Abstract
A unique arrangement of promoter elements was found upstream of the bacteriophage P1 particle maturation gene (mat). A P1-specific late-promoter sequence with conserved elements located at positions −22 and −10 was expected from the function of the gene in phage morphogenesis. In addition to a late-promoter sequence, a −35 element and an operator sequence for the major repressor protein, C1, were found. The −35 and −10 elements constituted an active Escherichia coli ς70 consensus promoter, which was converted into a P1-regulated early promoter by the superimposition of a C1 operator. This combination of early- and late-promoter elements regulates and fine-tunes the expression of the particle maturation gene. During lysogenic growth the gene is turned off by P1 immunity functions. Upon induction of lytic growth, the expression of mat starts simultaneously with the expression of other C1-regulated P1 early functions. However, while most of the latter functions are downregulated during late stages of lytic growth the expression of mat continues throughout the entire lytic growth cycle of bacteriophage P1. Thus, the maturation function has a head start on the structural components of the phage particle.
The genomes of bacteriophages contain the genetic information to reprogram bacterial host cells to produce viral particles rather than new bacterial cells. A successful phage infection depends on the precisely regulated expression of this genetic information. Intuitively, viral DNA amplification should occur prior to viral particle formation and DNA packaging, which in turn should precede host cell lysis. Even slight deviations from this developmental program would have dramatic effects on infection efficiency and phage viability. Complex regulatory networks have been elucidated for phages like T4, λ, P2/P4, Mu, and T7, among others. The investigations of various regulatory phage proteins like antiterminators (9, 32), repressors (18, 35), activators (1, 19, 26, 36), sigma factors (8), antisigma factors (15), and RNA polymerases (27) provided major contributions to our understanding of principal regulatory concepts.
For bacteriophage P1 only two major regulatory steps were described, early and late transcriptions (24). As a temperate phage, P1 has the ability to lysogenize its host (51). During lysogenic growth all lytic phage functions, many of which are toxic to the host, have to be silenced. To this end, P1 harbors a complex, tripartite immunity system with the major repressor protein, C1, as central regulator (for reviews on the P1 immunity system, see references 13 and 23). C1 is a DNA-binding repressor protein negatively regulating Escherichia coli consensus-like promoter sequences (12), which are superimposed by a C1 binding site (6, 41). Inactivation of C1 during lytic growth results in early transcription. Most P1 early functions are involved in phage DNA replication, but among them is also the late-promoter activator function gp10 (21, 24). gp10 in turn activates transcription from phage-specific late-promoter sequences, which control the expression of all morphogenetic, lysis control, and lysis functions (10, 22).
Over two decades ago Walker and Walker characterized a large set of P1 amber mutants, creating one of the first P1 linkage maps (43–45). Phages with amber mutations located in the linkage cluster I showed defects in the formation of both head and tail structures. Marker rescue experiments (38) mapped the gene at positions 3 to 4 on the circular P1 map (50). Based on its pleiotropic effect on P1 particle maturation, we termed the gene mat (formerly called gene 1) and considered it a likely candidate to be a late P1 gene. In this study we repeated the mapping experiments, cloned and determined the nucleotide sequence of the mat gene, and studied its transcriptional regulation. We show that mat is controlled by a hybrid promoter including both early and late P1 promoter elements. These results promote the idea that the P1 regulatory cascade is more complex than initially proposed, providing P1 with the ability to fine-tune the expression of its genetic information.
MATERIALS AND METHODS
Standard procedures and DNA sequencing.
Standard DNA techniques, liquid media, and agar plates were used as described by Sambrook et al. (33). Antibiotics were added as appropriate at concentrations of 100 μg/ml for ampicillin and 25 μg/ml for kanamycin. DNA-sequencing reactions were performed as described by Sanger et al. (34), using a Thermo Sequenase-based sequencing kit (Amersham). Restriction endonucleases were used as advised by the manufacturer (Fermentas).
Bacterial strains and bacteriophages.
The E. coli K-12 strains used were UT580 (supD lacZ) (14) and MC1061 (sup0 lacZ) (4). Bacteriophage P1 stocks were propagated as described by Iida and Arber (17). The P1 strains used were P1c1ts225 (25), P1Cm (20), P1 virs am 132 (45), and P1-15::Tn2680 (30).
