Abstract
Background:
Non-invasive monitoring of heart allograft health is important to improve clinical outcomes. MicroRNAs (miRs) are promising biomarkers of cardiovascular disease and limited studies suggest they can be used to non-invasively diagnose acute heart transplant rejection.
Methods:
The Genomic Research Alliance for Transplantation (GRAfT) is a multicenter prospective cohort study that phenotyped heart transplant patients from 5 mid-Atlantic centers. Patients who had no history of rejection after transplant were compared to patients with acute cellular rejection (ACR) or antibody-mediated rejection (AMR). Small RNA sequencing was performed on plasma samples collected at the time of an endomyocardial biopsy. Differential miR expression was performed with adjustment for clinical covariates. Regression was used to develop miR panels with high diagnostic accuracy for ACR and AMR. These panels were then validated in independent samples from GRAfT and Stanford University. Receiver operating characteristic curves were generated and area under the curve (AUC) statistics calculated. Distinct ACR and AMR clinical scores were developed to translate miR expression data for clinical use.
Results:
The GRAfT cohort had a median age of 52 years, with 35% females and 45% Black patients. Between GRAfT and Stanford, we included 157 heart transplant patients: 108 controls and 49 with rejection (50 ACR and 38 AMR episodes). After differential miR expression and regression analysis, we identified 12 miRs that accurately discriminate ACR and 17 miRs in AMR. Independent validation of the miR panels within GRAfT led to an ACR AUC 0.92 (95%CI: 0.86–0.98) and AMR AUC 0.82 (95%CI: 0.74–0.90). The externally validated ACR AUC was 0.72 (95% CI: 0.59–0.82). We developed distinct miR clinical scores for ACR and AMR (range 0–100), a score ≥ 65, identified ACR with 86% sensitivity, 76% specificity, and 98% negative predictive value, for AMR score performance was 82%, 84% and 97%, respectively.
Conclusions:
We identified novel miRs that had excellent performance to non-invasively diagnose acute rejection after heart transplantation. Once rigorously validated, the unique clinical ACR and AMR scores usher in an era whereby genomic biomarkers can be used to screen and diagnose the subtype of rejection. These novel biomarkers may potentially alleviate the need for an endomyocardial biopsy while facilitating the initiation of targeted therapy based on the non-invasive diagnosis of ACR or AMR.
Introduction
Heart transplantation remains the definitive therapy for patients with advanced heart failure and medically refractory symptoms. The median survival after heart transplant is 12.5 years, with only modest improvement in survival over the past two decades.1 Allograft rejection, both acute and chronic, is a leading cause of morbidity after heart transplantation and leads to graft dysfunction and death. Rejection has an incidence of 10–20% after transplant, but is often initially asymptomatic, thereby prompting routine surveillance with an endomyocardial biopsy (EMB). The EMB remains standard of care at many centers to screen for allograft rejection, in the first-year post-transplant the average heart transplant recipient is subject to ~10–17 biopsies. Recent work by our group, the Genomic Research Alliance for Transplantation (GRAfT) showed that only 5% of all EMB show evidence of allograft rejection.2 Distinguishing the two major subtypes of rejection (acute cellular rejection ACR and antibody-mediated rejection AMR), can be challenging in certain cases, and accurate and timely diagnosis of AMR is critical as it has distinct management implications, a higher rate of recurrence, and poorer long-term prognosis when compared to ACR.3, 4
MicroRNAs (miRs) are highly-conserved, non-coding, small (~22 nucleotide) sequences that negatively regulate gene expression by binding to the 3’ untranslated region of a complementary gene transcript.5, 6 Early data suggests that miRs are promising genomic biomarkers in cardiovascular medicine, have the potential ability to detect heart transplant rejection,7, 8 and may provide mechanistic insights into molecular pathways modulated during rejection. Prior studies have suggested that specific miRs are up- or down-regulated in the myocardium during rejection,8, 9 and that miRs may also be detected in the systemic circulation during rejection.8, 10, 11 Limitations of these prior studies include small sample size, absence of AMR patients, single-center analyses, annotation of a limited group of miRs by polymerase chain reaction (PCR), and/or absence of external validation.
Using a multicenter prospective cohort study, GRAfT, we performed small RNA sequencing of the circulating plasma in heart transplant patients and performed an integrated analysis with clinical data. To validate our miR panels, we included independent validation cohorts from GRAfT and Stanford University. The aims of this analysis were to: 1) determine the miR transcriptome of heart transplant recipients; 2) distinguish miR expression in the setting of ACR and AMR; 3) develop distinct miR panels that could be used to non-invasively screen for and diagnose ACR and AMR; and 4) create individual ACR and AMR miR scores that facilitate clinical decision-making and the non-invasive diagnosis of acute rejection. Our work begins an era whereby blood-based genomic biomarker testing is not only used to screen for rejection but allows for the non-invasive diagnosis of ACR or AMR and initiation of targeted therapy.
Methods
Multicenter Heart Transplant Cohort & Study Design
The GRAfT study (NCT #02423070) is a prospective, multi-center clinical study that enrolled heart transplant recipients while on the waitlist before transplant and follows them serially after transplant. The study is supported by a collaborative agreement between the National Heart, Lung and Blood Institute (NHLBI) and five regional transplant centers: Inova Heart and Vascular Institute (IHVI), Johns Hopkins University, University of Maryland, Virginia Commonwealth University and Medstar Washington Hospital Center. Patients 18 years of age or older were enrolled, those with a history of prior heart transplantation, and current pregnancy were excluded. GRAfT patients were enrolled from 2015 to 2020.
Routine post-transplant clinical care included surveillance and clinically indicated monitoring. Surveillance monitoring at pre-specified post-transplant time points included EMB for histopathology, right heart catheterization (RHC) hemodynamics, laboratory data to assess end-organ function, donor specific antibodies (DSA), cytomegalovirus (CMV) testing, as well as monitoring of immunosuppressive drug levels. The routine surveillance schedule for each of the 5 GRAfT centers is provided (Supplemental Table 1a–e). The study longitudinally tracked clinical data and collected blood samples coincident to both surveillance and clinically indicated monitoring. The 5 center(s) induction and immunosuppression protocols are provided (Supplemental Table 2).
We identified all GRAfT patients with ACR, AMR or mixed rejection during the study period. In patients with a history of both ACR and AMR on distinct EMB, the ACR and AMR samples were analyzed separately. Patients with mixed rejection were considered with the AMR cohort, per prior publications suggesting the mixed rejection miR signature is most similar to AMR.9 Controls were selected based on freedom from clinical- or histopathologic rejection during the entirety of their clinical follow-up. In the derivation analysis, no pre- and post-rejection samples were included. The center protocols for treatment of ACR and AMR are provided (Supplemental Table 3). The institutional review boards of all centers and the NHLBI approved the study. This study adheres to the principles of the Declaration of Helsinki and the International Society for Heart and Lung Transplantation statement on Transplant Ethics.
Histopathologic Definition of Rejection
The GRAfT Heart Steering Committee pre-specified the definitions of rejection that would trigger treatment at the GRAfT centers. The committee includes heart transplant cardiologists (n=6), immunogeneticists (n=2), pathologists (n=2), a statistician (n=1), and genomics experts (n=2). Rejection was defined by international histopathologic standards for grading ACR and AMR.12, 13 This includes histopathologic rejection: ACR grade ≥2R, AMR grade ≥1 (histologic or immunologic findings) or mixed rejection (with both ACR grade ≥2R and AMR grade ≥1). Rejection was defined based on biopsy interpretation by the individual center’s pathologists applying standardized criteria.
Independent Cardiac Pathologists
Given the previously reported high discordance between pathologists in grading rejection,14 we had two blinded cardiac pathologists (GJB and CM) review a subset of all histopathologic slides within GRAfT. Their interpretation was compared to the transplant center’s interpretation, and the analysis was repeated to assess miR performance against the blinded cardiac pathologist EMB interpretation.
Independent Validation Cohorts
Using distinct patient samples from GRAfT we performed an independent validation of our ACR and AMR miR panels in samples not included in the derivation analysis. Pre- and post-rejection samples are included as controls in the validation analysis. In addition, to permit external validation, we used plasma samples collected through the Stanford University Heart Transplant Biobank (NCT# 01985412) between 2011 and 2018. Stanford University samples were collected using different methodology then the GRAfT samples. Patients were recruited shortly after heart transplantation under similar exclusion criteria, and the study was approved locally. Through a collaboration (KK), we were able to identify patients with ACR and others with no history of rejection after transplant. The prevalence of AMR in the Stanford cohort was low; thus, validation was performed in ACR alone.
Details of the Sample Processing, Sequencing and Bioinformatic Analysis are provided in the Supplemental Methods
Biostatistical Analysis
Baseline patient characteristics were compared with the Wilcoxon Rank Sum test. Small RNA sequencing led to identification of ~1,900 expressed miRs in plasma. The average expression across all samples was 475 ± 124 miRs. Lowly expressed miRs, with less than 100 average mapped reads across all samples, were filtered out. In addition, miR 486–5p and miR-451a which are red blood cell derived miRs were removed from the analysis.15 The remaining 286 miRs were included in the analysis. We conducted a two-tier analysis to identify miRs with the strongest ability to discriminate rejection (ACR grade ≥2R or AMR grade ≥1) from no rejection (ACR grade 0 or 1, AMR grade 0). For the first-tier analysis, we screened the 286 miRs to identify differentially expressed miRs in ACR and AMR while adjusting for clinical covariates (age, sex, race, body-mass index) using DESeq2.16 We adjusted for blood group in a subset of patients with minimal change in miR profile.
For the second-tier analysis, we fitted a logistic regression models with a Least Absolute Shrinkage and Selection Operator (LASSO) penalty using the top differentially expressed miRs from the differential miR expression analysis.17 In the LASSO analysis, the reads per million counts were log-transformed to approximate normality and the log counts for each miR were standardized to have a mean of zero and variance of one. The tuning parameter of the LASSO penalty was chosen by 10-fold cross-validation to minimize model deviance.
Receiver operating characteristic (ROC) curves were generated and the area under the curves (AUC) were calculated to assess the performance of the LASSO-selected miRs in the independent validation cohort from GRAfT for ACR and AMR, and Stanford University for ACR. To assess the predictive ability of the miRs, AUC statistics were generated. Test performance characteristics are presented including sensitivity, specificity, negative predictive value (NPV) and positive predictive value (PPV).
Finally, we fitted a logistic regression model for ACR and AMR using the counts per million data for the LASSO-selected miRs. The ordinary logistic regression estimates are prone to bias due to our low sample size and the large observed difference in expression of some miRs (which causes the likelihood function to be flat near the maximum), particularly for AMR. We therefore used a bias reduced maximum likelihood estimator for the final logistic regression model parameters.18 These distinct ACR and AMR rejection scores are scaled from 0–100 based on the miR expression data for each patient sample. ROC curves of the individual patient scores were generated within the entire GRAfT cohort. Youden’s index was used to identify a threshold to maximize test performance of the score.19 The score was developed to facilitate clinical interpretation of blood miR expression data to support medical decision making about the likelihood of ACR or AMR.
