Abstract
TolQ, TolR, and TolA inner membrane proteins of Escherichia coli are involved in maintaining the stability of the outer membrane. They share homology with the ExbB, ExbD, and TonB proteins, respectively. The last is involved in energy transduction between the inner and the outer membrane, and its conformation has been shown to depend on the presence of the proton motive force (PMF), ExbB, and ExbD. Using limited proteolysis experiments, we investigated whether the conformation of TolA was also affected by the PMF. We found that dissipation of the PMF by uncouplers led to the formation of a proteinase K digestion fragment of TolA not seen when uncouplers are omitted. This fragment was also detected in ΔtolQ, ΔtolR, and tolA(H22P) mutants but, in contrast to the parental strain, was also seen in the absence of uncouplers. We repeated those experiments in outer membrane mutants such as lpp, pal, and Δrfa mutants: the behavior of TolA in lpp mutants was similar to that observed with the parental strain. However, the proteinase K-resistant fragment was never detected in the Δrfa mutant. Altogether, these results suggest that TolA is able to undergo a PMF-dependent change of conformation. This change requires TolQ, TolR, and a functional TolA N-terminal domain. The potential role of this energy-dependent process in the stability of the outer membrane is discussed.
The outer membrane of gram-negative bacteria acts as a permeability barrier protecting the cell against most antimicrobial agents. This is mainly due to the properties of its major components, the lipopolysaccharide (LPS) and the porins (24). Although most of the components of the outer membrane have been well characterized, we still have a limited understanding of how they cross the periplasm, are functionally integrated, and interact in the outer membrane (15).
The tol-pal mutants of Escherichia coli are altered in outer membrane stability, and their characterization may help in understanding some of the steps involved in outer membrane assembly. Homologues of the tol-pal genes have been found in many gram-negative bacteria (16, 25, 37, 48, 50). However, the effect of mutations in these genes has been extensively studied only for E. coli (33, 53), Vibrio cholerae (25), and Pseudomonas putida (37). tol-pal mutations lead to a pleiotropic phenotype including sensitivity to antibacterial agents like bile salts, release of periplasmic content into the external milieu (25, 33, 37, 53), formation of outer membrane vesicles at the cell surface (2, 37), reduced cell motility (37; our unpublished results), and impairment of cell division (39). Although these phenotypes strongly support an essential role of the Tol-Pal system in the maintenance of outer membrane integrity, the exact role of the Tol-Pal proteins in this process is still unknown. The TolQRA proteins are also required for the translocation of the filamentous bacteriophage DNA into the cytoplasm during the process of infection (25, 46, 53) and participate with TolB in the translocation of group A colicins across the cell envelope (6, 7, 23). In addition, some Tol-Pal proteins appear to play a role in bacterial virulence (8, 20).
The tol-pal operon encodes seven proteins, Orf1, TolQ, TolR, TolA, TolB, Pal, and Orf2. No role has been assigned yet to the cytoplasmic Orf1 and the periplasmic Orf2 proteins (49, 52).
TolQ, TolR, and TolA are inner membrane proteins. TolQ has three membrane-spanning fragments (51), and TolA and TolR have an N-terminal anchor, leaving most of the protein exposed to the periplasm (36, 40). In addition to the N-terminal anchoring region, TolA contains a large central domain with a high degree of α-helical content and a C-terminal domain interacting with the N-terminal domain of colicins and the g3p protein of filamentous phages (6, 14, 36, 46). The structure of the periplasmic domains of TolA has been recently established (19), as well as the crystal structure of the C-terminal domain of TolA in complex with the filamentous phage protein g3p (38). The crystal structure of the periplasmic protein TolB has also been solved (1, 11). This protein participates in the uptake of some group A colicins (6, 7). Pal is an outer membrane lipoprotein (34).
TolQ, TolR, and TolA interact with each other, but no ternary complex has been characterized (17, 22, 29). Pal and TolB also form a complex near the outer membrane (5, 13). It has been shown recently that Pal and TolA form a proton motive force (PMF)-dependent complex (12). The C-terminal part of Pal interacts with the C-terminal domain of TolB and the peptidoglycan (4, 45) The central domain of TolA and TolB interacts with porin trimers in vitro in the presence of sodium dodecyl sulfate (SDS) but not with OmpA (18, 47). TolB also interacts with Lpp and OmpA, two major peptidoglycan-associated outer membrane proteins (13).
