Abstract
Tetrastigma hemsleyanum Diels & Gilg, an herbal medicinal plant, is planted widely in bamboo forests in southern China to promote economic benefits. Volatile compounds (VOCs) of T. hemsleyanum from different geographical regions are difficult to identify in field forests. In this study, VOCs from leaf samples of different geographical origins were analyzed using an electronic nose with 10 different sensors. Principal component analysis (PCA), partial least-squares regression (PLS), hierarchical cluster analysis (HCA), and radial basis function (RBF) neural networks were used to determine differences among different local samples. The results demonstrated that PCA achieved an accurate discrimination percentage of 91.31% for different samples and HCA separated the samples into different groups. The RBF neural network was successfully applied to predict samples with no specified localities. T. hemsleyanum samples from geographically close regions tended to group together, whereas those from distant geographical regions showed obvious differences. These results indicate that an electronic nose is an effective tool for detecting VOCs and discriminating the geographical origins of T. hemsleyanum. This study provides insights for further studies on the fast detection of VOCs from plants and effect of forests and plant herbal medicines on improving air quality.
Keywords: Electronic nose, Volatiles, Geographical origin, T.hemsleyanum diels & gilg
Electronic nose; Volatiles; Geographical origin; T.hemsleyanum diels & gilg.
1. Introduction
Tetrastigma hemsleyanum Diels & Gilg, a traditional medicinal plant, is primarily distributed on moist hillsides and valleys of the southern and southwestern provinces of China and in bamboo forests. It has medicinal properties and high economic value. In traditional Chinese medicine, its root tubers and leaves are used to treat high fever, infantile febrile convulsion, pneumonia, asthma, hepatitis, rheumatism, menstrual disorders, sore throat, and circulatory problems (Hu et al., 2021; Shao et al., 2011). This species also has antiviral and antitumor properties and has been used as a folk remedy to boost the immune system and treat cancer in China (Ji et al., 2021). Recently, the market demand for T. hemsleyanum has sharply increased because of its high medicinal value and antitumor properties. However, its availability decreases severely owing to over-exploration and the specific growth environment. Therefore, the cultivation of T. hemsleyanum has attracted increasing attention, and is planned on a grand scale in provinces south of the Yangtze River (Wang et al., 2022). Geographical origin is important for cultivation techniques and affects its medical value (Farzaneh and Carvalho, 2015). The traditional method for classifying the geographical origin of T. hemsleyanum is based on its morphological characteristics. For example, T. hemsleyanum from different locations can be classified by stem color as purple cane and cyan cane (Ye et al., 2021). Purple cane plants are primarily found in Jiangxi, Hunan, and Zhejiang provinces, whereas cyan cane plants are predominant in Fujian, Sichuan, Guangdong, Guangxi, and Yunnan provinces. The purple cane type had better medicinal effects, with higher prices in markets than the cyan cane type. The introduction and cultivation of plants can obscure the provenance of the source material, but different genotypes may have different levels of bioactive compounds. Therefore, reliable methods are needed to discriminate among populations of important medicinal plants, such as T. hemsleyanum. Thus, establishing reliable, rapid, inexpensive, and practical identification techniques, is an urgent step for the exploration of T. hemsleyanum.
Odors have been used since ancient times to determine the quality of Chinese medicinal plants because each medicinal plant has a characteristic odor. Traditionally, samples with strong odors reflect a higher volatile content of bioactive compounds, such as coumarins, phenols, and acids, and are considered superior in quality. Therefore, the odor of a sample is traditionally used by doctors practicing Chinese medicine as an important standard to evaluate its quality. The electronic nose is an instrument that mimics the doctor's olfactory perception and provides an odor fingerprint of the sample. It is equipped with an array of nonselective and broad-spectrum chemical sensors useful for the analysis of the headspace of liquid or solid samples (Fang et al., 2011). Therefore, the electronic nose offers a fast and nondestructive alternative method for analyzing aromas that can be used to reflect the quality of Chinese medicine. Over the past few years, electronic nose technology has enhanced the possibility of exploiting information on aromas to assess medical quality, and it is gradually being applied in the quality assessment of Chinese medicine (Wojnowski et al., 2019; Xu et al., 2021; Ye et al., 2011). In many fields, this technology has become a promising method for identifying different samples based on various volatile components. Electronic nose technology, combined with several multiple mathematical algorithms, has been used for geoherbalism evaluation (Zheng et al., 2015) and quality assessment (Xu et al., 2009) of medical plants. However, there are few studies on the detection of geographical origins. In this study, we used an electronic nose with multiple mathematical algorithms to determine the geographical origin of T. hemsleyanum plants and to distinguish the samples. The hypothesis is as follows: (1) the electronic nose can be used to discriminate the geographical origin of T. hemsleyanum and (2) mathematical algorithms have been shown to improve the efficiency and reliability of electronic noses. This study lays a foundation for evaluating how the chemotypes of medicinal plants affect their aromatic properties in the future. This helps to promote techniques to detect VOCs in forest air, as forest air may be significantly affected by the forest.
