Abstract
Dual antiplatelet therapy (DAPT) is a therapeutic cornerstone to prevent stent thrombosis following percutaneous coronary intervention (PCI) for coronary artery disease (CAD). However, the longer the DAPT duration, the higher the incidence of bleeding and mortality. Since the advent of second-generation drug-eluting stents (DES), the continuous evolution of DES has reduced the thrombotic risk and allowed for a shorter DAPT duration. On the other hand, concerns on the elevated risk of bleeding during antithrombotic therapy have been further raised due to the growing number of elderly CAD patients with multiple comorbidities. The consequent debate topic over post-PCI antithrombotic therapy has shifted from simply reducing thrombotic risk to safely minimizing bleeding risk. Due to the significant impact of bleeding on clinical outcomes, including prognosis, current guidelines on antithrombotic therapy for CAD prioritize stratification of patients at a high bleeding risk (HBR) as the top consideration in determining post-PCI antithrombotic therapy. Achieving optimal antithrombotic therapy for each patient undergoing PCI requires a better understanding of the clinical variables constituting the balance of bleeding and thrombotic risk. This review highlights relevant evidence required to optimize antithrombotic therapy for HBR patients undergoing PCI.
Keywords: High Bleeding Risk (HBR), Antithrombotic Therapy, Percutaneous coronary intervention (PCI)
Introduction
Antithrombotic therapy effectively prevents recurrent thrombotic and ischemic events in patients with various clinical spectrums of coronary artery disease (CAD), which include acute coronary syndrome (ACS) 1 , 2) and chronic coronary syndrome (CCS) 3 , 4) , and after revascularization procedures, such as coronary artery bypass grafting (CABG) 5) and percutaneous coronary intervention (PCI) 6) . On the other hand, antithrombotic therapy increases the risk of bleeding to a greater or lesser extent 7 - 9) . Nearly 40% of patients undergoing PCI and requiring subsequent dual antiplatelet therapy (DAPT) are reported to be at a high bleeding risk (HBR) 10) , and the recent arrival of a super-aging society further accelerates the increased number of patients with HBR.
Maintaining antithrombotic efficacy without increasing bleeding events might be an ideal therapeutic goal; however, balancing between the risks of thrombosis and bleeding frequently includes dilemmas in practice 11) because the optimal tradeoff varies from patient to patient, and the “sweet spot” in the antithrombotic treatment can change over time from the acute to chronic phase even in the same patient 2) . Generally, the strategies to stratify patients at bleeding risk and modify the regimen and administration duration of antithrombotics according to bleeding risk-based strata can help avoid future bleeding events in treating thrombosis-related diseases 12) . Moreover, the importance of bleeding risk stratification strategies increases in clinical situations requiring intensified antithrombotics.
In the current treatment of CAD, the clinical use of DES with antithrombotic properties or the widespread optimal medical therapy, including intensified statin therapy, has contributed to the reduction of thrombotic and ischemic events after PCI. These therapeutic advances have significantly accelerated the shift of debate topic over antithrombotic therapy from the efficacy aspect of reducing the risk of thrombosis to the safety aspect of reducing the bleeding risk. As a result, shortening the duration of DAPT 13 , 14) or de-escalating antithrombotic therapy 15) to reduce bleeding and related mortality can now be considered.
This article highlights recent evidence regarding antithrombotic therapy in HBR patients undergoing PCI. We hope that this review contributes to the achievement of optimal antithrombotic therapy for CAD patients with HBR.
What is HBR? Consensus definition by Academic Research Consortium (ARC) for HBR
First, a better understanding of the clinical background in which the consensus definition of HBR emerged would enrich the debate on antithrombotic therapy in HBR patients. Most clinical trials that tested the efficacy of previously emerging DES have included only patients who could tolerate continued 12 months or more extended periods of DAPT following PCI, the duration recommended by guidelines at that time, resulting in the exclusion of many HBR patients from these trials 16 , 17) . Indeed, until recently, PCI with bare-metal stents (BMS) had the value of debate as one of the potent strategic options for HBR patients who could not use DAPT for at least 12 months as BMS requires only 1 month of DAPT 18) . The LEADERS FREE trial 19) and several other trials 18 , 20 , 21) were conducted to evaluate the efficacy of new DES, which are designed to be effective in HBR patients, given the clinical situations where a certain percentage of patients undergoing coronary stenting with DES have to discontinue DAPT due to bleeding complications, and this percentage shows an increasing trend with the increase in aging population with comorbidities. While each trial had shown favorable results, the lack of comparability between results due to wide variations in the definition of HBR patients and bleeding events across trials had been problematic. Moreover, the bleeding risk scores, such as PRECISE-DAPT (Predicting Bleeding Complications in Patients Undergoing Stent Implantation and Subsequent Dual Antiplatelet Therapy) score 22) , DAPT score 23) , PARIS (Patterns of Non-Adherence to Antiplatelet Regimen in Stented Patients) bleeding risk score 24) , CREDO-Kyoto (Coronary Revascularization Demonstrating Outcome Study in Kyoto) Risk Score 25) , and others, have demonstrated their usefulness in the prediction of bleeding events. However, the results from each bleeding risk prediction model also widely varied between studies due to differences in the definitions of bleeding and HBR patients, thus limiting clinical versatility of the above bleeding risk scores. Against these backgrounds, the ARC proposed a consensus definition of HBR, which was (1) a bleeding risk of ≥ 4% on Bleeding Academic Research Consortium bleeding criterion 3 or 5 or (2) a cerebral bleeding risk of ≥ 1% within 1 year after PCI. The ARC-HBR criteria consist of 20 major and minor clinical variables, of which patients with at least one major or two minor criteria were defined as HBR patients 16 , 17) . Since then, multiple validation studies of the ARC-HBR criteria have been conducted worldwide and confirmed their validities 26 , 27) . Natsuaki et al. demonstrated that the ARC-HBR criteria could identify patients with very HBR following PCI 28) . Nakamura et al. reported that the ARC-HBR criteria are appropriate for estimating bleeding risk in patients receiving second-generation (G2) DES implantation 29) . These studies proved that these criteria could be applied to an East Asian population at a lower risk of thrombosis and higher risk of bleeding than non-East Asians 30) , supporting the usefulness of the ARC-HBR definition and criteria as a global standard for HBR patients beyond racial groups.
What Determines Antithrombotic Therapy Following PCI in Patients with HBR?
DAPT is the therapeutic cornerstone after PCI with DES. The balance between the risks of thrombosis and bleeding determines the duration and intensity of the DAPT and influences the post-DAPT antithrombotic regimen. Specific clinical variables that determine this balance in patients with HBR would include the type of DES, complex PCI, whether the clinical presentation is ACS or CCS, the choice of P2Y12 inhibitor, and the concomitant use of anticoagulants (OACs). The concise depiction of the decision scheme and decision-making factors for antithrombotics after PCI is presented in Fig.1 . We will outline and discuss the relevant evidence on each determinant of antithrombotic therapy in PCI patients with HBR.
Fig.1. Decision scheme and decision-making factors for antithrombotic therapy after PCI.
