Abstract
Although systemic therapy is standard management for patients with metastatic disease, several recent reports have indicated that an addition of local therapies including stereotactic body radiation therapy (SBRT) for patients with oligometastatic disease (OMD) could improve survival. The lung is the most common site of distant metastasis from many solid tumors, and the strategy of SBRT, such as dose-fraction schedules, timing, etc., would be different depending on the type of primary tumor, location, and patterns of OMD. This review describes the role of SBRT with curative-intent for patients with pulmonary OMD for each of these variables. First, differences according to the type of primary tumor, for which many studies suggest that SBRT-mediated local control (LC) for patients with pulmonary OMD from colorectal cancer (CRC) is less successful than for those from non-CRC tumors. In addition, higher dose-fraction schedules seemed to correlate with higher LC; hence, different SBRT treatment strategies may be needed for patients with pulmonary OMD from CRC relative to other tumors. Second, differences according to location, where the safety of SBRT for peripheral pulmonary tumors has been relatively well established, but safety for central pulmonary tumors including pulmonary OMD is still considered controversial. To determine the optimal dose-fraction schedules, further data from prospective studies are still needed. Third, differences according to the patterns of OMD, the number of metastases and the timing of SBRT whereby 1–5 lesions in most patients and patients with synchronous or metachronous OMD are considered good candidates for SBRT. We conclude that there are still several problems in defining suitable indications for local therapy including SBRT, and that further prospective studies are required to resolve these issues.
Keywords: Stereotactic body radiation therapy (SBRT), Pulmonary oligometastatic disease (OMD)
Introduction
According to the 8th Edition of the Union for International Cancer Control (UICC) TNM classification of lung cancer, single metastatic lesions in a single distant organ result in an M1b classification, because of the significant prognostic relevance of M1b staging versus multiple metastatic lesions in a single or multiple distant organs (M1c) [1]. Thus, a small number of metastases, such as reflected by M1b staging of lung cancer, are considered as causing oligometastatic disease (OMD), which is an intermediate state between localized and systemically metastasized disease [2]. Although systemic therapy is standard management for patients with metastatic disease, several recent reports suggest that an addition of local therapies including radiation therapy, such as stereotactic body radiation therapy (SBRT), for patients with OMD could improve survival [3–5]. However, OMD encompasses heterogeneous patients, and it remains unknown which patients would be eligible for local therapies. To proceed further, for prospective studies to determine eligibility for and the role of local therapies, the European Society for Radiotherapy and Oncology (ESTRO) and European Organisation for Research and Treatment of Cancer (EORTC) provided a classification of OMD in 2020 [6]. First, OMD is divided into two groups based on the history of polymetastatic disease, namely, genuine OMD (no history of polymetastatic disease) and induced OMD (previous history of polymetastatic disease). The latter refers to limited metastatic lesions for which local treatment is possible together with systemic chemotherapy when multiple metastases are present at the time of diagnosis. Genuine OMD is subclassified into de-novo OMD (first time diagnosis of OMD) and repeated OMD (previous history of OMD), which is defined as limited metastatic lesions newly re-progressing after local treatment for OMD. Finally, de-novo OMD is further subclassified into synchronous OMD, which is defined as limited metastatic lesions present at the same time, usually within about 6 months, and metachronous OMD, which is defined as limited metastatic lesions newly progressing after local treatment for the primary site, also usually after 6 months or more.
The lung is the most common site of distant metastasis from many solid tumors, such as primary lung cancer itself, colorectal cancer (CRC), head and neck cancer, renal cell cancer, breast cancer, soft tissue sarcoma, and others. About 30% of all patients with cancer will develop lung metastases at some point in the course of their disease [7]. Local therapies for patients with pulmonary OMD include resection, radiation therapy, and radiofrequency ablation. Although resection is recommended as the first choice of treatment for patients with pulmonary OMD, especially from CRC [8], radiation therapy also plays an important role as a local therapy. Curative radiation therapy is very similar to SBRT, the strategy for which, such as dose-fraction schedules, timing, etc., differs according to the type of primary tumor, location, and patterns of OMD. This review describes the role of curative-intent SBRT for patients with pulmonary OMD separately for each situation.
Differences according to the primary tumor: non-lung primary malignancies or primary lung cancer
Treatment strategies for OMD are different for different primary tumors. Shultz et al. suggested that there are two scenarios, pulmonary metastases from non-lung primary malignancies and primary lung cancer [9].
SBRT for pulmonary OMD from non-lung primary malignancies
Table 1 summarizes the outcome of SBRT for pulmonary OMD. CRC is the most common tumor of origin for pulmonary OMD from non-lung primary malignancies. Many authors reported differences in treatment results for pulmonary CRC-OMD compared with other tumors [10–24]. Several retrospective studies including only patients with pulmonary OMD from CRC showed that 2- or 3-year local control (LC) and overall survival (OS) rates were 60–70% and 50–64%, respectively, with low toxicities when using various different dose-fractionation schedules [14, 15, 17, 18]. Takeda et al. reported a retrospective comparison of CRC and non-CRC origins using the same dose of 50 Gy in five fractions, reporting a significant difference of 2 year LC (72% in CRC, 94% in non-CRC, p < 0.05) [12]. Helou et al. reported a prospective cohort study comparing the results of treating OMD of CRC-versus-non-CRC origins [19]. Although higher dose-fraction schedules were used for patients with CRC-OMD, LC was significantly lower than for OMD of non-CRC origin (2 year LC 76.4% in CRC, 91.7% in non-CRC, p < 0.001). Yamamoto et al. reported a large retrospective study of 1378 patients, indicating that LC of CRC origin OMD was also significantly lower for non-CRC (3 year LC 65.6% in CRC, 86.8% in non-CRC, p < 0.001) [23]. In addition, multivariate analysis of factors affecting LC showed that a CRC origin was significantly associated with worse LC. On the other hand, Guckenberger et al. found no significant difference in the likelihood of tumor control between pulmonary metastases from primary tumors of lung (148 patients), CRC (133 patients), or kidney (56 patients) origin [25]. Further prospective studies are needed, but in general, it can be said that LC of pulmonary OMD from CRC is worse than for non-CRC, according to the many studies shown in Table 1. Given that, what are the optimal dose-fraction schedules for pulmonary CRC-OMD? Takeda et al. reported that 2 year LC was 100% for 21 patients (12 liver, 9 lung) with CRC-OMD when using a higher dose-fraction schedule, such as a maximum dose of 83–100 Gy in 5 fractions (50–60 Gy in 5 fractions, 60% isodose) [26]. Helou et al. compared LC in 56 patients who received < 60 Gy in 4–5 fractions and 45 patients who received 60 Gy in 4 fractions and showed that delivering 60 Gy in 4 fractions was independently associated with a lower hazard of local failure (subdistribution hazard ratio 0.271, 95% confidence interval 0.078–0.940, p = 0.040) [19]. According to a systematic review of SBRT for oligometastatic CRC, in which the biological effective dose (BED) 10 (α/β = 10) ranged from 51.3 to 262.5 Gy, higher BED10 seems to correlate with higher LC [27]. Figures 1 and 2 show complete responses and local recurrence, respectively, after SBRT in patients with pulmonary CRC-OMD.
