Abstract
BACKGROUND:
Early-life experiences have profound effects on functioning in adulthood. Altered cortical development may be one mechanism through which early-life experiences, including poverty and psychopathology symptoms, affect outcomes. However, there is little prospective research beginning early in development that combines clinician-rated psychopathology symptoms and multiwave magnetic resonance imaging to examine when these relationships emerge.
METHODS:
Children from the Preschool Depression Study who completed diagnostic interviews at three different developmental stages (preschool, school age, early adolescent) and up to three magnetic resonance imaging scans beginning in middle childhood participated in this study (N = 138). Multilevel models were used to calculate intercepts and slopes of cortical thickness within a priori cortical regions of interest. Linear regressions probed how early-life poverty and psychopathology (depression, anxiety, and externalizing symptoms at separate developmental periods) related to intercept/slope.
RESULTS:
Collectively, experiences during the preschool period predicted reduced cortical thickness, via either reduced intercept or accelerated thinning (slope). Early-life poverty predicted intercepts within sensory and sensory-motor integration regions. Beyond poverty, preschool anxiety symptoms predicted intercepts within the insula, subgenual cingulate, and inferior parietal cortex. Preschool externalizing symptoms predicted accelerated thinning within prefrontal and parietal cortices. Depression and anxiety/externalizing symptoms at later ages were not significant predictors.
CONCLUSIONS:
Early childhood is a critical period of risk; experiences at this developmental stage specifically have the potential for prolonged influence on brain development. Negative early experiences collectively predicted reduced cortical thickness, but the specific neural systems affected aligned with those typically implicated in these individual disorders/experiences.
INTRODUCTION
Negative early-life experiences, including poverty and experience of psychopathology, have long-term impacts on adaptive functioning in adulthood (1,2). Emerging evidence suggests that altered brain development may be a critical part of this risk pathway (3), particularly when these experiences occur early in life (4,5). However, as the majority of this literature either is cross-sectional or captures symptoms later in the course of development (e.g., school age and adolescence), it is unclear whether earlier (e.g., preschool) versus later (school age, adolescence) timing of these experiences have differential impacts on brain development. Understanding whether developmental timing of adversity affects brain outcomes is important for informing whether there are key periods when prevention or protection from adversity might be most critical (4). There is also emerging evidence that different domains of psychopathology (e.g., externalizing vs. internalizing symptoms—note that some studies use a more general internalizing symptom measure and others specifically isolate effects of depression and anxiety) affect cortical thickness development within dissociable neural systems during adolescence (6,7). For example, when considered as independent predictors of cortical thickness development, internalizing symptoms relate to limbic regions and externalizing symptoms relate to control and motor regions (6,7). In the past, studies tended to focus on a single disorder or symptom dimension when examining relationships with brain development, but more recently, this idea of examining multiple dimensions of psychopathology in the same study to isolate unique effects of different disorders/symptom domains has become more prominent (8). This is an important methodological consideration, particularly given the high rates of comorbidity across disorders (9), which will help identify how common and dissociable neural correlates of different symptom domains emerge across development. Such information will be important for future understanding of the developmental etiology and consequences of these symptoms.
There is a growing consensus that cortical thickness declines from early childhood through young adulthood in normative development (10-12). This thinning is thought to be driven by both synaptic pruning and myelination processes (13-15). However, the rate and timing of these thinning processes differ across cortices—these differences are driven in part by patterns of gene expression and relate to the evolutionary expansion of the cortex (16,17). For example, lower-order sensory areas, such as primary somatosensory or visual cortices, show less age-related change across adolescence (indicating earlier development) than higher-order association cortices, which show later onset of thinning and more pronounced changes over adolescence (18). This meaningful regional variation in cortical thickness can be used to define data-driven networks of regions that show coordinated cortical thickness development. Using these networks for investigations of brain behavior relationships is fundamentally similar to adopting a region of interest approach, except here, covariation patterns of cortical thickness data are used to define the region of interest instead of gyri and sulci. Non-negative matrix factorization (NMF) is one analytic technique for identifying such networks, often termed components. NMF is conceptually similar to principal component analysis, but where only positive loadings of voxels onto component(s) are allowed (19). Importantly, NMF has been applied in a large community sample of adolescents to characterize which parts of the cortex show similar thickness properties. Similar to functional connectivity networks, these structural networks or components tend to show related function or evolutionary development, and thickness in these components differs with adolescent development (16) and with psychopathology (8).
Anxiety, depression, and externalizing symptoms have all separately been linked to reduced cortical thickness (5,20-22). However, given that there is often significant comorbidity between disorders in childhood and adolescence (23), recent work has focused on the unique relationships between different transdiagnostic dimensions of psychopathology and cortical thickness. Notably, a recent cross-sectional paper using NMF techniques and a bifactor model of psychopathology highlighted relationships between reduced thickness across a large portion of the cortex with increased fear symptoms, above and beyond general psychopathology and other orthogonal symptom domains, which included anxious misery, psychosis, and behavioral dysfunction (8). However, another longitudinal study focusing on more general internalizing symptoms versus externalizing symptoms reported that these symptom domains relate to cortical thickness trajectories within separable networks of regions that are commonly implicated in that type of psychopathology, e.g., internalizing symptoms predicting thickness within limbic regions and externalizing symptoms predicting thickness within the postcentral gyrus (6). Such approaches, using separable domains of psychopathology as simultaneous predictors of cortical thickness development, highlight important differences in neural correlates across domains of psychopathology. Such findings may yield critical information regarding the specific neurodevelopmental mechanisms of these disorders, as well as the timing of when these symptoms/disorders might have their most powerful influence on neurodevelopment.
