Abstract
Advances in genome and tissue engineering have spurred significant progress and opportunity for innovation in cancer modeling. Human induced pluripotent stem cells (iPSCs) are an established and powerful tool to study cellular processes in the context of disease-specific genetic backgrounds; however, their application to cancer has been limited by the resistance of many transformed cells to undergo successful reprogramming. Here, we review the status of human iPSC modeling of solid tumors in the context of genetic engineering, including how base and prime editing can be incorporated into “bottom-up” cancer modeling, a term we coined for iPSC-based cancer models using genetic engineering to induce transformation. This approach circumvents the need to reprogram cancer cells while allowing for dissection of the genetic mechanisms underlying transformation, progression, and metastasis with a high degree of precision and control. We also discuss the strengths and limitations of respective engineering approaches and outline experimental considerations for establishing future models.
Introduction
The breakthrough discovery that somatic cells could be reprogrammed to pluripotency through the addition of four transcription factors (SOX2, KLF4, cMYC, and OCT4) was first reported nearly 15 years ago.1,2 In most non-transformed somatic cell types, the expression of these four factors induces global transcriptional and epigenetic changes that result in reversion of the somatic cell to a well-defined pluripotent state that is stably maintained throughout extended propagation in vitro.3,4 In the years after this discovery, methods to differentiate iPSC into a diverse array of somatic cell lineages were rapidly produced.5–7
With new differentiation methods continually being published, the fidelity of in vitro iPSC differentiation has only increased in efficiency and efficacy.8–12 Aside from a few cell lineages that lack effective differentiation protocols, iPSC-based modeling represents a promising approach to model many human cancers.13 Cellular reprogramming has already proven useful for modeling inherited genetic diseases, as patient-derived iPSC (pd-iPSC) stably capture genetic alterations that underlie disease phenotype and can be differentiated into somatic lineages that recapitulate disease pathology.14–18
In theory, reprogramming malignant cells could capture the genome of diverse human cancers, offering the opportunity to study the impact of genomic perturbations on cell development and function in the context of transformation.19,20 This renewable source of tumor cells could be especially advantageous to study rare, difficult-to-procure tumors. However, extensive efforts to reprogram malignant cells have been stymied by the apparent intransigence of many transformed cells to reprogramming.21,22 Aside from the few notable exceptions discussed later, efforts to reprogram cancer cells have been met with low efficiency or produced cells with an ill-defined, quasi-pluripotent phenotype.23,24
The iPSC technology is best suited for isolation of non-transformed cells, allowing for the investigation of cancer initiation and development with the ability to address how single and complex mutations influence the transformation process. This has provided the impetus to pursue alternative approaches to recapitulate the genetic basis of many cancers in human iPSC models.
Over the past decade, advancements in our ability to efficiently and precisely modify the human genome and differentiate stem cells into somatic lineages have provided an opportunity to recapitulate many of the complex genetic alterations linked to human cancers in cells of the proper developmental state.25,26 In an approach we coined “bottom-up” cancer modeling (Fig. 1), the combined use of iPSC and genetic engineering offers a new avenue to investigate discrete stages of cancer development in ways that can inform novel hypotheses and drive improved therapeutic development.
FIG. 1.
Bottom-up cancer modeling invokes the use of iPSC derived from somatic cells, which can then be differentiated into the cell-of-origin for a particular cancer alongside precise and timely genetic engineering to re-create cancer associated mutation/s. These models can be studied in many ways, including drug or genetic screens, used in organoid or mouse systems, or co-cultured with autologous or analogous immune cells to test new immunotherapies with the desired outcome of new therapeutics or new hypotheses. iPSC, induced pluripotent stem cell.
Here, we review bottom-up cancer models with a focus on solid tumors. We highlight the current challenges in iPSC cancer modeling and discuss the opportunities to further improve the development of these models. We also describe how new precision genome editing technologies, such as base and prime editing, can be combined with iPSC technology to study genomic alterations found in human cancer. For additional information on iPSC modeling of hematological cancers, we refer the reader to these excellent reviews.27–31
Current iPSC-Based Cancer Models
The value of iPSCs in cancer modeling has begun to emerge in recent years. As we move forward into a new era of cancer modeling, we turn to landmark studies to help shape and guide the field. Here, we provide a brief overview of cancer models developed using iPSC technology. We separate these models into three categories: (1) reprogramming of primary tumor cells, (2) reprogramming of patient somatic cells containing cancer-predisposition mutations, and (3) bottom-up cancer models, with a particular emphasis on the engineering technologies used to generate these models and their unique utility.
Reprogramming tumor cells
Most cancers, particularly solid tumors, seem refractory to the reprogramming process, limiting their potential for research.32–35 It is hypothesized that this is due to the underlying genomic and epigenetic alterations that prevent the cells from complete dedifferentiation, thus retaining the genetic and epigenetic state of the original cancer; pointing to why the initial attempts at reprogramming tumor cells into iPSC were largely unsuccessful.21,22 However, a handful of tumor types have now been reprogrammed successfully (Table 1). From these tumor cell derived iPSC, researchers have analyzed how various genetic mutations predispose to, cause, or maintain cancer.
Table 1.
Successful reprogramming of primary tumor cells
| Cancer diagnosis | Reprogramming method |
|---|---|
| Acute myeloid leukemia | Sendai virus,19 excisable lentivirus20 |
| Juvenile myelomonocytic leukemia | Lentivirus,241 sendai virus,40 lentivirus242 |
| Chronic myeloid leukemia | Episomal243 |
| Chronic myelomonocytic leukemia | Episomal,43 sendai virus244 |
| Solid plexiform neurofibroma | Retrovirus and/or sendai virus245 |
| Pancreatic ductal adenocarcinoma | Lentivirus38 |
Reprogramming cancer-predisposed patient somatic cells
One major benefit of iPSC technology is the ability to “capture” genetic backgrounds by generating stem cells from patient-derived somatic cells.36,37 Despite the limited success in generating iPSC from malignant cells, there have been many descriptions of iPSC derived from the somatic cells of patients with known germline cancer predisposition syndromes (Table 2). These pd-iPSC provide a novel method for in-depth studies of various genetic diseases that confer increased susceptibility to developing cancer.
Table 2.
Reprogramming of somatic cells from patients with cancer-predisposition syndromes
| Cancer predisposing disease | Mutant gene | Reprogramming method |
|---|---|---|
| Li-Fraumeni Syndrome | TP53 | Sendai virus39 |
| Rothmund-Thomson Syndrome | RECQL4 | Sendai virus246 |
| Werner Syndrome | WRN | Retrovirus,247,248 excisable lentivirus247,248 |
| Diamond-Blackfan Anemia | RPS19 and RPL5 | Excisable lentivirus,249,250 episomal and sendai virus249,250 |
| Myelodysplastic Syndrome | del(7q) | Excisable lentivirus184 |
| Chronic Myeloproliferative Disorders | KRAS, NRAS, GATA2, del(7q) | Excisable lentivirus20 |
| Congenital Neutropenia | ELANE | Excisable lentivirus45 |
| Noonan Syndrome | PTPN11 | Retrovirus251 |
| Gorlin Syndrome | PTCH1 | Sendai virus72–253 |
| Neurofibromatosis Type I | NF1 | Retrovirus and sendai virus245 |
| Multiple Endocrine Neoplasia Type 2 Syndrome | RET | Sendai virus254 |
| Hereditary Papillary Renal Cell Carcinoma | MET | Sendai virus46 |
| BRCA-Mutated Breast Cancer | BRCA1 | Non-integrating mRNA,47,255 sendai virus47,255 Non-integrating episomal plasmid256 |
| Familial Adenomatous Polyposis |
APC | Lentivirus216 |
| Down Syndrome | Trisomy 21 | Retrovirus257 |
NF1, neurofibromatosis type 1.
With these pd-iPSC lines, researchers have been able to study differentiation capacity, conduct drug screens, investigate disease mechanisms, and generate detailed gene expression profiles on cells derived from the iPSCs.38–47 However, iPSC derived from the somatic cells of individuals with cancer predisposing conditions are often devoid of obvious phenotypes, most likely due to the lack of secondary mutations required for transformation.39,45 Thus, even with cancer-predisposed pd-iPSCs, genetic engineering can be valuable in modeling the transformation process through the introduction of known cooperating driver mutations.
Genetically Engineered “Bottom-Up” iPSC Models
Despite the novelty in combining these two fields and the technical challenges faced, some iPSC-based cancer modeling studies have benefited from genetic engineering strategies.41,48–52 The feasibility of this approach has improved significantly through technological advances in gene editing methods, including reagent composition and delivery, allowing for more complex engineering approaches with higher efficiency.53,54 These improvements allow the controlled introduction of cancer-associated genetic alterations in wild-type iPSC and their derivatives, which has unique utility in elucidating the fundamental mechanisms underlying transformation.55
See Supplementary Table S1 for common cancer driving mutations and current gene editing tools that are suitable to introduce these mutations in bottom-up cancer models.56–66 Later, we review various efforts to create bottom-up models of solid tumors with an emphasis on highlighting the unique ways in which these iPSC-based models can be used.
To identify novel biomarkers of synovial sarcoma (SS), Hayakawa et al utilized a piggyBac (PB) transposon vector in iPSC to randomly insert and overexpress the SYT-SSX2 fusion cDNA found in a subset of SS.67 In contrast to previous findings, most genes were upregulated in iPSC by the fusion protein. To explain this confounding data, the group then induced SYT-SSX2 in iPSC and iPSC-derived neural crest cells (iNCC), a challenging cell type to obtain other than through iPSC differentiation. Expression of the SYT-SSX2 fusion protein in iNCC induced changes in gene expression similar to those observed in SS, unlike the iPSC findings, indicating that NCCs are the likely cell-of-origin for SS.68
Pancreatic ductal adenocarcinoma (PDAC) is an extremely deadly solid tumor that lacks effective therapies or surveillance protocol.69 In an attempt to describe novel drug targets or biomarkers, Huang et al used lentiviral expression of dominant negative TP53 and/or constitutively active KRAS to study PDAC genesis in iPSC.70 Genetically engineered iPSC were differentiated into 3D pancreatic progenitor organoids that exhibited differential localization (cytoplasm or nuclear) of Sox9 based on the mutational profile (mutant TP53 or KRAS). The localization of Sox9 was assessed in patient samples and was found to influence Sox9 localization in the same manner as iPSC-derived organoids. Patient outcome was also associated with Sox9 localization, thus providing a novel biomarker that is useful for predicting patient outcome.
As more driver genes are described in specific cancer types, it is now possible to divide cancers into genetic subtypes that guide the course of treatment. This includes medulloblastoma (MB) where an iPSC-derived organoid model was developed using PB transposon-mediated integration to express OTX2/cMYC (OM) or GFI1/cMYC (GM), which are commonly overexpressed in G3 MB.71 On implantation into nude mice, these organoids developed tumors at 100% penetrance for both genotypes.
Further analysis showed that methylation and gene expression patterns of the iPSC-derived tumors strongly correlated to G3 MB, but of two different G3 subtypes. Based on the second-generation molecular subgrouping of MB, the OM cells modeled subgroup IV, standard-risk G3 MB whereas the GM cells depicted the subgroup II, high-risk MB facilitating future studies to investigate further differences in these subgroups.
In another model of MB, human iPSC-derived neuroepithelial stem (NES) cells were transduced with a MYCN expression cassette and implanted orthotopically into immunocompromised mice.72 MYCN overexpression was sufficient to generate tumors that resembled human MB histologically, transcriptomically and epigenetically. In fact, the iPSC-derived MYCN NES cell tumors resembled sonic hedgehog (SHH) MB, whereas MYCN-driven mouse models of MB aligned with G3 MB, a subgroup that typically shows amplification of MYC rather than MYCN in human patients.73,74
To further investigate the phenotypic response to MYCN expression, iPSC and primary NES cells were transduced with a mutated MYCNT58A or MYCNWT lentivirus.75 Both cell types formed tumors in mice regardless of which MYCN cassette received, but the MYCNT58A iPSC-derived tumors formed faster with a more invasive phenotype. RNA-sequencing showed that MYCN expression in iPSC-derived or primary neuroepithelial cells developed into infant SHH-like MB with clinically relevant features validating the use of iPSC derived SHH-like MB models and reducing the need to obtain challenging primary cell types.
Glioblastoma (GBM) is the most common primary malignant tumor of the central nervous system,76 and historical mouse models of GBM have failed to capture intratumor heterogeneity seen in human GBM.77 To develop a novel model of GBM, Koga et al used CRISPR-Cas9 to knock out tumor suppressor genes (TSG) PTEN and NF1, or loss of TP53 and PDGFRA exons 8 and 9 resulting in a truncated constitutively active receptor.49 These alterations mimic those found in mesenchymal or proneural GBM subtypes, respectively. Engineered iPSC clones were then differentiated to neural progenitor cells (NPCs) and injected orthotopically into immunocompromised mice, with resulting tumors resembling their respective subtypes.
