Abstract
PCR mutagenesis and a unique enrichment scheme were used to obtain two mutants, each with a single lesion in fimH, the chromosomal gene that encodes the adhesin protein (FimH) of Escherichia coli type 1 pili. These mutants were noteworthy in part because both were altered in the normal range of cell types bound by FimH. One mutation altered an amino acid at a site previously shown to be involved in temperature-dependent binding, and the other altered an amino acid lining the predicted FimH binding pocket.
Type 1 pili are filamentous proteinaceous appendages produced by many members of the family Enterobacteriaceae. In Escherichia coli, the biosynthesis and binding properties of these pili have been well studied (reviewed in reference 30). Pili are made principally of a repeating monomer, FimA, the product of the fimA gene (13), that is arrayed helically to form a hollow-cored fiber (2). There are at least three minor pilus proteins that are organized into structures seen on the ends of pili (12) and may also be present in the pilus fiber (22). One of these minor components, FimH, the product of the fimH gene, is the molecule that actually binds to mannose-containing receptors on eucaryotic cells (14). The precise nature of the affinity of FimH for mannose is unclear. However, it has been known for some time that different arrangements of mannose monomers and substituent groups affect the affinity of FimH for these substrates (5). It is also known that other pilus components, ostensibly interacting with FimH, also affect the specificity of the interaction of FimH with mannose (16).
In this study, we have identified fimH mutants with changes in binding specificity. One especially novel feature of both mutants is the ability to bind and agglutinate yeast cells at parental levels but failed to bind macrophages any better than a fimH insertion mutant.
Bacterial strains, plasmids, and growth conditions.
The bacterial strains, which were all E. coli K-12 derivatives, and plasmids used are listed in Table 1. Media consisted of L broth, L agar (18), and maltose-tetrazolium agar (27). Antibiotic concentrations were as described previously (20).
TABLE 1.
Bacterial strains, phage, and plasmids used in the study
| Strain, phage, or plasmid | Description | Source or reference |
|---|---|---|
| E. coli | ||
| ORN115 | thr-1 leuB thi-1 Δ(argF-lac)U169 xyl-7 ara-13 mtl-2 gal-6 rpsL tonA2 supE44 pilG1 λr Pil+ (does not exhibit phase variation of piliation) | 31 |
| ORN174 | thr leu proA2 lacY1 galK his argE rpsL supE mtl xyl recBC sbcB tetR (inserted ca. 200 bp 3′ of the end of fimH) Pil+ | 24 |
| ORN178 | ORN115 except tetR inserted ca. 200 bp 3′ of the end of fimH, Pil+ | 24 |
| ORN201 | thr-1 leuB thi-1 Δ(argF-lac)U169 xyl-7 ara-13 mtl-2 gal-6 rpsL tonA2 supE44 pilG1 λrrecA13 Δ(fimBEACDEFGH) | 9 |
| ORN207 | ORN174 except fimH304::kan | Linear transformation of ORN174 with EcoRI-digested pORN164a |
| ORN208b | ORN115 except fimH304::kan with adjacent tetR, Nalr | P1 transduction from ORN207 to ORN115 and selection of a nalidixic acid-resistant variant |
| ORN209 | ORN115 except fimH165 allele and adjacent tetR gene | This study |
| ORN210 | ORN115 except fimH166 allele and adjacent tetR gene | This study |
| Bacteriophage | ||
| P1 | vir | Laboratory collection |
| Plasmids | ||
| pBR322 | ColE1 Apr Tcr | 1 |
| pKAS32c | oriR6K oriT rpsL Apr | 28 |
| pORN163d | pBR322 fimH Apr | Insertion of a 2-kb PvuII fragment containing fimH from pORN127 (17) into BamHI-cleaved pBR322 |
| pORN307 | pSH2 ΔfimH Cmr | 8 |
| pORN164 | pORN304 fimH304::kan, has kan gene from Tn5 inserted in the XhoI site created as part of deletion in fimH304 | Kan cassette inserted into XhoI site of pORN304 (8) |
| pORN165 | pORN163 except fimH165 | This study |
| pORN166 | pORN163 except fimH166 | This study |
Linear transformation and P1 transduction have been previously described (21).
Strain ORN208 was used as the recipient in a mating and allelic exchange protocol (28) that introduced mutant fimH alleles into the chromosome, replacing the fimH insertion mutation.
