Abstract
Classic and exertional heatstroke cause acute injury and damage across numerous organ systems. Moreover, heatstroke survivors may sustain long-term neurologic, cardiovascular, and renal complications with a persistent risk of death. In this context, biomarkers, defined as biological samples obtained from heatstroke patients, are needed to detect early organ injury, and predict outcomes to develop novel organ preservation therapeutic strategies. This narrative review provides preliminary insights that will guide the development and future utilization of these biomarkers. To this end, we have identified numerous biomarkers of widespread heatstroke-associated cellular injury, tissue damage and repair (extracellular heat shock proteins 72 and 60, high mobility group box protein 1, histone H3, and interleukin-1α), and other organ-specific biomarkers including those related to the cardiovascular system (cardiac troponin I, endothelium-derived factors, circulation endothelial cells, adhesion molecules, thrombomodulin, and von Willebrand factor antigen), the kidneys (plasma and urinary neutrophil gelatinase-associated lipocalin), the intestines (intestinal fatty acid-binding protein 2), the brain (serum s100β and neuron-specific enolase) and skeletal muscle (creatine kinase, myoglobin). No specific biomarkers have been identified so far for liver or lung injury in heatstroke. Before translating the identified biomarkers into clinical practice, additional preclinical and clinical prospective studies are required to further understand their clinical utility, particularly for the biomarkers related to long-term post-heatstroke health outcomes.
Keywords: Exertional heatstroke, classic heatstroke, biomarkers, organ systems, multiorgan failure, hyperthermia
Introduction
With global temperatures rising, heatwaves have become more frequent, intense, and longer in duration (IPCC, 2021), thereby exposing a greater proportion of the population to the deadly risk of extreme heat (Mora et al., 2017). Heat exposure elevates the risk of developing heat illness, which describes a continuum of pathologies ranging from relatively mild conditions associated with alterations in cardiovascular functioning (e.g., heat syncope, heat exhaustion) to potentially lethal heatstroke (Armstrong et al., 2007). Heatstroke is characterized by a rapidly rising core temperature (usually >40.1°C), central nervous system dysfunction, systemic inflammation, and organ system injury that can culminate in death (Bouchama et al., 2022a). Heatstroke manifests in two distinct forms, classic heatstroke (CHS) and exertional heatstroke (EHS) (Bouchama et al., 2022a). CHS typically occurs during resting exposures to hot and humid conditions, which limit the capacity for heat loss, and more frequently affects the very young, older adults, and populations with comorbidities (Bouchama et al., 2022a). EHS more often occurs in younger, physically active, healthy adults, such as military personnel and competitive or recreational athletes, and is characterized by the inability to adequately dissipate enough heat sufficient to offset the heat load caused by physical exertion (i.e., elevations in metabolic heat production) (Bouchama et al., 2022a). As a result, EHS can occur across a comparatively wide range of environmental conditions (Rae et al., 2008; Stacey et al., 2022). Therefore, the threat of heatstroke can impact the health and well-being of millions of people around the globe, and this risk will likely continue to increase in the coming decades (IPCC, 2021).
Heatstroke is diagnosed mainly through clinical signs and symptoms. Hallmarks of heatstroke include an elevated core temperature (often >40.1°C) and central nervous system alterations (e.g., confusion, irritability, altered consciousness) (Casa et al., 2015; Bouchama et al., 2022a) combined with a relevant patient history of exposure to hot and humid conditions and/or strenuous physical exertion (Leon & Bouchama, 2011). Notably, however, a core temperature less than 40.1°C should never rule out the diagnosis of heatstroke as the measurement of core temperature is often delayed, usually after the patient’s removal from environmental heat and/or cessation of the exercise and arrival of paramedics. With early diagnosis and rapid cooling, the prognosis for heatstroke victims is generally good (Demartini et al., 2015). In contrast, the risk of prolonged morbidity and mortality increases when heatstroke diagnosis and treatment are delayed, resulting in tissue damage and multiorgan dysfunction/failure, and eventually death. This evolution has been attributed to heat cytotoxicity, excessive systemic inflammation, and hypercoagulability (Leon & Bouchama, 2011; Casa et al., 2012; Bouchama et al., 2022a). The systemic nature of heatstroke pathology is exemplified in an analysis of 2,529 EHS episodes occurring in military personnel from 2008 to 2014, demonstrating abnormal clinical indices of liver, renal and hematological function and muscle damage that peak 0–4 days following diagnosis and persist for up to 16 days post injury (Ward et al., 2020). These findings corroborate animal literature demonstrating widespread damage across numerous organ systems following CHS (Leon et al., 2006b; Roberts et al., 2008a) and EHS (King et al., 2015). Moreover, observational evidence indicates that a single episode of heatstroke could have deleterious long-term all-cause mortality (Wallace et al., 2007), neurologic (Dematte et al., 1998; Argaud et al., 2007; Yang et al., 2017), cardiovascular (Wallace et al., 2007; Wang et al., 2019) and renal (Wang et al., 2019; Wu et al., 2021) complications with a continuous risk of death (Bouchama et al., 2022a). These observations have raised the need to develop tools that aid in detecting tissue and organ injury, monitoring recovery (or repair failure), and predicting long-term complications following heatstroke (Figure 1). Therefore, this narrative review provides an up-to-date overview of novel biological markers (biomarkers) that could help identify the patients at risk of organ injury or long-term complications following heatstroke. Our goal is not to provide recommendations for the clinical deployment of biomarkers because the literature base remains too underdeveloped for this to be reasonably accomplished. Rather, we will provide preliminary insights on biomarkers that could aid the prediction of short-term health outcomes in heatstroke and ultimately help develop novel preservation strategies to reduce organ damage and potentially prevent long-term sequela (Figure 2).
