Abstract
Background:
Feminizing hormonal therapy (FHT) and HIV potentially alter cardiovascular disease (CVD) risk in transgender women (TW).
Methods:
TW were enrolled in Los Angeles, CA and Houston, TX and frequency-matched to Multicenter AIDS Cohort Study cisgender men (CM) on age, race, substance use and abacavir use. Biomarkers of CVD risk and inflammation were assessed via ELISA. Wilcoxon rank sum and Fisher’s exact tests compared TW and CM. Multivariable linear regression assessed factors associated with biomarker concentrations.
Results:
TW (HIV+ n=75, HIV− n=47) and CM (HIV+ n=40, HIV− n=40) had mean age 43–45 years; TW/CM were 90%/91% non-Hispanic Black, Hispanic, or Multi-racial, 26%/53% obese, and 34%/24% current smokers; 67% of TW were on FHT. Among PLWH, TW had higher median extracellular newly-identified receptor for advanced glycation end-products (EN-RAGE), lipoprotein-associated phospholipase A2 (LpPLA2), oxidized LDL (oxLDL), soluble TNF receptor type (sTNFR) I/II, interleukin (IL)-8 and plasminogen activator inhibitor (PAI)-1, but lower soluble CD14, von Willebrand factor (vWF) and endothelin (ET)-1 levels than CM. Findings were similar for participants without HIV (all p<0.05). In multivariable analysis, TW had higher EN-RAGE, IL-6, IL-8, P selectin, PAI-1, oxLDL and sTNFRI/II concentrations, and lower vWF, independent of HIV serostatus and current FHT use. Both being a TW and a PLWH were associated with lower ET-1.
Conclusions:
Compared to matched cisgender men, trans women have altered profiles of biomarkers associated with systemic inflammation and CVD. Further work is needed to decipher the contributions of FHT to CVD risk in TW with HIV.
Keywords: Feminizing Hormonal Therapy, HIV, Cardiovascular Biomarkers, Trans Women
BACKGROUND
Feminizing hormone therapy (FHT) for transgender women (TW) can be critical to harmonizing gender identity and expression; however, FHT modulates inflammatory and coagulation pathways, causes fat gain, and may increase metabolic disease risk. Indeed, FHT is associated with increased cardiovascular disease (CVD) risk in persons assigned a female sex at birth and TW.[1–8] Cardiometabolic risk in TW is understudied, particularly with contemporary FHT regimens.
HIV is also associated with increased cardiometabolic disease risk, with CVD being a leading cause of morbidity and mortality among people living with HIV (PLWH) on suppressive antiretroviral therapy (ART). [9–14] The mechanisms underlying the heightened risk of CVD in PLWH are not well understood, but are believed to involve traditional as well as HIV− and ART-specific risk factors.[15–18] Chronic HIV is characterized by persistent inflammation and immune activation that can lead to tissue injury and dysfunction, with persistent monocyte activation playing an important role in the pathogenesis of end-organ disease.[19–22] Multiple coagulation pathway abnormalities have also been described.[23–25]
TW are disproportionately affected by HIV, [26] and TW have high rates of traditional CVD risk factors such as smoking,[27–31] but the intersections of chronic HIV−, ART- and modern FHT-induced alterations in immuno-metabolic pathways and their effects on cardiometabolic risk in TW have not been addressed. To better understand the intersections of HIV, ART and FHT with cardiometabolic risk among TW, we designed a cross-sectional, pilot study of inflammatory and cardiometabolic biomarker measurement in TW and matched cisgender males (CM).
METHODS
Study population
TW were recruited from community-based organizations and clinics in Los Angeles, California (APAIT) and Houston, Texas (Thomas Street Health Center) between 2016 and 2018. Participants were enrolled sequentially without regard to HIV serostatus. Inclusion criteria for TW included self-identification as a TW or transfeminine person and ability and willingness to provide informed consent. For TW with HIV, written documentation of HIV serostatus was required, and participants were required to be taking ART. Proof of HIV-1 RNA <50 copies/ml prior to blood draw was not required. Rather, participants were asked if they believed or had proof that they were undetectable at their last clinical visit. If yes, they were allowed to enroll. This was necessary due to the cross-sectional and community-based nature of the study. For TW believed to be HIV negative, documented testing within the last 90 days and/or testing immediately prior to enrollment was required (latter available at the enrollment sites and most persons were retested on the day of enrollment). TW were not required to be on FHT at time of enrollment.
CM were selected from men who have sex with men (MSM) enrolled in the former Multicenter AIDS Cohort Study (MACS). This cohort was chosen due to overlapping HIV risk behaviors with the communities of TW that we were enrolling and because the MACS included men with and without HIV. The MACS began in 1984 to study the natural history of HIV among MSM and to establish a repository of biologic specimens for future study.[32] Participants were enrolled from four sites (Pittsburgh, PA; Baltimore, MD/Washington, DC; Chicago, IL; Los Angeles, CA) over four time periods (1984/85, 1987/90, 2001/03, 2010+), and completed semi-annual visits that included a standardized medical history interview, clinical evaluations, laboratory tests and storage of specimens. CM were frequency-matched to TW on HIV serostatus, age within 5 years, race/ethnicity, recent recreational drug use (yes/no in last 90 days for TW and yes/no in last 6 months for CM) and abacavir use, eliminating the need for matched analyses. MACS men identifying on the transfeminine spectrum by self-reported gender identity were excluded from the pool of potential controls. Specimens and data from CM were collected between April 2012 and September 2014. This time frame was chosen due to: limited availability of samples from men without HIV beginning October 2014, all controls having available specimens during this time frame, and all specimens having been in storage <5 years.
Study Procedures
The study was approved by the institutional review boards of the University of California, Los Angeles (Los Angeles, CA) and UTHealth Houston (Houston, TX). Written informed consent was obtained from TW who were potential participants prior to the initiation of study procedures. Consent for MACS men was covered under their primary MACS consent.
For TW, blood was collected in the fasting state (nothing to eat or drink except water and medications for at least 8 hours) for measurement of comprehensive metabolic panel, lipid panel, and complete blood count according to local standardized practices, and transferred to LabCorp for real-time measurement of the above. CD4+ T lymphocyte count and HIV-1 RNA levels from TW living with HIV were collected from participant medical records (for those who provided consent to contact their primary provider), which were generally available within 90 days of entry.
