Abstract
Background
The Veterans Health Administration issued policy for lung cancer screening resources at eight Veterans Affairs Medical Centers (VAMCs) in a demonstration project (DP) from 2013 through 2015.
Research Question
Do policies that provide resources increase lung cancer screening rates?
Study Design and Methods
Data from eight DP VAMCs (DP group) and 20 comparable VAMCs (comparison group) were divided into before DP (January 2011-June 2013), DP (July 2013-June 2015), and after DP (July 2015-December 2018) periods. Coprimary outcomes were unique veterans screened per 1,000 eligible per month and those with 1-year (9-15 months) follow-up screening. Eligible veterans were estimated using yearly counts and the percentage of those with eligible smoking histories. Controlled interrupted time series and difference-in-differences analyses were performed.
Results
Of 27,746 veterans screened, the median age was 66.5 years and most were White (77.7%), male (95.6%), and urban dwelling (67.3%). During the DP, the average rate of unique veterans screened at DP VAMCs was 17.7 per 1,000 eligible per month, compared with 0.3 at comparison VAMCs. Adjusted analyses found a higher rate increase at DP VAMCs by 0.93 screening per 1,000 eligible per month (95% CI, 0.25-1.61) during this time, with an average facility-level difference of 17.4 screenings per 1,000 eligible per month (95% CI, 12.6-22.3). Veterans with 1-year follow-up screening also increased more rapidly at DP VAMCs during the DP, by 0.39 screening per 1,000 eligible per month (95% CI, 0.18-0.60), for an average facility-level difference of 7.2 more screenings per 1,000 eligible per month (95% CI, 5.2-9.2). Gains were not maintained after the DP.
Interpretation
In this cohort, provision of resources for lung cancer screening implementation was associated with an increase in veterans screened and those with 1-year follow-up screening. Screening gains associated with the DP were not maintained.
Key Words: early detection of cancer, implementation, lung cancer screening, lung neoplasm, policy, utilization
Abbreviations: CPT, Current Procedural Terminology; DP, Demonstration Project; LDCT, low-dose CT; NLST, National Lung Screening Trial; USPSTF, United States Preventive Services Task Force; VAMC, Veterans Affairs Medical Center; VHA, Veterans Health Administration
Take-home Points.
Study Question: Do policies that provide resources increase lung cancer screening?
Results: We found that medical centers with resources for lung cancer screening (personnel, clinical and educational tools, and support) screened 17.7 veterans per 1,000 eligible per month compared with 0.3 in centers without resources, with a rate increase higher by 0.93 screening per 1,000 eligible per month than the increase in centers without resources.
Interpretation: A policy to support resources for lung cancer screening was associated with increased implementation in the Veterans Health Administration.
Lung cancer screening using low-dose CT (LDCT) imaging reduces lung cancer mortality and is recommended widely.1, 2, 3, 4, 5, 6, 7, 8, 9 After publication of the National Lung Screening Trial (NLST) in 2011, the United States Preventive Services Task Force (USPSTF) recommended screening with LDCT imaging in 2013 for asymptomatic individuals 55 to 80 years of age who currently smoke cigarettes or formerly smoked and who quit within the past 15 years, have at least a 30-pack-year exposure to cigarette smoking, and are able to undergo curative treatment.3
The Veterans Health Administration (VHA) is the largest integrated health care system in the United States.10 Between the 2011 NLST publication and the 2013 USPSTF recommendation, lung cancer screening was not covered by private insurance or Medicare and largely was an unstructured process based on individual providers and patients. Rates were exceedingly low from 2011 through 2013 within and outside the VHA, with uncertainties about how to incorporate screening into clinical practice.11,12 Perceived barriers included identification of high-risk populations. It remained unknown if the screening-eligible population with significant comorbidities would benefit, what resources would be needed to meet demands, how to ensure adherence and safety, and whether real-world outcomes would reflect the NLST.13
Taking a middle-ground approach to implementation, the VHA announced a policy to conduct a staged and limited lung cancer screening implementation, the Lung Cancer Screening Demonstration Project (DP).13, 14, 15 Veterans Affairs Medical Centers (VAMCs) could apply to start VHA’s first lung cancer screening programs. VAMCs that applied committed to screening eligible patients in a phased-in approach, hiring a screening coordinator, and using standardized clinical algorithms and a patient management tool that integrated with the electronic health record.14,16 The DP ran from July 1, 2013, through June 30, 2015, and found that 32% of veterans 55 to 80 years of age met the USPSTF smoking criteria, with an estimated 900,000 veterans eligible for lung cancer screening.14,15 In the current study, we sought to test the hypothesis that lung cancer screening rates increased more rapidly at the DP VAMCs during and after the DP compared with similar VAMCs that applied to the DP, but were not selected.
Study Design and Methods
Data Source
Data were obtained from the VA Informatics and Computing Infrastructure and Observational Medical Outcomes Partnership databases, which contain information on clinical care in veterans’ medical records and dates and claims for services provided. This study was approved by the VA Tennessee Valley Healthcare System Institutional Review Board (approval no. 881295-26) with a waiver of informed consent. This report follows Strengthening the Reporting of Observational Studies in Epidemiology guidelines for cohort studies.
