Abstract
The pattern of peptidoglycan (murein) segregation in cells of Escherichia coli with impaired activity of the morphogenetic proteins penicillin-binding protein 2 and RodA has been investigated by the d-cysteine–biotin immunolabeling technique (M. A. de Pedro, J. C. Quintela, J.-V. Höltje, and H. Schwarz, J. Bacteriol. 179:2823–2834, 1997). Inactivation of these proteins either by amdinocillin treatment or by mutations in the corresponding genes, pbpA and rodA, respectively, leads to the generation of round, osmotically stable cells. In normal rod-shaped cells, new murein precursors are incorporated all over the lateral wall in a diffuse manner, being mixed up homogeneously with preexisting material, except during septation, when strictly localized murein synthesis occurs. In contrast, in rounded cells, incorporation of new precursors is apparently a zonal process, localized at positions at which division had previously taken place. Consequently, there is no mixing of new and old murein. Old murein is preserved for long periods of time in large, well-defined areas. We propose that the observed patterns are the result of a failure to switch off septal murein synthesis at the end of septation events. Furthermore, the segregation results confirm that round cells of rodA mutants do divide in alternate, perpendicular planes as previously proposed (K. J. Begg and W. D. Donachie, J. Bacteriol. 180:2564–2567, 1998).
The peptidoglycan (murein) sacculus is the principal stress-bearing and shape-maintaining element of the cell wall and plays an essential role in bacterial morphogenesis. Growth of Escherichia coli occurs by periodic alternation of elongation and division events (2, 14). Newborn cells elongate and then divide at midcell once the chromosome is replicated and the initial mass (length) is doubled (17, 20). Cell elongation demands the concomitant enlargement of the sacculus, and cell division requires the formation of a transverse septum at its center (2, 14, 40).
Elongation and septation of the sacculus require insertion of new precursors by the concerted action of murein biosynthetic and hydrolytic enzymes (25, 39, 46). Among the former, penicillin-binding proteins (PBPs) are of particular interest as the enzymes that actually polymerize monomeric subunits and cross-link the resulting new strands to the preexisting murein (15, 36, 48, 52). In E. coli, PBP2 and -3 have well-defined morphogenetic roles. PBP3 is specifically and absolutely required for septal murein synthesis. Inhibition of PBP3 activity blocks septation and leads to filament formation. Impairment of PBP2 activity leads to the generation of pleiomorphic, spherical cells (3, 18, 22, 41, 48, 49, 53, 55, 57, 58, 61). Enzymatically both proteins have dd-transpeptidase activities in vitro, but no transglycosylase activity has been reliably demonstrated for either of them (26, 56). To be fully functional, PBP2 apparently requires an active RodA protein (26). Impairment of either RodA or PBP2 leads to similar phenotypes. However, the enzymatic activity of RodA remains to be identified. Because the activities of both PBP2 and RodA are needed to keep the rod-like morphology of the cell, they are thought to be required for side wall murein synthesis during elongation of the cell wall (3, 11, 13).
Amdinocillin is a β-lactam antibiotic that binds with high affinity and specificity to PBP2. The effects of amdinocillin are generally attributed to its inhibitory action on PBP2, but the possibility of additional effects cannot be totally discounted (27, 43, 50). Inhibition of PBP2 by amdinocillin leads to important alterations in murein composition and rate of synthesis. Upon addition of amdinocillin, the rate of murein synthesis is reduced by about 50%, but the rate of growth and division remains constant for roughly one doubling in cell mass. Therefore, the amount of murein per cell drops to about one-half the normal value. Concomitantly cells become spherical, cell division is blocked, large multinucleated cells are generated, and viability is lost (11, 12, 31, 37, 47, 50). Spherical cells are also generated by mutations in the genes pbpA and rodA (coding for PBP2 and RodA, respectively). Thermosensitive mutations in both genes (pbpA45 and rodA52) were first obtained by using amdinocillin resistance as the selective criterion. At the restrictive temperature (42°C) both pbpA(Ts) and rodA(Ts) strains are viable, resistant to amdinocillin, and have a round cell morphology (28, 29, 37, 51, 55). At permissive temperatures (30°C), the rodA(Ts) mutant has normal morphology, but the pbpA(Ts)strain has a near-spherical shape in stationary-phase culture (45). Inactivation of either rodA or pbpA results in round cells that are unable to grow and divide in rich medium, although these round cells are viable in minimal medium. Both kinds of deletion mutants can, however, grow and divide well in rich medium if the level of FtsA and FtsZ division proteins is increased. This can result from either an increase in the copy number of these genes (on plasmid vectors) or mutations that increase transcription of these two genes (6). Division of rounded cells is efficient but abnormal in several respects (4, 6, 51, 55). The larger-than-normal diameter of the cell impedes formation of a complete FtsZ ring as required for normal septation (1, 7, 35, 54). Polymerization of FtsZ at the future division site leads instead to partial rings, which are nevertheless able to direct a lateralized invagination of the envelope (19, 28, 64). Progressive invagination eventually leads to cell separation. Interestingly, successive divisions seem to occur in perpendicularly alternating planes (4, 65). In addition, pbpA(Ts) mutants have a tendency to divide irregularly to give cells of different sizes (45).
Because PBP3 and PBP2 plus RodA are apparently involved in discrete morphological events, it has been proposed that together with additional proteins (such as FtsW, a close homologue of RodA) (9, 32, 57), each could be part of murein biosynthetic complexes active at alternating periods of the cell cycle (38). In these models, PBP3-containing complexes would be active exclusively at the septation period and PBP2-containing ones would be active during the elongation phase of cell growth or both elongation and division (3, 8, 10, 13, 16). An implication of such models is that murein synthesized upon PBP2 impairment should be made by complexes that are normally committed to septation and therefore should have the properties of septal murein (51).
Application of a new method to the analysis of murein segregation in E. coli (16) confirmed and complemented previous results that support “two-complex” models (8, 10, 40, 60, 62, 63). According to our interpretation, during cell elongation, new precursors are inserted in a diffuse fashion into the cylindrical part of the sacculus, but not at the polar caps, which do not undergo further expansion. However, shortly before septation actually starts, a strongly localized murein biosynthetic activity is triggered at the putative division site in an FtsZ-dependent process. Activation of septal murein synthesis (SMS) results in the generation of a ring of all-new murein around the cell, which in time develops into a circumferential invagination that is completed to form two new poles. Inhibition of cell division at any stage later than FtsZ ring formation leads to the generation of a ring of new murein, which grows for a defined period of time and then stops, generating an annular zone of new and inert murein. The studies of murein segregation suggest therefore some kind of periodic activation and inactivation of a septal murein biosynthesis complex, whose activity may alternate, or overlap, with that of complexes involved in lateral wall synthesis, which could in turn be directed by the PBP2 and RodA proteins.