2. Methods
2.1. Experimental materials
Twelve T. hemsleyanum samples were collected from different locations in southern China and the localities and ecological conditions are listed in Table 1. Leaf samples were collected in October and November of 2019. A dispersed sampling strategy was used to represent the population adequately. Samples were collected from three to five healthy individuals with little evidence of pests or diseases.
Table 1.
Geographical locations and ecological factors of test locations.
| No | Collection locality | Longitude (E) | Latitude (N) | Mean temperature (°C) | Annual precipitation (mm) | Frost-free period (d) | Soil color | Sample number |
|---|---|---|---|---|---|---|---|---|
| 1 | Fuyang, Zhejiang | 119°02′ | 25°56′ | 13.4 | 523.2 | 214 | Brown | 5 |
| 2 | Qianxinan, Guizhou | 113°30′ | 34°52′ | 14.2 | 651.0 | 214 | Red | 3 |
| 3 | Huaihua, Hunan | 104°54′ | 34°28′ | 12.4 | 591.8 | 207 | Brown | 4 |
| 4 | Jianyang, Fujian | 118°07′ | 27°21′ | 18.1 | 1742.0 | 322 | Yellow & red | 3 |
| 5 | Kaihua, Zhejiang | 118°01′ | 28°54′ | 16.4 | 1814.0 | 252 | Yellow | 4 |
| 6 | Linan, Zhejiang | 119.31′ | 30.10′ | 15.3 | 1425.0 | 235 | Yellow & red | 4 |
| 7 | Quzhou, Zhejiang | 119°05′ | 28°74′ | 17.3 | 1900.0 | 256 | Brown | 5 |
| 8 | Shaowu, Fujian | 117.27′ | 27.15′ | 18.7 | 1800.0 | 264 | Brown | 4 |
| 9 | Shunchang, Fujian | 117.52′ | 27.01′ | 18.9 | 1621.0 | 305 | Brown | 3 |
| 10 | Suichang, Zhejiang | 119.06′ | 28.31′ | 16.8 | 1521.0 | 251 | yellow | 5 |
| 11 | Taining, Fujian | 117.09′ | 26.50′ | 17.0 | 1725.0 | 300 | Brown | 5 |
| 12 | Zhoushan, Zhejiang | 122.25′ | 30.18′ | 16.0 | 1273.5 | 277 | Brown | 4 |
2.2. Electronic nose
The experiments were performed using a portable electronic nose (PEN3) with an enrichment and desorption unit (EDU) (Win Muster Airsense Analytics Inc., Schwerin, Germany). PEN3 consists of a sampling apparatus, detector unit containing the sensor array, and proprietary pattern recognition software for data recording and analysis. The sensor array system was composed of 10 metal oxide semiconductors (MOS) with different chemical compositions and thicknesses to provide selectivity for volatile compounds. Details of the sensor array are listed in Table 2. Repeatability of the measurements was evaluated using three parallel tests of the samples. The coefficients of variation (n = 3) for the MOS sensors were calculated. The results were all <1%, indicating high repeatability of the electronic nose response.
Table 2.
Sensors used and their main applications in PEN3 electronic nose.
| In array | Sensor name | Description | Reference material |
|---|---|---|---|
| 1 | W1C-aromatic | Aromatic compounds | Toluene, 10 ppm |
| 2 | W5S-broad-range | Broad-range sensitivity; reacts to nitrogen oxides and ozone, very sensitive, with negative signal | NO2, 10 ppm |
| 3 | W3C-aromatic | Ammonia, used as sensor for aromatic compounds | Benzene, 10 ppm |
| 4 | W6S-hydrogen | Mainly hydrogen; selective (respiration gases) | H2, 100 ppb |
| 5 | W5C-arom-aliph | Alkanes, aromatic compounds, less polar compounds | Propane, 1 ppm |
| 6 | W1S-broad-methane | Sensitive to methane (environmental) ca. 10 ppm; broad range, similar to #8 | CH4, 100 ppm |
| 7 | W1W-sulphur-organic | Reacts to sulfur compounds at H2S 0.1 ppm. Otherwise sensitive to many terpenes and sulfur organic compounds that are important for aroma, including limonene and pyrazine | H2S, 1 ppm |
| 8 | W2S-broad-alcohol | Detects alcohols, partially aromatic compounds; broad range | CO, 100 ppm |
| 9 | W2W-sulphur-chlor | Aromatic compounds, sulfur organic compounds | H2S, 1 ppm |
| 10 | W3S-methane-aliph | Reacts to high concentrations >100 ppm, sometimes very selective (methane) | CH4, 10 ppm |
2.3. Electronic procedure
The samples were prepared by placing 1 g of leaf tissue in 5 mL Pyrex® vials fitted with a pierceable silicon/TeflonTM disk in the cap. After equilibration for 1 h at room temperature, the samples were measured by pumping the headspace over the sensor surfaces for 60 s (injection time) at a flow rate of 400 mL/min. After sample analysis, the system was purged for 100 s with filtered air prior to the injection of the next sample to allow reestablishment of the instrument baseline.