Results
Patient Characteristics
During the study period, a total 116 heart transplant patients from GRAfT completed one-year follow-up after transplant and had plasma samples available for inclusion in the study. The median patient age was 52 years (IQR: 42–59), 35% of patients were female, and 45% were Black. The most common cause of heart failure was a non-ischemic cardiomyopathy (63%) and 67% of patients were bridged to transplant with a durable left ventricular assist device (LVAD, Table 1). The median follow-up time after transplantation was 3.73 patient-years (IQR: 3.23 – 4.15).
Table 1:
GRAfT Patient Characteristics at Time of Transplant with and without Acute Rejection
| Characteristic | GRAfT (N = 116) | Control (N = 85) | ACR (N = 20) | AMR (N = 14) | p-value for ACR vs AMR |
|---|---|---|---|---|---|
| Age, median (IQR) | 51.5 (42.0, 59.0) | 53.0 (44.0, 60.0) | 49.5 (39.0, 55.3) | 49.5 (35.3, 52.8) | 0.12 |
| Sex | 0.84 | ||||
| Female | 41 (35.3%) | 31 (36.5%) | 7 (35.0%) | 4 (28.6%) | |
| Male | 75 (64.7%) | 54 (63.5%) | 213 (65.0%) | 10 (71.4%) | |
| Race | 0.05 | ||||
| Black | 52 (44.8%) | 40 (47.1%) | 9 (45.0%) | 12 (85.7%) | |
| White | 58 (50%) | 39 (45.9%) | 11 (55.0%) | 2 (14.3%) | |
| Other | 6 (5.2%) | 6 (7.1%) | 0 (0.0%) | 0 (0%) | |
| Ethnicity | 0.67 | ||||
| Hispanic or Latino | 8 (6.9%) | 5 (5.9%) | 2 (10%) | 1 (7.1%) | |
| Not Hispanic or Latino | 108 (93.1%) | 80 (94.1%) | 18 (90%) | 12 (92.9%) | |
| Body-mass index, kg/m2 | 27.9 (24.8, 32.1) | 27.6 (24.0, 32.1) | 27.4 (25.2, 30.8) | 30.6 (28.4, 33.1) | 0.16 |
| Cardiomyopathy Type | 0.36 | ||||
| Ischemic | 23 (20.2%) | 15 (17.7%) | 4 (22.2%) | 4 (28.6%) | |
| Non-ischemic | 72 (63.2%) | 56 (65.9%) | 11 (61.1%) | 8 (43.2%) | |
| Other | 19 (16.7%) | 14 (16.4%) | 2 (16.8%) | 2 (14.3%) | |
| UNOS Status at Transplant | |||||
| 1A | 75 (72.1%) | 51 (68.9%) | 17 (85%) | 10 (76.9%) | 0.42 |
| 1B | 26 (25%) | 21 (28.4%) | 3 (15%) | 2 (15.4%) | |
| 2 | 3 (2.9%) | 2 (2.7%) | 0 (3%) | 1 (7.7%) | |
| Bridged to Transplant with LVAD | 70 (67.3%) | 52 (67.5%) | 9 (50%) | 11 (91.7%) | 0.06 |
| Medical Co-morbidities | |||||
| HTN | 79 (70.5%) | 62 (75.6%) | 10 (52.6%) | 9 (64.3%) | 0.12 |
| HLP | 42 (37.8%) | 30 (36.6%) | 6 (33.3%) | 8 (57.1%) | 0.30 |
| DM | 34 (30.6%) | 21 (25.9%) | 7 (36.8%) | 7 (50%) | 0.16 |
| Prior Smoking | 45 (38.8%) | 35 (41.2%) | 4 (20%) | 8 (57.1%) | 0.07 |
| Creatinine, mg/dl | 1.20 (1.00, 1.46) | 1.20 (1.00, 1.47) | 1.00 (0.86, 1.40) | 1.40 (1.20, 2.14) | 0.03 |
IQR: interquartile range, UNOS: United Network for Organ Sharing, LVAD: left ventricular assist device, HTN: hypertension; HLP: hyperlipidemia, DM: diabetes mellitus.3 patients had isolated episodes of ACR and AMR on distinct biopsies and are represented in each column. 2 patients had mixed ACR and AMR and are represented in the AMR column.
Within GRAfT, we previously reported an incidence of AR of 15.8%.2 Only an EMB with concomitant plasma miR sequencing that passed our quality thresholds, were included in this analysis. There were 85 patients with no history of rejection (controls), 20 patients with ACR, and 14 patients with AMR (2 had mixed rejection). Patients were divided into derivation and validation cohorts (Figure 1). In the derivation miR analysis we included 20 episodes of ACR (all grade 2R, no 3R episodes), 14 episodes of AMR (AMR 1, n = 8; AMR ≥ 2, n = 6). There were 3 patients with separate ACR and AMR events on different EMBs and each episode of rejection was analyzed separately. The median time after transplant to ACR was 146 days (IQR: 50 – 496) and AMR was 55 days (IQR: 21 – 171). There were 5 patients with recurrent ACR by histology and the median time to recurrent rejection was 77 days (range: 14 – 531). There were 6 patients with recurrent AMR by histology and the median time to recurrence was 8 days (range: 6 – 14). All ACR episodes were treated most typically using intravenous or oral pulse-dose steroids, with certain patients receiving thymoglobulin based on institutional protocol (Supplemental Table 4a). In AMR there was greater heterogeneity in treatment and most patients received treatment for their initial episode, but many recurrent AMR episodes were not treated (Supplemental Table 4b).
Figure 1. Study Flow Diagram Including Derivation and Validation Cohorts for ACR and AMR.
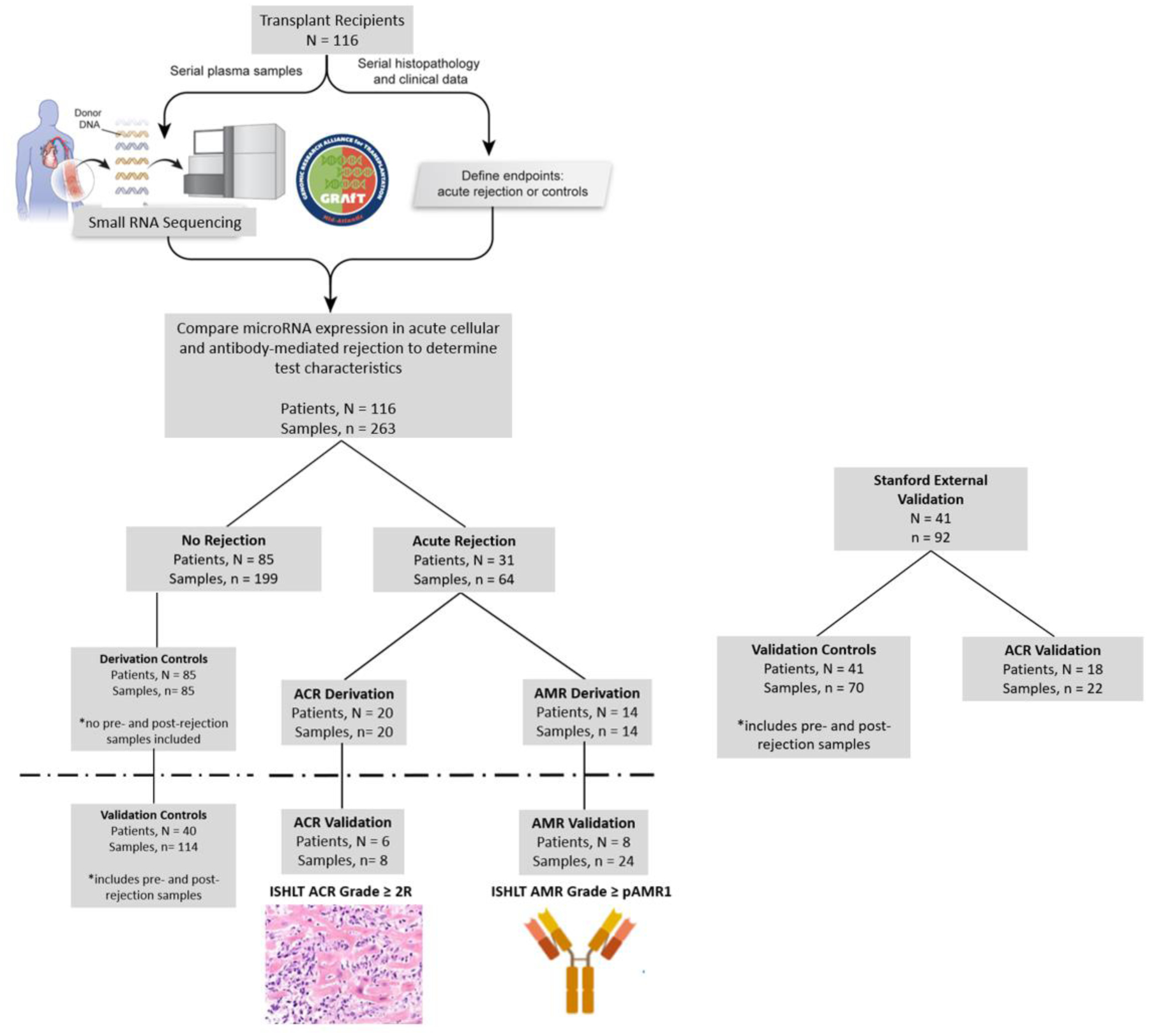
A total of 116 patients and 263 plasma samples from GRAfT were included in the analysis and 41 patients with 92 plasma samples from Stanford for validation. In the derivation analysis only samples from control patients with no history of rejection after transplant were included. In the validation analysis, control samples included patients without rejection as well as pre- and post-rejection samples for patients with a history of ACR or AMR. The first episode of ACR or AMR was included in the derivation cohort, repeat episodes of rejection were included in the validation analysis. Two episodes of mixed rejection were analyzed with the AMR validation cohort. Three patients had ACR or AMR on a distinct biopsy and the individual miR profiles are included in the ACR and AMR analysis.
Median patient age for patients with rejection was 49.5 years, younger than controls without rejection 53.0 years (Table 1). Black patients had a higher incidence of AMR (85.7%) compared to White patients (14.3%, p=0.05) and bridging to transplant with an LVAD was associated with a trend towards higher incidence of AMR compared to patients who went direct to transplant (91.7% v. 8.3%, p=0.06).
MicroRNA Sequencing
A total of 362 plasma samples underwent small RNA sequencing between GRAfT and Stanford University, 7 samples failed QC metrics and were excluded. On average, a total of 10.9 ± 3.1 million small RNA reads were generated per sample. This includes miR, piwi RNA, small nucleolar RNA, transfer RNA and unmapped RNA species. After filtering non-miR reads, each sample generated approximately 5.6 ± 2.7 million miR reads (Supplemental Table 5). There were on average 475 ± 124 individual miRs expressed per sample, with 286 ± 45 miRs detected at a depth of ≥ 100 reads per sample (Supplemental Figure 1). The top 20 miRs accounted for 75% of the total miR transcriptome in heart transplant patients, with miR-451a and miR-486–5p being the most abundant which were removed from the analysis due to their predominant origin from red blood cells (Supplemental Figure 2).