Table 1.
SBRT for pulmonary oligometastatic disease (OMD)
| Author/year | Study design | Patients | Lesions | (% CRC*) | Dose/fraction (Gy/fr) | Prescription | Local control | Overall survival | Toxicity grade≧3 |
|---|---|---|---|---|---|---|---|---|---|
| Norihisa 2005, Japan [10] | Retrospective | 34 | 43 | 26.5% | 42–60 Gy/3 fr | Isocenter | 90% (2y) | 84.3% (2y) | 3% |
| Rusthoven 2009, USA[11] | Phase I/II | 38 | 63 | 23.7% | 48- 60 Gy/ 3fr | 80–90% isodose | 96% (2y) | 39% (2y) | 7.9% |
| Takeda 2011, Japan [12] | Retrospective | 34 | 44 | CRC: 15 pts | 50 Gy/ 5fr | 80% isodose | CRC 72% (2y) | N.A | 3% |
| Non-CRC: 19 pts | Non-CRC 94% (2y) | N.A | |||||||
| Widder 2013, Nertherlands [13] | Retrospective | 42 | N.A.** | 73.8% | 60 Gy/ 3fr | N.A | 94% (2y) | 62% (3y) | 2.4% |
| Comito 2014, Italy [14] | Retrospective | 41 | 60 | 100% | 48–75 Gy/3-4fr | PTV D95% | 70% (3y) | 58% (3y) | 0% |
| Jung 2015, Korea [15] | Retrospective | 50 | 79 | 100% | 48 Gy/4 fr (median) | 85–90% isodose | 70.6% (3y) | 64% (3y) | 0% |
| Rieber 2016, Germany [16] | Retrospective | 700 | N.A | 21.9% | 3–33 Gy × 1-13fr | 88.7% isodose (median) | 81.2% (2y) | 54.4% (2y) | 6.5% |
| Agolli 2016, Germany [17] | Retrospective | 44 | 69 | 100% | 23–45 Gy/1-3fr | 95% isodose | 60.2% (2y) | 50.8% (3y) | 0% |
| Jingu, 2017, Japan [18] | Retrospective | 93 | 104 | 100% | 40-65 Gy/3-15fr | Isocenter (83%) | 65% (3y) | 56% (3y) | 2% |
| Helou, 2017, UK [19] | Prospective cohort | 120 | 184 | CRC: 101 pts | 56-60 Gy/4fr | PTV D95% | 76.4% (2y) | N.A | 1.7% |
| Non-CRC: 83 pts | 48-52 Gy/4fr | PTV D95% | 91.7% (2y) | N.A | |||||
| Osti, 2018, Italy [20] | Retrospective | 129 | 166 | 31.7% | 30 Gy/1fr | 95% isodose | 80.1% (3y) | 34% (3y) | 7.4% |
| Sharma, 2018, Nertherland [21] | Retrospective | 206 | 327 | 57.3% | 30-60 Gy/1-8fr | 70–90% isodose | 85% (2y) | 36% (2y) | 2% |
| Berkovic, 2020, Belgium [22] | Retrospective | 104 | 132 | 33.7% | 20-60 Gy/3 or 5fr | 80% isodose | 77.8% (3y) | 72% (3y) | 2% |
| Yamamoto 2020, Japan [23] | Retrospective | 1378 | 1547 | 25.3% | 48 Gy/4fr | Isocenter (71.3%) | 81.3% (3y) | 72% (3y) | 2.5% |
| Siva 2021, Australia [24] | Randomized Phase II | 45 | 69 | 46.7% | 28 Gy/ 1fr | PTV D99% | 64% (3y) | 81% (3y) | 5% |
| 45 | 69 | 46.7% | 48 Gy/ 4fr | PTV D99% | 80% (3y) | 67% (3y) | 3% |
*CRC colorectal cancer, **NA not available, #Grade≧2, # PTV D95%/99%: the dose covering 95%/99% of the planning target volume (PTV)
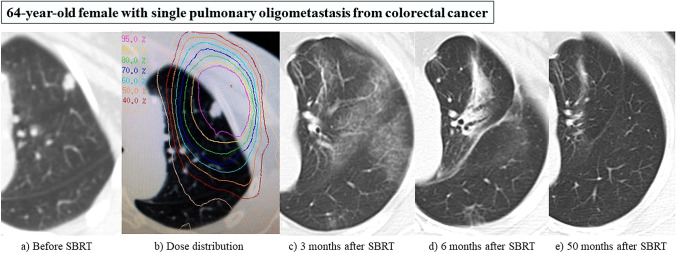
Fig. 1.
a 64-year-old female with single pulmonary oligometastasis from colorectal cancer. a Before SBRT. b Dose distribution: 48 Gy in 4 fractions, isocenter prescription (BED10 = 105.6 Gy). c 3 months after SBRT: Grade 1 radiation pneumonitis. d 6 months after SBRT: Post-irradiation change gradually shrinking. e 50 months after SBRT: Complete response
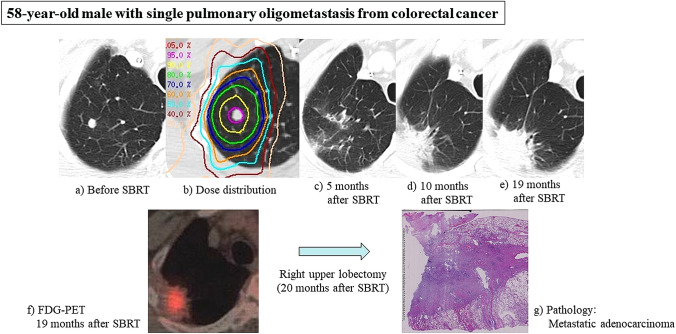
Fig. 2.