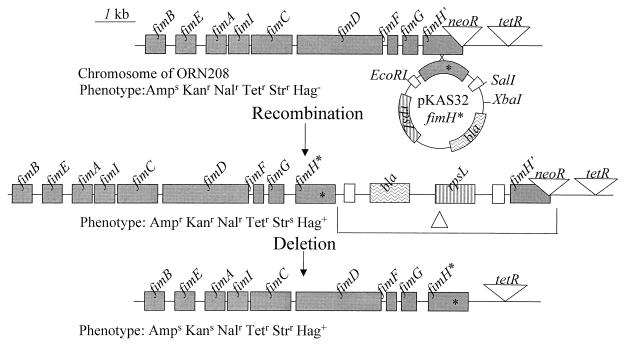
Introduction of the fimH mutant alleles into the chromosome was accomplished by first subcloning each mutant fimH allele carried on the ca. 1.8–kb SalI-EcoRI fragment of pORN163 into SalI-EcoRI-digested pGEM11ZF (Promega) and then removing an EcoRI-XbaI fragment containing the fimH allele. (The new restriction endonuclease sites on the ends of the fimH alleles were contributed by the polylinker in pGEM11ZF.) EcoRI-XbaI fragments containing the fimH alleles were introduced into EcoRI-XbaI-cleaved pKAS32 and then introduced into the chromosome of strain ORN208 by allelic exchange (28). Refer to Fig. 1 for a diagram.
PCR-generated mutant fimH genes were obtained by amplification using primers 5′GGGTTATTGTCTCATGAGCG and 5′CCGATCATCGTCGCGCTCCA flanking EcoRI and SalI sites in pBR322. PCR amplicons were digested with EcoRI and SalI, and the SalI-EcoRI fragments were isolated and ligated into SalI-EcoRI-cleaved pBR322. This ligation mixture was introduced into strain LE392 (19) by electroporation (25). Following electroporation, electroporant colonies were harvested and the plasmid DNA was extracted (4) and introduced into strain ORN201 harboring pORN307, as described in the text.
Receptor specificity mutant isolation.
Plasmid pORN163, containing the fimH gene flanked by the EcoRI and SalI restriction endonuclease sites, was used as a template for generating fimH PCR amplicons with a high proportion of mutations. Amplicons, obtained after 30 amplification cycles using a threefold-higher concentration of MgCl2 than called for by standard PCR conditions (32), were ligated into EcoRI- and SalI-cleaved pBR322. This mutant amplicon pool was subsequently introduced (by transformation [15]) into a Δfim strain (ORN201) containing a plasmid, pORN307, carrying all the genes for fimbriation except fimH. In a typical experiment, approximately 8,000 transformant colonies were pooled and subjected to enrichment for receptor specificity mutants (defined as those mutants that bound guinea pig erythrocytes in the presence of either of two inhibitors: 250 mM fructose or 0.25 mM p-nitrophenyl α-d-manno-pyranoside [N-phenyl mannose]). Enrichment was accomplished by mixing 0.5 ml of the transformant pool (ca. 5 × 108 cells) with 100 μl of settled guinea pig erythrocytes in phosphate-buffered saline (PBS) containing an inhibitor. After a 10-min room temperature incubation, erythrocytes were isolated by a 1-s centrifugation in a microcentrifuge and the supernatant was aspirated. The pellet was gently resuspended in 1.0 ml of PBS containing an inhibitor, and the erythrocytes were reisolated by centrifugation. After five more washing steps, the pelleted erythrocytes were resuspended in 1.0 ml of distilled water and diluted with 1.5 ml of L broth containing chloramphenicol and ampicillin, and the erythrocyte-bound population was expanded by overnight growth (with shaking) at 37°C. A second and third enrichment were typically employed. At the end of the procedure, broth cultures were streaked for colony isolation on L agar plates containing chloramphenicol and ampicillin. Individual colonies were screened for the ability to agglutinate erythrocytes in the presence of an inhibitor (10). Plasmids bearing candidate mutant fimH alleles were isolated (4) and reintroduced into strain ORN201 harboring pORN307 by transformation to confirm that the fimH-containing plasmid conferred the altered binding phenotype. One mutant per experiment was kept to ensure independent origin.
Characterization of the plasmid-borne alleles and introduction of fimH mutant alleles into the chromosome.
