Abstract
Objective:
To study the trajectories of metabolic parameters after bilateral oophorectomy.
Study design:
This population-based cohort study included a random sample of all premenopausal women who underwent bilateral oophorectomy at or before age 45 years from 1988 to 2007 in Olmsted County, Minnesota, and their age-matched (±1 year) referent women who did not undergo bilateral oophorectomy.
Main outcome measures:
The medical records of all women were reviewed to collect the metabolic parameters over a 10-year period. We compared three groups of women: 1) referent women (n = 270), 2) women who underwent bilateral oophorectomy and received estrogen therapy (n = 163), and 3) women who underwent bilateral oophorectomy and did not receive estrogen therapy (n = 107).
Results:
Over 10 years of follow-up, the three groups had significantly different mean values of diastolic blood pressure, weight, body mass index (BMI), total cholesterol, triglycerides, and high-density lipoprotein cholesterol (HDL-C). However, women with and without bilateral oophorectomy were already different at baseline for hyperlipidemia, systolic blood pressure, weight, and BMI. Nevertheless, the trajectories of change over 10 years were significant for weight (group by time interaction p = 0.03), BMI (p = 0.03), and HDL-C (p = 0.004). The changes occurred primarily in the initial 4–5 years. Women who received estrogen therapy after bilateral oophorectomy were comparable to the referent women with respect to the weight and BMI trends, and they experienced an increase in HDL-C over time.
Conclusion:
Women who underwent bilateral oophorectomy before menopause experienced unfavorable changes in some metabolic parameters possibly increasing their cardiovascular risk.
Keywords: Bilateral oophorectomy, menopause, weight, blood pressure, lipid profile, estrogen therapy
1. Introduction
We and other investigators have reported an increased risk of coronary artery disease (CAD) incidence or mortality after bilateral oophorectomy performed before the age of spontaneous menopause.[1–6] The effects of bilateral oophorectomy on body weight, body composition, lipid profile, and blood pressure in young women have also been reported.[1, 7, 8] However, it is not known how rapidly these metabolic parameters change after bilateral oophorectomy. In addition, it remains unclear which of these parameters are already different at baseline in women who undergo bilateral oophorectomy compared to women who do not.[9] Knowledge of these metabolic changes is clinically important because it may guide timely interventions.
Even though estrogen therapy (ET) may reduce the risk of several adverse health consequences that result from early oophorectomy, the effects of ET on the metabolic parameters in the immediate years following bilateral oophorectomy are not well known.[1] The current study was conducted to identify trajectories of change in blood pressure, weight, and lipid profiles over a 10-year period after bilateral oophorectomy performed in premenopausal women. A secondary objective of the study was to evaluate the effect of ET on these trajectories.
2. Methods
2.1. Study Population
The study participants were selected from the Mayo Clinic Cohort Study of Oophorectomy and Aging-2 (MOA-2). The methodology of MOA-2 and the clinical characteristics of the women included in MOA-2 have been previously described.[1, 9–11] Briefly, MOA-2 includes all premenopausal women who underwent bilateral oophorectomy before age 50 years in Olmsted County, Minnesota between January 1, 1988 and December 31, 2007 (n = 1,747). We excluded women who underwent oophorectomy at age 46–49 years (n = 622) and women who underwent oophorectomy for primary or metastatic ovarian cancer (n = 54), for the treatment of estrogen-sensitive malignancy (eg, breast cancer; n = 26), or because they carried a high genetic risk of cancer (eg, BRCA1 or BRCA2 carriers; n = 14). The medical records of these women were manually abstracted to confirm bilateral oophorectomy and to collect information on surgical indication, surgical pathology, and other clinical characteristics.[10, 11]
Bilateral oophorectomy was defined as the removal of both ovaries within the same surgical procedure or as the removal of the remaining ovary for women who had previously undergone unilateral oophorectomy. The index date was defined as the date of bilateral oophorectomy. Each woman who underwent bilateral oophorectomy was matched to a referent woman selected using simple random sampling among Olmsted County residents who were born in the same year (±1 year) and who had at least one ovary intact as of index date.[10]
In the current study, in order to reduce the amount of medical record abstraction, we selected a random sample of 270 women among a total of 1,031 women from MOA-2 who underwent bilateral oophorectomy at age ≤45 years. Because most women used ET for some time after oophorectomy, the random sampling was stratified by estrogen use and was weighted to obtain a similar number of women with and without ET (Figure 1; at least 1 year of ET). For each woman selected from the MOA-2 bilateral oophorectomy cohort, the corresponding age-matched referent woman was also selected, and was assigned the same index date. The sample size was derived from power calculations for detecting differences in trajectories of the metabolic parameters over 10 years of follow-up (interaction between group status and years). All data were collected using the Rochester Epidemiology Project (REP) medical records-linkage system. Extensive details about the REP have been reported elsewhere.[12–15] All research activities were approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards. This study follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
Figure 1.