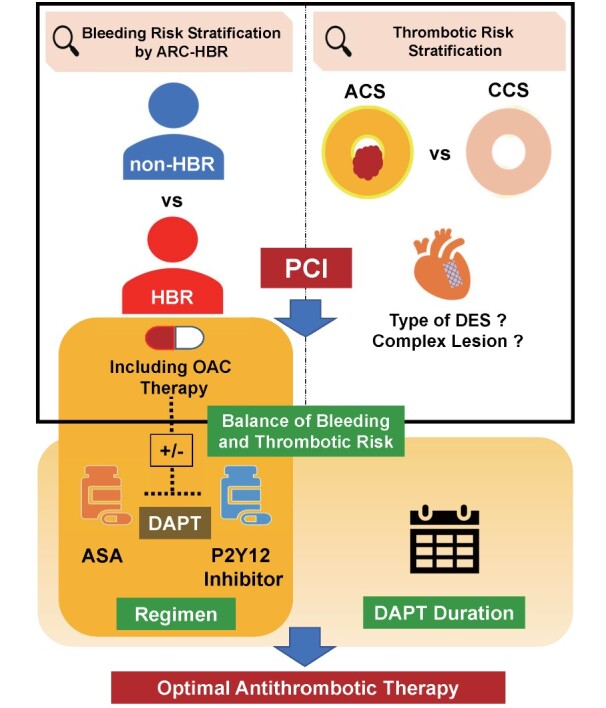
ARC-HBR, Academic Research Consortium for High Bleeding Risk; OAC, oral anticoagulant; ACS, acute coronary syndrome; CCS, chronic coronary syndrome; DES, drug-eluting stent; PCI, percutaneous coronary intervention; DAPT, dual antiplatelet therapy; ASA, acetylsalicylic acid
1) Type of DES
First-generation (G1) DES historically emerged to solve the issues of stent restenosis in BMS. While exhibiting a powerful preventative effect for stent restenosis 31 , 32) , this generation of DES had concerns on late and very late stent thrombosis (LST/VLST) after DAPT discontinuation 33 - 37) , partly due to excessive delay in re-endothelialization 38 , 39) , incomplete neointimal coverage 40 , 41) , and hypersensitivity to polymer 42 , 43) , requiring an extension of 1 year or more of DAPT duration. On the other hand, the longer the DAPT duration, the higher the incidence of bleeding events and mortality 44) . The G2 DES developed to resolve these issues has successfully preserved or improved efficacy for restenosis 45 , 46) and significantly reduced the incidence of LST/VLST observed in the G1 DES 47 , 48) . Dispelling concerns on LST/VLST through the recent advancement of DES provided the reason for the challenge to shorten the DAPT duration, which had been recommended for the continuation for 12 months or longer. Navarese et al. investigated the optimal duration of DAPT after PCI with DES, indicating that DAPT of less than 12 months after PCI, compared with the standard 12-month period, can reduce bleeding without increasing ischemic complications 49) . Recent meta-analyses have revealed that DAPT durations of 6 months or less and even shorter durations of 3 months or less compared with 12 months or more are associated with a lower risk of bleeding, comparable efficacy regardless of ACS or CCS, and a decreasing trend of overall mortality 50) . Currently, third-generation (G3) DES coated with bioabsorbable polymer is clinically available and has shown at least comparable efficacy and safety results of G2 DES 51) . Furthermore, the safety and efficacy of 1-month DAPT have been proved in newer DES for HBR patients undergoing PCI for ACS and CCS 20 , 52 - 54) . Due to the absolute decreased incidence of LST/VLST since the development of G2 DES, the impact of stent selection itself on thrombotic risks seems to be decreased relative to others and is no longer the highlight; however, its importance might be variable when considering further shortening of the DAPT duration and reduction of antithrombotic therapy.
2) Complex PCI
Complex PCI is usually defined as the composite of at least three stents implanted, at least three lesions treated, bifurcation with two stents implanted, total stent length >60 mm, and chronic total occlusion as a target lesion 55 , 56) . Patients undergoing complex PCI have significantly higher thrombotic and ischemic risks than those undergoing non-complex PCI 57) . The magnitude of the antithrombotic effect of prolonged DAPT progressively increases as the degree of PCI complexity is more significant 56) . This trend is similar in patients undergoing complex PCI with newer-generation DES 58) . On the other hand, Costa et al. reported that patients who underwent complex PCI received beneficial effects from the prolonged duration of DAPT only in non-HBR patients, not in HBR patients 57) . This finding indicates that pursuing antithrombotic efficacy by a more prolonged duration of DAPT, even after complex PCI, is not reasonable for HBR patients. It remains unclear whether complex PCI is merely an indicator of CAD severity in the background or whether the complexity of the PCI procedure itself increases the risk of thrombosis. However, the modern PCI strategies, especially in HBR patients, appear to be moving toward fewer complex strategies, i.e., as few stents as possible in number and length and shorter DAPT duration, through the better use of intravascular imaging tools. This strategic trend also includes stentless PCI for de novo lesions using drug-coated balloons, which is currently limited to in-stent restenosis or small vessel lesions 59) . Bleeding risk may play a pivotal role in the decision not only on the duration of DAPT but also on the procedural strategy in PCI.
3) ACS vs. CCS
Thrombus formation with aggregated platelets and fibrin on ruptured or eroded coronary plaques is one of the pathological hallmarks of ACS 60) . Moreover, the culprit lesions in patients with ACS show an increased thrombogenicity compared with patients with CCS 61 , 62) . Therefore, intensifying antithrombotic therapy in patients with ACS is reasonable to prevent subsequent recurrent thrombotic events. The CURE (Clopidogrel in Unstable Angina to Prevent Recurrent Events) trial demonstrated the antithrombotic and anti-ischemic efficacy of adding P2Y12 inhibitor clopidogrel to aspirin in patients with ACS without ST-segment elevation. However, these efficacies were at the expense of an increased risk of bleeding 1) . Since major bleeding significantly increases mortality in patients with ACS 63 , 64) , a better prognosis after ACS requires bleeding and thrombotic event prevention. The latest focused update on DAPT duration in the AHA (American Heart Association), ESC (European Society of Cardiology), and JCS (Japanese Circulation Society) guidelines all recommend a longer duration of DAPT in ACS than in CCS in the absence of HBR. However, in HBR patients, whether the clinical presentation is ACS or CCS is limited in its role as a determinant of DAPT duration 55 , 65 , 66) .
Recent evidence of DAPT after PCI with newer DES has shown that shortening the duration of DAPT to 1 month can reduce bleeding events without increasing thrombotic events. Investigation of this strategy extends to patients with high thrombotic risk, such as ACS. Valgimigli et al. demonstrated that patients with 1 month of DAPT had a similar rate of all-cause mortality or MI and significantly fewer bleeding events than those with 3 months of DAPT in the population in which approximately one-third of enrolled patients had ACS 67) . The result of the MASTER DAPT (Management of High-Bleeding-Risk Patients Post Bioresorbable Polymer-Coated Stent Implantation with an Abbreviated Versus Prolonged DAPT Regimen) trial in which 48.3% of enrolled patients were ACS indicated that the 1-month DAPT significantly reduced major or clinically relevant bleeding without increasing net adverse clinical events or major adverse cardiac or cerebral events compared with at least 3-month DAPT 53) . Conversely, the STOPDAPT-2 ACS (ShorT and OPtimal duration of Dual AntiPlatelet Therapy-2 study for patients with ACS) trial showed that 1 month of clopidogrel-based DAPT and subsequent clopidogrel monotherapy for 11 months failed to meet the criteria for noninferiority compared with the 12-month clopidogrel-based DAPT for the composite ischemic and bleeding endpoint in ACS patients undergoing PCI with everolimus-eluting stents 68) . The effect of 1-month DAPT after PCI on the efficacy and safety of patients with ACS is still unclear. HBR patients who developed ACS have coexisting risks of thrombosis and bleeding and require challenging management due to the antithrombotics’ narrow therapeutic “sweet spot” in efficacy and safety. Further relevant studies are required, which cover the drug choice of post-DAPT monotherapy or how to de-escalate antithrombotics for ACS.
4) Choice of P2Y12 Inhibitors in DAPT and Post-DAPT Monotherapies
The oral P2Y12 inhibitors that are currently used clinically include clopidogrel, prasugrel, and ticagrelor. The combination of clopidogrel and aspirin has been a standard regimen of DAPT following PCI, but clopidogrel has several limitations in clinical use, including variability in the antiplatelet response between patients and delayed onset of the drug effect. The attenuated antiplatelet effect in certain patients on clopidogrel can be explained partly by reduced hepatic biotransformation from a prodrug to an active form by the liver enzyme CYP2C19. Because the functional aberration of this enzyme is affected by the CYP2C19 genotype, selection of oral P2Y12 inhibitors considering the genotype seems reasonable for patients undergoing PCI 69) . Claassens et al. demonstrated that the CYP2C19 genotype-guided strategy for selecting oral P2Y12 inhibitors could reduce the bleeding risk without increasing thrombotic events in patients undergoing primary PCI 70) . In addition, given the differences in the antiplatelet potency among P2Y12 inhibitors, information on the CYP2C19 genotype may help in the selection of P2Y12 inhibitors for HBR patients. Contrarily, Pereira and colleagues reported that in patients with CYP2C19 loss-of-function alleles who developed ACS and CCS and underwent PCI, the genotype-guided oral P2Y12 inhibitor therapy failed to show statistically significant differences in the ischemic events and the related mortality at 12 months compared with the conventional clopidogrel treatment without point-of-care genotyping 71) . Furthermore, the results of genotype and functional testing by Hochholzer and colleagues indicated that the antiplatelet effect of clopidogrel is associated with the CYP2C19 genotype but that genotype alone or in combination with clinical factors does not fully explain the platelet reactivity to the clopidogrel 72) . The utility of the CYP2C19 genotype-guided strategy for selecting a P2Y12 inhibitor is still controversial, resulting in the updated guidelines of the AHA/ACC (American College of Cardiology), ESC, and JCS not recommending the routine use of genetic testing in clinical practice. However, there has been a drastic progress in the acquisition of genomic information and the ways to integrate genomics and clinical information. Further pharmacogenomic research is expected to determine optimal antithrombotic therapy following PCI.
