Abstract
Background
Asymmetries in craniofacial anomalies are commonly observed. In the facial skeleton, the left side is more commonly and/or severely affected than the right. Such asymmetries complicate treatment options. Mechanisms underlying variation in disease severity between individuals as well as within individuals (asymmetries) are still relatively unknown.
Results
Developmental reductions in fibroblast growth factor 8 (Fgf8) have a dosage dependent effect on jaw size, shape, and symmetry. Further, Fgf8 mutants have directionally asymmetric jaws with the left side being more affected than the right. Defects in lower jaw development begin with disruption to Meckel's cartilage, which is discontinuous. All skeletal elements associated with the proximal condensation are dysmorphic, exemplified by a malformed and misoriented malleus. At later stages, Fgf8 mutants exhibit syngnathia, which falls into two broad categories: bony fusion of the maxillary and mandibular alveolar ridges and zygomatico‐mandibular fusion. All of these morphological defects exhibit both inter‐ and intra‐specimen variation.
Conclusions
We hypothesize that these asymmetries are linked to heart development resulting in higher levels of Fgf8 on the right side of the face, which may buffer the right side to developmental perturbations. This mouse model may facilitate future investigations of mechanisms underlying human syngnathia and facial asymmetry.
Keywords: cardiopharyngeal, jaw asymmetry, malleus, non‐linear genotype‐phenotype, syngnathia
Key Findings
Reductions in Fgf8 have dosage‐dependent effects on proximal jaw and middle ear morphology
Defects in lower jaw development in Fgf8 mutants are directionally asymmetric, with the left side being more affected than the right
Fgf8 mutants exhibit the two types of syngnathia observed in humans: bony fusion of the maxillary and mandibular alveolar ridges and zygomatico‐mandibular fusion
Morphogenesis of pharyngeal epithelia is disrupted in Fgf8 mutants, causing further disruptions to gene expression in the pharyngeal arches
Asymmetry in jaw defects is associated with asymmetry in Fgf8 expression in the developing heart
1. INTRODUCTION
Among the most common and debilitating human congenital birth defects are those that affect the craniofacial complex. 1 Treatment of craniofacial malformations is often complicated by variation in disease severity. 2 , 3 Variation in severity can also occur within individuals, exhibited as left‐right asymmetry. Facial asymmetry is typical in healthy populations (nonpathologic asymmetry), with mandibular asymmetries being the most common. 4 In fact, recent studies indicate that more than half of the general population exhibits some degree of mandibular asymmetry and that right‐side dominance appears in up to 80% of individuals. 4 , 5 Congenital malformations exhibiting asymmetry include facial clefts, hemifacial microsomia, and craniosynostosis. Notably, unilateral clefts of the lip occur on the left side twice as often as clefts on the right side. 6 It is unclear what mechanisms underlie this directional asymmetry.
In a previous study, we found that phenotypic variation in craniofacial disease outcomes can be explained by a non‐linear relationship between gene expression and facial shape. 7 The non‐linear model posits that reduction in a particular developmental factor (e.g., gene expression) can be buffered until a point, but when molecular levels drop below the buffered level, high levels of phenotypic variance are observed. In other words, when molecular levels are high, differences in expression of 5%–10% (or even 50%) do not generate different phenotypes. However, when molecular levels are low, differences of 5%–10% cause quantitative differences in morphology (Figure 8E). This non‐linear model helps to explain both why most loss‐of‐function mutations are recessive (heterozygous individuals are unaffected) and why disease phenotypes exhibit differences in severity among affected individuals. 7 , 8 , 9 A further prediction of this model is that facial asymmetry derives from left to right differences in levels of important developmental factors.
FIGURE 8.
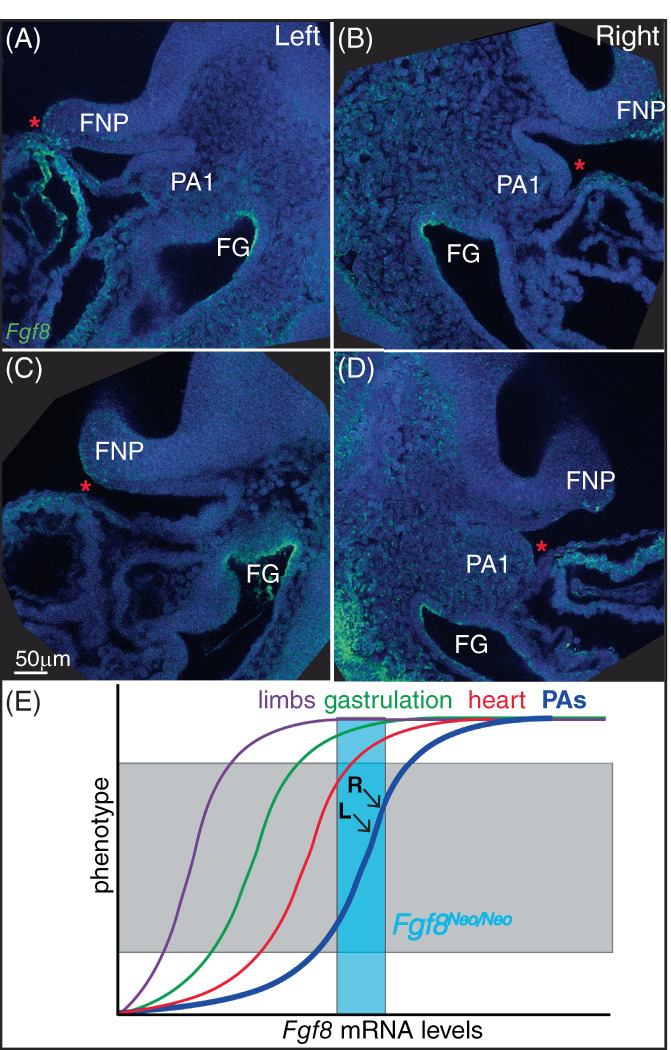
Fgf8 is expressed asymmetrically in the developing heart. (A‐D) Saggital sections of fluorescent in situ hybridization of Fgf8 (green) in E9.5 embryos. Left (A, C) and right (B, D) sides are shown for two separate specimens. Sections shown are those where the developing heart tube comes closest to the first pharyngeal arch (PA1) for each side. Nuclei are counterstained with dapi (blue). (E) Model for non‐linear relationships for Fgf8 levels and tissue morphogenesis. Teal box represents Fgf8 levels in Fgf8 Neo/Neo mice. L and R indicate levels in left and right sides of PA1 (see text for more details). Frontonasal process (FNP), foregut (FG)
The jaw develops from the growth and fusion of a series of facial primordia composed of neural crest‐ and mesoderm‐derived mesenchyme surrounded by several epithelia. The lower jaw forms from the paired mandibular processes of the first pharyngeal arch (PA1), while the upper jaw derives predominantly from the paired maxillary prominences with minor contributions from the frontonasal processes. The lower jaw, or mandible, is subsequently formed by the fusion of the left and right dentary bones, which develop largely independently from each other. The relative morphological simplicity of the lower jaw makes it a great system to study phenotypic variation. Additionally, the developmental independence of the left and right halves of the lower jaw allows the effects of subtle differences in developmental perturbations on morphology to be observed.
Fgf8 is expressed in several tissues important for craniofacial development, including the oral ectoderm of PA1, the lateral ectoderm of the pharyngeal clefts, the foregut endoderm of the pharyngeal pouches, and the anterior heart field mesoderm. To further investigate how Fgf8 dosage contributes to variation in craniofacial phenotypes, we utilized an allelic series of mutant mice that generates embryos expressing different levels of Fgf8 during development, including a mild and severe mutant. 10 Quantification of Fgf8 mRNA levels in E10.5 heads indicates that mice heterozygous for the Neo allele (Fgf8 Neo/+ ) express 90% of Fgf8 +/+ (wildtype; WT) levels, mice heterozygous for the Delta allele (Fgf8 Δ/+ ) express 60% of WT levels, mice homozygous for the Neo allele (Fgf8 Neo/Neo ) express 35% of WT levels, and compound mutants (Fgf8 Δ/Neo ) express 20% of WT levels. 7 Results reported here were generated using these mice, as well as an additional null allele, in which the Fgf8 coding sequence has been replaced by a LacZ cassette. The two null alleles are functionally equivalent 11 and both are referred to here as “Delta” alleles.
Our previous work quantitatively related differences in Fgf8 gene expression (molecular variation) to facial shape (morphological variation). Here, we further detail how Fgf8 dosage contributes to variation in disease severity, focusing on development of the lower jaw, particularly Meckel's cartilage (MC) and its derivatives. We describe not only inter‐individual variation in lower jaw defects, but also intra‐individual variation. Specifically, Fgf8 mutants exhibit directional asymmetry with the left side of the jaw more affected than the right. We hypothesize that cardio‐pharyngeal developmental interactions contribute to increased Fgf8 expression on the right side of PA1 during early development. This difference is likely to be small, but significant enough to buffer reductions in Fgf8 when levels are low (on the high slope of the non‐linear curve).
