Abstract
Objective:
To assess the relationship between walking for exercise and symptomatic and structural progression in those with knee osteoarthritis.
Methods:
We assessed a nested cohort of participants 50 years or older within the Osteoarthritis Initiative, a community-based observational study enrolled from 2004–2006. We focused on 4 dichotomous outcomes from baseline to 48-month visits using frequent knee pain and radiographic severity on posterior-anterior semi-flexed knee radiographs: 1) new frequent knee pain, 2) Kellgren-Lawrence grade worsening, 3) medial joint space narrowing, and 4) improved frequent knee pain. We used a modified version of the Historical Physical Activity Survey Instrument to ascertain walking for exercise after 50 years of age, administered at the 96-month visit (2012–2014).
Results:
Of 1212 participants, 73% walked for exercise, 45% were male, mean age = 63.2 (7.9) years, and mean body mass index = 29.4 (4.6) kg/m2. New frequent knee pain and medial joint space narrowing were less common in those who walked ((OR 0.6; 95%CI 0.4 – 0.8) and (OR 0.8; 95%CI 0.6 – 1.0), respectively).
Conclusion:
In individuals ≥ 50 years old with knee osteoarthritis, walking for exercise was associated with less development of frequent knee pain. These findings support that walking for exercise should be encouraged for people with knee osteoarthritis. Furthermore, we offer a proof of concept that walking for exercise could be disease modifying, which warrants further study.
Introduction:
Osteoarthritis is the most common type of arthritis in the United States and is a leading cause of pain for those affected1. Current pharmacologic therapies are limited to topical and oral nonsteroidal anti-inflammatory drugs, intra-articular corticosteroids, and analgesics such as tramadol, which only treat pain and do not modify structure2. The treatment recommendations by professional societies, including the American College of Rheumatology (ACR) and the Osteoarthritis Research Society International (OARSI), recommend a comprehensive care strategy incorporating educational, behavioral, and physical interventions3, 4.
Exercise is a physical intervention often touted as a treatment of osteoarthritis3–5. The ACR guidelines specifically mention walking as a reasonable means of obtaining such exercise4. Two clinical trials of land-based exercises that include walking as a treatment for knee osteoarthritis6, 7 and one trial of walking alone as an intervention (n = 92)8 support these recommendations. However, these trials were relatively short in duration. To address whether walking for exercise over time is beneficial in terms of long-term symptoms and structural progression, ascertainment of walking for exercise over many years and longer follow-up periods are required.
To our knowledge, the Osteoarthritis Initiative is the only cohort where walking for exercise over years is ascertained and where outcomes of knee osteoarthritis, including symptoms and structure are carefully characterized. Thus, we used data from the Osteoarthritis Initiative Cohort to address whether this exposure is beneficial or detrimental regarding changes in symptoms or radiographic disease severity in people who have knee osteoarthritis.
Patients and Methods:
Study Design
This is a nested cohort study within the Osteoarthritis Initiative, a multicenter prospective longitudinal observational study that enrolled people from 2004–2006 with and without symptomatic knee osteoarthritis. Staff at 4 clinical sites recruited participants: Memorial Hospital of Rhode Island, Ohio State University, the University of Pittsburgh, and the University of Maryland/Johns Hopkins. Participants attended annual evaluations from baseline to month 48 and every 2 years to month 96. Institutional review board approval was obtained at each clinical site, coordinating center, and Baylor College of Medicine. Each participant provided written informed consent.
All publicly available data can be accessed through the Osteoarthritis Initiative website (https://nda.nih.gov/oai/).
Study Timeline
The Historical Physical Activity Survey Instrument was administered at the OAI 96-month visit, as part of an ancillary study to the parent OAI. The radiographs and knee pain questions were planned as part of the parent study and ascertained at the OAI baseline and 48-month visits, the two time points with the largest number of outcomes available in this cohort. The timing of the exposure was not optimal since it was ascertained after the outcomes of interest; however, this was an unprecedented opportunity to capture information on physical activity over a lifetime in a cohort with highly characterized knee osteoarthritis outcomes, not available in any other cohort.
Inclusion Criteria
To be eligible, participants at the OAI baseline had to be age ≥ 50 years, with complete data on knee-specific pain and knee radiographs at the OAI baseline and the OAI 36- or 48- month visits, and who completed a modified version of the Historical Physical Activity Survey Instrument9 at the 96-month visit. Participants were required to have radiographic evidence of osteoarthritis (see section on Knee Radiographs) in at least one native knee at the time of enrollment.
Knee Radiographs
The largest number of funded radiographic readings within the Osteoarthritis Initiative occurred at the baseline and 48-month visits, so we chose these as the time points of interest for our study. If radiographs from the 48-month visit were missing, we used films from the 36-month visit instead. Bilateral, fixed-flexion, weight-bearing posteroanterior radiographs of knees were obtained at these visits. Kellgren-Lawrence grades (0–4)11 and medial joint space narrowing (JSN) were scored centrally based on the Osteoarthritis Research Society International Atlas12. The reliability for these readings (read-reread) was good (weighted kappa for intra-rater reliability was 0.71 [95% confidence interval 0.55–0.87])13. Radiographic osteoarthritis was defined as Kellgren-Lawrence ≥ 2.
