Abstract
Introduction:
Transmitted by ticks, Lyme disease is the most common vector-borne disease in the Northern hemisphere. Despite the geographical expansion of human Lyme disease cases, no effective preventive strategies are currently available. Developing an efficacious and safe vaccine is therefore urgently needed. Efforts have previously been taken to identify vaccine targets in the causative pathogen (Borrelia burgdorferi sensu lato) and arthropod vector (Ixodes spp.). However, progress was impeded due to a lack of consumer confidence caused by the myth of undesired off-target responses, low immune responses, a limited breadth of immune reactivity, as well as by the complexities of the vaccine process development.
Area covered:
In this review, we summarize the antigen engineering approaches that have been applied to overcome those challenges and the underlying mechanisms that can be exploited to improve both safety and efficacy of future Lyme disease vaccines.
Expert opinion:
Over the past two decades, several new genetically redesigned Lyme disease vaccine candidates have shown success in both preclinical and clinical settings and built a solid foundation for further development. These studies have greatly informed the protective mechanisms of reducing Lyme disease burdens and ending the endemic of this disease.
Keywords: Antigen design, Borrelia, genetic engineering, Lyme borreliae
1. Introduction
Lyme disease is the most common vector-borne disease in the Northern hemisphere [1,2]. Recent studies estimate approximately 476,000 people annually in the United States with the disease whereas 230,000 estimated human cases are found each year in Western Europe [3,4]. Lyme disease is spread by the bite of Ixodid ticks carrying spirochete bacteria, Borrelia burgdorferi sensu lato (also known as Borreliella burgdorferi sensu lato or Lyme borreliae) [2]. This species complex includes B. burgdorferi sensu stricto (hereafter B. burgdorferi), the human invasive species prevalent in both Eurasia and North America, and B. afzelii, B. garinii, B. spielmanii, and B. bavariensis, which are found in Eurasia [2]. Following a tick bite, while some spirochete strains do not cause systemic infection, others efficiently disseminate from the bite site through the skin and into the bloodstream to colonize distal tissues and organs, leading to severe systemic manifestations (i.e. arthritis, carditis, and neuroborreliosis) [2,5,6] (Figure 1). In the United States, for unknown reasons, a small population of these Lyme disease patients experiences persistent arthritic symptoms known as antibiotic-refractory Lyme arthritis even after both oral and intravenous antibiotic treatment [7]. Further, through similar tick feeding-mediated and hematogenous spreading mechanisms, some wild animals, such as small mammals, passerine birds, and reptiles, can also be infected by Lyme borreliae [8,9]. However, as reservoir hosts in nature, these animals were found to maintain the spirochetes persistently without developing manifestations [10]. The complexity of spirochetes interacting with ticks, humans, and reservoir hosts has been a significant challenge in the eradication of Lyme borreliae (Figure 1) [11].
Figure 1. The infection cycle of Lyme borreliae.
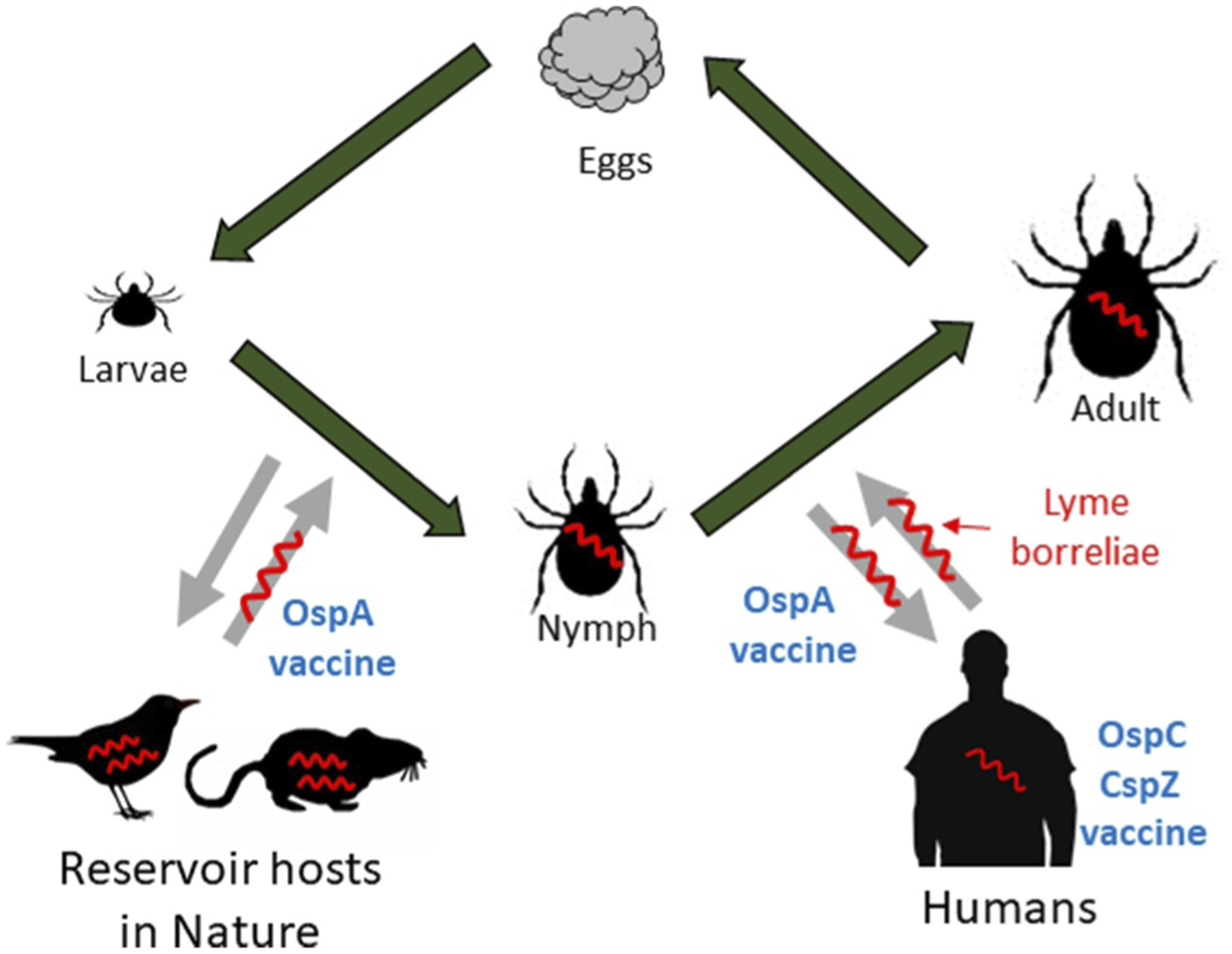
Lyme borreliae (shown in red) cannot be transovarially transmitted between ticks. The direction of arrows indicates the acquisition or transmission of Lyme borreliae through a blood meal. Though nymphs could also feed on reservoirs, and adults could also feed on humans, they are not shown here for brevity. The stages in the infection cycle that OspA-, OspC-, and CspZ-based vaccines target are shown in blue.
1.1. Outer surface protein A (OspA)
OspA is one of the best-studied Lyme borreliae preventative protein targets [12]. Its expression is upregulated when spirochetes reside in unfed ticks and downregulated after ticks feed on hosts [13–15], consistent with its essential role in promoting bacterial attachment to tick midguts and tick-to-host transmission of spirochetes [16–18]. In line with those findings, vaccinating with OspA or passively inoculating with anti-OspA antibodies has been documented to prevent spirochete migrating from mice to ticks as well as transmission from ticks to mice [17,19] (Figure 1, Table 1). Such findings in mice have also been recapitulated in two independent clinical trials [20,21], allowing the development of the first and only licensed Lyme disease vaccine (LYMErix) in 1998 by SmithKline Beecham (now GlaxoSmithKline) [12]. Regrettably, Gross et al. then reported that the sequences from OspA are partially homologous to those of a motif on a human leukocyte function-associated antigen-1 (hLFA-1) (Figure 2(a)) [22]. Based on a moderate degree of sequence homology, they proposed a molecular mimicry hypothesis that Lyme arthritis was driven via a mechanism where human T cells reacting to those OspA sequences would also recognize hLFA-1 motifs after B. burgdorferi infection [22]. That proposed concept raised concerns that OspA immunization could trigger T cell autoimmunity. However, it was demonstrated that sequence homology does not predict T cell receptor binding and that T cell receptors recognize epitopes based on conformation, not sequence [23,24]. Later studies also showed that T cell receptors are promiscuous, and that T cell cross-recognition alone does not automatically pose the risk of autoimmunity [25]. This conclusion is also supported by the observation that the immune responses induced by LYMErix do not replicate any Lyme disease-associated manifestations [26]. However, the loss of confidence from the general public led to the withdrawal of the vaccine from the market in 2002 (see [12] for details of OspA vaccine development).
Table 1.
The efficacy of Lyme borreliae antigens that have been tested as vaccines and the the phenotypes of Lyme borreliae strains deficient of each of these antigens in vivo.
| Tested Lyme borreliae antigens | The phenotypes of Lyme borreliae deficient of the antigen | Efficacy as vaccines preclinically against spirochetes introduced via | Reference | |
|---|---|---|---|---|
| Needle infection | Tick infection | |||
| OspA | No tick-to-mouse transmission after tick infectiona | No spirochete colonization at tissues and no Lyme disease-associated manifestationsd | No spirochete colonization at tissues and no Lyme disease-associated manifestations | [27–30] |
| OspC | No tick-to-mouse transmission. No colonization at initial infection sites and no dissemination in mice after tick or needle infectionb | No spirochete colonization at tissues and no Lyme disease-associated manifestations | No spirochete colonization. | [18,31,32,55–57,86] |
| CspZ/CspZ-YA | No defect of mouse infectivity after needle infection. No defect of bacteria acquired by ticks from mice. Reduced colonization at initial and distal sites of mice after needle infection with blood-treated spirochetes. | For CspZ: Spirochete colonization at tissues and arthritis remains, no different from the unvaccinated groups. For CspZ-YA: No spirochete colonization and arthritis | For CspZ: Spirochete colonization at tissues and arthritis remains, no different from the unvaccinated groups. For CspZ-YA: No spirochete colonization and arthritis | [76,78,80,119,149] |
| BB0172 | n.a.c | Reduced colonization only at low doses of needle infection. Arthritis remains, no different from the unvaccinated groups. | n.a. | [89] |
| BB0405 | Reduced colonization at initial and distal sites of mice after needle infection. Reduced levels of invasion of the salivary gland in feeding nymphs. No tick-to-mouse transmission after tick infection | n.a. | Reduced colonization at distal tissues after B. burgdorferi infection. No cross-protection to other species of Lyme borreliae. | [33,82] |
| BBA52 | Reduced levels of colonization in feeding nymphs and initial and distal sites of mice after tick infection. | n.a. | Reduced colonization of mouse tissues | [34,81] |
| BBI39 paralogs | n.a. | n.a. | Reduced colonization at feeding nymphs and mouse tissues | [83] |
| BBK32 | No defect of tick-to-mouse transmission and mouse infectivity after tick infection. No defect of mouse infectivity after needle infection of spirochetes at high doses. Reduced levels of colonization after needle infection of spirochetes at low doses | Reduced colonization at tissues when co-immunized with OspC and DbpA. | n.a. | [35,36,87] |
| DbpA | No defect of tick-to-mouse transmission and mouse infectivity after tick infection. Reduced levels of colonization after needle infection of spirochetes at low doses. No colonization at initial infection sites and no dissemination in mice after needle infection of spirochete at low doses. | No spirochete colonization. | Spirochete colonization at mouse tissues remains, no different from the unvaccinated groups. | [37–41,88] |
| RevA | Reduced colonization of the heart after needle infection. | Spirochete colonization at mouse tissues remains, no different from the unvaccinated groups. | Spirochete colonization at mouse tissues remains, no different from the unvaccinated groups. | [42,43] |
Lyme borreliae infection through the feeding of nymphs carrying Lyme borreliae
Lyme borreliae infection through intradermal inoculation of Lyme borreliae
No results available.