2. RESULTS
2.1. Reduction in Fgf8 causes dysmorphic and asymmetric jaws
To investigate the role of Fgf8 dosage on jaw size, we first evaluated the lower jaws of neonatal (P0) mice from the allelic series. Dentary bones in mice heterozygous for either Fgf8 Neo or Fgf8 Δ are phenotypically normal, suggesting that significant reductions in Fgf8 dosage are buffered in jaw development, consistent with previous work. 7 However, in both mutant genoytpes (Fgf8 Neo/Neo and Fgf8 Δ/Neo ), the dentary bones are hypoplastic and dysmorphic to varying degrees (Figures 1 and 2). Morphological defects are concentrated proximally. The distal most portion of the dentary is unaffected, with rostral process and incisors intact, consistent with the distal region of the jaw being Fgf8 independent. 12 Mandibles from Fgf8 Δ/Neo neonates exhibit severe phenotypes, while neonatal Fgf8 Neo/Neo mandibles are comparatively mild. In the mildest Fgf8 Neo/Neo mutants, only the coronoid process is missing while the rest of the dentary bone appears normal. However, in some Fgf8 Neo/Neo specimens, more severe phenotypes are apparent, including fusion of the dentary to the maxilla (Figure 2). When syngnathia occurs in Fgf8 Neo/Neo jaws, it is typically unilateral (11/34 Fgf8 Neo/Neo neonates exhibit unilateral fusion on the left side; 1/34 had bilaterally fused jaws; 22/34 exhibit no fusion). In Fgf8 Δ/Neo neonates, syngnathia is always bilateral (20/20).
FIGURE 1.
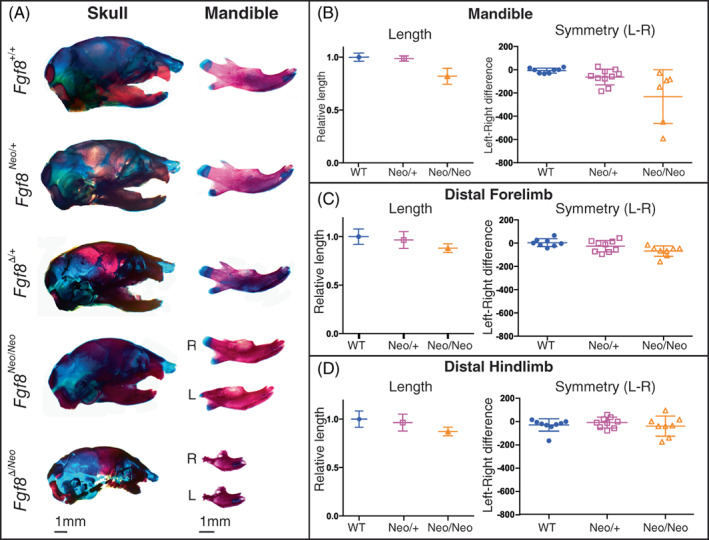
Mandibles exhibit dosage sensitivity and directional asymmetry. (A) Neonatal skulls (left) and isolated dentary bones (right) differentially stained for bone (red) and cartilage (blue). Representative images are shown for Fgf8 +/+ (WT), Fgf8 Neo/+ , Fgf8 Δ/+ , Fgf8 Neo/Neo , and Fgf8 Δ/Neo neonates. Right (R) and left (L) sides of the dentary are shown for the two mutant genotypes. (B) The length (left) of isolated dentaries were measured using arbitrary units and symmetry (right) was determined by subtracting the length of the right side from the length of the left side for each individual. (C, D) Isolated limb bones from Fgf8 Neo/+ and Fgf8 Neo/Neo neonatal mice were measured for length (left) and symmetry (right). n = 9 per genotype. 1 mm scale bars are shown for each respective column in (A)
FIGURE 2.
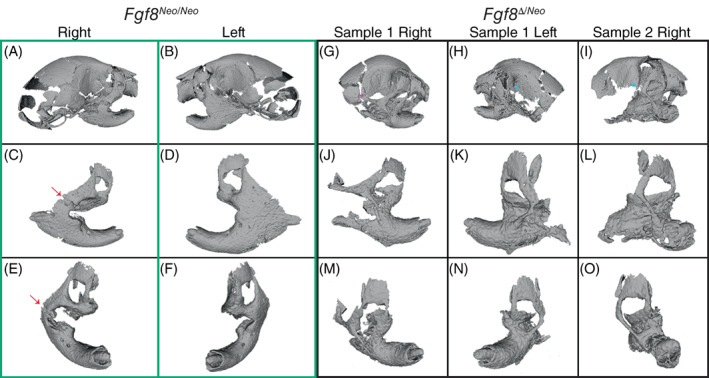
Fgf8‐mediated jaw fusion occurs in two major forms. Three‐dimensional reconstructions from micro‐CTs of (A‐F) Fgf8 Neo/Neo (green box) and (G‐O) Fgf8 Δ/Neo (black box) neonatal skulls and jaws. Panels (C‐F) and (J‐O) show individual outer, then frontal, aspects of a single dentary of the corresponding skull (shown in the upper panels). (A‐F) Fusion of Fgf8 Neo/Neo jaws occurs between a broadened zygomatic arch at the lateral side of the dentary and is more severe on the left side than the right side [arrows in (C) and (E) point to unfused zygomatic contact). (G‐O) Fgf8 Δ/Neo jaws exhibit an additional fusion between the dentary and maxillary process. The zygomatic fusion in Fgf8 Δ/Neo jaws occurs as a thinner bony element that is more distal and lateral on the dentary than what is observed in Fgf8 Neo/Neo jaws. Although the overall fusion is similar on both sides of the jaw in Fgf8 Δ/Neo , the squamosal process can be (G) present (purple asterisk) or (H, I) absent (red askterisks). Data shown are representative of eight Fgf8 Neo/Neo and 7 Fgf8 Δ/Neo neonatal skulls. CT, computed tomography
We observed two broad categories of syngnathia: (1) fusion of the maxillary zygomatic process and/or jugal to the dentary and (2) bony fusion of the maxilla and dentary (zygomatic process and jugal bones may be absent) (Figure 2). These two categories were most obvious in the Fgf8 Δ/Neo mandibles, where both types of fusion occur (Figure 2G‐O). In Fgf8 Neo/Neo neonates, we only observed fusion of upper and lower jaw elements on the dorso‐lateral side of the dentary, posterior to the molar alveolus. This fusion sometimes appears in a mild form with a jugal‐zygomatic process apparent. In other cases, the fusion forms a wide bony connection between the maxilla and mandible (Figure 2A‐F). In Fgf8 Δ/Neo neonates, fusion of the zygomatic process occurs more distal and lateral on the dentary than is observed in Fgf8 Neo/Neo neonates. Further, Fgf8 Δ/Neo neonates typically exhibit bony fusion between the maxilla and dentary that connects the entire dorsal side of the molar alveolar region of the dentary to the upper jaw (Figure 2G‐O). Notably, even in the most severe cases of fusion, rudimentary condylar and angular processes are typically present albeit dysmorphic (Figure 2).
Despite the wide variation in severity of dysmorphology between specimens, we also noted that within specimens, the left dentary bone was typically more severely disrupted than the right, which was especially apparent in Fgf8 Neo/Neo neonates. To further assess directional asymmetry, we measured the length of isolated dentary bones from WT and Fgf8 Neo/Neo neonates, comparing right versus left sides (Figure 1B). Our measurements indicate that the dentary bones of Fgf8 Neo/Neo neonates are shorter than in WT or Fgf8 Neo/+ neonates, and that the left side is on average shorter than the right. In contrast, skeletal elements from the limbs, while smaller in Fgf8 Neo/Neo mutants, are symmetrical (Figure 1C,D). Taken together, these data indicate that below an initial buffered level, Fgf8 has a dosage effect on jaw size and symmetry.
2.2. Loss of coronoid is independent of the temporalis
To further assess proximal defects of the mandible, we evaluated micro‐computed tomographies (micro‐CTs) of neonatal skulls (Figure 3). In all Fgf8 Neo/Neo mutants, the coronoid is absent or severely reduced bilaterally (Figure 3C). Notably, the temporalis muscle attaches to the dorsal region of the dentary where a rudimentary coronoid is sometimes present (Figure 3B). Since it has been shown that mechanical load from the temporalis muscle maintains growth and regulates size of the coronoid process, 13 , 14 we calculated the volume of the temporalis muscle from 3D reconstructions of micro‐CT scans of neonatal mouse heads to determine a potential role for the temporalis muscle in coronoid asymmetry. We found that the muscle volume is smaller in mutants than in WT, however, there is no bilateral difference in size (Figure 3D; n = 6 for WT, n = 3 for mutant). The attachment of the temporalis to the coronoid in the mutants suggests that an absence of mechanical signals from the temporalis is unlikely to explain the absence of the coronoid. The relative symmetry in the muscles further suggests that this mechanism is not responsible for morphological asymmetry.
FIGURE 3.