Differentially Expressed miRs in ACR and AMR
Using the GRAfT derivation cohort, differential gene expression analysis was performed comparing the miR profile of control patients without rejection to the first episode of ACR in patients with rejection. When comparing these patient populations, we identified 12 differentially expressed miRs with a p-value < 0.05 (Figure 2, Table 2). Target prediction analysis was performed with our ACR miRs using miRTarBase.20 Only previously published, experimentally validated miR-mRNA target interactions were included to ensure a high level of biologic relevance. A total of 294 genes were targeted by the ACR miRs (Supplemental Table 6) and their affected biological pathways are provided (Online Supplement ACR Pathway Analysis).
Figure 2: Volcano Plots of Differentially Expressed miRs in ACR and AMR.
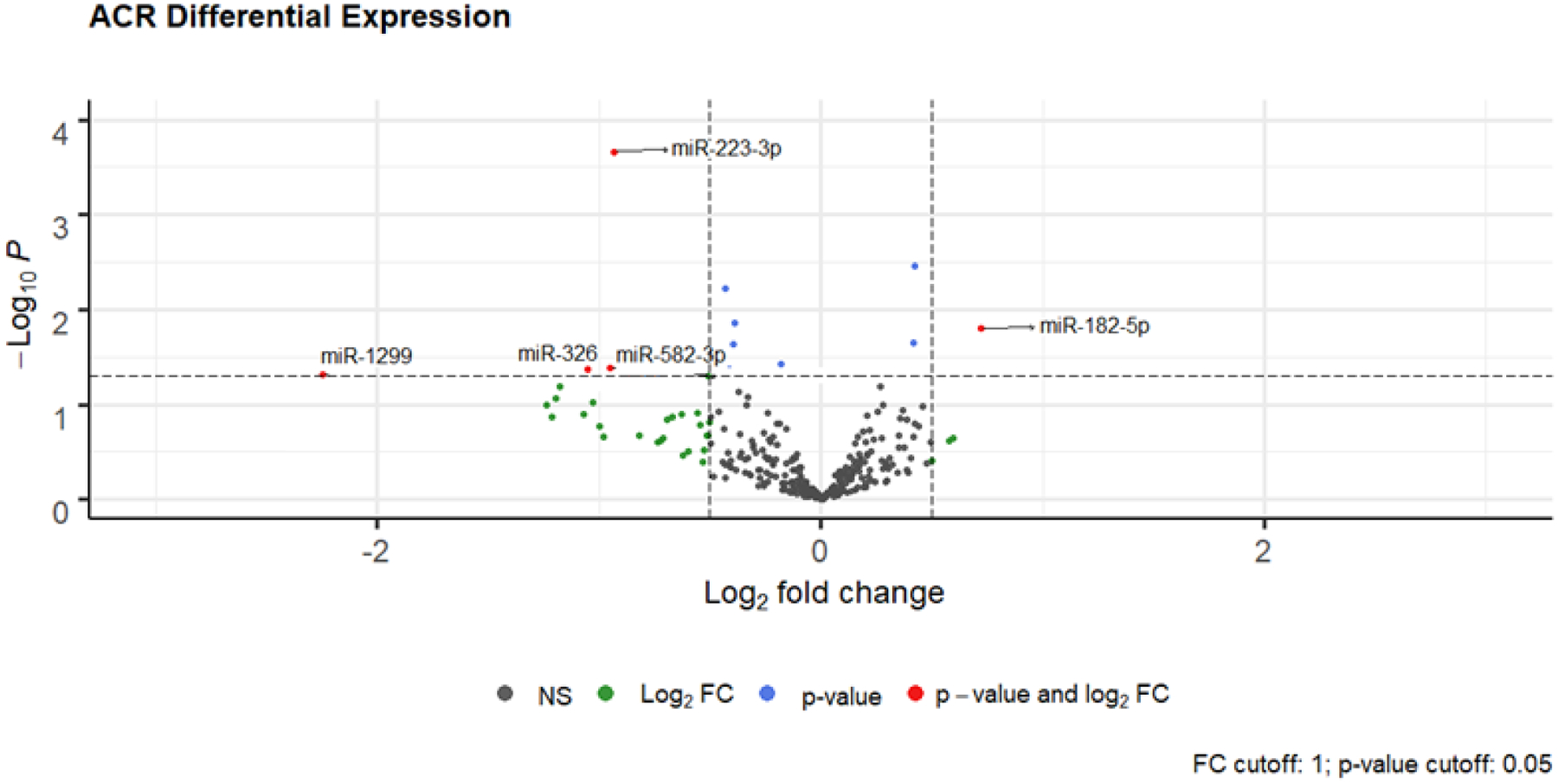
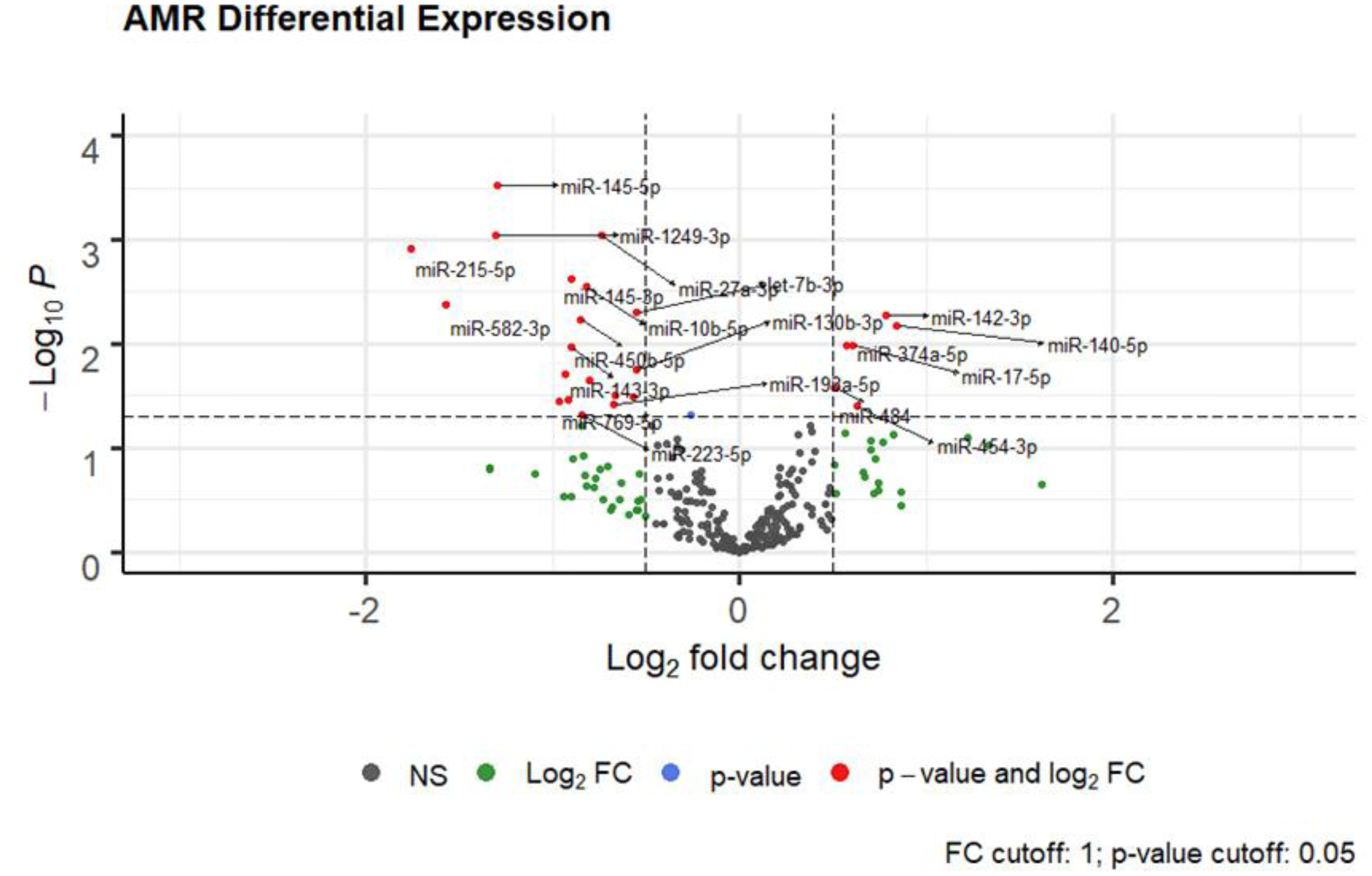
Differential microRNAs in ACR and AMR: x-axis line represents log fold +/− 0.5. y-axis line represents unadjusted p = 0.05. miRs with a log-fold change ≥ ± 0.5, but p-value < 0.05 are depicted by green circles; miRs with a p-value > 0.05, but log-fold change < ± 0.5 are depicted by blue circles; differentially expressed miRs with a log-fold change ≥ ± 0.5 and p-value < 0.05 are depicted by red circles for ACR and AMR respectively.
Table 2:
Differentially Expressed MicroRNAs in ACR and AMR
| ACR MicroRNAs | Mean Expression | log2 Fold Change | Fold Change | Standard Error | Statistic | P value |
|---|---|---|---|---|---|---|
| miR-223-3p | 45940 | −0.93 | 0.52 | 0.25 | −3.70 | 0.000 |
| miR-361-3p | 1049 | 0.42 | 1.34 | 0.14 | 2.93 | 0.003 |
| miR-3615 | 1364 | −0.43 | 0.74 | 0.16 | −2.75 | 0.006 |
| miR-24-3p | 37695 | −0.39 | 0.76 | 0.16 | −2.46 | 0.014 |
| miR-182-5p | 790 | 0.72 | 1.65 | 0.30 | 2.41 | 0.016 |
| miR-374a-5p | 883 | 0.42 | 1.34 | 0.18 | 2.28 | 0.023 |
| miR-23a-3p | 57985 | −0.39 | 0.76 | 0.17 | −2.27 | 0.023 |
| miR-30e-5p | 78017 | −0.18 | 0.88 | 0.09 | −2.09 | 0.037 |
| miR-582-3p | 340 | −0.95 | 0.52 | 0.46 | −2.04 | 0.041 |
| miR-130b-3p | 442 | −0.41 | 0.75 | 0.20 | −2.03 | 0.042 |
| miR-326 | 92 | −1.05 | 0.48 | 0.52 | −2.03 | 0.043 |
| miR-1299 | 100 | −2.25 | 0.21 | 1.14 | −1.98 | 0.048 |
| AMR MicroRNAs | Mean Expression | log2 Fold Change | Fold Change | Standard Error | Statistic | P value |
| miR-23a-3p | 58794 | −0.80 | 0.57 | 0.20 | −3.94 | 0.000 |
| miR-145-5p | 605 | −1.30 | 0.41 | 0.36 | −3.62 | 0.000 |
| miR-1249-3p | 411 | −1.31 | 0.40 | 0.39 | −3.32 | 0.001 |
| miR-27a-3p | 28287 | −0.74 | 0.60 | 0.22 | −3.32 | 0.001 |
| miR-215-5p | 815 | −1.76 | 0.30 | 0.54 | −3.23 | 0.001 |
| miR-145-3p | 1324 | −0.90 | 0.54 | 0.30 | −3.04 | 0.002 |
| miR-10b-5p | 24845 | −0.82 | 0.57 | 0.27 | −2.98 | 0.003 |
| miR-582-3p | 338 | −1.57 | 0.34 | 0.55 | −2.86 | 0.004 |
| let-7b-3p | 1349 | −0.55 | 0.68 | 0.20 | −2.80 | 0.005 |
| miR-142-3p | 1193 | 0.78 | 1.72 | 0.28 | 2.78 | 0.005 |
| miR-450b-5p | 1643 | −0.85 | 0.55 | 0.31 | −2.76 | 0.006 |
| miR-140-5p | 506 | 0.84 | 1.79 | 0.31 | 2.71 | 0.007 |
| miR-374a-5p | 906 | 0.57 | 1.48 | 0.22 | 2.57 | 0.010 |
| miR-17-5p | 1609 | 0.61 | 1.53 | 0.24 | 2.56 | 0.010 |
| miR-143-3p | 51349 | −0.90 | 0.54 | 0.35 | −2.55 | 0.011 |
| miR-130b-3p | 463 | −0.55 | 0.68 | 0.23 | −2.37 | 0.018 |
| miR-1-3p | 3288 | −0.93 | 0.52 | 0.40 | −2.34 | 0.019 |
| miR-542-3p | 738 | −0.80 | 0.57 | 0.35 | −2.28 | 0.022 |
| miR-484 | 9962 | 0.51 | 1.42 | 0.23 | 2.22 | 0.027 |
| miR-345-5p | 1217 | −0.67 | 0.63 | 0.31 | −2.16 | 0.031 |
| miR-125a-5p | 9648 | −0.57 | 0.67 | 0.27 | −2.14 | 0.032 |
| miR-338-5p | 879 | −0.92 | 0.53 | 0.43 | −2.11 | 0.035 |
| miR-769-5p | 351 | −0.97 | 0.51 | 0.46 | −2.10 | 0.036 |
| miR-193a-5p | 3018 | −0.67 | 0.63 | 0.32 | −2.08 | 0.038 |
| miR-454-3p | 858 | 0.63 | 1.55 | 0.31 | 2.06 | 0.040 |
| miR-223-5p | 2258 | −0.84 | 0.56 | 0.43 | −1.97 | 0.049 |
| let-7d-3p | 24761 | −0.26 | 0.84 | 0.13 | −1.97 | 0.049 |
We screened plasma miRs to identify differentially expressed miRs in ACR and AMR while adjusting for clinical covariates (age, sex, race, body-mass index). We adjusted for blood group in a subset of patients with minimal change in the miR profile. The table represents all differentially expressed miRs in ACR and AMR with an unadjusted p-value < 0.05.