We were therefore interested in studying murein segregation in strains with impaired activity of the PBP2 and RodA proteins. The mode of insertion of new materials under conditions in which cell wall growth is exclusively directed by septation complexes could be substantially different from that in normal cells and could help to explain the way in which viable dividing spherical cells are formed.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The E. coli strains used in this work were MC6RP1 (K-12 F− dra drm leuA lysA proA thi thr) (44), SP4500 [K-12 F− his pro purB thi mtl xyl galK lacY rpsL pbpA45(Ts)], SP5211 [K-12 F− his pro purB thi mtl xyl galK lacY rpsL rodA52(Ts)] (51), and KJB24 [K-12 IN(rrnD-rrnE)1 rodASui(Am) ddlB::Tn5] (4). Cultures were routinely grown in Luria-Bertani (LB) medium (34) at the appropriate temperature in gyratory water baths. Growth was monitored by measuring the optical density of the cultures at 550 nm (OD550).
d-Cysteine labeling of murein.
Labeling was performed as described previously (16). Flasks containing appropriate volumes of prewarmed medium were inoculated (OD550, ≈0.03) from overnight cultures of the selected bacterial strain and incubated until they had reached an OD550 of ≈0.06. At that moment, d-Cys was added to the cultures to a final concentration of 100 μg/ml, and cultures were further incubated for three doublings in cell mass as determined by measuring the OD550. To remove d-Cys, cultures were centrifuged (5 min, 20,000 × g) at the temperature used for growth, resuspended into an equal volume of d-Cys-free medium prewarmed at the growth temperature, centrifuged again as described above, and finally resuspended in the medium appropriate for each experiment. Cultures were further processed according to the specific requirements of individual experiments.
Purification, biotinylation, immunolabeling, and observation of sacculi.
Sacculi were purified, reduced with NaH4B, biotinylated at the free thiol groups, and immunolabeled for either epifluorescence or electron microscopy exactly as described previously (16). Observation and photographic work were performed with the same instruments used before (Zeiss Axioplan fluorescence microscope fitted with a 100×/1.3 Neofluar objective and a Philips CM10 transmission electron microscope at an acceleration voltage of 60 kV).
RESULTS
Effect of amdinocillin on murein segregation in E. coli MC6RP1.
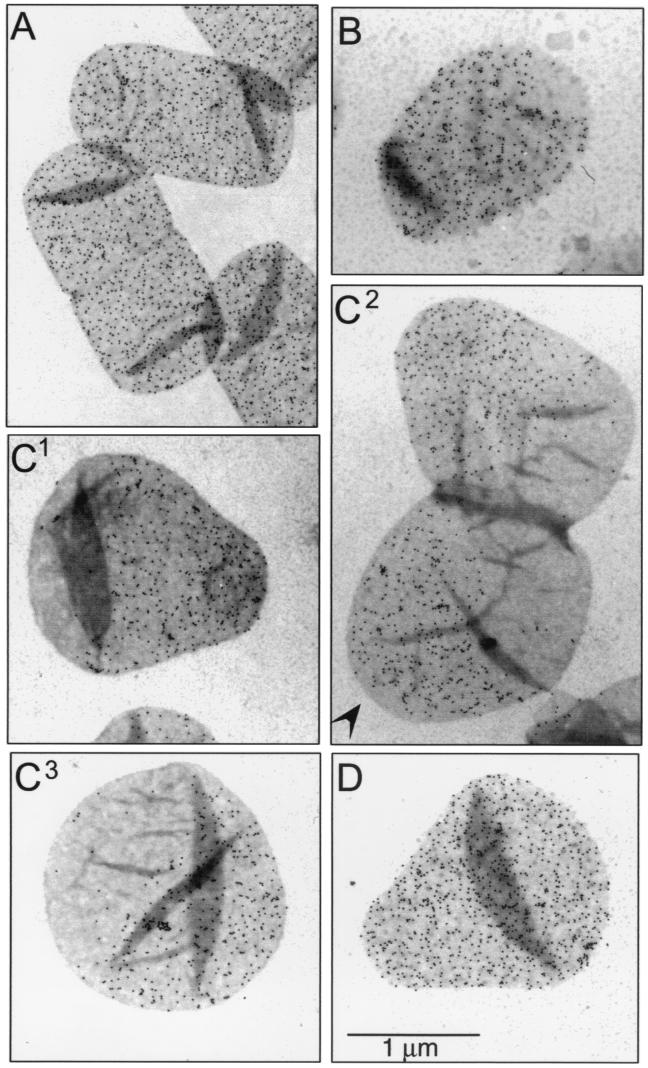
Cultures of E. coli MC6RP1 labeled with d-Cys were transferred to prewarmed d-Cys-free medium containing amdinocillin at 1 μg/ml. At increasing chase times, 25-ml samples were removed, and murein was purified and prepared for immunoelectron microscopy and immunofluorescence microscopy. Upon addition of amdinocillin, E. coli cells are able to complete the ongoing round of cell division, but are unable to complete any further divisions (11, 12, 47). Sacculi from nonchased cells (Fig. 1A) showed a homogeneous and dense distribution of gold grains over their surface. Sacculi from cells chased for one mass doubling time already showed clear signs of rounding. Most of them presented a gold grain-free pole, indicating that a division had taken place after the removal of label (Fig. 1B). After a longer chase (Fig. 1C), all sacculi had acquired aberrant shapes. Most were either pear shaped (with the older pole closing the small end of a conical section closed on the other side by a hemisphere) (Fig. 1C1) or hourglass shaped (Fig. 1C2). Only a few were actually spherical (Fig. 1C3). Most remarkable was that, in contrast to the observations with filaments and normal cells (16), large sharply delimited areas of labeled and unlabeled murein were present. The surface area covered by old (gold marked) and new (gold free) murein was roughly equal after two doubling times. In all instances, the large areas of all-new murein (unlabeled) appeared in places at which a division had already been completed or a new one had started. The distribution of gold grains in the labeled areas was essentially homogeneous. Interestingly, the hourglass-shaped sacculi had an additional small area free of gold grains in one of the poles (Fig. 1C2), indicative of an early division. Another interesting observation was that the total number of gold grains per sacculus (256 ± 66 grains/sacculus, n = 26) was about one-half of the value for cells at the beginning of the chase period (452 ± 110 grains/sacculus, n = 18). Because cells do divide once in the presence of the drug, these results indicate that most of the old murein is conserved throughout the chase period.
FIG. 1.
Immunoelectron microscopy of d-Cys-labeled murein in amdinocillin-treated cells of MC6RP1. Cells from a culture grown for three generations in LB medium supplemented with d-Cys were harvested by centrifugation and transferred to medium without d-Cys, but containing 1 μg of amdinocillin per ml. As a control, an aliquot was transferred to medium with both amdinocillin and d-Cys at the concentrations shown above. At the indicated chase times, samples were removed and further processed for murein purification, biotinylation, immunolabeling, and electron microscopy as described in Materials and Methods. Gold-conjugated (6-nm-diameter grains) protein A was used for the detection of antibodies. (A) Chase time zero. (B) Cells chased for one doubling in cell mass. (C) Cells chased for two doublings in cell mass. The arrowhead indicates a gold-free pole. (D) Control cells incubated for two doublings in cell mass in d-Cys plus amdinocillin. All pictures are at the same magnification.
