Abstract
This study aims to investigate the prognostic value of the systemic immune-inflammation index (SII)and its impact on survival in patients with metastatic renal cell carcinoma (mRCC). A total of 706patients with mRCC treated with tyrosine kinase inhibitors (TKIs)between January 2007 and June 2020 (i.e., sunitinib, pazopanib) were included in this study. SII was calculated in 621 patients with the following formula:[neutrophil (cellsx109/L) x platelet (cellsx109/L)] / lymphocyte (cellsx109/L).All patients were classified into SII-high and SII-low groups based on the cut-off value of SII at 756, which was the median SII level of our study group. The minimal follow-up duration was 10 months in all cohorts. The median age of patients was 60 (interquartile range (IQR):53–67) years. Three out of four patients were male. The majority of patients (85.7%) had clear cell histology, and sarcomatoid differentiation was observed in 16.9% of all patients. There were 311 and 310 patients in the SII-low and SII-high groups, respectively. In general, baseline characteristics were similar in each group. However, the rate of patients treated with sunitinib (63.3% vs. 49.0%, p < 0.001) and those who underwent nephrectomy (83.6% vs. 64.2%, p < 0.001) was higher in the SII-low group than in the SII-high group. On the other hand, patients with the IMDC poorrisk (31.6% vs. 8.0%, p < 0.001), those with bone (51.8% vs. 32.2%, p < 0.001) or central nervous system (12.9% vs. 5.8%, p = 0.026) metastasis, and those with Eastern Cooperative Oncology Group(ECOG) 2–4 performance score (28.1% vs.17.7%, p = 0.002) were more common in the SII-high group than in the SII-low group. The median overall survival (OS) was longer in the SII-low group than in the SII-high group (34.6 months vs. 14.5 months, p < 0.001). Similarly, the median progression-free survival (PFS) was longer in the SII-low group than in the SII-high group (18.0 months vs. 7.7 months, p < 0.001).In multivariableanalysis, SII was an independent prognostic factor for OS (hazard ratio (HR):1.39, 95% confidence interval (CI):1.05–1.85, p = 0.01) and PFS (HR:1.60, 95% CI:1.24–2.05, p < 0.001).Pre-treatment level of high SII might be considered a predictor of poor prognosisin patients with mRCC treated with TKIs.
Subject terms: Cancer, Tumour biomarkers, Urological cancer
Introduction
Renal cell carcinoma (RCC)accounts for 90–95% of all kidney cancers. In 2020, about 3% of all adult malignancies with an estimated 431,288new RCC cases were observed across the world1,2.More than 30% ofpatients diagnosed with RCC need systemic therapy for metastatic disease3.In the last decade, huge improvements have been observed in the mRCC treatment.Thus, immune checkpoint inhibitor (ICI) plus tyrosine kinase inhibitor (TKI)or ICI plus ICI combinations improved survival in patients with metastatic RCC (mRCC)4,5.
In parallel to the improvements in the treatment of mRCC, prognostic risk toolsbecame essential duringthe decision-making process in the treatment of mRCC patients. Thus, the International Metastatic RCC Database (IMDC)risk model is the standard for prognostic stratificationofpatients with mRCC treated with targeted therapies or ICIs6,7. The IMDC risk score is calculated by the following six parameters: Karnofsky performance status, time from diagnosis to the first systemic treatment, hemoglobin concentration, neutrophils,platelets, and corrected calcium levels. Althoughthe IMDC is a commonly usedprognostic scoring system, efforts to find a novel scoring system with fewer parameters are still continuing. Inflammatory-related peripheral cells (e.g., neutrophils, lymphocytes, platelets) derived from the peripheral blood were associated with tumor progression in various tumors. The prognostic significance of inflammatory cell parameters, such as neutrophil–lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR), C-reactive protein/albumin ratio, and systemic immune inflammation index (SII), were examined in many cancer types over the last ten years8–15. SIIis a combination based on the peripheral lymphocyte, neutrophil, and platelet counts. After Hu et al.showed its prognostic value in 2014, many studies established that SII couldbe a good prognostic marker in many cancer types8.
In this retrospective analysis, we aimed to evaluate the prognostic significance of SII in patients with mRCC treated with TKIs.
Methods
The local ethical committee (Ankara University Faculty of Medicine Human Research Ethics Committee, approval number: 01-79-19)approved this retrospective cohort study. Informed consent was waived by “Ankara University Faculty of Medicine Human Research Ethics Committee” due to the retrospective nature of the study. This study was conducted in compliance with the “Declaration of Helsinki”.