Plasmids constructed in this work.
The EcoRI-19 restriction fragments (2) of both P1c1ts225 and P1 virs am 132 were cloned into the standard cloning vector pUC19 (49). The resulting plasmids, pHAL255 and pHAL256, respectively, were used in marker rescue experiments as well as to determine the nucleotide sequences of the two restriction fragments. The 399-bp EcoRI/PvuII subfragment of EcoRI-19, cleaved out of pHAL255, was cloned into the EcoRI/SmaI restriction site of the lacZ fusion vector pNM480 (29). The resulting plasmid, pHAL257, expressed a β-galactosidase fusion protein under the control of the mat promoter.
Marker rescue experiments.
Host cells were grown to an optical density at 600 nm of 0.5 in Luria-Bertani (LB) medium supplemented with 20 mM CaCl2. Phage stocks were diluted appropriately in LB. One hundred microliters of a phage dilution was mixed with 100 μl of host culture in a 10-ml glass tube and incubated at room temperature for 15 min to allow phage adsorption. Five milliliters of molten top agar (28) was then added to the tube, thoroughly mixed with the phage and cell mixture, and then quickly spread on an agar plate. The top agar was allowed to set for 15 min before the plates were incubated overnight at 37°C. Regular phage plaques could be detected 16 to 20 h after infection.
EMSA.
To demonstrate that the C1 repressor protein specifically recognizes the operator sequence Op2b, located on the P1 EcoRI-19 fragment, an electrophoretic mobility shift assay (EMSA) was used. Purified C1 repressor protein was isolated following the protocol of Velleman and Parbus (42). The 1,043-bp P1 EcoRI-19 restriction fragment was cleaved into two subfragments, 399 and 644 bp in length, using the restriction endonuclease PvuII. Constant amounts of DNA were then incubated with increasing amounts of purified C1 protein in a nondenaturing buffer containing 20 mM Tris-HCl (pH 7.6), 50 mM EDTA, 1 mM dithiothreitol, 10% glycerol (vol/vol), 100 μg of bovine serum albumin per ml, and 2.5 mM MgCl2. Samples were incubated for 15 min at 37°C and then separated on a 2% agarose gel using 40 mM Tris-HCl (pH 7.9), 5 mM sodium acetate, and 1 mM EDTA as running buffer. The agarose gel was stained with ethidium bromide and destained in 5 mM MgSO4, and DNA bands were detected under UV light.
Detection of early and late promoter activities.
To study early promoter activity and the effect of C1 on the expression of mat, strains carrying the indicator plasmid pHAL257 were grown to exponential growth at 37°C in LB medium supplemented with the appropriate antibiotics. Aliquots were harvested and assayed for β-galactosidase activity according to the method of Miller (28) when the cultures reached an optical density at 600 nm of 0.6. To detect late promoter activity, P1 lysogenic strains carrying the prophage P1-15::Tn2680 and one of the three LacZ-indicator plasmids, pHAL257, pAW533 (10), and pAW919 (24), were grown at 30°C to an optical density at 600 nm of 0.2. Cultures were then evenly split and one half was incubated further at 30°C, while the second half was transferred to a 42°C water bath. This temperature shift induced the temperature-sensitive P1-15::Tn2680 prophage to lytic growth. Aliquots removed at various times after heat induction were assayed for β-galactosidase activity as above.
Nucleotide sequence accession number.
The nucleotide sequence determined in this study is part of the complete nucleotide sequence of the bacteriophage P1 genome and was deposited in GenBank under the accession number AF234173 (M. B. Lobocka, D. Rose, M. Rusin, A. Samojedny, M. B. Yarmolinsky, H. Lehnherr, and F. C. Blattner, unpublished results).
RESULTS
Mapping of the mat gene.