Arthritis and/or carditis
Figure 2. OspA-based vaccines in recent clinical trials.
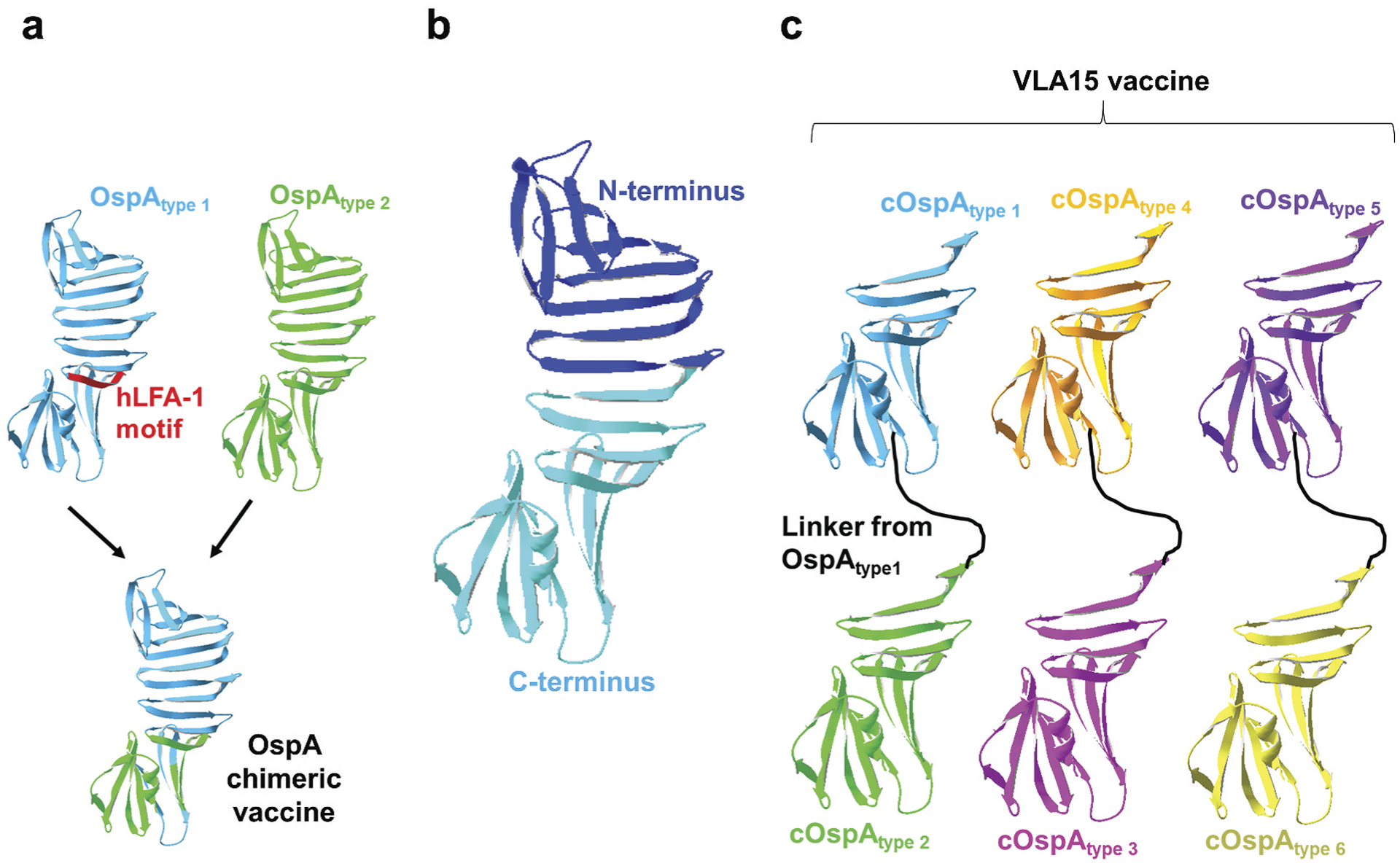
(a) Shown is the OspA chimeric vaccine from OspA variants of Borrelia burgdorferi (for OspA type 1) and B. afzelii (for OspA type 2) with the elimination of the autoimmune motif on OspA type 1 (human LFA-1 motif; hLFA-1 motif). (b) N-terminal (dark blue) and C-terminal (light blue) regions of OspA type 1 comprising amino acids 1 to 272 and 131 to 273, respectively, as defined in [133]. (c) Shown is the VLA15 vaccine that contains C-terminal OspA variants from any two of the six most commonly found human infectious OspA types of Lyme borreliae (OspA type 1 and 2, 3 and 4, and 5 and 6) connected with a linker from the sequences of OspA type 1.
Researchers have also proposed the concept of immunizing reservoir animals with OspA to prevent these hosts from carrying the spirochetes, reducing the risk of human exposure to Lyme borreliae [44–48] (Figure 1). Testing of this concept has already been successful [49–52] with one study showing that when OspA was subcutaneously introduced into wild-caught white-footed mice (Peromyscus leucopus), the most prevalent small mammal reservoir of Lyme borreliae in North America, the fraction of B. burgdorferi-carrying ticks was reduced by up to 42% within one year of releasing the vaccinated mice back into the field [53]. In another study, OspA was applied into the field as the bait for white-footed mice, leading to a 76% decline in the number of B. burgdorferi-positive ticks within five years [54], further strengthening the concept of reservoir-targeted prevention against Lyme borreliae. However, both studies showed significant site-to-site differences in the reduction of prevalence, suggesting that distinct structures of host populations among the sites may negatively impact the outcome of vaccination.
1.2. Outer surface protein C (OspC)
OspC is the other abundantly produced protein on the surface of Lyme borreliae; its expression is upregulated immediately after ticks feed and downregulated shortly after spirochetes disseminate into the bloodstream [14,15,55]. Such an expression profile is consistent with findings showing that this protein is essential for transmission to hosts, particularly for spirochete survival during the early stages of infection [18,55,56]. Supported by these roles, OspC was reported to bind to a tick salivary protein and several host ligands to promote early immune evasion and host tissue colonization [57–60]. Consistent with these, OspC vaccination or passive inoculation with anti-OspC antibodies prevented tick-to-mouse transmission [61–63] (Figure 1, Table 1). However, with as little as ~60% sequence identity among variants from different strains or species of Lyme borreliae, OspC is a highly polymorphic protein [64]. The allelic variation of OspC has been shown to cause low or no cross recognition of different variants of this protein by antibodies raised against a particular OspC variant [61]. Additionally, some protective epitopes of OspC contain polymorphic amino acids, further reducing the breadth of protection against a diverse range of Lyme borreliae species or strains [65].
1.3. Complement regulator acquiring surface protein 2 (CspZ)
As one of the innate immune defense mechanisms shared by vertebrate hosts, the complement system, once activated, is a cascade composed of several serum proteins to eradicate pathogens [66–68]. In the absence of pathogens, hosts produce a group of complement inhibitory proteins, such as Factor H (FH), to negatively modulate complement and prevent tissue damage caused by non-specific attacks [69]. Similar to other pathogens, the species, or strains of Lyme borreliae that can evade complement-mediated killing often generate a series of outer surface proteins to inhibit complement activation [70–72]. One of these proteins is the FH-binding protein, CspZ (also known as complement regulator acquiring surface protein 2 (CRASP-2) [73] (see [74] for all anti-complement proteins in Lyme borreliae). CspZ is upregulated after spirochetes invade the hosts, consistent with its role in promoting bacterial dissemination during early infection [75,76]. CspZ is highly conserved (>98% identity among different strains within individual Lyme borreliae species and >80% identity among different spirochete species) and is likely present in most human-infectious Lyme borreliae species and strains as most patients develop anti-CspZ antibodies [77,78]. While CspZ is therefore an attractive vaccine candidate (Figure 1, Table 1), sera from CspZ-immunized mice are not sufficient to protect against Lyme disease [78–80]. The investigations to enhance the efficacy of this protein are detailed in the sections 2.1 and 2.3.2.
1.4. Other Lyme borreliae proteins
In addition to OspA, OspC, and CspZ, other Lyme borreliae proteins have also been evaluated for their ability to prevent Lyme disease. Though Lyme borreliae deficient in some of these proteins have reduced levels of tick-to-host transmission, infection establishment, and/or persistence in vertebrate animals, immunization with each of these proteins did not prevent infection (Table 1) [81–88]. For other antigen candidates that appeared to have prevented the development of Lyme disease infection by needle challenge, the same could not be replicated via the physiologically more relevant infection routes of tick feeding (Table 1) [87,89]. Thus, the exact vaccine efficacy against Lyme borreliae transmitted by ticks warrants further investigations.
1.5. Tick proteins
One of the challenges in targeting spirochete proteins as a Lyme disease prevention strategy is the allelic variation of those proteins (e.g. OspC) [61]. Additionally, the tick genus (Ixodes) that carries Lyme borreliae is capable of carrying other human pathogens [90], which may not be eliminated by the vaccines targeting spirochete proteins. Therefore, a concept has been proposed to target the proteins in tick saliva, midgut, and hemolymph (ticks’ blood) and trigger the antibodies against those proteins to block the ability of ticks to acquire, maintain, and transmit Lyme borreliae (and other tickborne pathogens) [91,92]. For example, immunization with the tick gut protein, Is86, partially prevented tick feeding and reduced the levels of tick-to-host transmission of Lyme borreliae [93]. Similarly, efforts have been taken to examine transmission blocking by inoculating mice with antibodies against Ixodes tick proteins, Salp15 and TSLPI, which suppress a variety of host immune responses (i.e. anti-phagocytosis and/or complement inactivation). The reduction of B. burgdorferi burdens after transmission suggest the potential of targeting these proteins as vaccine antigen candidates against Lyme disease infection [57,94].