Twenty-five plasmid-borne fimH specificity mutants were initially isolated (15 using fructose and 10 using N-phenyl mannose). Nineteen of the fimH alleles conferring the strongest phenotypes were completely sequenced employing the methods of Russell and Orndorff (24). Seventeen of the alleles were unique, but 13 of the 17 had more than one mutation in the fimH coding sequence. Six of the plasmid-borne mutant alleles that had the fewest mutations were cloned into pKAS32 and introduced into the chromosome of ORN208 by allelic exchange (28) (diagrammed in Fig. 1).
FIG. 1.
Diagram of allelic exchange between a mutant fimH allele (fimH∗) carried on the nonreplicating vector pKAS32 and the fim region on the chromosome of strain ORN208. Following introduction of the plasmid by mating (28), recombinants were selected and scored for the phenotypic characteristics noted. Recombinants that had deletions (Δ) of the vector and the original fimH insertion mutation in ORN208 were identified by selection on streptomycin-containing agar and scored for the appropriate antibiotic and hemagglutination phenotype as indicated. Each fimH lesion introduced was more than 98% linked (by P1 transduction [18]) to the tetR insertion adjacent to fimH. Phenotypic designations denote sensitivity (s) or resistance (r) to antibiotics as follows: Nal, nalidixic acid; Kan, kanamycin; Tet, tetracycline; Amp, ampicillin; Str, streptomycin. Erythrocyte or yeast cell agglutination is collectively denoted phenotypically as Hag. Streptomycin sensitivity is conferred by the rpsL gene, kanamycin resistance is conferred by the neoR gene, ampicillin resistance is conferred by the bla gene, and tetracycline resistance is conferred by the tetR gene.
Interestingly, only two of the six fimH mutant alleles (both identified by enrichment in 250 mM fructose) conferred an altered binding specificity phenotype when chromosomally located even though all six of the chromosomal alleles were confirmed to have sequences identical to the starting plasmid-encoded alleles (as indicated by the sequencing of PCR-generated amplicons of the entire fimH gene; UNC Automated DNA Sequencing Facility, Chapel Hill, N.C.). The rest of the mutants produced either the parental phenotype (one of six) or a null binding phenotype (three of six) with regard to yeast cell and erythrocyte agglutination.
Altered phenotypes conferred by the two chromosomal fimH mutant alleles.
Both of the fimH chromosomal mutations that conferred altered binding specificities had single missense mutations predicted to cause an amino acid substitution within the first half of the mature FimH protein (alleles fimH165 and fimH166 from strains ORN209 and ORN210, respectively) (Table 2). Electron-microscopic examination using the methods of Hamrick et al. (8) revealed that strains ORN209 and ORN210 were similar to the parental strain in terms of pilus number per cell and in pilus morphology (data not shown). Also, they were similar to the parent in terms of the ability to agglutinate yeast cells in simple titration tests (Table 2). In contrast to the parent, the mutants retained approximately 50% of this ability in the presence of either of the two inhibitors used to initially isolate the mutants (Table 2). Both mutants also showed a somewhat reduced level of FimH cross-reacting material in antiserum agglutination reactions and a reduced ability to agglutinate guinea pig erythrocytes, and they were unable to bind to macrophages any better than a fimH insertion mutant (Table 2).
TABLE 2.
Summary of chromosomal fimH lesions and phenotypes associated with strains derived from the enrichment procedurea
| Strain | fimH allele | Lesion location (nucleotideb) | Amino acid change (positionc) | Agglutination and binding reactions (% of value for parental strain)d
|
Yeast agglutination (% of titer) in PBSe
|
||||
|---|---|---|---|---|---|---|---|---|---|
| FimH antiserum agglutination | Macrophage binding | Erythrocyte agglutination | Yeast agglutination | 0.25 mM N-phenyl mannose | 250 mM fructose | ||||
| ORN178 | Parental | None | None | (100) | (100) | (100) | (100) | 0 ± 0 | 0 ± 0 |
| ORN208 | fimH308′-kan | 836–935 deletion | Δ(Arg–Gln) (258–279) | (0) | 9.0 ± 3.8 | (0) | (0) | NA | NA |
| ORN209 | fimH165 | 236 T→C | Leu→Pro (58) | 58 ± 22 | 7.8 ± 3.8 | 44 ± 9 | 100 ± 20 | 37 ± 5 | 41 ± 2 |
| ORN210 | fimH166 | 488 T→C | Phe→Ser (142) | 68 ± 30 | 13.0 ± 5.7 | 44 ± 9 | 105 ± 13 | 53 ± 6 | 58 ± 20 |
Presence of FimH in mutant strains was determined by immunological and functional criteria. Polyclonal antiserum to a LacZ-FimH fusion protein was employed in bacterial agglutination reactions using serially diluted antiserum (8). Functional criteria for the presence of FimH consisted of measuring the ability of strains to agglutinate guinea pig erythrocytes and yeast as described by Hamrick et al. (8) with the following modifications. Flat-bottomed 96-well plates were used, and the bacteria and erythrocytes were gently mixed in order to effect agglutination, which took place within a few minutes. Yeast agglutination reactions employed 25 mg of baker's yeast/ml in PBS in place of erythrocytes.