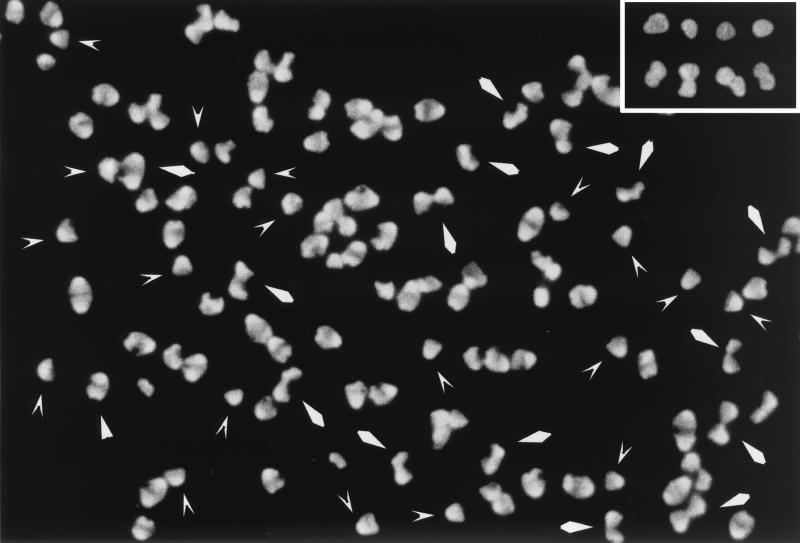
Fluorescence microscopy of the same sacculus samples gave similar results. In particular, the images corresponding to sacculi chased for two doubling times validated the electron microscopy ones discussed above. That the shapes shown in the electron microscopy pictures (Fig. 1) were by no means rare was clear from pictures like the one in Fig. 2. Again the labeled (old) murein appeared as a large area of essentially uniform brightness. The shapes of the bright areas clearly corresponded to the gold-labeled ones found in the electron microscopy pictures.
FIG. 2.
Immunofluorescence microscopy of d-Cys-labeled murein in amdinocillin-treated cells of MC6RP1. Aliquots of the same sacculi purified for the experiment shown in Fig. 1 were subjected to immunofluorescence microscopy for the detection of d-Cys-labeled areas with a fluorescein isothiocyanate-labeled goat anti-rabbit antibody. The picture shows sacculi chased for two doublings in cell mass, corresponding to Fig. 1C. V-shaped and rhomboidal arrowheads indicate sacculi similar to the ones in Fig. 1C1 and C2, respectively. The inset shows selected sacculi from the sample incubated for the same time in d-Cys plus amdinocillin (Fig. 1D).
Murein segregation in the E. coli pbpA(Ts) strain SP4500.
The pbpA(Ts) mutant strain SP4500 is amdinocillin resistant and has a round cell morphology at 42°C. To monitor murein segregation at 42°C, cells were first labeled with d-Cys and then chased in d-Cys-free medium before purification of the sacculi. The detailed pattern of segregation could not be as clearly established as that described above, although it was rather evident that incorporation of new material was essentially a localized event. Large areas of neatly delimited old and new murein were segregated during the chase period in accordance with the results for amdinocillin-treated cells (Fig. 3).
FIG. 3.
Immunoelectron microscopy of d-Cys-labeled murein in cells of the pbpA(Ts) mutant SP4500. Cells from a culture grown for three generations in LB medium supplemented with d-Cys at 42°C were harvested by centrifugation. One sample was immediately subjected to murein purification, and the rest of the cell suspension was transferred to prewarmed medium without d-Cys and further incubated for one and a half generations. Sacculi were purified, biotinylated, and immunolabeled as indicated in Materials and Methods. Gold-conjugated (6-nm-diameter grains) protein A was used for the detection of antibodies. (A) Nonchased sacculi. (B) Sacculi chased for one and a half doublings in mass. All pictures are at the same magnification.
Murein segregation in the E. coli rodA(Ts) mutant SP5211
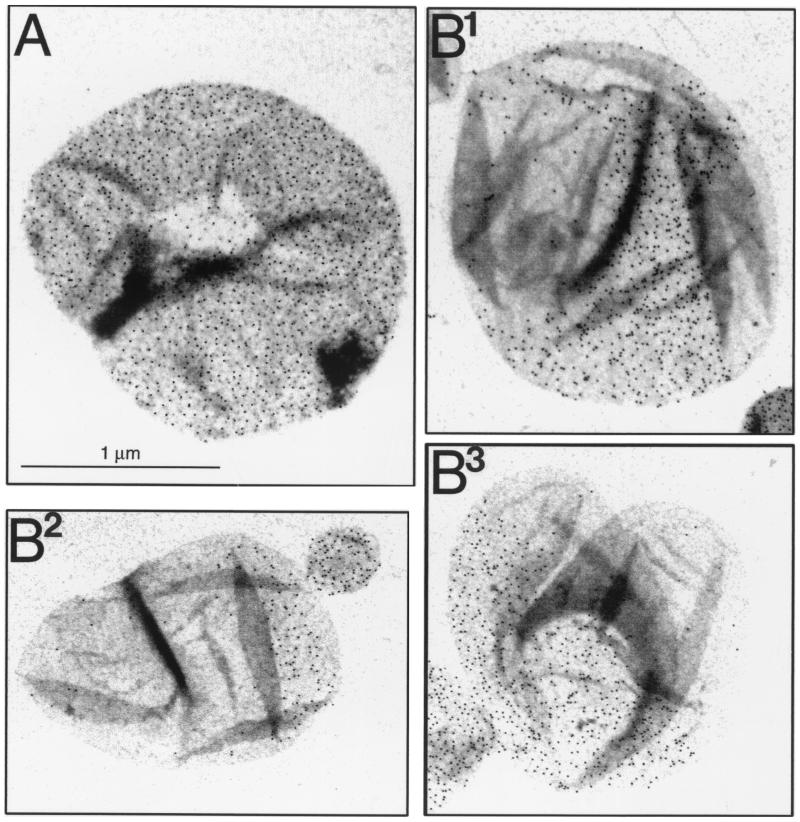
Cells of the rodA(Ts) mutant strain SP5211 have a normal rod shape at 30°C, which is lost upon transfer to 42°C, resembling the morphological change induced by amdinocillin. To investigate murein segregation in this mutant, cells were labeled with d-Cys at 30°C, and then d-Cys was removed by centrifugation and the cells were resuspended in d-Cys-free medium (prewarmed at 42°C) to initiate the chase period. Sacculi from cells chased at 42°C kept a rod-like morphology for the first doubling in mass, and up to this point, the segregation of murein corresponded to the observations in wild-type cells. Septal rings of all-new murein were clearly visible in some sacculi, and most of them had one pole without label, indicative of a division event (Fig. 4B). Transition into a round shape was a smoother process than in amdinocillin-treated cells, probably because SP5211 continues to divide at the restrictive temperature. As previously observed, once cells start getting rounded, as in Fig. 4B, septation starts as a localized invagination and proceeds asymmetrically (Fig. 4C1). After completion of division, cells were produced with sacculi very much like the ones generated by amdinocillin treatment (Fig. 4C2 to 4C4). Some had one large, densely labeled area and a gold-free one (Fig. 4C2 to 4C3), and others had a central belt of gold grains (Fig. 4C4). The former represent cells that conserved one of the poles of the initially labeled cell (Fig. 4C1, labeled pole), whereas the latter correspond to cells that inherited a new pole after each round of division (Fig. 4C1, unlabeled pole). Interestingly the latter kind of cells conserve more gold grains than corresponding sacculi from cells chased for the same time at 30°C (Fig. 4D). This suggests that dilution of old lateral wall material with new material was slower in the rounding cells. The end result was the accumulation of sacculi with large delimited areas of all-new and all-old murein, as in the previous cases. As described above, the distribution of gold grains in the areas of old murein was rather homogeneous. Cells chased at 30°C behaved like the wild type: that is, polar regions retained all of their original label, whereas label was progressively diluted in the rest of the sacculus, and septal rings of new murein were associated with division sites (data not shown).
