ABSTRACT
Perivascular spaces are fluid-filled spaces that surround the perforating vessels of the brain and are normal findings on brain imaging. These are usually asymptomatic and are considered a manifestation of aging. Perivascular spaces occasionally undergo significant enlargement and are referred to as tumefactive perivascular spaces, which are often indistinguishable from neoplastic lesions. Spontaneous regression of tumefactive perivascular spaces during follow-up is rare. We report the imaging findings and clinical course of a patient who showed spontaneous regression of tumefactive perivascular spaces in the anterior temporal lobe, together with a literature review and discussion regarding the characteristics and pathogenesis of spontaneous regression of tumefactive perivascular spaces. Most studies in the available literature report tumefactive perivascular spaces in the anterior temporal lobe; in our view, the characteristics of anterior temporal lobe tumefactive perivascular spaces may differ from those of tumefactive perivascular spaces that occur at other locations.
Key Words: tumefactive perivascular spaces, anterior temporal lobe, middle cerebral artery
INTRODUCTION
Perivascular spaces (PVS), also referred to as Virchow-Robin spaces are pia-lined interstitial fluid-filled spaces that surround the perforating vessels of the brain.1 Radiographically, PVS typically present as small-sized, well-circumscribed, homogeneous spaces, without surrounding signal changes in the brain parenchyma. PVS are asymptomatic in nearly all cases and are detected incidentally on radiological imaging, particularly in elderly individuals. However, PVS may rarely enlarge and become symptomatic; manifestations typically depend upon the site of involvement and extent of the ensuing mass effect.2 Enlarged PVS are referred to as tumefactive perivascular spaces (TPVS), which may be indistinguishable from neoplastic lesions.3 TPVS do not require any specific treatment, and spontaneous regression during follow-up has been reported in rare cases,2,4-6 but the characteristics and mechanisms underlying spontaneous regression remain unclear. In this report, we present the imaging findings and clinical course of a patient who showed spontaneous regression of TPVS. We have additionally presented a literature review and discussed the characteristics and pathogenesis of spontaneous regression of TPVS.