Figure 1:
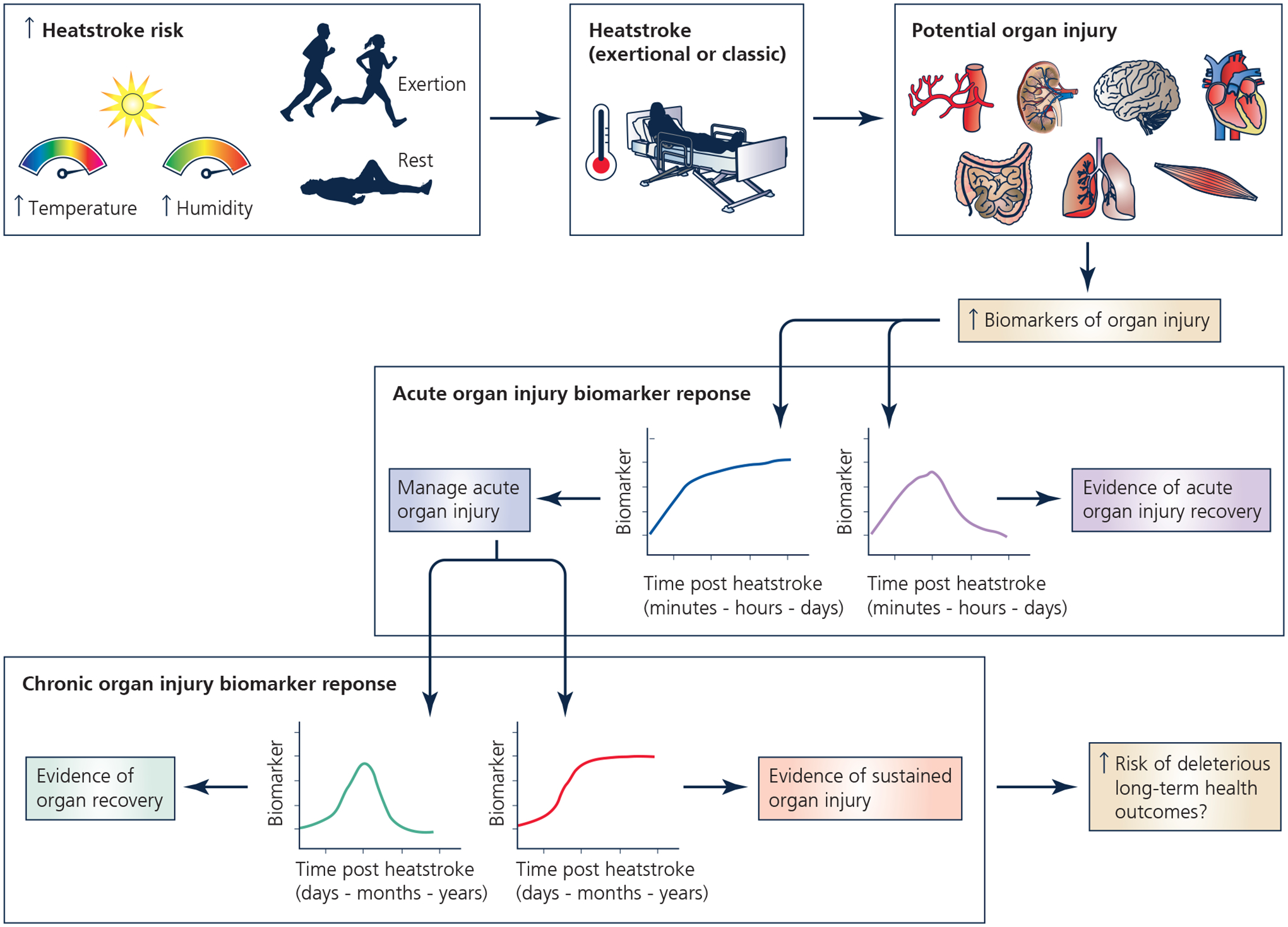
Potential utility of biomarkers to detect tissue and organ injury, monitor recovery, and predict long-term complications following heatstroke. The purpose of this narrative review is to identify biomarkers of heatstroke induced organ injury and repair. Created with BioRender.com.
Figure 2:
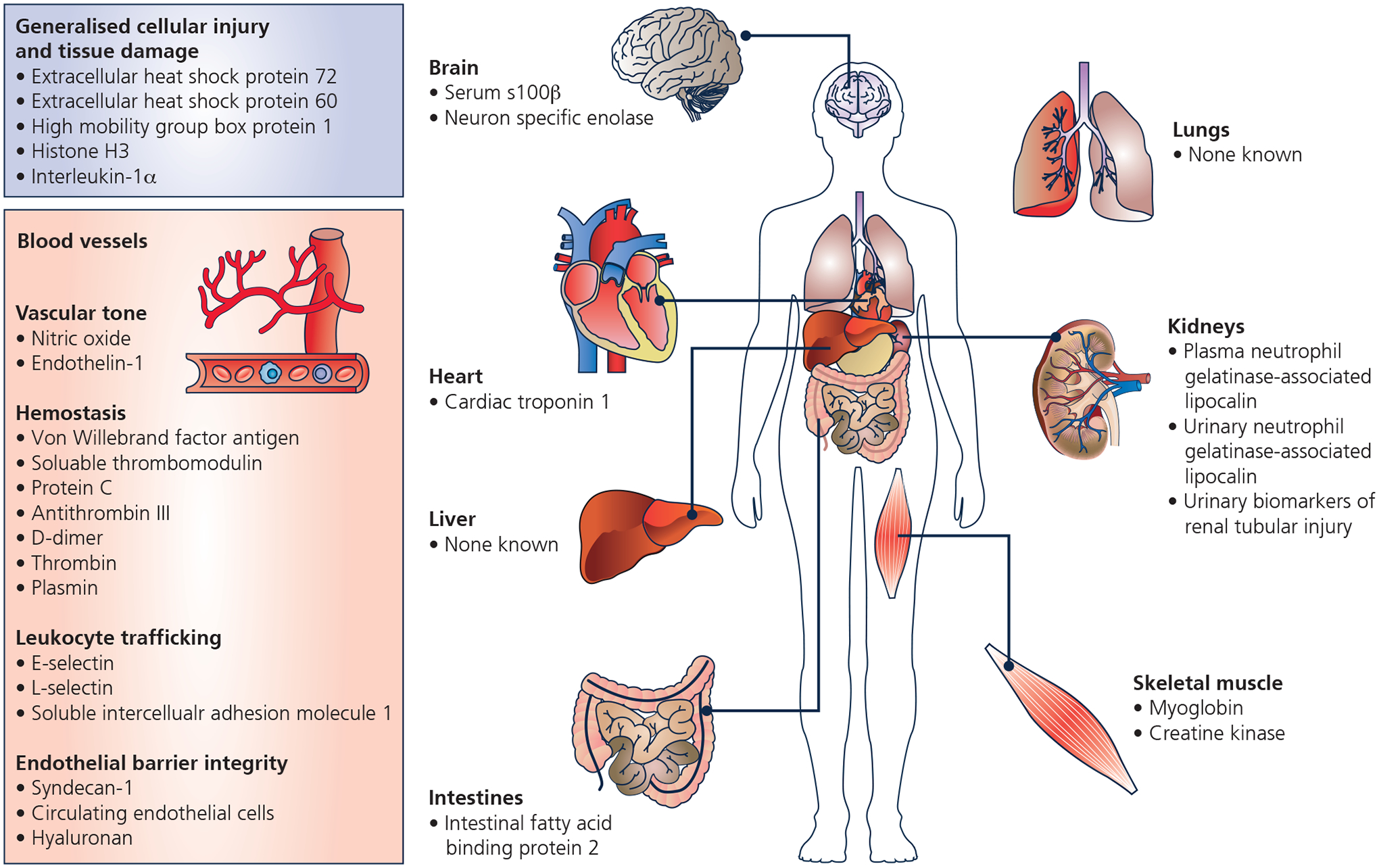
Identified biomarkers of heatstroke induced organ injury and/or recovery. Created with BioRender.com.
For this review, a biomarker is defined as a measurable substance derived from a bodily fluid that does not require assessment of clinical signs and symptoms. Notably, this definition purposely excludes standard clinical and biochemical tests that are helpful for patient care but are not specific to heatstroke. Furthermore, in this review, the term heatstroke will refer to observations that are consistent between CHS and EHS. At the same time, CHS and EHS will be used when referring to specific classic or exertional heatstroke observations or pathophysiology. Moreover, we will focus this review on tissues, organs and organ systems impacted by heatstroke. In the interest of conciseness, we will not provide an in-depth overview of the etiology of heatstroke, except where it is relevant to understanding injury biomarkers. Likewise, we will not be addressing risk predictive or diagnostic biomarkers of heatstroke, but we invite the interested reader to explore studies on these topics (e.g., (Lu et al., 2004a; Alele et al., 2021; Stacey et al., 2022)).
Generalized cellular injury and tissue damage
Heatstroke is associated with a complex innate immune response characterized by neutrophil and complement protein activation, production of pro- and anti- inflammatory cytokines, chemokines, and acute phase proteins (Bouchama et al., 2022b). The activation of the immune response has been attributed to endotoxin leaking from the heat-damaged gastrointestinal tract (Hall et al., 1999) and danger signal molecules or alarmins released in the circulation from damaged or dying cells (Gallucci & Matzinger, 2001). Alarmins, also known as damage-associated molecular patterns, play a crucial role in removing necrotic tissue debris and other repair mechanisms (Chen & Nuñez, 2010). Alarmins have been implicated in the excessive and sustained inflammatory response leading to the production of reactive oxygen species and proteolytic enzymes that cause further