FIG. 4.
Immunoelectron microscopy of d-Cys-labeled murein in cells of the rodA(Ts) mutant SP5211. Cells from a culture grown for three generations in LB medium supplemented with d-Cys (150 μg/ml) at 30°C were harvested and transferred to d-Cys-free medium prewarmed at 30 and 42°C. Cultures were further incubated at the respective temperature, and after one and two doublings in cell mass, samples were removed and further processed for sacculus purification, biotinylation, immunolabeling, and electron microscopy as indicated in Materials and Methods. Gold-conjugated (6-nm-diameter grains) protein A was used for the detection of antibodies. (A) Sacculi from control, nonchased cells. (B) Sacculi chased for one doubling in cell mass at 42°C. (C) Sacculi chased for two doublings in cell mass at 42°C. (D) Sacculi chased for two doublings in cell mass at 30°C. All pictures are at the same magnification.
Murein segregation in the E. coli rodA° mutant KJB24
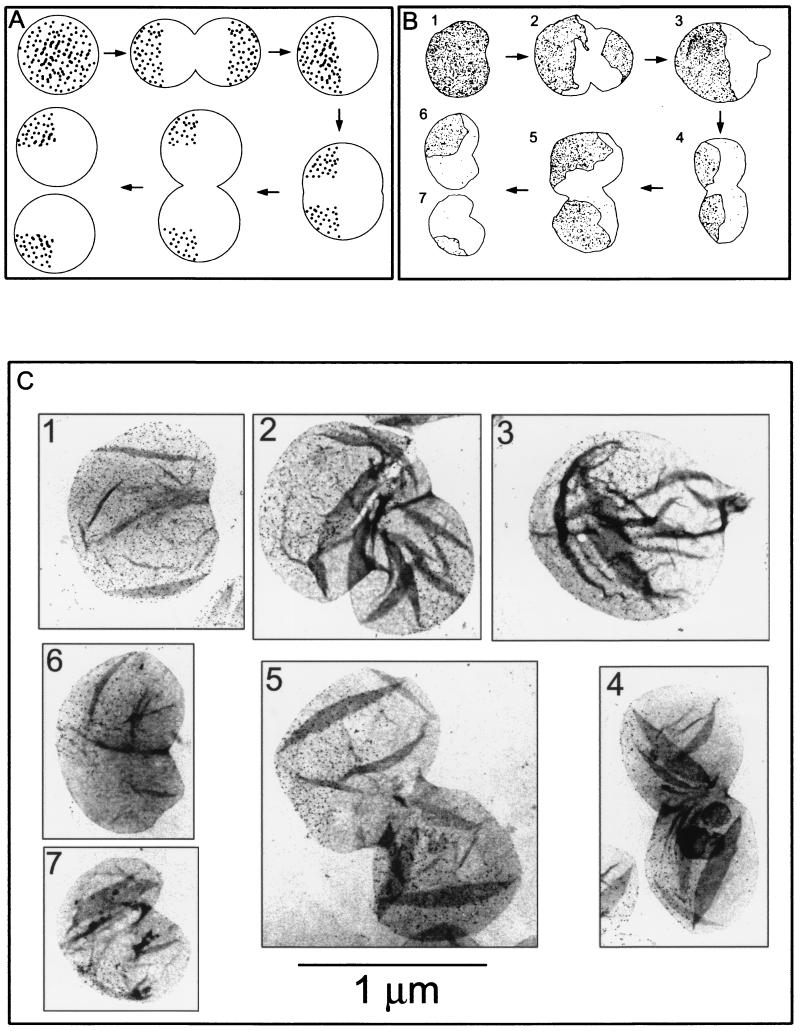
Although the RodA protein is required for normal cell shape, it is not strictly essential for cell viability. Null mutants are viable in minimal medium, in which growth is slow and the cell diameter is small, and plasmids or secondary mutations that increase the production of FtsA and FtsZ division proteins allow cell division in the larger cells formed in rich medium. In strain KJB24, a Tn5 insertion in the ddlB gene, immediately upstream of ftsQ, ftsA, and ftsZ, causes increased transcription of these genes and allows the strain to grow and divide well in rich medium (3). Null mutant cells are spherical in shape and have a wide range of sizes, although they keep the ability to divide at the central plane of the sphere. A point of particular interest is that successive division planes are perpendicular to each other (4). The results presented above suggest that disruption of the PBP2-RodA system results in localized and continuous synthesis of new murein at the division sites and in conservation of the old murein. Therefore, in strain KJB24, the sacculus of a newly born cell should consist of two hemispheres: one made of old murein and the other made of new murein. After a second round of division, at 90° to the preceding one, each daughter cell should have a 90° spherical sector of old murein and 270° of new murein (Fig. 5A). To check this prediction, KJB24 was labeled with d-Cys and chased for one and two mass doubling times as described above. Although collapsing and folding of the large spherical sacculi obscured observation in many instances, sacculi with the predicted distribution of gold grains for the first and the second rounds of division (Fig. 5B and C) were relatively frequent. In the sample chased longer, about 37% of sacculi were one-quarter labeled (Fig. 5C6), and about 25% were from cells dividing for the second time to give one-quarter-labeled sacculi (Fig. 5C4 and C5). Most other sacculi were either folded or oriented in ways that impeded a proper classification. The high proportion of sacculi with the predicted distribution of label supports the conclusions reached from observations of cell morphology. Incidentally, KJB24 sacculi appeared to be rather fragile, because a considerable proportion of them showed clear signs of degradation (data not shown), possibly because of lysis (4).
FIG. 5.
