply in the cell), selenoprotein S (regulates protein synthesis), and selenophosphate synthetase 2 (regulates biosynthesis of selenoproteins) [22], [29], [30], [31], [32], [33]. Selenium is a more potent antioxidant than tocopherol, vitamin C, and beta-carotene [31], [32]. Selenium and selenium-containing enzymes/proteins/food support the defense mechanism of the human body and play an essential biological role (antiviral, antioxidant, anti-inflammatory, immunity booster, etc.) for human health ( Fig. 1) [32], [33], [34], [35], [36], [37].
Fig. 1.
Sources and health benefits of selenium [32], [33], [34], [35], [36], [37].
The selenium content in its sources hangs on the selenium levels in the soil. The selenium content in soil differs from country to country. For example, the selenium content in the soil is relatively low in Europe, Italy, New Zealand, China, and some parts of the USA [18]. The dietary requirement of selenium varies with age, gender, and health status of the individual [17], [36] ( Table 1). Intake of higher concentrations can cause selenium toxicity (selenosis) [17], [36]. The average daily dose of selenium should not exceed 70 µg/day for adults, whereas a selenium dose of 400 µg/day may be toxic for an adult. The primary dietary bioavailable forms of selenium (≥ 90% bioavailability) are selenomethionine (SeMet) and selenocysteine (SeC) [32], [33]. The inorganic forms of selenium (selenate [SeO4]2-, selenite [SeO3]2-, etc.) also have good bioavailability (60–70% bioavailability) but less than SeMet and SeC [31], [38], [39]. SeMet is absorbed from the intestine, whereas the inorganic forms of selenium (selenate, selenite, etc.) are absorbed by the simple diffusion process. The absorbed selenium sources are converted to selenide (HSe2-) in the liver, which is utilized for the activation/generation of selenoenzymes [29], [31], [32], [39], [40], [41]. The inorganic forms of selenium are utilized as supplements to manage selenium deficiency. The liver is the chief storing organ for selenium and supplies selenium to other tissues on a need basis [29], [40], [41].
Table 1.
Dietary requirements of selenium.
| Age / Status | Upper Limit (µg/day) | Average requirement (µg/day) | Recommended dietary intake (µg/day) |
|---|---|---|---|
| 0–6 Months | 45 | – | – |
| 7–12 Months | 60 | – | – |
| 1–3 Years | 90 | 20 | 25 |
| 4–8 Years | 150 | 25 | 30 |
| Boys | |||
| 9–13 Years | 280 | 40 | 50 |
| 14–18 Years | 400 | 60 | 70 |
| Girls | |||
| 9–13 Years | 280 | 40 | 50 |
| 14–18 Years | 400 | 50 | 60 |
| Men | |||
| 19–70 Years | 400 | 60 | 70 |
| Women | |||
| 19–70 Years | 400 | 50 | 60 |
| Pregnancy | 400 | 55 | 65 |
| Lactation | 400 | 65 | 75 |
Selenium is vital for the preservation of complete health. Its deficiency affects 0.5–1 billion people around the globe due to its poor intake [42]. A low selenium deficiency causes cognitive decline, involving confusion, anxiety, and depressive mood. The moderate to severe deficiency of selenium may lead to thyroid disorder, oxidative stress, deformity of bones (Kashin-Beck), weakness of heart muscles, myo-degenerative diseases, deficiency of vitamin E, infertility in men, prostate cancer, reduced adaptive immunity, increased chances of infection, neurological disorders, and Keshan disease [43]. Among these diseases, Keshan disease (weakness of heart muscles and cardiomyopathy) is endemic in Keshan, China, and is generally accompanied by Coxsackie B3 enterovirus infection [42], [43], [44]. One Chinese report has stated that selenium deficiency makes the individual more susceptible to COVID-19, whereas the supplementation of selenium decreases the cure time of COVID-19 patients. This report also stated a faster recovery of the COVID-19 patients with a higher hair selenium concentration [44]. Selenium deficiency can be managed by increasing the intake of selenium-rich food and its supplements [45]. However, selenium has a narrow therapeutic window [42]. Therefore, an excess intake of selenium or its supplements can cause toxicity (selenosis, garlic odor in the mouth, gastrointestinal upset, hair loss, fatigue, sloughing of nails, neurological damage, and irritability). The United States Food and Drug Administration (USFDA) recognized one such case of the selenium toxicity epidemic in 2008, wherein more than 200 individuals suffered from selenium toxicity due to nutritional supplements containing an excess of selenium [46].
2.1. USFDA approved selenium products
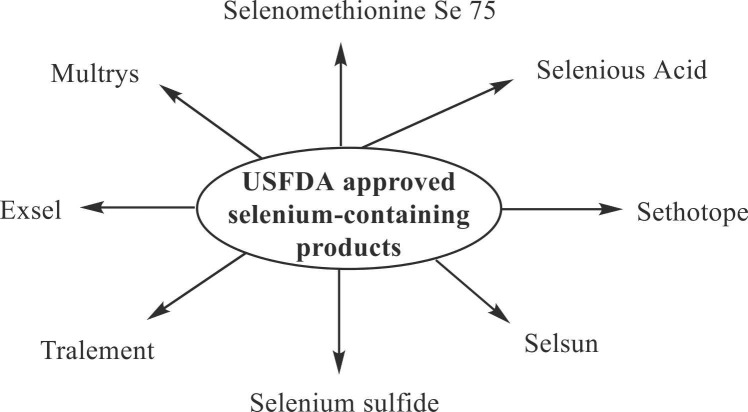
A search for the USFDA-approved selenium-containing products on the USFDA’s Orange Book database [47] was conducted on May 17, 2022. This search revealed eleven selenium products, out of which seven have been discontinued, and 4 are prescription products ( Table 2) ( Fig. 2). Two prescription products (Multrys and Selenious Acid) are used as a source of selenium for parenteral nutrition. In contrast, others are indicated to treat tinea versicolor/seborrheic dermatitis/dandruff and selenium deficiency.
Table 2.
