Abstract
Morphogenesis, the process by which tissues develop into functional shapes, requires coordinated mechanical forces. Most current literature ascribes contractile forces derived from actomyosin networks as the major driver of tissue morphogenesis. Recent works from diverse species have shown that pressure derived from fluids can generate deformations necessary for tissue morphogenesis. In this review, we discuss how hydrostatic pressure is generated at the cellular and tissue level and how the pressure can cause deformations. We highlight and review findings demonstrating the mechanical roles of pressures from fluid-filled lumens and viscous gel-like components of the extracellular matrix. We also emphasise the interactions and mechanochemical feedbacks between extracellular pressures and tissue behaviour in driving tissue remodelling. Lastly, we offer perspectives on the open questions in the field that will further our understanding to uncover new principles of tissue organisation during development.
Keywords: Hydrostatic pressure, epithelial lumen, hyaluronan, tissue morphogenesis, mechanochemical feedback, epithelial fluid transport
1. Introduction
Water is fundamental to life and is the most abundant molecule in our bodies. Active fluxes of water and ions in cells create osmotic and hydrostatic pressure gradients across cell membranes and epithelia. Mechano-hydraulic coupling allows steady balancing of hydrostatic pressure and is essential for regulating cell shape, volume, motility, and behaviour. At the tissue level, water can be actively confined in extracellular and intercellular spaces such as in epithelial lumens, thereby generating hydrostatic pressure. Most studies on developmental processes describe how tissue morphogenesis is driven by intracellular forces generated from cytoskeletal networks–F-actin, microtubules—and molecular motors[1–5]. Recent work from several tissues across species has demonstrated the role of hydrostatic pressure in applying sufficient mechanical forces to drive behaviours such as growth, deformations, size control, and cell fate decisions[6–14].
In the following sections, we review recent findings that describe how hydrostatic pressure, derived from the incompressible nature of water, is generated at cellular and tissue scales. We highlight our current knowledge of how fluid movement occurs across cells and tissues. We then discuss how hydrostatic pressure is harnessed for driving and regulating developmental processes such as tissue growth, morphogenesis, and patterning. We also describe examples invoking feedback interactions between tissue hydraulics and mechanics providing an integrative framework for tissue morphogenesis. We conclude with our perspectives on the emerging questions in the field.
2. The nuts and bolts of pressure generation
Biological cells can be simplistically perceived as a water-based soup of solutes—ions, proteins, nucleic acids, and other small and macromolecules—enclosed by a semipermeable plasma membrane. At all times, there is an exchange of solutes through the plasma membrane via active (energy-dependent) or passive (non-energy-dependent) transport, for example actively maintaining Na+ and K+ cytosolic concentration via Na+/K+ ATPase[15]. Any imbalance of solutes generates a pressure that leads to the flow of water molecules from lower solute to higher solute concentrations across the cell membrane[16–19] (Fig. 1). Utilising solute concentration imbalances to trigger the movement of water can also happen at tissue scales, for example during lumen formation by epithelial tissues (described in detail in later sections).
Fig. 1: Schematic representation for generation of osmotic and hydrostatic pressure.
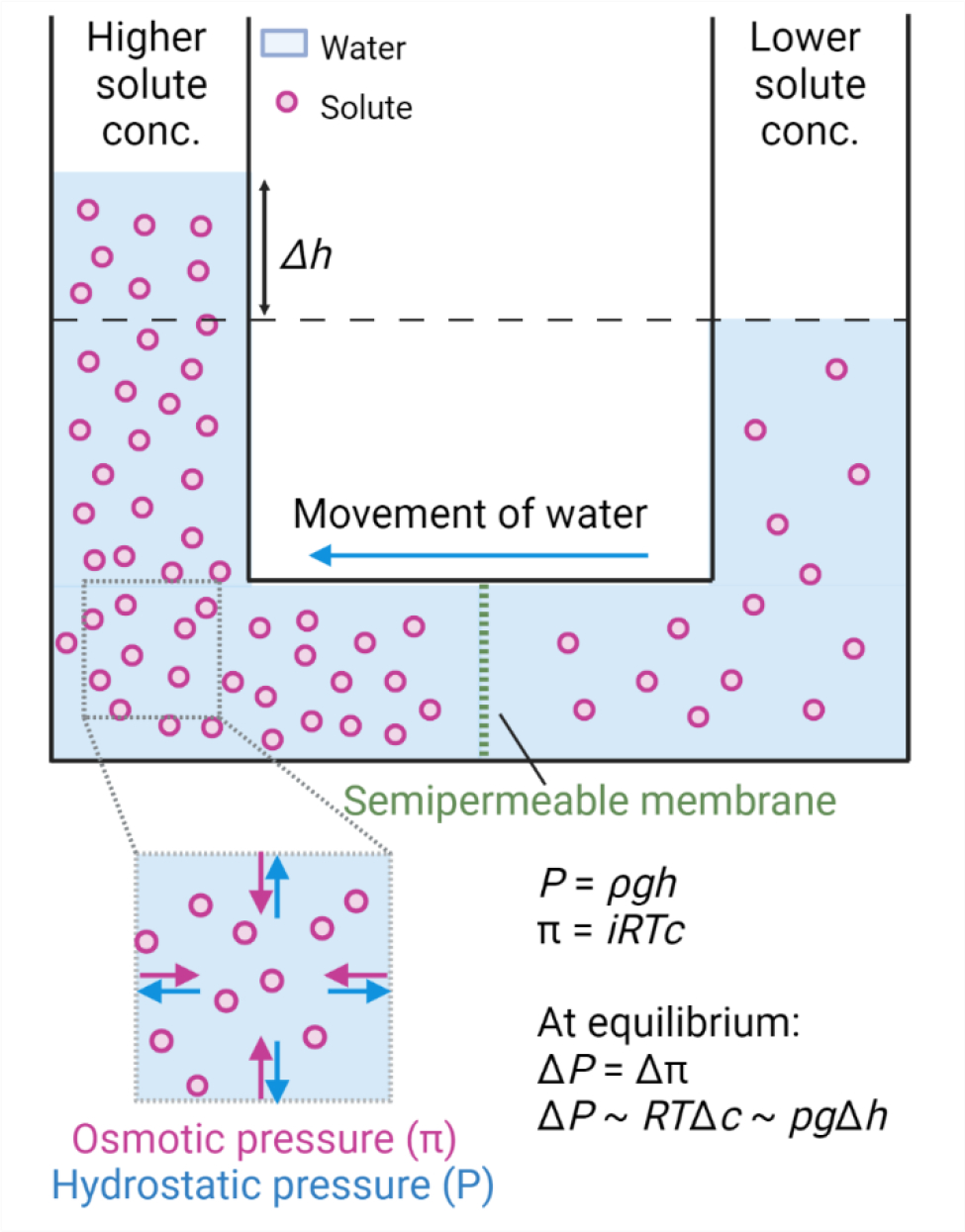
Imbalance in the solute concentration across the semipermeable membrane generates osmotic pressure π leading to the flow of water from lower solute concentration to higher solute concentration. π = iRTc, where i is the van’t Hoff factor, R is the gas constant, T is the absolute temperature and c is the solute concentration. The counteractive pressure to osmotic pressure is hydrostatic pressure P = ρgh, where ρ is the density of the liquid, g is gravity, and h is the height of the liquid. At chemical equilibrium, osmotic pressure is equal to hydrostatic pressure.
The force exerted by the solute molecules per unit area to drive water movement is called osmotic pressure and is given by van’t Hoff’s equation π = iRTc, where i is the van’t Hoff factor, R is the gas constant, T is the absolute temperature and c is the molar solute concentration[16,19]. The counteracting pressure to the osmotic pressure is the hydrostatic pressure. The balance of these pressures can be seen in the example of a “U” shaped tube open at both ends with a semipermeable membrane at the bottom (Fig. 1). In this setup, hydrostatic pressure is given by P = ρgh, where ρ is the density of the liquid, g is force due to gravity, and h is the height difference of the liquid. Although both are entropically governed, it is important to note that hydrostatic pressure is not the same as osmotic pressure. Intuitively, osmotic pressure can be seen as an inward exerting or pulling force that drives the water molecules towards higher solute concentration (Fig. 1). On the other hand, hydrostatic pressure can be seen as an outward exerting or pushing force, owing to the incompressible nature of water (Fig. 1). At equilibrium, osmotic pressure is equal to hydrostatic pressure[16]. In a biological setting, hydrostatic pressure builds because of the tension in the barrier (e.g. cell membrane or epithelium) that contains the water.
In practice, due to sustained exchange of ions, there is typically an osmotic and hydrostatic pressure gradient across cells, which is steadily maintained[20]. The hydrostatic pressure gradient is balanced by the mechanical tension on the cell surface (cytoskeleton network at the cortex, tension in the plasma membrane, and cell surface protein network such as glycoproteins - reviewed in[19]). In contrast to animal cells, plant cells maintain an incredibly high hydrostatic pressure in their cytoplasm due to the presence of stiff cell walls[21–24]. The pressure inside a single plant cell is on the order of a few (0.5–4) megapascals, which is much higher than a car tire at 0.2 megapascals[21,24–26]. Mammalian cells maintain a pressure of about a few hundred to a thousand pascals because their cell membrane and cortex are much less stiff[21,24–26]. As a comparison, this is lower than physiological blood pressure which in humans is 10–20 kilopascals. Abrupt or large changes in the solute concentration can lead to changes in cortical stress and hydrostatic pressure gradients, resulting in water flux across the cell. In the next section, we will review findings that describe how water moves across cells and tissues.
3. Movement of water across the membrane barrier
3.1. Water transport across cellular barriers
Water movement across the plasma membrane is accomplished in multiple ways. Diffusion of water molecules through the plasma membrane is based on solubility, where water molecules enter the lipid bilayer from one side and exit on the other. However, this rate is very low due to the hydrophobicity of the plasma membrane[18]. Aquaporins are transmembrane proteins that selectively transport water molecules in response to an osmotic or hydrostatic pressure gradient (Fig. 2a)[27,28]. Aquaporins (AQPs) are important for fluid transport in epithelial cells and can increase epithelial membrane permeability by a factor of 10[19,29,30]. For example, AQP1 is involved in the secretion of aqueous humour in the eye by the ocular ciliary epithelium and of cerebrospinal fluid (CSF) from the choroid plexus in the brain[31,32]. However, how central aquaporins are to overall water flux isn’t well understood. Mice depleted of AQP1 in the choroid plexus exhibit reduced CSF production by 25% but reduced osmotic permeability by 80% suggesting a role for aquaporins in enriching the osmotic permeability of cells[31–33]. Plant cells also utilise AQPs for the transport of water across the membrane[34].
Fig. 2: Proposed routes of water movement across epithelial cell and tissue barriers.