Study Population
To calculate screening rates, we obtained the number of screenings (numerator) and the number of eligible veterans (denominator) at each VAMC. The numerators were monthly totals of screening examinations from January 2011 through December 2018. We excluded examinations for patients with a previous diagnosis of thoracic malignancy, conducted outside the eligibility age, or within 31 days of the previous examination. LDCT imaging screenings were identified by Current Procedural Terminology (CPT) codes G0297 and 71250 and a description of “screening,” “lung cancer screening,” or “LCS” (lung cancer screening) (e-Table 1). This algorithm has a positive predictive value of 92% (95% CI, 0.81-0.97) to detect an LDCT imaging examination performed for lung cancer screening.11 For the denominator, we obtained the annual counts of unique inpatients or outpatients 55 to 80 years of age seen at each VAMC from 2011 through 2018. To obtain the monthly VAMC count, we divided each annual count by 12. Because VHA records do not capture pack-years of smoking history, we multiplied each monthly count by 32% to estimate the number of age-eligible veterans who would meet the USPSTF smoking history criteria as reported in the DP report.14,15
DP and Comparison Groups
The VHA planned to select six to eight VAMCs to participate in the DP, and 35 applied.13 The competitive selection was based on geography (rural vs urban); ongoing research; leadership and staff support; equipment and resources, including an on-site radiologist; multidisciplinary lung cancer program; availability of tobacco cessation services; willingness to name a clinical coordinator; commitment to offer screening to all eligible patients; and agreement to follow clinical algorithms and use a tracking system.14,15 Eight VAMCs were selected: Ann Arbor, Charleston, Cincinnati, Durham, Minneapolis, New York Harbor, Portland, and San Francisco. Each hired a coordinator; used clinical reminders (to collect smoking history and to prompt providers for initial and annual screening), clinical algorithms, and a management tool; received patient and provider educational materials; and participated in a national support network. Coordinators conducted screening coordination, including eligibility confirmation, shared decision-making, tobacco cessation, ordering the screening, communicating results, and ensuring that next screening steps were taken (annual repeat screening, short-term follow-up scan, or referral for diagnostic evaluation).14,15
We assumed in our analyses that observed screening rates in the comparison VAMCs represent rates that would have been observed at the DP VAMCs had the DP not taken place.17 All DP VAMCs were complexity level 1a or 1b, indicating highest complexity based on patient volume, patient cases, number and type of clinical services, presence and size of residency programs, and research (60 of 141 VAMCs are level 1a or 1b).18 Accordingly, we included in the comparator group only the level 1a or 1b VAMCs that applied but were not selected to participate in the DP, because these VAMCs were comparable with DP VAMCs in motivation to implement lung cancer screening and complexity level.
Time Periods
Our study examined monthly screening rates across three time periods: (1) before the DP (January 1, 2011-June 30, 2013), (2) during the DP (July 1, 2013-June 30, 2015), and (3) after the DP (July 1, 2015-December 31, 2018).15
Outcomes: LDCT Imaging Screening, Follow-up Examinations, Diagnosis of Thoracic Malignancy
The coprimary outcomes were facility-level rates of unique veterans screened and those screened who also underwent 1-year follow-up screening. One-year follow-up screening was defined as a screening conducted 9 to 15 months after the initial scan. All rates are reported per 1,000 eligible veterans per month.
The secondary outcome was the number of veterans who received a diagnosis of a thoracic malignancy within 12 months after a screening examination, defined using International Classification of Diseases, Ninth Revision, CPT, Systematized Nomenclature of Medicine, and Logical Observation Identifiers Names and Codes codes (e-Table 2).
To examine the robustness of our findings to variations in our screening definition, we conducted sensitivity analyses that expanded the definition to include all examinations associated with descriptions of “screening,” “lung cancer screening,” “LCS,” “low-dose,” “LDCT,” or “VCAR” (volume-computed algorithm). Volume-computed algorithm is a radiology software package used to analyze screening LDCT scans for three-dimensional volumetric assessment.11 To investigate whether increases in screenings solely may be the result of improved coding, we compared the number of screenings with the total number of scans over time at each site.
Additional Data
Reported characteristics of screened veterans included screening age, sex, race or ethnicity, diagnosis of COPD or coronary artery disease, and home location. International Classification of Diseases, Ninth and Tenth Revisions, CPT, and Systematized Nomenclature of Medicine codes were used to define the above characteristics. We collected tobacco history, defined as diagnosed tobacco-related conditions and diagnosis of emphysema within 2 years before screening (e-Table 2). Facility characteristics included complexity score and geographic region defined by the US Census and classified by Veterans Integrated Service Networks (e-Table 3).
Statistical Analysis
The main analysis was a controlled interrupted time series (additional detailed e-Appendix 1). We examined differences in linear trends in monthly screening rates across the three periods. The linear regression adjusted for facility complexity level, with robust SEs clustered at the facility level, and assumed no abrupt increases or decreases in screening rates at the beginning or end of the demonstration project. To summarize results without assumptions about time trend, we also conducted a difference-in-differences analysis that examined differences in mean monthly rates across periods without regard to within-period trend and used linear regression adjusted for facility complexity level, with robust SEs clustered at the facility level. All statistical analysis was performed using R version 4.0.4 software (R Foundation for Statistical Computing).