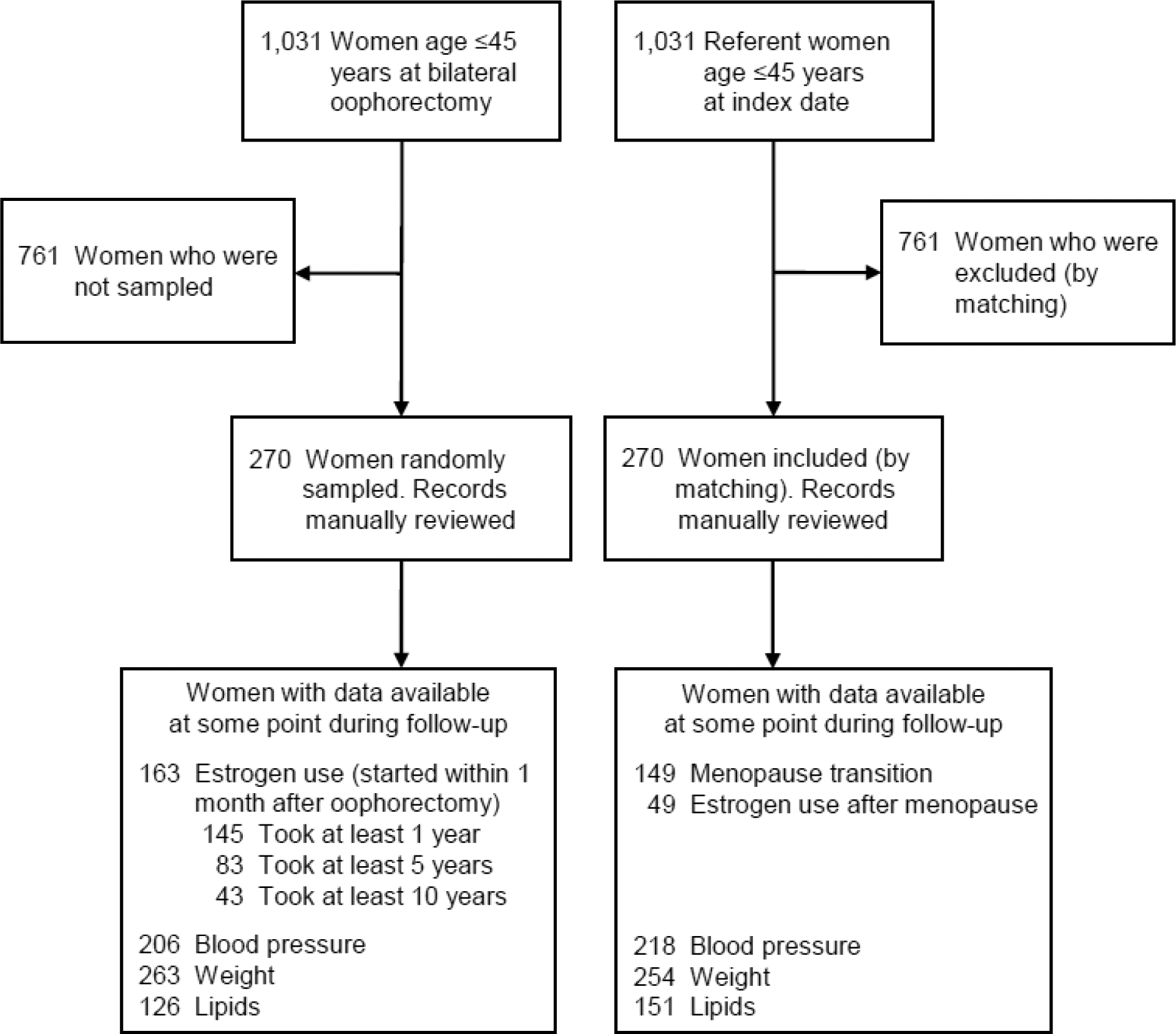
Flow chart of the two study cohorts. All women who underwent bilateral oophorectomy at age ≤45 years and age-matched (±1 year) referent women were identified from the Mayo Clinic Cohort Study of Oophorectomy and Aging-2 (MOA-2). A random sample stratified by estrogen therapy was selected among women who underwent bilateral oophorectomy. The corresponding age-matched referent women were passively included (by virtue of the matching). The medical records for the sampled women were abstracted for metabolic parameters using the Rochester Epidemiology Project medical records-linkage system.
2.2. Data collection
Metabolic parameters were manually abstracted from the medical records in the REP medical records-linkage system by two physicians (EK and LGR) and three nurse abstractors. The metabolic parameters included outpatient systolic and diastolic blood pressure, height, weight, and fasting lipids including total cholesterol, triglycerides, low-density lipoprotein cholesterol, (LDL-C), and high-density lipoprotein cholesterol (HDL-C). For height, we used the measurement closest to and before bilateral oophorectomy or index date (single adult height measure). All other parameters were abstracted from medical records, one value per year for up to 3 years before the index date, and for up to 10 years after. If multiple measurements were available in the same year, the measurement closest to July 1st (mid-point of year) was chosen. However, we did not include any blood pressure or lipid measurements taken during an acute illness, emergency department visit, or hospitalization. Body mass index (BMI) was calculated for each weight measurement available using a single adult height measurement for each woman. Additional data were also collected on the onset of antihypertensive medication use (for any indication including migraine prevention) and of lipid lowering medication use at any time within 3 years before the index date or up to 10 years after.
Hypertension onset was defined as the earliest date of systolic blood pressure >140 mmHg and/or diastolic blood pressure >90 mmHg, or the onset of antihypertensive medication use. Hyperlipidemia onset was defined as the earliest date of total cholesterol ≥200 mg/dL, triglycerides ≥150 mg/dL, HDL-C ≤50 mg/dL, or the onset of lipid lowering medication use.
The complete medical records of women in both the bilateral oophorectomy and referent cohorts were manually abstracted for demographics and life habits information (eg, tobacco use). Detailed information on ET use by women in the oophorectomy cohort was also collected, including the type, dose, route, and duration of treatment.[10, 11]
2.3. Statistical methods
Women were followed passively through the abstraction of medical records in the records-linkage system from the index date to the earliest occurring of 3 endpoints: death, last visit with a REP provider, or December 31st of the tenth year after index. Women who started ET within one month after bilateral oophorectomy were classified as “on ET” if they were still taking ET at the date of measurement of each parameter. Women who discontinued ET were moved from the “on ET” group to the “not on ET” group for that measurement time-point, and they remained in the “not on ET group” to the end of follow-up. Each parameter (blood pressure, weight, BMI, and lipids) was recorded only once per year after the index date, and we used a simple time scale of 0 to 10 years.
Generalized estimating equation (GEE) linear models were used to compare the trends in metabolic measures after the index date for bilateral oophorectomy on ET, bilateral oophorectomy not on ET, and referent women. For each model, we reported mean values for these 3 groups. Variables for group status, years after index, and the interaction of these two variables were included in the models and 3 separate p-values were reported. The models were also adjusted for age at index, years of education (≤12, 13–16, >16), and smoking status (current or former vs never). Age and smoking status were available for all women, and women missing years of education were assigned to the reference group (≤12 years). Since the metabolic measures were collected as part of routine medical care, each woman could have a different number of measurements available during the study period, and the GEE models in the primary analyses were limited to these available measurements. An autoregressive correlation structure was used to account for the repeated measurements within women. This correlation structure assumed that measurements within a single woman were correlated, with higher correlation for the measurements that were closer in time versus lower correlation for the measurements that were farther apart in time.
Women with hypertension before the index date were excluded from models for systolic and diastolic blood pressure, and women with hyperlipidemia before the index date were excluded from the models for lipids. For women who developed hypertension within ten years after index and were treated, the last blood pressure value before the initiation of treatment was carried forward in the analysis (for any subsequent years). A similar approach was used for women who developed hyperlipidemia during follow-up. If pre-treatment measurements were not available, the values were imputed as 140 mmHg for systolic and 90 mmHg for diastolic blood pressure, or as 200 mg/dL for total cholesterol, 150 mg/dL for triglycerides, 50 mg/dL for HDL-C, and 100 mg/dL for LDL-C.
Figures were generated to show the smoothed trajectories over time of the mean parameter values predicted from the GEE models. All analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC), and tests of statistical significance were conducted at the two-tailed alpha level of 0.05.