Cortical development is also related to exposure to poverty early in life (24). In particular, reduced thickness is routinely observed within sensory integration networks when poverty is experienced in childhood. Important emerging work suggests that relationships between early poverty and later academic/cognitive functioning are explained in part by the impact of poverty on cortical development (25,26). Critically, although poverty and psychopathology are related (27), studies investigating effects of psychopathology on cortical development do not always include measures of poverty or socioeconomic status in models. As such, it is unclear to what extent relationships between psychopathology and cortical development are explained by poverty and correlated risk factors, particularly when experienced early in development.
To address these gaps in the literature, we capitalize on a rich longitudinal dataset where cortical thickness was assessed repeatedly beginning in middle childhood and psychopathology was assessed via semistructured clinical interviews beginning in preschool and continuing through adolescence. These data allow for examining three key questions: 1) whether relationships between early-life experiences, including poverty and psychopathology symptoms (externalizing, anxiety, and depression), and cortical thickness are already observable by middle childhood or emerge across adolescent development; 2) whether relationships between these experience types and cortical development are dissociable across both experience type and brain region/system; and 3) whether timing of experience (e.g., symptoms experienced in preschool vs. school age or adolescence) relates to brain development in different ways.
In this study, we build on previous work showing the utility of using NMF as a data-driven data reduction tool that facilitates examination of the unique relationships of different dimensions of psychopathology to cortical thickness across development (8,16). In our unique dataset—where early poverty was assessed alongside psychopathology symptoms (beginning in preschool and continuing through adolescence) and cortical thickness trajectories (beginning at school age and continuing through adolescence)—we can prospectively examine the importance of early versus later childhood life experiences in relation to cortical thickness development. We hypothesize that early life is a sensitive period, such that experience of poverty and psychopathology symptoms at this period will show the strongest relationships with cortical thickness. We further hypothesize that adversity (either financial or experience of psychopathology symptoms) will predict reduced cortical thickness, either at baseline (intercept) or through accelerated thinning trajectories (slope). Finally, there is evidence for both widespread thinning of the cortex with increased fear symptoms in cross-sectional work (8) and more regionally specific effects of different types of psychopathology symptoms in longitudinal work (6). However, given the similarity in sample ages and longitudinal design between our study and the study by Whittle et al. (6), we also hypothesize that different symptom domains will predict thickness within dissociable sets of regions that have been functionally related to that domain, e.g., limbic regions involved in affective processing (insula and subgenual anterior cingulate) will relate specifically to anxiety and/or depression, whereas regions involved in executive function (dorsolateral prefrontal cortex [DLPFC]) will relate specifically to externalizing symptoms.
METHODS AND MATERIALS
Participants
Children/adolescents from the Preschool Depression Study (PDS) who completed at least one magnetic resonance imaging (MRI) scan during middle childhood/early adolescence and diagnostic interviews beginning in preschool participated (N = 138). Most participants (n = 125) completed three MRI scans. See Table 1 for demographic and symptom descriptive statistics.
Table 1.
Descriptive Statistics
| Measure | Mean | SD | Minimum | Maximum | n |
|---|---|---|---|---|---|
| Externalizing Symptoms—Mean PS | 6.78 | 6.31 | 0 | 31 | – |
| Externalizing Symptoms—Mean SA | 6.00 | 5.77 | 0 | 27 | – |
| Externalizing Symptoms—Mean EA | 4.44 | 5.30 | 0 | 25 | – |
| Anxiety Symptoms—Mean PS | 2.17 | 2.63 | 0 | 12.5 | – |
| Anxiety Symptoms—Mean SA | 1.43 | 2.11 | 0 | 10 | – |
| Anxiety Symptoms—Mean EA | 0.67 | 1.40 | 0 | 9.5 | – |
| Depression Symptoms—Mean PS | 2.44 | 1.65 | 0 | 7 | – |
| Depression Symptoms—Mean SA | 2.32 | 1.49 | 0 | 6.67 | – |
| Depression Symptoms—Mean EA | 2.30 | 1.57 | 0 | 8 | – |
| Baseline Income-to-Needs Ratio | 2.07 | 1.15 | 0 | 3.93 | – |
| Age at Scan 1, Years | 9.80 | 1.31 | 6 | 12 | 138 |
| Age at Scan 2, Years | 11.30 | 1.15 | 9 | 14 | 135 |
| Age at Scan 3, Years | 12.58 | 1.26 | 10 | 15 | 127 |
| n | % | ||||
| Parent Report of Child Gender | |||||
| Male | 73 | 52.9% | |||
| Female | 65 | 47.1% | |||
| Parent Report of Child Race | |||||
| Black | 48 | 34.8% | |||
| White | 76 | 55.1% | |||
| Other | 14 | 10.1% | |||
EA, early adolescent (ages 10 years to 14 years 11 mo); PS, preschool (ages 3 years to 5 years 11 mo); SA, school age (ages 6 years to 9 years 11 mo).
The PDS is a longitudinal study at Washington University School of Medicine in St. Louis where children aged 3 years to 5 years 11 months were recruited from primary caregivers, daycares, and preschools oversampling for depression using the Preschool Feelings Checklist (28). The Preschool Feelings Checklist is sensitive to screen for preschool depressive symptoms but, due to comorbidities, also identifies children with other disorders, including anxiety and externalizing disorders (29). Children with either elevated Preschool Feelings Checklist scores (≥3) or scores of 0 (presumed healthy) were contacted for participation. Children with autism spectrum disorder, chronic illness, clinically significant speech, language or cognitive delays, or neurologic disorders were excluded. Diagnostic assessments were conducted approximately annually over the next 12 to 15 years; these data were used to create dimensional symptom scores at specific developmental stages (more detail below). MRI was added to the PDS protocol in middle childhood, when children were aged 6 to 12 years. An additional two scans were administered at the next two in-person follow-up sessions. Details of the PDS and MRI exclusions have been reported elsewhere (5,30).