Each curve represents each sensor's conductivity induced by electrovalve action as volatile gases reached the measurement chamber (Liu et al., 2014). The sensor response to samples, or the odor intensity, was calculated using the following expression (Concina et al., 2010):
| R = G0/G |
where R is the response, G0 is the conductance of the sensor in air, and G is the conductance of the sensor in sample gas.
2.4. Statistical processing
Raw data were analyzed by principal component analysis (PCA) and partial least-squares regression (PLS) using the Simca-p software (version 11.5; Umetrics AB, Umeå, Sweden) after component screening and data dimension reduction (Zou et al., 2015). PCA and PLS were applied to determine whether the MOS sensor array could extract appropriate features for evaluating the samples. The same dataset was processed via hierarchical cluster analysis (HCA) to assess classification accuracy. To control for the cross-sensitivity of MOS sensors, a radial basis function (RBF) neural network was introduced to classify and predict unknown samples. The network contains one layer of input nodes, one hidden radial-basis function layer, and one output linear layer. The training dataset used 10 sensors as inputs, 12 units in the hidden layer, and 12 units in the output layer. The activation functions were softmax in the hidden layer, and identity functions were used in the output layer. Thirty-six samples (three duplicates for each sample) were divided into two groups: 18 samples each (50 percent of samples) for the training and testing datasets. The functions used to assess the prediction accuracy were mean square error (MSE), root mean square error (RMSE), and standard error of prediction.
3. Results
3.1. Electronic nose response to samples
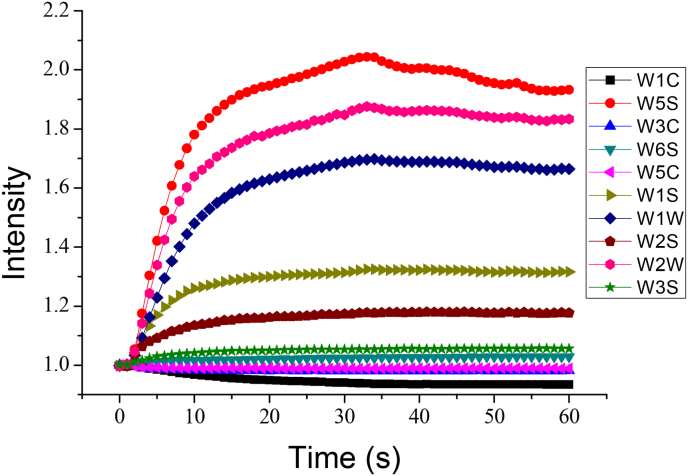
Figure 1 shows the typical signal of MOS sensors with one sample of T. hemsleyanum. Each line represents the signals of a T. hemsleyanum sample from one of the 10 MOS sensors. The horizontal axis represents the timeline, with a total of 60 s, and the vertical axis represents the response value of the MOS sensor. The curves represent the resistance of each sensor against the time from the electrovalve action when the volatile compounds reached the detection chamber. The response value of each sensor was initially low, increased for a few seconds, and then stabilized. In this study, the signals of each sensor 50 s after the electrovalve action were extracted and analyzed individually. Figure 2 shows radar charts of the responses of the MOS sensors for the samples at 50 s.
Figure 1.
A typical response of 10 sensors during the measurement of one T. hemsleyanum sample. W1C, W5S, and W3C are the names of the sensors and the detailed descriptions are given in Table 2.
Figure 2.
Radar plots of the 10 MOS sensor responses for the samples. W1C, W5S, W3C et al., are the names of the sensors and detailed descriptions are given in Table 2.