Similarly, we compared the miR profile from the first episode of AMR to control patients without rejection and identified 27 differentially expressed miRs with a p-value < 0.05 (Figure 2, Table 2). A total of 478 genes were targeted by the AMR miRs (Supplemental Table 7) and their affected biological pathways are provided (Online Supplement AMR Pathway Analysis).
Using LASSO regression with 10-fold internal cross-validation, we identified a panel of 12 miRs that accurately discriminated ACR from controls. Similarly, we identified 17 miRs that accurately discriminated AMR from controls. The internal correlation between these ACR and AMR miRs was low (data not presented). Only 2 miRs were common to both the ACR and AMR panels (miR-130b-3p and miR-374a-5p). The unsupervised hierarchal clustering of patients based on the ACR and AMR miRs is presented (Supplemental Figures 3 and 4).
miR Diagnostic Performance in Validation Cohorts
To discriminate performance of our selected miR panels to non-invasively diagnose ACR and AMR, we performed validation in an independent cohort of GRAfT used the remaining GRAfT samples which were not used in the derivation analysis (Figure 1). This includes 114 control samples which includes pre- and post-rejection samples as well as 8 ACR and 24 AMR episodes. The performance characteristics were excellent with an ACR AUC of 0.92 (95% CI: 0.85–0.97) and an AMR AUC of 0.82 (95% CI: 0.74–0.90, Figure 3). This leads to a sensitivity ranging between 92%–100%, and specificity ranging between 63–79%.
Figure 3: Receiver Operating Characteristic Curve of LASSO-selected ACR and AMR miRs in Validation Cohorts.
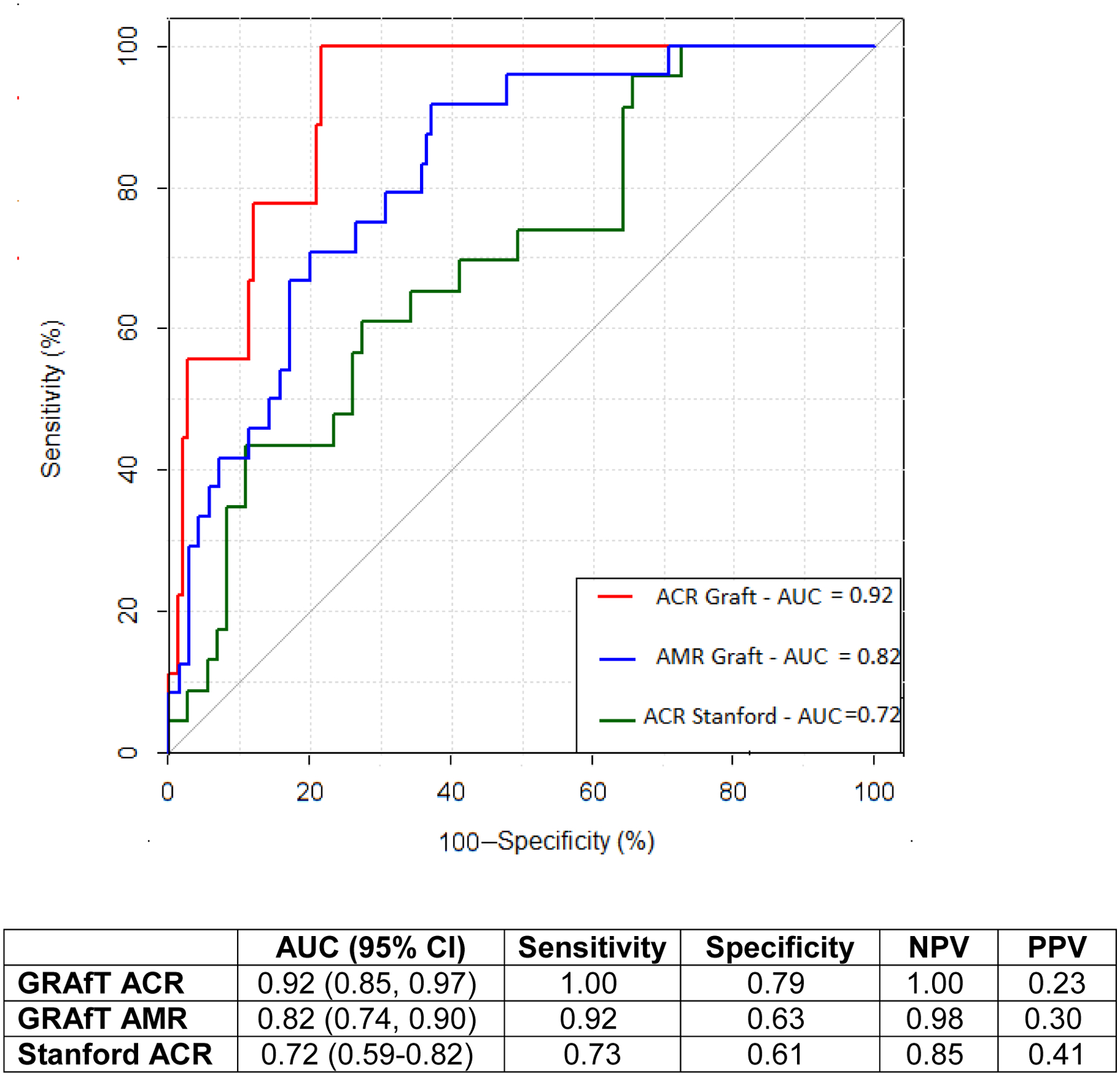
Receiver-Operating-Characteristic Curve for ACR and AMR miR panels in distinguishing the presence of acute rejection. The diagnostic performance was assessed in 2 independent validation cohorts: 1) GRAfT validation for ACR and AMR and 2) Stanford for ACR. When pre- and post-rejections samples are removed from the Stanford analysis, the AUC improves to 0.91 (95% CI: 0.82–0.99).
To perform additional external validation, we sequenced the miR transcriptome in a transplant patient cohort from Stanford University (n = 41; 22 ACR episodes, 70 control samples, Figure 1). Stanford University patients tended to be older than GRAfT (57.2 years v. 51.5 years, p=0.10), had a lower proportion of female patients (16.2% v. 35.3%, p=0.05), Black patients (13.5% v. 44.8%, p<0.001) and more renal dysfunction prior to transplant (1.50mg/dl v. 1.20mg/dl, p=0.02, Supplemental Table 8). Other patient characteristics were similar between the cohorts. When all sequenced Stanford samples were included, the AUC of the ACR miR panel in the Stanford cohort was 0.72 (95% CI: 0.59–0.82, Figure 3), corresponding to an NPV of 85% and PPV of 41%. When pre- and post-rejection samples were excluded from the Stanford analysis the AUC improved to 0.91 (95% CI: 0.82–0.99), the corresponding sensitivity was 74%, specificity 99%, NPV 81% and PPV of 99%.
Independent Cardiac Pathologist Analysis
Two blinded cardiac pathologists (GJB and CM) reviewed a subset of EMB histopathologic slides from GRAfT patients enrolled prior to 2018 to verify the presence of ACR and AMR. Of the 263 biopsies included in the analysis, 116 (44%) were re-reviewed for ACR and 93 (35%) for AMR. We compared overall concordance between the core cardiac pathologists and the institutional pathologist histopathologic interpretation of the EMB. For all reviewed biopsies, the overall concordance for ACR was 92%, for biopsies without ACR by the center read concordance was 95%, and for ACR grade ≥2R concordance was 73%. Overall concordance for AMR was 82%, for biopsies without AMR by the center read concordance was 95%, and for AMR grade ≥1 concordance was 28% (Table 3).
Table 3:
Comparison of Center and Blinded Cardiac Pathologists Histopathologic Interpretation of Heart Biopsy Slides for ACR and AMR
| ACR GRADE | CENTER READ | ||
|---|---|---|---|
| 2R | 1R or 0 | ||
| BLINDED CARDIAC PATHOLOGISTS | 2R | 11 | 5 |
| 1R or 0 | 4 | 96 | |
| AMR GRADE | CENTER READ | ||
| pAMR 1, 2 or 3 | pAMR0 | ||
| BLINDED CARDIAC PATHOLOGISTS | pAMR 1, 2 or 3 | 5 | 4 |
| pAMR0 | 13 | 71 | |
Using our ACR and AMR miR panels, we assessed performance for detection of acute rejection based on the blinded cardiac pathologists EMB interpretation. The selected ACR miR panel had an AUC of 0.94 (95% CI: 0.89–1.00), and the AMR miR panel had an AUC of 0.92 (95% CI: 0.76–1.00).