Caregivers completed informed consent, and child verbal/written assent was obtained before study participation. The Washington University School of Medicine Institutional Review Board approved all procedures.
Measures
Early-Life Psychopathology.
Dimensional depression, anxiety, and externalizing severity scores were created using diagnostic interviews of caregivers. The Preschool-Age Psychiatric Assessment (31) through age 7 and the caregiver and child report were obtained. The Childhood and Adolescent Psychiatric Assessment (32) was obtained from ages 8 and older. Anxiety severity scores include symptoms from anxious affect, worries, life events, posttraumatic stress disorder, somatization, separation anxiety, and sleep modules. Externalizing symptom severity scores include items from attention-deficit/hyperactivity disorder, oppositional defiant disorder, and conduct disorder modules. Depression symptom severity scores include items that went into the major depressive disorder diagnosis/severity score from major depressive disorder, food, sleep, and hyperactivity modules.
To create symptom scores specific to each developmental epoch, means for each symptom dimension were calculated using all diagnostic interviews conducted when participants were within each of the following age ranges: preschool age, <6 years; school age, 6 years to 9 years 11 months; early adolescence, 10 years to 14 years 11 months. Notably, MRI scans occurred during the school age and early adolescent epochs (Figure S1), meaning that preschool symptoms preceded all scan waves, whereas scanning was underway during school age and early adolescent symptom assessment.
Early-Life Income-to-Needs Ratio.
At baseline, caregivers reported on family income. Income-to-needs ratio was calculated as total family income divided by federal poverty level, based on family size at the time of collection (33). A family with an income-to-needs ratio of 1 is at the federal poverty level.
MRI Data Collection and Processing
At each of the three scanning waves, participants underwent neuroimaging with an MRI scanner (model 3.0T Tim Trio; Siemens Healthcare GmbH) that included two magnetization prepared rapid acquisition gradient-echo (MPRAGE) T1-weighted images with the following parameters: 1-mm isotropic resolution; repetition time, 2.4 ms; echo time, 3.16 ms; 160 slices; field of view, 256 × 256 × 224 mm; flip angle, 8°; matrix, 256 × 224 mm. The two MPRAGE scans were assessed visually, and the best one was selected for further processing by blinded raters (see Supplemental Methods for greater detail). The longitudinal FreeSurfer processing stream (version 5.3, http://surfer.nmr.mgh.harvard.edu/) was used on the selected MPRAGE [see (5) for further details] to generate cortical thickness maps. Cortical thickness maps were spatially normalized to the fsaverage5 template and smoothed using an isotropic Gaussian filter kernel with full width at half maximum size of 20 mm. The 18-component solution from (2) (Table 2 and Figure S2) was applied to these data, and the thickness for each component was extracted for each participant from each scan. See Supplemental Methods for explanation of NMF.
Table 2.
Standardized Betas and FDR-Corrected p Values From Regressions Predicting Either Thickness Intercept (PS Anxiety, Income/Need) or Slope (PS Extl) for Each Structural Component
| Component | Label | PS Anxiety | Income/Need | PS Extl |
|---|---|---|---|---|
| 1 | Anterior PFC | −0.242 (0.056) | −0.068 (0.813) | −0.419 (0.036)a |
| 2 | Inferior lateral parietal cortex | −0.32 (0.018)a | 0.137 (0.337) | −0.34 (0.108) |
| 3 | sgACC, entorhinal cortex, and medial temporal pole | −0.302 (0.018)a | −0.048 (0.88) | 0.151 (0.513) |
| 4 | Occipital cortex | −0.31 (0.018)a | 0.215 (0.042)a | −0.134 (0.525) |
| 5 | Superior frontal cortex | −0.166 (0.142) | 0.017 (0.906) | −0.570 (0.009)a |
| 6 | Superior parietal cortex | −0.317 (0.018)a | 0.225 (0.042)a | −0.503 (0.018)a |
| 7 | Temporoparietal junction | −0.213 (0.077) | 0.249 (0.036)a | −0.021 (0.902) |
| 8 | Primary motor cortex | −0.05 (0.624) | 0.293 (0.018)a | −0.473 (0.027)a |
| 9 | Cingulate cortex | −0.082 (0.475) | 0.040 (0.881) | −0.277 (0.188) |
| 10 | Inferior and anterior middle temporal lobe | −0.254 (0.056) | −0.035 (0.88) | −0.101 (0.581) |
| 11 | Primary somatosens cortex | −0.229 (0.056) | 0.226 (0.042)a | −0.121 (0.534) |
| 12 | Lingual gyrus | −0.231 (0.056) | 0.274 (0.018)a | −0.295 (0.173) |
| 13 | Dorsolateral PFC | −0.171 (0.138) | −0.031 (0.88) | −0.591 (0.018)a |
| 14 | Left posterior middle and superior temporal lobe | −0.184 (0.114) | 0.078 (0.813) | −0.134 (0.525) |
| 15 | Insula | −0.308 (0.018)a | −0.008 (0.927) | −0.197 (0.394) |
| 16 | Precuneus | −0.203 (0.091) | 0.074 (0.813) | −0.422 (0.036)a |
| 17 | Inferior frontal cortex | −0.204 (0.091) | −0.019 (0.927) | −0.151 (0.513) |
| 18 | Right lateral frontal cortex, inferior temporal cortex | −0.19 (0.111) | 0.032 (0.88) | −0.191 (0.394) |
Extl, externalizing; FDR, false discovery rate; PFC, prefrontal cortex; PS, preschool; sgACC, subgenual anterior cingulate cortex.