Blood was collected from TW and stored at −70°C until batched biomarker measurement could occur in the lab of Dr. Nicholas Funderburg (The Ohio State University, Columbus, OH). Control serum and plasma samples were sent from the MACS repository (NIH, Frederick, MD) to Dr. Funderburg’s lab. Soluble CD14 (sCD14), soluble CD163 (sCD163), interleukin-6 (IL-6), interleukin-8 (IL-8), soluble TNF receptor type (sTNFR) I/II, P selectin, high molecular weight adiponectin, endothelin-1 (ET-1), extracellular newly-identified receptor for advanced glycation end-products (EN-RAGE), lipoprotein-associated phospholipase A2 (LpPLA2), vascular cell adhesion molecule-1 (VCAM-1) (R&D Systems, Minneapolis, MN, USA), oxidized LDL (oxLDL) (Mercodia, Uppsala, Sweden), plasminogen activator inhibitor-1 (PAI-1), von Willebrand factor (vWF) (Abcam, Cambridge, United Kingdom), and d dimer (Stago, Asnières-sur-Seine, France) were measured on all samples via ELISA. These biomarkers were chosen for their known relationships to cardiovascular disease, inflammatory processes and/or coagulation abnormalities. Twenty percent of all samples were run in duplicate to assess variability, which was consistently <15%.
TW self-reported basic demographic information, medical history including substance use history and current medication usage. A separate FHT questionnaire assessed FHT usage, including current or past usage, FHT type and frequency, and mode of acquisition. For MACS CM, variables equivalent to those available for TW were selected.
Analysis
Demographic and clinical characteristics were summarized as median (interquartile range, IQR) or frequency. Given the inflammatory and metabolic perturbations associated with HIV, biomarker results were stratified first by HIV serostatus and then by gender. Wilcoxon rank sum and Fisher’s exact tests compared differences between TW and CM. Multivariable linear regression analyses assessed factors associated with individual natural log-transformed biomarker concentrations after adjusting for HIV serostatus, gender, age, race/ethnicity, body mass index (BMI), and smoking. For regression analyses, the population of TW with HIV was limited to those with confirmed HIV-1 RNA <50 copies/ml (undetectable; n=51). All CM with HIV also had undetectable HIV-1 RNA. Statistical significance was defined as a two-sided p<0.05. For this hypothesis-generating, pilot study, no adjustments were made for multiple comparisons. Analyses were conducted in SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Study population
Demographic and clinical characteristics are presented in Table 1. Briefly, 122 TW (HIV+ n=75, HIV− n=47) were enrolled and had available samples for analysis. Eighty CM (HIV+ n=40, HIV− n=40) were selected as frequency-matched controls. Overall, the population of TW and CM: had mean age of 43 and 45 years, 90% and 91% self-identified as non-Hispanic Black, Hispanic, or Multi-racial; 26% and 53% were obese (body mass index ≥30 kg/m2), and 34% and 24% were current smokers, respectively. PLWH had current median CD4+ T lymphocyte count 609 cells/uL; 67% of TW were currently on FHT (68% HIV+, 66% HIV−). ART use included 30% non-nucleoside reverse transcriptase inhibitor (NNRTI)−, 29% protease inhibitor (PI)-, and 37% integrase strand transfer inhibitor (INSTI)-based regimens. TW were less likely to be on NNRTI-based ART (among PLWH), be obese, and have smoking history or hypertension, but were more likely to be on INSTI-based ART, have heavy alcohol intake, and be current smokers. Upon medical record review, 32% of TW had recent detectable HIV-1 RNA compared to 0% of CM.
Table 1.
Clinical and Demographic Characteristics
| CM, HIV− (N=40) | CM, HIV+ (N=40) | TW, HIV− (N=47) | TW, HIV+* (N=75) | |
|---|---|---|---|---|
| Age | 43 (35, 52) | 48 (43, 54) | 40 (32, 48) | 43 (37, 52) |
| Black race | 13% | 23% | 13% | 27% |
| Hispanic ethnicity | 65% | 60% | 64% | 57% |
| Overweight/obese (BMI >25 kg/m2) | 58% | 67% | 30% | 24% |
| Former smoker | 60% | 58% | 22% | 11% |
| Current smoker | 20% | 28% | 28% | 37% |
| Recreational drug use | 28% | 48% | 23% | 41% |
| >=14 drinks/week | 5% | 3% | 11% | 8% |
| Hypertension | 40% | 39% | 17% | 33% |
| Diabetes mellitus | 13% | 19% | 15% | 13% |
| INSTI-based | 13% | 49% | ||
| CD4+ T lymphocyte count (cells/μL) | 650 (466,846) | 580 (391,841) | ||
| CD4+ T lymphocyte nadir <200 (cells/μL) | 48% | 54% | ||
| Detectable HIV-1 RNA (≥50 copies/ml) | 0% | 32% | ||
| Any AIDS diagnosis | 8% | 36% | ||
| Feminizing Hormonal Therapy | 66% | 68% |
Frequency or median (interquartile range) presented.
When restricting to TW with undetectable HIV-1 RNA, age, current smoking and high-risk alcohol use frequency more closely resembled CM. TW=transgender women, CM=cisgender males, NNRTI= non-nucleoside reverse transcriptase inhibitors, PI=protease inhibitors, INTI=integrase strand transfer inhibitors
Biomarker Results
Among participants without HIV, TW had significantly higher EN-RAGE, oxidized LDL, sTNFRI/II, IL-6, P selectin, IL-8 and PAI-1 concentrations. Only ET-1 levels were lower in TW (all p<0.05, Table 2). Similarly, TW with HIV had higher EN-RAGE, oxidized LDL, sTNFRI/II, LpPLA2, IL-8, and PAI-1 concentrations than CM with HIV. A trend was seen for higher P selectin concentrations among TW. IL-6 levels were similar by gender among participants with HIV. ET-1 concentrations were lower in TW than CM with HIV, but unlike participants without HIV, so were sCD14 and vWF (p<0.05, Table 2).