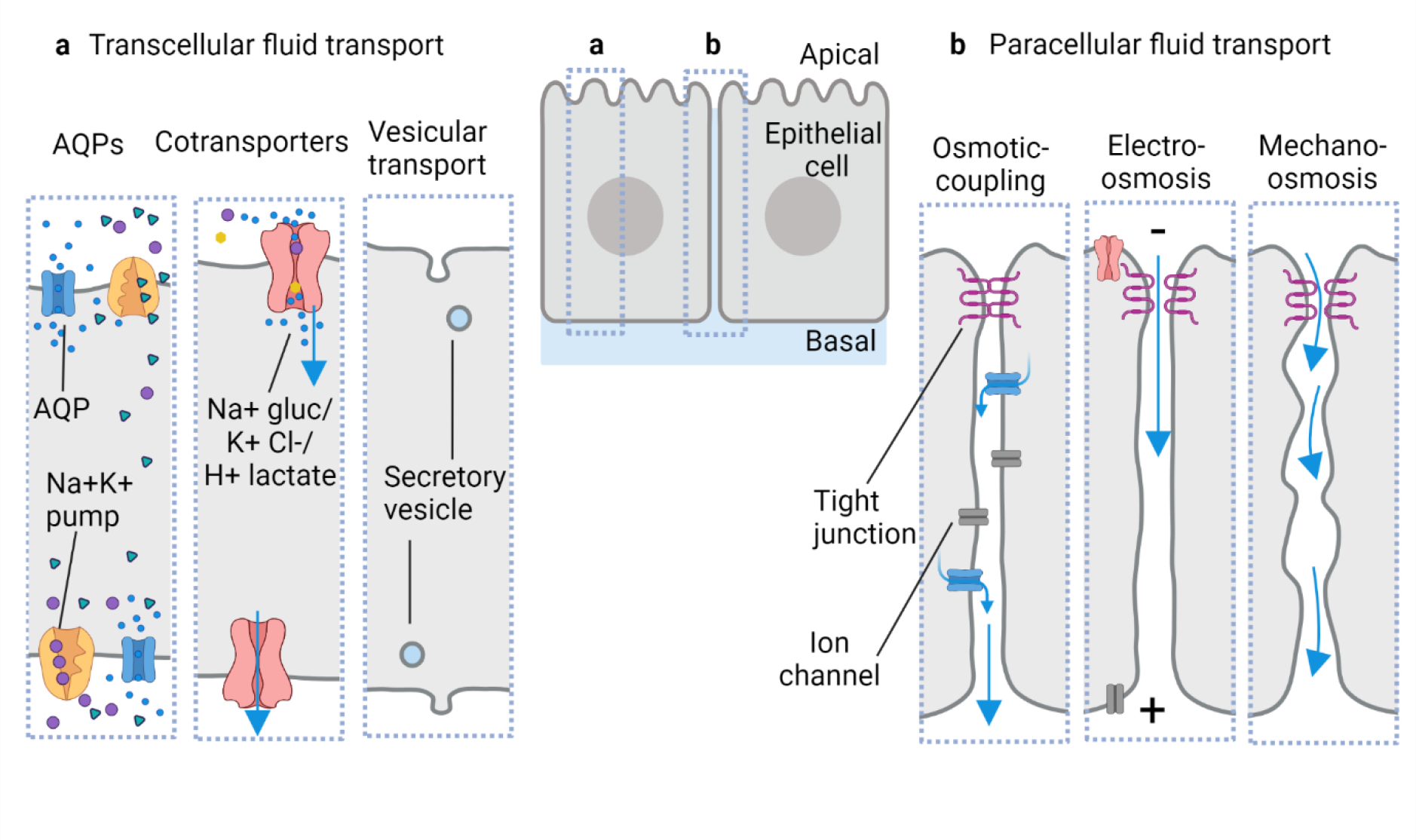
a) Transcellular transport of water through the cells can be achieved via multiple modes: (left to right) solute imbalances within the cell for example due to the Na+/K+ pump can lead to a flow of water through aquaporins (AQPs) and membrane diffusion; water can also be transported via cotransporters such as Na+/glucose, H+/lactate, and K+/Cl− and vesicular transport such as pinocytosis and exocytosis can also enable water transport. b) Paracellular transport of water has been proposed across diverse epithelia: (left to right) the osmotic coupling model suggests water transport by following the ionic flux (osmosis) in the lateral intercellular spaces; electro-osmosis shown in the rabbit corneal endothelium suggests electric potential generation across the epithelial cells via differential ion pumping that eventually leads to flow of counterion current with water; mechano-osmosis model suggests an active role of cellular junctions and lateral membranes in transporting water flow by generating micro-peristalsis movements. Blue arrows represent the flow of water.
Other membrane channels and carriers have also been identified that cotransport water molecules together with another solute moiety (Fig. 2a). Examples of water molecules hitchhiking along with the K+/Cl− cotransporter, Na+/glucose cotransporter, and H+/lactate cotransporter have been reported in animal cells while additional cotransporters have been identified in plants[35–40]. In the human intestine, the Na+/glucose pump transports roughly 250 water molecules along with 2 sodium ions and 1 glucose molecule[38,41]. Cotransporters can move water molecules against the osmotic gradient by utilising a chemical gradient[39]. Our understanding of water movement through cotransporters is still limited.
Another way of moving water molecules is through pinocytosis or “cell drinking”. Pinocytosis is a non-selective bulk transport involving uptaking/endocytosis of solutes such as proteins and ions, and water molecules by membrane invaginations. Pinocytosis is the proposed mechanism during the absorption of nutrients by the intestinal epithelial barrier and in dendritic cells[42,43]. However, the relevance of pinocytosis in water transport specifically has been previously questioned[42]. Conversely, secretion of cytoplasmic vesicles and apical membrane remodelling, for example, by the Madin-Darby canine kidney (MDCK) cells and mouse blastocyst is known to contribute to fluid accumulation in epithelial lumens (Fig. 2a)[44–46].
3.2. Water transport across the tissue barrier
We now change scales and move our attention to the transport of water across epithelial tissues. Sheets of polarised epithelia line our organs, body cavities, and lumens where they act as functional and protective barriers. Such epithelia need to selectively regulate passage of solutes and water, and some epithelia have a tremendous competence for absorption or secretion of water[47]. Our current understanding of how water moves across epithelia is surprisingly lacking given its fundamental importance in physiology. The simplest model is osmosis, where imbalances in ionic concentrations driven by ion pumps lead to the passive transport of water via AQPs. However, there are reasons as to why this intuitive model might not be sufficient or true[48]. Most theories of water movement are based on investigations of solute-water coupling studies in the mammalian gallbladder, intestinal, and kidney epithelia investigated in the second half of the twentieth century[42,48–54]. Several models for water movement across epithelia have been proposed, but their accuracy in mature physiology and especially in development remain unclear[48].
Due to their polarised nature and the route of water transport, epithelia can be described as forward-facing (apical to basolateral transport of water) and backward-facing (basolateral to apical transport of water)[55]. Forward-facing epithelia include absorptive systems such as intestine, kidney proximal tubule, and gallbladder. Backward-facing epithelia include secretory systems such as salivary glands, mammary glands, choroid plexus, and epithelial lumens. However, de novo lumens can also be formed basolaterally such as in the zebrafish blastoderm and mammalian blastocyst[7,8,12].
At the cellular level, water movement across epithelia is categorised into two major routes, transcellular and paracellular[42,50,51]. The transcellular route implies water movement through the interior of epithelial cells along their apicobasal or basoapical axis via membrane diffusion, AQPs, cotransporters, vesicular trafficking, or their combination (Fig. 2a). The paracellular route implies water movement through the intercellular or lateral spaces with or without involving junctional complexes. Models for paracellular water transport include osmotic gradients along the lateral spaces, electrical potential across the epithelia, and active water transport due to cellular mechanics (Fig. 2b). Although the importance of both routes has been debated since the 1960s, the discrepancies could arise from the chosen epithelial tissues. For example, work from Manchen, Frömter, and Diamond in the 1970s categorised epithelial tissues as leaky, intermediate, or tight based on the relative permeability to small ions through the tight junctions[42,56,57]. By measuring electrical resistance across such epithelia, some would have a high electrical conductance (leaky) while others had a low conductance (tight). Experimentally some epithelia that transport isotonically such as intestinal, gallbladder, kidney proximal tubule, and corneal endothelium are electrically leaky[42] thereby likely favouring paracellular water transport via tight junctions. The AQP knockout mice is another example of ambiguity as the water flux does not greatly differ from controls suggesting paracellular water movement might be at play[31,32,55,58].
The proposition of the osmotic coupling/standing gradient model and electro-osmosis has helped favour the paracellular route of water transport[33,48,59] (Fig. 2). The osmotic coupling model relies on ion accumulation in the lateral spaces driving water flow by osmosis, and creating a standing concentration gradient and thus, giving rise to quasi-isotonic fluid flow[48]. The osmotic coupling model has also been adapted with transporters and cotransporters (reviewed in[48]). Electro-osmosis on the other hand relies upon the electric potential across the epithelial cells to drive fluid flow. Experimentally rabbit corneal endothelium generates an ionic current in the intercellular space that forces movement of fluid from the basal and lateral space to the apical aqueous humour[60]. The driving force for the electro-osmosis is the electric potential difference set by Na+ deposition via Na+ pumps along the lateral membranes and potentially Na+/3HCO3− cotransporters on the apical side[60]. In fact, 80% of the total water transport in the rabbit corneal endothelium is attributed to electro-osmosis through the paracellular space and 20% to the osmotic transcellular route[60]. If this is also true in other absorptive or secretory epithelial tissues and organisms remains to be seen.
Another paracellular favouring model proposed 20 years ago by Adrian Hill and colleagues is mechano-osmosis, based on radioactive dextran probe experiments in Necturus (salamander), rat, and rabbit epithelia[48,55] (Fig. 2b). The model argues paracellular transport cannot be osmotic because the epithelia are leaky and transport water at high rates. Based on the lack of sufficiency of other mechanisms, Hill hypothesised micro-peristalsis movements possibly powered via actomyosin on the lateral membranes and involving junctional complexes could conduct water transport while AQPs in the cells would aid as osmotic sensors. No direct data supporting this intriguing, yet speculative model have been presented yet.
Two critical realisations from both the electro-osmosis and mechano-osmosis models are 1) an expense of energy or active transport is needed for water flux across the intercellular junction, 2) since both models favour a lesser contribution of osmotic forces in overall water flux, they suggest alternate ways to create water transport and thus hydrostatic pressure, in cells, in the intercellular space, and lumens. Although tight junctions might be leaky, there may also be transient rupturing during the active flow of water. Indeed recent reports from mouse blastocysts and in vitro MDCK epithelial clusters suggest build-up of microlumens in the intercellular junctions with high hydrostatic pressure sufficient to break tight junctions and create paracellular water transport to create lumens[8,61,62]. This rupturing process is referred to as hydraulic fracturing and will be discussed further in the review in the context of luminogenesis. Moreover, similar cellular mechanics alone or by virtue of cell movement or fluctuations in cell membranes could allow for water transport. Indeed a recent report shows that fluid transport during cytokinesis via the cleavage furrow contributes to pre-amniotic lumen formation and expansion in mice[62]. It is rather thrilling that as current work explores the role of hydrostatic pressure in developing tissues with advanced optical and spatiotemporal tools, there is also an opportunity to revisit and gather critical insights into the basic mechanisms of water transport across epithelial tissues.
Similar to animals, there are two major routes of water movement in plant cells[21,24]: 1) symplast – volume confined within the plasma membrane where water flux relies upon aquaporins in the plasma membrane and cytoplasmic intercellular bridges termed plasmodesmata, 2) and apoplast – volume confined by the cell walls and the intercellular spaces. Apoplast pathways govern the high conductivity of water movement via pressure gradients made accessible by the permeable and poroelastic nature of the organised cell walls[21,24].
As previously discussed, the net result of forming osmotic gradients and transporting water across cells or tissues is the establishment of hydrostatic pressure. In the following sections, we will review the effects of hydrostatic pressure at cellular and tissue scales, and how pressure is harnessed for driving many developmental processes.
4. Cells under pressure
In both plants and animal cells, there is a coupling between osmotic potential, hydrostatic pressure, and mechanical tension (Fig. 3a–b). Such mechano-hydraulic coupling enables cells to maintain specific volume and shape. Any changes in the water flux or mechanical tension lead to changes in hydrostatic pressure, thereby causing cellular deformations by affecting processes involving cell volume and shape or vice-versa. For example, turgor pressure in plant cells is balanced by the compressive stress from the cell wall[23,63,64]. Cell wall relaxation or mechanical imbalance, leads to a drop in the turgor pressure, thereby, reducing the water potential in the cell and creating more water influx, which results in cell elongation, a key aspect of cell growth[64,65].
Fig. 3: Comparative role of mechano-hydraulic coupling in animal and plant cells.