3.2. Discrimination of samples using PCA and PLS
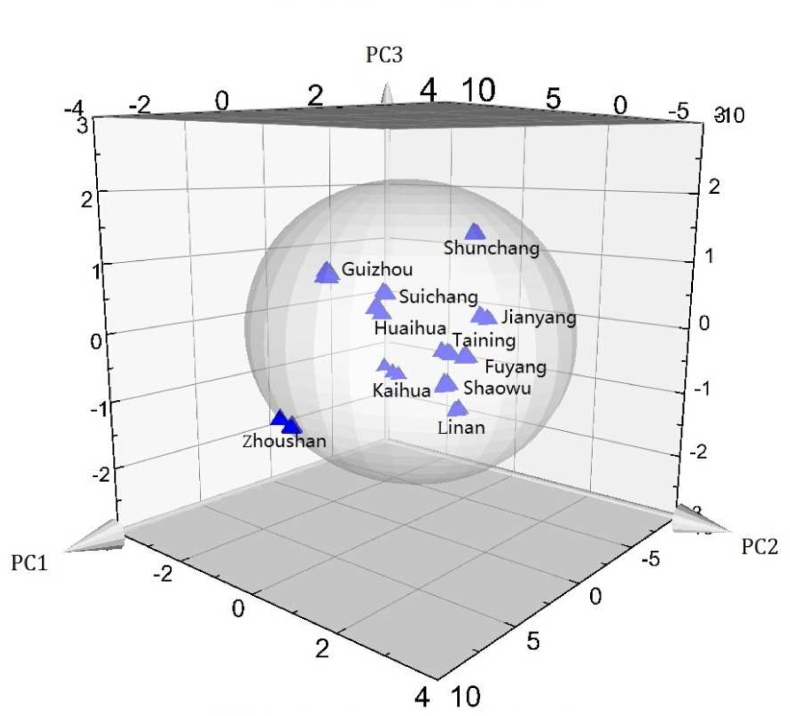
Data from all T. hemsleyanum samples were analyzed using PCA. The first two principal components accounted for >85% of the variance. The plots of the first (PC 1) and second (PC 2) principal components are presented in Figure 3a, and the loading plots are presented in Figure 3b. PC 1 and PC2 explained 74.18% and 17.13% of the variance, respectively. The samples were clustered into separate groups in a PCA plot. W5S (broad-range) and W2W (sulf-chlor) exhibited the highest contributions. W1W (sulfur-organic), W1S (methane), W2S (broad-alcohol), and W1C (aromatic) had low signal values, indicating low contents of these gases. W3C (ammonia), W6S (hydrogen), W5C (arom-aliph), and W3S (methane-aliph) were too low to indicate a significant response to T. hemsleyanum. Figure 4 shows a three-dimensional scatter plot of PCA.
Figure 3.
a. Plot of the first two principal components for electronic nose response signals of samples. Points or groups of points are labeled by province. Principal component 1 = 0.741773 and principal component 2 = 0.17126. The ellipse indicates Hotelling's T2 (0.95). b. Loadings plot of 10 sensors for 12 samples. Points are labeled by sensors. Principal component 1 = 0.8109, and principal component 2 = 0.1785. W1C, W5S, W3C et al., are the names of the sensors and the descriptions are given in Table 2.
Figure 4.
Three-dimensional plot of the first three components for electronic nose response signals of samples. Points or point clusters are labeled by province. The ellipse indicates Hotelling's T2 (0.95).
The PLS plot is shown in Figure 5. As illustrated in a two-dimensional scatter plot (Figure 5) and a three-dimensional scatter plot (Figure 6), T. hemsleyanum samples could be successfully classified such that those from geographically proximate regions tended to group together, while those from distant localities showed obvious differences.
Figure 5.
Two-dimensional scatter plot of PLS for electronic nose response signals of samples. The ellipse indicates Hotelling's T2 (0.95).
Figure 6.
Three-dimensional scatter plot of PLS for electronic nose response signals of samples. The ellipse indicates Hotelling's T2 (0.95).
3.3. Discrimination of samples using HCA
Figure 7 depicts the HCA dendrogram, which has two major groups. The first group included replicates of sample 12 from Zhoushan Island, which has an oceanic climate, whereas the second group included the other 11 samples. Within this group, samples 1, 4, 7, 8, 10, and 11 were very similar owing to the presence of similar volatiles. All samples were collected from Wuyi Mountain and the surrounding areas. Sample 5 was grouped with sample 6, both of which came from moist hillsides and valleys in Zhejiang Province. Samples 2 and 3 were collected from southwestern and south-central China, both of which are adjacent, but they are not in a separate grouping, which can be affected by genetic resources through drift, mating systems, fertility and viability selection, and migration (Buiteveld et al., 2007). In general, T. hemsleyanum samples from geographically proximate regions tended to group together, whereas those from distant geographical regions showed obvious differences.
Figure 7.