However, since ticks can generate multiple functionally redundant proteins, blocking individual proteins failed [91,92,95,96]. Thus, the concept of simultaneously vaccinating with several tick proteins (or the corresponding DNA or mRNA) or immunizing with a combination of tick and bacterial proteins has been examined to block spirochete transmission to the hosts [94,97–100] (Figure 1). Further, a model of ‘acquired tick immunity’ has recently been investigated as a new preventive strategy [101,102]: this model is based on the finding that certain mammalian hosts produce antibodies against some tick proteins during the initial tick feeding, and those antibodies then facilitate the resistance [103–105]. These findings raise the possibility of targeting those proteins that could induce such antibodies, resulting in acquired tick immunity to prevent Lyme borreliae transmission. This concept has been tested using several DNA, mRNA, and protein-based vaccines [97,106], and the initial success shed light on the potential of using these vaccines to combat multiple tickborne diseases, including Lyme disease. The details of the development of vaccines targeting tick proteins have been reviewed recently [92,101,107].
2. Antigen engineering to enhance vaccine efficacy: targeting immunologically subdominant epitopes
Many early generations of protein vaccines were unable to prevent infection, incapable of inducing sufficient levels of protective antibodies, and/or triggered undesired antibody responses [108]. With some of these vaccine candidates, the antibodies preferentially were raised against non-neutralizing epitopes, making other, often protective, epitopes immunologically subdominant [109]. That finding led to the concept of ‘immunodominance’ during immunization [109]. Though the molecular basis of immunodominance remains unclear, there is evidence that this phenomenon occurs during inter-clonal competition of B cells in the thymus, resulting in a hierarchy of the population for the B cells that produce the antibodies to recognize each epitope [110].
Antigen engineering is an extensively investigated strategy to enhance the protective antibody responses by altering the hierarchy of those B cell populations to change the antibody repertoires against the antigen of interest [109]. Strategically editing protective and/or non-protective epitopes has been shown to 1) magnify overall B cell responses, 2) minimize the production of unwanted ‘off-target’ antibodies, and 3) enhance the population of antibodies against protective epitopes. Here, we discuss some commonly used antigen engineering strategies with Lyme disease antigen candidates as examples to elucidate the potential underlying mechanisms of these strategies to improve vaccine efficacy.
2.1. Magnification of the humoral response through the multimeric display of antigens
The repetition of antigens or particular immunogenic epitopes has been shown to generate more robust antibody responses than individually presented antigens [111]. This finding led to the practice of conjugating the antigens of interest to carrier molecules or genetically co-produce these antigens with scaffolds (e.g. virus-like particles, liposomes, or nanoparticles) to trigger robust antibody responses, an approach known as ‘Multimeric displaying strategy’ [112–114]. This concept has been tested by covalently conjugating hepatitis B virus core protein-derived capsid-like particles with several previously reported candidates of tickborne transmission-blocking vaccines, e.g. Salp15 (tick salivary proteins 15) or tHRF (tick histamine-releasing factors) [115]. The resulting conjugates induced higher antigen-specific antibody titers than the free proteins [115]. Similarly, different types of liposomes composed of cobalt or nickel-containing phospholipids were linked to OspA or OspC, enhancing the levels and longevity of the resulting antibody response in immunized mice even preventing the infection caused by Lyme borreliae [116,117]. This antigen engineering strategy was also applied to ferritin-derived nanoparticles genetically fused with OspA. Compared to control mice and monkeys immunized with free protein, those animals vaccinated with OspA-nanoparticles generated greater levels of anti-OspA antibodies with a longer duration of the immune response and generally enhanced protection against Lyme disease infection [118].
However, this antigen engineering strategy did not always increase antibody titers, as mice immunized with a bacteriophage virus-like particle (VLP)-conjugated with CspZ yielded no higher titers of anti-CspZ antibodies than the free antigen [119]. Despite that, the ‘quality’ of protective antibodies (i.e. the levels of bactericidal activities for those antibodies) appeared to be consistently improved with the engineered proteins. This was supported by the fact that more efficient Lyme borreliae killing was seen by sera from mice immunized with liposome-conjugated OspA or VLP-conjugated CspZ, compared to the respective unconjugated proteins [116,119]. Previous findings of robust neutralizing antibodies in a nanoparticle-fused influenza antigen suggest a model where the multivalent presentation of antigens alters the protein topology and the hierarchy of each epitope-specific antibody, skewing antibody repertoires towards protective epitopes [120,121]. However, a few caveats of the multimeric displaying strategy have been noticed: the complexity of chemical conjugation can lead to inaccurate stoichiometric ratios between scaffold molecules and antigens, resulting in batch-to-batch differences in the protective reagents [119]. Moreover, including scaffold molecules as immunogens could introduce additional epitopes, resulting in interconal competition from the B cells that recognize these newly introduced scaffold molecule-generated epitopes, possibly taking priority away from desirable subdominant epitopes. Further, since some of these strategies require coupling antigens to adjuvant particles, other factors, such as coupling efficiency and additional purification steps to remove excess particles or antigens, may complicate the vaccine production process. These have to be taken into consideration when a multimeric displaying strategy is considered.
2.2. Prevention of the myth of ‘off-target’ antibodies by eliminating undesired epitopes
Some antigen epitopes may yield unwanted immune responses (i.e. antibody-mediated side effects). As the number of epitopes is expected to be proportional to size, the inter-clonal competition of larger antigen-activated B cells would be more apparent than for smaller proteins [122,123]. Thus, the concept of reducing the size or truncating the undesired epitopes has been a strategy to eliminate the side effects caused by those epitopes. For the OspA antigen from Lyme borreliae, the association of this protein with autoimmune responses was proven false (see sections 1.1 and [12] for details of OspA vaccine development). However, the efforts for antigen engineering of OspA were taken for non-scientific purposes, possibly to enhance consumers’ confidence, particularly focusing on the proposed T cell autoimmune motif (amino acids 165 to 173 of OspA from B. burgdorferi strain B31) [22] (Figure 2(a)). The concept of removing undesired epitopes was tested by generating an intact OspA eliminating this motif [118,124]. Mice vaccinated with a truncated OspA were protected from infection caused by multiple Lyme borreliae species and strains, similarly to wild-type OspA-immunized mice [124]. Further, since the C-terminal epitopes on OspA are surface accessible and associated with in vitro bactericidal and in vivo protective antibodies [125–127], the C-terminal region (amino acids 130 to 273 from a B. burgdorferi strain B31) with mutations of three additional amino acids for structural stabilization was generated [128]. This truncation did not encompass the proposed autoimmune motif and could still be recognized by OspA-specific protective antibodies. Regrettably, the C-terminal sequences of OspA are allelically variable, grouping Lyme borreliae species and strains into distinct ‘OspA types’ [129]. That finding raises the possibility of limited breadth for protectivity provided by that truncated antigen. Therefore, a chimeric protein of OspA from two different OspA types of Lyme borreliae (B. burgdorferi and B. afzelii) with the elimination of the proposed autoimmune motif was generated and proved its efficacy in blocking multiple spirochete species to spread to mice [130] (Figure 2(a)). Though not yet commercially available, the Lyme disease vaccine based on that chimeric OspA antigen yielded promising safety and efficacious results in clinical trials supported by Baxter Inc [131,132]. In another independent study to increase the breadth of OspA-based vaccines, a chimeric OspA antigen was generated to remove the proposed autoimmune motif but contain a linker to combine C-terminal OspA proteins from any two of the six most commonly found human infectious OspA types of Lyme borreliae (see Figure 2(b) to highlight the N- and C-terminal regions of OspA) [133]. The formulation of an equal ratio for three resulting bivalent OspA chimera (from OspA type 1 and 2, 3 and 4, and 5 and 6), also known as ‘VLA15,’ has been shown to protect against the infection caused by multiple OspA types of Lyme borreliae in mice [134,135] (Figure 2(c)). Supported by Velneva, Inc and Pfizer, Inc, the results from phase 1 and undergoing phase 2 clinical trials are promising, giving hope to the idea of reintroducing a new and potent human Lyme disease vaccine to the market [136].
2.3. Amplification of the antibody responses targeting preferred epitopes
2.3.1. Increasing the breadth of antibody responses by epitope grafting and resurfacing
Like other pathogens, Lyme borreliae include multiple species and strains, genotyped based on different loci that encode proteins with substantial sequence variation (e.g. OspA; with extensive C-terminal variation as mentioned above) [129], or overall polymorphism (e.g. OspC) [64]. Generating a vaccine antigen that elicits cross-reactive serum to multiple Lyme borreliae strains and species would be ideal. Simultaneously inoculating with multiple variants of a particular antigen or with multivalent, chimeric antigens has been an approach to address this challenge [130]. However, producing a vaccine with multiple protein components and/or multivalent proteins would significantly increase the complexity of chemical manufacturing controls. Moreover, new species and strains of Lyme borreliae are continuously found, leading to the need of adding more variants to the vaccine regimens, thus further increasing manufacturing complexity. Therefore, the concept of grafting the protective epitopes from multiple variants into one protein and/or altering the location of epitopes on the resulting antigen to enhance their surface accessibility has been proposed as one of the engineering strategies to amplify those antibodies that target preferred epitopes and maintain the breadth of the immune response [137]. In a test-of-concept study, multiple C-terminus-targeting monoclonal antibodies to OspA were successfully applied to identify surface-accessible epitopes [138]. That information then allowed exchanging the amino acids of these epitopes on a scaffold OspA variant to the equivalent amino acids from several other OspA variants, resulting in a single OspA protein that induced broadly protective antibodies [138].
In another study, bactericidal and protective linear epitopes on loop 5 and helix 5 of the polymorphic OspC protein were identified [65,139]. Different types of OspC from human invasive Lyme borreliae strains were characterized (types A, B, K, D, E, N, I, and C) [6,65], and tetra- and octavalent chimeric antigens were generated [140,141] (Figure 3). The cross-recognition and bactericidal abilities of the resulting anti-OspC antibodies after immunization proved the feasibility of this epitope grafting concept [140–143]. The next generation of these multivalent, epitope-grafting OspC antigens has already been generated by swapping the order of loop 5 and helix 5 in some of the variants and modifying the length of the linkers [144]. That engineering resulted in several new OspC-based ‘chimeritope vaccinogens’ with epitopes from nine different OspC variants [144]. The improvement of antibody-mediated bactericidal activities and OspC recognition for each of these newly generated chimeric antigens elucidated the importance of the epitopes’ order and the length of the linkers for the overall antigenicity of the epitope grafting vaccines [144] Note that the risks of introducing immunodominant neoepitopes remain and may lead to off-target effects. However, the success of developing one of the commercially available canine Lyme disease vaccines (VANGUARD®crLyme) based on the above-mentioned potent OspC chimeritopes further suggests such an epitope grafting approach as a pivotal direction for antigen engineering [145].
Figure 3. Tetravalent and octavalent OspC chimeric vaccinogens.