An earlier study by Sternberg localized a gene 1 amber mutation to the P1 EcoRI-19 restriction fragment (38). A marker rescue experiment was performed in order to confirm this result for the P1 virs am 132 phage in our hands. The P1 EcoRI-19 restriction fragment was cloned into the cloning vector pUC19, resulting in plasmid pHAL255. A stock solution of P1 virs am 132 was then titrated on derivatives of the sup0 strain MC1061, harboring either no plasmid, the cloning vector pUC19, or the plasmid pHAL255. Complementation can be expected in the presence of pHAL255 if the entire mat gene is present on pHAL255 and expression occurs during the infection with P1 virs am 132. Stable marker rescue can occur by a recombination event between pHAL255 and the replicating P1 virs am 132 genome even if only a part of the mat reading frame, covering the location of the amber mutation, is present on pHAL255. The results of these experiments are shown in Table 1. The reversion rate of P1 virs am 132 was about 1 in 105, seen as difference in plating efficiency on the strains UT580 (supD) and MC1061 (sup0). In the presence of pHAL255, P1 virs am 132 grew almost as efficiently as on the suppressor host UT580. To distinguish between complementation and stable marker rescue, single plaques grown on MC1061/pHAL255 were picked and the phages contained within were analyzed for their respective phenotypes. The plaques contained a mixture of phages with a ratio of about 1 wild-type phage per 100 amber mutants. That the majority of the progeny phages were phenotypically amber indicated that complementation occurred. Thus, a functional copy, if not the entire mat gene, had to be present on the P1 EcoRI-19 restriction fragment.
TABLE 1.
Marker rescue experimenta
| Host strain | No. of PFU observed after infection with:
|
|
|---|---|---|
| P1c1ts | P1 virs am 132 | |
| UT580 (supD) | 6 × 1010 | 1 × 1010 |
| MC1061 (sup0) | 2 × 1010 | 3 × 105 |
| MC1061/pUC19 | 4 × 1010 | 3 × 105 |
| MC1061/pHAL255 | 6 × 1010 | 2 × 109 |
Stock solutions of the phages P1c1ts and P1 virs am 132 were titrated on the indicated host strains. Phage plaques were counted after overnight incubation of the agar plates at 37°C. Values represent averages of six independent plating experiments.
The nucleotide sequence of the P1 EcoRI-19 restriction fragment was determined in order to identify the open reading frame of the mat gene and locate its promoter region. Figure 1 shows the nucleotide sequence of the entire EcoRI-19 restriction fragment. A 219-amino-acid open reading frame was found to be flanked by the ends of the genes ref (48) and res (16). That this open reading frame corresponded to the mat gene was confirmed by the presence of a single mismatch to the wild-type sequence in the fragment cloned from P1 virs am 132. A transition from C to T at position 571 (Fig. 1) changed a CAG glutamine codon to a TAG amber stop codon. The Mat protein has a calculated molecular mass of 25.6 kDa, a net charge of +4 at neutral pH, and an isoelectric point of 9.7. Thorough sequence homology searches did not result in any significant match between mat and any gene currently present in publicly available databases.
FIG. 1.
Nucleotide sequence of the P1 EcoRI-19 restriction fragment. The recognition sequences of the EcoRI restriction endonuclease are underlined at both ends of the nucleotide sequence. Promoter elements and the start codon of the mat gene are in bold. The transition leading to an amber stop codon in P1 virs am 132 is indicated by a T above the underlined wild-type CAG codon. Open reading frames are translated into the single-letter amino acid code below the sequence. The genes ref and mat are oriented clockwise, while the res gene is oriented counterclockwise.
The intergenic region between the genes ref and mat revealed an unusual assortment of promoter elements. E. coli −10 and −35 standard promoter elements, with a suboptimal spacing of 19 bp (12), a putative binding site for the major P1 repressor protein C1 (Op2b) (7), and a putative late P1-specific −22 operator sequence (22), were present at appropriate positions upstream of the mat gene. The composite nature of the promoter sequence suggested that the mat gene could be expressed both early and late during lytic growth and that it could be repressed by C1 during lysogenic growth.
The expression of mat is regulated by P1 immunity functions.