Patient population and data extraction
The Turkish Oncology Group Kidney Cancer Consortium (TKCC) database consists of approximately 1,000 patients aged 18 years and older with mRCC from 13 cancer centers in Turkey. Patientswith mRCC treated with sunitinib or pazopanib in the first-line setting were extracted from the TKCC database. Patients treated with TKIs between January 2007 and June 2020 were included in the study. The minimum follow-up duration in all patients was 10 months.
Demographic data (e.g., date of birth, gender, comorbidities, medications), date of diagnosis with RCC, the initial date of systemic treatment in the metastatic setting, Eastern Cooperative Oncology Group (ECOG) performance score, laboratory findings (e.g., neutrophil, platelet, lymphocyte counts, hemoglobin concentration, corrected calcium level),start and end dates of TKIs, and dates of progression and death were extracted from the TKCC database.
SII was calculated by using the following formula:[neutrophil (cellsx109/L) x platelet (cellsx109/L)] / lymphocyte (cellsx109/L). All values were obtained from a complete blood count (CBC) up to 30 days before the first dose of TKIs. If there were more than one CBC result, the closest one to the initiation of TKI was used. The best cut-off value for SII was determined by using the median value of 756.In this regard, patients were divided into two groups: SII-high (≥ 756) and SII-low (< 756). The primary outcome was overall survival (OS), and the secondary outcome was progression-free survival (PFS).
Statistical analyses
To summarize data, median with interquartile range (IQR) or mean with standard deviationand percentages were used for continuous and categorical variables, respectively. The independent sample t-test or Mann–Whitney Uand chi-squaretests were performed to compare continuous and categorical variables, respectively. Survival curves were estimated using the Kaplan–Meier method, and the differences between groups were analyzed by using thelog-rank test. Cox proportional hazards regression model was used for multivariable analyses of parameters associated with OS and PFS.OS was calculated from the initial date of TKIs to death.PFS was calculated from the initial date of TKIsto disease progression or death. Hazard ratio (HR) and 95% confidence interval (CI) were used to describe the risk factors.Harrell’s concordance index (C-index) was calculated to compare the predictive value of SII and the IMDC risk scores for OS and PFS. Differences were considered significant if the p-value was less than 0.05.All statistical analyses were performed using the SPSS 27.0 for Mac (IBM Corp., Armonk, NY).
Results
Baseline characteristics
A total of 706 patientswith mRCC were included in this study and SII was calculated in 621 patients. The median age of patients was 60 (IQR: 53–67) years. Three out of four patients were male.Most patients (85.7%) had clear cell histology,and 16.9%of all patients had sarcomatoid differentiation.The ECOG PS was 0 or 1 in most patients (83.5%). Approximately one out of four patients were in the IMDC poor-risk group.404 (57.2%) and 302 (42.8%)patients were treated with sunitinib and pazopanib,respectively.Approximately half of the patientsreceived interferon before TKI treatment.About three out of four patients underwent nephrectomybeforestarting systemic treatment. The lung was the most common metastatic site (51.4%).
There were 311 and 310 patients in the SII-low and SII-high groups, respectively. The rate of patients who underwent nephrectomy was higher in the SII-low group than in the SII-high group (83.9% vs. 64.4%, p < 0.001). Similarly, the rate of patients treated with sunitinib was higher in the SII-low group than in the SII-high group (63.3% vs. 49.0%, p < 0.001).The IMDC poor-risk patients’ rate was higher in the SII-high group than in the SII-low group (34.6% vs. 8.8%, p < 0.001). Allbaseline characteristics of the included patients are shown in Table 1.
Table 1.
Baseline characteristics.