CASE PRESENTATION
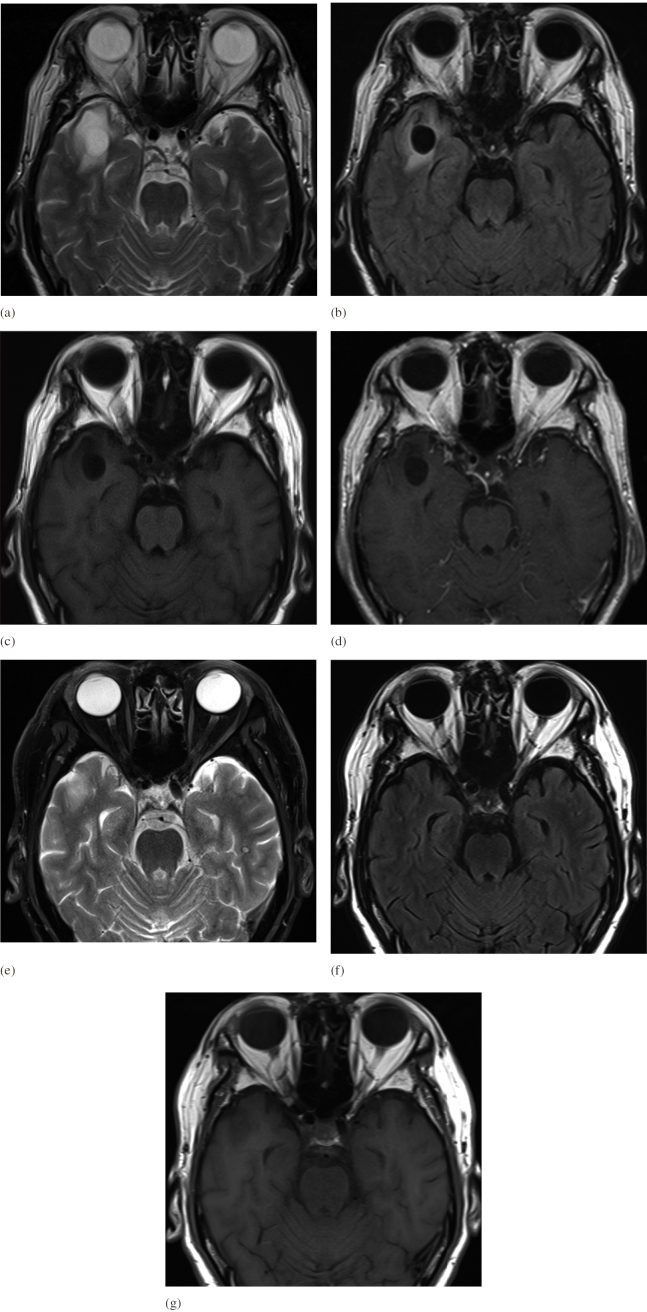
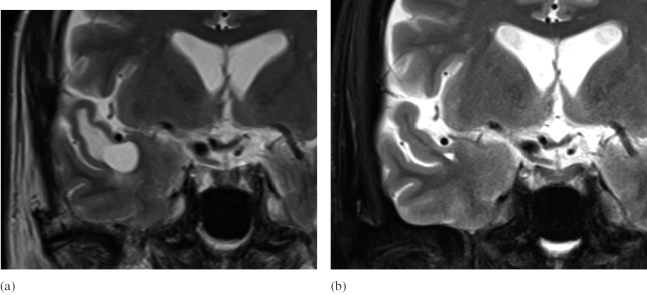
A 68-year-old man was referred to our department for evaluation of a cystic lesion that was incidentally detected on screening head magnetic resonance imaging (MRI) performed prior to cardiovascular surgery. He denied headache or a history of epileptic seizures, and neurological examination showed non-focal findings. Head MRI revealed a cystic lesion (diameter 1.5 cm) in the anterior pole of the right temporal lobe. The cyst was isointense to cerebrospinal fluid, and T2-weighted and fluid-attenuated inversion recovery images (FLIAR) revealed pericystic edematous changes (Fig. 1a-c). Gadolinium-enhanced T1-weighted images showed no enhancement (Fig. 1d). We strongly suspected TPVS because a branch of the middle cerebral artery (MCA) ran above the cyst (Fig. 2a). Differential diagnoses included neoplastic lesions such as ganglioglioma, a metastatic tumor, and inflammatory diseases, such as parasitic infestations. The patient showed no neurological symptoms; therefore, follow-up MRI was performed at 3-, 6-, and 12-month intervals. The size of the cyst remained unchanged during follow-up; however, MRI performed at the age of 72 years (4 years after the initial radiological evaluation) revealed spontaneous regression of the cyst (Fig. 1e-g, Fig. 2b). The patient had no neurological abnormalities during follow-up, and based on his clinical course, we diagnosed spontaneous regression of TPVS in this case.
Fig. 1.
Axial brain MRI images of the cystic lesion
Fig. 1a-c: Axial MRI images at the time of the initial diagnosis [(a) T2, (b) FLAIR, (c) T1] showed cystic lesions in the right temporal lobe with surrounding edematous signal change.
Fig. 1d: Gadolinium-enhanced T1 axial MRI at the time of the initial diagnosis showed a cystic lesion without contrast enhancement.
Fig. 1e-g: Four years later, MRI images showed complete disappearance of the dilated perivascular spaces [(e) T2, (f) FLAIR, (g) T1].
Fig. 2.
Comparison of coronal MRI images before and after the resolution of the cystic lesions
Fig. 2a: Coronal T2 weighted image at the time of initial diagnosis showed the branch of the middle cerebral artery (M2) runs above the cyst.
Fig. 2b: Coronal T2 weighted image showed the cyst had disappeared.
