Abstract
Background:
Hysterectomy is the most common non-obstetric medical procedure performed in U.S. women. Evaluating hysterectomy prevalence trends and determinants is important for estimating gynecologic cancer rates and management of uterine conditions.
Objective:
Our objective was to assess hysterectomy prevalence trends and determinants using the Behavioral Risk Factor Surveillance System (BRFSS; 2006–2016).
Study Design:
We estimated crude hysterectomy prevalences and multivariable-adjusted odds ratios (ORs) and 95% confidence intervals (CIs) for associations of race/ethnicity, age group (5-year), body mass index (BMI; categorical), smoking status, education, insurance, income, and U.S. region with hysterectomy. Missing data were imputed. The number of women in each survey year ranged from 220,302 in 2006 to 275,631 in 2016.
Results:
While overall hysterectomy prevalence changed little between 2006–2016 (21.4% and 21.1%, respectively), hysterectomy prevalence was lower in 2016 than 2006 among women aged 40 years and older, particularly among non-Hispanic Black and Hispanic women. Current smoking (OR 1.38, 95% CI:1.35–1.41), increasing age (OR 1.40, 95% CI:1.39–1.40), living in the South compared to the Midwest (OR 1.36, 95% CI:1.34–1.39), higher BMI (OR 1.26, 95% CI:1.25–1.27), Black race compared to White (OR 1.10, 95% CI:1.07–1.13), and having insurance compared to being uninsured (OR 1.26, 95% CI:1.22–1.30) were most strongly associated with increased prevalence. Hispanic ethnicity and living in the Northeast were most strongly associated with decreased prevalence (OR 0.73, 95% CI:0.70–0.76; OR 0.67, 95% CI:0.65–0.69).
Conclusions:
Nationwide, hysterectomy prevalence decreased among women aged 40 years and older from 2006 to 2016, particularly among non-Hispanic Black and Hispanic women. Age, non-Hispanic Black race, having insurance, current smoking, and living in the South were associated with increased odds of hysterectomy, even after accounting for possible explanatory factors. Further research is needed to better understand associations of race and ethnicity and region with hysterectomy prevalence.
Keywords: hysterectomy, gynecologic epidemiology, gynecologic surgery, gynecologic cancer
Condensation:
Hysterectomy prevalence in United States has decreased in recent years among most age and racial and ethnic strata, and is strongly associated with age, smoking, and region.
Introduction
Approximately 600,000 hysterectomy procedures are performed annually in the United States (U.S.), making it the most common non-obstetric surgical procedure among women1,2. Characterizing the epidemiology of hysterectomy is important for understanding its trends over time, quantifying possible adverse effects of hysterectomy on a population level, and properly estimating gynecologic cancer rates. Recent reports based on national claims data suggest that hysterectomy rates are decreasing3,4; however, these data do not account for outpatient hysterectomy procedures, which have increased in recent years5,6, and vary by race, ethnicity, and other factors7–13.
Previous studies reported higher rates of hysterectomy and related complications among Black women compared to White and Hispanic women1,14–19.These racial and/or ethnic differences are not fully understood; however, Black women are known to have a higher prevalence of uterine fibroids, the most common benign indication for hysterectomy1,8,20,21. A higher frequency of hysterectomy in the South compared to the Northeast has also been observed, but the extent to which region and race and/or ethnicity are independently associated with hysterectomy prevalence is unclear2,22,23.
A better understanding of hysterectomy prevalence over time and its determinants also has important implications for accurate estimation of gynecologic cancer incidence and mortality rates. Women who undergo total hysterectomy are no longer at risk for developing cervical and endometrial cancer; failure to remove these women from the population at-risk can bias racial and/or ethnic and geographic comparisons, and changes in rates over time19,22,24–28, to the extent that hysterectomy prevalence varies by these factors. Further, hysterectomy prevalence estimates are essential for risk prediction models that estimate gynecologic cancer risks.
We conducted an up-to-date analysis using nationally representative data from the Behavioral Risk Factor Surveillance System (BRFSS) to evaluate hysterectomy prevalence from 2006 to 2016, and to assess determinants of hysterectomy prevalence among U.S. women.
Methods
Data Sources
Behavioral Risk Factor Surveillance System
The Centers for Disease Control and Prevention (CDC) Behavioral Risk Factor Surveillance System (BRFSS) is a nationally representative, cross-sectional survey of health-related risk factors, behaviors, chronic health conditions, and use of preventive services in the noninstitutionalized adult civilian U.S. population aged 18 years and older. BRFSS is a stratified survey using a dual-frame sample design, conducting both landline and cell phone surveys using random digit dialing. Data collection is decentralized by state and surveys are managed by the state health department. BRFSS data can be weighted back to represent the U.S. population, as previously reported30. Self-reported hysterectomy status is ascertained every other year by the question, “Have you had a hysterectomy?”.
Statistical Analyses
We evaluated the proportion of women missing hysterectomy information in each dataset (2006, 2008…2016); hysterectomy status was missing most frequently in 2016 (62,070/275,631; 22.5% unweighted, 18.5% weighted). Women with and without missing hysterectomy information were generally similar (data not shown). Datasets for each year were combined and missing data on variables included in the multivariable model were imputed using sequential regression imputation method implemented in IVEware (http://www.isr.umich.edu/src/smp/ive)31. Missingness for covariates ranged from weighted percentages of 0.2% to 16.9% in all years, with the highest percent missing observed for BMI and income. We obtained five imputations from the models that included interaction terms between year and BMI, hysterectomy status, income, and smoking. The survey weights were also included in the imputation model. Survey weighted prevalence estimates (PROC SURVEYMEANS) and odds ratios (ORs; PROC SURVEYLOGISTIC; SAS 9.4) were obtained for each of the five imputed datasets and results were combined using PROC MIANALYZE; SAS 9.4.
To assess unadjusted hysterectomy prevalence over time, we fit weighted logistic regression models accounting for the stratified sampling design with time in single years coded as a trend using PROC SURVEYLOGISTIC to each imputed data set and then combined estimates using PROC MIANALYZE. We computed prevalence estimates using the final estimates for the intercept and year log-odds ratio coefficients in logistic models and calculated the corresponding variance using the delta method. Results were identical to those obtained using PROC SURVEYMEANS. We evaluated the unadjusted prevalence of hysterectomy over time for all women, by race and/or ethnicity, and by U.S. region. Because age is strongly associated with hysterectomy prevalence and the age distribution varies by race and/or ethnicity (Supplement Table 1) we evaluated hysterectomy prevalence over time by race and ethnicity, stratified by age (<50 ≥and 50 years, as a proxy for menopausal status). We report descriptive statistics of the baseline population using imputed data for years 2006 (the earliest year) and 2016 (the latest year) to characterize the study population and evaluate any changes in the distribution of variables over time.
The multivariable model included data from each year combined, with the following variables: age group (5-year intervals; ordinal predictor), race and/or ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, Asian/Pacific Islander, and other), body mass index (BMI, underweight [<18.5 kg/m2], normal [18.5 – 24.9 kg/m2], overweight [25 – 29.9 kg/m2], and obese [30+ kg/m2]; ordinal predictor), smoking status (current, former, never), education (did not graduate from high school, graduated from high school, attended some college or technical school, graduated college or technical school), insurance status (uninsured, insured), yearly household income (<$50,000, ≥$50,000), U.S. region (Midwest, South, West, Northeast, and US Territories including Puerto Rico, Guam, and the Virgin Islands), and survey year(ordinal predictor). We used mutually-adjusted weighted logistic regression models to estimate ORs and 95% confidence intervals (95% CIs) for associations with hysterectomy status. In a secondary analysis, we stratified the multivariable model by insurance status because we hypothesized that insurance, as an indicator of access to care, could potentially modify associations.
Analyses were performed in SAS 9.4. Two-sided P-values of <0.05 were considered statistically significant.