Pain Assessment
Participants were assessed for symptoms using an assessment of frequent knee pain using the question, “During the last 12 months, have you had pain, aching, or stiffness in or around your right/left knee on most days for at least one month? By most days, we mean more than half the days of a month”14.
Knee Arthroplasty
Knee replacement (partial or total) was reported or observed on radiographs at or before the 9-year Osteoarthritis Initiative visit (>96% adjudicated). A knee replacement was recorded if 1 of the following 3 criteria for a partial or total knee replacement was met: 1) the knee replacement was centrally adjudicated (medical records reviewed by two adjudicators and a physician adjudicator if there was a disagreement between the first two), 2) the knee replacement was observed on a study x-ray, or 3) the knee replacement was self-reported (even if the self-reported replacement had not gone through the adjudication process).
Static Alignment
We defined static alignment using hip-knee-ankle angles measured on long-limb films. Long-limb films were acquired between months 12–48 based on participant availability. Staff obtained bilateral films with participants standing with the tibial tubercle forward. Hip-knee-ankle angles were calculated at the intersection of two lines: 1) from the ankle talar surface center to the tibial interspinous sulcus base and 2) from the femoral head and intercondylar notch centers15, 16. Alignment was classified as varus if ≤ −2°, valgus if ≥ 2°, and neutral among those in between17. These radiographs were obtained as part of a separate ancillary grant to the Osteoarthritis Initiative. As a result, these radiographs were not obtained at the baseline visit; they were collected at month 12 (40%), month 24 (41%), month 36 (18%), and month 48 (2%) visits. 985 people of 1212 participants had long limb films (81%) – 1484 knees of 1808 (82%) that we included.
Historical Physical Activity Survey Instrument
To ascertain the exposure of walking for exercise, we administered a modified version of the Historical Physical Activity Survey Instrument9. This survey questionnaire was mailed to participants. We altered the survey so that a participant could complete it as a take-home survey, similar to what was previously done by Chasan-Taber10. If the survey was incomplete at the time of the closest in person follow-up clinic visit, the 96-month visit, clinic staff asked participants to complete it at the clinic visit; clinic staff assistance was available if requested. These data were acquired between September 12, 2012 and October 31, 2014.
The walking for exercise exposure was assessed using the following questions, “When you were 50 and older, did you walk for exercise at least 10 times? Please include walking outdoors and walking on a treadmill or track.” Those who answered “yes” were considered walkers. Those who answered “no” or “don’t know” (n = 25) were considered non-walkers. For those who responded to the questionnaire overall but had missing data on walking (n = 17), we coded them as non-walkers as well.
Those who answered “yes” were then asked further questions about the amount they walked for exercise: “While you were 50 and older, did you walk for exercise at least 20 minutes within a given day (these do not have to be consecutive minutes).” If the answer was affirmative, then they were asked the question, “How many years did you walk for exercise? These do not have to be consecutive years.” The choices they were given were 1–5 years, 6–10 years, 11–20 years, >20 years, or don’t know. Then they were also asked, “How many months per year did you walk for exercise? Again, these do not have to be consecutive months.” The choices they were given were 1–4 months per year, 5–8 months per year, 9–12 months per year or don’t know. And finally, they were asked, “How many times per month did you walk for exercise?” The choices they were given were 1–3 times per month, 4–8 times per month, 9 or more times per month, or don’t know. Using the median answers to each of these questions, an estimate was made regarding the number of times that the participant has walked for exercise since turning age 50.
Covariates
Date of birth and date of the baseline visit were used to calculate participant ages. Body mass index (BMI) was calculated as weight divided by height squared (kg/m2) measured at the baseline visit.
Outcome Measures
New frequent knee pain was defined using the FREQUENT knee pain question as defined under “Pain Assessment,” as a knee without frequent knee pain at baseline but with frequent knee pain at the 48-month visit. To be explicit, the question focused on frequent knee pain, not any knee pain. Improved frequent knee pain was similarly defined using the FREQUENT knee pain question, specifically as a knee having frequent knee pain at baseline but not at the 48-month visit. Medial JSN worsening was defined as an increase in medial JSN score from baseline to the 48-month visit, including within-grade worsening18. We chose to evaluate medial JSN worsening as most of the loading within the knee passes through the medial tibiofemoral compartment. Kellgren-Lawrence grade worsening was defined as an increase in Kellgren-Lawrence grade over the same time. For all outcomes, if data from the 48-month visit was not available, data from the 36-month visit was carried forward, which occurred for 8% of the participants.