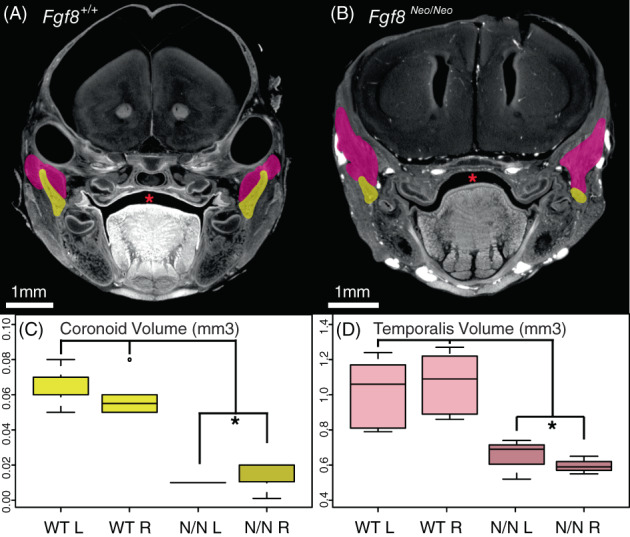
Fgf8 Neo/Neo mutants have small temporalis muscles and absent coronoid processes. Cross‐sections from micro‐CT scans of (A) Fgf8 +/+ (WT) and (B) Fgf8 Neo/Neo neonatal skulls highlighting the coronoid (yellow) and temporalis muscles (pink). (C) Coronoid volume and (D) temporalis volume are represented in mm3. The section where the coronoid was the largest was chosen for each sample in (A, B). The temporalis appears larger in this particular section of the Fgf8 Neo/Neo embryo because shape changes in the muscle related to the smaller jaw and proximal shift in the location of the coronoid relative to the rest of the skull condense the temporalis. Red asterisks highlight normal palate formation in WT and the cleft palate in Fgf8 Neo/Neo . 1 mm scale bars are shown for each panel. *P‐value <.05, Tukey's HSD Test. n = 6 for WT, n = 3 for Fgf8 Neo/Neo . CT, computed tomography
2.3. Fgf8 mutants exhibit discontinuous MC
In addition to hypoplasia of the jaw bones, disruptions to MC exhibit Fgf8 dosage sensitivity. In both Fgf8 Neo/Neo and Fgf8 Δ/Neo mutants, MC is discontinuous near its proximal end where it appears as two separate condensations forming proximal and distal segments (Figure 4). This disruption to MC is observed in 100% of mutant embryos of both genotypes (observed in 6/6 embryos of each genotype) and occurs early in skeletal development, as it is apparent by E14.5. In the mildest mutants, this discontinuity is unilateral, occurring only on the left side (observed in one embryo), while in specimens where the discontinuity is bilateral, the gap is typically greater on the left side (data not shown). These data are consistent with our observation of directional asymmetry in the dentary bones. Further, the distance between the distal and proximal segments of MC is greater in Fgf8 Δ/Neo than it is in Fgf8 Neo/Neo embryos, suggesting an Fgf8 dosage effect (data not shown).
FIGURE 4.
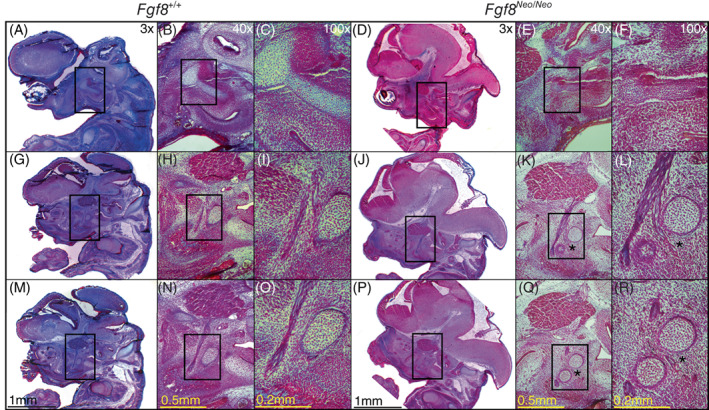
Meckel's cartilage of Fgf8 Neo/Neo mutants is separated into two non‐continuous condensations. Trichrome stained cross‐sections of Fgf8 +/+ (WT) and Fgf8 Neo/Neo E14.5 embryonic skulls. Sections are arranged from lateral (top row) to medial (bottom row). Laterally, Meckel's cartilage (MC) in (A‐C) Fgf8 +/+ and (D‐F) Fgf8 Neo/Neo is connected to the malleus in the proximal region. (G‐L) Once the trigeminal branches into the Lingual and Inferior Alveolar Nerve, MC is discontinuous in Fgf8 Neo/Neo creating a proximal and distal segment. (M‐R) MC is proximal to the branching nerve(s) in Fgf8 +/+ while in Fgf8 Neo/Neo the distal segment is between the branches. Asterisks denote the separation of the proximal and distal condensations of MC in Fgf8 Neo/Neo . Discontinuity at E14.5 was seen bilaterally in 2/2 Fgf8 Neo/Neo heads. Scale bars shown in (M‐R) apply to all images in each respective column
In all cases, the discontinuity in MC consistently occurs at the proximal end of the cartilage. In younger embryos (E14.5), MC is separated at the point where the trigeminal nerve branches into the Lingual and Inferior Alveolar Nerve (Figure 4K,L). In neonatal (P0) embryos, this discontinuity can be observed behind the developing molars, in the proximal plane of the lower jaw near where the coronoid would normally form. Related to this discontinuity, the most proximal end of MC fails to extend medially and instead curves laterally.
2.4. MC defects contribute to abnormalities of the middle ear and jaw joint
Fgf8 reductions result in a truncated and discontinuous MC. The proximal condensation of MC is malformed and mis‐oriented, contributing defects of the malleus and middle ear (Figure 5). The head of the malleus is reduced in Fgf8 Neo/Neo neonates and in Fgf8 Δ/Neo mutants it is nearly lost. In contrast, the manubrium of the malleus is enlarged and rotated dorsally. As a consequence, the tympanic membrane contacts the malleus at the process brevis in addition to the manubrium (compare Figure 5A,B to Figure 5C). The enlarged process brevis of the malleus lies ventrally on a shortened tympanic membrane (Figure 5C), whereas in WT, the manubrium of the malleus connects to the middle of the tympanic membrane at a focal point generating a V‐shape in the membrane (Figure 5A,B). Additionally, the tensor tympani muscle is smaller in Fgf8 Neo/Neo mutants (Figure 5, compare J‐K) and absent in Fgf8 Δ/Neo mutants (Figure 5L).
FIGURE 5.
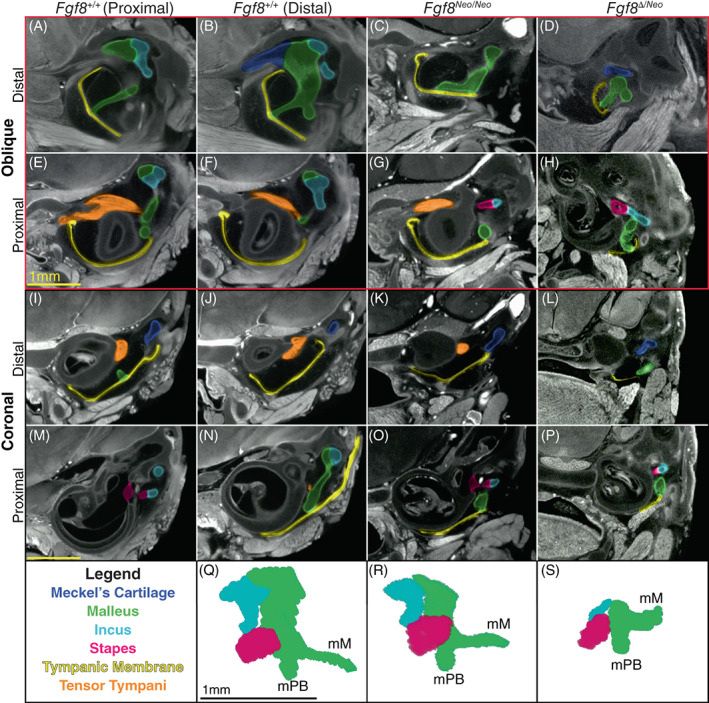
Fgf8 reductions condense the malleus and middle ear. Micro‐CT scans of the middle ear of Fgf8 +/+ (WT; two left columns), Fgf8 Neo/Neo (third column) and Fgf8 Δ/Neo (fourth right column) neonatal skulls. WT sections in the first column are proximal to WT sections in column 2. Rows 1 and 2 (red rectangle) are oblique sections through the malleus and rows 3 and 4 are coronal sections through similar regions. Distal sections are shown in rows 1 and 3; more proximal sections of each sample are shown in rows 2 and 4. (A, B) In WT, the manubrium of the malleus (green) is shown where it contacts the tympanic membrane (TM; yellow) creating a V shape at the point of contact. (C, D) In, Fgf8 Neo/Neo and Fgf8 Δ/Neo the manubrium is dorsally rotated away from the TM, which is small and dysmorphic. (E, F) In WT, the tensor tympani muscle (orange) curves around the sphenoid‐temporal articulation. The tensor tympani muscle is reduced in (G) Fgf8 Neo/Neo , and possibly absent in (H) Fgf8 Δ/Neo [see also (K, L)]. (I, J) In WT, the tympanic cavity is large separating the middle ear and cochlea, but in (K) Fgf8 Neo/Neo the cavity is reduced in size and becomes smaller in (L) Fgf8 Δ/Neo . (M, N) The incus (light blue) articulates with the stapes (pink) at the oval window of the otic capsule proximal to the malleus [panel (N) is distal to (M)]. (O, P) In the mutants, the middle ear is compressed, such that all three middle ear bones lie in one plane, with the incus and stapes rostral to the malleus. The stapes appears morphologically normal in all dosage levels. (Q‐S) Three‐dimensional reconstructions of the middle ear of (Q) Fgf8 +/+ (WT), (R) Fgf8 Neo/Neo , and (S) Fgf8 Δ/Neo ; malleus, incus and stapes. Manubrium of Malleus (mM),process brevis of the malleus (mPB). Scale bar in (E) applies to (A‐H), scale bar in (M) applies to (I‐P), and scale bar in (Q) applies to (Q, S). CT, computed tomography
In Fgf8 Neo/Neo mutants, the tympanic cavity is reduced in size (compare Figure 5I,J to Figure 5K). In Fgf8 Δ/Neo mutants, the tympanic cavity is severely reduced and sometimes absent (Figure 5H,L; see also Figure 6). In some Fgf8 Δ/Neo mutants, the tympanic cavity opens into the oral cavity (Figure 6G). This abnormal connection between the tympanic and oral cavities was observed bilaterally in three neonates, although the proximal‐distal plane varies from side to side so that it appears unilateral in any particular cross‐section.