Prasugrel and ticagrelor solve the drawbacks of clopidogrel as new P2Y12 inhibitors. The TRITON-TIMI (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel-Thrombolysis in Myocardial Infarction) 38 trial showed that prasugrel that has more potent antiplatelet effect, compared with clopidogrel, significantly reduced cardiovascular events, including stent thrombosis, but at the expense of increased bleeding events in patients with STEMI 73) . In the PRASFIT-ACS (PRASugrel compared with clopidogrel For Japanese patIenTs with ACS undergoing PCI) 74) and PRASFIT-Elective (PRASugrel For Japanese PatIenTs with Coronary Artery Diseases Undergoing Elective PCI) 75) trials, the efficacy and safety of prasugrel were examined at a reduced dose setting (20-mg loading dose and 3.75-mg maintenance dose) equivalent to about one-third that was tested in the TRITON-TIMI38 study. The PRASFIT-ACS and PRASFIT-Elective trials demonstrated that adjustment of the dosing regimen of prasugrel for Japanese patients minimized the bleeding events and preserved efficacy equivalent to that of the TRITON-TIMI38 trial. Moreover, these two studies supported the concept that lowering the dose of new P2Y12 inhibitors might be one of the new ways to reduce the bleeding risk while preserving ischemic efficacy.
Wallentin and colleagues reported that ticagrelor, another new P2Y12 inhibitor, is more effective than clopidogrel, with no increase in major bleeding events observed in patients with ACS 76) . However, subsequent clinical trials of ticagrelor conducted at the same drug doses in Asia as in the USA and Europe reported increased bleeding events. Furthermore, the ISAR-REACT (Intracoronary Stenting and Antithrombotic Regimen: Rapid Early Action for Coronary Treatment) 5 trial compared the efficacy and safety of prasugrel and ticagrelor in patients with ACS and reported fewer thrombotic events and similar bleeding events in the prasugrel group 77) . In patients with ACS, a potent new P2Y12 inhibitor, prasugrel, appears to be more effective than clopidogrel, but ticagrelor appears to have limited use in clinical practice.
A lifelong aspirin administration has been recommended as single antiplatelet monotherapy (SAPT) after DAPT. However, which drug is required as SAPT is still unclear because aspirin has concerns on the increased risk of gastrointestinal bleeding 78) , especially in HBR patients. Chiarito et al. reported that a P2Y12 inhibitor is more effective than aspirin as SAPT for secondary prevention in patients with established atherosclerosis 79) . Furthermore, a recent systematic review and meta-analysis demonstrated the safety and efficacy of the discontinuation of aspirin 1 to 3 months after PCI with continued use of P2Y12 inhibitors 80) . The TWILIGHT (Ticagrelor with Aspirin or Alone in High-Risk Patients after Coronary Intervention) trial demonstrated a significant reduction of bleeding events without worsening of the ischemic events over 1 year by a 3-month DAPT and subsequent ticagrelor monotherapy compared with 12-month combination therapy of aspirin and ticagrelor 81) .
The consensus on the choice of P2Y12 inhibitors for DAPT and post-DAPT monotherapies in CAD patients with HBR will require further studies and careful discussions on the points, including the balance between thrombotic and bleeding risks, clopidogrel metabolism, and application of de-escalation strategies.
5) Concomitant Use of OACs
Atrial fibrillation (AF) is a common comorbidity in patients with CAD and requires long-term treatment with oral anticoagulant (OAC) to prevent stroke or systemic thromboembolism. Previous guidelines had recommended antithrombotic therapy with DAPT plus OAC as the standard care for AF-complicated CAD patients undergoing PCI. However, this triple antithrombotic therapy significantly increased the incidence of severe bleeding 82 , 83) . The ARC-HBR criteria include the long-term use of anticoagulants as the major criteria of HBR 16 , 17) . Given the severe concern on the triple therapy for bleeding outcomes, the WOEST (What is the Optimal antiplatElet and anticoagulant therapy in patients with oral anticoagulation and coronary StenTing) trial was conducted, which showed that clopidogrel monotherapy significantly reduced bleeding events without increasing thrombotic events at 1 year compared with the clopidogrel and aspirin combination therapy in patients undergoing PCI while taking OAC 84) . After the emergence of direct oral anticoagulant (DOAC), several clinical trials have demonstrated that DOAC, in combination with a P2Y12 inhibitor, improves bleeding outcomes in patients undergoing PCI compared with triple therapy with warfarin 85 - 88) . In these trials, more than half of the patients had ACS. In both ACS and CCS, the combination of DOAC and clopidogrel had better outcomes than the triple therapy. A short-term triple therapy within 2 weeks perioperatively recommended by the 2020 JCS guideline, followed by at least 12 months of antithrombotic therapy with clopidogrel/prasugrel and DOAC, is suggested for patients with ACS/CCS and AF.
What is the appropriate regimen of antithrombotic therapy for AF patients beyond 1 year after coronary stenting? The OAC-ALONE (Optimizing Antithrombotic Care in patients with AtriaL fibrillatiON and coronary stEnt) trial challenged this question. This study investigated whether oral anticoagulation monotherapy is non-inferior to the combination of oral anticoagulation treatment and a single antiplatelet agent in AF patients at more than 1 year after stenting for CAD. This study was terminated early due to delayed enrollment and failure to answer the above question conclusively 89) . The AFIRE (Atrial Fibrillation and Ischemic Events with Rivaroxaban in Patients with Stable Coronary Artery Disease) trial demonstrated that rivaroxaban monotherapy significantly improved bleeding and thrombotic outcomes as well as all-cause mortality compared with the combination of antiplatelet agents and OAC 90) . Based on this result, the 2020 JCS guideline recommends OAC monotherapy as standard therapy in the subsequent long-term management for stable CAD comorbid with AF.
Conclusions and Future Perspectives
Antithrombotic therapy for CAD has recently shifted from a thrombotic risk-first approach to a bleeding risk-first one. With the increasing number of elderly CAD patients suffering from multiple comorbidities, strategies for identifying HBR patients and optimizing the duration and regimen of antithrombotic therapy are being emphasized. However, because bleeding and thrombotic risks often coexist in the same patient, management of the tradeoff between these risks is challenging for the clinicians. Against these backgrounds, while clinical monitoring systems for assessing the efficacy and safety of antiplatelet and anticoagulation therapies are currently limited, there is a growing interest in tools for quantifying and visualizing the “sweet spot” of antithrombotic therapy. For example, the recently developed Total Thrombus-Formation Analysis System (T-TAS), a microchip-based system for assessing thrombus formation in whole blood, offers the potential for efficiently monitoring bleeding risk as an easy-to-use quantitative analysis system for thrombus formation 91) .
The current post-PCI antithrombotic therapy applies a “treat without setting of target” strategy after stratifying bleeding risk and determining the treatment protocol for each stratum. In other words, until bleeding occurs after the initiation of antithrombotic therapy, it is unknown whether the patient has crossed the bleeding-prone threshold. In the future, a strategy that visualizes the threshold of increased bleeding risk itself and applies the concept of “treat not beyond target” might be ideal for optimizing further antithrombotic therapy in HBR patients undergoing PCI. Further advances to precisely assess bleeding risk are warranted to realize this strategy.
Conflict of Interest (COI)
K.K. has received remuneration for lectures from Bayer Yakuhin, Daiichi-Sankyo, Novartis Pharma, and Otsuka Pharmaceutical; has received trust research/joint research funds from Bayer Yakuhin and Daiichi-Sankyo; and has received scholarship funds from Abbott Medical. The remaining authors have no conflicts of interest to declare.
