Structured Abstract
Objective:
To determine the association of pathogen type with mortality, functional status, and health-related quality of life (HRQL) among children at hospital discharge/one month following hospitalization for septic shock
Design:
Secondary database analysis of a prospective, descriptive cohort investigation
Setting:
Twelve academic pediatric intensive care units (PICU) in the United States
Patients:
Critically ill children, aged 1 month to 18 years, enrolled 2013–2017
Interventions:
None
Measurements and Main Results:
Association of clinical outcomes with pathogen type was assessed for all patients and separately for surviving patients enrolled in the primary LAPSE investigation. For this secondary analysis, we predicted that age would be associated with pathogen type and outcomes, and accordingly it was incorporated as a confounding variable in primary analyses. Among 389 children enrolled with septic shock, at one month/hospital discharge, we observed no statistically significant differences in relation to pathogen types for the composite outcome mortality or substantial new functional morbidity: no causative organism identified (27% [28/103]); pure viral infections (26% [24/91]); pure bacterial/fungal infections (25% [31/125]); bacterial/fungal+viral co-infections (33% [23/70]). Similarly, we observed no statistically significant differences in relation to pathogen types for the composite outcome, mortality or persistent serious deterioration of HRQL: no causative organism identified (43% [44/103]); pure viral infections (33% [30/91]); pure bacterial/fungal infections (46% [57/125]); bacterial/fungal+viral co-infections (43% [30/70]). However, we did identify statistically significant associations between pathogen type and the outcomes ventilator free days (p = 0.0083) and PICU free days (0.0238).
Conclusions:
This secondary analysis of the LAPSE database identified no statistically significant association of pathogen type with composite mortality and morbidity outcomes. However, pathogen type may be associated with PICU resources employed to treat sepsis organ dysfunction. Ultimately, pediatric septic shock was frequently associated with adverse patient-centered, clinically meaningful outcomes regardless of infectious disease pathogen type.
Keywords: septic shock, infection pathogens, patient-centered outcomes, functional status, health-related quality of life
Introduction
In 2016 the (adult) Sepsis-3 working group defined sepsis as life-threatening organ dysfunction caused by a dysregulated host response to infection (1). Notably no distinction was made regarding the type of infection initiating sepsis. Viral pandemics involving influenza in 1918 and the novel coronavirus in 2019 ascertain that viral sepsis can be deadly, albeit sometimes complicated with bacterial infection co-infection (2, 3). Similarly, infection negative sepsis is commonly encountered in clinical medicine (4). Although mortality from pediatric sepsis has declined substantially over the past several decades in resource rich countries (5), sepsis remains a major cause of childhood death throughout the world (6). While pediatric sepsis is characterized by increasing prevalence and decreasing mortality overall, children and families surviving sepsis face a substantial burden of long-term morbidity (7). For example, the prospective, descriptive cohort investigation, Life After Pediatric Sepsis Evaluation (LAPSE) reported that 35% of children encountering septic shock had not yet regained their baseline health-related quality of life (HRQL) one year following hospital admission (8).
The Surviving Sepsis Campaign has identified predictors of sepsis long-term mortality and morbidity as research priorities (9). Although numerous possible risk factors for adverse outcomes following sepsis exist, no pediatric studies have evaluated the potential impact of pathogen type. Accordingly, the primary aim of this secondary analysis of the LAPSE database was to examine the association of pathogen type with mortality, functional status, and HRQL all assessed at hospital discharge/one month following hospitalization for septic shock. We hypothesized that patients with culture-positive bacterial/fungal infections would demonstrate poorer outcomes than patients with documented pure viral infections or patients with no causative organism identified.
Methods
This secondary analysis queried publicly available databases [https://www.cpccrn.org/study-datasets/2855-2/; https://dash.nichd.nih.gov/explore/study?q=LAPSE&filters=[]&page=1&sortBy=relevance&asc=true&size=50] for LAPSE (NCT01415180, R01HD073362), a prospective, observational cohort study of critically ill children with community-acquired septic shock, aged 0.1–18 years, who were admitted to 12 tertiary pediatric intensive care units (PICU) in the United States and enrolled 2013–2017. Details of the LAPSE study protocol were published previously (8, 10). Under institutional review board oversight (Supplemental Digital Content [SDC], eText 1), documented, informed parental permission was obtained before any LAPSE study procedures were undertaken.
All children enrolled in LAPSE demonstrated documented or highly suspected infection and septic shock and received all the following: antimicrobials, volume resuscitation, positive pressure pulmonary support, and vasoactive-inotropic medication infusion. Complete inclusion/exclusion criteria for the primary investigation were previously detailed (8) and are reproduced in SDC, eText 2. This secondary analysis was conducted according to Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for reporting observational studies (11) (SDC, eTable 1).
We identified four infection pathogen types among LAPSE subjects through review of uploaded Day 0–1 study microbiology data: 1) no causative organism identified (sterile bacterial culture(s) and negative surveillance polymerase chain reaction(s) [PCR]); 2) pure viral infections; 3) pure bacterial/fungal infections; and 4) bacterial/fungal and viral co-infections. Fourteen patients demonstrated concurrent fungal infections.
In the primary LAPSE investigation, research coordinators collected data describing demographics, infection characteristics, chronic comorbid conditions (Pediatric Medical Complexity Algorithm) (12), illness severity (Pediatric Risk of Mortality, version III [PRISM-III], employing data collection 2 hours prior to PICU admission to 4 hours post PICU admission) (13), organ dysfunction (Pediatric Logistic Organ Dysfunction score, version 2 [PELOD-2]) (14), and vasoactive-inotropic support, mechanical ventilation, PICU and hospital free days, truncated at study Day 28. Patients were serially assessed for functional status utilizing the Functional Status Scale (FSS) (15) at study entry (reflecting baseline, pre-sepsis status during the month prior to PICU admission), study Day 7, and hospital discharge/one month, whichever occurred first. Similarly, participating families completed serial parent-proxy assessments of their child’s HRQL utilizing the Pediatric Quality of Life Inventory (PedsQL™) (16, 17) or the Stein-Jessop Functional Status Scale (short form, 14 item, double element, FSII-R) (18), according to parental preference based on assessment of their child’s developmental status (8).