In gram-negative bacteria, the Tol and Ton systems are involved in the uptake of macromolecules across the envelope. The Ton system is required for the uptake of iron-siderophore complexes and vitamin B12 and the entry of group B colicins and phages like φ80 or T1 (43). The TonB-ExbBD proteins which constitute the Ton system present some similarities to the TolQRA proteins of the Tol system (31). TolQ and TolR are structurally and functionally homologous to ExbB and ExbD, respectively (9, 10). While ExbB and ExbD have been shown previously to form homomultimers (26), only TolR homodimers have been detected so far (29). TolA and TonB are homologous only in their N-terminal transmembrane domains, where they both have a well-conserved S-X(3)-H-X(6)-L-X(3)-S motif (22, 30). Despite both having an elongated conformation, the periplasmic domains of TolA and TonB share no sequence homologies: the central domain of TolA has an α-helical structure (19, 36), whereas that of TonB has a stretch of X-Pro repeats (44).
The TonB-ExbBD complex appears to mediate the energy transfer of the electrochemical potential from the cytoplasmic membrane to the outer membrane (28, 30). TonB is thought to open outer membrane channels via an energy-dependent conformational change, either from the cytoplasmic membrane or by shuttling between the two membranes (32, 35). The importance of the TonB transmembrane anchor in energy transduction was demonstrated elsewhere (30). Replacement of the TonB transmembrane anchor by the TolA corresponding domain produces a TolQ-TolR-dependent chimera with TonB function (30). These data suggest that the physiological function of the TolQ-TolR-TolA complex may be dependent on the PMF (32). The present study was undertaken to test whether TolA was able to undergo an energy-dependent change of conformation in the periplasmic space.
MATERIALS AND METHODS
Strains and plasmids.
Strains were all E. coli K-12 derivatives of JC188 (Hfr P4X, metB lacI pstS). JC188 Δorf1 tolQRA (22), JC188 tolB2(H147D), JC188 pal892, JC188 Δlpp, JC188 lpp916, and JC188 ΔtolB pal were from the lab collection. JC188 pal892 has a stop mutation after Met41 of the mature Pal protein. The lpp and tolB mutations have been described elsewhere (13). JC188 Δrfa carried the Δrfa1 deletion [Δrfa(GPSBI)::Cm] (41). pJEL-1QRA has been described elsewhere (22). pJEL-1QA and pJEL-1RA were pJEL-250 derivatives of pT7-1QA and pT7-1RA, respectively (22). pJEL-1QRA derivatives carrying the tolA (S18L), tolA (H22P), and tolA (H22R) derivatives were constructed from plasmids carrying the tolA mutations (22). pJEL-BP2 contained a NotI-EcoRV 3,310-bp fragment carrying the 3′ end of tolA, tolB, pal, and orf2 cloned into pJEL-250.
Spheroplast formation.
All the experiments were performed at 4°C. Exponentially growing cells were collected by centrifugation for 1 min at 12,000 × g and converted to spheroplasts after resuspension in 10 mM Tris (pH 7.5)–5 mM EDTA–20% (wt/vol) sucrose–0.1 mg of lysozyme/ml (spheroplast medium). Spheroplast formation was monitored by using alkaline phosphatase and β-galactosidase as periplasmic and cytoplasmic markers, respectively (34). Cells were centrifuged for 5 min at 10,000 × g. The supernatant contained the periplasm. The cell pellet was resuspended in 10 mM Tris (pH 7.5)–5 mM EDTA and incubated at −70°C for 5 min and then at 37°C for 5 min in the presence of Benzonase (Merck, Darmstadt, Germany). Cells were centrifuged at 10,000 × g for 30 min. The supernatant contained the cytoplasm, and the pellet was called the total membrane fraction. A correct spheroplast formation was ascertained by the presence of more than 90% alkaline phosphatase in the periplasmic plus extracellular fractions and at least 90% β-galactosidase in the cytoplasm.
Proteinase K accessibility assays.
Exponentially growing cells were treated for 3 min with 50 μM carbonylcyanide m-chlorophenylhydrazone (CCCP) or 1 mM 2,4-dinitrophenol (DNP) directly added to the culture media. Cells were then collected by centrifugation for 1 min at 10,000 rpm and converted to spheroplasts after resuspension in the spheroplast medium supplemented with 50 μM CCCP, 1 mM DNP, or an equal volume of carrier ethanol. Samples were then incubated with 10 μg of proteinase K/ml for 0 or 5 min at 4°C, treated for 2 min with 1 mM phenylmethylsulfonyl fluoride to inactivate proteinase K, and then precipitated by 1 volume of 10% trichloroacetic acid and incubated for 30 min on ice. After centrifugation (10 min at 10,000 × g and 4°C), the pellets were washed with 10 mM Tris (pH 7.5) and resuspended in loading buffer. The equivalent of 3 × 108 cells was loaded on an SDS-polyacrylamide gel (12% acrylamide).