Results
Population Characteristics
Characteristics of women surveyed in the years 2006 and 2016 are shown in Table 1. Overall, populations were similar with respect to mean age (47.years in 2006, 48.5 years in 2016). The percent of non-Hispanic Whites declined from 2006 (70.2%) to 2016 (62.9%). In 2006, 41.6% of women had a normal BMI versus 35.0% in 2016. Fewer women reported never smoking (60.9%) and having insurance (85.7%) in 2006 compared to 2016 (64.4% % and 89.6%, respectively), whereas annual income and education were relatively stable. In both years, most women lived in the South (35.9% in 2006 and 38.3% in 2016).
Table 1.
Study Population Characteristics in the 2006 and 2016 Behavioral Risk Factor Surveillance System (BRFSS), weighted percents with 95% CI
| 2006 (N=211,659) | 2016 (N=213,561) | |||||
|---|---|---|---|---|---|---|
| Total | Hysterectomy | No Hysterectomy | Total | Hysterectomy | No Hysterectomy | |
|
| ||||||
| Age | ||||||
| Mean | 47.3 (47.1–47.5) | 61.4 (61.2–61.6) | 43.4 (43.2–43.6) | 48.5 (48.4–48.7) | 62.6 (62.4–62.8) | 44.7 (44.5–44.9) |
| Race | ||||||
| NH White | 70.2 (69.7–70.7) | 76.0 (75.1–76.8) | 68.6 (68–69.2) | 62.9 (62.5–63.4) | 71.7 (70.8–72.6) | 60.5 (60–61.1) |
| NH Black | 9.8 (9.6–10.1) | 10.3 (9.8–10.8) | 9.7 (9.4–10.0) | 12.2 (11.8–12.5) | 12.5 (11.9–13.1) | 12.1 (11.7–12.4) |
| Hispanic | 13.8 (13.4–14.3) | 8.7 (8.1–9.4) | 15.2 (14.7–15.8) | 16.0 (15.6–16.4) | 9.6 (9.0–10.2) | 17.8 (17.3–18.2) |
| Asian/Pacific Islander | 2.5 (2.2–2.7) | 1.0 (0.8–1.3) | 2.9 (2.6–3.1) | 4.9 (4.6–5.2) | 2.2 (1.7–2.8) | 5.6 (5.3–6.0) |
| Other | 3.0 (2.9–3.2) | 3.3 (3.0–3.6) | 2.9 (2.8–3.1) | 2.5 (2.4–2.6) | 2.3 (2.1–2.5) | 2.6 (2.4–2.7) |
| Missing | 0.7 (0.6–0.8) | 0.7 (0.7–0.9) | 0.7 (0.6–0.8) | 1.6 (1.5–1.7) | 1.8 (1.5–2.1) | 1.5 (1.4–1.7) |
| BMI (kg/m2) | ||||||
| Underweight (<18.5) | 1.6 (1.5–1.7) | 1.4 (1.2–1.6) | 1.6 (1.5–1.8) | 2.5 (2.3–2.6) | 1.7 (1.5–1.9) | 2.7 (2.5–2.9) |
| Normal (18.5–25) | 41.6 (41.1–42.0) | 33.4 (32.6–34.1) | 43.9 (43.3–44.4) | 35.0 (34.6–35.5) | 27.7 (26.9–28.4) | 37.1 (36.6–37.6) |
| Overweight (25–30) | 27.6 (27.2–28.0) | 31.6 (30.8–32.4) | 26.5 (26.0–27.0) | 27.1 (26.7–27.5) | 29.5 (28.7–30.3) | 26.4 (25.9–26.9) |
| Obese (>30) | 22.9 (22.5–23.2) | 28.0 (27.3–28.7) | 21.4 (21.0–21.9) | 27.4 (27.0–27.8) | 32.7 (31.9–33.6) | 25.9 (25.5–26.4) |
| Missing | 6.4 (6.2–6.7) | 5.7 (5.4–6.1) | 6.6 (6.3–6.9) | 8.1 (7.8–8.3) | 8.4 (8–8.9) | 8.0 (7.7–8.3) |
| Smoking Status | ||||||
| Current | 17.7 (17.4–18.1) | 17.3 (16.6–17.9) | 17.9 (17.5–18.3) | 14.3 (14.0–14.6) | 14.7 (14.1–15.3) | 14.1 (13.8–14.5) |
| Former | 21.1 (20.7–21.4) | 27.5 (26.8–28.2) | 19.3 (18.9–19.7) | 20.8 (20.4–21.1) | 28.3 (27.5–29.0) | 18.7 (18.4–19.1) |
| Never | 60.9 (60.4–61.3) | 54.8 (54.0–55.6) | 62.6 (62.1–63.1) | 64.4 (64.0–64.8) | 56.5 (55.6–57.3) | 66.6 (66.1–67.1) |
| Missing | 0.3 (0.3–0.4) | 0.5 (0.4–0.6) | 0.3 (0.2–0.3) | 0.6 (0.5–0.6) | 0.6 (0.5–0.7) | 0.6 (0.5–0.7) |
| Insurance | ||||||
| Do not have | 14.0 (13.5–14.4) | 8.4 (7.9–8.9) | 15.5 (15.0–16.0) | 9.9 (9.6–10.2) | 5.3 (4.9–5.8) | 11.2 (10.8–11.6) |
| Have | 85.7 (85.3–86.1) | 91.5 (90.9–92.0) | 84.2 (83.7–84.6) | 89.6 (89.3–90.0) | 94.5 (93.9–94.9) | 88.3 (87.9–88.7) |
| Missing | 0.3 (0.2–0.4) | 0.2 (0.1–0.2) | 0.3 (0.3–0.5) | 0.5 (0.4–0.5) | 0.2 (0.2–0.3) | 0.5 (0.4–0.6) |
| Annual Income | ||||||
| <$50,000 | 48.7 (48.2–49.1) | 55.1 (54.3–55.9) | 46.9 (46.3–47.4) | 46.6 (46.1–47) | 51.0 (50.1–51.8) | 45.3 (44.8–45.9) |