Statistical Analysis
We performed unadjusted and adjusted knee-based logistic regression analyses, using generalized estimating equations to account for correlation within person19, where the predictor was walking for exercise. Walking for exercise was defined dichotomously (walkers and non-walkers).
The outcomes were New frequent knee pain, Kellgren-Lawrence worsening, Medial JSN worsening, and Improved frequent knee pain; adjusted analyses included age, sex, and baseline Kellgren-Lawrence grade as covariates. For the pain analyses, we excluded knees that already had the outcomes of interest from those respective analyses (e.g., those with baseline frequent knee pain were excluded from new frequent knee pain analyses). We included knees with Kellgren-Lawrence grade 4 and medial JSN grade 3 in the structural analyses as they still had the potential of having an interval knee replacement that would have allowed for the outcomes of Kellgren-Lawrence worsening and medial JSN worsening, respectively. We also performed analyses stratified by baseline age (50 to 59, 60 to 69, 70 to 79 years) to address the possibility of reverse causation. Finally, we examined the frequency of each outcome among walkers and non-walkers stratified by knee alignment (varus, neutral, valgus). No statistical analyses stratified by alignment were performed because of a limited sample size in some strata.
The prospective protocol describing the statistical analysis plan for this study is included as a supplemental appendix.
Because there were situations of missing data on walking, we performed a simplistic non-responder imputation sensitivity analysis with a best and worst case imputation to provide a manual “extreme case”, where all those who had missing walking status were assumed to be walkers and then again as non-walkers.
Results:
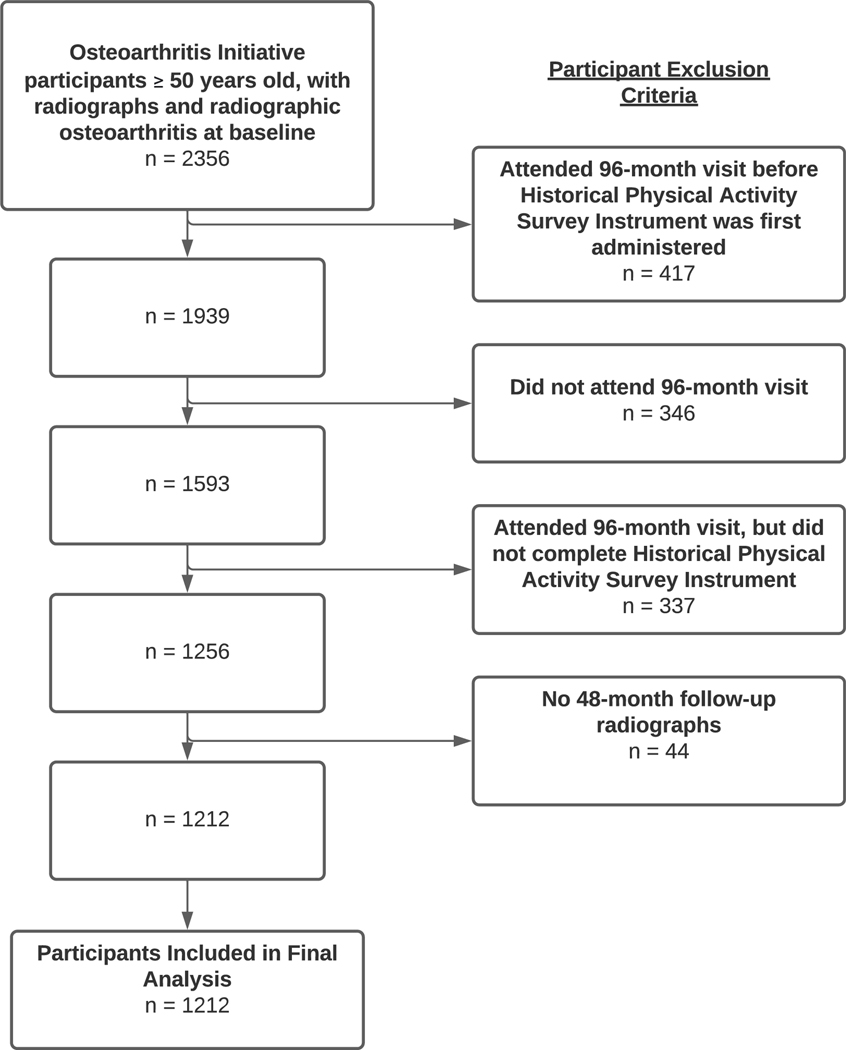
Of the original 4796 participants enrolled into the OAI, 549 were younger than age 50 at the time of OAI baseline visit, leaving 4,247 participants. Of these, 267 had missing baseline radiographs, leaving 3,980 participants who were evaluated for evidence of radiographic OA at the OAI baseline visit. Of these, 1,624 did not have evidence of radiographic OA, leaving 2,356 who would have been eligible to have been surveyed at the 96-month visit, which we will call the “Intent-to-survey” group. Figure 1 illustrates how we arrived at the sample that was included in our study. Of the 2,356 in the “Intent-to-survey” group, 417 participants attended the 96-month visit prior to the date the Historical Physical Activity Survey Instrument was first administered within the OAI, leaving 1,939 participants. 346 participants didn’t attend the 96-month visit at all, leaving 1,593 participants. Of these, 337 people attended the 96-month visit when the survey was being administered but chose not to complete the questionnaire, leaving 1256 who had exposure data to participate in this study. Of these, 44 did not have follow up radiographs.