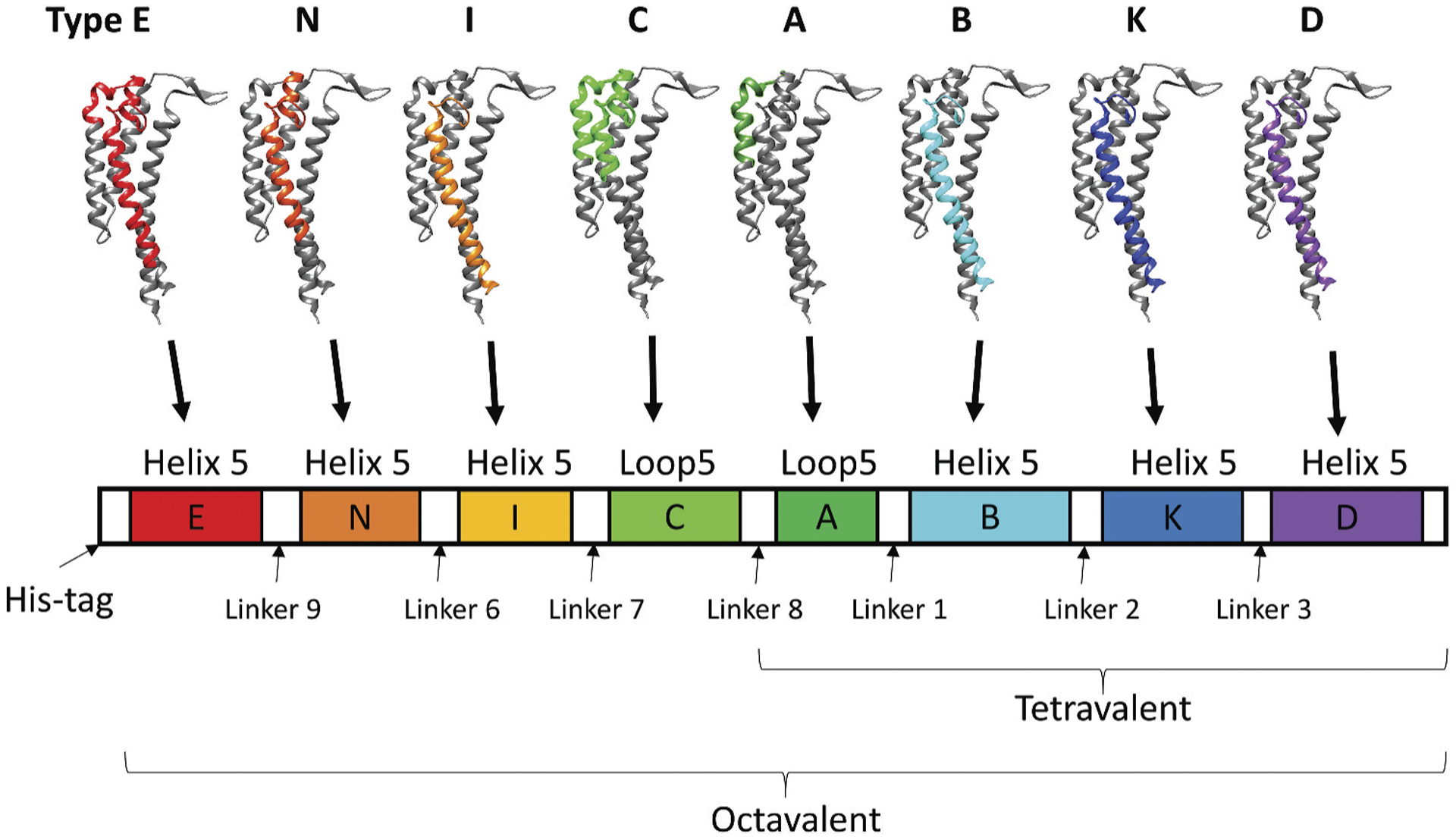
Tetravalent OspC chimeric vaccinogen contains the helix 5 or loop 5 of OspC variants from types A, B, K, and D, while octavalent OspC chimeric vaccinogen contains helix or loop 5 of OspC from types E, N, I, C, A, B, K, and D.
2.3.2. Enhancing protective antibody responses by unmasking normally occluded epitopes
One explanation for the low or null antibody response towards protective epitopes after vaccination is the structural occlusion of these epitopes. In this case, removing the factors that cause this structure hindrance could allow the alteration of the hierarchy of B cell repertoires, increasing the levels of antibodies that recognize protective and immunologically subdominant epitopes [146]. This concept has also been demonstrated by eliminating the glycans that mask the protective epitopes of several viral antigens to cause elevated protective antibody responses [147,148]. In Lyme borreliae, bactericidal antibody titers induced after vaccination of mice with wild-type CspZ protein (Figure 4(a)) were not sufficient to protect mice from bacterial colonization and Lyme disease-associated manifestations [78,80,119,149]. However, mutating two amino acids essential for the ability of CspZ to bind to its binding partner, FH, resulted in the removal of the FH-binding activity of this protein [119,149] (Figure 4(b)). The mutated CspZ-YA protein protected mice from infection with different species and strains of Lyme borreliae [119,149]. Though the titers of the anti-CspZ antibodies from CspZ-YA-immunized mice are comparable with those from CspZ-immunized mice, the former displayed 5.5-fold greater levels of bactericidal activities [149]. As FH is present in the vertebrate blood at a high concentration (~500 μg/ml) [150], wild-type CspZ after vaccination likely binds to FH, which might mask the epitopes around CspZ. Thus, eliminating the FH-binding site may make those neoepitopes more accessible, raising the possibility that they will enhance the protective antibody response (Figure 4). Similar to the epitope grafting approach described above, this epitope unmasking approach may not always fully expose the protective epitopes. Even if the neoepitopes in the engineered proteins were protective, exposing new epitopes would introduce interclonal competition of B cells that recognize these epitopes, which does not always yield a favorable and protective antibody response
Figure 4. CspZ and CspZ-YA and the antibodies triggered by both proteins.
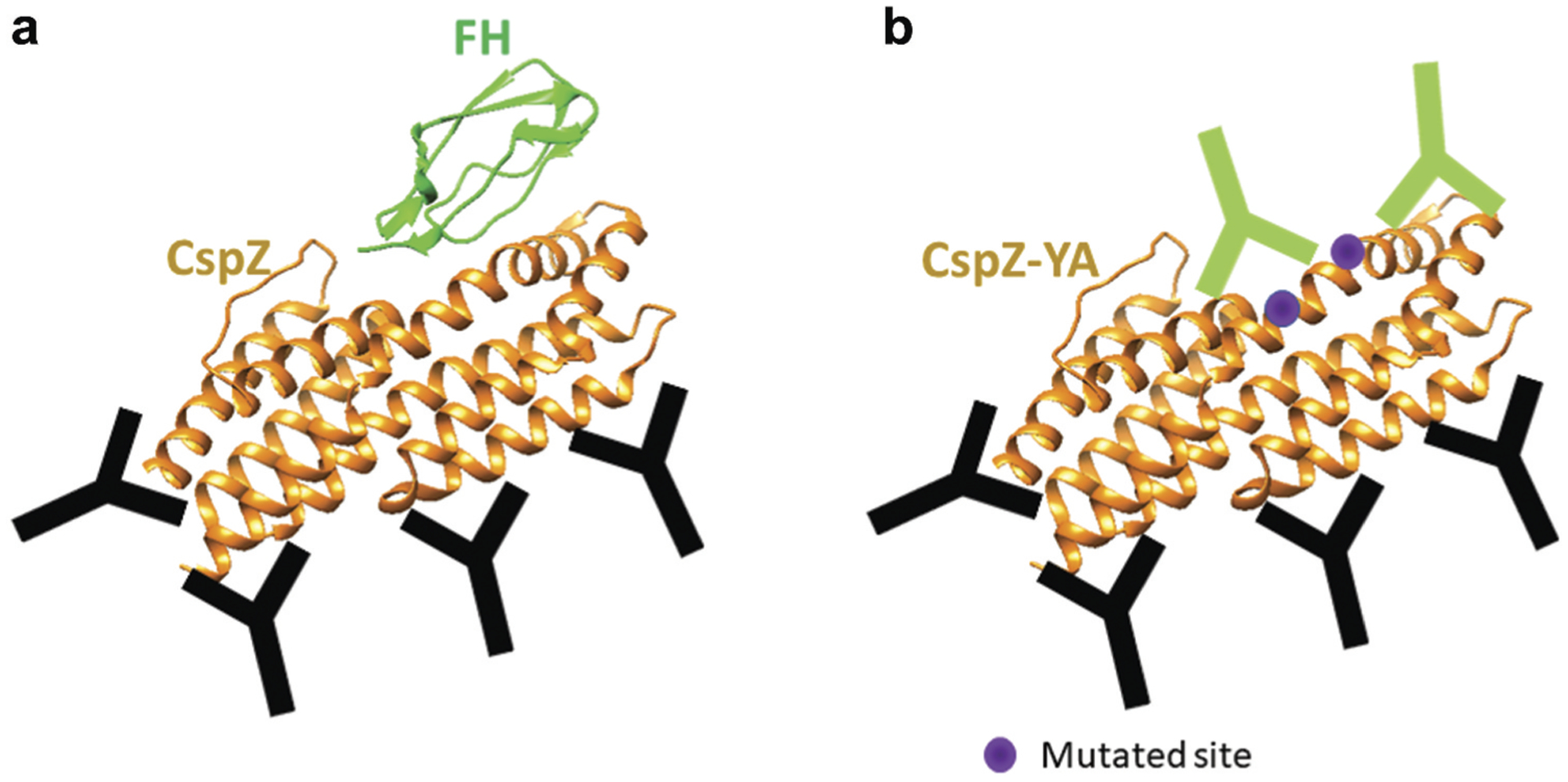
The Y207 and Y211 that are mutated in CspZ-YA are in purple. The antibodies that bind to CspZ FH-binding sites and other sites are in green and black, respectively.
3. Antigen engineering to improve stability and/or facilitate process development
When an antigen is produced recombinantly, antigen engineering may facilitate process development. As the tertiary structure of the protective epitopes is often critical for the immunogenicity of an antigen, improving the stability or retaining the proper antigen folding, similar to the native protein, is important. Specifically, to maintain certain conformation and keep cooperative interdomain interactions, proper linkers to connect different fragments are required. Including additional tags [151] or mutations [128] has also been applied as antigen engineering approaches to increase the stability of Lyme disease antigens, resulting in the improvement of the antigen production process. For instance, Dunn et al. designed a truncated OspA construct by removing the lipidation signal sequence and successfully increased this antigen’s solubility and production yield in E. coli [152]. More examples will be detailed in sections 3.1 to 3.3.
3.1. Linkers to ensure proper folding
When generating a chimeric or fusion antigen, the choice of a suitable linker to join the multiple antigens can be critical for production yields [153], antigen folding [154], and/or function or bioactivity [155]. Several studies and tools have guided the design of linkers [156–158]. Based on the sequences of the linkers present in between functional domains of many proteins, proline-rich linkers are considered rigid and would preclude undesired interactions between adjacent proteins [159]. In addition, glycine-rich linkers have also been commonly used to connect two antigens because of their greater flexibility [160]. For the OspA-based vaccine candidate VLA15 (see section 2.2), a linker consisting of the sequences of two loop-regions of OspA type 1 was used to connect two types of OspA C-termini [133] (Figure 2(c)). Additionally, for the tetravalent and octavalent chimeric OspC proteins that were generated as Lyme borreliae vaccine candidates (see section 2.3.1), the six amino acid linkers were designed in silico to be unstructured and protease-resistant [156] (Figure 3). Similarly, the newly generated OspC-based chimeritope vaccinogens (see section 2.3.1) also contain linkers from helix 4 and loop 6 of OspC to connect different OspC peptides [144]. All these linkers appear to increase the stability and solubility of the resulting chimeric Lyme borreliae vaccine candidates.