Table 2:
Biomarker Concentrations by Gender and HIV Serostatus
| PLWH | PWOH | |||||
|---|---|---|---|---|---|---|
| TW (N=75) | CM (N=40) | P-value | TW (N=47) | CM (N=40) | P-value | |
| Adiponectin (ng/mL) | 3499.2 (1965.5–5335.0) | 2926.0 (1323.3–4454.1) | 0.215 | 3034.7 (1696.8–5015.4) | 2723.0 (1307.0–3678.9) | 0.344 |
| Human ENRAGE (pg/mL) | 9498.6 (6875.6–12461.0) | 912.9 (787.4–2797.4) | <0.001 | 9971.1 (8182.2–10796.5) | 2272.5 (877.6–3325.0) | <0.001 |
| LpPLA2(ng/mL) | 242.6 (208.6–309.4) | 220.8 (194.1–254.3) | 0.013 | 243.5 (197.8–289.4) | 250.7 (234.7–279.8) | 0.268 |
| Oxidized LDL (mU/L) | 51166.2 (34108.9–60422.8) | 37248.1 (31470.8–46385.6) | 0.007 | 52260.8 (38632.2–67630.7) | 34833.5 (29078.1–48335.0) | <0.001 |
| sCD14 (ng/mL) | 1878.2 (1524.5–2200.9) | 2024.0 (1783.6–2353.3) | 0.026 | 1687.7 (1296.3–1928.3) | 1638.8 (1459.1–1868.6) | 0.517 |
| sCD163 (ng/mL) | 608.1 (384.4–944.0) | 727.0 (576.1–843.6) | 0.178 | 500.7 (407.5–682.1) | 534.1 (407.0–668.1) | 0.583 |
| sTNFR I (pg/mL) | 1369.0 (1176.0–1604.5) | 989.4 (787.7–1194.0) | <0.001 | 1057.5 (920.6–1492.2) | 980.5 (814.0–1120.4) | 0.037 |
| sTNFR II (pg/mL) | 3243.1 (2656.9–4344.5) | 2669.8 (2041.4–3718.3) | 0.005 | 2698.4 (2242.2–3450.7) | 2220.1 (1971.1–2806.3) | 0.008 |
| VCAM-1 (ng/mL) | 730.0 (576.5–1098.0) | 766.9 (613.9–867.4) | 0.577 | 717.5 (505.4–879.1) | 683.4 (509.9–759.4) | 0.501 |
| VWF (mU/mL) | 1797.9 (1410.4–2910.9) | 2719.1 (1916.8–3890.7) | 0.002 | 1736.4 (1100.6–2308.6) | 1957.1 (1481.2–3737.0) | 0.057 |
| P-Selectin (ug/mL) | 116.0 (91.5–149.4) | 97.8 (79.0–129.0) | 0.094 | 113.9 (91.6–144.6) | 90.0 (73.1–108.1) | 0.010 |
| Endothelin-1 (pg/mL) | 3.7 (1.9–5.0) | 5.9 (3.0–7.4) | 0.005 | 4.6 (3.0–6.8) | 7.6 (5.3–8.6) | 0.004 |
| IL-6 (pg/mL) | 2.5 (1.5–7.7) | 3.2 (2.0–7.8) | 0.368 | 4.5 (1.7–9.4) | 1.9 (1.2–4.8) | 0.048 |
| IL-8 (pg/mL) | 31.3 (19.7–54.3) | 10.7 (5.6–17.4) | <0.001 | 49.6 (31.3–310.5) | 7.1 (3.9–12.8) | <0.001 |
| PAI-1 (pg/mL) | 191640.1 (152676.2–260532.7) | 96152.4 (75255.7–150325.9) | <0.001 | 206907.8 (177575.5–252684.4) | 124326.9 (93968.2–146791.6) | <0.001 |
Median and interquartile range presented, TW=transgender women, CM=cisgender males, PLWH=people living with HIV, PWOH=people without HIV
Upon further stratifying TW by current FHT use, several stepwise changes were observed going from CM to TW not on FHT to TW on FHT (Table 3), with additional increases in ENRAGE, oxLDL, sTNFRI, and PAI-1 (Figure 1), and decreases in vWF and ET-1 meeting statistical significance for a difference across groups. However, when comparing biomarker concentrations between TW not on FHT to TW on FHT, no significant differences were observed by FHT status either when pooling TW or stratifying by HIV serostatus.
Table 3:
Biomarker Concentrations by Gender, FHT Use and HIV Serostatus*