FIGURE 6.
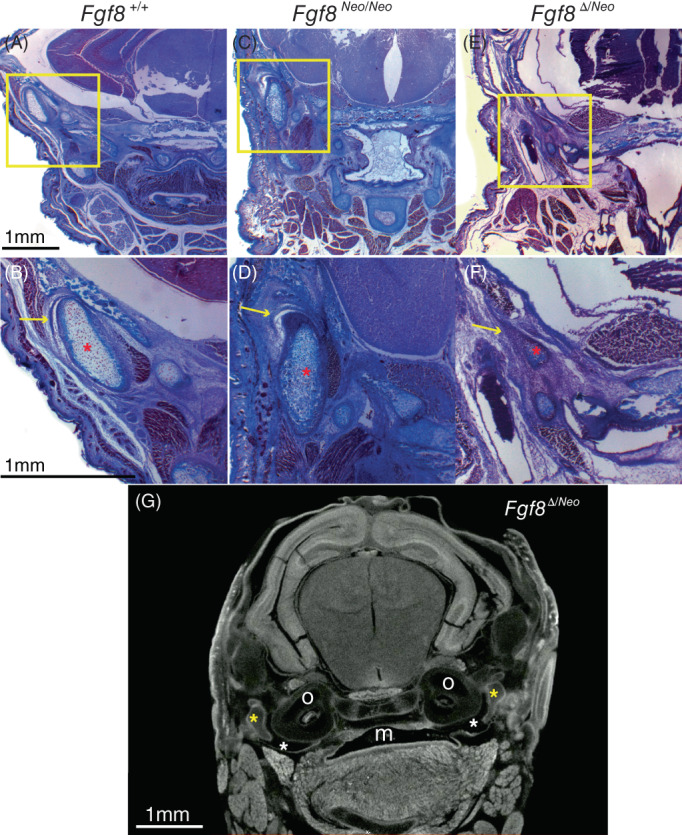
Severe reductions of Fgf8 deform the temporomandibular jaw joint (TMJ). Trichrome stained coronal sections of Fgf8 +/+ (WT; left column), Fgf8 Neo/Neo (middle column), and Fgf8 Δ/Neo (right column) neonatal (P0) skulls. Yellow boxes in upper panels are shown magnified in lower panels. (A, B) In WT, the TMJ has a joint disc [yellow arrow in (B)] below a defined glenoid fossa creating an upper joint cavity. (C, D) The condyle in Fgf8 Neo/Neo mice is dysmorphic and rotated vertically and the upper joint cavity is reduced, but an articular disk [yellow arrow in (D)] is still present. (E, F) Fgf8 Δ/Neo neonates have a severely reduced glenoid fossa and condylar process, and the joint cavity and articular disc are absent [yellow arrow in (F)]. Red asterisks highlight the condylar process. (G) micro‐CT scans of Fgf8 Δ/Neo show the oral cavity opening into the tympanic cavity (white asterisk). Yellow asterisks highlight the malleus. Mouth (m), otic capsule (o). CT, computed tomography
Truncation of MC contributes to increased cranial base flexion in the mutant skulls relative to WT (Figure 1). This flexion results in the cochlea lying behind (caudal and ventral) the middle ear. As a result, there is rostral shift of the jaw joint and middle ear relative to the rostral‐caudal axis of the head. This contributes to compression of the middle ear in the mutants (Figure 5M‐P), In WT, the articulation between the stapes and incus (Figure 5M) is proximal to the malleus (Figure 5N). In contrast, in both mutants, the stapes‐incus articulation occurs in the same proximal‐distal plane where the malleus contacts the tympanic membrane (Figure 5O,P). In this plane, the malleus is lateral to the incus and stapes at the oval window of the otic capsule rather than lying distal to the oval window (Figure 5M‐P).
To better visualize defects of the middle ear bones, we generated 3D models of the middle ears extracted from the micro‐CT scans of each genotype (Figure 5Q,R). As noted above, the head of the malleus is reduced in the mutants in a dosage sensitive manner. In the Fgf8 Δ/Neo mutants, the head of the malleus is nearly lost, which contributes to the articulation between malleus and incus being severely reduced.
We also observed an Fgf8 dosage effect on jaw joint formation. In mammals, the jaw articulates at the temporomandibular joint (TMJ) between the condylar process of the dentary and the glenoid fossa of the squamosal bone. In WT (Fgf8 +/+ ) neonatal mice, the TMJ is characterized by an articular disc sitting below the glenoid fossa creating a defined joint cavity (Figure 6A,B). In Fgf8 Neo/Neo neonates, the condyle is rotated vertically and the upper joint cavity is reduced. An articular disk is present, albeit slightly dysmorphic (Figure 6C,D). However, in Fgf8 Δ/Neo neonates, the glenoid fossa, condylar process and joint cavity are severely reduced and malformed, and the articular disc is absent (Figure 6E,F).
2.5. Apoptosis of neural crest is not asymmetric
Reductions in Fgf8 have previously been reported to increase apoptosis in migrating and post‐migratory neural crest. 15 , 16 , 17 , 18 Such reductions in neural crest numbers have been hypothesized to contribute to the hypoplasia associated with craniofacial defects in Fgf8 mutant mice. We also evaluated apoptosis in E9.5 and E10.5 Fgf8 Neo/Neo heads to investigate if any asymmetry was present that might contribute to the directional asymmetry we observe in the lower jaw. In WT embryos, apoptosis appears as an isolated cluster in the proximal hinge region of PA1, where apoptosis is known to occur in the developing epibranchial placodes. 19 In contrast, in Fgf8 Neo/Neo embryos, apoptosis occurs in large streams of neural crest migrating from the neural tube and the focal cluster in the epibranchial placodes is absent. However, apoptosis in PA1 of Fgf8 Neo/Neo embryos is minor (Figure 7P,Q). Previously, Frank et al. obtained similar results from a different hypomorphic Fgf8 mouse line. 18 In contrast, in Fgf8;Nestin‐Cre embryos, in which Fgf8 is completely absent in the oral ectoderm, cell death in PA1 is extensive and the mandibular arch is severely hypoplastic by E10.5. 12 These data suggest that sufficient Fgf8 levels exist in the oral ectoderm of Fgf8 Neo/Neo embryos to maintain survival of post‐migratory neural crest. Notably, we find no evidence suggesting that left‐right asymmetry in apoptosis is a driving factor in mandibular asymmetry (data not shown).
FIGURE 7.
