Abstract
Objective
Mindfulness-based interventions are widely used to target pain, yet their neural mechanisms of action are insufficiently understood. We studied neural and subjective response in a randomized active-control trial of Mindfulness-Based Stress Reduction (MBSR) alongside long-term mindfulness practitioners (LTMs).
Method
Healthy participants (N=115) underwent functional neuroimaging during a thermal acute pain task before and after random assignment to MBSR (n=28), an active control condition (Health Enhancement Program; HEP; n=32), or waitlist (n=31). LTMs (N=30) completed the same neuroimaging paradigm. Pain response was measured via self-reported intensity and unpleasantness, and neurally via two multi-voxel machine-learning derived signatures: The Neurologic Pain Signature (NPS), emphasizing nociceptive pain processing, and the Stimulus Intensity Independent Pain Signature-1 (SIIPS1), emphasizing stimulus-independent neuromodulatory processes.
Results
The MBSR group showed a decrease in NPS response relative to HEP (d=−0.43, p=.050, two-tailed) and from pre- to post-intervention (d=−0.47, p=.023). The MBSR group also showed small, marginal decreases in NPS relative to waitlist (d=−0.36, p=.096), and in SIIPS1 relative to both groups (HEP: d=−0.37, p=.089; waitlist: d=−0.37, p=.087). For subjective unpleasantness, MBSR and HEP also showed modest reductions versus waitlist (d=−0.45, p=.031; d=−0.55, p=.005). LTMs reported lower pain than non-meditators (p’s < .001) but did not differ in neural response. Within LTMs, cumulative practice during intensive retreat was associated with reduced SIIPS1 (r=−.65, p=.027) whereas daily practice was not.
Conclusions
Mindfulness training showed associations with pain reduction that implicate differing neural pathways depending on extent and context of practice. Use of neural pain signatures in randomized trials offers promise for guiding the application of mindfulness interventions to pain treatment.
ClinicalTrials.gov Identifier:
Introduction
Understanding the neurocognitive mechanisms of efficacy in non-pharmacological pain interventions is a high-priority objective for improving pain treatment (1). Mindfulness-based interventions are a category of non-pharmacological intervention which trains participants in awareness and acceptance of mental experience, commonly implemented via 8-week structured group programs such as Mindfulness-Based Stress Reduction (MBSR; 2). Despite growing popularity and demonstrated benefits for a range of pain-related conditions and outcomes, understanding of neural mechanisms underlying mindfulness interventions for pain remains limited (3).
Available mechanistic evidence on mindfulness-related pain modulation comes predominantly from brief laboratory interventions and cross-sectional study of long-term meditation practitioners (4). Together, these studies suggest that mindfulness training may be associated with alterations in sensory processing circuitry as well as cognitive-emotional regulatory networks (4–6). However, no such study has yet been conducted on a standardized, full length, and widely used clinical intervention such as MBSR.
To address this gap in evidence, we conducted a neuroimaging-based mechanistic investigation of pain response within a randomized, actively controlled trial of MBSR. To maximize the clinical interpretability of our findings, we applied a recently developed and well-suited method for analyzing functional neuroimaging of pain: behaviorally validated neural signature responses (7, 8). These neural signatures comprise multivoxel patterns of neural activity which have been previously optimized for sensitivity and specificity to pain experience using machine-learning techniques (7–9). This approach offers several potential advantages for clinical mechanistic research. Relationships between signature response and subjective pain outcomes have been empirically established, addressing the common problem of “reverse inference” (10) in conventional neuroimaging analysis. Thus, pain-related neural signatures are well-suited to provide mechanistic insights that bridge neural and psychological levels of analysis. Reflecting their status as potential neural biomarkers for pain, each signature also provides a single unidimensional response which can be analyzed alongside other outcomes such as lab assays or symptom scales using established clinical trial methodology (11). Finally, traditional mass-univariate analysis for neuroimaging typically involves dozens or thousands of parallel hypothesis tests, requiring stringent corrections to control false positives and complicating estimations of effect size. For neural signature-based analysis, only a single such test is required per signature, increasing statistical power without inflating false positive rates, and furthermore allowing for unbiased estimates of effect size (11).
In this study, we examined the effects of mindfulness training on two distinct aspects of pain processing using a complementary pair of signatures: the Neurologic Pain Signature (NPS; 7) and the Stimulus Intensity Independent Pain Signature-1 (SIIPS-1; 8). The NPS was trained and subsequently validated to track mainly stimulus-dependent aspects of pain, i.e., the intensity of pain reports induced by variations in noxious stimulus intensity. It is comprised of brain regions which show most consistent activation to painful stimuli, especially those directly receiving afferent pain signals from the body. The NPS is reliably activated by multiple types of pain while responding minimally or not at all to other salient, emotionally evocative stimuli or to cognitive modulators of pain such as placebo treatment (8, 9, 12). By contrast, the SIIPS1 is designed to track stimulus-independent aspects of pain, specifically, variation in pain reports not accounted for stimulus intensity or NPS response. The SIIPS1 is not constrained to neural regions directly associated with nociceptive activity and thus incorporates a broader range of cognitive and emotional modulatory circuits. Importantly, the SIIPS1 shows sensitivity to psychological modulators of pain processing in previous studies, including expectancy cueing and changes in perceived control, and thus tracks cognitive elaborative processes which modulate pain experience independent of the sensory stimulus itself (8).
To experimentally study the effects of mindfulness training, healthy, meditation-naïve participants (MNP) were randomly assigned to either a standardized 8-week MBSR course, a matched active control intervention validated in previous research (Health Enhancement Program [HEP] 13), or a waitlist condition (WL). Because much existing evidence in this area has been derived on long-term mindfulness practitioners, and because the pain-related cognitive processing involved has been theorized to change with mindfulness practice experience, we also examined a cross-sectional comparison sample of North American long-term mindfulness practitioners. The inclusion of this sample alongside MBSR practitioners using a consistent study protocol and pain task paradigm was designed to allow for side-by-side study of short-term (MBSR) and long-term training effects.