| PLWH | PWOH | |||||||
|---|---|---|---|---|---|---|---|---|
| TW on FHT (N=51) | TW not on FHT (N=24) | CM (N=40) | P-value | TW on FHT (N=31) | TW not on FHT (N=16) | CM (N=40) | P-value | |
| ENRAGE (pg/mL) | 9642.3 (6834.2–12857.7) | 8596.5 (7345.9–10696.7) | 912.9 (787.4–2797.4) | <0.001 | 10021.4 (8182.2–11471.4) | 9652.8 (8018.3–10254.8) | 2272.5 (877.6–3325.0) | <0.001 |
| LpPLA2 (ng/mL) | 235.9 (208.6–304.3) | 263.6 (199.5–318.8) | 220.8 (194.1–254.3) | 0.040 | 243.5 (195.5–287.4) | 241.5 (217.5–305.2) | 250.7 (234.7–279.8) | 0.499 |
| Oxidized LDL (mU/L) | 54347.4 (34837.7–60737.1) | 44096.3 (32866.8–55587.9) | 37248.1 (31470.8–46385.6) | 0.023 | 52924.3 (38632.2–67630.7) | 50779.3 (39381.7–65883.7) | 34833.5 (29078.1–48335.0) | 0.004 |
| sTNFRI (pg/mL) | 1385.7 (1212.3–1604.5) | 1315.2 (992.9–1574.3) | 989.4 (787.7–1194.0) | <0.001 | 1071.4 (941.6–1575.1) | 1022.6 (880.6–1197.8) | 980.5 (814.0–1120.4) | 0.061 |
| sTNFRII (pg/mL) | 3225.9 (2634.0–4396.9) | 3300.2 (2801.3–4205.0) | 2669.8 (2041.4–3718.3) | 0.017 | 2698.4 (2242.2–3921.1) | 2842.9 (2318.5–3380.6) | 2220.1 (1971.1–2806.3) | 0.031 |
| VWF (mU/mL) | 1750.7 (1289.6–2644.3) | 1901.0 (1599.4–3444.1) | 2719.1 (1916.8–3890.7) | 0.005 | 1573.5 (940.5–2186.8) | 1970.3 (1343.1–2449.5) | 1957.1 (1481.2–3737.0) | 0.078 |
| Endothelin-1 (pg/mL) | 3.6 (1.3–4.8) | 4.1 (2.1–5.2) | 5.9 (3.0–7.4) | 0.012 | 4.5 (2.8–6.1) | 5.1 (3.0–8.3) | 7.6 (5.3–8.6) | 0.007 |
| IL-8 (pg/mL) | 31.3 (20.6–45.1) | 31.3 (16.4–90.4) | 10.7 (5.6–17.4) | <0.001 | 35.4 (31.3–297.0) | 89.8 (31.3–355.6) | 7.1 (3.9–12.8) | <0.001 |
| PAI-1 (pg/mL) | 184436.3 (148166.6–259048.4) | 193237.3 (169630.9–267712.5) | 96152.4 (75255.7–150325.9) | <0.001 | 213929.0 (177999.5–254385.6) | 198819.6 (165473.8–250659.3) | 124326.9 (93968.2–146791.6) | <0.001 |
Limited to subset of biomarkers with statistical significance across at least 1 HIV serostatus
Median and interquartile range presented, FHT=feminizing hormonal therapy, TW=transgender women, CM=cisgender males, PLWH=people living with HIV, PWOH=people without HIV
Figure 1:
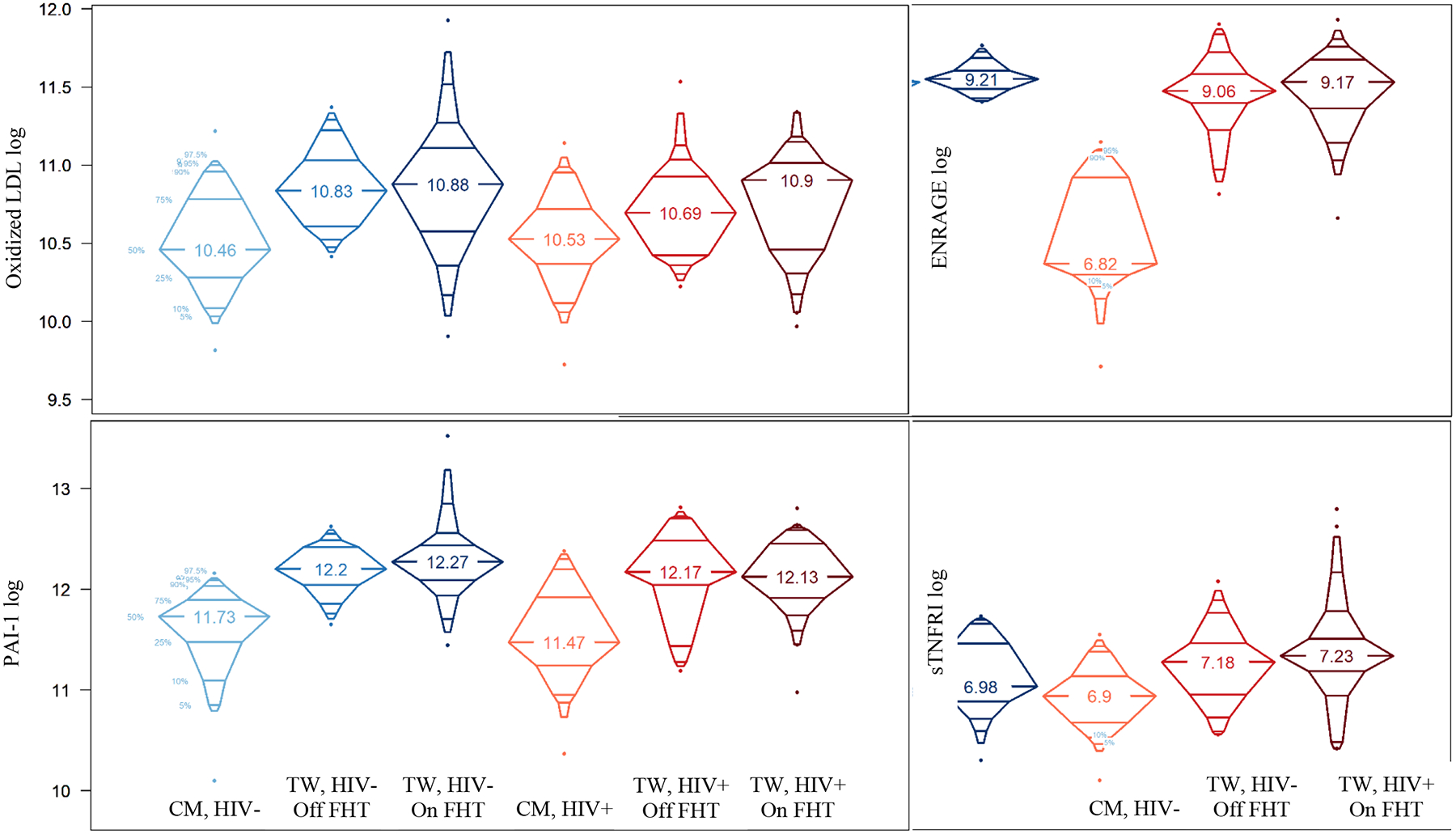
Differences in Biomarker Concentrations by Gender, FHT Use and HIV Serostatus
FHT=feminizing hormonal therapy, TW=transgender women, CM=cisgender males
In multivariable analysis adjusting for HIV serostatus, age, race/ethnicity, BMI, and smoking status, being a TW was associated with higher ENRAGE, oxLDL, sTNFRI/II, P selectin, IL-6, IL-8 and PAI-1 concentrations, and lower sCD14, sCD163, vWF and ET-1 concentrations (Table 4). A positive HIV serostatus was associated with higher sCD14 and lower ET-1 concentrations (both p<0.02). In a model restricted to TW and adding FHT use status as an additional covariate, current FHT use was not independently associated with any of the biomarkers concentrations (data not shown).