DISCUSSION
PVS are pia-lined, fluid-filled structures that surround the small perforating arterioles and venules that penetrate the brain from the subarachnoid space. The arteries and veins that run in the subarachnoid space are surrounded by a pial sheath. The intraparenchymal arterioles also show an inner pial sheath that closely surrounds the adventitia. The ultrastructure of the perivascular space shows a single or double layer of invaginated pia with endothelial, pial, and glial cell layers, with a basement membrane that delineates each component.7 The outer wall of the PVS is formed by the glia limitans, a glial membrane that covers the brain parenchyma. The PVS disappears at the capillary level as the basement membrane of the glia fuses with the outer vascular membrane.8 PVS contain interstitial fluid and do not directly communicate with the subarachnoid space.7
PVS are benign expansions of normal anatomical structures distributed across characteristic sites, such as in the inferior basal ganglia along the anterior commissure.9 Giant PVS (1.5 cm in diameter) most commonly occur within the mesencephalothalamic region.10 Patients with TPVS are typically asymptomatic but may occasionally present with nonspecific symptoms, such as headache. In a case series of 37 patients investigated by Salzman et al, headache was the most common symptom that occurred in approximately 50% of patients. Other symptoms include dizziness, dementia, visual changes, seizures, syncope, memory deficits, poor balance and concentration.10 Many studies have reported symptomatic TPVS; however, spontaneous regression of TPVS is rare. To our knowledge, only six case reports have described this rare condition (Table 1); three patients had spontaneous regression of TPVS and three showed resolution following treatment for intracranial masses located at remote sites.2,4-6 Two patients underwent surgical treatment for a pituitary macroadenoma and a parasagittal meningioma, and one patient showed resolution of TPVS following medical therapy for pituitary apoplexy.
Table 1:
The characterizes of the cases with spontaneous regression of perivascular spaces
| Authors, Year |
Age/
Sex |
Presenting Symptoms | Location of cystric lesion | Size of cystric lesion | complication | MRI findings | Regression |
| Cerase et al,4 2009 | 62
/M |
not described | anterior left temporal lobe | no image | nonfunctioning pituitary macroadenoma | no image | surgical resection of the macroadenoma |
| Cerase et al,5 2010 | 76
/F |
right hemiparesis and motor aphasia | anterior left temporal lobe | 16mm | left meningioma of the cranial vault infiltrating the superior sagittal sinus | edema(+) | surgical resection of the meningioma |
| 70
/M |
left hemiparesis and unconsciousness | anterior right temporal lobe & left anterior perforate substance | 11mm & 12mm | sellar and suprasellar expansile lesion | edema(-) | Medical therapy (mannitol, pituitary substitutive therapy, etc.) | |
| 78
/M |
generalized seizure | anterior right temporal lobe | 11mm | (-) | edema(-) | Spontaneous regression | |
| Tortora et al,6 2012 | 28
/F |
generalized seizure | pre-rolandic right cortex | 23mm | (-) | edema(+) | Spontaneous regression |
| Eluvathingal Muttikkal et al,2 2014 | 47
/M |
holocranial headache | anterior left temporal lobe | 12mm | (-) | edema(+) | Spontaneous regression |
| Our case, 2021 | 68
/M |
none | anterior right temporal lobe | 14mm | (-) | edema(+) | Spontaneous regression |
The mechanism underlying spontaneous resolution of TPVS remains unclear, although several hypotheses have been proposed in the literature, including atrophy or ischemic injury of the surrounding brain tissue (which results in an “ex vacuo” phenomenon), mechanical trauma from cerebrospinal fluid pulsation or vascular ectasia, increased arteriolar tortuosity, altered arterial wall permeability, inflammatory cell infiltration, fibrosis, or obstruction of lymphatic drainage pathways.11,12 Cerase et al hypothesized that resolution of the mass effect resulted in improved cerebrospinal fluid flow and better drainage of interstitial fluid in patients with large meningiomas and pituitary lesions. Communication between the interstitial and the subarachnoid space allows drainage of interstitial fluid and TPVS regression.4,5 Tortora et al proposed that inflammation likely played a significant role in TPVS formation.6 Thomas et al described a case of TPVS regression, with recurrence observed 2 years later. Recurrent TPVS could be attributed to reocclusion of the communication between the perivascular and the subarachnoid space. Spontaneous resolution and recurrence suggest that local obstruction of the perivascular space secondary to inflammation or fibrosis may be implicated as a likely pathophysiological contributor.2