Figure 1.
Flow Diagram Illustrating Participant Eligibility and Sample Size for Final Analysis
This left 1212 participants (who contributed 1808 knees) included in our study, of whom 887/1212 (73%) walked for exercise, 45% were male, and on average they were 63.2 (7.9) years of age with a mean body mass index of 29.5 (4.6) kg/m2 (Table 1). The knee-based descriptors of this sample included that at OAI baseline, 64%, 29%, and 7% had Kellgren-Lawrence grades 2, 3 and 4 respectively; 65% had some medial joint space narrowing; 37% reported frequent symptoms. Rates of TKR over the 48-month follow up period were similar in those who did and did not walk for exercise (Table 1). For 151 (12.6%) participants, 36-month follow-up data were used instead of 48-month data. The characteristics of the participants who were not included in the study were similar to those who were (Supplemental Table 1).
Table 1.
Baseline and 48-month Characteristics of Non-Walkers, Walkers, and All Included Participants.
| Baseline Characteristics | Non-Walkers | Walkers | All Included Participants |
|---|---|---|---|
|
| |||
| Person-Based Characteristics | (n = 325) | (n = 887) | (n = 1212) |
| Age (years) (mean (SD)) | 64.5 (8.3) | 62.7 (7.7) | 63.2 (7.9) |
| Sex (% Male) | 167/325 (51%) | 381/887 (43%) | 548/1212 (45%) |
| Body mass index (kg/m2) (mean (SD)) | 30.2 (4.6) | 29.2 (4.6) | 29.4 (4.6) |
| Estimated total days people walked for exercise after age 50 | |||
| minimum | 0 | 13 | |
| 25th percentile | 0 | 337 | |
| median | 0 | 845 | |
| 75th percentile | 0 | 1417 | |
| maximum | 0 | 2100 | |
| Knee-Based Characteristics (n (%)) | (n = 503) | (n = 1305) | (n = 1808) |
| Kellgren-Lawrence Grade | |||
| 2 | 284/503 (56%) | 869/1305 (67%) | 1153/1808 (64%) |
| 3 | 175/503 (35%) | 354/1305 (27%) | 529/1808 (29%) |
| 4 | 44/503 (9%) | 82/1305 (6%) | 126/1808 (7%) |
| Medial Joint Space Narrowing Grade | |||
| 0 | 151/503 (30%) | 487/1305 (37%) | 638/1808 (35%) |
| 1 | 173/503 (34%) | 475/1305 (36%) | 648/1808 (36%) |
| 2 | 144/503 (29%) | 293/1305 (22%) | 437/1808 (24%) |
| 3 | 35/503 (7%) | 50/1305 (4%) | 85/1808 (5%) |
| Frequent Knee Symptoms (%) | 223/503 (44%) | 453/1305 (35%) | 676/1808 (37%) |
| Static Alignment | (n = 418) | (n = 1066) | (n = 1484) |
| Varus | 227/418 (54%) | 480/1066 (45%) | 707/1484 (48%) |
| Neutral | 129/418 (31%) | 407/1066 (38%) | 536/1484 (36%) |
| Valgus | 62/418 (15%) | 179/1066 (17%) | 241/1484 (16%) |
| 48-month Knee-Based Characteristics | |||
| Frequent Knee Symptoms (%) | 233/503 (46%) | 496/1305 (38%) | 729/1808 (40%) |
| Knee Replacement (%) | 27/503 (5%) | 50/1305 (4%) | 77/1808 (4%) |
Those who walked for exercise had a 40% decreased odds of new frequent knee pain compared to non-walkers with an adjusted OR of 0.6 (0.4 – 0.8) (Table 2). The adjusted OR for the outcome of medial JSN progression was 0.8 (0.6 – 1.0) (Table 2). No other outcomes were statistically significant. Stratified analyses based on age groups were similar to that of the whole group for all ages and did not provide any suggestion of reverse causation (data not shown). When performing the sensitivity analyses, assuming all who had missing data on walking were non-walkers, the results were similar (Supplemental Table 2). When assuming all who had missing data on walking were walkers, the results were also similar though the point estimates for medial joint space narrowing grade worsening were closer to 1 and no longer statistically significant (Supplemental Table 3). Importantly for the outcome of new frequent knee pain, the finding was robust in both imputation models.
