Abstract
Aim: We investigated the relationship between small dense low-density cholesterol (sdLDL-C) and risk of major adverse cardiovascular events (MACE) in patients treated with high- or low-dose statin therapy.
Methods: This was a prospective case-cohort study within the Randomized Evaluation of Aggressive or Moderate Lipid-Lowering Therapy with Pitavastatin in Coronary Artery Disease (REAL-CAD) study, a randomized trial of high- or low-dose (4 or 1 mg/d pitavastatin, respectively) statin therapy, in patients with stable coronary artery disease (CAD). Serum sdLDL-C was determined using an automated homogenous assay at baseline (randomization after a rule-in period, >1 month with 1 mg/d pitavastatin) and 6 months after randomization, in 497 MACE cases, and 1543 participants randomly selected from the REAL-CAD study population.
Results: High-dose pitavastatin reduced sdLDL-C by 20% than low-dose pitavastatin (p for interaction <0.001). Among patients receiving low-dose pitavastatin, baseline sdLDL-C demonstrated higher MACE risk independent of LDL-C (hazard ratio [95% confidence interval], 4th versus 1st quartile, 1.67 [1.04–2.68];p for trend=0.034). High-dose (versus low-dose) pitavastatin reduced MACE risk by 46% in patients in the highest baseline sdLDL-C quartile (>34.3 mg/dL; 0.54 [0.36–0.81];p=0.003), but increased relative risk by 40% in patients with 1st quartile (≤ 19.5 mg/dL; 1.40 [0.94–2.09];p=0.099) and did not alter risk in those in 2nd and 3rd quartiles (p for interaction=0.002).
Conclusions: These findings associate sdLDL-C and cardiovascular risk, independent of LDL-C, in statin-treated CAD patients. Notably, high-dose statin therapy reduces this risk in those with the highest baseline sdLDL-C.
Keywords: Small dense LDL cholesterol, Coronary artery disease, Statin therapy, Secondary prevention
See editorial vol. 29: 1425-1426
Clinical Trial Registration: https//www.clinicaltrials.gov. Unique identifier: NCT01042730.
Abbreviations: CAD, coronary artery disease; CI, confidence intervals; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; HR, hazard ratio; hsCRP, high-sensitivity C-reactive protein; LDL-C, low-density lipoprotein cholesterol; MACE, major adverse cardiovascular events; non-HDL-C, non-high-density lipoprotein cholesterol; REAL-CAD, Randomized Evaluation of Aggressive or Moderate Lipid-Lowering Therapy with Pitavastatin in Coronary Artery Disease; sdLDL-C, small dense low-density lipoprotein cholesterol; TRL-C, triglyceride-rich lipoprotein cholesterol.
Introduction
Low-density lipoprotein cholesterol (LDL-C) is a primary risk factor for cardiovascular disease 1) and lowering LDL-C levels with medications, such as statins, has proved effective for primary and secondary prevention 2 - 4) . However, even when LDL-C levels drop to optimal levels, the risk of cardiovascular events exists, and an additional option may be necessary to reduce these events 5 , 6) .
Small dense LDL-C (sdLDL-C) levels may partly account for this residual risk. Because sdLDL particles contain less cholesterol and are smaller, increased sdLDL-C levels represent an increase in numbers of atherogenic LDL particles, which LDL-C levels may not reflect 7) . These particles are considered more atherogenic than large buoyant LDL particles due to their lower binding affinity for LDL receptors, increased penetration into the arterial wall, longer plasma residence time, and greater susceptibility to oxidation 8 - 12) . There have been demonstrations in recent large cohort studies using a simple homogeneous sdLDL-C assay of the interrelationship between higher sdLDL-C level and cardiovascular risk regardless of LDL-C level 7 , 13 - 17) . Recently, the prospective Framingham Offspring Study documented that sdLDL-C may be the best measure of cardiovascular risk in atherogenic lipid markers, including LDL triglycerides, large buoyant LDL-C, triglyceride-rich lipoprotein cholesterol (TRL-C), remnant lipoprotein particle cholesterol, and lipoprotein (a) 18) . Thus, optimizing sdLDL-C may reduce cardiovascular risk. The statin therapy lowered the levels of LDL-C as well as sdLDL-C, primarily by enhancing apolipoprotein B catabolism 19 - 21) . However, the role of sdLDL-C level in the prognosis of statin-treated patients remains undefined.
The Randomized Evaluation of Aggressive or Moderate Lipid-Lowering Therapy with Pitavastatin in Coronary Artery Disease (REAL-CAD) study is currently the largest randomized trial. It compared high- and low-dose pitavastatin therapy in patients with stable coronary artery disease (CAD) 22 , 23) and used a study design similar to the previously reported Treating to New Targets study 24 , 25) . The REAL-CAD study demonstrated that, compared with low-dose, high-dose pitavastatin, safely reduced the risk of major adverse cardiovascular events (MACE) by 19% in Japanese patients with stable CAD, who commonly receive low-intensity statin therapy 23) .
Aim
In this prospective case-cohort study, within the REAL-CAD study, we investigated the relationship between sdLDL-C level determined by a simple homogeneous assay 26) and subsequent MACE in statin-treated patients with stable CAD. We evaluated whether more intensive statin therapy attenuates this risk.
Methods
Study Participants and Case-Cohort Design
The REAL-CAD study is a prospective, multicenter, randomized trial that compared the efficacies of high- and low-dose (4 and 1 mg/d pitavastatin, respectively) statin therapy for secondary prevention 13,054 Japanese patients with stable CAD. The patients achieved LDL-C levels <120 mg/dL during a run-in period of >1 month with 1 mg/d of pitavastatin 22 , 23) . This study was a prospective case-cohort study within the REAL-CAD study. MACE was the study’s primary endpoint, comprising nonfatal myocardial infarction, cardiovascular death, unstable angina, and nonfatal ischemic stroke, requiring emergency hospitalization. Importantly, for the REAL-CAD study, fasting serum samples were obtained at baseline (randomization) after at least a 1-month run-in period on 1 mg/d pitavastatin and 6 months after randomization on 1 mg/d or 4 mg/d pitavastatin.
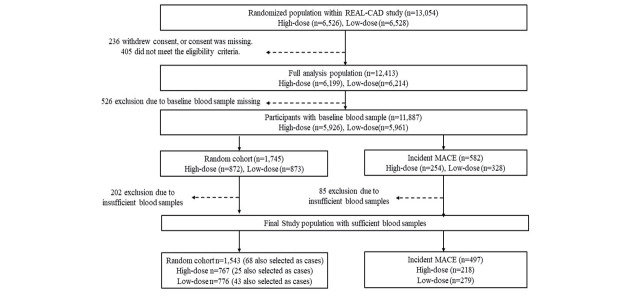
There were 11,887 participants with baseline blood samples in the full analysis population of the REAL-CAD study. We conducted a case-cohort analysis based on 582 cases who developed MACE during a median follow-up period of 3.9 years and a random cohort sample of 1,745 participants (three times the number of MACE cases). After excluding 202 participants with insufficient serum samples, the random cohort comprised 1,543 patients (high-dose, n=767; low-dose, n=776). Similarly, exclusion of 85 MACE cases with insufficient serum samples resulted in 497 cases (high-dose, n=218; low-dose, n=279). Of these, 68 MACE cases were duplicated in the random cohort. A case-cohort data set comprising 1,972 participants was created ( Supplementary Fig.1 ) .
Supplementary Fig.1. Case-cohort design within REAL-CAD study.
Abbreviations; MACE, major adverse cardiovascular events; REAL-CAD, Randomized Evaluation of Agressive or Moderate Lipid Lowering Therapy With Pitavastatin in Coronary Artery Disease.
Ethics approval for the REAL-CAD study was granted by the Public Health Research Foundation ethics review committee and by ethics committees at all participating sites. All participants provided written informed consent. The study complied with the Declaration of Helsinki. The ethics committee of Fujita Health University also approved the present case-cohort study.
Laboratory Analysis
Fasting serum samples for sdLDL-C measurement were collected at baseline and 6 months after randomization. They were stored at −70℃ or colder. Serum sdLDL-C level was directly measured on a LABOSPECT 006 automated chemistry analyzer using a homogenous assay (sdLDL-EX “SEIKEN;” Denka Seiken, Tokyo, Japan) 26) . The central laboratory measured serum triglycerides, total cholesterol, and high-density lipoprotein cholesterol (HDL-C) levels. Serum LDL-C was calculated with the Friedewald formula 27) unless the triglyceride level was ≥ 400 mg/dL, when LDL-C was directly measured by a homogenous assay. Non-HDL-C was defined as total cholesterol minus HDL-C. TRL-C level was defined as the difference between non-HDL-C and LDL-C. These calculated TRL-C levels have been reported to closely approximate triglyceride levels 28 , 29) .