The primary aim of this investigation was to determine any association of pathogen type with mortality and magnitude of functional status or HRQL deterioration from baseline, reasonably attributable to the septic shock event. The primary composite endpoints examined were: 1) mortality or new substantial functional morbidity and 2) mortality or persistent, serious deterioration of HRQL. Mortality was defined as death within the first month of index hospitalization. New substantial functional morbidity was defined as an increase in FSS ≥ 3 points from baseline assessed at hospital discharge/one-month. Persistent, serious deterioration of HRQL was defined as a HRQL score ≥ 25% below baseline assessed at one-month (21–42 days) (10). Secondary endpoints examined associations of pathogen type with in-hospital mortality, absolute changes in FSS, PedsQL™, and FSII-R comparing baseline and hospital discharge/one-month (as detailed above), and vasoactive-inotropic infusion, mechanical ventilation, PICU and hospital free days. Exploratory endpoints included deterioration of HRQL and functional status, expressed as percent change, again comparing baseline and hospital discharge/one-month assessments (ΔFSS, ΔHRQL). The entire LAPSE cohort is considered in these latter analyses. Those who died were assigned a one-month HRQL score of 0 and FSS score of 30.
Patient and clinical characteristics were summarized using frequencies and percentages or median [interquartile range (IQR)] in relation to the four pathogen types. All percentages are based on denominators reflecting patients with non-missing values for HRQL assessments as indicated in the tables. Imputation was not invoked for missing data. Associations of baseline characteristics with pathogen type were examined using Fisher’s exact test for categorical variables and the Kruskal-Wallis test for continuous and ordinal variables. Associations of clinical outcomes with pathogen types were assessed for all subjects, as well as for survivors only. Continuous outcomes were assessed by rank regression and binary outcomes were assessed by logistic regression, with pathogen type as the primary predictor. Age-adjusted models incorporated age (≤ 1 year, > 1 year) as a covariate because a priori we expected that age would impact both pathogen type and outcomes. P values are reported based on a 2-sided alternative and considered statistically significant when <0.05. No corrections were made for multiple comparisons. Analyses were performed using SAS software v9.4 (Cary, NC).
Results
A flow diagram for the patients included in this analysis is provided as Figure 1. Table 1 summarizes baseline demographics, clinical characteristics, and measures of initial illness severity, for each of the four pathogen types. While patient age was distributed broadly and was significantly associated with pathogen group (p = 0.039), race and ethnicity were not. Baseline clinical characteristics including immunosuppression status, PMCA category, PRISM-III, and PELOD-2, were not differentially distributed among pathogen types. Table 2 summarizes the most common microbes for each pathogen group. In total 14 patients encountered fungal infections, 9 with concurrent bacterial infections, 4 with concurrent bacterial+viral infections, and 1 with a concurrent viral infection.
Figure 1.
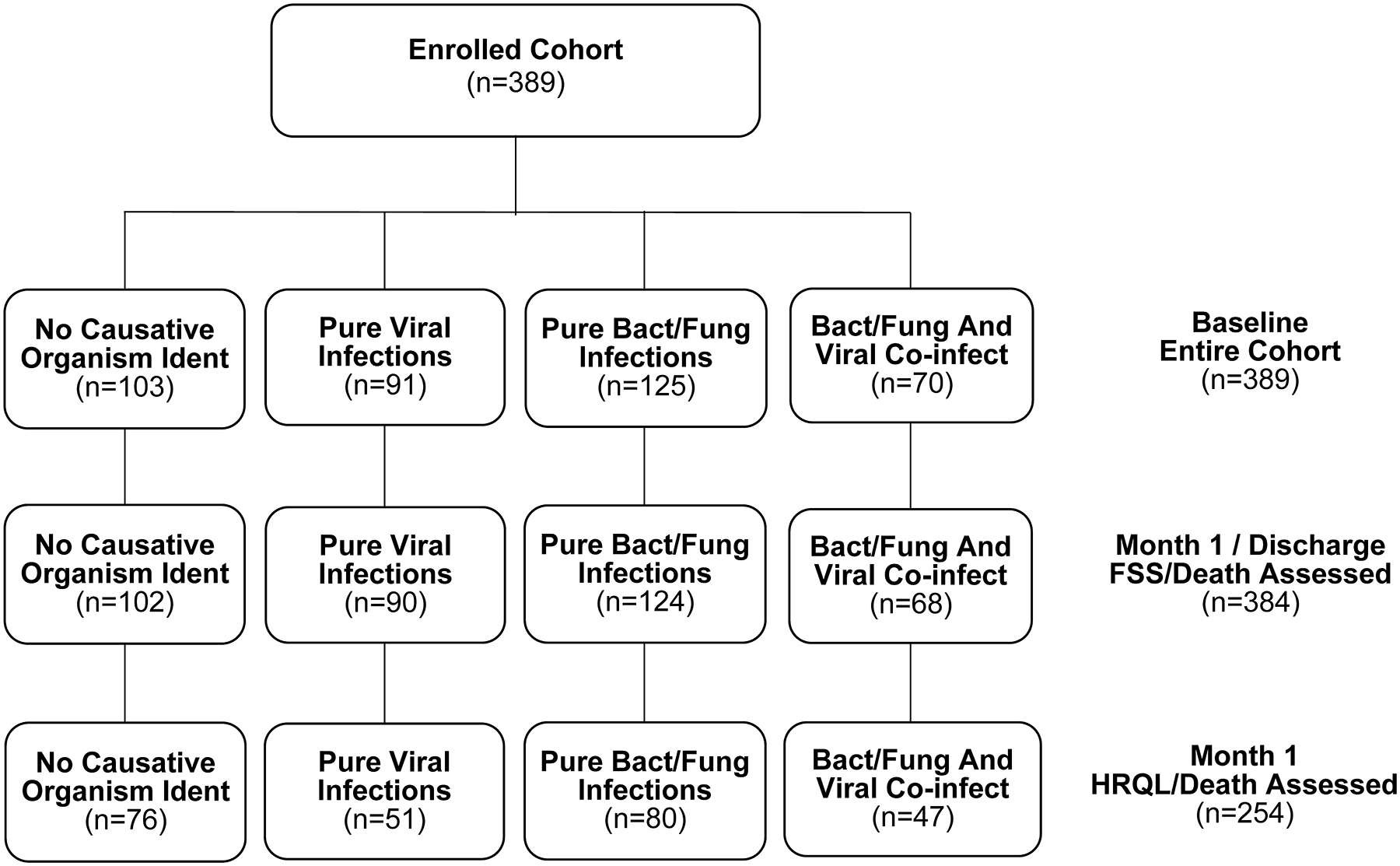
Patient Flow Diagram
Pathogen Type (infection status at admission) data were collated from uploaded microbiology reports. Only reports collected on or before Day 1 are considered. If a patient had no upload on or before Day 1, then the patient was classified as not having an infection at PICU admission.