| >$50,000 | 35.9 (35.5–36.4) | 26.9 (26.2–27.7) | 38.4 (37.9–39.0) | 36.6 (36.1–37) | 30.3 (29.5–31.1) | 38.3 (37.8–38.8) |
| Missing | 15.4 (15.1–15.8) | 18.0 (17.3–18.6) | 14.7 (14.3–15.1) | 16.9 (16.5–17.2) | 18.7 (18.1–19.4) | 16.4 (16.0–16.8) |
| Education | ||||||
| Did not graduate high | 11.4 | 14.7 | 10.5 | 13.3 | 15.6 | 12.6 |
| school | (11.1–11.8) | (14.0–15.4) | (10.1–10.9) | (12.9–13.6) | (14.9–16.3) | (12.2–13.0) |
| Graduated high | 29.3 | 35.7 | 27.5 | 26.7 | 31.3 | 25.5 |
| school | (28.9–29.7) | (35.0–36.5) | (27.0–28.0) | (26.3–27.1) | (30.5–32.1) | (25–25.9) |
| Attended college or | 28.0 | 27.6 | 28.1 | 32.9 | 34.1 | 32.5 |
| technical school | (27.6–28.4) | (26.9–28.3) | (27.6–28.6) | (32.4–33.3) | (33.3–35) | (32.0–33.0) |
| Graduated college or | 31.1 | 21.8 | 33.7 | 26.9 | 18.7 | 29.2 |
| technical school | (30.7–31.6) | 21.1–22.5) | (33.2–34.3) | (26.6–27.3) | (18.2–19.3) | (28.7–29.6) |
| Missing | 0.2 (0.1–0.2) | 0.2 (0.1–0.2) | 0.2 (0.1–0.2) | 0.3 (0.2–0.3) | 0.3 (0.2–0.4) | 0.3 (0.2–0.3) |
| US Region | ||||||
| Northeast | 18.8 (18.5–19.1) | 14.1 (13.6–14.7) | 20.1 (19.7–20.5) | 17.1 (16.9–17.4) | 12.6 (12.2–13.2) | 18.4 (18.0–18.7) |
| Midwest | 22.4 (22.0–22.8) | 22.0 (21.3–22.6) | 22.5 (22.1–23.0) | 23.3 (23.0–23.5) | 23.9 (23.4–24.5) | 23.1 (22.8–23.4) |
| South | 35.9 (35.5–36.4) | 42.6 (41.8–43.4) | 34.1 (33.6–34.6) | 38.3 (37.9–38.7) | 44.8 (44.0–45.6) | 36.5 (36.0–36.9) |
| West | 21.5 (21.0–22.0) | 20.2 (19.4–21.0) | 21.9 (21.3–22.4) | 19.9 (19.5–20.2) | 17.2 (16.5–18.0) | 20.6 (20.2–21.0) |
| Territories | 1.4 (1.3–1.4) | 1.2 (1.1–1.3) | 1.4 (1.4–1.5) | 1.4 (1.4–1.5) | 1.4 (1.3–1.5) | 1.5 (1.4–1.5) |
*95% CI: 95% confidence intervals
*Total number (N) corresponds with unweighted, unimputed population counts
Hysterectomy Prevalence Over Time
Among all women, the unadjusted hysterectomy prevalence ranged from 21.4% (95% CI 21.0–21.7%) in 2006 to 21.1% (95% CI 20.8–21.4%) in 2016 (Figure 1). In analyses stratified by race and ethnicity, hysterectomy prevalence increased slightly between 2006 and 2016 from 23.2% (95% CI 22.9–23.6%) to 24.2% (95% CI 23.9–24.6%) among non-Hispanic White women and decreased slightly from 22.6% (95% CI 21.4–23.9%) to 21.7% (95% CI 20.6–22.9%) among non-Hispanic Black women, and from 13.2% (95% CI 12.1–14.3%) to 12.8% (95% CI 12.0–13.6%) in Hispanic women (Figure 2). Among those aged 50 years and older, non-Hispanic Black women had significantly higher prevalence of hysterectomy across all years compared to non-Hispanic White and Hispanic women (Figure 3). Except for 2010, there was a steady decrease of hysterectomy prevalence by year in non-Hispanic Black and Hispanic women; prevalence remained stable among non-Hispanic White women. We observed similar patterns of hysterectomy prevalence over time by region, with slight declines in the Northeast (16.2% to 15.6%), South (25.4% to 24.6%), and West (19.8% to 19.2%), and a slight increase in the Midwest (21.0% to 21.7%)(Supplement Figure 1).
Figure 1. Hysterectomy Prevalence in the Behavioral Risk Factor Surveillance System (BRFSS), 2006–2016.
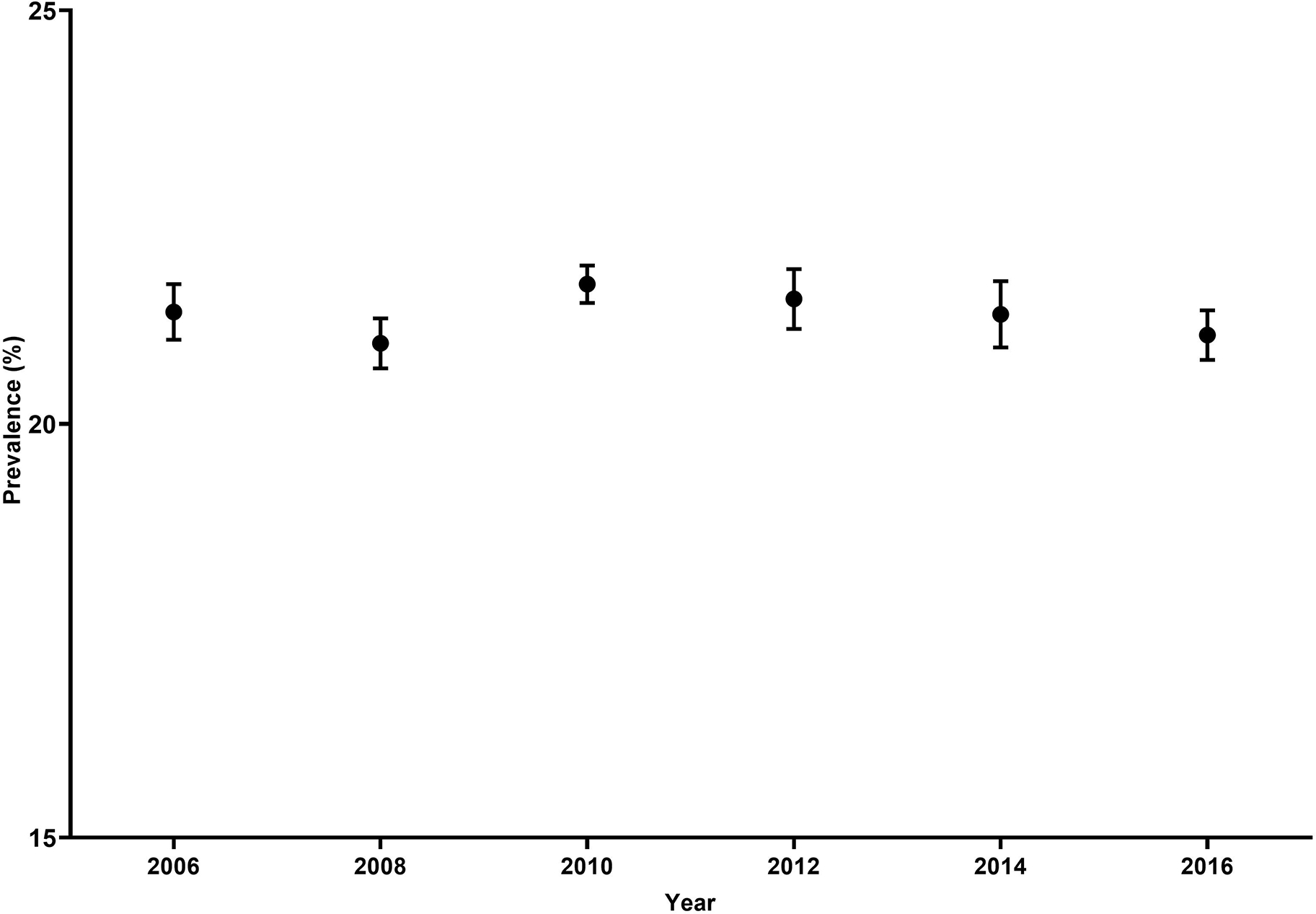
Unadjusted prevalence of self-reported hysterectomy with 95% confidence intervals among U.S. women aged 18 to 80 years in the Behavioral Risk Factor Surveillance System every two years from 2006 to 2016. Prevalences are shown in percentages.
Figure 2. Hysterectomy Prevalence in the Behavioral Risk Factor Surveillance System (BRFSS), 2006–2016, by Race/Ethnicity (A), and by Race/Ethnicity and Age Group (years; B).