Statistical Analysis
All analyses were performed using SAS software, version 9.4 (SAS Institute Inc). The random cohort’s differences in the mean (standard deviation) or median (interquartile range) in high- and low-dose groups were tested by Wilcoxon rank-sum test. Similarly, the frequency of risk factors in the two groups was tested by Fisher’s exact or Chi-square test. Trends in the mean (standard deviation) or median (interquartile range) and frequency of risk factors across quartiles of sdLDL-C levels at baseline were tested by the Jonckheere–Terpstra test and Cochran–Mantel–Haenszel correlation test, respectively. The association between sdLDL-C and other lipid markers was assessed using Spearman’s rank correlation coefficient. Absolute and relative changes from baseline to 6 months after randomization in sdLDL-C, LDL-C, triglycerides, and percentage of sdLDL-C in LDL-C were tested with Wilcoxon signed-rank test, compared with Wilcoxon rank-sum test between treatment groups, and tested by an analysis of covariance (ANCOVA) with individual baseline covariate between treatment groups.
The subjects were categorized into quartiles for each baseline lipid marker according to the distribution in the random cohort. Hazard ratios (HR) and 95% confidence intervals (CI) of MACE concerning quartiles were calculated using Cox proportional hazard models with Barlow’s methods 30) to account for the case-cohort design. Multivariable models accounted for the following covariates: Model 1 was adjusted for standard risk factors (i.e., age [≥ 65 and <65 years], male sex, body mass index, systolic blood pressure, heart rate, hypertension, diabetes mellitus, smoking status [current versus not current], myocardial infarction history, percutaneous coronary intervention history, coronary artery bypass grafting history, atrial fibrillation, heart failure, malignant disease, and peripheral artery disease), creatinine-based estimated glomerular filtration rate (eGFR), hemoglobin A1c, high-sensitivity C-reactive protein (hsCRP), and HDL-C at baseline. Model 2 was adjusted for model 1 variables and LDL-C at baseline. Model 3 was adjusted for model 1 variables and triglycerides at baseline. Model 4 was adjusted for model 1 variables and TRL-C at baseline. Similarly, the subjects were categorized into quartiles for sdLDL-C level after 6 months and absolute and relative changes from baseline to 6 months in sdLDL-C level according to the distribution in the random cohort. The HR and 95% CI of MACE regarding quartiles were calculated using Cox proportional hazard models with Barlow’s methods 30) . Multivariable models were created adjusting for established risk factors, eGFR, hemoglobin A1c, hsCRP, triglycerides, and HDL-C at 6 months. Participants with MACE before 6 months were excluded from these analyses.
The intertreatment (high- versus low-dose pitavastatin) differences in endpoint in subgroups, according to quartile of serum sdLDL-C at baseline, were examined with the weighted Kaplan–Meier method and compared with Barlow’s log-rank test. The weights were the inverse of patients’ sampling probability of this case-cohort design. The interaction of statin intensity (high- versus low-dose pitavastatin) by subgroup according to the quartile of serum sdLDL-C, LDL-C, triglycerides, TRL-C, non-HDL-C, and HDL-C levels at baseline, were examined separately in the same model with the interaction term. Multivariable models were created adjusting for standard risk factors, eGFR, hemoglobin A1c, hsCRP, LDL-C, HDL-C, and triglycerides at baseline (excluding the subgroup classifier variable).
Results
Baseline Characteristic in Random Cohort
Among the random cohort (n=1,543), there were no significant differences in baseline characteristics between high- and low-dose groups except for HDL-C (p=0.01), triglycerides (p=0.04), and TRL-C levels (p=0.04; Table 1 ). On the baseline characteristics of the random cohort according to the quartile of serum sdLDL-C level, mean body mass index, diastolic blood pressure, total cholesterol, LDL-C, hemoglobin A1c, and eGFR; median triglycerides, TRL-C, and hsCRP; and frequency of male sex and current smoking increased significantly with higher sdLDL-C levels at baseline ( Supplementary Table 1 ) . Conversely, mean age and HDL-C; and frequency of atrial fibrillation and malignant disease decreased significantly with higher sdLDL-C levels at baseline ( Supplementary Table 1 ) . The sdLDL-C level at baseline was more strongly correlated with baseline TRL-C (r=0.63, p<0.001), triglyceride (r=0.61, p<0.001), and non-HDL-C (r=0.71, p<0.001) levels compared with baseline LDL-C (r=0.33, p<0.001) and HDL-C (r=−0.13, p<0.001) levels. TRL-C level at baseline was strongly correlated with triglyceride level (r=0.94, p<0.001).
Table 1. Patient characteristics at baseline in high- and low-dose groups among random cohort population.
| High-dose (n = 767) | Low-dose (n = 776) | p-value | |
|---|---|---|---|
| Age, y | 68.5 (8.4) | 68.0 (8.4) | 0.29 |
| Male, n (%) | 649 (84.6) | 631 (81.3) | 0.08 |
| Body mass index, kg/m2 | 24.8 (3.3) | 24.7 (3.4) | 0.55 |
| Systolic blood pressure, mmHg | 128 (16.3) | 128 (16.3) | 0.82 |
| Diastolic blood pressure, mmHg | 72.7 (10.7) | 72.8 (10.7) | 0.53 |
| Heart rate, bpm | 69.8 (11.2) | 69.6 (11.8) | 0.79 |
| Left ventricular ejection fraction, % | 60.0 (12.4) | 60.5 (12.0) | 0.77 |
| Cardiovascular history | |||
| Myocardial infarction, n (%) | 372 (48.5) | 388 (50.0) | 0.84 |
| Percutaneous coronary intervention, n (%) | 633 (82.5) | 639 (82.3) | 1.00 |
| Coronary artery bypass grafting, n (%) | 92 (12.0) | 106 (13.7) | 0.62 |
| Congestive heart failure, n (%) | 41 (5.3) | 42 (5.4) | 0.61 |
| Atrial fibrillation, n (%) | 48 (6.3) | 47 (6.1) | 0.99 |
| Ischemic stroke, n (%) | 56 (7.3) | 63 (8.1) | 0.59 |
| Hemorrhagic stroke, n (%) | 12 (1.6) | 11 (1.4) | 0.81 |
| Peripheral artery disease, n (%) | 46 (6.0) | 67 (8.6) | 0.08 |
| Current smoking, n (%) | 122 (15.9) | 113 (14.6) | 0.76 |
| Diabetes mellitus, n (%) | 320 (41.7) | 313 (40.3) | 0.58 |
| Hypertension, n (%) | 585 (76.3) | 587 (75.6) | 0.96 |
| Family history of coronary artery disease, n (%) | 130 (16.9) | 148 (19.1) | 0.66 |
| History of malignant disease, n (%) | 42 (5.5) | 40 (5.7) | 0.96 |
| Blood examinations | |||
| Total cholesterol, mg/dL | 166 (23.1) | 166 (25.1) | 0.50 |
| LDL-C, mg/dL | 87.8 (18.0) | 88.0 (18.9) | 0.91 |
| HDL-C, mg/dL | 49.6 (12.3) | 51.6 (13.1) | 0.01 |
| Triglycerides, mg/dL, median | 125 (92, 180) | 120 (87, 168) | 0.04 |
| sdLDL-C, mg/dL | 28.5 (12.3) | 27.4 (11.4) | 0.09 |
| TRL-C, mg/dL, median | 25.0 (18.6, 36.0) | 24.0 (17.4, 33.6) | 0.04 |
| hsCRP, mg/L, median | 0.53 (0.25, 1.19) | 0.52 (0.23, 1.19) | 0.51 |
| Glucose, mg/dL | 127 (41.7) | 125 (43.0) | 0.21 |
| Hemoglobin A1c, % | 5.91 (0.93) | 5.87 (0.87) | 0.42 |
| eGFR, mL/min/1.73 m2 | 66.0 (16.2) | 66.5 (30.2) | 0.61 |
| Chronic kidney disease, n (%) | 0.93 | ||
| Stage 1 | 58 (7.6) | 61 (7.9) | |
| Stage 2 | 412 (53.7) | 428 (55.2) | |
| Stage 3 | 275 (35.9) | 269 (34.7) | |
| Stage 4 | 10 (1.3) | 9 (1.2) | |
| Stage 5 | 0 (0) | 0 (0) | |
| Medications, n (%) | |||
| Aspirin | 664 (86.6) | 660 (85.1) | 0.68 |
| Thienopyridine | 342 (44.6) | 338 (43.6) | 0.74 |
| Dual antiplatelet therapy | 316 (41.2) | 320 (41.2) | 0.75 |
| β-blocker | 284 (37.0) | 297 (38.3) | 0.60 |
| ACEI and/or ARB | 479 (62.5) | 496 (63.9) | 0.50 |
Abbreviations: ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; hsCRP, high-sensitivity C-reactive protein; LDL-C, low-density lipoprotein cholesterol; sdLDL-C, small dense low-density lipoprotein cholesterol; TRL-C, triglyceride-rich lipoprotein cholesterol. Data are n (%), mean (standard deviation) or median (interquartile range).