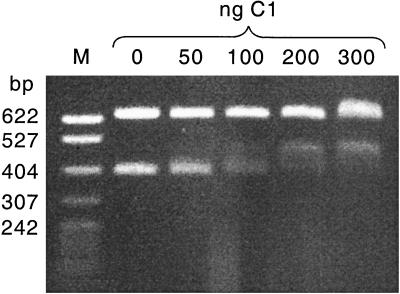
To test whether the putative operator sequence Op2b was a true binding site for the P1 C1 repressor protein, a mobility shift assay was used (Fig. 2). Two DNA fragments, 399 and 644 bp in length, were incubated with increasing concentrations of purified C1 repressor protein and then separated by gel electrophoresis. The larger DNA fragment did not contain a C1 binding site and served as a control to detect nonspecific DNA-binding activity of C1. The migration of this fragment was relatively undisturbed even in the presence of high concentrations of C1. The 399-bp DNA fragment contained Op2b, and its migration was specifically retarded in the presence of C1. Thus, Op2b was a bona fide C1 binding site. That the interaction between C1 and Op2b also had an effect on the expression of the mat gene was shown using a β-galactosidase activity assay. An indicator plasmid, pHAL257, was constructed, expressing a β-galactosidase fusion protein under the control of the mat promoter (see Materials and Methods). Expression from pHAL257 was then assayed in the absence or presence of P1 immunity functions. Table 2 lists the results of these experiments. In the absence of any P1 regulatory functions the mat promoter turned out to be constitutively active. This activity indicated that the −35 and −10 elements seen in Fig. 1 constitute an active E. coli promoter. This result identified the mat promoter as an early phage promoter, given that any phage promoter with the potential to be expressed immediately after phage infection falls into this category. In the presence of a plasmid-borne copy of the c1 gene the expression level of the mat promoter dropped about 30-fold, demonstrating a direct effect of C1 on mat expression. In the presence of a P1 lysogen, an autoregulated immunity control circuit involving all P1 immunity functions was established in the cell (23) and the expression from the mat promoter was completely turned off. These results allowed us to conclude that the mat gene is expressed from an immunity-regulated, early promoter.
FIG. 2.
EMSA with purified C1 protein. The restriction endonuclease PvuII was used to cleave the P1 EcoRI-19 DNA fragment into two subfragments of 644 and 399 bp. The smaller DNA fragment contained the Op2b putative binding site for the C1 repressor protein. A constant amount of DNA (2 μg per reaction) was incubated with increasing amounts of C1 protein in a total reaction volume of 20 μl. A standard size marker with fragment sizes indicated in base pairs was loaded on the left side of the gel.
TABLE 2.
Early expression of the mat promotera
| Strain | P1 immunity function(s) | β-Galactosidase activity (Miller units) |
|---|---|---|
| UT580/pNM480 | 2 ± 0.9 | |
| UT580/pHAL257 | 177 ± 59 | |
| UT580/pHAL257, pAM2b | c1 | 6 ± 0.8 |
| UT580/pHAL257, P1Cm | All | 2 ± 0.9 |
Strains were assayed for β-galactosidase activity as described in Materials and Methods. Values represent averages of at least four independent measurements.
Temporal expression pattern of the mat gene.
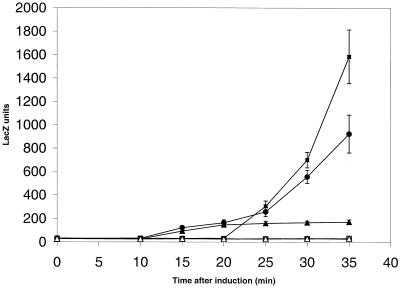
The presence of a late relative operator sequence (22) with an optimal spacing to the −10 region of the mat promoter (Fig. 1) indicated that the mat promoter might also be active late during P1 development. To test this possibility, a temporal expression profile of the mat promoter was established (Fig. 3.). In strains harboring a temperature-sensitive P1 prophage and either the indicator plasmid pHAL257 or one of the two control plasmids, pAW533 (10) and pAW919 (24), the prophage was induced to lytic growth. The first control plasmid, pAW533, expressed lacZ from the well-studied P1 tail fiber promoter Ps (10, 22), while the second control plasmid, pAW919, expressed lacZ from the early promoter Pr94, controlling gene 10 (21, 24). A typical late-promoter activation profile with an onset of promoter activity 20 to 30 min following phage induction was observed for Ps. During the second half of the lytic growth cycle the expression profile of the mat promoter was very similar to this profile. Differences, however, could be observed at the onset of promoter activity. Low levels of mat promoter activity could already be detected between 10 and 20 min following induction, matching the initial profile of the expression of gene 10 (Fig. 3) and other studied P1 early functions (37, 39). During lysogenic growth, i.e., when assayed at 30°C, all three promoters were repressed. These results showed that the P1 mat gene was expressed from a hybrid promoter, containing both early and late promoter elements.