tissue damage and the progression to multiorgan failure (Chen & Nuñez, 2010). Several alarmins have been identified in heatstroke patients (Huisse et al., 2008; Ruell et al., 2014) and preclinical models (Dehbi et al., 2010; Bruchim et al., 2015). These include extracellular heat shock protein (eHSP)72 (Huisse et al., 2008; Dehbi et al., 2010; Ruell et al., 2014; Bruchim et al., 2015) and eHSP60 (Huisse et al., 2008; Dehbi et al., 2010), high mobility group box protein 1 (HMGB1) (Tong et al., 2011b; Dehbi et al., 2012), histone H3 (Bruchim et al., 2017; Li et al., 2021), and interleukin-1α (IL-1α) (Bouchama et al., 1991).
Heat shock proteins are a family of proteins produced by cells when exposed to stressful conditions, including extreme heat. Heat shock proteins are produced intracellularly and function as chaperones to prevent heat-induced protein misfolding or aggregation (Kultz, 2005). eHSPs do not exert a chaperone function but act as cytokines engaging pattern-recognition receptors, such as toll-like receptors, to elicit an inflammatory response (Chen & Nuñez, 2010). eHSP72 is released in a sustained manner for up to 72 hours in non-human primate models of severe heatstroke and was associated with multiorgan damage and death, suggesting a role as a prognostic biomarker (Dehbi et al., 2010). Moreover, high eHSP70 and eHSP60 were documented in the plasma of heatstroke patients, and the former correlated with plasma interleukin-8 concentrations (Huisse et al., 2008). HMGB1, histone, and IL-1α are chromatin-associated nuclear proteins that are released by injured or dying cells (Cohen et al., 2010). HMGB1 was demonstrated as an early mediator of inflammation, tissue injury and lethality in rodent model of heatstroke (Dehbi et al., 2012), and plasma HMGB1 is associated with heatstroke severity and mortality (Huisse et al., 2008). Likewise, histone H3 was linked to coagulopathy, and heatstroke severity and lethality (Bruchim et al., 2017; Li et al., 2021). Finally, plasma concentrations of IL-1α were increased in small cohort of CHS patients (Bouchama et al., 1991). Notably, IL-1α functions intracellularly as transcription factor in healthy cells, but upon release in circulation behave as proinflammatory cytokine and contributes to tissue remodeling and repair (Cohen et al., 2010). Overall, alarmins are promising biomarkers of heatstroke-associated tissue damage and repair that deserve further validation studies.