| All patients | SII-low patients | SII-high patients | p | ||||
|---|---|---|---|---|---|---|---|
| n = 706 | (%) | n = 311 | (%) | n = 310 | (%) | ||
| Age-years, median (IQR) | 60 (53–67) | 60 (53–69) | 60 (53–70) | 0.710 | |||
| Sex | 0.317 | ||||||
| Male | 531 | 75.2 | 229 | 73.6 | 239 | 77.1 | |
| Female | 175 | 24.8 | 82 | 26.4 | 71 | 22.9 | |
| Histological Type | 0.196 | ||||||
| Clear Cell | 563 | 79.7 | 241 | 77.5 | 257 | 82.9 | |
| Non-clear Cell | 94 | 13.3 | 46 | 14.8 | 36 | 11.6 | |
| Missing | 49 | 6.9 | 24 | 7.7 | 17 | 5.5 | |
| Sarcomatoid Feature | 0.830 | ||||||
| Yes | 83 | 11.8 | 35 | 11.3 | 39 | 12.6 | |
| No | 407 | 57.6 | 182 | 58.5 | 192 | 61.9 | |
| Missing | 216 | 30.6 | 94 | 30.2 | 79 | 25.5 | |
| Fuhrman Grade | 0.076 | ||||||
| 1–2 | 124 | 17.6 | 63 | 20.3 | 43 | 13.9 | |
| 3–4 | 297 | 42.1 | 129 | 41.4 | 133 | 42.9 | |
| Missing | 285 | 40.4 | 119 | 38.3 | 134 | 43.2 | |
| Previous Nephrectomy | < 0.001 | ||||||
| Yes | 525 | 74.4 | 260 | 83.6 | 199 | 64.2 | |
| No | 177 | 25.1 | 50 | 16.1 | 110 | 35.5 | |
| Missing | 4 | 0.6 | 1 | 0.3 | 1 | 0.3 | |
| Systemic Treatment | < 0.001 | ||||||
| Sunitinib | 404 | 57.2 | 197 | 63.3 | 152 | 49.0 | |
| Pazopanib | 302 | 42.8 | 114 | 36.7 | 158 | 51.0 | |
| IMDC Risk | < 0.001 | ||||||
| Favorable | 116 | 16.4 | 83 | 26.7 | 33 | 10.6 | |
| Intermediate | 332 | 47.0 | 175 | 56.3 | 152 | 49.0 | |
| Poor | 128 | 18.1 | 25 | 8.0 | 98 | 31.6 | |
| Missing | 130 | 18.4 | 28 | 9.0 | 27 | 8.7 | |
| MSKCC Risk | < 0.001 | ||||||
| Favorable | 91 | 12.9 | 64 | 20.6 | 27 | 8.7 | |
| Intermediate | 279 | 39.5 | 148 | 47.6 | 128 | 41.3 | |
| High | 87 | 12.3 | 27 | 8.7 | 59 | 19.0 | |
| Missing | 249 | 35.3 | 72 | 23.2 | 96 | 31.0 | |
| Previous Cytokine Use | 0.032 | ||||||
| Yes | 334 | 47.3 | 152 | 48.9 | 125 | 40.3 | |
| No | 372 | 52.7 | 159 | 51.1 | 185 | 59.7 | |
| Metastatic Sites | |||||||
| Lung | 319 | 51.4 | 161 | 51.8 | 158 | 51.0 | 0.886 |
| Bone | 259 | 41.7 | 100 | 32.2 | 159 | 51.3 | < 0.001 |
| Liver | 92 | 14.8 | 42 | 13.5 | 50 | 16.1 | 0.252 |
| CNS | 58 | 9.3 | 18 | 5.8 | 40 | 12.9 | 0.026 |
| Performance Status | 0.002 | ||||||
| ECOG 0–1 | 515 | 72.9 | 243 | 78.1 | 207 | 66.8 | |
| ECOG 2–3-4 | 149 | 21.1 | 55 | 17.7 | 87 | 28.1 | |
| Missing | 42 | 5.9 | 13 | 4.2 | 16 | 5.2 | |
ECOG eastern cooperative oncology group, IMDC international metastatic renal cell carcinoma database consortium, IQR interquartile range, MSKCC memorial sloan kettering cancer center.
Significant values are in bold.