Results
Study Population
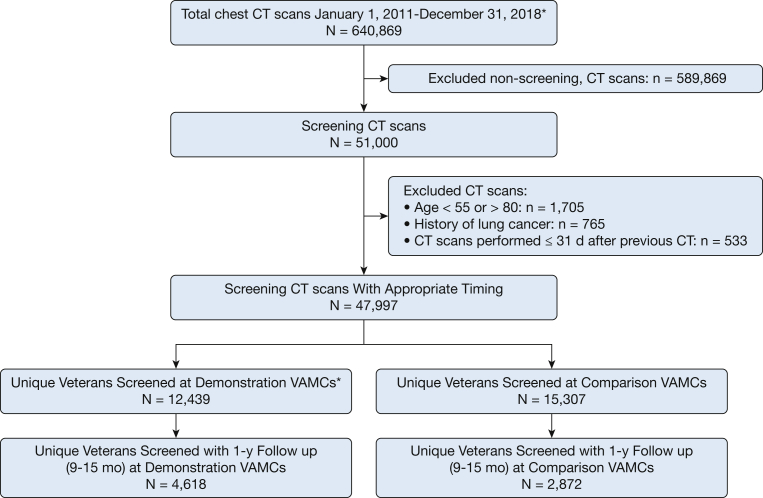
Beginning with 640,869 chest CT scan examinations performed at DP or comparison VAMCs between January 1, 2011, and December 31, 2018, with CPT code G0297 or 71250, we excluded 589,869 examinations without screening descriptors, 1,705 examinations of veterans outside the 55-to-80-year age range, 765 examinations in patients with a history of lung cancer, and 533 examinations carried out within 31 days of a previous examination. The analytic cohort contained 47,997 screening LDCT scan examinations among 27,746 unique veterans (Fig 1).
Figure 1.
Cohort flow chart. VAMC = Veterans Affairs Medical Center.
Veterans at initial screening had a mean age of 66.5 years, 95.6% were men, 77.7% were White, 32.7% were living in rural or highly rural areas, 54.8% had a diagnosis of tobacco use, 18.4% had a diagnosis of coronary artery disease, and 38.2% had a diagnosis of COPD (Table 1).18
Table 1.
Characteristics of Veterans at Initial Screening and Characteristics of Facilities
| Veteran Characteristics | Total Sample (N = 27,746) | Demonstration Project VAMCsa (n = 12,439) | Comparison VAMCsb (n = 15,307) |
|---|---|---|---|
| Age, y | 66.5 (61.7-69.9) | 66.1 (61.6-69.4) | 66.9 (61.8-70.2) |
| Sex | |||
| Male | 26,539 (95.6) | 11,889 (95.6) | 14,650 (95.7) |
| Female | 1,207 (4.4) | 550 (4.4) | 657 (4.3) |
| Ethnicity | |||
| Hispanic or Latino | 797 (2.9) | 369 (3.0) | 428 (2.8) |
| Not Hispanic or Latino | 26,228 (94.5) | 11,744 (94.4) | 14,484 (94.6) |
| Missing | 721 (2.6) | 326 (2.6) | 395 (2.6) |
| Race | |||
| American Indian or Alaska Native | 171 (0.4) | 92 (0.7) | 79 (0.5) |
| Asian | 124 (0.4) | 90 (0.7) | 34 (0.2) |
| Black | 4,404 (15.9) | 2,095 (16.8) | 2,309 (15.1) |
| Native Hawaiian or other Pacific Island | 125 (0.5) | 65 (0.5) | 60 (0.4) |
| White | 21,545 (77.7) | 9,174 (73.8) | 12,371 (80.8) |
| Missing | 1,377 (5.0) | 923 (7.4) | 454 (3.0) |
| Home address location | |||
| Highly rural | 324 (1.2) | 81 (0.7) | 243 (1.6) |
| Rural | 8,733 (31.5) | 4,071 (32.7) | 4,662 (30.5) |
| Urban | 18,673 (67.3) | 8,282 (66.6) | 10,391 (67.9) |
| Missing | 16 (0.1) | 5 (0.0) | 11 (0.1) |
| Diagnosed tobacco usec | 15,211 (54.8) | 7,583 (61.0) | 7,628 (49.8) |
| COPD | 10,586 (38.2) | 4,280 (34.4) | 6,306 (41.2) |
| Coronary artery disease | 5,102 (18.4) | 2,301 (18.5) | 2,801 (18.3) |
| Facility characteristics | |||
| US Census region | |||
| Midwest (VISNs 10, 12, 15, and 23) | 7 | 3 | 4 |
| Northeast (VISNs 1, 2, and 4) | 6 | 1 | 5 |
| South (VISNs 5, 6, 7, 8, 9, 16, and 17) | 8 | 2 | 6 |
| West (VISNs 19, 20, 21, and 22) | 7 | 2 | 5 |
| Complexity leveld | |||
| 1a | 19 | 6 | 13 |
| 1b | 9 | 2 | 7 |
Data are presented as No. (%), No., or median (interquartile range). VAMC = Veterans Affairs Medical Center; VISN = Veterans Integrated Service Network.
VAMCs that participated in the Veterans Affairs Lung Cancer Screening Demonstration Project, which included: Ann Arbor, Charleston, Cincinnati, Durham, Minneapolis, New York Harbor, Portland, and San Francisco.
The 20 VAMCs with complexity level 1a or 1b that applied but were not selected to participate in the Demonstration Project.
Diagnosed tobacco use defined as up to 730 d before the lung cancer screening examination date using International Classification of Diseases, Ninth Revision, Clinical Modification; Current Procedural Terminology; Systematized Nomenclature of Medicine; and Logical Observation Identifiers Names and Codes codes (e-Table 2).
Consists of five complexity levels: 1a, 1b, 1c, 2, and 3, where 1a is the most complex and 3 is the least complex. This ranking system takes the following into consideration: (1) volume and patient case mix, (2) clinical services provided, (3) patient risk calculated from Veterans Affairs patient diagnosis, (4) total resident slots, (5) an index of multiple residency programs at a single facility, (6) total amount of research dollars, and (7) the number of specialized clinical services.18
The geographic distribution differed slightly between DP and comparison VAMCs. Only one DP VAMC was in the Northeast, with at least two VAMCs in each of the other three regions. The comparison VAMCs were distributed more evenly (four to six VAMCs per region).