a 58-year-old male with single pulmonary oligometastasis from colorectal cancer. a Before SBRT. b Dose distribution: 56 Gy in 4 fractions, isocenter prescription (BED10 = 134.4 Gy). c 5 months after SBRT: Grade 1 radiation pneumonitis. d 10 months after SBRT: Post-irradiation change was mass-like appearance. e 19 months after SBRT: Mass-like appearance gradually increasing. f FDG-PET at 19 months after SBRT: Increased accumulation in mass-like appearance. g Pathology (right upper lobectomy in 20 months after SBRT9: Diagnosed as metastatic adenocarcinoma
SBRT for oligometastatic lung cancer
The SBRT treatment strategy for pulmonary OMD from primary lung cancer is generally considered to be the same as for early-stage non-small cell lung cancer (NSCLC). For clinical stage IA (≦3 cm) NSCLC, the Japan Clinical Oncology Group (JCOG) 0403 Phase II trial showed that 3-year OS and LC was 76.5% and 85.4% in 64 operable patients, and 59.9% and 87.3% in 100 inoperable patients, with no severe toxicities when using 48 Gy in 4 fractions prescribed for the isocenter [28]. This dose-fraction schedule is considered equivalent to 42 Gy in 4 fractions prescribed as the dose covering 95% of the planning target volume (PTV) (D95%) using a superposition algorithm [29] and is the standard dose-fraction schedule for patients with c-stage IA NSCLC in Japan. For further improvement of treatment results and determination of the optimal dose-fraction schedule, a randomized Phase III trial (JCOG1408) is ongoing. This is comparing treatment outcomes of SBRT for patients with medically inoperable stage IA NSCLC and small lung lesions (tumor diameter within 3 cm) clinically diagnosed as NSCLC with 42 Gy in 4 fractions versus 55 Gy in 4 fractions at the D95%, 80% isodose of the PTV [30]. For c-T2N0M0 (tumor diameter 3–5 cm) NSCLC, a Phase I trial of SBRT, JCOG0702, recommended 55 Gy in 4 fractions for tumors with PTV < 100 cc [31], and 50 Gy in 4 fractions for tumors with PTV ≧100 cc [32]. For all c-T1-2 N0M0 NSCLC, National Comprehensive Cancer Network (NCCN) guidelines recommend several dose-fraction schedules, such as 25–34 Gy in one fraction, 45–60 Gy in 3 fractions, 48–50 Gy in 4 fractions and 50–55 Gy in 5 fractions, for peripheral tumors [33]. In Western countries, 54 Gy in 3 fractions is commonly used in routine practice and for clinical trials (RTOG0236/0618) [34, 35]. Another dose-fraction schedule, 34 Gy in one fraction, is also considered a good option (RTOG0915) [36]. Onishi et al. analyzed various dose-fraction schedules for 257 patients with c-stage I NSCLC and reported that the LC and survival rates were better with a BED10≧100 Gy than with < 100 Gy [37]. At least, a BED10≧100 Gy would be needed to achieve satisfactory LC and better survival [33].
Impact of LC on survival
Theoretically, prevention of the proliferation of tumor cells and their invasion through adjacent tissues and basement membranes by LC could reduce the incidence of lymph node metastasis and distant metastasis in many primary cancers [37]. SBRT is one of the local therapies that help to achieve LC, and several reports on its use for primary lung cancer and pulmonary OMD indicate that better local control improves OS [23, 38, 39]. In a multivariate analysis, Yamamoto et al. showed that LC was one of the significant factors for OS in 1378 patients with pulmonary OMD who received SBRT [23]. In addition, several retrospective studies showed that higher SBRT doses could improve not only LC but also OS [38, 39]. Further prospective studies are warranted.
Differences according to tumor location: peripheral-versus-central
The safety of SBRT for peripheral pulmonary tumors has been relatively clearly established, but for central pulmonary tumors, which are typically located close to organs such as the proximal bronchial tree, esophagus, or great vessels, it is still considered controversial. Several prospective studies of SBRT for centrally located stage I NSCLC have been reported. Thus, Kimura et al. reported the phase I study (JROSG10-1) that recommended a dose of 60 Gy in 8 fractions at the isocenter, which is considered equivalent to 50 Gy in 8 fractions prescribed at PTV D95%, without grade ≧3 adverse effects for patients with T1 (≦ 3 cm) N0M0 centrally located NSCLC [40]. Bezjak et al. reported a phase I/II study (RTOG0813) that recommended a dose of 60 Gy in 5 fractions prescribed at D95% with 7.2% grade ≧3 adverse effects for patients with T1 or 2 (≦ 5 cm) N0M0 centrally located NSCLC [41]. Patients in the high-dose group in that study (57.5 Gy or 60 Gy in 5 fractions) had a similar OS as patients with peripheral tumors with high rates of tumor control. However, considering that instances of fatal toxicity have been reported [42], the use of SBRT for central pulmonary tumors should be carefully considered. This applies even more to ultracentral pulmonary tumors, which are defined as lesions whose gross tumor volume (GTV) or PTV abuts the proximal bronchial tree and/or other mediastinal structures [43]. Hypofractionated schedules may be considered in these cases. Karasawa et al. reported that accelerated hypofractionated radiotherapy with 75 Gy in 25 fractions at the isocenter, which is considered equivalent to 62.5 Gy in 25 fractions prescribed at PTV D95%, is promising in that it can achieve LC and survival results similar to SBRT, and it can control both central and peripheral stage I NSCLC without any serious organ toxicities [44].
On the other hand, there is also no consensus on the application of SBRT for centrally located pulmonary OMD. Table 2 shows the details of several studies [21, 45–51]. The number of fractions tended to increase (5–10 fractions) compared to the SBRT for peripheral OMD, as shown in Table 1. In addition, the incidence of grade 5 toxicities also tended to increase. Lindberg et al. reported the HILUS Trial, a prospective Nordic multicenter phase II study of ultracentral lung tumors treated with SBRT of 56 Gy in 8 fractions, prescribed to the PTV-encompassing isodose [51]. Of a total of 65 patients, grade 3 to 5 toxicity was observed in 22 (34%), including 10 cases of treatment-related death (15%). Therefore, the authors concluded that this dose-fraction schedule should not be used for tumors located within 1 cm of the main bronchi and trachea. According to the American Society for Radiation Oncology (ASTRO) guidelines, when using SBRT for centrally located tumors, physicians should endeavor to meet the dose constraints that have been utilized in prospective or other studies, given the severe toxicities that have been reported [52]. To determine the optimal dose-fraction schedules, further data from prospective studies are needed.
Table 2.