Supplementary Table 1. Patient characteristics according to baseline sdLDL-C quartiles in random cohort population.
| sdLD-C quartile Range, mg/dL |
1st ≤ 19.5 (n = 386) |
2nd > 19.5 to 25.7 (n = 398) |
3rd > 25.7 to 34.3 (n = 374) |
4th > 34.3 (n = 385) |
p for trend |
|---|---|---|---|---|---|
| High-dose statin group, n (%) | 175 (45.3) | 206 (51.8) | 183 (48.9) | 203 (52.7) | 0.09 |
| Age, y | 70.0 (7.6) | 69.2 (8.2) | 67.6 (8.4) | 66.2 (8.0) | <0.01 |
| Male, n (%) | 304 (78.8) | 333 (83.7) | 317 (84.8) | 326 (84.7) | 0.03 |
| Body mass index, kg/m2 | 24.0 (3.41) | 24.5 (3.23) | 24.7 (3.21) | 25.7 (3.33) | <0.01 |
| Systolic blood pressure, mmHg | 128 (17.5) | 126 (17.4) | 127 (15.9) | 128 (14.1) | 0.09 |
| Diastolic blood pressure, mmHg | 71.9 (10.9) | 71.1 (10.8) | 72.9 (10.4) | 75.0 (10.3) | <0.01 |
| Heart rate, bpm | 69.7 (11.6) | 69.5 (11.6) | 69.1 (11.4) | 70.3 (11.2) | 0.50 |
| Left ventricular ejection fraction, % | 60.1 (12.7) | 60.0 (12.3) | 61.1 (12.0) | 59.9 (11.9) | 0.94 |
| Cardiovascular history | |||||
| Myocardial infarction, n (%) | 196 (50.8) | 187 (47.0) | 196 (52.4) | 181 (47.0) | 0.64 |
| Percutaneous coronary intervention, n (%) | 318 (82.4) | 324 (81.4) | 313 (83.7) | 317 (82.3) | 0.73 |
| Coronary artery bypass grafting, n (%) | 63 (16.3) | 41 (10.3) | 42 (11.2) | 52 (13.5) | 0.34 |
| Congestive heart failure, n (%) | 16 (4.1) | 27 (6.8) | 16 (4.3) | 24 (6.2) | 0.46 |
| Atrial fibrillation, n (%) | 32 (8.3) | 23 (5.8) | 23 (6.1) | 17 (4.4) | 0.04 |
| Ischemic stroke, n (%) | 42 (10.9) | 32 (8.0) | 27 (7.2) | 20 (5.2) | 0.80 |
| Hemorrhagic stroke, n (%) | 8 (2.1) | 6 (1.5) | 7 (1.9) | 2 (0.5) | 0.75 |
| Peripheral artery disease, n (%) | 30 (7.8) | 30 (7.5) | 23 (6.1) | 30 (7.8) | 0.83 |
| Current smoking, n (%) | 45 (11.7) | 63 (15.8) | 54 (14.4) | 73 (19.0) | 0.01 |
| Diabetes mellitus, n (%) | 171 (44.3) | 168 (42.2) | 131 (35.0) | 163 (42.3) | 0.25 |
| Hypertension, n (%) | 291 (75.4) | 303 (76.1) | 281 (75.1) | 287 (77.1) | 0.60 |
| Family history of coronary artery disease, n (%) | 70 (18.1) | 67 (16.8) | 66 (17.4) | 76 (19.7) | 0.47 |
| History of malignant disease, n (%) | 31 (8.0) | 23 (5.8) | 17 (4.5) | 11 (2.9) | <0.01 |
| Blood examinations | |||||
| Total cholesterol, mg/dL | 147 (19.0) | 162 (20.2) | 172 (19.5) | 183 (21.7) | <0.01 |
| LDL-C, mg/dL | 76.1 (14.0) | 87.3 (16.5) | 92.6 (18.0) | 93.7 (19.6) | <0.01 |
| HDL-C, mg/dL | 52.1 (12.9) | 51.5 (12.6) | 51.0 (13.7) | 47.8 (11.4) | <0.01 |
| Triglycerides, mg/dL, median | 89.0 (66, 114) | 108.5 (86, 140) | 130 (100, 171) | 192 (147, 260) | <0.01 |
| TRL-C, mg/dL, median | 17.8 (13.2, 22.8) | 21.8 (17.4, 28.0) | 26.0 (20.4, 34.2) | 38.2 (29.4, 51.4) | <0.01 |
| hsCRP, mg/L, median | 0.49 (0.18, 1.32) | 0.50 (0.22, 1.22) | 0.54 (0.26, 0.98) | 0.56 (0.30, 1.21) | 0.03 |
| Glucose, mg/dL | 125 (44.8) | 122 (37.1) | 124 (39.6) | 131 (46.9) | 0.06 |
| Hemoglobin A1c, % | 5.82 (0.84) | 5.85 (0.78) | 5.86 (0.93) | 6.03 (1.02) | <0.01 |
| eGFR, mL/min/1.73m2 | 63.5 (16.7) | 66.7 (39.2) | 66.7 (16.0) | 68.1 (15.2) | <0.01 |
| Chronic kidney disease, n (%) | <0.01 | ||||
| Stage 1 | 25 (6.5) | 29 (7.3) | 32 (8.6) | 33 (8.6) | |
| Stage 2 | 193 (50.0) | 215 (54.0) | 206 (55.1) | 226 (58.7) | |
| Stage 3 | 156 (40.4) | 145 (36.4) | 126 (33.7) | 117 (30.4) | |
| Stage 4 | 9 (2.3) | 6 (1.5) | 2 (0.5) | 2 (0.5) | |
| Stage 5 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Medications, n (%) | |||||
| Aspirin | 327 (84.7) | 336 (84.4) | 328 (87.7) | 333 (86.5) | 0.23 |
| Thienopyridine | 166 (43.0) | 171 (43.0) | 163 (43.6) | 180 (46.8) | 0.31 |
| Dual antiplatelet therapy | 156 (40.4) | 159 (39.9) | 153 (40.9) | 168 (43.6) | 0.36 |
| β-blocker | 131 (33.9) | 159 (39.9) | 138 (36.9) | 153 (39.7) | 0.21 |
| ACEI and/or ARB | 254 (65.8) | 252 (63.3) | 222 (59.4) | 247 (64.2) | 0.34 |
Abbreviations: ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; hsCRP, high-sensitivity C-reactive protein; LDL-C, low-density lipoprotein cholesterol; sdLDL-C, small dense low-density lipoprotein cholesterol; TRL-C, triglyceride-rich lipoprotein cholesterol.
Data are n (%), mean (standard deviation) or median (interquartile range).
Changes in Lipid Markers in High- and Low-dose Groups
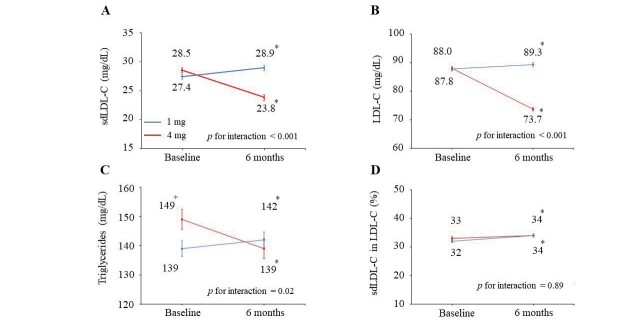
In the random cohort, mean sdLDL-C levels at baseline were 28.5 and 27.4 mg/dL in the high- and low-dose groups, respectively ( Fig.1A ) . The sdLDL-C level in the high-dose group decreased by 9.9% (p<0.001) at 6 months when compared with the value at baseline, whereas in the low-dose group, an increase by 10% at 6 months was noted (p<0.001). The high-dose pitavastatin significantly reduced sdLDL-C levels by 20% (p for interaction <0.001) than low-dose pitavastatin.
Fig.1. Changes in lipid makers from baseline to 6 months in high- and low-dose groups in random cohort population.
Data are mean (standard error) for sdLDL-C (A), LDL-C (B), triglycerides (C), and percentage of sdLDL-C in LDL-C (D). +p<0.05 vs. low-dose (1 mg/d) group. *p<0.05 vs. baseline.
Similar to sdLDL-C, the high-dose pitavastatin reduced LDL-C levels by 19% (p for interaction < 0.001; Fig.1B ) and triglycerides by 7.5% (p for interaction=0.02; Fig.1C ). The percentage of sdLDL-C in LDL-C in the high- and low-dose statin groups increased at 6 months (p=0.02 and p=0.004, respectively) when compared with baseline ( Fig.1D ) . There was no significant change in the percentage of sdLDL-C in LDL-C with high-dose (versus low-dose) statin treatment.