Refers to the position for E. coli K-12 fimH in GenBank. Our parental allele, while not originally from E. coli K-12, has an identical sequence.
Follows the convention of Sokurenko et al. (29) where the 279 amino acids of the mature FimH protein are numbered sequentially from 1 to 279.
In the case of agglutination reactions, the log2 of the highest bacterial or antiserum dilution in which agglutination was witnessed was calculated so as to produce a linear scale of agglutination values which were normalized to the value for the parental strain and expressed as a percentage. Values in parentheses indicate defined values. The insertion mutant (strain ORN208) showed no agglutination titer under any conditions. All mutant values were statistically significantly different from the parental values but not from each other, as determined in the two-tailed unpaired t test as previously described (8). The assay for the ability of type 1 piliated E. coli to bind BALB/c mouse resident peritoneal macrophages has been described previously (8). P1 transduction was used to introduce the appropriate fimH mutant alleles into the genetically marked strains used in the assay. Values are expressed as means ± standard deviations.
The log2 of the highest dilution of bacteria in which agglutination was witnessed in the presence of an inhibitor was calculated so as to produce a linear scale of agglutination values, which were normalized to the agglutination titer in the absence of inhibitor and expressed as a percentage. Both inhibitors completely blocked erythrocyte and yeast agglutination by the parental strain even at the lowest dilution of bacteria. All mutant values were statistically significantly different from the parental values but not from each other. Methods for determining the statistical significance of mean differences were as previously described (8). NA, not applicable. Values are expressed as means ± standard deviations.
Conclusions.
Our results describe the utilization of mannose analogs to enrich mutants with different FimH binding specificities. The isolated mutants retained yeast cell binding activity in the presence of normally inhibitory mannose analogs and were additionally altered in the range of cell types they normally bound. Most striking was the complete loss of macrophage binding and the contrasting retention of parental levels of yeast cell agglutination.
Neither of the two single-site fimH mutations described here (fimH165 and fimH166) have been previously reported. However, the lesion at amino acid position 142 (fimH166) has been noted by Schembri et al. (26). Unfortunately, the allele described by Schembri et al. had an additional lesion. Consequently, the two alleles cannot be directly compared. Also, Schembri et al. utilized recombinant plasmid-borne fimH alleles. In the mutants we isolated, only one-third displayed the same phenotype when the plasmid-borne lesions were introduced into the chromosome. It is possible that overproduction (or other effects) attendant with transcription from a recombinant plasmid, when combined with certain lesions, effected an altered binding phenotype that was not exhibited in the chromosome. We chose to examine only those fimH alleles whose mutant binding phenotypes were manifested in the chromosome.
Of the two chromosomal fimH mutants described here, the one with the fimH165 allele (a Leu→Pro substitution at position 58 of FimH) was of particular interest because a mutant with a lesion at the same nucleotide (conferring a Leu→Arg change) has been noted to confer a temperature-dependent binding phenotype (8). The present Leu→Pro substitution effected a complete absence of macrophage binding but the mutant retained full ability to agglutinate yeast and partial ability to agglutinate erythrocytes. In contrast, the Leu→Arg mutant retains macrophage binding ability at the restrictive temperature (albeit with an altered specificity) but erythrocyte binding ability is lost (8). The previous and present results indicate that the type of amino acid at position 58 influences two important traits: (i) the conditions under which FimH is active and (ii) the specificity of that interaction. The lesion we found at amino acid position 142 (fimH166) changes an amino acid predicted to line the FimH binding pocket (3). The location of this lesion would appear to make a good deal of structural sense (i.e., a change in receptor specificity attributable to an amino acid lining the binding pocket).
Quite a variety of eucaryotic cells bind type 1 piliated E. coli via FimH. However, the FimH receptor on each eucaryotic cell type differs (6, 7, 11). Consequently, FimH mutants of the type reported here (i.e., those that have retained the affinity for one cell type but not another) may be particularly useful in better understanding issues such as bacterial tissue trophism (23) and intra- and intercellular signaling initiated by the interaction of FimH with particular types of eucaryotic cells (11).