Counts and Average Rates of Screening Across the Entire Study Period
Over the entire study period, the eight DP VAMCs screened 12,439 veterans, with little variation between sites (e-Fig 1). Of the 9,916 for whom 15 months of follow-up was available, 47% (n = 4,618) had a 1-year follow-up screening. The 20 comparison VAMCs screened 15,307 veterans, 8,791 of whom had 15 months of follow-up available. Of these, 33% (n = 2,872) underwent a 1-year follow-up screening.
Over the study period, the average numbers of veterans screened per 1,000 eligible veterans per VAMC per month in the DP and comparison VAMCs were 15.0 and 6.7, respectively; the average numbers of veterans screened with a 1-year follow-up per 1,000 eligible veterans per VAMC per month were 6.1 and 1.5.
Unadjusted Screening Rates and Controlled Interrupted Time Series
Trends before the DP in screening use between DP and comparison VAMCs were nearly identical (0 vs 1 screening) (Table 2). During the DP, the average rate of veterans screened per 1,000 eligible per month increased in both groups: to 17.7 at DP VAMCS vs 0.3 at comparison VAMCs. The steeper increase at DP VAMCs is reflected in an adjusted difference in slopes of 0.93 (95% CI, 0.25-1.61). During the DP, the average rate of screenings with 1 year of follow-up also increased in both groups (to 7.2 and 0.1, respectively). The adjusted difference of 0.39 (95% CI, 0.18-0.60) reflects the steeper increase in the DP group.
Table 2.
Lung Cancer Screening Use: Unadjusted Results and Difference-in-Differences Analysis
| Period | VAMC Statusa |
|
|---|---|---|
| Demonstration Project | Comparison | |
| Before DP (January 2011-June 2013) | ||
| Average no. eligible per VAMC per month | 1,104 | 1,231 |
| Unique veterans screened (initial screenings) | 0 | 1 |
| Initial screenings with follow-upb | 0 | 1 |
| Unadjusted average monthly VAMC-level rates per 1,000 eligible | ||
| Initial screenings | 0 | 0 |
| Initial screenings with follow-up | 0 | 0 |
| DP period (July 2013-June 2015) | ||
| Average no. eligible per VAMC per month | 1,170 | 1,250 |
| Unique veterans screened (initial screenings) | 3,559 | 132 |
| Initial screenings with follow-up | 1,581 | 28 |
| Unadjusted average monthly VAMC-level rates per 1,000 eligible | ||
| Initial screenings | 17.7 | 0.3 |
| Initial screenings with follow-up | 7.2 | 0.1 |
| Adjusted resultsc for monthly VAMC-level rates per 1,000 eligible | ||
| Initial screenings: change since last period | 17.7 | 0.3 |
| Initial screenings: DID with 95% CId | 17.4 (12.6-22.3) | |
| Initial screenings with follow-up: change since last period | 7.2 | 0.1 |
| Initial screenings with follow-up: DID with 95% CI | 7.2 (5.2-9.2) | |
| After DP (July 2015-December 2018) | ||
| Average no. eligible per VAMC per month | 1,214 | 1,284 |
| Unique veterans screened (initial screenings) | 8,880 | 15,174 |
| Initial screenings with follow-up | 3,037 | 2,844 |
| Unadjusted average monthly VAMC-level rates per 1,000 eligible | ||
| Initial screenings | 24.1 | 15.2 |
| Initial screenings with follow-up | 11.8 | 4.3 |
| Adjusted results for monthly VAMC-level rates per 1,000 eligible | ||
| Initial screenings: change since last period | 6.3 | 14.9 |
| Initial screenings: DID with 95% CI | –8.6 (–19.5 to 2.3) | |
| Initial screenings with follow-up: change since last period | 4.6 | 4.3 |
| Initial screenings with follow-up: DID with 95% CI | 0.3 (–4.8 to 5.4) | |
Data are presented as No., unless otherwise indicated. DID = difference-in-differences; DP = Demonstration Project; VAMC = Veterans Affairs Medical Center.
Demonstration Project VAMCs were Ann Arbor, Charleston, Cincinnati, Durham, Minneapolis, New York Harbor, Portland, and San Francisco; Comparison VAMCs were the 20 VAMCs of complexity level 1a or 1b that applied but were not selected to participate in the Demonstration Project.
Initial screenings that eventually were followed by a 1-year follow-up examination 9 to 15 months later.
Analyses adjusted for VAMC complexity score and used robust SEs clustered at the VAMC level.
The model-based (adjusted) difference between the change since last period for the DP group and the change since last period for the control group; positive values favor the DP group.
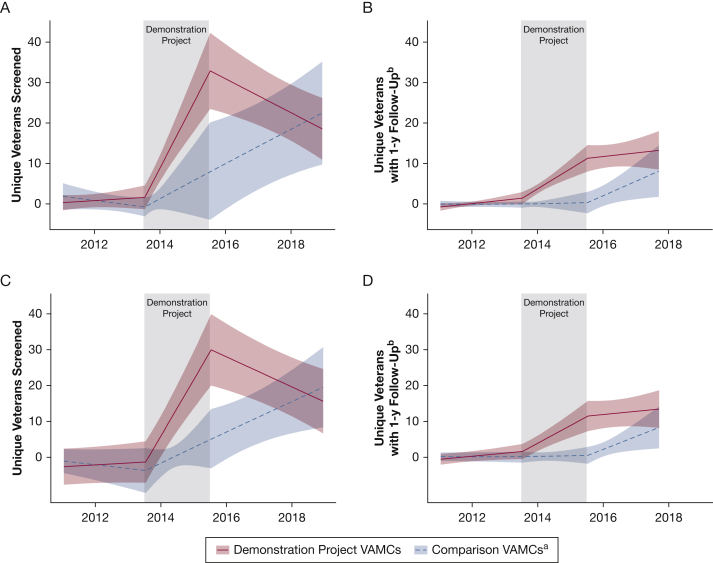
During the period after the DP, the average rate of unique veterans screened at DP VAMCs decreased slightly, whereas the average rate at comparison VAMCs continued to increase (unadjusted rates of 24.1 and 15.2, respectively; adjusted difference in slopes, –0.70; 95% CI, –1.08 to –0.32). Average rates of screening examinations with 1 year of follow-up increased in both groups, with a more prominent increase at comparison VAMCs (unadjusted rates of 11.8 and 4.3, respectively; adjusted difference in slopes, –0.22; 95% CI, –0.56 to 0.11) (Fig 2).