References
- 1).Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK and Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators: Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med, 2001; 345: 494-502 [DOI] [PubMed] [Google Scholar]
- 2).Rodriguez F and Harrington RA: Management of Antithrombotic Therapy after Acute Coronary Syndromes. N Engl J Med, 2021; 384: 452-460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Antithrombotic Trialists’ Collaboration: Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ, 2002; 324: 71-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Patrono C, Garcia Rodriguez LA, Landolfi R and Baigent C: Low-dose aspirin for the prevention of atherothrombosis. N Engl J Med, 2005; 353: 2373-2383 [DOI] [PubMed] [Google Scholar]
- 5).Mangano DT and Multicenter Study of Perioperative Ischemia Research Group: Aspirin and mortality from coronary bypass surgery. N Engl J Med, 2002; 347: 1309-1317 [DOI] [PubMed] [Google Scholar]
- 6).Leon MB, Baim DS, Popma JJ, Gordon PC, Cutlip DE, Ho KK, Giambartolomei A, Diver DJ, Lasorda DM, Williams DO, Pocock SJ and Kuntz RE: A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. Stent Anticoagulation Restenosis Study Investigators. N Engl J Med, 1998; 339: 1665-1671 [DOI] [PubMed] [Google Scholar]
- 7).Hallas J, Dall M, Andries A, Andersen BS, Aalykke C, Hansen JM, Andersen M and Lassen AT: Use of single and combined antithrombotic therapy and risk of serious upper gastrointestinal bleeding: population based case-control study. BMJ, 2006; 333: 726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Toyoda K, Yasaka M, Iwade K, Nagata K, Koretsune Y, Sakamoto T, Uchiyama S, Gotoh J, Nagao T, Yamamoto M, Takahashi JC, Minematsu K and Bleeding with Antithrombotic Therapy Study Group: Dual antithrombotic therapy increases severe bleeding events in patients with stroke and cardiovascular disease: a prospective, multicenter, observational study. Stroke, 2008; 39: 1740-1745 [DOI] [PubMed] [Google Scholar]
- 9).Toyoda K, Yasaka M, Nagata K, Nagao T, Gotoh J, Sakamoto T, Uchiyama S, Minematsu K and Bleeding with Antithrombotic Therapy Study Group: Antithrombotic therapy influences location, enlargement, and mortality from intracerebral hemorrhage. The Bleeding with Antithrombotic Therapy (BAT) Retrospective Study. Cerebrovasc Dis, 2009; 27: 151-159 [DOI] [PubMed] [Google Scholar]
- 10).Ueki Y, Bar S, Losdat S, Otsuka T, Zanchin C, Zanchin T, Gragnano F, Gargiulo G, Siontis GCM, Praz F, Lanz J, Hunziker L, Stortecky S, Pilgrim T, Heg D, Valgimigli M, Windecker S and Raber L: Validation of the Academic Research Consortium for High Bleeding Risk (ARC-HBR) criteria in patients undergoing percutaneous coronary intervention and comparison with contemporary bleeding risk scores. EuroIntervention, 2020; 16: 371-379 [DOI] [PubMed] [Google Scholar]
- 11).Alexander JH, Wojdyla D, Vora AN, Thomas L, Granger CB, Goodman SG, Aronson R, Windecker S, Mehran R and Lopes RD: Risk/Benefit Tradeoff of Antithrombotic Therapy in Patients With Atrial Fibrillation Early and Late After an Acute Coronary Syndrome or Percutaneous Coronary Intervention: Insights From AUGUSTUS. Circulation, 2020; 141: 1618-1627 [DOI] [PubMed] [Google Scholar]
- 12).Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ and Lip GY: A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest, 2010; 138: 1093-1100 [DOI] [PubMed] [Google Scholar]
- 13).Tarantini G, Nai Fovino L, Tellaroli P, Chieffo A, Barioli A, Menozzi A, Frasheri A, Garbo R, Masotti-Centol M, Salvatella N, Dominguez JF, Steffanon L, Presbitero P, Pucci E, Fraccaro C, Mauri J, Giustino G, Sardella G and Colombo A: Optimal duration of dual antiplatelet therapy after second-generation drug-eluting stent implantation in patients with diabetes: The SECURITY (Second-Generation Drug-Eluting Stent Implantation Followed By Six- Versus Twelve-Month Dual Antiplatelet Therapy)-diabetes substudy. Int J Cardiol, 2016; 207: 168-176 [DOI] [PubMed] [Google Scholar]
- 14).Feres F, Costa RA, Abizaid A, Leon MB, Marin-Neto JA, Botelho RV, King SB, 3rd, Negoita M, Liu M, de Paula JE, Mangione JA, Meireles GX, Castello HJ, Jr., Nicolela EL, Jr., Perin MA, Devito FS, Labrunie A, Salvadori D, Jr., Gusmao M, Staico R, Costa JR, Jr., de Castro JP, Abizaid AS, Bhatt DL and OPTIMIZE Trial Investigators: Three vs twelve months of dual antiplatelet therapy after zotarolimus-eluting stents: the OPTIMIZE randomized trial. JAMA, 2013; 310: 2510-2522 [DOI] [PubMed] [Google Scholar]
- 15).Sinnaeve PR and Adriaenssens T: Dual Antiplatelet Therapy De-escalation Strategies. Am J Cardiol, 2021; 144 Suppl 1: S23-S31 [DOI] [PubMed] [Google Scholar]
- 16).Urban P, Mehran R, Colleran R, Angiolillo DJ, Byrne RA, Capodanno D, Cuisset T, Cutlip D, Eerdmans P, Eikelboom J, Farb A, Gibson CM, Gregson J, Haude M, James SK, Kim HS, Kimura T, Konishi A, Laschinger J, Leon MB, Magee PFA, Mitsutake Y, Mylotte D, Pocock S, Price MJ, Rao SV, Spitzer E, Stockbridge N, Valgimigli M, Varenne O, Windhoevel U, Yeh RW, Krucoff MW and Morice MC: Defining High Bleeding Risk in Patients Undergoing Percutaneous Coronary Intervention. Circulation, 2019; 140: 240-261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Urban P, Mehran R, Colleran R, Angiolillo DJ, Byrne RA, Capodanno D, Cuisset T, Cutlip D, Eerdmans P, Eikelboom J, Farb A, Gibson CM, Gregson J, Haude M, James SK, Kim HS, Kimura T, Konishi A, Laschinger J, Leon MB, Magee PFA, Mitsutake Y, Mylotte D, Pocock S, Price MJ, Rao SV, Spitzer E, Stockbridge N, Valgimigli M, Varenne O, Windhoevel U, Yeh RW, Krucoff MW and Morice MC: Defining high bleeding risk in patients undergoing percutaneous coronary intervention: a consensus document from the Academic Research Consortium for High Bleeding Risk. Eur Heart J, 2019; 40: 2632-2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Ariotti S, Adamo M, Costa F, Patialiakas A, Briguori C, Thury A, Colangelo S, Campo G, Tebaldi M, Ungi I, Tondi S, Roffi M, Menozzi A, de Cesare N, Garbo R, Meliga E, Testa L, Gabriel HM, Ferlini M, Vranckx P, Valgimigli M and ZEUS Investigators: Is Bare-Metal Stent Implantation Still Justifiable in High Bleeding Risk Patients Undergoing Percutaneous Coronary Intervention?: A Pre-Specified Analysis From the ZEUS Trial. JACC Cardiovasc Interv, 2016; 9: 426-436 [DOI] [PubMed] [Google Scholar]
- 19).Urban P, Meredith IT, Abizaid A, Pocock SJ, Carrie D, Naber C, Lipiecki J, Richardt G, Iniguez A, Brunel P, Valdes-Chavarri M, Garot P, Talwar S, Berland J, Abdellaoui M, Eberli F, Oldroyd K, Zambahari R, Gregson J, Greene S, Stoll HP, Morice MC and LEADERS FREE Investigators: Polymer-free Drug-Coated Coronary Stents in Patients at High Bleeding Risk. N Engl J Med, 2015; 373: 2038-2047 [DOI] [PubMed] [Google Scholar]