3.2. Affinity tags to ease protein purification
Affinity tags have been widely used in the production process of recombinant proteins. While affinity purification might be costly, the great selectivity of this approach has provided a straightforward solution to isolate many recombinant proteins, allowing fast process development and high protein recovery. During early development, hexahistidine tags (His-tag) have been fused with various LD vaccine antigens, such as OspA [128,133], OspC [140], and CspZ [119,149], and these his-tagged versions of antigens retain their protection in mice. In addition to His-tags, glutathione-S-transferase (GST) tags have also been commonly used, as GST can facilitate the folding and solubility of proteins, which thus has been used to fuse with the C-terminal OspA fragment as vaccine antigens in mice [151]. However, affinity tags are typically considered undesirable for use in human vaccines because of safety concerns due to their potential to trigger unwanted immune responses [161]. The only exception of a fusion as an approach for process development is to fuse the Fc region of human IgG to the protein of interest, as evidenced by 13 FDA- or EMA-approved Fc-fusion biotherapeutics for human use [162]. Fc has been demonstrated to increase solubility, plasma half-life, simplify the purification process, and promote immunogenicity. Several vaccine antigens against HIV, MERS-CoV, and SARS-CoV-2, have been fused with Fc and evaluated in preclinical and clinical testing [161,163,164], illustrating the promise of applying this approach to the development of Lyme disease vaccines.
3.3. Site-directed mutagenesis to improve stability
Site-directed mutagenesis of proteins has been another approach to increase the stability of vaccine antigen candidates [165,166]. A general strategy is to evaluate the Gibbs free energy of protein folding and optimize the intra- and intermolecular interactions of involved residues to create a more thermodynamically favorable condition for protein folding [165,166]. This antigen engineering approach has been applied to the Lyme disease vaccine antigen, OspA, by mutating three amino acids within this protein. The resulting triple mutant (OspA (130–273), E139M, E160Y, K189M) had significantly enhanced stability compared to wild-type OspA while maintaining the protective antibody profile of the wild-type version [128]. Similarly, the OspA-based vaccine candidate, VLA15, had cysteine residues added to its sequences to enhance structural stability by introducing intramolecular disulfide bonds. However, antigens that contained free cysteine residues sometimes formed oligomers via intermolecular disulfide bonds, causing a stability issue. Thus, mutating the free cysteine to serine or alanine has also been employed to prevent oligomerization [167,168].
4. Expert opinion
As the cases of human Lyme disease have been increasing, and the geographical distribution of these cases is expanding both in North America and Europe, developing efficacious and safe Lyme disease vaccines is likely to be the ultimate solution. Numerous efforts have been taken to develop the next generation of Lyme disease vaccines. Both new and previously tested antigens from Lyme borreliae or ticks have been engineered and tested for their efficacy in Lyme disease prevention. However, researchers have continuously identified new species of Lyme borreliae over the years [169–171], which poses a challenge for using antigens from the spirochete as exploring an effective vaccine candidate with a single consensus sequence will be difficult. In addition to Lyme borreliae, additional tick-borne pathogens are reported. The fact that individual ticks could carry Lyme borreliae and other tickborne pathogens suggests that developing pan-tickborne pathogen vaccines would be the next significant direction to advance this field. Conversely, there are concerns about using tick proteins as vaccine antigens, e.g. while some are functionally redundant, others may not always be immunogenic; moreover, genetic diversity among tick populations also makes it more challenging to use single antigens as vaccines [172]. Therefore, innovative antigen engineering strategies are crucial to designing a more universal and immunogenic tick vaccine candidate. Additionally, many vaccines that have had recent success in Lyme disease prevention (i.e. OspA-targeted vaccine and anti-tick vaccine) do not target the proteins that are produced during infection in humans, and thus, the memory immunity built into such antigens cannot trigger efficacious titers of antibodies after exposure to Lyme borreliae. Therefore, the choice of the antigen that could trigger greater memory immunity and/or further engineering of these proteins to enhance long-lasting antibody production would also be a pivotal direction to move forward in Lyme disease vaccine development. Since the removal of the LYMErix vaccine from the market nearly 20 years ago, several new vaccines have entered preclinical or clinical development. The efficacy of these vaccines is not yet known, and multiple strategies may have to be taken to further improve these vaccines for optimal safety and efficacy. However, the current success of several Lyme disease vaccine candidates in both preclinical and clinical settings has built a solid foundation for these further studies. Such regained efforts in Lyme vaccine development are an encouraging sign that an effective vaccine may soon enter the market. The information from the current work would thus inform the development of Lyme disease vaccines and provide insights into the protective mechanisms and reduce the disease burdens and end this disease.
Article highlights.
Lyme disease, caused by the pathogen Borrelia burgdorferi sensu lato, is the most common vector-borne disease in the Northern hemisphere, yet no effective preventive strategies are currently available.
Vaccine candidates originating in the pathogen (Borrelia burgdorferi sensu lato) and in the vector (Ixodes spp.) have been evaluated; however, several challenges, such as low consumer confidence caused by the myth of off-target responses, a limited breadth of immune reactivity, low-level immune responses and complexities within the vaccine process development, have impacted progress.
Antigen engineering was shown to not only improve the immune response targeting the desired immunologically subdominant epitopes but also prevent the production of off-target antibodies.
Antigen editing was shown to simplify the production process and improve the stability of vaccine candidates.
Acknowledgments
We thank Klemen Strle for critical reading and editing the manuscript.
Funding
This work was supported by NIH R21AI144891, DoD TB190048.
Footnotes
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Disclosure statement
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as either of interest (•) or ofconsiderable interest (••) to readers.
- 1.Radolf JD, Caimano MJ, Stevenson B, et al. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat Rev Microbiol. 2012;10(2):87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steere AC, Strle F, Wormser GP, et al. Lyme borreliosis. Nat Rev Dis Primers. 2016;2:16090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sykes RA, Makiello P. An estimate of Lyme borreliosis incidence in Western Europedagger. J Public Health (Oxf). 2017;39(1):74–81. [DOI] [PubMed] [Google Scholar]
- 4.Kugeler KJ, Schwartz AM, Delorey MJ, et al. Estimating the frequency of Lyme disease diagnoses, United States, 2010–2018. Emerg Infect Dis. 2021;27(2):616–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strle K, Jones KL, Drouin EE, et al. Borrelia burgdorferi RST1 (OspC type A) genotype is associated with greater inflammation and more severe Lyme disease. Am J Pathol. 2011;178(6):2726–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dykhuizen DE, Brisson D, Sandigursky S, et al. The propensity of different Borrelia burgdorferi sensu stricto genotypes to cause disseminated infections in humans. Am J Trop Med Hyg. 2008;78(5):806–810. [PMC free article] [PubMed] [Google Scholar]
- 7.Steere AC, Strle K, and Drouin EE. Infection and autoimmunity. Yehuda Shoenfeld NA-L, Noel RR, eds. London, UK: Academic Press: 2015. [Google Scholar]
- 8.Wolcott KA, Margos G, Fingerle V, et al. Host association of Borrelia burgdorferi sensu lato: a review. Ticks Tick-Borne Dis. 2021;12 (5):101766. [DOI] [PubMed] [Google Scholar]
- 9.Tufts DM, Hart TM, Chen GF, et al. Outer surface protein polymorphisms linked to host-spirochete association in Lyme borreliae. Mol Microbiol. 2019;111(4):868–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helble JD, McCarthy JE, Hu LT. Interactions between Borrelia burgdorferi and its hosts across the enzootic cycle. Parasite Immunol. 2021;43(5):e12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Bier NS, Hatke AL, Camire AC, et al. Human and veterinary vaccines for Lyme disease. Curr Issues Mol Biol. 2020;42:191–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dattwyler RJ, and Gomes-Solecki M. The year that shaped the outcome of the OspA vaccine for human Lyme disease. NPJ Vaccines. 2022;7(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This article summarize the development of OspA-based vaccines, the only vaccine that was in the market. and how this vaccine was withdrawn from the market, focusing on the history of this vaccine.