Table 2.
Odds Ratios of Outcomes Based on Walking Status
| Prevalence of Outcome | Unadjusted Odds Ratios (95% CI) | Adjusted Odds Ratios (95% CI)* | |
|---|---|---|---|
|
| |||
| Worsened Outcomes | |||
| Outcome: New Frequent Knee Pain | |||
| Non-Walkers | 103/280 (37%) | Referent | Referent |
| Walkers (Y/N) | 223/852 (26%) | 0.6 (0.4 – 0.8) | 0.6 (0.4 – 0.8) |
| Outcome: Kellgren-Lawrence Worsening | |||
| Non-Walkers | 105/503 (21%) | Referent | Referent |
| Walkers (Y/N) | 234/1305 (18%) | 0.8 (0.6 – 1.1) | 0.8 (0.6 – 1.1) |
| Outcome: Medial Joint Space Narrowing Grade Worsening | |||
| Non-Walkers | 137/503 (27%) | Referent | Referent |
| Walkers (Y/N) | 281/1305 (22%) | 0.7 (0.6 – 1.0) | 0.8 (0.6 – 1.0) |
| Improved Outcome | |||
| Outcome: Improved Frequent Knee Pain | |||
| Non-Walkers | 93/223 (42%) | Referent | Referent |
| Walkers (Y/N) | 180/453 (40%) | 0.9 (0.7 – 1.3) | 0.8 (0.6 – 1.2) |
Note:
95% CI = 95% confidence interval, Y/N = Yes/No, Bold = statistically significant
adjusted for age, sex, and baseline Kellgren-Lawrence grade.
In stratified results based on alignment (Table 3), walkers with varus alignment less frequently developed new frequent knee pain (28% vs. 39%), Kellgren-Lawrence grade worsening (20% vs. 26%), and medial JSN grade worsening (31% vs. 39%) compared to non-walkers with varus alignment. Walkers with neutral alignment less frequently developed new frequent knee pain (23% vs. 36%) and more frequently experienced improved frequent knee pain (47% vs. 38%) than non-walkers with neutral alignment. However, they more frequently experienced medial JSN worsening (17% vs. 11%). Interestingly, walkers with valgus alignment more frequently had Kellgren-Lawrence grade worsening (20% vs. 15%) and less frequently had improved frequent knee pain (35% vs. 48%) than non-walkers. Thus, there did appear to potentially be a differential effect of walking for exercise based on knee static alignment.
Table 3.
Frequency of Outcomes Stratified by Static Alignment
| Varus knees | Neutral knees | Valgus knees | |
|---|---|---|---|
|
| |||
| Outcome: New Frequent Knee Pain | |||
| Non-Walkers | 47/120 (39%) | 28/77 (36%) | 9/31 (29%) |
| Walkers (Y/N) | 82/290 (28%) | 60/266 (23%) | 40/122 (33%) |
| Outcome: Kellgren-Lawrence Grade Worsening | |||
| Non-Walkers | 59/227 (26%) | 13/129 (10%) | 9/62 (15%) |
| Walkers (Y/N) | 97/480 (20%) | 58/407 (14%) | 35/179 (20%) |
| Outcome: Medial Joint Space Narrowing Grade Worsening | |||
| Non-Walkers | 88/227 (39%) | 14/129 (11%) | 3/62 (5%) |
| Walkers (Y/N) | 149/480 (31%) | 69/407 (17%) | 16/179 (9%) |
| Outcome: Improved Frequent Knee Pain | |||
| Non-Walkers | 43/107 (40%) | 20/52 (38%) | 15/31 (48%) |
| Walkers (Y/N) | 74/190 (39%) | 66/141 (47%) | 20/57 (35%) |
Note: Y/N = Yes/No
Discussion:
Findings from our study support the idea that walking is beneficial from both a structural and symptomatic perspective of knee osteoarthritis. Specifically, we found that those who walked for exercise were less likely to develop new frequent knee pain; however, we found no relationship with improved frequent knee pain. Hence, it may be especially beneficial to advise people to walk for exercise to help prevent the onset of frequent knee pain. These findings also offer the first reported evidence that walking may be an effective treatment to slow the structural progression of osteoarthritis. Our findings highlight the possibility that biomechanical interventions may hold the key to the elusive treatments in this disease that might provide benefit from both a structure and symptom perspective. This is potentially an important paradigm shift in the field of osteoarthritis research.
To our knowledge, this is the first study to explore the effects of walking stratified by static alignment. Specifically, we observed that walking might be related to less symptomatic and structural progression among knees with varus alignment (48% of cohort), less symptomatic progression among knees with neutral alignment (36% of cohort), and possibly little benefit among knees with valgus alignment (16% of cohort). There is a wealth of data indicating that knee osteoarthritis is largely biomechanically driven17, 20–28, so it is not surprising that we found that static alignment could be an important effect measure modifier in evaluating the association between walking and knee osteoarthritis progression. It will be important to replicate these analyses in other epidemiologic studies with larger groups of people who have neutral and valgus alignment to confirm these findings.