FDR-corrected p < .05.
Statistical Analyses
Longitudinal Multilevel Linear Models.
Longitudinal multilevel linear models were implemented in SAS, version 9.3 (SAS Institute Inc.). The growth curve model for each of the 18 components included both random intercept and random slope components (with either a variance component or autoregressive covariance matrix between the two components, depending on best model fit); model fit did generally improve when slope was modeled as a fixed effect, but modeling slope as a random effect is essential for asking questions about individual differences in thickness trajectories. Time was coded as age. Models included age (centered at mean = 12 years) and female gender; model fit improved for two components, 5-superior frontal cortex and 9-anterior cingulate cortex, with inclusion of quadratic effect of age, and thus, age2 was also included for these components. Intercepts and slopes from these multilevel linear models were used as the dependent variables in further analyses. Relationships between poverty/psychopathology and intercepts would indicate that these vulnerabilities affect cortical thickness similarly across development, whereas relationships with slope would indicate that the impact of vulnerabilities on cortical thickness emerges from middle childhood into adolescence.
Regressions Predicting Brain Development.
Regressions for each of the 18 components (intercept and slope dependent variables for separate tests) were conducted with income-to-needs ratio, preschool (ages 3 years to 5 years 11 months) depression, school age (ages 6 years to 9 years 11 months) depression, early adolescent (ages 10 years to 14 years 11 months) depression, preschool anxiety, school-age anxiety, early adolescent anxiety, preschool externalizing, school-age externalizing, and early adolescent externalizing as predictors of interest. Gender (male = 1, female = 2) and age in months at scan 1 were included as covariates (age at scan 3 was also included for slope regressions because time between scans 1 and 3 varied across participants). Before conducting these regressions, data were checked for multivariate outliers using Mahalanobis distances; two sets of distances were calculated using the independent variables predicting intercepts or slopes. No individuals were identified as significant multivariate outliers with p > .001 for all individuals for both sets of predictors.
Bivariate correlations between significant predictors of interest and components are reported in Table S3, and multicollinearity statistics are reported in Table S4.
Multiple Comparisons Correction.
A false discovery rate correction (q = .05) was applied across the 18 components for a particular effect, e.g., preschool anxiety. This means that for each dependent variable, we corrected across the 18 parallel regressions (one for each component). This approach has been used previously (8).
RESULTS
Relationships Between Poverty and Psychopathology Domains Across Development
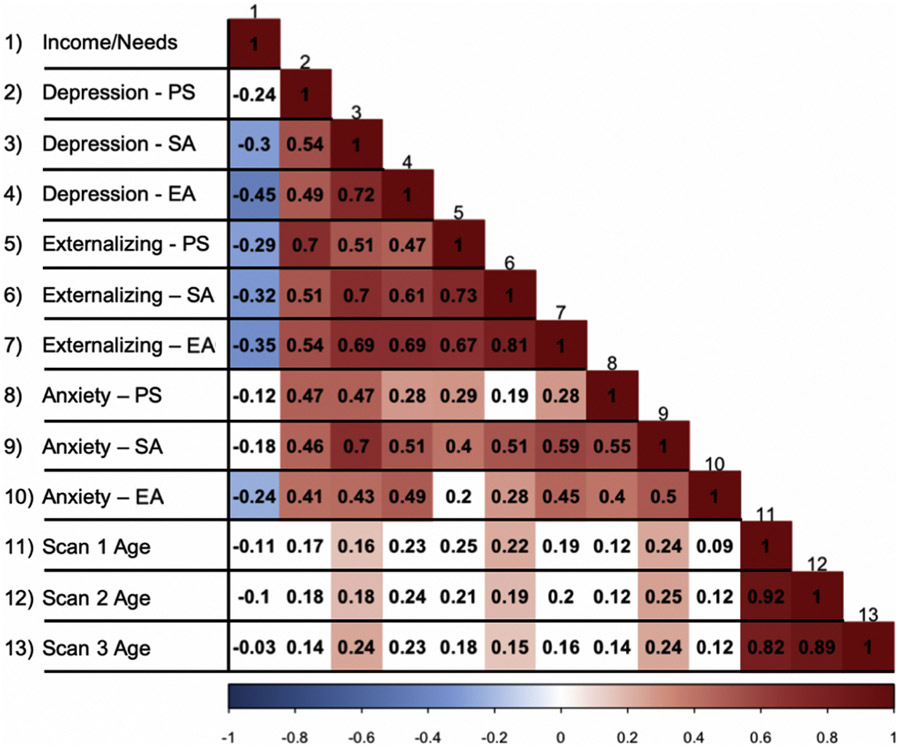
Correlations between predictors are reported in Figure 1. Symptoms were positively related within and across domains, whereas higher income-to-needs ratio (i.e., greater advantage) was related to reduced symptoms across domains. Of note, externalizing symptoms showed the strongest within-symptom domain relationships across the three developmental periods, followed by depression symptoms and then anxiety symptoms, which were only moderately related across time with r ~0.4. Depression and externalizing symptoms also showed the strongest relationship between symptoms at a given point in development; this was true across developmental periods. Relationships between anxiety and the other symptom dimensions strengthened over development, but these relationships remained modest in size and were not a source of problematic collinearity (Table S4). Finally, income-to-needs ratio showed the strongest relationship to preschool externalizing and early adolescent depression symptoms.