Sample analyses.
Samples were separated by SDS-polyacrylamide gel electrophoresis and subjected to immunodetection as previously described (22). Purified anti-TolA polyclonal antibodies directed against the TolA soluble fraction (central and C-terminal domains) or the TolA C-terminal domain (a gift from Robert Webster) were used. For an optimal resolution, a colored marker was used, and the migration was stopped when the band corresponding to a molecular mass of 36.4 kDa reached the bottom of the gel.
RESULTS AND DISCUSSION
TolA conformation depends on the PMF.
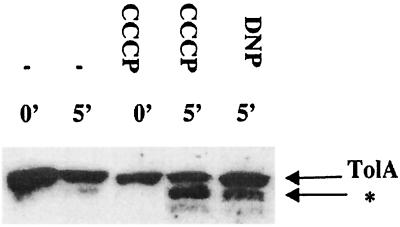
Patterns of limited proteolytic digestion by proteinase K were used to characterize TolA conformation. After spheroplast formation, TolA was progressively degraded (Fig. 1). Dissipation of the PMF by the uncoupler CCCP or DNP led to the presence of a novel proteinase K-resistant product (Fig. 1). This change in the proteolytic pattern indicated that TolA could adopt a new conformation in the absence of the PMF. To determine whether the effect of CCCP was reversible, cells treated with CCCP were washed twice with and resuspended in fresh medium. Alternatively, 1 mM bovine serum albumin, to which CCCP adsorbs, was added to the medium (42). In both conditions, the proteinase K-resistant product was no longer detected, showing that the action of the CCCP could be reversed (data not shown). We propose that TolA is able to achieve a PMF-dependent conformation in the periplasm.
FIG. 1.
Identification of a PMF-responsive TolA conformation. Immunoblots of spheroplasts from JC188 treated or not treated with CCCP or DNP are shown. After digestion by proteinase K for 0 or 5 min, samples were resolved on SDS–12% polyacrylamide gels, transferred to nitrocellulose membranes, and probed with a polyclonal antibody, diluted 1/2,000, raised against the periplasmic domain TolA (residues 42 to 420). The positions of TolA and its proteinase K-resistant form (∗) are indicated in all the figures.
Proteinase K accessibility of TolA in the absence of TolQ or TolR.
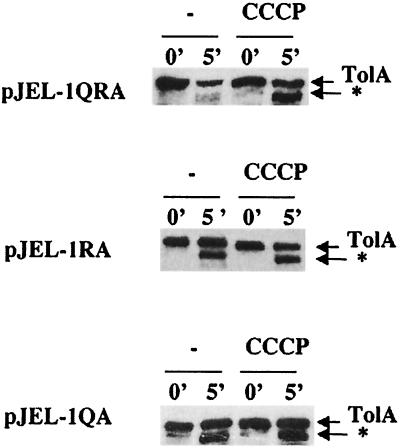
As TolA interacts with TolQ and TolR via its N-terminal domain, the possibility that these proteins play a role in the proteinase accessibility of TolA was investigated. Experiments were done with JC188Δ1QRA derivatives carrying the tolQRA genes cloned on a low-copy-number plasmid. The level of expression of TolA in those strains was similar to that observed for the wild-type JC188 (data not shown). The pattern of TolA digestion in the absence of TolQ or TolR is shown in Fig. 2. The proteinase K-resistant product could be visualized in the tolQ and tolR strains in all the experimental conditions. Thus, TolA conformation depended on TolQ and TolR and was no longer PMF dependent when one of these proteins was absent.
FIG. 2.
The proteinase K-resistant product of TolA is present in ΔtolQ and ΔtolR strains independently of the presence of CCCP. Immunoblots of spheroplasts from strain JC188Δ1QRA transformed with pJEL-1QRA, pJEL-1RA, or pJEL-1QA were probed as for Fig. 1.
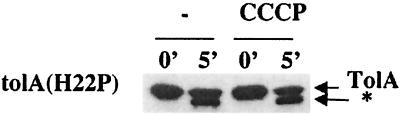
We have previously isolated tolA mutants affected in the N-terminal domain of TolA (22). In these mutants, TolA could not be cross-linked with TolQ. The pattern of proteinase K accessibility of the corresponding TolA altered proteins was similar to that obtained for strains lacking TolQ and TolR. A typical pattern obtained in the presence of the tolA(H22P) mutation is given in Fig. 3. Here again, the pattern of proteinase K digestion was no longer dependent on the presence of CCCP. The same results were obtained with the tolA(S18L), tolA(H22Y), and tolA(H22L) mutations (data not shown).