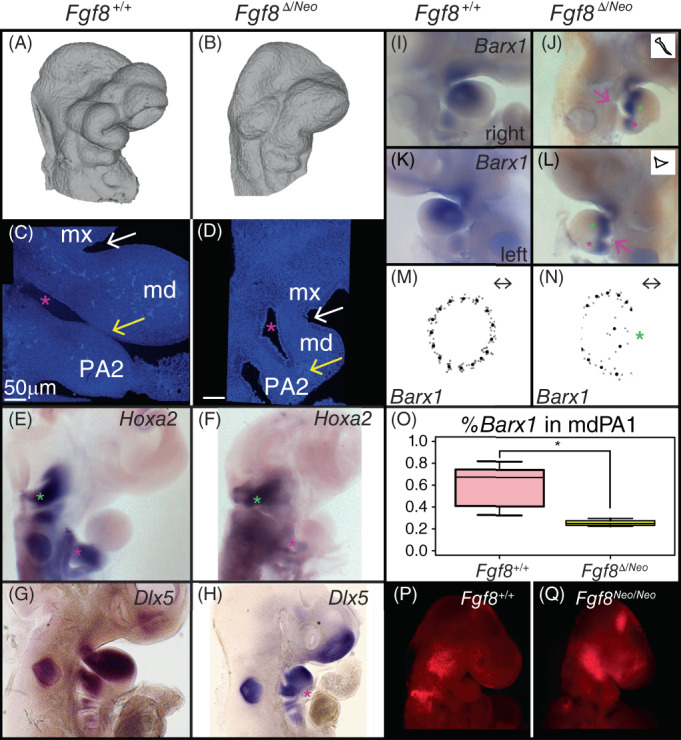
Reductions in Fgf8 dosage alter pharyngeal tissue relationships and patterning. (A, B) Lateral views of micro‐CT images of E10.5 (A) Fgf8 +/+ (WT) and (B) Fgf8 Δ/Neo embryos show reduction of the first pharyngeal arch (PA1) in the mutants. Lateral views of nuclear staining of E10.5 (C) Fgf8 +/+ (WT) and (D) Fgf8 Δ/Neo embryos show alterations to the mandibular (md) and maxillary (mx) portions of PA1. The oral ectoderm does not extend into the hinge region of PA1 (white arrow). The first pharyngeal cleft (pink asterisk) fails to extend in the mutants and the first and second arches fail to separate distally (yellow arrow). Whole‐mount in situ hybridization in E10.5 embryos for (E, F) Hoxa2, (G, H) Dlx5, and (I‐L) Barx1 show alterations to gene expression in Fgf8 Δ/Neo mutants. In WT, Hoxa2 expression in PA2 [pink asterisk in (E)] is equivalent to that in the hindbrain (green asterisk), whereas in Fgf8 Δ/Neo embryos, Hoxa2 is reduced in PA2 [pink asterisk in (F)]. Dlx5 and Barx1 expression domains are continuus across PA1 and PA2 where the arches are abnormally continugous [pink asterisks in (H, J, L)]. Additionally, Barx1 separates proximally in the mutants [green asterisks in (J, L, N)]. The cleft on the left side of the embryo exhibits less extension compared to the right (pink arrow; outline of cleft highlighted in inset of (J, L). (M, N) The average shape of Barx1 expression in PA1 is circular in (M) WT and more crescent shaped in (N) Fgf8 Δ/Neo . (O) Barx1 expression is reduced in PA1 of Fgf8 Δ/Neo compared to WT embryos. (P, Q) Lysotracker (red) staining of apoptotic cells is shown in E9.0 (P) WT and (Q) Fgf8 Neo/Neo embryos. All in situs were performed on at least two embryos per genotype. *P‐value <.0001, Welch Two Sample t‐test. CT, computed tomography
2.6. Reduction in Fgf8 disrupts PA1 patterning
The alterations to the distribution of cell death discussed above suggested that early embryonic patterning may be disrupted when Fgf8 dosage is reduced. To further assess alterations to PA1 patterning, we investigated the expression of several genes known to be important to early patterning of the jaw. At E10.5, the first pharyngeal arch is typically well developed with a deep invagination of the oral ectoderm separating the maxillary (mx) and mandibular (md) portions of PA1. However, in Fgf8 Δ/Neo embryos, the oral ectoderm is severely reduced and the hinge region of PA1 is underdeveloped such that the maxillary and mandibular processes are only separated at the most distal end of the arch (Figure 7A‐D). Further, the pharyngeal cleft, which normally separates the first and second arches, fails to extend in Fgf8 Δ/Neo embryos. As a consequence, the mesenchyme of PA1 and PA2 is continuous (yellow arrows in Figure 7C,D).
We found that the expression of Hoxa2 is severely reduced in PA2 in Fgf8 Δ/Neo embryos (Figure 7E,F). This PA1‐like expression pattern may be related to its altered association to signaling centers in the pharyngeal arches. Within PA1, we find that Dlx5 and Barx1 are also altered in Fgf8 Δ/Neo E10.5 embryos (Figure 7G‐J). Dlx5 does not extend past the hinge region in mutant embryos and is continuous across PA1 and PA2 in the region where the cleft fails to extend (pink asterisk in Figure 7H). Similar to Dlx5, Barx1 expression in Fgf8 Δ/Neo embryos is continuous across PA1 and PA2 (pink asterisks in Figure 7I‐L). Further, Barx1 expression is reduced in Fgf8 Δ/Neo embryos and appears to bifurcate at the distal end of its expression domain (green asterisks in Figure 7J,L). We quantified this difference in Barx1 expression in two ways. First, we used a morphometric approach to evaluate shape of the expression domain (Figure 7M,N). In WT, Barx1 expression is essentially circular, appearing round at the distal end (Figure 7K,M). However, in Fgf8 Δ/Neo embryos the expression domain is crescent in shape, with a concave distal end (Figure 7L,N). Additionally, these embryos show that failure of cleft extension on the left side is more severe than on the right side (pink arrows and insets in Figure 6J,L). These data are consistent with an alteration to patterning of the pharyngeal arches in Fgf8 mutants.
2.7. Fgf8 is expressed asymmetrically in the developing heart
Fgf8 is expressed in several focal areas of the pharyngeal arches, specifically the proximal oral ectoderm, the lateral ectoderm rostral to the pharyngeal clefts, and the endodermal pouches, where it is critical to survival and patterning of neural crest cells. 10 , 12 , 20 Expression in these domains, especially the oral ectoderm, may exhibit fluctuating asymmetry in early pharyngeal arch development, however, it equilibrates bilaterally by E10.5. 21 Therefore, we do not consider Fgf8 expression in these domains to be a causative factor underlying directional asymmetry in the mandible. However, Fgf8 expression is bilaterally asymmetric in the anterior heart field mesoderm precursors of the outflow tract. 22 This asymmetry is a byproduct of heart looping which shifts the outflow tract to the right side of the pharyngeal region, adjacent to the first and second pharyngeal arches.
To further characterize Fgf8 expression in this domain relative to the developing first arch, we performed fluorescent in situ hybridization for Fgf8 on E9.5 embryos. We found that ectoderm overlying the developing heart contacts the first arch ectoderm on the right side, but not the left. Instead, on the left side of the embryo, regions of the heart approach the frontonasal process (Figure 8A‐D). Fgf8 expression within the developing heart at early developmental stages may, therefore, contribute to PA1 development. These tissue relationships provide a possible explanation for directional asymmetry of the jaw, via increased Fgf8 levels on the right side of the embryo at a stage that is critical for mandibular patterning.
We did further test this hypothesis by investigating mandibular asymmetry in tissue‐specific Fgf8 knock‐outs by crossing mice with a floxed Fgf8 allele with tissue‐specific cre mice. Loss of Fgf8 in the ectoderm using an Ap2α‐Cre generates a severely truncated jaw similar to that reported for Fgf8;Nestin‐Cre, with some fluctuating asymmetry (data not shown). Removal of Fgf8 from the mesoderm using Mesp1‐Cre had no obvious affect on jaw morphology. Because of buffering effects, it is possible that directional asymmetry only occurs when Fgf8 is reduced globally. If so, a tissue‐specific knock‐out in a global hypomorphic background would be required to test this hypothesis. Such a mouse model is not currently available.
3. DISCUSSION
3.1. Jaw defects are caused by disruptions to pharyngeal patterning
To investigate the impact of Fgf8 dosage on jaw morphology, we examined the morphology of the lower jaw in mice expressing various levels of Fgf8 during development. The allelic series of mice we used includes two mutant genotypes, Fgf8 Neo/Neo (mild mutants) and Fgf8 Δ/Neo (severe mutants). These two genotypes are distinguished by their average level of Fgf8 expression as well as the overall severity of defects. Nevertheless, specimens within each mutant genotype also express variable phenotypes (Figures 1 and 2). For example, the mildest Fgf8 Neo/Neo neonates have relatively normal mandibles that only lack a coronoid process. More severe Fgf8 Neo/Neo neonates exhibit unilateral fusion of the upper and lower jaws. Overall, we find that reductions in Fgf8 expression below about 40% of WT expression levels result in truncated and dysmorphic lower jaws.
The morphological defects observed in mutant embryos are focal, being localized to the proximal portion of the jaw. Specifically, MC is bifurcated near the TMJ, and all derivatives of MC proximal to the point of bifurcation are dysmorphic. The distal end of MC and its derivatives, including the rostral process and incisor portion of the dentary are grossly morphologically normal, although reduced in size to varying degrees. Proximal defects of MC are exemplified by a dysmorphic malleus (Figures 4, 5, 6). Defects of the middle ear are compounded by increased cranial base flexion which compresses the middle ear on the rostral‐caudal axis (Figure 5).
Although the most severe mutant mandibles are small, many mild mutants have relatively normal dentaries in size and shape, lacking only a coronoid process. These mild mutants maintain muscle attachment (Figure 3), and are therefore not likely to be caused by deficiencies in mechanical signaling. 14 Additionally, despite an increase in apoptosis in migrating neural crest, Fgf8 mutants nonetheless have well‐populated pharyngeal arches. Cell death is observed in migrating neural crest but is not detected within PA1 at E9.5 (Figure 6Q). Because neural crest are not regionally specified until E10.5, 20 this suggests that the available neural crest should respond to appropriate patterning signals if present. Furthermore, previous work has shown that PA1 can regulate proliferation to account for significant reductions in progenitor numbers. 23 Although we cannot rule out a contribution of apoptosis to some of the defects, size of the neural crest progenitor population is not likely to explain the specificity of the defects to the proximal region of the jaw nor the asymmetry. Instead, the localization of the defects around the jaw hinge region suggests that reductions in Fgf8 dosage disrupt jaw patterning.
In PA1, proximal‐distal patterning is conferred by signals from the oral epithelia and Fgf8 has previously been identified as a key contributor to this patterning. 12 , 15 , 20 , 24 In particular, it has been hypothesized that patterning and polarity of the jaw are regulated by the interaction of signals emanating from the oral ectoderm and the first pharyngeal plate, which is formed where the ectodermal cleft contacts the first pharyngeal pouch endoderm. 24 , 25 Fgf8 is expressed in all three of these epithelia, and these tissues are underdeveloped and mis‐aligned in mutant embryos (Figure 7). The disruption of these tissue interactions is associated with alterations to pharyngeal morphology as well as the expression of key genes associated with pharyngeal patterning. Future work investigating how Fgf8 regulates the morphogenesis of these epithelia will be important to understand how patterning of the jaw is established.