Table 4:
Adjusted Associations Between Gender (TW vs CM) and Biomarker Concentrations
| Difference in Log Biomarker (95% CI) | P-value | |
|---|---|---|
| Adiponectin (ng/mL) | 0.06 (−0.20, 0.33) | 0.648 |
| ENRAGE (pg/mL) | 1.82 (1.58, 2.06) | <0.001 |
| LpPLA2 (ng/mL) | 0.06 (−0.03, 0.15) | 0.209 |
| Oxidized LDL (mU/L) | 0.26 (0.12, 0.39) | <0.001 |
| sCD14 (ng/mL) | −0.09 (−0.19, −0.00) | 0.048 |
| sCD163 (ng/mL) | −0.27 (−0.47, −0.08) | 0.007 |
| sTNFR I (pg/mL) | 0.20 (0.07, 0.34) | 0.003 |
| sTNFR II (pg/mL) | 0.17 (0.03, 0.30) | 0.017 |
| VCAM-1 (ng/mL) | −0.05 (−0.17, 0.07) | 0.425 |
| VWF (mU/mL) | −0.41 (−0.63, −0.19) | <0.001 |
| P-Selectin (ug/mL) | 0.26 (0.08, 0.45) | 0.006 |
| Endothelin-1 (pg/mL) | −0.35 (−0.64, −0.05) | 0.021 |
| IL-6 (pg/mL) | 0.53 (0.12, 0.94) | 0.012 |
| IL-8 (pg/mL) | 2.08 (1.49, 2.67) | <0.001 |
| PAI-1 (pg/mL) | 0.67 (0.50, 0.83) | <0.001 |
Adjusted for HIV serostatus, age, race/ethnicity, BMI, and smoking status; TW=transgender women, CM=cisgender males
Discussion
In this cohort of TW both on and off FHT, significant aberrations in biomarkers reflecting inflammation, cardiovascular health and coagulation pathways were observed compared to matched CM and independent of HIV serostatus. Although pathophysiology cannot be inferred from this cross-sectional assessment, these provocative data highlight the potential for adverse cardiovascular events among TW and the need for further research.
Importantly, both FHT and HIV are associated with increased cardiovascular and thrombo-embolic event risk. [1–14] However, in this cohort TW demonstrated significant alterations in biomarker profiles vs matched CM regardless of FHT use and independent of HIV serostatus. Demographic and clinical factors potentially affecting biomarker profiles include BMI and smoking status, with TW less likely to be overweight or obese and more likely to be current smokers than CM. Diabetes mellitus frequency was similar between TW and CM, and rates of hypertension were lower among TW. Supporting our belief that observed differences in this cohort are not due to traditional components of CVD risk is an analysis from The Behavioral Risk Factor Surveillance System, in which no significant differences in rates of myocardial infarction were observed in TW compared to CM after adjusting for traditional CVD risk factors.[33]
Unmeasured factors, however, could contribute. For example, in this group of predominantly TW of color, multiple intersecting contributors to minority stress could predispose TW to poorer health outcomes,[34] some possibly mediated by the pathways reflected in the measured biomarkers. Indeed, The Intersectional Transgender Multilevel Minority Stress Model illustrates how multiple marginalized identities intersect with social determinants of health to impact cardiovascular health.[35] Despite this knowledge, the physiologic mechanisms of non-traditional cardiovascular risk remain poorly defined, yet their understanding is critical to optimizing health for transgender persons.
The most striking differences in biomarker concentrations between TW and CM were EN-RAGE, oxLDL and PAI-1 concentrations. EN-RAGE is a pro-inflammatory ligand of the receptor for advanced glycation end products (RAGE) and Toll-like receptor 4 (TLR4) that has been associated with incident CVD events independently of traditional CVD risk factors and associated biomarkers of inflammation.[36–38] OxLDL plays a key role in atherogenic and pro-apoptotic processes.[39] PAI-1 is the principal inhibitor of plasminogen, with elevations linked to hypofibrinolytic states and acute cardiovascular events.[40] Complex relationships between these molecules exist: EN-RAGE and oxLDL both bind CD36 and activate TLR4, EN-RAGE may regulate CD36 expression, and oxLDL can bind RAGE, all leading to and reinforcing inflammatory pathways involved in CVD.[41] Several stressor pathways, including IL-6 release,[42] lead to increased PAI-1 expression. OxLDL induces IL-6 release,[43] and IL-6 leads to increases in EN-RAGE.[44] The tight pathologic relationships between these biomarkers lends credence to the hypothesis that elevations of these biomarkers in TW are related to and represent increased cardiovascular risk. Additionally, sexual dimorphism in EN-RAGE concentrations has not been reported, suggesting elevated EN-RAGE levels in this group of TW cannot be accounted for by increased estradiol levels/relative testosterone deficiency.[45, 46]
ET-1 plays a diverse role in CVD development and is modulated by sex hormones. ET-1 is lower in adults assigned female at birth than adults assigned male at birth, and increases throughout the menopausal transition. The role of testosterone is more complex: people assigned female at birth with hyperandrogenism have higher ET-1 concentrations, but people assigned male at birth with hypogonadism have higher ET-1 levels that decline with testosterone supplementation.[47] Given higher estrogen levels and relative hypogonadism in our TW vs CM, it is not surprising that we observed lower ET-1 among TW. Additionally, though differences in ET-1 levels were not statistically different by FHT use status in our cohort (likely due to small sample size), TW on FHT did have lower ET-1 levels than those not on FHT (data not shown).
We also observed lower sCD14 and vWF concentrations among TW than CM among PLWH only. A sexual dimorphism in sCD14 levels has been documented, with higher levels generally observed in people assigned female at birth that are not greatly affected by menopause.[48] Additionally, in one study of transgender people, feminizing therapies increased vWF whereas testosterone treatment decreased vWF levels.[49] Thus, our findings cannot readily be explained by sex hormone differences between TW and CM, and HIV complicates the picture, with sCD14 and vWF levels elevated among PLWH.[50, 51] Future studies are needed to confirm or deny these findings.