2.4. Sensitivity analyses
We performed 4 sets of sensitivity analyses. First, to study the effect of age at estrogen deprivation, we repeated the GEE models stratified by age at oophorectomy or index date (<40 years vs. 40–45 years). Second, to study the possible effect of missing values, we repeated the GEE models after using multiple imputation methods to impute metabolic measurements after the index date that were missing.[16] Third, to study the effect of a longer duration of ET use, we repeated the GEE models in women with ≥5 years of ET after bilateral oophorectomy, women who did not receive ET or with <5 years of ET after bilateral oophorectomy, and referent women. The length of follow-up was restricted to measurements collected 5–10 years after index. Fourth, to study the effect of differences preceding the index date, we included in the GEE models both the measurements up to 3 years before the index date and the measurements up to 10 years after. This fourth set of sensitivity analyses only involved 2 groups, women who did or did not undergo bilateral oophorectomy, because ET use did not apply before the index date. Women with hypertension or hyperlipidemia more than 3 years before index date were excluded from the corresponding analyses.
3. Results
3.1. Characteristics at the index date
In the MOA-2 study, there were 1,031 women who underwent bilateral oophorectomy at age ≤45 years and 1,031 age-matched referent women. A weighted random sample of 270 women who underwent bilateral oophorectomy was selected, among whom 163 (60.4%) started ET within one month after oophorectomy and 107 (39.6%) did not (Figure 1). Women who were randomly selected had demographic and clinical characteristics similar to women who were not selected (data not shown). The corresponding 270 age-matched referent women were also selected. Most women had data available for outpatient blood pressure and weight at some point during the 10-year follow-up (Figure 1). By contrast, data for lipids were available only for a subset of women in both groups.
Table 1 shows the baseline characteristics of the randomly sampled matched pairs. The women who underwent bilateral oophorectomy were less educated, were more often smokers, and were more likely to have had a prior unilateral oophorectomy. Only 6 referent women (2.2%) had undergone a unilateral oophorectomy before the index date. Finally, most women underwent hysterectomy concurrently with bilateral oophorectomy. Women who underwent bilateral oophorectomy had significantly more hyperlipidemia and significantly higher systolic blood pressure, weight, and BMI at the index date compared to the referent women.
Table 1.
Demographic and clinical characteristics at baseline among women who underwent bilateral oophorectomy and age-matched referent women.
| Characteristicsa | Bilateral oophorectomy (n=270) | Referent women (n=270) | p-valueb | ||
|---|---|---|---|---|---|
| N | % or median (IQR) | N | % or median (IQR) | ||
| Age at index (years) | -- | ||||
| <40 | 34.1 | 34.1 | |||
| 40–45 | 65.9 | 65.9 | |||
| Calendar year at index | -- | ||||
| 1988–1997 | 44.8 | 44.8 | |||
| 1998–2007 | 55.2 | 55.2 | |||
| Race | 0.21 | ||||
| White | 97.0 | 93.7 | |||
| Black | 1.5 | 2.6 | |||
| Asian | 0.7 | 3.7 | |||
| Other | 0.7 | 0.0 | |||
| Hispanic ethnicity | 1.5 | 1.5 | >0.99 | ||
| Years of education | 0.003 | ||||
| ≤12 | 37.9 | 24.2 | |||
| 13–16 | 50.9 | 56.8 | |||
| >16 | 11.2 | 18.9 | |||
| Household incomec | 0.17 | ||||
| <$42,000 | 31.1 | 21.6 | |||
| $42,000–56,999 | 28.5 | 25.6 | |||
| $57,000–71,999 | 19.5 | 25.6 | |||
| ≥$72,000 | 21.0 | 27.1 | |||
| Smoking status | 0.009 | ||||
| Never | 50.4 | 58.5 | |||
| Former | 21.5 | 25.2 | |||
| Current | 28.1 | 16.3 | |||
| Hysterectomy status | <0.001 | ||||
| No | 1.5 | 94.4 | |||
| Before | 10.7 | 5.6 | |||
| Concurrent | 87.8 | ||||
| Prior unilateral oophorectomy | 13.3 | 2.2 | <0.001 | ||
| Indication for oophorectomyd | -- | ||||
| Benign ovarian condition | 50.7 | ||||
| No ovarian condition | 49.3 | ||||
| Hypertensione | 20.7 | 14.4 | 0.053 | ||
| Hyperlipidemiaf | 43.0 | 34.4 | 0.04 | ||
| Systolic blood pressure (mmHg)g | 252 | 120.0 (111.0–130.0) | 239 | 120.0 (110.0–128.0) | 0.04 |
| Diastolic blood pressure (mmHg)g | 252 | 76.0 (70.0–82.0) | 239 | 74.0 (68.0–82.0) | 0.09 |
| Weight (kg)g | 246 | 72.5 (61.9–92.8) | 234 | 70.2 (60.9–79.4) | 0.002 |
| Body mass index (kg/m2)g | 246 | 26.7 (23.2–33.6) | 234 | 25.1 (22.4–29.5) | <0.001 |
| Total cholesterol (mg/dL)g | 161 | 197.0 (170.0–221.0) | 143 | 188.0 (167.0–211.0) | 0.49 |
| Triglycerides (mg/dL)g | 156 | 110.0 (73.0–159.0) | 133 | 97.0 (64.0–141.0) | 0.053 |
| HDL cholesterol (mg/dL)g | 144 | 50.0 (44.0–59.0) | 127 | 54.0 (44.0–66.0) | 0.32 |
| LDL cholesterol (mg/dL)g | 119 | 115.0 (96.0–139.0) | 107 | 107.0 (91.0–130.0) | 0.75 |
IQR = interquartile range.
Women with unknown data were not included in the tests for differences between the groups..
The p-values were calculated using tests which accounted for the matched pairs (McNemar’s tests or Bowker’s tests of symmetry for categorical variables or Wilcoxon signed rank tests for continuous variables). Non-parametric tests were used for the continuous variables because the paired differences for systolic blood pressure, triglycerides, and LDL cholesterol were not normally distributed.
Income was derived from the 2000 United States Census. Each woman was assigned the median household income for the census block group in which she lived at the index date.
The indication was listed by the gynecologist in the medical record at the time of oophorectomy. Benign ovarian conditions include benign tumors, cyst, or endometriosis in either ovary. No ovarian condition includes women without a benign ovarian condition in either ovary. Historically, the terms ‘prophylactic’, ‘elective’, or ‘incidental’ oophorectomy were used; however, we avoided these terms.
Hypertension was defined as systolic blood pressure >140 mmHg, diastolic blood pressure >90 mmHg, or onset of antihypertensive medication use before the index date.
Hyperlipidemia was defined as total cholesterol ≥200 mg/dL, triglycerides ≥150 mg/dL, HDL cholesterol ≤50 mg/dL, or onset of lipid lowering medication use before the index date.
Measurements closest to and within 3 years before in the index date.
3.2. Follow-up and primary results
Virtually all women underwent hysterectomy either before oophorectomy or concurrent with oophorectomy. Therefore, virtually all women took ET alone. Among 163 women who started ET within one month after oophorectomy, 99 women (60.7%) used only oral ET, 20 women (12.3%) used only transdermal ET, and 44 women (27.0%) used both oral and transdermal ET. Among the 143 women (87.7%) who used oral ET, conjugated equine estrogen was the most frequent type (n = 98) and was most commonly used at a dose of 0.625 mg (n = 76). Oral estradiol was used by 18 women, most commonly at a dose of 1 mg (n = 14).