USFDA approved selenium-containing products.
| Active Ingredient (Proprietary Name) | Dosage Form (Route of Administration) | Strength (Marketing Status) | Approval Date | Applicant |
|---|---|---|---|---|
| Selenomethionine Se-75 (Sethotope) |
Injectable (Injection) |
85–550 uCi/ml (Discontinued) |
Before January 1, 1982 | Bracco Diagnostics |
| Selenomethionine Se 75 (Selenomethionine Se 75) |
Injectable (Injection) |
100 uCi/ml (Discontinued) |
Before January 1, 1982 | Mallinckrodt |
| Selenious Acid (Selenious Acid) |
Solution (Intravenous) |
600 mcg selenium/10 ml (Prescription) |
April 30, 2019 | American Regent |
| Cupric sulfate; Manganese sulfate; Selenious acid; Zinc sulfate (Multrys; Tralement) |
Solution (Intravenous) |
6 mcg selenium/ml along with other ingredients (Prescription) |
July 2, 2020 | American Regent |
| Selenium sulfide (Selsun) |
Lotion/Shampoo (Topical) |
2.5 % (Discontinued) |
Before January 1, 1982 | Chattem |
| Selenium sulfide (Selenium sulfide) |
Lotion/Shampoo (Topical) |
2.5 % (Discontinued) |
Before January 1, 1982 | Ivax Pharmaceuticals |
| Selenium sulfide (Selenium sulfide) |
Lotion/Shampoo (Topical) |
2.5 % (Discontinued) |
Before January 1, 1982 | Cosette Pharmaceuticals |
| Selenium sulfide (Selenium sulfide) |
Lotion/Shampoo (Topical) |
2.5 % (Discontinued) |
Before January 1, 1982 | Actavis Mid Atlantic |
| Selenium sulfide (Exsel) |
Lotion/Shampoo (Topical) |
2.5 % (Discontinued) |
Before January 1, 1982 | Allergan Herbert Div Allergan |
| Selenium sulfide (Selenium Sulfide) |
Lotion/Shampoo (Topical) |
2.5 % (Prescription) |
September 1, 1983 | Wockhardt |
| Selenium sulfide (Selenium sulfide) |
Lotion/Shampoo (Topical) |
2.5 % (Prescription) |
January 10, 1991 | Padagis US |
Fig. 2.
USFDA approved selenium products [47].
2.2. Selenium and COVID-19
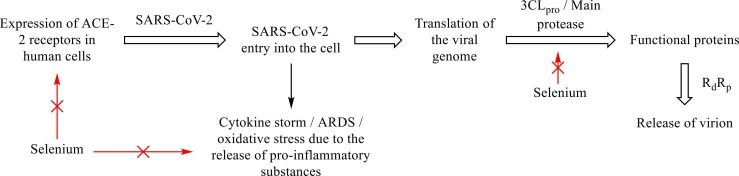
SARS-CoV-2 penetrates the human cells via Angiotensin-converting enzyme 2 receptor (ACE-2) [2], [3], [4]. This initiates the discharge of pro-inflammatory substances, cytokine storm, acute respiratory distress syndrome (ARDS), and oxidative stress in the human body [2], [3], [4]. The replication of SARS-CoV-2 takes place through 3CLPro (main protease enzyme) and RNA-dependent RNA-Polymerase (RdRp) [2], [3], [4]. Selenium (selenoproteins and selenium-containing organic compounds) exerts its anti-COVID-19 action by decreasing the expression of ACE-2 receptor (prevents SARS-CoV-2 entry into the cell); lowering the release of pro-inflammatory substances (inhibits cytokine storm, oxidative stress, ARDS, etc.), and inhibiting the 3CLPro (main protease) of SARS-CoV-2 [17], [18], [19], [20], [21], [22], [23], [24], [26], [27] ( Fig. 3). Selenium also inhibits papain-like protease (PLpro) of SARS-CoV-2, an essential enzyme for its replication [17], [21]. One report also hypothesizes that selenium interacts with the sulfhydryl group of viral protein (disulfide isomerase) and changes the active sulfhydryl group to an inactive disulfide group. This also prevents the virus entry into the cell [19], [20]. Selenium also has anti-thrombotic and anti-platelet effects, which also help combat symptoms and complications of COVID-19 [15], [20], [28].
Fig. 3.
Anti-SARS-CoV-2 and anti-COVID-19 mechanism of action of selenium.
2.3. Clinical trials/studies on selenium
A search for the selenium-related clinical studies (CS) was performed on the clinical trial database [48] on June 27, 2022, utilizing the keywords “selenium”, “selenium + COVID-19″, and “selenium + SARS-CoV-2″. This search revealed 369 CSs in which selenium has been/is being assessed clinically for many conditions/disorders., including diseases related to infection, immunity, respiratory system, musculoskeletal system, blood, heart, diabetes, and cancer. This search also unveiled 13 CSs for the prevention/treatment of COVID-19 employing selenium. However, in two CSs, selenium was used as a comparator rather than an intervention. Accordingly, the summary of 11 CSs is mentioned in Table 3.
Table 3.
Summary of the COVID-19 related CSs on selenium.
| Short title / objective / intervention | Primary purpose (Allocation/Type; Status; Phase; Number enrolled; Results; Comparison group; Primary outcome) | NCT number (Sponsor; Location; Start date; Completion date) |
|---|---|---|
| Selenium efficacy among hospitalized COVID-19 patients (Loading dose of selenious acid infusion (2000 µg) on day 1, and maintenance dose (1000 µg per day) for 2–14 days along with standard care) |
Treatment (Randomized; Not yet recruiting; 2; 100; Not available; Standard care + placebo; Mean change in the ordinal scale and rate of hospital discharges/deaths) |
NCT04869579 (Christus Health and Pharco Pharmaceuticals; United States; August 15, 2021; December 15, 2021) |
| Evaluation of the plasma concentration of micronutrients (selenium, zinc, copper, etc.) in elderly COVID-19 patients and its correlation with the prognosis of COVID-19 | Observational (Cohort; Completed; Not mentioned; 229; Not available; COVID-19 patients of > 50 years; The plasma concentration of selenium, zinc, copper, and vitamin A, D, and E) |
NCT04877509 (Hospices Civils de Lyon; France; March 1, 2020; May 1, 2021) |
| Assessment of the ABBC1 (a nutritional supplement containing selenium and zinc) benefits among volunteers taking influenza and COVID-19 vaccine. The supplementation starts 30 and 35 days after taking the influenza, and COVID-19 vaccine, respectively. | Other (Randomized; Recruiting; Not applicable; 90; Published; Influenza/COVID-19 vaccine + placebo; Change in the immune response and selenium/zinc level after supplementation) |
NCT04798677 (AB Biotek; Spain; October 29, 2020; July 2021) |
| To determine the serum concentration of trace elements (selenium, zinc, copper, etc.) in COVID-19 patients before starting the treatment and to compare the results with a healthy individual | Prospective (Randomized; Completed; Not mentioned; 40; Not available; Healthy individuals; Levels of serum trace elements) |