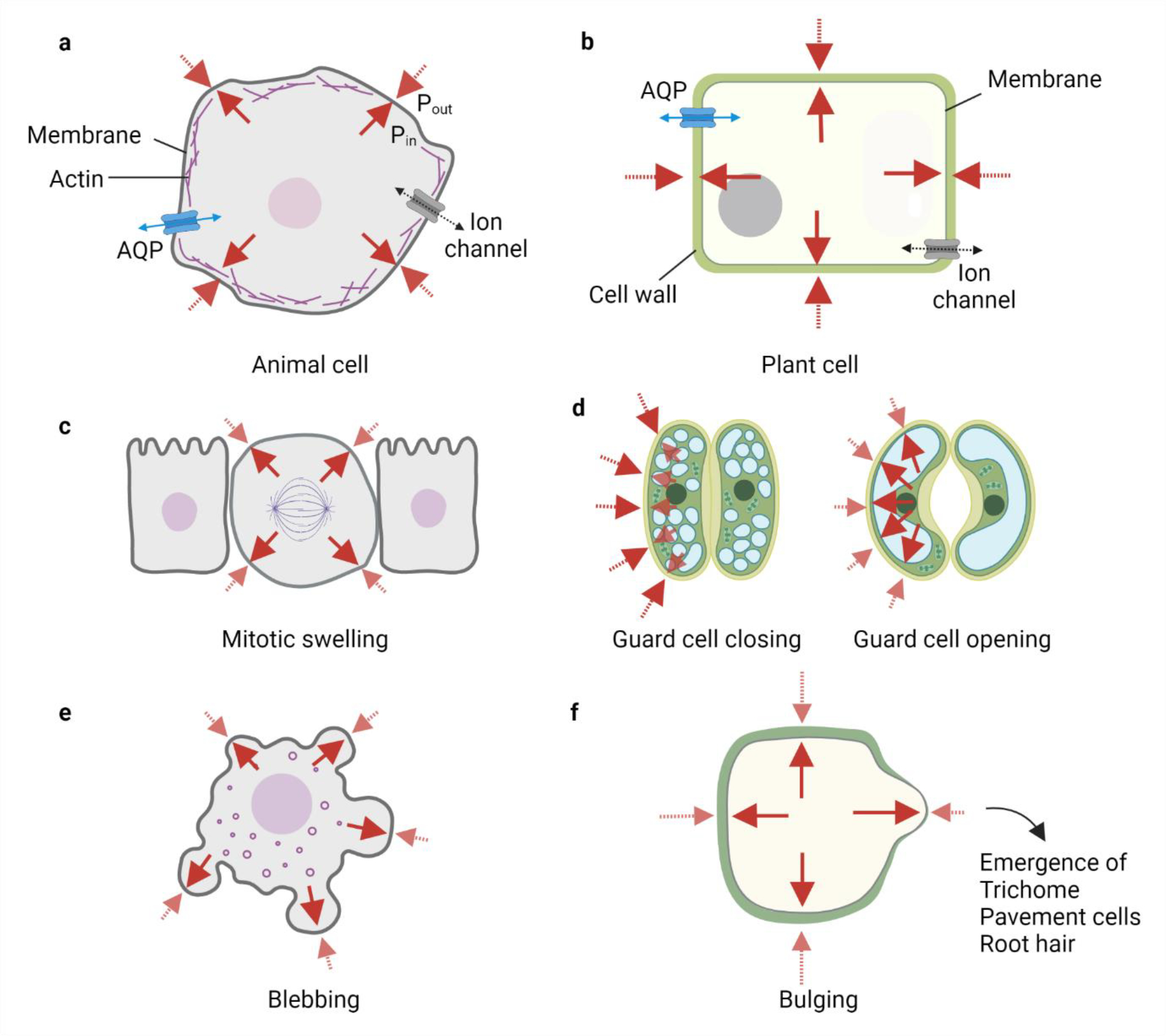
a-b) Animal and plant cell shape and volume are regulated by steady maintenance of ion and water flux through the cells, i.e. there is always an osmotic and hydrostatic pressure difference across the cell. The hydrostatic pressure gradient is balanced by the mechanical tension in the membrane, cell surface proteins, and cytoskeletal (actomyosin) cortex in animal cells as depicted by the red arrows. Solid red arrows represent Pin and dotted red arrows represent Pout. Blue arrows represent water flux. Plant cells have a high turgor pressure which is primarily balanced by the compressive forces from the stiff cell walls. c-f) Changes in mechano-hydraulic coupling can lead to alteration in hydrostatic pressure, cell shape, and cell volume and are important for cellular function. c) Animal cells undergo mitotic swelling due to increased hydrostatic pressure which is potentially useful for faithful segregation of chromosomes. d) Plant (guard) cells utilise swelling to open and close the stomatal apparatus for transpiration. e) Localised increases in the hydrostatic pressure in areas of the weaker cytoskeleton can lead to an inflation of animal membranes called blebbing, which is seen in various cellular processes such as cell division or cell death. f) Localised increases in the hydrostatic pressure due to weaker or thinner cell wall areas in plant cells can also lead to bulges which are important in the emergence of plant structures such as trichomes or root hairs
This is also recently shown to be true for mammalian cells, where alterations in water/ion content led to changes in hydrostatic pressure and cortical cytoskeletal dynamics[66–68]. Changes in water flux underlie the phenomenon of mitotic swelling, where cells swell during cell division due to increased internal hydrostatic pressure[68–70] (Fig. 3c). Increased hydrostatic pressure enables rounding up of cells during mitosis that creates space by pushing neighbours and potentially favouring faithful segregation of chromosomes. Interestingly, such swelling within confined cell walls enables plants to bend in the direction of the sun, where one side of the stem swells (high hydrostatic pressure) and the other side shrinks (low hydrostatic pressure)[71]. Such reversible swelling and shrinking of plant cell volumes are also the basis for gas exchange and transpiration in the stomata[72] (Fig. 3d).
Besides volume, mechano-hydraulic coupling also regulates cell shape and motility, a core aspect of morphogenesis. The cytoplasm can be seen as a fluid-soaked poroelastic sponge, where the contractile network is defined by the cytoskeleton and organelles immersed with cytosolic fluid consisting of water molecules, ions, and soluble proteins. The cytoplasm is also proposed to be heterogeneous implying any change in hydraulic pressure does not propagate uniformly across the cytoplasm but rather depends on the hydraulic and electrochemical conductivity of the network[73]. Therefore, upon alteration in the steady-state mechano-hydraulic coupling, the hydrostatic pressure might be locally governed or regulated. For example, compression in the actomyosin network can lead to a local increase in hydrostatic pressure like squeezing a fluid-filled sponge at a corner will extrude water in that region. Such localised hydrostatic pressure increase can deform the cell membrane by inflation causing blebbing[73,74] (Fig. 3e). However, blebbing can also be caused by an influx of water into the cell[75]. Analogously, local bulges of increased hydrostatic pressure in the areas of the weaker cellular cortex have been modelled for polarised growth of plant root hairs and trichomes (Fig. 3f)[76]. Since the cytoskeletal constituents such as actomyosin also behave like a viscous fluid on a longer time scale (>10s), the cytoplasm can be rendered with two fluids with different dynamic and hydraulic properties. While blebbing can be explained with the two-fluid model (reviewed in[19]), we are currently understanding the functional implications of such dynamic behaviour.
Changes in the hydrostatic pressure can also cause cell movement and motility. One proposed mechanism for cell motility is the osmotic engine model (OEM), where cells in confined channels utilise a polarised distribution of AQPs and other ion channels at the leading and the trailing edge to create a constant flux of water and ions through the cell enabling cell movement[77]. Such OEM based motility has been experimentally shown for mouse fibrosarcoma (S180) and human metastatic breast cancer cells (MDA-MB-231) in a confined microenvironment. When S180 and MDA-MB-231 cells are confined in narrow channels, they can migrate even when their actin network is disrupted[77]. The MDA-MB-231 cells display polarised localisation of AQPs and Na+ and H+ exchanger in accordance with the OEM model[77,78]. Interestingly, the expression of AQP9 in motile cells has been shown to promote filopodia formation[79]. Cell motility and hydrostatic pressure are highly relevant in solid tumours as high interstitial fluid pressure (7 kPa, confined within the intracellular spaces of a solid tumour), is shown to increase metastasis/cell migration and epithelial-mesenchymal transition (EMT)[80–82]. Using 3D tumour-engineered models of breast cancer cells, it has been shown that high hydrostatic pressure in the tumour interstitium leads to genetic changes associated with EMT requisite for collective invasion[81,83]. With or without the genetic changes, if the invading cancer cells utilise an osmotic engine or similar model for motility by generating a polarised flux of ions is an exciting frontier and remains to be seen.
5. Tissues under pressure
Epithelial cells, as previously mentioned, have an incredible ability for water movement, and many epithelial tissues form a de novo lumen. The molecular components for lumen formation of epithelia have been studied[46,84–87]. Ion channels and AQPs have been suggested to play a role in setting osmotic gradients and inward water flux. Yet, their exact contribution and whether alternate mechanisms are at play remains unknown (see section 3.2). The directed flow of water and its compartmentalisation in epithelial cavities creates hydrostatic pressure. Recent studies from the mouse blastocyst[7,8,45], the zebrafish inner ear (otic vesicle, endolymphatic sac, and the buds of the semicircular canals)[6,9,10], lung explants[14], and organoids of the mammary gland, intestine, and MDCK cells[44,88–91] have provided evidence for the valuable role of hydrostatic pressure during development.
Direct measurement of hydrostatic pressure in vivo is hard but has been achieved using micropressure probes, traction microscopy, and gel deformation assays. Recently, Mosaliganti et al. measured luminal pressure dynamics in the zebrafish inner ear using a piezo-based solid sensor devised for accurate pressure measurement in small volumes of liquid[6]. Similarly, luminal pressure in mammalian blastocyst has been measured using a micropressure probe[7]. Other indirect methods to measure luminal pressure are reviewed in[92]. Nevertheless, the pressures in the zebrafish otic vesicle and the mammary gland organoid range between 100–300 Pa[6,93]. When taken into account the cell surface area, the force translates as 50–100 nN per cell, which is consistent with actomyosin based forces generated by the cells when measured using traction microscopy[19,94]. Similarly, pressure has been measured in mouse blastocyst with and without the zona pellucida[7,95]. The luminal pressure with the zona pellucida averages about 1500 Pa using both hydrogel and micropressure probe assays[7,95]. Whereas without the zona pellucida the mouse blastocyst luminal pressure averages about 500 Pa consistent with the magnitude in zebrafish and mammary organoids[7,95]. These data suggest that indeed hydrostatic pressure can generate sufficient forces at tissue scales for driving morphological changes.
In the following sections, we will describe a framework, which demonstrates the role of hydraulics in regulating tissue growth and size, driving tissue morphogenesis, and specifying cell fate during embryonic development.
5.1. Hydrostatic pressure regulates tissue growth and size
Robust patterning and morphogenesis of organs requires embryonic tissues of a critical size. While earlier work proposed size control was achieved primarily by cell proliferation[96–98], recent studies have shown that hydrostatic pressure of lumens can also contribute towards achieving correct tissue size (Fig. 4a, c)[6,7,99]. For example, the intricate adult inner ear, consisting of the semicircular canals required for sensing balance and acceleration, the cochlea required for hearing, and the endolymphatic duct and sac that likely maintains pressure, forms from an embryonic tissue called the otic placode. In zebrafish, the mesenchymal cells of the otic placode cavitate and develop to form a lumenized epithelial cyst called the otic vesicle (OV)[6,99,100]. The OV continues to grow and expand before it is remodelled to form the complex shape of the mature ear[10,100]. It was shown that ion transporters are involved in fluid flux into the lumen[6] through the correlation of lumen inflation with genetic mutants and drugs that affect transporters. Whether fluid flux into the lumen is simply a result of osmotic swelling remains to be demonstrated. Water, being incompressible and contained in this epithelium with tight junctions, applies hydrostatic pressure on the surrounding otic tissue. Hydrostatic pressure causes the otic epithelial cells to stretch in the plane normal to the surface. While the otic epithelial cells do proliferate contributing to tissue growth, Mosaliganti et al. showed that the expansion of the OV by the hydrostatic pressure from the lumen and the resultant stretch in the epithelium is the dominating driver of otic vesicle growth (Fig. 4a, d).