Survival outcomes
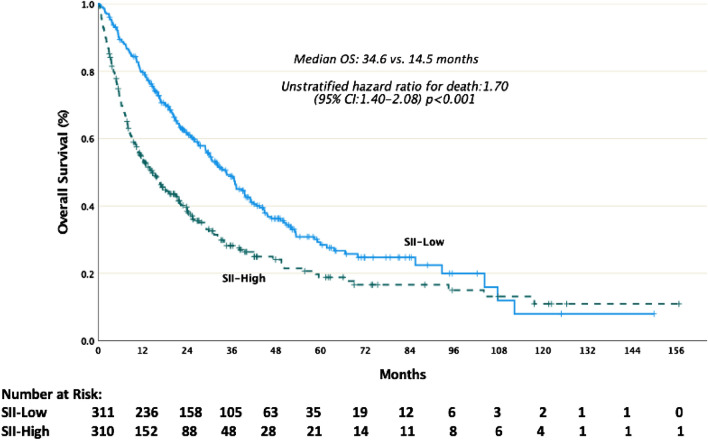
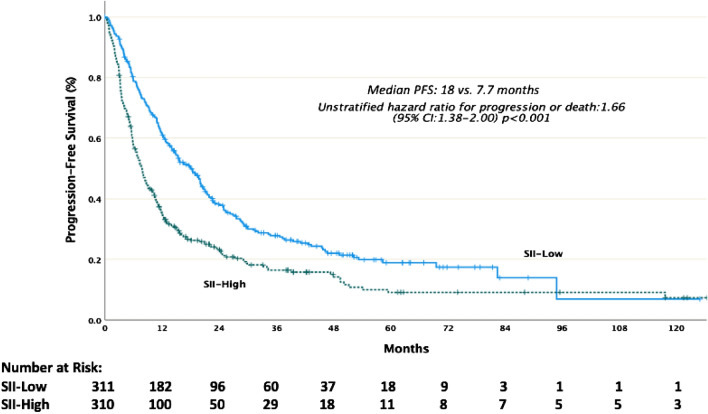
At the median follow-up of 48.6 months, the median OS and PFS were26.1 months (95% CI: 22.5–29.7) and 11.9 months (95% CI: 10.5–13.3), respectively.The median OS was longer in the SII-low group than in the SII-high group (34.6 months vs. 14.5 months, p < 0.001). Similarly, the median PFS was longer in the SII-low group than in the SII-high group (18.0 months vs. 7.7 months, p < 0.001). Kaplan–Meier estimates of OS and PFS are shown in Figs. 1,2
Figure 1.
Kaplan-Meier estimates of overall survival (OS). SII systemic immune inflammation index.
Figure 2.
Kaplan-Meier estimates of progression-free survival (PFS). SII systemic immune inflammation index.
After adjusting for confounding factors (age, sarcomatoid feature, nephrectomy, systemic treatment with sunitinib or pazopanib, anemia, hypercalcemia, LDH elevation, ECOG PS, time from diagnosis to systemic treatment, the total number of systemic treatment (except for IFN), and presence of bone or central nervous system (CNS) metastasis for OS; sarcomatoid feature, nephrectomy, anemia, hypercalcemia, LDH elevation, ECOG PS, time from diagnosis to systemic treatment, and presence of bone or CNS metastasis for PFS), SII was an independent prognostic factor for OS (HR:1.41, 95% CI: 1.06–1.87, p = 0.018) and PFS (HR:1.64, 95% CI:1.28–2.10, p < 0.001).Uni-and multivariable analyses of OS and PFS are shown in Tables 2, 3.
Table 2.
Univariable and multivariable analysis of overall survival.
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| hazard ratio | 95% CI | p | hazard ratio | 95% CI | p | |
| Age | 0.003 | 0.141 | ||||
| < 65 | 1 | 1 | ||||
| ≥ 65 | 1.34 | 1.10–1.63 | 1.23 | 0.93–1.64 | ||
| Sarcomatoid Feature | 0.018 | 0.003 | ||||
| No | 1 | 1 | ||||
| Yes | 1.41 | 1.06–1.89 | 1.72 | 1.21–2.46 | ||
| Nephrectomy | < 0.001 | 0.018 | ||||
| No | 1.94 | 1.58–2.39 | 1.53 | 1.08–2.22 | ||
| Yes | 1 | 1 | ||||
| Systemic Treatment | 0.034 | 0.704 | ||||
| Sunitinib | 1 | 1 | ||||
| Pazopanib | 1.22 | 1.01–1.48 | 1.05 | 0.79–1.39 | ||
| Anemia | < 0.001 | 0.014 | ||||
| No | 1 | 1 | ||||
| Yes | 1.93 | 1.59–2.35 | 1.41 | 1.07–1.86 | ||
| Hypercalcemia | < 0.001 | 0.894 | ||||
| No | 1 | 1 | ||||
| Yes | 2.21 | 1.52–3.21 | 1.04 | 0.53–2.06 | ||
| LDH Elevation | < 0.001 | 0.190 | ||||
| No | 1 | 1 | ||||
| Yes | 1.97 | 1.47–2.64 | 1.32 | 0.87–2.00 | ||
| ECOG Performance Score | < 0.001 | < 0.001 | ||||
| ECOG 0–1 | 1 | 1 | ||||
| ECOG 2–3-4 | 3.18 | 2.58–3.91 | 2.75 | 2.04–3.71 | ||
| Time to Systemic Treatment | < 0.001 | 0.411 | ||||
| < 1 year | 1.59 | 1.30–1.96 | 1.14 | 0.82–1.59 | ||
| ≥ 1 year | 1 | 1 | ||||
| Previous Cytokine Use | 0.491 | |||||
| No | 1 | |||||
| Yes | 1.06 | 0.88–1.28 | ||||
| Bone or CNS Metastasis | < 0.001 | 0.141 | ||||
| No | 1 | 1 | ||||
| Yes | 1.60 | 1.28–2.00 | 1.23 | 0.93–1.64 | ||
| Number of Systemic Treatment* | 0.061 | 0.289 | ||||
| 1 | 1.19 | 0.99–1.44 | 1.16 | 0.88–1.52 | ||
| > 1 | 1 | 1 | ||||
| SII | < 0.001 | 0.018 | ||||
| Low | 1 | 1 | ||||
| High | 1.70 | 1.40–2.08 | 1.41 | 1.06–1.87 | ||
CI confidence interval, CNS central nervous system, ECOG eastern cooperative oncology group, LDH lactate dehydrogenase, SII systemic immune-inflammation index.