Human cells were isolated from the tumors and cultured as neurospheres similar to previous GBM patient-derived xenograft experiments.78 Tumor-derived cells showed the ability to self-renew in extreme limiting dilution assays and were able to form tumors in a secondary engraftment with significantly shortened latency. Interestingly, the researchers monitored cancer initiation and tumor evolution by performing single cell RNA-seq on primary and secondary spheres and tumors. They found modest levels of intratumor heterogeneity and differences between genotypes, which only became apparent over time. This study could aid in the creation of more accurate cell-based phenotypic drug discovery approaches in GBM reviewed elsewhere.79
Historically, modeling the genetic cancer predisposing disease neurofibromatosis type 1 (NF1) employed immortalized human Schwann cells, patient derived xenografts, and several mouse models.80 Recently, iPSC-based models have been shown to recapitulate several symptoms associated with NF1 syndrome. Anastasaki and Wegscheid et al used CRISPR-Cas9 to engineer NF1 patient-derived germline mutations into iPSC. Differentiation of these NF1 mutant iPSC clones to NPCs led to increased RAS-controlled proliferation.81
Using a cerebral organoid culture system, they found that a subset of NF1 patient germline NF1 mutations had normal levels of NPC proliferation, but reduced apoptotic activity, resulting in an 80% reduction in immature neurons. Dissecting the intricacies of “first hit” NF1 mutations in an isogenic system will allow us to better understand the genotype-to-phenotype correlations in NF1 patients.
Using a similar approach, Mo et al created an isogenic iPSC line harboring patient-specific NF1 mutations.82 Although NF1−/− cells readily formed tumors in mice when differentiated into Schwann cells before injection, NF1−/+ and wild-type cells did not. Tumors that developed in this system were found to exhibit high SOX10 expression, leading the investigators to conclude that SOX10 expressing cells are the cell of origin for neurofibromas. To confirm this, the group inactivated Nf1 in Sox10+ expressing cells in mice and observed tumors that closely recapitulated the human disease.
The success of these studies has spurred a surge of interest in the use of iPSC to model cancer development and progression. As of July 2022, our analysis of the NIH RePORTER database showed 328 active projects using the keywords “iPSC and cancer,” and 101 when searching “iPSC and cancer and CRISPR”; the numbers we anticipate to increase.83 As additional iPSC-based modeling systems are developed, consideration must be given to the types of mutations that are installed and the developmental stage at which they are introduced. Depending on the malignancy or disease, there are several established and emerging genome editing tools available to precisely create the desired genetic alterations.
Overview of Genetic Engineering Strategies
Broadly, genetic engineering is employed to change or manipulate the genome through installation of a desired DNA sequence, but the methods utilized to accomplish this have evolved as new technologies are developed.84,85 Early methods of genetic engineering relied on plasmids, viruses, and later transposons, to insert genetic cargo randomly into the genome with limited control of where the cargo was inserted.86–88 Although the advent of zinc-finger nucleases, transcription activator-like effector nucleases, and engineered meganucleases initiated the era of precision genome engineering, these methods were challenging to design and implement without specialized expertise, limiting their widespread use.89,90
In 2013, the CRISPR-Cas9 system was shown to produce targeted double-strand breaks in mammalian cells in a significantly more user-friendly fashion than prior technologies.91–94 Further developments expanded the utility of CRISPR-Cas to include the introduction of single base changes and the insertion of genetic cargo without introducing double-strand breaks.95–98 Here, we highlight select CRISPR-Cas-based systems and their utility in developing bottom-up cancer models in iPSC. For a more detailed description of these and other genetic engineering tools, please refer to these other reviews.99–103
CRISPR-Cas9
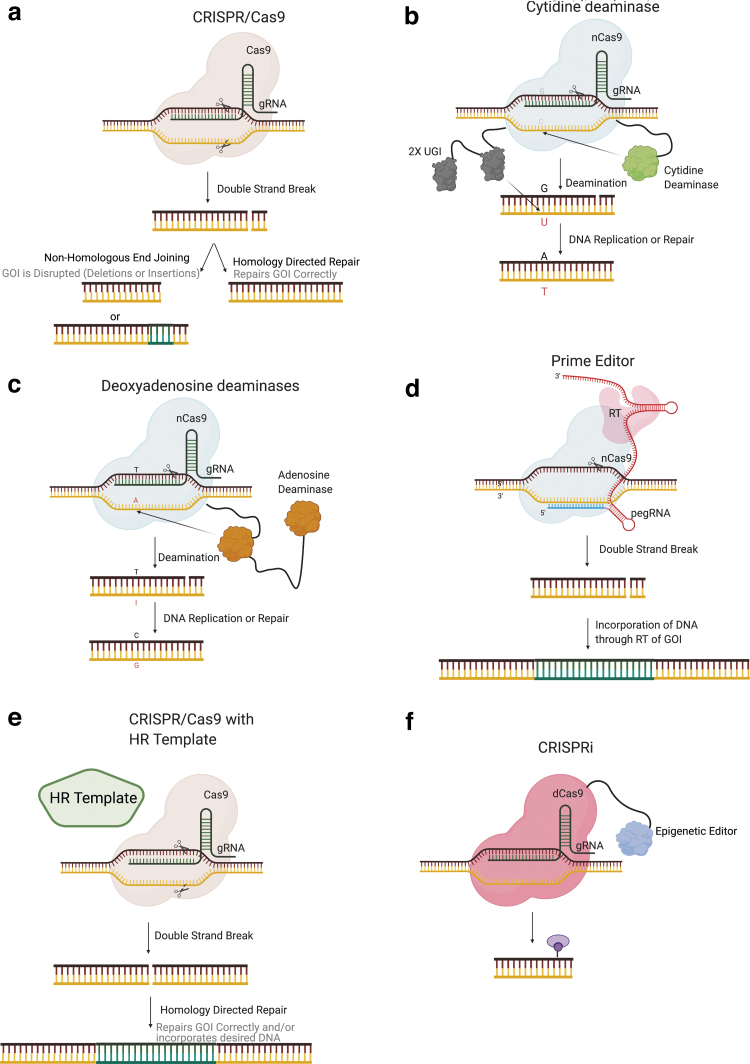
CRISPR-Cas9 is an RNA-guided nuclease derived from a prokaryotic adaptive immune system that can be utilized to induce targeted Double strand breaks (DSBs) in human cells.91,92 The CRISPR-Cas9 system commonly used today consists of a ∼100 bp chimeric single guide RNA (sgRNA) that is bound by the Cas9 protein and localizes to the complementary DNA sequence triggering DNA cleavage by the Cas9 enzyme (Fig. 2a). The sgRNA is a combination of the unique ∼20 bp CRISPR RNA (crRNA) complementary to the target genomic locus and the tracr RNA, which provides the required scaffolding for interaction with the Cas9 enzyme.91,92
FIG. 2.
Precision genetic engineering tools. (a) CRISPR-Cas9 causes double-strand breaks when the sgRNA and DNA complement, activating the Cas9 enzyme. (b) CBE produces C → T transitions through the tethered cytidine deaminase to the nCas9 protein. (c) ABE produces A → G transitions via the tethered lab evolved DNA active adenosine deaminase to nCas9. (d) PE has a tethered RT to Cas9 and elongated sgRNA (pegRNA) such that the pegRNA is the template for the RT to incorporate into the DNA during repair. (e) CRISPRa/i has either an activator or a repressor tethered to a dCas9 to allow for site-specific gene activation or repression, respectively. (f) In many cases, an HR template will be desired for proper genetic engineering. These HR templates can be DNA plasmids, ssODNs, and AAV, among many other options. AAV, adeno-associated virus; ABE, Adenine base editor; CBE, Cytosine base editor; HR, homologous recombination; pegRNA, prime editor guide RNA; RT, reverse transcriptase; CRISPRa, CRISPR activation; CRISPRi, CRISPR interference; PE, prime editor; sgRNA, single guide RNA.
One restriction of the original CRISPR-Cas9 system is the requirement of a protospacer-adjacent motif (PAM) tri-nucleotide sequence where N stands for any nucleotide (ACGT) and GG stands for guanine adjacent to the crRNA complementary DNA sequence.91 However, evolved spCas9 and other Cas orthologs have been discovered with broadened or reduced PAM requirements, thereby increasing the scope of targetable sequences.104–108 For the scope of this review, we will be discussing Streptococcus pyogenes (SpCas9), which remains the most common Cas system employed for genome engineering.
For information on additional Cas variants and/or uses, refer to these other reviews.103,109,110 Site-specific Cas9 induced DSBs are repaired in human cells primarily via non-homologous end joining (NHEJ), leaving small insertions and/or deletions (indels) at the target site.91,92 Conversely, homologous recombination (HR) can be engaged at the DSB site when a donor template or the sister chromatid is used as a repair substrate.91 Variants of the Cas9 enzyme have been developed to create only single-strand breaks (Cas9 nickase, nCas9) or no DNA strand breaks (dead Cas9, dCas9), allowing for expanded functionality.91,111 Broadly speaking, NHEJ is typically leveraged for gene knockout, whereas HR is used to specifically change endogenous DNA sequences or introduce heterologous sequences.
CRISPR-derived DNA editors
Recently, new technologies have emerged to edit DNA without the necessity of a DSB or DNA donor template, namely Cas9 base editors (BEs) (Fig. 2b, c) and prime editors (PEs) (Fig. 2d).95–98 By replacing Cas9 nuclease with the BE/PE technology, the introduction of specific gene edits can be performed safely and in most cases, more efficiently.95,96,112 The major advantages of using BE or PE editing technologies over traditional nuclease mediated HR is the high efficiency of site-specific editing.95,96,112 This is particularly true for BE, where depending on experimental optimization and desired outcome, high genomic editing efficiencies—in some cases exceeding 95%—can be achieved with regularity.113–117 Further benefits are the lack of donor DNA, and critically, reduced or eliminated DSB, both of which are known to be toxic to primary stem cells.118–120
Cytosine BEs (CBEs) consist of a cytidine deaminase fused to the amino terminus of nCas9 and a uracil glycosylase inhibitor tethered to its carboxyl terminus.96 A separate sgRNA then complexes with nCas9 and targets the CBE to the specified genomic locus, where the cytidine deaminase acts on cytosines within a specific region of the sgRNA target site (positions 4–8), converting them to uracil. To increase efficiency, the nCas9 targets the opposite strand as the desired C → T transition causing mismatch repair pathways to select the nicked strand as divergent and read the uracil as a thymine, resulting in a stable C → T transition.
A similar configuration was used to develop an adenine base editor (ABE).95 Using directed evolution of a TadA deoxyadenosine deaminase, the Liu lab created a DNA adenosine deaminase that was tethered to nCas9 (ABE).95 The resulting ABE can efficiently deaminate adenosine to inosine within a defined editing window analogous to CBE. The inosine is read as guanosine by DNA polymerase, and again, nicking of the non-edited strand promotes the introduction of adenosine by mismatch repair, and subsequent replication resulted in the transition of an A → G in the absence of DSBs. Ongoing efforts are underway to narrow or manipulate the editing window to minimize non-target editing.97,121
Recently, the use of an imperfect-guide RNA approach was reported to promote single base editing using CBE and ABE, thereby improving the probability of installing a desired single nucleotide polymorphism.122
The more recently described PE platform, on the other hand, utilizes nCas9 fused to an optimized Moloney murine leukemia virus reverse transcriptase for so-called “search and replace” editing.98 Here, the nCas9 cleaves one strand of the “R loop” such that a 3′ extended sgRNA, termed prime editor guide RNA (pegRNA), can anneal with a freed strand based on sequence complementarity and prime reverse transcription, thereby incorporating a designer sequence included in the 3′ pegRNA extension (Fig. 2d).
The nascent DNA strand created during reverse transcription is subsequently incorporated into the target site during DNA repair. As with BE, preferential incorporation of the desired edit during DNA repair can be stimulated through the introduction of a proximal DNA nick on the opposite strand using a second gRNA.98 The primary benefit of PE over BE is the significantly broader diversity of desired edits that are possible using PE, including the ability to introduce transversion and transition mutations as well as defined deletions and insertions up to 80 and 44 bp, respectively.98 At present, the primary limitation to PE is the lower efficiency of editing compared with BE; however, new versions continue to emerge and efficiency is likely to improve in the coming years.98,123,124 Additional considerations surrounding the use of BE- and PE-derived technologies such as potential off-target effects, immune response, and delivery options are thoroughly reviewed elsewhere.103,125
CRISPR-derived transcriptional modulators
Beyond its ability to alter DNA sequences, the CRISPR platform has also been adapted to regulate endogenous gene expression at the transcriptional level. Early research identified that dCas9 targeted to gene regulatory elements was capable of inhibiting transcription by interfering with transcriptional regulatory protein association with DNA, causing downregulation of target gene expression by up to ∼40%, a process coined CRISPR interference (CRISPRi).111 Subsequent iterations of CRISPRi utilized dCas9 fused to transcriptional effector molecules, most commonly the KRAB repressor.126–128
Alternatively, the fusion of dCas9 to transcriptional activators such as VP16 can induce endogenous gene expression at a defined locus, a process termed CRISPR activation (CRISPRa) (Fig. 2e).129–132 These systems work by targeting dCas9 near the transcriptional start site, thus allowing the transcriptional effector molecule to have local impacts on gene regulation in up to ∼99% of cells tested.130 These technologies may find utility in bottom-up cancer models where permanent gene modification is not desired, or where the functional effects of epigenetic changes to specific genomic loci are being investigated.
For example, epigenetic modifiers may prove useful to untangling mechanisms underlying metastasis, which has been increasingly associated with epigenetic alterations and their influence on the transient nature of the epithelial-to-mesenchymal transition.133,134 When considering CRISPRi/a systems it should be considered that effect can take up to 7 h for full activation/repression of your target gene, and in settings of transient delivery, the desired effect will remain stable for 48 h dropping to baseline after 5–6 days post-electroporation.130 Some applications may benefit from stable integration of CRISPRi/a machinery, with further control offered by including drug-regulated activity that can be used to modulate function of CRISPRa/i systems.
