SUMMARY
Small poikilotherms such as the fruit fly Drosophila depend on absolute temperature measurements to identify external conditions that are above (hot) or below (cold) their preferred range and to react accordingly. Hot and cold temperatures have a different impact on fly activity and sleep, but the circuits and mechanisms that adjust behavior to specific thermal conditions are not well understood. Here, we use patch-clamp electrophysiology to show that internal thermosensory neurons located within the fly head capsule (the AC neurons1) function as a thermometer active in the hot range. ACs exhibit sustained firing rates that scale with absolute temperature – but only for temperatures above the fly’s preferred ~25°C (i.e. “hot” temperature). We identify ACs in the fly brain connectome and demonstrate that they target a single class of circadian neurons, the LPNs2. LPNs receive excitatory drive from ACs and respond robustly to hot stimuli, but their responses do not exclusively rely on ACs. Instead, LPNs receive independent drive from thermosensory neurons of the fly antenna via a new class of second-order projection neurons (TPN-IV). Finally, we show that silencing LPNs blocks the restructuring of daytime “siesta” sleep which normally occurs in response to persistent heat. Our previous work described a distinct thermometer circuit for cold temperature3. Together, the results demonstrate that the fly nervous system separately encodes and relays absolute hot and cold temperature information, show how patterns of sleep and activity can be adapted to specific temperature conditions, and illustrate how persistent drive from sensory pathways can impact behavior on extended temporal scales.
eTOC, In Brief
Alpert et al. uncover a circuit that functions as a thermometer for hot temperature in Drosophila. Internal sensors located within the fly head display persistent activity proportional to temperature above the fly’s preferred range. These cells target circadian centers in the brain, adjusting daytime “siesta” sleep specifically to hot conditions
Graphical Abstract

RESULTS
We have recently reported the existence of a specialized “cold thermometer” circuit in the fruit fly Drosophila melanogaster: a circuit encoding absolute temperature in the cold range that selectively regulates daytime sleep in response to cold temperature. This circuit is composed of cold activated thermosensory receptor neurons (TRNs) of the fly antenna and of a specific second-order thermosensory projection neuron cell type (TPN-II). This unique TPN displays persistent activity scaling with temperature, but only for absolute temperatures lower than the fly’s favorite 25°C (i.e. “cold”). TPN-IIs directly target and inhibit DN1as, a small cluster of circadian neurons, adjusting daytime activity and sleep patterns specifically to cold conditions3. Is there a parallel thermometer circuit in the fly brain for hot temperature? Here, our first goal was to identify candidate receptors neurons that may convey persistent signals scaling with external temperature in the hot temperature range (>25°C).
AC neuron firing rates scale with absolute temperature in the hot range
The last antennal segment, the arista, contains three thermosensory sensilla, each housing one hot- and one cold-activated TRN4. The sacculus, a pit-like organ in the second antennal segment, contains additional cold sensors3, but no sacculus heat-sensors have been described. Interestingly, flies also possess “internal” thermosensory neurons housed in the head capsule. “Anterior Cell” or AC neurons located next to the base of the antennal nerve (Figure 1A) respond to heat1, while similarly located “Anterior Cold Cell” neurons respond to cold3.
Figure 1: AC neurons display persistent firing which scales with absolute temperature in the hot range.
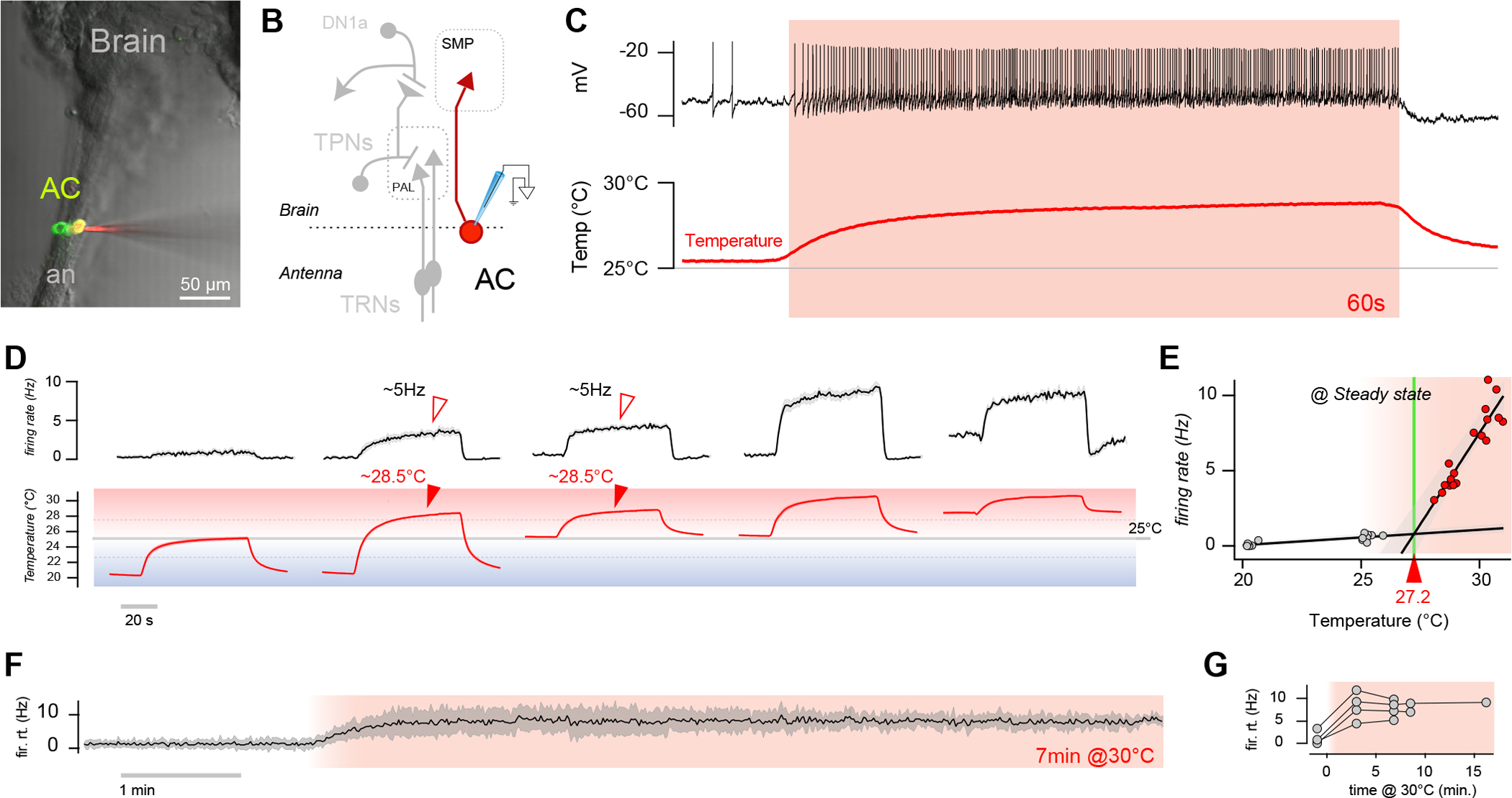
(A) 2-photon frame of GFP fluorescence overlaid with a Dodt gradient contrast image demonstrating the targeting of ACs for recording. AC expresses GFP, while the electrode is filled with Alexafluor 594 (an = antennal nerve). (B) Diagram of ACs and other thermosensory circuit components (TRNs = thermosensory receptor neurons; PAL = posterior antennal lobe; TPNs = thermosensory projection neurons; SMP = superior median protocerebrum). (C) Representative whole-cell current clamp recording from an AC neuron in response to a hot step (~3°C; 1 min) demonstrating persistent responses. (D) Firing rate histograms from ACs in response to hot steps of different sizes and settling on distinct absolute temperatures above or below 25°C (gray line), showing that persistent activity only appears in the hot range (above 25°C; 9 cells/6 animals, ±SEM; temp. trace av. of 9, ±SEM). Note that the firing rate at a similar hot temperature is comparable, independent of stimulus history (arrowheads). (E) AC responses appear to have a distinct threshold of ~27.2°C. Circles represent 9 cells/6 animals (linear fit ± 95% CI; for 20–25°C r2=0.7; for ~28–33°C r2=0.83). (F-G) AC firing rates show no evidence of adaptation in hot temperature. (F) Representative firing rate histogram from an AC subjected to extended hot steps (AV±SD of 4 sweeps) and (G) responses from 4 different cells/animals (circles are average firing rates at stable temperature, connected circles are recordings from the same cell).