PVS most commonly occur along the lenticulostriate arteries in the basal ganglia region, above the anterior perforate substance and adjacent to the anterior commissure, followed by the midbrain, subcortical white matter, subinsular cortex, extreme capsule, thalamus, cerebellum, corpus callosum, and cingulate gyrus, although PVS can occur at any site throughout the brain in elderly individuals.11,13 PVS are more common in the mesencephalothalamic region and occur less frequently in the anterior temporal lobe.10 Nevertheless, six of seven studies (including our study) have reported PVS regression in the anterior temporal lobe (Table 1).2,4-6 David et al14 proposed the expression “opercular perivascular cysts” to refer to a new subtype of TPVS, based on a novel association between the opercular or insular segment of the MCA, which may be observed in the anterior temporal or opercular frontal lobes. Recent studies have described a large (≥5 mm in diameter) anterior temporal white matter TPVS that can mimic cystic tumors. In a clinical case series of 39 patients with large anterior temporal white matter TPVS, Anthony et al15 reported that the potential unique radiological features were indicative of focal cortical distortion by an adjacent branch of the MCA that is expected to traverse the PVS. The authors were of the view that tortuous arterial branches may cause focal distortion of the overlying cortex, which may consequently obstruct small tracts that connect the perivascular and subarachnoid spaces in a few patients. The mechanical obstruction may result in secondary dilatation of the PVS. They also observed that the cystic lesion surrounded the branches of the MCA. In our case, a branch of the MCA was observed to run above the TPVS. Although other studies have not implicated the MCA, based on the site of the lesions, it is reasonable to conclude that a branch of the MCA was involved in the development of TPVS in our patient. The vessel that coursed above the dilated PVS measured approximately 3 mm in diameter in our case; it was clearly thicker than the lenticulostriate artery, a common site at which PVS are known to occur. Based on this difference in vessel thickness observed in our study, we conclude that TPVS that occur in the anterior temporal lobe may show characteristics that differ from those of TPVS detected at other sites.
TPVS in the anterior temporal lobe also show characteristic imaging findings. In a case series of 37 patients who showed dilated PVS, Salzman et al10 reported that 21 (57%) PVS were located in the mesencephalothalamic region and only 8 (22%) were detected within the subcortical white matter. Interestingly, of all lesions located within the white matter, 50% showed surrounding perilesional signal change on fluid-attenuated inversion recovery/T2-weighted MRI sequences, although no mesencephalothalamic lesion showed this association. In our case, dilated PVS were associated with perifocal edema in the brain parenchyma. Owing to accompanying edematous changes, TPVS are often indistinguishable from neoplastic lesions, such as ganglioglioma, metastatic tumors, or inflammatory diseases such as parasitic infestations.
CONCLUSION
In this report, we described a rare case of spontaneous regression of asymptomatic TPVS. Most studies in the literature have described spontaneous regression primarily in the anterior temporal lobe; it is our opinion that characteristics of PVS at this site differ from those of PVS distributed across other regions.
CONFLICT OF INTEREST
We have no conflict of interest to declare.