Figure 2.
Graphs showing interrupted time series analyses. A, Facility-level average number of unique veterans screened per 1,000 eligible veterans per month at Veterans Affairs Medical Centers (VAMCs) with complexity level 1a. B, Facility-level average number of unique veterans screened with eventual 1-year follow-up (9-15 months) per 1,000 eligible veterans per month at VAMCs with complexity level 1a. C, Facility-level average number of unique veterans screened per 1,000 eligible veterans per month at VAMCs with complexity level 1b. D, Facility-level average number of unique veterans screened with eventual 1-year follow-up (9-15 months) per 1,000 eligible veterans per month at VAMCs with complexity level 1b. aDemonstration Project refers to the Veterans Affairs Lung Cancer Screening Demonstration Project that took place from 2013 through 2015 at these eight VAMCs: Ann Arbor, Charleston, Cincinnati, Durham, Minneapolis, New York Harbor, Portland, and San Francisco. Comparison VAMCs refer to comparison VAMCs with complexity level 1a that applied but were not selected to participate in the Demonstration Project. bDefined as a subsequent scan 9 to 15 months after the initial scan.
Difference-in-Differences Analysis
Compared with rate before the DP, the mean facility-level rates of veterans screened were higher during the DP period in DP and comparison VAMCs. The mean increase among DP sites was higher than the mean increase among comparison VAMCs by 17.4 veterans per 1,000 eligible veterans per month (95% CI, 12.6-22.3). The number of veterans who underwent the 1-year follow-up screening demonstrated a similar pattern, with a difference of 7.2 more examinations per 1,000 eligible veterans per month (95% CI, 5.2-9.2) (Table 2).
Compared with the DP period, the mean facility-level screening rates were higher in both groups after the DP, but the mean increase among comparison VAMCs was higher than the mean among the DP VAMCs by 8.6 per 1,000 eligible veterans per month (95% CI, –2.3 to 19.5). For the number of veterans who underwent 1-year follow-up screening, the mean increases were similar, with a difference of 0.3 more screenings per 1,000 eligible veterans per month in the DP group (95% CI, –4.8 to 5.4) (Table 2).
Secondary Outcome: Diagnosis of Thoracic Malignancy Among Screened Veterans
Among screened veterans with 12 months of follow-up, the proportion with a diagnosed thoracic malignancy within 12 months after screening was 2.5% vs 1.8% in DP and comparison VAMCs, respectively.
Sensitivity Analyses
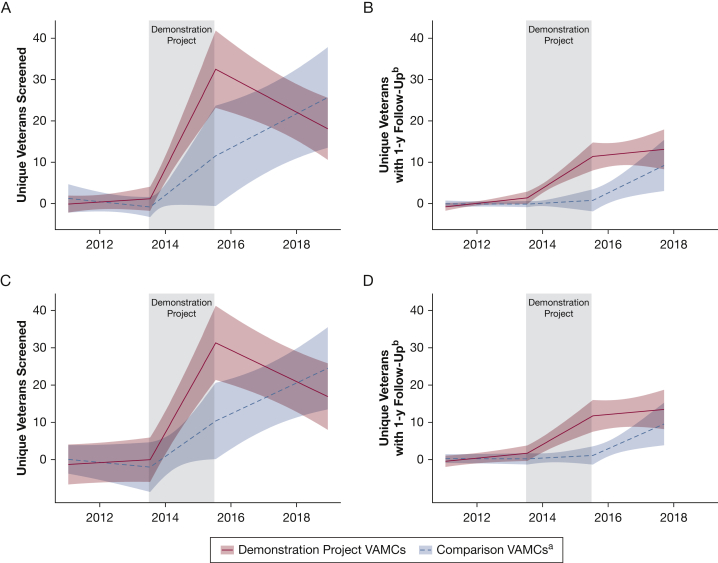
Including possible screening examinations in the definition of our primary outcome resulted in an increase in the unique veterans screened (n = 32,762) and screenings conducted (n = 55,716). Results were consistent with the main analysis in both direction and magnitude (Fig 3, e-Table 4). To assess changes in procedure codes at VAMCs over time, e-Figure 2 demonstrates increases in both total CT scan studies (purple line) and screening studies (yellow line) across all three periods at most VAMCs. It is unlikely that changes in coding solely explain the increases in screening rates observed because a concurrent rise was found in the total number of chest CT scans and screenings performed over time.
Figure 3.