We predicted that mindfulness training would reduce pain response on both subjective report and neural measures, with effects specific to duration of training. We tested predictions from prior research (4) via competing hypotheses. If mindfulness training modulates direct sensory signals, then reductions should be observed primarily in NPS response. Alternatively, if mindfulness training influences more elaborative stages of cognitive processing (5) then reductions should be observed primarily in SIIPS1 response. We predicted that effects of short-term mindfulness training should be observed in the MBSR but not HEP or waitlist conditions, while non-specific effects should be common to both MBSR and HEP but not the waitlist group. With regard to practice experience, we hypothesized that modulation of sensory signals, as observed in reported pain intensity and NPS response, should occur primarily in the early stages of training, due to greater use of effortful attentional mechanisms which may produce a gating effect on incoming nociceptive signals. In contrast, long-term training has been proposed to rely on less effortful mechanisms and more on consolidated changes in cognitive appraisal and elaboration, rather than sensory experience of pain (4). We therefore hypothesized that greater differences in long-term practitioners would be observed in more stimulus-independent measures of pain unpleasantness and SIIPS1 response. Lastly, among long-term practitioners, we predicted that pain modulation should be related to the extent of practice experience. Given that our previous analyses have shown differing effects of mindfulness training according to the training context (14, 15), we also separately examined the effects of practice experience accumulated through day-to-day practice and through intensive meditation retreats (16), with the prediction that intensive retreat practice should produce stronger effects than routine daily practice.
Methods
Participant recruitment
Healthy human subjects were recruited and enrolled by logistical study personnel. Of these, 127 were meditation-naïve participants (MNP), who were assigned to one of three groups: an 8-week MBSR course, an 8-week HEP course as an active control group, or a waitlist control group (WL) with no intervention. Assignment was performed by a logistical staff member using computerized random number generation. Study measures were collected by experimenters who remained blind to group assignment during data collection. An additional 31 long-term meditators (LTM) were recruited for cross-sectional comparison with non-meditators.
All participants were screened for cardiovascular and neurological health issues and history of psychiatric diagnosis or psychotropic medication use. Additional exclusion criteria for the MNP group included prior experience with meditation or mind-body techniques, physical limitations, or extensive engagement in physical exercise. Inclusion in the LTM group required at least 3 years of formal meditation experience, including multiple intensive retreats and ongoing daily practice. Data were collected as part of a larger study on mindfulness interventions for emotions and well-being at the University of Madison-Wisconsin from November 2009 to March 2012 (14, 15, 17). The enrolled sample size, targeting n=30 per group allowing for dropout, was chosen based on findings from previous studies of neural changes associated with MBSR to allow detection of medium effects (d=.5) with power of .80 at an alpha level of .05. Participants provided written informed consent for study procedures that were approved by the UW-Madison Health Sciences Institutional Review Board. Further details on study recruitment are provided in Supplemental Methods.
Interventions
The primary intervention, MBSR, is an 8-week course consisting of instruction and practice in cultivating continuous focused attention on the breath, bodily sensations, and mental content while in seated postures, walking, and yoga (18, 19). The active comparison intervention, HEP, is a non-mindfulness-based course matched with MBSR on length, structure, and non-specific therapeutic elements including supportive group atmosphere, expert instruction, and positive expectancy for benefit (13). Further details on the interventions are provided in Supplemental Methods.
Task design
A total of 20 thermal stimuli lasting 12 seconds, including an 8-second plateau at peak temperature, were delivered using thermal stimulation to the inside of the left wrist (see full task details in supplement). Thermal stimulations were separated by a distractor task and intervals for cued anticipation, recovery, and subjective ratings of intensity and unpleasantness. Participants rated the intensity and unpleasantness of each thermal stimulus on a 0 to 20 scale (20). An equal number of thermal stimuli were delivered for two conditions, painful heat and non-painful warmth, in counterbalanced order. During painful heat trials, participants received stimulation at a temperature previously calibrated to correspond to a rating of 14/20, adjusted downward for tolerability at time of scan in 1° increments if needed (range, 42°C to 49°C). During non-painful warmth trials, participants received stimulation calibrated to be detectable but not painful (range, 36° to 43°C). Further details on task design are provided in Supplemental Methods.
Data acquisition
Images were acquired on a GE X750 3.0 Tesla MRI scanner device with an 8-channel head coil. Respiration belt signals were recorded continuously during imaging runs using BIOPAC equipment and AcqKnowledge software. Further details on acquisition sequences and image processing are provided in Supplemental Methods.
Neural signatures
Both NPS and SIIPS1 neural signatures were independently derived (7, 8) and applied to this novel data set without further refinement. Preprocessed MRI data were prepared for neural signature analysis by contrasting neural responses to painful hot versus non-painful warm stimuli collapsed across all 20 thermal stimuli, yielding one contrast image per participant. Computation of per-participant NPS and SIIPS1 responses was performed using a publicly available analysis script without modifications (apply_mask.m, available in the CANlab Core toolbox on Github; see https://github.com/canlab/). Processing for the two signatures differed only in which of the corresponding signature response maps was used in the script. Scaling of the signature response depends on both study-specific parameters (as with all BOLD fMRI studies) and specific properties of each signature and is thus reported in arbitrary units (a.u.). The NPS is available for non-commercial research use with a signed Material Transfer Agreement from Dr. Wager. The SIIPS1 is freely available for download from https://github.com/canlab/Neuroimaging_Pattern_Masks. Further details on development of the signatures, previous validation, component brain regions, and their availability to researchers are provided in Supplemental Methods.
Statistical analysis
Final statistical analysis was conducted using R 3.3.2 (https://www.r-project.org/). Intervention effects on pain response were modeled using analysis of covariance (ANCOVA), regressing pre-post change on pre-intervention values. Potential covariates of age and gender were examined for associations with pain outcomes at baseline and included in intervention models when such associations were present. Effects of between-group differences for LTM and MNP and log-scaled lifetime practice hours for LTM were computed using ordinary least squares regression. Age, gender, mean respiration rate, and pain tolerance (maximum tolerable thermode temperature as determined by the calibration procedure) were examined as potential confounds for significant effects of practice hours. For neural pain signature responses, standardized effects are presented. For analyses of pain ratings (intensity and unpleasantness), respiration rate and pain tolerance, effects are presented on the scale of measurement. Validation of pain signatures was performed using receiver operating characteristic (ROC) analysis and an area-under-curve (AUC) metric (21) in addition to raw accuracy, with binomial tests versus chance performance. Standardized effect sizes are reported using Cohen’s d. The threshold for statistical significance was set at p ≤ .05, two-tailed. For purposes of potential further investigation, hypothesis-consistent effects with p values above the significance threshold but below a threshold of .1 are noted as marginal and discussed separately.