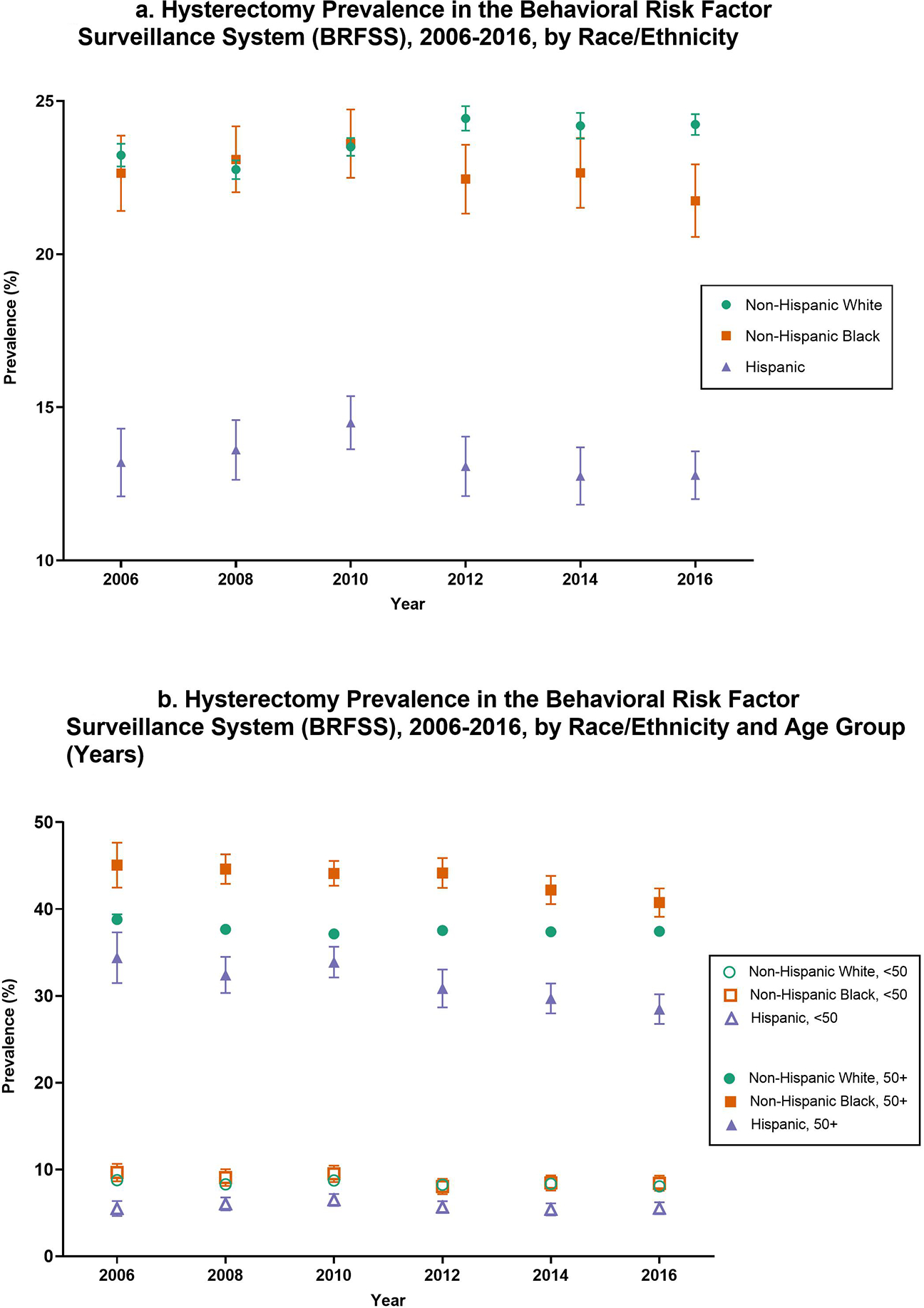
Unadjusted prevalence of self-reported hysterectomy with 95% confidence intervals among U.S. women aged 18 to 80 years in the Behavioral Risk Factor Surveillance System every two years from 2006 to 2016. Prevalences (A) are shown in percentages and are displayed separately among Non-Hispanic White (green circle), Non-Hispanic Black (orange square), and Hispanic (purple triangle) women. Prevalences (B) are further stratified by age group, showing hysterectomy prevalence among Non-Hispanic White women aged <50 years (open green circle), Non-Hispanic Black women aged <50 years (open orange square), Hispanic women aged <50 years (open purple triangle), Non-Hispanic white women aged 50+ years (closed green circle), Non-Hispanic Black women aged 50+ years (closed orange square), and Hispanic women aged 50+ years (closed purple triangle).
Figure 3. Hysterectomy Prevalence in the Behavioral Risk Factor Surveillance System (BRFSS), 2006 and 2016, by Age (Years) and Race/Ethnicity.
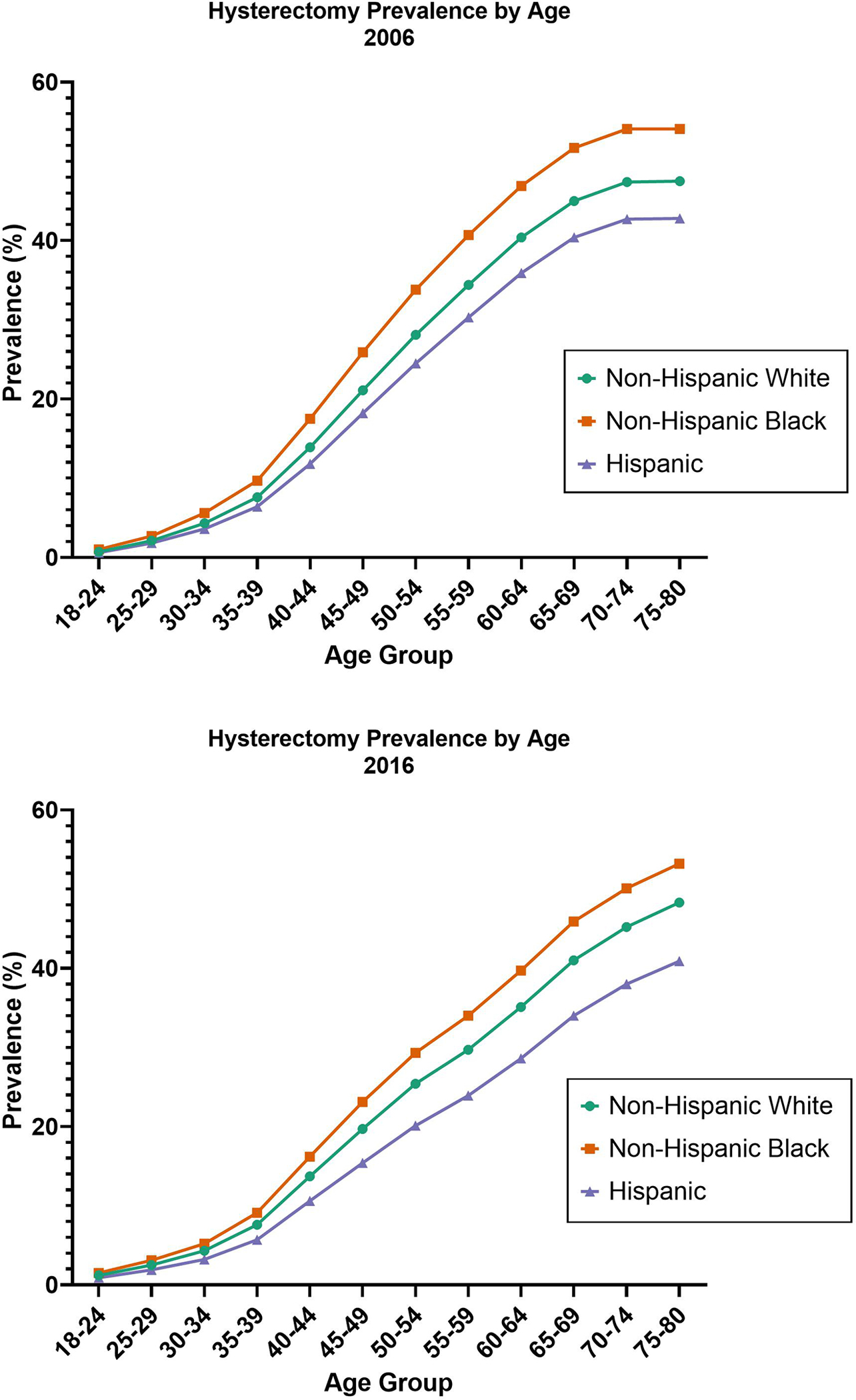
Unadjusted prevalence of self-reported hysterectomy among U.S. women aged 18 to 80 years in the Behavioral Risk Factor Surveillance System in 2006 and 2016. Prevalences are shown in percentages at 12 age groupings and are displayed separately among Non-Hispanic White (green circle), Non-Hispanic Black (orange square), and Hispanic (purple triangle) women.