- 13.Schwan TG. Temporal regulation of outer surface proteins of the Lyme-disease spirochaete Borrelia burgdorferi. Biochem Soc Trans. 2003;31(Pt 1):108–112. [DOI] [PubMed] [Google Scholar]
- 14.Schwan TG, Piesman J, Golde WT, et al. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proceedings of the National Academy of Sciences of the United States of America, 92(7), 2909–2913 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hodzic E, Feng S, Freet KJ, et al. Borrelia burgdorferi population kinetics and selected gene expression at the host-vector interface. Infect Immun. 2002;70(7):3382–3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang XF, Pal U, Alani SM, et al. Essential role for OspA/B in the life cycle of the Lyme disease spirochete. J Exp Med. 2004;199(5):641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pal U, de Silva AM, Montgomery RR, et al. Attachment of Borrelia burgdorferi within Ixodes scapularis mediated by outer surface protein A. J Clin Invest. 2000;106(4):561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pal U, Yang X, Chen M, et al. OspC facilitates Borrelia burgdorferi invasion of Ixodes scapularis salivary glands. J Clin Invest. 2004;113 (2):220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pal U, Montgomery RR, Lusitani D, et al. Inhibition of Borrelia burgdorferi-tick interactions in vivo by outer surface protein antibody. J Iimmunol. 2001;166(12):7398–7403. [DOI] [PubMed] [Google Scholar]
- 20.Steere AC, Sikand VK, Meurice F, et al. Vaccination against Lyme disease with recombinant Borrelia burgdorferi outer-surface lipoprotein A with adjuvant. Lyme Disease Vaccine Study Group. N Engl J Med. 1998;339(4):209–215. [DOI] [PubMed] [Google Scholar]
- 21.Sigal LH, Zahradnik JM, Lavin P, et al. A vaccine consisting recombinant Borrelia burgdorferi outer-surface protein A to prevent Lyme disease. recombinant outer-surface protein A Lyme disease vaccine study consortium. N Engl J Med. 1998;339 (4):216–222. [DOI] [PubMed] [Google Scholar]
- 22.Gross DM, Forsthuber T, Tary-Lehmann M, et al. Identification LFA-1 as a candidate autoantigen in treatment-resistant Lyme arthritis. Science. 1998;281(5377):703–706. [DOI] [PubMed] [Google Scholar]
- 23.Hemmer B, Vergelli M, Gran B, et al. Predictable TCR antigen recognition based on peptide scans leads to the identification agonist ligands with no sequence homology. J Iimmunol. 1998;160 (8):3631–3636. [PubMed] [Google Scholar]
- 24.Hemmer B, Vergelli M, Pinilla C, et al. Probing degeneracy in T-cell recognition using peptide combinatorial libraries. Immunol Today. 1998;19(4):163–168. [DOI] [PubMed] [Google Scholar]
- 25.Maier B, Molinger M, Cope AP, et al. Multiple cross-reactive self-ligands for Borrelia burgdorferi-specific HLA-DR4-restricted cells. Eur J Immunol. 2000;30(2):448–457. [DOI] [PubMed] [Google Scholar]
- 26.Steere AC, Drouin EE, Glickstein LJ. Relationship between immunity to Borrelia burgdorferi outer-surface protein A (OspA) and Lyme arthritis. Clinical Infectious Diseases : an Official Publication of Infectious Diseases Society of America. 2011;52(3):s259–265. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fikrig E, Barthold SW, Kantor FS, et al. Protection of mice against the Lyme disease agent by immunizing with recombinant OspA. Science. 1990;250(4980):553–556. [DOI] [PubMed] [Google Scholar]
- 28.Fikrig E, Telford SR 3rd, Barthold SW, et al. Elimination of Borrelia burgdorferi from vector ticks feeding on OspA-immunized mice. Proceedings of the National Academy of Sciences of the United States of America, 89(12), 5418–5421 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Silva AM, Telford SR 3rd, Brunet LR, et al. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J Exp Med. 1996;183(1):271–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pal U, Li X, Wang T, et al. TROSPA, an Ixodes scapularis receptor Borrelia burgdorferi. Cell. 2004;119(4):457–468. [DOI] [PubMed] [Google Scholar]
- 31.Preac-Mursic V, Wilske B, Patsouris E, et al. Active immunization with pC protein of Borrelia burgdorferi protects gerbils against B. burgdorferi infection. Infection. 1992;20(6):342–349. [DOI] [PubMed] [Google Scholar]
- 32.Probert WS, LeFebvre RB. Protection of C3H/HeN mice from challenge with Borrelia burgdorferi through active immunization with OspA, OspB, or OspC, but not with OspD or the 83-kilodalton antigen. Infection and Immunity. 1994;62(5):1920–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kung F, Kaur S, Smith AA, et al. A Borrelia burgdorfer surface-exposed transmembrane protein lacking detectable immune responses supports pathogen persistence and constitutes a vaccine target. J Infect Dis. 2016;213(11):1786–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar M, Yang X, Coleman AS, et al. BBA52 facilitates Borrelia burgdorferi transmission from feeding ticks to murine hosts. J Infect Dis. 2010;201(7):1084–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seshu J, Esteve-Gassent MD, Labandeira-Rey M, et al. Inactivation of the fibronectin-binding adhesin gene bbk32 significantly attenuates the infectivity potential of Borrelia burgdorferi. Mol Microbiol. 2006;59(5):1591–1601. [DOI] [PubMed] [Google Scholar]
- 36.Li X, Liu X, Beck DS, et al. Borrelia burgdorferi lacking BBK32, a fibronectin-binding protein, retains full pathogenicity. Infect Immun. 2006;74(6):3305–3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanson MS, Cassatt DR, Guo BP, et al. Active and passive immunity against Borrelia burgdorferi decorin binding protein A (DbpA) protects against infection. Infect Immun. 1998;66(5):2143–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi Y, Xu Q, McShan K, et al. Both decorin-binding proteins A and B are critical for the overall virulence of Borrelia burgdorferi. Infect Immun. 2008;76(3):1239–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi Y, Xu Q, Seemanapalli SV, et al. The dbpBA locus of Borrelia burgdorferi is not essential for infection of mice. Infect Immun. 2006;74(11):6509–6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weening EH, Parveen N, Trzeciakowski JP, et al. Borrelia burgdorferi lacking DbpBA exhibits an early survival defect during experimental infection. Infect Immun. 2008;76(12):5694–5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blevins JS, Hagman KE, Norgard MV. Assessment of decorin-binding protein A to the infectivity of Borrelia burgdorferi in the murine models of needle and tick infection. BMC Microbiol. 2008;8(82). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Byram R, Gaultney RA, Floden AM, et al. Borrelia burgdorferi RevA significantly affects pathogenicity and host response in the mouse model of Lyme disease. Infect Immun. 2015;83(9):3675–3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Floden AM, Gonzalez T, Gaultney RA, et al. Evaluation of RevA, a fibronectin-binding protein of Borrelia burgdorferi, as a potential vaccine candidate for lyme disease. Clin vaccin immunol. 2013;20 (6):892–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gomes-Solecki M Blocking pathogen transmission at the source: reservoir targeted OspA-based vaccines against Borrelia burgdorferi. Front Cell Infect Microbiol. 2014;4:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Voordouw MJ, Tupper H, Onder O, et al. Reductions in human Lyme disease risk due to the effects of oral vaccination on tick-to-mouse and mouse-to-tick transmission. Vector Borne Zoonotic Dis. 2013;13(4):203–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsao K, Fish D, Galvani AP. Predicted outcomes of vaccinating wildlife to reduce human risk of Lyme disease. Vector Borne Zoonotic Dis. 2012;12(7):544–551. [DOI] [PubMed] [Google Scholar]
- 47.Gomes-Solecki M, Arnaboldi PM, and Backenson PB, et al. Protective immunity and new vaccines for Lyme disease. Clin Infect Dis. 2020;70(8):1768–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This review paper summarized the concepts that have been proposed to prevent Lyme disease infection in both human vaccines and reservoir-targeted approaches. The efforts to test these concepts were also described in this review paper.
- 48.Embers ME, and Narasimhan S. Vaccination against Lyme disease: past, present, and future. Front Cell Infect Microbiol. 2013;3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meirelles Richer L, Aroso M, Contente-Cuomo T, et al. Reservoir targeted vaccine for lyme borreliosis induces a yearlong, neutralizing antibody response to OspA in white-footed mice. Clin Vaccine Immunol. 2011;18(11):1809–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bhattacharya D, Bensaci M, Luker KE, et al. Development of a baited oral vaccine for use in reservoir-targeted strategies against Lyme disease. Vaccine. 2011;29(44):7818–7825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kurtenbach K, Dizij A, Voet P, et al. Vaccination of natural reservoir hosts with recombinant lipidated OspA induces a transmission-blocking immunity against Lyme disease spirochaetes associated with high levels of LA-2 equivalent antibodies. Vaccine. 1997;15(15):1670–1674. [DOI] [PubMed] [Google Scholar]
- 52.Scheckelhoff MR, Telford SR, Hu LT. Protective efficacy of an oral vaccine to reduce carriage of Borrelia burgdorferi (strain N40) in mouse and tick reservoirs. Vaccine. 2006;24(11):1949–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsao JI, Wootton JT, Bunikis J, et al. An ecological approach to preventing human infection: vaccinating wild mouse reservoirs intervenes in the Lyme disease cycle. Proceedings of the National Academy of Sciences of the United States of America, 101(52), 18159–18164 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Richer LM, Brisson D, Melo R, et al. Reservoir targeted vaccine against Borrelia burgdorferi: a new strategy to prevent Lyme disease transmission. J Infect Dis. 2014;209(12):1972–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grimm D, Tilly K, Byram R, et al. Outer-surface protein C of the Lyme disease spirochete: a protein induced in ticks for infection of mammals. Proceedings of the National Academy of Sciences of the United States of America, 101(9), 3142–3147 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tilly K, Krum JG, Bestor A, et al. Borrelia burgdorferi OspC protein required exclusively in a crucial early stage of mammalian infection. Infect Immun. 2006;74(6):3554–3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramamoorthi N, Narasimhan S, Pal U, et al. The Lyme disease agent exploits a tick protein to infect the mammalian host. Nature. 2005;436(7050):573–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Caine JA, Lin YP, Kessler JR, et al. Borrelia burgdorferi outer surface protein C (OspC) binds complement component C4b and confers bloodstream survival. Cell Microbiol. 2017;19:e12786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lin YP, Tan X, Caine JA, et al. Strain-specific joint invasion and colonization by Lyme disease spirochetes is promoted by outer surface protein C. PLoS Pathog. 2020;16(5):e1008516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lagal V, Portnoi D, Faure G, et al. Borrelia burgdorferi sensu stricto invasiveness is correlated with OspC-plasminogen affinity. Microbes Infect. 2006;8(3):645–652. [DOI] [PubMed] [Google Scholar]