Table 2 shows the mean values predicted by the GEE linear models for the metabolic parameters within 10 years after bilateral oophorectomy or the index date among the three groups. Although significant differences between the groups were observed for diastolic blood pressure, the magnitude of the differences was small, and the interaction between group and years after index was not significant. As shown in Figure 2, the differences for diastolic blood pressure were similar over time. By contrast, a significant interaction was found for weight and BMI, and the changes occurred primarily in the initial 4–5 years. Women who underwent bilateral oophorectomy and were not taking ET at the measurement dates had higher values, compared to women who took ET. Women who took ET had modest increases over time, which were similar to the increases observed in the referent women (Table 2, Figure 2).
Table 2.
Predicted mean values of biomarkers within 10 years after bilateral oophorectomy or index date (generalized estimating equation [GEE] linear models).
| Biomarker | N women | Predicted mean values (95% CI) from adjusted modelsb | p-valuesc | ||||
|---|---|---|---|---|---|---|---|
| (N values)a | Overall | 1 year | 3 years | 5 years | 10 years | ||
| Systolic blood pressure (mmHg)d | 0.11 | ||||||
| Oophorectomy, not on ET | 157 (906) | 118.3 (116.7–119.8) | 116.6 (112.9–120.3) | 120.0 (116.6–123.4) | 119.5 (116.3–122.7) | 121.5 (118.3–124.7) | 0.01 |
| Oophorectomy, on ET | 122 (599) | 118.2 (116.2–120.2) | 116.4 (113.2–119.7) | 116.4 (112.8–120.0) | 116.1 (112.1–120.1) | 123.2 (118.0–128.5) | 0.42 |
| Referent | 218 (1,574) | 116.4 (115.2–117.7) | 115.8 (113.0–118.5) | 116.1 (113.4–118.8) | 115.0 (112.2–117.7) | 119.3 (116.4–122.1) | |
| Diastolic blood pressure (mmHg)d | 0.002 | ||||||
| Oophorectomy, not on ET | 157 (906) | 75.1 (74.0–76.1) | 74.1 (71.5–76.7) | 75.5 (73.2–77.8) | 75.5 (73.3–77.8) | 77.2 (75.0–79.5) | 0.40 |
| Oophorectomy, on ET | 122 (599) | 74.0 (72.6–75.3) | 73.4 (71.1–75.6) | 74.1 (71.7–76.6) | 73.0 (70.3–75.8) | 74.5 (70.9–78.1) | 0.76 |
| Referent | 218 (1,574) | 72.7 (71.8–73.5) | 72.2 (70.3–74.1) | 72.4 (70.5–74.2) | 72.4 (70.5–74.3) | 73.4 (71.4–75.3) | |
| Weight (kg) | <0.001 | ||||||
| Oophorectomy, not on ET | 204 (1,122) | 84.2 (81.2–87.1) | 83.6 (79.2–88.0) | 85.7 (81.6–89.9) | 86.4 (82.2–90.5) | 84.6 (80.4–88.8) | <0.001 |
| Oophorectomy, on ET | 146 (659) | 77.3 (73.8–80.8) | 75.5 (70.9–80.1) | 76.3 (71.6–81.1) | 77.3 (72.4–82.2) | 77.4 (71.9–82.8) | 0.03 |
| Referent | 254 (1,741) | 76.2 (73.6–78.8) | 74.4 (70.3–78.5) | 75.7 (71.5–79.8) | 77.2 (73.2–81.3) | 77.4 (73.3–81.5) | |
| Body mass index (kg/m2) | <0.001 | ||||||
| Oophorectomy, not on ET | 204 (1,122) | 30.9 (29.8–31.9) | 30.7 (29.2–32.3) | 31.6 (30.1–33.1) | 31.8 (30.3–33.2) | 31.1 (29.6–32.6) | <0.001 |
| Oophorectomy, on ET | 146 (659) | 28.7 (27.5–29.9) | 28.1 (26.4–29.7) | 28.3 (26.7–30.0) | 28.8 (27.0–30.5) | 28.8 (26.9–30.7) | 0.03 |
| Referent | 254 (1,741) | 27.9 (27.0–28.8) | 27.2 (25.8–28.7) | 27.7 (26.2–29.1) | 28.2 (26.8–29.7) | 28.3 (26.8–29.8) | |
| Total cholesterol (mg/dL)e | 0.009 | ||||||
| Oophorectomy, not on ET | 86 (261) | 207.7 (200.4–214.9) | 208.2 (191.1–225.3) | 206.0 (193.3–218.7) | 208.8 (196.5–221.0) | 222.6 (210.7–234.5) | 0.008 |
| Oophorectomy, on ET | 60 (153) | 212.9 (203.8–221.9) | 198.5 (182.9–214.2) | 207.2 (192.4–222.0) | 218.0 (203.9–232.0) | 213.6 (195.4–231.7) | 0.06 |
| Referent | 151 (443) | 198.3 (192.9–203.8) | 189.9 (178.4–201.4) | 193.0 (182.3–203.6) | 195.2 (184.4–206.0) | 210.8 (200.0–221.6) | |
| Triglycerides (mg/dL)e | <0.001 | ||||||
| Oophorectomy, not on ET | 85 (255) | 137.0 (123.4–150.7) | 124.8 (91.3–158.3) | 138.5 (113.8–163.2) | 135.8 (112.2–159.3) | 163.4 (140.4–186.3) | 0.005 |
| Oophorectomy, on ET | 59 (151) | 138.9 (121.7–156.1) | 110.0 (79.9–140.1) | 133.0 (104.3–161.6) | 124.0 (96.8–151.1) | 169.5 (134.3–204.6) | 0.13 |
| Referent | 149 (432) | 109.7 (99.4–120.0) | 115.0 (92.5–137.5) | 106.6 (85.9–127.4) | 103.7 (83.0–124.4) | 114.6 (93.8–135.4) | |
| HDL cholesterol (mg/dL)e | <0.001 | ||||||
| Oophorectomy, not on ET | 86 (253) | 62.2 (59.0–65.4) | 66.8 (59.2–74.4) | 60.9 (55.4–66.4) | 58.5 (53.2–63.7) | 59.2 (54.0–64.3) | 0.41 |
| Oophorectomy, on ET | 58 (148) | 70.9 (67.0–74.9) | 67.5 (60.6–74.3) | 73.2 (66.9–79.6) | 73.4 (67.4–79.5) | 71.6 (63.9–79.3) | 0.004 |
| Referent | 150 (432) | 62.2 (59.8–64.5) | 56.4 (51.3–61.4) | 62.3 (57.7–66.9) | 61.0 (56.4–65.6) | 66.0 (61.3–70.6) | |
| LDL cholesterol (mg/dL)e | 0.56 | ||||||
| Oophorectomy, not on ET | 84 (245) | 118.3 (111.1–125.4) | 116.3 (99.9–132.8) | 116.7 (104.5–128.9) | 122.1 (110.7–133.5) | 130.6 (119.2–142.0) | 0.36 |
| Oophorectomy, on ET | 58 (142) | 113.7 (103.7–123.6) | 117.4 (102.5–132.3) | 109.7 (95.8–123.5) | 121.5 (108.2–134.8) | 110.7 (93.1–128.2) | 0.13 |
| Referent | 144 (409) | 113.7 (108.3–119.1) | 105.1 (93.1–117.0) | 110.6 (100.4–120.8) | 113.7 (103.5–124.0) | 122.1 (111.7–132.4) | |
CI = confidence interval; ET = estrogen therapy; HDL = high-density lipoprotein; LDL = low-density lipoprotein.