Hysterectomy Prevalence by Age Group Over Time
We evaluated hysterectomy prevalence by 5-year age groups and race and/ethnicity in 2006 and 2016. Overall hysterectomy prevalence was lowest among those aged <35 years (7.5% in 2006 and 7.1% in 2016), after which there was a steady increase in 50–54-year-olds (28.1% in 2006 and 24.7% in 2016) and 70–74-year-olds (47.5% in 2006 and 44.5% in 2016; Table 2, Figure 3). Hysterectomy prevalence was highest in non-Hispanic Black women and lowest in Hispanic women across age groups at both time points. Among all groups aged 40 years and older, prevalence decreased from 2006 to 2016; particularly for non-Hispanic Black and Hispanic women aged 50–74 years. In a multivariable-adjusted logistic model combining all years (2006–2016), we observed a significant interaction between race and age-group, although the effect size for the interaction term was small (OR 1.05, 95% CI 1.04–1.06).
Table 2.
Hysterectomy Prevalence (%) by Age Group and Race in the 2006 and 2016 Behavioral Risk Factor Surveillance System (BRFSS)
| 2006 (N=220,302) | 2016 (N=275,631) | |||||||
|---|---|---|---|---|---|---|---|---|
| Age | Total | Non Hispanic White | Non Hispanic Black | Hispanic | Total | Non Hispanic White | Non Hispanic Black | Hispanic |
|
| ||||||||
| 18–24 | 0.7 | 0.7 | 1.0 | 0.6 | 1.2 | 1.2 | 1.5 | 0.9 |
| 25–29 | 2.1 | 2.1 | 2.7 | 1.8 | 2.4 | 2.5 | 3.1 | 1.9 |
| 30–34 | 4.2 | 4.3 | 5.6 | 3.6 | 4.1 | 4.3 | 5.2 | 3.2 |
| 35–39 | 7.5 | 7.6 | 9.7 | 6.4 | 7.1 | 7.6 | 9.1 | 5.7 |
| 40–44 | 13.9 | 13.9 | 17.5 | 11.8 | 13.1 | 13.7 | 16.2 | 10.6 |
| 45–49 | 21.1 | 21.1 | 25.9 | 18.2 | 18.9 | 19.7 | 23.1 | 15.4 |
| 50–54 | 28.1 | 28.1 | 33.8 | 24.5 | 24.7 | 25.4 | 29.3 | 20.1 |
| 55–59 | 34.5 | 34.4 | 40.7 | 30.3 | 29.0 | 29.7 | 34.0 | 23.9 |
| 60–64 | 40.4 | 40.4 | 46.9 | 35.9 | 34.3 | 35.1 | 39.7 | 28.6 |
| 65–69 | 45.0 | 45.0 | 51.7 | 40.4 | 40.4 | 41.0 | 45.9 | 34.0 |
| 70–74 | 47.5 | 47.4 | 54.1 | 42.7 | 44.5 | 45.2 | 50.1 | 38.0 |
| 75–80 | 47.5 | 47.5 | 54.1 | 42.8 | 47.8 | 48.3 | 53.2 | 40.9 |
*Total number (N) corresponds with unweighted, imputed population counts
Determinants of Hysterectomy Prevalence
Multivariable-adjusted associations of determinants of hysterectomy prevalence using aggregate data from the entire period of 2006–2016 are shown in Table 3. We observed a significant association for year, such that for each 2-year increase from 2006 to 2016, the odds of hysterectomy decreased by 2% (OR 0.98, 95% CI, 0.98–0.98). Compared to non-Hispanic White women, non-Hispanic Black women had significantly increased odds of hysterectomy (OR 1.10, 95% CI, 1.07–1.13) whereas Hispanic women had significantly decreased odds (OR 0.73, 95% CI 0.70–0.76). Compared to women living in the Midwest, women living in the South and Territories had significantly greater odds of hysterectomy (OR 1.36, 95% CI 1.34–1.39 and OR 1.27, 95% CI 1.20–1.35; respectively) while women living in the Northeast had significantly lower odds (OR 0.67, 95% CI 0.65–0.69). Higher BMI was significantly associated with hysterectomy (OR 1.26, 95% CI 1.25–1.27). Compared to women who did not graduate high school, women who attended or graduated college or technical school were less likely to have a hysterectomy (OR 0.66, 95% CI 0.64–0.69). Current and former smokers had significantly higher odds of having a hysterectomy compared to non-smokers (OR 1.38, 95% CI 1.35–1.41 and OR 1.06, 95% CI, 1.04–1.08, respectively). Insured women (OR 1.26, 95% CI 1.22–1.30) were more likely than uninsured women to have a hysterectomy. Income was not associated with hysterectomy.
Table 3.
Multivariable-Adjusted Associations with Hysterectomy Prevalence in the Behavioral Risk Factor Surveillance System (BRFSS)
| Total OR (95% CI) | Uninsured OR (95% CI) | Insured OR (95% CI) | |
|---|---|---|---|
| Year | 0.98 (0.98–0.98) | 0.97 (0.96–0.98) | 0.98 (0.98–0.98) |
| Age Group | 1.40 (1.39–1.40) | 1.50 (1.48–1.52) | 1.39 (1.38–1.39) |
| Race | |||
| NH White | 1.00 | 1.00 | 1.00 |
| NH Black | 1.10 (1.07–1.13) | 1.03 (0.95–1.12) | 1.1 (1.07–1.14) |
| Hispanic | 0.73 (0.7–0.76) | 0.66 (0.6–0.73) | 0.75 (0.72–0.78) |
| Asian/Pacific Islander | 0.56 (0.5–0.62) | 0.56 (0.37–0.83) | 0.56 (0.5–0.62) |
| Other | 1.07 (1.02–1.12) | 1.07 (0.92–1.24) | 1.06 (1.01–1.11) |
| BMI (kg/m2) | 1.26 (1.25–1.27) | 1.21 (1.17–1.25) | 1.26 (1.25–1.27) |
| Smoking Status | |||
| Current | 1.38 (1.35–1.41) | 1.41 (1.31–1.51) | 1.37 (1.34–1.4) |
| Former | 1.06 (1.04–1.08) | 1.17 (1.08–1.28) | 1.06 (1.04–1.08) |
| Never | 1.00 | 1.00 | 1.00 |
| Insurance | |||
| Do Not Have | 1.00 | - | - |
| Have | 1.26 (1.22–1.3) | - | - |
| Income | |||
| <$50,000 | 1.00 | 1.00 | 1.00 |
| >$50,000 | 1.00 (0.98–1.02) | 0.92 (0.84–1.01) | 1.00 (0.98–1.02) |
| Education | |||
| Did not graduate high school | 1.00 | 1.00 | 1.00 |
| Graduated high school | 0.99 (0.96–1.02) | 1.00 (0.92–1.1) | 0.98 (0.95–1.01) |
| Attended college or technical school | 0.96 (0.93–0.99) | 1.02 (0.93–1.12) | 0.95 (0.91–0.98) |
| Graduated college or technical school | 0.66 (0.64–0.69) | 0.74 (0.66–0.84) | 0.65 (0.63–0.68) |
| US Region | |||
| Northeast | 0.67 (0.65–0.69) | 0.65 (0.58–0.73) | 0.67 (0.65–0.69) |
| Midwest | 1.00 | 1.00 | 1.00 |
| South | 1.36 (1.34–1.39) | 1.25 (1.16–1.35) | 1.37 (1.35–1.4) |
| West | 1.06 (1.03–1.08) | 0.96 (0.87–1.06) | 1.06 (1.04–1.09) |
| Territories | 1.27 (1.2–1.35) | 0.98 (0.73–1.32) | 1.25 (1.17–1.33) |
*OR (95% CI): odds ratio (95% confidence interval)
*Adjusted for all covariates shown
Similar results were observed in models stratified by insurance (Table 3), except for non-Hispanic Black race which was only significantly associated with hysterectomy (vs. non-Hispanic White) among insured women (OR 1.10, 95% CI 1.07–1.14).