Abbreviations: Ident, Identified; Bact/Fung, Bacterial/Fungal; Co-infect, Co-infection
FSS, Functional Status Scale; HRQL, health-related quality of life
TABLE 1.
Baseline Characteristics of the Study Population by Pathogen Type
| Pathogen Type1 | |||||
|---|---|---|---|---|---|
| Characteristic | No Causative Organism Identified (N=103) |
Pure Viral Infections (N=91) |
Pure Bacterial/Fungal Infections (N=125) |
Bacterial/Fungal And Viral Co-infections (N=70) |
P-value |
| Demographics | |||||
| Female [n (%)] | 53 (51.5) | 41 (45.1) | 58 (46.4) | 26 (37.1) | 0.3374 |
| Age [n (%)] | 0.0394 | ||||
| 0–12 months | 16 (15.5) | 19 (20.9) | 15 (12.0) | 17 (24.3) | |
| 13–24 months | 7 (6.8) | 14 (15.4) | 9 (7.2) | 10 (14.3) | |
| 2–4 years | 19 (18.4) | 18 (19.8) | 23 (18.4) | 10 (14.3) | |
| 5–7 years | 11 (10.7) | 11 (12.1) | 11 (8.8) | 10 (14.3) | |
| 8–12 years | 17 (16.5) | 10 (11.0) | 35 (28.0) | 10 (14.3) | |
| 13–17 years | 33 (32.0) | 19 (20.9) | 32 (25.6) | 13 (18.6) | |
| Age, Median [Q1, Q3] | 7.7 [2.6, 13.5] | 4.3 [1.1, 10.6] | 8.3 [3.1, 13.3] | 4.1 [1.0, 10.7] | 0.0065 |
| Clinical Characteristics | |||||
| Immunocomp [n (%)] | 16 (15.5) | 11 (12.1) | 29 (23.2) | 12 (17.1) | 0.1854 |
| PMCA Category2 [n (%)] | 0.4304 | ||||
| Non-Chronic | 52 (50.5) | 46 (50.5) | 60 (48.0) | 31 (44.3) | |
| Non-Complex Chronic | 5 (4.9) | 8 (8.8) | 3 (2.4) | 4 (5.7) | |
| Complex Chronic | 45 (43.7) | 37 (40.7) | 62 (49.6) | 35 (50.0) | |
| PRISM III3 (mean ± SD) | 11.4 ± 8.4 | 11.6 ± 8.1 | 12.9 ± 8.3 | 11.8 ± 7.5 | 0.5605 |
| PELOD-2 (mean ± SD) | 8.7 ± 3.8 | 8.5 ± 3.2 | 9.1 ± 3.9 | 9.5 ± 4.3 | 0.8255 |
Abbreviations: PCR, polymerase chain reaction; Bact, bacterial; Infect, infection; Amer, American; Immunocomp, immunocompromised; PMCA, Pediatric Medical Complexity Algorithm category; PRISM III, Pediatric Risk of Mortality, Version III; PELOD-2, Pediatric Logistic Organ Dysfunction, Version 2.
The entire LAPSE cohort is considered in this table (n=389). In total 14 patients encountered fungal infections, 9 with concurrent bacterial infections, 4 with concurrent bacterial+viral infections, and 1 with a concurrent viral infection.
Chronic comorbid conditions were assessed using the Pediatric Medical Complexity Algorithm (PMCA) for a three-year period prior to and including the index septic shock admission.
PRISM III variables were collected during a modified 6-hour window of 2 hours prior to PICU admission through 4 hours post PICU admission.
Fisher’s exact test
Kruskal-Wallis test
Pathogen Type (infection status at admission) data were collated from uploaded microbiology reports. Only reports collected on or before Day 1 are considered. If a patient had no upload on or before Day 1, then the patient is classified as not having an infection at PICU admission.
TABLE 2.
Most Commonly Documented Organisms by Admission Pathogen Groups
| Pathogen Type | Count (%) |
|---|---|
| Viral | 91 |
| Rhinovirus / Enterovirus | 45 (49%) |
| Influenza | 17 (19%) |
| Respiratory syncytial virus | 13 (14%) |
| Parainfluenza | 8 (9%) |
| Metapneumovirus | 7 (8%) |
| Bacterial / Fungal | 125 |
| Staphylococcus aureus | 23 (18%) |
| Methicillin resistant Staphylococcus aureus | 16 (13%) |
| Streptococcus pyogenes | 14 (11%) |
| Escherichia coli | 12 (10%) |
| Klebsiella pneumoniae | 10 (8%) |
| Pseudomonas aeruginosa | 10 (8%) |
| Bacterial / Fungal with Viral | 70 |
| Bacterial / Fungal | |
| Staphylococcus aureus | 19 (27%) |
| Pseudomonas aeruginosa | 13 (19%) |
| Streptococcus pyogenes | 12 (17%) |
| Hemophilus influenzae | 7 (10%) |
| Streptococcus pneumoniae | 5 (7%) |
| Viral | |
| Rhinovirus / Enterovirus | 30 (43%) |
| Respiratory syncytial virus | 17 (24%) |
| Influenza | 11 (16%) |
| Adenovirus | 9 (13%) |
| Coronavirus | 6 (9%) |
Tables 3 and 4 detail baseline and clinical outcomes at hospital discharge/one-month for all patients and survivors only respectively, including mortality, functional status, HRQL, and resource utilization. Among 389 children enrolled with septic shock, at one month/hospital discharge, we observed no statistically significant differences in relation to pathogen types for the composite outcome mortality or substantial new functional morbidity: no causative organism identified (27% [28/103]); pure viral infections (26% [24/91]); pure bacterial/fungal infections (25% [31/125]); bacterial/fungal+viral co-infections (33% [23/70]). Similarly, we observed no statistically significant differences in relation to pathogen types for the composite outcome, mortality or persistent, serious deterioration of HRQL: no causative organism identified (43% [44/103]); pure viral infections (33% [30/91]); pure bacterial/fungal infections (46% [57/125]); bacterial/fungal+viral co-infections (43% [30/70]). Regardless of pathogen type, pediatric septic shock was associated with risk for significant deterioration of functional status and HRQL comparing baseline and hospital discharge/one-month paired assessments.