We reasoned that the AC neurons could represent an excellent candidate for a cell type that displays persistent responses to heat and therefore regulate behavior (such as sleep) that unfolds on extended temporal scales:
silencing AC neurons does not impair rapid heat navigation5, but effects of AC silencing on sleep and activity rhythms have been previously reported6–8.
When imaged using calcium indicators such as GCaMP, ACs respond to heating ramps with calcium transients that appear to have distinct thresholds at the edge of the hot range (>25°C)1,9.
Genetically encoded calcium indicators (GECI) such as GCaMP report responses to rapid stimuli relatively faithfully. Yet, GECIs cannot distinguish sub-threshold calcium fluctuations from changes that reflect action potential firing and are therefore less suited to estimate the properties of a neuron in stable conditions.
To overcome these limitations, and to obtain a more direct readout of ACs action potential firing under conditions of persistent heat, we performed patch-clamp electrophysiology by targeting ACs under a two-photon microscope using GFP expression as a guide (under the control of the AC driver SH-Gal41). As before3, we designed a stimulation protocol that consists of a rapid temperature change (~2°C/s) followed by extended stable hot conditions (1–15 min), again followed by a rapid return to baseline.
Our observations confirm the notion that the AC neurons may indeed function as a thermometer selectively active in the hot range (Figure 1A–G). First, patch-clamp recordings revealed little AC firing at or below 25°C (<1Hz, Figure 1C, D, E). ACs only displayed robust activity in the hot range (Figure 1C, E), independent of the pre-stimulus baseline temperature (Figure 1D) or of the magnitude of the temperature step the preparation experienced (Figure 1D, arrowheads). In fact, robust AC firing only appeared above an apparent threshold of ~27°C (Figure 1E; note that we saw no evidence of a two-step threshold for AC responses9). Importantly, above ~27°C, AC’s steady-state firing rate was remarkably proportional to absolute temperature, consistent with their potential role as a “thermometer”. Finally, AC firing rates showed little or no evidence of adaptation even after minutes of exposure to stable heat (Figure 1F–G).
Together, our results support the idea that AC neurons may constitute the hot “thermometer” that parallels the cold TRN→TPN-II circuit we previously described. AC neurons are unusual in that they serve a sensory role but also directly send axons deep into the fly brain. Our next objective was to identify the AC targets in the brain and to test the hypothesis that persistent drive from ACs may regulate behavior under hot conditions.
ACs target the LPN cluster of circadian neurons
The AC neurons have been suggested to directly target a number of alternative cell types in the Drosophila brain: the s-LNVs10 or the DN1p cluster of dorsal neurons6,7, distinct cell types that are both considered core components of the circadian network (reviewed in 11). To clarify the connectivity of AC neurons, we first identified their reconstructed volumes in the publicly available EM fly hemibrain connectome12. Our search was facilitated by their well-described anatomy13; in addition, we reasoned that, because of the unusual position of their cell bodies (outside of the brain), their volumes were likely to be annotated as partial reconstructions. Our search led to the identification of 4 AC neuron volumes, 2 near-complete on the right side of the brain (the focus of the EM reconstruction) and 2 less complete volumes on the left side (Figure 2A, and see methods for details). This is consistent with the fact that two distinct AC neurons have been described on each side of the fly brain1,13.
Figure 2: Direct drive from ACs imparts sustained heat responses to LPN circadian neurons.

(A-C) LPNs are a major synaptic target of ACs. (A) Partial EM brain volume reconstruction showing reconstructed ACs, LPNs and relevant brain regions (the mushroom body, MB, is shown as a reference; see below for abbreviations). (B) Pie charts representing synaptic connectivity between AC and LPN (each slice corresponds to a cell type, cell type names are abbreviated and clustered by region where possible, see Table S2 for full names and details). (C) Connectivity diagram illustrating drive from the four ACs to the three left hemisphere LPNs (right hemisphere LPNs are not included in the EM brain volume). (D, E) Optogenetic activation of LPNs can drive action potential firing in ACs. (D) Experimental schematic. ACs selectively express CsChrimson (driven by SH-Gal4), while LPNs are targeted for recording by independent GFP expression (under 65D05-LexA). (E) Left: example recording and firing rate histogram (6 cells/3 animals, ±SEM; temp. trace is shown as control). Right: max. response from 8 cells/4 animals for experimental and 3 cells/ 3 animals for control (control: no Cs Chrimson; empty circles: max firing rate during light stimulation; Filled circles AV±SD; 1-way, 2-sample t-test, asterisk: p<0.05). (F-L) Heat responses of LPNs can be explained by AC input and additional input from the antenna. (F) Circuit diagram for LPN hot drive. (G) Representative whole-cell current clamp recording from a control LPN (top trace), and from an LPN in an animal were ACs had been independently silenced (by expression of Kir2.1; bottom trace). The initial burst of activity (arrowheads) is not affected by AC silencing but persistent firing in hot conditions is no longer elevated compared to the baseline at 25°C (grey boxes). (H) Quantification of the effects of AC silencing. Top: firing rate histograms and corresponding temperature stimuli (control: 6 cells/4 animals ±SEM;-AC: 6 cells/2 animals ±SEM). Bottom: firing rates at stable temperatures (circles: average firing rates at stable temperature, connected circles: recordings from the same cell, color line: AV±SEM; 1-way, 2-sample t-test, asterisk: p<0.01). (I) Partial EM brain volume reconstruction showing hot receptors of the arista, TPN-IV and LPNs. (J) Connectivity diagram. (K) Removal of the antennae in AC silenced animals abolishes all heat responses in LPNs. Top: firing rate histograms and corresponding temperature stimuli (5 cells/2 animals ±SEM). Bottom: firing rates at stable temperatures (circles: average firing rates at stable temperature, connected dots: recordings from the same cell, color line: AV±SEM; 1-way, 2-sample t-test, asterisk: p<0.01). (L) LPN’s display heating responses from a “cold” baseline (<25°C; see arrowhead). Top: firing rate histograms and corresponding temperature stimuli (5 cells/N animals ±SEM). Bottom: firing rates at stable temperatures (circles: average firing rates at stable temperature, connected circles: recordings from the same cell, color line: AV±SEM;). (Abbreviations, MB: mushroom body, PAL: posterior antennal lobe, SLP: superior lateral protecerebrum, SMP: superior median protocerebrum; ACR/L: AC of the right/left hemisphere; LPN: lateral posterior neuron;TRNs: thermosensory receptor neurons; TPNs: projection neurons; arHC: hot cell of the arista).