Changes in sdLDL-C in Subgroups According to sdLDL-C Quartile at Baseline
In all baseline sdLDL-C quartile, high-dose pitavastatin significantly reduced sdLDL-C levels further than low-dose pitavastatin (all, p for interaction <0.001; Table 2 ). Both absolute and relative changes in sdLDL-C from baseline to 6 months were associated with increased baseline sdLDL-C quartiles (p for trend < 0.001).
Table 2. Changes in sdLDL-C from baseline to 6 months in high- and low-dose groups according to baseline sdLDL-C quartiles.
| Baseline sdLDL-C quartiles | High-dose | Low-dose | p-value |
|---|---|---|---|
| 1st (≤ 19.5 mg/dL) | |||
| Baseline, mg/dL | 15.2 (3.1) | 15.3 (2.8) | 0.84 |
| 6 months, mg/dL | 16.2 (6.5) | 19.5 (7.2) ** | <0.001 |
| Absolute change, mg/dL, median | −0.30 (−3.3, 3.7) | 3.4 (−0.25, 6.8) | <0.001 |
| Relative change, %, median | −2.1 (−21.3, 22.4) | 20.3 (−2.0, 48.6) | <0.001 |
| 2nd (> 19.5 to 25.7 mg/dL) | |||
| Baseline, mg/dL | 22.7 (1.8) | 22.7 (1.8) | 0.94 |
| 6 months, mg/dL | 19.8 (5.9) ** | 25.6 (8.5) ** | <0.001 |
| Absolute change, mg/dL, median | −3.1 (−7.2, 0.50) | 2.2 (−2.5, 6.3) | <0.001 |
| Relative change, %, median | −14.6 (−31.5, 2.6) | 9.1 (−10.7, 26.5) | <0.001 |
| 3rd (> 25.7 to 34.3 mg/dL) | |||
| Baseline, mg/dL | 29.6 (2.5) | 29.8 (2.5) | 0.74 |
| 6 months, mg/dL | 25.1 (8.6) ** | 30.3 (9.7) | <0.001 |
| Absolute change, mg/dL, median | −6.1 (−9.9, −0.50) | −0.60 (−5.1, 4.9) | <0.001 |
| Relative change, %, median | −20.7 (−33.0, −1.8) | −1.8 (−16.9, 16.5) | <0.001 |
| 4th (> 34.3 mg/dL) | |||
| Baseline, mg/dL | 45.0 (9.8) | 43.9 (8.3) | 0.43 |
| 6 months, mg/dL | 34.7 (12.0)** | 41.3 (12.5)** | <0.001 |
| Absolute change, mg/dL, median | −11.2 (−16.2, −3.6) | −2.8 (−9.75, 2.95) | <0.001 |
| Relative change, %, median | −25.2 (−37.5, −8.8) | −6.2 (−21.4, 6.7) | <0.001 |
Abbreviations: sdLDL-C, small dense low-density lipoprotein cholesterol.
Absolute and relative changes in sdLDL-C level were defined as (sdLDL-C value at 6 months – at baseline) and (absolute change
/ baseline)×100, respectively. Data are mean (standard deviation) for sdLDL-C at baseline and 6 months, and are median
(interquartile range) for absolute and relative changes in sdLDL-C.
**p<0.001 vs. baseline.
MACE Risk and Lipid Markers
In the low-dose statin group, the sdLDL-C level at baseline was significantly associated with risk of subsequent MACE (HR quartile 4 vs. 1, 1.76; 95% CI, 1.15–2.71; p for trend=0.009) after adjustment for standard risk factors, eGFR, hemoglobin A1c, hsCRP, and HDL-C at baseline ( Table 3 ) . These associations remained significant even after additional adjustment for LDL-C level (HR quartile 4 vs. 1, 1.67; 95% CI, 1.04–2.68; p for trend=0.034), triglyceride level (HR quartile 4 vs. 1, 1.78; 95% CI, 1.06–2.97; p for trend=0.024) or TRL-C level (HR quartile 4 vs. 1, 1.82; 95% CI, 1.09–3.05; p for trend=0.019). In the high-dose group, the sdLDL-C level was inversely associated with increased risk of MACE in the univariate model (p for trend=0.042). However, this association did not remain significant after adjustment for established risk factors, eGFR, hemoglobin A1c, hsCRP, and HDL-C at baseline ( Table 3 ) .
Table 3. Risk of MACE according to baseline sdLDL-C quartiles in low- (A) and high-dose groups (B).
| Unadjusted | Model 1 |
Model 2 (Model 1 + LDL-C) |
Model 3 (Model 1 + triglycerides) |
Model 4 (Model 1 + TRL-C) |
|
|---|---|---|---|---|---|
| A. Low-dose group | |||||
| sdLDL-C, mg/dL | |||||
| 1st quartile ≤ 19.5 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 2nd quartile > 19.5 to 25.7 | 1.29 (0.87, 1.93) | 1.21 (0.76, 1.94) | 1.18 (0.73, 1.91) | 1.22 (0.75, 1.96) | 1.22 (0.76, 1.98) |
| 3rd quartile > 25.7 to 34.3 | 1.34 (0.90, 1.98) | 1.40 (0.88, 2.24) | 1.34 (0.81, 2.19) | 1.41 (0.87, 2.28) | 1.43 (0.88, 2.31) |
| 4th quartile 34.3< | 1.61 (1.09, 2.37) | 1.76 (1.15, 2.71) | 1.67 (1.04, 2.68) | 1.78 (1.06, 2.97) | 1.82 (1.09, 3.05) |
| p for trend | 0.020 | 0.009 | 0.034 | 0.024 | 0.019 |
| B. High-dose group | |||||
| sdLDL-C, mg/dL | |||||
| 1st quartile ≤ 19.5 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 2nd quartile > 19.5 to 25.7 | 0.67 (0.41, 1.01) | 0.68 (0.41, 1.13) | 0.64 (0.38, 1.09) | 0.68 (0.41, 1.13) | 0.68 (0.41, 1.13) |
| 3rd quartile > 25.7 to 34.3 | 0.69 (0.46, 1.06) | 0.87 (0.54, 1.41) | 0.80 (0.49, 1.33) | 0.88 (0.53, 1.46) | 0.87 (0.51, 1.48) |
| 4th quartile 34.3< | 0.62 (0.41, 0.95) | 0.71 (0.43, 1.17) | 0.64 (0.37, 1.11) | 0.72 (0.40, 1.31) | 0.71 (0.38, 1.33) |
| p for trend | 0.042 | 0.325 | 0.201 | 0.466 | 0.455 |
Abbreviations: eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; hsCRP, high-sensitivity C-reactive protein; LDL-C, low-density lipoprotein cholesterol; MACE, major adverse cardiovascular events; sdLDL-C, small dense low-density lipoprotein cholesterol; TRL-C, triglyceride-rich lipoprotein cholesterol.
Data are hazard ratio (95% confidence interval). Model 1 was adjusted for standard risk factors (i.e., age [≥ 65 and <65 years], male sex, body mass index, systolic blood pressure, heart rate, hypertension, diabetes mellitus, smoking status [current versus not current], myocardial infarction history, percutaneous coronary intervention history, coronary artery bypass grafting history, atrial fibrillation, heart failure, malignant disease, and peripheral artery disease), eGFR, hemoglobin A1c, hsCRP, and HDL-C at baseline. Model 2 was adjusted for model 1 variables and LDL-C. Model 3 was adjusted for model 1 variables and triglycerides. Model 4 was adjusted for model 1 variables and TRL-C.
On the contrary, the level of LDL-C at baseline was not significantly associated with the risk of subsequent MACE in low- and high-dose statin groups ( Supplementary Table 2 ) . The analysis of TRL-C and triglyceride levels and risk of MACE provided qualitatively similar findings to sdLDL-C levels after multivariate adjustment for standard risk factors, eGFR, hemoglobin A1c, hsCRP, and HDL-C at baseline in low-dose statin group, although these were not statistically significant (p for trend=0.088 and 0.091, respectively; Supplementary Table 2 ).