Murein segregation in the rodA° mutant KJB24 as observed by immunoelectron microscopy. Cells from a culture grown for three generations in LB medium supplemented with d-Cys were harvested and transferred to prewarmed d-Cys-free medium. Cultures were further incubated, and after one and two doublings in cell mass, samples were removed and further processed for sacculus purification, biotinylation, immunolabeling, and electron microscopy as indicated in Materials and Methods. Gold-conjugated (6-nm-diameter grains) protein A was used for the detection of antibodies. (A) Hypothetical segregation pattern. (B) Schematic representation of the actual segregation pattern as found in the images shown in panel C. The profile, position, and number of dots were drawn from the real pictures by using digitized images and PhotoShop software. (C) Immunolabeling of d-Cys in selected sacculi after no chase (panel 1) or after chase for one (panels 2 and 3) and two (panels 4 to 7) doublings in cell mass. The numbers in panels B and C correspond. All pictures are at the same magnification.
DISCUSSION
Previous studies of murein segregation by the d-amino acid labeling technique revealed two features relevant to an understanding of cell wall growth in E. coli. First, the polar caps of the cell sacculus are metabolically inert, and second, at the initiation of septation, there is local activation of murein synthesis at the future division site, the place at which new poles will be generated (16). Therefore, it seems quite straightforward to propose a direct relationship between the two phenomena: localized synthesis at the division site produces septal (later polar) murein, which is inert either because of some intrinsic property or because of topological influences. Investigation of murein segregation upon impairment of PBP2 or RodA function further supports this idea.
Inhibition of PBP2 by amdinocillin led to a profoundly altered segregation pattern. After one doubling in mass, most sacculi were slightly ovoid in shape and had gone through a division event. The distributions of gold grains at this stage in amdinocillin-treated and untreated sacculi were similar. Further incubation of cells with amdinocillin resulted in the generation of large sacculi with bizarre morphologies and distribution of gold grains. Most frequently, chased sacculi had a distinct conical shape with a relatively narrow apex and a large hemispherical base. The apex and lateral (i.e., conical) areas retained a large proportion of old murein, as indicated by their heavy labeling upon immunodetection of d-Cys residues. In contrast, the large basal area seems to be made exclusively of new murein, because no d-Cys was detected by immunomicroscopy. A significant proportion of cells were apparently able to reach a rather advanced stage of a second division round and generated sacculi in which two units similar to the one described above were connected by the wide ends. The connection was often displaced to one side instead of being central as previously described (3, 4). In these sacculi, segregation of old murein in each half-cell followed a pattern similar to the one described above for single cells, resulting in sacculi with two large areas of labeled murein at the former poles separated by a large area of all-new murein divided by a furrow. Therefore, inhibition of PBP2 by amdinocillin results in a modified pattern of murein segregation. In this pattern, most or all of the old murein is conserved in two equal domains corresponding to the two hemispheres of the original cell and two all-new half-cells are formed between them to generate a pair of sister cells. Thus, each sister cell consists of two equal hemispheres, one of which consists of all-new murein and the other of which consists of conserved old murein. This is the classical pattern for natural coccal species such as Enterococcus faecalis (24), in which growth consists entirely of the formation of new cell halves. In Enterococcus and, we believe, in coccal mutants of E. coli, cell division (i.e., the formation of new cell halves) is synonymous with cell growth.
According to the measurements of the numbers of gold grains in sacculi at the end of the labeling period and after two mass doublings in the presence of amdinocillin, most of the old murein seems to be conserved. Indeed, as cells are able to go through one division event, the amount of grains per sacculus should be around one-half of the value at the initiation of the chase, which is remarkably similar to the actual values. Therefore, recycling seems to have been reduced to a minimum under these conditions. If this were not so, a significant part of the old, labeled murein should have been lost (about one-half, assuming a moderate turnover rate of 30% per generation) (23, 30, 42). The reduction in murein recycling is also consistent with the interpretation that round cells consist entirely of septal murein. As previously reported, septal (polar) murein turns over extremely slowly, if at all (16, 33).
Because PBP2 is the target for amdinocillin, murein segregation was also studied with the pbpA(Ts) mutant SP4500. However, it is important to realize that the segregation patterns for amdinocillin-treated cells and the pbpA(Ts) mutant cannot be directly compared because of the very different experimental conditions used. Amdinocillin-treated cells were in a morphological transition to rounded forms and were the result of a single division event. In contrast, the pbpA(Ts) mutant was spherical, showed a large dispersion in cell size, and could divide more than once, often in an asymmetric way (Fig. 3B). Because pbpA(Ts) cells are spherical during the labeling period, no “polar” regions can be defined. Evaluation of the number of divisions a cell has gone through is also uncertain. Dividing cells are very disperse in size, and asymmetric divisions generate an unequal distribution of old and new murein between daughter cells. Nevertheless, old (labeled) and new murein segregated in all instances as large, well-delimited domains on the surface of the sacculi, which supports a zonal mode of murein synthesis. This result is consistent with the observations in amdinocillin-treated cells and reinforces the idea that impairment of PBP2 activity permanently blocks the diffuse mode of murein growth responsible for cell wall elongation.
When the rodA(Ts) mutant SP5211 is transferred to restrictive conditions, the cells undergo a morphological change similar to that observed in amdinocillin-treated wild-type cells (51). However, acquisition of the round shape is more gradual, possibly because division continues in spite of cell deformation. During the first doubling in cell mass at the restrictive temperature, murein segregation was similar to that in the wild type or in the mutant itself at 30°C, except that cells were slightly more rounded (Fig. 4B). However, upon further incubation, sacculi became progressively more rounded and had a distribution of label similar to the ones observed in amdinocillin-treated cells. Most sacculi had large areas where new and old murein did not intermix and therefore appeared as discrete labeled and unlabeled regions (Fig. 4C).