Nucleotide sequence accession numbers.
The fimH165 and fimH166 alleles have been submitted to GenBank. They have been given the following accession numbers: fimH165, AF306535; and fimH166, AF306536.
Acknowledgments
We thank Craig Altier for a critical reading of the manuscript.
This work was supported by a grant from the National Institutes of Health (AI22223).
REFERENCES
- 1.Bolivar F. Construction and characterization of new cloning vehicles. III. Derivatives of plasmid pBR322 carrying unique EcoRI sites for selection of EcoRI-generated recombinant molecules. Gene. 1978;4:121–136. doi: 10.1016/0378-1119(78)90025-2. [DOI] [PubMed] [Google Scholar]
- 2.Brinton C C., Jr The structure, function, synthesis and genetic control of bacterial pili and a molecular model for DNA and RNA transport in gram negative bacteria. Trans N Y Acad Sci. 1965;27:1003–1054. doi: 10.1111/j.2164-0947.1965.tb02342.x. [DOI] [PubMed] [Google Scholar]
- 3.Choudhury D, Thompson A, Stojanoff V, Langermann S, Pinkner J, Hultgren S J, Knight S D. X-ray structure of the FimC-FimH chaperone-adhesin complex from uropathogenic Escherichia coli. Science. 1999;285:1061–1066. doi: 10.1126/science.285.5430.1061. [DOI] [PubMed] [Google Scholar]
- 4.Davis R W, Botstein D, Roth J R. Advanced bacterial genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1980. p. 90. [Google Scholar]
- 5.Firon N, Ashkenazi S, Mirelman D, Ofek I, Sharon N. Aromatic alpha-glycosides of mannose are powerful inhibitors of the adherence of type 1 fimbriated Escherichia coli to yeast and intestinal epithelial cells. Infect Immun. 1987;55:472–476. doi: 10.1128/iai.55.2.472-476.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gbarah A, C, Gahmberg G, Ofek I, Jacobi U, Sharon N. Identification of the leukocyte adhesion molecules CD11 and CD18 as receptors for type 1-fimbriated (mannose-specific) Escherichia coli. Infect Immun. 1991;59:4524–4530. doi: 10.1128/iai.59.12.4524-4530.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giampapa C S, Abraham S N, Chiang T M, Beachey E H. Isolation and characterization of a receptor for type 1 fimbriae of Escherichia coli from guinea pig erythrocytes. J Biol Chem. 1988;263:5362–5367. [PubMed] [Google Scholar]
- 8.Hamrick T S, Harris S L, Spears P A, Havell E A, Horton J R, Russell P W, Orndorff P E. Genetic characterization of Escherichia coli type 1 pilus adhesin mutants and identification of a novel binding phenotype. J Bacteriol. 2000;182:4012–4021. doi: 10.1128/jb.182.14.4012-4021.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamrick T S, Havell E A, Horton J R, Orndorff P E. Host and bacterial factors involved in the innate ability of mouse macrophages to eliminate internalized unopsonized Escherichia coli. Infect Immun. 2000;68:125–132. doi: 10.1128/iai.68.1.125-132.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris S L, Elliott D A, Blake M C, Must L M, Messenger M, Orndorff P E. Isolation and characterization of mutants with lesions affecting pellicle formation and erythrocyte agglutination by type 1 piliated Escherichia coli. J Bacteriol. 1990;172:6411–6418. doi: 10.1128/jb.172.11.6411-6418.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hedlund M, Frendeus B, Wachtler C, Hang L, Fischer H, Svanborg C. Type 1 fimbriae deliver an LPS- and TLR4-dependent activation signal to CD14-negative cells. Mol Microbiol. 2001;39:542–552. doi: 10.1046/j.1365-2958.2001.02205.x. [DOI] [PubMed] [Google Scholar]
- 12.Jones C H, Pinkner J S, Roth R, Heuser J, Nicholes A V, Abraham S N, Hultgren S J. FimH adhesin of type 1 pili is assembled into a fibrillar tip structure in the Enterobacteriaceae. Proc Natl Acad Sci USA. 1995;92:2081–2085. doi: 10.1073/pnas.92.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klemm P. The fimA gene encoding the type-1 fimbrial subunit of Escherichia coli. Nucleotide sequence and primary structure of the protein. Eur J Biochem. 1984;143:395–399. doi: 10.1111/j.1432-1033.1984.tb08386.x. [DOI] [PubMed] [Google Scholar]