Graphs showing interrupted time series sensitivity analyses. A, Facility-level average number of unique veterans screened per 1,000 eligible veterans per month at Veterans Affairs Medical Centers (VAMCs) with complexity level 1a. B, Facility-level average number of unique veterans screened with eventual 1-year follow-up (9-15 months) per 1,000 eligible veterans per month at VAMCs with complexity level 1a. C, Facility-level average number of unique veterans screened per 1,000 eligible veterans per month at VAMCs with complexity level 1b. D, Facility-level average number of unique veterans screened with eventual 1-year follow-up (9-15 months) per 1,000 eligible veterans per month at VAMCs with complexity level 1b. aDemonstration Project refers to the Veterans Affairs Lung Cancer Screening Demonstration Project that took place from 2013 through 2015 at these eight VAMCs: Ann Arbor, Charleston, Cincinnati, Durham, Minneapolis, New York Harbor, Portland, and San Francisco. Comparison VAMCs refer to comparison VAMCs with complexity level 1a that applied but were not selected to participate in the Demonstration Project. bDefined as a subsequent scan 9 to 15 months after the initial scan.
Discussion
This study evaluated policy that implemented system-level resources for lung cancer screening in the largest integrated US health care system. We report that VAMCs provided with system-level resources (program coordinator, educational and clinical tools including a management system, clinical algorithms, reminders, and support network) were associated with increased rates of veterans screened and screenings with 1-year follow-up. It is important to evaluate policies that involve the funding of large, national programs, such as the VHA’s Clinical Lung Cancer Demonstration Project, because this informs future resource allocation. Based on our main results, we would expect that future VHA financial investments (appropriated by the US Congress) for lung cancer screening would be associated with an increase in unique veterans screened, annual screening adherence, and cancers diagnosed.
An earlier report of the DP reported screening 2,106 veterans during the project’s 2-year period; we found a slightly higher number (n = 3,559) that may reflect screenings performed outside of the screening programs during the DP period.15 Before the current evaluation, DP screening rates had not been compared with rates at other VAMCs. We found that each DP VAMC screened, on average, 17.4 more veterans per 1,000 eligible per month during the DP period vs comparator VAMCs. This increase, although small, required each DP VAMC to build infrastructure from scratch, which takes time (well beyond the 2 years of the DP project) to optimize workflows and outcomes for all stakeholders involved.14,15 However, this is the start needed to reach > 1 million veterans eligible for lung cancer screening.11,15 During the DP, we also found an average of 7.2 more veterans with 1-year follow-up per 1,000 eligible per month among DP VAMCs vs comparison VAMCs. This finding suggests that resources provided in the DP encouraged annual adherence. Thus, our findings suggest that VHA policies that increase system-level resources likely would increase the number of veterans screened and adherence to annual screening.
The VHA is unique in its ability to implement and evaluate clinical programs that reach a national population. Program workflows and tools, such as clinical reminders, can be standardized and deployed for use across VAMCs. Clinical outcomes can be studied at a population level using data from a national electronic health record system for program evaluation.15,19 Furthermore, national policy can standardize practices across the organization. Health care systems in the US private sector likely encounter different challenges to lung cancer screening implementation compared with the VHA, such as reimbursement requirements including prior authorizations, shared decision-making visits, and participation in a national registry. However, many health care organizations outside of VHA have started lung cancer screening programs and report outcomes similar to the NLST.20, 21, 22, 23, 24, 25, 26 Thus, our findings that system-level resources improve screening likely could impact non-VHA health care system settings positively.
The relative gains in unique veterans screened at DP VAMCs diminished after the DP ended, with an increase at comparison VAMCs. The reasons for this are unclear because it is unknown which resources remained in use at each VAMC after the DP conclusion. It is plausible that navigators maximized their effort with patients already enrolled in their programs, which is suggested by the decline in the rate of unique veterans screened and maintenance in the rate of screenings with 1-year follow-up among DP VAMCs after the DP.
The rate of screening unique veterans increased among comparison VAMCs after the DP. This may suggest that comparison VAMCs eventually found resources outside of the DP to start lung cancer screening programs or to implement other system-level support for screening. In fact, VHA supports multiple initiatives on lung cancer screening, including policy recommendations on starting screening programs, some of which could have influenced screening rates after the DP in 2017 and 2018.19,27, 28, 29 The degree to which comparison VAMCs acquired resources for lung cancer screening is unknown. It is also possible that adoption among individual providers increased at comparison VAMCs as awareness of key policy changes3,30 increased. These results also could suggest regression to the mean in the period after the DP. Our finding of increases of unique veterans screened in the comparison VAMCs and declines in DP VAMCs was unexpected and is hypothesis generating. It underscores the need for qualitative studies to uncover system-level reasons for changes in screening rates.
We found that DP VAMCs diagnosed more thoracic malignancies within 12 months of a screening examination among unique veterans relative to comparison VAMCs from 2011 through 2018. This promising finding suggests an increase in screen-detected lung cancers that may reflect the increased follow-up screenings at DP VAMCs.
The use of a comparison group is a strength of our analyses, because their use rates represent what might have occurred at the DP VAMCs had the DP not existed. The VAMCs selected for the comparison group resembled DP VAMCs in health care services offered, volume and patient type, academic medical center affiliation, and research. All 28 VAMCs applied to participate in the DP, showing similar motivation to start lung cancer screening programs. The nearly identical use rates before the DP offer further evidence that the selected comparison VAMCs are an appropriate comparison group.
This study had limitations. First, because our analysis evaluated resources as a bundle, it remains unclear which individual resources (ie, coordinator vs tracking system vs clinical reminders) are associated with increased screening or whether the entire bundle was essential. Second, misclassification of LDCT scan examinations could have occurred: providers may have intended for an examination recorded as a screening to be diagnostic and vice versa. Third, our algorithm, which classifies an LDCT scan as screening only if its descriptor contained certain key words (LCS, screening, lung cancer screening) may undercount the number of examinations. Sensitivity analyses added CT scans described as “low-dose” or “LDCT,” with unchanged results. Fourth, care received outside of VHA is not captured; therefore, screening rates may be a systematic underestimate. No reason exists to believe that this underestimate occurred differentially between DP and comparison VAMCs. Fifth, selection bias may have occurred because of the lack of random assignment. Sixth, the denominator of veterans eligible is imperfect and does not account for those with symptoms or significant comorbidities. The estimated rates are assumed to reflect appropriate screening (ie, which patients met the USPSTF criteria for lung cancer screening). Our estimation of 32% eligible veterans is based on previous research and national estimates.11,15 Finally, our results may not be generalizable outside of the VHA.