Fig. 4: Hydrostatic pressure drives multiscale remodelling.
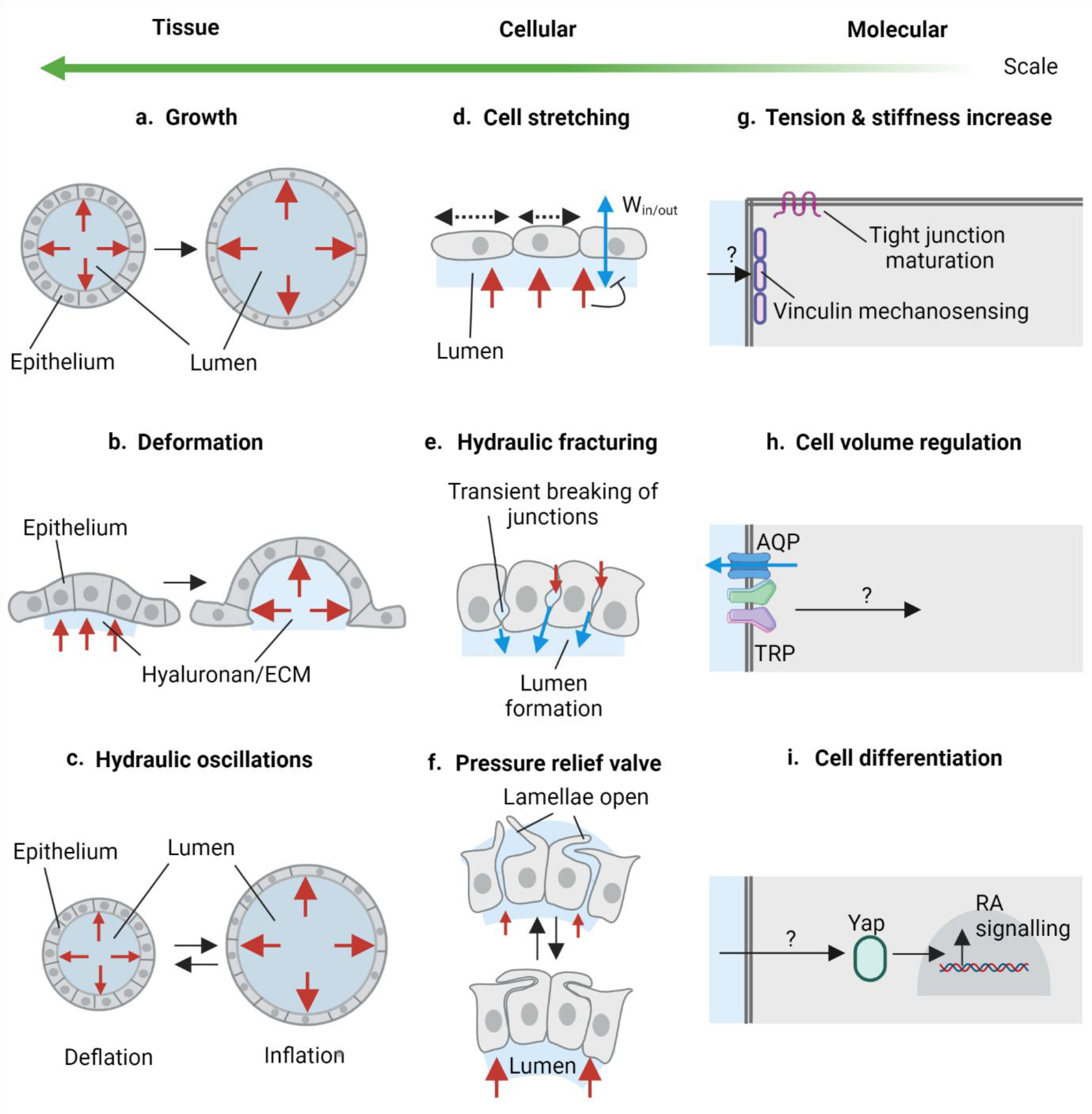
a-c) Hydrostatic pressure in dynamic tissue changes. a) Increase in the hydrostatic pressure in the epithelial lumen or lumen expansion as shown in mammalian blastocysts, zebrafish inner ear, and organoids regulate growth and size of the organ. b) Hydrostatic pressure arising due to hyaluronan hydrogel (water-dependent swelling of hyaluronan) in the ECM causes tissue deformation as shown during semicircular canal morphogenesis in the zebrafish inner ear. c) Inflation-deflation cycles of luminal pressure called hydraulic oscillations can regulate pressure in the lumen and control the size of the organ as demonstrated in mammalian blastocysts and organoids. d-f) Effects of hydrostatic pressure at the cellular scale. d) Hydrostatic pressure can cause epithelial cell stretching, which in zebrafish inner ear has been shown to negatively regulate water influx into the lumen. e) Pressurised microlumens in the lateral intercellular space can cause transient breakage of cellular junctions. This process called hydraulic fracturing has been demonstrated in mouse blastocoel formation. f) Oscillations in the luminal pressure can lead to quick release or build-up of pressure in the epithelial lumens. Epithelial cells can respond to pressure oscillations via membrane protrusions such as lamellae in the pressure relief valve in the zebrafish endolymphatic sac. g-i) Molecular perception and response to hydrostatic pressure. g) Hydrostatic pressure can elicit increased cell tension and stiffness and can allow pressure mechanosensing via vinculin and allow maturation of tight junctions as demonstrated in the mouse blastocyst. h) Cells can perceive hydrostatic pressure via stretch sensitive TRP family of proteins and/or Piezo proteins. TRP can physically interact with AQPs which can modulate water flux in the cell. This is a possible mechanism with extracellular hyaluronan during cell volume regulation during zebrafish heart valve morphogenesis. i) Hydrostatic pressure can also elicit a transcriptional response in the responding cells such as RA signalling activation via Yap nuclear localisation in the lung organoids. Red arrows represent hydrostatic pressure exerted by the lumen (a-f) and hyaluronan-rich ECM (b). Blue arrows represent water flux through the cells (d) and through the paracellular spaces via fracturing (e).
In addition, the authors demonstrate a negative hydraulic feedback loop for tissue size control. Through mechanical puncturing of the OV, the authors observed an immediate reduction in the size of the OV showing loss of pressure. After a rapid repair of the wounded epithelial barrier, the punctured OV lumen expands at a rate faster than unpunctured OV, a phenomenon described as catch-up growth. Together with theoretical modelling, the authors show negative hydraulic feedback, where pressure build up negatively regulates further water flux, potentially buffering natural size variations during development.
How does the pressure build up inside the inner ear regulated? A striking set of observations by Swinburne et al., show that the endolymphatic duct and sac, a dead-end epithelial tube connected to the inner ear, inflates with the OV luminal fluid, followed by deflation[9]. Deflation, or release of pressure, is facilitated by the opening of overlapping basal lamellae produced by cells in the endolymphatic sac (ES). Following pressure release, the lamellae close back, thereby behaving like a pressure relief valve (Fig. 4c, f).
A similar feedback system regulates early mouse embryo size. The first few days of mouse development involve the formation of a fluid-filled cavity, the blastocoel. Blastocoel lumen formation requires hydrostatic pressure that ruptures the cell-cell contacts called hydraulic fracturing (Fig 4e) and creates hundreds of microsized water pockets[8]. These microlumens coalesce to form a single large lumen. Similar to the zebrafish OV, the mouse blastocoel lumen continues to expand through hydrostatic pressure, thereby causing the blastocyst cells to stretch (Fig 4d)[7]. As pressure continues to build up, tight junctions between the blastocyst cells fracture during mitosis, thereby releasing the pressure. Tight junctions reseal upon completion of mitosis and the lumen pressure begins to build up again, thereby creating a feedback loop manifested in the form of cycles of blastocyst inflation and deflation.
Hydrostatic pressure arising from the cerebrospinal fluid (CSF) has also been shown to drive brain enlargement and shaping in chick embryos [101]. The closure of the neural tube coincides with the onset of enlargement of the brain and thus positive CSF pressure leads to expansion of brain ventricles like a blown-up balloon [101].
5.2. Hydrostatic pressure drives tissue deformations
So far, we’ve discussed examples where hydrostatic pressure builds as a result of confining water in lumenized tissues. Hydrostatic pressure can also be harnessed by confining water in poroelastic or viscoelastic gels[73,102–104]. A unique example is the ECM component, hyaluronic acid, or HA. HA is an anionic biopolymer that is synthesised as long strands by HA synthases on the plasma membrane[105,106]. Similar to osmotic potential generated by ion transporters during lumen formation, the negative charges on HA polymeric chains attract Na+ and Ca2+ ions, and thereby water, resulting in the formation of a swollen viscoelastic hydrogel in the extracellular space (Fig. 4b)[106,107]. Interestingly, the morphogenesis of the semicircular canals in the inner ear utilises this physical property of HA[10,108]. As previously discussed, the mature inner ear forms from the embryonic otic epithelium. Munjal et al. showed that in the zebrafish otic epithelium, six stereotypical regions express HA synthase 3, resulting in the local synthesis of HA in the ECM beneath these regions. The swollen HA hydrogel applies hydrostatic pressure on the overlying tissue causing it to deform into buds (Fig. 4b) which go on to extend and fuse to form the hubs of the semicircular canals.
An important concept to be drawn here is the response of epithelial tissues to pressures and the time scale of deformations. It is now well known that embryonic tissues have viscoelastic material properties. In other words, depending on the time scales of applied mechanical stresses, the cells either respond in a reversible elastic manner (on short-time scales) or in an irreversible viscous manner (on long-time scales)[109]. For example, puncturing and the consequent collapse of the OV[6], digestion of HA and the consequent collapse of the otic epithelial buds[10], and the fracturing of junctions during mitosis in the mouse blastocyst and the consequent deflation of the blastocoel[7], are all instances of elastic responses: at cellular scale–the cells lose their stretching, and at tissue scale–their deformations. However, for morphogenesis to successfully happen, epithelial deformations need to be irreversible. In all the above examples, epithelial tissues respond to the mechanical stresses of hydrostatic pressure by tissue stiffening, whereby cells exhibit accumulation of cytoskeletal proteins at the interface with pressure, apically in case of OV lumen and ES, basally in case of inner ear budding, and at the tight junctions in the case of the mouse blastocyst. Although stiffening is seemingly counterintuitive to the viscous fluid-like properties of epithelia at long-time scales, the turnover of cytoskeleton proteins and their regulators, and/or the dynamic behaviour of the subcellular structures formed by them, likely ensures tissue fluidity. Other mechanisms such as cell flow and division can also contribute to a viscous response at long time scales. Such feedback interactions between hydrostatic pressure and tissue material properties likely ensure the maintenance of epithelial integrity during morphogenesis.
Another potential outcome of coupling interactions between hydrostatic pressure and tissue material properties is attaining an anisotropic shape. Most, if not all, tissues undergoing morphogenesis exhibit anisotropy. Obeying Pascal’s law for static fluids, hydrostatic pressure within lumens or the ECM is usually isotropic in nature. Therefore, unlike deformations produced by intracellular forces, where anisotropy is usually attained by the polarised distribution of cytoskeleton proteins, morphogenesis driven by hydrostatic pressure may utilise spatially patterned mechanical properties of the tissue to attain anisotropic shapes. For example, during zebrafish canal morphogenesis, the budding tissue exhibits anisotropic stiffness to drive directed growth from the ECM-generated isotropic hydrostatic pressure[10,108]. The ovoid shape of the zebrafish otic epithelium is likely derived from anisotropic stiffness of the surrounding tissues. Not only can tissue material properties differ spatially and directionally but they can also be responsive to applied forces, setting up feedback loops as discussed in the next section.