NCT04694716 (Izmir Bakircay University; Turkey; January 6, 2021; August 15, 2021) |
| Efficacy of micronutrient supplements (once a day oral supplementation comprising vitamins, iron (5 mg), zinc (10 mg), selenium (110 mg), and copper (0.9 mg)) in lowering hospital admissions of COVID-19 patients | Treatment (Randomized; Not yet recruiting; Not applicable; 300; Not available; Placebo dietary supplement; Need for hospital admission) |
NCT04751669 (Fundació Institut Germans Trias i Pujol; Spain; April 1, 2021; December 31, 2021) |
| Understanding the course of COVID-19 among patients treated with an Immuno-Formulation (Immuno-TF) that consists of selenium yeast (48 mg, equivalent to 96 μg of selenium), zinc, ferulic acid, resveratrol, spirulina, N-acetylcysteine, and vitamins along with other ingredients | Observational (Retrospective cohort; Completed; Not mentioned; 40; Not available; COVID-19 patients; Clinical symptoms duration) |
NCT04666753 (Fagron Iberica S.A.U.; Spain; July 2, 2020; September 29, 2020) |
| Reducing the COVID-19 severity by utilizing once a day oral capsule comprising selenium (15 ug), zinc (7.5 mg), vitamin A (1500 mcg), vitamin C (250 mg), and vitamin E (90 mg) for 7–14 days | Supportive Care (Randomized Recruiting 2/3; 40; Not available; cellulose-containing placebo capsules; Change in the serum level of ferritin, Interleukin-6, C-reactive protein, Tumor necrosis factor-α, and monocyte chemoattractant protein 1) |
NCT04323228 (King Saud University; Saudi Arabia; September 1, 2020; December 30, 2020) |
| Understanding the change in the serum concentration of trace elements (selenium, zinc, magnesium, copper, etc.) in COVID-19 patients before and after COVID-19 treatment | Observational (Cohort; Completed; Not mentioned; 15; Not available; Not mentioned; Change of the levels of Trace Element at baseline and discharge) |
NCT04694703 (Izmir Bakircay University; Turkey; January 6, 2021; August 29, 2021) |
| Determination of the oxidative stress indicators (Superoxide dismutase and malondialdehyde), trace elements (selenium, zinc, potassium, sodium, magnesium, and copper), and quality of life among healthy women before and COVID-19 vaccine |
Screening (Not applicable; Not yet recruiting; Not applicable; 20; Not available; Not mentioned; Change of the levels serum trace element) |
NCT04751721 (Izmir Bakircay University; Turkey; February 2021; April 2021) |
| Determination of the oxidative stress indicators (Superoxide dismutase and malondialdehyde), trace elements (selenium, zinc, potassium, sodium, magnesium, and calcium), and quality of life among healthy men before and COVID-19 vaccine |
Screening (Not applicable; Not yet recruiting; Not applicable; 20; Not available; Not mentioned; Change of the levels serum trace element) |
NCT04751695 (Izmir Bakircay University; Turkey; February 2021; April 2021) |
| Determination of the concentration of trace elements (selenium, zinc, and magnesium) along with other parameters to assess their involvement in the generation of fatigue associated with COVID-19 | Diagnostic (Non-Randomized; Recruiting; Not applicable; 102; Not available; Not mentioned; Voluntary maximum force reduction) |
NCT04363606 (Centre Hospitalier Universitaire de Saint Etienne; France; May 27, 2020; December 2022) |
It is evident from the data in Table 3 that selenium (as selenious acid) monotherapy and the nutritional supplement containing selenium/selenium yeast in combination with vitamins (A, C, and E), iron, zinc, copper, ferulic acid, resveratrol, spirulina, N-acetylcysteine, and vitamins are a target for research in the context of prevention/treatment of COVID-19. It can also be observed that the maximum number of clinical studies have been conducted in Turkey (4 studies), followed by Spain (3), France (2), the United States (1), and Saudi Arabia (1). Interestingly, only one study (NCT04798677) mentioned in Table 3 has been published, whereas the results of other studies have not been publicized yet.
The PubMed search also revealed two important studies involving selenium and COVID-19. One study on 50 COVID-19 patients proved a lower level of selenium in COVID-19 patients (77.8 ± 13.9 µg/L) in comparison to healthy individuals (91.7 ± 16.7 µg/L) [49]. This study also stated that a lower selenium concentration might increase the chances of getting SARS-CoV-2 infection or COVID-19 but is silent about the relationship between serum selenium levels and severity/mortality associated with COVID-19. Another study published the results of NCT04798677 [50]. The relevant information about NCT04798677 is mentioned in Table 3. This study demonstrated that supplementation of ABBC1 increased the level of selenium, zinc, CD4 +T cells, CD3 +T lymphocytes, CD8 +T lymphocytes, IgG, and IgM compared to placebo. These findings confirm the immunity booster effects of ABBC1.
2.4. Patent literature
The patent literature search was performed on Sci-Finder, Espacenet, Patentscope, and USPTO databases on June 27, 2022 [51], [52], [53], [54], [55]. The keywords “selenium + COVID-19″ and “selenium + SARS-CoV-2″ were selected for patent searching on different patent databases (Espacenet, USPTO, Scifinder, and Patentscope). The search on Espacenet (selenium + COVID-19 = 22 hits; selenium + SARS-CoV-2 = 26 hits), USPTO (selenium + COVID-19 = 25 hits; selenium + SARS-CoV-2 = 27 hits), Sci-finder (selenium + COVID-19 = 19 hits; selenium + SARS-CoV-2 = 22 hits), Patentscope (selenium + COVID-19 = 25 hits; selenium + SARS-CoV-2 = 21 hits) explored many patent applications. The duplicate patents/patent applications of the same patent family were removed. The patents/patent applications that did not explicitly claim or did not exemplify the use of selenium or its composition to prevent/treat COVID-19 were excluded. The summary of the finalized patent application is provided in Table 4.
Table 4.
Summary of the patent applications claiming the use of selenium to prevent/treat COVID-19.