Interpretation
A policy that implemented system-level resources was associated with an increase in veterans screened and 1-year follow-up screening during the DP. Policies that increase system-level resources within the VHA could improve lung cancer screening reach and sustainability.
Acknowledgments
Author contributions: J. A. L. is responsible for all content of the manuscript. J. A. L. contributed to conceptualization, methodology, investigation, project administration, formal analysis, validation, visualization, funding acquisition, writing of the original draft, and reviewing and editing later drafts. L. R. S. contributed to methodology, formal analysis, validation, visualization, and reviewing and editing drafts. J. D. contributed to software, data gathering, validation, and reviewing and editing drafts. M. E. M. contributed to methodology, data gathering, resources, and review and editing drafts. A. M. contributed to conceptualization, resources, and reviewing and editing drafts. C. G. S. contributed to methodology and reviewing and editing drafts. E. G. contributed to resources and reviewing and editing drafts. J. K. contributed to methodology and reviewing and editing drafts. R. H. S. contributed to conceptualization, methodology, resources, and reviewing and editing drafts. R. S. D. contributed to resources, reviewing and editing drafts, and funding acquisition. P. P. M. contributed to methodology, validation, and reviewing and editing drafts. L. K. contributed to conceptualization, methodology, and reviewing and editing drafts. C. L. R. contributed to conceptualization, methodology, investigation, validation, supervision, writing the original draft, and reviewing and editing drafts. S. N. contributed to conceptualization, methodology, and reviewing and editing drafts.
Funding/support: This study was supported in part by the Vanderbilt CTSA [Grant UL1 TR000445] from National Center for Advancing Translational Sciences at the National Institutes of Health, US Department of Veterans Affairs (VA) Office of Rural Health, VA Office of Academic Affiliations, and the VA National Quality Scholars Program, with resources and use of facilities at VA Tennessee Valley Healthcare System, Nashville, TN, and the VA Portland Health Care System, Portland, OR. The study was also supported in part by a Conquer Cancer The ASCO Foundation Young Investigator Award, 2021YIA9865677411, a LUNGevity Foundation VA Research Scholars Award, 2021-08, the Vanderbilt-Ingram Cancer Center [Grant CA68485], and the Vanderbilt Scholars in T4 Translational Research (VSTTaR) K12 Program, funded by the National Heart, Lung, and Blood Institute [Grant K12HL137943].
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: C. G. S. and J. A. L. are medical codirectors of their VA’s lung cancer screening programs, but they do not receive financial compensation for these roles. J. A. L. is a founding board member of the Rescue Lung Rescue Life Society, but does not receive financial compensation for this role. None declared (L. R. S., J. D., M. E. M., A. M., E. G., J. K., R. H. S., R. S. D., P. P. M., L. K., C. L. R., S. N.).
Role of sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data. The VA Office of Rural Health had the opportunity to read the manuscript and make comments prior to submission.
Other contributions: Jason Denton and Pierre P. Massion, MD, are deceased. This work is dedicated to Jason Denton, who died of COVID-19 before its completion. The authors thank Ms. Kathy Pittman for her assistance with the VA Lung Cancer Screening Clinical Demonstration Project.
Additional information: The e-Appendix, e-Figures, and e-Tables are available online under “Supplementary Data.”
Supplementary Data
References
- 1.National Lung Screening Trial Research Team. Aberle D.R., Adams A.M., et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Koning H.J., van der Aalst C.M., de Jong P.A., et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382(6):503–513. doi: 10.1056/NEJMoa1911793. [DOI] [PubMed] [Google Scholar]
- 3.Moyer V.A., United States Preventive Services Task Force Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330–338. doi: 10.7326/M13-2771. [DOI] [PubMed] [Google Scholar]
- 4.Wender R., Fontham E.T., Barrera E., Jr., et al. American Cancer Society lung cancer screening guidelines. CA Cancer J Clin. 2013;63(2):107–117. doi: 10.3322/caac.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wood DE, Kazerooni EA, et al. NCCN Clinical Practices Guidelines in Oncology (NCCN Guidelines) for lung cancer screening version 1.2021. 2021. National Comprehensive Cancer Network website. Accessed January 3, 2021. https://www.nccn.org/
- 6.Jaklitsch M.T., Jacobson F.L., Austin J.H., et al. The American Association for Thoracic Surgery guidelines for lung cancer screening using low-dose computed tomography scans for lung cancer survivors and other high-risk groups. J Thorac Cardiovasc Surg. 2012;144(1):33–38. doi: 10.1016/j.jtcvs.2012.05.060. [DOI] [PubMed] [Google Scholar]
- 7.American Lung Association Providing guidance on lung cancer screening to patients and physicians. American Lung Association website. https://www.lung.org/getmedia/0f9f6821-8817-4444-a647-e6ca0c82104c/lung-cancer-screening-report.pdf.pdf
- 8.Mazzone P.J., Silvestri G.A., Patel S., et al. Screening for lung cancer: CHEST guideline and expert panel report. Chest. 2018;153(4):954–985. doi: 10.1016/j.chest.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 9.National Center for Health Promotion and Disease Prevention, U.S. Department of Veterans Affairs. Get recommended screening tests and immunizations for women. National Center for Health Promotion and Disease Prevention, U.S. Department of Veterans Affairs website. https://www.prevention.va.gov/Healthy_Living/Get_Recommended_Screening_Tests_and_Immunizations_for_Women.asps
- 10.Veterans Health Administration. U.S. Department of Veterans Affairs U.S. Veterans Health Administration, Department of Veterans Affairs, website. https://www.va.gov/health/
- 11.Lewis J.A., Samuels L.R., Denton J., et al. National lung cancer screening utilization trends in the Veterans Health Administration. JNCI Cancer Spectr. 2020;4(5):pkaa053. doi: 10.1093/jncics/pkaa053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jemal A., Fedewa S.A. Lung cancer screening with low-dose computed tomography in the United States 2010 to 2015. JAMA Oncol. 2017;3(9):1278–1281. doi: 10.1001/jamaoncol.2016.6416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kinsinger L.S., Atkins D., Provenzale D., Anderson C., Petzel R. Implementation of a new screening recommendation in health care: the Veterans Health Administration’s approach to lung cancer screening. Ann Intern Med. 2014;161(8):597–598. doi: 10.7326/M14-1070. [DOI] [PubMed] [Google Scholar]
- 14.Jackson GL, King HA, McNeil RB, et al. Evaluation of the VA Lung Cancer Screening Clinical Demonstration Project—report submitted to the Veterans Affairs Office of the Under Secretary for Health. 2016. March 2016.