5.3. Mechanochemical feedback loops
Mechanochemical feedback loops are essentially the communicating language between the mechanical forces, here hydrostatic pressure, and the biochemical signalling, one evoking the other. Much work has been described in cytoskeleton and adhesion-based mechanics for coordinating mechanical and biochemical signalling, but we have just started to explore how hydrostatic pressure might regulate biochemical signalling or vice-versa[1,110,111]. Cells must first perceive the pressure via mechanosensitive proteins or changes in cell shape and then elicit a response that may or may not utilise transcriptional changes. Piezo and TRP are two stretch-sensitive ion channels that have been shown to perceive mechanical forces in many developmental contexts[112,113]. In zebrafish heart valve morphogenesis, mechanical forces due to blood flow are perceived by the endocardial cells via mechano- and osmosensitive TRP channels[114]. TRPV4 channels then regulate cell volume via possible interaction with aquaporins as TRPV4 has been shown to physically interact with aquaporins (Fig. 4h)[115]. Additionally, the accumulation of hyaluronic acid in the cardiac jelly underneath the endocardial cells establishes an osmotic gradient, which attracts water thereby enabling cell volume regulation (Fig. 4h)[114]. If the cell volume regulation here involves any transcriptional change hasn’t been established. It has been recently shown that hydrostatic pressure can elicit cell stretching and govern growth in the zebrafish otic vesicle by setting a negative feedback loop, where lumen expansion inhibits water influx into the lumen[6]. Similarly, in the mouse blastocyst, luminal pressure sets a positive feedback loop for organ size control[7]. Increased luminal pressure results in increased cortical tension and stiffness leading to vinculin mechanosensing and tight junction maturation of trophectoderm cells (Fig. 4g). However, in both these cases, how the pressure is perceived, and the downstream biochemical signalling is not well understood. A recent study using mouse lung explants reports that hydrostatic pressure activates retinoic acid (RA) signalling via nuclear localisation of YAP (Yes-associated protein)[116], and pharmacological inhibition of RA signalling leads to decreased lung branching illustrating pressure-elicited downstream signalling (Fig. 4i). RA is known to regulate many aspects of organ development including inner ear formation[100,117], so it will be interesting to explore if RA is downstream of hydrostatic pressure in other scenarios. Interestingly, YAP and TAZ (transcriptional coactivator with PDZ binding motif) have also been shown to modulate cell volume in cell culture studies[26]. On the other hand, if signalling molecules can control hydrostatic pressure isn’t known yet. We do know besides applying pressure, epithelial lumens can also act as signalling hubs[92]. For example in zebrafish, the lateral line epithelial rosette cells secrete and trap fibroblast growth factor (FGF) molecules in its microlumen, setting up a positive feedback loop for robust patterning of mechanosensory organs[118]. FGF signalling in the mouse blastocyst lumen is also shown to regulate cell fate specification of the inner cell mass[45]. Theoretical models of mechanochemical feedback have been proposed. For example in Hydra, osmotically driven shape oscillations along with tissue mechanics and Wnt morphogens can help break symmetry (of the main body-axis)[119,120]. Indeed, this hydraulic oscillation and Wnt-dependent symmetry breaking in Hydra has been recently shown experimentally, demonstrating the combined power of theoretical models and experimental validation[121].
Can studies of turgor pressure in plants teach us anything about mechanotransduction? Turgor pressure is also perceived by stretch-activated or force-gated ion channels[122]. Some of them are membrane small conductance like (MSL) channels that respond to changes in membrane tension, reduced hyperosmolarity-induced calcium increase (OSCA) that regulates calcium flux with changes in osmolarity, and defective kernel 1 (DEK1) which is a calcium-dependent cysteine protease that regulates calcium flux with changes in pressure[122,123]. DEK is a member of the calpain protein family, which in mammals are involved in adhesion (integrin) and shear stress-based motility[124]. If calpain proteins can sense hydrostatic pressure in animals isn’t known yet. However, microtubules are the torch-bearers of mechanosensing in plants as they are proposed to be connected to the cellulose microfibrils and might offer a spatial continuum for transmission of mechanical forces[125–127]. Microtubules are at the interplay of mechanical forces, cell and tissue geometry, and cellular decisions such as division orientation[127–130]. Downstream signalling is also less understood in plants, but reports suggest an involvement of plant hormones such as auxin [119,124].
5.4. Hydrostatic pressure in cell fate specification and patterning
Luminal pressure-driven tissue morphogenesis can involve decisions of cellular patterning and fate. Such modulation of cellular decisions is often governed by mechanotransduction and mechanochemical feedback loops. During mammalian lung morphogenesis, flow of amniotic fluid into the branching lungs leads to stretching and differentiation of alveolar epithelial type 1 (AT1) cells[131]. Similarly, in mouse lung explants the transmural pressure activates RA signalling which regulates smooth muscle wrapping and differentiation and epithelial proliferation, thereby promoting lung branching[116]. The hydraulic oscillations (inflation-deflation cycles) of lumens have also been shown to pattern the epithelium in intestinal organoids. The continuous stem cell zones (SCZ) in the intestinal epithelium splits and differentiates by fission due to lumen inflation[89] which may be regulated by the mechanotransducer Piezo1.
In the mammalian blastocyst, lumen expansion has been shown to guide epiblast and primitive endoderm cell fate specification and inner cell mass (ICM) sorting[45]. Luminal signalling containing FGF molecules is shown to expedite lineage specification suggesting mechanical and biochemical roles of the lumen in cell fate specification. Interestingly, lumen expansion can also induce cell specification by altering tissue geometry. Lumen reduced mouse blastocysts exhibit more asymmetric divisions in the trophectoderm which thereby affects cell allocation and fate in the ICM[7].
6. Conclusions and emerging questions in the field
Progress within the last decade has demonstrated the mechanical role of water movement and induced hydrostatic pressure in cell and tissue morphogenesis, yet our conceptual and experimental understanding of hydrostatic pressure is inadequate in comparison to our knowledge of cytoskeletal and adhesion-based mechanics. With recent discoveries we are realising hydrostatic pressure is an efficient driver of tissue deformation and tissue behaviour, but many questions remain. Within a cell, pressure can create a spatiotemporal field, eliciting local responses[73]. It will be important to determine how quickly pressure equilibrates in different cell types and how that relates to cellular function. It is also critical to address how isotropic pressure is modulated in tissue lumens and extracellular spaces to cause anisotropy in tissue deformation and cellular decisions of fate, proliferation, death, and growth. Conceptually, we need to ask what advantages hydrostatic pressure has over cytoskeletal and adhesion-based tissue morphogenesis and how both are spatiotemporally coordinated.
New developments in methods and technology are important for continued progress. We currently lack reliable tools for directly measuring pressure and osmolarity in epithelial lumens, especially in the in vivo context. A few emerging sensors for direct measurement of luminal pressure[6,7,132] and osmolarity[133] have been utilised recently but remain complicated to use and adapt. Direct measurements allow us to have a true characterisation of the system and measure its response to perturbations. As a corollary, we also need advances in methods and tools that will allow us to reliably control the pressure and osmolarity in vivo and investigate the resulting tissue behaviour.
The routes of water movement, transcellular and paracellular, across the epithelial membrane have been researched since the early 1900s, however, the mechanisms remain debated. Although molecular components of these pathways are known, the localisation for example of aquaporins, other pumps and co/transporters in tissue context is still limited. The observation of hydraulic fracturing in epithelial cells in vitro and in mouse blastocyst lumen expansion is exciting[8,61] and may support a paracellular mechano-osmosis model[55] but the detailed characterisation at the cellular, molecular, and temporal scale needs to be carried out. Such characterisation and direction of work can be leveraged with better spatiotemporal imaging advances and data analysis methods. Nevertheless, with our progressive understanding, we are bound to tackle again questions once posed by Hill and others in the 1970s, i.e. ‘Is there a basic mechanism which operates to allow isotonic transfer in all fluid transporting epithelia as there is, for example, a basic mechanism in nerve conduction and in muscle contraction?’ For example, hydraulic fracturing so far has been observed along the basal to the apical epithelial axis, however, we do not know if it is a general luminogenesis mechanism. The integration of theoretical models in understanding luminogenesis stages–nucleation, coalescence, expansion–and water movement as well as hydrostatic pressure regulation is a valuable complementary approach. However, these models are based on assumptions of primarily osmotic driven cavitation by water and standardised properties of epithelial cells[8,134,135]. It will be important to incorporate alternate models of water transport in theoretical models and experimentally validate model predictions in a tissue-specific manner. Such a quantitative framework would allow us to tease apart the fundamental mechanisms of water transport and luminogenesis in diverse epithelia and to compare the water transport mechanisms in mature absorptive and secretory epithelia versus epithelia undergoing morphogenesis in a developmental context.
In plants, the timescale of water transport at the cellular and tissue level limits the maximal speed of mechanical movements achieved in response to stimuli[104]. Such coupling between water flux and growth has been demonstrated in the zebrafish otic vesicle[6] but if such a physical basis is also governed in other animal systems to set spatiotemporal deformation and/or response remains to be investigated. Moreover, fluid flow in the developing tissues is important and isn’t well understood. Most of our current understanding of fluid flow in morphogenesis is based on active flows generated by cilia such as in the Kupffer’s vesicle for left-right axis determination[136]. A collaborative understanding of hydrostatic pressure and fluid flow in development will be a cornerstone in tackling pertinent pathological states such as in tumours, where interstitial pressure and fluid flow drive cellular identity transition and metastasis[80].
The perception of hydrostatic pressure by tissue and its subsequent response is key to cellular behaviour and decisions. Our understanding of such pressure elicited feedback is beginning to emerge. A recent report that pressure activates retinoic acid signalling via the mechanosensor YAP in mouse lung explants, thereby regulating epithelial branching and smooth muscle wrapping is thrilling[116]. Although we have a couple of reports of pressure based feedback on tissue behaviour such as growth in the otic vesicle[6] and mouse blastocyst lumen[7], we need further exploration. It is known that microlumens such as in the lateral line in zebrafish and mouse blastocyst trap fibroblast growth factor (FGF) thereby, serving as a luminal signalling hub[45,118]. However, if trapped molecules can modulate pressure in the lumen or vice-versa is not known. Many organoid systems generate a central lumen and may provide experimental advantages such as better accessibility for imaging, measurement, and perturbation. Beyond lumens, we have seen the relevance of the ECM component hyaluronan in physically deforming tissues in zebrafish semicircular canal morphogenesis[10] and heart morphogenesis[114] It will be important to see if hyaluronan and other ECM components act to generate hydrostatic pressure in other morphogenetic contexts.
In a broader context, tissue morphogenesis encapsulates complex interplay between cellular decisions of fate, proliferation, death, shape, and rearrangements powered by mechanical forces, biochemical signalling, geometry, and cellular material properties. This interaction at the cellular level gives rise to tissue wide physical properties such as rigidity or fluidity. These physical properties can dictate “tissue pressure” that is distinct from the hydrostatic pressure reviewed here. Recent work during zebrafish, chicken, and Drosophila morphogenesis has shown that tissues exhibit fluid-like phase transition allowing for control of tissue flow and pressure[137–140]. Integrating concepts of tissue pressure with hydrostatic pressure derived from ECM or water in lumens is an important future direction. Such physical understanding of biological tissues is an emerging field and prompts us to investigate what hydraulics might mean in the multiscale development of organisms.