Connectomic analysis of these 4 volumes revealed that the only circadian neurons directly targeted by the ACs are the Lateral Posterior clock Neurons or LPNs2,14,15. The majority of the targets of AC axons in the brain are in the Superior Medial Protocerebrum (SMP) and Superior Lateral Protocerebrum (SLP; Figure 2A, B; and see16). In fact, LPNs represent one of the top synaptic outputs of ACs receiving 14% of all of the AC axonal synapses (136 synapses; Figure 2B). LPNs’ inputs are also mainly in the SMP and SLP, and synapses from ACs represent the top input for LPNs (7% of their input synapses, Figure 2B; and see Table S2 for complete lists). We note that ACs in fact robustly target 2 out of the 3 annotated LPNs, but LPNs are also inter-connected (Figure 2C). We also note that LPNs receive additional potential thermosensory drive from a hitherto un-characterized TPN (which we name TPN-IV, Figure 2B,16,17 and see below).
Are LPNs functional targets of ACs? To test this idea directly, we expressed the red-light activated channel Cs.Chrimson18 in ACs (under the control of SH-Gal4) and targeted LPNs for recordings (i.e. independently labeling their cell bodies using 65D05-LexA19 and Aop-GFP). Our results confirm that optogenetic activation of ACs is sufficient to evoke firing in LPNs (Figure 2D).
Here, we also attempted to resolve the possibility that only two of the three LPNs may be functional AC targets (see above) by recording from multiple LPN cell bodies in a single animal (e.g. the plot in Figure 2E represents 8 cells from 4 animals, 2 cells/animal on the same side of the brain –recording all 3 LPNs from the same animal proved prohibitive). While we saw a spread of LPN responses (open circles in the plot), we did not record from any LPN that was completely refractory to activation (Figure 2E). We conclude that either LPN-LPN inter-connectivity can spread AC drive to the three LPNs, or that, for some unknown reason, our recording may be biased towards the connected LPNs.
LPNs display both acute and persistent activity in response to heat
LPNs have been previously implicated in the synchronization of activity to temperature cycles15,16,20. Yet calcium indicators did not record temperature responses in these cells21. Next, we used electrophysiology to test the possibility that LPN firing rate may be modulated by heat, and that AC drive may contribute to their heat responses. We again used two-photon guided patch clamp, and targeted LPNs under the microscope using GFP expression as a guide (under the control of 65D05-Gal419). We first used stimuli that produce reliable persistent activity in ACs (rapid heating from 25°C, followed by stable hot conditions for ~1 minute, again followed by a return to baseline).
LPNs responded robustly to heat stimulation. Upon heating, LPNs fired a burst of action potentials (Figure 2G, arrowhead), following which their firing settled to a stable rate (Figure 2G, box). Their firing rate in the hot range was invariably higher than that of their baseline at 25°C (Figure 2G and see H for quantification). To test the potential contribution of ACs to LPN heat responses, we next performed LPN recordings in animals in which ACs had been genetically silenced (by expression of the hyperpolarizing agent Kir2.122 under SH-Gal4). In these animals, LPNs’ heating-evoked bursts of activity remained intact (arrowhead in Figure 2H), but the effect of hot temperature on LPNs’ persistent response was abolished, so that when the temperature reached stable hot conditions (>25°C, boxes in Figure 2G and H) LPNs’ firing settled to a rate comparable to that of the baseline at 25°C (Figure 2G and see H for quantification). We conclude that LPNs display both a dynamic response to heating (which is independent of AC) and a persistent increase in firing in the hot range which depends on drive from ACs.
LPNs receive input from hot cells of the arista via the TPN-IV projection neuron
Where do the dynamic (and AC-independent) LPN responses to heating originate? As we noted above an additional prominent input of LPNs is a projection neuron (TPN-IV) that in turn receives input in the posterior antennal lobe (PAL), a region of the fly brain where “hot” and “cold” glomeruli are innervated by the axons of hot and cold receptors of the antenna and by their post-synaptic partners4,23. Connectomic analysis confirmed that TPN-IV’s dendrites mainly innervate the hot glomerulus (also known as VP217, Figure 2I), and that TPN-IV receives abundant synaptic input from the three hot receptor cells of the arista (HCs, 218 synapses) providing a potential drive to LPN that is comparable to that of the ACs (121 vs 136 synapses; Figure 2J).
While our efforts to obtain reagents to selectively label and manipulate TPN-IV have been unsuccessful, two lines of evidence suggest that input from the HC→TPN-IV circuit may explain the dynamic LPN responses to heating:
We have shown above that genetic silencing of AC neurons abolishes LPN’s persistent responses to heat but leaves intact LPNs’ heating-evoked bursts in activity. Surgical resection of the antennae in AC-silenced animals completely abolished all heat responses in LPNs, consistent with an independent drive from the antenna (Figure 2K).
While AC neurons respond to hot stimuli only above a defined threshold of ~27°C, LPNs display dynamic responses to heating even in the cold range (<25°C; Figure 2L, arrowhead), a feature again consistent with drive from the arista HCs (and note their limited modulation in the cold, Figure 2L).
ACs and LPNs mediate daytime sleep restructuring by hot temperature
Having established that ACs display persistent responses in the hot range, and that their main targets in the clock network are LPNs, our next goal was to explore if this persistent activity may help shape behavior impacted by hot temperature on extended temporal scales. We choose daytime sleep patterns as read-out for the effects of persistent hot conditions on behavior for two reasons: as a parallel with our experiments on cold temperature3, and because recent work has already implicated ACs and LPNs in the control of daytime sleep16,19.
In the laboratory, daytime sleep and activity patterns respond robustly to changes in external temperature in Drosophila, likely reflecting adaptation to day-to-day or seasonal changes that would be encountered in the natural environment. Here, we used a baseline temperature of 25°C and a hot temperature that activates ACs and LPNs (30°C), but that is below the threshold for noxious heat responses in Drosophila (>35°C5). The effects of heat on daytime sleep have been characterized before, but often using “cold” baselines of ~20°C (e.g. 6,7,16); unlike previous studies, our experiments were guided by knowledge of the thermal range that selectively activates LPNs through ACs.
At the normal rearing temperature of 25°C (and under 12 hr light:12 hr dark cycles), fly behavior is characteristically crepuscular: distinct peaks of activity correspond to the late night-early morning transition (“morning peak”) and to the end of the day (“evening peak”; see Figure 3A, gray bar graph). Fly sleep is measured as inactivity that persists for 5 minutes or longer24,25, and the lack of activity during the mid-day corresponds to a peak in daytime sleep (also known as mid-day siesta26; Figure 3A, black plot). This mid-day rest has been suggested to help flies avoid potentially hot/dry conditions that are more likely to occur at high noon26.
Figure 3: ACs and LPNs are required for daytime sleep restructuring by hot temperature.

Drosophila melanogaster wild type and control flies respond to 30°C heat by increasing morning and afternoon sleep, while genetic silencing of ACs or LPNs impairs normal responses to heat. (A,D,F,H) Flies of the noted genotype were entrained to a LD 12:12 cycle at 25°C before the temperature was increased to 30°C for the subsequent day or time-delimited as illustrated (during the first or second half of the day). Top drawing in A is an outline of the temperature and light/dark protocol used for the experiments. For each panel (D,F), the schematic to the left is a circuit diagram representing the specific manipulation. Activity and sleep were quantified in 30-minute bins using one day per condition. Graphs represent the averages ± SEM of 2 consecutive days of sleep (top) or activity (bar graphs, bottom) at 25°C (black/gray) and 30°C (overlaid in red); filled circles in sleep plots and black dots above activity bars indicate timepoints that are significantly different between 25° and 30°C conditions (p < 0.05, paired two-sided t-test). Dark shading indicates lights off (night); red shading indicates 30°C; ZT = zeitgeber time. Specific sleep quantifications are presented in (B,C,G).