3.2. Fgf8 dosage effects and phenotypic variation
We have previously described how Fgf8 mediates non‐linear dosage effects on craniofacial shape. 7 Here, we focus on the impact of Ffg8 dosage on jaw development, and more specifically, mandibular development. We have found that reductions in Fgf8 have a dosage dependent effect on jaw size, shape, and symmetry. On average, Fgf8 Neo/Neo E10.5 embryos express 35% of WT levels of Fgf8, while Fgf8 Δ/Neo embryos express roughly 20% of WT Fgf8 levels. 7 This indicates that the entire range of morphological variation seen in the jaws of both mutants occurs within a molecular range 15% of Fgf8 expression. On the other hand, the difference between Fgf8 expression in WT and heterozygous (Fgf8 Δ/+ ) embryos is 40%, yet these two genotypes are indistinguishable morphologically. These data are consistent with the hypothesis that developmental defects exhibit non‐linear genotype‐phenotype relationships (Figure 8E). Interestingly, some tissues appear to be more sensitive to reductions in Fgf8 dosage.
We have shown that Fgf8 Neo/Neo neonates have small but normal limbs, consistent with previous reports. 10 However, Fgf8 is required for limb development as tissue‐specific loss of Fgf8 in the apical ectodermal ridge (AER) of the developing limb results in reduction or loss of skeletal elements. 26 , 27 Nonetheless, limb development is more robust to loss of Fgf8 than is craniofacial development, likely due to compensation by other Fgfs expressed in the AER. Global Fgf8 knock‐outs fail to gastrulate, but 80% of Fgf8 Δ/Neo embryos complete gastrulation, 17 indicating that gastrulation has a low threshold requirement for Fgf8. In contrast, both heart and craniofacial development are more sensitive to Fgf8 levels. Abu‐Issa et al. describe abnormal heart development in 66% of Fgf8 Δ/Neo mutants, while roughly one‐third of these severe mutants have grossly normal hearts. Furthermore, only 2% exhibit situs invs, suggesting that these heart defects are related to heart‐specific Fgf8 expression and not associated with defective left‐right axis formation deriving from gastrulation related Fgf8 expression at the node. 17 In contrast, 100% of Fgf8 Neo/Neo (mild mutants) exhibit craniofacial defects including cleft palate. 10 , 17 These data suggest that craniofacial development is particularly sensitive to Fgf8 dosage (Figure 8E). These differences in sensitivity may reflect different evolutionary pressures for canalization vs evolvability in tissue development. Selection favoring evolvability in craniofacial development may underlie the adaptive radiation of vertebrates, but may also result in increased susceptibility to disease. 28
3.3. Directional asymmetry and cardiopharyngeal developmental interactions
The relatively mild defects observed in some Fgf8 Neo/Neo mutants suggests that this genotype expresses a range of Fgf8 that is near the upper part of the genotype‐phenotype curve (teal box in Figure 8E). Further, the directional asymmetry observed in this genotype is suggestive of subtle, but consistent, left‐right differences in Fgf8 expression (or Fgf signaling) in jaw development. We hypothesize that directional asymmetry in Fgf8 mutants results from asymmetric contributions of Fgf8 (or some other Fgf family member) to PA1 development from heart progenitor populations. The jaw and heart share progenitors within the pharyngeal mesoderm. 29 Thus, the directional asymmetry may derive from asymmetry in these migrating populations and/or subsequent heart morphogenesis. Heart looping results in the right side of PA1 experiencing more proximate relationships with the developing heart than the left side (Figure 8A‐D). Either of these tissue interactions may allow for asymmetric contributions of morphogens expressed in heart progenitors to PA1 development. We attempted to further evaluate this hypothesis by generating tissue‐specific Fgf8 loss‐of‐function embryos. Another potential approach to resolving this issue is to cross the Fgf8 allelic series into the situs inversus (iv mutation) mouse line. In this model, asymmetry would be predicted to fluctuate due to the randomization of visceral asymmetry caused by this mutation. 30
Directional asymmetry of the mandible, with the left side being smaller than the right, has also been reported in zebrafish heterozygous for Fgf8. 31 Interestingly, zebrafish that are hypomorphic for Fgf8 (ace mutants, which are comparable to Fgf8 Δ/Neo mutants), exhibit directional asymmetry in ceratobranchial cartilages, which derive from pharyngeal arches 3–7, but not the mandible. 32 In the hypomorphic mutants, the right side is more severely affected than the left. It is not clear why Fgf8 would have different bilateral roles in zebrafish adults and embryos or why the mandible would be affected with mild (heterozygous) reductions in Fgf8, but not more severe reductions (ace mutants). However, directional asymmetry of ceratobranchial cartilages in Fgf8 hypomorphic embryos only occurs in embryos with Kupffer's vesicle, a ciliated organ similar to the node that is associated with establishing left‐right asymmetry of the viscera. When Kupffer's vesicle is absent, these cartilages are symmetric. 32 These data suggest that: (1) Fgf8 is required for Kupffer's vesicle formation and establishment of the left‐right body axis, (2) defects in Kupffer's vesicle formation exhibit variable penetrance in Fgf8 hypomorphic mutants, and (3) the caudal pharyngeal arches (PAs 3–7) are sensitive to left‐right body axis signals, but the rostral arches (PAs 1 and 2) are not. 32 Therefore, the directional asymmetry observed in the mandibles of heterozygous Fgf8 adult zebrafish may be caused by asymmetries occurring after the establishment of left‐right asymmetry (such as heart looping) and may only be observed in individuals with sufficient Fgf8 levels to complete heart morphogenesis. This also suggests that zebrafish have different thresholds for Fgf8‐mediated tissue morphogenesis than mice, which would not be surprising given the much smaller size of zebrafish embryos.
Further investigation of how Fgf signaling is shared between craniofacial and cardiac progenitor populations may help elucidate disease mechanisms as well as asymmetries in congenital disorders. Human craniofacial anomalies exhibit similar directional trends to those reported here, and several craniofacial syndromes including Chromosome 22q11 deletion (22q11del) syndrome and Charge syndrome are characterized by both craniofacial and heart developmental defects. 4 , 5 , 6 Because Fgf signaling has been implicated in 22q11del syndrome, 18 , 33 , 34 it may therefore have more widespread involvement in congenital dysmorphology and asymmetry, which remains to be elucidated.
3.4. Fgf8 reduction models human syngnathia and TMJ defects
In humans, two broad categories of syngnathia exist: bony fusion of the maxillary and mandibular alveolar ridges and zygomatico‐mandibular fusion. 35 , 36 , 37 In the majority of the latter cases, the TMJ is intact. We have observed a similar pattern in our Fgf8 mutants. A role for Fgf8 in syngnathia and jaw joint formation has previously been reported. 38 In that study, it was hypothesized that loss of Foxc1 results in downregulation of Fgf8 in the oral ectoderm to 80% of WT levels (although this may also have included Fgf8 expression in the pharyngeal plate). Our data suggest that this is not a sufficient reduction to cause skeletal defects and the syngathia observed in Foxc1 mutants is likely due to some other function of this gene. This would not be surprising given the difference in specific aspects of the fusion, which include hyperplasia rather than hypoplasia, and the broader expression of Foxc1 in the proximal PA1 mesenchyme. Nonetheless, genetic interaction between Fgf8 and Foxc1, and potentially many other genes expressed in the pharyngeal region, may further contribute to variation in syngnathia phenotypes. However, the particular model we present here, especially the mild mutant (Fgf8 Neo/Neo ), provides an excellent system for future investigations of molecular and cellular mechanisms underlying syngnathia and TMJ defects that closely model those observed in human disease.
4. EXPERIMENTAL PROCEDURES
4.1. Experimental animals
The Fgf8 allelic series involves three viable, adult genotypes that can be crossed to generate five different embryonic genotypes. 10 These mice and embryos were genotyped as previously reported. 7 , 10 , 15 Embryos were collected and staged based on the number of days after the observation of a postcoital plug at E0.5. Neonates (P0) were collected at birth. Mouse experiments were approved by the University of Massachusetts Lowell (Neo series) and the University of Utah (CRE series) Institutional Animal Care and Use Committees. The tissue specific knock‐out lines were generated and genotyped as previously described. 22 , 39 Embryos were dissected on ice and fixed in 4% paraformaldehyde (PFA; for histology, gene expression and μCT) or 95% ethanol (for skeletal preparation).
4.2. Bone and cartilage staining
Differential bone and cartilage staining was performed using standard procedures. 40 Briefly, after ethanol fixation, embryos were skinned and then stained with 0.1% Alizarin Red and 0.3% Alcian Blue in ethanol for 3 days. After staining, the remaining non‐skeletal tissue was digested with 1% KOH. Skeletal tissue was then cleared in glycerol prior to imaging.
4.3. Quantification of jaw and limb length
Dentary and limb bones were isolated from neonates after differential bone and cartilage staining. Isolated bones were imaged on a dissecting microscope at 1x magnification. The length of the bones was measured using arbitrary units. The average length of wild‐type bones was set to 1 and then each individual bone was quantified relative to that average. Symmetry was determined by subtracting the length of the right bone from the left bone for each specimen.