Supplementary Table 2. Risk of MACE according to baseline lipid marker quartiles in low- and high-dose groups.
| Unadjusted | Adjusted | |
|---|---|---|
| A. LDL-C, mg/dL | ||
| Low-dose group | ||
| 1st quartile ≤ 75.2 | 1.00 | 1.00 |
| 2nd quartile > 75.2 to 88.0 | 0.99 (0.68, 1.47) | 1.23 (0.78, 1.93) |
| 3rd quartile > 88.0 to 101.4 | 1.04 (0.71, 1.52) | 1.26 (0. 80, 1.98) |
| 4th quartile 4 > 101.4 | 1.13 (0.78, 1.65) | 1.46 (0.90, 2.38) |
| p for trend | 0.501 | 0.131 |
| High-dose group | ||
| 1st quartile ≤ 75.2 | 1.00 | 1.00 |
| 2nd quartile > 75.2 to 88.0 | 0.78 (0.51, 1.19) | 0.64 (0.39, 1.05) |
| 3rd quartile 3 > 88.0 to 101.4 | 1.00 (0.66, 1.51) | 0.96 (0.60, 1.55) |
| 4th quartile 4 > 101.4 | 0.84 (0.55, 1.27) | 0.85 (0.52, 1.39) |
| p for trend | 0.671 | 0.928 |
| B. Triglycerides, mg/dL | ||
| Low-dose group | ||
| 1st quartile ≤ 90.0 | 1.00 | 1.00 |
| 2nd quartile > 90.0 to 124 | 1.40 (0.95, 2.04) | 1.30 (0.81, 2.05) |
| 3rd quartile > 124 to 175 | 1.47 (1.00, 2.18) | 1.32 (0.84, 2.13) |
| 4th quartile > 175 | 1.59 (1.06, 2.37) | 1.55 (0.93, 2.58) |
| p for trend | 0.024 | 0.091 |
| High-dose group | ||
| 1st quartile ≤ 90.0 | 1.00 | 1.00 |
| 2nd quartile > 90.0 to 124 | 0.75 (0.49, 1.15) | 0.73 (0.43, 1.23) |
| 3rd quartile > 124 to 175 | 1.02 (0.68, 1.54) | 0.94 (0.56, 1.58) |
| 4th quartile > 175 | 0.68 (0.45, 1.05) | 0.64 (0.38, 1.08) |
| p for trend | 0.229 | 0.205 |
| C. TRL-C, mg/dL | ||
| Low-dose group | ||
| 1st quartile ≤ 18.0 | 1.00 | 1.00 |
| 2nd quartile > 18.1 to 24.8 | 1.41 (0.96, 2.07) | 1.31 (0.83, 2.07) |
| 3rd quartile > 24.8 to 35.0 | 1.49 (1.00, 2.20) | 1.34 (0.84, 2.15) |
| 4th quartile > 35.0 | 1.61 (1.09, 2.39) | 1.57 (0.95, 2.59) |
| p for trend | 0.019 | 0.088 |
| High-dose group | ||
| 1st quartile ≤ 18.0 | 1.00 | 1.00 |
| 2nd quartile > 18.1 to 24.8 | 0.74 (0.49, 1.13) | 0.73 (0.43, 1.23) |
| 3rd quartile > 24.8 to 35.0 | 1.00 (0.67, 1.51) | 0.93 (0.55, 1.57) |
| 4th quartile > 35.0 | 0.68 (0.44, 1.04) | 0.65 (0.39, 1.09) |
| p for trend | 0.214 | 0.210 |
Abbreviations: eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; hsCRP, high-sensitivity C-reactive protein; LDL-C, low-density lipoprotein cholesterol; MACE, major adverse cardiovascular events; TRL-C, triglyceride- rich lipoprotein cholesterol.
Data are hazard ratio (95% confidence interval). Multivariable model was adjusted for standard risk factors (i.e., age [≥ 65 and <65 years], male sex, body mass index, systolic blood pressure, heart rate, hypertension, diabetes mellitus, smoking status [current versus not current], myocardial infarction history, percutaneous coronary intervention history, coronary artery bypass grafting history, atrial fibrillation, heart failure, malignant disease, and peripheral artery disease), eGFR, hemoglobin A1c, hsCRP, and HDL-C at baseline.
In the low-dose statin group, the HDL-C level at baseline was associated with the risk of subsequent MACE (HR quartile 4 vs. 1, 0.67; 95% CI, 0.42–1.08; p for trend=0.086) after adjustment for standard risk factors, eGFR, hemoglobin A1c, hsCRP, LDL-C, and triglycerides at baseline. The non-HDL-C level at baseline was significantly associated with risk of subsequent MACE (HR quartile 4 vs. 1, 1.57; 95% CI, 0.99–1.62; p for trend=0.041) after adjustment for standard risk factors, eGFR, hemoglobin A1c, hsCRP, and HDL-C at baseline. On the other hand, the level of sdLDL-C at 6 months and absolute and relative changes from baseline to 6 months in sdLDL-C level were not significantly associated with the risk of MACE among all study patients ( Supplementary Table 3 ) .
Supplementary Table 3. Risk of MACE according to quartiles of sdLDL-C at 6 months and absolute and relative changes from baseline to 6 months in all patients.
| Unadjusted | Adjusted | |
|---|---|---|
| A. sdLDL-C at 6 months, mg/dL | ||
| 1st quartile ≤ 17.6 | 1.00 | 1.00 |
| 2nd quartile > 17.6 to 23.8 | 1.11 (0.83, 1.50) | 1.30 (0.91, 1.85) |
| 3rd quartile > 23.8 to 32.5 | 1.11 (0.83, 1.50) | 1.30 (0.91, 1.86) |
| 4th quartile 4 > 32.5 | 0.90 (0.67, 1.23) | 0.93 (0.62, 1.41) |
| p for trend | 0.541 | 0.758 |
| B. Absolute change from baseline to 6 months in sdLDL-C level, mg/dL | ||
| 1st quartile ≤ -6.9 | 1.00 | 1.00 |
| 2nd quartile > -6.9 to -1.7 | 0.90 (0.66, 1.24) | 0.94 (0.65, 1.34) |
| 3rd quartile > -1.7 to 3.5 | 0.95 (0.70, 1.30) | 0.97 (0.69, 1.38) |
| 4th quartile > 3.5 | 0.90 (0.66, 1.23) | 0.88 (0.61, 1.27) |
| p for trend | 0.599 | 0.549 |
| C. Relative change from baseline to 6 months in sdLDL-C level, % | ||
| 1st quartile ≤ -25.2 | 1.00 | 1.00 |
| 2nd quartile > -25.2 to -7.1 | 0.80 (0.52, 1.25) | 0.73 (0.44, 1.20) |
| 3rd quartile > -7.1 to 15.7 | 1.05 (0.66, 1.66) | 0.86 (0.50, 1.50) |
| 4th quartile > 15.7 | 0.98 (0.58, 1.65) | 0.88 (0.47, 1.65) |
| p for trend | 0.800 | 0.829 |
Abbreviations: eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; hsCRP, high-sensitivity C-reactive protein; MACE, major adverse cardiovascular events; sdLDL-C, small dense low-density lipoprotein cholesterol.
Absolute and relative changes in sdLDL-C level were defined as (sdLDL-C value at 6 months – at baseline) and (absolute change / baseline)×100,
respectively. Data are hazard ratio (95% confidence interval). Multivariable model was adjusted for standard risk factors (i.e., age [≥ 65 and <65 years], male sex, body mass index, systolic blood pressure, heart rate, hypertension, diabetes mellitus, smoking status [current versus not current], myocardial infarction history, percutaneous coronary intervention history, coronary artery bypass grafting history, atrial fibrillation, heart failure, malignant disease, and peripheral artery disease), eGFR, hemoglobin A1c, hsCRP, triglycerides, and HDL-C at 6 months.
MACE Risk and Treatment Groups According to Quartile of Baseline Lipid Markers
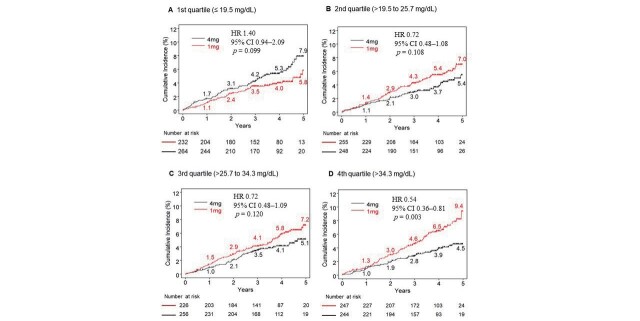
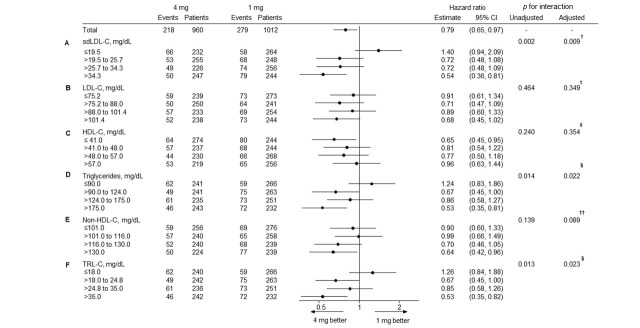
If the findings for low-dose statin therapy were compared to high-dose statin therapy, the latter significantly reduced absolute risk in 5-year cumulative incidence by 4.9% and relative risk by 46% (p=0.003) in patients with 4th quartile (>34.3 mg/dL) sdLDL-C levels at baseline. However, it increased absolute risk in 5-year cumulative incidence by 2.1% and relative risk by 40% (p=0.099) in patients with 1st quartile (≤ 19.5 mg/dL) sdLDL-C levels at baseline and did not alter the MACE risk in patients with 2nd and 3rd quartile of sdLDL-C levels at baseline ( Fig.2 ) . The treatment interaction by sdLDL-C level at baseline was significant (p for interaction=0.002; Fig.3A ), but that by LDL-C ( Fig.3B ) or HDL-C ( Fig.3C ) at baseline was not statistically significant. Similar to sdLDL-C, TRL-C (p for interaction=0.013) and triglycerides (p for interaction=0.014) had a statistically significant effect in patients with 4th quartile levels at baseline. The effect with non-HDL-C (p for interaction=0.139) was not statistically significant at baseline ( Fig.3 ) . In addition, considering the covariate imbalance (HDL-C [p=0.01], triglycerides [p=0.04], and TRL-C levels [p=0.04]) at baseline between the low- and high-dose groups, we assessed effects of high-dose statin therapy on baseline lipid markers by multivariate analyses. However, after multivariable adjustment for standard risk factors, eGFR, hemoglobin A1c, hsCRP, LDL-C, HDL-C, and triglycerides (excluding the subgroup classifier variable), identical results were obtained observed as in univariate analyses ( Fig.3 ) .