Hierarchical clustering analysis (HCA) dendrogram for the samples.
3.4. Discrimination of samples using RBF neural network
The 36 samples (three replicates for each locality) were equally divided into training and testing datasets. Figure 8 illustrates the structure of the RBF neural network and shows how the input and output data are organized. The RBF neural network performed very well in the samples, with an MSE of 2.677 × 10−14 and a 100% rate of successful prediction.
Figure 8.
The architecture of three layers of an RBF neural network for training and identification. Sensors are on the left, the hidden layer is in the middle, and the collecting localities are on the right. W1C, W5S, W3C et al., are the names of the sensors and the detailed descriptions are given in Table 2.
4. Discussion
The electronic nose technique is a low-cost, rapid, noninvasive, and accurate method for identifying the geographical origin of T. hemsleyanum. Different geographical origins show different bioactivities and treatment effects. For example, in Chinese herbal medicine plant markets, most T. hemsleyanum tubers are primarily sourced from wild populations, and tubers from Zhejiang Province and Jiangsu Province are better than those produced in other areas. Thus, researchers have focused on the taxonomy of Tetrastigma and evolution of Vitaceae (Habib et al., 2017; Obico et al., 2021; Rossetto et al., 2007). Traditional species-level studies are not suitable for distinguishing population differences or identifying the geographical origins of Tetrastigma tubers (Fu et al., 2011). Moreover, newer technologies may be effective in identifying population-level variations. DNA fingerprinting and microsatellites (including restriction fragment length polymorphisms and amplified fragment length polymorphisms) are effective tools for distinguishing between plant populations. Fingerprinting analysis of traditional Chinese medicine (TCM) represents a comprehensive qualitative approach to species authentication and quality evaluation, and ensures a consistent quality of TCMs (Wang et al., 2011; Xu et al., 2014). Gas chromatography (GC) is the preferred method for the analysis of volatiles because of its high reproducibility, while all other methods involve high capital costs, complex operations, and are time consuming (Tao et al., 2014; Yang et al., 2016). The electronic nose, which has already been applied in various fields in recent decades, is a very promising method for identifying different samples based on their volatile components. The electronic nose has been used for geoherbalism evaluation (Zheng et al., 2015), Chinese herbal medicine classification (Luo et al., 2015), and rice plant identification (Timsorn et al., 2017). The electronic nose can produce a distinguishable response pattern for each separate type of analyte or mixture with an array of sensors (Bartlett et al., 1997). For Chinese herbal medicines, the initial and unique chemical form of the volatile components could be reflected by their response to the electronic nose, which can be used to identify different Chinese herbal medicines (Zhan et al., 2018). Therefore, it is an effective tool for evaluating how the chemotypes of medicinal plants affect their aromatic properties. Unlike gas chromatography (GC), gas chromatography-mass spectrometry (GC–MS), high-performance liquid chromatography-mass spectrometry (HPLC–MS), and the electronic-nose technique does not destroy the samples used for analysis. This technology is being gradually adopted in the agricultural, environmental, medical, biotechnological, and food sectors (Ali et al., 2020; Chen et al., 2013; Jiang et al., 2017). This is the first report on the identification of T. hemsleyanum using an electronic nose. The results indicate that electronic nose can be used to identify the geographical origin of T. hemsleyanum.
This study tested herbs from 12 different locations, mainly from Zhejiang, Fujian, Hunan, and Guizhou provinces, which are the main areas producing Moso bamboo (Ouyang et al., 2022). Moso bamboo is widely used and has high ecological value (Bian et al., 2020). T. hemsleyanum is shade-tolerant and is suitable for cultivation in bamboo forests. It is an important economic and excellent medicinal plant that contains bioactive constituents (Ji et al., 2021). Different geographical and ecological factors such as soil, climate, altitude, terrain, sunshine, and rainfall have different effects on plant growth, resulting in different chemical components (Li et al., 2020). Therefore, the place of origin is closely related to the quality of traditional medicinal plants. This is meaningful for herbal medicine research because the chemical composition of herbal medicines grown in different locations and climatic conditions is often different (Zhan et al., 2019), which affects the origin and quality of medical plants. We studied the use of electronic noses to quantify the geographical origins of plants by quantifying their odor characteristics. The experimental results showed that it is feasible to identify Chinese herbs from different geographical sources using electronic nose technology and appropriate statistical methods.