TABLE 3.
Baseline Status and Outcomes at Hospital Discharge/One Month By Pathogen Group For All Patients
| Pathogen Group1 | P Values | |||||
|---|---|---|---|---|---|---|
| Functional Status, Health Related Quality of Life Measure |
No Causative Organism Identified (N=103) |
Pure Viral Infections (N=91) |
Pure Bacterial/Fungal Infections (N=125) |
Bacterial/Fungal And Viral Co-infections (N=70) |
Unadjusted P Value |
Age-adjusted P value |
| Baseline FS, HRQL | ||||||
| FSS | 7 [6, 11] | 6 [6, 12] | 6 [6, 13] | 7 [6, 12] | 0.6979 | 0.7496 |
| HRQL, Median [IQR] (n) | 75 [64, 90] (96) | 78 [61, 91] (84) | 74 [61, 90] (112) | 75 [64, 86] (66) | 0.5354 | 0.7183 |
| PedsQL™, Median [IQR] (n) | 77 [68, 93] (60) | 83 [71, 93] (50) | 78 [62, 92] (71) | 77 [64, 87] (41) | 0.3332 | 0.3530 |
| FSII-R, Median [IQR] (n) | 73 [61, 83] (36) | 71 [57, 86] (34) | 68 [61, 81] (41) | 75 [64, 82] (25) | 0.8617 | 0.8655 |
| Outcomes At Hosp Dc/One Month | ||||||
| Alive at 1 Month (%) | 94 (91.3) | 83 (91.2) | 117 (93.6) | 65 (92.9) | 0.8893 | 0.9091 |
| 2 Mortality or New FSS morbidity (%) | 28 (27.2%) | 24 (26.4%) | 31 (24.8%) | 23 (32.9%) | 0.6786 | 0.7657 |
| 3 Mortality or PSD-HRQL (%) | 0.4117 | 0.4372 | ||||
| No | 34 (33.0) | 21 (23.1) | 26 (20.8) | 17 (24.3) | ||
| Yes | 44 (42.7) | 30 (33.0) | 57 (45.6) | 30 (42.9) | ||
| No Month 1 HRQL Follow-up | 25 (24.3) | 40 (44.0) | 42 (33.6) | 23 (32.9) | ||
| 4 FSS, Median [IQR] (n) | 9 [6, 16] (102) | 10 [6, 15] (90) | 10 [6, 15] (124) | 10 [8, 18] (68) | 0.1135 | 0.0178 |
| HRQL, Median [IQR] (n) | 68 [44, 82] (79) | 69 [43, 85] (52) | 63 [41, 78] (85) | 66 [45, 80] (48) | 0.7229 | 0.3441 |
| 5 PedsQL™, Median [IQR] (n) | 61 [37, 83] (49) | 68 [45, 82] (31) | 58 [38, 72] (54) | 53 [31, 73] (29) | 0.5350 | 0.2687 |
| 6 FSII-R, Median [IQR] (n) | 72 [61, 82] (30) | 78 [41, 89] (20) | 71 [57, 85] (30) | 68 [61, 82] (19) | 0.9730 | 0.7686 |
| ΔFSS, Median [IQR] (n) | 0 [0, 3] (102) | 0 [0, 3] (90) | 0 [0, 3] (124) | 1 [0, 4] (68) | 0.2059 | 0.0691 |
| Δ HRQL, Median [IQR] (n) | −4 [−27, 8] (76) | −7 [−33, 7] (51) | −11 [−32, 5] (80) | −7 [−27, 7] (47) | 0.4982 | 0.5957 |
| ΔPedsQL™, Median [IQR] (n) | −11 [−35, 7] (46) | −12 [−40, 2] (31) | −17 [−39, 0] (51) | −19 [−31, 3] (28) | 0.3741 | 0.5139 |
| ΔFSII-R, Median [IQR] (n) | −2 [−14, 13] (30) | 2 [−23, 23] (20) | −4 [−11, 7] (29) | −3 [−7, 11] (19) | 0.9750 | 0.7620 |
| %ΔFSS, Median [IQR] (n) | 0 [0, 33] (102) | 0 [0, 33] (90) | 0 [0, 33] (124) | 14 [0, 50] (68) | 0.1923 | 0.0724 |
| %ΔHRQL, Median [IQR] (n) | −6 [−36, 12] (76) | −8 [−42, 9] (51) | −12 [−40, 7] (80) | −9 [−36, 9] (47) | 0.5147 | 0.5830 |
| %ΔPedsQL™, Median [IQR] (n) | −13 [−40, 9] (46) | −15 [−42, 3] (31) | −28 [−41, 0] (51) | −22 [−53, 4] (28) | 0.3726 | 0.4816 |
| %ΔFSII-R, Median [IQR] (n) | −2 [−17, 17] (30) | 2 [−31, 36] (20) | −4 [−16, 11] (29) | −4 [−9, 14] (19) | 0.9783 | 0.7982 |
| Ventilator Free Days, Median [IQR] | 21 [15, 25] | 19 [12, 23] | 20 [14, 23] | 17 [4, 22] | 0.0013 | 0.0083 |
| V-IS Free Days, Median [IQR] | 25 [22, 27] | 25 [22, 26] | 25 [22, 26] | 25 [21, 26] | 0.0372 | 0.1719 |
| PICU Free Days, Median [IQR] | 21 [16, 25] | 20 [14, 23] | 18 [13, 23] | 19 [10, 24] | 0.0134 | 0.0238 |
| Hospital Free Days, Median [IQR] | 18 [9, 23] | 16 [9, 21] | 17 [9, 25] | 16 [5, 24] | 0.1029 | 0.5001 |
Binary outcomes were analyzed using logistic regression, and continuous outcomes were analyzed with rank regression. The primary predictor in all models was the pathogen type. Adjusted p-values are based on analogous models that additionally incorporate age (≤ 1 year, > 1 year) as a covariate. Summaries reported are counts and percentages for binary outcomes and median [interquartile range (IQR)] for continuous outcomes.