Results
Sample characteristics
Baseline analyses included 115 MNP and 30 LTM with valid pain task data. LTM and baseline MNP samples did not differ in age, gender, level of education, or socio-economic status measured with the Hollingshead index (22) (all p>.05) and had minimal psychiatric history (see Table 1 and Supplemental Results). Intervention analyses included 91 participants (MBSR: 28, HEP: 32, WL: 31) with valid pain task data at both sessions. Analyses involving respiration measures were based on 74 MNP (MBSR: n=24, HEP: n=27, WL: n=23) and 25 LTM participants with valid respiration data. See Figure S1 for CONSORT chart.
Table 1.
Demographic characteristics of study participants.
| Characteristic | MBSR (N=28) | HEP (N=32) | WL (N=31) | Baseline (N=115) | LTM (N=30) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
|
| ||||||||||
| Age | 50.9 | 9.7 | 49.0 | 12.3 | 48.6 | 10.9 | 48.3 | 11.0 | 51.4 | 9.6 |
| SES (Hollingshead)a | 55.8 | 11.9 | 57.3 | 12.6 | 62.0 | 7.6 | 58.3 | 11.1 | 62.8 | 7.4 |
| N | % | N | % | N | % | N | % | N | % | |
|
| ||||||||||
| Female | 18 | 64.3 | 18 | 56.2 | 21 | 67.7 | 71 | 61.7 | 17 | 56.7 |
| Race/Ethnicity: | ||||||||||
| Hispanic or Latino | 1 | 3.6 | 1 | 3.1 | 6 | 19.4 | 10 | 8.7 | 0 | 0.0 |
| White | 25 | 89.3 | 30 | 93.8 | 28 | 90.3 | 101 | 87.8 | 27 | 90.0 |
| Black or African American | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 3 | 2.6 | 0 | 0.0 |
| Asian | 1 | 3.6 | 1 | 3.1 | 0 | 0.0 | 3 | 2.6 | 2 | 6.7 |
| American Indian or Alaska Native | 1 | 3.6 | 0 | 0.0 | 0 | 0.0 | 2 | 1.7 | 0 | 0.0 |
| Multiple races | 1 | 3.6 | 1 | 3.1 | 1 | 3.2 | 4 | 3.5 | 1 | 3.3 |
| Declined to respond | 0 | 0.0 | 0 | 0.0 | 2 | 6.5 | 2 | 1.7 | 0 | 0.0 |
| Education: | ||||||||||
| High school or GED | 4 | 14.3 | 1 | 3.1 | 0 | 0.0 | 7 | 6.1 | 0 | 0.0 |
| Some college | 0 | 0.0 | 1 | 3.1 | 2 | 6.5 | 5 | 4.3 | 2 | 6.7 |
| Undergraduate degree | 9 | 32.1 | 17 | 53.1 | 10 | 32.3 | 45 | 39.1 | 13 | 43.3 |
| Graduate degree | 15 | 53.6 | 13 | 40.6 | 19 | 61.3 | 58 | 50.4 | 15 | 50.0 |
| Income: | ||||||||||
| <$70,000 | 15 | 53.6 | 13 | 40.6 | 8 | 25.8 | 47 | 40.9 | 12 | 40.0 |
| $70,000–$150,000 | 9 | 32.1 | 14 | 43.8 | 23 | 74.2 | 55 | 47.8 | 11 | 36.7 |
| >$150,000 | 4 | 14.3 | 5 | 15.6 | 0 | 0.0 | 13 | 11.3 | 7 | 23.3 |
| Psychiatric history b | ||||||||||
| Depression, ≥5 years prior | 2 | 7.1 | 2 | 6.3 | 1 | 3.2 | 5 | 4.3 | 0 | 0.0 |
| Other diagnosis or <5 years prior | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
Notes: Baseline refers to pre-randomization sample of non-meditators used to validate neural signature properties.
Calculated using Hollingshead two-factor index (22).
Non-exclusionary.
Abbreviations: MBSR = Mindfulness-Based Stress Reduction; HEP = Health Enhancement Program; WL = waitlist control; LTM = long-term meditator; SES: socio-economic status.
Validation of neural signatures
Performance of the neural signatures was validated at the baseline session. Both signatures showed good performance in discriminating between painful heat and non-painful warmth at the participant level (SIIPS1: accuracy=0.77; NPS: accuracy=0.77; both p<.001 versus chance; see supplemental results for additional details). Discrimination was similar or better for painful heat versus anticipation, recovery, and retrospective pain reporting periods (all p<.001; see supplemental results for additional details). Both signatures were positively associated with thermode temperature (SIIPS1: r=0.18, p=.028; NPS: r=0.36, p<.001), with SIIPS1 response positively associated with subjective reports (intensity: r=0.19, p=.026; unpleasantness: r=0.18, p=0.27), while NPS response was positively associated with thermode temperature after controlling for SIIPS1 response (r=.34, p<.001).
Baseline characteristics
Across all participants, age was negatively associated with both NPS response (r=−0.26, p=.001), and SIIPS1 response (r=−0.31, p<.001), and was therefore included as a covariate in subsequent analyses of neural signatures. Age was not associated with subjective pain reports, and gender was not associated with either neural signature or subjective reports. Full details are presented in Table S1. Overall, findings for training effects on both pain signatures and subjective pain ratings were unchanged by the inclusion or omission of age, gender, and respiration rate as covariates, except where noted.
Short-term training
Neural signature response
The MBSR group showed a decrease in NPS response relative to HEP (d=−0.43, p=.050) and a decrease in NPS response from pre- to post-intervention (d=−0.43, p=.023; see Table 2 and Figure 1 for all intervention effects). The MBSR group also showed marginal decreases in NPS relative to waitlist (d=−0.36, p=.096), and in SIIPS1 relative to both groups (HEP: d=−0.37, p=.089; waitlist: d=−0.37, p=.087). The HEP group also showed a marginal decrease in SIIPS1 response relative to waitlist (HEP: d=−0.37, p=.087). No other between-group or within-group effects were observed for neural signatures.