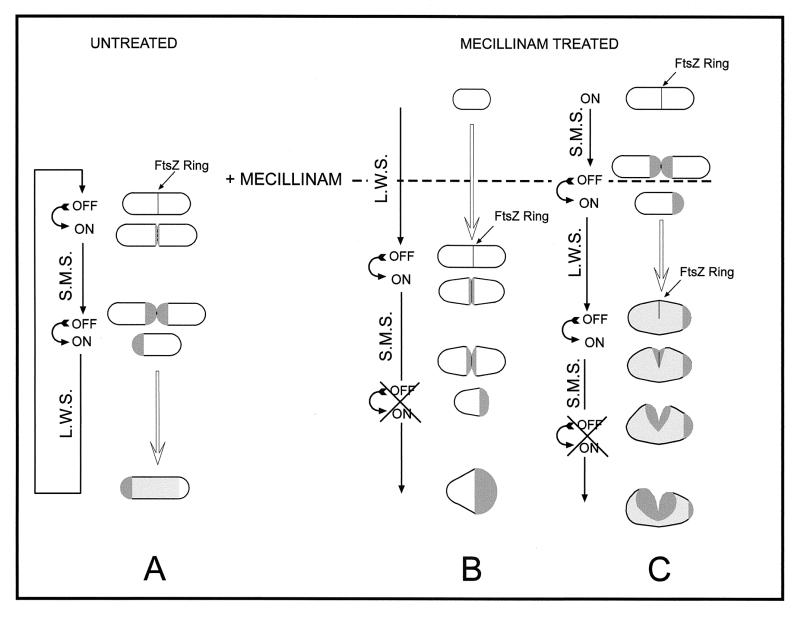
The observed patterns of segregation and morphological evolution suggest the following model. In normal cells (Fig. 6A), septation would occur by the FtsZ-dependent activation of SMS at the potential division site and the concomitant inhibition of lateral wall murein synthesis. Upon septum completion, SMS is shut off, murein at the new poles becomes metabolically inert, and PBP2-promoted lateral wall murein synthesis proceeds until the next division cycle by diffuse incorporation of precursors all over the cylindrical surface of the sacculus (8, 10, 12, 21). The consequences of amdinocillin treatment depend on the age of individual cells at the time of drug addition. Elongating but nondividing cells (Fig. 6B) complete the ongoing period of lateral wall synthesis, SMS is triggered, and septation proceeds to completion. However, inhibition of PBP2 activity prevents SMS from being switched off. Because the SMS system is localized and makes inert murein, large areas of all-new murein are generated at the new polar regions. Incidentally, a molecular interaction between RodA and the septal peptidoglycan synthetase PBP3 has been proposed on the basis of genetic complementation experiments (5). Cells that are dividing at the time of drug addition (Fig. 6C) might be able to switch from septal to lateral wall synthesis before amdinocillin actually blocks PBP2 activity. Lateral wall expansion is started, but because of PBP2 inactivation, the regular rod shape cannot be properly maintained. Cells increase in diameter, and FtsZ assembles as incomplete rings, which are nevertheless competent to trigger SMS (1). The septation event is started as a lateral furrow, which grows into a bilobed surface of new murein for an undefined period of time, generating the peculiar morphology and segregation pattern shown in Fig. 1C2. Thermosensitive mutations in pbpA and rodA or null mutations in rodA would generate spherical cells by essentially the same mechanism. That is, they would generate such cells by suppressing lateral wall murein synthesis and keeping SMS permanently on. In these mutant spherical cells, each round of SMS would end with the completion of the new hemispherical sections and, in the absence of the system for lateral wall synthesis, be succeeded by the immediate initiation of a new round of SMS. Thus, sacculus growth and cell division would continue essentially in the same way as in natural gram-negative cocci such as Neisseria spp. (59).
FIG. 6.
Diagrammatic representation of the generation of rounded cells by the action of amdinocillin (mecillinam). Dark gray areas show newly synthesized septal murein; Light gray areas show newly made lateral wall murein. L.W.S., lateral wall murein synthesis.
Septation in round E. coli cells, as in Neisseria, is an asymmetric process (3, 4, 59, 64, 65). The analysis of sacculi reported here shows that septal invagination in spherical sacculi starts as a lateral furrow that produces a two-lobed sacculus. The lateral furrow then progresses inwards, making the two lobes deeper until eventually two new spherical cells are released. An essentially identical series of events has been proposed on the basis of cell morphology and FtsZ ring formation in spherical cells (1, 64).
Previous studies of the growth and division of rodA° mutants showed that the lack of RodA protein leads to generation of spherical, viable cells, which divided in alternate perpendicular planes (3, 4). The results of murein segregation experiments in such a strain were consistent with both the proposed mode of cell division and with the model proposed for growth of the sacculus upon disturbance of the PPB2-RodA system. Based on the assumptions that (i) division planes were central and alternated by 90° and (ii) murein synthesis would take place exclusively at the division furrow to generate two new hemispherical half-sacculi (Fig. 5A), cells that had gone through one division during the chase period should have sacculi consisting of sharply delimited labeled and unlabeled halves. Likewise, sacculi from cells that have divided twice after removal of d-Cys should consist of a 90° sector of labeled murein and a 270° sector of all-new murein. Sacculi with the expected distributions of new and old murein were indeed frequently found after appropriate chase times (Fig. 5), a result strongly supporting both assumptions.
To summarize, our segregation studies indicate that impairment of PPB2 or RodA function leads to inactivation of cylindrical sacculus extension and permanent activation of the septal murein biosynthetic system, which is normally activated for only part of each cell cycle.
ACKNOWLEDGMENTS
This work was supported by grant PM97–0148 from the PGC, a grant from the CSIC-MPG bilateral cooperation program, and an institutional grant from Fundación Ramón Areces to M.A.D.P.
Footnotes
This paper is dedicated to J. de la Rosa for the many years of hard work that she has dedicated to our group at Centro de Biología Molecular “Severo Ochoa.”
REFERENCES
- 1.Addinall S G, Lutkenhaus J. FtsZ-spirals and -arcs determine the shape of the invaginating septa in some mutants of Escherichia coli. Mol Microbiol. 1996;22:231–237. doi: 10.1046/j.1365-2958.1996.00100.x. [DOI] [PubMed] [Google Scholar]
- 2.Ayala J A, Garrido T, de Pedro M A, Vicente M. Molecular biology of bacterial septation. In: Ghuysen J M, Hakenbeck R, editors. Bacterial cell wall. Amsterdam, The Netherlands: Elsevier; 1994. pp. 73–101. [Google Scholar]
- 3.Begg K J, Donachie W D. Cell shape and division in Escherichia coli: experiments with shape and division mutants. J Bacteriol. 1985;163:615–622. doi: 10.1128/jb.163.2.615-622.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Begg K J, Donachie W D. Division planes alternate in spherical cells of Escherichia coli. J Bacteriol. 1998;180:2564–2567. doi: 10.1128/jb.180.9.2564-2567.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Begg K J, Spratt B G, Donachie W D. Interaction between membrane proteins PBP3 and RodA is required for normal cell shape and division in Escherichia coli. J Bacteriol. 1986;167:1004–1008. doi: 10.1128/jb.167.3.1004-1008.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Begg K J, Takasuga A, Edwards D H, Dewar S J, Spratt B G, Adachi H, Ohta T, Matsuzawa H, Donachie W D. The balance between different peptidoglycan precursors determines whether Escherichia coli cells will elongate or divide. J Bacteriol. 1990;172:6697–6703. doi: 10.1128/jb.172.12.6697-6703.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bi E F, Lutkenhaus J. FtsZ ring structure associated with division in Escherichia coli. Nature. 1991;354:161–164. doi: 10.1038/354161a0. [DOI] [PubMed] [Google Scholar]