Acknowledgements
We thank Megason lab members for support, discussions, and feedback during this review. We thank Sean McGeary for feedback on the manuscript. This work was supported by NIH R00 HD098918 to A.M. and NIH R01DC015478 and R01GM107733 to S.G.M. We apologise to the authors whose work we could not include due to spatial restraints. Figures were created with BioRender.com.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- [1].Lecuit T, Lenne P-F, Munro E, Force generation, transmission, and integration during cell and tissue morphogenesis, Annu. Rev. Cell Dev. Biol 27 (2011) 157–184. 10.1146/annurev-cellbio-100109-104027. [DOI] [PubMed] [Google Scholar]
- [2].Matis M, The Mechanical Role of Microtubules in Tissue Remodeling, Bioessays. 42 (2020) e1900244. 10.1002/bies.201900244. [DOI] [PubMed] [Google Scholar]
- [3].Mathur J, Hülskamp M, Microtubules and microfilaments in cell morphogenesis in higher plants, Curr. Biol 12 (2002) R669–76. 10.1016/s0960-9822(02)01164-8. [DOI] [PubMed] [Google Scholar]
- [4].LeGoff L, Lecuit T, Mechanical Forces and Growth in Animal Tissues, Cold Spring Harb. Perspect. Biol 8 (2015) a019232. 10.1101/cshperspect.a019232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Heisenberg C-P, Bellaïche Y, Forces in tissue morphogenesis and patterning, Cell. 153 (2013) 948–962. 10.1016/j.cell.2013.05.008. [DOI] [PubMed] [Google Scholar]
- [6].Mosaliganti KR, Swinburne IA, Chan CU, Obholzer ND, Green AA, Tanksale S, Mahadevan L, Megason SG, Size control of the inner ear via hydraulic feedback, Elife. 8 (2019). 10.7554/eLife.39596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chan CJ, Costanzo M, Ruiz-Herrero T, Mönke G, Petrie RJ, Bergert M, Diz-Muñoz A, Mahadevan L, Hiiragi T, Hydraulic control of mammalian embryo size and cell fate, Nature. 571 (2019) 112–116. 10.1038/s41586-019-1309-x. [DOI] [PubMed] [Google Scholar]
- [8].Dumortier JG, Le Verge-Serandour M, Tortorelli AF, Mielke A, de Plater L, Turlier H, Maître J-L, Hydraulic fracturing and active coarsening position the lumen of the mouse blastocyst, Science. 365 (2019) 465–468. 10.1126/science.aaw7709. [DOI] [PubMed] [Google Scholar]
- [9].Swinburne IA, Mosaliganti KR, Upadhyayula S, Liu T-L, Hildebrand DGC, Tsai TY-C, Chen A, Al-Obeidi E, Fass AK, Malhotra S, Engert F, Lichtman JW, Kirchhausen T, Betzig E, Megason SG, Lamellar projections in the endolymphatic sac act as a relief valve to regulate inner ear pressure, Elife. 7 (2018). 10.7554/eLife.37131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Munjal A, Hannezo E, Tsai TY-C, Mitchison TJ, Megason SG, Extracellular hyaluronate pressure shaped by cellular tethers drives tissue morphogenesis, Cell. 184 (2021) 6313–6325.e18. 10.1016/j.cell.2021.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhao R, Cui S, Ge Z, Zhang Y, Bera K, Zhu L, Sun SX, Konstantopoulos K, Hydraulic resistance induces cell phenotypic transition in confinement, Sci Adv. 7 (2021). 10.1126/sciadv.abg4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Petridou NI, Grigolon S, Salbreux G, Hannezo E, Heisenberg C-P, Fluidization-mediated tissue spreading by mitotic cell rounding and non-canonical Wnt signalling, Nat. Cell Biol. 21 (2019) 169–178. 10.1038/s41556-018-0247-4. [DOI] [PubMed] [Google Scholar]
- [13].Navis A, Marjoram L, Bagnat M, Cftr controls lumen expansion and function of Kupffer’s vesicle in zebrafish, Development. 140 (2013) 1703–1712. 10.1242/dev.091819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nelson CM, Gleghorn JP, Pang M-F, Jaslove JM, Goodwin K, Varner VD, Miller E, Radisky DC, Stone HA, Microfluidic chest cavities reveal that transmural pressure controls the rate of lung development, Development. 144 (2017) 4328–4335. 10.1242/dev.154823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gadsby DC, Ion channels versus ion pumps: the principal difference, in principle, Nat. Rev. Mol. Cell Biol. 10 (2009) 344–352. 10.1038/nrm2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Atkins P, Atkins PW, de Paula J, Atkins’ Physical Chemistry, OUP Oxford, 2014. [Google Scholar]
- [17].Farinas J, Verkman AS, Cell volume and plasma membrane osmotic water permeability in epithelial cell layers measured by interferometry, Biophys. J 71 (1996) 3511–3522. 10.1016/S0006-3495(96)79546-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Reuss L, Water transport across cell membranes, eLS. (2012). 10.1002/9780470015902.a0020621.pub2. [DOI] [Google Scholar]
- [19].Li Y, Konstantopoulos K, Zhao R, Mori Y, Sun SX, The importance of water and hydraulic pressure in cell dynamics, J. Cell Sci. 133 (2020). 10.1242/jcs.240341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hoffmann EK, Lambert IH, Pedersen SF, Physiology of cell volume regulation in vertebrates, Physiol. Rev 89 (2009) 193–277. 10.1152/physrev.00037.2007. [DOI] [PubMed] [Google Scholar]
- [21].Dumais J, Forterre Y, “Vegetable Dynamicks”: The Role of Water in Plant Movements, Annu. Rev. Fluid Mech 44 (2012) 453–478. 10.1146/annurev-fluid-120710-101200. [DOI] [Google Scholar]
- [22].Zonia L, Munnik T, Life under pressure: hydrostatic pressure in cell growth and function, Trends Plant Sci. 12 (2007) 90–97. 10.1016/j.tplants.2007.01.006. [DOI] [PubMed] [Google Scholar]
- [23].Hernández-Hernández V, Benítez M, Boudaoud A, Interplay between turgor pressure and plasmodesmata during plant development, J. Exp. Bot 71 (2020) 768–777. 10.1093/jxb/erz434. [DOI] [PubMed] [Google Scholar]
- [24].Taiz L, Taiz Emeritus of MCD Biology Lincoln, Zeiger E, Plant Physiology, Sinauer Associates, 2002. [Google Scholar]
- [25].Petrie RJ, Koo H, Yamada KM, Generation of compartmentalized pressure by a nuclear piston governs cell motility in a 3D matrix, Science. 345 (2014) 1062–1065. 10.1126/science.1256965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Perez-Gonzalez NA, Rochman ND, Yao K, Tao J, Le M-TT, Flanary S, Sablich L, Toler B, Crentsil E, Takaesu F, Lambrus B, Huang J, Fu V, Chengappa P, Jones TM, Holland AJ, An S, Wirtz D, Petrie RJ, Guan K-L, Sun SX, YAP and TAZ regulate cell volume, J. Cell Biol. 218 (2019) 3472–3488. 10.1083/jcb.201902067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Fischbarg J, Water channels and their roles in some ocular tissues, Mol. Aspects Med. 33 (2012) 638–641. 10.1016/j.mam.2012.07.016. [DOI] [PubMed] [Google Scholar]
- [28].Agre P, Kozono D, Aquaporin water channels: molecular mechanisms for human diseases, FEBS Lett. 555 (2003) 72–78. 10.1016/s0014-5793(03)01083-4. [DOI] [PubMed] [Google Scholar]
- [29].Nielsen S, Smith BL, Christensen EI, Agre P, Distribution of the aquaporin CHIP in secretory and resorptive epithelia and capillary endothelia, Proc. Natl. Acad. Sci. U. S. A 90 (1993) 7275–7279. 10.1073/pnas.90.15.7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Farinas J, Kneen M, Moore M, Verkman AS, Plasma membrane water permeability of cultured cells and epithelia measured by light microscopy with spatial filtering, J. Gen. Physiol 110 (1997) 283–296. 10.1085/jgp.110.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Oshio K, Watanabe H, Song Y, Verkman AS, Manley GT, Reduced cerebrospinal fluid production and intracranial pressure in mice lacking choroid plexus water channel Aquaporin-1, FASEB J. 19 (2005) 76–78. 10.1096/fj.04-1711fje. [DOI] [PubMed] [Google Scholar]
- [32].Kuang K, Yiming M, Wen Q, Li Y, Ma L, Iserovich P, Verkman AS, Fischbarg J, Fluid transport across cultured layers of corneal endothelium from aquaporin-1 null mice, Exp. Eye Res. 78 (2004) 791–798. 10.1016/j.exer.2003.11.017. [DOI] [PubMed] [Google Scholar]
- [33].Fischbarg J, Diecke FPJ, Iserovich P, Rubashkin A, The Role of the Tight Junction in Paracellular Fluid Transport across Corneal Endothelium. Electro-osmosis as a Driving Force, J. Membr. Biol 210 (2006) 117–130. 10.1007/s00232-005-0850-8. [DOI] [PubMed] [Google Scholar]
- [34].Maurel C, Boursiac Y, Luu D-T, Santoni V, Shahzad Z, Verdoucq L, Aquaporins in Plants, Physiol. Rev 95 (2015) 1321–1358. 10.1152/physrev.00008.2015. [DOI] [PubMed] [Google Scholar]
- [35].Zeuthen T, Hamann S, la Cour M, Cotransport of H+, lactate and H2O by membrane proteins in retinal pigment epithelium of bullfrog, J. Physiol 497 ( Pt 1) (1996) 3–17. 10.1113/jphysiol.1996.sp021745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Loo DD, Zeuthen T, Chandy G, Wright EM, Cotransport of water by the Na+/glucose cotransporter, Proc. Natl. Acad. Sci. U. S. A 93 (1996) 13367–13370. 10.1073/pnas.93.23.13367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Duquette P-P, Bissonnette P, Lapointe J-Y, Local osmotic gradients drive the water flux associated with Na+/glucose cotransport, Proc. Natl. Acad. Sci. U. S. A 98 (2001) 3796–3801. 10.1073/pnas.071245198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Loo DDF, Wright EM, Zeuthen T, Water pumps, J. Physiol 542 (2002) 53–60. 10.1113/jphysiol.2002.018713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Mollajew R, Zocher F, Horner A, Wiesner B, Klussmann E, Pohl P, Routes of epithelial water flow: aquaporins versus cotransporters, Biophys. J 99 (2010) 3647–3656. 10.1016/j.bpj.2010.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wegner LH, Cotransport of water and solutes in plant membranes: The molecular basis, and physiological functions, AIMS Biophys. 4 (2017) 192–209. 10.3934/biophy.2017.2.192. [DOI] [Google Scholar]
- [41].Zeuthen T, Meinild A-K, Loo DDF, Wright EM, Klaerke DA, Isotonic transport by the Na -glucose cotransporter SGLT1 from humans and rabbit, The Journal of Physiology. 531 (2001) 631–644. 10.1111/j.1469-7793.2001.0631h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Fischbarg J, Fluid transport across leaky epithelia: central role of the tight junction and supporting role of aquaporins, Physiol. Rev 90 (2010) 1271–1290. 10.1152/physrev.00025.2009. [DOI] [PubMed] [Google Scholar]
- [43].Moreau HD, Blanch-Mercader C, Attia R, Maurin M, Alraies Z, Sanséau D, Malbec O, Delgado M-G, Bousso P, Joanny J-F, Voituriez R, Piel M, Lennon-Duménil A-M, Macropinocytosis Overcomes Directional Bias in Dendritic Cells Due to Hydraulic Resistance and Facilitates Space Exploration, Dev. Cell 49 (2019) 171–188.e5. 10.1016/j.devcel.2019.03.024. [DOI] [PubMed] [Google Scholar]