Significant values are in bold.
*Except for interferon.
Table 3.
Univariable and multivariable analysis of progression-free survival.
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| hazard ratio | 95% CI | p | hazard ratio | 95% CI | p | |
| Age | 0.116 | |||||
| < 65 | 1 | |||||
| ≥ 65 | 1.15 | 0.96–1.37 | ||||
| Sarcomatoid Feature | 0.006 | 0.013 | ||||
| No | 1 | 1 | ||||
| Yes | 1.45 | 1.11–1.89 | 1.49 | 1.08–2.04 | ||
| Nephrectomy | < 0.001 | 0.142 | ||||
| No | 1.77 | 1.46–2.14 | 1.26 | 0.92–1.73 | ||
| Yes | 1 | 1 | ||||
| Systemic Treatment | 0.289 | |||||
| Sunitinib | 1 | |||||
| Pazopanib | 0.91 | 0.76–1.08 | ||||
| Anemia | < 0.001 | 0.008 | ||||
| No | 1 | 1 | ||||
| Yes | 1.65 | 1.38–1.98 | 1.39 | 1.09–1.79 | ||
| Hypercalcemia | 0.001 | 0.565 | ||||
| No | 1 | 1 | ||||
| Yes | 1.79 | 1.25–2.57 | 1.15 | 0.70–1.91 | ||
| LDH Elevation | 0.010 | 0.848 | ||||
| No | 1 | 1 | ||||
| Yes | 1.44 | 1.09–1.91 | 1.04 | 0.64–1.70 | ||
| ECOG Performance Score | < 0.001 | < 0.001 | ||||
| ECOG 0–1 | 1 | 1 | ||||
| ECOG 2–3-4 | 2.24 | 1.84–2.74 | 1.82 | 1.37–2.41 | ||
| Time to Systemic Treatment | < 0.001 | 0.937 | ||||
| < 1 year | 1.51 | 1.26–1.82 | 1.01 | 0.75–1.34 | ||
| ≥ 1 year | 1 | 1 | ||||
| Previous Cytokine Use | 0.567 | |||||
| No | 1 | |||||
| Yes | 1.05 | 0.88–1.24 | ||||
| Bone or CNS Metastasis | < 0.001 | 0.552 | ||||
| No | 1 | 1 | ||||
| Yes | 1.48 | 1.25–1.75 | 1.07 | 0.84–1.38 | ||
| SII | < 0.001 | < 0.001 | ||||
| Low | 1 | 1 | ||||
| High | 1.66 | 1.38–2.00 | 1.64 | 1.28–2.10 | ||
CI confidence interval, CNS central nervous system, ECOG eastern cooperative oncology group, LDH lactate dehydrogenase, SII systemic immune-inflammation index.
Significant values are in bold.
In the subgroup analysis of patients who were not treated with IFN, the median OS was longer in the SII-low group than in the SII-high group (36.4 months vs. 16.6 months, p = 0.001 in patients previously untreated with interferon). Similarly, the median PFS was also longer in the SII-low group than in the SII-high group(19.7 months vs. 8.1 months, p < 0.001) (Figures S1 and S2).
Harrell’s C-index with SII, IMDC, and MSKCC risk scoreswas 0.60, 0.63, 0.63for OS, and 0.59, 0.60, 0.61 for PFS, respectively.
Discussion
In this multicenter study,we investigated the prognostic value of SII in patients with mRCC treated with TKIs. To the best of our knowledge, our study has the largest number of patients among studies examining the relationship between SII and survival outcomes in patients with mRCC16–20. The results showed that low (< 756) and high(≥ 756) SII levels had a statistically significant difference in terms of OS and PFS. Thus, SII might have a prognostic value in patients with mRCC treated with TKIs.