HR templates
To model many cancer-associated alterations, it is necessary to introduce larger cargo, such as conditionally regulated gene expression cassettes, which may be several kilobases (kb) in length. Site-specific integration of larger cargo is typically accomplished using HR from an exogenous DNA donor. This process is highly inefficient in human cells, but it can be enhanced substantially by the introduction of a DSB at the target site, typically using CRISPR-Cas9 or other targeted nucleases (Fig. 2f).135,136
Common HR donor vector designs entail a cargo of interest flanked by regions of homology (homology arms, HA) flanking the target locus. The HA lengths of ∼1 kb are commonly employed; however, recent methodologies such as microhomology-mediated end joining and homology mediated end joining (HMEJ) can utilize substantially smaller HA (<50 bp).137–142 Recently, several groups have utilized recombinant adeno-associated virus (rAAV) as a donor template for HR-mediated site specific integration in human iPSC.143,144
One potential benefit of rAAV is its broad tropism in human tissues, including several serotypes with demonstrated tissue specificity, which may facilitate the transduction of developmental intermediates, or allow the selective engineering of subsets within heterogeneous cell populations.145 One disadvantage to using rAAV is the small carrying capacity (4.7 kb), which limits the size of cargo that can be delivered, particularly considering the capacity that must be dedicated to HA sequences.
Single-stranded DNA oligonucleotides (ssODNs) are another HR template with particular utility for introducing small sequence changes, including single base mutations.146–148 The ssODNs have been shown to have lower unintended integration events compared with double-stranded DNA templates, and ssODNs have been shown to induce point mutations at rates of 70% and insertion of LoxP sites in 40% of cells, making this a reliable HR template for some instances.149,150 However, limitations in synthesis technologies continue to hinder the utility of ssODN for the introduction of larger and more complex DNA sequences.
Cancer Driving Mutations Possible with Genetic Engineering
The types of genetic modifications that can result in oncogenesis, as described in the hallmarks of cancer, are very diverse and include both small and large alterations.151 Here, we discuss many common types of mutations with the potential to drive cancer along with the ways these mutations can be genetically engineered into wild type iPSC to develop bottom-up cancer modeling systems (Fig. 3).
FIG. 3.
Common mutations found in cancer and suggested genetic engineering tools for induction.
Loss of TSG
Loss of TSG such as TP53, RB1, or BRCA1/2 is often required for cancer initiation and development.152 Thus, to effectively model transformation, it is pertinent that these mutations be accurately introduced in bottom-up cancer models, especially when germline mutations are involved in the development of cancer. The basic CRISPR-Cas9 approach for the introduction of DSBs will result in high efficiency gene knockout as a result of indel formation due to error-prone NHEJ DNA repair.153–156
An additional method to induce the loss of TSG in iPSC is the use of ABE/CBE through the targeting of splice sites and the introduction of premature stop codons.113,114,157–159 Targeting splice sites can disrupt the reading frame of the protein of interest, resulting in truncation of the normal protein sequence. This is highly effective at creating protein knock-outs, but much lower efficiencies were found when a truncated protein was desired. Another method to cause loss of gene expression is to disrupt transcription through the use of CRISPRi.129,160
Generation of single nucleotide variants
For some TSG, recurrent point mutations drive oncogenesis; for example, TP53 missense mutations with dominant negative function, or the truncating nonsense or frameshift mutations common of the APC gene.161–163 For these cases, the use of CBE, ABE, and PE allows for the introduction of specific desired mutations without induction of a DSB. If the point mutation is a C → T or A → G mutation, CBE and ABE can be used, respectively, although it must be considered that bystander bases within the editing window are susceptible to deamination.95
The use of editors with altered PAM requirements to shift the editing window and/or clonal isolation and screening may be required to isolate correctly altered clones. Unlike CBE/ABE, the use of prime editing can eliminate potential non-target editing within the editing window.98 By using a user-defined RNA template to insert the desired mutation, PE allows more flexibility in the types of modification that can be introduced compared with standard ABE/CBE.
Activation of oncogenes
Many cancers are driven or maintained by the overexpression of oncogenes. Oncogene overexpression/activation can be induced by a variety of gene alterations, including but not limited to: changes in gene copy number, sequence or methylation changes in the promoters of the oncogene, missense single nucleotide variants resulting in a constitutively active protein, and chromosomal translocations, described in the next section. Some modern genetic engineering tools can aid in the induction of efficient and specific oncogene overexpression, such as the use of CRISPRa that was designed specifically for gene activation.130
In addition, the use of an HR template alongside CRISPR-Cas9 induced DSBs allows for efficient insertion of an entire gene of interest or strong promoter upstream of the oncogene. These approaches can increase both copy number and expression level of oncogenes.164 If the overexpression of an oncogene of interest is caused by a specific point mutation(s) in the promoter, such as is the case for TERT, ABE/CBE/PE could be used to replicate specific promoter mutations as well.165,166
Translocations
Several types of cancer are driven by specific translocations that produce oncogenic fusion proteins or recruit enhancers and/or promoters into the vicinity of an oncogene, causing dysregulated expression.167–170 The simplest method to reproduce this type of alteration is the use of dual CRISPR sgRNAs targeting each desired breakpoint simultaneously. A subset of cells will harbor chromosomal rearrangements and the desired translocation, but the frequency of this event is typically low.171–174 The inclusion of a donor template or single stranded oligodeoxynucleotides (ssODN) with homology arms spanning sequences on two target chromosomes can increase the frequency of translocation.174,175
In addition, a selectable marker flanked by loxP sites can be added within the homology arms to allow drug-based selection of translocation positive populations.176,177 An effective way to introduce the donor template in iPSC may be through the use of rAAV6 or HMEJ, both of which can carry the homology arm cassette in addition to other genetic cargo such as a lox-stop-lox or fluorescent markers to allow tracking or conditional control over translocation activity.178,179
It must be taken into account, when using the two-guide method, with or without a homology arm template, that there is a potential for repair of the individual DSBs at their respective loci, resulting in indel formation, and potentially the induction of inversions or deletions of chromosomal segments. Thorough sequencing should be utilized to rule out potential chromosomal rearrangements in the engineered cells before experimental use. Although PE-mediated translocations have not been explicitly demonstrated, the simultaneous insertion of attP and attB sites by PE and treatment with Bxb1 integrase has been shown to mediate large inversions (40 kb) and one can speculate that the development of similar technology could be used to create translocations.180
Chromosomal deletions
Using the CRISPR-Cas9 system employing a single sgRNA targeting chromosome-specific repetitive sequences or multiple guides targeting adjacent regions on the chromosome can result in deletion of the intervening sequence and even entire chromosome loss if enough sites are targeted by CRISPR-Cas9.181,182 It should be considered that with any multiple guide strategy there is a potential for inversions to occur; thus, this outcome should be evaluated by comprehensive sequencing of the target region.
In addition, the use of an HR template containing recombination sequences, such as inverted loxP sites, allows for Cre-mediated excision of the internal sequence, including large chromosomal deletions, with the ability to control timing of the genetic change.183 This method was used to recreate the MDS associated del(7q) in wild type cells by Kotini et al.184 Further, a recent study demonstrated precise deletion of up to 10 kb in cells using two PE simultaneously, where the 3′ pegRNA template of one PE encoded complementary DNA sequences to the nCas9 target sequence of the second PE and vice versa (PRIME-Del).180,185,186 Advances in this area demonstrate feasible deletion of large chromosomal segments, although the deletion of entire chromosomes or chromosomal arms has not been reported to date and represents an opportunity to further broaden the scope of current cancer models.
Chromosomal duplications
Chromosomal duplications are commonly identified in tumors, particularly tumors that utilize alternative lengthening of telomeres for telomere maintenance or undergo chromothripsis.187,188 Many chromosomal amplifications found in cancer are identified as double minutes (dmin), or extrachromosomal segments of DNA packaged as circular chromosomes that have been shown to independently replicate, resulting in duplications.189,190 One hypothesis for the generation of dmin is through the breakage-fusion-bridge pathway or DNA excision and repair via NHEJ. Thus, to recreate this process in a cell, CRISPR-C was employed to determine whether dmin are created using the CRISPR system.191
CRISPR-C uses dual CRISPR guides to cause simultaneous DSB and in a portion of the cells the two ends of the DNA fragment liberated by paired DSB join, forming a circle through NHEJ activity. Using this system, a chromosome 18-derived ring structure was created in ∼2% of cells tested.191 Currently, this method is limited by dilution of the circular chromosome, suggesting that additional mutations may be required to stably retain the dmin.
Inversions
Gene inversions are commonly found in cancer and will be important to recreate in new bottom-up modeling systems. The creation of specific inversions can be accomplished through the introduction of two simultaneous DSBs, resulting in the inversion of the intervening sequence.192 The iPSC populations harboring such inversions can be screened to isolate desired clones through polymerase chain reaction analysis and sequencing. In a recent study by the Liu Lab, the use of TwinPE was able to induce inversions up to 40 kb and we anticipate this technology could be further developed to induce larger inversions.180
Another method to reproduce inversions is through the addition of loxP sites on the same strand oriented in opposite directions, such that the addition of Cre-recombinase will result in an inversion.193,194 The CRISPR-Cas9 system can be used to induce a double-strand break in combination with an HR template carrying the loxP site and homology arms to the target region. The main advantage to using loxP sites is the ability to control timing of the inversion through the addition of Cre-recombinase at various stages of cell differentiation, allowing investigation into the cell stage-specific responses to the genetic insult.
Temporal control of Cre expression can be accomplished with the tamoxifen inducible Cre-ERT2 fusion or doxycycline-inducible tTA/rtTA systems, where the addition of tamoxifen or doxycycline to the cell culture allows for the activation or suppression of Cre.195–197 Developmental regulation of Cre expression can also be achieved by placing the Cre transgene under the control of a tissue-specific promoter within a transgene or by knocking at an endogenous locus.198–200 These kinds of loxP systems require clonal selection and extensive characterization to confirm insertion of the loxP sites in trans, as well as faithful inversion after the addition of Cre-recombinase.
Initiation of chromothripsis
Chromothripsis results from a single punctuated event in which dozens to thousands of chromosomal rearrangements occur.201 This event is common in genetically complex cancers, such as osteosarcoma, and is believed to drive cellular transformation through the loss of TSG and overexpression of oncogenes.188 Although the precise mechanisms driving chromothripsis remain unclear, one current hypothesis is that during the first cell cycle after the induction of a DSB, chromosome misalignment results in missegregation of the chromosome into a micronuclei.202
During the following cell cycle, the micronucleus malfunctions, resulting in envelope disruption and chromosomal shattering. The resulting chromosomal pieces are randomly ligated back together by NHEJ in a canonical LIG4-dependent manner, resulting in the genomic rearrangements that are a hallmark of chromothripsis. Another proposed mechanism involves telomere fusion followed by chromosome bridge formation during cell division, resulting in chromosomal breaks on segregation followed by DNA fusion at the next cell cycle.201
Currently, inducing chromothripsis on a genome wide scale using genetic engineering has not been accomplished, although there are several methods that have shown promise. One possibility is to disrupt genes involved in replication stress responses followed by DSB induction to promote aberrant replication fork collapse after treatment with hydroxyurea.203 An alternative method could involve disrupting kinetochore function, which could promote missegregation and an increase in micronuclei formation, thereby creating an environment that is conducive to chromothriptic events.202
The expression of Cas9 and 100 s of sgRNAs simultaneously targeting discrete regions throughout the genome or, alternatively, a single sgRNA targeting repetitive sequences found throughout the genome could conceivably induce a chromothripsis-like event, including mass genomic rearrangements and translocations, allowing for the study of cell behavior during and after global DNA rearrangement.114 It was recently shown that even on-target Cas9 editing with a single sgRNA could potentially initiate local chromothripsis, or genomic rearrangements on the target chromosome in proximity to the cut site, illustrating the feasibility of inducing local and genome wide chromothripsis events using the CRISPR system.63
Conditional systems to model developmental cancers
One of the major benefits of using iPSC in investigating cancer is that iPSC allows access to developmental intermediate cell types that are not accessible in adult tissues. Mouse models of pediatric cancers have affirmed that developmental tumors can originate in stem or progenitor cells during specific developmental windows of time.204–210 It is hypothesized that the cell of origin for developmental cancers can inform the precise cellular state permissive to the initial oncogenic insults that can promote self-renewal at the expense of orchestrated differentiation pathways.211
In this context, iPSC models provide a novel tool to understand the developmental, temporal, and sequential order of events en route to a mutational landscape permissive to oncogenesis. As has been done in mice, oncogene expression can be restricted to specific developmental time points using tamoxifen-inducible Cre or doxycycline-inducible tTA/rtTA systems, as well as through the use of tissue-specific promoters.195–197 In this way, oncogene expression can be tailored to occur within specific lineages and/or specific cell-states, expanding the capacity to investigate links between development and transformation.
Remaining Challenges and Future Directions
Bottom-up cancer models show promise in recapitulating human disease. These models provide several advantages over current model systems, providing: (1) a renewable source of healthy non-tumor human cells to evaluate candidate genetic drivers of tumorigenesis, (2) isogenic control cell lines for iPSC-derived tumors undergoing chemical or genetic screening to filter out non-specific activity, (3) providing systems to investigate the role of chromosome copy number changes in tumorigenesis, and (4) to identify the cell types and target cells that can give rise to a specific tumor, including embryonic tissues.