(A) In WT flies, daytime hot conditions suppress activity and increase both morning (ZT0-3) and afternoon sleep (ZT6-12; n=32). Effects are replicated during time-delimited heat steps (n = 31 flies for morning and n=29 for afternoon; black arrowheads denote the afternoon increase). (B) Quantification of the effects of heat on total sleep. Box edges: 25th and 75th percentiles; thick lines: median; whiskers: data range; gray dots: individual data points/flies; *p < 0.05 in paired two-sided t-test comparing 25°C versus 30°C within genotype. (C) Quantification of per-fly sleep increases demonstrate a stronger effect of heat on afternoon sleep (arrowhead). Outer box edges: one standard deviation range; inner colored box edges: 95% confidence interval of the mean; thick lines: mean; gray dots: individual data points/flies; *p < 0.05 different from zero, one-sample t-test. (D) Silencing AC synaptic output via expression of tetanus toxin light chain perturbs afternoon sleep restructuring by hot temperature (empty arrowhead; n=30; see G and H for quantifications and controls). (E-F) Silencing LPN synaptic output under the control of a selective driver impairs afternoon sleep restructuring by heat (n = 63; empty arrowhead); E is a two-photon z stack from the brain of a fly expressing GFP under the control of LPNsplit, overlaid with a 3D reconstruction of EM volumes for ACs and LPNs for comparison. (G) Quantification of per-fly sleep increases for silenced and control flies. Outer box edges: one standard deviation range; inner colored box edges: 95% confidence interval of the mean; thick lines: mean; gray dots: individual data points/flies; **p < 0.05 for differences between silenced and control genotypes, 2-way ANOVAs with a Bonferroni correction. (H) Control genotypes for D and F (n = 25 for AC/+, n = 55 for LPN/+, and n = 62 for TNT/+).
In our experiments, increasing the temperature from 25°C to 30°C invariably increased daytime sleep: we observed a modest increase in morning sleep (ZT0-6) and a more pronounced increase in afternoon sleep (ZT6-12; Figure 3A–C), resulting in an overall widening of the peak of mid-day siesta. This result is largely consistent with what has been previously reported7,26,27.
We note that the heat-dependent increase in morning sleep was less pronounced and less robust across genotypes both in our experiments and in the literature7,26,27. For these reasons, we chose to further focus on the effects of heat on afternoon sleep (ZT6-12, arrowheads in Figure 3A–C). Our results suggest that the effects of hot temperature on afternoon sleep are both acute and persistent, as they can be readily reproduced by delimited heat steps (Figure 3A–C). Overall, both delimited heat steps and all-day heat increased afternoon sleep by an average ~75 minutes (Figure 3C, arrowheads).
Our next goal was to establish if the AC-LPN circuit may be involved in the restructuring of daytime sleep observed in hot conditions. To test this idea, we first genetically silenced AC output by expression of a blocker of synaptic transmission (tetanus toxin light chain28). Our data show that AC silencing largely attenuated the increase in afternoon sleep which normally results from heat exposure (Figure 3D, and see G, H for quantifications and control plots). Silencing LPN output (using a selective split-Gal4 driver produced for this study, Figure 3E and see methods for details) also abolished daytime sleep restructuring by hot temperature (Figure 3F, G, H and see Figure S1 for quantifications of baseline sleep in the various genotypes). We conclude that the normal restructuring of daytime sleep by hot temperature requires the persistent drive of AC onto LPNs.
Parallel thermometer circuits for hot and cold temperature in the Drosophila brain
Our results so far confirm that the fly brain contains a distinct thermometer circuit that functions in the hot range, a circuit that is therefore in principle analogous to the cold thermometer we previously described3. Interestingly, both the hot-responding AC neurons and the cold TRN→TPN-II circuit regulate daytime sleep, but they do so by targeting different cell types (LPNs and DN1as, respectively) and exert different effects on their activity (excitation and inhibition, respectively). TPN-II also independently targets neurons of the mushroom body (MB; 17), an additional site of sleep regulation29–31 (Figure 4).
Figure 4: Distinct thermometer circuits for hot and cold temperature in the Drosophila brain.

Cold-inhibited DN1as and hot-activated LPNs are characterized by largely non-overlapping connectivity. (A) Pie charts representing the major synaptic input and output pathways of DN1a and LPN as reconstructed from the EM connectome hemibrain dataset (each slice corresponds to a cell type, cell type names are abbreviated and clustered by region where possible, see Table S2 for tables and methods for details). Inputs: DN1a’s major input is from the cold-activated inhibitory projection neuron TPN-II (blue slice, 300 synapses, 17% of total input). DN1a also receive significant input from TPN-IV and TPN-V (hot TPNs, gold and orange slices -respectively) and in addition from a number of less characterized PNs (including potential hygrosensory PNs). LPNs’ top input is from ACs (136 synapses, 7% of total; red slice), but they also receive additional hot input from TPN-IV (121 synapses, 6%; gold slice). Outputs: DN1a and LPN outputs are largely non-overlapping. DN1a has a number of targets in the LHPV/LHAV region, in the SLP and aMe, while the large majority of the synaptic targets of LPNs are in the SMP. The only common target appears to be SLP266. Both DN1a and LPNs demonstrate some reciprocal connectivity and some connectivity with DN3a (brown slices). (B) Circuit diagram illustrating how cold and hot temperature are relayed by distinct “thermometer” circuits that are active in a non-overlapping thermal range (<25°C for cold-TRNs>TPN-IIs, and >27°C for ACs, respectively) and drive adjustments in daytime sleep by directly modulating the activity of DN1a and LPNs.
Abbreviations: TRN thermosensory receptor neurons, TPN thermosensory projection neuron, AC anterior cell, PN projection neuron, PAL posterior antennal lobe, MB mushroom body, CA Calyx, SLP/SMP superior lateral/medial protocerebrum, LHA/P V lateral horn anterior/posterior ventral, AVLP anterior ventrolateral protocerebrum, aMe accessory Medulla, WED wedge, CL claw; “unc” stands for uncharacterized. Clock neurons: DN1a, LPN, DN3a, LNd, s-LNv, DN1pB.
These observations raise a number of interesting questions. First, why are AC and TPN-II targets different? One explanation may be that hot and cold have only partially overlapping effects on daytime sleep (both hot and cold temperatures increase morning sleep, but while cold temperature decreases afternoon sleep, hot temperature increases it; Figure 4). Yet, eventually, both hot and cold influences have to converge on the regulation of sleep drive; it is therefore conceivable that LPN and DN1a may share at least some common targets. To explore this question, we charted the full connectivity of DN1as and LPNs, looking for potential common targets (and/or inputs) in the EM connectome (Figure 4A and see Table S2).
Overall, connectomic analysis suggests that DN1as are more directly embedded in the circadian network than LPNs. DN1as are inter-connected with DN3as (note that DN3s have also been shown to be modulated by cold temperature32) and provide significant input to s-LNvs and LNds (and note that in return DN1as are activated by the neuropeptide PDF released by s-LNvs3,33,34). LPNs also receive direct synaptic input from DN3a, but their main synaptic output are many uncharacterized cells of the SMP (Figure 4A). One caveat is that, because LPNs express as many as 3 different neuropeptides16, their effect on neighboring neurons may not be limited by traditional synaptic connections (e.g. the ones readily identified in the EM volume).