Table 2.
Neural and subjective responses to thermal pain in relation to short-term mindfulness training.
| Measure | Comparison | df | b | 95% CI | t | p | d | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| NPS | Group by time | MBSR-HEP | 86 | −0.44 | [−0.89, 0.00] | −1.99 | .050 * | −0.43 |
| MBSR-WL | 86 | −0.38 | [−0.82, 0.07] | −1.68 | .096 † | −0.36 | ||
| HEP-WL | 86 | 0.07 | [−0.37, 0.50] | 0.31 | .760 | 0.07 | ||
|
|
||||||||
| Within group | MBSR | 26 | −0.46 | [−0.86, −0.07] | −2.41 | .023 * | −0.47 | |
| HEP | 30 | 0.20 | [−0.16, 0.57] | 1.14 | .265 | 0.21 | ||
| WL | 29 | −0.06 | [−0.43, 0.30] | −0.37 | .718 | −0.07 | ||
|
| ||||||||
| SIIPS1 | Group by time | MBSR-HEP | 86 | −0.01 | [−0.48, 0.45] | −0.06 | .949 | −0.01 |
| MBSR-WL | 86 | −0.40 | [−0.87, 0.06] | −1.72 | .089 † | −0.37 | ||
| HEP-WL | 86 | −0.39 | [−0.84, 0.06] | −1.73 | .087 † | −0.37 | ||
|
|
||||||||
| Within group | MBSR | 26 | −0.12 | [−0.50, 0.27] | −0.61 | .546 | −0.12 | |
| HEP | 30 | −0.21 | [−0.57, 0.15] | −1.18 | .246 | −0.22 | ||
| WL | 29 | 0.14 | [−0.22, 0.51] | 0.79 | .434 | 0.15 | ||
|
| ||||||||
| Intensity | Group by time | MBSR-HEP | 87 | −0.14 | [−1.00, 0.72] | −0.32 | .751 | −0.08 |
| MBSR-WL | 87 | −0.54 | [−1.40, 0.32] | −1.24 | .217 | −0.28 | ||
| HEP-WL | 87 | −0.40 | [−1.23, 0.44] | −0.95 | .344 | −0.21 | ||
|
|
||||||||
| Within group | MBSR | 27 | −0.46 | [−1.06, 0.15] | −1.55 | .133 | −0.30 | |
| HEP | 31 | −0.59 | [−1.17, −0.01] | −2.08 | .046 * | −0.38 | ||
| WL | 30 | 0.00 | [−0.80, 0.80] | 0.00 | .997 | −0.00 | ||
|
| ||||||||
| Unpleasantness | Group by time | MBSR-HEP | 87 | 0.12 | [−1.02, 1.25] | 0.21 | .838 | 0.04 |
| MBSR-WL | 87 | −1.02 | [−2.16, 0.12] | −1.78 | .078 † | −0.39 | ||
| HEP-WL | 87 | −1.14 | [−2.24, −0.04] | −2.06 | .043 * | −0.44 | ||
|
|
||||||||
| Within group | MBSR | 27 | −0.97 | [−1.82, −0.12] | −2.33 | .028 * | −0.45 | |
| HEP | 31 | −1.20 | [−2.05, −0.35] | −2.88 | .007 * | −0.55 | ||
| WL | 30 | 0.06 | [−0.99, 1.11] | 0.12 | .906 | 0.02 | ||
Notes: Effects represent comparisons between Mindfulness-Based Stress duction (MBSR, n=28); Health Enhancement Program (HEP, n=32) and waitlist control (WL, n=31). Group by time effects represent two-sampled t-test of relative change from pre- to post-intervention, adjusting for pre-intervention. Within group effects represent absolute change from pre- to post-intervention with paired t-tests, two-tailed. Effects for pain signatures (NPS and SIIPS1) are standardized and adjusted for age. Effects for subjective reports (intensity and unpleasantness) are on the outcome scale of measurement.
Abbreviations: NPS = Neural Pain Signature; SIIPS1 = Stimulus Intensity Independent Pain Signature; df = degrees of freedom; d = standardized effect size (Cohen’s d); CI = confidence interval.
indicates p ≤ .05.
Indicates p ≤ .1.
Figure 1. Neural signature and subjective responses to thermal pain pre- and post-intervention, by intervention group (MBSR: n=28; HEP: n=32; Waitlist: n=31).