- 20).Varenne O, Cook S, Sideris G, Kedev S, Cuisset T, Carrie D, Hovasse T, Garot P, El Mahmoud R, Spaulding C, Helft G, Diaz Fernandez JF, Brugaletta S, Pinar-Bermudez E, Mauri Ferre J, Commeau P, Teiger E, Bogaerts K, Sabate M, Morice MC, Sinnaeve PR and SENIOR investigators: Drug-eluting stents in elderly patients with coronary artery disease (SENIOR): a randomised single-blind trial. Lancet, 2018; 391: 41-50 [DOI] [PubMed] [Google Scholar]
- 21).de Belder A, de la Torre Hernandez JM, Lopez-Palop R, O’Kane P, Hernandez Hernandez F, Strange J, Gimeno F, Cotton J, Diaz Fernandez JF, Carrillo Saez P, Thomas M, Pinar E, Curzen N, Baz JA, Cooter N, Lozano I, Skipper N, Robinson D, Hildick-Smith D and XIMA Investigators: A prospective randomized trial of everolimus-eluting stents versus bare-metal stents in octogenarians: the XIMA Trial (Xience or Vision Stents for the Management of Angina in the Elderly). J Am Coll Cardiol, 2014; 63: 1371-1375 [DOI] [PubMed] [Google Scholar]
- 22).Costa F, van Klaveren D, James S, Heg D, Raber L, Feres F, Pilgrim T, Hong MK, Kim HS, Colombo A, Steg PG, Zanchin T, Palmerini T, Wallentin L, Bhatt DL, Stone GW, Windecker S, Steyerberg EW, Valgimigli M and PRECISE-DAPT Study Investigators: Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet, 2017; 389: 1025-1034 [DOI] [PubMed] [Google Scholar]
- 23).Yeh RW, Secemsky EA, Kereiakes DJ, Normand SL, Gershlick AH, Cohen DJ, Spertus JA, Steg PG, Cutlip DE, Rinaldi MJ, Camenzind E, Wijns W, Apruzzese PK, Song Y, Massaro JM, Mauri L and DAPT Study Investigators: Development and Validation of a Prediction Rule for Benefit and Harm of Dual Antiplatelet Therapy Beyond 1 Year After Percutaneous Coronary Intervention. JAMA, 2016; 315: 1735-1749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Baber U, Mehran R, Giustino G, Cohen DJ, Henry TD, Sartori S, Ariti C, Litherland C, Dangas G, Gibson CM, Krucoff MW, Moliterno DJ, Kirtane AJ, Stone GW, Colombo A, Chieffo A, Kini AS, Witzenbichler B, Weisz G, Steg PG and Pocock S: Coronary Thrombosis and Major Bleeding After PCI With Drug-Eluting Stents: Risk Scores From PARIS. J Am Coll Cardiol, 2016; 67: 2224-2234 [DOI] [PubMed] [Google Scholar]
- 25).Natsuaki M, Morimoto T, Yamaji K, Watanabe H, Yoshikawa Y, Shiomi H, Nakagawa Y, Furukawa Y, Kadota K, Ando K, Akasaka T, Hanaoka KI, Kozuma K, Tanabe K, Morino Y, Muramatsu T, Kimura T, Credo-Kyoto PCI/CABG Registry Cohort 2, RESET, and NEXT trial investigators: Prediction of Thrombotic and Bleeding Events After Percutaneous Coronary Intervention: CREDO-Kyoto Thrombotic and Bleeding Risk Scores. J Am Heart Assoc, 2018; 7: e008708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Cao D, Mehran R, Dangas G, Baber U, Sartori S, Chandiramani R, Stefanini GG, Angiolillo DJ, Capodanno D, Urban P, Morice MC, Krucoff M, Goel R, Roumeliotis A, Sweeny J, Sharma SK and Kini A: Validation of the Academic Research Consortium High Bleeding Risk Definition in Contemporary PCI Patients. J Am Coll Cardiol, 2020; 75: 2711-2722 [DOI] [PubMed] [Google Scholar]
- 27).Corpataux N, Spirito A, Gragnano F, Vaisnora L, Galea R, Svab S, Gargiulo G, Zanchin T, Zanchin C, Siontis GCM, Praz F, Lanz J, Hunziker L, Stortecky S, Pilgrim T, Raber L, Capodanno D, Urban P, Pocock S, Heg D, Windecker S and Valgimigli M: Validation of high bleeding risk criteria and definition as proposed by the academic research consortium for high bleeding risk. Eur Heart J, 2020; 41: 3743-3749 [DOI] [PubMed] [Google Scholar]
- 28).Natsuaki M, Morimoto T, Shiomi H, Yamaji K, Watanabe H, Shizuta S, Kato T, Ando K, Nakagawa Y, Furukawa Y, Tada T, Nagao K, Kadota K, Toyofuku M and Kimura T: Application of the Academic Research Consortium High Bleeding Risk Criteria in an All-Comers Registry of Percutaneous Coronary Intervention. Circ Cardiovasc Interv, 2019; 12: e008307 [DOI] [PubMed] [Google Scholar]
- 29).Nakamura M, Kadota K, Takahashi A, Kanda J, Anzai H, Ishii Y, Shibata Y, Yasaka Y, Takamisawa I, Yamaguchi J, Takeda Y, Harada A, Motohashi T, Iijima R, Uemura S, Murakami Y and PENDULUM Investigators*: Relationship Between Platelet Reactivity and Ischemic and Bleeding Events After Percutaneous Coronary Intervention in East Asian Patients: 1-Year Results of the PENDULUM Registry. J Am Heart Assoc, 2020; 9: e015439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Kang J, Park KW, Palmerini T, Stone GW, Lee MS, Colombo A, Chieffo A, Feres F, Abizaid A, Bhatt DL, Valgimigli M, Hong MK, Jang Y, Gilard M, Morice MC, Park DW, Park SJ, Jeong YH, Park J, Koo BK and Kim HS: Racial Differences in Ischaemia/Bleeding Risk Trade-Off during Anti-Platelet Therapy: Individual Patient Level Landmark Meta-Analysis from Seven RCTs. Thromb Haemost, 2019; 119: 149-162 [DOI] [PubMed] [Google Scholar]
- 31).Morice MC, Serruys PW, Sousa JE, Fajadet J, Ban Hayashi E, Perin M, Colombo A, Schuler G, Barragan P, Guagliumi G, Molnar F, Falotico R, for the RAVEL Study Group: A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med, 2002; 346: 1773-1780 [DOI] [PubMed] [Google Scholar]
- 32).Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O’Shaughnessy C, Caputo RP, Kereiakes DJ, Williams DO, Teirstein PS, Jaeger JL, Kuntz RE and SIRIUS Investigators: Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med, 2003; 349: 1315-1323 [DOI] [PubMed] [Google Scholar]
- 33).McFadden EP, Stabile E, Regar E, Cheneau E, Ong AT, Kinnaird T, Suddath WO, Weissman NJ, Torguson R, Kent KM, Pichard AD, Satler LF, Waksman R and Serruys PW: Late thrombosis in drug-eluting coronary stents after discontinuation of antiplatelet therapy. Lancet, 2004; 364: 1519-1521 [DOI] [PubMed] [Google Scholar]
- 34).Iakovou I, Schmidt T, Bonizzoni E, Ge L, Sangiorgi GM, Stankovic G, Airoldi F, Chieffo A, Montorfano M, Carlino M, Michev I, Corvaja N, Briguori C, Gerckens U, Grube E and Colombo A: Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA, 2005; 293: 2126-2130 [DOI] [PubMed] [Google Scholar]
- 35).Pfisterer M, Brunner-La Rocca HP, Buser PT, Rickenbacher P, Hunziker P, Mueller C, Jeger R, Bader F, Osswald S, Kaiser C and BASKET-LATE Investigators: Late clinical events after clopidogrel discontinuation may limit the benefit of drug-eluting stents: an observational study of drug-eluting versus bare-metal stents. J Am Coll Cardiol, 2006; 48: 2584-2591 [DOI] [PubMed] [Google Scholar]
- 36).Natsuaki M, Morimoto T, Furukawa Y, Nakagawa Y, Kadota K, Yamaji K, Ando K, Shizuta S, Shiomi H, Tada T, Tazaki J, Kato Y, Hayano M, Abe M, Tamura T, Shirotani M, Miki S, Matsuda M, Takahashi M, Ishii K, Tanaka M, Aoyama T, Doi O, Hattori R, Kato M, Suwa S, Takizawa A, Takatsu Y, Shinoda E, Eizawa H, Takeda T, Lee JD, Inoko M, Ogawa H, Hamasaki S, Horie M, Nohara R, Kambara H, Fujiwara H, Mitsudo K, Nobuyoshi M, Kita T, Kimura T: Late adverse events after implantation of sirolimus-eluting stent and bare-metal stent: long-term (5-7 years) follow-up of the Coronary Revascularization Demonstrating Outcome study-Kyoto registry Cohort-2. Circ Cardiovasc Interv, 2014; 7: 168-179 [DOI] [PubMed] [Google Scholar]