The only common target of LPNs and DN1as in the connectome is a single uncharacterized cell type of the superior lateral protocerebrum, SLP266. The fly brain connectome contains 6 cell IDs annotated as SLP266, and they receive a combined 205 synapses from DN1as and 67 from LPNs (Figure 4A and Figure S2). SLPs are an interesting cell type as they are a common output of DN1a and LPN, but also because they provide some input to both (7 and 16 synapses, respectively) and are additionally interconnected with clock neurons such as DN1p, s-LNvs, LNds, etc. (Figure S2). Future work will reveal if they too are involved in the regulation of sleep by temperature.
When it comes to potential shared inputs, connectomic analysis of DN1a revealed that these cells are not only targeted by the cold-activated TPN-II, but also by the hot-activated TPN-IV (described above as an input to LPNs) and by an additional TPN that also originates in the hot glomerulus (termed TPN-V), as well as by additional potentially hygrosensory PNs (Figure 4A and Table S2). Our previous recordings demonstrated that DN1a is robustly inhibited by cold via TPN-II, but, curiously, we recorded only a limited modulation by heat. We did observe bursts of activity in response to heating3, which are compatible with direct drive from TPN-IV. We conclude that DN1a is at least one point of convergence for the two circuits: while it is persistently inhibited by cold via TPN-II, it can be transiently activated by heating likely through TPN-IV (and potentially further modulated by TPN-V in the hot range; Figure 4B).
DISCUSSION
As homeotherms, our body temperature is relatively independent from that of the environment, and we therefore define hot and cold in relation to it: we perceive temperatures higher than our skin as hot, lower as cold. The internal temperature of poikilotherms such as the insects is instead largely at the mercy of external conditions. In the absence of a stable endogenous reference point, selective responses to “hot” or “cold” (temperatures above or below the preferred range, respectively) have to depend on absolute, rather than relative (i.e. heating/cooling from a reference point) temperature measurements.
Our work demonstrates the existence of largely independent thermometer circuits for absolute hot and cold temperature in the Drosophila brain. These circuits relay information about temperature to higher brain centers, where their persistent drive impacts daytime sleep, a response that unfolds on temporal scales from minutes to hours.
Our results show that hot and cold signals enter the circadian network at distinct nodes (DN1a for cold and LPN for hot temperature, respectively). This organization may allow for different behavioral responses to hot and cold conditions, and for the appropriate integration of temperature signals with light and endogenous rhythms –upstream of the regulation of sleep.
We note that the robust persistent responses to temperature of both DN1as and LPNs has been missed by calcium-based reporters of activity21, and that the threshold of the AC neurons was estimated at ~25°C9 based on what may be a sub-threshold calcium raise that anticipates action potential firing at ~27°C. This underscores the limitations of calcium-based reporters in revealing thresholds of activation and firing properties in stable conditions.
Persistent hot or cold conditions are well known to bring about distinct changes in both physiology and behavior, but –unlike other environmental cues, external temperature can directly influence biochemical and physiological processes throughout the fly body. The need for specific sensory mechanisms to mediate the persistent effects of temperature is therefore not obvious. We speculate that the mechanism we describe here maybe important to bridge the gap between responses on the timescale of minutes to hours and physiological changes that may take days to set in. The fact that persistent signaling in thermosensory circuits can have a profound and persistent impact on behavior may prove of more general significance, and help explain the myriad of interactions between external temperature, behavior and physiology that occur on diverse temporal scales across the animal kingdom.
STAR METHODS
RESOURCE AVAILABILITY
Lead Contact
Further information and requests for reagents should be directed to and will be fulfilled by the Lead Contact, Marco Gallio (marco.gallio@northwestern.edu).
Materials Availability
This study did not generate new unique reagents. Requests for fly stocks be directed to and will be fulfilled by the Lead Contact, Marco Gallio (marco.gallio@northwestern.edu).
Data and Code Availability
All data reported in this paper will be shared by the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Fly Strains
Drosophila melanogaster strains were reared on cornmeal agar medium under 12:12 LD cycles at 25°C. Stocks were obtained from Bloomington Drosophila Stock Center (BDSC). The following stocks were used: TRPA1SH Gal4 (from P. Garrity), 10XUAS-IVS-mCD8::GFP (BDSC 32186) 20XUAS-IVS-CsChrimson.mVenus (BDSC 55136), Aop.CD2::GFP (35), UAS Kir2.1 (BDSC 6595). 65D05 Gal4 (BDSC 39351), 65D05 LexA (BDSC 53625), 26A08 AD (BDSC 70150), 65D05 DBD (BDSC 69667) UAS-TNT (BDSC 28837). A full description of genotypes used in each figure can be found in Table S1.
METHOD DETAILS
Characterization of LPN split-Gal4 driver
R26A08 AD ∩ R65D05 DBD is active in 3 LPNs per hemisphere and in no other cell in the brain, as assessed by 2-photon microscopy (n=10 brains) as follows. Two-photon imaging of GFP-labeled neurons was performed on a Prairie Ultima two-photon microscope with a Coherent Chameleon Ti:Sapphire laser tuned to 945nm, GaAsP PMTs and an Olympus 40X 0.9NA water immersion objective at 512×512 pixel resolution and 1X or 2X optical zoom. Maximum projections were obtained from stacks taken at 1μm steps and processed using Fiji.
Electrophysiology
Whole-cell patch clamp electrophysiology experiments were performed on 2–3 days old flies. Flies were anaesthetized by brief cold exposure in an ice bath (~0°C) for ~1 min. Using a dissection microscope (Nikon SMZ1000), a small window in the head cuticle was opened and the underlying perineural sheath was gently removed using fine forceps (Moria Surgical). Brain tissue was exposed, and bathed in artificial hemolymph (AHL) solution containing the following (in mM): 103 NaCl, 3 KCl, 26 NaHCO3, 1 NaH2PO4, 8 trehalose dihydrate, 10 dextrose, 5 TES, 4 MgCl2, adjusted to 270–275 mOSm. For experiments, 1.5 mM CaCl2 was included and the solution was continuously bubbled with 95% O2 5% CO2 to pH 7.3 and perfused over the brain at a flow rate of 1–2 mL/min. To target neurons for patching under the 2-photon microscope, Gal4 or LexA lines expressing GFP for neuron targeting were excited at 840 nm and detected using a photomultiplier tube (PMT) through a bandpass filter (490–560 nm) using an Ultima 2-photon laser scanning microscope (Bruker, formerly Prairie Technologies). The microscope is equipped with galvanometers driving a Coherent Chameleon laser and a Dodt detector was used to visualize neural tissue/somata. Images were acquired with an upright Zeiss Examiner.Z1 microscope with a Zeiss W Plan-Apochromat 40×0.9 numerical aperture water immersion objective at 512 pixels × 512 pixels resolution using PrairieView software v. 5.2 (Bruker). Current clamp recordings were performed with pipettes pulled (Sutter P-97) using borosilicate capillary tubes (WPI Cat # 1B150F-4) with open tip resistances of 20 +/− 3 MΩ filled with internal solution containing the following (in mM): 140 K-aspartate, 1 KCl, 1 EGTA, 10 HEPES, 4 Mg-ATP, 0.5 Na3-GTP, pH 7.3, 265 mOsm. To visualize the electrode and fill the cell after recording to confirm GFP co-localization, Alexa Fluor 594 Hydrazide (5 μM; Thermofisher Scientific Cat. # A10438) was added into the intracellular solution, excited using the 2-photon microscope at 840 nm, and detected with a second PMT through a bandpass filter (580–630 nm). Recordings were made using Axopatch 200B patch-clamp amplifier and CV203BU headstage (Axon Instruments), lowpass filtered at 2 KHz, scaled to a 20x output gain, digitized with a Digidata 1320 A, and acquired with Clampex software v.9.2.1.9 (Axon Instruments).