Abbreviations: Outcomes At Hosp Dc/One Month, outcomes at hospital discharge/one month; PCR, polymerase chain reaction; PICU, pediatric intensive care unit; VIS, vasoactive-inotropic support; PedsQL™, Pediatric Quality of Life Inventory; FSII-R, Stein–Jessup Functional Status Score; HRQL, combined PedsQL™ and FSII-R data analyzed together; PSD-HRQL, persistent, serious deterioration in HRQL;.
In total 14 patients encountered fungal infections, 9 with concurrent bacterial infections, 4 with concurrent bacterial+viral infections, and 1 with a concurrent viral infection.
New FSS Morbidity is defined as death or ΔFSS ≥ 3 comparing hospital discharge/one month and baseline
PSD-HRQL is defined as a HRQL score persisting > 25% below baseline value, as assessed at Month 1 (Day 21–42)
Patients who died before one month or hospital discharge were assigned a FSS score of 30
Patients who died before one month or hospital discharge were assigned a PedsQL™ score of 0
Patients who died before one month or hospital discharge were assigned a FSII-R score of 0
TABLE 4.
One Month/Hospital Discharge Outcomes By Pathogen Group Among Survivors
| Pathogen Group | P Values | |||||
|---|---|---|---|---|---|---|
| Functional Status, Health Related Quality of Life Measure |
No Causative Organism Identified (N=91) |
Pure Viral Infections (N=80) |
Pure Bacterial/Fungal Infections1 (N=111) |
Bacterial/Fungal And Viral Co-infection1 (N=56) |
Unadjusted P Value |
Age-adjusted P value |
| Month 1 Outcomes | ||||||
| ΔFSS, Median [IQR] (n) | 0 [0, 2] (90) | 0 [0, 1] (79) | 0 [0, 2] (110) | 0 [0, 2] (54) | 0.1854 | 0.2595 |
| ΔHRQL, Median [IQR] (n) | 0 [−21, 12] (67) | −3 [−18, 7] (42) | −10 [−30, 7] (71) | −5 [−18, 6] (35) | 0.2988 | 0.3773 |
| ΔPedsQL™, Median [IQR] (n) | −3 [−27, 8] (40) | −6 [−30, 3] (26) | −15 [−37, 0] (47) | −11 [−24, 5] (20) | 0.2592 | 0.2406 |
| ΔFSII-R, Median [IQR] (n) | 4 [−13, 14] (27) | 5 [−13, 23] (16) | 1 [−11, 10] (24) | −4 [−7, 7] (15) | 0.9894 | 0.5821 |
| %ΔFSS, Median [IQR] (n) | 0 [0, 27] (90) | 0 [0, 17] (79) | 0 [0, 29] (110) | 0 [0, 33] (54) | 0.1623 | 0.2550 |
| %ΔHRQL, Median [IQR] (n) | 0 [−31, 17] (67) | −4 [−21, 10] (42) | −12 [−36, 8] (71) | −9 [−21, 7] (35) | 0.3675 | 0.4313 |
| %ΔPedsQL™, Median [IQR] (n) | −4 [−36, 12] (40) | −7 [−33, 4] (26) | −27 [−40, 0] (47) | −15 [−31, 7] (20) | 0.3748 | 0.3819 |
| %ΔFSII-R, Median [IQR] (n) | 4 [−16, 22] (27) | 6 [−16, 36] (16) | 1 [−12, 15] (24) | −4 [−9, 10] (15) | 0.9944 | 0.6424 |
| 2New FSS Morbidity (%) | 18 (19.8%) | 14 (17.5%) | 21 (18.9%) | 12 (21.4%) | 0.9499 | 0.9596 |
| 3PSD-HRQL (%) | 33 (36.3%) | 22 (27.5%) | 47 (42.3%) | 22 (39.3%) | 0.1810 | 0.1897 |
| Ventilator Free Days, Median [IQR] | 22 [17, 25] | 20 [15, 23] | 20 [16, 23] | 19 [12, 23] | 0.0054 | 0.0804 |
| VIS Free Days, Median [IQR] | 26 [23, 27] | 25 [23, 27] | 25 [24, 26] | 25 [24, 27] | 0.0953 | 0.4848 |
| PICU Free Days, Median [IQR] | 22 [17, 25] | 20 [15, 23] | 18 [13, 23] | 19 [15, 24] | 0.0104 | 0.0164 |
| Hospital Free Days, Median [IQR] | 19 [10, 24] | 17 [13, 22] | 18 [9, 28] | 16 [12, 24] | 0.2798 | 0.8425 |
Binary outcomes were analyzed using logistic regression, and continuous outcomes were analyzed with rank regression. The primary predictor in all models was the pathogen type. Adjusted p-values are based on analogous models that additionally incorporate age (≤ 1 year, > 1 year) as a covariate. Summaries reported are counts and percentages for binary outcomes and median [interquartile range (IQR)] for continuous outcomes.
Abbreviations: PCR, polymerase chain reaction; PICU, pediatric intensive care unit; PSD-HRQL, persistent, severe deterioration of health-related quality of life; VIS, vasoactive-inotropic support; PedsQL™, Pediatric Quality of Life Inventory; FSII-R, Stein–Jessup Functional Status Score; HRQL, combined PedsQL™ and FSII-R data analyzed together; PSD-HRQL, persistent, serious deterioration of HRQL.