Many previous studies have widely investigated the relationship between inflammation and cancer. Inflammatory cells (e.g., neutrophils, macrophages, lymphocytes ) and cytokines are effective in transformation, proliferation, and metastasis in all tumor stages21. Neutrophils can secrete cytokines related to the stimulation of the tumor microenvironment and have a tumor-promoting activity, including cancer cell survival and proliferation, angiogenesis, and metastasis13. Conversely, lymphocytes inhibit tumor cell proliferation by secreting cytokines.On the other hand, platelets regulate cancer invasion, migration, and angiogenesis by secretion of numerous chemokines and growth factors 22. In 2014, Hu et al. developed SII to predict the prognosis of patients who underwent curative resection for hepatocellular carcinoma and established that a high SII score (> 330 × 109 cells/L) indicated a poor outcome for those patients8. Subsequently, SII has been investigated as a marker to predict cancer survival in various tumors, such as gastric cancer, germ-cell tumor, and prostate cancer6–12.. A recentstudy that evaluated the impact of SII on the survival of patients with mRCC treated with TKI was published in 2020. In this study, Teishima et al. showed that low SII was associated with poorer survival in 179 patients with mRCC treated with TKI16. Furthermore, the pre-treatment SII cut-off value was determined as 730 × 109 cells/L in the study of Teishima et al., which was numerically close to SII cut-off value of our study. It should be noted that the number of patients was higher in our study.Another study that investigated the relationship between SII and survival in patients with mRCC treated with TKIs was reported by Lolli et al. They included 335 patients with mRCC and concluded that pretreatment SII was an independent prognostic factor OS12.
In addition to prognostic value in patients with mRCC, SII was also evaluated as a prognostic marker inearly RCC patients. The studies concluded that SII was an accurate prognostic marker irrespective of disease stage in RCC19,20. Actually, this result may be associated with the role of immune system in the clinical course of RCC irrespective of clinical stage23.
We have several approved prognostic scoring systemsin patients with mRCC. The IMDC and Memorial Sloan Kettering Cancer Center (MSKCC) were the most popularrisk models24,25.However, the IMDC and MSKCC risk scores are calculated by using six and five parameters, respectively. In our study, C-index values were almost similar for OS and PFS in SII, IMDC, and MSKCC risk scores. SII could provide the same prognostic accuracy as the IMDC and MSKCC, despite only including neutrophils, platelets, and lymphocytes in the equation.About the prognostic value of IMDC risk score and SII combination, Chrom et al.showed thatreplacement of neutrophil and platelet counts with SII in the IMDC risk model increased the accuracy of the IMDC risk model.It should be noticed that they also used a cut-off value of 730 × 109 cells/L for SII, which is almost the same asour study26.Furthermore, as a result of efforts to find a novel prognostic marker in patients with mRCC, Başal et al. showed that SII could predict survival in each IMDC risk group27.
Our survival results were also compatible with the pivotal study of sunitinib,including previously untreated patients with mRCC. They reported that the median OS was 26.4 months and PFS was 11 months in patients with mRCC receiving sunitinib, which was also numerically close to our study’s survival results28.
Our study has several limitations due to its retrospective nature. First, we had a lack of data to calculate SII in some patients. Because of this reason, we had to exclude those patients from our study. Second,the time interval betweenobtaining laboratory values to calculate SII and the initial date of TKIs might be different in each included center.Third, mRCC patients treated with interferon before TKI treatment were included in our study. ICI plus TKI or ICI plus ICI combinationsare accepted as the standard of care in the first-line treatment of patients with mRCC. Althoughcombinationsare considered standard treatment, there is still a subgroup of patients who benefit from TKI alone. ICI plus TKI studies concluded that no clear difference between the sunitinib and combination arms in survival outcomes in the IMDC favorable risk group29–31. All these findings suggest that we cannot completely abandon TKIs in the treatment of patients with mRCC.
In conclusion, our study showed the prognostic value of SII in mRCC patients treated with TKIs. In this context, SII, an easily accessible marker, might lead to establishing novel therapeutic strategies or risk models in patients with mRCC treated with TKIs.Although -studies evaluated prognostic effect of SII on patients treated with ICI,the relationship of ICIs plus TKIs combinations with SII has not been investigated yet32,33. SII may be a potential prognostic marker for RCC patients treated with ICI and TKIs combination from a future perspective.