FIG. 3.
Temporal expression pattern of the mat promoter. Strains carrying the temperature-sensitive P1 prophage P1-15::Tn2680 and one of the three indicator plasmids, pHAL257 (circles), pAW533 (boxes), and pAW919 (triangles), were assayed for β-galactosidase activity under inducing (42°C, filled symbols) and noninducing (30°C, open symbols) conditions. The values shown represent averages of six independent measurements and standard deviations are indicated.
DISCUSSION
During phage infection the energy resources of the host are optimally diverted towards the production of progeny particles. The complexity of this task is adequately reflected by the precisely regulated and finely tuned developmental programs specified by bacteriophages. A basic model for the lytic development of bacteriophage P1 has been proposed (23). Upon P1 infection a set of early functions battle for the switch between lytic and lysogenic growth (13). If this battle results in the inactivation of the major repressor protein, C1, all P1 early proteins are expressed and lytic DNA replication is initiated (11). One of the early proteins, gp10, then mediates a direct switch from early to late transcription (24). The results presented here allowed us to refine this model. Using a combination of early and late promoter elements, bacteriophage P1 is able to express the particle maturation function during both transcriptional stages, thus blurring a strict contrast between early and late transcriptions.
Such a strategy to organize gene expression is reminiscent of bacteriophage T4. The T4 transcriptional program (31), though more intricately complex and much better understood than the one of P1, is subdivided into three stages, early (46), middle (40), and late (47). However, several T4 genes and operons are under multiple transcriptional controls (31). Both T4 early and middle (3), as well as early and late (5), promoters can overlap in sequence or are positioned appropriately to sequentially express a common set of genes (31). There is evidence that at least two more P1 genes are also under dual transcriptional control. The P1 pacase proteins PacA and PacB were shown to be expressed early from a promoter located upstream of gene 10 (24, 37). After the switch from early to late transcription the pacase proteins are then also expressed late from a promoter located directly upstream of pacA (H. Lehnherr, unpublished data). A careful analysis of the complete nucleotide sequence of the bacteriophage P1 genome is in progress (Lobocka, Rose, Rusin, Samojedny, Yarmolinsky, Lehnherr, and Blattner, unpublished). It might reveal whether other functions show expression profiles similar to those found for mat or pac.
The dual expression provides the Mat protein with a head start on P1 late proteins expressed exclusively from late promoter sequences and allows the continued expression of Mat compared to proteins expressed only from early promoters. Speculations about the biological significance of such an expression profile are hampered by the absence of information about the function of Mat during phage morphogenesis. The amber mutations characterized by Walker and Walker (45) showed complete phage particles with empty heads and unstable tails, with tail sheaths in various stages of contraction. However, it is unknown whether the Mat protein is a structural component of the final P1 particle or is only transiently required during the assembly process. No further information could be gained from in silico analyses, as Mat did not show any significant match to proteins in accessible databases. A biochemical characterization of the Mat protein will thus be a prerequisite to fathom the reasons behind the presence of the complex arrangement of promoter elements found upstream of the mat gene.
ACKNOWLEDGMENTS
We thank J. T. Walker for generously providing us with an entire set of P1 amber mutants, among them the P1 virs am 132 phage used in this study. We also thank T. V. Ilyina for critical comments on the manuscript.