- 37).Stone GW, Moses JW, Ellis SG, Schofer J, Dawkins KD, Morice MC, Colombo A, Schampaert E, Grube E, Kirtane AJ, Cutlip DE, Fahy M, Pocock SJ, Mehran R and Leon MB: Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med, 2007; 356: 998-1008 [DOI] [PubMed] [Google Scholar]
- 38).Finn AV, Joner M, Nakazawa G, Kolodgie F, Newell J, John MC, Gold HK and Virmani R: Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation, 2007; 115: 2435-2441 [DOI] [PubMed] [Google Scholar]
- 39).Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, Kutys R, Skorija K, Gold HK and Virmani R: Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol, 2006; 48: 193-202 [DOI] [PubMed] [Google Scholar]
- 40).Kotani J, Awata M, Nanto S, Uematsu M, Oshima F, Minamiguchi H, Mintz GS and Nagata S: Incomplete neointimal coverage of sirolimus-eluting stents: angioscopic findings. J Am Coll Cardiol, 2006; 47: 2108-2111 [DOI] [PubMed] [Google Scholar]
- 41).Awata M, Kotani J, Uematsu M, Morozumi T, Watanabe T, Onishi T, Iida O, Sera F, Nanto S, Hori M and Nagata S: Serial angioscopic evidence of incomplete neointimal coverage after sirolimus-eluting stent implantation: comparison with bare-metal stents. Circulation, 2007; 116: 910-916 [DOI] [PubMed] [Google Scholar]
- 42).Virmani R, Guagliumi G, Farb A, Musumeci G, Grieco N, Motta T, Mihalcsik L, Tespili M, Valsecchi O and Kolodgie FD: Localized hypersensitivity and late coronary thrombosis secondary to a sirolimus-eluting stent: should we be cautious? Circulation, 2004; 109: 701-705 [DOI] [PubMed] [Google Scholar]
- 43).Nebeker JR, Virmani R, Bennett CL, Hoffman JM, Samore MH, Alvarez J, Davidson CJ, McKoy JM, Raisch DW, Whisenant BK, Yarnold PR, Belknap SM, West DP, Gage JE, Morse RE, Gligoric G, Davidson L and Feldman MD: Hypersensitivity cases associated with drug-eluting coronary stents: a review of available cases from the Research on Adverse Drug Events and Reports (RADAR) project. J Am Coll Cardiol, 2006; 47: 175-181 [DOI] [PubMed] [Google Scholar]
- 44).Palmerini T, Benedetto U, Bacchi-Reggiani L, Della Riva D, Biondi-Zoccai G, Feres F, Abizaid A, Hong MK, Kim BK, Jang Y, Kim HS, Park KW, Genereux P, Bhatt DL, Orlandi C, De Servi S, Petrou M, Rapezzi C and Stone GW: Mortality in patients treated with extended duration dual antiplatelet therapy after drug-eluting stent implantation: a pairwise and Bayesian network meta-analysis of randomised trials. Lancet, 2015; 385: 2371-2382 [DOI] [PubMed] [Google Scholar]
- 45).Stone GW, Rizvi A, Newman W, Mastali K, Wang JC, Caputo R, Doostzadeh J, Cao S, Simonton CA, Sudhir K, Lansky AJ, Cutlip DE, Kereiakes DJ and SPIRIT IV Investigators: Everolimus-eluting versus paclitaxel-eluting stents in coronary artery disease. N Engl J Med, 2010; 362: 1663-1674 [DOI] [PubMed] [Google Scholar]
- 46).Dangas GD, Serruys PW, Kereiakes DJ, Hermiller J, Rizvi A, Newman W, Sudhir K, Smith RS, Jr., Cao S, Theodoropoulos K, Cutlip DE, Lansky AJ and Stone GW: Meta-analysis of everolimus-eluting versus paclitaxel-eluting stents in coronary artery disease: final 3-year results of the SPIRIT clinical trials program (Clinical Evaluation of the Xience V Everolimus Eluting Coronary Stent System in the Treatment of Patients With De Novo Native Coronary Artery Lesions). JACC Cardiovasc Interv, 2013; 6: 914-922 [DOI] [PubMed] [Google Scholar]
- 47).Baber U, Mehran R, Sharma SK, Brar S, Yu J, Suh JW, Kim HS, Park SJ, Kastrati A, de Waha A, Krishnan P, Moreno P, Sweeny J, Kim MC, Suleman J, Pyo R, Wiley J, Kovacic J, Kini AS and Dangas GD: Impact of the everolimus-eluting stent on stent thrombosis: a meta-analysis of 13 randomized trials. J Am Coll Cardiol, 2011; 58: 1569-1577 [DOI] [PubMed] [Google Scholar]
- 48).Tada T, Byrne RA, Simunovic I, King LA, Cassese S, Joner M, Fusaro M, Schneider S, Schulz S, Ibrahim T, Ott I, Massberg S, Laugwitz KL and Kastrati A: Risk of stent thrombosis among bare-metal stents, first-generation drug-eluting stents, and second-generation drug-eluting stents: results from a registry of 18,334 patients. JACC Cardiovasc Interv, 2013; 6: 1267-1274 [DOI] [PubMed] [Google Scholar]
- 49).Navarese EP, Andreotti F, Schulze V, Kolodziejczak M, Buffon A, Brouwer M, Costa F, Kowalewski M, Parati G, Lip GY, Kelm M and Valgimigli M: Optimal duration of dual antiplatelet therapy after percutaneous coronary intervention with drug eluting stents: meta-analysis of randomised controlled trials. BMJ, 2015; 350: h1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50).Bularga A, Meah MN, Doudesis D, Shah ASV, Mills NL, Newby DE and Lee KK: Duration of dual antiplatelet therapy and stability of coronary heart disease: a 60 000-patient meta-analysis of randomised controlled trials. Open Heart, 2021; 8: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51).El-Hayek G, Bangalore S, Casso Dominguez A, Devireddy C, Jaber W, Kumar G, Mavromatis K, Tamis-Holland J and Samady H: Meta-Analysis of Randomized Clinical Trials Comparing Biodegradable Polymer Drug-Eluting Stent to Second-Generation Durable Polymer Drug-Eluting Stents. JACC Cardiovasc Interv, 2017; 10: 462-473 [DOI] [PubMed] [Google Scholar]
- 52).Mehran R, Cao D, Angiolillo DJ, Bangalore S, Bhatt DL, Ge J, Hermiller J, Makkar RR, Neumann FJ, Saito S, Picon H, Toelg R, Maksoud A, Chehab BM, De la Torre Hernandez JM, Kunadian V, Sardella G, Thiele H, Varenne O, Vranckx P, Windecker S, Zhou Y, Krucoff MW, Ruster K, Wang J, Valgimigli M: 3- or 1-Month DAPT in Patients at High Bleeding Risk Undergoing Everolimus-Eluting Stent Implantation. JACC Cardiovasc Interv, 2021; 14: 1870-1883 [DOI] [PubMed] [Google Scholar]
- 53).Valgimigli M, Frigoli E, Heg D, Tijssen J, Juni P, Vranckx P, Ozaki Y, Morice MC, Chevalier B, Onuma Y, Windecker S, Tonino PAL, Roffi M, Lesiak M, Mahfoud F, Bartunek J, Hildick-Smith D, Colombo A, Stankovic G, Iniguez A, Schultz C, Kornowski R, Ong PJL, Alasnag M, Rodriguez AE, Moschovitis A, Laanmets P, Donahue M, Leonardi S, Smits PC and MASTER DAPT Investigators: Dual Antiplatelet Therapy after PCI in Patients at High Bleeding Risk. N Engl J Med, 2021; 385: 1643-1655 [Google Scholar]
- 54).Watanabe H, Domei T, Morimoto T, Natsuaki M, Shiomi H, Toyota T, Ohya M, Suwa S, Takagi K, Nanasato M, Hata Y, Yagi M, Suematsu N, Yokomatsu T, Takamisawa I, Doi M, Noda T, Okayama H, Seino Y, Tada T, Sakamoto H, Hibi K, Abe M, Kawai K, Nakao K, Ando K, Tanabe K, Ikari Y, Hanaoka KI, Morino Y, Kozuma K, Kadota K, Furukawa Y, Nakagawa Y, Kimura T and STOPDAPT-2 Investigators: Effect of 1-Month Dual Antiplatelet Therapy Followed by Clopidogrel vs 12-Month Dual Antiplatelet Therapy on Cardiovascular and Bleeding Events in Patients Receiving PCI: The STOPDAPT-2 Randomized Clinical Trial. JAMA, 2019; 321: 2414-2427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55).Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, Juni P, Kastrati A, Kolh P, Mauri L, Montalescot G, Neumann FJ, Petricevic M, Roffi M, Steg PG, Windecker S, Zamorano JL, Levine GN: 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J, 2018; 39: 213-260 [Google Scholar]