Supplementary Information
Author contributions
Conceptualization: K.B.Y., E.Y., S.K., D.T., İ.E., C.E., Ö.E., N.Ş.Ö., Ç.A., G.U., A.K., Ö.N.S., S.K., O.Ç., S.C.Y., B.Ö., N.K., M.A.Ş., Y.Ü. Methodology: K.B.Y., E.Y., Y.Ü. Software: E.Y. Data Curation: K.B.Y., E.Y., S.K., D.T., İ.E., C.E., Ö.E., N.Ş.Ö., Ç.A., G.U., A.K., Ö.N.S., S.K., O.Ç., S.C.Y., B.Ö., N.K., M.A.Ş., Y.Ü.. Writing—Original Draft: K.B.Y., E.Y. Writing–Review and Editing: K.B.Y., E.Y., S.K., D.T., İ.E., C.E., Ö.E., N.Ş.Ö., Ç.A., G.U.,A.K., Ö.N.S., S.K., O.Ç., S.C.Y., B.Ö., N.K., M.A.Ş., Y.Ü. Supervision: S.K., D.T., İ.E., C.E., Ö.E., N.Ş.Ö., Ç.A., G.U.,A.K., Ö.N.S., S.K., O.Ç., S.C.Y., B.Ö., N.K., M.A.Ş, Y.Ü.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-20056-3.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA a cancer j clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA a cancer j. clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 3.Lam JS, Shvarts O, Leppert JT, et al. Renal cell carcinoma 2005: New frontiers in staging, prognostication and targeted molecular therapy. J. Urol. 2005;173:1853–1862. doi: 10.1097/01.ju.0000165693.68449.c3. [DOI] [PubMed] [Google Scholar]
- 4.Tannir, N.M., Frontera, O.A., Hammers, H.J., et al. Thirty-month follow-up of the phase III CheckMate 214 trial of first-line nivolumab+ ipilimumab (N+ I) or sunitinib (S) in patients (pts) with advanced renal cell carcinoma (aRCC). Am. Soc. Clin. Oncol. (2019).
- 5.Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 2019;380:1116–1127. doi: 10.1056/NEJMoa1816714. [DOI] [PubMed] [Google Scholar]
- 6.Motzer RJ, Mazumdar M, Bacik J, et al. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J. Clin. Oncol. 1999;17:2530–2530. doi: 10.1200/JCO.1999.17.8.2530. [DOI] [PubMed] [Google Scholar]
- 7.Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor–targeted agents: results from a large, multicenter study. J. Clin. Oncol. 2009;27:5794–5799. doi: 10.1200/JCO.2008.21.4809. [DOI] [PubMed] [Google Scholar]
- 8.Hu B, Yang X-R, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin. Cancer Res. 2014;20:6212–6222. doi: 10.1158/1078-0432.CCR-14-0442. [DOI] [PubMed] [Google Scholar]
- 9.Zhong J-H, Huang D-H, Chen Z-Y. Prognostic role of systemic immune-inflammation index in solid tumors: A systematic review and meta-analysis. Oncotarget. 2017;8:75381. doi: 10.18632/oncotarget.18856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen J-H, Zhai E-T, Yuan Y-J, et al. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J. Gastroenterol. 2017;23:6261. doi: 10.3748/wjg.v23.i34.6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tong Y-S, Tan J, Zhou X-L, et al. Systemic immune-inflammation index predicting chemoradiation resistance and poor outcome in patients with stage III non-small cell lung cancer. J. Transl. Med. 2017;15:1–10. doi: 10.1186/s12967-017-1326-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lolli C, Caffo O, Scarpi E, et al. Systemic immune-inflammation index predicts the clinical outcome in patients with mCRPC treated with abiraterone. Front. Pharmacol. 2016;7:376. doi: 10.3389/fphar.2016.00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Templeton, A.J., McNamara, M.G., Šeruga, B., et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. JNCI: J. Natl. Cancer Inst. 106, (2014). [DOI] [PubMed]
- 14.Rossi L, Santoni M, Crabb SJ, et al. High neutrophil-to-lymphocyte ratio persistent during first-line chemotherapy predicts poor clinical outcome in patients with advanced urothelial cancer. Ann. Surg. Oncol. 2015;22:1377–1384. doi: 10.1245/s10434-014-4097-4. [DOI] [PubMed] [Google Scholar]
- 15.Templeton AJ, Knox JJ, Lin X, et al. Change in neutrophil-to-lymphocyte ratio in response to targeted therapy for metastatic renal cell carcinoma as a prognosticator and biomarker of efficacy. Eur. Urol. 2016;70:358–364. doi: 10.1016/j.eururo.2016.02.033. [DOI] [PubMed] [Google Scholar]