SBRT for centrally located pulmonary oligometastatic disease (OMD)
| Author/year | Study design | Patients | Lesions | Lesions of OMD (%) | Dose/fraction (Gy/fr) | Prescription | Local control | Overall survival | Toxicity grade≧3 (Grade 5) |
|---|---|---|---|---|---|---|---|---|---|
| Milano 2009, USA [45] | Retrospective | 53 | 63 | 34 (54) | 30–60 Gy/ 4–18 fr | 80% isodose | 73% (2y) | 44% (2y) | N.A. (19%) |
|
Rowe 2012, USA [46] (USA, IN) |
Retrospective | 47 | 51 | 21 (41%) | 50 Gy/ 4fr | 70–90% isodose | 94% (2y) | N.A* | 13% (2%) |
| Davis, 2015, USA [47] | Retrospective | 64 | 66 | 66 (100%) | 37.5 Gy/ 3fr (median) | N.A | 69.8% (2y) | 49.6% (2y) | 0% |
| Lischalk, 2016, USA [48] | Retrospective | 20 | 20 | 20 (100%) | 35 or 40 Gy/ 5fr | PVT D95%** | 57.4% (2y) | 40% (2y) | 10% (0%) |
| Figlia, 2018, Irtaly [49] | Retrospective | 39 | 39 | 13 (33%) | 40–70 Gy/ 8–10 fr | PVT D95% | 92.9% (2y) | 83.9% (2y) | 0% |
| Chang, 2018, Australia [50] | Retrospective | 107 | 107 | 107 (100%) | 30–50 Gy1-3 fr | 83% isodose | 96.6%/ 95.7% (2y)# | 55.1% (2y) | 5.6% (2.8%) |
| Sharma, 2018, Netherland [21] | Retrospective | N.A | 83 | 83 (100%) | 45-60 Gy/5fr | 70–90% isodose | 82% (3y) | N.A | 2% (0%) |
| Lindberg, 2021, Sweden [51] | Phase II | 65 | 68 | 14 (22%) | 56 Gy/8fr | 67% isodose | 83% (3y) | 50% (3y) | 34% (15%) |
*N.A not available, **D95%: the dose covering 95% of the planning target volume (PTV), # 96.6% in central tumors and 95.7% in ultracentral tumors trails of
Differences according to the patterns of OMD: the number of metastases and the timing of SBRT
Eligibility criteria for applying SBRT for pulmonary OMD are considered to include operability, tumor location, number of metastases and the timing of treatment. The tumor location, such as peripheral or central, has already been discussed above. Operability is judged by pulmonary function, comorbidities, previous history of thoracic resection, and other factors in the same manner as early-stage lung cancer. Regarding the number of metastases and the timing of treatment, several randomized phase II studies, which compared local therapies including SBRT and maintenance therapies or observation for patients with OMD, provide us with useful information [3–5] (Table 3). These trials showed that local therapies including SBRT improved progression-free survival or OS compared with maintenance therapies. The eligibility criteria determining the number of metastases were three metastatic sites plus primary NSCLC in Gometz’s study [3], five metastatic sites plus primary NSCLC in Iyengar’s study [4] and five metastatic sites within three organs plus various primary tumors in SABR-COMET [5], respectively. According to the European Society for Radiotherapy and Oncology (ESTO)-American Society for Radiation Oncology (ASTRO) consensus document, OMD was defined as a limited number of metastases, 3 to 5, or less [53]. In fact, the results of these studies showed that the number of metastases was 1 to 3 lesions in most of the patients.
Table 3.
SBRT for pulmonary oligometastatic disease (OMD)
| Author/year | Primary | Eligibility | Patients | Number of lung lesions | Dose/fractions for lung lesions (Gy) | OS | PFS | Treatment-related death |
|---|---|---|---|---|---|---|---|---|
| Gometz 2016, USA | Stage IV NSCLC | Stable with 4≧cycles first-line chemotherapy or 3≧ months of EGFR✝/ALK¶ inhibitors. Primary plus up to 3 metastatic sites | 49 (Local therapy: 25 vs maintenance: 24) | Local therapy: 27 vs maintenance: 27 | 5 lesions: 50 Gy/4fr | N.A.** | MST: 11.9 M vs 3.9 M (p = 0.0054) | None |
| 3 lesions: 52.5 Gy/15fr, 66 Gy/30fr | ||||||||
| 2 lesions: 60 Gy/30fr, 70 Gy/10fr, 45 Gy/15fr | ||||||||
| 1 lesion: 66 Gy/33fr, 60 Gy/15fr, 67.5 Gy/27fr, 60 Gy/8fr, 48 Gy/4fr, 55 Gy/15fr | ||||||||
| Iyengar, 2017, USA | Stage IV NSCLC | Stable with 4 to 6 cycles first-line chemotherapy (EGFR/ALK inhibitors were excluded). Primary plus up to 5 metastatic sites | 29 (SBRT:14 vs maintenance: 15) | SBRT:16 vs maintenance: 18 | 10 lesions: 18–21 Gy/1fr | N.A | MST: 9.7 M vs 3.5 M (p = 0.01) | None |
| 5 lesions: 45 Gy/15fr | ||||||||
| 1 lesion: 33 Gy/3fr | ||||||||
| Palma 2020, Canada | Lung:18 ptsBreast 18 pts. CRC: 18 pts. Prostate: 16 pts. Others: 29 pts | Stable with standard systemic therapy for 3 months or more. Controlled primary plus up to 5 metastatic sites (within 3 organs) | 99 (SBRT:66 vs maintenance: 33) | SBRT: 55 vs maintenance: 34 | 54 Gy/3fr, 55 Gy/5fr, 60 Gy/8fr | 42.3% vs 17.7% (5y) (p = 0.006) | 17.3% vs 3.2% (5y) (p = 0.001) | 3 pts (4.5%) in SBRT |
*including primary lung cancer, **N.A not available, ✝EGFR epidermal growth factor receptor, ¶ ALK anaplastic lymphoma kinase
What of the timing of treatment? In general, the timing of local therapies has not been established obviously. The trials shown in Table 3 were considered as investigating synchronous OMD. For patients with synchronous OMD, local therapies including SBRT are usually intervened after the completion of the first-line chemotherapy. De-novo metachronous OMD is also a good candidate for SBRT because most patients are administered curative treatment for the primary tumor after 6 months or more. In particular, the goal of SBRT for metachronous rather than synchronous OMD is considered to be to achieve control of metastatic sites [53]. For patients with repeated OMD, SBRT is also one of the possible local therapies. The timing of intervention of SBRT for the repeated OMD is usually considered at the time when new OMD is found as well as that of metachronous OMD. However, the importance of the maintenance treatment after the completion of SBRT for repeated OMD would increase comparing to that of metachronous OMD. Figure 3 shows a case of SBRT for repeated OMD. This patient had repeatedly undergone resection for pulmonary metachronous OMD, but three pulmonary and pleural metastases rapidly recurred after resection and the patient was diagnosed as having repeated OMD. Although SBRT was performed for these lesions and controlled the disease locally, multiple metastases at axillary lymph nodes, skin and lungs were observed at the same time 7 months after completion of SBRT. It is important that local therapies including SBRT should be applied before repeated OMD develops into polymetastatic disease, as is the case for induced OMD. The timing of intervention using local therapies for patients with repeated or induced OMD has to be judged individually after careful consideration in each case. In addition, the trials shown in Table 3 focused on the addition of local therapies including SBRT, but the combination of SBRT and immune checkpoint inhibitors (ICI) was not referred. Sharabi et al. described that SBRT can produce immune-mediated systemic responses and induce an "abscopal effect", therefore, SBRT, combined with ICI, increases tumor cell susceptibility to immune-mediated cell death. [54]. In a clinical setting, Tang et al. reported a phase I trial testing SBRT with cytotoxic T lymphocyte antigen 4 (CTLA-4) and ipilimumab for patients with metastatic solid tumors of the liver or lung refractory to standard therapies. They concluded that combining SBRT and ipilimumab was safe with a 10% partial response in non-irradiated lesions, and irradiation to the liver produced greater T-cell activation than did irradiation to the lung [55]. From these evidences, the combination of SBRT and ICI could improve survival more than SBRT alone for patients with OMD.”