4.4. Micro‐CT imaging
For analysis of soft tissues, neonatal heads were stained using a 0.7% phosphotungstic acid (PTA) solution following published methods. 41 Imaging was performed on paraffin wrapped samples. For analysis of isolated bone, publicly available scans of neonatal heads were used from a previously published study. 7 Scans of PTA‐stained embryos were visualized using Dragonfly software (version 2021.1 build 977, Object Research Systems). Scans of isolated bone were visualized with MeshLab.
4.5. Quantification of temporalis and coronoid volumes
Temporalis and coronoid volumes were quantified within the Dragonfly software. micro‐CT scans of each head were read into the program as tiff stacks. The pitch and yaw were adjusted to achieve a coronal view for each sample. A region‐of‐interest (ROI) mask was created separately for each measured structure of each sampled head. The round brush within the ROI Painter tool was used to label each voxel corresponding to the sections of each separate structure(s). By choosing separate masks and labeling the structure's voxels, a 3D image can be created for each structure, based on the morphology within the sample. Once the voxels of each structure were labeled, the Dragonfly software automatically calculates the total number of voxels labeled (based on the ROI mask chosen) and gives a volume of the chosen mask. By using the underlying morphologies to dictate, which voxels were labeled, we used this method to calculate 3D volumes of the temporalis and coronoid within each sample given as mm3. One‐way ANOVAs were used to evaluate the difference between genotypes and side.
4.6. Histology/trichrome staining
Neonatal and E14.5 heads were dehydrated in ethanol after overnight PFA fixation. The heads then were processed with Clear‐Rite, a xylene substitute, to remove ethanol, and finally embedded in paraffin (Surgipath Paraplast Plus, Leica (Epredia, Fisher Scientific)) inside a vacuum drying oven (ADP 31, Yamato). Embryo heads were sectioned at 10um using a microtome. Sections were stained using Milligan's trichrome, 42 mounted with Permount, and imaged under a bright field microscope.
4.7. In situ hybridization
Probes for in situ hybridization were generated from RNA isolated from E9.5 and E10.5 embryos. cDNA was produced using the Maxima first strand synthesis kit (ThermoFisher; K1641). The cDNA was then used as a PCR template to amplify the gene of interest (GOI). Select PCR primers had linkers (Fw:5′‐ggccgcgg‐3′; Rv:5′‐cccggggc‐3′) to allow for a nested PCR TOPO cloning. PCR purification of these templates used a gene specific forward primer (GSFP) and the T7 Universal primer to amplify the initial GOI template. Primers lacking linkers were TOPO cloned into TOP10 competent E. coli cells using a PCR4 topo vector (ThermoFisher). Colonies were screened to ensure correct band length. Once purified, samples were sent to Genewiz to be sequenced to ensure the correct GOI was amplified and direction of the insertion. A second PCR was used to amplify the GOI using M13 primers after plasmid verification. Second PCR product lengths were verified on a 1% agarose gel. Products from the secondary PCR were used as the template for the anti‐sense mRNA probe. Probes were made by using a dig RNA labeling mix (Sigma‐Aldrich; #11277073910). Fluorescent probes were created with A555 fluorescent tyramine following publicly available protocols (https://sites.google.com/site/helobdellaprotocols/histology/tsa). Fluorescent samples were counter‐stained using Hoechst's O.N. (1:1,000 dilution of 10 mg/mL stock) and were imaged using a 40x oil immersion objective lens on a Leica sp8 confocal microscope.
4.8. Apoptosis staining
Apoptotic cells were identified using Lysotracker Red (ThermoFisher). Unfixed E9.5 or E10.5 embryos were stained for 30 min in Lysotracker solution as previously described. 43 Stained embryos were clarified with methanol and then fixed in 4% PFA.
4.9. Quantification of Barx1 expression domain size and shape
Lateral images of E10.5 embryos processed for Barx1 in situ hybridization were taken at 5× magnification. Four landmarks were digitized of each image denoting the most anterior, distal, ventral, and proximal aspect of the Barx1 expression within the first pharyngeal arch (PA1). Based on curves that were added between each permanent landmark (four total curves), five sliding semi landmarks were added per curve (note: no curve was added that would cross over the expression area). The StereoMorph (version 1.6.3) package within RStudio (version 1.2.1335) was used to digitize these landmarks on each image. A generalized Procrustes analysis and Procrustes analysis of variance (ANOVA) were used in the geomorph package (version 3.3.2) to test if the shape of the expression was different based on genotype. Barx1 expression size was quantified by measuring the Barx1 positive area and total area of PA1 using imageJ. The area of the Barx1 positive region was divided by the size of the total area of PA1.
4.10. Specimens analyzed
Specimens were evaluated at E9.5, E10.5, E14.5, and P0 (neonatal) as described in Table 1.
TABLE 1.
Description of samples used in experiments described in this study
| Figure | Experiment | Non‐mutant | Mutant | Age |
|---|---|---|---|---|
| 1 | Skeletal preps | 25 | 23 | P0 |
| 1 | Length measurements | 18 | 9 | P0 |
| 2 | Mesh scans | ‐ | 15 | P0 |
| 3 | Micro‐CT/volumes | 6 | 3 | P0 |
| 4 | Histology | 2 | 2 | E14.5 |
| 5 | Micro‐CT | 6 | 5 | P0 |
| 6 | Histology | 3 | 6 | P0 |
| 6 | Micro‐CT | 0 | 2 | P0 |
| 7 | Morphology | 4 | 4 | E10.5 |
| 7 | Hoxa2 in situ | 3 | 4 | E10.5 |
| 7 | Dlx5 in situ | 3 | 3 | E10.5 |
| 7 | Barx1 in situ | 3 | 3 | E10.5 |
| 7 | Barx1 shape/size | 3 | 3 | E10.5 |
| 7 | Apoptosis | 5 | 3 | E9.5 |
| 8 | Fgf8 in situ | 2 | ‐ | E9.5 |
Note: Embryo numbers and stages per experiment are listed.
Abbreviation: CT, computed tomography.
AUTHOR CONTRIBUTIONS
Nathaniel Zbasnik: Data curation (equal); formal analysis (lead); investigation (lead); project administration (supporting); writing – original draft (supporting); writing – review and editing (supporting). Katie Dolan: Data curation (supporting); formal analysis (supporting). Stephanie A. Buczkowski: Data curation (supporting). Rebecca M. Green: Data curation (supporting). Benedikt Hallgrímsson: Funding acquisition (supporting); resources (supporting); writing – review and editing (supporting). Ralph S. Marcucio: Funding acquisition (supporting); resources (supporting); writing – review and editing (supporting). Anne M. Moon: Formal analysis (supporting); investigation (supporting); resources (supporting). Jennifer L. Fish: Conceptualization (lead); data curation (supporting); formal analysis (supporting); funding acquisition (lead); project administration (lead); resources (lead); supervision (lead); writing – original draft (lead); writing – review and editing (lead).
ACKNOWLEDGMENTS
We would like to thank Evelyn Schwager for technical support. This work was supported by the National Institutes of Health: R03 DE028984 to JLF and R01 DE019638 to BH and RSM.