The numbers represent the number of unique women and the number of total values across all time points for each group separately.
Predicted mean values, confidence intervals, and p-values for each biomarker were obtained from generalized estimating equation linear models including variables for group status (bilateral oophorectomy not on ET, bilateral oophorectomy on ET, and referent women), years after index date (0–10; as a categorical variable), and the interaction between group and years after index. Each model was adjusted for age at index (as a continuous variable), years of education (missing or ≤12, 13–16, >16), and smoking (current or former vs never). Predicted mean values for each group at each time were calculated as the predicted mean value across all women in the corresponding group.
Three p-values are stacked vertically for each model in the following order: 1) the p-value for the overall difference across the three groups of women, 2) the p-value for the years after the index date, and 3) the p-value for the interaction between group and years after index date.
Models for blood pressure values excluded 95 women (30 bilateral oophorectomy not on ET, 26 bilateral oophorectomy on ET, and 39 referent women) who had hypertension at index.
Models for lipid values excluded 209 women (45 bilateral oophorectomy not on ET, 71 bilateral oophorectomy on ET, and 93 referent women) who had hyperlipidemia at index.
Figure 2.
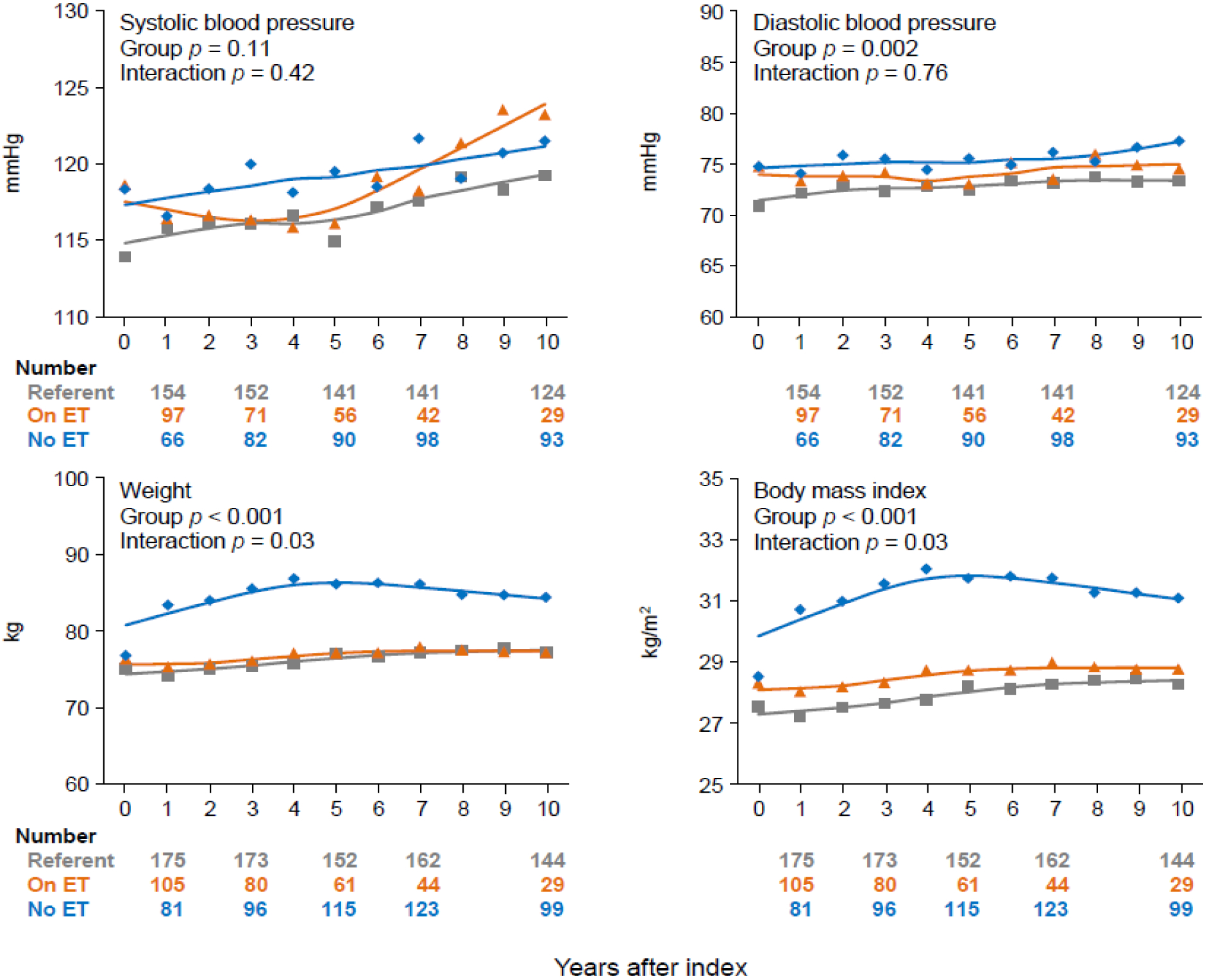
Smoothed curves of mean values predicted by generalized estimating equation (GEE) linear models for outpatient systolic and diastolic blood pressure, weight, and body mass index over ten years after bilateral oophorectomy or index date. The predicted values were adjusted by age at index, years of education, and cigarette smoking. Women with hypertension before the index date were excluded from the analyses for systolic and diastolic blood pressure. Weight and body mass index had significant overall group differences and significant interactions with years after index. For both parameters, we also conducted pairwise group comparisons, and the differences were significant for women who did not receive estrogen therapy after bilateral oophorectomy compared to women who did receive estrogen therapy (both p ≤ 0.005) and compared to referent women (both p < 0.001).
Significant group differences were found for fasting total cholesterol and triglycerides, however, the interactions between group and years after index were not significant (Figure 3). By contrast, we found a significant overall group difference and a significant interaction with years after index date for HDL-C. Once again, the changes occurred primarily in the initial 4–5 years. Women who took ET after bilateral oophorectomy experienced an increase in HDL-C over time, whereas women who did not take ET experienced a decrease over time.