REFERENCES:
- 1.Artsimovitch I, Murakami K, Ishihama A, Howe M M. Transcription activation by the bacteriophage Mu Mor protein requires the C-terminal regions of both alpha and sigma70 subunits of Escherichia coli RNA polymerase. J Biol Chem. 1996;271:32343–32348. doi: 10.1074/jbc.271.50.32343. [DOI] [PubMed] [Google Scholar]
- 2.Bächi B, Arber W. Physical mapping of BglII, BamHI, EcoRI, HindIII and PstI restriction fragments of bacteriophage P1 DNA. Mol Gen Genet. 1977;153:311–324. doi: 10.1007/BF00431596. [DOI] [PubMed] [Google Scholar]
- 3.Brody E, Rabussay D, Hall D H. Regulation of transcription of prereplicative genes. In: Mathews C K, Kutter E M, Mosig G, Berget P B, editors. Bacteriophage T4. Washington, D.C.: American Society for Microbiology; 1983. pp. 174–183. [Google Scholar]
- 4.Casadaban M J, Cohen S N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980;138:179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- 5.Chang A. Initiation of DNA replication at bacteriophage T4 origin. Nashville, Tenn: Vanderbilt University; 1992. [Google Scholar]
- 6.Dreiseikelmann B, Velleman M, Schuster H. The c1 repressor of bacteriophage P1. Isolation and characterization of the repressor protein. J Biol Chem. 1988;263:1391–1397. [PubMed] [Google Scholar]
- 7.Eliason J L, Sternberg N L. Characterization of the binding sites of c1 repressor of bacteriophage P1. Evidence for multiple asymmetric sites. J Mol Biol. 1987;198:281–293. doi: 10.1016/0022-2836(87)90313-5. [DOI] [PubMed] [Google Scholar]
- 8.Geiduschek E P. Regulation of expression of the late genes of bacteriophage T4. Annu Rev Genet. 1991;25:437–460. doi: 10.1146/annurev.ge.25.120191.002253. [DOI] [PubMed] [Google Scholar]
- 9.Greenblatt J, Mah T F, Legault P, Mogridge J, Li J, Kay L E. Structure and mechanism in transcriptional antitermination by the bacteriophage lambda N protein. Cold Spring Harbor Symp Quant Biol. 1998;63:327–336. doi: 10.1101/sqb.1998.63.327. [DOI] [PubMed] [Google Scholar]
- 10.Guidolin A, Zingg J-M, Lehnherr H, Arber W. Bacteriophage P1 tail-fibre and dar operons are expressed from homologous phage-specific late promoter sequences. J Mol Biol. 1989;208:615–622. doi: 10.1016/0022-2836(89)90152-6. [DOI] [PubMed] [Google Scholar]
- 11.Hansen E B. Structure and regulation of the lytic replicon of phage P1. J Mol Biol. 1989;207:135–149. doi: 10.1016/0022-2836(89)90445-2. [DOI] [PubMed] [Google Scholar]
- 12.Hawley D K, McClure W R. Compilation and analysis of Escherichia coli promoter and DNA sequences. Nucleic Acids Res. 1983;11:2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heinrich J, Velleman M, Schuster H. The tripartite immunity system of phages P1 and P7. FEMS Microbiol Rev. 1995;17:121–126. doi: 10.1111/j.1574-6976.1995.tb00193.x. .EcoP15 mod gene and some EcoP1 mod mutants. J. Mol. Biol. 200:23–29. [DOI] [PubMed] [Google Scholar]
- 14.Hübner P, Haffter P, Iida S, Arber W. Bent DNA is needed for recombinational enhancer activity in the site-specific recombination system Cin of bacteriophage P1. The role of FIS protein. J Mol Biol. 1989;205:493–500. doi: 10.1016/0022-2836(89)90220-9. [DOI] [PubMed] [Google Scholar]
- 15.Hughes K T, Mathee K. The anti-sigma factors. Annu Rev Microbiol. 1998;52:231–286. doi: 10.1146/annurev.micro.52.1.231. [DOI] [PubMed] [Google Scholar]
- 16.Humbelin M, Suri B, Rao D N, Hornby D P, Eberle H, Pripfl T, Kenel S, Bickle T A. Type III DNA restriction and modification systems EcoP1 and EcoP15. Nucleotide sequence of the EcoP1 operon, the EcoP15 mod gene and some EcoP1 mod mutants. J Mol Biol. 1988;200:23–29. doi: 10.1016/0022-2836(88)90330-0. [DOI] [PubMed] [Google Scholar]