Cardiovascular system
Heart
Elevations in body temperature have profound effects on the cardiovascular system to support the increases in skin blood flow necessary to promote heat loss (Crandall & Wilson, 2014). The heart responds by increasing cardiac output, mediated mainly through increases in heart rate and the maintenance of stroke volume, which is made possible by increases in cardiac contractility (Crandall & Wilson, 2014). Given these cardiovascular effects, particularly when paired with the profuse hematological and vascular effects during and following heatstroke, it is not surprising that a single episode of heatstroke increases the risk of all-cause mortality by 40% (Wallace et al., 2007). More specifically, patients with EHS display a higher incidence of cardiovascular disease, myocardial infarction, ischemic heart disease, and acute ischemic stroke as early as 14 years following the initial heatstroke event (Wallace et al., 2007; Wang et al., 2019).
Evidence of potential myocardial injury during heatstroke was preliminarily identified in a case report published in 2006 of a dog presenting with suspected EHS arnd elevations in cardiac troponin I (Mellor et al., 2006), which increases when myocardial cells become damaged. These findings were corroborated in a subsequent case report in humans (Whiticar et al., 2008), including one case report reporting evidence of cardiomyopathy following heatstroke (Chen et al., 2012). Indeed, EHS has recently been shown to provoke myocardial metabolic alterations consistent with the presence of myocardial injury that persists for up to two weeks following heatstroke (Laitano et al., 2020) and marked immunosuppression that last at least one month but likely longer (Murray et al., 2021). Interestingly, these observations were specific to female mice (Laitano et al., 2020; Murray et al., 2021), which was attributed to greater heat tolerance resulting in a higher net heat load than male mice before EHS occurred. Given the consistency of cardiac ramifications during and after heatstroke, a cardiac-specific biomarker likely has utility. To this end, there is a growing body of evidence supporting cardiac troponin I as a sensitive biomarker of myocardial injury during and following heatstroke. For instance, and consistent with the aforementioned case reports (Mellor et al., 2006; Whiticar et al., 2008), graded increases in circulating cardiac troponin I were observed with increased severity of heatstroke 24 hours following heatstroke in a rodent model of CHS (Quinn et al., 2014). Moreover, a point-of-care assessment of cardiac troponin I predicted heatstroke severity (r2=0.83) in a rodent model of CHS (Audet et al., 2015). Finally, circulating cardiac troponin I may have longer-term prognostic utility. For example, higher levels of cardiac troponin I are associated with a higher death rate following emergency department admission during heatwaves (Hausfater et al., 2010). Particularly relevant for EHS, is that cardiac troponin I can be elevated during prolonged exercise (e.g., marathon) (Shave et al., 2007). Thus, the specificity of cardiac troponin I to heatstroke induced cardiac injury remains to be determined.
Blood vessels
Clinical and experimental studies suggest that changes in vascular endothelium and coagulation functions are characteristic features of heatstroke (Sohal et al., 1968; Shieh et al., 1995; Bouchama et al., 1996b; Roberts et al., 2008b). Moreover, functional alterations in these systems are also strongly associated with an increased risk of cardiovascular morbidity and mortality (Hadi et al., 2005; Lowe & Rumley, 2014). Thus, it is not surprising that the risk of cardiovascular disease is elevated following a single heatstroke occurrence (Wang et al., 2019). Postmortem studies in heatstroke patients revealed extensive endothelial damage and hemorrhagic thrombosis in most body organs (Malamud et al., 1946a; Sohal et al., 1968; Chao et al., 1981). These findings were replicated and expanded in non-human primate models of heatstroke, demonstrating that endothelial cell, inflammation, and coagulation activation precede endothelial damage, thrombosis, and bleeding, which culminates in death (Sohal et al., 1968; Roberts et al., 2008b). Hence the capacity to detect early changes in endothelial function may have clinical utility (Bouchama et al., 2005; Roberts et al., 2008b). Several studies have investigated potential circulating biomarkers of endothelial dysfunction, including their link with heatstroke-induced organ damage, severity, and outcomes (Sohal et al., 1968; Shieh et al., 1995; Bouchama et al., 1996b; Hammami et al., 1998a; Alzeer et al., 1999; Roberts et al., 2008b). These include mediators of vascular tone, hemostasis, leucocyte trafficking, and endothelium barrier integrity.
Vascular tone
A series of elegant experimental studies by Hall, et al., demonstrated a central role of nitric oxide in the progression from heat stress to heatstroke (Hall et al., 1994; Hall et al., 2001). Indeed, hyperthermia stimulates nitric oxide synthesis and reactive oxygen species production in the rodent splanchnic circulation, resulting in intestinal barrier dysfunction, cardiovascular collapse, and heatstroke. An imbalance consisting of a marked release of endothelin-1 concomitant to reduced nitric oxide production in the liver sinusoid was also demonstrated in rodents (Zhang et al., 2018). High levels of nitric oxide, assessed by nitrate and nitrite were identified in a small cohort of heatstroke patients that correlated with the severity of heatstroke on admission and mortality (Alzeer et al., 1999). Endothelin-1, another endothelium-derived factor with dual properties including vasoconstrictive and vasodilating effects at higher and lower concentrations, was also detected in the splanchnic circulation of heatstroke patients although its clinical significance remains unknown (Ohlstein et al., 1990; Bouchama et al., 1996b). Taken together, these findings suggest that mediators of vascular tone are implicated in the pathogenesis of heatstroke and may warrant further evaluation as biomarkers of disease activity and survival.