Despite these benefits, significant and complex challenges continue to limit the realization of accurate stem cell models of human cancer. Many of these challenges are outside the scope of this perspective and will be discussed briefly but have been reviewed extensively elsewhere.212,213
Any effort to apply bottom-up cancer modeling in iPSC must consider the amenability of the specific malignancy to this approach. Tumors with known genetic drivers that arise from a well described cell-of-origin for which defined iPSC differentiation protocols are available are the most straightforward to model using this method. Some examples include several brain tumors, melanoma, translocation-driven sarcomas, and colorectal cancers.71,214–217
Cancers that are driven by punctuated mass genomic instability and complex mutational profiles such as osteosarcoma and liposarcoma are more difficult as replicating complex, less understood events such as chromothripsis and kataegis presents a significant challenge.218 Similarly, cancers driven by environmental influences or cancers where the cell-of-origin is unknown or does not have an established method for generation from iPSC may present additional challenges.
It is important to consider that the biological variation between individual iPSC lines may influence results. It was previously found that out of 711 iPSC cell lines derived from 301 individuals, differences between individual donors were identified as the largest source of iPSC heterogeneity.219 To circumvent this issue, iPSC donors from the different ends of polygenic risk of a particular cancer may be used to discern such influences. Additional considerations should include the potential for sex-specific differences, necessitating the study of tumor models created from iPSC lines derived from both sexes.
To avoid confounding results due to the reprogramming factors themselves, the use of non-integrating approaches to reprogramming should be prioritized to remove the potential for the re-expression of reprogramming transgenes and unintended gene alterations arising from rom random and stable transgene insertion.220,221
Directed differentiation of human iPSC has been informed by decades of developmental biology knowledge and has been shown to faithfully recapitulate cellular development across numerous cell lineages; however, some limitations remain and should be considered.5,222–225 The process of in vitro differentiation typically involves the provision of specific growth factors, small molecules, and extracellular matrices in the appropriate temporal sequence that is necessary to first specify the germ layer (e.g., endoderm, mesoderm, ectoderm) followed by a lineage of interest. In vitro, this occurs on a much shorter timeframe than that of human gestation, and in some cases results in a more immature or fetal phenotype.226–229
Despite this, the resultant cells can still serve as a valuable model as most of the key functions and phenotypic attributes of the target lineage are present. Protocol efficiency and heterogeneity within the final cell population should also be considered; however, the use of immunomagnetic separation or fluorescence activated cell sorting can minimize the impact of such issues. The high complexity of some differentiation protocols can sometimes pose a challenge to reproducibility. This was aptly illustrated in a study where the same two iPSC lines were differentiated to neurons at five different laboratories with divergent results and concluded that the laboratory in which the cells were differentiated represented the largest source of variability.230
Thus, multi-center collaborations, or at minimum replicate differentiations using multiple iPSC lines should be considered to limit the influence of differentiation protocol and/or iPSC source. Finally, appropriate molecular, phenotypic, and functional assays should be performed to validate cells derived during differentiation.
Tumors are highly heterogeneous, and this heterogeneity is a determinant of therapeutic response and disease pathology.231,232 How well tumors derived from iPSC models mimic this intratumoral heterogeneity is not well understood. However, heterogeneity may be forced by creating multiple iPSC-derivative tumors with differing genotypes that can be mixed to robustly evaluate the efficacy of treatment strategies. Additional methods to recapitulate tumor heterogeneity in genetically engineered human stem cell models of cancer will be critical to future model development.
In addition, although iPSC-derived cancer cells can be transplanted into immunocompromised animals, such models fail to recapitulate immune infiltration and the heterogeneous microenvironment of primary tumors.233 In particular, the lack of a functional immune system precludes the ability to evaluate immunotherapeutic approaches in vivo, although specialized murine models with a humanized immune system may overcome this limitation in the future.234
Recently, human stem cell models were generated in immunocompetent mice, but the human cells were implanted into gastrulating mouse embryos, before development of the immune system.235 Humanized mice, however, are costly and complex making it difficult for most research labs to routinely use these methods, and because the human immune cells and tumor cells did not arise from the same patient, there is still the possibility of rejection in a humanized mouse model.
Alternatively, using iPSC from the same person to generate both the tumor lines and immune cells will facilitate an in vitro co-culture environment that potentially mimics the microenvironment better than the humanized mouse model. The potential utility of immune cells, including T lymphocytes, natural killer cells, and macrophages, derived from human Embryonic Stem Cells (ESC) or iPSC for research of tumor biology and cancer immunotherapy has been extensively reviewed elsewhere.236,237
Another level of genetic regulation in cancer is that of the epigenome. Investigation of epigenetic regulation during cancer initiation, development, and progression will be greatly aided by the use of bottom-up cancer models and the newly developed CRISPR-based epigenetic technologies. One such use is the use of dCas9 targeted to specific promoters to study the impacts of demethylation or histone modifications.238,239 For additional information on epigenetic editing using CRISPR-based technology, here is an excellent review.240 The ability to edit the epigenome will be essential as we continue to develop more accurate and predictive cancer models.
Concluding Remarks
Historically, modeling cancer development beginning with normal cells has been studied using genetically engineered mouse models (GEMMs). The GEMMs have provided a breadth of knowledge and methods that we can now leverage to usher in a new era of cancer modeling in human iPSC. The capacity of iPSC to differentiate into nearly any somatic cell-type coupled with their amenability to manipulation at the genetic level using new genetic engineering strategies represents an unprecedented opportunity to expand our mechanistic understanding of human cancer development.
Further, the ability to derive iPSC from easily accessible somatic cell-types has facilitated the establishment and continued expansion of large iPSC banks from diverse genetic backgrounds, providing an unprecedented opportunity to fuse epidemiology and genomics with basic mechanistic studies at scales not previously possible. Realizing this potential will necessitate interdisciplinary collaboration spanning stem cell biology, bioengineering, cancer biology, genomics, and genome engineering. Moving forward, we envision bottom-up cancer modeling in iPSC as a highly flexible and powerful approach to glean novel mechanistic insights into the fundamental biology of human cancers.
Supplementary Material
Authors' Contributions
K.L.B., G.M.D., B.R.W., and B.S.M. conceived the scope of the review, wrote, and edited the article. R.A.M., T.K., M.H., and W.A.W. contributed to sections on model applications to specific cancers. M.G.K. assimilated data on mutational profiles of relevant cancers and identified optimal genetic engineering approaches to introduce mutations as outlined in Figure 3 and Supplementary Table S1. L.G.S. contributed epidemiological insights to sections pertaining to genetic diversity of iPSC lines. All authors reviewed and approved of the final article.
Author Disclosure Statement
D.A.L. is the co-founder and co-owner of several biotechnology companies, including NeoClone Biotechnologies, Inc., Discovery Genomics, Inc. (recently acquired by Immusoft, Inc.), B-MoGen Biotechnologies, Inc. (recently acquired by Biotechne Corporation), and Luminary Therapeutics, Inc. D.A.L. holds equity in, serves as a Senior Scientific Advisor for and a Board of Director member for Recombinetics, a genome editing company. D.A.L. consults for Genentech, Inc., which is funding some of his research. W.A.W. is a co-founder of StemSynergy Therapeutics and holds equity in Nalo Therapeutics and Auron Therapeutics. The business of all these companies is unrelated to the contents of this article.
Funding Information
B.R.W. acknowledges funding from NIH grants R21CA237789, R21AI163731, P01CA254849 Alex's Lemonade Stand Foundation, Children's Cancer Research Fund, and Rein in Sarcoma. Research in the Moriarity Lab is supported by NIH grants P50CA136393, P01CA254849, R01AI161017, and R01AI146009. D.A.L. acknowledges funding from the American Cancer Society Research Professor Award and the National Institutes of Health (R01NS115438). M.H. acknowledges funding from the Wright Trust Foundation, The Saban Research Institute, and National Institutes of Health (R00CA197484). Research in the Weiss lab is supported by NIH grants R01CA255369, R01NS106155, R01CA221969, P01CA217959, P30CA082103, P50CA097257, U01CA217864, U54CA243125, Alex's Lemonade Stand, The Brain Tumour Charity, Cancer Research UK grant A28592, St. Baldrick, and Samuel G. Waxman Foundations; and the Evelyn and Mattie Anderson Chair. Figures were created using BioRender (BioRender.com).
Supplementary Material
References
- 1. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126(4):663–676; doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 2. Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007;131(5):861–872; doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 3. Brambrink T, Foreman R, Welstead GG, et al. Sequential expression of pluripotency markers during direct reprogramming of mouse somatic cells. Cell Stem Cell 2008;2(2):151–159; doi: 10.1016/j.stem.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maherali N, Sridharan R, Xie W, et al. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell 2007;1(1):55–70; doi: 10.1016/j.stem.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 5. Hu B-Y, Weick JP, Yu J, et al. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc Natl Acad Sci U S A 2010;107(9):4335–4340; doi: 10.1073/pnas.0910012107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Agarwal S, Holton KL, Lanza R. Efficient differentiation of functional hepatocytes from human embryonic stem cells. Stem Cells 2008;26(5):1117–1127; doi: 10.1634/stemcells.2007-1102. [DOI] [PubMed] [Google Scholar]
- 7. Wong AP, Rossant J. Generation of lung epithelium from pluripotent stem cells. Curr Pathobiol Rep 2013;1(2):137–145; doi: 10.1007/s40139-013-0016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abdal Dayem A, Lee SB, Kim K, et al. Production of mesenchymal stem cells through stem cell reprogramming. Int J Mol Sci 2019;20(8); doi: 10.3390/ijms20081922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abou-Saleh H, Zouein FA, El-Yazbi A, et al. The march of pluripotent stem cells in cardiovascular regenerative medicine. Stem Cell Res Ther 2018;9(1):201; DOI: 10.1186/s13287-018-0947-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hansel MC, Davila JC, Vosough M, et al. The use of induced pluripotent stem cells for the study and treatment of liver diseases. Curr Protoc Toxicol 2016;67:14.13.1–14.13.27; DOI: 10.1002/0471140856.tx1413s67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Calvert BA, Ryan Firth AL. Application of iPSC to modelling of respiratory diseases. Adv Exp Med Biol 2020;1237:1–16; DOI: 10.1007/5584_2019_430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ebrahimi M, Forouzesh M, Raoufi S, et al. Differentiation of human induced pluripotent stem cells into erythroid cells. Stem Cell Res Ther 2020;11(1):483; DOI: 10.1186/s13287-020-01998-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Efthymiou AG, Chen G, Rao M, et al. Self-renewal and cell lineage differentiation strategies in human embryonic stem cells and induced pluripotent stem cells. Expert Opin Biol Ther 2014;14(9):1333–1344; doi: 10.1517/14712598.2014.922533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Park I-H, Arora N, Huo H, et al. Disease-specific induced pluripotent stem cells. Cell 2008;134(5):877–886; doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dimos JT, Rodolfa KT, Niakan KK, et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science 2008;321(5893):1218–1221; doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- 16. Israel MA, Yuan SH, Bardy C, et al. Probing sporadic and familial Alzheimer's disease using induced pluripotent stem cells. Nature 2012;482(7384):216–220; doi: 10.1038/nature10821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rashid ST, Corbineau S, Hannan N, et al. Modeling inherited metabolic disorders of the liver using human induced pluripotent stem cells. J Clin Invest 2010;120(9):3127–3136; doi: 10.1172/JCI43122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Müller LUW, Milsom MD, Harris CE, et al. Overcoming reprogramming resistance of fanconi anemia cells. Blood 2012;119(23):5449–5457; doi: 10.1182/blood-2012-02-408674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chao MP, Gentles AJ, Chatterjee S, et al. Human AML-iPSCs reacquire leukemic properties after differentiation and model clonal variation of disease. Cell Stem Cell 2017;20(3):329–344.e7; doi: 10.1016/j.stem.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kotini AG, Chang C-J, Chow A, et al. Stage-specific human induced pluripotent stem cells map the progression of myeloid transformation to transplantable leukemia. Cell Stem Cell 2017;20(3):315–328.e7; doi: 10.1016/j.stem.2017.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Izgi K, Canatan H, Iskender B. Current status in cancer cell reprogramming and its clinical implications. J Cancer Res Clin Oncol 2017;143(3):371–383; doi: 10.1007/s00432-016-2258-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bang JS, Choi NY, Lee M, et al. Reprogramming of cancer cells into induced pluripotent stem cells questioned. Int J Stem Cells 2019;12(3):430–439; doi: 10.15283/ijsc19067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moore JB, 4th, Loeb DM, Hong KU, et al. Epigenetic reprogramming and re-differentiation of a ewing sarcoma cell line. Front Cell Dev Biol 2015;3:15; DOI: 10.3389/fcell.2015.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chao H-M, Chern E. Patient-derived induced pluripotent stem cells for models of cancer and cancer stem cell research. J Formos Med Assoc 2018;117(12):1046–1057; doi: 10.1016/j.jfma.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 25. Moses C, Garcia-Bloj B, Harvey AR, et al. Hallmarks of cancer: The CRISPR generation. Eur J Cancer 2018;93:10–18; DOI: 10.1016/j.ejca.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 26. Glaumann H. Crinophagy as a means for degrading excess secretory proteins in rat liver. Revis Biol Celular 1989;20:97–110. [PubMed] [Google Scholar]