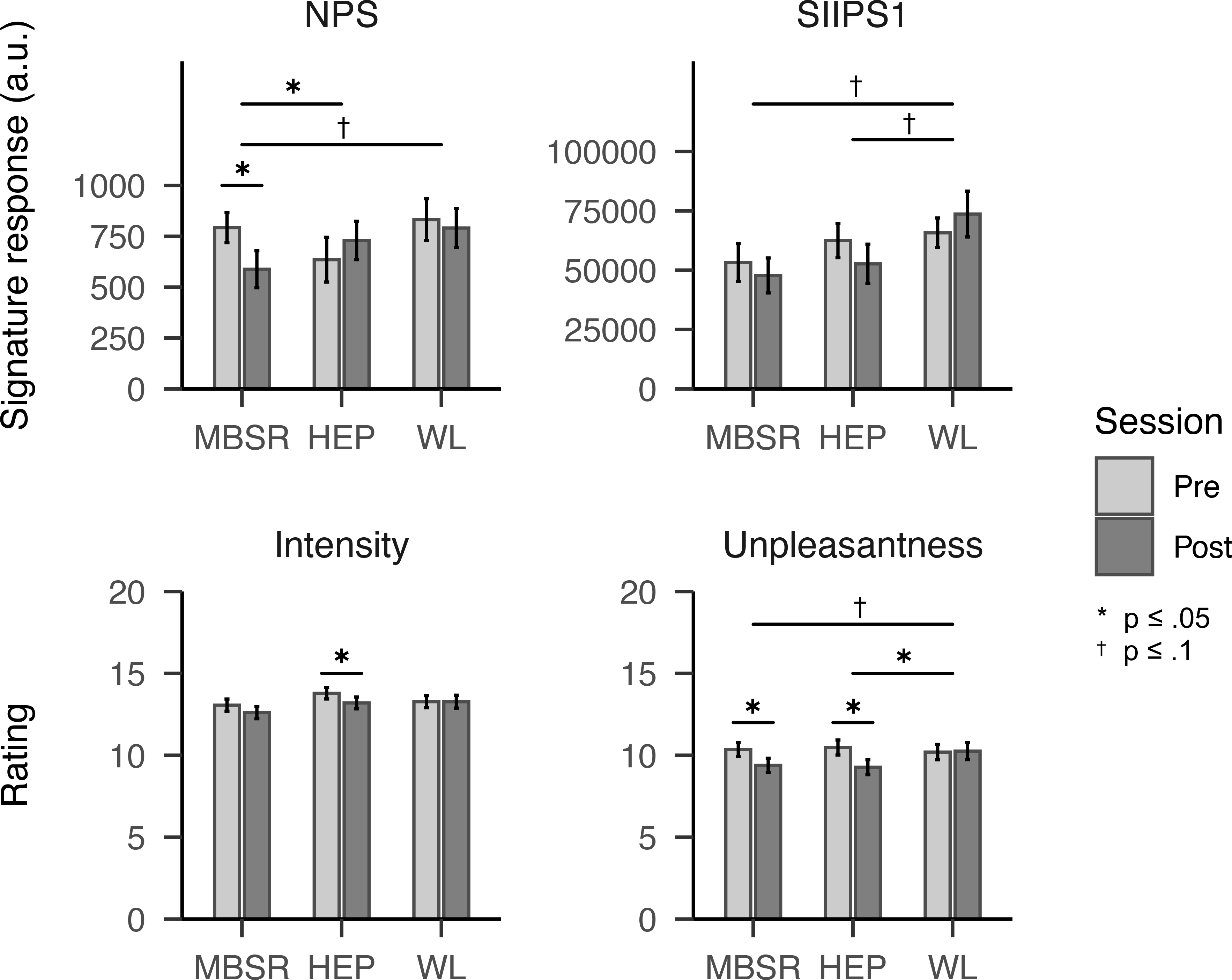
Notes: Horizontal crossbars between groups, in black, represent between-group difference at post-intervention adjusting for pre-intervention and participant age. Crossbars within groups demarcate pre-post changes. Error bars represent standard errors. Abbreviations: MBSR = Mindfulness-based stress reduction; HEP = Health Enhancement Program; WL = Waitlist; NPS = Neural Pain Signature; SIIPS1 = Stimulus Intensity Independent Pain Signature-1; a.u. = arbitrary units. * indicates p ≤ .05; † indicates p ≤ .1.
Subjective report
The MBSR group showed a marginal decrease relative to waitlist (d=−0.39, p=.078) and within the group from pre- to post-intervention (d=−0.38, p=.028; see Table 2 and Figure 1 for all intervention effects). The HEP group showed a decrease in unpleasantness relative to waitlist (d=−0.44, p=.043) and an decrease within the group in both reported intensity and unpleasantness (intensity: d=−0.38, p=.046; unpleasantness d=−0.55, p=.007). There were no other between-group differences in subjective report from pre- to post-intervention. There were no differences between or within groups in pain tolerance (see Table S2).
Respiration
Within the MBSR group, mean respiration rate showed a decrease of 0.61 breaths/minute from pre- to post-intervention, (95% CI [−1.21, −0.01], t(23)= −2.10, p=.047). However, decreases in respiration rate within the MBSR group were not significantly correlated with decreased neural or subjective pain response (see Table S2). There were no other differences between or within groups in mean respiration rate from pre- to post-intervention during the pain task (see Table S2).
Long-term training
Neural signature response
No mean differences were observed between LTM and MNP samples in neural signature response (see Table 3). Among long-term meditators, SIIPS1 response showed an inverse relationship with retreat hours (r=−.65, p=.027; see Table 3 and Figure 2), which remained similar after further adjusting for gender and mean respiration rate (r=−.45, p=.031). No other relationships were observed between neural signature response and practice hours (see Table 3).
Table 3.
Neural and subjective responses to pain in relation to long-term mindfulness training.
| Measure | Comparison | df | b | 95% CI | t | p | d | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| NPS | Between groups | LTM-MNP | 142 | −0.17 | [−0.57, 0.22] | −0.86 | .394 | −0.14 |
|
|
||||||||
| Practice hours | Retreat | 27 | −0.07 | [−0.65, 0.50] | −0.26 | .797 | −0.10 | |
| Daily | 27 | 0.36 | [−0.89, 1.61] | 0.59 | .557 | 0.23 | ||
| Total | 27 | −0.08 | [−1.16, 1.00] | −0.16 | .876 | −0.06 | ||
|
| ||||||||
| SIIPS-1 | Between groups | LTM-MNP | 142 | 0.15 | [−0.24, 0.54] | 0.75 | .455 | 0.13 |
|
|
||||||||
| Practice hours | Retreat | 27 | −0.65 | [−1.22, −0.08] | −2.34 | .027 * | −0.90 | |
| Daily | 27 | 0.13 | [−1.23, 1.50] | 0.20 | .842 | 0.08 | ||
| Total | 27 | −0.95 | [−2.06, 0.17] | −1.74 | .093 † | −0.67 | ||
|
| ||||||||
| Intensity | Between groups | LTM-MNP | 143 | −1.56 | [−2.51, −0.61] | −3.26 | .001 * | −0.54 |
|
|
||||||||
| Practice hours | Retreat | 28 | −1.91 | [−3.78, −0.04] | −2.09 | .046 * | −0.79 | |
| Daily | 28 | 4.00 | [−0.08, 8.07] | 2.01 | .054 † | 0.76 | ||
| Total | 28 | −1.21 | [−4.96, 2.53] | −0.66 | .513 | −0.25 | ||
|
| ||||||||
| Unpleasantness | Between groups | LTM-MNP | 143 | −3.58 | [−4.71, −2.45] | −6.26 | <.001 * | −1.05 |
|
|
||||||||
| Practice hours | Retreat | 28 | −1.84 | [−3.95, 0.27] | −1.79 | .085 † | −0.67 | |
| Daily | 28 | 1.81 | [−2.96, 6.58] | 0.78 | .444 | 0.29 | ||
| Total | 28 | −1.89 | [−6.00, 2.22] | −0.94 | .355 | −0.36 | ||
Notes: Between-groups difference represents cross-sectional comparison of long-term meditators (LTM, n=30) versus meditation-naive practitioners (MNP, n=115) at baseline. Lifetime practice hours represents regression of pain response against cumulative lifetime practice hours (log-scaled) in the LTM sample across categories of intensive retreat, daily practice, and combined total. Effects for neural pain signatures (NPS and SIIPS1) are standardized; effects for subjective reports (intensity and unpleasantness) are on the outcome scale of measurement. All analyses are adjusted for participant age.