FIG. 3.
Identification of the proteinase K-resistant product of TolA in a tolA(H22P) derivative. Immunoblots of spheroplasts from strain JC188Δ1QRA transformed with pJEL-1QRA(H22P) treated or not treated with CCCP were probed as for Fig. 1.
It was concluded that TolA conformation was dependent on TolQ, TolR, and its own transmembrane component. When TolQ or TolR was absent, or when residue Ser18 or His22 of TolA was altered, TolA conformation was no longer PMF dependent.
This study provides evidence that the conformation of TolA depends on the PMF, TolQ, TolR, and the transmembrane domain of TolA. Using similar methods, a PMF-dependent conformational change was also previously demonstrated for TonB (32). It should be noted that the pattern of proteinase K digestion of TolA observed for a tolQ or tolR strain and the mutants affected in the TolA transmembrane domain corresponded to that obtained for the wild-type strain after the dissipation of the PMF. In the case of the Ton system, the opposite situation occurs, since the pattern of proteinase K digestion of TonB in an exbB or exbD strain is the same as the one obtained for the wild-type strain in the presence of the PMF (32). In this case, it is possible that the fragment of TonB formed in the wild-type strain in the presence of CCCP is sensitive to the interaction with ExbB and ExbD: in the exbBD strain, in the presence of CCCP, this fragment would be formed but would be further cleaved by proteinase K due to a lack of interaction with ExbB or ExbD.
The PMF-dependent conformation of TonB leads to the active transport of iron and vitamin B12 through interactions with outer membrane receptors. Concerning TolA, the purpose of the conformational change described above remains elusive. By analogy, we propose that TolA is able to generate an energy-dependent change of conformation of some outer membrane components. This is supported by the instability of TolA in a ΔtolB pal background seen in this study (see below) and by the recent description of an energy-dependent interaction between TolA and Pal (12). However, TolA, in contrast to TonB, does not seem to shuttle between the outer and cytoplasmic membranes (22).
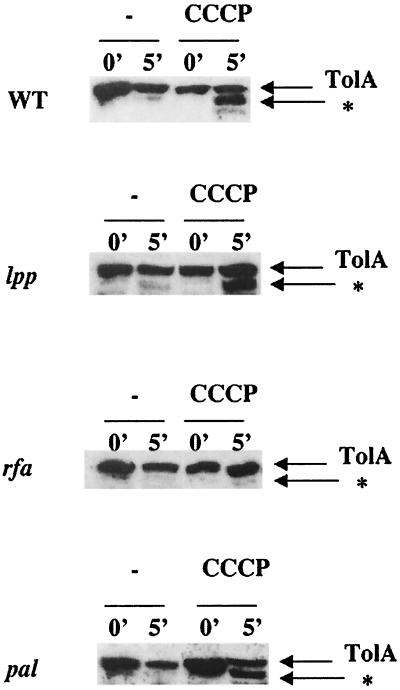
Conformation of TolA in tol, pal, lpp, and rfa mutants.
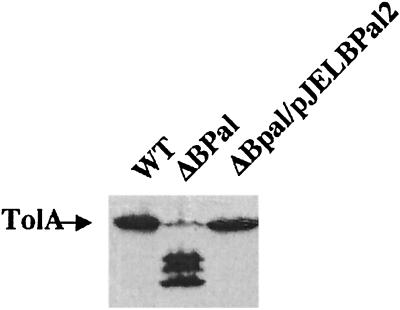
As alteration or deletion of the tolQ, tolR, and tolA genes leads to a change in outer membrane permeability, the possibility that the tol phenotype could be responsible for the presence of the novel proteinase K-resistant product was investigated. We first used tolB or pal point mutations as well as a ΔtolB pal deletion. The pattern of proteinase K digestion of TolA in strains carrying tolB or pal point mutations was the same as the one obtained for the parental strain. A typical pattern obtained with a JC188 strain carrying a pal892 nonsense mutation is given in Fig. 4. We concluded that the alteration of the outer membrane by tolQ, tolR, or tolA mutations is not the cause of the presence of the proteinase K-resistant fragment. During the course of this study, we observed that TolA was highly unstable in exponentially growing cells carrying the ΔtolB pal deletion, since the protein was degraded in total cell extracts without any addition of exogenous proteinase (Fig. 5). This was not the case for tolB or pal mutants. This phenotype was fully complemented by a low-copy-number plasmid carrying the tolB, pal, and orf2 genes. Therefore, the absence of both TolB and Pal leads to the instability of TolA.
FIG. 4.