| Patent application number (Applicant; Filing country; Publication date; Status) | Status (Family members as of June 27, 2022) | Summary of the claimed invention |
|---|---|---|
|
US2021322464A1 (Ghoweba Mohamed Samir Elsayed; United States; October 21, 2021) |
Under examination (WO2021216581A1) |
A method of treating COVID-19 by administering a bolus dose (1000–6000 μg/ day) of selenium in the form of its pharmaceutically acceptable form (selenium hydride, sodium selenite, selenic acid, selenium dioxide, selenium, SeMet, SeC, selenodiglutathione, dimethyl selenoxide, selenocystamine, selenomethyl SeC, selenated yeasts, etc.) followed by a continuous dose of selenium (1000–1600 μg/day), and monitoring the patient for certain parameters (levels of selenium, oxygen, enzymes, platelets, ferritin, total bilirubin, white blood cell counts, complete blood counts, interleukin-1, interleukin-6, tumor necrosis factor-alpha, SARS-CoV-2 polymerase chain reaction). The patent application states the utilization of selenium as an antioxidant, cytokine modulator, antiviral, immune booster, anti-apoptotic, and anticoagulant to treat/prevent COVID-19. This study provides prophetic examples for the intravenous/oral use of selenium (as Selenious Acid) to treat COVID-19 patients[56]. |
|
WO2021263206A1 (Prothione; United States; December 30, 2021) |
No national phase entry (None) |
A composition comprising glutathione precursor (glycine, L-cystine, and a glutamate source) and a selenium compound (SeMet, selenite, methyl SeC, or selenium nanoparticles)[57]. |
|
US2022047545A1 (Rath Matthias W; United States; February 17, 2022) |
Under examination (WO2022034549A1) |
A micronutrient-based composition containing many nutrients (selenium, polyphenols, plant extracts, alkaloids, stilbenes, terpenes, volatile oils, vitamins, fatty acids, and amino acids) to treat COVID-19. This patent application demonstrates the effect of the selenium (2–500 µg) containing composition on the expression of the ACE-2 receptor and replication of SARS-CoV-2[58]. |
|
WO2022035869A1 (HSIA, Houn Simon; United States; February 17, 2022) |
No national phase entry (None) |
A composition containing fish oil (up to 20 g/day) and selenium (1000–10000 pg/day) to treat COVID-19. This composition may optionally contain coenzyme Q10. The patent application demonstrates apoptotic effects against SARS-CoV-2 and anti-inflammatory effects of the claimed composition against pro-inflammatory substances[59]. |
|
CN113004358A (Niuao Weite Chengdu Biotechnology Company Limited; China; June 22, 2021) |
Under examination (None) |
Selenothymidine-5′-triphosphate compounds[60]. |
|
CN112914109A (Chen Yuxiang; China; June 8, 2021) |
Under examination (None) |
Peptide nano-selenium compounds[61] |
|
CN111248231A (Baishan Fengshou Bee Products Technology Development Company Limited; China; June 9, 2020) |
Application withdrawn (None) |
Selenium-based disinfectants[62] |
|
US2022105110A1 (Lalvani Kartar Singh; Europe; April 7, 2022) |
Under examination (None) |
A composition containing aspirin, promethazine, and niacinamide, which may optionally contain selenium, zinc, vitamins, etc.[63] |
|
US2022040228A1 (Gaertner Frank; United States; February 10, 2022) |
Under examination (None) |
A composition consisting of zinc, elderberry fruit extract blend, vitamins (A, C, D3, E, B6, B12, folate), selenium, Echinacea, garlic allicin, and elderberry fruit extract[64]. |
|
CN113208020A (Guo Lifeng; China; August 6, 2021) |
Under examination (None) |
An immune booster herbal drink containing larch extract, echinacea, and Ganoderma lucidum, which may optionally contain vitamin D, vitamin E, zinc, selenium, and taurine[65]. |
|
RU2763189C1 (Smart Aqua; Russia; December 28, 2021) |
Patented case (None) |
An immune booster liquid composition comprising vitamins (C, D3, B6, B9, B12), succinic acid, selenium, zinc, magnesium citrate, and Bor[66]. |
|
CN113831302A (Shandong University; China; December 24, 2021) |
Under examination (None) |
Benzisoselenazolone derivatives[67] |
|
CN112515162A (Song Jiaoyu; China; March 19, 2021) |
Under examination (None) |
A selenium-enriched herbal composition comprising many herbs, including areca nut, cinnamon oil, and sweet potato[68] |
|
CN111631404A (Chongqing Zhenminrun Technology Company Limited; China; September 8, 2020) |
Under examination (None) |
A palm oil-linseed oil-based nutritional composition comprising fat-soluble vitamins (vitamin A and fat-soluble vitamin D), and minerals (selenium, zinc, and copper)[69] |
|
CN111333667B (Zhejiang University of Technology; China; June 26, 2020) |
Patented case (None) |
Selenium-containing heterocyclic naphthalimide derivative[70] |
|
FR3109299A1 (Hamdan Sami; France; October 22, 2021) |
Lapsed (None) |
A composition comprising arthemeter, azithromycin, vitamin C, selenium, zinc, and vitamin D[71] |
|
DE202021105157U1 (Rittinghausen Reiner; Germany; October 13, 2021) |
Under examination (None) |
An immunity booster composition of Lactobacillus coryniformis, selenium, and zinc[72] |
|
WO2021255464A1 (Hahn Norman; United Kingdom; December 23, 2021) |
No national phase entry (None) |
A composition obtained by combining curcumin powder, vitamin C, grape seed extract, quercetin, vitamin D3, vitamin K2, zinc gluconate, Astragalus root extract, L-SeMet, vitamin B12, vitamin A acetate, glutathione, Boswellia resin extract, potassium sorbate, stevia, and flavor (mango)[73]. |
|
US2021315910A1 (Stafford Vivi Robyn; United States; October 14, 2021) |
Abandoned (None) |
A composition containing doxycycline and immunity booster (selenium, zinc, quercetin, copper, vitamins, etc.)[74] |
|
WO2021191864A1 (Dound Yogesh; India; September 30, 2021) |
No national phase entry (None) |
A composition of selenium, zinc, vitamin C, vitamin K2–7, and curcumin, which may optionally contain other vitamins (A, B, and D)[75] |
| US11278520B2 (Hazan Sabine; United States; September 30, 2021) | Patented case (None) |
Composition of vitamin C, vitamin D, and zinc, that may optionally contain selenium[76] |
|
WO2021074706A1 (Alsec Alimentos Secos; Columbia; April 22, 2021) |
Entered in Columbia and is under examination (CO2021008245A2) |
An immunomodulatory herbal food product that may optionally contain selenium[77] |
|
US2022040228A1 (Gaertner Frank; United States; February 10, 2022) |
Under examination (None) |
A zinc composition that may optionally contain additional components like additional components including a zinc ionophore, vitamins, folate, selenium, garlic, and/or Echinacea. The zinc ionophore can be elderberry. The vitamins can include vitamins (A, C, D3, E, B6, and B12)[78] |
|
WO2022051713A1 (The University of Chicago; United States; March 10, 2022) |
None (No national phase entry) |
A composition of amphiphilic block copolymer (ABC) (poloxamer 108, poloxamer 188, poloxamer 238, and poloxamine T1107) and an antioxidant (ascorbate, selenium, tocopherol, glutathione, N-acetyl-cysteine, lipoic acid, coenzyme Q-10, lycopene, lutein, etc.). This application does not exemplify the outcome of the combination of selenium and ABC against SARS-CoV-2[79]. |
|
WO2022031744A2 (Gowey Research Group; United States; February 10, 2022) |
No national phase entry (WO2022031744A3) |
A composition comprising keratin extracts and a cofactor (selenium, magnesium, zinc, chromium, vitamins, etc.). Keratins are used as a carrier to transfer cofactors into the target site (cell and nucleus). There is no example demonstrating anti-COVID-19 activity of the composition of selenium and keratins[80]. |
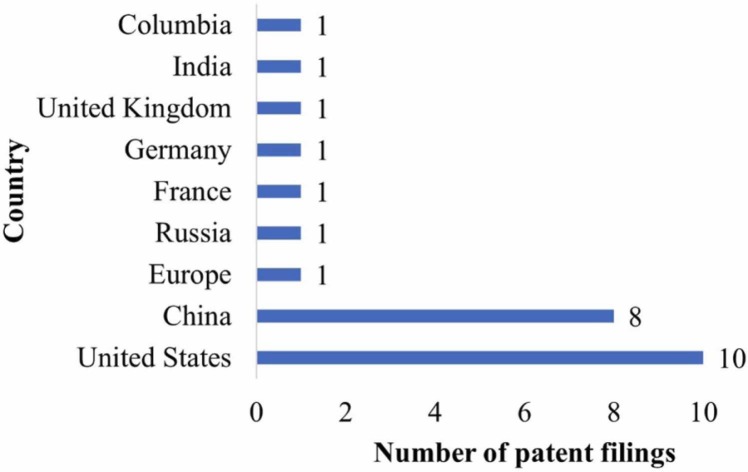
The patent literature search exposed 25 patent applications connected to selenium or its composition to treat COVID-19 or associated diseases (Table 4). The patents/patent applications relate to treating COVID-19 using selenium or its compositions with other nutrients (keratin extracts and fish oil). These documents also cover antiviral and immunomodulatory compositions of selenium with other nutrients (zinc and vitamins) and selenium-containing novel organic compounds. Most patent applications are filed in the United States and China ( Fig. 4). The patent applications are also filed in Europe, Russia, France, Germany, the United Kingdom, India, and Columbia.