Hemostasis
The coagulopathy of heatstroke progresses from the initial activation of coagulation and fibrinolysis followed by late fibrinolytic inhibition, resulting in a hypercoagulable state. Clinically, the patients with heatstroke display low platelet count, fibrinogen levels and increasing coagulation times, and D-dimer levels (al-Mashhadani et al., 1994; Bouchama et al., 1996a). However, except for D-dimer, these conventional coagulation parameters are not likely sensitive enough or specific to be used as biomarkers of organ injury in heatstroke. When assessed in research settings using sensitive assays such as thrombin-antithrombin and plasmin-antiplasmin complexes, one can detect early rise in thrombin and plasmin, with concomitant decrease of natural anticoagulant proteins (Protein C, S, and antithrombin III) before the alteration of coagulation tests used in clinical practice. Thrombin (r=0.635, p=0.01), plasmin (r=0.577, p=0.02), and D-dimer (r=0.76, p=0.003) correlate significantly with the magnitude of hyperthermia in CHS (Bouchama et al., 1996a), whereas the levels of protein C and antithrombin III discriminate outcome severity in heatstroke, with lower concentrations of these biomarkers being associated with poorer outcomes (i.e., requiring therapeutic blood product intervention) (al-Mashhadani et al., 1994). Hence, further research is needed to assess the value of pro-and anticoagulant molecules as prognostic biomarkers of heatstroke provoked organ injury/recovery using highly sensitive assays.
Von Willebrand factor antigen (vWfAg) and soluble thrombomodulin are likely the most studied biomarkers of endothelial cell injury activation or injury in heatstroke (Shieh et al., 1995; Bouchama et al., 1996b; Bouchama et al., 2005; Huisse et al., 2008; Tong et al., 2014; Zhang et al., 2018; Proctor et al., 2020). vWfAg is synthesized and stored in endothelial cells and megakaryocytes, and mediates platelet aggregation and adhesion at vascular injury sites and endothelial damage (Lip & Blann, 1997). In an experimental model of heatstroke, immunostaining analysis revealed higher expression of vWfAg in most organs of the body, suggesting that the vascular endothelium activation is systemic (Roberts et al., 2008b). However, no association between organ injury and survival was demonstrated in humans with heatstroke (r2=0.37, p>0.05) (Bouchama et al., 1996b).
Thrombomodulin is a transmembrane proteoglycan located on the surface of the vascular endothelium that functions as an anticoagulant (Martin et al., 2013). There is also a soluble form of thrombomodulin released from membrane-bound thrombomodulin that can be released in the circulation through proteolytic cleavage and is considered a marker of endothelial cell damage (Martin et al., 2013). Thrombomodulin activates coagulation and inhibits tissue plasminogen activator-induced fibrinolysis following binding to thrombin and thrombin-activatable fibrinolysis inhibitor, respectively (Esmon & Owen, 1981; Bajzar, 2000). Recombinant thrombomodulin given to heat-stressed rodents prevented heatstroke by inhibition of HMGB1, a damage-associated molecular pattern indicating that thrombomodulin also has an anti-inflammatory function in heatstroke (Hagiwara et al., 2010). Several clinical and experimental studies demonstrated an increased plasma soluble thrombomodulin concentration in heatstroke associated with severity and survival in some studies, but this was not consistently observed (Shieh et al., 1995; Bouchama et al., 2005; Huisse et al., 2008; Tong et al., 2014; Zhang et al., 2018). Hence, further studies are needed to clarify the prognostic utility of soluble thrombomodulin in heatstroke.
Leucocyte trafficking
A central step in the complex crosstalk between inflammation, coagulation, and endothelial cells in heatstroke is the adherence of leukocytes and platelets to the endothelium via cell adhesion molecules. Ex-vivo study of leucocytes obtained from CHS patients showed that this process is mediated by the up-regulation of β2 integrin and down-regulation of L-selectin (Huisse et al., 2008). Changes in lymphocyte β2 integrin expression were also demonstrated in vivo in both CHS and EHS (Hammami et al., 1998b; Lu et al., 2004b). Notably, soluble forms of adhesion molecules are released in the circulation and correlate with the concentration of molecules expressed on the cells and various diseases activity. Thus, soluble adhesion molecules may constitute more practical biomarkers in clinical settings (Gearing & Newman, 1993). In CHS patients, elevated plasma concentrations of soluble intercellular adhesion molecule 1, E-selectin, and L-selectin adhesion molecules have been documented (Bouchama et al., 1996b; Hammami et al., 1998a). L-selectin correlated significantly with E-selectin (r=0.68, p=0.0002) and the level of consciousness (r=−0.45, p=0.03) in heatstroke patients (Hammami et al., 1998a). Compared with other soluble adhesion molecules, E-selectin is probably the most specific among these biomarkers of endothelial cell dysfunction and hence merits further study (Gearing & Newman, 1993).