Temperature stimulation
For temperature stimulation, preparations were continuously perfused with Ca2+-containing AHL (as described above). AHL was gravity fed through a 3-way valve (Lee company, part # LHDA1231315H) and flow rate was adjusted through a flow regulator. Following the valve, temperature was precisely regulated through 2 in-line solution heater/coolers (Warner, cat. # SC-20) in parallel with by a dual channel bipolar temperature controller (Warner Instruments, Cl-200A). Excess heat produced by each SC-20 Peltier was dissipated through a liquid cooling system (Koolance, Cat. # EXT-1055). To circumvent changes in resistivity and voltage offsets from changing the temperature of the bathing solution, the reference Ag-Cl pellet electrode was placed in an isolated well adjacent to the recording chamber (Warner Instruments, Cat. # RC-24N), filled with identical AHL and connected via a borosilicate capillary tube filled by 2% agar in 3 M KCl. The bath temperature was precisely recorded using a custom Type T thermocouple with an exposed tip (Physitemp, Cat. # T-384A) connected to a thermometer (BAT-12, Physitemp) with an analogue output connected to the digitizer and sampled at 10 kHz. The tip of thermocouple was threaded through a borosilicate capillary tube and precisely placed near the antennae using a micromanipulator (MP-225, Sutter Instruments).
Optogenetic Stimulation
All-trans retinal powder (RET, Sigma-Aldrich) was mixed with ethanol to prepare a 100mM stock solution. 1mL of stock was then mixed with 250mL of molasses and cornmeal medium to produce 400μM food. To optogenetically activate ACs, TRPA1SH Gal4;UAS-CsChrimson males were crossed to 65D05LexA;Aop.GFP females on food laced with all-trans RET, covered in foil and placed in a DigiTherm incubator (Trikinetics). Progeny (2–3 days old) for experimentation were then dissected (as above) and placed in perfusate un the 2-photon microscope for LPN recording. ACs expressing Cs.Chrimson were stimulated with a red LED light (660nm, Thorlabs M660FP1) placed near the brain with a fiberoptic cable (Thorlabs M28L01). After establishing a whole cell recodring in an LPN, 5s light stimuli were triggered using a TTL pulse with pClamp software delivered to the LED driver (Thorlabs, LEDD1B). A one-tailed, 2-sample t-test was used to test for significance between light evoked firing rates in Cs.Chrimson expressing and control (no Chrimson) flies and significance (*) was defined as p<0.05).
Antenna Ablation
To remove both AC and antennal input while recording from LPNs, TRPA1SH Gal4>UAS.Kir; 65D05 LexA>Aop.GFP flies were collected shortly after eclosion and anesthetized on ice. Fine forceps were used to gently pluck the antennae from the flies. Removal of the entire third segment and arista was confirmed visually. Flies were allowed to recover overnight before dissecting and making whole-cell recordings of LPNs under the 2-photon microscope. A 2-sample, 1-tailed t-test was used to test for significance (*) between WT (control) flies and AC silenced; -antennae at 28°C both 30°C, p<0.01.
Sleep Experiments
All flies were reared at 25°C in 12 hr light: 12 hr dark (LD) conditions). Male flies (2–5 days old) were loaded into 65mm × 5mm tubes containing 5% sucrose 2% agar food medium. Locomotor activity was recorded using the Drosophila Activity Monitoring System (DAMS, Trikinetics) in 1-minute intervals. Temperature, light, and humidity were controlled using a DR-36NL incubator (Percival Scientific). To suppress synaptic output of AC Gal4 (TRPA1SH Gal4) and LPN split-Gal4 (R26A08 AD ∩ R65D05 DBD), males were crossed to virgin UAS-TNT females, and male progeny were assayed. Sleep was defined as ≥5 consecutive minutes of inactivity. Activity and sleep analysis was performed using MatLab 2020b software (Mathworks). Sleep and activity are displayed in 30-minute bins represented as mean ± SEM. For 25°C-30°C behavior comparisons (Fig. 3A, D, F, H), flies were entrained for 3 days to 12:12 LD at 25°C, then gradually transferred to 30°C during the 3rd night (12 hr +0.4°C/hr linear heating ramp, ZT12-0) for one day. 25°C activity and sleep data are from the 3rd day. For heat step experiments (Fig. 3 A–C), flies were entrained for 3 days to 12:12 LD at 25°C. On the 4th day, the temperature was raised to 30°C either from ZT0-3 or ZT6-12 (+0.5°C/min). Quantifications were made between the 30°C day and the preceding 25°C day.
Connectomics
To identify ACs in the connectome, we queried the EM hemibrain using Python scripts and Neuprint v.1.2.1 (https://neuprint.janelia.org/). We reasoned (see text) that AC neurons would be annotated as fragments as their somas are located on the antennal nerve. Candidate ACs skeletons were selected based on projections in AL/PAL region near where the antennal nerve enters the brain and based on bilateral output in the SMP. Candidate skeletons were further validated by visual comparison with AC light microscopy images. AC skeleton IDs: 789330613, 1912436017, 572408934, 635523991. LPN skeleton IDs: 45034902 (R1), 480029788 (R2), 356818551(R3). DN1a skeleton IDs: 5813022274, 264083994. TPN-IV skeleton ID: 1858901026. TPN-V skeleton ID: 1975878958.To reconstruct brain volume images, neuron skeletons and brain region ROIs were downloaded from the hemibrain dataset using Python scripts and reconstructed in Paraview. To determine synaptic connectivity from neurons of interest, we used Python scripts to query Neuprint. Pie charts in Figures 2 and 4 were constructed by first excluding any synaptic connection with less than 5 synapses (i.e. weight <5). Neurons were then grouped according to type, following which we applied a secondary group exclusion criterion of less than a summed weight of 10. See supplemental Figures for a complete list of neuron types/IDs that were included in each pie chart. Color maps (Figure S2) were constructed using no exclusion criteria.
QUANTIFICATION AND STATISTICAL ANALYSIS
Detection of Action Potentials
Membrane potential recordings were made in current clamp mode sampled at 10 kHz. Data was analyzed offline using Axograph and Igor Pro. Action potentials (spikes) were detected using custom scripts in Igor Pro using Neuromatic v2.6i plug-in. A first derivative transformation was performed on the membrane potential trace defining dV/dt. Then a constant dV/dt threshold was used to detect individual spikes. Peristimulus time histograms (PTH) of firing rate were made by binning detected spikes in 1s bins, defining spikes/s (Hz). In experiments in which single trials from individual cells were averaged, the line and shading indicate the mean firing rate (Hz) ± SD. For experiments in which multiple sweeps were performed at a given stimulus temperature, average PTH were calculated per cell; the line and shading indicate the mean firing rate (Hz) ± SD; then across cells the line and shading indicate the mean firing rate (Hz) ± SEM.
Temperature Stimulation
In experiments in which single trials from individual cells were averaged, the line and shading indicate the mean temperature (°C) ± SEM. For experiments in which multiple sweeps were performed at a given stimulus temperature, the line and shading indicate the mean temperature (°C) ± SD across cells. Separate linear fits were performed (from 20°C to 25°C and from 28°C to 30°C) in Igor Pro to identify a relationship between firing rates and absolute temperature for ACs (plot in Fig. 1E). The 95% CI bands are displayed along with the r2 correlation value for each fit line. To test for a significant relationship between temperature and firing rate for LPNs (Fig. 2 H,I,K), a 1-way, 2-sample t-test was performed and significance (*) defined as p < 0.01. (*) indicates independent comparisons between either 28°C or 30°C between control, -AC, and -AC/-antennae conditions.