Fig. 4.
Number of patent filings in different countries.
3. Discussion
Selenium is an essential structural part of many necessary enzymes (selenoproteins), which are implicated in developing a solid defense mechanism for the human body [17], [18], [19], [20], [21], [22], [23], [24]. Most selenoproteins help prevent viral infections and positively impact immunity due to their anti-inflammatory and antioxidant nature [17], [18], [19]. It is documented that selenium deficiency makes an individual vulnerable to SARS-CoV-2 infection and is linked with the cure rate of COVID-19 patients [17], [19], [49]. Selenium deficiency also relates to the pathogenicity of the virus [22]. As stated above, the content of selenium in the soil is relatively low in Europe, Italy, China, and some parts of the USA [18], [42], [43], [44]. This fact may be one of the reasons that people of these regions suffered from COVID-19 more than other countries. Some immunopathological conditions (sepsis) are also there in which selenium deficiency is a marker. Patients suffering from such diseases are also prone to COVID-19 [19]. Older, hypertensive, diabetic, and immunocompromised people are susceptible to selenium deficiency, making these patients a high-risk group for developing COVID-19 [17], [18], [19], [20].
The adequate level of selenium in the human body avoids the cytokine storm owing to its anti-inflammatory and antioxidant effects, stops the doorway of SARS-CoV-2 into the human body by depressing the expression of ACE-2 receptors, and hindering the enzyme of SARS-CoV-2 (main protease and papain-like protease) [17], [18], [19], [20], [21], [22], [23], [24], [26], [27]. The anti-thrombotic and anti-platelet activity of selenium is also reported [20]. Respiratory failure, septic shock, thromboembolism, and multiorgan failure are the foremost reasons for fatality among severe COVID-19 patients [2]. The studies suggest that selenium supplements (oral and intravenous) can improve the treatment outcomes of COVID-19 [17], [18], [19], [20], [56]. This effect may be due to the antiviral, antioxidant, anti-inflammatory, and anti-thrombotic effects of selenium [17], [18], [19], [20]. The clinical study data of selenium (Table 3) also evidenced that selenium as a monotherapy and as a combination therapy with vitamins (A, C, and E), zinc, copper, iron, ferulic acid, resveratrol, spirulina, N-acetylcysteine, and vitamins may be adequate to prevent/treat COVID-19. The selenium compositions with herbal medicine (ginseng) and minerals (zinc) are also supposed to display beneficial antiviral effects on patients [19], [21], [22]. However, this effect needs confirmation. New variants of SARS-CoV-2 have been identified [1], [3]. The efficacy of selenium against these variants is yet to be established.
The inorganic forms of selenium, like selenious acid (H2SO3), and the combination of selenium with other nutrients/compounds are in clinical studies against COVID-19 (Table 3) [48]. The patent literature also discloses some selenium-containing compositions and organic compounds (organoselenium compounds) as antiviral/anti-COVID-19 agents (Table 4) [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80]. Ebselen ( Fig. 5) is an organoselenium compound that has demonstrated anti-SARS-CoV-2 activity and antioxidant/anti-inflammatory activity [18], [20]. Ebselen has also been shown to hinder the main protease enzyme of SARS-CoV-2 [19]. This indicates that discovering more organoselenium compounds may be an option for developing drugs against COVID-19. The authors believe that the computational studies on already reported organoselenium compounds may also provide some potential lead compounds as anti-SAR-CoV-2 compounds. The patenting of new Selenium-based compositions and dosage forms (spray, inhalers, control release dosage forms, etc.) are also foreseeable to the authors.
Fig. 5.
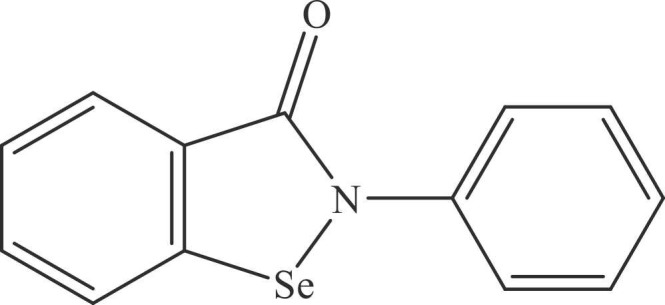
Chemical structure of ebselen.
The USFDA has approved two prescription products containing selenium to treat selenium deficiency (Table 2). These products are cheap and readily available [23]. Since selenium has promising anti-COVID-19 properties, its supplementation can be started in the initial phases of COVID-19. However, selenium has a narrow therapeutic window, and its unnecessary supplementation in individuals having adequate selenium concentration may cause toxicity [17], [46]. Therefore, it is crucial to adhere to the optimal dose of selenium while starting selenium supplementation [17], [22]. According to the postulated mechanism of selenium toxicity, selenium compounds like selenite react with the thiol group of proteins and generate selenotrisulfides. These selenotrisulfides produce superoxides and hydrogen peroxides that cause oxidative damage and symptoms of selenium toxicity [46]. Accordingly, the chemical interaction of selenium with other ingredients of its compositions must also be considered for safety and efficacy.
4. Conclusion
Selenium owns noticeable anti-COVID-19 activity. The data of clinical studies, inventive pharmaceutic compositions, and patent literature support selenium's prophylactic and therapeutic ability against COVID-19. However, there is a need to generate more evidence via randomized controlled clinical trials. The authors foresee the development and commercialization of Selenium-based composition to battle COVID-19 and other viral diseases. The therapeutic window of selenium is narrow and can also show chemical interactions with other excipients of the compositions. This makes it essential to consider these two factors before developing selenium-based compositions. We believe that countless selenium-based inventive antiviral and anti-COVID-19 compositions are yet to be explored. Accordingly, there is good scope for scientists to work on developing novel and inventive selenium-based compositions to fight against COVID-19 and other viral infections.