Circulating MicroRNA Clinical Rejection Scores for ACR and AMR
To facilitate clinical interpretation of miR expression, we developed distinct ACR and AMR clinical rejection scores which were scaled from 0–100 using logistic regression. An individual miR ACR and AMR score was calculated for each biopsy time point in the entire GRAfT patient cohort. ROC curves were generated, and Youden’s Index was used to identify the threshold of the score to maximize the AUC and test performance characteristics (Figure 4). The point that maximized sensitivity and specificity was 65. An ACR score threshold of 65 led to an AUC of 0.86 (95% CI: 0.79 – 0.93), the associated test characteristics were a sensitivity of 86%, specificity of 78%, NPV of 98% and PPV of 32%. For AMR, the AUC was 0.84 (95% CI: 0.78 – 0.91). An AMR score threshold of 65 led to a sensitivity of 82%, specificity of 84%, NPV of 97% and PPV of 37%. The score threshold can be increased or decreased to maximize test sensitivity and specificity as demonstrated in the figure.
Figure 4: Receiver Operating Characteristic Curve of ACR and AMR Scores in GRAfT.
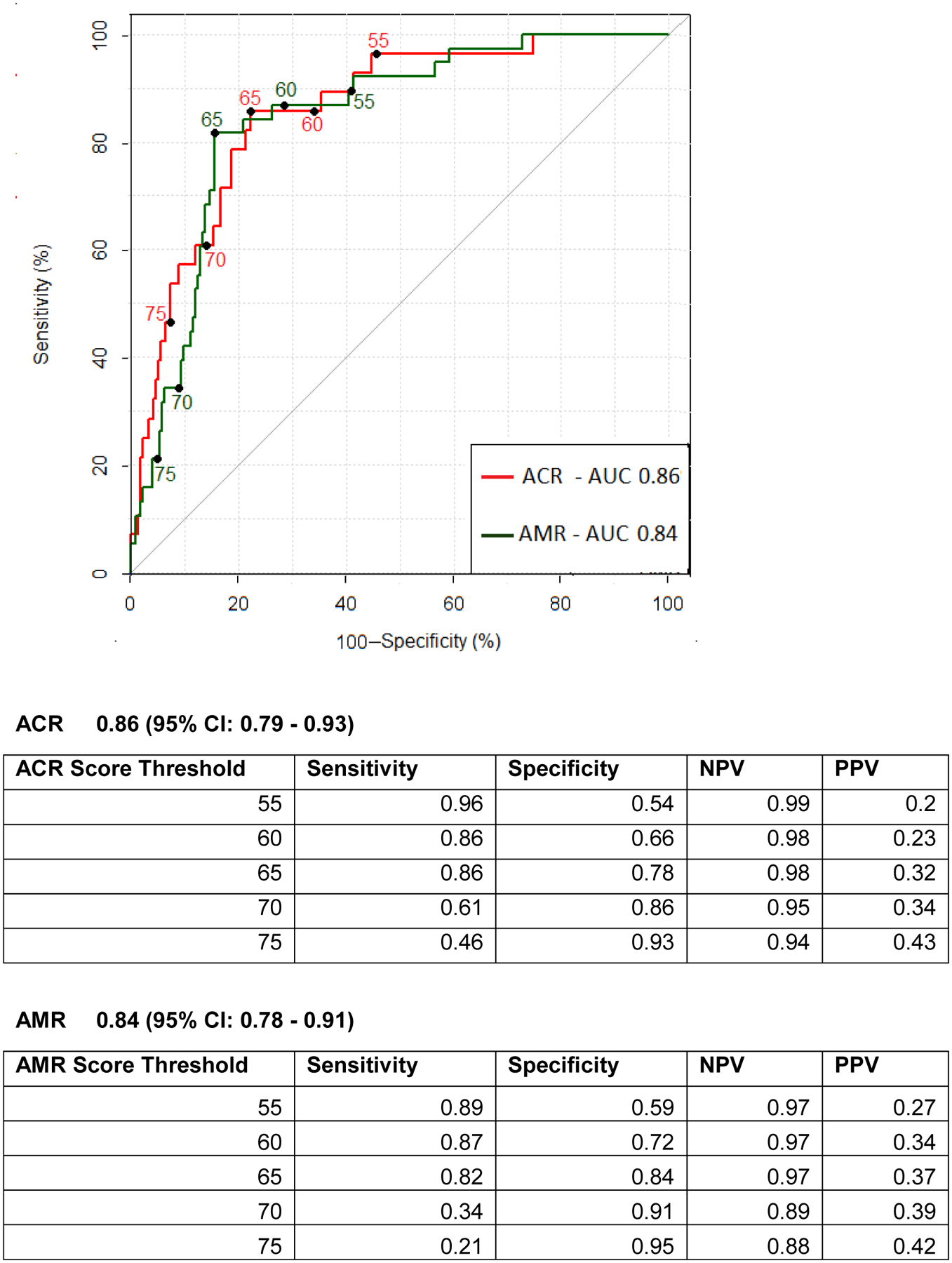
Receiver-Operating-Characteristic Curve for Various Cutoff Levels of the ACR and AMR clinical risk scores in distinguishing the presence of ACR and AMR compared to no rejection.
ACR and AMR Scores Before and After Rejection
To understand whether the ACR and AMR scores could be used to predict patients at risk for future rejection or understand the response to therapy, the calculated ACR and AMR scores were evaluated in a small subset of patients with a pre- or post-rejection sample. We included only the first episode of rejection in this analysis. In ACR, pre-rejection samples were collected a median of 35 days before the rejection episode and the scores are mildly elevated (53) prior to the rejection diagnosis (74) and 14 days after treatment improve (59) but remain elevated compared to controls (50, p < 0.001, Figure 5). In AMR, similarly the scores are elevated a median of 13 days before the rejection episode (75), rise at time of rejection (82) and then 57 days later improve after treatment for AMR (67, Figure 5).
Figure 5: ACR and AMR Scores in GRAfT Before, During and After Acute Rejection.
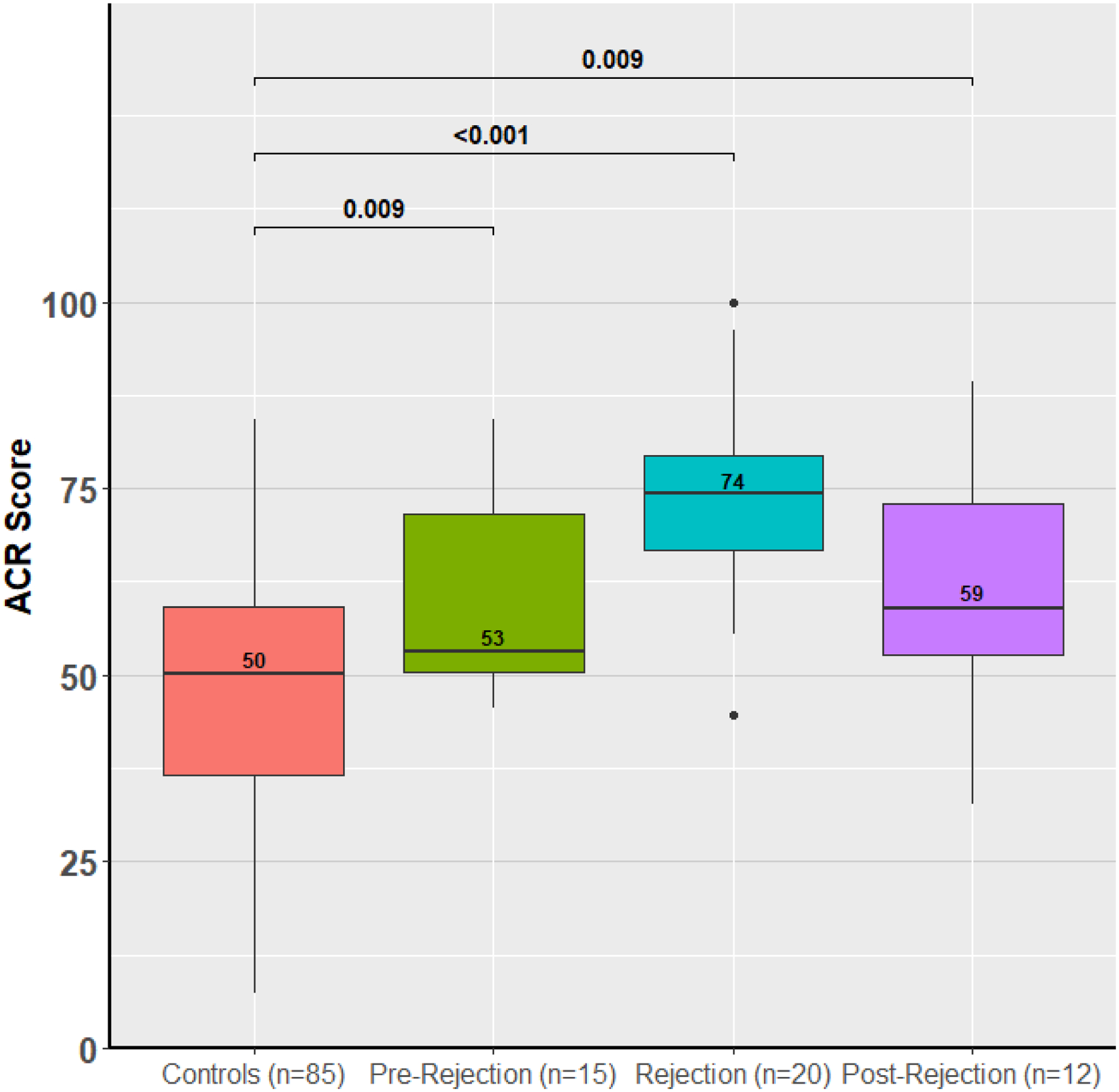
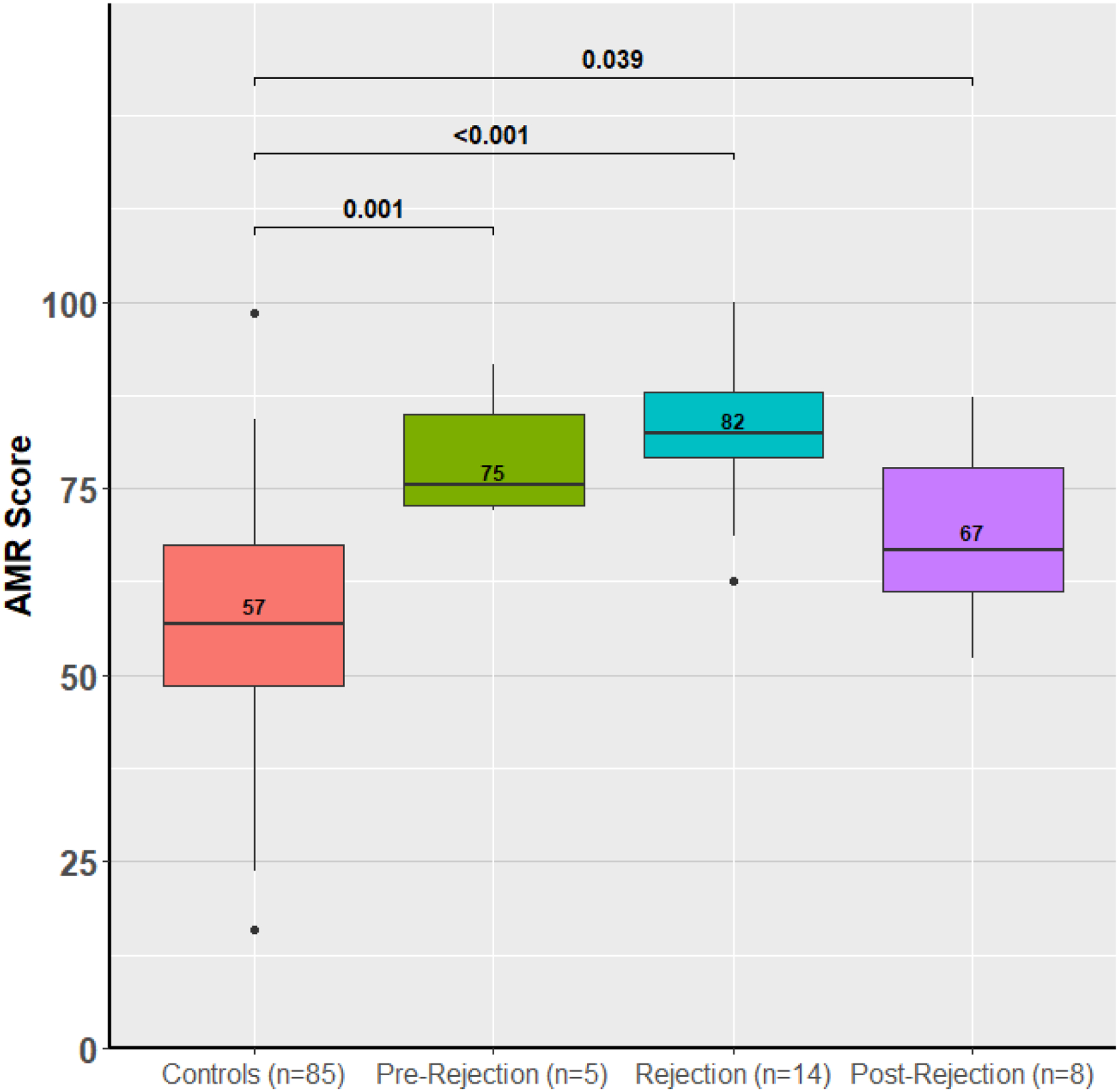
ACR and AMR miR scores are contrasted before, during and after a rejection episode compared to control patients. Pre- and post-rejection samples are within 3 months of the rejection episode.