- [44].Vasquez CG, Vachharajani VT, Garzon-Coral C, Dunn AR, Physical basis for the determination of lumen shape in a simple epithelium, Nat. Commun 12 (2021) 5608. 10.1038/s41467-021-25050-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ryan AQ, Chan CJ, Graner F, Hiiragi T, Lumen Expansion Facilitates Epiblast-Primitive Endoderm Fate Specification during Mouse Blastocyst Formation, Dev. Cell 51 (2019) 684–697.e4. 10.1016/j.devcel.2019.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Datta A, Bryant DM, Mostov KE, Molecular regulation of lumen morphogenesis, Curr. Biol 21 (2011) R126–36. 10.1016/j.cub.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Urry LA, Cain ML, Wasserman SA, Minorsky PV, Reece JB, Campbell biology, Pearson Education, Incorporated, 2017. [Google Scholar]
- [48].Hill AE, Fluid transport: a guide for the perplexed, J. Membr. Biol 223 (2008) 1–11. 10.1007/s00232-007-9085-1. [DOI] [PubMed] [Google Scholar]
- [49].Spring KR, Routes and mechanism of fluid transport by epithelia, Annu. Rev. Physiol 60 (1998) 105–119. 10.1146/annurev.physiol.60.1.105. [DOI] [PubMed] [Google Scholar]
- [50].Spring KR, Epithelial Fluid Transport—A Century of Investigation, Physiology. 14 (1999) 92–98. 10.1152/physiologyonline.1999.14.3.92. [DOI] [PubMed] [Google Scholar]
- [51].Diamond JM, THE MECHANISM OF ISOTONIC WATER TRANSPORT, J. Gen. Physiol 48 (1964) 15–42. 10.1085/jgp.48.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Diamond JM, The mechanism of water transport by the gall-bladder, J. Physiol 161 (1962) 503–527. 10.1113/jphysiol.1962.sp006900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Diamond JM, Bossert WH, Standing-gradient osmotic flow. A mechanism for coupling of water and solute transport in epithelia, J. Gen. Physiol 50 (1967) 2061–2083. 10.1085/jgp.50.8.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Bentzel CJ, Davies M, Scott WN, Zatzman M, Solomon AK, Osmotic volume flow in the proximal tubule of Necturus kidney, J. Gen. Physiol 51 (1968) 517–533. 10.1085/jgp.51.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Shachar-Hill B, Hill AE, Paracellular fluid transport by epithelia, Int. Rev. Cytol 215 (2002) 319–350. 10.1016/s0074-7696(02)15014-5. [DOI] [PubMed] [Google Scholar]
- [56].Frömter E, Diamond J, Route of Passive Ion Permeation in Epithelia, Nat. New Biol. 235 (1972) 9–13. 10.1038/newbio235009a0. [DOI] [PubMed] [Google Scholar]
- [57].Machen TE, Erlij D, Wooding FB, Permeable junctional complexes. The movement of lanthanum across rabbit gallbladder and intestine, J. Cell Biol. 54 (1972) 302–312. 10.1083/jcb.54.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Hill AE, Shachar-Hill B, Shachar-Hill Y, What are aquaporins for?, J. Membr. Biol 197 (2004) 1–32. 10.1007/s00232-003-0639-6. [DOI] [PubMed] [Google Scholar]
- [59].Diamond JM, Tormey JM, Role of Long Extracellular Channels in Fluid Transport across Epithelia, Nature. 210 (1966) 817–820. 10.1038/210817a0. [DOI] [PubMed] [Google Scholar]
- [60].Sánchez JM, Li Y, Rubashkin A, Iserovich P, Wen Q, Ruberti JW, Smith RW, Rittenband D, Kuang K, Diecke FPJ, Fischbarg J, Evidence for a Central Role for Electro-Osmosis in Fluid Transport by Corneal Endothelium, The Journal of Membrane Biology. 187 (2002) 37–50. 10.1007/s00232-001-0151-9. [DOI] [PubMed] [Google Scholar]
- [61].Casares L, Vincent R, Zalvidea D, Campillo N, Hydraulic fracture during epithelial stretching, Nat. Mater (2015). https://www.nature.com/articles/nmat4206. [DOI] [PMC free article] [PubMed]
- [62].Kim YS, Fan R, Kremer L, Kuempel-Rink N, Mildner K, Zeuschner D, Hekking L, Stehling M, Bedzhov I, Deciphering epiblast lumenogenesis reveals proamniotic cavity control of embryo growth and patterning, Sci Adv. 7 (2021). 10.1126/sciadv.abe1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Cosgrove DJ, Cell wall yield properties of growing tissue : evaluation by in vivo stress relaxation, Plant Physiol. 78 (1985) 347–356. 10.1104/pp.78.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Schopfer P, Biomechanics of plant growth, Am. J. Bot 93 (2006) 1415–1425. 10.3732/ajb.93.10.1415. [DOI] [PubMed] [Google Scholar]
- [65].Cosgrove DJ, Growth of the plant cell wall, Nat. Rev. Mol. Cell Biol. 6 (2005) 850–861. 10.1038/nrm1746. [DOI] [PubMed] [Google Scholar]
- [66].He L, Tao J, Maity D, Si F, Wu Y, Wu T, Prasath V, Wirtz D, Sun SX, Role of membrane-tension gated Ca2+ flux in cell mechanosensation, J. Cell Sci. 131 (2018). 10.1242/jcs.208470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Zhao R, Afthinos A, Zhu T, Mistriotis P, Li Y, Serra SA, Zhang Y, Yankaskas CL, He S, Valverde MA, Sun SX, Konstantopoulos K, Cell sensing and decision-making in confinement: The role of TRPM7 in a tug of war between hydraulic pressure and cross-sectional area, Sci Adv. 5 (2019) eaaw7243. 10.1126/sciadv.aaw7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Stewart MP, Helenius J, Toyoda Y, Ramanathan SP, Muller DJ, Hyman AA, Hydrostatic pressure and the actomyosin cortex drive mitotic cell rounding, Nature. 469 (2011) 226–230. 10.1038/nature09642. [DOI] [PubMed] [Google Scholar]
- [69].Zlotek-Zlotkiewicz E, Monnier S, Cappello G, Le Berre M, Piel M, Optical volume and mass measurements show that mammalian cells swell during mitosis, J. Cell Biol. 211 (2015) 765–774. 10.1083/jcb.201505056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Son S, Kang JH, Oh S, Kirschner MW, Mitchison TJ, Manalis S, Resonant microchannel volume and mass measurements show that suspended cells swell during mitosis, J. Cell Biol. 211 (2015) 757–763. 10.1083/jcb.201505058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Kroeger JH, Zerzour R, Geitmann A, Regulator or driving force? The role of turgor pressure in oscillatory plant cell growth, PLoS One. 6 (2011) e18549. 10.1371/journal.pone.0018549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Buckley TN, The control of stomata by water balance, New Phytol. 168 (2005) 275–292. 10.1111/j.1469-8137.2005.01543.x. [DOI] [PubMed] [Google Scholar]
- [73].Charras GT, Yarrow JC, Horton MA, Mahadevan L, Mitchison TJ, Non-equilibration of hydrostatic pressure in blebbing cells, Nature. 435 (2005) 365–369. 10.1038/nature03550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Charras GT, A short history of blebbing, J. Microsc 231 (2008) 466–478. 10.1111/j.1365-2818.2008.02059.x. [DOI] [PubMed] [Google Scholar]
- [75].Taloni A, Kardash E, Salman OU, Truskinovsky L, Zapperi S, La Porta CAM, Volume Changes During Active Shape Fluctuations in Cells, Phys. Rev. Lett 114 (2015) 208101. 10.1103/PhysRevLett.114.208101. [DOI] [PubMed] [Google Scholar]
- [76].Mathur J, Local interactions shape plant cells, Curr. Opin. Cell Biol. 18 (2006) 40–46. 10.1016/j.ceb.2005.12.002. [DOI] [PubMed] [Google Scholar]
- [77].Stroka KM, Jiang H, Chen S-H, Tong Z, Wirtz D, Sun SX, Konstantopoulos K, Water permeation drives tumor cell migration in confined microenvironments, Cell. 157 (2014) 611–623. 10.1016/j.cell.2014.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Shoji K, Kawano R, Osmotic-engine-driven liposomes in microfluidic channels, Lab Chip. 19 (2019) 3472–3480. 10.1039/c9lc00788a. [DOI] [PubMed] [Google Scholar]
- [79].Loitto VM, Huang C, Sigal YJ, Jacobson K, Filopodia are induced by aquaporin-9 expression, Exp. Cell Res. 313 (2007) 1295–1306. 10.1016/j.yexcr.2007.01.023. [DOI] [PubMed] [Google Scholar]
- [80].Jain RK, Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy, Science. 307 (2005) 58–62. 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- [81].Kao Y-C, Jheng J-R, Pan H-J, Liao W-Y, Lee C-H, Kuo P-L, Elevated hydrostatic pressure enhances the motility and enlarges the size of the lung cancer cells through aquaporin upregulation mediated by caveolin-1 and ERK1/2 signaling, Oncogene. 36 (2017) 863–874. 10.1038/onc.2016.255. [DOI] [PubMed] [Google Scholar]
- [82].Choi HY, Yang G-M, Dayem AA, Saha SK, Kim K, Yoo Y, Hong K, Kim J-H, Yee C, Lee K-M, Cho S-G, Hydrodynamic shear stress promotes epithelial-mesenchymal transition by downregulating ERK and GSK3β activities, Breast Cancer Res. 21 (2019) 6. 10.1186/s13058-018-1071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Piotrowski-Daspit AS, Tien J, Nelson CM, Interstitial fluid pressure regulates collective invasion in engineered human breast tumors via Snail, vimentin, and E-cadherin, Integr. Biol. 8 (2016) 319–331. 10.1039/c5ib00282f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Alvers AL, Ryan S, Scherz PJ, Huisken J, Bagnat M, Single continuous lumen formation in the zebrafish gut is mediated by smoothened-dependent tissue remodeling, Development. 141 (2014) 1110–1119. 10.1242/dev.100313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Bryant DM, Roignot J, Datta A, Overeem AW, Kim M, Yu W, Peng X, Eastburn DJ, Ewald AJ, Werb Z, Mostov KE, A molecular switch for the orientation of epithelial cell polarization, Dev. Cell 31 (2014) 171–187. 10.1016/j.devcel.2014.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Bryant DM, Datta A, Rodríguez-Fraticelli AE, Peränen J, Martín-Belmonte F, Mostov KE, A molecular network for de novo generation of the apical surface and lumen, Nat. Cell Biol. 12 (2010) 1035–1045. 10.1038/ncb2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Sigurbjörnsdóttir S, Mathew R, Leptin M, Molecular mechanisms of de novo lumen formation, Nat. Rev. Mol. Cell Biol. 15 (2014) 665–676. 10.1038/nrm3871. [DOI] [PubMed] [Google Scholar]
- [88].Piotrowski-Daspit AS, Simi AK, Pang M-F, Tien J, Nelson CM, A 3D Culture Model to Study How Fluid Pressure and Flow Affect the Behavior of Aggregates of Epithelial Cells, Methods Mol. Biol 1501 (2017) 245–257. 10.1007/978-1-4939-6475-8_12. [DOI] [PubMed] [Google Scholar]
- [89].Tallapragada NP, Cambra HM, Wald T, Keough Jalbert S, Abraham DM, Klein OD, Klein AM, Inflation-collapse dynamics drive patterning and morphogenesis in intestinal organoids, Cell Stem Cell. 28 (2021) 1516–1532.e14. 10.1016/j.stem.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Yang Q, Xue S-L, Chan CJ, Rempfler M, Vischi D, Maurer-Gutierrez F, Hiiragi T, Hannezo E, Liberali P, Cell fate coordinates mechano-osmotic forces in intestinal crypt formation, Nat. Cell Biol. 23 (2021) 733–744. 10.1038/s41556-021-00700-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Ruiz-Herrero T, Alessandri K, Gurchenkov BV, Nassoy P, Mahadevan L, Organ size control via hydraulically gated oscillations, Development. 144 (2017) 4422–4427. 10.1242/dev.153056. [DOI] [PubMed] [Google Scholar]
- [92].Chan CJ, Hiiragi T, Integration of luminal pressure and signalling in tissue self-organization, Development. 147 (2020). 10.1242/dev.181297. [DOI] [PubMed] [Google Scholar]