Funding
This work is supported by the Deputyship for Research and Innovation, Ministry of Education, Saudi Arabia.
CRediT authorship contribution statement
The study was conceptualized by Mohammed Kanan Alshammari, Waseem Fatima, and Mohd Imran. The study design was made by Mohammed Kanan Alshammari, Mohd Imran, and Syed Mohammed Basheeruddin Asdaq. The acquisition of data and drafting of the article was performed by Waseem Fatima Reem Ahmed Alraya, Reem Saud Alshammari, Sarah Ayad Alshammari, Lina Mohammed Alharbi, Norah Saad Alsubaie, Rakan Bijad Alosaimi, and Mehnaz Kamal. The analysis and interpretation of data were performed by A. Khuzaim Alzahrani, Reem Ahmed Alraya, and Mehnaz Kamal. The manuscript was reviewed and edited by Mohammed Kanan Alshammari, Waseem Fatima, Mohd Imran, A. Khuzaim Alzahrani, and Syed Mohammed Basheeruddin Asdaq. All the authors approved of the version to be submitted.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgment
The authors extend their appreciation to the Deputyship for Research and Innovation, Ministry of Education, Saudi Arabia, for funding this work through the project number IF-2020-NBU-226. All the authors are also thankful to their respective institutes for providing support for this article.
Ethical approval
It is not required for the review article.
Data Availability
The data presented in this review are available in various databases (PubMed, Orange Book, Clinical trials, Sci-Finder, Espacenet, Patentscope, and USPTO). These databases have also been cited in the text and references.
References
- 1.Cascella M, Rajnik M, Aleem A, Dulebohn SC, Napoli RD. Features, evaluation, and treatment of coronavirus (COVID-19. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. Available from: https://www.ncbi.nlm.nih.gov/books/NBK554776/ (Accessed on June 27, 2022). [PubMed]
- 2.Imran M., Fatima W., Alzahrani A.K., Suhail N., Alshammari M.K., Alghitran A.A., et al. Development of therapeutic and prophylactic zinc compositions for use against COVID-19: a glimpse of the trends, inventions, and patents. Nutrients. 2022;14(6):1227. doi: 10.3390/nu14061227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imran M., Thabet H.K., Alaqel S.I., Alzahrani A.R., Abida A., Alshammari M.K., et al. The therapeutic and prophylactic potential of quercetin against COVID-19: an outlook on the clinical studies, inventive compositions, and patent literature. Antioxidants. 2022;11(5):876. doi: 10.3390/antiox11050876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imran M., Khan S.A., Abida, Alshammari M.K., Alkhaldi S.M., Alshammari F.N., et al. Nigella sativa L. and COVID-19: a glance at the anti-COVID-19 chemical constituents, clinical trials, inventions, and patent literature. Molecules. 2022;27(9):2750. doi: 10.3390/molecules27092750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization, https://covid19.who.int/, 2022 (Accessed on June 27, 2022).
- 6.Imran M., Alshrari A.S., Asdaq S.M.B. Abida. Trends in the development of remdesivir based inventions against COVID-19 and other disorders: a patent review. J Infect Public Health. 2021;14(8):1075–1086. doi: 10.1016/j.jiph.2021.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imran M., Arora M.A., Asdaq S.M.B., Khan S.A., Alaqel S.I., Alshammari M.K., et al. Discovery, development, and patent trends on molnupiravir: A prospective oral treatment for COVID-19. Molecules. 2021;26(19):5795. doi: 10.3390/molecules26195795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reina J., Iglesias C. Nirmatrelvir plus ritonavir (Paxlovid) a potent SARS-CoV-2 3CLpro protease inhibitor combination. Rev Esp Quim. 2022 doi: 10.37201/req/002.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alshrari A.S., Hudu S.A., Imran M., Asdaq S.M.B., Ali A.M., Rabbani S.I. Innovations and development of COVID-19 vaccines: a patent review. J Infect Public Health. 2022;15(1):123–131. doi: 10.1016/j.jiph.2021.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asdaq S.M.B., Jomah S., Rabbani S.I., Alamri A.M., Alshammari S.K.S., Duwaidi B.S., et al. Insight into the advances in clinical trials of SARS-CoV-2 vaccines. Can J Infect Dis Med Microbiol. 2022;2022 doi: 10.1155/2022/6913772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashwlayan V.D., Antlash C., Imran M., Asdaq S.M.B., Alshammari M.K., Alomani M., et al. Insight into the biological impact of COVID-19 and its vaccines on human health. Saudi J Biol Sci. 2022;29(5):3326–3337. doi: 10.1016/j.sjbs.2022.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization, 〈https://www.who.int/emergencies/diseases/novel-coronavirus-2019〉, 2022 (Accessed on May 17, 2022).
- 13.Balboni E., Zagnoli F., Filippini T., Fairweather-Tait S.J., Vinceti M. Zinc and selenium supplementation in COVID-19 prevention and treatment: a systematic review of the experimental studies. J Trace Elem Med Biol. 2022;71 doi: 10.1016/j.jtemb.2022.126956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steinbrenner H., Duntas L.H., Rayman M.P. The role of selenium in type-2 diabetes mellitus and its metabolic comorbidities. Redox Biol. 2022;50 doi: 10.1016/j.redox.2022.102236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang L., Qi M., Du X., Xia Z., Fu G., Chen X., et al. Selenium concentration is associated with occurrence and diagnosis of three cardiovascular diseases: a systematic review and meta-analysis. J Trace Elem Med Biol. 2022;70 doi: 10.1016/j.jtemb.2021.126908. [DOI] [PubMed] [Google Scholar]