TolA is highly unstable in a ΔtolB pal background. Strains JC188, JC188 ΔtolB pal, and JC188 ΔtolB pal transformed with pJEL-BP2 were grown to mid-log phase. Immunoblots of intact cells were probed as for Fig. 1. WT, wild type.
FIG. 5.
Accessibility of TolA to proteinase K after spheroplast formation in Δlpp, Δrfa, and pal892 strains. Immunoblots of spheroplasts from strains JC188, JC188 Δlpp, JC188 Δrfa, and JC188 pal892 were probed as for Fig. 1. The amount of cells loaded for JC188 pal892 is twice those for other strains.
It is now well established that the TolQRA proteins are involved in outer membrane stability, but the underlying mechanisms are still unknown. To better understand the participation of the PMF-dependent conformation of TolA in the organization of the outer membrane, the conformation of TolA was characterized for various outer membrane mutants such as rfa and lpp mutants. The pattern of proteinase K digestion of TolA in a strain carrying an Δlpp or an lpp916 nonsense mutation was like that in the wild type (Fig. 4). No proteinase K-resistant band was visible in the Δrfa mutant. Prolonged incubation of the samples in the presence of proteinase K led gradually to a total digestion of TolA. At no time could the proteinase K-dependent band be detected (data not shown). The Δrfa1mutant used in this experiment was able to synthesize only a minimal LPS core composed of 2-keto-3-deoxyoctulosonic acid and one heptose (53).
This raises the possibility that the Tol system may be involved in some step of the biogenesis, assembly, or transport of the bacterial LPS. Evidence already suggests a role of the Tol proteins in porin and/or LPS translocation or assembly (18, 21, 47). However, rfa mutants are highly pleiotropic, and the fact that no proteinase K-resistant fragment of TolA is detected may be due to a variety of things. Clearly, more work is needed to understand this phenomenon. We concluded that the presence of the PMF-dependent TolA fragment was not affected by the presence of many mutations leading to a defect in outer membrane stability, except in rfa strains, where TolA was resistant to proteinase K.
We suspect that the characterization of the physiological role of the Tol system has been difficult because most of the studies have been performed on the laboratory strain E. coli K-12. An extensive physiological analysis of the role of the Tol system in naturally occurring strains should help to elucidate its role; it would be especially important to know whether the Tol proteins are involved in the formation of outer membrane vesicles, which have been suggested elsewhere to play a critical role in bacterial virulence (3, 27).
ACKNOWLEDGMENTS
We thank John Klenna for the Δrfa strain, Robert Webster for some of the TolA antibodies, and Kathleen Postle for helpful suggestions.
This work was supported by the Life Sciences Department of the CNRS and the MENRT. M.-C.R. has an MENRT fellowship.
REFERENCES
- 1.Abergel C, Bouveret E, Claverie J M, Brown K, Rigal A, Lazdunski C, Benedetti H. Structure of the Escherichia coli TolB protein determined by MAD methods at 1.95 A resolution. Struct Fold Des. 1999;7:1291–1300. doi: 10.1016/s0969-2126(00)80062-3. [DOI] [PubMed] [Google Scholar]
- 2.Bernadac A, Gavioli M, Lazzaroni J C, Raina S, Lloubes R. Escherichia coli tol-pal mutants form outer membrane vesicles. J Bacteriol. 1998;180:4872–4878. doi: 10.1128/jb.180.18.4872-4878.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beveridge T J. Structures of gram-negative cell walls and their derived membrane vesicles. J Bacteriol. 1999;181:4725–4733. doi: 10.1128/jb.181.16.4725-4733.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouveret E, Benedetti H, Rigal A, Loret E, Lazdunski C. In vitro characterization of peptidoglycan-associated lipoprotein (PAL)-peptidoglycan and PAL-TolB interactions. J Bacteriol. 1999;181:6306–6311. doi: 10.1128/jb.181.20.6306-6311.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouveret E, Derouiche R, Rigal A, Lloubes R, Lazdunski C, Benedetti H. Peptidoglycan-associated lipoprotein-TolB interaction. A possible key to explaining the formation of contact sites between the inner and outer membranes of Escherichia coli. J Biol Chem. 1995;270:11071–11077. doi: 10.1074/jbc.270.19.11071. [DOI] [PubMed] [Google Scholar]