Discussion
Using GRAfT, a multicenter prospective cohort study of heart transplant recipients, we sequenced the circulating plasma miR transcriptome to identify differentially regulated miRs during acute allograft rejection. The major findings of this analysis include identification of distinct miR panels that can be used to screen for and non-invasively diagnose ACR and AMR from a peripheral blood sample with excellent test performance characteristics. These miR panels were independently validated within GRAfT using distinct patient samples for ACR and AMR and for ACR using an external validation cohort from Stanford University. Due to significant variability in EMB interpretation between pathologists, we reassessed performance of the miR panels using blinded cardiac pathologists and found improved diagnostic performance. Finally, we created clinical ACR and AMR scores that translate the miR expression data into a readily interpretable report for clinicians to employ while managing heart transplant patients (Figure 6). Since there are distinct ACR and AMR scores, it allows for a non-invasive blood test to be used to not only screen for rejection, but also diagnose the subtype of rejection present. This type of testing, once rigorously validated, distinguishes acute rejection from no rejection, ACR from AMR, and facilitates clinical decision making about the subtype of rejection present, enabling the initiation of targeted therapy while awaiting additional diagnostic testing.
Figure 6: Clinical Diagnostic Scores for ACR and AMR.
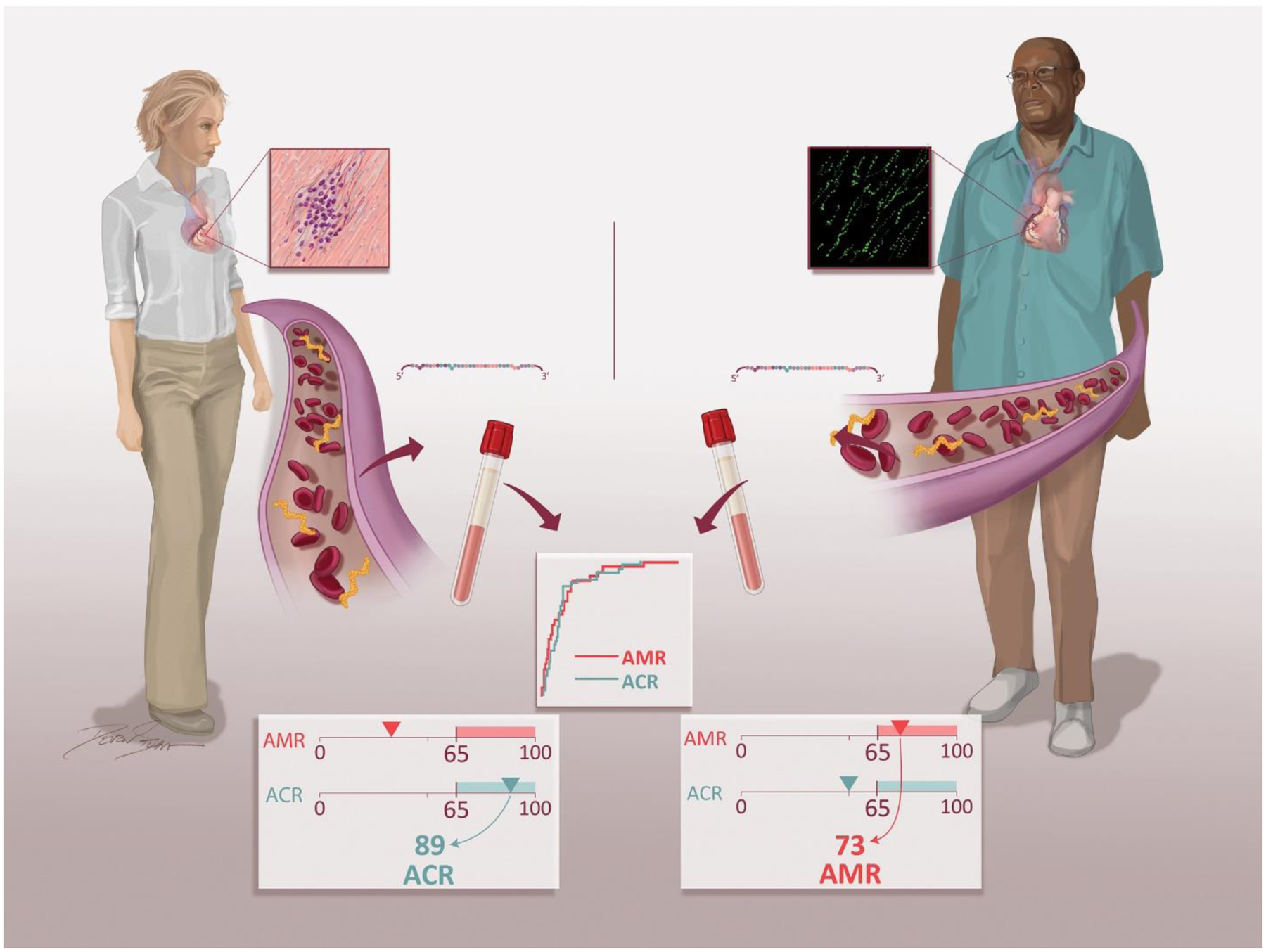
Blood samples are collected in transplant patients during routine surveillance or at the time of clinically suspected rejection. Plasma is isolated, small RNA is extracted, and microRNAs are quantitated. Expression of individual ACR and AMR microRNAs in each sample is determined to calculate the individual patient ACR and AMR score. The distinct ACR and AMR scores allow for initiation of targeted therapy for the subtype of rejection present.
Genomic biomarkers in heart transplantation
The transplant community has spent the greater part of the past 3 decades searching for reliable, non-invasive biomarkers to detect acute allograft rejection. Current biomarkers include gene expression profiling (GEP), soluble protein biomarkers, donor-derived cell-free DNA (dd-cfDNA), and T-cell immune function assays.21–24 Widescale implementation and reliance on GEP testing has been limited by its poor PPV (~10%) and inability to detect AMR.22 More recently, through sequencing of a panel of highly informative single-nucleotide polymorphisms (SNPs) in circulating, cell-free DNA, SNP mismatches between donor and recipient DNA can be used to quantify the donor-derived portion of cell-free DNA (dd-cfDNA).24 Prior work by our group and others has validated dd-cfDNA as a highly sensitive non-invasive biomarker of both ACR and AMR.2, 25, 26 However, a critical limitation of dd-cfDNA in its current application is it cannot distinguish ACR from AMR, and a follow-up EMB is still required to diagnose rejection and to establish a treatment pathway.
In this work we identified unique circulating miR subsets that discriminate the presence of ACR and AMR from patients without rejection with an excellent NPV (~98%). These ACR and AMR miR panels can be used as part of a post-transplant non-invasive surveillance strategy. Since there are unique ACR and AMR miR scores, clinicians can potentially use the test results to start targeted therapy (e.g., pulse corticosteroids for ACR v. plasmapheresis and intravenous immunoglobulin for AMR), while awaiting results from other diagnostic testing: DSA, echocardiogram, EMB, and/or dd-cfDNA. This strategy, once rigorously validated, would permit an entirely non-invasive approach to detect and diagnose acute rejection after heart transplant, i.e., the veritable “liquid biopsy.”
MicroRNAs in heart transplant rejection
Initial work by Wei et al. identified myocardium- and CD3+/CD4+ T-lymphocyte specific miRs that were up- or down- regulated with acute rejection.27, 28 Duong Van Huyen and colleagues reported on 14 pre-selected miRs that were characterized in the serum and EMB tissue specimens of heart transplant patients with ACR and AMR using RT-PCR.8 The group identified four miRs (miR-10a, -31, -92a, and -155) that were differentially expressed in both the myocardium and serum of rejecting patients.8 The University of Padova group using sequencing of EMB samples, identified unique miRs in ACR and AMR that could potentially enhance the pathologic diagnosis of rejection.9 Finally, using a case-control approach in ACR, Constanso-Conde identified miR-181a-5p as a potential biomarker of ACR.10
We did assess the performance of the prior published miRs to diagnose ACR and AMR and found limited performance, with individuals AUCs ranging from 0.50 to 0.70 (Supplemental Table 9). There is significant variability amongst prior miR biomarker studies in methodology used to detect miRs implicated in rejection. This includes (but is not limited to) variation in blood sample collection and storage (serum vs. plasma vs. whole blood), extraction of small RNA, miR detection methodology (PCR vs. microarray vs. sequencing), absence of external validation, and finally patient population, as most have focused on ACR with limited inclusion of AMR patients. We found 12 differentially expressed miRs in ACR and 27 in AMR. Using a rigorous statistical approach while controlling for clinical co-variates, we selected a subset of miRs that accurately diagnosed ACR and AMR. Further these miRs had minimal correlation with each other (data not presented), suggesting that they are individually informative of rejection. Although, prior gene expression assays are sensitive to the time post-transplant, our miR scores were developed using rejection episodes as early as 1-week and as late as 2.5 years after transplant. Finally, our performance characteristics were reported in independent samples of ACR and AMR patients from GRAfT and overall performance was excellent (AUC 0.82 to 0.92). In the Stanford cohort we saw modest performance for the ACR miRs, which improved significantly once pre- and post-rejection samples were excluded. Some of the variation in performance may be due to differences in sample handling and processing between GRAfT and Stanford.