Abbreviations: NPS = Neural Pain Signature; SIIPS1 = Stimulus Intensity Independent Pain Signature-1; df = degrees of freedom; d = standardized effect size (Cohen’s d); CI = confidence interval.
indicates p ≤ .05.
Indicates p ≤ .1.
Figure 2. Correlations between neural and subjective pain response and log-scaled lifetime practice experience among long-term meditators (N=30) across practice contexts: intensive retreat practice (Retreat), routine daily practice (Daily), and combined total (Total).
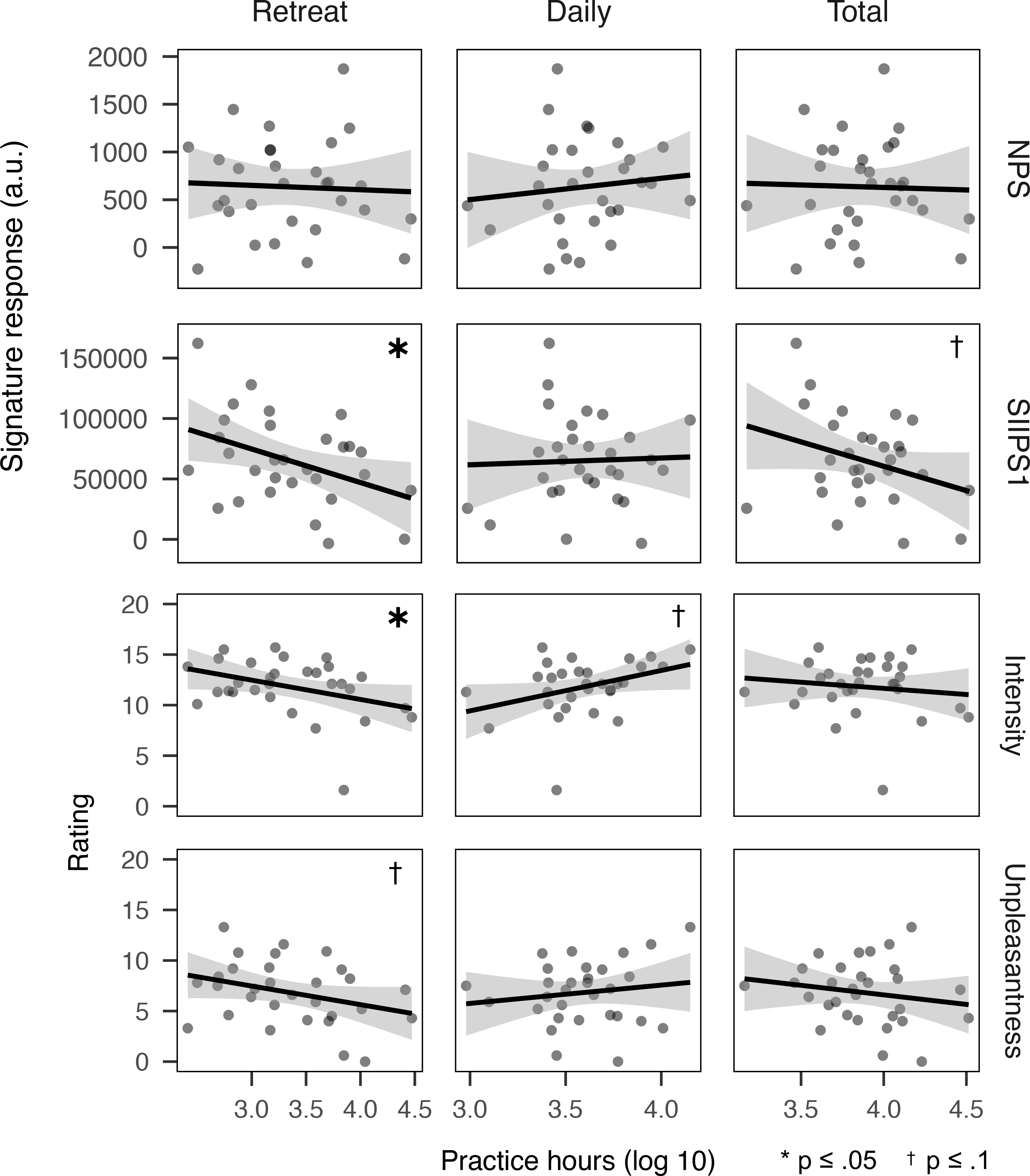
Note: Shaded regions represent standard error. Pain responses adjusted for participant age. Abbreviations: NPS =Neural Pain Signature; SIIPS1 = Stimulus Intensity Independent Pain Signature-1; a.u. = arbitrary units. * indicates p ≤ .05; † indicates p ≤ .1.
Subjective report
LTM participants reported less pain than MNPs (intensity: d=−0.54, p=.001; unpleasantness: d=−1.05, p<.001; see Table 3, Figure 2). Also, among LTM participants, more retreat practice hours were associated with reduced pain (intensity: r=−.37, p=.046; unpleasantness: r=−0.32, p=.085); these relationships remained similar after further adjusting for gender and respiration rate (intensity: r=−.46, p=.031; unpleasantness: −.50, p=.012; see Table 3, Figure 2). Daily practice hours showed a marginal association with increased pain intensity, contrary to hypotheses (r=.36, p=.054); however, unlike findings for Retreat practice, this was not robust to adjusting for gender and respiration rate (r=.32, p=.171). No other associations were observed between subjective pain reports and practice hours (see Table 3). Pain tolerance did not differ between LTM and MNP samples and was not associated with practice hours among LTMs (see Table S2).