In total 14 patients encountered fungal infections, 9 with concurrent bacterial infections, 4 with concurrent bacterial+viral infections, and 1 with a concurrent viral infection
FSS ≥ 3 comparing hospital discharge/one month and baseline assessments
HRQL scores > 25% below baseline comparing one month and baseline assessments
Our exploratory outcomes, percent deterioration of FSS or HRQL from baseline assessed at hospital discharge/one-month were also not statistically different among pathogen types. For ΔFSS: bacterial/fungal+viral co-infections (14% [0%, 50%]) versus no causative organism identified, pure viral infections, and pure bacterial/fungal infections, (all 0% [0%, 33%]). For ΔHRQL: no causative organism identified (−6% [−36%, 12%]); pure viral infections, (−8% [−42%, 9%]); pure bacterial/fungal infection (−12% [−40%, 7%]); and bacterial/fungal+viral co-infection (−9% [−36%, 9%]).
Pathogen type was significantly associated with mechanical ventilation-free days, independent of age, albeit without correction for multiple comparisons: no causative organism identified (21 [15, 25]); pure viral infections (19 [12, 23]); pure bacterial/fungal infections (20 [14, 23]); and bacterial/fungal+viral co-infections (17 [4, 22]), p=0.0083. Only in unadjusted analysis (all subjects and surviving subjects only) did we observe a statistically significant relationship between vasoactive-inotropic support free days and pathogen type, p=0.0372. However, this relationship was age-dependent, and in age-adjusted analysis, the relationship became statistically insignificant, p = 0.1719. As duration of vasoactive-inotropic support and particularly mechanical ventilation are plausibly associated with duration of PICU stay, it is not surprising that pathogen type was also significantly associated with PICU-free days (again without correction for multiple comparisons), independent of age: no causative organism identified (21 [16, 25]); pure viral infections (20 [14, 23]); pure bacterial/fungal infections (18 [13, 23]); and bacterial/fungal+viral co-infections (19 [10, 24]), p = 0.0238. In analyzing hospital-free days, we found no significant differences among pathogen types.
Discussion
In this secondary analysis of the LAPSE database, no causative organism identified, pure viral infections, pure bacterial/fungal infections, and bacterial/fungal+viral co-infections were observed in 25%, 23%, 32%, and 18% of the LAPSE study cohort respectively (n=389) (8). The composite outcomes of mortality or substantial functional status deterioration occurred in 106/389 (27%) and mortality or persistent, serious deterioration of HRQL occurred in 161/259 (62%) of children encountering septic shock as assessed at hospital discharge/one-month following PICU admission. No statistically significant differences were seen in the proportion of patients with these outcomes among the four pathogen types, although multiple comparisons without statistical correction and small numbers in subgroups increase the risk of both type 1 and type 2 errors for these analyses. Patients with no causative organism identified appeared to enjoy more mechanical ventilation and PICU free days compared to patients with documented bacterial/fungal infection, albeit without statistic correction for multiple comparisons.
In a prospective study of 763 children presenting with sepsis in southeast Asia, 11.8%, 36.0% and 7.1% encountered infections involving bacteria alone, virus alone, and bacteria plus virus respectively; no pathogens were identified in 44.3% (19). In the Sepsis PRevalence, OUtcomes, and Therapies (SPROUT) point prevalence investigation, among 567 children with severe sepsis, a bacterial pathogen was identified in 65.4% and viral pathogen in 20.9% (20). Similar to our findings, SPROUT reported rhinovirus, respiratory syncytial virus (RSV), and adenovirus as commonly detected viruses in children with severe sepsis (20). Another epidemiologic study from Australia/New Zealand identified RSV, cytomegalovirus, Epstein-Barr virus, herpes simplex virus, varicella zoster virus and influenza as common viruses associated with pediatric sepsis (21). Influenza mediated pediatric sepsis confers a particularly high risk for mortality (22) and may be associated with secondary bacterial infections (23). While exogenous viruses may incite sepsis, endogenous DNAs from multiple viruses have been detected in children with sepsis, and this finding was strongly associated with preexisting immunosuppression and risk for secondary infection (24). Epidemiology, pathophysiology, clinical manifestations, diagnostic testing, and management of pediatric viral sepsis have been thoroughly reviewed (25, 26).
No causative organism identified sepsis is commonly encountered in critically ill adults, children and especially neonates (27). Sepsis pathogens may never be identified in this so called “forgotten cohort” for at least four reasons (28): 1) administration of antibiotics before cultures; 2) lack of testing for viruses; 3) limitations of current clinical diagnostic tools; and 4) contribution of a non-infectious systemic inflammatory response. A large (n=1001) prospective cohort study of adult sepsis patients reported culture-negative sepsis in 41.5%. In adjusted analyses, identification of a pathogen was not independently associated with mortality in this study (29). A 2016 epidemiologic investigation that included 6,843,279 patients hospitalized for sepsis reported negative cultures in 47.1%. As compared to patients with culture documented infection, those with culture negative sepsis had more comorbidities, acute organ dysfunctions and higher risk of mortality (OR, 1.75; 95% CI, 1.72–1.77) (30). Similarly, another large epidemiology study of 5,033,257 patients hospitalized with severe sepsis reported that patients with unknown or unreported organisms (61.5% of the cohort), had a mortality of 36% as compared to 27% among patients with identified pathogens (28). Such data challenge antimicrobial de-escalation and antimicrobial stewardship programs (27), and provide impetus for development of novel pathogen detection diagnostics based on the host-response to infection, e.g. (31–34).
Both pure viral infections (e.g.SARS-CoV2, herpes simplex) and pure bacterial infections (e.g. N. meningitides, methicillin resistant S. aureus, multiple drug resistant organisms) can be deadly and long-term debilitating, but related to different clinical contexts and pathophysiologies. In a retrospective cohort investigation that examined 19,361 adults hospitalized for respiratory infection, 32.9%, 17.8%, and 5.6% demonstrated laboratory confirmed pure viral infection, pure bacterial infection, and viral-bacterial co-infection respectively. In the latter cohort, after propensity matching, need for ICU admission and 30-day mortality were significantly higher (35). A generally (but not exclusively) more vigorous host-response (dysoxia; inflammation) to bacterial/fungal infections compared to viral infections may explain potentially greater PICU resource use to support dysfunctional organs in bacterial/fungal septic shock.