While the aforementioned differences between TW and CM were observed, additional statistically significant differences within TW by FHT use status were not seen in this cohort. This may be due, in part, to the relatively small sample sizes of the groups, particularly when additionally stratified by HIV serostatus, and/or the high rates of current FHT use (67%). However, most data on CVD in TW on FHT is in the context of 17-α-ethinyl estradiol use, which is no longer recommended for FHT due to its perturbations of inflammatory and coagulation biomarker and lipid concentrations.[52] 17-β estradiol has milder effects on inflammatory and atherosclerotic pathways, and is the current standard of care. However, 17-α-ethinyl estradiol use is still observed, and TW engaging in medically-unsupervised FHT use may not have access to standard of care therapies. In this study, incomplete reporting of current FHT components prevented further exploration of biomarker levels by FHT type.
Limitations of this study include its cross-sectional design and lack of detailed FHT use information. FHT use was self-reported with confirmation by medical record where available. However, confirmation was not available for all participants, and some participants were taking medically-unsupervised FHT, the true components of which were unknown. Additionally, serum estradiol and testosterone concentrations were not available for this cohort, and we cannot completely exclude the possibility that an acute HIV acquisition was missed by rapid testing of persons believed to be HIV-negative. Lastly, time in storage was a few years longer for MACS men. However, samples had not previously been thawed and biomarkers we chose are not anticipated to have had substantial additional degradation during the additional storage period.
Although the sample size is fairly small, it is large among studies of TW. Strengths include having data from TW with and without HIV, on and off FHT, and from diverse racial, ethnic and geographic backgrounds. Well-matched controls are another significant strength. However, future research is needed to further characterize differences in cardiovascular risk by gender and FHT use and type, including research on surrogate measures of risk, such as circulating biomarkers.
Conclusions
Compared to matched CM, TW have altered profiles of biomarkers associated with systemic inflammation and CVD, even after adjusting for key risk factors. HIV and FHT may further complicate CVD risk. Although pathophysiology cannot be inferred from these cross-sectional assessments, these provocative data highlight the potential for adverse cardiovascular events among TW. Clinical data and longitudinal studies are needed to understand the mechanisms of CVD risk among TW.
Acknowledgements and sources of funding
This work was funded by NIH grants K23 AI110532 and R01 DK126042 to JEL, and the Gilead Sciences, Inc. Research Scholars Program to JEL. TTB is supported in part by K24 AI120834.
Data in this manuscript were collected by the Multicenter AIDS Cohort Study (MACS), now the MACS/WIHS Combined Cohort Study (MWCCS). The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). MWCCS (Principal Investigators): Atlanta CRS (Ighovwerha Ofotokun, Anandi Sheth, and Gina Wingood), U01-HL146241; Baltimore CRS (Todd Brown and Joseph Margolick), U01-HL146201; Bronx CRS (Kathryn Anastos and Anjali Sharma), U01-HL146204; Brooklyn CRS (Deborah Gustafson and Tracey Wilson), U01-HL146202; Data Analysis and Coordination Center (Gypsyamber D’Souza, Stephen Gange and Elizabeth Golub), U01-HL146193; Chicago-Cook County CRS (Mardge Cohen and Audrey French), U01-HL146245; Chicago-Northwestern CRS (Steven Wolinsky), U01-HL146240; Northern California CRS (Bradley Aouizerat, Jennifer Price, and Phyllis Tien), U01-HL146242; Los Angeles CRS (Roger Detels and Matthew Mimiaga), U01-HL146333; Metropolitan Washington CRS (Seble Kassaye and Daniel Merenstein), U01-HL146205; Miami CRS (Maria Alcaide, Margaret Fischl, and Deborah Jones), U01-HL146203; Pittsburgh CRS (Jeremy Martinson and Charles Rinaldo), U01-HL146208; UAB-MS CRS (Mirjam-Colette Kempf, Jodie Dionne-Odom, and Deborah Konkle-Parker), U01-HL146192; UNC CRS (Adaora Adimora and Michelle Floris-Moore), U01-HL146194. The MWCCS is funded primarily by the National Heart, Lung, and Blood Institute (NHLBI), with additional co-funding from the Eunice Kennedy Shriver National Institute Of Child Health & Human Development (NICHD), National Institute On Aging (NIA), National Institute Of Dental & Craniofacial Research (NIDCR), National Institute Of Allergy And Infectious Diseases (NIAID), National Institute Of Neurological Disorders And Stroke (NINDS), National Institute Of Mental Health (NIMH), National Institute On Drug Abuse (NIDA), National Institute Of Nursing Research (NINR), National Cancer Institute (NCI), National Institute on Alcohol Abuse and Alcoholism (NIAAA), National Institute on Deafness and Other Communication Disorders (NIDCD), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute on Minority Health and Health Disparities (NIMHD), and in coordination and alignment with the research priorities of the National Institutes of Health, Office of AIDS Research (OAR). MWCCS data collection is also supported by UL1-TR000004 (UCSF CTSA), UL1-TR003098 (JHU ICTR), UL1-TR001881 (UCLA CTSI), P30-AI-050409 (Atlanta CFAR), P30-AI-073961 (Miami CFAR), P30-AI-050410 (UNC CFAR), P30-AI-027767 (UAB CFAR), and P30-MH-116867 (Miami CHARM).
The authors gratefully acknowledge the contributions of the study participants and dedication of the staff at the MACS sites.
Funding:
This work was funded by NIH grants K23 AI110532 and R01 DK126042 to JEL, and the Gilead Sciences, Inc. Research Scholars Program to JEL. TTB is supported in part by K24 AI120834. Some data in this manuscript were collected by the Multicenter AIDS Cohort Study (MACS). Full funding information for the MACS can be found in the acknowledgements section of the manuscript.