- 8.Botta G A, Park J T. Evidence for involvement of penicillin-binding protein 3 in murein synthesis during septation but not during cell elongation. J Bacteriol. 1981;145:333–340. doi: 10.1128/jb.145.1.333-340.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyle D S, Khattar M M, Addinall S G, Lutkenhaus J, Donachie W D. ftsW is an essential cell-division gene in Escherichia coli. Mol Microbiol. 1997;24:1263–1273. doi: 10.1046/j.1365-2958.1997.4091773.x. [DOI] [PubMed] [Google Scholar]
- 10.Burman L G, Raichler J, Park J T. Evidence for diffuse growth of the cylindrical portion of the Escherichia coli murein sacculus. J Bacteriol. 1983;155:983–988. doi: 10.1128/jb.155.3.983-988.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Canepari P, Botta G, Satta G. Inhibition of lateral wall elongation by mecillinam stimulates cell division in certain cell division conditional mutants of Escherichia coli. J Bacteriol. 1984;157:130–133. doi: 10.1128/jb.157.1.130-133.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Canepari P, Di Stefano C F, Lleo M M, Satta G. Peptidoglycan synthesis and its fine chemical composition in dividing and not dividing Klebsiella pneumoniae cocci. New Microbiol. 1993;16:165–170. [PubMed] [Google Scholar]
- 13.Canepari P, Signoretto C, Boaretti M, Del Mar L. Cell elongation and septation are two mutually exclusive processes in Escherichia coli. Arch Microbiol. 1997;168:152–159. doi: 10.1007/s002030050481. [DOI] [PubMed] [Google Scholar]
- 14.Cooper S. Bacterial growth and division. San Diego, Calif: Academic Press, Inc.; 1991. [Google Scholar]
- 15.Denome S A, Elf P K, Henderson T A, Nelson D E, Young K D. Escherichia coli mutants lacking all possible combinations of eight penicillin binding proteins: viability, characteristics, and implications for peptidoglycan synthesis. J Bacteriol. 1999;181:3981–3993. doi: 10.1128/jb.181.13.3981-3993.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Pedro M A, Quintela J C, Höltje J-V, Schwarz H. Murein segregation in Escherichia coli. J Bacteriol. 1997;179:2823–2834. doi: 10.1128/jb.179.9.2823-2834.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donachie W D. Relationship between cell size and time of initiation of DNA replication. Nature. 1968;219:1077–1079. doi: 10.1038/2191077a0. [DOI] [PubMed] [Google Scholar]
- 18.Donachie W D. The cell cycle of Escherichia coli. Annu Rev Microbiol. 1993;47:199–230. doi: 10.1146/annurev.mi.47.100193.001215. [DOI] [PubMed] [Google Scholar]
- 19.Donachie W D, Addinall S, Begg K. Cell shape and chromosome partition in prokaryotes, or why E. coli is rod-shaped and haploid. Bioessays. 1995;17:569–576. doi: 10.1002/bies.950170616. [DOI] [PubMed] [Google Scholar]
- 20.Donachie W D, Begg K J, Vicente M. Cell length, cell growth and cell division. Nature. 1976;264:328–333. doi: 10.1038/264328a0. [DOI] [PubMed] [Google Scholar]
- 21.Garcia del Portillo F, de Pedro M A. Penicillin-binding protein 2 is essential for the integrity of growing cells of Escherichia coli ponB strains. J Bacteriol. 1991;173:4530–4532. doi: 10.1128/jb.173.14.4530-4532.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goffin C, Fraipont C, Ayala J, Terrak M, Nguyen-Distèche M, Ghuysen J-M. The non-penicillin-binding module of the tripartite penicillin-binding protein 3 of Escherichia coli is required for folding and/or stability of the penicillin-binding module and the membrane-anchoring module confers cell septation activity on the folded structure. J Bacteriol. 1996;178:5402–5409. doi: 10.1128/jb.178.18.5402-5409.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goodell E W. Recycling of murein by Escherichia coli. J Bacteriol. 1985;163:305–310. doi: 10.1128/jb.163.1.305-310.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higgins M L, Shockman G D. Study of a cycle of cell wall assembly in Streptococcus faecalis by three-dimensional reconstructions of thin sections of cells. J Bacteriol. 1976;127:1346–1358. doi: 10.1128/jb.127.3.1346-1358.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Höltje J V, Schwarz U. Biosynthesis and growth of the murein sacculus. In: Nanninga N, editor. Molecular cytology of Escherichia coli. New York, N.Y: Academic Press, Inc.; 1985. pp. 77–119. [Google Scholar]
- 26.Ishino F, Park W, Tomioka S, Tamaki S, Takase I, Kunugita K, Matsuzawa H, Asoh S, Ohta T, Spratt B G. Peptidoglycan synthetic activities in membranes of Escherichia coli caused by overproduction of penicillin-binding protein 2 and rodA protein. J Biol Chem. 1986;261:7024–7031. [PubMed] [Google Scholar]
- 27.Ishino F, Tamaki S, Spratt B G, Matsuhashi M. A mecillinam-sensitive peptidoglycan crosslinking reaction in Escherichia coli. Biochem Biophys Res Commun. 1982;109:689–696. doi: 10.1016/0006-291x(82)91995-7. [DOI] [PubMed] [Google Scholar]
- 28.Iwaya M, Goldman R, Tipper D J, Feingold B, Strominger J L. Morphology of an Escherichia coli mutant with a temperature-dependent round cell shape. J Bacteriol. 1978;136:1143–1158. doi: 10.1128/jb.136.3.1143-1158.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iwaya M, Jones C W, Khorana J, Strominger J L. Mapping of the mecillinam-resistant, round morphological mutants of Escherichia coli. J Bacteriol. 1978;133:196–202. doi: 10.1128/jb.133.1.196-202.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacobs C, Huang L J, Bartowsky E, Normark S, Park J T. Bacterial cell wall recycling provides cytosolic muropeptides as effectors for beta-lactamase induction. EMBO J. 1994;13:4684–4694. doi: 10.1002/j.1460-2075.1994.tb06792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.James R, Haga J Y, Pardee A B. Inhibition of an early event in the cell division cycle of Escherichia coli by FL1060, an amidinopenicillanic acid. J Bacteriol. 1975;122:1283–1292. doi: 10.1128/jb.122.3.1283-1292.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khattar M M, Begg K J, Donachie W D. Identification of FtsW and characterization of a new ftsW division mutant of Escherichia coli. J Bacteriol. 1994;176:7140–7147. doi: 10.1128/jb.176.23.7140-7147.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koch A L, Woldringh C L. The metabolic inertness of the pole wall of a Gram negative rod. J Theor Biol. 1994;171:415–425. [Google Scholar]
- 34.Lennox E S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955;1:190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- 35.Lutkenhaus J. FtsZ ring in bacterial cytokinesis. Mol Microbiol. 1993;9:403–409. doi: 10.1111/j.1365-2958.1993.tb01701.x. [DOI] [PubMed] [Google Scholar]
- 36.Matsuhashi M. Utilization of lipid-linked precursors and the formation of peptidoglycan in the process of cell growth and division: membrane enzymes involved in the final steps of peptidoglycan synthesis and the mechanism of their regulation. In: Ghuysen J M, Hakenbeck R, editors. Bacterial cell wall. Amsterdam, The Netherlands: Elsevier; 1994. pp. 55–102. [Google Scholar]