- 27. Arai S, Miyauchi M, Kurokawa M. Modeling of hematologic malignancies by iPS technology. Exp Hematol 2015;43(8):654–660; doi: 10.1016/j.exphem.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 28. Kim H, Schaniel C. Modeling hematological diseases and cancer with patient-specific induced pluripotent stem cells. Front Immunol 2018;9:2243; DOI: 10.3389/fimmu.2018.02243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Papapetrou EP. Modeling myeloid malignancies with patient-derived iPSCs. Exp Hematol 2019;71:77–84; DOI: 10.1016/j.exphem.2018.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sidhu I, Barwe SP, Pillai RK, et al. Harnessing the power of induced pluripotent stem cells and gene editing technology: Therapeutic implications in hematological malignancies. Cells 2021;10(10):2698; DOI: 10.3390/cells10102698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Donada A, Basso-Valentina F, Arkoun B, et al. Induced pluripotent stem cells and hematological malignancies: A powerful tool for disease modeling and drug development. Stem Cell Res 2020;49:102060; DOI: 10.1016/j.scr.2020.102060. [DOI] [PubMed] [Google Scholar]
- 32. Yamasaki AE, King NE, Matsui H, et al. Two iPSC lines generated from the bone marrow of a relapsed/refractory AML patient display normal karyotypes and myeloid differentiation potential. Stem Cell Res 2019;41:101587; DOI: 10.1016/j.scr.2019.101587. [DOI] [PubMed] [Google Scholar]
- 33. Wuputra K, Lin C-S, Tsai M-H, et al. Cancer cell reprogramming to identify the genes competent for generating liver cancer stem cells. Inflamm Regen 2017;37:15; DOI: 10.1186/s41232-017-0041-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chu P-Y, Hou M-F, Lai J-C, et al. Cell reprogramming in tumorigenesis and its therapeutic implications for breast cancer. Int J Mol Sci 2019;20(8); doi: 10.3390/ijms20081827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang X, Cruz FD, Terry M, et al. Terminal differentiation and loss of tumorigenicity of human cancers via pluripotency-based reprogramming. Oncogene 2013;32(18):2249–2260, 2260.e1–e21; doi: 10.1038/onc.2012.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang Y, Schmid B, Nikolaisen NK, et al. Patient iPSC-derived neurons for disease modeling of frontotemporal dementia with mutation in CHMP2B. Stem Cell Rep 2017;8(3):648–658; doi: 10.1016/j.stemcr.2017.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lukovic D, Artero Castro A, Delgado ABG, et al. Human iPSC derived disease model of MERTK-associated retinitis pigmentosa. Sci Rep 2015;5:12910; DOI: 10.1038/srep12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kim J, Hoffman JP, Alpaugh RK, et al. An iPSC line from human pancreatic ductal adenocarcinoma undergoes early to invasive stages of pancreatic cancer progression. Cell Rep 2013;3(6):2088–2099; doi: 10.1016/j.celrep.2013.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee D-F, Su J, Kim HS, et al. Modeling familial cancer with induced pluripotent stem cells. Cell 2015;161(2):240–254; doi: 10.1016/j.cell.2015.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shigemura T, Matsuda K, Kurata T, et al. Essential role of PTPN11 mutation in enhanced haematopoietic differentiation potential of induced pluripotent stem cells of juvenile myelomonocytic leukaemia. Br J Haematol 2019;187(2):163–173; doi: 10.1111/bjh.16060. [DOI] [PubMed] [Google Scholar]
- 41. Chang C-J, Kotini AG, Olszewska M, et al. Dissecting the contributions of cooperating gene mutations to cancer phenotypes and drug responses with patient-derived iPSCs. Stem Cell Rep 2018;10(5):1610–1624; doi: 10.1016/j.stemcr.2018.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pearson S, Guo B, Pierce A, et al. Proteomic analysis of an induced pluripotent stem cell model reveals strategies to treat juvenile myelomonocytic leukemia. J Proteome Res 2020;19(1):194–203; doi: 10.1021/acs.jproteome.9b00495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Taoka K, Arai S, Kataoka K, et al. Using patient-derived iPSCs to develop humanized mouse models for chronic myelomonocytic leukemia and therapeutic drug identification, including liposomal clodronate. Sci Rep 2018;8(1):15855; DOI: 10.1038/s41598-018-34193-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kim H, Yoo S, Zhou R, et al. Oncogenic role of SFRP2 in p53-mutant osteosarcoma development via autocrine and paracrine mechanism. Proc Natl Acad Sci U S A 2018;115(47):E11128–E11137; doi: 10.1073/pnas.1814044115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dannenmann B, Zahabi A, Mir P, et al. Human iPSC-based model of severe congenital neutropenia reveals elevated UPR and DNA damage in CD34+ cells preceding leukemic transformation. Exp Hematol 2019;71:51–60; DOI: 10.1016/j.exphem.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 46. Hwang JW, Desterke C, Féraud O, et al. iPSC-derived embryoid bodies as models of c-met-mutated hereditary papillary renal cell carcinoma. Int J Mol Sci 2019;20(19):4867; DOI: 10.3390/ijms20194867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Soyombo AA, Wu Y, Kolski L, et al. Analysis of induced pluripotent stem cells from a BRCA1 mutant family. Stem Cell Rep 2013;1(4):336–349; doi: 10.1016/j.stemcr.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lanikova L, Babosova O, Prchal JT. Experimental modeling of myeloproliferative neoplasms. Genes 2019;10(10):813; DOI: 10.3390/genes10100813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Koga T, Chaim IA, Benitez JA, et al. Longitudinal assessment of tumor development using cancer avatars derived from genetically engineered pluripotent stem cells. Nat Commun 2020;11(1):550; DOI: 10.1038/s41467-020-14312-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ogawa J, Pao GM, Shokhirev MN, et al. Glioblastoma model using human cerebral organoids. Cell Rep 2018;23(4):1220–1229; doi: 10.1016/j.celrep.2018.03.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Koga T, Chen CC, Furnari FB. Genome engineering evolves brain tumor modeling. Neurol Med Chir 2020;60(7):329–336; doi: 10.2176/nmc.ra.2020-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang T, Pine AR, Kotini AG, et al. Sequential CRISPR gene editing in human iPSCs charts the clonal evolution of myeloid leukemia and identifies early disease targets. Cell Stem Cell 2021;28(6):1074.e7–1089.e7; DOI: 10.1016/j.stem.2021.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Filippova J, Matveeva A, Zhuravlev E, et al. Guide RNA modification as a way to improve CRISPR/Cas9-based genome-editing systems. Biochimie 2019;167:49–60; DOI: 10.1016/j.biochi.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 54. Zhang S, Shen J, Li D, et al. Strategies in the delivery of Cas9 ribonucleoprotein for CRISPR/Cas9 genome editing. Theranostics 2021;11(2):614–648; doi: 10.7150/thno.47007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhang M, Vandana JJ, Lacko L, et al. Modeling cancer progression using human pluripotent stem cell-derived cells and organoids. Stem Cell Res 2020;49:102063; DOI: 10.1016/j.scr.2020.102063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cosmic. COSMIC; 2020. https://cancer.sanger.ac.uk/cosmic [Last accessed: July 19, 2022].
- 57. Qin Z, Li X, Han P, et al. Association between polymorphic CAG repeat lengths in the androgen receptor gene and susceptibility to prostate cancer: A systematic review and meta-analysis. Medicine 2017;96(25):e7258; DOI: 10.1097/MD.0000000000007258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sturm D, Orr BA, Toprak UH, et al. New brain tumor entities emerge from molecular classification of CNS-PNETs. Cell 2016;164(5):1060–1072; doi: 10.1016/j.cell.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yamazaki H, Ohba Y, Tamaoki N, et al. A deletion mutation within the ligand binding domain is responsible for activation of epidermal growth factor receptor gene in human brain tumors. Jpn J Cancer Res 1990;81(8):773–779; doi: 10.1111/j.1349-7006.1990.tb02644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nowell P, Hungerford D, Nowell PC. A minute chromosome in human chronic granulocytic leukemia; n.d. Available from: https://www.scienceopen.com/document?vid=f287f1cc-cb6a-4eec-9a02-d8baf5240bb0 [Last accessed: May 14, 2021].
- 61. Tseng Y-Y, Moriarity BS, Gong W, et al. PVT1 dependence in cancer with MYC copy-number increase. Nature 2014;512(7512):82–86; doi: 10.1038/nature13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Knuutila S, Elonen E, Teerenhovi L, et al. Trisomy 12 in B cells of patients with B-cell chronic lymphocytic leukemia. N Engl J Med 1986;314(14):865–869; doi: 10.1056/NEJM198604033141401. [DOI] [PubMed] [Google Scholar]
- 63. Leibowitz ML, Papathanasiou S, Doerfler PA, et al. Chromothripsis as an on-target consequence of CRISPR-Cas9 genome editing. Nat Gen 2021;53(6):895–905; doi: 10.1038/s41588-021-00838-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Vousden KH, Doniger J, DiPaolo JA, et al. The E7 open reading frame of human papillomavirus type 16 encodes a transforming gene. Oncogene Res 1988;3(2):167–175. [PubMed] [Google Scholar]
- 65. Yuan X, Larsson C, Xu D. Mechanisms underlying the activation of TERT transcription and telomerase activity in human cancer: Old actors and new players. Oncogene 2019;38(34):6172–6183; doi: 10.1038/s41388-019-0872-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bushman FD. Retroviral insertional mutagenesis in humans: Evidence for four genetic mechanisms promoting expansion of cell clones. Mol Ther 2020;28(2):352–356; doi: 10.1016/j.ymthe.2019.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hayakawa K, Ikeya M, Fukuta M, et al. Identification of target genes of synovial sarcoma-associated fusion oncoprotein using human pluripotent stem cells. Biochem Biophys Res Commun 2013;432(4):713–719; doi: 10.1016/j.bbrc.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 68. Tamaki S, Fukuta M, Sekiguchi K, et al. SS18-SSX, the oncogenic fusion protein in synovial sarcoma, is a cellular context-dependent epigenetic modifier. PLoS One 2015;10(11):e0142991; DOI: 10.1371/journal.pone.0142991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Grossberg AJ, Chu LC, Deig CR, et al. Multidisciplinary standards of care and recent progress in pancreatic ductal adenocarcinoma. CA Cancer J Clin 2020;70(5):375–403; doi: 10.3322/caac.21626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Huang L, Holtzinger A, Jagan I, et al. Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell- and patient-derived tumor organoids. Nat Med 2015;21(11):1364–1371; doi: 10.1038/nm.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ballabio C, Anderle M, Gianesello M, et al. Modeling medulloblastoma in vivo and with human cerebellar organoids. Nat Commun 2020;11(1):583; DOI: 10.1038/s41467-019-13989-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Huang M, Tailor J, Zhen Q, et al. Engineering genetic predisposition in human neuroepithelial stem cells recapitulates medulloblastoma tumorigenesis. Cell Stem Cell 2019;25(3):433–446.e7; doi: 10.1016/j.stem.2019.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Swartling FJ, Grimmer MR, Hackett CS, et al. Pleiotropic role for MYCN in medulloblastoma. Genes Dev 2010;24(10):1059–1072; doi: 10.1101/gad.1907510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Northcott PA, Buchhalter I, Morrissy AS, et al. The whole-genome landscape of medulloblastoma subtypes. Nature 2017;547(7663):311–317; doi: 10.1038/nature22973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Čančer M, Hutter S, Holmberg KO, et al. Humanized stem cell models of pediatric medulloblastoma reveal an Oct4/mTOR axis that promotes malignancy. Cell Stem Cell 2019;25(6):855.e11–870.e11; DOI: 10.1016/j.stem.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Cloughesy TF, Cavenee WK, Mischel PS. Glioblastoma: From molecular pathology to targeted treatment. Annu Rev Pathol 2014;9:1–25; DOI: 10.1146/annurev-pathol-011110-130324. [DOI] [PubMed] [Google Scholar]
- 77. Lenting K, Verhaak R, Ter Laan M, et al. Glioma: Experimental models and reality. Acta Neuropathol 2017;133(2):263–282; doi: 10.1007/s00401-017-1671-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Akhavan D, Pourzia AL, Nourian AA, et al. De-repression of PDGFRβ transcription promotes acquired resistance to EGFR tyrosine kinase inhibitors in glioblastoma patients. Cancer Discov 2013;3(5):534–547; doi: 10.1158/2159-8290.CD-12-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. O'Duibhir E, Carragher NO, Pollard SM. Accelerating glioblastoma drug discovery: Convergence of patient-derived models, genome editing and phenotypic screening. Mol Cell Neurosci 2017;80:198–207; DOI: 10.1016/j.mcn.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Williams KB, Largaespada DA. New model systems and the development of targeted therapies for the treatment of neurofibromatosis type 1-associated malignant peripheral nerve sheath tumors. Genes 2020;11(5):477; DOI: 10.3390/genes11050477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Anastasaki C, Wegscheid ML, Hartigan K, et al. Human iPSC-derived neurons and cerebral organoids establish differential effects of germline NF1 gene mutations. Stem Cell Rep 2020;14(4):541–550; doi: 10.1016/j.stemcr.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Mo J, Anastasaki C, Chen Z, et al. Humanized neurofibroma model from induced pluripotent stem cells delineates tumor pathogenesis and developmental origins. J Clin Invest 2021;131(1):e139807; DOI: 10.1172/JCI139807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Anonymous. RePORT > RePORTER; n.d. Available from: https://reporter.nih.gov/ [Last accessed: April 13, 2021].