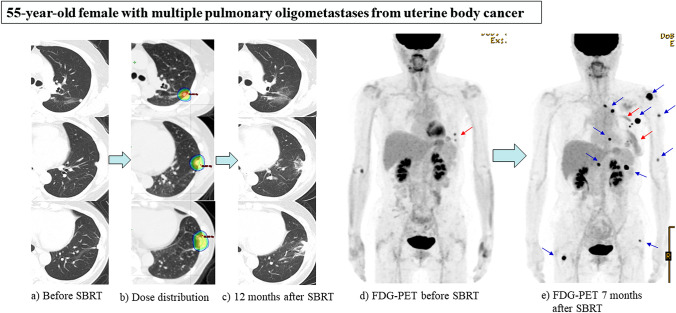
Fig. 3 A.
55-year-old female with multiple pulmonary and pleural oligometastases from uterine body cancer. This patient had repeatedly undergone resection for pulmonary metachronous oligometastatic disease (OMD), but three pulmonary and pleural metastases rapidly recurred after resection resulting in a diagnosis of repeated OMD. a Before SBRT. b Dose distribution: 56 Gy in 4 fractions, D95% prescription, 80% isodose (BED10 = 134.4 Gy, BED10max = 192.5 Gy). c 12 months after SBRT: Post-irradiation change (Grade 1 radiation pneumonitis). Diagnosed as local control. d FDG-PET before SBRT: Accumulation at two pulmonary and pleural oligometastases (red arrow). e FDG-PET at 7 months after SBRT: Although the three irradiated lesions were controlled locally (red arrow), multiple metastases at axillary lymph node, skin and lungs were observed at the same time 7 months after the completion of SBRT (blue arrow)
For multiple pulmonary lesions, the optimal dose-fraction schedules of curative-intent SBRT remain controversial. Table 3 also shows the various dose-fraction schedules for 1 to 5 metastatic lesions plus primary site, which would be considered as radical dose setting. Kobiela et al. described that a higher number of lesions might correlate with lower LC and, therefore, OS in their systematic review of SBRT for pulmonary OMD from CRC [56]. Chmura et al. reported results of the NRG-BR001 phase I trial, which was to establish safety of SBRT dose-fraction schedules in patients with 3–4 metastases or 2 metastases in close proximity to each other [57]. In that trial, 45 Gy in 3 fractions and 50 Gy in 5 fractions were used for peripheral and centrally located pulmonary OMD, respectively. There was no dose-limiting toxicity and the authors concluded that these dose-fraction schedules could be recommended for patients with multiple metastases with acceptable short-term toxicities using curative-intent SBRT developed for a single metastasis or primary tumors. On the other hand, Palma et al. suggested that the goal of using SBRT for all lesions should not be cure, but merely a temporary tumor growth arrest with minimization of toxicities, an approach they called “Ablative Radiation Therapy to Restrain Everything Safely Treatable (ARREST)” [58]. In fact, the dose-fraction schedules of 54 to 60 Gy in 3 to 8 fractions were recommended for pulmonary OMD in the SABR-COMET trial [5], but these have been reduced to doses of 20 to 35 Gy in 1 to 5 fractions in the ongoing SABR-COMET-10 trial, which treats 4–10 oligometastatic tumors [59]. However, the evaluation of this ARREST concept has just started by Phase I study [60], and there is no evidence at this moment. We should aim to cure the limited number of OMD using curative-intent SBRT to improve the survival for patients with OMD, as described this manuscript.
Finally, what is the expected benefit of SBRT for patients with pulmonary OMD? Lehrer et al. reported the results of a meta-analysis of 21 studies comprising 943 patients with 1290 oligometastases (≦5 lesions of extracranial disease), for which SBRT was administered in ≦8 fractions with ≧5 Gy per fraction [61]. The lesions treated by SBRT included those in the lung in 29.2% of patients. One-year LC and OS rates were 94.7% (95% CI 88.6–98.6%) and 85.4% (95% CI 77.1–92.0%), respectively. Acute and late grade 3 to 5 toxicities occurred in 1.2% (95% CI 0–3.8%) and 1.7% (95% CI 0.2–4.6) of cases, respectively. Ongoing and planned prospective studies are needed to confirm these results.
Conclusions
This review discussed different strategies for employing SBRT for patients with pulmonary OMD according to the type of primary tumor, location, and patterns of OMD. Considering these differences, it is concluded that curative-intent SBRT for limited pulmonary OMD does offer the possibility of improving not only LC but also OS, according to the results of the studies reviewed here. Recently, indications for different treatment strategies for OMD including pulmonary lesions are expanding, such as for treating increasing numbers of lesions, due to the development of radiation therapy and ICI therapies. Therefore, the goal of SBRT may be changing from curative intent to the aim of temporarily causing tumor growth arrest with minimization of toxicities. There are still several problems for determining the most appropriate indications for local therapy interventions, and further prospective studies are expected to resolve these issues.
Funding
This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (Grant no. 17K10478).
Declarations
Conflict of interest
Lecture fee from AstraZeneca Co., Ltd. (Tomoki Kimura).