- 61.Bockenstedt LK, Hodzic E, Feng S, et al. Borrelia burgdorferi strain-specific OspC-mediated immunity in mice. Infect Immun. 1997;65(11):4661–4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gilmore RD Jr., Piesman J, Moore RN. Inhibition of Borrelia burgdorferi migration from the midgut to the salivary glands following feeding by ticks on OspC-immunized mice. Infect Immun. 2000;68 (1):411–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mbow ML, Gilmore RD Jr., Titus RG. An OspC-specific monoclonal antibody passively protects mice from tick-transmitted infection by Borrelia burgdorferi B31. Infect Immun. 1999;67(10):5470–5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wilske B, Preac-Mursic V, Jauris S, et al. Immunological and molecular polymorphisms of OspC, an immunodominant major outer surface protein of Borrelia burgdorferi. Infect Immun. 1993;61 (5):2182–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Earnhart CG, Buckles EL, Dumler JS, et al. Demonstration of OspC type diversity in invasive human lyme disease isolates and identification of previously uncharacterized epitopes that define the specificity of the OspC murine antibody response. Infect Immun. 2005;73(12):7869–7877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reis ES, Mastellos DC, Hajishengallis G, et al. New insights into the immune functions of complement. Nat Rev Immunol. 2019;19 (8):503–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zipfel PF, Hallstrom T, Riesbeck K. Human complement control and complement evasion by pathogenic microbes–tipping the balance. Mol Immunol. 2013;56(3):152–160. [DOI] [PubMed] [Google Scholar]
- 68.Blom AM. The role of complement inhibitors beyond controlling inflammation. J Intern Med. 2017;282(2):116–128. [DOI] [PubMed] [Google Scholar]
- 69.Zipfel PF, Skerka C. Complement regulators and inhibitory proteins. Nat Rev Immunol. 2009;9(10):729–740. [DOI] [PubMed] [Google Scholar]
- 70.Skare JT, Garcia BL. Complement evasion by Lyme disease spirochetes. Trends Microbiol. 2020;28(11):889–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lin YP, Diuk-Wasser MA, Stevenson B, et al. Complement evasion contributes to Lyme Borreliae-host associations. Trends Parasitol. 2020;36(7):634–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dulipati V, Meri S, Panelius J. Complement evasion strategies of Borrelia burgdorferi sensu lato. FEBS Lett. 2020;594(16):2645–2656. [DOI] [PubMed] [Google Scholar]
- 73.Hartmann K, Corvey C, Skerka C, et al. Functional characterization of BbCRASP-2, a distinct outer membrane protein of Borrelia burgdorferi that binds host complement regulators factor H and FHL-1. Mol Microbiol. 2006;61(5):1220–1236. [DOI] [PubMed] [Google Scholar]
- 74.Lin YP, Frye AM, Nowak TA, et al. New Insights Into CRASP-mediated complement evasion in the Lyme disease enzootic cycle. Front Cell Infect Microbiol. 2020;10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bykowski T, Woodman ME, Cooley AE, et al. Coordinated expression of Borrelia burgdorferi complement regulator-acquiring surface proteins during the Lyme disease spirochete’s mammal-tick infection cycle. Infect Immun. 2007;75(9):4227–4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marcinkiewicz AL, Dupuis AP, Zamba-Campero M, et al. Blood treatment of Lyme borreliae demonstrates the mechanism of CspZ-mediated complement evasion to promote systemic infection in vertebrate hosts. Cell Microbiol. 2019;21(2):e12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kraiczy P, Seling A, Brissette CA, et al. Borrelia burgdorferi complement regulator-acquiring surface protein 2 (CspZ) as a serological marker of human Lyme disease. Clin Vaccin Immunol. 2008;15 (3):484–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rogers EA, Abdunnur SV, McDowell JV, et al. Comparative analysis of the properties and ligand binding characteristics of CspZ, a factor H binding protein, derived from Borrelia burgdorferi isolates of human origin. Infect Immun. 2009;77(10):4396–4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rogers EA, Marconi RT. Delineation of species-specific binding properties of the CspZ protein (BBH06) of Lyme disease spirochetes: evidence for new contributions to the pathogenesis of Borrelia spp. Infect Immun. 2007;75(11):5272–5281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Coleman AS, Yang X, Kumar M, et al. Borrelia burgdorferi complement regulator-acquiring surface protein 2 does not contribute to complement resistance or host infectivity. PloS one. 2008;3 (8):3010e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kumar M, Kaur S, Kariu T, et al. Borrelia burgdorferi BBA52 is a potential target for transmission blocking Lyme disease vaccine. Vaccine. 2011;29(48):9012–9019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Klouwens MJ, Trentelman JJ, Ersoz JI, et al. Investigating BB0405 as a novel Borrelia afzelii vaccination candidate in Lyme borreliosis. Sci Rep. 2021;11(1):4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Singh P, Verma D, Backstedt BT, et al. Borrelia burgdorferi BBI39 paralogs, targets of protective immunity, reduce pathogen persistence either in hosts or in the vector. J Infect Dis. 2017;215 (6):1000–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brandt KS, Gilmore RD. Immunization of mice with Borrelia burgdorferi lp54 gene encoded recombinant proteins does not provide protection against tick transmitted infectious challenge. Vaccine. 2017;35(40):5310–5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brandt KS, Patton TG, Allard AS, et al. Evaluation of the Borrelia burgdorferi BBA64 protein as a protective immunogen in mice. Clin vaccin immunol. 2014;21(4):526–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gilmore RD Jr., Kappel KJ, Dolan MC, et al. Outer surface protein C (OspC), but not P39, is a protective immunogen against a tick-transmitted Borrelia burgdorferi challenge: evidence for a conformational protective epitope in OspC. Infect Immun. 1996;64(6):2234–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brown EL, Kim JH, Reisenbichler ES, et al. Multicomponent Lyme vaccine: three is not a crowd. Vaccine. 2005;23(28):3687–3696. [DOI] [PubMed] [Google Scholar]
- 88.Hagman KE, Yang X, Wikel SK, et al. Decorin-binding protein A (DbpA) of Borrelia burgdorferi is not protective when immunized mice are challenged via tick infestation and correlates with the lack of DbpA expression by B. burgdorferi in ticks. Infect Immun. 2000;68(8):4759–4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Small CM, Ajithdoss DK, Rodrigues Hoffmann A, et al. Immunization with a Borrelia burgdorferi BB0172-derived peptide protects mice against lyme disease. PloS one. 2014;9(2):e88245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Eisen L Vector competence studies with hard ticks and Borrelia burgdorferi sensu lato spirochetes: a review. Ticks Tick-Borne Dis. 2020;11(3):101359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schuijt TJ, Hovius JW, van der Poll T, et al. Lyme borreliosis vaccination: the facts, the challenge, the future. Trends Parasitol. 2011;27(1):40–47. [DOI] [PubMed] [Google Scholar]
- 92.Rego ROM, Trentelman JJA, and Anguita J, et al. Counterattacking the tick bite: towards a rational design of anti-tick vaccines targeting pathogen transmission. Parasit Vectors. 2019;12(1):229. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This review paper summarized the concepts of targeting tick proteins in preventing transmission of Lyme disease bacteria to hosts and listed the target proteins that have been used to test this concept preclinically.
- 93.Koci J, Bista S, Chirania P, et al. Antibodies against EGF-like domains in Ixodes scapularis BM86 orthologs impact tick feeding and survival of Borrelia burgdorferi. Sci Rep. 2021;11(1):6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dai J, Wang P, Adusumilli S, et al. Antibodies against a tick protein, Salp15, protect mice from the Lyme disease agent. Cell Host Amp; Microbe. 2009;6(5):482–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gomes-Solecki M, Arnaboldi PM, Backenson PB, et al. Protective immunity and new vaccines for Lyme disease. Clin Infect Dis. 2019;68:1052–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sprong H, Trentelman J, Seemann I, et al. ANTIDotE: anti-tick vaccines to prevent tick-borne diseases in Europe. Parasit Vectors. 2014;7:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sajid A, Matias J, Arora G, et al. mRNA vaccination induces tick resistance and prevents transmission of the Lyme disease agent. Sci Transl Med. 2021;13(620):eabj9827. [DOI] [PubMed] [Google Scholar]
- 98.Ullmann AJ, Dolan MC, Sackal CA, et al. Immunization with adenoviral-vectored tick salivary gland proteins (SALPs) in a murine model of Lyme borreliosis. TicksTick-Borne Dis. 2013;4 (1–2):160–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dai J, Narasimhan S, Zhang L, et al. Tick histamine release factor is critical for Ixodes scapularis engorgement and transmission of the lyme disease agent. PLoS Pathogens. 2010;6(11): e1001205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Klouwens MJ, Trentelman JJA, Wagemakers A, et al. Tick-tattoo: DNA vaccination Against B. burgdorferi or Ixodes scapularis tick proteins. Front Immunol. 2021;12:615011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Narasimhan S, Kurokawa C, DeBlasio M, et al. Acquired tick resistance: the trail is hot. Parasite Immunol. 2021;43(5):e12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kitsou C, Fikrig E, Pal U. Tick host immunity: vector immunomodulation and acquired tick resistance. Trends Immunol. 2021;42(7):554–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lynn GE, Diktas H, DePonte K, et al. Naturally acquired resistance to Ixodes scapularis elicits partial immunity against other tick vectors in a laboratory host. Am J Trop Med Hyg. 2021;104(1):175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cerny J, Lynn G, DePonte K, et al. Fractionation of tick saliva reveals proteins associated with the development of acquired resistance to Ixodes scapularis. Vaccine. 2020;38(51):8121–8129. [DOI] [PubMed] [Google Scholar]
- 105.Kurokawa C, Narasimhan S, Vidyarthi A, et al. Repeat tick exposure elicits distinct immune responses in Guinea pigs and mice. Ticks Tick-Borne Dis. 2020;11(6):101529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Matias J, Kurokawa C, Sajid A, et al. Tick immunity using mRNA, DNA and protein-based Salp14 delivery strategies. Vaccine. 2021;39 (52):7661–7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.de la Fuente J, Estrada-Pena A, Contreras M. Modeling tick vaccines: a key tool to improve protection efficacy. Expert Rev Vaccines. 2020;19(3):217–225. [DOI] [PubMed] [Google Scholar]
- 108.Caradonna TM, and Schmidt AG. Protein engineering strategies for rational immunogen design. NPJ Vaccines. 2021;6(1):154. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This review paper summarized the concepts of engineering and their potential mechanisms to enhance the vaccine efficacy. Though most of the work described in this study is not relevant to Lyme disease vaccines, some concepts of antigen engineering in this review paper have been applied to edit the vaccine antigens of Lyme disease bacteria to enhance the efficacy.