- 84. Tamura R, Toda M. Historic overview of genetic engineering technologies for human gene therapy. Neurol Med Chir 2020;60(10):483–491; doi: 10.2176/nmc.ra.2020-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Rogers S, Pfuderer P. Use of viruses as carriers of added genetic information. Nature 1968;219(5155):749–751; doi: 10.1038/219749a0. [DOI] [PubMed] [Google Scholar]
- 86. Szybalska EH, Szybalski W. Genetics of human cell line. IV. DNA-mediated heritable transformation of a biochemical trait. Proc Natl Acad Sci U S A 1962;48(12):2026–2034; doi: 10.1073/pnas.48.12.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Rosenberg SA, Aebersold P, Cornetta K, et al. Gene transfer into humans—Immunotherapy of patients with advanced melanoma, using tumor-infiltrating lymphocytes modified by retroviral gene transduction. N Engl J Med 1990;323(9):570–578; doi: 10.1056/NEJM199008303230904. [DOI] [PubMed] [Google Scholar]
- 88. Ivics Z, Hackett PB, Plasterk RH, et al. Molecular reconstruction of sleeping beauty, a Tc1-like transposon from fish, and its transposition in human cells. Cell 1997;91(4):501–510; doi: 10.1016/s0092-8674(00)80436-5. [DOI] [PubMed] [Google Scholar]
- 89. Kim YG, Cha J, Chandrasegaran S. Hybrid restriction enzymes: Zinc finger fusions to Fok I cleavage domain. Proc Natl Acad Sci U S A 1996;93(3):1156–1160; doi: 10.1073/pnas.93.3.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Christian M, Cermak T, Doyle EL, et al. Targeting DNA double-strand breaks with TAL effector nucleases. Genetics 2010;186(2):757–761; doi: 10.1534/genetics.110.120717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Cong L, Ran FA, Cox D, et al. Multiplex genome engineering using CRISPR/Cas systems. Science 2013;339(6121):819–823; doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Mali P, Yang L, Esvelt KM, et al. RNA-guided human genome engineering via Cas9. Science 2013;339(6121):823–826; doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Cho SW, Kim S, Kim JM, et al. Targeted genome engineering in human cells with the Cas9 RNA-guided endonuclease. Nat Biotechnol 2013;31(3):230–232; doi: 10.1038/nbt.2507. [DOI] [PubMed] [Google Scholar]
- 94. Jinek M, East A, Cheng A, et al. RNA-programmed genome editing in human cells. Elife 2013;2:e00471; DOI: 10.7554/eLife.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Gaudelli NM, Komor AC, Rees HA, et al. Programmable base editing of A•T to G•C in genomic DNA without DNA cleavage. Nature 2017;551(7681):464–471; doi: 10.1038/nature24644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Komor AC, Kim YB, Packer MS, et al. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 2016;533(7603):420–424; doi: 10.1038/nature17946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Komor AC, Zhao KT, Packer MS, et al. Improved base excision repair inhibition and bacteriophage Mu Gam protein yields C:G-to-T:A base editors with higher efficiency and product purity. Sci Adv 2017;3(8):eaao4774; DOI: 10.1126/sciadv.aao4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Anzalone AV, Randolph PB, Davis JR, et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 2019;576(7785):149–157; doi: 10.1038/s41586-019-1711-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kim H, Kim J-S. A guide to genome engineering with programmable nucleases. Nat Rev Genet 2014;15(5):321–334; doi: 10.1038/nrg3686. [DOI] [PubMed] [Google Scholar]
- 100. Carroll D. Genome engineering with targetable nucleases. Annu Rev Biochem 2014;83:409–439; DOI: 10.1146/annurev-biochem-060713-035418. [DOI] [PubMed] [Google Scholar]
- 101. Gaj T, Gersbach CA, Barbas CF 3rd. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol 2013;31(7):397–405; doi: 10.1016/j.tibtech.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Pal M, Herold MJ. CRISPR base editing applications for identifying cancer-driving mutations. Biochem Soc Trans 2021;49(1):269–280; doi: 10.1042/BST20200550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Anzalone AV, Koblan LW, Liu DR. Genome editing with CRISPR-Cas nucleases, base editors, transposases and prime editors. Nat Biotechnol 2020;38(7):824–844; doi: 10.1038/s41587-020-0561-9. [DOI] [PubMed] [Google Scholar]
- 104. Ran FA, Cong L, Yan WX, et al. In vivo genome editing using staphylococcus aureus Cas9. Nature 2015;520(7546):186–191; doi: 10.1038/nature14299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Gasiunas G, Young JK, Karvelis T, et al. A catalogue of biochemically diverse CRISPR-Cas9 orthologs. Nat Commun 2020;11(1):5512; DOI: 10.1038/s41467-020-19344-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kleinstiver BP, Prew MS, Tsai SQ, et al. Engineered CRISPR-Cas9 nucleases with altered PAM specificities. Nature 2015;523(7561):481–485; doi: 10.1038/nature14592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Chatterjee P, Lee J, Nip L, et al. A Cas9 with PAM recognition for adenine dinucleotides. Nat Commun 2020;11(1):2474; DOI: 10.1038/s41467-020-16117-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Gao L, Cox DBT, Yan WX, et al. Engineered Cpf1 variants with altered PAM specificities. Nat Biotechnol 2017;35(8):789–792; doi: 10.1038/nbt.3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Lino CA, Harper JC, Carney JP, et al. Delivering CRISPR: A review of the challenges and approaches. Drug Deliv 2018;25(1):1234–1257; doi: 10.1080/10717544.2018.1474964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Gupta D, Bhattacharjee O, Mandal D, et al. CRISPR-Cas9 system: A new-fangled dawn in gene editing. Life Sci 2019;232:116636; DOI: 10.1016/j.lfs.2019.116636. [DOI] [PubMed] [Google Scholar]
- 111. Qi LS, Larson MH, Gilbert LA, et al. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 2013;152(5):1173–1183; doi: 10.1016/j.cell.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Adikusuma F, Lushington C, Arudkumar J, et al. Optimized nickase- and nuclease-based prime editing in human and mouse cells. Nucleic Acids Res 2021;49(18):10785–10795; doi: 10.1093/nar/gkab792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Kluesner MG, Lahr WS, Lonetree C-L, et al. CRISPR-Cas9 cytidine and adenosine base editing of splice-sites mediates highly-efficient disruption of proteins in primary and immortalized cells. Nat Commun 2021;12(1):2437; DOI: 10.1038/s41467-021-22009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Webber BR, Lonetree C-L, Kluesner MG, et al. Highly efficient multiplex human T cell engineering without double-strand breaks using Cas9 base editors. Nat Commun 2019;10(1):5222; DOI: 10.1038/s41467-019-13007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Hsieh J, Becklin KL, Givens S, et al. Myosin heavy chain converter domain mutations drive early-stage changes in extracellular matrix dynamics in hypertrophic cardiomyopathy. Front Cell Dev Biol 2022;10:894635; DOI: 10.3389/fcell.2022.894635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Osborn MJ, Newby GA, McElroy AN, et al. Base editor correction of COL7A1 in recessive dystrophic epidermolysis bullosa patient-derived fibroblasts and iPSCs. J Invest Dermatol 2020;140(2):338.e5–347.e5; DOI: 10.1016/j.jid.2019.07.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Zeng J, Wu Y, Ren C, et al. Therapeutic base editing of human hematopoietic stem cells. Nat Med 2020;26(4):535–541; doi: 10.1038/s41591-020-0790-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Chapman JR, Taylor MRG, Boulton SJ. Playing the end game: DNA double-strand break repair pathway choice. Mol Cell 2012;47(4):497–510; doi: 10.1016/j.molcel.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 119. Khanna KK, Jackson SP. DNA double-strand breaks: Signaling, repair and the cancer connection. Nat Genet 2001;27(3):247–254; doi: 10.1038/85798. [DOI] [PubMed] [Google Scholar]
- 120. June CH, Blazar BR, Riley JL. Engineering lymphocyte subsets: Tools, trials and tribulations. Nat Rev Immunol 2009;9(10):704–716; doi: 10.1038/nri2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Kim YB, Komor AC, Levy JM, et al. Increasing the genome-targeting scope and precision of base editing with engineered Cas9-cytidine deaminase fusions. Nat Biotechnol 2017;35(4):371–376; doi: 10.1038/nbt.3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Zhao D, Jiang G, Li J, et al. Imperfect guide-RNA (igRNA) enables CRISPR single-base editing with ABE and CBE. Nucleic Acids Res 2022;50(7):4161–4170; doi: 10.1093/nar/gkac201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Chen PJ, Hussmann JA, Yan J, et al. Enhanced prime editing systems by manipulating cellular determinants of editing outcomes. Cell 2021;184(22):5635.e29–5652.e29; DOI: 10.1016/j.cell.2021.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Velimirovic M, Zanetti LC, Shen MW, et al. Peptide fusion improves prime editing efficiency. Nat Commun 2022;13(1):3512; DOI: 10.1038/s41467-022-31270-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Porto EM, Komor AC, Slaymaker IM, et al. Base editing: Advances and therapeutic opportunities. Nat Rev Drug Discov 2020;19(12):839–859; doi: 10.1038/s41573-020-0084-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Zalatan JG, Lee ME, Almeida R, et al. Engineering complex synthetic transcriptional programs with CRISPR RNA scaffolds. Cell 2015;160(1–2):339–350; doi: 10.1016/j.cell.2014.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Mandegar MA, Huebsch N, Frolov EB, et al. CRISPR interference efficiently induces specific and reversible gene silencing in human iPSCs. Cell Stem Cell 2016;18(4):541–553; doi: 10.1016/j.stem.2016.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Parsi KM, Hennessy E, Kearns N, et al. Using an inducible CRISPR-dCas9-KRAB effector system to dissect transcriptional regulation in human embryonic stem cells. Methods Mol Biol 2017;1507:221–233; DOI: 10.1007/978-1-4939-6518-2_16. [DOI] [PubMed] [Google Scholar]
- 129. Thakore PI, D'Ippolito AM, Song L, et al. Highly specific epigenome editing by CRISPR-Cas9 repressors for silencing of distal regulatory elements. Nat Methods 2015;12(12):1143–1149; doi: 10.1038/nmeth.3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Jensen TI, Mikkelsen NS, Gao Z, et al. Targeted regulation of transcription in primary cells using CRISPRa and CRISPRi. Genome Res 2021;31(11):2120–2130; doi: 10.1101/gr.275607.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Maeder ML, Linder SJ, Cascio VM, et al. CRISPR RNA-guided activation of endogenous human genes. Nat Methods 2013;10(10):977–979; doi: 10.1038/nmeth.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Balboa D, Weltner J, Eurola S, et al. Conditionally stabilized dCas9 activator for controlling gene expression in human cell reprogramming and differentiation. Stem Cell Rep 2015;5(3):448–459; doi: 10.1016/j.stemcr.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Cheng PF, Shakhova O, Widmer DS, et al. Methylation-dependent SOX9 expression mediates invasion in human melanoma cells and is a negative prognostic factor in advanced melanoma. Genome Biol 2015;16(1):42; DOI: 10.1186/s13059-015-0594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Pastushenko I, Brisebarre A, Sifrim A, et al. Identification of the tumour transition states occurring during EMT. Nature 2018;556(7702):463–468; doi: 10.1038/s41586-018-0040-3. [DOI] [PubMed] [Google Scholar]
- 135. Jasin M, de Villiers J, Weber F, et al. High frequency of homologous recombination in mammalian cells between endogenous and introduced SV40 genomes. Cell 1985;43(3 Pt 2):695–703; doi: 10.1016/0092-8674(85)90242-9. [DOI] [PubMed] [Google Scholar]
- 136. Jasin M, Berg P. Homologous integration in mammalian cells without target gene selection. Genes Dev 1988;2(11):1353–1363; doi: 10.1101/gad.2.11.1353. [DOI] [PubMed] [Google Scholar]
- 137. Kim S-I, Matsumoto T, Kagawa H, et al. Microhomology-assisted scarless genome editing in human iPSCs. Nat Commun 2018;9(1):939; DOI: 10.1038/s41467-018-03044-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Grajcarek J, Monlong J, Nishinaka-Arai Y, et al. Genome-wide microhomologies enable precise template-free editing of biologically relevant deletion mutations. Nat Commun 2019;10(1):4856; DOI: 10.1038/s41467-019-12829-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Nakade S, Tsubota T, Sakane Y, et al. Microhomology-mediated end-joining-dependent integration of donor DNA in cells and animals using TALENs and CRISPR/Cas9. Nat Commun 2014;5:5560; DOI: 10.1038/ncomms6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Aida T, Nakade S, Sakuma T, et al. Gene cassette knock-in in mammalian cells and zygotes by enhanced MMEJ. BMC Genom 2016;17(1):979; DOI: 10.1186/s12864-016-3331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Zhang J-P, Li X-L, Li G-H, et al. Efficient precise knockin with a double cut HDR donor after CRISPR/Cas9-mediated double-stranded DNA cleavage. Genome Biol 2017;18(1):35; DOI: 10.1186/s13059-017-1164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Yao X, Wang X, Hu X, et al. Homology-mediated end joining-based targeted integration using CRISPR/Cas9. Cell Res 2017;27(6):801–814; doi: 10.1038/cr.2017.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Khan IF, Hirata RK, Wang P-R, et al. Engineering of human pluripotent stem cells by AAV-mediated gene targeting. Mol Ther 2010;18(6):1192–1199; doi: 10.1038/mt.2010.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Fu Y-W, Dai X-Y, Wang W-T, et al. Dynamics and competition of CRISPR-Cas9 ribonucleoproteins and AAV donor-mediated NHEJ, MMEJ and HDR editing. Nucleic Acids Res 2021;49(2):969–985; doi: 10.1093/nar/gkaa1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Large EE, Silveria MA, Zane GM, et al. Adeno-associated virus (AAV) gene delivery: Dissecting molecular interactions upon cell entry. Viruses 2021;13(7):1336; DOI: 10.3390/v13071336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Wei R, Yang J, Cheng C-W, et al. CRISPR-targeted genome editing of human induced pluripotent stem cell-derived hepatocytes for the treatment of Wilson's disease. JHEP Rep 2022;4(1):100389; DOI: 10.1016/j.jhepr.2021.100389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Wen W, Quan Z-J, Li S-A, et al. Effective control of large deletions after double-strand breaks by homology-directed repair and dsODN insertion. Genome Biol 2021;22(1):236; DOI: 10.1186/s13059-021-02462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Wattanapanitch M. Correction of hemoglobin E/Beta-thalassemia patient-derived iPSCs using CRISPR/Cas9. Methods Mol Biol 2021;2211:193–211; DOI: 10.1007/978-1-0716-0943-9_14. [DOI] [PubMed] [Google Scholar]