A strength of this LAPSE ancillary investigation involves data prospectively collected from 12 tertiary PICUs in the United States. We report associations of sepsis pathogen groups with PICU resource utilization for treatment of sepsis, in addition to patient-centered, clinically meaningful outcomes including mortality as well as functional status and HRQL morbidity during the first month following PICU admission for septic shock. The key limitation of this secondary analysis of the LAPSE cohort, is near certainty of type 2 statistical errors in terms of identifying differences among pathogen type groups with relatively small numbers for the primary outcome measures. For the total LAPSE cohort of 389, mortality or HRQL scores persisting more than 25% below baseline occurred in 37% of the cohort. To assess an intervention with a relative treatment effect (difference) of 25%, α 0.05 and power 0.9, (and a single comparison) would require 568 patients in each treatment arm (comparison group). Similarly, multiple comparisons without correction increase the likelihood of type 1 statistical errors. Another important limitation of the current study relates to significant number of missing HRQL assessments at the one-month follow-up. For example, we specify that 62% of patients died or exhibited persistent, serious HRQL one-month following hospital admission for sepsis, but this percentage is based on a denominator of 259. Regrettably, we do not know the status of 130 patients with no HRQL information at follow-up. Because we combined the various viral and bacterial/fungal pathogens due to the limited number of patients with each individual pathogen group, we were unable to address differential outcomes attributable to specific pathogens. Additionally, we did not discriminate the focus of infection as a potential confounder in the current analysis. Children enrolled in LAPSE all carried diagnoses of community-acquired septic shock. Accordingly, the present report may not be applicable to hospital-acquired septic shock.
In conclusion, this secondary analysis of the LAPSE database identified no statistically significant association of sepsis pathogen types with composite mortality and morbidity outcomes assessed approximately one-month following PICU admission for septic shock. However, pathogen type may be associated with PICU resources employed to treat sepsis organ dysfunction. Significant mortality and functional or HRQL morbidity, were noted among all sepsis pathogen types, including children with sterile cultures and negative PCR surveillance for potential viral pathogens.
Supplementary Material
Research In Context.
Septic shock is classically associated with bacterial infection but can also occur with viral infection and in the absence of documented infection.
Detailed pediatric septic shock clinical and outcomes data have not been reported in relation to pathogen type.
We hypothesized that septic shock involving documented bacterial infections would be associated with increased illness severity, critical care resource utilization, and mortality and morbidity assessed one month following hospital admission.
At The Bedside.
Septic shock may occur with bacterial, or viral infections or in the absence of documented infection.
Regardless of pathogen group, children with septic shock exhibit significant deterioration of functional status and HRQL.
Septic shock involving bacterial infections may be associated with greater PICU resource utilization.
Acknowledgements:
The authors gratefully acknowledge patients and families who agreed to participate in the LAPSE investigation, performance site research personnel for their diligence in enrolling study participants and collecting a wealth of information, and the LAPSE Data Coordinating Center personnel who designed and verified the rich LAPSE database that permitted the current ancillary investigation.
Funding:
Life After Pediatric Sepsis Evaluation was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, R01HD073362, and was supported, in part, by the following cooperative agreements: UG1HD050096, UG1HD049981, UG1HD049983, UG1HD063108, UG1HD083171, UG1HD083166, UG1HD083170, U10HD050012, U10HD063106, and U01HD049934. For secondary analyses of the LAPSE database, Dr Reeder is supported by R03HD104001.
Copyright Form Disclosure:
Drs. Salud, Banks, Carcillo, and Zimmerman’s institutions received funding from the National Institute of Child Health and Human Development. Drs. Salud, Reeder, Banks, Meert, Berg, Zuppa, Newth, Hall, Sapru, Carcillo, McQuillen, Mourani, Varni, and Zimmerman received support for article research from the National Institutes of Health (NIH). Drs. Reeder, Meert, Berg, Zuppa, Hall, McQuillen, Mourani, and Varni’s institutions received funding from the NIH. Dr. Banks disclosed government work. Dr. Newth received funding from Philips Research North America, Hamilton Medical, and Nihon Kohden. Dr. Hall received funding from Abbvie, La Jolla Pharmaceuticals, and Kiadis. Dr. Carcillo’s institution received funding from and the National Institute of General Medical Sciences. Dr. Varni disclosed that he holds the copyright and the trademark for the PedsQL and receives financial compensation from the Mapi Research Trust. Dr. Zimmerman’s institution received funding Immunexpress; he received funding from Elsevier Publishing. Dr. Quasney has disclosed that he does not have any potential conflicts of interest.
Footnotes
Disclosures: No performance site investigators disclose financial interests, activities, relationships, or affiliations that could be construed as real or potential conflicts of interest related to the manuscript or the related investigation. Dr. Varni holds the copyright and the trademark for the Pediatric Quality of Life Inventory™ (PedsQL™) and receives financial compensation from the Mapi Research Trust, which is a nonprofit research institute that charges distribution fees to for-profit companies that use the PedsQL™. Dr Varni provided consultation on original study design and final manuscript edits, but he played no role in data acquisition or analysis.