- 37.Matsuhashi M, Kamiryo T, Blumberg P M, Linnet P, Willoughby E, Strominger J L. Mechanism of action and development of resistance to a new amidino penicillin. J Bacteriol. 1974;117:578–587. doi: 10.1128/jb.117.2.578-587.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsuhashi M, Wachi M, Ishino F. Machinery for cell growth and division: penicillin-binding proteins and other proteins. Res Microbiol. 1990;141:89–103. doi: 10.1016/0923-2508(90)90101-u. [DOI] [PubMed] [Google Scholar]
- 39.Nanninga N. Cell division and peptidoglycan assembly in Escherichia coli. Mol Microbiol. 1991;5:791–795. doi: 10.1111/j.1365-2958.1991.tb00751.x. [DOI] [PubMed] [Google Scholar]
- 40.Nanninga N, Woldringh C L. Cell growth, genome duplication and cell division. In: Nanninga N, editor. Molecular cytology of Escherichia coli. New York, N.Y: Academic Press, Inc.; 1985. pp. 259–318. [Google Scholar]
- 41.Nguyen-Disteche M, Fraipont C, Buddelmeijer N, Nanninga N. The structure and function of Escherichia coli penicillin-binding protein 3. Cell Mol Life Sci. 1998;54:309–316. doi: 10.1007/s000180050157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park J T. Why does Escherichia coli recycle its cell wall peptides? Mol Microbiol. 1995;17:421–426. doi: 10.1111/j.1365-2958.1995.mmi_17030421.x. [DOI] [PubMed] [Google Scholar]
- 43.Park J T, Burman L. FL-1060: a new penicillin with a unique mode of action. Biochem Biophys Res Commun. 1973;51:863–868. doi: 10.1016/0006-291x(73)90006-5. [DOI] [PubMed] [Google Scholar]
- 44.Prats R, de Pedro M A. Normal growth and division of Escherichia coli with a reduced amount of murein. J Bacteriol. 1989;171:3740–3745. doi: 10.1128/jb.171.7.3740-3745.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodriguez M C, de Pedro M A. Initiation of growth in pbpAts and rodAts mutants of Escherichia coli. FEMS Microbiol Lett. 1990;60:235–239. doi: 10.1016/0378-1097(90)90378-4. [DOI] [PubMed] [Google Scholar]
- 46.Schwarz U, Ryter A, Rambach A, Hellio R, Hirota Y. Process of cellular division in Escherichia coli: differentiation of growth zones in the sacculus. J Mol Biol. 1975;98:749–759. doi: 10.1016/s0022-2836(75)80008-8. [DOI] [PubMed] [Google Scholar]
- 47.Signoretto C, Di Stefano F, Canepari P. Modified peptidoglycan chemical composition in shape-altered Escherichia coli. Microbiology. 1996;142:1919–1926. doi: 10.1099/13500872-142-8-1919. [DOI] [PubMed] [Google Scholar]
- 48.Spratt B G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci USA. 1975;72:2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spratt B G. Properties of the penicillin-binding proteins of Escherichia coli K12. Eur J Biochem. 1977;72:341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- 50.Spratt B G. The mechanism of action of mecillinam. J Antimicrob Chemother. 1977;3(Suppl. B):13–19. doi: 10.1093/jac/3.suppl_b.13. [DOI] [PubMed] [Google Scholar]
- 51.Spratt B G, Boyd A, Stoker N. Defective and plaque-forming lambda transducing bacteriophage carrying penicillin-binding protein-cell shape genes: genetic and physical mapping and identification of gene products from the lip-dacA-rodA-pbpA-leuS region of the Escherichia coli chromosome. J Bacteriol. 1980;143:569–581. doi: 10.1128/jb.143.2.569-581.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spratt B G, Cromie K D. Penicillin-binding proteins of gram-negative bacteria. Rev Infect Dis. 1988;10:699–711. doi: 10.1093/clinids/10.4.699. [DOI] [PubMed] [Google Scholar]
- 53.Stoker N G, Broome-Smith J K, Edelman A, Spratt B G. Organization and subcloning of the dacA-rodA-pbpA cluster of cell shape genes in Escherichia coli. J Bacteriol. 1983;155:847–853. doi: 10.1128/jb.155.2.847-853.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun Q, Margolin W. FtsZ dynamics during the division cycle of live Escherichia coli cells. J Bacteriol. 1998;180:2050–2056. doi: 10.1128/jb.180.8.2050-2056.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tamaki S, Matsuzawa H, Matsuhashi M. Cluster of mrdA and mrdB genes responsible for the rod shape and mecillinam sensitivity of Escherichia coli. J Bacteriol. 1980;141:52–57. doi: 10.1128/jb.141.1.52-57.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Heijenoort Y, Gomez M, Derrien M, Ayala J, van Heijenoort J. Membrane intermediates in the peptidoglycan metabolism of Escherichia coli: possible roles of PBP 1b and PBP 3. J Bacteriol. 1992;174:3549–3557. doi: 10.1128/jb.174.11.3549-3557.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang L, Khattar M K, Donachie W D, Lutkenhaus J. FtsI and FtsW are localized to the septum in Escherichia coli. J Bacteriol. 1998;180:2810–2816. doi: 10.1128/jb.180.11.2810-2816.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weiss D S, Chen J C, Ghigo J-M, Boyd D, Beckwith J. Localization of FtsI (PBP3) to the septal ring requires its membrane anchor, the Z ring, FtsA, FtsQ, and FtsL. J Bacteriol. 1999;181:508–520. doi: 10.1128/jb.181.2.508-520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Westling-Häggström B, Elmros T, Normark S, Winblad B. Growth pattern and cell division in Neisseria gonorrhoeae. J Bacteriol. 1977;129:333–342. doi: 10.1128/jb.129.1.333-342.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wientjes F B, Nanninga N. Rate and topography of peptidoglycan synthesis during cell division in Escherichia coli: concept of a leading edge. J Bacteriol. 1989;171:3412–3419. doi: 10.1128/jb.171.6.3412-3419.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wientjes F B, Nanninga N. On the role of the high molecular weight penicillin-binding proteins in the cell cycle of Escherichia coli. Res Microbiol. 1991;142:333–344. doi: 10.1016/0923-2508(91)90049-g. [DOI] [PubMed] [Google Scholar]
- 62.Wientjes F B, Pas E, Taschner P E M, Woldringh C L. Kinetics of uptake and incorporation of meso-diaminopimelic acid in different Escherichia coli strains. J Bacteriol. 1985;164:331–337. doi: 10.1128/jb.164.1.331-337.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Woldringh C L, Huls P, Pas E, Brakenhoff G H, Nanninga N. Topography of peptidoglycan synthesis during elongation and polar cap formation in a cell division mutant of Escherichia coli. J Gen Microbiol. 1987;133:575–586. [Google Scholar]
- 64.Zaritsky A, Van Geel A, Fishov I, Pas E, Einav M, Woldringh C L. Visualizing multiple constrictions in spheroidal Escherichia coli cells. Biochimie. 1999;81:897–900. doi: 10.1016/s0300-9084(99)00206-0. [DOI] [PubMed] [Google Scholar]
- 65.Zaritsky A, Woldringh C L, Fishov I, Vischer N O E, Einav M. Varying division planes of secondary constrictions in spheroidal Escherichia coli cells. Microbiology. 1999;145:1015–1022. doi: 10.1099/13500872-145-5-1015. [DOI] [PubMed] [Google Scholar]