References
- 1.Wierckx K, Elaut E, Declercq E, Heylens G, De Cuypere G, Taes Y, et al. Prevalence of cardiovascular disease and cancer during cross-sex hormone therapy in a large cohort of trans persons: a case-control study. Eur J Endocrinol 2013; 169(4):471–478. [DOI] [PubMed] [Google Scholar]
- 2.Asscheman H, Giltay EJ, Megens JA, de Ronde WP, van Trotsenburg MA, Gooren LJ. A long-term follow-up study of mortality in transsexuals receiving treatment with cross-sex hormones. Eur J Endocrinol 2011; 164(4):635–642. [DOI] [PubMed] [Google Scholar]
- 3.Dhejne C, Lichtenstein P, Boman M, Johansson AL, Langstrom N, Landen M. Long-term follow-up of transsexual persons undergoing sex reassignment surgery: cohort study in Sweden. PLoS One 2011; 6(2):e16885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson HD, Humphrey LL, Nygren P, Teutsch SM, Allan JD. Postmenopausal hormone replacement therapy: scientific review. JAMA 2002; 288(7):872–881. [DOI] [PubMed] [Google Scholar]
- 5.Venous thromboembolic disease and combined oral contraceptives: results of international multicentre case-control study. World Health Organization Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Lancet 1995; 346(8990):1575–1582. [PubMed] [Google Scholar]
- 6.Sare GM, Gray LJ, Bath PM. Association between hormone replacement therapy and subsequent arterial and venous vascular events: a meta-analysis. Eur Heart J 2008; 29(16):2031–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gillum LA, Mamidipudi SK, Johnston SC. Ischemic stroke risk with oral contraceptives: A meta-analysis. JAMA 2000; 284(1):72–78. [DOI] [PubMed] [Google Scholar]
- 8.Lidegaard O, Lokkegaard E, Jensen A, Skovlund CW, Keiding N. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med 2012; 366(24):2257–2266. [DOI] [PubMed] [Google Scholar]
- 9.Masia M, Padilla S, Alvarez D, Lopez JC, Santos I, Soriano V, et al. Risk, predictors, and mortality associated with non-AIDS events in newly diagnosed HIV-infected patients: role of antiretroviral therapy. AIDS. [DOI] [PubMed] [Google Scholar]
- 10.Weber R, Ruppik M, Rickenbach M, Spoerri A, Furrer H, Battegay M, et al. Decreasing mortality and changing patterns of causes of death in the Swiss HIV Cohort Study. HIV Med. [DOI] [PubMed] [Google Scholar]
- 11.Deeken JF, Tjen ALA, Rudek MA, Okuliar C, Young M, Little RF, et al. The Rising Challenge of Non-AIDS-Defining Cancers in HIV-Infected Patients. Clin Infect Dis; 55(9):1228–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Currier JS, Taylor A, Boyd F, Dezii CM, Kawabata H, Burtcel B, et al. Coronary heart disease in HIV-infected individuals. J Acquir Immune Defic Syndr 2003; 33(4):506–512. [DOI] [PubMed] [Google Scholar]
- 13.Dolan SE, Hadigan C, Killilea KM, Sullivan MP, Hemphill L, Lees RS, et al. Increased cardiovascular disease risk indices in HIV-infected women. J Acquir Immune Defic Syndr 2005; 39(1):44–54. [DOI] [PubMed] [Google Scholar]
- 14.Oliviero U, Bonadies G, Apuzzi V, Foggia M, Bosso G, Nappa S, et al. Human immunodeficiency virus per se exerts atherogenic effects. Atherosclerosis 2009; 204(2):586–589. [DOI] [PubMed] [Google Scholar]
- 15.Nou E, Lo J, Hadigan C, Grinspoon SK. Pathophysiology and management of cardiovascular disease in patients with HIV. The Lancet Diabetes & Endocrinology 2016; 4(7):598–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah ASV, Stelzle D, Lee KK, Beck EJ, Alam S, Clifford S, et al. Global Burden of Atherosclerotic Cardiovascular Disease in People Living With HIV: Systematic Review and Meta-Analysis. Circulation 2018; 138(11):1100–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsue PY, Waters DD. HIV infection and coronary heart disease: mechanisms and management. Nat Rev Cardiol 2019; 16(12):745–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feinstein MJ, Hsue PY, Benjamin LA, Bloomfield GS, Currier JS, Freiberg MS, et al. Characteristics, Prevention, and Management of Cardiovascular Disease in People Living With HIV: A Scientific Statement From the American Heart Association. Circulation 2019; 140(2):e98–e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Funderburg NT, Zidar DA, Shive C, Lioi A, Mudd J, Musselwhite LW, et al. Shared monocyte subset phenotypes in HIV-1 infection and in uninfected subjects with acute coronary syndrome. Blood 2012; 120(23):4599–4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anzinger JJ BT, Angelovich TA, Crowe SM, Palmer CS. Monocytes as regulators of inflammation and HIV-related comorbidities during cART. J Immunol Res 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang H, Xie Z, Shen T. Monocyte activation and cardiovascular disease in HIV infection. Cell Mol Immunol 2017; 14(12):960–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teer E, Joseph DE, Glashoff RH, Faadiel Essop M. Monocyte/Macrophage-Mediated Innate Immunity in HIV-1 Infection: From Early Response to Late Dysregulation and Links to Cardiovascular Diseases Onset. Virol Sin 2021; 36(4):565–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Funderburg NT, Mayne E, Sieg SF, Asaad R, Jiang W, Kalinowska M, et al. Increased tissue factor expression on circulating monocytes in chronic HIV infection: relationship to in vivo coagulation and immune activation. Blood 2010; 115(2):161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baker JV, Brummel-Ziedins K, Neuhaus J, Duprez D, Cummins N, Dalmau D, et al. HIV replication alters the composition of extrinsic pathway coagulation factors and increases thrombin generation. J Am Heart Assoc 2013; 2(4):e000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Funderburg NT, Lederman MM. Coagulation and morbidity in treated HIV infection. Thromb Res 2014; 133 Suppl 1:S21–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.CIA. HIV/AIDS-Adult Prevalence Rate. 2014.
- 27.Lee JG, Griffin GK, Melvin CL. Tobacco use among sexual minorities in the USA, 1987 to May 2007: a systematic review. Tob Control 2009; 18(4):275–282. [DOI] [PubMed] [Google Scholar]
- 28.Berger I, Mooney-Somers J. Smoking Cessation Programs for Lesbian, Gay, Bisexual, Transgender, and Intersex People: A Content-Based Systematic Review. Nicotine Tob Res 2017; 19(12):1408–1417. [DOI] [PubMed] [Google Scholar]
- 29.Buchting FO, Emory KT, Scout, Kim Y, Fagan P, Vera LE, et al. Transgender Use of Cigarettes, Cigars, and E-Cigarettes in a National Study. Am J Prev Med 2017; 53(1):e1–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Office on Smoking and Health NCfCDPaHP, Centers for Disease Control and Prevention. Lesbian, Gay, Bisexual, and Transgender (LGBT) People. In; March 17, 2022.