Figure 3.
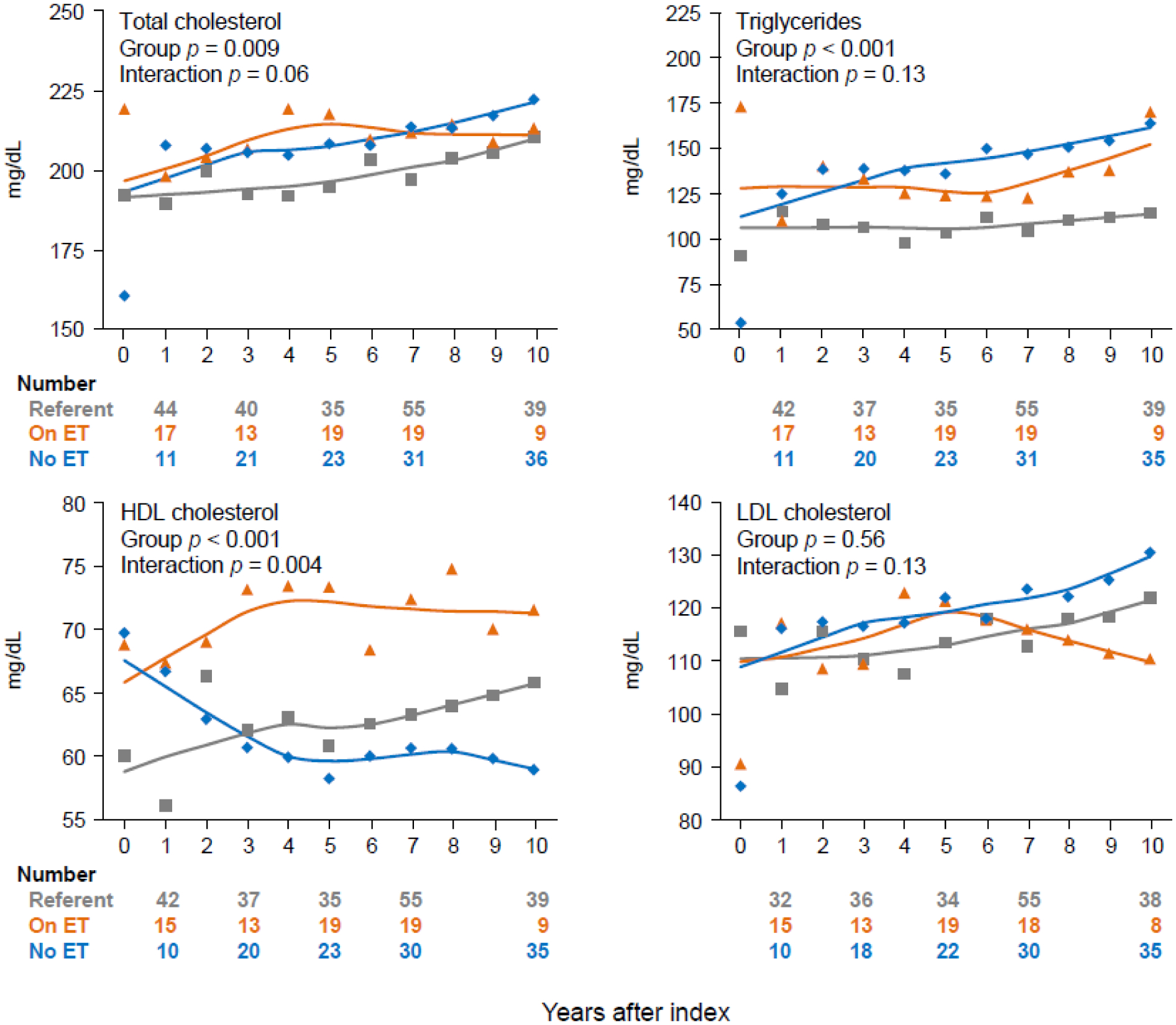
Smoothed curves of mean values predicted by generalized estimating equation (GEE) linear models for fasting lipid values over ten years after bilateral oophorectomy or index date. The predicted values were adjusted by age at index, years of education, and cigarette smoking. Women with hyperlipidemia before the index date were excluded from the analyses for the fasting lipids. High-density lipoprotein cholesterol had a significant overall group difference and a significant interaction with years after index. The pairwise group differences were significant for women who received estrogen therapy after bilateral oophorectomy compared to women who did not receive estrogen therapy (p < 0.001) and to referent women (p < 0.001).
3.3. Results from sensitivity analyses
Supplementary Table 1 shows the predicted mean values for the metabolic parameters within 10 years after bilateral oophorectomy or the index date stratified by age at oophorectomy or index date. In this first set of sensitivity analyses, significant interactions between group and years after index were found for total cholesterol, triglycerides, and HDL-C among women age <40 years at index date; and for weight and BMI among women age 40–45 years at index date.
In a second set of sensitivity analyses, we used multiple imputation methods to impute all possible values for the metabolic parameters within 10 years after index. The results were similar to the primary analysis; however, the confidence intervals were smaller due to the increased sample size (data not shown).
In a third set of sensitivity analyses, we studied the mean values for the metabolic parameters among referent women, women with ≥5 years of ET after bilateral oophorectomy, and women with no ET or <5 years of ET after bilateral oophorectomy. The results were generally similar to the primary analysis. However, because the follow-up data was limited to 5–10 years after the index date, the interaction between group and years after index remained significant for only HDL-C (p = 0.04, data not shown).
Supplementary Table 2 shows the predicted mean values for the metabolic parameters within 3 years before index and within 10 years after index for referent women and women who underwent bilateral oophorectomy. In this fourth set of sensitivity analyses, significant differences between the two groups were noted for almost all measures (Supplementary Figures 1 and 2), however, the interaction between group and years after index was only significant for total cholesterol and for LDL-C.
4. Discussion
4.1. Principal findings
Over the 10 years of follow-up, women who underwent oophorectomy and took ET, women who underwent oophorectomy and did not take ET, and referent women had significantly different mean values for diastolic blood pressure, weight, BMI, total cholesterol, triglycerides, and HDL-C. However, women with and without oophorectomy were already different at the index date for hyperlipidemia, systolic blood pressure, weight, and BMI. Nevertheless, the trajectories of change over 10 years after bilateral oophorectomy were significantly different for weight, BMI, and HDL-C. The changes occurred primarily in the initial 4–5 years.