- 16.Teishima J, Inoue S, Hayashi T, et al. Impact of the systemic immune-inflammation index for the prediction of prognosis and modification of the risk model in patients with metastatic renal cell carcinoma treated with first-line tyrosine kinase inhibitors. Can. Urol. Assoc. J. 2020;14:E582. doi: 10.5489/cuaj.6413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laukhtina, E., Pradere, B., D'Andrea, D., et al. Prognostic effect of preoperative systemic immune-inflammation index in patients treated with cytoreductive nephrectomy for metastatic renal cell carcinoma. Minerva Urol. Nephrol. (2021) [DOI] [PubMed]
- 18.Fukuda H, Takagi T, Kondo T, et al. Predictive value of inflammation-based prognostic scores in patients with metastatic renal cell carcinoma treated with cytoreductive nephrectomy. Oncotarget. 2018;9:14296. doi: 10.18632/oncotarget.24507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ozbek E, Besiroglu H, Ozer K, et al. Systemic immune inflammation index is a promising non-invasive marker for the prognosis of the patients with localized renal cell carcinoma. Int. Urol. Nephrol. 2020;52:1455–1463. doi: 10.1007/s11255-020-02440-y. [DOI] [PubMed] [Google Scholar]
- 20.Hu X, Shao Y-X, Yang Z-Q, et al. Preoperative systemic immune-inflammation index predicts prognosis of patients with non-metastatic renal cell carcinoma: a propensity score-matched analysis. Cancer Cell Int. 2020;20:1–9. doi: 10.1186/s12935-020-01320-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 22.Schumacher D, Strilic B, Sivaraj KK, et al. Platelet-derived nucleotides promote tumor-cell transendothelial migration and metastasis via P2Y2 receptor. Cancer Cell. 2013;24:130–137. doi: 10.1016/j.ccr.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Yin C, Geng L, et al. immune infiltration landscape in clear cell renal cell carcinoma implications. Front Oncol. 2020;10:491621. doi: 10.3389/fonc.2020.491621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J. Clin. Oncol. 2009;27:5794–5799. doi: 10.1200/JCO.2008.21.4809. [DOI] [PubMed] [Google Scholar]
- 25.Motzer RJ, Bukowski RM, Figlin RA, et al. Prognostic nomogram for sunitinib in patients with metastatic renal cell carcinoma. Cancer. 2008;113:1552–1558. doi: 10.1002/cncr.23776. [DOI] [PubMed] [Google Scholar]
- 26.Chrom P, Zolnierek J, Bodnar L, et al. External validation of the systemic immune-inflammation index as a prognostic factor in metastatic renal cell carcinoma and its implementation within the international metastatic renal cell carcinoma database consortium model. Int. J. Clin. Oncol. 2019;24:526–532. doi: 10.1007/s10147-018-01390-x. [DOI] [PubMed] [Google Scholar]
- 27.Bugdayci Basal F, Karacin C, Bilgetekin I, et al. Can systemic immune-inflammation index create a new perspective for the IMDC scoring system in patients with metastatic renal cell carcinoma? Urol. Int. 2021;105:666–673. doi: 10.1159/000513456. [DOI] [PubMed] [Google Scholar]
- 28.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N. Engl. J. Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 29.Choueiri TK, Powles T, Burotto M, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 2021;384:829–841. doi: 10.1056/NEJMoa2026982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 2019;380:1116–1127. doi: 10.1056/NEJMoa1816714. [DOI] [PubMed] [Google Scholar]
- 31.Motzer R, Alekseev B, Rha SY, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N. Engl. J. Med. 2021;384:1289–1300. doi: 10.1056/NEJMoa2035716. [DOI] [PubMed] [Google Scholar]
- 32.De Giorgi U, Procopio G, Giannarelli D, et al. Association of systemic inflammation index and body mass index with survival in patients with renal cell cancer treated with nivolumab. Clin. Cancer Res. 2019;25:3839–3846. doi: 10.1158/1078-0432.CCR-18-3661. [DOI] [PubMed] [Google Scholar]
- 33.Iinuma K, Enomoto T, Kawada K, et al. Utility of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and systemic immune inflammation index as prognostic, predictive biomarkers in patients with metastatic renal cell carcinoma treated with nivolumab and ipilimumab. J. Clin. Med. 2021;10:5325. doi: 10.3390/jcm10225325. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.