Since the odor fingerprints of herbs from different origins may show little difference, it is more difficult to distinguish the origin of herbs than that of categories. The electronic nose technique was applied using multiple mathematical algorithms to identify geographical origins. A key topic is the selection of an efficient discriminative model for the application of an electronic nose. In many studies, various multiple mathematical algorithms, such as diffusion maps or PCA, have been combined with the electronic nose (Hu et al., 2019; Luo et al., 2015; Zheng et al., 2015). PCA is a statistical method that can be used to analyze, classify, and reduce the dimensionality of numerical datasets in multivariate analysis (Abdi and Williams, 2010; Zou et al., 2006). To support the PCA results, PLS was used to discriminate between samples. The PLS is an important algorithm derived from multivariate linear regression analysis, canonical correlation analysis, and PCA. The aim of HCA is to divide samples into specific groups based on similarity criteria (Hidayat et al., 2010). An RBF neural network was used to identify the samples from unknown localities. In this study, coupled with PCA, PLS, HCA, and an RBF neural network, an electronic nose was able to objectively analyze and successfully differentiate samples of T. hemsleyanum. PCA accurately discriminated 91.31% of the samples, consistent with other studies (Concina et al., 2010; Dong et al., 2013; Zou et al., 2015). HCA was also successful in separating samples into different groups by geographic origin. The RBF neural network successfully predicted the samples from unknown localities. The electronic nose did not need to be modified to detect volatile compounds, particularly T. hemsleyanum, and was superior to traditional methods used to identify the provenance of this species. This method provides a rapid, noninvasive, and accurate method for the categorization of complex aroma mixtures to protect the rights of patients and avoid the use of inferior herbal medicines as fake drugs.
5. Conclusions
The electronic nose is an effective tool to identify the geographical origin of T. hemsleyanum when combined with PCA, PLS, and HCA. T. hemsleyanum samples from geographically proximate regions tended to group together, whereas those from distant geographical regions showed obvious differences. Electronic noses do not need to be specially designed to detect certain volatile compounds and do not require reference compounds; therefore, they should be able to discriminate among chemotypes of medicinal plants. This research provides insights for further studies, such as selecting sensors that are more sensitive to volatile components in Chinese herbal medical plants, to improve the reliability and efficiency of the electronic nose in combination with mathematical algorithms. It is also helpful to study the air quality in forestland, which is mixed with herbal medical plants and used for human recuperate tourism.
Declarations
Author contribution statement
Zhizhuang Wu, Xiaodan Ye: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Fangyuan Bian: Analyzed and interpreted the data; Wrote the paper.
Ganglei Yu, Guibing Gao, Jiande Ou, Yukui Wang, Yueqiao Li: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Xuhua Du: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
Xuhua DU was supported by People's Government of Zhejiang Province-Chinese Academy of Forestry cooperative project [2019SY01], Fundamental Research Funds for the Central Non-Profit Research Institution of CAF [CAFYBB2019MB005], National Natural Science Foundation of China [31600448].
Yukui Wang was supported by key research and development program of Zhejiang Province [2020C02008].
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Contributor Information
Fangyuan Bian, Email: bianfangyuan@yeah.net.
Xuhua Du, Email: duxh@caf.ac.cn.
References
- Abdi H., Williams L.J. Principal component analysis. Wiley interdisciplinary reviews: Comput. Stat. 2010;2:433–459. [Google Scholar]
- Ali M.M., Hashim N., Abd Aziz S., Lasekan O. Principles and recent advances in electronic nose for quality inspection of agricultural and food products. Trends Food Sci. Technol. 2020;99:1–10. [Google Scholar]
- Bartlett P.N., Elliott J.M., Gardner J.W. Electronic noses and their application in the food industry. Food Technol. 1997;51:44–48. [Google Scholar]