- 56).Giustino G, Chieffo A, Palmerini T, Valgimigli M, Feres F, Abizaid A, Costa RA, Hong MK, Kim BK, Jang Y, Kim HS, Park KW, Gilard M, Morice MC, Sawaya F, Sardella G, Genereux P, Redfors B, Leon MB, Bhatt DL, Stone GW and Colombo A: Efficacy and Safety of Dual Antiplatelet Therapy After Complex PCI. J Am Coll Cardiol, 2016; 68: 1851-1864 [DOI] [PubMed] [Google Scholar]
- 57).Costa F, Van Klaveren D, Feres F, James S, Raber L, Pilgrim T, Hong MK, Kim HS, Colombo A, Steg PG, Bhatt DL, Stone GW, Windecker S, Steyerberg EW, Valgimigli M and PRECISE-DAPT Study Investigators: Dual Antiplatelet Therapy Duration Based on Ischemic and Bleeding Risks After Coronary Stenting. J Am Coll Cardiol, 2019; 73: 741-754 [DOI] [PubMed] [Google Scholar]
- 58).Hemetsberger R, Abdelghani M, Toelg R, Garcia-Garcia HM, Farhan S, Mankerious N, Elbasha K, Allali A, Windecker S, Lefevre T, Saito S, Kandzari D, Waksman R and Richardt G: Complex vs. non-complex percutaneous coronary intervention with newer-generation drug-eluting stents: an analysis from the randomized BIOFLOW trials. Clin Res Cardiol, 2022; [DOI] [PubMed] [Google Scholar]
- 59).Jeger RV, Eccleshall S, Wan Ahmad WA, Ge J, Poerner TC, Shin ES, Alfonso F, Latib A, Ong PJ, Rissanen TT, Saucedo J, Scheller B, Kleber FX and International DCB Consensus Group: Drug-Coated Balloons for Coronary Artery Disease: Third Report of the International DCB Consensus Group. JACC Cardiovasc Interv, 2020; 13: 1391-1402 [DOI] [PubMed] [Google Scholar]
- 60).Sato Y, Hatakeyama K, Yamashita A, Marutsuka K, Sumiyoshi A and Asada Y: Proportion of fibrin and platelets differs in thrombi on ruptured and eroded coronary atherosclerotic plaques in humans. Heart, 2005; 91: 526-530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61).Kaikita K, Ogawa H, Yasue H, Takeya M, Takahashi K, Saito T, Hayasaki K, Horiuchi K, Takizawa A, Kamikubo Y and Nakamura S: Tissue factor expression on macrophages in coronary plaques in patients with unstable angina. Arterioscler Thromb Vasc Biol, 1997; 17: 2232-2237 [DOI] [PubMed] [Google Scholar]
- 62).Matsuura Y, Yamashita A, Iwakiri T, Sugita C, Okuyama N, Kitamura K and Asada Y: Vascular wall hypoxia promotes arterial thrombus formation via augmentation of vascular thrombogenicity. Thromb Haemost, 2015; 114: 158-172 [DOI] [PubMed] [Google Scholar]
- 63).Moscucci M, Fox KA, Cannon CP, Klein W, Lopez-Sendon J, Montalescot G, White K and Goldberg RJ: Predictors of major bleeding in acute coronary syndromes: the Global Registry of Acute Coronary Events (GRACE). Eur Heart J, 2003; 24: 1815-1823 [DOI] [PubMed] [Google Scholar]
- 64).Segev A, Strauss BH, Tan M, Constance C, Langer A, Goodman SG and Canadian Acute Coronary Syndromes Registries Investigators: Predictors and 1-year outcome of major bleeding in patients with non-ST-elevation acute coronary syndromes: insights from the Canadian Acute Coronary Syndrome Registries. Am Heart J, 2005; 150: 690-694 [DOI] [PubMed] [Google Scholar]
- 65).Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, Granger CB, Lange RA, Mack MJ, Mauri L, Mehran R, Mukherjee D, Newby LK, O’Gara PT, Sabatine MS, Smith PK and Smith SC, Jr.: 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention, 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery, 2012 ACC/AHA/ACP /AATS/PCNA /SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease, 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction, 2014 AHA/ACC Guideline for the Management of Patients With Non-ST-Elevation Acute Coronary Syndromes, and 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation, 2016; 134: e123-155 [DOI] [PubMed] [Google Scholar]
- 66).Nakamura M, Kimura K, Kimura T, Ishihara M, Otsuka F, Kozuma K, Kosuge M, Shinke T, Nakagawa Y, Natsuaki M, Yasuda S, Akasaka T, Kohsaka S, Haze K and Hirayama A: JCS 2020 Guideline Focused Update on Antithrombotic Therapy in Patients With Coronary Artery Disease. Circ J, 2020; 84: 831-865 [DOI] [PubMed] [Google Scholar]
- 67).Valgimigli M, Cao D, Angiolillo DJ, Bangalore S, Bhatt DL, Ge J, Hermiller J, Makkar RR, Neumann FJ, Saito S, Picon H, Toelg R, Maksoud A, Chehab BM, Choi JW, Campo G, De la Torre Hernandez JM, Kunadian V, Sardella G, Thiele H, Varenne O, Vranckx P, Windecker S, Zhou Y, Krucoff MW, Ruster K, Zheng Y, Mehran R: Duration of Dual Antiplatelet Therapy for Patients at High Bleeding Risk Undergoing PCI. J Am Coll Cardiol, 2021; 78: 2060-2072 [DOI] [PubMed] [Google Scholar]
- 68).Watanabe H, Morimoto T, Natsuaki M, Yamamoto K, Obayashi Y, Ogita M, Suwa S, Isawa T, Domei T, Yamaji K, Tatsushima S, Watanabe H, Ohya M, Tokuyama H, Tada T, Sakamoto H, Mori H, Suzuki H, Nishikura T, Wakabayashi K, Hibi K, Abe M, Kawai K, Nakao K, Ando K, Tanabe K, Ikari Y, Morino Y, Kadota K, Furukawa Y, Nakagawa Y, Kimura T and STOPDAPT-2 ACS Investigators: Comparison of Clopidogrel Monotherapy After 1 to 2 Months of Dual Antiplatelet Therapy With 12 Months of Dual Antiplatelet Therapy in Patients With Acute Coronary Syndrome: The STOPDAPT-2 ACS Randomized Clinical Trial. JAMA Cardiol, 2022; 7: 407-417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69).Holmes MV, Perel P, Shah T, Hingorani AD and Casas JP: CYP2C19 genotype, clopidogrel metabolism, platelet function, and cardiovascular events: a systematic review and meta-analysis. JAMA, 2011; 306: 2704-2714 [DOI] [PubMed] [Google Scholar]
- 70).Claassens DMF, Vos GJA, Bergmeijer TO, Hermanides RS, van ‘t Hof AWJ, van der Harst P, Barbato E, Morisco C, Tjon Joe Gin RM, Asselbergs FW, Mosterd A, Herrman JR, Dewilde WJM, Janssen PWA, Kelder JC, Postma MJ, de Boer A, Boersma C, Deneer VHM and Ten Berg JM: A Genotype-Guided Strategy for Oral P2Y12 Inhibitors in Primary PCI. N Engl J Med, 2019; 381: 1621-1631 [DOI] [PubMed] [Google Scholar]
- 71).Pereira NL, Farkouh ME, So D, Lennon R, Geller N, Mathew V, Bell M, Bae JH, Jeong MH, Chavez I, Gordon P, Abbott JD, Cagin C, Baudhuin L, Fu YP, Goodman SG, Hasan A, Iturriaga E, Lerman A, Sidhu M, Tanguay JF, Wang L, Weinshilboum R, Welsh R, Rosenberg Y, Bailey K and Rihal C: Effect of Genotype-Guided Oral P2Y12 Inhibitor Selection vs Conventional Clopidogrel Therapy on Ischemic Outcomes After Percutaneous Coronary Intervention: The TAILOR-PCI Randomized Clinical Trial. JAMA, 2020; 324: 761-771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72).Hochholzer W, Trenk D, Fromm MF, Valina CM, Stratz C, Bestehorn HP, Buttner HJ and Neumann FJ: Impact of cytochrome P450 2C19 loss-of-function polymorphism and of major demographic characteristics on residual platelet function after loading and maintenance treatment with clopidogrel in patients undergoing elective coronary stent placement. J Am Coll Cardiol, 2010; 55: 2427-2434 [DOI] [PubMed] [Google Scholar]