Figure 1.
Bivariate correlations between symptom measures and demographic variables. Bivariate correlations for all predictors of interest. Those relationships that were associated at an uncorrected p value of .05 are shown in color. The darkness of color indicates strength of relationship (the darker the color, the stronger the relationship), and the hue indicates direction of relationship (blue indicates negative; red indicates positive). EA, early adolescent; PS, preschool; SA, school age.
Longitudinal Multilevel Linear Models
All regions showed a significant negative slope indicating cortical thinning from middle childhood through early adolescence (Table S1 and Figure S3). As expected, older age predicted thinner cortex intercepts. Gender was not a significant predictor of any component’s intercept/slope.
Early Poverty and Anxiety, but Not Depression or Externalizing, Symptoms Predict Reduced Cortical Thickness Intercepts
Income-to-Needs Ratio.
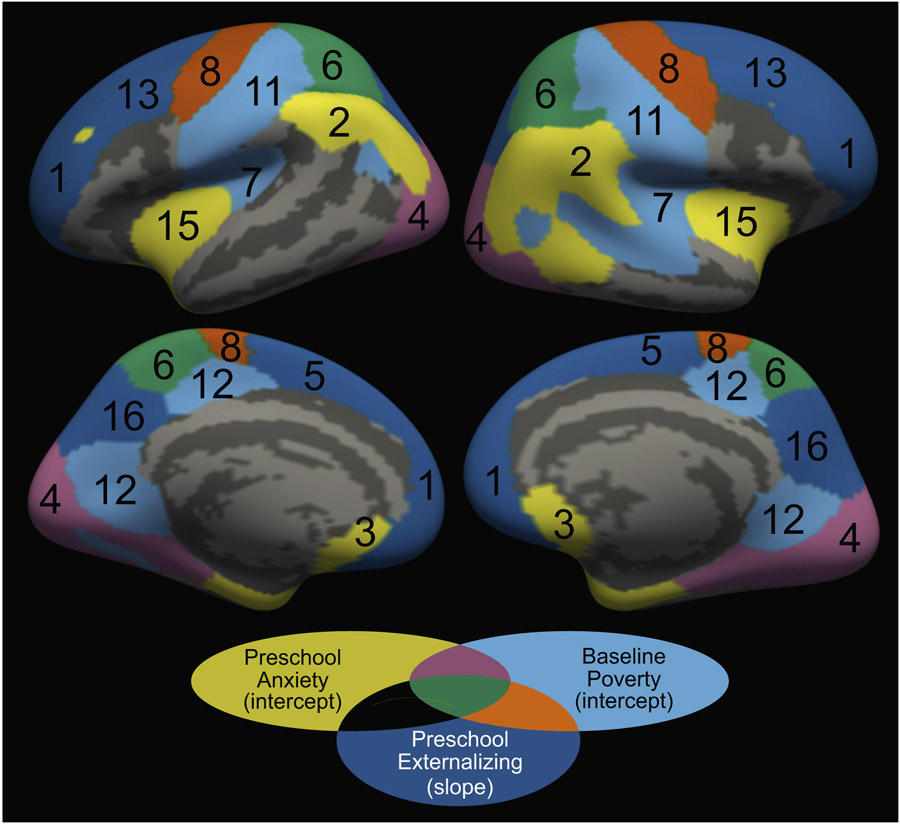
Low income-to-needs ratio, i.e., increased poverty early in life, predicted reduced cortical thickness intercepts within several components that include regions of parietal and occipital cortices and primary motor/somatosensory cortices (Table 2, Figure 2, and Figure S4) but did not predict slopes of any components.
Figure 2.
Components showing significant relationships between early-life experiences and the components’ intercept or slope. Baseline poverty (income-to-needs ratio) and preschool anxiety symptoms predicted intercepts only, whereas preschool externalizing symptoms predicted slopes only. Components are numbered according to Table 2.
Anxiety Symptoms.
Anxiety symptoms specifically experienced during the preschool period (3–5 years) predicted reduced cortical thickness intercepts within several components that include regions implicated in affective function, including the insula, subgenual anterior cingulate cortex, and inferior lateral parietal cortex (Table 2, Figure 2, and Figure S4). Notably, both early poverty and preschool anxiety were unique predictors of cortical thickness within occipital and superior parietal cortices. Anxiety symptoms experienced at school age (7–9 years) and early adolescence (10–14 years) did not significantly predict intercept or slope for any component above and beyond other symptom and demographic measures (Table S2).
Early Externalizing, but Not Early Poverty, Depression, or Anxiety, Symptoms Predict Accelerated Cortical Thinning
Externalizing symptoms specifically experienced during the preschool period (3–5 years) predicted accelerated thinning in components that include the DLPFC, superior PFC, and association and motor/somatosensory cortices (Table 2, Figure 2, and Figure S4). Preschool externalizing symptoms did not significantly predict intercept for any component (Table S2). Externalizing symptoms experienced at other developmental stages did not significantly predict intercept or slope for any component above and beyond other symptom and demographic measures (Table S2).
DISCUSSION
Here, we capitalize on a rich prospective dataset in which both semistructured clinician-rated psychopathology and brain development were assessed longitudinally beginning early in development. This allowed for an investigation of whether different symptom dimensions, experienced at key periods in development, and early poverty might uniquely relate to longitudinal change in cortical thickness. We observed reduced cortical thickness in children who experienced greater adversity (including poverty and psychopathology). However, the pattern of reduced thickness—either reduced thickness consistently observed over development (intercept effects) and/or more rapid thinning over development (slope effects)—and the neural systems affected (limbic, sensory-motor, prefrontal) showed important differences across domains of adversity and psychopathology. Finally, across poverty and symptom domains, relationships between the developmental timing of those experiences and neural outcomes suggest that early childhood is a critical period because adversity experienced specifically during the preschool years predicted adverse neural outcomes, some of which worsened over time.