Circadian Behavior
To test for significant differences in both sleep and activity bins at 25°C vs 30°C (Fig. 3A, D, F, H), paired two-sided paired t-tests were used; p<0.05. To test for significant changes in sleep from 25°C to 30°C (Fig. 3B), paired two-sided paired t-tests were used; * = p<0.05. To test for significant changes in sleep within fly from 25°C to 30°C (Fig. 3C), one-sample t-tests were used; * = p<0.05. When comparing change in sleep across genotypes (Fig. 3G), significance differences between experimental and the appropriate control genotypes were identified using a 2-way Analysis of Variance (ANOVA) with a post-hoc Bonferroni test for multiple comparisons; * = p<0.05.
Supplementary Material
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, Peptides, and Recombinant Proteins | ||
| Alexa Fluor 594 Hydrazide | Thermo Fisher Scientific | Cat. # A10438 |
| all trans-Retinal | Sigma Aldrich Inc | R2500 |
| NaCl | Sigma Aldrich Inc | S9888 |
| KCl | Sigma Aldrich Inc | P9333 |
| NaHCO3 | Sigma Aldrich Inc | S5761-1KG |
| NaH2PO4 | Sigma Aldrich Inc | S8282 |
| trehalose dihydrate | Sigma Aldrich Inc | T5251 |
| dextrose | Sigma Aldrich Inc | G8270 |
| TES | Sigma Aldrich Inc | T1375 |
| MgCl2 | Sigma Aldrich Inc | M9272 |
| CaCl2 | Sigma Aldrich Inc | C1016 |
| L-Aspartic acid potassium salt | Sigma Aldrich Inc | A6558 |
| EGTA | Sigma Aldrich Inc | 03777 |
| Mg-ATP | Sigma Aldrich Inc | A9187 |
| Na3-GTP | Sigma Aldrich Inc | G3776 |
| Yellow cornmeal | Genesee Scientific | 62–101 |
| Yeast | Genesee Scientific | 62–103 |
| Nutri-Fly® Drosophila Agar, Gelidium | Genesee Scientific | 66–104 |
| Molasses | Genesee Scientific | 62–118 |
| Propionic acid | Fisher Scientific | A258-500 |
| Tegosept | Genesee Scientific | 20–259 |
| Experimental Models: Organisms/Strains | ||
| D. melanogaster: 20XUAS-IVS-CsChrimson.mVenus | Bloomington Drosophila Stock Center | BDSC: 55136; Flybase: FBti0160804 |
| D. melanogaster: 10XUAS-IVS-mCD8::GFP | Bloomington Drosophila Stock Center | BDSC: 32186; Flybase: FBst0032186 |
| D. melanogaster: UAS-TNT.E2 | Bloomington Drosophila Stock Center | BDSC: 28837; Flybase: FBti0038528 |
| D. melanogaster: 65D05 LexA | Bloomington Drosophila Stock Center | BDSC: 53625 Flybase: FBst0053625 |
| D. melanogaster: 13x LexAop-CD2::GFP | 35 | |
| D. melanogaster: SH-Gal4 | 1 | |
| D. melanogaster: R26A08 AD | Bloomington Drosophila Stock Center | BDSC:70150 Flybase: FBst0070150 |
| D. melanogaster: 65D05 DBD | Bloomington Drosophila Stock Center | BDSC:69667 Flybase: FBst0069667 |
| D. melanogaster: UAS.Kir 2.1 | Bloomington Drosophila Stock Center | BDSC:6595 Flybase: FBst0006595 |
| Software and Algorithms | ||
| MATLAB | The Mathworks | http://www.mathworks.com |
| Fiji | 36 | http://fiji.sc |
| Pclamp (Clampex software v.9.2.1.9) | Axon Instruments/Molecular Devices | https://www.moleculardevices.com/ |
| Igor Pro v.6.37 | Wavemetrics, Inc. | https://www.wavemetrics.com/ |
| Neuromatic v2.6i plug-in for Igor Pro | 37 | http://www.neuromatic.thinkrandom.com/ |
| Axograph v.1.70 | Axograph | https://axograph.com |
| Python v.3.7.7 | Python Software foundation | https://www.python.org/psf/ |
| Paraview v.5.9.0 | Kitware, Inc. | https://www.paraview.org/ |
| Neuprint v.1.2.1 | 12 | https://neuprint.janelia.org/ |
Highlights.
-
d
Internal AC sensors function as thermometers for hot temperature in Drosophila
-
d
ACs firing is proportional to temperature above the fly’s preferred range of 25°C
-
d
ACs drive the LPN clock neurons, increasing daytime siesta sleep in hot conditions
-
d
AC→LPN run parallel to cold circuitry allowing selective sleep adjustment to hot/cold
ACKNOWLEDGMENTS
We thank Evan Kaspi, Eileen Ni and Ramzy Issa for technical assistance, Josh Levy for scripts and Ravi Allada for generously providing access to equipment, Ravi Allada, Nilay Yapici, Matthew Meiselman, Dominic Frank and members of the Gallio Lab for comments on the manuscript. Work in the Gallio lab is supported by NIH grants R01NS086859, R21EY031849, a Pew Scholars Program in the Biomedical Sciences and a McKnight Technological Innovations in Neuroscience Awards (to M.G). M.H.A. was supported by training grant NIH T32HL007909. Authors declare no competing interests. All data is available in the main text or the supplementary materials.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Hamada FN, Rosenzweig M, Kang K, Pulver SR, Ghezzi A, Jegla TJ, and Garrity PA (2008). An internal thermal sensor controlling temperature preference in Drosophila. Nature 454, 217–220. 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shafer OT, Helfrich-Forster C, Renn SC, and Taghert PH (2006). Reevaluation of Drosophila melanogaster’s neuronal circadian pacemakers reveals new neuronal classes. J Comp Neurol 498, 180–193. 10.1002/cne.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alpert MH, Frank DD, Kaspi E, Flourakis M, Zaharieva EE, Allada R, Para A, and Gallio M (2020). A Circuit Encoding Absolute Cold Temperature in Drosophila. Curr Biol 30, 2275–2288 e2275. 10.1016/j.cub.2020.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallio M, Ofstad TA, Macpherson LJ, Wang JW, and Zuker CS (2011). The coding of temperature in the Drosophila brain. Cell 144, 614–624. 10.1016/j.cell.2011.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simoes JM, Levy JI, Zaharieva EE, Vinson LT, Zhao P, Alpert MH, Kath WL, Para A, and Gallio M (2021). Robustness and plasticity in Drosophila heat avoidance. Nat Commun 12, 2044. 10.1038/s41467-021-22322-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin X, Tian Y, Zhang ZC, Gu P, Liu C, and Han J (2021). A subset of DN1p neurons integrates thermosensory inputs to promote wakefulness via CNMa signaling. Curr Biol 31, 2075–2087 e2076. 10.1016/j.cub.2021.02.048. [DOI] [PubMed] [Google Scholar]
- 7.Lamaze A, Ozturk-Colak A, Fischer R, Peschel N, Koh K, and Jepson JE (2017). Regulation of sleep plasticity by a thermo-sensitive circuit in Drosophila. Sci Rep 7, 40304. 10.1038/srep40304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Das A, Holmes TC, and Sheeba V (2016). dTRPA1 in Non-circadian Neurons Modulates Temperature-dependent Rhythmic Activity in Drosophila melanogaster. J Biol Rhythms 31, 272–288. 10.1177/0748730415627037. [DOI] [PubMed] [Google Scholar]