- Bian F.Y., Zhong Z.K., Zhang X.P., Yang C.B., Gai X. Bamboo–an untapped plant resource for the phytoremediation of heavy metal contaminated soils. Chemosphere. 2020;246 doi: 10.1016/j.chemosphere.2019.125750. [DOI] [PubMed] [Google Scholar]
- Buiteveld J., Vendramin G., Leonardi S., Kamer K., Geburek T. Genetic diversity and differentiation in European beech (Fagus sylvatica L.) stands varying in management history. For. Ecol. Manag. 2007;247:98–106. [Google Scholar]
- Chen S., Wang Y., Choi S. 2013. Applications and Technology of Electronic Nose for Clinical Diagnosis. [Google Scholar]
- Concina I., Bornšek M., Baccelliere S., Falasconi M., Gobbi E., Sberveglieri G. Alicyclobacillus spp.: detection in soft drinks by electronic nose. Food Res. Int. 2010;43:2108–2114. [Google Scholar]
- Dong Q., Du L., Zhuang L., Li R., Liu Q., Wang P. A novel bioelectronic nose based on brain–machine interface using implanted electrode recording in vivo in olfactory bulb. Biosens. Bioelectron. 2013;49:263–269. doi: 10.1016/j.bios.2013.05.035. [DOI] [PubMed] [Google Scholar]
- Fang Q., Zhang M., Yang Y., Zhou X., Jia H., Fu P., Huang L. Discrimination of 11 Chinese Materia Medica from umbelliferae by electronic nose. Chin. Med. 2011;2:143. [Google Scholar]
- Farzaneh V., Carvalho I.S. A review of the health benefit potentials of herbal plant infusions and their mechanism of actions. Ind. Crop. Prod. 2015;65:247–258. [Google Scholar]
- Fu Y., Jiang W., Fu C. Identification of species within Tetrastigma (miq.) planch.(vitaceae) based on DNA barcoding techniques. J. Systemat. Evol. 2011;49:237–245. [Google Scholar]
- Habib S., Dang V.-C., Ickert-Bond S.M., Zhang J.-L., Lu L.-M., Wen J., Chen Z.-D. Robust phylogeny of Tetrastigma (Vitaceae) based on ten plastid DNA regions: implications for infrageneric classification and seed character evolution. Front. Plant Sci. 2017;8:590. doi: 10.3389/fpls.2017.00590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidayat W., Shakaff A.Y.M., Ahmad M.N., Adom A.H. Classification of agarwood oil using an electronic nose. Sensors. 2010;10:4675–4685. doi: 10.3390/s100504675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W., Wan L., Jian Y., Ren C., Jin K., Su X., Bai X., Haick H., Yao M., Wu W. Electronic noses: from advanced materials to sensors aided with data processing. Advanced Materials Technologies. 2019;4 [Google Scholar]
- Hu W., Zheng Y., Xia P., Liang Z. The research progresses and future prospects of Tetrastigma hemsleyanum Diels et Gilg: a valuable Chinese herbal medicine. J. Ethnopharmacol. 2021;271 doi: 10.1016/j.jep.2021.113836. [DOI] [PubMed] [Google Scholar]
- Ji T., Ji W.W., Wang J., Chen H.J., Peng X., Cheng K.J., Qiu D., Yang W.J. A comprehensive review on traditional uses, chemical compositions, pharmacology properties and toxicology of Tetrastigma hemsleyanum. J. Ethnopharmacol. 2021;264 doi: 10.1016/j.jep.2020.113247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J., Moore J.A., Priyadarshi A., Classen A.T. Plant-mycorrhizal interactions mediate plant community coexistence by altering resource demand. Ecology. 2017;98:187–197. doi: 10.1002/ecy.1630. [DOI] [PubMed] [Google Scholar]
- Li J., Shao Y., Yao Y., Yu Y., Cao G., Zou H., Yan Y. A novel quality evaluation method for magnolia bark using electronic nose and colorimeter data with multiple statistical algorithms. J. Tradit. Chinese Med. Sci. 2020;7:221–227. [Google Scholar]
- Liu J., Wang W., Yang Y., Yan Y., Wang W., Wu H., Ren Z. A rapid discrimination of authentic and unauthentic Radix Angelicae Sinensis growth regions by electronic nose coupled with multivariate statistical analyses. Sensors. 2014;14:20134–20148. doi: 10.3390/s141120134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo D., Chen H., Yu H., Sun Y. A novel approach for classification of Chinese herbal medicines using diffusion maps. Int. J. Pattern Recogn. Artif. Intell. 2015;29 [Google Scholar]
- Obico J.J.A., Barcelona J.F., Bonhomme V., Hale M., Pelser P.B. Resolving the Tetrastigma loheri sl species complex (Vitaceae) in the Philippines: No evidence for recognizing more than one species. Syst. Bot. 2021;46:750–763. [Google Scholar]
- Ouyang M., Yang C., Tian D., Pan J., Chen G., Su H., Yan Z., Ji C., Tang Z., Fang J. A field-based estimation of moso bamboo forest biomass in China. For. Ecol. Manag. 2022;505 [Google Scholar]