Comment
Principal Findings
In this study, we analyzed hysterectomy prevalence over 10 years using nationally representative BRFSS survey data from 2006 to 2016. While the prevalence of hysterectomy appeared stable from 2010 to 2016 in the overall population, prevalence decreased among women 40 to 74 years of age, especially in non-Hispanic Black and Hispanic women aged 50 years and older. These age-specific differences suggest that hysterectomy prevalence has decreased over time, particularly in postmenopausal women, and the apparent stable overall estimates are likely due to an aging population. Several factors were strongly associated with increased odds of hysterectomy, including age, smoking, Black race, having insurance, and living in the South.
Results in the Context of What is Known
There have been no recent U.S. population-based studies evaluating hysterectomy prevalence over time. Prior studies based on claims data have shown declines in utilization of inpatient hysterectomy, which is becoming less common in favor of minimally invasive outpatient hysterectomy techniques5. However, because national claims data do not capture outpatient procedures, these studies may underestimate the true prevalence of hysterectomy procedures in the U.S., particularly to the extent that utilization of these procedures varies by patient demographic and clinical characteristics2,3,7,15,16,33–37. In 1987, the National Center for Health Statistics reported a hysterectomy prevalence of 37% among women aged 55–59 years32. This estimate is the most cited hysterectomy prevalence statistic in the current literature. In comparison to these historical data, we observed a slightly lower hysterectomy prevalence of 29% in women aged 55–59 years in 2016, and a higher prevalence among women over 65 years (40.4–47.8% in 2016 vs. 33.4–43.2% in 1987), likely explained by an aging population. Our study therefore provides an updated analysis of hysterectomy prevalence in the U.S. population, capturing self-reported hysterectomy, which encompasses both in- and outpatient procedures.
Few recent studies have evaluated predictors of hysterectomy prevalence in a nationally representative population. Our multivariable analysis revealed several significant associations with hysterectomy prevalence, including age, current smoking, geographic region, BMI, race and/or ethnicity, and insurance status. In line with earlier studies2,33,35, women living in the Southern U.S. were more likely to undergo hysterectomy compared to women living in other regions, whereas women living in the Northeast were least likely. Importantly, these regional differences persisted even after accounting for potential explanatory factors such as race and ethnicity, income, and education. It is not clear why the prevalence of hysterectomy varies widely by region. Some studies have suggested that non-clinical factors such as the regional cultural values, patient-physician relationship, and provider-preference may influence regional variability2,8,33,38,39.
Consistent with our unadjusted age-stratified analysis, which revealed higher prevalence in non-Hispanic Black women, results from our fully adjusted model suggest that the odds of hysterectomy are 10% higher in non-Hispanic Black women compared to White women. In analyses stratified by insurance status, the effect of non-Hispanic Black race was only significant among those who were insured; however, the lack of significance among underinsured individuals could be due to lack of power (i.e., much fewer individuals in the uninsured strata). Some studies have shown stronger associations between Black race and hysterectomy; our findings may be different from those of previous studies due to differences in study period, analytic approach, study populations (e.g., age range of participants, region) and availability of covariate data1,43. For example, a study restricted to women under 45 years of age reported much stronger positive associations of Black race with hysterectomy (OR of 3.7; unweighted) compared to White women.14. It has been suggested that non-Hispanic Black women may undergo hysterectomy at younger ages than non-Hispanic White women21, which may emphasize racial and/or ethnic differences in studies of younger populations.
Hispanic women were consistently less likely to have hysterectomy than non-Hispanic White women in all analyses. There is limited literature regarding hysterectomy prevalence and predictors among Hispanic women, and much of it is conflicting. Our findings of decreased odds of hysterectomy in Hispanic women align with some studies’ findings19,22,44, but not all18. It has been suggested that level of acculturation, the primary language spoken, and other cultural and psychosocial factors may contribute to lower hysterectomy prevalence among Hispanic women18,39,45,46. As the Hispanic population continues to grow in the U.S., future studies will be important to clarify these differences.
Clinical Implications
Hysterectomy is the most common non-obstetric surgical procedure performed on women in the U.S.; however, the extent to which clinical practice has changed over time is unclear since the majority of information regarding hysterectomy prevalence comes from studies conducted within inpatient settings. Our findings regarding the regional variation of hysterectomy prevalence even after controlling for patient characteristics suggests that perhaps other provider, institutional, or geographic factors may be influencing these associations. More research is needed to better understand how geographic differences, patient-provider relationships, and provider-preferences affect the clinical practice of this procedure within different settings.
Research Implications
Accounting for hysterectomy prevalence is important when estimating gynecologic cancer rates, particularly when reporting trends over time and making comparisons across race and/or ethnicity and by U.S. regions24,29. Hysterectomy prevalence estimates are also crucial for the development of risk prediction models for gynecologic cancers29. We observed that some risk factors for cervical and endometrial cancer, such as obesity, are also associated with hysterectomy. Obesity is also a known risk factor for uterine fibroids and for other non-malignant uterine diseases8,47–49. Our data suggest that women undergoing hysterectomy are more likely to have obesity, and thus would have had an elevated risk of endometrial and cervical cancer had they not had a hysterectomy. This has important implications for the estimation of population-based hysterectomy-corrected gynecologic cancer rates, which involves correcting the population so that women with hysterectomy are removed from the population at-risk (i.e., the denominator). For example, if hysterectomy prevalence declines over time or within certain groups, it may lead to apparent increases in endometrial and/or cervical cancer incidence, as more women would remain in the population at-risk. Results from our study provide an important reference for investigators conducting such epidemiologic studies, as there are no publicly available resources for accessing these data, and accurate estimation requires computationally intensive methods.
Strengths and Limitations
Major strengths of our study include a large, nationally representative sample of women and use of multiple imputation methods to account for missing data. Additionally, while BRFSS does not collect data on surgical methods or settings, it is likely that self-reported hysterectomy status encompasses a more complete picture of hysterectomy prevalence compared to those limited to specific settings or surgical routes. However, some limitations of our analysis should be considered. First, the median response rate of BRFSS is approximately 47% (ranging, 31%−65%, 2006–2016)30. Second, hysterectomy prevalence was obtained via self-report; however, the validity of self-reported data is high, with 99% accuracy compared to ultrasound-confirmed hysterectomy14. Third, the BRFSS does not collect clinical indication for hysterectomy and healthcare system-level factors that could account for differences observed by race and ethnicity and geographic region. BRFSS additionally does not collect data on incident hysterectomy; therefore, this analysis is focused on risk factors associated with prevalent hysterectomy. Fourth, although we observed strong regional differences, analyses of multi-state regions may mask other contributing factors and trends only seen on a smaller scale (e.g., county or census tract level). Expansion of publicly available county and sub-county data would enable future studies to elucidate factors that may underlie regional differences. Finally, small, albeit significant, effect sizes reported in our study should be interpreted with caution and could be due to unmeasured confounding.
Conclusions
Our study suggests that hysterectomy prevalence in the U.S. has decreased among women aged 40 years and older from 2006 to 2016, particularly among non-Hispanic Black and Hispanic women, but that overall prevalence has remained stable in an aging population. Consistent with previous studies, non-Hispanic Black women were more likely to have a hysterectomy compared to non-Hispanic White women. The prevalence of hysterectomy was particularly high in the South, even after accounting for individual-level characteristics. Associations of gynecologic cancer risk factors with hysterectomy prevalence in our study, including obesity, suggest that correction for hysterectomy prevalence may remove women at high risk for cervical and endometrial cancers from the population at-risk, which could have important implications for estimates of cancer rates if hysterectomy prevalence changes over time or when comparing risks across populations that vary widely in hysterectomy prevalence.
Supplementary Material
Supplement Figure 1. Hysterectomy Prevalence in the Behavioral Risk Factor Surveillance System (BRFSS), 2006–2016, by United States Region. Unadjusted prevalence of self-reported hysterectomy with 95% confidence intervals among U.S. women aged 18 to 80 years in the Behavioral Risk Factor Surveillance System every two years from 2006 to 2016. Prevalences are shown in percentages and are displayed separately among women living in the U.S. Northeast (red circle), Midwest (blue circle ), South (green circle), and West (purple circle) regions, and the U.S. Territories (orange circle).