Circulating microRNAs as biomarkers
MicroRNAs are found in the circulation contained within exosomes, microvesicles and apoptotic bodies; making them extremely stable biomarkers, even when subject to rapid freeze-thaw cycles or prolonged room temperature exposure.29 In our analysis we were focused on detection of circulating plasma miRs which could include extravesicular miRs, protein-bound miRs and/or exosomal miRs. There is a great interest in exosomal specific miRs, which are implicated in cell to cell communication, but the process of isolating these exosomes prior to small RNA extraction and sequencing is laborious, requires a larger plasma volume, and it remains unclear whether use of exosomal miRs will improve diagnostic performance for rejection compared to circulating miRs.30 We adjusted our analysis for miR-451a and miR-486–5p which are highly expressed in the plasma, originate from erythroid cells and are potential hemolysis markers.15 Small RNA sequencing is subject to adapter ligation, reverse transcription and amplification biases.31 We used random adapters to reduce ligation bias prior to sequencing. The miR panels will need to be orthogonally validated using quantitative reverse transcription polymerase chain reaction (RT-PCR). We selected a small panel of miRs which permits easier adaptation of miR testing to high-throughput, widely available, lower cost technologies such as RT-PCR, and even a point of care (POC) assay such as an isothermal nucleic acid amplification test (iNAAT).32
Interpretation of the ACR and AMR Scores
To facilitate clinical decision making, we developed distinct ACR and AMR scores using miR expression data. These scores could be used to screen for ACR and AMR, and a positive score could trigger an EMB to confirm the diagnosis or the ACR/AMR scores can be combined with existing genomic biomarkers (e.g., dd-cfDNA) to create a multi-marker approach that could enhances overall sensitivity and specificity without an EMB. Given that the scores were elevated before rejection, especially in AMR, they could be used to predict patients at risk for future rejection. The miR scores, once rigorously validated, could permit the initiation of ACR or AMR-specific treatment pathways alleviating the need for an EMB. Finally, the scores could be used to monitor response to rejection therapy. Figure 6 demonstrates the potential clinical application of these scores. To help clinically contextualize the ACR and AMR scores, a few patient examples from GRAfT are included in the Supplemental materials.
It is critical to note that test performance characteristics were assessed against the EMB which has significant heterogeneity in its histopathologic interpretation and should be considered a reference standard as opposed to gold standard. We did, however, evaluate performance of a subset of EMB samples using blinded cardiac pathologists and found slightly better performance of our miR panels for AMR and ACR. The overall concordance between the center read and blinded cardiac pathologists was high for non-rejection samples 92–95%, but low at 28% for episodes of AMR. This observation is like prior publications,14 and suggests that caution should be taken when interpreting test performance characteristics that are linked to an imperfect reference standard.
In addition, in our prior work we saw elevations in dd-cfDNA that precede AMR by months--this parallels our current analyses demonstrating dysregulation of certain miRs even before EMB confirmed a diagnosis of AMR. This unfortunately leads to poor test performance characteristics as the specificity and PPV of the test are lower, because the concurrent EMB was initially negative, then positive several months later.
Racial Differences in Antibody-Mediated Rejection
To enhance the robustness and reproducibility of our results, we used a prospective multicenter cohort of heart transplant patients with a high proportion of female sex (35%) and Black race (45%) transplant patients. Each center had its own clinical management protocols after transplant to enhance translation to other worldwide heart transplant centers. Although the incidence of ACR was similar between Black and White transplant patients, we found a higher incidence of AMR in Black patients. This parallels a study by Cole et al., where Black patients were 4 times more likely to develop de novo DSA and 5 times more likely to have AMR than non-Black patients.33 We found that the risk of AMR appeared to be higher in patients supported with a left ventricular assist device prior to transplant (91.7% vs. 67.5%), this is consistent with prior analyses and the intersection between race, prior left ventricular assist device support and AMR needs to be explored further.33 Social determinants of health are known to contribute to disparate outcomes between racial groups after transplant.34–37 Prior work by our group using GEP has found that, despite similar calcineurin inhibitor drug levels, immune system activity varies more significantly in Black heart transplant patients compared to non-Black patients.38 In this analysis, we found that the miR transcriptome was altered before the clinical diagnosis of AMR, supporting the concept of an altered alloimmune response and miRs may explain the higher incidence of AMR in Black heart transplant recipients. Additional insights may be gained from exploring miRs across diverse transplant patient populations.
Limitations
GRAfT has enrolled over 200 heart transplant patients to date and has over 2,000 serial samples available for analysis, but due to resource limitations we selected a subset of patients and therefore our diagnostic test performance characteristics (e.g., sensitivity, specificity, PPV and NPV) will require additional validation in larger cohorts. Due to the low incidence of severe rejection (e.g., ACR Grade 3R or pAMR 3), we were unable to quantify whether expression patterns change in patients with moderate vs. severe rejection. We relied on the center assessment of ACR and AMR to develop our miR panels, which is known to vary from center to center.14 We did, however, assess test characteristics of our miR panels on a subset of the endomyocardial biopsy samples using a blinded cardiac pathologists and found higher performance as compared to the institutional pathology read. Clinical treatment of AMR relies on complementary information from the EMB, DSA testing, echocardiography, hemodynamics, and clinical signs/symptoms.39 We found significant heterogeneity across GRAfT sites in the decision to treat AMR and how to treat it. Since our goal was to identify miR biomarkers of histopathologic AMR, we did include episodes of both treated and untreated AMR. Our findings suggest that these AMR scores need to be used in conjunction with other complimentary tests to facilitate clinical management.
Each center within GRAfT uses different induction, immunosuppression, and post-transplant graft surveillance protocols. These are provided in the supplemental materials and reflect the heterogeneity seen across transplant centers worldwide. Future studies will need to focus on the interplay between miR expression and immunosuppressant drug levels (e.g., steroid dose or tacrolimus level). The ACR and AMR clinical scores were only internally validated within the GRAfT cohort, and similar scores could be created for the external validation cohort. Although the miRs would be the same the coefficients may be different in magnitude due to several pre-analytic differences in sample collection and processing that affect miR expression.
Our differentially expressed miRs were not adjusted for multiple hypothesis testing, but we did use LASSO regression to select our miRs of interest. Variable selection using LASSO does not rely on the significance of the p-value; rather it uses a penalized model that automatically shrinks the unimportant variables to zero.40 The model is built to incorporate a high number of covariates and is not affected by multiple hypothesis testing.
Further analyses could focus on comparing our miR panels with other markers of allograft injury such as dd-cfDNA, EMB mRNA or miR signatures, or using a multi-marker approach that combines dd-cfDNA and miRs.9, 41
Conclusion
Using small RNA sequencing, we identified novel miRs that are differentially regulated with ACR and AMR and were independently validated. By creating miR panels, we were able to non-invasively identify ACR and AMR with excellent test performance characteristics. Our unique ACR and AMR scores, once appropriately validated, usher in an era for the use of non-invasive biomarkers to not only screen for acute allograft rejection, but also diagnose ACR or AMR, permitting the initiation of targeted therapy based on the veritable “liquid biopsy.”
Supplementary Material
Acknowledgements
The authors express great gratitude to Drs. Jun Zhu and Ramaswamy Iyer, who were pivotal in establishing the laboratory methodology for the miR sequencing. Marina Provenzano, Michael Harpole, Erick McNair, Ryan Fassnacht, Quang Phan, Lopa Mehta, and Vincent Chou, for their support in the lab where the miR sequencing pipeline was developed. Elizabeth Adams and the other GRAfT study coordinators for their tireless work to recruit patients. As well as Dr. Kevin D. Allen who assisted in developing our bioinformatics pipeline for these analyses.
Funding
Work supported by American Heart Association / Enduring Hearts Foundation Scientist Development Grant 17SDG33660431, Inova Translational Research Fund and NIH K23 Career Development Award 1K23HL143179 awarded to P.S.; HV and SAE are supported by the NHLBI Division of Intramural Research (HHSN268201300001C); KK is supported by an NIH RC4AI092673 and the Gottlieb Charitable Foundation.
Declaration of Competing Interest
Inova Health System has secured a provisional patent no. 63/283,053 for the methods, microRNA biomarkers and clinical rejection scores described in this study. PS = Unrelated grant support paid to institution from Merck, Bayer, Roche, and Abbott. Unrelated consulting for Merck and Procyrion. KBS = Consultant for Eidos and Grant Reviewer for Pfizer. KK = CareDx scientific advisor, speaker, grant recipient. All other authors report no relevant disclosures.