Footnotes
The original publication has been corrected to update Tables 1 and 2.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
9/26/2022
A Correction to this paper has been published: 10.1007/s11604-022-01342-6
References
- 1.Eberhardt WEE, Mitchell A, Crowley J, Kondo H, Kim YT, Turrisi A, III, et al. The IASLC lung cancer staging project proposals for the revision of the M descriptors in the forthcoming eighth edition of the TNM classification of lung cancer. J Thorac Oncol. 2015;10:1515–1522. doi: 10.1097/JTO.0000000000000673. [DOI] [PubMed] [Google Scholar]
- 2.Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995;13:8–10. doi: 10.1200/JCO.1995.13.1.8. [DOI] [PubMed] [Google Scholar]
- 3.Gomez DR, Blumenschein GR, Jr, Lee JJ, Hernandez M, Ye R, Camidge DR, et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after fi rst-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol. 2016;17:1672–1682. doi: 10.1016/S1470-2045(16)30532-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iyengar P, Wardak Z, Gerber DE, Tumati V, Ahn C, Hughes RS, et al. Consolidative radiotherapy for limited metastatic non–small-cell lung cancer A phase 2 randomized clinical trial. JAMA Oncol. 2018;4:e173501. doi: 10.1001/jamaoncol.2017.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, et al. Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: Long-term results of the SABR-COMET phase II randomized trial. J Clin Oncol. 2020;38:2830–2838. doi: 10.1200/JCO.20.00818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guckenberger M, Lievens Y, Bouma AB, Collette L, Dekker A, deSouza NM, et al. Characterisation and classification of oligometastatic disease: a European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol. 2020;21:e18–28. doi: 10.1016/S1470-2045(19)30718-1. [DOI] [PubMed] [Google Scholar]
- 7.XuL BurkeAP. Pulmonary oligometastases: Histological features and difficulties in determining site of origin. Int J Surg Pathol. 2012;20:577–588. doi: 10.1177/1066896912449039. [DOI] [PubMed] [Google Scholar]
- 8.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Colon Cancer ver. 1. 2022 [homepage on the internet]. Fort Washington, PA: National Comprehensive Cancer Network®; [updated 2022 Feb 25; cited 2022 Apr 4]. Available from https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf
- 9.Shultz DB, Filippi AR, Thariat J, Mornex F, Loo BW, Jr, Ricardi U. Stereotactic ablative radiotherapy for pulmonary oligometastases and oligometastatic lng cancer. J Thorac Oncol. 2014;9:1426–1433. doi: 10.1097/JTO.0000000000000317. [DOI] [PubMed] [Google Scholar]
- 10.Norihisa Y, Nagata Y, Takayama K, Matsuo Y, Sakamoto T, Sakamoto M, et al. Stereotactic body radiotherapy for oligometastatic lung tmors. Int J Radiat Oncol Biol Phys. 2008;72:398–403. doi: 10.1016/j.ijrobp.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Rusthoven KE, Kavanagh BD, Burri SH, Chen C, Cardenes H, Chidel MA, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for lung metastases. J Clin Oncol. 2009;27:1579–1584. doi: 10.1200/JCO.2008.19.6386. [DOI] [PubMed] [Google Scholar]
- 12.Takeda A, Kunieda E, Ohashi T, Aoki Y, Koike N, Takeda T. Stereotactic body radiotherapy (SBRT) for oligometastatic lung tumors from colorectal cancer and other primary cancers in comparison with primary lung cancer. Radiother Oncol. 2011;101:255–259. doi: 10.1016/j.radonc.2011.05.033. [DOI] [PubMed] [Google Scholar]
- 13.Widder J, Klinkenberg TJ, Ubbels JF, Wiegman EM, Groen HJ, Langendijk JA. Pulmonary oligometastases: metastasectomy or stereotactic ablative radiotherapy? Radiother Oncol. 2013;107:409–413. doi: 10.1016/j.radonc.2013.05.024. [DOI] [PubMed] [Google Scholar]
- 14.Comito T, Cozzi L, Clerici E, Campisi MC, Liardo RLE, Navarria P, et al. Stereotactic ablative radiotherapy (SABR) in inoperable oligometastatic disease from colorectal cancer: a safe and effective approach. BMC Cancer. 2014;14(1):619. doi: 10.1186/1471-2407-14-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jung J, Song SY, Kim JH, Yu CS, Kim JC, Kim TW, et al. Clinical efficacy of stereotactic ablative radiotherapy for lung metastases arising from colorectal cancer. Radiat Oncol. 2015;10:238. doi: 10.1186/s13014-015-0546-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rieber J, Streblow J, Uhlmann L, Flentje M, Duma M, Ernst I, et al. Stereotactic body radiotherapy (SBRT) for medically inoperable lung metastases-A pooled analysis of the German working group "stereotactic radiotherapy". Lung Cancer. 2016;97:51–58. doi: 10.1016/j.lungcan.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Agolli L, Bracci S, Nicosia L, Valeriani M, De Sanctis V, Osti MF. Lung metastases treated with stereotactic ablative radiation therapy in oligometastatic colorectal cancer patients: Outcomes and prognostic factors after long-term follow-up. Clin Colorectal Cancer. 2017;16:58–64. doi: 10.1016/j.clcc.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Jingu K, Matsuo Y, Onishi H, Yamamoto T, Aoki M, Murakami Y, et al. Dose escalation improves outcome in stereotactic body radiotherapy for pulmonary oligometastases from colorectal cancer. Anticancer Res. 2017;37:2709–2713. doi: 10.21873/anticanres.11621. [DOI] [PubMed] [Google Scholar]
- 19.Helou J, Thibault I, Poon I, Chiang A, Jain S, Soliman H, et al. Stereotactic ablative radiation therapy for pulmonary metastases: Histology, dose, and indication matter. Int J Radiat Oncol Biol Phys. 2017;98:419–427. doi: 10.1016/j.ijrobp.2017.02.093. [DOI] [PubMed] [Google Scholar]
- 20.Osti MF, Agolli L, Valeriani M, Reverberi C, Bracci S, Marinelli L, et al. 30 Gy single dose stereotactic body radiation therapy (SBRT): Report on outcome in a large series of patients with lung oligometastatic disease. Lung Cancer. 2018;122:165–170. doi: 10.1016/j.lungcan.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 21.Sharma A, Duijm M, Oomen-de Hoop E, Aerts JG, Verhoef C, Hoogeman M, et al. Factors affecting local control of pulmonary oligometastases treated with stereotactic body radiotherapy. Acta Oncol. 2018;57:1031–1037. doi: 10.1080/0284186X.2018.1445285. [DOI] [PubMed] [Google Scholar]