- 31.Malhotra A, Kort S, Lauther T, Mann N, Skopicki HA, Parikh PB. Prevalence and Predictors of Cardiovascular Disease and Risk Factors in Transgender Persons in the United States. Crit Pathw Cardiol 2022; 21(1):42–46. [DOI] [PubMed] [Google Scholar]
- 32.Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo CR Jr. The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol 1987; 126(2):310–318. [DOI] [PubMed] [Google Scholar]
- 33.Alzahrani T, Nguyen T, Ryan A, Dwairy A, McCaffrey J, Yunus R, et al. Cardiovascular Disease Risk Factors and Myocardial Infarction in the Transgender Population. Circ Cardiovasc Qual Outcomes 2019; 12(4):e005597. [DOI] [PubMed] [Google Scholar]
- 34.Testa RJ, Habarth J, Peta J, Balsam K, Bockting W. Development of the Gender Minority Stress and Resilience Measure. In. Psychology of Sexual Orientation and Gender Diversity; 2015. pp. 65–77. [Google Scholar]
- 35.Streed CG, Beach LB, Caceres BA, Dowshen NL, Moreau KL, Mukherjee M, et al. Assessing and Addressing Cardiovascular Health in People Who Are Transgender and Gender Diverse: A Scientific Statement From the American Heart Association. Circulation 2021; 144(6):e136–e148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell 1999; 97(7):889–901. [DOI] [PubMed] [Google Scholar]
- 37.Foell D, Wittkowski H, Kessel C, Lüken A, Weinhage T, Varga G, et al. Proinflammatory S100A12 can activate human monocytes via Toll-like receptor 4. Am J Respir Crit Care Med 2013; 187(12):1324–1334. [DOI] [PubMed] [Google Scholar]
- 38.Ligthart S, Sedaghat S, Ikram MA, Hofman A, Franco OH, Dehghan A. EN-RAGE: a novel inflammatory marker for incident coronary heart disease. Arterioscler Thromb Vasc Biol 2014; 34(12):2695–2699. [DOI] [PubMed] [Google Scholar]
- 39.Varghese DS, Ali BR. Pathological Crosstalk Between Oxidized LDL and ER Stress in Human Diseases: A Comprehensive Review. Front Cell Dev Biol 2021; 9:674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morrow GB, Whyte CS, Mutch NJ. A Serpin With a Finger in Many PAIs: PAI-1’s Central Function in Thromboinflammation and Cardiovascular Disease. Front Cardiovasc Med 2021; 8:653655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farokhzadian J, Mangolian Shahrbabaki P, Bagheri V. S100A12-CD36 axis: A novel player in the pathogenesis of atherosclerosis? Cytokine 2019; 122:154104. [DOI] [PubMed] [Google Scholar]
- 42.Kang S, Tanaka T, Inoue H, Ono C, Hashimoto S, Kioi Y, et al. IL-6 trans-signaling induces plasminogen activator inhibitor-1 from vascular endothelial cells in cytokine release syndrome. Proc Natl Acad Sci U S A 2020; 117(36):22351–22356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lubrano V, Gabriele M, Puntoni MR, Longo V, Pucci L. Relationship among IL-6, LDL cholesterol and lipid peroxidation. Cell Mol Biol Lett 2015; 20(2):310–322. [DOI] [PubMed] [Google Scholar]
- 44.Hasegawa T, Kosaki A, Kimura T, Matsubara H, Mori Y, Okigaki M, et al. The regulation of EN-RAGE (S100A12) gene expression in human THP-1 macrophages. Atherosclerosis 2003; 171(2):211–218. [DOI] [PubMed] [Google Scholar]
- 45.Larsen A, Bronstein IB, Dahl O, Wentzel-Larsen T, Kristoffersen EK, Fagerhol MK. Quantification of S100A12 (EN-RAGE) in blood varies with sampling method, calcium and heparin. Scand J Immunol 2007; 65(2):192–201. [DOI] [PubMed] [Google Scholar]
- 46.Manolakis AC, Kapsoritakis AN, Georgoulias P, Tzavara C, Valotassiou V, Kapsoritaki A, et al. Moderate performance of serum S100A12, in distinguishing inflammatory bowel disease from irritable bowel syndrome. BMC Gastroenterol 2010; 10:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gohar EY, Giachini FR, Pollock DM, Tostes RC. Role of the endothelin system in sexual dimorphism in cardiovascular and renal diseases. Life Sci 2016; 159:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Looby SE, Kantor A, Burdo TH, Currier JS, Fichtenbaum CJ, Overton ET, et al. Factors Associated with Systemic Immune Activation Indices in a Global Primary Cardiovascular Disease Prevention Cohort of People with HIV on Antiretroviral Therapy. Clin Infect Dis 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Kesteren PJ, Kooistra T, Lansink M, van Kamp GJ, Asscheman H, Gooren LJ, et al. The effects of sex steroids on plasma levels of marker proteins of endothelial cell functioning. Thromb Haemost 1998; 79(5):1029–1033. [PubMed] [Google Scholar]
- 50.de Larrañaga GF, Bocassi AR, Puga LM, Alonso BS, Benetucci JA. Endothelial markers and HIV infection in the era of highly active antiretroviral treatment. Thrombosis Research 2003; 110(2–3):93–98. [DOI] [PubMed] [Google Scholar]
- 51.Novelli S, Lecuroux C, Goujard C, Reynes J, Villemant A, Blum L, et al. Persistence of monocyte activation under treatment in people followed since acute HIV-1 infection relative to participants at high or low risk of HIV infection. EBioMedicine 2020; 62:103129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gooren LJ, Wierckx K, Giltay EJ. Cardiovascular disease in transsexual persons treated with cross-sex hormones: reversal of the traditional sex difference in cardiovascular disease pattern. Eur J Endocrinol 2014; 170(6):809–819. [DOI] [PubMed] [Google Scholar]