As supported by our study, the current guidelines advocate that walking is beneficial for knee osteoarthritis. Investigators who conducted a systematic review to inform the 2018 Physical Activity Guidelines for Americans29 reported that while there is moderate evidence of safety up to 10,000 steps/day, there was limited evidence that more than 10,000 steps/day may adversely affect knee osteoarthritis progression30. It is unclear if our findings address those concerns since we focused on walking for exercise and less than 14% of participants in a US-based cohort, similar to the Osteoarthritis Initiative, exceed 10,000 steps/day31. There are two moderate to large-sized epidemiologic studies, including those with and without knee osteoarthritis, suggesting harm related to walking32, 33. These studies used step counts from activity monitors over 7 days to quantify daily step counts. This is the standard method of using physical activity monitoring data; however, this is potentially problematic as the exposure ascertainment time frame is very short, not specific to walking for exercise, and people are known to modify their activity when they know they are being monitored. Thus, these measures may not be a true reflection of the amount people walk for exercise over an extended time. In our study, we used a retrospective, self-reported measure of walking. While our measurement method has limitations due to recall bias, a benefit to our method of ascertaining walking is that it provides an average amount of walking for exercise over a much longer time period.
There are some limitations to our study. This is an observational study wherein the walkers were self-selected. There is the possibility that the association observed may result from reverse causation; people might walk more because they have less OA, not that walking is protective of osteoarthritis. We performed age-stratified analyses that were similar to that of the whole group making this possibility less likely based on the idea that people self-selected walking for exercise; if the situation of reverse causation existed then those who had pain at a younger age when they did walk would presumably stop over time because they had pain, which was not observed in our study. Another limitation is that, as mentioned previously, the walking exposure was ascertained retrospectively, which raises concerns about recall bias. Beyond the idea that people had to think back over their life to estimate their walking exposure, the addition of the Historical Physical Activity Survey Instrument was part of an ancillary study to the main OAI study, so the ascertainment of the exposure of physical activity was performed after the outcomes of interest. Admittedly, this is not ideal; however, since the participants were reviewing their lifetime of physical activity which was already a retrospective activity, it is less likely that the timing of the administration of the instrument detracts from the observed findings. Of important note, participants were unaware of our specific study questions when they completed the surveys, making the retrospective aspects of the ascertainment less likely to impact our results. Another limitation of our study is that this study used participants who had radiographic osteoarthritis and who were ages 50 and older; therefore, it is not clear if these results apply to those without osteoarthritis and in younger age groups. Finally, because of the nature of the cohort, static alignment was not ascertained at the same time for all participants, although static alignment is unlikely to change rapidly over time. Future studies should make a particular effort to assess static alignment at baseline evaluations.
This study only addresses walking for exercise. It does not address the situation of compulsory walking such as occupational walking (e.g., mail carriers) or walking for transportation. Although the study assesses walking for exercise over years, it was based on self-report, not step counts from an activity monitor; thus, specific statements about extremely high exposures to walking cannot be made.
In conclusion, the findings from our study provide a glimmer of hope that there may be an inexpensive intervention that modifies the structure and symptoms related to osteoarthritis, the most common type of arthritis and a source of substantial disability. Clinicians should encourage patients to walk and consider in-person or web-based walking programs like “Walk with Ease,” which has durable benefits over one year34 35. Our findings support recommendations by professional societies that walking for exercise should be encouraged. Beyond a benefit in symptoms for osteoarthritis, the findings from our study also suggest that walking may also provide a structural benefit for a large portion of the community with osteoarthritis. A randomized controlled trial of walking in those with knee osteoarthritis stratified by alignment is warranted.
Supplementary Material
Acknowledgments
Dr. Lo was supported by K23 AR062127, an NIH/NIAMS funded mentored award, providing support for the design and conduct of the study, analysis, interpretation of the data, and preparation and review of this work. This work was supported in part with resources at the VA HSR&D Center for Innovations in Quality, Effectiveness, and Safety (#CIN 13-413), at the Michael E. DeBakey VA Medical Center, Houston, TX. This research was supported in part by generous donations to the Tupper Research Fund at Tufts Medical Center. The Osteoarthritis Initiative is a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the Osteoarthritis Initiative Study Investigators. Private funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the Osteoarthritis Initiative is managed by the Foundation for the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the National Institutes of Health, or the Department of Veterans Affairs.
Footnotes
The authors have no other financial interests that could create a potential conflict of interest regarding this work.