Fig.2. Cumulative incidence of MACE stratified by treatment groups in subgroups according to baseline sdLDL-C quartile.
Numbers at risk of respective groups are described at the bottom of the figure.
Fig.3. Risk of MACE and treatment groups by subgroups according to baseline lipid marker quartile.
†adjusted for standard risk factors (i.e., age [≥ 65 and <65 years], male sex, body mass index, systolic blood pressure, heart rate, hypertension, diabetes mellitus, smoking status [current versus not current], myocardial infarction history, percutaneous coronary intervention history, coronary artery bypass grafting history, atrial fibrillation, heart failure, malignant disease, and peripheral artery disease), eGFR, hemoglobin A1c, hsCRP, HDL-C, and triglycerides at baseline. ‡adjusted for standard risk factors, eGFR, hemoglobin A1c, hsCRP, LDL-C, and triglycerides at baseline. §adjusted for standard risk factors, eGFR, hemoglobin A1c, hsCRP, LDL-C, and HDL-C at baseline. ††adjusted for standard risk factors, eGFR, hemoglobin A1c, hsCRP, and HDL-C at baseline.
Discussion
The present case-cohort study obtained the following main findings. First, high-dose statin therapy (4 mg/d pitavastatin) reduced sdLDL-C levels by 20% than low-dose statin therapy (1 mg/d). Second, among patients receiving low-dose statin therapy, the higher sdLDL-C level was associated with an increased risk of MACE independent of LDL-C level. Finally, compared with low-dose statin therapy, high-dose statin therapy caused a greater reduction of MACE risk in patients with the highest sdLDL-C level at baseline. Our findings support evidence from observational studies 7 , 13 - 18) suggesting that sdLDL-C is a risk factor for cardiovascular disease.
Among patients targeted for secondary prevention, the prognostic role of the sdLDL-C level was less investigated than patients targeted for primary prevention. A recent prospective cohort study of 4,148 Chinese patients with stable CAD (mean age 60 years, 63.8% baseline statin use) showed a significant association between sdLDL-C level determined using a simple homogeneous assay and risk of MACE regardless of LDL-C level, for secondary prevention 31) . However, the prognostic role of the sdLDL-C level has not been fully evaluated in statin-treated patients. Thus, we demonstrated the association between sdLDL-C level and risk of MACE, independent of LDL-C level, in statin-treated patients with stable CAD. Furthermore, we found novel evidence for a greater cardiovascular benefit from more intensive statin therapy in patients with stable CAD who had higher sdLDL-C levels. Thus, sdLDL-C may aid clinical decisions regarding whether to implement more intensive statin therapy or not. In fact, Japanese treatment guidelines recommend a therapeutic target of LDL-C <100 mg/dL for secondary prevention 32) . However, the present study suggests that it is clinically important to implement more intensive statin therapy in patients with higher sdLDL-C levels for secondary prevention, even when LDL-C levels drop to optimal levels (<100 mg/dL). Compared with low-dose, high-dose pitavastatin, patients in the lowest (≤ 19.5 mg/dL) baseline sdLDL-C quartile tended to increase MACE risk. Thus, intensive statin therapy may not be necessary to the lowest sdLDL-C subgroup. Moreover, these findings support the hypothesis that intensive lipid-lowering medication in patients with higher sdLDL-C levels effectively reduces cardiovascular risk. Further studies are warranted to determine whether the combination of statins with ezetimibe or proprotein convertase subtilisin/kexin type 9 inhibitors may lead to additional benefits based on baseline sdLDL-C level.
On the analysis of sdLDL-C level at 6 months and absolute and relative changes from baseline to 6 months in sdLDL-C level, all these variables were not significantly associated with subsequent MACE risk. In the present study, there was no placebo arm and all study patients achieved LDL-C levels <120 mg/dL with 1 mg/d pitavastatin for at least 1 month before randomization. Also, statin intensity in the high-dose statin group (4 mg/d pitavastatin) is generally considered moderate-intensity statin therapy. Furthermore, in the present study, high-dose statin therapy’s effect on sdLDL-C levels was evaluated at 6 months. However, this period might be too short to determine the high-dose statin therapy effect on sdLDL-C levels. These factors may make it difficult to evaluate these variables’ prognostic ability for MACE risk.
In the high-dose statin group, the sdLDL-C level at baseline was inversely associated with subsequent cardiovascular risk in the univariate Cox proportional hazard model with Barlow’s methods. Regarding the baseline characteristics of the random cohort, eGFR increased significantly with a higher sdLDL-C at baseline. Conversely, age and frequency of atrial fibrillation decreased significantly with higher sdLDL-C levels at baseline, possibly because of statin-treated patients with stable CAD. These confounding factors may lead to the inverse association of sdLDL-C level with cardiovascular risk in the high-dose group. This could be why there was no significant association between the sdLDL-C level at baseline and cardiovascular risk in the multivariable model. Additionally, LDL-C was an established risk factor 1 - 4) . However, the present study did not show a significant association between LDL-C level and MASE risk because of statin-treated patients.
Previous studies have suggested that sdLDL-C lowering effects of statin treatment may be due to the decrease in the LDL-C level rather than their effects on LDL particle size 19 - 21) . In the present study, although the high-dose statin therapy reduced sdLDL-C level by 20%, when compared with low-dose statin therapy, it brought about no change in the percentage of sdLDL-C in LDL-C from baseline to 6 months. Thus, high-dose statin therapy may not have any additional effect on LDL particle size compared with low-dose statin therapy. While lower triglyceride levels are generally expected to increase the size of LDL particles 19 - 21) , the mild (but significant) reduction in triglycerides (8 mg/dL) by high-dose compared with low-dose pitavastatin in the present study might be insufficient to bring about this effect. In phase 2 trials, in the case of subjects with residual dyslipidemia (high triglycerides and non-HDL-C levels) on background statin therapy, a novel selective peroxisome proliferator-activated receptor alpha modulator—pemafibrate—shifted the distribution of LDL particle size to larger LDL particles, with a 20% reduction in sdLDL-C level 17 , 33) . Whether the combination of statins with pemafibrate may lead to additional benefits, based on baseline sdLDL-C levels, requires further research.
There are some limitations to our study. The study population enrolled only Japanese statin-treated patients with stable CAD. Therefore, our results may not generally apply to other populations. Also, there was no placebo arm, and all patients were treated with 1 mg/d pitavastatin for at least 1 month before randomization, which has known effects on lipid markers such as sdLDL-C level. Furthermore, the high-dose statin group (4 mg/d pitavastatin) is generally considered moderate-intensity statin therapy 22 , 23) . This may have attenuated the changes in these lipid markers. In addition, high-intensity statin therapy (e. g. 80 mg/d atorvastatin) is not covered by Japanese national health insurance and is thus seldom used 22 , 23) .
Conclusion
The sdLDL-C level, determined using a simple homogeneous assay, was associated with cardiovascular risk independent of LDL-C in statin-treated CAD patients. Notably, high-dose statin therapy reduced this risk in those with the highest baseline sdLDL-C level. Thus, sdLDL-C can be a useful risk marker and a predictive marker for the therapeutic benefit of intensive statin therapy. The present results provide the rationale for future studies of intensive lipid-lowering medication for secondary prevention, based on baseline sdLDL-C level.
Acknowledgements
The authors thank all patients and investigators who participated in this study; Associate professor Dr Kenichi Aizawa and other members of the Division of Clinical Pharmacology, Department of Pharmacology, Jichi Medical University for their work on stored serum samples; and Yoji Mitadera, Yuka Nakajima, and other members of the Public Health Research Foundation for their administrative assistance.