Endothelial barrier integrity
Damage to the endothelium resulting in loss of its barrier function and vascular leaks were demonstrated in human and non-human primate models of heatstroke (Sohal et al., 1968; Roberts et al., 2008b). The luminal surface of the endothelium is covered by the glycocalyx, a network of membrane-bound proteoglycans and glycoproteins that binds and incorporates soluble molecules derived from the plasma and endothelium. Heatstroke disrupts the endothelial glycocalyx as assessed by plasma levels of syndecan-1 and hyaluronan in laboratory animals (Kobayashi et al., 2018; Umemura et al., 2018; Zhang et al., 2018). Syndecan-1 is the principal component of the endothelial glycocalyx and hyaluronan maintains vascular integrity through endothelial glycocalyx modulation (Lennon & Singleton, 2011). Other biomarkers of endothelial cell activation and loss of integrity include the detachment of endothelial cells and their release into the peripheral circulation. An ~2.5 fold increase in circulating endothelial cells in an experimental heatstroke rat model has been demonstrated, suggesting it can be useful as a marker of endothelial injury (Tong et al., 2014). Overall, biomarkers of vascular barrier function in heatstroke were primarily established experimentally and deserve further translational studies
Brain
Heatstroke encephalopathy is a universal primary manifestation of heatstroke (Leon & Bouchama, 2011). Heatstroke induced encephalopathy is characteristically an early event in the natural course of this condition, preceding the dysfunction of other organ-systems. Further, heatstroke encephalopathy is associated with long-term cognitive and motor disability in heatstroke survivors (Dematte et al., 1998; Argaud et al., 2007; Yang et al., 2017). These findings suggest that the brain is extraordinary vulnerable to heatstroke. The neurologic signs and symptoms are not specific and include, altered mental status, delirium, and coma (Leon & Bouchama, 2011; Casa et al., 2012; Bouchama et al., 2022a). There are no lateralizing signs on physical examination and brain imaging at presentation is normal with no evidence of structural damage (Leon & Bouchama, 2011). In contrast, follow-up brain imaging performed months after heatstroke in patients with persistent central nervous system dysfunction can reveal damage to the cerebellum, prefrontal cortex, or hippocampus (Leon & Bouchama, 2011). Hence, this indicates that early brain injury leading to delayed brain damage may not be readily detectable with standard diagnostic approaches. In this context, biomarkers would aid in the early detection of injury and predict delayed brain damage would be crucial. Notably, S100 calcium-binding protein β (S100β) (Chun et al., 2019; Li et al., 2020) and neuron-specific enolase (NSE) (Li et al., 2020) have been recently proposed as potential biomarkers of heatstroke encephalopathy.
S100β, a glial-specific protein expressed by astrocytes and Schwann cells, functions as a neurotrophic and neuronal survival factor in the developing brain. S100β can be released in the circulation from injured cells (Van Eldik et al., 1986). Accordingly, S100β levels in serum and cerebrospinal fluid have been used as biomarkers of brain and blood brain barrier injury in many conditions, including traumatic brain injury, subarachnoid hemorrhage, and stroke (Persson et al., 1987). Exercise in the heat increases circulating S100β indicating that heat stress may alter blood-brain permeability (Watson et al., 2005). However, additional work refutes these findings during exercise in the heat (Cheuvront et al., 2008) and passive heat stress (Shepley et al., 2021) models in humans. Nonetheless, two recent studies in heatstroke patients demonstrated elevated S100β protein in the serum and cerebrospinal fluid of heatstroke patients (Chun et al., 2019; Li et al., 2020), with one study finding that the heatstroke patients with poor prognosis (defined as not being able to live without assistance at hospital discharge) having serum S100β concentrations ~5 times higher than in heatstroke patients with a good prognosis (Chun et al., 2019). NSE is a dimeric isoform of the glycolytic enzyme enolase expressed mainly in neurons (Kaiser et al., 1989). NSE is upregulated in injured neuronal cells and can spill over into the circulation from injured or dying cells (Persson et al., 1987). Elevated NSE levels in the serum and cerebrospinal fluid have been reported in various brain pathologies, such as head trauma and vascular stroke (Persson et al., 1987). In heatstroke patients, NSE levels strongly correlated with neurological outcomes for up to seven days post-heatstroke (all days r≥−0.624, all days p<0.05), suggesting that NSE could be helpful as diagnostic and prognostic biomarker of heatstroke encephalopathy (Li et al., 2020). Overall, these observations indicate that S100β and NSE are promising biomarkers for early detection or follow-up of heatstroke associated brain injury and thus merit larger prospective studies to determine their sensitivity and specificity.
Kidneys
Kidney damage is well recognized as one of the hallmarks of multiorgan injury caused by heatstroke (Leon et al., 2006b; Roberts et al., 2008a; King et al., 2015). Indeed, patients diagnosed with heatstroke often suffer from acute kidney injury (e.g., (Gauss & Meyer, 1917; Kew et al., 1967; Schrier et al., 1970)), characterized by elevations in serum creatinine, which is indicative of reductions in glomerular filtration rate, measured as part of the basic clinical bloodwork (Ward et al., 2020). A recent analysis of 187 EHS cases in southern China identified that ~44% of patients developed acute kidney injury and that ~27% of the patients with acute kidney injury died within 90 days (Wu et al., 2021). Thus, the management of acute kidney injury following the diagnosis of heatstroke is an essential consideration. It is important to note, however, that acute kidney injury is not ubiquitous such that other observations indicate that in most heatstroke cases, elevations in serum creatinine (Ward et al., 2020) and other markers of renal dysfunction (Kew et al., 1967; Schrier et al., 1970) subsided as soon as 16 days following heatstroke.
Data in animal models of heatstroke indicate that kidney injury is contributed to by vascular congestion, hemorrhage, and thrombi distributed throughout renal tissues (Leon et al., 2006b; Roberts et al., 2008a; King et al., 2015). This likely occurs secondary to the heatstroke-induced systemic inflammation and coagulation activation that catalyze hemorrhagic and thrombotic events within the kidneys (and other organs), similar to sepsis (Ma et al., 2019). Recent findings support that the etiology of kidney injury during heatstroke also includes injury to the renal tubules (Lin & Zhang, 2019). This study additionally identified serum neutrophil gelatinase-associated lipocalin (NGAL) as a potential early biomarker of kidney injury secondary to EHS, such that elevations in serum NGAL were almost 10 times higher post EHS compared to a non-heated control group (Lin & Zhang, 2019). Such observations are consistent with findings in humans undertaking exercise in the heat (Schlader et al., 2017). While NGAL, which functions as a bacteriostatic agent secondary to tissue injury, is primarily expressed in renal tissues, it is also expressed in liver and heart tissues, suggesting that elevations in circulating NGAL may not be specific to the kidneys (Chapman et al., 2020a). This issue may be overcome by examining urinary NGAL or other markers of urinary tubular injury, such as kidney injury molecule-1, insulin-like growth factor binding protein-7, and/or tissue inhibitor metalloproteinase-2 (Chapman et al., 2020a). Indeed, urinary NGAL is elevated in dogs suffering from heatstroke (Segev et al., 2015). Whether this observation translates to humans and whether urinary markers of kidney injury can be used as biomarkers of kidney injury during or following heatstroke is unknown and warrants further study. It is also worth highlighting that obtaining urine samples during/following heatstroke may be challenged because urine production is reduced by elevations in body temperature and hypovolemia (Chapman et al., 2020a). Thus, urinary kidney injury biomarkers may only be available upon urinary catheterization. Moreover, the specificity of plasma or urinary NGAL towards heatstroke should be examined, as they are both temporarily elevated during heat stress in humans without heatstroke and these increases resolve within 24 hours (Schlader et al., 2017; Chapman et al., 2020b).