- 6.Bouveret E, Rigal A, Lazdunski C, Benedetti H. Distinct regions of the colicin A translocation domain are involved in the interaction with TolA and TolB proteins upon import into Escherichia coli. Mol Microbiol. 1998;27:143–157. doi: 10.1046/j.1365-2958.1998.00667.x. [DOI] [PubMed] [Google Scholar]
- 7.Bouveret E, Rigal A, Lazdunski C, Benedetti H. The N-terminal domain of colicin E3 interacts with TolB which is involved in the colicin translocation step. Mol Microbiol. 1997;23:909–920. doi: 10.1046/j.1365-2958.1997.2751640.x. [DOI] [PubMed] [Google Scholar]
- 8.Bowe F, Lipps C J, Tsolis R M, Groisman E, Heffron F, Kusters J G. At least four percent of the Salmonella typhimurium genome is required for fatal infection of mice. Infect Immun. 1998;66:3372–3377. doi: 10.1128/iai.66.7.3372-3377.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braun V. The structurally related exbB and tolQ genes are interchangeable in conferring tonB-dependent colicin, bacteriophage, and albomycin sensitivity. J Bacteriol. 1989;171:6387–6390. doi: 10.1128/jb.171.11.6387-6390.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braun V, Herrmann C. Evolutionary relationship of uptake systems for biopolymers in Escherichia coli: cross-complementation between the TonB-ExbB-ExbD and the TolA-TolQ-TolR proteins. Mol Microbiol. 1993;8:261–268. doi: 10.1111/j.1365-2958.1993.tb01570.x. [DOI] [PubMed] [Google Scholar]
- 11.Carr S, Penfold C N, Bamford V, James R, Hemmings A M. The structure of TolB, an essential component of the tol-dependent translocation system, and its protein-protein interaction with the translocation domain of colicin E9. Struct Fold Des. 2000;8:57–66. doi: 10.1016/s0969-2126(00)00079-4. [DOI] [PubMed] [Google Scholar]
- 12.Cascales E, Gavioli M, Sturgis J N, Lloubes R. Proton motive force drives the interaction of the inner membrane TolA and outer membrane Pal proteins in Escherichia coli. Mol Microbiol. 2000;38:904–915. doi: 10.1046/j.1365-2958.2000.02190.x. [DOI] [PubMed] [Google Scholar]
- 13.Clavel T, Germon P, Vianney A, Portalier R, Lazzaroni J C. TolB protein of Escherichia coli K-12 interacts with the outer membrane peptidoglycan-associated proteins Pal, Lpp and OmpA. Mol Microbiol. 1998;29:359–367. doi: 10.1046/j.1365-2958.1998.00945.x. [DOI] [PubMed] [Google Scholar]
- 14.Click E M, Webster R E. The TolQRA proteins are required for membrane insertion of the major capsid protein of the filamentous phage f1 during infection. J Bacteriol. 1998;180:1723–1728. doi: 10.1128/jb.180.7.1723-1728.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Danese P N, Silhavy T J. Targeting and assembly of periplasmic and outer-membrane proteins in Escherichia coli. Annu Rev Genet. 1998;32:59–94. doi: 10.1146/annurev.genet.32.1.59. [DOI] [PubMed] [Google Scholar]
- 16.Dennis J J, Lafontaine E R, Sokol P A. Identification and characterization of the tolQRA genes of Pseudomonas aeruginosa. J Bacteriol. 1996;178:7059–7068. doi: 10.1128/jb.178.24.7059-7068.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Derouiche R, Benedetti H, Lazzaroni J C, Lazdunski C, Lloubes R. Protein complex within Escherichia coli inner membrane. TolA N-terminal domain interacts with TolQ and TolR proteins. J Biol Chem. 1995;270:11078–11084. doi: 10.1074/jbc.270.19.11078. [DOI] [PubMed] [Google Scholar]
- 18.Derouiche R, Gavioli M, Benedetti H, Prilipov A, Lazdunski C, Lloubes R. TolA central domain interacts with Escherichia coli porins. EMBO J. 1996;15:6408–6415. [PMC free article] [PubMed] [Google Scholar]
- 19.Derouiche R, Lloubes R, Sasso S, Bouteille H, Oughideni R, Lazdunski C, Loret E. Circular dichroism and molecular modeling of the E. coli TolA periplasmic domains. Biospectroscopy. 1999;5:189–198. doi: 10.1002/(SICI)1520-6343(1999)5:3<189::AID-BSPY8>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 20.Fortney K R, Young R S, Bauer M E, Katz B P, Hood A F, Munson R S, Spinola S M. Expression of peptidoglycan-associated lipoprotein is required for virulence in the human model of Haemophilus ducreyi infection. Infect Immun. 2000;68:6441–6448. doi: 10.1128/iai.68.11.6441-6448.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaspar J A, Thomas J A, Marolda C L, Valvano M A. Surface expression of O-specific lipopolysaccharide in Escherichia coli requires the function of the TolA protein. Mol Microbiol. 2000;38:262–275. doi: 10.1046/j.1365-2958.2000.02094.x. [DOI] [PubMed] [Google Scholar]