Sources of Funding
The Randomized Evaluation of Aggressive or Moderate Lipid-Lowering Therapy with Pitavastatin in Coronary Artery Disease (REAL-CAD) study was funded by the Comprehensive Support Project for Clinical Research of Lifestyle-Related Disease of the Public Health Research Foundation. The company manufacturing the study drug (Kowa Pharmaceutical Co. Ltd.) was one of the entities providing financial support for Public Health Research Foundation projects but was not involved in the design, analysis, data interpretation, or manuscript preparation. This work was supported by JSPS KAKENHI (21K07403).
Conflicts of Interest
Dr J. Ishii received research grant from Sysmex Corp. and LSI Medience Corp., and honoraria from Siemens Healthineers Japan, and LSI Medience Corp.
Dr Kashiwabara reports no conflicts.
Dr Ozaki received research grants from Takeda Pharmaceutical Co. Ltd., Sanofi KK, Mitsubishi Tanabe Pharma Corp., Otsuka Pharmaceutical Co. Ltd., Bayer Yakuhin, Ltd., Daiichi Sankyo Co. Ltd., Sumitomo Dainippon Pharma Co. Ltd., and Public Health Research Foundation.
Mr Takahashi, Mr Kitagawa, and Dr Nishimura report no conflicts.
Dr H. Ishii received honoraria from Astellas Pharma Inc., AstraZeneca KK, Bayer Yakuhin, Ltd., Chugai Pharmaceutical Co. Ltd., Daiichi Sankyo Co. Ltd., and MSD KK.
Dr Iimuro, Dr Kawai, Dr Muramatsu, and Dr Naruse report no conflicts.
Dr Iwata received honoraria from Kowa Pharmaceutical Co. Ltd.
Dr Tanizawa-Motoyama reports no conflicts.
Dr Ito received research fundings from Hitachi High-Tech Coropration and Kawasaki Heavy Industries, Ltd.
Dr Watanabe received honoraria from Daiichi Sankyo Co. Ltd. and Biotronik Japan.
Dr Matsuyama reports no conflicts.
Dr Fukumoto received research fundings from Actelion Pharmaceuticals Japan Inc., Alnylam Phramaceuticals Inc., Kyowa Kirin Co. Ltd., Daiichi Sankyo Co. Ltd., and Nippon Shinyaku Co. Ltd., and scholarship grants from Astellas Pharma Inc., Abbott Medical Japan LLC, Eisai Co. Ltd., MSD KK, Takeda Pharmaceutical Co. Ltd., Teijin Pharma Ltd., Japan Lifeline Co. Ltd., Bayer Yakuhin, Ltd., and Mochida Pharmaceutical Co. Ltd., and honoraria from Actelion Pharmaceuticals Japan Inc., AstraZeneca KK, Otsuka Pharmaceutical, Co. Ltd., Ono Pharmaceutical Co. Ltd., Kowa Pharmaceutical, Co. Ltd., Daiichi Sankyo Co. Ltd. TOA EIYO LTD., Nippon Boehringer Ingelheim Co. Ltd., Novartis Pharma KK, Bayer Yakuhin, Ltd., and Janssen Pharmaceutical KK.
Dr Sakuma received research fundings from National Cerebral and Cardiovascular Center and Medical Informatics Study Group, Takeda Pharmaceutical Co. Ltd., GlaxoSmithKline KK, AstraZeneca KK, and Boehringer Ingelheim Japan Inc.
Dr Nakagawa received honoraria from Kowa Pharmaceutical Co. Ltd., Takeda Pharmaceutical Co. Ltd., Bayer Yakuhin, Ltd., Sanofi KK, Daiichi Sankyo Co. Ltd., Shionogi & Co. Ltd., Astellas Pharma Inc., MSD KK, Mitsubishi Tanabe Pharma Corp., Sumitomo Dainippon Pharma Co. Ltd., AstraZeneca KK, Amgen Astellas BioPharma KK, Eisai Co. Ltd., Otsuka Pharmaceutical Co. Ltd., and Pfizer Japan Inc.
Dr Hibi received honoraria from Kowa Pharmaceutical Co. Ltd.
Dr Hiro received research fundings from Astellas Pharma Inc., Eisai Co. Ltd, MSD KK, Otsuka Pharmaceutical Co. Ltd., Sanofi KK, Shionogi & Co. Ltd., Daiichi Sankyo Co. Ltd, Sumitomo Dainippon Pharma Co. Ltd., Takeda Pharmaceutical Co. Ltd., Mitsubishi Tanabe Pharma Corp., Bayer Yakuhin, Ltd., Pfizer Japan Inc., and AstraZeneca KK, and a course (Department of Advanced Cardiovascular Imaging, Nihon University School of Medicine) endowed by Boston-Scientific Japan Co. Ltd.
Dr Hokimoto reports no conflicts.
Dr Miyauchi received honoraria from Sanofi KK, Daiichi Sankyo Co. Ltd., Amgen Astellas BioPharma KK, Bayel Yakuhin, Ltd., Bristol-Meyers Squibb Co. Ltd., and MSD KK.
Dr Ohtsu reports no conflicts.
Dr Izawa received research grants from Takeda Pharmaceutical Co. Ltd., Shionogi & Co. Ltd., Otsuka Pharmaceutical Co. Ltd., Pfizer Japan Inc., Teijin Pharma Ltd., Ono Pharmaceutical Co. Ltd., Mochida Pharmaceutical Co. Ltd., and Daiichi Sankyo Co. Ltd. and honoraria from Otsuka Pharmaceutical Co. Ltd. and Daiichi Sankyo Co. Ltd.
Dr Ogawa reports no conflicts.
Dr Daida received honoraria from Amgen Astellas BioPharma KK, Daiichi Sankyo Co. Ltd., Kowa Pharmaceutical Co. Ltd., and MSD KK, research grant from Canon Medical Systems Corp., and scholarship grant from Otsuka Pharmaceutical Co. Ltd., Sanofi KK, MSD KK, Daiichi Sankyo Co. Ltd., Pfizer Japan Inc., Mitsubishi Tanabe Pharma Corp., Astellas Pharma Inc., Takeda Pharmaceutical Co. Ltd., Teijin Pharma Ltd., Shionogi & Co. Ltd., Actelion Pharmaceuticals Ltd., Kowa Pharmaceutical Co. Ltd., Bayer Yakuhin, Ltd., Boehringer Ingelheim Japan Inc., and courses endowed by Philips Japan Inc., Resmed Japan, Fukuda Denshi Co. Ltd., Asahi Kasei Corp., Inter Reha Co. Ltd., and Toho Holdings Co. Ltd.
Dr. Shimokawa and Dr Saito report no conflicts.
Dr Kimura received research grants from Astellas Pharma Inc., Edwards Lifesciences Corp., Pfizer Japan Inc., Kowa Company, Limited, DAIICHI SANKYO ESPHA CO. LTD., EP-CRSU Co. Ltd. and honoraria from Abbott Medical Japan LLC, Kowa Company, Limited, Bristol Myers Squibb, and Boston Scientific Corp.
Dr Matsuzaki reports no conflicts
Dr Nagai received a research funding from Kowa Pharmaceutical Co. Ltd. and a scholarship grant from Tanaka Industry.