- 109.Angeletti D, Yewdell JW. Understanding and manipulating viral immunity: antibody immunodominance enters center stage. Trends Immunol. 2018;39(7):549–561. [DOI] [PubMed] [Google Scholar]
- 110.Yeh CH, Nojima T, Kuraoka M, et al. Germinal center entry not selection of B cells is controlled by peptide-MHCII complex density. Nat Commun. 2018;9(1):928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bachmann MF, Zinkernagel RM. Neutralizing antiviral B cell responses. Annu Rev Immunol. 1997;15:235–270. [DOI] [PubMed] [Google Scholar]
- 112.Tornesello AL, Tagliamonte M, Buonaguro FM, et al. Virus-like particles as preventive and therapeutic cancer vaccines. Vaccines (Basel). 2022;10(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ramos TI, Villacis-Aguirre CA, Lopez-Aguilar KV, et al. The hitchhiker’s guide to human therapeutic nanoparticle development. Pharmaceutics. 2022;14(2):247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sriwidodo UAK, Wathoni N, Zothantluanga JH, et al. Liposome-polymer complex for drug delivery system and vaccine stabilization. Heliyon. 2022;8(2):e08934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kolb P, Wallich R, Nassal M. Whole-chain tick saliva proteins presented on hepatitis B virus capsid-like particles induce high-titered antibodies with neutralizing potential. PloS one. 2015;10(9): e0136180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Federizon J, Frye A, Huang WC, et al. Immunogenicity of the Lyme disease antigen OspA, particleized by cobalt porphyrin-phospholipid liposomes. Vaccine. 2020;38(4):942–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Krupka M, Masek J, Bartheldyova E, et al. Enhancement of immune response towards non-lipidized Borrelia burgdorferi recombinant OspC antigen by binding onto the surface of metallochelating nanoliposomes with entrapped lipophilic derivatives of norAbuMDP. J Control Release. 2012;160(2):374–381. [DOI] [PubMed] [Google Scholar]
- 118.Kamp HD, Swanson KA, Wei RR, et al. Design of a broadly reactive Lyme disease vaccine. NPJ Vaccines. 2020;5(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Marcinkiewicz AL, Lieknina I, Kotelovica S, et al. Eliminating factor H-binding activity of Borrelia burgdorferi CspZ combined with virus-like particle conjugation enhances its efficacy as a Lyme disease vaccine. Front Immunol. 2018;9:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kanekiyo M, Wei CJ, Yassine HM, et al. Self-assembling influenza nanoparticle vaccines elicit broadly neutralizing H1N1 antibodies. Nature. 2013;499(7456):102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Darricarrere N, Pougatcheva S, Duan X, et al. Development of a Pan-H1 influenza vaccine. J Virol. 2018;92(22). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kuraoka M, Schmidt AG, Nojima T, et al. Complex antigens drive permissive clonal selection in germinal centers. Immunity. 2016;44 (3):542–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Finney J, Yeh CH, Kelsoe G, et al. Germinal center responses to complex antigens. Immunol Rev. 2018;284(1):42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Willett TA, Meyer AL, Brown EL, et al. An effective second-generation outer surface protein A-derived Lyme vaccine that eliminates a potentially autoreactive T cell epitope. Proceedings of the National Academy of Sciences of the United States of America, 101(5), 1303–1308 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ding W, Huang X, Yang X, et al. Structural identification of a key protective B-cell epitope in Lyme disease antigen OspA. J Mol Biol. 2000;302(5):1153–1164. [DOI] [PubMed] [Google Scholar]
- 126.Schubach WH, Mudri S, Dattwyler RJ, et al. Mapping antibody-binding domains of the major outer surface membrane protein (OspA) of Borrelia burgdorferi. Infect Immun. 1991;59(6):1911–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Golde WT, Piesman J, Dolan MC, et al. Reactivity with a specific epitope of outer surface protein A predicts protection from infection with the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun. 1997;65(3):882–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Koide S, Yang X, Huang X, et al. Structure-based design of a second-generation Lyme disease vaccine based on a C-terminal fragment of Borrelia burgdorferi OspA. J Mol Biol. 2005;350 (2):290–299. [DOI] [PubMed] [Google Scholar]
- 129.Wilske B, Preac-Mursic V, Gobel UB, et al. An OspA serotyping system for Borrelia burgdorferi based on reactivity with monoclonal antibodies and OspA sequence analysis. J Clin Microbiol. 1993;31 (2):340–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Livey I, O’Rourke M, Traweger A, et al. A new approach to a Lyme disease vaccine. Clin Infect Dis. 2011;52(3):s266–270. [DOI] [PubMed] [Google Scholar]
- 131.Wressnigg N, Pollabauer EM, Aichinger G, et al. Safety and immunogenicity of a novel multivalent OspA vaccine against Lyme borreliosis in healthy adults: a double-blind, randomised, dose-escalation phase 1/2 trial. Lancet Infect Dis. 2013;13 (8):680–689. [DOI] [PubMed] [Google Scholar]
- 132.Wressnigg N, Barrett PN, Pollabauer EM, et al. A Novel multivalent OspA vaccine against Lyme borreliosis is safe and immunogenic in an adult population previously infected with Borrelia burgdorferi sensu lato. Clin vaccin immunol. 2014;21(11):1490–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Comstedt P, Hanner M, Schuler W, et al. Design and development of a novel vaccine for protection against Lyme borreliosis. PloS one. 2014;9(11):e113294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Comstedt P, Schuler W, Meinke A, et al. The novel Lyme borreliosis vaccine VLA15 shows broad protection against Borrelia species expressing six different OspA serotypes. PloS one. 2017;12(9): e0184357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Comstedt P, Hanner M, Schuler W, et al. Characterization and optimization of a novel vaccine for protection against Lyme borreliosis. Vaccine. 2015;33(44):5982–5988. [DOI] [PubMed] [Google Scholar]
- 136.Valneva and Pfizer report further positive phase 2 data for Lyme disease vaccine candidate. (Ed.^(Eds). 2022.
- 137.Bajic G, Maron MJ, Caradonna TM, et al. Structure-guided molecular grafting of a complex broadly neutralizing viral epitope. ACS Infect Dis. 2020;6(5):1182–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nayak A, Schuler W, Seidel S, et al. Broadly protective multivalent OspA vaccine against lyme borreliosis, developed based on surface shaping of the C-terminal fragment. Infect Immun. 2020;88(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Buckles EL, Earnhart CG, Marconi RT. Analysis of antibody response in humans to the type A OspC loop 5 domain and assessment of the potential utility of the loop 5 epitope in Lyme disease vaccine development. Clin vaccin immunol. 2006;13(10):1162–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Earnhart CG, Buckles EL, Marconi RT. Development of an OspC-based tetravalent, recombinant, chimeric vaccinogen that elicits bactericidal antibody against diverse Lyme disease spirochete strains. Vaccine. 2007;25(3):466–480. [DOI] [PubMed] [Google Scholar]
- 141.Earnhart CG, Marconi RT. An octavalent lyme disease vaccine induces antibodies that recognize all incorporated OspC type-specific sequences. Hum Vaccines. 2007;3(6):281–289. [DOI] [PubMed] [Google Scholar]
- 142.Oliver LD Jr., Earnhart CG, Virginia-Rhodes D, et al. Antibody profiling of canine IgG responses to the OspC protein of the Lyme disease spirochetes supports a multivalent approach in vaccine and diagnostic assay development. Vet J. 2016;218:27–33. [DOI] [PubMed] [Google Scholar]
- 143.Earnhart CG, Marconi RT. Construction and analysis of variants of a polyvalent Lyme disease vaccine: approaches for improving the immune response to chimeric vaccinogens. Vaccine. 2007;25 (17):3419–3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Izac JR, O’Bier NS, Oliver LD Jr., et al. Development and optimization of OspC chimeritope vaccinogens for Lyme disease. Vaccine. 2020;38(8):1915–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Marconi RT, Garcia-Tapia D, Hoevers J, et al. VANGUARD(R)crLyme: a next generation Lyme disease vaccine that prevents B. burgdorferi infection in dogs. Vaccine X. 2020; 6: 100079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ringe RP, Pugach P, Cottrell CA, et al. Closing and opening holes in the glycan shield of HIV-1 envelope glycoprotein SOSIP trimers can redirect the neutralizing antibody response to the newly unmasked epitopes. J Virol. 2019;93(4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dubrovskaya V, Tran K, Ozorowski G, et al. Vaccination with glycan-modified HIV NFL envelope trimer-liposomes elicits broadly neutralizing antibodies to multiple sites of vulnerability. Immunity. 2019;51(5):915–929 e917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Liu WC, Jan JT, Huang YJ, et al. Unmasking stem-specific neutralizing epitopes by abolishing N-linked glycosylation sites of influenza virus hemagglutinin proteins for vaccine design. J Virol. 2016;90 (19):8496–8508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Marcinkiewicz AL, Lieknina I, Yang X, et al. The factor H-binding site of CspZ as a protective target against multistrain, tick-transmitted lyme disease. Infect Immun. 2020;88(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ferreira VP, Pangburn MK, Cortes C. Complement control protein factor H: the good, the bad, and the inadequate. Mol Immunol. 2010;47(13):2187–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bockenstedt LK, Fikrig E, Barthold SW, et al. Inability of truncated recombinant Osp A proteins to elicit protective immunity to Borrelia burgdorferi in mice. J Iimmunol. 1993;151(2):900–906. [PubMed] [Google Scholar]
- 152.Dunn JJ, Lade BN, Barbour AG. Outer surface protein A (OspA) from the Lyme disease spirochete, Borrelia burgdorferi: high level expression and purification of a soluble recombinant form of OspA. Protein Expr Purif. 1990;1(2):159–168. [DOI] [PubMed] [Google Scholar]
- 153.Amet N, Lee HF, Shen WC. Insertion of the designed helical linker led to increased expression of tf-based fusion proteins. Pharm Res. 2009;26(3):523–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhao HL, Yao XQ, Xue C, et al. Increasing the homogeneity, stability and activity of human serum albumin and interferon-alpha2b fusion protein by linker engineering. Protein Expr Purif. 2008;61(1):73–77. [DOI] [PubMed] [Google Scholar]
- 155.Bai Y, Shen WC. Improving the oral efficacy of recombinant granulocyte colony-stimulating factor and transferrin fusion protein by spacer optimization. Pharm Res. 2006;23(9):2116–2121. [DOI] [PubMed] [Google Scholar]
- 156.Crasto CJ, Feng JA. LINKER: a program to generate linker sequences for fusion proteins. Protein Eng. 2000;13(5):309–312. [DOI] [PubMed] [Google Scholar]
- 157.Reddy Chichili VP, Kumar V, Sivaraman J. Linkers in the structural biology of protein-protein interactions. Protein Sci. 2013;22(2):153–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chen X, Zaro JL, Shen WC. Fusion protein linkers: property, design and functionality. Adv Drug Deliv Rev. 2013;65(10):1357–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Radford SE, Laue ED, Perham RN, et al. Segmental structure and protein domains in the pyruvate dehydrogenase multienzyme complex of Escherichia coli. Genetic reconstruction in vitro and 1H-n.m.r. spectroscopy. Biochem J. 1987;247(3):641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lubkowski J, Hennecke F, Pluckthun A, et al. Filamentous phage infection: crystal structure of g3p in complex with its coreceptor, the C-terminal domain of TolA. Structure. 1999;7(6):711–722. [DOI] [PubMed] [Google Scholar]
- 161.Nyon MP, Du L, Tseng CK, et al. Engineering a stable CHO cell line for the expression of a MERS-coronavirus vaccine antigen. Vaccine. 2018;36(14):1853–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Duivelshof BL, Murisier A, Camperi J, et al. Therapeutic Fc-fusion proteins: current analytical strategies. J Sep Sci. 2021;44(1):35–62. [DOI] [PubMed] [Google Scholar]
- 163.Sun S, He L, Zhao Z, et al. Recombinant vaccine containing an RBD-Fc fusion induced protection against SARS-CoV-2 in nonhuman primates and mice. Cell Mol Immunol. 2021;18(4):1070–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lu L, Palaniyandi S, Zeng R, et al. A neonatal Fc receptor-targeted mucosal vaccine strategy effectively induces HIV-1 antigen-specific immunity to genital infection. J Virol. 2011;85(20):10542–10553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Vizcarra CL, Mayo SL. Electrostatics in computational protein design. Curr Opin Chem Biol. 2005;9(6):622–626. [DOI] [PubMed] [Google Scholar]
- 166.Wunderlich M, Schmid FX. The correlation between protein stability and dipole moment: a critical test. Protein Eng Des Sel. 2006;19(8):355–358. [DOI] [PubMed] [Google Scholar]
- 167.Seid CA, Jones KM, Pollet J, et al. Cysteine mutagenesis improves the production without abrogating antigenicity of a recombinant protein vaccine candidate for human chagas disease. Hum Vaccin Immunother. 2017;13(3):621–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chen WH, Wei J, Kundu RT, et al. Genetic modification to design a stable yeast-expressed recombinant SARS-CoV-2 receptor binding domain as a COVID-19 vaccine candidate. Biochim Biophys Acta Gen Subj. 2021;1865(6):129893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Margos G, Vollmer SA, Cornet M, et al. A new Borrelia species defined by multilocus sequence analysis of housekeeping genes. Appl Environ Microbiol. 2009;75(16):5410–5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Pritt BS, Mead PS, Johnson DKH, et al. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: a descriptive study. Lancet Infect Dis. 2016;16(5):556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Binetruy F, Garnier S, Boulanger N, et al. A novel Borrelia species, intermediate between Lyme disease and relapsing fever groups, in neotropical passerine-associated ticks. Sci Rep. 2020;10 (1):10596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Shaffer L Inner workings: Lyme disease vaccines face familiar challenges, both societal and scientific. Proceedings of the National Academy of Sciences of the United States of America, 116(39), 19214–19217 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]