We observed reduced thickness (intercept) for children experiencing increased anxiety symptoms during the preschool period and those experiencing poverty early in life. Previous work using similar NMF methods and contrasting different dimensions of psychopathology in a large cross-sectional sample of adolescents reported specific relationships between fear symptoms and reduced cortical thickness; this effect was observed broadly but was strongest in the posterior cingulate cortex and temporoparietal junction (8). In this work, thickness within these regions was predicted by early-life poverty rather than anxiety symptoms. Poverty was not included (3). Our finding that poverty predicted reduced cortical thickness within regions of the parietal, occipital, and primary motor/somatosensory cortices is similar to other work focusing on poverty (25,34). These results also suggest that the relationship between poverty and the brain emerges early—greater income-to-need ratio early in life predicted greater cortical thickness at baseline (intercept) but did not predict the trajectory of thinning over development (slope). Although this conclusion is common in the literature (26,35), with effects of poverty on brain development observed in infancy (3,36), here we extend this literature to identify unique effects of early poverty, independent of psychopathology. This is an important addition to the literature because psychopathology and poverty are often related (27) but are less frequently used as independent predictors of brain outcomes. Given this known relationship and the similar direction of poverty and anxiety effects reported here, future work investigating cortical thickness correlates of early anxiety or other psychopathology should aim to also account for early-life poverty.
Anxiety symptoms experienced in preschool predicted reduced intercepts within the subgenual anterior cingulate cortex, insula, and inferior parietal cortex—all regions functionally implicated in affective disorders (37) and where fear symptoms predicted reduced thickness (3). Although there were differences between our two studies, both studies attempted to isolate the unique relationships between different symptom domains and cortical thickness. Kaczkurkin et al. (3) used a bifactor modeling approach to create orthogonal symptom dimensions, as well as a general psychopathology or p-factor, and included all factors as simultaneous predictors of thickness. In this data-driven bifactor model, items that traditionally fall under anxiety broadly were assigned to either a fear factor or anxious misery, which also included traditional depression items. We also included different symptom domains as simultaneous predictors of thickness in our work, although we used more traditional anxiety, depression, and externalizing symptom scores. Given this approach, and given that the study by Hanson et al. (3) also found relationships between thickness in these regions and anxiety specific (i.e., fear factor) versus internalizing more broadly (i.e., anxious misery factor), the similarity in the findings’ specificity to anxiety symptoms is quite striking. However, our data were also able to provide critical within-subject developmental context not available in prior work. In fact, our results suggest that relationships between anxiety and reduced thickness within these systems is evident relatively early in development, rather than emerging over adolescence. Future work is still needed to investigate whether these relationships are observed even earlier, for example, in preschool.
Externalizing symptoms, specifically those experienced in preschool, predicted accelerated thinning from middle childhood through early adolescence within the PFC, somatosensory and motor cortices, precuneus, and superior parietal cortex. The PFC and parietal regions in particular have been implicated in cognitive control and decision making—processes known to be affected in externalizing disorders (38). Although less longitudinal work has focused on relationships between externalizing symptoms and longitudinal change in cortical thickness, our results do largely fit within the extant literature. For example, reduced thickness within the prefrontal and parietal cortices is predicted by impulsivity (but not depression) in adolescence (7), attention-deficit/hyperactivity disorder in adults (39), and attention-deficit/hyperactivity disorder in children/adolescents (40). However, some studies report attenuated cortical thinning or more protracted neurodevelopment in externalizing psychopathology/symptoms (22,41). The inconsistency in this literature may relate to sample sizes, ages of participants, or other comorbidities. These findings underscore the need to attend to externalizing symptoms arising in the preschool period to prevent deleterious effects on brain development.
Notably, we did not observe any significant relationships between depression symptoms and cortical thickness. This may seem to stand in contrast to our previous work demonstrating links between early depression symptoms, but not trauma or familial depression, and accelerated whole-brain cortical thinning (5). However, it is important to note that these analyses focused on the relative impact of different domains of psychopathology and early poverty on regional cortical thickness (not whole brain). Another caveat is that our analyses focused on cortices. There is long-established literature linking depression to alterations in subcortical volume, particularly the putamen (42,43). It may be that the relative effects of depression, compared with anxiety and externalizing symptoms, are maximal in the subcortex or relate to volume rather than cortical thickness.
Although this study has many strengths, there are also limitations. Multicollinearity is always a concern when correlated measures are included in the same model because multicollinearity can affect the precision of estimated coefficients and the reliability of associated p values. All significant relationships identified in regression analyses were also evident in bivariate correlations, and the variance inflation factor was tolerable for all variables of interest. However, symptom measures were correlated across domain and developmental stage, and this may have influenced their estimated effects on cortical thickness; future studies may use other factoring approaches to minimize correlation between symptom domains. A further limitation is the generalizability of these findings, given that this sample was enriched for early depression, which, because of comorbidity, also increased the incidence of preschool anxiety and externalizing symptoms at preschool. Because of the heightened early symptoms, there was a general regression to the mean, i.e., an improvement in symptoms over time. Although this increased variation in early symptoms likely improves our ability to detect relationships with brain development, it may reduce the application of our findings to the population at large and findings need to be replicated in community samples. However, the lack of depression effects somewhat mitigates this limitation. Finally, nonlinear relationships between symptoms and thickness were not considered but should be investigated in future studies.