References
- 1.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016; 315: 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bilinski A, Emanuel EJ. COVID-19 and Excess All-Cause Mortality in the US and 18 Comparison Countries. JAMA 2020; 324: 2100–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taubenberger JK, Morens DM. 1918 Influenza: the mother of all pandemics. Emerg Infect Dis 2006; 12: 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Prost N, Razazi K, Brun-Buisson C. Unrevealing culture-negative severe sepsis. Crit Care 2013; 17: 1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hartman ME, Linde-Zwirble WT, Angus DC, et al. Trends in the epidemiology of pediatric severe sepsis. Pediatr Crit Care Med 2013; 14: 686–693. [DOI] [PubMed] [Google Scholar]
- 6.Tan B, Wong JJ, Sultana R, et al. Global Case-Fatality Rates in Pediatric Severe Sepsis and Septic Shock: A Systematic Review and Meta-analysis. JAMA Pediatr 2019; 173: 352–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kyosti E, Ala-Kokko TI, Ohtonen P, et al. Factors associated with health-related quality of life 6 years after ICU discharge in a Finnish paediatric population: a cohort study. Intensive Care Med 2018; 44: 1378–1387. [DOI] [PubMed] [Google Scholar]
- 8.Zimmerman JJ, Banks R, Berg RA, et al. Life After Pediatric Sepsis Evaluation: Trajectory of Mortality and Health-Related Quality of Life Morbidity Following Community-Acquired Pediatric Septic Shock. Crit Care Med 2020; 48: 329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coopersmith CM, De Backer D, Deutschman CS, et al. Surviving Sepsis Campaign: Research Priorities for Sepsis and Septic Shock. Crit Care Med 2018; 46: 1334–1356. [DOI] [PubMed] [Google Scholar]
- 10.Zimmerman JJ, Banks R, Berg RA, et al. Life After Pediatric Sepsis Evaluation: Critical Illness Factors Associated With Long-Term Mortality and Health-Related Quality of Life Morbidity Following Community-Acquired Pediatric Septic Shock. Crit Care Med 2020; 48: 319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008; 61: 344–349. [DOI] [PubMed] [Google Scholar]
- 12.Simon TD, Cawthon ML, Stanford S, et al. Pediatric medical complexity algorithm: a new method to stratify children by medical complexity. Pediatrics 2014; 133: e1647–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pollack MM, Patel KM, Ruttimann UE. The Pediatric Risk of Mortality III--Acute Physiology Score (PRISM III-APS): a method of assessing physiologic instability for pediatric intensive care unit patients. J Pediatr 1997; 131: 575–581. [DOI] [PubMed] [Google Scholar]
- 14.Leteurtre S, Duhamel A, Salleron J, et al. PELOD-2: an update of the PEdiatric logistic organ dysfunction score. Crit Care Med 2013; 41: 1761–1773. [DOI] [PubMed] [Google Scholar]
- 15.Pollack MM, Holubkov R, Glass P,et al. Functional status scale: new pediatric outcome measure. Pediatrics 2009; 124: e18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varni JW, Limbers CA The Pediatric Quality of Life Inventory: Measureing pediatric health-related quality of life from the perspective of children and their parents. Pediat Clin N Am 2009; 56: 843–863. [DOI] [PubMed] [Google Scholar]
- 17.Varni JW, Limbers CA, Neighbors K, et al. The PedsQL Infant Scales: feasibility, internal consistency reliability, and validity in healthy and ill infants. Qual Life Res 2011; 20: 45–55. [DOI] [PubMed] [Google Scholar]
- 18.Stein RE, Jessop DJ. Functional status II(R). A measure of child health status. Med Care 1990; 28: 1041–1055. [DOI] [PubMed] [Google Scholar]
- 19.Southeast Asia Infectious Disease Clinical Research Network. Causes and outcomes of sepsis in southeast Asia: a multinational multicentre cross-sectional study. Lancet Glob Health 2017; 5: e157–e167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiss SL, Fitzgerald JC, Pappachan J, et al. Global epidemiology of pediatric severe sepsis: the sepsis prevalence, outcomes, and therapies study. Am J Respir Crit Care Med 2015; 191: 1147–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schlapbach LJ, Straney L, Alexander J, et al. Mortality related to invasive infections, sepsis, and septic shock in critically ill children in Australia and New Zealand, 2002–13: a multicentre retrospective cohort study. The Lancet Infectious diseases 2015; 15: 46–54. [DOI] [PubMed] [Google Scholar]
- 22.Bhat N, Wright JG, Broder KR, et al. Influenza-associated deaths among children in the United States, 2003–2004. N Engl J Med 2005; 353: 2559–2567. [DOI] [PubMed] [Google Scholar]
- 23.Hendaus MA, Jomha FA, Alhammadi AH. Virus-induced secondary bacterial infection: a concise review. Ther Clin Risk Manag 2015; 11: 1265–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davila S, Halstead ES, Hall MW, et al. Viral DNAemia and Immune Suppression in Pediatric Sepsis. Pediatr Crit Care Med 2018; 19: e14–e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta N, Richter R, Robert S, et al. Front Pediatr 2018; 6: 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin GL, McGinley JP, Drysdale SB, et al. Epidemiology and Immune Pathogenesis of Viral Sepsis. Front Immunol 2018; 9: 2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thorndike J, Kollef MH. Culture-negative sepsis. Curr Opin Crit Care 2020; 26: 473–477. [DOI] [PubMed] [Google Scholar]
- 28.Jacob JT. Elucidating the known unknowns of sepsis. Crit Care Med 2015; 43: 237–238. [DOI] [PubMed] [Google Scholar]
- 29.Phua J, Ngerng W, See K, et al. Characteristics and outcomes of culture-negative versus culture-positive severe sepsis. Crit Care 2013; 17: R202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta S, Sakhuja A, Kumar G, et al. Culture-Negative Severe Sepsis: Nationwide Trends and Outcomes. Chest 2016; 150: 1251–1259. [DOI] [PubMed] [Google Scholar]
- 31.Herberg JA, Kaforou M, Wright VJ, et al. Diagnostic Test Accuracy of a 2-Transcript Host RNA Signature for Discriminating Bacterial vs Viral Infection in Febrile Children. JAMA 2016; 316: 835–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahajan P, Kuppermann N, Mejias A, et al. Association of RNA Biosignatures With Bacterial Infections in Febrile Infants Aged 60 Days or Younger. JAMA 2016; 316: 846–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zimmerman JJ, Sullivan E, Yager TD, et al. Diagnostic Accuracy of a Host Gene Expression Signature That Discriminates Clinical Severe Sepsis Syndrome and Infection-Negative Systemic Inflammation Among Critically Ill Children. Crit Care Med 2017; 45: e418–e425. [DOI] [PubMed] [Google Scholar]
- 34.Mohammed A, Cui Y, Mas VR, et al. Differential gene expression analysis reveals novel genes and pathways in pediatric septic shock patients. Sci Rep 2019; 9: 11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Y, Ling L, Wong SH, et al. Outcomes of respiratory viral-bacterial co-infection in adult hospitalized patients. E Clin Med 2021; 37: 100955. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