- 22.Berkovic P, Gulyban A, Defraene G, Swenen L, Dechambre D, Nguyen PV, et al. Stereotactic robotic body radiotherapy for patients with oligorecurrent pulmonary metastases. BMC Cancer. 2020;20:402. doi: 10.1186/s12885-020-06906-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamamoto T, Niibe Y, Aoki M, Shintani T, Yamada K, Kobayashi M, et al. Analyses of the local control of pulmonary oligometastases after stereotactic body radiotherapy and the impact of local control on survival. BMC Cancer. 2020;20:997. doi: 10.1186/s12885-020-07514-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siva S, Bressel M, Mai T, Le H, Vinod S, de Silva H, et al. Single-fraction vs multifraction stereotactic ablative body radiotherapy for pulmonary oligometastases (SAFRON II): the trans tasman radiation oncology group 13 01 phase 2 randomized clinical trial. JAMA Oncol. 2021;7:1476–1485. doi: 10.1001/jamaoncol.2021.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guckenberger M, Klement RJ, Allagauer M, Andratschke N, Blanck O, Boda-Heggemann J, at al. Local tumor control probability modeling of primary and secondary lung tumors in stereotactic body radiotherapy. Radiother Oncol. 2016;118:485–491. doi: 10.1016/j.radonc.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 26.Takeda A, Sanuki N, Tsurugai Y, Oku Y, Aoki Y. Stereotactic body radiotherapy for patients with oligometastases from colorectal cancer: risk adapted dose prescription with a maximum dose of 83–100 Gy in five fractions. J radiat Res. 2016;57:400–405. doi: 10.1093/jrr/rrw029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kobiela J, Spychalski P, Marvaso G, Ciardo D, Dell’Acqua V, Kraja F, et al. Ablative stereotactic radiotherapy for oligometastatic colorectal cancer: Systematic review. Crit Rev Oncol Hematol. 2018;129:91–101. doi: 10.1016/j.critrevonc.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Nagata Y, Hiraoka M, Shibata T, Onishi H, Kokubo M, Karasawa K, et al. Prospective trial of stereotactic body radiation therapy for both operable and inoperable T1N0M0 non-smallcell lung cancer: Japan Clinical Oncology Group Study JCOG0403. Int J Radiat Oncol Biol Phys. 2015;93:989–996. doi: 10.1016/j.ijrobp.2015.07.2278. [DOI] [PubMed] [Google Scholar]
- 29.Kawahara D, Ozawa S, Kimura T, Saito A, Nishio T, Nakashima T, et al. Marginal prescription equivalent to the isocenter prescription in lung stereotactic body radiotherapy: preliminary study for Japan Clinical Oncology Group trial (JCOG1408) J Radiat Res. 2017;58:149–154. doi: 10.1093/jrr/rrw096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kimura T, Nagata Y, Eba J, Ozawa S, Ishikura S, Shibata T, et al. A randomized Phase III trial of comparing two dose-fractionations stereotactic body radiotherapy (SBRT) for medically inoperable Stage IA non-small cell lung cancer or small lung lesions clinically diagnosed as primary lung cancer: Japan Clinical Oncology Group Study JCOG1408 (J-SBRT trial) Jpn J Clin Oncol. 2017;47:277–281. doi: 10.1093/jjco/hyw198. [DOI] [PubMed] [Google Scholar]
- 31.Onimaru R, Shirato H, Shibata T, Hiraoka M, Ishikura S, Karasawa K, et al. Phase I study of stereotactic body radiation therapy for peripheral T2N0M0 non-small cell lung cancer with PTV < 100 cc using a continual reassessment method (JCOG0702) Radiother Oncol. 2015;116:276–280. doi: 10.1016/j.radonc.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 32.Onimaru R, Onishi H, Shibata T, Hiraoka M, Ishikura S, Karasawa K, et al. Phase I study of stereotactic body radiation therapy for peripheral T2N0M0 non-small cell lung cancer (JCOG0702): Results for the group with PTV ≧ 100 cc. Radiother Oncol. 2017;122:281–285. doi: 10.1016/j.radonc.2016.11.022. [DOI] [PubMed] [Google Scholar]
- 33.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Non-Small Cell Lung Cancer version 3. Available at: https://www.nccn.org/login?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Accessed 13 Apr 2022.
- 34.Timmerman R, Paulus R. GalvinJ, Michalski J, Straube W, Bradley J, et al Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Timmerman R, Paulus R, Pass HI, Gore EM, Edelman MJ, Galvin J, et al. Stereotactic body radiation therapy for operable early stage lung cancer Findings from the NRG Oncology RTOG 0618 trial. JAMA Oncol. 2018;4:1263–1266. doi: 10.1001/jamaoncol.2018.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gregory MM, et al. Randomized phase 2 study comparing 2 stereotactic body radiation therapy schedules for medically inoperable patients with stage I peripheral non-small cell lung cancer: NRG oncology RTOG 0915 (NCCTG N0927) Int J Radiat Oncol Biol Phys. 2015;93:757–764. doi: 10.1016/j.ijrobp.2015.07.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hunter KW, Crawford NPS, Alsarraj J. Mechanisms of metastasis. Breast Cancer Res. 2008;10:S2. doi: 10.1186/bcr1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Onishi H, Shirato H, Nagata Y, Hiraoka M, Fujino M, Gomi K, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: Updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol. 2007;2:S94–S100. doi: 10.1097/JTO.0b013e318074de34. [DOI] [PubMed] [Google Scholar]
- 39.Tateishi Y, Takeda A, Horita N, Tsurugai Y, Eriguchi T, Kibe Y, et al. Stereotactic body radiation therapy with a high maximum dose improves local control, cancer-specific death, and overall survival in peripheral early-stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2021;111:143–151. doi: 10.1016/j.ijrobp.2021.04.014. [DOI] [PubMed] [Google Scholar]
- 40.Kimura T, Nagata Y, Harada H, Matsuo Y, Takanaka T, Kokubo M, et al. Phase I study of stereotactic body radiation therapy for centrally located stage IA non-small cell lung cancer (JROSG10-1) Int J Clin Oncol. 2017;22:849–856. doi: 10.1007/s10147-017-1125-y. [DOI] [PubMed] [Google Scholar]