- 16.Barchielli G., Capperucci A., Tanini D. The role of selenium in pathologies: an updated review. Antioxidants. 2022;11(2):251. doi: 10.3390/antiox11020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J., Saad R., Taylor E.W., Rayman M.P. Selenium and selenoproteins in viral infection with potential relevance to COVID-19. Redox Biol. 2020;37 doi: 10.1016/j.redox.2020.101715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bermano G., Méplan C., Mercer D.K., Hesketh J.E. Selenium and viral infection: are there lessons for COVID-19? Br J Nutr. 2021;125(6):618–627. doi: 10.1017/S0007114520003128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khatiwada S., Subedi A. A mechanistic link between selenium and coronavirus disease 2019 (COVID-19. Curr Nutr Rep. 2021;10(2):125–136. doi: 10.1007/s13668-021-00354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hiffler L., Rakotoambinina B. Selenium and RNA virus interactions: potential implications for SARS-CoV-2 infection (COVID-19) Front Nutr. 2020;7:164. doi: 10.3389/fnut.2020.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomo S., Saikiran G., Banerjee M., Paul S. Selenium to selenoproteins - role in COVID-19. Excli J. 2021;20:781–791. doi: 10.17179/excli2021-3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schomburg L. Selenium deficiency due to diet, pregnancy, severe illness, or COVID-19-a preventable trigger for autoimmune disease. Int J Mol Sci. 2021;22(16):8532. doi: 10.3390/ijms22168532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alexander J., Tinkov A., Strand T.A., Alehagen U., Skalny A., Aaseth J. Early nutritional interventions with zinc, selenium and vitamin D for raising anti-viral resistance against progressive COVID-19. Nutrients. 2020;12(8):2358. doi: 10.3390/nu12082358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bae M., Kim H. Mini-review on the roles of vitamin C, vitamin D, and selenium in the immune system against COVID-19. Molecules. 2020;25(22):5346. doi: 10.3390/molecules25225346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fakhrolmobasheri M., Mazaheri-Tehrani S., Kieliszek M., Zeinalian M., Abbasi M., Karimi F., et al. COVID-19 and selenium deficiency: a systematic review. Biol Trace Elem Res. 2021:1–12. doi: 10.1007/s12011-021-02997-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kieliszek M., Lipinski B. Selenium supplementation in the prevention of coronavirus infections (COVID-19. Med Hypotheses. 2020;143 doi: 10.1016/j.mehy.2020.109878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kieliszek M. Selenium in the prevention of SARS-CoV-2 and other viruses. Biol Trace Elem Res. 2022:1–8. doi: 10.1007/s12011-022-03208-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kieliszek M., Bano I., Zare H. A comprehensive review on selenium and its effects on human health and distribution in middle eastern countries. Biol Trace Elem Res. 2022;200(3):971–987. doi: 10.1007/s12011-021-02716-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kieliszek M., Błażejak S. Selenium: significance, and outlook for supplementation. Nutrition. 2013;29(5):713–718. doi: 10.1016/j.nut.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 30.Rayman M.P. The importance of selenium to human health. Lancet. 2000;356(9225):233–241. doi: 10.1016/S0140-6736(00)02490-9. [DOI] [PubMed] [Google Scholar]
- 31.Kieliszek M. Selenium-Fascinating microelement, properties and sources in food. Molecules. 1924;7:1298. doi: 10.3390/molecules24071298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kieliszek M., Błażejak S. Current knowledge on the importance of selenium in food for living organisms: a review. Molecules. 2016;21(5):609. doi: 10.3390/molecules21050609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kieliszek M., Lipinski B. Pathophysiological significance of protein hydrophobic interactions: an emerging hypothesis. Med Hypotheses. 2018;110:15–22. doi: 10.1016/j.mehy.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 34.Kieliszek M., Lipinski B., Błażejak S. Application of sodium selenite in the prevention and treatment of cancers. Cells. 2017;6(4):39. doi: 10.3390/cells6040039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Avery J.C., Hoffmann P.R. Selenium, selenoproteins, and immunity. Nutrients. 2018;10(9):1203. doi: 10.3390/nu10091203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmutzler C., Mentrup B., Schomburg L., Hoang-Vu C., Herzog V., Köhrle J. Selenoproteins of the thyroid gland: expression, localization and possible function of glutathione peroxidase 3. Biol Chem. 2007;388(10):1053–1059. doi: 10.1515/BC.2007.122. [DOI] [PubMed] [Google Scholar]
- 37.Fairweather-Tait S.J., Bao Y., Broadley M.R., Collings R., Ford D., Hesketh J.E., et al. Selenium in human health and disease. Antioxid Redox Signal. 2011;14(7):1337–1383. doi: 10.1089/ars.2010.3275. [DOI] [PubMed] [Google Scholar]
- 38.Stoffaneller R., Morse N.L. A review of dietary selenium intake and selenium status in Europe and the Middle East. Nutrients. 2015;7(3):1494–1537. doi: 10.3390/nu7031494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Köhrl J., Brigelius-Flohé R., Böck A., Gärtner R., Meyer O., Flohé L. Selenium in biology: facts and medical perspectives. Biol Chem. 2000;381(9–10):849–864. doi: 10.1515/BC.2000.107. [DOI] [PubMed] [Google Scholar]
- 40.Navarro-Alarcón M., López-Martínez M.C. Essentiality of selenium in the human body: relationship with different diseases. Sci Total Environ. 2000;249(1–3):347–371. doi: 10.1016/s0048-9697(99)00526-4. [DOI] [PubMed] [Google Scholar]
- 41.National Health and Medical Research Council, 〈https://www.nhmrc.gov.au/about-us/publications/nutrient-reference-values-australia-and-new-zealand-including-recommended-dietary-intakes〉, 2022 (Accessed on May 17, 2022).
- 42.Shreenath A.P., Ameer M.A., Dooley J. Selenium deficiency 2021. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing 2022. PMID: 29489289. [PubMed]
- 43.Rayman M.P. Selenium and human health. Lancet. 2012;379(9822):1256–1268. doi: 10.1016/S0140-6736(11)61452-9. [DOI] [PubMed] [Google Scholar]
- 44.Zhang J., Taylor E.W., Bennett K., Saad R., Rayman M.P. Association between regional selenium status and reported outcome of COVID-19 cases in China. Am J Clin Nutr. 2020;111(6):1297–1299. doi: 10.1093/ajcn/nqaa095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kipp A.P., Strohm D., Brigelius-Flohé R., Schomburg L., Bechthold A., Leschik-Bonnet E., et al. Revised reference values for selenium intake. J Trace Elem Med Biol. 2015;32:195–199. doi: 10.1016/j.jtemb.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 46.Aldosary B.M., Sutter M.E., Schwartz M., Morgan B.W. Case series of selenium toxicity from a nutritional supplement. Clin Toxicol. 2012;50(1):57–64. doi: 10.3109/15563650.2011.641560. [DOI] [PubMed] [Google Scholar]
- 47.United States Food and Drug Administration, 〈https://www.accessdata.fda.gov/scripts/cder/ob/index.cfm2022〉 (Accessed on May 17, 2022).
- 48.National Library of Medicine. Selenium, 〈https://www.clinicaltrials.gov/〉 (Accessed on June 27, 2022).