AJOG at a Glance:
A. Why was this study conducted?
Hysterectomy is the most common non-obstetric medical procedure performed in U.S. women.
The existing body of research is not nationally representative and does not reflect recent surgical trends.
Evaluating nationally representative hysterectomy prevalence trends and determinants is important for estimating gynecologic cancer rates and management of uterine conditions.
B. What were the main findings?
Between 2010 and 2016 hysterectomy prevalence in the U.S. decreased among most age and race and/or ethnic strata.
Age, non-Hispanic Black race, smoking, and living in the South were associated with increased odds of hysterectomy.
C. What does this study add to what is already known?
This study provides more recent nationally representative trends and predictors of hysterectomy in the U.S.
This study provides basis for a better understanding of estimating gynecologic cancer risk among various demographics, as well as accurate adjustment for hysterectomy when estimating gynecologic cancer rates.
Source of Funding
This work was supported by the Intramural Research Program of the US National Cancer Institute, National Institutes of Health, Department of Health and Human Services.
Footnotes
Conflicts of Interest
The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Keshavarz H, Hillis S, Kieke BA, Marchbanks PA. Hysterectomy surveillance – United States, 1994–1999. MMWR Surveill Summ. 2002;51:1–8. [PubMed] [Google Scholar]
- 2.Wu JM, Wechter ME, Geller EJ, Nguyen TV, Visco AG. Hysterectomy Rates in the United States, 2003. Obstetrics & Gynecology. 2007;110(5):1091–1095. doi: 10.1097/01.AOG.0000285997.38553.4b [DOI] [PubMed] [Google Scholar]
- 3.Cohen SL, Vitonis AF, Einarsson JI. Updated Hysterectomy Surveillance and Factors Associated With Minimally Invasive Hysterectomy. JSLS. 2014;18(3):e2014.00096. doi: 10.4293/JSLS.2014.00096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wright JD, Herzog TJ, Tsui J, et al. Nationwide Trends in the Performance of Inpatient Hysterectomy in the United States. Obstetrics & Gynecology. 2013;122(2):233–241. doi: 10.1097/AOG.0b013e318299a6cf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morgan DM, Kamdar NS, Swenson CW, Kobernik EK, Sammarco AG, Nallamothu B. Nationwide trends in the utilization of and payments for hysterectomy in the United States among commercially insured women. American Journal of Obstetrics and Gynecology. 2018;218(4):425.e1–425.e18. doi: 10.1016/j.ajog.2017.12.218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen SL, Ajao MO, Clark NV, Vitonis AF, Einarsson JI. Outpatient Hysterectomy Volume in the United States. Obstetrics & Gynecology. 2017;130(1):130–137. doi: 10.1097/AOG.0000000000002103 [DOI] [PubMed] [Google Scholar]
- 7.Robinson WR, Cheng MM, Howard AG, Carpenter WR, Brewster WR, Doll KM. For U.S. Black women, shift of hysterectomy to outpatient settings may have lagged behind White women: a claims-based analysis, 2011–2013. BMC Health Serv Res. 2017;17(1):526. doi: 10.1186/s12913-017-2471-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacoby VL, Fujimoto VY, Giudice LC, Kuppermann M, Washington AE. Racial and ethnic disparities in benign gynecologic conditions and associated surgeries. American Journal of Obstetrics and Gynecology. 2010;202(6):514–521. doi: 10.1016/j.ajog.2010.02.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehta A, Xu T, Hutfless S, et al. Patient, surgeon, and hospital disparities associated with benign hysterectomy approach and perioperative complications. American Journal of Obstetrics and Gynecology. 2017;216(5):497.e1–497.e10. doi: 10.1016/j.ajog.2016.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Callegari LS, Katon JG, Gray KE, et al. Associations between Race/Ethnicity, Uterine Fibroids, and Minimally Invasive Hysterectomy in the VA Healthcare System. Women’s Health Issues. 2019;29(1):48–55. doi: 10.1016/j.whi.2018.08.005 [DOI] [PubMed] [Google Scholar]
- 11.Abenhaim HA, Azziz R, Hu J, Bartolucci A, Tulandi T. Socioeconomic and Racial Predictors of Undergoing Laparoscopic Hysterectomy for Selected Benign Diseases: Analysis of 341487 Hysterectomies. Journal of Minimally Invasive Gynecology. 2008;15(1):11–15. doi: 10.1016/j.jmig.2007.07.014 [DOI] [PubMed] [Google Scholar]
- 12.Jacoby VL, Autry A, Jacobson G, Domush R, Nakagawa S, Jacoby A. Nationwide Use of Laparoscopic Hysterectomy Compared With Abdominal and Vaginal Approaches. Obstetrics & Gynecology. 2009;114(5):1041–1048. doi: 10.1097/AOG.0b013e3181b9d222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel PR, Lee J, Rodriguez AM, et al. Disparities in Use of Laparoscopic Hysterectomies: A Nationwide Analysis. Journal of Minimally Invasive Gynecology. 2014;21(2):223–227. doi: 10.1016/j.jmig.2013.08.709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bower JK, Schreiner PJ, Sternfeld B, Lewis CE. Black–White Differences in Hysterectomy Prevalence: The CARDIA Study. Am J Public Health. 2009;99(2):300–307. doi: 10.2105/AJPH.2008.133702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kjerulff K, Guzinski GM, Langenberg PW, Stolley PD, Adler Moye NE, Kazandjian VA. Hysterectomy and race. Obstetrics and Gynecology. 1993;82(5):757–764. [PubMed] [Google Scholar]
- 16.Lonky NM, Mohan Y, Chiu VY, et al. Hysterectomy for benign conditions: Complications relative to surgical approach and other variables that lead to post-operative readmission within 90 days of surgery. Womens Health (Lond Engl). 2017;13(2):17–26. doi: 10.1177/1745505717714657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merrill RM. Impact of Hysterectomy and Bilateral Oophorectomy on Race-Specific Rates of Corpus, Cervical, and Ovarian Cancers in the United States. Annals of Epidemiology. 2006;16(12):880–887. doi: 10.1016/j.annepidem.2006.06.001 [DOI] [PubMed] [Google Scholar]
- 18.Powell LH, Meyer P, Weiss G, et al. Ethnic differences in past hysterectomy for benign conditions. Women’s Health Issues. 2005;15(4):179–186. doi: 10.1016/j.whi.2005.05.002 [DOI] [PubMed] [Google Scholar]
- 19.Sherman ME, Carreon JD, Lacey JV, Devesa SS. Impact of Hysterectomy on Endometrial Carcinoma Rates in the United States. JNCI: Journal of the National Cancer Institute. 2005;97(22):1700–1702. doi: 10.1093/jnci/dji378 [DOI] [PubMed] [Google Scholar]
- 20.Day Baird D, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: Ultrasound evidence. American Journal of Obstetrics and Gynecology. 2003;188(1):100–107. doi: 10.1067/mob.2003.99 [DOI] [PubMed] [Google Scholar]
- 21.Kjerulff KH, Langenberg P, Seidman JD, Stolley PD, Guzinski GM. Uterine leiomyomas. Racial differences in severity, symptoms and age at diagnosis. J Reprod Med. 1996;41(7):483–490. [PubMed] [Google Scholar]
- 22.Siegel RL, Devesa SS, Cokkinides V, Ma J, Jemal A. State-Level Uterine Corpus Cancer Incidence Rates Corrected for Hysterectomy Prevalence, 2004 to 2008. Cancer Epidemiol Biomarkers Prev. 2013;22(1):25–31. doi: 10.1158/1055-9965.EPI-12-0991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whiteman MK, Hillis SD, Jamieson DJ, et al. Inpatient hysterectomy surveillance in the United States, 2000–2004. American Journal of Obstetrics and Gynecology. 2008;198(1):34.e1–34.e7. doi: 10.1016/j.ajog.2007.05.039 [DOI] [PubMed] [Google Scholar]