- 73).Montalescot G, Wiviott SD, Braunwald E, Murphy SA, Gibson CM, McCabe CH, Antman EM and investigators T-T: Prasugrel compared with clopidogrel in patients undergoing percutaneous coronary intervention for ST-elevation myocardial infarction (TRITON-TIMI 38): double-blind, randomised controlled trial. Lancet, 2009; 373: 723-731 [DOI] [PubMed] [Google Scholar]
- 74).Saito S, Isshiki T, Kimura T, Ogawa H, Yokoi H, Nanto S, Takayama M, Kitagawa K, Nishikawa M, Miyazaki S and Nakamura M: Efficacy and safety of adjusted-dose prasugrel compared with clopidogrel in Japanese patients with acute coronary syndrome: the PRASFIT-ACS study. Circ J, 2014; 78: 1684-1692 [DOI] [PubMed] [Google Scholar]
- 75).Isshiki T, Kimura T, Ogawa H, Yokoi H, Nanto S, Takayama M, Kitagawa K, Nishikawa M, Miyazaki S, Ikeda Y, Nakamura M, Saito S and Investigators PR-E: Prasugrel, a third-generation P2Y12 receptor antagonist, in patients with coronary artery disease undergoing elective percutaneous coronary intervention. Circ J, 2014; 78: 2926-2934 [DOI] [PubMed] [Google Scholar]
- 76).Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA, Freij A, Thorsen M; PLATO Investigators: Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med, 2009; 361: 1045-1057 [DOI] [PubMed] [Google Scholar]
- 77).Schupke S, Neumann FJ, Menichelli M, Mayer K, Bernlochner I, Wohrle J, Richardt G, Liebetrau C, Witzenbichler B, Antoniucci D, Akin I, Bott-Flugel L, Fischer M, Landmesser U, Katus HA, Sibbing D, Seyfarth M, Janisch M, Boncompagni D, Hilz R, Rottbauer W, Okrojek R, Mollmann H, Hochholzer W, Migliorini A, Cassese S, Mollo P, Xhepa E, Kufner S, Strehle A, Leggewie S, Allali A, Ndrepepa G, Schuhlen H, Angiolillo DJ, Hamm CW, Hapfelmeier A, Tolg R, Trenk D, Schunkert H, Laugwitz KL, Kastrati A and ISAR-REACT 5 Trial Investigators: Ticagrelor or Prasugrel in Patients with Acute Coronary Syndromes. N Engl J Med, 2019; 381: 1524-1534 [Google Scholar]
- 78).Sorensen HT, Mellemkjaer L, Blot WJ, Nielsen GL, Steffensen FH, McLaughlin JK and Olsen JH: Risk of upper gastrointestinal bleeding associated with use of low-dose aspirin. Am J Gastroenterol, 2000; 95: 2218-2224 [DOI] [PubMed] [Google Scholar]
- 79).Chiarito M, Sanz-Sanchez J, Cannata F, Cao D, Sturla M, Panico C, Godino C, Regazzoli D, Reimers B, De Caterina R, Condorelli G, Ferrante G and Stefanini GG: Monotherapy with a P2Y12 inhibitor or aspirin for secondary prevention in patients with established atherosclerosis: a systematic review and meta-analysis. Lancet, 2020; 395: 1487-1495 [DOI] [PubMed] [Google Scholar]
- 80).O’Donoghue ML, Murphy SA and Sabatine MS: The Safety and Efficacy of Aspirin Discontinuation on a Background of a P2Y12 Inhibitor in Patients After Percutaneous Coronary Intervention: A Systematic Review and Meta-Analysis. Circulation, 2020; 142: 538-545 [DOI] [PubMed] [Google Scholar]
- 81).Mehran R, Baber U, Sharma SK, Cohen DJ, Angiolillo DJ, Briguori C, Cha JY, Collier T, Dangas G, Dudek D, Dzavik V, Escaned J, Gil R, Gurbel P, Hamm CW, Henry T, Huber K, Kastrati A, Kaul U, Kornowski R, Krucoff M, Kunadian V, Marx SO, Mehta SR, Moliterno D, Ohman EM, Oldroyd K, Sardella G, Sartori S, Shlofmitz R, Steg PG, Weisz G, Witzenbichler B, Han YL, Pocock S and Gibson CM: Ticagrelor with or without Aspirin in High-Risk Patients after PCI. N Engl J Med, 2019; 381: 2032-2042 [DOI] [PubMed] [Google Scholar]
- 82).Hess CN, Peterson ED, Peng SA, de Lemos JA, Fosbol EL, Thomas L, Bhatt DL, Saucedo JF and Wang TY: Use and Outcomes of Triple Therapy Among Older Patients With Acute Myocardial Infarction and Atrial Fibrillation. J Am Coll Cardiol, 2015; 66: 616-627 [DOI] [PubMed] [Google Scholar]
- 83).Hansen ML, Sorensen R, Clausen MT, Fog-Petersen ML, Raunso J, Gadsboll N, Gislason GH, Folke F, Andersen SS, Schramm TK, Abildstrom SZ, Poulsen HE, Kober L and Torp-Pedersen C: Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med, 2010; 170: 1433-1441 [DOI] [PubMed] [Google Scholar]
- 84).Dewilde WJ, Oirbans T, Verheugt FW, Kelder JC, De Smet BJ, Herrman JP, Adriaenssens T, Vrolix M, Heestermans AA, Vis MM, Tijsen JG, van ‘t Hof AW, ten Berg JM and WOEST study investigators: Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet, 2013; 381: 1107-1115 [DOI] [PubMed] [Google Scholar]
- 85).Gibson CM, Mehran R, Bode C, Halperin J, Verheugt FW, Wildgoose P, Birmingham M, Ianus J, Burton P, van Eickels M, Korjian S, Daaboul Y, Lip GY, Cohen M, Husted S, Peterson ED and Fox KA: Prevention of Bleeding in Patients with Atrial Fibrillation Undergoing PCI. N Engl J Med, 2016; 375: 2423-2434 [DOI] [PubMed] [Google Scholar]
- 86).Cannon CP, Bhatt DL, Oldgren J, Lip GYH, Ellis SG, Kimura T, Maeng M, Merkely B, Zeymer U, Gropper S, Nordaby M, Kleine E, Harper R, Manassie J, Januzzi JL, Ten Berg JM, Steg PG, Hohnloser SH: Dual Antithrombotic Therapy with Dabigatran after PCI in Atrial Fibrillation. N Engl J Med, 2017; 377: 1513-1524 [DOI] [PubMed] [Google Scholar]
- 87).Vranckx P, Valgimigli M, Eckardt L, Tijssen J, Lewalter T, Gargiulo G, Batushkin V, Campo G, Lysak Z, Vakaliuk I, Milewski K, Laeis P, Reimitz PE, Smolnik R, Zierhut W and Goette A: Edoxaban-based versus vitamin K antagonist-based antithrombotic regimen after successful coronary stenting in patients with atrial fibrillation (ENTRUST-AF PCI): a randomised, open-label, phase 3b trial. Lancet, 2019; 394: 1335-1343 [DOI] [PubMed] [Google Scholar]
- 88).Lopes RD, Heizer G, Aronson R, Vora AN, Massaro T, Mehran R, Goodman SG, Windecker S, Darius H, Li J, Averkov O, Bahit MC, Berwanger O, Budaj A, Hijazi Z, Parkhomenko A, Sinnaeve P, Storey RF, Thiele H, Vinereanu D, Granger CB, Alexander JH and AUGUSTUS Investigators: Antithrombotic Therapy after Acute Coronary Syndrome or PCI in Atrial Fibrillation. N Engl J Med, 2019; 380: 1509-1524 [DOI] [PubMed] [Google Scholar]
- 89).Matsumura-Nakano Y, Shizuta S, Komasa A, Morimoto T, Masuda H, Shiomi H, Goto K, Nakai K, Ogawa H, Kobori A, Kono Y, Kaitani K, Suwa S, Aoyama T, Takahashi M, Sasaki Y, Onishi Y, Mano T, Matsuda M, Motooka M, Tomita H, Inoko M, Wakeyama T, Hagiwara N, Tanabe K, Akao M, Miyauchi K, Yajima J, Hanaoka K, Morino Y, Ando K, Furukawa Y, Nakagawa Y, Nakao K, Kozuma K, Kadota K, Kimura K, Kawai K, Ueno T, Okumura K, Kimura T and OAC-ALONE Study Investigators: Open-Label Randomized Trial Comparing Oral Anticoagulation With and Without Single Antiplatelet Therapy in Patients With Atrial Fibrillation and Stable Coronary Artery Disease Beyond 1 Year After Coronary Stent Implantation. Circulation, 2019; 139: 604-616 [DOI] [PubMed] [Google Scholar]
- 90).Yasuda S, Kaikita K, Akao M, Ako J, Matoba T, Nakamura M, Miyauchi K, Hagiwara N, Kimura K, Hirayama A, Matsui K, Ogawa H and AFIRE Investigators: Antithrombotic Therapy for Atrial Fibrillation with Stable Coronary Disease. N Engl J Med, 2019; 381: 1103-1113 [Google Scholar]
- 91).Kaikita K, Hosokawa K, Dahlen JR and Tsujita K: Total Thrombus-Formation Analysis System (T-TAS): Clinical Application of Quantitative Analysis of Thrombus Formation in Cardiovascular Disease. Thromb Haemost, 2019; 119: 1554-1562 [DOI] [PubMed] [Google Scholar]