References
- 1.Zhang Y, Jordan JM. Epidemiology of osteoarthritis [Review]. Clin Geriatr Med. Aug 2010;26(3):355–69. doi: 10.1016/j.cger.2010.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kolasinski SL, Neogi T, Hochberg MC, Oatis C, Guyatt G, Block J, et al. 2019. American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Rheumatol.2326–5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bannuru RR, Osani MC, Vaysbrot EE, Arden NK, Bennell K, Bierma-Zeinstra SMA, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis [Review]. Osteoarthritis Cartilage. Nov 2019;27(11):1578–1589. doi: 10.1016/j.joca.2019.06.011 [DOI] [PubMed] [Google Scholar]
- 4.Kolasinski SL, Neogi T, Hochberg MC, Oatis C, Guyatt G, Block J, et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Rheumatol. Feb 2020;72(2):220–233. doi: 10.1002/art.41142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson AE, Allen KD, Golightly YM, Goode AP, Jordan JM. A systematic review of recommendations and guidelines for the management of osteoarthritis: The chronic osteoarthritis management initiative of the U.S. bone and joint initiative [Review]. Semin Arthritis Rheum. Jun 2014;43(6):701–12. doi: 10.1016/j.semarthrit.2013.11.012 [DOI] [PubMed] [Google Scholar]
- 6.Ettinger WH Jr., Burns R, Messier SP, Applegate W, Rejeski WJ, Morgan T, et al. A randomized trial comparing aerobic exercise and resistance exercise with a health education program in older adults with knee osteoarthritis. The Fitness Arthritis and Seniors Trial (FAST). JAMA. 1997;277(1):25–31. [PubMed] [Google Scholar]
- 7.Bautch JC, Malone DG, Vailas AC. Effects of exercise on knee joints with osteoarthritis: a pilot study of biologic markers. Arthritis Care Res. Feb 1997;10(1):48–55. doi: 10.1002/art.1790100108 [DOI] [PubMed] [Google Scholar]
- 8.Kovar PA, Allegrante JP, MacKenzie CR, Peterson MG, Gutin B, Charlson ME. Supervised fitness walking in patients with osteoarthritis of the knee. A randomized, controlled trial. Ann Intern Med. Apr 1 1992;116(7):529–34. doi: 10.7326/0003-4819-116-7-529 [DOI] [PubMed] [Google Scholar]
- 9.Friedenreich CM, Courneya KS, Bryant HE. The lifetime total physical activity questionnaire: development and reliability. Med Sci Sports Exerc. Feb 1998;30(2):266–74. doi: 10.1097/00005768-199802000-00015 [DOI] [PubMed] [Google Scholar]
- 10.Chasan-Taber L, Erickson JB, McBride JW, Nasca PC, Chasan-Taber S, Freedson PS. Reproducibility of a self-administered lifetime physical activity questionnaire among female college alumnae. Am J Epidemiol. Feb 1 2002;155(3):282–9. [DOI] [PubMed] [Google Scholar]
- 11.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. Dec 1957;16(4):494–502. doi: 10.1136/ard.16.4.494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Altman RD, Gold GE. Atlas of individual radiographic features in osteoarthritis, revised. Osteoarthritis Cartilage. 2007;15 Suppl A:A1–56. doi: 10.1016/j.joca.2006.11.009 [DOI] [PubMed] [Google Scholar]
- 13.Project Osteoarthritis I. 15 Test-Retest Reliability of Semi-quantitative Readings from Knee Radiographs. Updated November 22, 2016. Accessed February 3, 2017. https://nda.nih.gov/oai/study_documentation.html
- 14.O’Reilly SC, Muir KR, Doherty M. Screening for pain in knee osteoarthritis: which question? Ann Rheum Dis. Dec 1996;55(12):931–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sled EA, Sheehy LM, Felson DT, Costigan PA, Lam M, Cooke TDV. Reliability of lower limb alignment measures using an established landmark-based method with a customized computer software program. Rheumatol Int. 2011/01/01 2011;31(1):71–77. doi: 10.1007/s00296-009-1236-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooke TDV, Sled EA, Scudamore RA. Frontal plane knee alignment: a call for standardized measurement [Editorial]. J Rheumatol. 2007;34(9):1796–1801. [PubMed] [Google Scholar]
- 17.Sharma L, Song J, Dunlop D, Felson D, Lewis CE, Segal N, et al. Varus and valgus alignment and incident and progressive knee osteoarthritis. Ann Rheum Dis. Nov 2010;69(11):1940–5. doi:ard.2010.129742 [pii] 10.1136/ard.2010.129742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Felson DT, Nevitt MC, Yang M, Clancy M, Niu J, Torner JC, et al. A new approach yields high rates of radiographic progression in knee osteoarthritis. J Rheumatol. Oct 2008;35(10):2047–54. [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Glynn RJ, Felson DT. Musculoskeletal disease research: should we analyze the joint or the person? J Rheumatol. 1996;23(7):1130–4. [PubMed] [Google Scholar]