- 17.Iida S, Arber W. Plaque forming specialized transducing phage P1: isolation of P1CmSmSu, a precursor of P1Cm. Mol Gen Genet. 1977;153:259–269. doi: 10.1007/BF00431591. [DOI] [PubMed] [Google Scholar]
- 18.Johnson A D, Poteete A R, Lauer G, Sauer R T, Ackers G K, Ptashne M. lambda Repressor and cro—components of an efficient molecular switch. Nature. 1981;294:217–223. doi: 10.1038/294217a0. [DOI] [PubMed] [Google Scholar]
- 19.Julien B, Pountney D, Christie G E, Calendar R. Mutational analysis of a satellite phage activator. Gene. 1998;223:129–134. doi: 10.1016/s0378-1119(98)00161-9. [DOI] [PubMed] [Google Scholar]
- 20.Kondo E, Mitsuhashi S. Drug resistance of enteric bacteria. IV. Active transducing bacteriophage P1CM produced by the recombination of R factor with bacteriophage P1. J Bacteriol. 1964;88:1266–1276. doi: 10.1128/jb.88.5.1266-1276.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lehnherr H, Guidolin A, Arber W. Bacteriophage P1 gene 10 encodes a trans-activating factor required for late gene expression. J Bacteriol. 1991;173:6438–6445. doi: 10.1128/jb.173.20.6438-6445.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lehnherr H, Guidolin A, Arber W. Mutational analysis of the bacteriophage P1 late promoter sequence Ps. J Mol Biol. 1992;228:101–107. doi: 10.1016/0022-2836(92)90494-5. [DOI] [PubMed] [Google Scholar]
- 23.Lehnherr H, Meyer J. Enterobacteria phage P1 (Myoviridae) In: Granoff A, Webster R G, editors. Encyclopedia of virology. London, United Kingdom: Academic Press Ltd.; 1999. pp. 455–461. [Google Scholar]
- 24.Lehnherr H, Velleman M, Guidolin A, Arber W. Bacteriophage P1 gene 10 is expressed from a promoter-operator sequence controlled by C1 and Bof proteins. J Bacteriol. 1992;174:6138–6144. doi: 10.1128/jb.174.19.6138-6144.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lennox E S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955;1:190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- 26.Margolin W, Howe M M. Activation of the bacteriophage Mu lys promoter by Mu C protein requires the sigma 70 subunit of Escherichia coli RNA polymerase. J Bacteriol. 1990;172:1424–1429. doi: 10.1128/jb.172.3.1424-1429.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McAllister W T, Raskin C A. The phage RNA polymerases are related to DNA polymerases and reverse transcriptases. Mol Microbiol. 1993;10:1–6. doi: 10.1111/j.1365-2958.1993.tb00897.x. [DOI] [PubMed] [Google Scholar]
- 28.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 29.Minton N P. Improved plasmid vectors for the isolation of translational lac gene fusions. Gene. 1984;31:269–273. doi: 10.1016/0378-1119(84)90220-8. [DOI] [PubMed] [Google Scholar]
- 30.Mollet B, Iida S, Arber W. An active variant of the prokaryotic transposable element IS903 carries an amber stop codon in the middle of an open reading frame. Mol Gen Genet. 1985;199:534–536. doi: 10.1007/BF00330770. [DOI] [PubMed] [Google Scholar]
- 31.Mosig G, Hall D H. Gene expression: a paradigm of integrated circuits. In: Karam J D, editor. Molecular biology of bacteriopage T4. Washington, D.C.: ASM Press; 1994. pp. 127–131. [Google Scholar]
- 32.Roberts J W, Yarnell W, Bartlett E, Guo J, Marr M, Ko D C, Sun H, Roberts C W. Antitermination by bacteriophage lambda Q protein. Cold Spring Harbor Symp Quant Biol. 1998;63:319–325. doi: 10.1101/sqb.1998.63.319. [DOI] [PubMed] [Google Scholar]