- Rossetto M., Crayn D.M., Jackes B.R., Porter C. An updated estimate of intergeneric phylogenetic relationships in the Australian Vitaceae. Botany. 2007;85:722–730. [Google Scholar]
- Shao Q., Deng Y., Fang H., Zhao X. Optimization of polysaccharides extraction from Tetrastigma hemsleyanum Diels et Gilg using response surface methodology. Int. J. Biol. Macromol. 2011;49:958–962. doi: 10.1016/j.ijbiomac.2011.08.015. [DOI] [PubMed] [Google Scholar]
- Tao N.-P., Wu R., Zhou P.-G., Gu S.-Q., Wu W. Characterization of odor-active compounds in cooked meat of farmed obscure puffer (Takifugu obscurus) using gas chromatography–mass spectrometry–olfactometry. J. Food Drug Anal. 2014;22:431–438. doi: 10.1016/j.jfda.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timsorn K., Lorjaroenphon Y., Wongchoosuk C. Identification of adulteration in uncooked Jasmine rice by a portable low-cost artificial olfactory system. Measurement. 2017;108:67–76. [Google Scholar]
- Wang J., Qian Q., Zhang F., Jia X., He J. The possible future changes in potential suitable habitats of Tetrastigma hemsleyanum (Vitaceae) in China predicted by an ensemble model. Global Ecology and Conservation. 2022;35 [Google Scholar]
- Wang L., Pan J., Yang M., Wu J., Yang J. Chromatographic fingerprint analysis and simultaneous determination of eight lignans in Justicia procumbens and its compound preparation by HPLC-DAD. J. Separ. Sci. 2011;34:667–674. doi: 10.1002/jssc.201000781. [DOI] [PubMed] [Google Scholar]
- Wojnowski W., Dymerski T., Gębicki J., Namieśnik J. Electronic noses in medical diagnostics. Curr. Med. Chem. 2019;26:197–215. doi: 10.2174/0929867324666171004164636. [DOI] [PubMed] [Google Scholar]
- Xu G., Liao C., Ren X., Zhang X., Zhang X., Liu S., Fu X., Wu H., Huang L., Liu C. Rapid assessment of quality of deer antler slices by using an electronic nose coupled with chemometric analysis. Revista Brasileira de Farmacognosia. 2014;24:716–721. [Google Scholar]
- Xu J., Liu K., Zhang C. Electronic nose for volatile organic compounds analysis in rice aging. Trends Food Sci. Technol. 2021;109:83–93. [Google Scholar]
- Xu S., Yang L., Tian R., Wang Z., Liu Z., Xie P., Feng Q. Species differentiation and quality assessment of Radix Paeoniae Rubra (Chi-shao) by means of high-performance liquid chromatographic fingerprint. J. Chromatogr. A. 2009;1216:2163–2168. doi: 10.1016/j.chroma.2008.04.064. [DOI] [PubMed] [Google Scholar]
- Yang Y., Kong W., Feng H., Dou X., Zhao L., Xiao Q., Yang M. Quantitative and fingerprinting analysis of Pogostemon cablin based on GC-FID combined with chemometrics. J. Pharm. Biomed. Anal. 2016;121:84–90. doi: 10.1016/j.jpba.2016.01.012. [DOI] [PubMed] [Google Scholar]
- Ye H., Li C., Ye W., Zeng F., Liu F., Liu Y., Wang F., Ye Y., Fu L., Li J. Common Chinese Materia Medica. Springer; 2021. Medicinal angiosperms of Vitaceae; pp. 461–518. [Google Scholar]
- Ye T., Jin C., Zhou J., Li X., Wang H., Deng P., Yang Y., Wu Y., Xiao X. Can odors of TCM be captured by electronic nose? The novel quality control method for musk by electronic nose coupled with chemometrics. J. Pharm. Biomed. Anal. 2011;55:1239–1244. doi: 10.1016/j.jpba.2011.03.018. [DOI] [PubMed] [Google Scholar]
- Zhan X., Guan X., Wu R., Wang Z., Wang Y., Li G. Discrimination between alternative herbal medicines from different categories with the electronic nose. Sensors. 2018;18:2936. doi: 10.3390/s18092936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan X., Guan X., Wu R., Wang Z., Wang Y., Li G. Paper Presented at: 2019 IEEE 7th International Conference on Bioinformatics and Computational Biology (ICBCB) (IEEE) 2019. Feature engineering in discrimination of herbal medicines from different geographical origins with electronic nose. [Google Scholar]
- Zheng S., Ren W., Huang L. Geoherbalism evaluation of Radix Angelica sinensis based on electronic nose. J. Pharm. Biomed. Anal. 2015;105:101–106. doi: 10.1016/j.jpba.2014.10.033. [DOI] [PubMed] [Google Scholar]
- Zou H.-Q., Lu G., Liu Y., Bauer R., Tao O., Gong J.-T., Zhao L.-Y., Li J.-H., Ren Z.-Y., Yan Y.-H. Is it possible to rapidly and noninvasively identify different plants from Asteraceae using electronic nose with multiple mathematical algorithms? J. Food Drug Anal. 2015;23:788–794. doi: 10.1016/j.jfda.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou H., Hastie T., Tibshirani R. Sparse principal component analysis. J. Comput. Graph Stat. 2006;15:265–286. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.