- 149. Bier E, Harrison MM, O'Connor-Giles KM, et al. Advances in engineering the fly genome with the CRISPR-Cas system. Genetics 2018;208(1):1–18; doi: 10.1534/genetics.117.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Kagita A, Lung MSY, Xu H, et al. Efficient ssODN-mediated targeting by avoiding cellular inhibitory RNAs through precomplexed CRISPR-Cas9/sgRNA ribonucleoprotein. Stem Cell Rep 2021;16(4):985–996; doi: 10.1016/j.stemcr.2021.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell 2011;144(5):646–674; doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 152. Wang L-H, Wu C-F, Rajasekaran N, et al. Loss of tumor suppressor gene function in human cancer: An overview. Cell Physiol Biochem 2018;51(6):2647–2693; doi: 10.1159/000495956. [DOI] [PubMed] [Google Scholar]
- 153. Geng B-C, Choi K-H, Wang S-Z, et al. A simple, quick, and efficient CRISPR/Cas9 genome editing method for human induced pluripotent stem cells. Acta Pharmacol Sin 2020;41(11):1427–1432; doi: 10.1038/s41401-020-0452-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Zhang F, Wen Y, Guo X. CRISPR/Cas9 for genome editing: Progress, implications and challenges. Hum Mol Genet 2014;23(R1):R40–R46; doi: 10.1093/hmg/ddu125. [DOI] [PubMed] [Google Scholar]
- 155. Li X-L, Li G-H, Fu J, et al. Highly efficient genome editing via CRISPR-Cas9 in human pluripotent stem cells is achieved by transient BCL-XL overexpression. Nucleic Acids Res 2018;46(19):10195–10215; doi: 10.1093/nar/gky804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Bertero A, Pawlowski M, Ortmann D, et al. Optimized inducible shRNA and CRISPR/Cas9 platforms for in vitro studies of human development using hPSCs. Development 2016;143(23):4405–4418; doi: 10.1242/dev.138081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Wang X, Liu Z, Li G, et al. Efficient gene silencing by adenine base editor-mediated start codon mutation. Mol Ther 2020;28(2):431–440; doi: 10.1016/j.ymthe.2019.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Wu Y, He Y, Sretenovic S, et al. CRISPR-BETS: A base-editing design tool for generating stop codons. Plant Biotechnol J 2022;20(3):499–510; doi: 10.1111/pbi.13732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Kuscu C, Parlak M, Tufan T, et al. CRISPR-STOP: Gene silencing through base-editing-induced nonsense mutations. Nat Methods 2017;14(7):710–712; doi: 10.1038/nmeth.4327. [DOI] [PubMed] [Google Scholar]
- 160. Larson MH, Gilbert LA, Wang X, et al. CRISPR interference (CRISPRi) for sequence-specific control of gene expression. Nat Protoc 2013;8(11):2180–2196; doi: 10.1038/nprot.2013.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Parrales A, Iwakuma T. Targeting oncogenic mutant p53 for cancer therapy. Front Oncol 2015;5:288; DOI: 10.3389/fonc.2015.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Vieler M, Sanyal S. p53 isoforms and their implications in cancer. Cancers 2018;10(9):288; DOI: 10.3390/cancers10090288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Aghabozorgi AS, Bahreyni A, Soleimani A, et al. Role of adenomatous polyposis coli (APC) gene mutations in the pathogenesis of colorectal cancer; current status and perspectives. Biochimie 2019;157:64–71; DOI: 10.1016/j.biochi.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 164. Kang JG, Park JS, Ko J-H, et al. Regulation of gene expression by altered promoter methylation using a CRISPR/Cas9-mediated epigenetic editing system. Sci Rep 2019;9(1):11960; DOI: 10.1038/s41598-019-48130-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Liu R, Xing M. TERT promoter mutations in thyroid cancer. Endocr Relat Cancer 2016;23(3):R143–R155; doi: 10.1530/ERC-15-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Patel B, Taiwo R, Kim AH, et al. TERT, a promoter of CNS malignancies. Neurooncol Adv 2020;2(1):vdaa025; DOI: 10.1093/noajnl/vdaa025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Zöllner SK, Amatruda JF, Bauer S, et al. Ewing sarcoma-diagnosis, treatment, clinical challenges and future perspectives. J Clin Med Res 2021;10(8):1685; DOI: 10.3390/jcm10081685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Glassman AB, Hopwood V, Hayes KJ. Cytogenetics as an aid in the diagnosis of lymphomas. Ann Clin Lab Sci 2000;30(1):72–74. [PubMed] [Google Scholar]
- 169. Cleary ML, Smith SD, Sklar J. Cloning and structural analysis of cDNAs for Bcl-2 and a hybrid Bcl-2/immunoglobulin transcript resulting from the t(14;18) translocation. Cell 1986;47(1):19–28; doi: 10.1016/0092-8674(86)90362-4. [DOI] [PubMed] [Google Scholar]
- 170. Shtivelman E, Lifshitz B, Gale RP, et al. Fused transcript of Abl and Bcr genes in chronic myelogenous leukaemia. Nature 1985;315(6020):550–554; doi: 10.1038/315550a0. [DOI] [PubMed] [Google Scholar]
- 171. Sarrou E, Richmond L, Carmody RJ, et al. CRISPR gene editing of murine blood stem and progenitor cells induces MLL-AF9 chromosomal translocation and MLL-AF9 leukaemogenesis. Int J Mol Sci 2020;21(12):4266; DOI: 10.3390/ijms21124266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Letuchikh AA. [Colpitis: Pathogenesis, classification and diagnosis (I)]. Akush Ginekol 1985;(2):61–66. [PubMed] [Google Scholar]
- 173. Spraggon L, Martelotto LG, Hmeljak J, et al. Generation of conditional oncogenic chromosomal translocations using CRISPR-Cas9 genomic editing and homology-directed repair. J Pathol 2017;242(1):102–112; doi: 10.1002/path.4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Torres-Ruiz R, Martinez-Lage M, Martin MC, et al. Efficient recreation of t(11;22) EWSR1-FLI1+ in human stem cells using CRISPR/Cas9. Stem Cell Rep 2017;8(5):1408–1420; doi: 10.1016/j.stemcr.2017.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Vanoli F, Jasin M. Generation of chromosomal translocations that lead to conditional fusion protein expression using CRISPR-Cas9 and homology-directed repair. Methods 2017;121–122:138–145; DOI: 10.1016/j.ymeth.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Smith AJ, De Sousa MA, Kwabi-Addo B, et al. A site-directed chromosomal translocation induced in embryonic stem cells by Cre-loxP recombination. Nat Genet 1995;9(4):376–385; doi: 10.1038/ng0495-376. [DOI] [PubMed] [Google Scholar]
- 177. Vanoli F, Tomishima M, Feng W, et al. CRISPR-Cas9-guided oncogenic chromosomal translocations with conditional fusion protein expression in human mesenchymal cells. Proc Natl Acad Sci U S A 2017;114(14):3696–3701; doi: 10.1073/pnas.1700622114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Martin RM, Ikeda K, Cromer MK, et al. Highly efficient and marker-free genome editing of human pluripotent stem cells by CRISPR-Cas9 RNP and AAV6 donor-mediated homologous recombination. Cell Stem Cell 2019;24(5):821.e5–828.e5; DOI: 10.1016/j.stem.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 179. Wierson WA, Welker JM, Almeida MP, et al. Efficient targeted integration directed by short homology in zebrafish and mammalian cells. Elife 2020;9:e53968; DOI: 10.7554/eLife.53968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Anzalone AV, Gao XD, Podracky CJ, et al. Programmable deletion, replacement, integration and inversion of large DNA sequences with twin prime editing. Nat Biotechnol 2022;40(5):731–740; doi: 10.1038/s41587-021-01133-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Zuo E, Huo X, Yao X, et al. CRISPR/Cas9-mediated targeted chromosome elimination. Genome Biol 2017;18(1):224; DOI: 10.1186/s13059-017-1354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Cheng LH, Liu Y, Niu T. [Chromosomal large fragment deletion induced by CRISPR/Cas9 gene editing system]. Zhonghua Xue Ye Xue Za Zhi 2017;38(5):427–431; doi: 10.3760/cma.j.issn.0253-2727.2017.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Ramírez-Solis R, Liu P, Bradley A. Chromosome engineering in mice. Nature 1995;378(6558):720–724; doi: 10.1038/378720a0. [DOI] [PubMed] [Google Scholar]
- 184. Kotini AG, Chang C-J, Boussaad I, et al. Functional analysis of a chromosomal deletion associated with myelodysplastic syndromes using isogenic human induced pluripotent stem cells. Nat Biotechnol 2015;33(6):646–655; doi: 10.1038/nbt.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Choi J, Chen W, Suiter CC, et al. Precise genomic deletions using paired prime editing. Nat Biotechnol 2022;40(2):218–226; doi: 10.1038/s41587-021-01025-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Jiang T, Zhang X-O, Weng Z, et al. Deletion and replacement of long genomic sequences using prime editing. Nat Biotechnol 2022;40(2):227–234; doi: 10.1038/s41587-021-01026-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Raftopoulou C, Roumelioti F-M, Dragona E, et al. Karyotypic flexibility of the complex cancer genome and the role of polyploidization in maintenance of structural integrity of cancer chromosomes. Cancers 2020;12(3):591; DOI: 10.3390/cancers12030591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Stephens PJ, Greenman CD, Fu B, et al. Massive genomic rearrangement acquired in a single catastrophic event during cancer development. Cell 2011;144(1):27–40; doi: 10.1016/j.cell.2010.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Storlazzi CT, Lonoce A, Guastadisegni MC, et al. Gene amplification as double minutes or homogeneously staining regions in solid tumors: Origin and structure. Genome Res 2010;20(9):1198–1206; doi: 10.1101/gr.106252.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Carroll SM, DeRose ML, Gaudray P, et al. Double minute chromosomes can be produced from precursors derived from a chromosomal deletion. Mol Cell Biol 1988;8(4):1525–1533; doi: 10.1128/mcb.8.4.1525-1533.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Møller HD, Lin L, Xiang X, et al. CRISPR-C: Circularization of genes and chromosome by CRISPR in human cells. Nucleic Acids Res 2018;46(22):e131; DOI: 10.1093/nar/gky767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Xiao A, Wang Z, Hu Y, et al. Chromosomal deletions and inversions mediated by TALENs and CRISPR/Cas in zebrafish. Nucleic Acids Res 2013;41(14):e141; DOI: 10.1093/nar/gkt464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Oberdoerffer P, Otipoby KL, Maruyama M, et al. Unidirectional Cre-mediated genetic inversion in mice using the mutant loxP pair lox66/lox71. Nucleic Acids Res 2003;31(22):e140; DOI: 10.1093/nar/gng140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Han P, Ma Y, Fu Z, et al. A DNA inversion system in eukaryotes established via laboratory evolution. ACS Synth Biol 2021;10(9):2222–2230; doi: 10.1021/acssynbio.1c00132. [DOI] [PubMed] [Google Scholar]
- 195. Whitfield J, Littlewood T, Evan GI, et al. The estrogen receptor fusion system in mouse models: A reversible switch. Cold Spring Harb Protoc 2015;2015(3):227–234; doi: 10.1101/pdb.top069815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Kim H, Kim M, Im S-K, et al. Mouse Cre-LoxP system: General principles to determine tissue-specific roles of target genes. Lab Anim Res 2018;34(4):147–159; doi: 10.5625/lar.2018.34.4.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Jeong JH. Inducible mouse models for cancer drug target validation. J Cancer Prev 2016;21(4):243–248; doi: 10.15430/JCP.2016.21.4.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007;449(7165):1003–1007; doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 199. Geltzeiler M, Li G, Abraham J, et al. The case for primary salivary rhabdomyosarcoma. Front Oncol 2015;5:74; DOI: 10.3389/fonc.2015.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Jaluvka V, Albig M, Hamm B. [Magnetic resonance tomography in the differential diagnosis of ovarian tumor and mucocele of the appendix]. Dtsch Med Wochenschr 1989;114(33):1245–1247; doi: 10.1055/s-2008-1066749. [DOI] [PubMed] [Google Scholar]
- 201. Marcozzi A, Pellestor F, Kloosterman WP. The genomic characteristics and origin of chromothripsis. Methods Mol Biol 2018;1769:3–19; DOI: 10.1007/978-1-4939-7780-2_1. [DOI] [PubMed] [Google Scholar]
- 202. Ly P, Teitz LS, Kim DH, et al. Selective Y centromere inactivation triggers chromosome shattering in micronuclei and repair by non-homologous end joining. Nat Cell Biol 2017;19(1):68–75; doi: 10.1038/ncb3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Hanada K, Budzowska M, Davies SL, et al. The structure-specific endonuclease Mus81 contributes to replication restart by generating double-strand DNA breaks. Nat Struct Mol Biol 2007;14(11):1096–1104; doi: 10.1038/nsmb1313. [DOI] [PubMed] [Google Scholar]