References
- 1).Wilson PWF, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, and Kannel WB: Prediction of coronary heart disease using risk factor categories. Circulation, 1998; 97: 1837-1847 [DOI] [PubMed] [Google Scholar]
- 2).Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, and Simes R: For the Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet, 2005; 366: 1267-1278 [DOI] [PubMed] [Google Scholar]
- 3).Nakamura H, Arakawa K, Itakura H, Kitabatake A, Goto Y, Toyota T, Nakaya N, Nishimoto S, Muranaka M, Yamamoto A, Mizuno K, and Ohashi Y: For the MEGA study group. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet, 2006; 368: 1155-1163 [DOI] [PubMed] [Google Scholar]
- 4).Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, and Collins R: For the Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet, 2010; 376: 1670-1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Mora S, Wenger NK, Demicco DA, Breazna A, Boekholdt SM, Arsenault BJ, Deedwania P, Kastelein JJ, and Waters DD: Determinants of residual risk in secondary prevention patients treated with high- versus low-dose statin therapy: the Treating to New Targets (TNT) study. Circulation, 2012; 125: 1979-1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Sachdeva A, Cannon CP, Deedwania PC, Labresh KA, Smith SC Jr, Dai D, Hernandez A, and Fonarow GC: Lipid levels in patients hospitalized with coronary artery disease: an analysis of 136,905 hospitalizations in Get With The Guidelines. Am Heart J, 2009; 157: 111-117 [DOI] [PubMed] [Google Scholar]
- 7).Hoogeveen RC, Gaubatz JW, Sun W, Dodge RC, Crosby JR, Jiang J, Couper D, Virani SS, Kathiresan S, Boerwinkle E, and Ballantyne CM: Small dense low-density lipoprotein-cholesterol concentrations predict risk for coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) study. Arterioscler Thromb Vasc Biol, 2014; 34: 1069-1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).de Graaf J, Hak-Lemmers HL, Hectors MP, Demacker PN, Hendriks JC, and Stalenhoef AF: Enhanced susceptibility to in vitro oxidation of the dense low-density lipoprotein subfraction in healthy subjects. Arterioscler Thromb Vasc Biol, 1991; 11: 298-306 [DOI] [PubMed] [Google Scholar]
- 9).Chancharme L, Therond P, Nigon F, Lepage S, Couturier M, and Chapman MJ: Cholesteryl ester hydroperoxide lability is a key feature of the oxidative susceptibility of small, dense LDL. Arterioscler Thromb Vasc Biol, 1999; 19: 810-820 [DOI] [PubMed] [Google Scholar]
- 10).Griffin BA: Lipoprotein atherogenicity: an overview of current mechanisms. P Nutr Soc, 199; 58: 163-169 [DOI] [PubMed] [Google Scholar]
- 11).Berneis KK, and Krauss RM: Metabolic origins and clinical significance of LDL heterogeneity. J Lipid Res, 2002; 43: 1363-1379 [DOI] [PubMed] [Google Scholar]
- 12).Brunzell JD: Increased ApoB in small dense LDL particles predicts premature coronary artery disease. Arterioscler Thromb Vasc Biol, 2005; 25: 474-475 [DOI] [PubMed] [Google Scholar]
- 13).Arai H, Kokubo Y, Watanabe M, Sawamura T, Ito Y, Minagawa A, Okamura T, and Miyamato Y: Small dense low-density lipoproteins cholesterol can predict incident cardiovascular disease in an urban Japanese cohort: the Suita Study. J Atheroscler Thromb, 2013; 20: 195-203 [DOI] [PubMed] [Google Scholar]
- 14).Tsai MY, Steffen BT, Guan W, McClelland RL, Warnick R, McConnell J, Hoefner DM, and Remaley AT: New automated assay of small dense low-density lipoprotein cholesterol identifies risk of coronary heart disease: the multi-ethnic study of atherosclerosis. Arterioscler Thromb Vasc Biol, 2014; 34: 196-201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Higashioka M, Sakata S, Honda T, Hata J, Yoshida D, Hirakawa Y, Shibata M, Goto K, Kitazono T, Osawa H, and Ninomiya T: Small dense low-density lipoprotein cholesterol and the risk of coronary heart disease in a Japanese community. J Atheroscler Thromb, 2020; 27: 669-682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Balling M, Nordestgaard BG, Langsted A, Varbo A, Kamstrup PR, and Afzal S: Small dense low-density lipoprotein cholesterol predicts atherosclerotic cardiovascular disease in the Copenhagen General Population Study. J Am Coll Cardiol, 2020; 75: 2873-2875 [DOI] [PubMed] [Google Scholar]
- 17).Duran EK, Aday AW, Cook NR, Buring JE, Ridker PM, and Pradhan AD: Triglyceride-rich lipoprotein cholesterol, small dense LDL cholesterol, and incident cardiovascular disease. J Am Coll Cardiol, 2020; 75: 2122-2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Ikezaki H, Lim E, Cupples LA, Liu CT, Asztalos BF, and Schaefer EJ: Small dense low-density lipoprotein cholesterol is the most atherogenic lipoprotein parameter in the prospective Framingham offspring study. J Am Heart Assoc, 2021; 10: e019140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Tokuno A, Hirano T, Hayashi T, Mori Y, Yamamoto T, Nagashima M, Shiraishi Y, Ito Y, and Adachi M: The effects of statin and fibrate on lowering small dense LDL- cholesterol in hyperlipidemic patients with type 2 diabetes. J Atheroscler Thromb, 2007; 14: 128-132 [DOI] [PubMed] [Google Scholar]
- 20).Ai M, Otokozawa S, Asztalos BF, Nakajima K, Stein E, Jones PH, and Schaefer EJ: Effects of maximal doses of atorvastatin versus rosuvastatin on small dense low-density lipoprotein cholesterol levels. Am J Cardiol, 2008; 101: 315-318 [DOI] [PubMed] [Google Scholar]
- 21).Thongtang N, Diffenderfer MR, Ooi EMM, Barrett PHR, Turner SM, Le NA, Brown WV, and Schaefer EJ: Metabolism and proteomics of large and small dense LDL in combined hyperlipidemia: effects of rosuvastatin. J Lipid Res, 2017; 58: 1315-1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Miyauchi K, Kimura T, Shimokawa H, Daida H, Iimuro S, Iwata H, Ozaki Y, Sakuma I, Nakagawa Y, Hibi K, Hiro T, Fukumoto Y, Hokimoto S, Ohashi Y, Ohtsu H, Saito Y, Matsuzki M, and Nagai R: For the REAL-CAD trial investigators. Rationale and design of randomized evaluation of aggressive or moderate lipid lowering therapy with pitavastatin in coronary artery disease (REAL-CAD) trial. Int Heart J, 2018; 59: 315-320 [DOI] [PubMed] [Google Scholar]
- 23).Taguchi I, Iimuro S, Iwata H, Takashima H, Abe M, Amiya E, Ogawa T, Ozaki Y, Sakuma I, Nakagawa Y, Hibi K, Hiro T, Fukumoto Y, Hokimoto S, Miyauchi K, Yamazaki T, Ito H, Otsuji Y, Kimura K, Takahashi J, Hirayama A et al.: High-dose versus low-dose pitavastatin in Japanese patients with stable coronary artery disease (REAL-CAD): a randomized superiority trial. Circulation, 2018; 137: 1997-2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Waters DD, Guyton JR, Herrington DM, McGowan MP, Wenger NK, and Shear C: Treating to New Targets (TNT) Study: does lowering low-density lipoprotein cholesterol levels below currently recommended guidelines yield incremental clinical benefit? Am J Cardiol, 2004; 93: 154-158 [DOI] [PubMed] [Google Scholar]
- 25).LaRosa JC, Grundy SM, Waters DD, Shear C, Barter P, Fruchart JC, Gotto AM, Greten H, Kastelein JJ, Shepherd J, and Wenger NK: Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med, 2005; 352: 1425-1435 [DOI] [PubMed] [Google Scholar]
- 26).Ito Y, Fujimura M, Ohta M, and Hirano T: Development of a homogeneous assay for measurement of small dense LDL cholesterol. Clin Chem, 2011; 57: 57-65 [DOI] [PubMed] [Google Scholar]
- 27).Friedewald WT, Levy RI, and Fredrickson DS: Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem, 1972; 18: 499-502 [PubMed] [Google Scholar]
- 28).Jones SR, Martin SS, and Brinton EA: Letter by Jones et al regarding article, “Elevated remnant cholesterol causes both low-grade inflammation and ischemic heart disease, whereas elevated low-density lipoprotein cholesterol causes ischemic heart disease without inflammation.” Circulation, 2014; 129: e655 [DOI] [PubMed] [Google Scholar]
- 29).Vallejo-Vaz AJ, Fayyad R, Boekholdt SM, Hovingh GK, Kastelein JJ, Melamed S, Barter P, Waters DD, and Ray KK: Triglyceride-rich lipoprotein cholesterol and risk of cardiovascular events among patients receiving statin therapy in the TNT trial. Circulation, 2018; 138: 770-781 [DOI] [PubMed] [Google Scholar]
- 30).Barlow WE, Ichikawa L, Rosner D, and Izumi S: Analysis of case-cohort designs. J Clin Epidemiol, 1999; 52: 1165-1172 [DOI] [PubMed] [Google Scholar]
- 31).Jin JL, Zhang HW, Cao YX, Liu HH, Hua Q, Li YF, Zhang Y, Wu NQ, Zhu CG, Xu RX, Gao Y, Li XL, Cui CL, Liu G, Sun J, Dong Q, Guo YL, and Li JJ: Association of small dense low-density lipoprotein with cardiovascular outcome in patients with coronary artery disease and diabetes: a prospective, observational cohort study. Cardiovasc Diabetol, 2020; 19: 45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).Kinoshita M, Yokote K, Arai H, Iida M, Ishigaki Y, Ishibashi S, Umemoto S, Egusa G, Ohmura H, Okamura T, Kihara S, Koba S, Saito I, Shoji T, Daida H, Tsukamoto K, Deguchi J, Dobashi K, Hamaguchi et al: For the committee for epidemiology and clinical management of atherosclerosis. Japan Atherosclerosis Society (JAS) guidelines for prevention of atherosclerotic cardiovascular diseases 2017. J Atheroscler Thromb, 2018; 25: 846-984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Arai H, Yamashita S, Yokote K, Araki E, Suganami H, and Ishibashi S: For the K-877 study group. Efficacy and safety of K-877, a novel selective peroxisome proliferator-activated receptor α modulator (SPPARMα), in combination with statin treatment: two randomised, double-blind, placebo-controlled clinical trials in patients with dyslipidaemia. Atherosclerosis, 2017; 261: 144-152 [DOI] [PubMed] [Google Scholar]