4.2. Comparison with other studies
Earlier age at menopause, whether spontaneous or surgically induced via bilateral oophorectomy, has been associated with an increased risk for CAD and cardiovascular mortality.[3, 5, 6, 17, 18] Premature menopause, defined as menopause occurring prior to the age of 40 years, as opposed to early menopause, defined as menopause occurring between the ages of 40–45 years, is associated with an even greater risk,[4] and is now recognized by the American College of Cardiology/American Heart Association as a risk factor for CAD.[19] Therefore, the importance of understanding and managing the risk factors for CAD in this clinical setting cannot be overstated.[20, 21]
Despite the well-recognized increase in risk of CAD after bilateral oophorectomy performed prior to spontaneous menopause, the mechanisms and the risk factors mediating this association are not known. Unfavorable changes in weight, body fat distribution, BMI, blood pressure and lipid profile that have been previously reported after bilateral oophorectomy could potentially increase the risk of CAD.[1, 7, 8] However, the trajectory of these changes following bilateral oophorectomy has not been examined. Even though prospective studies might provide the ideal setting to evaluate the trends in these biomarkers, such studies are difficult to conduct because of the long-term follow-up required. Data registries are therefore the next best alternative to examine such research questions.
This is the first study to report on the trajectory of change in important and modifiable metabolic parameters related to risk of CAD, including weight, BMI, and dyslipidemia after bilateral oophorectomy performed in younger women. Identifying the timeline of change in these metabolic parameters is crucial for counseling patients as well as planning and timing appropriate mitigation strategies. The changes in weight and lipids seem to occur early after bilateral oophorectomy, and women should be informed of this risk, monitored for changes, and offered potential management options, including lifestyle counseling and medications (particularly statin therapy). Timely interventions can mitigate the risk of CAD and the associated mortality in this group of patients.
The other novel finding from this study pertains to the effect of ET on the modifiable metabolic parameters related to risk of CAD. Even though ET has been shown to mitigate the risk of CAD in women with premature menopause,[4, 17, 22] its effect on the trajectory of modifiable CAD risk factors after bilateral oophorectomy is less well known. Again, even though this question is best investigated in a prospective randomized controlled clinical trial setting with comparisons drawn among different regimens of ET versus no ET, such clinical studies are difficult to conduct because of the long-term follow-up required. Therefore, a large data registry with longitudinal follow-up such as the REP medical records-linkage system provides a nearly ideal setting to investigate this question. In the current study, ET favorably impacted weight, BMI, and lipid profiles in young women after bilateral oophorectomy, confirming the results from some smaller previous studies.[4, 23] It remains unclear whether the effect of ET on these metabolic biomarkers is the only mechanism involved in reducing the risk of CAD in this patient population. It is likely that other pathways in the pathogenesis of CAD are also favorably impacted by ET use, including improvement in insulin sensitivity[22, 24] and vascular reactivity,[25] culminating in an overall reduced risk. The lack of an effect of ET on blood pressure points towards an alternative mechanism mediating blood pressure control in young women after bilateral oophorectomy.
4.3. Strengths and limitations
This study has several strengths. First, the bilateral oophorectomy cohort and the referent cohort from which the samples were randomly derived were representative of a geographically-defined population.[10] Second, details about the bilateral oophorectomy, baseline characteristics, ET, and the metabolic parameters were obtained through abstraction of medical records from a medical records-linkage system, thus limiting recall bias.[10] None of the data were self-reported.
However, limitations also warrant consideration. The first and most important limitation was the source of the metabolic parameters. We considered only measures that were recorded in the medical records as part of routine medical care. Contrary to measures collected as part of a longitudinal research project, our measures may be incomplete and may lack standardization. Longitudinal data were available in most women for blood pressure and weight. However, the measures were only available on a subset of women for lipids. Unfortunately, lipid profiles are not done routinely in medical practice, and may be performed for a specific indication. On the other hand, over a long follow-up, these limitations should apply symmetrically to both cohorts of women. Therefore, any error should be non-differential and should not bias our conclusions. A set of sensitivity analyses involving imputation for missing values showed similar results. Similarly, it would have been desirable to include fasting glucose as a parameter in this study; however, fasting glucose was not routinely available for most of the participants.
Second, the measures were abstracted by 2 physicians and 3 nurse abstractors. Different abstractors may have performed differently. However, the measures were mainly numerical values that did not require interpretation, and errors should be non-differential. Third, women participants were predominantly white, and all resided in Olmsted County, Minnesota. Thus, results may not be generalizable to other populations with different racial or ethnic characteristics.[13] Fourth, the observational nature of our study limits causal inference, and unknown confounding variables may have been present. For example, women who chose to use ET after bilateral oophorectomy may have had some health behavioral characteristics that were different from women who did not. Fifth, despite the initial sample size estimation, the study had limited power to study some of the group by time interactions, especially in analyses for lipids (missing data and exclusion of hyperlipidemia at the index date). On the other hand, the sample was limited to reduce the amount of medical record abstraction. Sixth, because we considered only 10 years of follow-up, the women in our study were still relatively young at the end of follow-up. It is possible that we would observe additional significant differences if the women were followed for a longer time. We plan to continue to follow our cohorts. Seventh, our study excluded women who carry high-risk genetic variants that increase the risk of ovarian cancer. Finally, we did not stratify our analyses based on the specific ET regimens and doses utilized by our cohort because there was a wide variability in ET regimens. The effect of different ET regimens on metabolic biomarkers might be best investigated in a randomized controlled clinical trial. In addition, because a majority of the participants in our study had a concurrent hysterectomy at the time of bilateral oophorectomy, progestogen therapy did not apply, and was not studied. Future studies should focus on the effect of progestogen therapy on these parameters.
5. Conclusion
Women undergoing bilateral oophorectomy prior to the age of spontaneous menopause experience unfavorable changes in several metabolic parameters possibly related to the risk of CAD within the first few years following the surgery. Some of these changes can be reduced in magnitude by using ET. Future studies are needed to determine the effect of dose and formulation of ET on the trajectory of these metabolic parameters after bilateral oophorectomy. Recognizing the timeline of change in these parameters after bilateral oophorectomy is crucial for counseling patients and for planning timed interventions geared toward managing these risk factors, and ultimately, reducing the risk of CAD.
Supplementary Material
HIGHLIGHTS.
The trajectories of metabolic parameters after bilateral oophorectomy remain unknown.
Weight, body mass index (BMI), and high-density lipoprotein cholesterol (HDL-C) changed significantly over a 10-year period after surgery.
The unfavorable changes in metabolic parameters occurred primarily within 4–5 years after surgery.
Some of these changes can be reduced in magnitude by estrogen therapy.
Our findings may inform the clinical management of women after bilateral oophorectomy.
Funding
The Mayo Clinic Cohort Study of Oophorectomy and Aging (MOA-2) is partly supported by the National Institute on Aging (NIA grant U54 AG044170) and uses the resources of the Rochester Epidemiology Project (REP) medical records-linkage system, which is supported by the National Institute on Aging (NIA grant AG 058738), by the Mayo Clinic Research Committee, and by fees paid annually by REP users. However, the content of this article is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health (NIH) or of the Mayo Clinic. Walter A. Rocca was partly funded by the Ralph S. and Beverley E. Caulkins Professorship of Neurodegenerative Diseases Research of the Mayo Clinic.