- 22.Germon P, Clavel T, Vianney A, Portalier R, Lazzaroni J C. Mutational analysis of the Escherichia coli K-12 TolA N-terminal region and characterization of its TolQ-interacting domain by genetic suppression. J Bacteriol. 1998;180:6433–6439. doi: 10.1128/jb.180.24.6433-6439.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gokce I, Raggett E M, Hong Q, Virden R, Cooper A, Lakey J H. The TolA-recognition site of colicin N. ITC, SPR and stopped-flow fluorescence define a crucial 27-residue segment. J Mol Biol. 2000;304:621–632. doi: 10.1006/jmbi.2000.4232. [DOI] [PubMed] [Google Scholar]
- 24.Hancock R E. The bacterial outer membrane as a drug barrier. Trends Microbiol. 1997;5:37–42. doi: 10.1016/S0966-842X(97)81773-8. [DOI] [PubMed] [Google Scholar]
- 25.Heilpern A J, Waldor M K. CTXφ infection of Vibrio cholerae requires the tolQRA gene products. J Bacteriol. 2000;182:1739–1747. doi: 10.1128/jb.182.6.1739-1747.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higgs P I, Myers P S, Postle K. Interactions in the TonB-dependent energy transduction complex: ExbB and ExbD form homomultimers. J Bacteriol. 1998;180:6031–6038. doi: 10.1128/jb.180.22.6031-6038.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horstman A L, Kuehn M J. Enterotoxigenic Escherichia coli secretes active heat-labile enterotoxin via outer membrane vesicles. J Biol Chem. 2000;275:12489–12496. doi: 10.1074/jbc.275.17.12489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jaskula J C, Letain T E, Roof S K, Skare J T, Postle K. Role of the TonB amino terminus in energy transduction between membranes. J Bacteriol. 1994;176:2326–2338. doi: 10.1128/jb.176.8.2326-2338.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Journet L, Rigal A, Lazdunski C, Benedetti H. Role of TolR N-terminal, central, and C-terminal domains in dimerization and interaction with TolA and TolQ. J Bacteriol. 1999;181:4476–4484. doi: 10.1128/jb.181.15.4476-4484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karlsson M, Hannavy K, Higgins C F. A sequence-specific function for the N-terminal signal-like sequence of the TonB protein. Mol Microbiol. 1993;8:379–388. doi: 10.1111/j.1365-2958.1993.tb01581.x. [DOI] [PubMed] [Google Scholar]
- 31.Koebnik R. The molecular interaction between components of the TonB-ExbBD-dependent and of the TolQRA-dependent bacterial uptake systems. Mol Microbiol. 1993;9:219. doi: 10.1111/j.1365-2958.1993.tb01683.x. [DOI] [PubMed] [Google Scholar]
- 32.Larsen R A, Thomas M G, Postle K. Protonmotive force, ExbB and ligand-bound FepA drive conformational changes in TonB. Mol Microbiol. 1999;31:1809–1824. doi: 10.1046/j.1365-2958.1999.01317.x. [DOI] [PubMed] [Google Scholar]
- 33.Lazzaroni J C, Germon P, Ray M C, Vianney A. The Tol proteins of Escherichia coli and their involvement in the uptake of biomolecules and outer membrane stability. FEMS Microbiol Lett. 1999;177:191–197. doi: 10.1111/j.1574-6968.1999.tb13731.x. [DOI] [PubMed] [Google Scholar]
- 34.Lazzaroni J C, Portalier R. The excC gene of Escherichia coli K-12 required for cell envelope integrity encodes the peptidoglycan-associated lipoprotein (PAL) Mol Microbiol. 1992;6:735–742. doi: 10.1111/j.1365-2958.1992.tb01523.x. [DOI] [PubMed] [Google Scholar]
- 35.Letain T E, Postle K. TonB protein appears to transduce energy by shuttling between the cytoplasmic membrane and the outer membrane in Escherichia coli. Mol Microbiol. 1997;24:271–283. doi: 10.1046/j.1365-2958.1997.3331703.x. [DOI] [PubMed] [Google Scholar]
- 36.Levengood S K, Beyer W F, Webster R E. TolA: a membrane protein involved in colicin uptake contains an extended helical region. Proc Natl Acad Sci USA. 1991;88:5939–5943. doi: 10.1073/pnas.88.14.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Llamas M A, Ramos J L, Rodriguez-Herva J J. Mutations in each of the tol genes of Pseudomonas putida reveal that they are critical for maintenance of outer membrane stability. J Bacteriol. 2000;182:4764–4772. doi: 10.1128/jb.182.17.4764-4772.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lubkowski J, Hennecke F, Pluckthun A, Wlodawer A. Filamentous phage infection: crystal structure of g3p in complex with its coreceptor, the C-terminal domain of TolA. Struct Fold Des. 1999;7:711–722. doi: 10.1016/s0969-2126(99)80092-6. [DOI] [PubMed] [Google Scholar]