- 41.Bezjak A, Paulus R, Gaspar LE, Timmerman RD, Straube WL, Ryan WF, et al. Safety and efficacy of a five-fraction stereotactic body radiotherapy schedule for centrally located non–small-cell lung cancer: NRG Oncology/RTOG 0813 Trial. J Clin Oncol. 2019;37:1316–1325. doi: 10.1200/JCO.18.00622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corradetti MN, Haas AR, Rengan R. Central-airway necrosis after stereotactic body radiation therapy. N Engl J Med. 2012;366:2327–2329. doi: 10.1056/NEJMc1203770. [DOI] [PubMed] [Google Scholar]
- 43.Chen H, Laba JM, Zayed S, Boldt RG, Palma DA, Louie AV. Safety and effectiveness of stereotactic ablative radiotherapy for ultra-central lung lesions: A systematic review. J Thorac Oncol. 2019;14:1332–1342. doi: 10.1016/j.jtho.2019.04.018. [DOI] [PubMed] [Google Scholar]
- 44.Karasawa K, Hayakawa S, Machitori Y, Shibata Y, Ogawa H, Ito K, et al. Accelerated hypofractionated radiotherapy versus stereotactic body radiotherapy for the treatment of stage I nonsmall cell lung cancer - A single institution experience with long-term follow-up. Technol Cancer Res Treat. 2018;17:1–8. doi: 10.1177/1533033818806318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Milano MT, Chen Y, Katz AW, Philip A, Schell MC, Okunieff P. Central thoracic lesions treated with hypofractionated stereotactic body radiotherapy. Radiother Oncol. 2009;91:301–306. doi: 10.1016/j.radonc.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 46.Rowe BP, Boffa DJ, Wilson LD, Kim AW, Detterbeck FC, Decker RH. Stereotactic body radiotherapy for central lung tumors. J Thorac Oncol. 2012;7:1394–1399. doi: 10.1097/JTO.0b013e3182614bf3. [DOI] [PubMed] [Google Scholar]
- 47.Davis JN, Medbery C, Sharma S, Pablo J, Kimsey F, Perry D, et al. Stereotactic body radiotherapy for centrally located early-stage non-small cell lung cancer or lung metastases from the RSSearch® patient registry. Radiat Oncol. 2015;10:113. doi: 10.1186/s13014-015-0417-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lischalk JW, Malik RM, Collins SP, Collins BT, Matus IA, Anderson ED. Stereotactic body radiotherapy (SBRT) for high-risk central pulmonary metastases. Radiat Oncol. 2016;11:28. doi: 10.1186/s13014-016-0608-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Figlia V, Mazzola R, Cuccia F, Alongi F, Mortellaro G, Cespuglio D, et al. Hypo-fractionated stereotactic radiation therapy for lung malignancies by means of helical tomotherapy: report of feasibility by a single-center experience. Radiol Med (Torino) 2018;123:406–414. doi: 10.1007/s11547-018-0858-7. [DOI] [PubMed] [Google Scholar]
- 50.Chang JH, Poon I, Erler D, Zhang L, Cheung P. The safety and effectiveness of stereotactic body radiotherapy for central versus ultracentral lung tumors. Radiother Oncol. 2018;129:277–283. doi: 10.1016/j.radonc.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 51.Lindberg K, Grozman V, Karlsson K, Lindberg S, Lax I, Wersall P, et al. The HILUS-Trial—a Prospective Nordic multicenter phase 2 study of ultracentral lung tumors treated with stereotactic body radiotherapy. J Thorac Oncol. 2021;16:1200–1210. doi: 10.1016/j.jtho.2021.03.019. [DOI] [PubMed] [Google Scholar]
- 52.Videtic GMM, Donington J, Giuliani M, Heinzerling J, Kelsey KTZ, CR,, et al. Stereotactic body radiation therapy for early stage non-small cell lung cancer: executive summary of an ASTRO evidence-based guideline. Pract Radiat Oncol. 2017;7:292–301. doi: 10.1016/j.prro.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 53.Lievens Y, Guckenberger M, Gomez D, Hoyer M, Iyengar P, Kindts I, et al. Defining oligometastatic disease from a radiation oncology perspective: An ESTRO-ASTRO consensus document. Radiother Oncol. 2020;148:157–166. doi: 10.1016/j.radonc.2020.04.003. [DOI] [PubMed] [Google Scholar]
- 54.Kobiela J, Spychalski P, Marvaso G, Ciardo D, Dell’Acqua V, Kraja F, et al. Ablative stereotactic radiotherapy for oligometastatic colorectal cancer: Systematic review. Crit Rev in Oncol hematol. 2018;129:91–101. doi: 10.1016/j.critrevonc.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 55.Sharabi AB, Lim M, DeWeese TL, Drake CG. Radiation and checkpoint blockade immunotherapy: radiosensitization and potential mechanisms of synergy. Lancet Oncol. 2015;16:e489–509. doi: 10.1016/S1470-2045(15)00007-8. [DOI] [PubMed] [Google Scholar]
- 56.Tang C, Welsh JW, Groot P, Massarelli E, Chang JY, Hess KR, et al. Ipilimumab with stereotactic ablative radiationtherapy: phase i results and immunologic correlates from peripheral T cells. Clin Can Res. 2016;23:1388–1396. doi: 10.1158/1078-0432.CCR-16-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chmura S, Winter KA, Robinson C, Pisansky TM, Borges V, Al-Hallaq H, et al. Evaluation of safety of stereotactic body radiotherapy for the treatment of patients with multiple metastases Findings from the NRG-BR001 phase 1 trial. JAMA Oncol. 2021;7:845–852. doi: 10.1001/jamaoncol.2021.0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Palma DA, Bauman GS, Rodrigues GB. Beyond oligometastases. Int J Clin Oncol. 2020;107:253–256. doi: 10.1016/j.ijrobp.2019.12.023. [DOI] [PubMed] [Google Scholar]
- 59.Palma DA, Olson R, Harrow S, Correa RJM, Schneiders F, Haasbeek CJA,, et al. Stereotactic ablative radiotherapy for the comprehensive treatment of 4–10 oligometastatic tumors (SABR-COMET-10): study protocol for a randomized phase III trial. BMC Cancer. 2019;19:816. doi: 10.1186/s12885-019-5977-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bauman GS, Corkum MT, Fakir H, Nguyen TK, Palma DA. Ablative radiation therapy to restrain everything safely treatable (ARREST): study protocol for a phase I trial treating polymetastatic cancer with stereotactic radiotherapy. BMC Cancer. 2021;21:405. doi: 10.1186/s12885-021-08020-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lehrer EJ, Singh R, Wang M, Chinchillo VM, Trifiletti DM, Ost P, et al. Safety and survival rates associated with ablative stereotactic radiotherapy for patients with oligometastatic cancer A systematic review and meta-analysis. JAMA Oncol. 2021;7:92–106. doi: 10.1001/jamaoncol.2020.6146. [DOI] [PMC free article] [PubMed] [Google Scholar]