Abbreviations
- PVS
perivascular spaces
- TPVS
tumefactive perivascular spaces
- MRI
magnetic resonance imaging
- MCA
middle cerebral artery
- FLAIR
fluid-attenuated inversion recovery
REFERENCES
- 1.Kwee RM, Kwee TC. Virchow-Robin spaces at MR imaging. Radiographics. 2007;27(4):1071–1086. doi: 10.1148/rg.274065722. [DOI] [PubMed]
- 2.Eluvathingal Muttikkal TJ, Raghavan P. Spontaneous regression and recurrence of a tumefactive perivascular space. Neuroradiol J. 2014;27(2):195–202. doi: 10.15274/NRJ-2014-10034. [DOI] [PMC free article] [PubMed]
- 3.Kwee RM, Kwee TC. Tumefactive Virchow-Robin spaces. Eur J Radiol. 2019;111:21–33. doi: 10.1016/j.ejrad.2018.12.011. [DOI] [PubMed]
- 4.Cerase A, Venturi C, Rubenni E, Tarantini B, Pacini F. Complete regression of a temporal stem perivascular space following resection of a pituitary nonfunctioning macroadenoma. AJNR Am J Neuroradiol. 2009;30(1):E4–E5. doi: 10.3174/ajnr.A1244. [DOI] [PMC free article] [PubMed]
- 5.Cerase A, Vallone IM, Muccio CF, Petrini C, Signori G, Venturi C. Regression of dilated perivascular spaces of the brain. Surg Radiol Anat. 2010;32(6):555–561. doi: 10.1007/s00276-009-0603-y. [DOI] [PubMed]
- 6.Tortora F, Cirillo M, Belfiore MP, et al. Spontaneous regression of dilated Virchow-Robin spaces. Neuroradiol J. 2012;25(1):40–44. doi: 10.1177/197140091202500106. [DOI] [PubMed]
- 7.Zhang ET, Inman CB, Weller RO. Interrelationship of the pia mater and the perivascular (Virchow-Robin) spaces in the human cerebrum. J Anat. 1990;170:111–123. [PMC free article] [PubMed]
- 8.Taoka T, Naganawa S. Imaging for central nervous system (CNS) interstitial fluidopathy: disorders with impaired interstitial fluid dynamics. Jpn J Radiol. 2021;39(1):1–14. doi: 10.1007/s11604-020-01017-0. [DOI] [PMC free article] [PubMed]
- 9.Adachi M, Hosoya T, Haku T, Yamaguchi K. Dilated Virchow-Robin spaces: MRI pathological study. Neuroradiology. 1998;40(1):27–31. doi: 10.1007/s002340050533. [DOI] [PubMed]
- 10.Salzman KL, Osborn AG, House P, et al. Giant tumefactive perivascular spaces. AJNR Am J Neuroradiol. 2005;26(2):298–305. [PMC free article] [PubMed]
- 11.Fanous R, Midia M. Perivascular spaces: normal and giant. Can J Neurol Sci. 2007;34(1):5–10. doi: 10.1017/s0317167100005722. [DOI] [PubMed]
- 12.Ahmad FU, Garg A, Singh M, Mishra NK. Giant mesencephalothalamic Virchow-Robin spaces causing obstructive hydrocephalus: A case report. Neuroradiol J. 2007;20(3):303–306. doi: 10.1177/197140090702000310. [DOI] [PubMed]
- 13.Groeschel S, Chong WK, Surtees R, Hanefeld F. Virchow-Robin spaces on magnetic resonance images: normative data, their dilatation, and a review of the literature. Neuroradiology. 2006;48(10):745–754. doi: 10.1007/s00234-006-0112-1. [DOI] [PubMed]
- 14.Tobias McArdle DJ, Haynes Lovell TJ, Lekgabe E, Gaillard F. Opercular perivascular cysts: A proposed new subtype of dilated perivascular spaces. Eur J Radiol. 2020;124:108838. doi: 10.1016/j.ejrad.2020.108838. [DOI] [PubMed]
- 15.Lim AT, Chandra RV, Trost NM, McKelvie PA, Stuckey SL. Large anterior temporal Virchow-Robin spaces: unique MR imaging features. Neuroradiology. 2015;57(5):491–499. doi: 10.1007/s00234-015-1491-y. [DOI] [PubMed]