- 39.Meury J, Devilliers G. Impairment of cell division in tolA mutants of Escherichia coli at low and high medium osmolarities. Biol Cell. 1999;91:67–75. [PubMed] [Google Scholar]
- 40.Muller M M, Vianney A, Lazzaroni J C, Webster R E, Portalier R. Membrane topology of the Escherichia coli TolR protein required for cell envelope integrity. J Bacteriol. 1993;175:6059–6061. doi: 10.1128/jb.175.18.6059-6061.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parker C T, Kloser A W, Schnaitman C A, Stein M A, Gottesman S, Gibson B W. Role of the rfaG and rfaP genes in determining the lipopolysaccharide core structure and cell surface properties of Escherichia coli K-12. J Bacteriol. 1992;174:2525–2538. doi: 10.1128/jb.174.8.2525-2538.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Possot O M, Letellier L, Pugsley A P. Energy requirement for pullulanase secretion by the main terminal branch of the general secretory pathway. Mol Microbiol. 1997;24:457–464. doi: 10.1046/j.1365-2958.1997.3451726.x. [DOI] [PubMed] [Google Scholar]
- 43.Postle K. TonB protein and energy transduction between membranes. J Bioenerg Biomembr. 1993;25:591–601. doi: 10.1007/BF00770246. [DOI] [PubMed] [Google Scholar]
- 44.Postle K, Good R F. DNA sequence of the Escherichia coli tonB gene. Proc Natl Acad Sci USA. 1983;80:5235–5239. doi: 10.1073/pnas.80.17.5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ray M C, Germon P, Vianney A, Portalier R, Lazzaroni J C. Identification by genetic suppression of Escherichia coli TolB residues important for TolB-Pal interaction. J Bacteriol. 2000;182:821–824. doi: 10.1128/jb.182.3.821-824.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Riechmann L, Holliger P. The C-terminal domain of TolA is the coreceptor for filamentous phage infection of E. coli. Cell. 1997;90:351–360. doi: 10.1016/s0092-8674(00)80342-6. [DOI] [PubMed] [Google Scholar]
- 47.Rigal A, Bouveret E, Lloubes R, Lazdunski C, Benedetti H. The TolB protein interacts with the porins of Escherichia coli. J Bacteriol. 1997;179:7274–7279. doi: 10.1128/jb.179.23.7274-7279.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sen K, Sikkema D J, Murphy T F. Isolation and characterization of the Haemophilus influenzae tolQ, tolR, tolA and tolB genes. Gene. 1996;178:75–81. doi: 10.1016/0378-1119(96)00338-1. [DOI] [PubMed] [Google Scholar]
- 49.Sun T P, Webster R E. Nucleotide sequence of a gene cluster involved in entry of E colicins and single-stranded DNA of infecting filamentous bacteriophages into Escherichia coli. J Bacteriol. 1987;169:2667–2674. doi: 10.1128/jb.169.6.2667-2674.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tibor A, Weynants V, Denoel P, Lichtfouse B, De Bolle X, Saman E, Limet J N, Letesson J J. Molecular cloning, nucleotide sequence, and occurrence of a 16.5-kilodalton outer membrane protein of Brucella abortus with similarity to PAL lipoproteins. Infect Immun. 1994;62:3633–3639. doi: 10.1128/iai.62.9.3633-3639.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vianney A, Lewin T M, Beyer W F, Lazzaroni J C, Portalier R, Webster R E. Membrane topology and mutational analysis of the TolQ protein of Escherichia coli required for the uptake of macromolecules and cell envelope integrity. J Bacteriol. 1994;176:822–829. doi: 10.1128/jb.176.3.822-829.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vianney A, Muller M M, Clavel T, Lazzaroni J C, Portalier R, Webster R E. Characterization of the tol-pal region of Escherichia coli K-12: translational control of tolR expression by TolQ and identification of a new open reading frame downstream of pal encoding a periplasmic protein. J Bacteriol. 1996;178:4031–4038. doi: 10.1128/jb.178.14.4031-4038.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Webster R E. The tol gene products and the import of macromolecules into Escherichia coli. Mol Microbiol. 1991;5:1005–1011. doi: 10.1111/j.1365-2958.1991.tb01873.x. [DOI] [PubMed] [Google Scholar]