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study, and all authors had final responsibility for the decision to submit for publication.
Declaration of competing interest
Ekta Kapoor is a consultant for Mithra Pharmaceuticals, Astellas Pharmaceuticals and Womaness. Michelle M. Mielke receives funding from the National Institutes of Health and unrestricted research grants from Biogen, and she consults for Brain Protection Company. Walter A. Rocca receives funding from the National Institutes of Health.
Abbreviations:
- CAD
coronary artery disease
- ET
estrogen therapy
- MOA-2
Mayo Clinic Cohort Study of Oophorectomy and Aging-2
- REP
Rochester Epidemiology Project
- LDL-C
low-density lipoprotein cholesterol
- HDL-C
high-density lipoprotein cholesterol
- BMI
body mass index
- GEE
generalized estimating equation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethical approval
All research activities were approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards.
Research data (data sharing and collaboration)
There are no linked research data sets for this paper. Additional details can be sought by contacting the corresponding author, Ekta Kapoor, MBBS (kapoor.ekta@mayo.edu). Deidentified participant data that underlie the reported results in this article (ie, text, tables, and figures) can be made available beginning at 9 months and ending at 36 months after article publication for individual participant data meta-analysis, if the proposed use has been approved by an independent review committee. Proposals should be directed Ekta Kapoor, MBBS (kapoor.ekta@mayo.edu). To gain access, data requestors will need to sign a data access agreement.
References
- 1.Rocca WA, et al. , Accelerated Accumulation of Multimorbidity After Bilateral Oophorectomy: A Population-Based Cohort Study. Mayo Clin Proc, 2016. 91(11): p. 1577–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rivera CM, et al. , Increased cardiovascular mortality after early bilateral oophorectomy. Menopause, 2009. 16(1): p. 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Honigberg MC, et al. , Association of Premature Natural and Surgical Menopause With Incident Cardiovascular Disease. JAMA, 2019. 322(24): p. 2411–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Panay N, et al. , Premature ovarian insufficiency: an International Menopause Society White Paper. Climacteric, 2020. 23(5): p. 426–446. [DOI] [PubMed] [Google Scholar]
- 5.Ley SH, et al. , Duration of Reproductive Life Span, Age at Menarche, and Age at Menopause Are Associated With Risk of Cardiovascular Disease in Women. J Am Heart Assoc, 2017. 6(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muka T, et al. , Association of Age at Onset of Menopause and Time Since Onset of Menopause With Cardiovascular Outcomes, Intermediate Vascular Traits, and All-Cause Mortality: A Systematic Review and Meta-analysis. JAMA Cardiol, 2016. 1(7): p. 767–776. [DOI] [PubMed] [Google Scholar]
- 7.Michelsen TM, et al. , Metabolic syndrome after risk-reducing salpingo-oophorectomy in women at high risk for hereditary breast ovarian cancer: a controlled observational study. Eur J Cancer, 2009. 45(1): p. 82–9. [DOI] [PubMed] [Google Scholar]
- 8.Franklin RM, Ploutz-Snyder L, and Kanaley JA, Longitudinal changes in abdominal fat distribution with menopause. Metabolism, 2009. 58(3): p. 311–5. [DOI] [PubMed] [Google Scholar]
- 9.Rocca WA, et al. , Bilateral Oophorectomy and Accelerated Aging: Cause or Effect? J Gerontol A Biol Sci Med Sci, 2017. 72(9): p. 1213–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rocca WA, et al. , Cohort profile: the Mayo Clinic Cohort Study of Oophorectomy and Aging-2 (MOA-2) in Olmsted County, Minnesota (USA). BMJ Open, 2017. 7(11): p. e018861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rocca WA, et al. , Personal, reproductive, and familial characteristics associated with bilateral oophorectomy in premenopausal women: A population-based case-control study. Maturitas, 2018. 117: p. 64–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rocca WA, et al. , History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc, 2012. 87(12): p. 1202–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.St Sauver JL, et al. , Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc, 2012. 87(2): p. 151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.St Sauver JL, et al. , Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol, 2012. 41(6): p. 1614–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.St Sauver JL, et al. , Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol, 2011. 173(9): p. 1059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee KJ, et al. , Framework for the treatment and reporting of missing data in observational studies: The Treatment And Reporting of Missing data in Observational Studies framework. J Clin Epidemiol, 2021. 134: p. 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu D, et al. , Age at natural menopause and risk of incident cardiovascular disease: a pooled analysis of individual patient data. Lancet Public Health, 2019. 4(11): p. e553–e564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roeters van Lennep JE, et al. , Cardiovascular disease risk in women with premature ovarian insufficiency: A systematic review and meta-analysis. Eur J Prev Cardiol, 2016. 23(2): p. 178–86. [DOI] [PubMed] [Google Scholar]
- 19.Arnett DK, et al. , 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation, 2019. 140(11): p. e596–e646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elder P, et al. , Identification of female-specific risk enhancers throughout the lifespan of women to improve cardiovascular disease prevention. Am J Prev Cardiol, 2020. 2: p. 100028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Young L and Cho L, Unique cardiovascular risk factors in women. Heart, 2019. 105(21): p. 1656–1660. [DOI] [PubMed] [Google Scholar]
- 22.Tsiligiannis S, Panay N, and Stevenson JC, Premature Ovarian Insufficiency and Long-Term Health Consequences. Curr Vasc Pharmacol, 2019. 17(6): p. 604–609. [DOI] [PubMed] [Google Scholar]
- 23.Archer DF, et al. , Long-term safety of drospirenone-estradiol for hormone therapy: a randomized, double-blind, multicenter trial. Menopause, 2005. 12(6): p. 716–27. [DOI] [PubMed] [Google Scholar]
- 24.Guttmann H, et al. , Choosing an oestrogen replacement therapy in young adult women with Turner syndrome. Clin Endocrinol (Oxf), 2001. 54(2): p. 159–64. [DOI] [PubMed] [Google Scholar]
- 25.Kalantaridou SN, et al. , Impaired endothelial function in young women with premature ovarian failure: normalization with hormone therapy. J Clin Endocrinol Metab, 2004. 89(8): p. 3907–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
There are no linked research data sets for this paper. Additional details can be sought by contacting the corresponding author, Ekta Kapoor, MBBS (kapoor.ekta@mayo.edu). Deidentified participant data that underlie the reported results in this article (ie, text, tables, and figures) can be made available beginning at 9 months and ending at 36 months after article publication for individual participant data meta-analysis, if the proposed use has been approved by an independent review committee. Proposals should be directed Ekta Kapoor, MBBS (kapoor.ekta@mayo.edu). To gain access, data requestors will need to sign a data access agreement.