- 20.Sharma L, Song J, Felson DT, Cahue S, Shamiyeh E, Dunlop DD. The role of knee alignment in disease progression and functional decline in knee osteoarthritis. JAMA. Jul 11 2001;286(2):188–95. [DOI] [PubMed] [Google Scholar]
- 21.Felson DT, McLaughlin S, Goggins J, LaValley MP, Gale ME, Totterman S, et al. Bone marrow edema and its relation to progression of knee osteoarthritis. Ann Intern Med. Sep 2 2003;139(5 Pt 1):330–6. doi: 10.7326/0003-4819-139-5_part_1-200309020-00008 [DOI] [PubMed] [Google Scholar]
- 22.Hunter DJ, Zhang Y, Niu J, Goggins J, Amin S, Lavalley MP, et al. Increase in bone marrow lesions associated with cartilage loss: A longitudinal magnetic resonance imaging study of knee osteoarthritis. Arthritis Rheum. 2006;54(5):1529–1535. [DOI] [PubMed] [Google Scholar]
- 23.Chang A, Hayes K, Dunlop D, Hurwitz D, Song J, Cahue S, et al. Thrust during ambulation and the progression of knee osteoarthritis. Arthritis Rheum. Dec 2004;50(12):3897–903. [DOI] [PubMed] [Google Scholar]
- 24.Shakoor N, Block JA, Shott S, Case JP. Nonrandom evolution of end-stage osteoarthritis of the lower limbs. Arthritis Rheum. 2002;46(12):3185–9. [DOI] [PubMed] [Google Scholar]
- 25.Lo GH, Harvey WF, McAlindon TE. Associations of varus thrust and alignment with pain in knee osteoarthritis. Arthritis Rheum. Jul 2012;64(7):2252–9. doi: 10.1002/art.34422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lo GH, Hunter DJ, Nevitt M, Lynch J, McAlindon TE. Strong association of MRI meniscal derangement and bone marrow lesions in knee osteoarthritis: data from the osteoarthritis initiative. Osteoarthritis Cartilage. Jun 2009;17(6):743–7. DOI: 10.1016/j.joca.2008.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lo GH, Hunter DJ, Zhang Y, McLennan CE, Lavalley MP, Kiel DP, et al. Bone marrow lesions in the knee are associated with increased local bone density. Arthritis Rheum. Sep 2005;52(9):2814–21. doi: 10.1002/art.21290 [DOI] [PubMed] [Google Scholar]
- 28.Lo GH, Niu J, McLennan CE, Kiel DP, McLean RR, Guermazi A, et al. Meniscal damage associated with increased local subchondral bone mineral density: a Framingham study. Osteoarthritis Cartilage. Feb 2008;16(2):261–7. doi: 10.1016/j.joca.2007.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Physical Activity Guidelines for Americans. In: U.S. Department of Health and Human Services PHS, editor. 2nd edition ed. Washington, D.C.; 2018. [Google Scholar]
- 30.Kraus VB, Sprow K, Powell KE, Buchner D, Bloodgood B, Piercy K, et al. Effects of Physical Activity in Knee and Hip Osteoarthritis: A Systematic Umbrella Review [Review]. Med Sci Sports Exerc. Jun 2019;51(6):1324–1339. doi: 10.1249/mss.0000000000001944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.White DK, Tudor-Locke C, Zhang Y, Fielding R, LaValley M, Felson DT, et al. Daily walking and the Risk of Incident Functional Limitation in Knee Osteoarthritis: An Observational Study. Arthritis Care Res (Hoboken). 2014;66(9):1328–1336. doi: 10.1002/acr.22362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Voinier D, Neogi T, Stefanik JJ, Guermazi A, Roemer FW, Thoma LM, et al. Using Cumulative Load to Explain How Body Mass Index and Daily Walking Relate to Worsening Knee Cartilage Damage Over Two Years: The MOST Study. Arthritis Rheumatol. Jun 2020;72(6):957–965. doi: 10.1002/art.41181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dore DA, Winzenberg TM, Ding C, Otahal P, Pelletier JP, Martel-Pelletier J, et al. The association between objectively measured physical activity and knee structural change using MRI. Ann Rheum Dis. Jul 2013;72(7):1170–5. doi: 10.1136/annrheumdis-2012-201691 [DOI] [PubMed] [Google Scholar]
- 34.Callahan LF, Shreffler JH, Altpeter M, Schoster B, Hootman J, Houenou LO, et al. Evaluation of group and self-directed formats of the Arthritis Foundation’s Walk With Ease Program. Arthritis Care Res (Hoboken). Aug 2011;63(8):1098–107. doi: 10.1002/acr.20490 [DOI] [PubMed] [Google Scholar]
- 35.Talbot LA, Gaines JM, Huynh TN, Metter EJ. A home-based pedometer-driven walking program to increase physical activity in older adults with osteoarthritis of the knee: a preliminary study. J Am Geriatr Soc. Mar 2003;51(3):387–92. doi: 10.1046/j.1532-5415.2003.51113.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.