ABBREVIATIONS:
- ACR
acute cellular rejection
- AMR
antibody-mediated rejection
- AUC
area under the curve
- EMB
endomyocardial biopsy
- LVAD
left ventricular assist device
- miR
microRNA
- NGS
next-generation sequencing
- NPV
negative predictive value
- PPV
positive predictive value
- ROC
receiver operating characteristic
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Khush KK, Potena L, Cherikh WS, Chambers DC, Harhay MO, Hayes D Jr., Hsich E, Sadavarte A, Singh TP, Zuckermann A, Stehlik J, International Society for H and Lung T. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: 37th adult heart transplantation report-2020; focus on deceased donor characteristics. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agbor-Enoh S, Shah P, Tunc I, Hsu S, Russell S, Feller E, Shah K, Rodrigo ME, Najjar SS, Kong H, Pirooznia M, Fideli U, Bikineyeva A, Marishta A, Bhatti K, Yang Y, Mutebi C, Yu K, Kyoo Jang M, Marboe C, Berry GJ, Valantine HA and Investigators GR. Cell-Free DNA to Detect Heart Allograft Acute Rejection. Circulation. 2021;143:1184–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colvin MM, Cook JL, Chang P, Francis G, Hsu DT, Kiernan MS, Kobashigawa JA, Lindenfeld J, Masri SC, Miller D, O’Connell J, Rodriguez ER, Rosengard B, Self S, White-Williams C, Zeevi A, American Heart Association Heart F, Transplantation Committee of the Council on Clinical C, American Heart Association Heart F, Transplantation Committee of the Council on Cardiopulmonary Critical Care P, Resuscitation, American Heart Association Heart F, Transplantation Committee of the Council on Cardiovascular Disease in the Y, American Heart Association Heart F, Transplantation Committee of the Council on Clinical Cardiology CoC, Stroke N, American Heart Association Heart F, Transplantation Committee of the Council on Cardiovascular R, Intervention, American Heart Association Heart F, Transplantation Committee of the Council on Cardiovascular S and Anesthesia. Antibody-mediated rejection in cardiac transplantation: emerging knowledge in diagnosis and management: a scientific statement from the American Heart Association. Circulation. 2015;131:1608–39. [DOI] [PubMed] [Google Scholar]
- 4.Wu GW, Kobashigawa JA, Fishbein MC, Patel JK, Kittleson MM, Reed EF, Kiyosaki KK and Ardehali A. Asymptomatic antibody-mediated rejection after heart transplantation predicts poor outcomes. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2009;28:417–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee RC, Feinbaum RL and Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–54. [DOI] [PubMed] [Google Scholar]
- 6.Shah P, Bristow MR and Port JD. MicroRNAs in Heart Failure, Cardiac Transplantation, and Myocardial Recovery: Biomarkers with Therapeutic Potential. Curr Heart Fail Rep. 2017;14:454–464. [DOI] [PubMed] [Google Scholar]
- 7.Sukma Dewi I, Torngren K, Gidlof O, Kornhall B and Ohman J. Altered serum miRNA profiles during acute rejection after heart transplantation: potential for non-invasive allograft surveillance. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2013;32:463–6. [DOI] [PubMed] [Google Scholar]
- 8.Duong Van Huyen JP, Tible M, Gay A, Guillemain R, Aubert O, Varnous S, Iserin F, Rouvier P, Francois A, Vernerey D, Loyer X, Leprince P, Empana JP, Bruneval P, Loupy A and Jouven X. MicroRNAs as non-invasive biomarkers of heart transplant rejection. European heart journal. 2014;35:3194–202. [DOI] [PubMed] [Google Scholar]
- 9.Di Francesco A, Fedrigo M, Santovito D, Natarelli L, Castellani C, De Pascale F, Toscano G, Fraiese A, Feltrin G, Benazzi E, Nocco A, Thiene G, Valente M, Valle G, Schober A, Gerosa G and Angelini A. MicroRNA signatures in cardiac biopsies and detection of allograft rejection. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2018;37:1329–1340. [DOI] [PubMed] [Google Scholar]
- 10.Constanso-Conde I, Hermida-Prieto M, Barge-Caballero E, Nunez L, Pombo-Otero J, Suarez-Fuentetaja N, Paniagua-Martin MJ, Barge-Caballero G, Couto-Mallon D, Pan-Lizcano R, Vazquez-Rodriguez JM and Crespo-Leiro MG. Circulating miR-181a-5p as a new biomarker for acute cellular rejection in heart transplantation. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2020;39:1100–1108. [DOI] [PubMed] [Google Scholar]
- 11.Guo S, Guo X, Wang S, Nie Q, Ni G and Wang C. Role of miR-29 as marker of risk of acute rejection after heart transplant. Br J Biomed Sci. 2017;74:187–192. [DOI] [PubMed] [Google Scholar]
- 12.Stewart S, Winters GL, Fishbein MC, Tazelaar HD, Kobashigawa J, Abrams J, Andersen CB, Angelini A, Berry GJ, Burke MM, Demetris AJ, Hammond E, Itescu S, Marboe CC, McManus B, Reed EF, Reinsmoen NL, Rodriguez ER, Rose AG, Rose M, Suciu-Focia N, Zeevi A and Billingham ME. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2005;24:1710–20. [DOI] [PubMed] [Google Scholar]
- 13.Berry GJ, Burke MM, Andersen C, Bruneval P, Fedrigo M, Fishbein MC, Goddard M, Hammond EH, Leone O, Marboe C, Miller D, Neil D, Rassl D, Revelo MP, Rice A, Rene Rodriguez E, Stewart S, Tan CD, Winters GL, West L, Mehra MR and Angelini A. The 2013 International Society for Heart and Lung Transplantation Working Formulation for the standardization of nomenclature in the pathologic diagnosis of antibody-mediated rejection in heart transplantation. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2013;32:1147–62. [DOI] [PubMed] [Google Scholar]
- 14.Crespo-Leiro MG, Zuckermann A, Bara C, Mohacsi P, Schulz U, Boyle A, Ross HJ, Parameshwar J, Zakliczynski M, Fiocchi R, Stypmann J, Hoefer D, Lehmkuhl H, Deng MC, Leprince P, Berry G, Marboe CC, Stewart S, Tazelaar HD, Baron HM, Coleman IC and Vanhaecke J. Concordance among pathologists in the second Cardiac Allograft Rejection Gene Expression Observational Study (CARGO II). Transplantation. 2012;94:1172–7. [DOI] [PubMed] [Google Scholar]
- 15.Sun L, Yu Y, Niu B and Wang D. Red Blood Cells as Potential Repositories of MicroRNAs in the Circulatory System. Front Genet. 2020;11:442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Love MI, Huber W and Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedman J, Hastie T and Tibshirani R. Regularization Paths for Generalized Linear Models via Coordinate Descent. J Stat Softw. 2010;33:1–22. [PMC free article] [PubMed] [Google Scholar]
- 18.Firth D Bias Reduction of Maximum Likelihood Estimates. Biometrika. 1993;80:27–38. [Google Scholar]
- 19.Fluss R, Faraggi D and Reiser B. Estimation of the Youden Index and its associated cutoff point. Biom J. 2005;47:458–72. [DOI] [PubMed] [Google Scholar]
- 20.Huang HY, Lin YC, Li J, Huang KY, Shrestha S, Hong HC, Tang Y, Chen YG, Jin CN, Yu Y, Xu JT, Li YM, Cai XX, Zhou ZY, Chen XH, Pei YY, Hu L, Su JJ, Cui SD, Wang F, Xie YY, Ding SY, Luo MF, Chou CH, Chang NW, Chen KW, Cheng YH, Wan XH, Hsu WL, Lee TY, Wei FX and Huang HD. miRTarBase 2020: updates to the experimentally validated microRNA-target interaction database. Nucleic acids research. 2020;48:D148–D154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allegra A, Alonci A, Campo S, Penna G, Petrungaro A, Gerace D and Musolino C. Circulating microRNAs: new biomarkers in diagnosis, prognosis and treatment of cancer (review). Int J Oncol. 2012;41:1897–912. [DOI] [PubMed] [Google Scholar]
- 22.Deng MC, Eisen HJ, Mehra MR, Billingham M, Marboe CC, Berry G, Kobashigawa J, Johnson FL, Starling RC, Murali S, Pauly DF, Baron H, Wohlgemuth JG, Woodward RN, Klingler TM, Walther D, Lal PG, Rosenberg S, Hunt S and Investigators C. Noninvasive discrimination of rejection in cardiac allograft recipients using gene expression profiling. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2006;6:150–60. [DOI] [PubMed] [Google Scholar]
- 23.Kobashigawa JA, Kiyosaki KK, Patel JK, Kittleson MM, Kubak BM, Davis SN, Kawano MA and Ardehali AA. Benefit of immune monitoring in heart transplant patients using ATP production in activated lymphocytes. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2010;29:504–8. [DOI] [PubMed] [Google Scholar]
- 24.Snyder TM, Khush KK, Valantine HA and Quake SR. Universal noninvasive detection of solid organ transplant rejection. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:6229–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Vlaminck I, Valantine HA, Snyder TM, Strehl C, Cohen G, Luikart H, Neff NF, Okamoto J, Bernstein D, Weisshaar D, Quake SR and Khush KK. Circulating cell-free DNA enables noninvasive diagnosis of heart transplant rejection. Science translational medicine. 2014;6:241ra77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khush KK, Patel J, Pinney S, Kao A, Alharethi R, DePasquale E, Ewald G, Berman P, Kanwar M, Hiller D, Yee JP, Woodward RN, Hall S and Kobashigawa J. Noninvasive detection of graft injury after heart transplant using donor-derived cell-free DNA: A prospective multicenter study. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2019;19:2889–2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei L, Wang M, Qu X, Mah A, Xiong X, Harris AG, Phillips LK, Martinez OM and Krams SM. Differential expression of microRNAs during allograft rejection. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012;12:1113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei L, Kaul V, Qu X, Xiong X, Lau AH, Iwai N, Martinez OM and Krams SM. Absence of miR-182 Augments Cardiac Allograft Survival. Transplantation. 2017;101:524–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Creemers EE, Tijsen AJ and Pinto YM. Circulating microRNAs: novel biomarkers and extracellular communicators in cardiovascular disease? Circulation research. 2012;110:483–95. [DOI] [PubMed] [Google Scholar]
- 30.Hu RW, Korutla L, Reddy S, Harmon J, Zielinski PD, Bueker A, Molina M, Romano C, Margulies K, McLean R, Lal P and Vallabhajosyula P. Circulating Donor Heart Exosome Profiling Enables Noninvasive Detection of Antibody-mediated Rejection. Transplant Direct. 2020;6:e615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wright C, Rajpurohit A, Burke EE, Williams C, Collado-Torres L, Kimos M, Brandon NJ, Cross AJ, Jaffe AE, Weinberger DR and Shin JH. Comprehensive assessment of multiple biases in small RNA sequencing reveals significant differences in the performance of widely used methods. BMC Genomics. 2019;20:513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dave VP, Ngo TA, Pernestig AK, Tilevik D, Kant K, Nguyen T, Wolff A and Bang DD. MicroRNA amplification and detection technologies: opportunities and challenges for point of care diagnostics. Lab Invest. 2019;99:452–469. [DOI] [PubMed] [Google Scholar]
- 33.Cole RT, Gandhi J, Bray RA, Gebel HM, Yin M, Shekiladze N, Young A, Grant A, Mahoney I, Laskar SR, Gupta D, Bhatt K, Book W, Smith A, Nguyen D, Vega JD and Morris AA. Racial differences in the development of de-novo donor-specific antibodies and treated antibody-mediated rejection after heart transplantation. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2018;37:503–512. [DOI] [PubMed] [Google Scholar]
- 34.Kilic A, Higgins RS, Whitson BA and Kilic A. Racial disparities in outcomes of adult heart transplantation. Circulation. 2015;131:882–9. [DOI] [PubMed] [Google Scholar]
- 35.Singh TP, Givertz MM, Semigran M, Denofrio D, Costantino F and Gauvreau K. Socioeconomic position, ethnicity, and outcomes in heart transplant recipients. Am J Cardiol. 2010;105:1024–9. [DOI] [PubMed] [Google Scholar]
- 36.Morris AA, Kransdorf EP, Coleman BL and Colvin M. Racial and ethnic disparities in outcomes after heart transplantation: A systematic review of contributing factors and future directions to close the outcomes gap. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2016;35:953–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lewsey SC and Breathett K. Racial and ethnic disparities in heart failure: current state and future directions. Curr Opin Cardiol. 2021;36:320–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khush KK, Pham MX, Teuteberg JJ, Kfoury AG, Deng MC, Kao A, Anderson AS, Cotts WG, Ewald GA, Baran DA, Hiller D, Yee J and Valantine HA. Gene expression profiling to study racial differences after heart transplantation. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2015;34:970–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chih S, Tinckam KJ and Ross HJ. A survey of current practice for antibody-mediated rejection in heart transplantation. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013;13:1069–1074. [DOI] [PubMed] [Google Scholar]
- 40.Tibshirani R Regression Shrinkage and Selection via the Lasso. Journal of the Royal Statistical Society Series B (Methodological). 1996;58:267–288. [Google Scholar]
- 41.Loupy A, Duong Van Huyen JP, Hidalgo L, Reeve J, Racape M, Aubert O, Venner JM, Falmuski K, Bories MC, Beuscart T, Guillemain R, Francois A, Pattier S, Toquet C, Gay A, Rouvier P, Varnous S, Leprince P, Empana JP, Lefaucheur C, Bruneval P, Jouven X and Halloran PF. Gene Expression Profiling for the Identification and Classification of Antibody-Mediated Heart Rejection. Circulation. 2017;135:917–935. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