- 15.Kinsinger L.S., Anderson C., Kim J., et al. Implementation of lung cancer screening in the Veterans Health Administration. JAMA Intern Med. 2017;177(3):399–406. doi: 10.1001/jamainternmed.2016.9022. [DOI] [PubMed] [Google Scholar]
- 16.Schoenhard W. Memorandum: Lung Cancer Screening Clinical Demonstration Project. In: Department of Veterans Affairs; 2012.
- 17.Daw J.R., Hatfield L.A. Matching and regression to the mean in difference-in-differences analysis. Health Serv Res. 2018;53(6):4138–4156. doi: 10.1111/1475-6773.12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.U.S. Department of Veterans Affairs. Veterans Health Administration. Summary of VHA facility complexity model. Veterans Health Administration website. https://www.vendorportal.ecms.va.gov/FBODocumentServer/DocumentServer.aspx?DocumentId=2793591&FileName=VA118-16-R-1059-A00002002.docx
- 19.Lewis J.A., Spalluto L.B., Henschke C.I., et al. Protocol to evaluate an enterprise-wide initiative to increase access to lung cancer screening in the Veterans Health Administration. Clin Imaging. 2020;73:151–161. doi: 10.1016/j.clinimag.2020.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cattaneo S.M., 2nd, Meisenberg B.R., Geronimo M.C.M., Bhandari B., Maxted J.W., Brady-Copertino C.J. Lung cancer screening in the community setting. Ann Thorac Surg. 2018;105(6):1627–1632. doi: 10.1016/j.athoracsur.2018.01.075. [DOI] [PubMed] [Google Scholar]
- 21.Bhandari S., Tripathi P., Pham D., Pinkston C., Kloecker G. Performance of community-based lung cancer screening program in a Histoplasma endemic region. Lung Cancer. 2019;136:102–104. doi: 10.1016/j.lungcan.2019.08.026. [DOI] [PubMed] [Google Scholar]
- 22.McKee B.J., McKee A.B., Flacke S., Lamb C.R., Hesketh P.J., Wald C. Initial experience with a free, high-volume, low-dose CT lung cancer screening program. J Am Coll Radiol. 2013;10(8):586–592. doi: 10.1016/j.jacr.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 23.Walker B.L., Williamson C., Regis S.M., et al. Surgical outcomes in a large, clinical, low-dose computed tomographic lung cancer screening program. Ann Thorac Surg. 2015;100(4):1218–1223. doi: 10.1016/j.athoracsur.2015.04.112. [DOI] [PubMed] [Google Scholar]
- 24.Henschke C.I., Yip R., Shaham D., et al. The regimen of computed tomography screening for lung cancer: lessons learned over 25 years from the International Early Lung Cancer Action Program. J Thorac Imaging. 2021;36(1):6–23. doi: 10.1097/RTI.0000000000000538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raghavan D., Wheeler M., Doege D., et al. Initial results from mobile low-dose computerized tomographic lung cancer screening unit: improved outcomes for underserved populations. Oncologist. 2020;25(5):e777–e781. doi: 10.1634/theoncologist.2019-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaminetzky M., Milch H.S., Shmukler A., et al. Effectiveness of Lung-RADS in reducing false-positive results in a diverse, underserved, urban lung cancer screening cohort. J Am Coll Radiol. 2019;16(4 pt A):419–426. doi: 10.1016/j.jacr.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 27.Department of Veterans Affairs. Memorandum: lung cancer screening with low dose computed tomography (VAIQ: 7845332). November 27, 2017.
- 28.U.S. Department of Veterans Affairs. National Oncology Program Office, National Precision Oncology Program. US Department of Veterans Affairs website. https://www.cancer.va.gov/CANCER/NPOP.asp
- 29.VA and GO2 Foundation for Lung Cancer partner to improve outcomes for veterans at risk for lung cancer [press release]. GO2 Foundation for Lung Cancer website. https://go2foundation.org/
- 30.Centers for Medicare and Medicaid Services. U.S. Department of Health and Human Services Decision memo for screening for lung cancer with low dose computed tomography (LDCT) (CAG-00439N). Centers for Medicare and Medicaid Services website. https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.