- [93].Yang J, Duan X, Fraser AK, Choudhury MI, Ewald AJ, Li R, Sun SX, Microscale pressure measurements based on an immiscible fluid/fluid interface, Sci. Rep 9 (2019) 20044. 10.1038/s41598-019-56573-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Style RW, Boltyanskiy R, German GK, Hyland C, MacMinn CW, Mertz AF, Wilen LA, Xu Y, Dufresne ER, Traction force microscopy in physics and biology, Soft Matter. 10 (2014) 4047–4055. 10.1039/C4SM00264D. [DOI] [PubMed] [Google Scholar]
- [95].Leonavicius K, Royer C, Preece C, Davies B, Biggins JS, Srinivas S, Mechanics of mouse blastocyst hatching revealed by a hydrogel-based microdeformation assay, Proc. Natl. Acad. Sci. U. S. A 115 (2018) 10375–10380. 10.1073/pnas.1719930115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Yang X, Xu T, Molecular mechanism of size control in development and human diseases, Cell Res. 21 (2011) 715–729. 10.1038/cr.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Powell AE, Lenhard M, Control of organ size in plants, Curr. Biol 22 (2012) R360–7. 10.1016/j.cub.2012.02.010. [DOI] [PubMed] [Google Scholar]
- [98].Yu F-X, Zhao B, Guan K-L, Hippo Pathway in Organ Size Control, Tissue Homeostasis, and Cancer, Cell. 163 (2015) 811–828. 10.1016/j.cell.2015.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Hoijman E, Rubbini D, Colombelli J, Alsina B, Mitotic cell rounding and epithelial thinning regulate lumen growth and shape, Nat. Commun 6 (2015) 7355. 10.1038/ncomms8355. [DOI] [PubMed] [Google Scholar]
- [100].Whitfield TT, Development of the inner ear, Curr. Opin. Genet. Dev 32 (2015) 112–118. 10.1016/j.gde.2015.02.006. [DOI] [PubMed] [Google Scholar]
- [101].Desmond ME, Jacobson AG, Embryonic brain enlargement requires cerebrospinal fluid pressure, Dev. Biol 57 (1977) 188–198. 10.1016/0012-1606(77)90364-5. [DOI] [PubMed] [Google Scholar]
- [102].Mitchison TJ, Charras GT, Mahadevan L, Implications of a poroelastic cytoplasm for the dynamics of animal cell shape, Semin. Cell Dev. Biol 19 (2008) 215–223. 10.1016/j.semcdb.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Chaudhuri O, Cooper-White J, Janmey PA, Mooney DJ, Shenoy VB, Effects of extracellular matrix viscoelasticity on cellular behaviour, Nature. 584 (2020) 535–546. 10.1038/s41586-020-2612-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Skotheim JM, Mahadevan L, Physical Limits and Design Principles for Plant and Fungal Movements, Science. 308 (2005) 1308–1310. 10.1126/science.1107976. [DOI] [PubMed] [Google Scholar]
- [105].Liu M, Tolg C, Turley E, Dissecting the Dual Nature of Hyaluronan in the Tumor Microenvironment, Front. Immunol 10 (2019) 947. 10.3389/fimmu.2019.00947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Toole BP, Hyaluronan in morphogenesis, Semin. Cell Dev. Biol 12 (2001) 79–87. 10.1006/scdb.2000.0244. [DOI] [PubMed] [Google Scholar]
- [107].Cowman MK, Schmidt TA, Raghavan P, Stecco A, Viscoelastic Properties of Hyaluronan in Physiological Conditions, F1000Res. 4 (2015) 622. 10.12688/f1000research.6885.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Haddon CM, Lewis JH, Hyaluronan as a propellant for epithelial movement: the development of semicircular canals in the inner ear of Xenopus, Development. 112 (1991) 541–550. 10.1242/dev.112.2.541. [DOI] [PubMed] [Google Scholar]
- [109].Lecuit T, Lenne P-F, Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis, Nat. Rev. Mol. Cell Biol. 8 (2007) 633–644. 10.1038/nrm2222. [DOI] [PubMed] [Google Scholar]
- [110].Hannezo E, Heisenberg C-P, Mechanochemical Feedback Loops in Development and Disease, Cell. 178 (2019) 12–25. 10.1016/j.cell.2019.05.052. [DOI] [PubMed] [Google Scholar]
- [111].Verger S, Long Y, Boudaoud A, Hamant O, A tension-adhesion feedback loop in plant epidermis, Elife. 7 (2018). 10.7554/eLife.34460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Xiao R, Xu XZS, Mechanosensitive channels: in touch with Piezo, Curr. Biol 20 (2010) R936–8. 10.1016/j.cub.2010.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Christensen AP, Corey DP, TRP channels in mechanosensation: direct or indirect activation?, Nat. Rev. Neurosci 8 (2007) 510–521. 10.1038/nrn2149. [DOI] [PubMed] [Google Scholar]
- [114].Vignes H, Vagena-Pantoula C, Prakash M, Fukui H, Norden C, Mochizuki N, Jug F, Vermot J, Extracellular mechanical forces drive endocardial cell volume decrease during zebrafish cardiac valve morphogenesis, Dev. Cell 57 (2022) 598–609.e5. 10.1016/j.devcel.2022.02.011. [DOI] [PubMed] [Google Scholar]
- [115].Benfenati V, Caprini M, Dovizio M, Mylonakou MN, Ferroni S, Ottersen OP, Amiry-Moghaddam M, An aquaporin-4/transient receptor potential vanilloid 4 (AQP4/TRPV4) complex is essential for cell-volume control in astrocytes, Proc. Natl. Acad. Sci. U. S. A 108 (2011) 2563–2568. 10.1073/pnas.1012867108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Jaslove JM, Goodwin K, Sundarakrishnan A, Spurlin JW, Mao S, Košmrlj A, Nelson CM, Transmural pressure signals through retinoic acid to regulate lung branching, Development. 149 (2022). 10.1242/dev.199726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Cunningham TJ, Duester G, Mechanisms of retinoic acid signalling and its roles in organ and limb development, Nat. Rev. Mol. Cell Biol. 16 (2015) 110–123. 10.1038/nrm3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Durdu S, Iskar M, Revenu C, Schieber N, Kunze A, Bork P, Schwab Y, Gilmour D, Luminal signalling links cell communication to tissue architecture during organogenesis, Nature. 515 (2014) 120–124. 10.1038/nature13852. [DOI] [PubMed] [Google Scholar]
- [119].Mercker M, Köthe A, Marciniak-Czochra A, Mechanochemical Symmetry Breaking in Hydra Aggregates, Biophys. J 108 (2015) 2396–2407. 10.1016/j.bpj.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Hobmayer B, Rentzsch F, Kuhn K, Happel CM, von Laue CC, Snyder P, Rothbächer U, Holstein TW, WNT signalling molecules act in axis formation in the diploblastic metazoan Hydra, Nature. 407 (2000) 186–189. 10.1038/35025063. [DOI] [PubMed] [Google Scholar]
- [121].Ferenc J, Papasaikas P, Ferralli J, Nakamura Y, Smallwood S, Tsiairis CD, Mechanical oscillations orchestrate axial patterning through Wnt activation in Hydra, Sci Adv. 7 (2021) eabj6897. 10.1126/sciadv.abj6897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Sampathkumar A, Mechanical feedback-loop regulation of morphogenesis in plants, Development. 147 (2020). 10.1242/dev.177964. [DOI] [PubMed] [Google Scholar]
- [123].Hamant O, Traas J, The mechanics behind plant development, New Phytol. 185 (2010) 369–385. 10.1111/j.1469-8137.2009.03100.x. [DOI] [PubMed] [Google Scholar]
- [124].Wells A, Huttenlocher A, Lauffenburger DA, Calpain proteases in cell adhesion and motility, Int. Rev. Cytol 245 (2005) 1–16. 10.1016/S0074-7696(05)45001-9. [DOI] [PubMed] [Google Scholar]
- [125].Malivert A, Hamant O, Ingram G, The contribution of mechanosensing to epidermal cell fate specification, Curr. Opin. Genet. Dev 51 (2018) 52–58. 10.1016/j.gde.2018.06.011. [DOI] [PubMed] [Google Scholar]
- [126].Paredez AR, Somerville CR, Ehrhardt DW, Visualization of cellulose synthase demonstrates functional association with microtubules, Science. 312 (2006) 1491–1495. 10.1126/science.1126551. [DOI] [PubMed] [Google Scholar]
- [127].Hamant O, Inoue D, Bouchez D, Dumais J, Mjolsness E, Are microtubules tension sensors?, Nat. Commun 10 (2019) 2360. 10.1038/s41467-019-10207-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Livanos P, Müller S, Division Plane Establishment and Cytokinesis, Annu. Rev. Plant Biol. 70 (2019) 239–267. 10.1146/annurev-arplant-050718-100444. [DOI] [PubMed] [Google Scholar]
- [129].Chugh M, Reißner M, Bugiel M, Lipka E, Herrmann A, Roy B, Müller S, Schäffer E, Phragmoplast Orienting Kinesin 2 Is a Weak Motor Switching between Processive and Diffusive Modes, Biophys. J 115 (2018) 375–385. 10.1016/j.bpj.2018.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Bringmann M, Bergmann DC, Tissue-wide Mechanical Forces Influence the Polarity of Stomatal Stem Cells in Arabidopsis, Curr. Biol 27 (2017) 877–883. 10.1016/j.cub.2017.01.059. [DOI] [PubMed] [Google Scholar]
- [131].Li J, Wang Z, Chu Q, Jiang K, Li J, Tang N, The Strength of Mechanical Forces Determines the Differentiation of Alveolar Epithelial Cells, Dev. Cell 44 (2018) 297–312.e5. 10.1016/j.devcel.2018.01.008. [DOI] [PubMed] [Google Scholar]
- [132].Choudhury MI, Li Y, Mistriotis P, Vasconcelos A, Trans-epithelial fluid pumping performance of renal epithelial cells and mechanics of cystic expansion, (2021). https://www.researchsquare.com/article/rs-524708/latest.pdf.
- [133].Krens SFG, Veldhuis JH, Barone V, Čapek D, Maître J-L, Brodland GW, Heisenberg C-P, Interstitial fluid osmolarity modulates the action of differential tissue surface tension in progenitor cell segregation during gastrulation, Development. 144 (2017) 1798–1806. 10.1242/dev.144964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Dasgupta S, Gupta K, Zhang Y, Viasnoff V, Prost J, Physics of lumen growth, Proc. Natl. Acad. Sci. U. S. A 115 (2018) E4751–E4757. 10.1073/pnas.1722154115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Duclut C, Prost J, Jülicher F, Hydraulic and electric control of cell spheroids, Proc. Natl. Acad. Sci. U. S. A 118 (2021). 10.1073/pnas.2021972118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Navis A, Bagnat M, Developing pressures: fluid forces driving morphogenesis, Curr. Opin. Genet. Dev 32 (2015) 24–30. 10.1016/j.gde.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Mongera A, Rowghanian P, Gustafson HJ, Shelton E, Kealhofer DA, Carn EK, Serwane F, Lucio AA, Giammona J, Campàs O, A fluid-to-solid jamming transition underlies vertebrate body axis elongation, Nature. 561 (2018) 401–405. 10.1038/s41586-018-0479-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Bénazéraf B, Francois P, Baker RE, Denans N, Little CD, Pourquié O, A random cell motility gradient downstream of FGF controls elongation of an amniote embryo, Nature. 466 (2010) 248–252. 10.1038/nature09151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Wang X, Merkel M, Sutter LB, Erdemci-Tandogan G, Manning ML, Kasza KE, Anisotropy links cell shapes to tissue flow during convergent extension, Proc. Natl. Acad. Sci. U. S. A 117 (2020) 13541–13551. 10.1073/pnas.1916418117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Stooke-Vaughan GA, Campàs O, Physical control of tissue morphogenesis across scales, Curr. Opin. Genet. Dev 51 (2018) 111–119. 10.1016/j.gde.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