Gastrointestinal system
Intestines
The systemic immune response to heatstroke has been partially attributed to the development of intestinal cellular dysfunction, making the gastrointestinal tract leaky, and allowing for the translocation of endotoxin into the systemic circulation (Hall et al., 1999; Novosad et al., 2013). The mechanisms by which the gastrointestinal tract may contribute to heatstroke etiology are outside of the scope of this review. Here, however, we consider the consequences of heatstroke on biomarkers of intestinal injury and recovery. To this end, it is notable that diarrhea is consistently observed in heatstroke (Hart et al., 1980), which typically subsides with cooling (Leon & Bouchama, 2011; Bouchama et al., 2022a). Data from preclinical models of CHS show severe intestinal injury in the duodenum, jejunum, and ileum (Novosad et al., 2013) that is characterized by cellular apoptosis (Roberts et al., 2008a) and interstitial edema (Miyamoto et al., 2021). Whether these findings are consistent in the clinical setting remains unknown (Bouchama et al., 2022a). However, circulating intestinal fatty acid-binding protein 2 (I-FABP), a protein found in enterocytes of the small intestinal epithelium that when found in the circulation is often interpreted as evidence of increased intestinal permeability and/or injury (Wells et al., 2017). I-FABP is elevated in patients with heatstroke, with peak responses occurring three days post-heatstroke and reduced with treatment (Zhang et al., 2015), suggesting that I-FABP may be a biomarker of gastrointestinal injury associated with heatstroke. The experimental demonstration of an increased I-FABP gene expression in intestinal mucosa up to 7 h post-passive heat exposure (Miyamoto et al., 2021) (Miyamoto et al., 2021) and elevations in plasma I-FABP protein levels for 24 h after EHS (King et al., 2015), all of which were associated with intestinal histopathological damage, lend support to this interpretation. Given these I-FABP observations, other biomarkers of increased intestinal permeability (e.g., urinary lactulose/rhamnose ratio) may also be considered as intestinal injury biomarkers following heatstroke. However, data from recent meta-analyses indicate that hyperthermia (Pires et al., 2017) and exercise (Chantler et al., 2021), in the absence of heatstroke, consistently increases markers of intestinal permeability, including I-FABP, casting doubt on the value of I-FABP and other markers of intestinal permeability on the specificity of heatstroke-induced intestinal injury or recovery.
Liver
Liver dysfunction and injury are relatively common following heatstroke (Leon & Bouchama, 2011). Indeed, increases in circulating alanine aminotransferase (ALT) and aspartate aminotransferase (AST), standard clinical tests of liver function, are ubiquitous in heatstroke patients (Ward et al., 2020). Moreover, data from a mouse model of EHS (King et al., 2015) and a baboon model of CHS (Roberts et al., 2008a) indicate that heatstroke induces vascular congestion and hemorrhagic thrombosis in liver tissues, while postmortem clinical findings provide support for liver tissue ischemia and necrosis (Malamud et al., 1946b). Notably, these pathological findings also identified that the extent of liver damage was greater when survival following heatstroke exceeded 30 hours (Malamud et al., 1946b), suggesting that the etiology of liver damage initiated by heat cytotoxicity is likely amplified (or worsened) by ischemia-reperfusion injury and an exacerbated systemic inflammatory response. This interpretation is supported by the timing of changes in ALT and AST following heatstroke, with peak concentrations being observed two to three days post-heatstroke (Ward et al., 2020; Ji et al., 2021). There are no formally identified biomarkers, as defined herein, quantifying the extent of liver damage or recovery post-heatstroke. Nevertheless, it is important to recognize that recent evidence supports that increased circulating clinical markers of hepatic dysfunction, including ALT and AST, are associated with an increased likelihood of EHS mortality (Ji et al., 2021; Li et al., 2022). However, it is unknown whether this is due to hepatic causes per se or simply reflects the more severe clinical condition of these patients (Ji et al., 2021). This conclusion is supported by evidence that heatstroke elicits liver injury via interleukin-1β mechanisms (Geng et al., 2015) and that pretreatment with specific antibodies that inhibit circulating HMGB1 protein, which is at least partially implicated in the inflammatory response during EHS (Tong et al., 2011a), alleviates CHS-provoked liver pathology (Tong et al., 2013).