- 14.Krogfelt K A, Bergmans H, Klemm P. Direct evidence that the FimH protein is the mannose-specific adhesin of Escherichia coli type 1 fimbriae. Infect Immun. 1990;58:1995–1998. doi: 10.1128/iai.58.6.1995-1998.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lederberg E M, Cohen S N. Transformation of Salmonella typhimurium by plasmid deoxyribonucleic acid. J Bacteriol. 1974;119:1072–1074. doi: 10.1128/jb.119.3.1072-1074.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madison B, Ofek I, Clegg S, Abraham S N. Type 1 fimbrial shafts of Escherichia coli and Klebsiella pneumoniae influence sugar-binding specificities of their FimH adhesins. Infect Immun. 1994;62:843–848. doi: 10.1128/iai.62.3.843-848.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maurer L, Orndorff P E. A new locus, pilE, required for the binding of type 1 piliated Escherichia coli to erythrocytes. FEMS Microbiol Lett. 1985;30:59–66. [Google Scholar]
- 18.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 19.Murray N E, Brammer W J, Murray K. Lambdoid phages that simplify the recovery of in vitro recombinants. Mol Gen Genet. 1977;150:53–61. doi: 10.1007/BF02425325. [DOI] [PubMed] [Google Scholar]
- 20.Orndorff P E, Falkow S. Organization and expression of genes responsible for type 1 piliation in Escherichia coli. J Bacteriol. 1984;159:736–744. doi: 10.1128/jb.159.2.736-744.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orndorff P E, Spears P A, Schauer D, Falkow S. Two modes of control of pilA, the gene encoding type 1 pilin in Escherichia coli. J Bacteriol. 1985;164:321–330. doi: 10.1128/jb.164.1.321-330.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ponniah S, Endres R O, Hasty D L, Abraham S N. Fragmentation of Escherichia coli type 1 fimbriae exposes cryptic d-mannose-binding sites. J Bacteriol. 1991;173:4195–4202. doi: 10.1128/jb.173.13.4195-4202.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pouttu R, Puustinen T, Virkola R, Hacker J, Klemm P, Korhonen T K. Amino acid residue Ala-62 in the FimH fimbrial adhesin is critical for the adhesiveness of meningitis-associated Escherichia coli to collagens. Mol Microbiol. 1999;31:1747–1757. doi: 10.1046/j.1365-2958.1999.01311.x. [DOI] [PubMed] [Google Scholar]
- 24.Russell P W, Orndorff P E. Lesions in two Escherichia coli type 1 pilus genes alter pilus number and length without affecting receptor binding. J Bacteriol. 1992;174:5923–5935. doi: 10.1128/jb.174.18.5923-5935.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 26.Schembri M A, Sokurenko E V, Klemm P. Functional flexibility of the FimH adhesin: insights from a random mutant library. Infect Immun. 2000;68:2638–2646. doi: 10.1128/iai.68.5.2638-2646.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silhavy T J, Berman M L, Enquist L W. Experiments with gene fusions. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1984. [Google Scholar]
- 28.Skorupski K, Taylor R K. Positive selection vectors for allelic exchange. Gene. 1996;169:47–52. doi: 10.1016/0378-1119(95)00793-8. [DOI] [PubMed] [Google Scholar]
- 29.Sokurenko E V, Courtney H S, Ohman D E, Klemm P, Hasty D L. FimH family of type 1 fimbrial adhesins: functional heterogeneity due to minor sequence variations among fimH genes. J Bacteriol. 1994;176:748–755. doi: 10.1128/jb.176.3.748-755.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soto G E, Hultgren S J. Bacterial adhesins: common themes and variations in architecture and assembly. J Bacteriol. 1999;181:1059–1071. doi: 10.1128/jb.181.4.1059-1071.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spears P A, Schauer D, Orndorff P E. Metastable regulation of type 1 piliation in Escherichia coli and isolation and characterization of a phenotypically stable mutant. J Bacteriol. 1986;168:179–185. doi: 10.1128/jb.168.1.179-185.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou Y, Zhang X, Ebright R H. Random mutagenesis of genome-sized DNA molecules by use of PCR with Taq DNA polymerase. Nucleic Acids Res. 1991;19:6052. doi: 10.1093/nar/19.21.6052. [DOI] [PMC free article] [PubMed] [Google Scholar]