In conclusion, we observed that negative early experiences (externalizing, anxiety, poverty) predicted thinner cortices through either reductions in thickness early in life or accelerated thinning trajectories across adolescence. However, the neural systems affected differed depending on symptom dimension and align with neural systems traditionally implicated in these disorders. Our findings further suggest that early childhood is a critical period of risk, and experiences at this developmental stage specifically have the potential for prolonged influence on brain development.
Supplementary Material
ACKNOWLEDGMENTS AND DISCLOSURES
This study was supported by National Institutes of Health Grant Nos. 2R01 MH064769 and R01 MH090786 (to JLL and DMB) and Grant No. R01 AG067103 (to AS).
Data supporting these findings are available from the corresponding author by request.
Footnotes
JLL receives royalties from Guilford Press. All other authors report no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsc.2021.06.013.
Contributor Information
Katherine R. Luking, Department of Psychological and Brain Sciences, Washington University in St. Louis
Robert J. Jirsaraie, Division of Computational and Data Sciences, Washington University in St. Louis
Rebecca Tillman, Department of Psychiatry, Washington University School of Medicine, St. Louis, Missouri..
Joan L. Luby, Department of Psychiatry, Washington University School of Medicine, St. Louis, Missouri.
Deanna M. Barch, Department of Psychological and Brain Sciences, Washington University in St. Louis; Department of Psychiatry, Washington University School of Medicine, St. Louis, Missouri.
Aristeidis Sotiras, Department of Radiology and Institute for Informatics, Washington University School of Medicine, St. Louis, Missouri..
REFERENCES
- 1.Staff RT, Murray AD, Ahearn TS, Mustafa N, Fox HC, Whalley LJ (2012): Childhood socioeconomic status and adult brain size: Childhood socioeconomic status influences adult hippocampal size. Ann Neurol 71:653–660. [DOI] [PubMed] [Google Scholar]
- 2.Cohen RA, Grieve S, Hoth KF, Paul RH, Sweet L, Tate D, et al. (2006): Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biol Psychiatry 59:975–982. [DOI] [PubMed] [Google Scholar]
- 3.Hanson JL, Hair N, Shen DG, Shi F, Gilmore JH, Wolfe BL, Pollak SD (2013): Family poverty affects the rate of human infant brain growth. PLoS One 8:e80954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luby JL, Baram TZ, Rogers CE, Barch DM (2020): Neurodevelopmental optimization after early-life adversity: Cross-species studies to elucidate sensitive periods and brain mechanisms to inform early intervention. Trends Neurosci 43:744–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luby JL, Belden AC, Jackson JJ, Lessov-Schlaggar CN, Harms MP, Tillman R, et al. (2016): Early childhood depression and alterations in the trajectory of gray matter maturation in middle childhood and early adolescence. JAMA Psychiatry 73:31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whittle S, Vijayakumar N, Simmons JG, Allen NB (2020): Internalizing and externalizing symptoms are associated with different trajectories of cortical development during late childhood. J Am Acad Child Adolesc Psychiatry 59:177–185. [DOI] [PubMed] [Google Scholar]
- 7.Merz EC, He X, Noble KG, Pediatric Imaging, Neurocognition, and Genetics Study (2018): Anxiety, depression, impulsivity, and brain structure in children and adolescents. Neuroimage Clin 20:243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaczkurkin AN, Park SS, Sotiras A, Moore TM, Calkins ME, Cieslak M, et al. (2019): Evidence for dissociable linkage of dimensions of psychopathology to brain structure in youths. Am J Psychiatry 176:1000–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L, et al. (2010): Lifetime prevalence of mental disorders in U.S. adolescents: Results from the National comorbidity Survey Replication—Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry 49:980–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amlien IK, Fjell AM, Tamnes CK, Grydeland H, Krogsrud SK, Chaplin TA, et al. (2016): Organizing principles of human cortical development—Thickness and area from 4 to 30 years: Insights from comparative primate neuroanatomy. Cereb Cortex 26:257–267. [DOI] [PubMed] [Google Scholar]
- 11.Walhovd KB, Fjell AM, Giedd J, Dale AM, Brown TT (2017): Through thick and thin: A need to reconcile contradictory results on trajectories in human cortical development. Cereb Cortex 27:1472–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ducharme S, Albaugh MD, Nguyen TV, Hudziak JJ, Mateos-Pérez JM, Labbe A, et al. (2016): Trajectories of cortical thickness maturation in normal brain development—The importance of quality control procedures. Neuroimage 125:267–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huttenlocher PR (1979): Synaptic density in human frontal cortex—Developmental changes and effects of aging. Brain Res 163:195–205. [DOI] [PubMed] [Google Scholar]
- 14.Petanjek Z, Judaš M, Šimic G, Rasin MR, Uylings HB, Rakic P, Kostovic I (2011): Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci U S A 108:13281–13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parker N, Patel Y, Jackowski AP, Pan PM, Salum GA, Pausova Z, et al. (2020): Assessment of neurobiological mechanisms of cortical thinning during childhood and adolescence and their implications for psychiatric disorders. JAMA Psychiatry 77:1127–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sotiras A, Toledo JB, Gur RE, Gur RC, Satterthwaite TD, Davatzikos C (2017): Patterns of coordinated cortical remodeling during adolescence and their associations with functional specialization and evolutionary expansion. Proc Natl Acad Sci U S A 114:3527–3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fjell AM, Grydeland H, Krogsrud SK, Amlien I, Rohani DA, Ferschmann L, et al. (2015): Development and aging of cortical thickness correspond to genetic organization patterns. Proc Natl Acad Sci U S A 