- 24.Clarke MA, Devesa SS, Harvey SV, Wentzensen N. Hysterectomy-Corrected Uterine Corpus Cancer Incidence Trends and Differences in Relative Survival Reveal Racial Disparities and Rising Rates of Nonendometrioid Cancers. JCO. 2019;37(22):1895–1908. doi: 10.1200/JCO.19.00151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hammer A, Rositch AF, Kahlert J, Gravitt PE, Blaakaer J, Søgaard M. Global epidemiology of hysterectomy: possible impact on gynecological cancer rates. American Journal of Obstetrics and Gynecology. 2015;213(1):23–29. doi: 10.1016/j.ajog.2015.02.019 [DOI] [PubMed] [Google Scholar]
- 26.Jamison PM, Noone AM, Ries LAG, Lee NC, Edwards BK. Trends in Endometrial Cancer Incidence by Race and Histology with a Correction for the Prevalence of Hysterectomy, SEER 1992 to 2008. Cancer Epidemiol Biomarkers Prev. 2013;22(2):233–241. doi: 10.1158/1055-9965.EPI-12-0996 [DOI] [PubMed] [Google Scholar]
- 27.Rositch AF, Nowak RG, Gravitt PE. Increased age and race-specific incidence of cervical cancer after correction for hysterectomy prevalence in the United States from 2000 to 2009: Hysterectomy-Corrected Cervical Cancer Incidence. Cancer. 2014;120(13):2032–2038. doi: 10.1002/cncr.28548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Temkin SM, Minasian L, Noone AM. The End of the Hysterectomy Epidemic and Endometrial Cancer Incidence: What Are the Unintended Consequences of Declining Hysterectomy Rates? Front Oncol. 2016;6. doi: 10.3389/fonc.2016.00089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pfeiffer RM, Park Y, Kreimer AR, et al. Risk Prediction for Breast, Endometrial, and Ovarian Cancer in White Women Aged 50 y or Older: Derivation and Validation from Population-Based Cohort Studies. Franco EL, ed. PLoS Med. 2013;10(7):e1001492. doi: 10.1371/journal.pmed.1001492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Center for Disease Control and Prevention (CDC). Behavioral Risk Factor Surveillance System Survey Data. Published August 31, 2020. Accessed May 24, 2021. https://www.cdc.gov/brfss/index.html
- 31.Raghunathan T, Lepkowski J, Hoewyk J, Solenberger P. A Multivariate Technique for Multiply Imputing Missing Values Using a Sequence of Regression Models. Survey Methodology. 2000;27. [Google Scholar]
- 32.Vital and Health Statistics; Series 13, No. 92 (12/87). :39.
- 33.Dicker RC. Hysterectomy Among Women of Reproductive Age: Trends in the United States, 1970–1978. JAMA. 1982;248(3):323. doi: 10.1001/jama.1982.03330030029020 [DOI] [PubMed] [Google Scholar]
- 34.Pollack LM, Olsen MA, Gehlert SJ, Chang SH, Lowder JL. Racial/Ethnic Disparities/Differences in Hysterectomy Route in Women Likely Eligible for Minimally Invasive Surgery. Journal of Minimally Invasive Gynecology. 2020;27(5):1167–1177.e2. doi: 10.1016/j.jmig.2019.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walker AM, Jick H. TEMPORAL AND REGIONAL VARIATION IN HYSTERECTOMY RATES IN THE UNITED STATES, 1970–1975. American Journal of Epidemiology. 1979;110(1):41–46. doi: 10.1093/oxfordjournals.aje.a112785 [DOI] [PubMed] [Google Scholar]
- 36.Carlson KJ. Outcomes of Hysterectomy: Clinical Obstetrics and Gynecology. 1997;40(4):939–946. doi: 10.1097/00003081-199712000-00029 [DOI] [PubMed] [Google Scholar]
- 37.Lone F Evidence Based Review of Hysterectomy and Sexuality. In: Alkatout I, Mettler L, eds. Hysterectomy. Springer International Publishing; 2018:133–138. doi: 10.1007/978-3-319-22497-8_9 [DOI] [Google Scholar]
- 38.Geller SE, Burns LR, Brailer DJ. The impact of nonclinical factors on practice variations: the case of hysterectomies. Health Serv Res. 1996;30(6):729–750. [PMC free article] [PubMed] [Google Scholar]
- 39.Lewis CE, Groff JY, Herman CJ, McKeown RE, Wilcox LS. Overview of Women’s Decision Making Regarding Elective Hysterectomy, Oophorectomy, and Hormone Replacement Therapy. Journal of Women’s Health & Gender-Based Medicine. 2000;9(supplement 2):5–14. doi: 10.1089/152460900318722 [DOI] [PubMed] [Google Scholar]
- 40.Gaskin DJ, Dinwiddie GY, Chan KS, McCleary RR. Residential Segregation and the Availability of Primary Care Physicians. Health Serv Res. 2012;47(6):2353–2376. doi: 10.1111/j.1475-6773.2012.01417.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gaskin DJ, Dinwiddie GY, Chan KS, McCleary R. Residential Segregation and Disparities in Healthcare Services Utilization. Med Care Res Rev. 2012;69(2):158–175. doi: 10.1177/1077558711420263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohen RA, Terlizzi EP, Cha AE, Martinez ME. Health Insurance Coverage: Early Release of Estimates From the National Health Interview Survey, January–June 2020. National Center for Health Statistics (U.S.), ed. National Health Interview Survey (NHIS). Published online January 29, 2021. https://stacks.cdc.gov/view/cdc/100469 [Google Scholar]
- 43.Kjerulff K, Langenberg P, Guzinski G. The socioeconomic correlates of hysterectomies in the United States. Am J Public Health. 1993;83(1):106–108. doi: 10.2105/AJPH.83.1.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brett KM, Higgins JA. Hysterectomy Prevalence by Hispanic Ethnicity: Evidence From a National Survey. Am J Public Health. 2003;93(2):307–312. doi: 10.2105/AJPH.93.2.307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hautaniemi SI, Leidy Sievert L. Risk factors for hysterectomy among Mexican-American women in the U.S. southwest. Am J Hum Biol. 2003;15(1):38–47. doi: 10.1002/ajhb.10110. [DOI] [PubMed] [Google Scholar]
- 46.Tortolero-Luna G, Byrd T, Groff JY, Linares AC, Mullen PD, Cantor SB. Relationship between English Language Use and Preferences for Involvement in Medical Care among Hispanic Women. Journal of Women’s Health. 2006;15(6):774–785. doi: 10.1089/jwh.2006.15.774 [DOI] [PubMed] [Google Scholar]
- 47.Clarke MA, Fetterman B, Cheung LC, et al. Epidemiologic Evidence That Excess Body Weight Increases Risk of Cervical Cancer by Decreased Detection of Precancer. JCO. 2018;36(12):1184–1191. doi: 10.1200/JCO.2017.75.3442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Onstad MA, Schmandt RE, Lu KH. Addressing the Role of Obesity in Endometrial Cancer Risk, Prevention, and Treatment. JCO. 2016;34(35):4225–4230. doi: 10.1200/JCO.2016.69.4638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Setiawan VW, Yang HP, Pike MC, et al. Type I and II Endometrial Cancers: Have They Different Risk Factors? JCO. 2013;31(20):2607–2618. doi: 10.1200/JCO.2012.48.2596 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement Figure 1. Hysterectomy Prevalence in the Behavioral Risk Factor Surveillance System (BRFSS), 2006–2016, by United States Region. Unadjusted prevalence of self-reported hysterectomy with 95% confidence intervals among U.S. women aged 18 to 80 years in the Behavioral Risk Factor Surveillance System every two years from 2006 to 2016. Prevalences are shown in percentages and are displayed separately among women living in the U.S. Northeast (red circle), Midwest (blue circle ), South (green circle), and West (purple circle) regions, and the U.S. Territories (orange circle).