- 204. Funato K, Major T, Lewis PW, et al. Use of human embryonic stem cells to model pediatric gliomas with H3.3K27M histone mutation. Science 2014;346(6216):1529–1533; doi: 10.1126/science.1253799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Pathania M, De Jay N, Maestro N, et al. H3.3K27M cooperates with Trp53 loss and PDGFRA gain in mouse embryonic neural progenitor cells to induce invasive high-grade gliomas. Cancer Cell 2017;32(5):684.e9–700.e9; DOI: 10.1016/j.ccell.2017.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Li Z, Godinho FJ, Klusmann J-H, et al. Developmental stage-selective effect of somatically mutated leukemogenic transcription factor GATA1. Nat Genet 2005;37(6):613–619; doi: 10.1038/ng1566. [DOI] [PubMed] [Google Scholar]
- 207. Huntly BJP, Shigematsu H, Deguchi K, et al. MOZ-TIF2, but not BCR-ABL, confers properties of leukemic stem cells to committed murine hematopoietic progenitors. Cancer Cell 2004;6(6):587–596; doi: 10.1016/j.ccr.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 208. Ng JMY, Martinez D, Marsh ED, et al. Generation of a mouse model of atypical teratoid/rhabdoid tumor of the central nervous system through combined deletion of Snf5 and p53. Cancer Res 2015;75(21):4629–4639; doi: 10.1158/0008-5472.CAN-15-0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Han Z-Y, Richer W, Fréneaux P, et al. The occurrence of intracranial rhabdoid tumours in mice depends on temporal control of Smarcb1 inactivation. Nat Commun 2016;7:10421; DOI: 10.1038/ncomms10421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Vitte J, Gao F, Coppola G, et al. Timing of Smarcb1 and Nf2 inactivation determines schwannoma versus rhabdoid tumor development. Nat Commun 2017;8(1):300; DOI: 10.1038/s41467-017-00346-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Filbin M, Monje M. Developmental origins and emerging therapeutic opportunities for childhood cancer. Nat Med 2019;25(3):367–376; doi: 10.1038/s41591-019-0383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Smith RC, Tabar V. Constructing and deconstructing cancers using human pluripotent stem cells and organoids. Cell Stem Cell 2019;24(1):12–24; doi: 10.1016/j.stem.2018.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Ohnuki M, Takahashi K. Present and future challenges of induced pluripotent stem cells. Philos Trans R Soc Lond B Biol Sci 2015;370(1680):20140367; DOI: 10.1098/rstb.2014.0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Conway JR, Dietlein F, Taylor-Weiner A, et al. Integrated molecular drivers coordinate biological and clinical states in melanoma. Nat Genet 2020;52(12):1373–1383; doi: 10.1038/s41588-020-00739-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Ohta S, Imaizumi Y, Okada Y, et al. Generation of human melanocytes from induced pluripotent stem cells. PLoS One 2011;6(1):e16182; DOI: 10.1371/journal.pone.0016182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Crespo M, Vilar E, Tsai S-Y, et al. Colonic organoids derived from human induced pluripotent stem cells for modeling colorectal cancer and drug testing. Nat Med 2017;23(7):878–884; doi: 10.1038/nm.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Nakano K, Takahashi S. Translocation-related sarcomas. Int J Mol Sci 2018;19(12):3784; DOI: 10.3390/ijms19123784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Cortés-Ciriano I, Lee JJ-K, Xi R, et al. Comprehensive analysis of chromothripsis in 2,658 human cancers using whole-genome sequencing. Nat Genet 2020;52(3):331–341; doi: 10.1038/s41588-019-0576-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Kilpinen H, Goncalves A, Leha A, et al. Corrigendum: Common genetic variation drives molecular heterogeneity in human iPSCs. Nature 2017;546(7660):686; DOI: 10.1038/nature23012. [DOI] [PubMed] [Google Scholar]
- 220. Chen I-P, Fukuda K, Fusaki N, et al. Induced pluripotent stem cell reprogramming by integration-free Sendai virus vectors from peripheral blood of patients with craniometaphyseal dysplasia. Cell Reprogr 2013;15(6):503–513; doi: 10.1089/cell.2013.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Borgohain MP, Haridhasapavalan KK, Dey C, et al. An insight into DNA-free reprogramming approaches to generate integration-free induced pluripotent stem cells for prospective biomedical applications. Stem Cell Rev Rep 2019;15(2):286–313; doi: 10.1007/s12015-018-9861-6. [DOI] [PubMed] [Google Scholar]
- 222. Babiarz JE, Ravon M, Sridhar S, et al. Determination of the human cardiomyocyte mRNA and miRNA differentiation network by fine-scale profiling. Stem Cells Dev 2012;21(11):1956–1965; doi: 10.1089/scd.2011.0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Morizane R, Lam AQ, Freedman BS, et al. Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat Biotechnol 2015;33(11):1193–1200; doi: 10.1038/nbt.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Bukong TN, Lo T, Szabo G, et al. Novel developmental biology-based protocol of embryonic stem cell differentiation to morphologically sound and functional yet immature hepatocytes. Liver Int 2012;32(5):732–741; doi: 10.1111/j.1478-3231.2011.02743.x. [DOI] [PubMed] [Google Scholar]
- 225. Ng WH, Johnston EK, Tan JJ, et al. Recapitulating human cardio-pulmonary co-development using simultaneous multilineage differentiation of pluripotent stem cells. Elife 2022;11:e67872; DOI: 10.7554/eLife.67872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Koivumäki JT, Naumenko N, Tuomainen T, et al. Structural immaturity of human iPSC-derived cardiomyocytes: In silico investigation of effects on function and disease modeling. Front Physiol 2018;9:80; DOI: 10.3389/fphys.2018.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Baxter M, Withey S, Harrison S, et al. Phenotypic and functional analyses show stem cell-derived hepatocyte-like cells better mimic fetal rather than adult hepatocytes. J Hepatol 2015;62(3):581–589; doi: 10.1016/j.jhep.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Hrvatin S, O'Donnell CW, Deng F, et al. Differentiated human stem cells resemble fetal, not adult, β cells. Proc Natl Acad Sci U S A 2014;111(8):3038–3043; doi: 10.1073/pnas.1400709111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Steg LC, Shireby GL, Imm J, et al. Novel epigenetic clock for fetal brain development predicts prenatal age for cellular stem cell models and derived neurons. Mol Brain 2021;14(1):98; DOI: 10.1186/s13041-021-00810-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Volpato V, Smith J, Sandor C, et al. Reproducibility of molecular phenotypes after long-term differentiation to human iPSC-derived neurons: A multi-site omics study. Stem Cell Rep 2018;11(4):897–911; doi: 10.1016/j.stemcr.2018.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Dagogo-Jack I, Shaw AT. Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol 2018;15(2):81–94; doi: 10.1038/nrclinonc.2017.166. [DOI] [PubMed] [Google Scholar]
- 232. Ramón Y Cajal S, Sesé M, Capdevila C, et al. Clinical implications of intratumor heterogeneity: Challenges and opportunities. J Mol Med 2020;98(2):161–177; doi: 10.1007/s00109-020-01874-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Papapetrou EP. Patient-derived induced pluripotent stem cells in cancer research and precision oncology. Nat Med 2016;22(12):1392–1401; doi: 10.1038/nm.4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Walsh NC, Kenney LL, Jangalwe S, et al. Humanized mouse models of clinical disease. Annu Rev Pathol 2017;12:187–215; DOI: 10.1146/annurev-pathol-052016-100332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Cohen MA, Zhang S, Sengupta S, et al. Formation of human neuroblastoma in mouse-human neural crest chimeras. Cell Stem Cell 2020;26(4):579.e6–592.e6; DOI: 10.1016/j.stem.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Bernareggi D, Pouyanfard S, Kaufman DS. Development of innate immune cells from human pluripotent stem cells. Exp Hematol 2019;71:13–23; DOI: 10.1016/j.exphem.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Nianias A, Themeli M. Induced pluripotent stem cell (iPSC)-derived lymphocytes for adoptive cell immunotherapy: Recent advances and challenges. Curr Hematol Malig Rep 2019;14(4):261–268; doi: 10.1007/s11899-019-00528-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Sapozhnikov DM, Szyf M. Unraveling the functional role of DNA demethylation at specific promoters by targeted steric blockage of DNA methyltransferase with CRISPR/dCas9. Nat Commun 2021;12(1):1–26; doi: 10.1038/s41467-021-25991-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Li J, Mahata B, Escobar M, et al. Programmable human histone phosphorylation and gene activation using a CRISPR/Cas9-based chromatin kinase. Nat Commun 2021;12(1):896; DOI: 10.1038/s41467-021-21188-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Nakamura M, Gao Y, Dominguez AA, et al. CRISPR technologies for precise epigenome editing. Nat Cell Biol 2021;23(1):11–22; doi: 10.1038/s41556-020-00620-7. [DOI] [PubMed] [Google Scholar]
- 241. Gagne AL, Maguire JA, Gandre-Babbe S, et al. Generation of a human juvenile myelomonocytic leukemia iPSC line, CHOPi001-A, with a mutation in CBL. Stem Cell Res 2018;31:157–160; DOI: 10.1016/j.scr.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Tasian SK, Casas JA, Posocco D, et al. Mutation-specific signaling profiles and kinase inhibitor sensitivities of juvenile myelomonocytic leukemia revealed by induced pluripotent stem cells. Leukemia 2019;33(1):181–190; doi: 10.1038/s41375-018-0169-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Miyauchi M, Koya J, Arai S, et al. ADAM8 is an antigen of tyrosine kinase inhibitor-resistant chronic myeloid leukemia cells identified by patient-derived induced pluripotent stem cells. Stem Cell Rep 2018;10(3):1115–1130; doi: 10.1016/j.stemcr.2018.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Beke A, Laplane L, Riviere J, et al. Multilayer intraclonal heterogeneity in chronic myelomonocytic leukemia. Haematologica 2020;105(1):112–123; doi: 10.3324/haematol.2018.208488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Carrió M, Mazuelas H, Richaud-Patin Y, et al. Reprogramming captures the genetic and tumorigenic properties of neurofibromatosis type 1 plexiform neurofibromas. Stem Cell Rep 2019;12(2):411–426; doi: 10.1016/j.stemcr.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Jewell BE, Liu M, Lu L, et al. Generation of an induced pluripotent stem cell line from an individual with a heterozygous RECQL4 mutation. Stem Cell Res 2018;33:36–40; DOI: 10.1016/j.scr.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Shimamoto A, Kagawa H, Zensho K, et al. Reprogramming suppresses premature senescence phenotypes of werner syndrome cells and maintains chromosomal stability over long-term culture. PLoS One 2014;9(11):e112900; DOI: 10.1371/journal.pone.0112900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Cheung H-H, Liu X, Canterel-Thouennon L, et al. Telomerase protects werner syndrome lineage-specific stem cells from premature aging. Stem Cell Rep 2014;2(4):534–546; doi: 10.1016/j.stemcr.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Garçon L, Ge J, Manjunath SH, et al. Ribosomal and hematopoietic defects in induced pluripotent stem cells derived from diamond blackfan anemia patients. Blood 2013;122(6):912–921; doi: 10.1182/blood-2013-01-478321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Doulatov S, Vo LT, Macari ER, et al. Drug discovery for diamond-blackfan anemia using reprogrammed hematopoietic progenitors. Sci Transl Med 2017;9(376):eaah5645; DOI: 10.1126/scitranslmed.aah5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Mulero-Navarro S, Sevilla A, Roman AC, et al. Myeloid dysregulation in a human induced pluripotent stem cell model of PTPN11-associated juvenile myelomonocytic leukemia. Cell Rep 2015;13(3):504–515; doi: 10.1016/j.celrep.2015.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Ikemoto Y, Miyashita T, Nasu M, et al. Gorlin syndrome-induced pluripotent stem cells form medulloblastoma with loss of heterozygosity in PTCH1. Aging 2020;12(10):9935–9947; doi: 10.18632/aging.103258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Susanto E, Marin Navarro A, Zhou L, et al. Modeling SHH-driven medulloblastoma with patient iPS cell-derived neural stem cells. Proc Natl Acad Sci U S A 2020;117(33):20127–20138; doi: 10.1073/pnas.1920521117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Hadoux J, Féraud O, Griscelli F, et al. Generation of an induced pluripotent stem cell line from a patient with hereditary multiple endocrine neoplasia 2A (MEN2A) syndrome with RET mutation. Stem Cell Res 2016;17(1):154–157; doi: 10.1016/j.scr.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 255. Griscelli F, Oudrhiri N, Feraud O, et al. Generation of induced pluripotent stem cell (iPSC) line from a patient with triple negative breast cancer with hereditary Exon 17 deletion of BRCA1 gene. Stem Cell Res 2017;24:135–138; DOI: 10.1016/j.scr.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 256. Yucer N, Ahdoot R, Workman MJ, et al. Human iPSC-derived fallopian tube organoids with BRCA1 mutation recapitulate early-stage carcinogenesis. Cell Rep 2021;37(13):110146; DOI: 10.1016/j.celrep.2021.110146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Barwe SP, Sidhu I, Kolb EA, et al. Modeling transient abnormal myelopoiesis using induced pluripotent stem cells and CRISPR/Cas9 technology. Mol Ther Methods Clin Dev 2020;19:201–209; DOI: 10.1016/j.omtm.2020.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.