- 49.Younesian O., Khodabakhshi B., Abdolahi N., Norouzi A., Behnampour N., Hosseinzadeh S., et al. Decreased serum selenium levels of COVID-19 patients in comparison with healthy individuals. Biol Trace Elem Res. 2022;200(4):1562–1567. doi: 10.1007/s12011-021-02797-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodriguez J.A.M., Bifano M., Roca Goma E., Plasencia C.M., Torralba A.O., Font M.S., Millán P.R. Effect and tolerability of a nutritional supplement based on a synergistic combination of β-glucans and selenium- and zinc-enriched Saccharomyces cerevisiae (ABB-C1®) in volunteers receiving the influenza or the COVID-19 vaccine: A randomized, double-blind, placebo-controlled study. Nutrients. 2021;13(12):4347. doi: 10.3390/nu13124347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Imran M., Alshrari S.A., Tauseef M., Khan S.A., Hudu S.A. Abida. Mucormycosis medications: a patent review. Expert Opin Ther Pat. 2021;31(11):1059–1074. doi: 10.1080/13543776.2021.1939308. [DOI] [PubMed] [Google Scholar]
- 52.Imran M., Alshrari S.A., Thabet H.K., Abida, Bakht M.A. Synthetic molecules as DprE1 inhibitors: a patent review. Expert Opin Ther Pat. 2021;31(8):759–772. doi: 10.1080/13543776.2021. [DOI] [PubMed] [Google Scholar]
- 53.Imran M., Asdaq S.M.B., Khan S.A., Unnikrishnan M.D., Alamri A.S., Alsanie W.F., et al. Innovations and patent trends in the development of USFDA approved protein kinase inhibitors in the last two decades. Pharmaceuticals. 2021;14(8):710. doi: 10.3390/ph14080710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Imran M., Khan S.A., Alshammari M.K., Alreshidi M.A., Alreshidi A.A., Alghonaim R.S., et al. Discovery, development, inventions, and patent trends on mobocertinib succinate: the first-in-class oral treatment for NSCLC with EGFR exon 20 insertions. Biomedicines. 2021;9(12):1938. doi: 10.3390/biomedicines9121938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Imran M., Khan S.A., Alshammari M.K., Alqahtani A.M., Alanazi T.A., Kamal M., et al. Discovery, development, inventions and patent review of fexinidazole: the first all-oral therapy for human African Trypanosomiasis. Pharmaceuticals. 2022;15(2):128. doi: 10.3390/ph15020128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ghoweba, M.S.E. Method of treating and preventing coronavirus disease 19 (COVID-19) using a selenium administration. United States Patent Application Publication Number US2021322464A1, October 21, 2021. Application number US202117235592A.
- 57.Crum A., Lile L. Compositions and methods for the treatment of COVID-19. PCT Patent Application Publication Number WO2021263206A1, December 30, 2021. Application number US2021039251W.
- 58.Niedzwiecki A., Rath M.W., Ivanov V., Goc A. Micronutrient combination to inhibit coronavirus cell infection. United States Patent Application Publication Number US2022047545A1, February 17, 2022. Application number US202117402396A.
- 59.Hsia H.S. Compositions and methods for treating viral infection. PCT Patent Application Publication Number WO2022035869A1, February 17, 2022. Application number US2021045399W.
- 60.Huang Z., Li Y., Hu R. Selenium or thiothymine nucleoside-5 '-triphosphoric acid and synthesis method thereof. Chinese Patent Application Publication Number CN113004358A, June 22, 2021. Application number CN202110321200A.
- 61.Chen Y., Huang Z., He J. Preparation method and application of peptide nano-selenium. Chinese Patent Application Publication Number CN112914109A, June 8, 2021. Application number CN202110160008A.
- 62.Pei F., Zhang L., Pei F. Selenium-enriched plant-derived disinfection and killing agent, and preparation method and application thereof. Chinese Patent Application Publication Number CN111248231A, June 9, 2020. Application number CN202010240786A.
- 63.Lalvani K.S., Taylor R., Shelatkar R. Composition for the treatment of COVID-19. United States Patent Application Publication Number US2022105110A1, April 7, 2022. Application number US202117157107A.
- 64.Gaertner F. Materials and methods for stopping respiratory virus infections. United States Patent Application Publication Number US2022040228A1, February 10, 2022. Application number US202117444809A.
- 65.Guo L. Plant health-care beverage capable of improving human immunity. Chinese Patent Application Publication Number CN113208020A, August 6, 2021. Application number CN202110369475A.
- 66.Bolotin M.G. Functional drinking water “Smart Aqua” to increase immunity. Russian Patent Number RU2763189C1, December 28, 2021. Application number RU2020142189A.
- 67.Zhan P., Li J., Liu X., Du R., Gao S., Jiang X., et al. Benzisoselenazolone derivative and preparation method and application thereof. Chinese Patent Application Publication Number CN113831302A, December 24, 2021. Application number CN202111224728A.
- 68.Song J. Anti-corona virus disease selenium-enriched additive composition for areca nuts and preparation method thereof. Chinese Patent Application Publication Number CN112515162A, March 19, 2021. Application number CN202010275654A.
- 69.Yan H., Yi M., Yan X. Medicinal and edible composite vitamin trace element palm oil-linseed oil nutritional oil and preparation method thereof. Chinese Patent Application Publication Number CN111631404A, September 8, 2020. Application number CN202010539741A.
- 70.Zhang W., Wu Y., Yu K., Shen L. Selenium heterocyclic ring-containing naphthalimide derivative, preparation method and antiviral application thereof. Chinese Patent Number CN111333667B, April 27, 2021. Application number CN202010290793A.
- 71.Hamdan S. Combination of artemether and azithromycin in the treatment of coronavirus type epidemics. French Patent Application Publication Number FR3109299A1, October 22, 2021. Application number FR2003801A.
- 72.Rittinghausen R. Composition for nutritional supplementation in case of susceptibility to infections and weakened immune system. German Patent Application Publication Number DE202021105157U1, October 13, 2021. Application number DE202021105157U.
- 73.Hahn N. Nutraceutical composition. PCT Patent Application Publication Number WO2021255464A1, December 23, 2021. Application number GB2021051542W.
- 74.Stafford V.R. Method to mitigate morbidity and mortality in virally induced forms of ACE2 receptor pathology progressing to SARS or ARDS. United States Patent Application Publication Number US2021315910A1, October 14, 2021. Application number US202016842787A.
- 75.Dound Y., Dound B., Kokane A. Food supplements for the prevention of COVID-19. PCT Patent Application Publication Number WO2021191864A1, September 30, 2021. Application number IB2021052534W.
- 76.Hazan S. Method of preventing COVID-19 infection. United States Patent Number US11278520B2, March 22, 2022. Application number US202017114271A.
- 77.Vargas U.A.M. Food product with immunomodulator effect. PCT Patent Application Publication Number WO2021074706A1, April 22, 2021. Application number IB2020056982W.
- 78.Gaertner F. Materials and methods for stopping respiratory virus infections. United States Patent Application Publication Number US2022040228A1, February 10, 2022. Application number US202117444809A.
- 79.Raphael C., Ling M.X., Nguyen M., Mccollum K.J., Bigdelle V.A. Materials and methods of treating viral infection with amphiphilic block copolymers. PCT Patent Application Publication Number WO2022051713A1, March 10, 2022. Application number US2021049266W.
- 80.Gowey B. Method and composition of upregulating RNA interference process. PCT Patent Application Publication Number WO2022031744A2, February 10, 2022. Application number US2021044399W.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this review are available in various databases (PubMed, Orange Book, Clinical trials, Sci-Finder, Espacenet, Patentscope, and USPTO). These databases have also been cited in the text and references.