Respiration
Mean respiration rate during the pain task did not differ between LTM and MNP samples and was not associated with practice hours among LTMs (see Table S2).
Discussion
We used neural pain signatures to identify the effects of mindfulness training on pain regulation. We confirmed in our sample that these signatures provided valid, objective measures of brain physiology related to pain and differentiated between two components of pain processing: direct, stimulus-related nociceptive activity and stimulus-independent elaborative cognition. Our first aim was to investigate the effects associated with short-term mindfulness training in the form of a standardized MBSR course. Our second aim was to look at practice-related differences in pain processing side-by-side for MBSR practitioners and a comparison sample of long-term meditators.
For MBSR training, as hypothesized, we observed a decrease in stimulus-dependent neural pain response (NPS) within the group as well as relative to the active control condition, and marginally relative to waitlist. This decrease in neural response was paralleled by reduction in subjective pain unpleasantness, again both in within the group from pre- to post-intervention and marginally relative to waitlist. In both MBSR and HEP groups, only marginal decreases were observed for stimulus-independent response (SIIPS1) relative to waitlist. No within-group changes in SIIPS1 response were observed, leaving ambiguous to which group any such changes in response could be attributed. For long-term meditators, although subjective pain report differed from non-meditators, neural response did not. However, among long-term meditators, greater retreat practice experience was associated with reduced neural pain response as well as reduced subjective pain; again, these changes were robust to the covariates examined. Overall, standardized effect sizes for short-term training fell in the small to medium range, while effect sizes associated with long-term training fell in the medium to large range.
Decreased response in the nociception signature (NPS) for MBSR aligns with earlier evidence suggesting that in early phases of training, mindfulness practice may have specific effects on stimulus-dependent sensory components of pain processing due to the common use of physical sensation as the basis for developing mindfulness skills. In particular, components of MBSR which emphasize body awareness, including body scanning practice and mindful movement may play a role in this phenomenon. For the stimulus-independent pain processing response (SIIPS1), we observed potential reductions across both MBSR and the active control condition (HEP). Effects shared by both interventions could reflect non-specific effects on pain response, which is known to be influenced by multiple social processes and expectancies found in in-person intervention settings (23).
Although long-term meditators reported less subjective pain than non-meditators, we did not find any group differences in neural response, whereas within the long-term meditator group, we did observe an association with cumulative practice. This somewhat surprising pattern is nevertheless consistent with previous findings on other measures; one potential explanation is that long-term commitment to meditation practice results in part from self-selection due to individual differences at baseline, which may then be remediated or reversed through extended practice. Among long-term meditators, we observed association with stimulus-independent pain response (SIIPS1), but not with stimulus-dependent response (NPS). This pattern was distinct from that seen in MBSR and suggests a possible shift in long-term mindfulness training, especially in intensive settings, from sensory-focused effects on pain toward more indirect, elaborative cognitive processes. Consistent with this pattern, evidence from previous studies of brief (1 week or less) interventions has suggested a transitional model whereby, initially, mindfulness training promotes modulation of both nociceptive sensation and of elaborative, pain-related cognition, while in advanced training, sensory modulation decreases and cognitive modulation increasingly predominates (4). Here, we extended these findings to the context of an MBSR intervention and provided further support for this model through side-by-side comparison of novice and experienced meditators using a common study design and consistent experimental paradigm. Finally, we note that in long-term training, differences in neural pain response were associated with intensive retreat practice but not routine daily meditation. We have previously reported on distinct relationships between daily and retreat practice and other psychological and neurophysiological outcomes in long-term meditators (14, 15, 24). One possible explanation for this finding is that on an hour-by-hour basis, intensive retreat practice, by facilitating longer sustained periods of practice and more intensive instruction, may support better consolidation of pain-relevant changes in cognition than shorter daily practice intervals (3).
Alongside neural responses, we examined relationships between mindfulness training and subjective pain report, as well as considering the potential role of respiratory physiology in these findings. Although the effects we observed were modest, MBSR and long-term retreat practice were both associated with reductions in pain unpleasantness, as was the active control intervention. Long-term meditators also reported lower pain intensity relative to non-meditators. Notably, for subjective reports, while we observed potential reductions for both interventions, we did not observe differentiation in between MBSR and the active control. Thus, the MBSR-specific reduction in neural signature response may reveal an intervention-specific and psychologically interpretable change in pain response which is not accessible solely through subjective report. Finally, across conditions, respiration rate did not account for differences in pain response related to mindfulness training, reinforcing evidence that such differences are subserved by cognitive and affective processes and not solely physiological ones.
There were some limitations associated with this study. For practical reasons, analyses of long-term mindfulness training relied on a cross-sectional practitioner sample and are thus associative in nature. Regarding clinical applicability, the study was conducted in a healthy community population, which may play a role in the modest size of observed effects. The current observations provide foundational estimates and ranges for the effects studied; however, as in any mechanistic clinical research, precise pinpointing requires cumulative evidence from multiple studies. Finally, our experimental approach relied on an acute pain paradigm optimized for compatibility with neuroimaging. The SIIPS1 signature incorporates activation in multiple regions associated with the transition from acute to chronic pain (25); however, as yet, neural signatures specific to chronic pain have not been developed. Thus, future study will be needed to confirm how these findings apply in the specific contexts of chronic pain, pain-related medical conditions, and the presence of other psychiatric comorbidities.
Better understanding of non-pharmacological interventions for pain is an urgent challenge for clinical neuroscience. Furthermore, because pain is a complex biopsychological phenomenon, research is needed which integrates validated measures at both neurological and psychological levels to study individual aspects of pain. Here, we applied this approach in a randomized actively controlled study design to provide the first neuroimaging study of changes in pain processing associated with a standardized mindfulness intervention in wide clinical use. Behaviorally validated neural pain signatures provided novel evidence for mechanisms of pain modulation in MBSR and differences in pain modulation between short-term and long-term mindfulness training. These findings advance understanding of pain regulation mechanisms associated with non-pharmacological interventions. They also expose specific targets which can be leveraged in future research to optimize the efficacy of mindfulness-based interventions for pain.