112:15462–15467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, et al. (2008): Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci 28:3586–3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sotiras A, Resnick SM, Davatzikos C (2015): Finding imaging patterns of structural covariance via non-negative matrix factorization. Neuroimage 108:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newman E, Thompson WK, Bartsch H, Hagler DJ Jr, Chen CH, Brown TT, et al. (2016): Anxiety is related to indices of cortical maturation in typically developing children and adolescents. Brain Struct Funct 221:3013–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McLaughlin KA, Sheridan MA, Winter W, Fox NA, Zeanah CH, Nelson CA (2014): Widespread reductions in cortical thickness following severe early-life deprivation: A neurodevelopmental pathway to attention-deficit/hyperactivity disorder. Biol Psychiatry 76:629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ducharme S, Hudziak JJ, Botteron KN, Albaugh MD, Nguyen TV, Karama S, et al. (2012): Decreased regional cortical thickness and thinning rate are associated with inattention symptoms in healthy children. J Am Acad Child Adolesc Psychiatry 51:18–27.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Willner CJ, Gatzke-Kopp LM, Bray BC (2016): The dynamics of internalizing and externalizing comorbidity across the early school years. Dev Psychopathol 28:1033–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blair C, Raver CC (2016): Poverty, stress, and brain development: New directions for prevention and intervention. Acad Pediatr 16(suppl):S30–S36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDermott CL, Seidlitz J, Nadig A, Liu S, Clasen LS, Blumenthal JD, et al. (2019): Longitudinally mapping childhood socioeconomic status associations with cortical and subcortical morphology. J Neurosci 39:1365–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hair NL, Hanson JL, Wolfe BL, Pollak SD (2015): Association of Child Poverty, brain development, and academic achievement. JAMA Pediatr 169:822–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wadsworth ME, Achenbach TM (2005): Explaining the link between low socioeconomic status and psychopathology: Testing two mechanisms of the social causation hypothesis. J Consult Clin Psychol 73:1146–1153. [DOI] [PubMed] [Google Scholar]
- 28.Luby JL, Heffelfinger A, Koenig-McNaught AL, Brown K, Spitznagel E (2004): The Preschool Feelings Checklist: A brief and sensitive screening measure for depression in young children. J Am Acad Child Adolesc Psychiatry 43:708–717. [DOI] [PubMed] [Google Scholar]
- 29.Belden AC, Thomson NR, Luby JL (2008): Temper tantrums in healthy versus depressed and disruptive preschoolers: Defining tantrum behaviors associated with clinical problems. J Pediatr 152:117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barch DM, Shirtcliff EA, Elsayed NM, Whalen D, Gilbert K, Vogel AC, et al. (2020): Testosterone and hippocampal trajectories mediate relationship of poverty to emotion dysregulation and depression. Proc Natl Acad Sci U S A 117:22015–22023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Egger HL, Erkanli A, Keeler G, Potts E, Walter BK, Angold A (2006): Test-retest reliability of the preschool age psychiatric assessment (PAPA). J Am Acad Child Adolesc Psychiatry 45:538–549. [DOI] [PubMed] [Google Scholar]
- 32.Angold A, Costello EJ (2000): The child and adolescent psychiatric assessment (CAPA). J Am Acad Child Adolesc Psychiatry 39:39–48. [DOI] [PubMed] [Google Scholar]
- 33.McLoyd VC (1998): Socioeconomic disadvantage and child development. Am Psychol 53:185–204. [DOI] [PubMed] [Google Scholar]
- 34.King LS, Dennis EL, Humphreys KL, Thompson PM, Gotlib IH (2020): Cross-sectional and longitudinal associations of family income-to-needs ratio with cortical and subcortical brain volume in adolescent boys and girls. Dev Cogn Neurosci 44:100796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noble KG, Houston SM, Brito NH, Bartsch H, Kan E, Kuperman JM, et al. (2015): Family income, parental education and brain structure in children and adolescents. Nat Neurosci 18:773–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luby J, Allen N, Estabrook R, Pine DS, Rogers C, Krogh-Jespersen S, et al. (2019): Mapping infant neurodevelopmental precursors of mental disorders: How synthetic cohorts & computational approaches can be used to enhance prediction of early childhood psychopathology. Behav Res Ther 123:103484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Etkin A, Wager TD (2007): Functional neuroimaging of anxiety: A meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 164:1476–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arnsten AF (2009): The emerging neurobiology of attention deficit hyperactivity disorder: The key role of the prefrontal association cortex. J Pediatr 154:I–S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Makris N, Biederman J, Valera EM, Bush G, Kaiser J, Kennedy DN, et al. (2007): Cortical thinning of the attention and executive function networks in adults with attention-deficit/hyperactivity disorder. Cereb Cortex 17:1364–1375. [DOI] [PubMed] [Google Scholar]
- 40.Kumar U, Arya A, Agarwal V (2017): Neural alterations in ADHD children as indicated by voxel-based cortical thickness and morphometry analysis. Brain Dev 39:403–410. [DOI] [PubMed] [Google Scholar]
- 41.Vaidya CJ (2012): Neurodevelopmental abnormalities in ADHD. Curr Top Behav Neurosci 9:49–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sacchet MD, Camacho MC, Livermore EE, Thomas EAC, Gotlib IH (2017): Accelerated aging of the putamen in patients with major depressive disorder. J Psychiatry Neurosci 42:164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whittle S, Lichter R, Dennison M, Vijayakumar N, Schwartz O, Byrne ML, et al. (2014): Structural brain development and depression onset during adolescence: A prospective longitudinal study. Am J Psychiatry 171:564–571. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.