- 33.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 34.Sanger F, Nicklen S, Coulsen A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sauer R T, Jordan S R, Pabo C O. Lambda repressor: a model system for understanding protein-DNA interactions and protein stability. Adv Protein Chem. 1990;40:1–61. doi: 10.1016/s0065-3233(08)60286-7. [DOI] [PubMed] [Google Scholar]
- 36.Sharma M, Marshall P, Hinton D M. Binding of the bacteriophage T4 transcriptional activator, MotA, to T4 middle promoter DNA: evidence for both major and minor groove contacts. J Mol Biol. 1999;290:905–915. doi: 10.1006/jmbi.1999.2928. [DOI] [PubMed] [Google Scholar]
- 37.Skorupski K, Pierce J C, Sauer B, Sternberg N. Bacteriophage P1 genes involved in the recognition and cleavage of the phage packaging site (pac) J Mol Biol. 1992;223:977–989. doi: 10.1016/0022-2836(92)90256-j. [DOI] [PubMed] [Google Scholar]
- 38.Sternberg N. A characterization of bacteriophage P1 DNA fragments cloned in a lambda vector. Virology. 1979;96:129–142. doi: 10.1016/0042-6822(79)90179-x. [DOI] [PubMed] [Google Scholar]
- 39.Sternberg N, Sauer B, Hoess R, Abremski K. Bacteriophage P1 cre gene and its regulatory region. Evidence for multiple promoters and for regulation by DNA methylation. J Mol Biol. 1986;187:197–212. doi: 10.1016/0022-2836(86)90228-7. [DOI] [PubMed] [Google Scholar]
- 40.Stitt B, Hinton D M. Regulation of middle-mode transcription. In: Karam J D, editor. Molecular biology of bacteriophage T4. Washington, D.C.: ASM Press; 1994. pp. 142–160. [Google Scholar]
- 41.Velleman M, Dreiseikelmann B, Schuster H. Multiple repressor binding sites in the genome of bacteriophage P1. Proc Natl Acad Sci USA. 1987;84:5570–5574. doi: 10.1073/pnas.84.16.5570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Velleman M, Parbus S. Purification of the C1 repressor of bacteriophage P1 by fast protein liquid chromatography. J Chromatogr. 1992;625:41–46. doi: 10.1016/0021-9673(92)87219-x. [DOI] [PubMed] [Google Scholar]
- 43.Walker D H, Jr, Walker J T. Genetic studies of coliphage P1. III. Extended genetic map. J Virol. 1976;20:177–187. doi: 10.1128/jvi.20.1.177-187.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walker J T, Walker D H., Jr Mutations in coliphage P1 affecting host cell lysis. J Virol. 1980;35:519–530. doi: 10.1128/jvi.35.2.519-530.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walker J T, Walker D H., Jr Coliphage P1 morphogenesis: analysis of mutants by electron microscopy. J Virol. 1983;45:1118–1139. doi: 10.1128/jvi.45.3.1118-1139.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilkens K, Rüger W. Transcription from early promoters. In: Karam J D, editor. Molecular biology of bacteriophage T4. Washington, D.C.: ASM Press; 1994. pp. 132–141. [Google Scholar]
- 47.Williams K P, Kassavetis G A, Herendeen D R, Geiduschek E P. Regulation of late-gene expression. In: Karam J D, editor. Molecular biology of bacteriophage T4. Washington, D.C.: ASM Press; 1994. pp. 161–175. [Google Scholar]
- 48.Windle B E, Laufer C S, Hays J B. Sequence and deletion analysis of the recombination enhancement gene (ref) of bacteriophage P1: evidence for promoter-operator and attenuator-antiterminator control. J Bacteriol. 1988;170:4881–4889. doi: 10.1128/jb.170.10.4881-4889.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 50.Yarmolinsky M B, Lobocka M B. Bacteriophage P1. In: O'Brian S J, editor. Locus maps of complex genomes. New York, N.Y: Cold Spring Harbor Laboratory Press; 1993. pp. 1.50–1.61. [Google Scholar]
- 51.Yarmolinsky M B, Sternberg N L. Bacteriophage P1. In: Calendar R, editor. The bacteriophages. New York, N.Y: Plenum Publishing Corp.; 1988. pp. 291–438. [Google Scholar]