Supplementary Material
Acknowledgments
This work was supported by the National Center for Complementary and Alternative Medicine P01AT004952 to RJD, grants from the National Institute of Mental Health R01-MH43454, P50-MH084051 to RJD, grants from the Fetzer Institute [2407] and the John Templeton Foundation [21337] to RJD, a core grant to the Waisman Center from the National Institute of Child Health and Human Development [P30 HD003352-449015] to Albee Messing. JW and TRAK were supported by the National Institute of Mental Health award number T32MH018931. JW was additionally supported during the preparation of this manuscript by the Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship Program in Mental Illness Research and Treatment, the Medical Research Service of the VA Palo Alto Health Care System, and the Department of Veterans Affairs Sierra Pacific Mental Illness Research, Education, and Clinical Center (MIRECC).
We would like to thank Michael Anderle, Ron Fisher, Lisa Angelos, Heather Hessenthaler, Trina Nelson, Amelia Cayo, Michael Kruepke, Jon Brumbaugh, and Sarah Christens and Andrew Schoen for assistance with data collection, and Diane E. Stodola, John Ollinger, Rasmus Birn, John Koger, and Nate Vack for technical assistance. Finally, we express our appreciation to the teachers of the MBSR and HEP protocols.
Footnotes
Disclosures
Dr. Davidson is the founder, president, and serves on the board of directors for the non-profit organization, Healthy Minds Innovations, Inc. No donors, either anonymous or identified, have participated in the design, conduct, or reporting of research results in this manuscript. Drs. Wielgosz, Kral, Perlman, Mumford, and Wager report no financial relationships with commercial interests.
References
- 1.Interagency Pain Research Coordinating Committee: National Pain Strategy [Internet]. Washington, DC, U.S. Department of Health and Human Services, 2016[cited 2016 Jul 25] Available from: https://iprcc.nih.gov/National_Pain_Strategy/NPS_Main.htm [Google Scholar]
- 2.Kabat-Zinn J, Lipworth L, Burney R: The clinical use of mindfulness meditation for the self-regulation of chronic pain. J Behav Med 1985; 8:163–190 [DOI] [PubMed] [Google Scholar]
- 3.Wielgosz J, Goldberg SB, Kral TRA, et al. : Mindfulness meditation and psychopathology. Annu Rev Clin Psychol 2019; 15:285–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeidan F, Vago DR: Mindfulness meditation–based pain relief: A mechanistic account. Ann N Y Acad Sci 2016; 1373:114–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grant JA: Meditative analgesia: the current state of the field. Ann N Y Acad Sci 2014; 1307:55–63 [DOI] [PubMed] [Google Scholar]
- 6.Zeidan F, Grant JA, Brown CA, et al. : Mindfulness meditation-related pain relief: Evidence for unique brain mechanisms in the regulation of pain. Neurosci Lett 2012; 520:165–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wager TD, Atlas LY, Lindquist MA, et al. : An fMRI-based neurologic signature of physical pain. N Engl J Med 2013; 368:1388–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woo C-W, Schmidt L, Krishnan A, et al. : Quantifying cerebral contributions to pain beyond nociception. Nat Commun 2017; 8:14211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zunhammer M, Bingel U, Wager TD: Placebo effects on the neurologic pain signature: a meta-analysis of individual participant functional magnetic resonance imaging data. JAMA Neurol 2018; 75:1321–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poldrack RA: Inferring mental states from neuroimaging data: from reverse inference to large-scale decoding. Neuron 2011; 72:692–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reddan MC, Wager TD: Modeling pain using fMRI: From regions to biomarkers. Neurosci Bull 2017; 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.López-Solà M, Woo C-W, Pujol J, et al. : Towards a neurophysiological signature for fibromyalgia. Pain 2017; 158:34–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacCoon DG, Imel ZE, Rosenkranz MA, et al. : The validation of an active control intervention for Mindfulness Based Stress Reduction (MBSR). Behav Res Ther 2012; 50:3–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kral TRA, Schuyler BS, Mumford JA, et al. : Impact of short- and long-term mindfulness meditation training on amygdala reactivity to emotional stimuli. NeuroImage 2018; 181:301–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wielgosz J, Schuyler BS, Lutz A, et al. : Long-term mindfulness training is associated with reliable differences in resting respiration rate. Sci Rep 2016; 6:27533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khoury B, Knäuper B, Schlosser M, et al. : Effectiveness of traditional meditation retreats: A systematic review and meta-analysis. J Psychosom Res 2017; 92:16–25 [DOI] [PubMed] [Google Scholar]
- 17.Korponay C, Dentico D, Kral TRA, et al. : The effect of mindfulness meditation on impulsivity and its neurobiological correlates in healthy adults. Sci Rep 2019; 9:1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kabat-Zinn J: An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: Theoretical considerations and preliminary results. Gen Hosp Psychiatry 1982; 4:33–47 [DOI] [PubMed] [Google Scholar]
- 19.Kabat-Zinn J: Mindfulness-based interventions in context: Past, present, and future. Clin Psychol Sci Pract 2003; 10:144–156 [Google Scholar]
- 20.Gracely RH, McGrath P, Dubner R: Ratio scales of sensory and affective verbal pain descriptors. Pain 1978; 5:5–18 [DOI] [PubMed] [Google Scholar]
- 21.Fawcett T: An introduction to ROC analysis. Pattern Recognit Lett 2006; 27:861–874 [Google Scholar]
- 22.Hollingshead AB: Four factor index of social status. 1975 [Google Scholar]
- 23.Chen P-HA, Cheong JH, Jolly E, et al. : Socially transmitted placebo effects. Nat Hum Behav 2019; 3:1295–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferrarelli F, Smith R, Dentico D, et al. : Experienced mindfulness meditators exhibit higher parietal-occipital EEG gamma activity during NREM sleep. PLOS ONE 2013; 8:e73417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bushnell MC, Čeko M, Low LA: Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci 2013; 14:502–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