- 9.Tang X, Platt MD, Lagnese CM, Leslie JR, and Hamada FN (2013). Temperature integration at the AC thermosensory neurons in Drosophila. J Neurosci 33, 894–901. 10.1523/JNEUROSCI.1894-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang X, Roessingh S, Hayley SE, Chu ML, Tanaka NK, Wolfgang W, Song S, Stanewsky R, and Hamada FN (2017). The role of PDF neurons in setting the preferred temperature before dawn in Drosophila. Elife 6. 10.7554/eLife.23206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Top D, and Young MW (2018). Coordination between Differentially Regulated Circadian Clocks Generates Rhythmic Behavior. Cold Spring Harb Perspect Biol 10. 10.1101/cshperspect.a033589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scheffer LK, Xu CS, Januszewski M, Lu Z, Takemura SY, Hayworth KJ, Huang GB, Shinomiya K, Maitlin-Shepard J, Berg S, et al. (2020). A connectome and analysis of the adult Drosophila central brain. Elife 9. 10.7554/eLife.57443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shih HW, and Chiang AS (2011). Anatomical characterization of thermosensory AC neurons in the adult Drosophila brain. J Neurogenet 25, 1–6. 10.3109/01677063.2011.571323. [DOI] [PubMed] [Google Scholar]
- 14.Kaneko M, and Hall JC (2000). Neuroanatomy of cells expressing clock genes in Drosophila: transgenic manipulation of the period and timeless genes to mark the perikarya of circadian pacemaker neurons and their projections. J Comp Neurol 422, 66–94. . [DOI] [PubMed] [Google Scholar]
- 15.Yoshii T, Heshiki Y, Ibuki-Ishibashi T, Matsumoto A, Tanimura T, and Tomioka K (2005). Temperature cycles drive Drosophila circadian oscillation in constant light that otherwise induces behavioural arrhythmicity. Eur J Neurosci 22, 1176–1184. 10.1111/j.1460-9568.2005.04295.x. [DOI] [PubMed] [Google Scholar]
- 16.Reinhard N, Bertolini E, Saito A, Sekiguchi M, Yoshii T, Rieger D, and Helfrich-Forster C (2022). The lateral posterior clock neurons of Drosophila melanogaster express three neuropeptides and have multiple connections within the circadian clock network and beyond. J Comp Neurol 530, 1507–1529. 10.1002/cne.25294. [DOI] [PubMed] [Google Scholar]
- 17.Marin EC, Buld L, Theiss M, Sarkissian T, Roberts RJV, Turnbull R, Tamimi IFM, Pleijzier MW, Laursen WJ, Drummond N, et al. (2020). Connectomics Analysis Reveals First-, Second-, and Third-Order Thermosensory and Hygrosensory Neurons in the Adult Drosophila Brain. Curr Biol 30, 3167–3182 e3164. 10.1016/j.cub.2020.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klapoetke NC, Murata Y, Kim SS, Pulver SR, Birdsey-Benson A, Cho YK, Morimoto TK, Chuong AS, Carpenter EJ, Tian Z, et al. (2014). Independent optical excitation of distinct neural populations. Nat Methods 11, 338–346. 10.1038/nmeth.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ni JD, Gurav AS, Liu W, Ogunmowo TH, Hackbart H, Elsheikh A, Verdegaal AA, and Montell C (2019). Differential regulation of the Drosophila sleep homeostat by circadian and arousal inputs. Elife 8. 10.7554/eLife.40487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyasako Y, Umezaki Y, and Tomioka K (2007). Separate sets of cerebral clock neurons are responsible for light and temperature entrainment of Drosophila circadian locomotor rhythms. J Biol Rhythms 22, 115–126. 10.1177/0748730407299344. [DOI] [PubMed] [Google Scholar]
- 21.Yadlapalli S, Jiang C, Bahle A, Reddy P, Meyhofer E, and Shafer OT (2018). Circadian clock neurons constantly monitor environmental temperature to set sleep timing. Nature 555, 98–102. 10.1038/nature25740. [DOI] [PubMed] [Google Scholar]
- 22.Baines RA, Uhler JP, Thompson A, Sweeney ST, and Bate M (2001). Altered electrical properties in Drosophila neurons developing without synaptic transmission. J Neurosci 21, 1523–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frank DD, Jouandet GC, Kearney PJ, Macpherson LJ, and Gallio M (2015). Temperature representation in the Drosophila brain. Nature 519, 358–361. 10.1038/nature14284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hendricks JC, Finn SM, Panckeri KA, Chavkin J, Williams JA, Sehgal A, and Pack AI (2000). Rest in Drosophila is a sleep-like state. Neuron 25, 129–138. 10.1016/s0896-6273(00)80877-6. [DOI] [PubMed] [Google Scholar]
- 25.Shaw PJ, Cirelli C, Greenspan RJ, and Tononi G (2000). Correlates of sleep and waking in Drosophila melanogaster. Science 287, 1834–1837. 10.1126/science.287.5459.1834. [DOI] [PubMed] [Google Scholar]
- 26.Cao W, and Edery I (2015). A novel pathway for sensory-mediated arousal involves splicing of an intron in the period clock gene. Sleep 38, 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parisky KM, Agosto Rivera JL, Donelson NC, Kotecha S, and Griffith LC (2016). Reorganization of Sleep by Temperature in Drosophila Requires Light, the Homeostat, and the Circadian Clock. Curr Biol 26, 882–892. 10.1016/j.cub.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sweeney ST, Broadie K, Keane J, Niemann H, and O’Kane CJ (1995). Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron 14, 341–351. 10.1016/0896-6273(95)90290-2. [DOI] [PubMed] [Google Scholar]
- 29.Joiner WJ, Crocker A, White BH, and Sehgal A (2006). Sleep in Drosophila is regulated by adult mushroom bodies. Nature 441, 757–760. 10.1038/nature04811. [DOI] [PubMed] [Google Scholar]
- 30.Pitman JL, McGill JJ, Keegan KP, and Allada R (2006). A dynamic role for the mushroom bodies in promoting sleep in Drosophila. Nature 441, 753–756. 10.1038/nature04739. [DOI] [PubMed] [Google Scholar]
- 31.Sitaraman D, Aso Y, Jin X, Chen N, Felix M, Rubin GM, and Nitabach MN (2015). Propagation of Homeostatic Sleep Signals by Segregated Synaptic Microcircuits of the Drosophila Mushroom Body. Curr Biol 25, 2915–2927. 10.1016/j.cub.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meiselman MR, Alpert MH, Cui X, Shea J, Gregg I, Gallio M, and Yapici N (2022). Recovery from cold-induced reproductive dormancy is regulated by temperature-dependent AstC signaling. Curr Biol 32, 1362–1375 e1368. 10.1016/j.cub.2022.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Helfrich-Forster C (1995). The period clock gene is expressed in central nervous system neurons which also produce a neuropeptide that reveals the projections of circadian pacemaker cells within the brain of Drosophila melanogaster. Proc Natl Acad Sci U S A 92, 612–616. 10.1073/pnas.92.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shafer OT, Kim DJ, Dunbar-Yaffe R, Nikolaev VO, Lohse MJ, and Taghert PH (2008). Widespread receptivity to neuropeptide PDF throughout the neuronal circadian clock network of Drosophila revealed by real-time cyclic AMP imaging. Neuron 58, 223–237. 10.1016/j.neuron.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lai SL, and Lee T (2006). Genetic mosaic with dual binary transcriptional systems in Drosophila. Nat Neurosci 9, 703–709. 10.1038/nn1681. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data reported in this paper will be shared by the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.