Zbasnik N, Dolan K, Buczkowski SA, et al. Fgf8 dosage regulates jaw shape and symmetry through pharyngeal‐cardiac tissue relationships. Developmental Dynamics. 2022;251(10):1711‐1727. doi: 10.1002/dvdy.501
Funding information National Institutes of Health, Grant/Award Numbers: R01 DE019638, R03 DE028984
REFERENCES
- 1. Passos‐Bueno MR, Ornelas CC, Fanganiello RD. Syndromes of the first and second pharyngeal arches: a review. Am J Med Genet A. 2009;149A(8):1853‐1859. doi: 10.1002/ajmg.a.32950 [DOI] [PubMed] [Google Scholar]
- 2. McDonald‐McGinn DM, Sullivan KE. Chromosome 22q11.2 deletion syndrome (DiGeorge syndrome/velocardiofacial syndrome). Medicine (Baltimore). 2011;90(1):1‐18. doi: 10.1097/MD.0b013e3182060469 [DOI] [PubMed] [Google Scholar]
- 3. Dudakovic A, Nam HK, Wijnen AJV, Hatch NE. Genetic background dependent modifiers of craniosynostosis severity. J Struct Biol. 2020;212(3):107629. doi: 10.1016/j.jsb.2020.107629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thiesen G, Gribel BF, Kim KB, Pereira KCR, Freitas MPM. Prevalence and associated factors of mandibular asymmetry in an adult population. J Craniofac Surg. 2017;28(3):e199‐e203. doi: 10.1097/SCS.0000000000003371 [DOI] [PubMed] [Google Scholar]
- 5. Haraguchi S, Iguchi Y, Takada K. Asymmetry of the face in orthodontic patients. Angle Orthod. 2008;78(3):421‐426. doi: 10.2319/022107-85.1 [DOI] [PubMed] [Google Scholar]
- 6. Gundlach KK, Maus C. Epidemiological studies on the frequency of clefts in Europe and world‐wide. J Craniomaxillofac Surg. 2006;34(suppl 2):1‐2. doi: 10.1016/S1010-5182(06)60001-2 [DOI] [PubMed] [Google Scholar]
- 7. Green RM, Fish JL, Young NM, et al. Developmental nonlinearity drives phenotypic robustness. Nat Commun. 2017;8(1):1970. doi: 10.1038/s41467-017-02037-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Young NM, Chong HJ, Hu D, Hallgrímsson B, Marcucio RS. Quantitative analyses link modulation of sonic hedgehog signaling to continuous variation in facial growth and shape. Development Oct 2010;137(20):3405‐9. 10.1242/dev.052340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fridman H, Yntema HG, Magi R, et al. The landscape of autosomal‐recessive pathogenic variants in European populations reveals phenotype‐specific effects. Am J Hum Genet. 2021;108(4):608‐619. doi: 10.1016/j.ajhg.2021.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Meyers EN, Lewandoski M, Martin GR. An Fgf8 mutant allelic series generated by Cre‐ and Flp‐mediated recombination. Nat Genet. 1998;18(2):136‐141. doi: 10.1038/ng0298-136 [DOI] [PubMed] [Google Scholar]
- 11. Grieshammer U, Cebrian C, Ilagan R, Meyers E, Herzlinger D, Martin GR. FGF8 is required for cell survival at distinct stages of nephrogenesis and for regulation of gene expression in nascent nephrons. Development. 2005;132(17):3847‐3857. doi: 10.1242/dev.01944 [DOI] [PubMed] [Google Scholar]
- 12. Trumpp A, Depew MJ, Rubenstein JL, Bishop JM, Martin GR. Cre‐mediated gene inactivation demonstrates that FGF8 is required for cell survival and patterning of the first branchial arch. Genes Dev. 1999;13(23):3136‐3148. doi: 10.1101/gad.13.23.3136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Avis V. The relation of the temporal muscle to the form of the coronoid process. Am J Phys Anthropol. 1959;17(2):99‐104. doi: 10.1002/ajpa.1330170204 [DOI] [PubMed] [Google Scholar]
- 14. Anthwal N, Peters H, Tucker AS. Species‐specific modifications of mandible shape reveal independent mechanisms for growth and initiation of the coronoid. Evodevo. 2015;6:35. doi: 10.1186/s13227-015-0030-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Griffin JN, Compagnucci C, Hu D, et al. Fgf8 dosage determines midfacial integration and polarity within the nasal and optic capsules. Dev Biol. 2013;374(1):185‐197. doi: 10.1016/j.ydbio.2012.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Storm EE, Garel S, Borello U, et al. Dose‐dependent functions of Fgf8 in regulating telencephalic patterning centers. Development. 2006;133(9):1831‐1844. doi: 10.1242/dev.02324 [DOI] [PubMed] [Google Scholar]
- 17. Abu‐Issa R, Smyth G, Smoak I, Yamamura K, Meyers EN. Fgf8 is required for pharyngeal arch and cardiovascular development in the mouse. Development. 2002;129(19):4613‐4625. [DOI] [PubMed] [Google Scholar]
- 18. Frank DU, Fotheringham LK, Brewer JA, et al. An Fgf8 mouse mutant phenocopies human 22q11 deletion syndrome. Development. 2002;129(19):4591‐4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Washausen S, Obermayer B, Brunnett G, Kuhn HJ, Knabe W. Apoptosis and proliferation in developing, mature, and regressing epibranchial placodes. Dev Biol. 2005;278(1):86‐102. doi: 10.1016/j.ydbio.2004.10.016 [DOI] [PubMed] [Google Scholar]
- 20. Ferguson CA, Tucker AS, Sharpe PT. Temporospatial cell interactions regulating mandibular and maxillary arch patterning. Development. 2000;127(2):403‐412. [DOI] [PubMed] [Google Scholar]
- 21. Fish JL, Villmoare B, Kobernick K, et al. Satb2, modularity, and the evolvability of the vertebrate jaw. Evol Dev. 2011;13(6):549‐564. doi: 10.1111/j.1525-142X.2011.00511.x [DOI] [PubMed] [Google Scholar]
- 22. Park EJ, Ogden LA, Talbot A, et al. Required, tissue‐specific roles for Fgf8 in outflow tract formation and remodeling. Development. 2006;133(12):2419‐2433. doi: 10.1242/dev.02367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fish JL, Sklar RS, Woronowicz KC, Schneider RA. Multiple developmental mechanisms regulate species‐specific jaw size. Development. 2014;141(3):674‐684. doi: 10.1242/dev.100107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Depew MJ, Compagnucci C. Tweaking the hinge and caps: testing a model of the organization of jaws. J Exp Zool B Mol Dev Evol. 2008;310(4):315‐335. doi: 10.1002/jez.b.21205 [DOI] [PubMed] [Google Scholar]
- 25. Depew MJ, Simpson CA. 21st century neontology and the comparative development of the vertebrate skull. Dev Dyn. 2006;235(5):1256‐1291. doi: 10.1002/dvdy.20796 [DOI] [PubMed] [Google Scholar]
- 26. Moon AM, Capecchi MR. Fgf8 is required for outgrowth and patterning of the limbs. Nat Genet. 2000;26(4):455‐459. doi: 10.1038/82601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lewandoski M, Sun X, Martin GR. Fgf8 signalling from the AER is essential for normal limb development. Nat Genet. 2000;26(4):460‐463. doi: 10.1038/82609 [DOI] [PubMed] [Google Scholar]
- 28. Fish JL. Evolvability of the vertebrate craniofacial skeleton. Semin Cell Dev Biol. 2019;91:13‐22. doi: 10.1016/j.semcdb.2017.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Diogo R, Kelly RG, Christiaen L, et al. A new heart for a new head in vertebrate cardiopharyngeal evolution. Nature. 2015;520(7548):466‐473. doi: 10.1038/nature14435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Layton WM Jr. Random determination of a developmental process: reversal of normal visceral asymmetry in the mouse. J Hered. 1976;67(6):336‐338. doi: 10.1093/oxfordjournals.jhered.a108749 [DOI] [PubMed] [Google Scholar]
- 31. Albertson RC, Yelick PC. Fgf8 haploinsufficiency results in distinct craniofacial defects in adult zebrafish. Dev Biol. 2007;306(2):505‐515. doi: 10.1016/j.ydbio.2007.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Albertson RC, Yelick PC. Roles for fgf8 signaling in left‐right patterning of the visceral organs and craniofacial skeleton. Dev Biol. 2005;283(2):310‐321. doi: 10.1016/j.ydbio.2005.04.025 [DOI] [PubMed] [Google Scholar]
- 33. Moon AM, Guris DL, Seo JH, et al. Crkl deficiency disrupts Fgf8 signaling in a mouse model of 22q11 deletion syndromes. Dev Cell. 2006;10(1):71‐80. doi: 10.1016/j.devcel.2005.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guo C, Sun Y, Zhou B, et al. A Tbx1‐Six1/Eya1‐Fgf8 genetic pathway controls mammalian cardiovascular and craniofacial morphogenesis. J Clin Invest. 2011;121(4):1585‐1595. doi: 10.1172/JCI44630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Laster Z, Temkin D, Zarfin Y, Kushnir A. Complete bony fusion of the mandible to the zygomatic complex and maxillary tuberosity: case report and review. Int J Oral Maxillofac Surg. 2001;30(1):75‐79. doi: 10.1054/ijom.2000.0009 [DOI] [PubMed] [Google Scholar]
- 36. Rubio‐Palau J, Prieto‐Gundin A, de Abreu Graterol LM, Vercruysse H Jr. Maxillomandibular syngnathia: 3D planning and review of the literature. Craniomaxillofac Trauma Reconstr. 2018;11(2):124‐130. doi: 10.1055/s-0037-1606248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fallahi HR, Naeini M, Mahmoudi M, Javaherforoosh F. Congenital zygomatico‐maxillo‐mandibular fusion: a brief case report and review of literature. Int J Oral Maxillofac Surg. 2010;39(9):930‐933. doi: 10.1016/j.ijom.2010.04.003 [DOI] [PubMed] [Google Scholar]
- 38. Inman KE, Purcell P, Kume T, Trainor PA. Interaction between Foxc1 and Fgf8 during mammalian jaw patterning and in the pathogenesis of syngnathia. PLoS Genet. 2013;9(12):e1003949. doi: 10.1371/journal.pgen.1003949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Macatee TL, Hammond BP, Arenkiel BR, Francis L, Frank DU, Moon AM. Ablation of specific expression domains reveals discrete functions of ectoderm‐ and endoderm‐derived FGF8 during cardiovascular and pharyngeal development. Development. 2003;130(25):6361‐6374. doi: 10.1242/dev.00850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Depew MJ. Analysis of skeletal ontogenesis through differential staining of bone and cartilage. Methods Mol Biol. 2008;461:37‐45. doi: 10.1007/978-1-60327-483-8_5 [DOI] [PubMed] [Google Scholar]
- 41. Lesciotto KM, Motch Perrine SM, Kawasaki M, et al. Phosphotungstic acid‐enhanced microCT: optimized protocols for embryonic and early postnatal mice. Dev Dyn. 2020;249(4):573‐585. doi: 10.1002/dvdy.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Milligan M. Trichrome stain for formalin‐fixed tissue. Am J Clin Pathol. 1946;10(6):184. doi: 10.1093/ajcp/16.11_ts.184 [DOI] [PubMed] [Google Scholar]
- 43. Fogel JL, Thein TZ, Mariani FV. Use of LysoTracker to detect programmed cell death in embryos and differentiating embryonic stem cells. J Vis Exp. 2012;11(68):4254. doi: 10.3791/4254 [DOI] [PMC free article] [PubMed] [Google Scholar]