Lungs
Acute lung injury (ALI) is common and is associated with excessive systemic inflammation and coagulation activation in heatstroke (El-Kassimi et al., 1986). However, whether ALI is a direct consequence of excessive heat, or a secondary consequence of systemic inflammation and coagulation activation remains unclear. Several studies in rats used ambient heating to induce CHS and the associated multiorgan failure syndrome that is commonly observed in clinical patients (Yang et al., 2009; Hsi-Hsing et al., 2010). The specific experimental manipulation included exposure of the rats to a thermally stressful environment of 42°C and 80% relative humidity, in which the ambient air had a heat content of approximately 153 kJ/kg. With 146 kJ/kg of heat content at the respiratory surface (assuming 100% RH and a normal body temperature of 37.9°C), this experimental design not only effectively prevents any heat dissipation from the experimental subjects but may also result in net heat absorption from the environment into the respiratory tissues. This pattern of preferentially heating the respiratory tract, with the rest of the body accumulating heat due to perfusion of heated blood from the respiratory tract, is likely consistent with the instances of CHS but differs from EHS. Particularly relevant in animal models of heatstroke is that the mechanism of selective brain cooling relies on cooling blood in the upper respiratory tract. Thus, it can be speculated that this arrangement may lead to faster central nervous system heating and signs of heatstroke than in typical clinical situations in which the primary source of heat is other tissues in the body. In contrast, studies of CHS using a lower ambient temperature failed to show any histological evidence of lung injury (Leon et al., 2006a), supporting the contention that early ALI in the development of heatstroke may be a function of selective injury to the lung during conditions favoring heat uptake through that organ. In contrast, ALI developing later in the disease process may be secondary to systemic inflammatory processes and not specific to heat injury. As a result, biomarkers specific to ALI are probably relevant only in this context.
Skeletal muscle
As the principal source of excess heat, skeletal muscle plays a central role in EHS. Biomarkers of skeletal muscle injury, such as myoglobin and creatine kinase, are prominent in cases of exertional heatstroke, with reported increases of 2 to 4.5 times, respectively, above normal limits in a retrospective study of cases of EHS (Laitano et al., 2021). However, cases of exertional rhabdomyolysis without associated heatstroke can demonstrate similar or higher serum concentrations of these biomarkers (Lippi, 2019), making these biomarkers specific to muscle injury but not specific for the underlying cause of muscle injury. Similarly, serum cytokine concentrations lack specificity regarding skeletal muscle injury in heatstroke since it has been well-established that, skeletal muscle has considerable secretory activity during exercise (Pedersen & Febbraio, 2008). Given that skeletal muscle activity is inherently associated with increased skeletal muscle temperature due to the metabolic activity needed to support skeletal muscle contraction, it is impossible to separate the effects of exercise from the thermal consequences in this tissue. Furthermore, the same cytokines released by active skeletal muscles can also be released by other tissues, making it less certain that the cascade of cytokines commonly identified in cases of heatstroke is of muscular origin. Thus, these biomarkers may be more specific to exertion (and perhaps the heating of the skeletal muscle tissue per se) than whole-body hyperthermia secondary to an inability to dissipate the heat produced by the skeletal muscle. That said, an understanding of the extent of skeletal muscle injury may have important implications regarding the management of kidney injury during or following heatstroke.
Skeletal muscle also can suffer injury and presumably release biomarkers of that injury during CHS. A specific role for mitochondria in heat-induced muscle injury is suggested by the fact that lesions induced by passive heating are more prominent in muscles with higher oxidative capacity (Sharma et al., 2021). Increased temperature has been shown to increase mitochondrial leak respiration in skeletal muscle in rats (Jarmuszkiewicz et al., 2015), dogs (Davis & Barrett, 2021), horses (Davis et al., 2020), and humans (Fiorenza et al., 2019). Increased leak respiration will decrease the efficiency of ATP synthesis and as a result increase heat production through oxidative metabolism is necessary to maintain the desired rate of ATP synthesis. If heat dissipation from the tissue is not increased, a cycle will develop resulting in skeletal muscle overheating and metabolic failure. Additional possible consequences of skeletal muscle heating that may result in damage are the production of reactive oxygen species, which is slightly increased during phosphorylating respiration in hyperthermic rat muscle (Jarmuszkiewicz et al., 2015). Finally, tissue hyperthermia may cause failure of cytoplasmic calcium regulation due to heat-induced dysfunction of the sarcoplasmic calcium-mediated calcium channel (aka, ryanodine receptor RyR1) (Capacchione & Muldoon, 2009), contributing to a syndrome of malignant hyperthermia. Due to these mechanisms, the presence of biomarkers of skeletal muscle injury in the absence of a history of recent exertion could support the passive accumulation of heat – and by extension, systemic heat stress or heatstroke – as an important differential diagnosis.
Summary
Based on observational evidence indicating that a single episode of heatstroke could have harmful acute and chronic long-term health outcomes across numerous organ systems, this narrative review aimed to identify biomarkers of heatstroke-induced organ injury and repair that could be used to guide further development and utilization. This includes as a potential aid to initiate specific organ-protective strategies and prognostication (Figure 1). To this end, we have identified numerous biomarkers related to many aspects of generalized heatstroke induced cellular injury and tissue damage, and heatstroke provoked cardiovascular, renal, cerebral, intestinal, and skeletal muscle injury (Figure 2). The literature supporting biomarkers related to vascular endothelium dysfunction following heatstroke appears to be the most extensive, which may be related to the uniquely multi-functional nature of the endothelium. Moreover, no novel biomarkers were identified for liver or lung injury, which may be because it remains unclear whether injury to these organs is specific to heatstroke or generalized systemic inflammation. In general, there was evidence that the identified biomarkers were specific to acute organ injury. However, the kinetic profile of these biomarkers post-heatstroke, and their reproducibility and specificity to predict organ recovery or failure and long-term outcomes remain relatively unexplored. Collectively, before the translation of the identified biomarkers into clinical practice, additional preclinical and clinical prospective studies are required to better understand the clinical utility of these biomarkers. These studies should likely focus on the relations between the identified biomarkers and how they relate to long-term post-heatstroke health outcomes.
What is the topic of this review?
This narrative review provides an up-to-date overview of the status and potential role of novel biological markers (biomarkers) that can help identify the patients at risk of organ injury or long-term complications following heatstroke.
What advances does it highlight?
This narrative review has identified numerous biomarkers related to many aspects of generalized heatstroke induced cellular injury and tissue damage, and heatstroke provoked cardiovascular, renal, cerebral, intestinal, and skeletal muscle injury. No novel biomarkers were identified for liver or lung injury.
Acknowledgements
This work was supported by awards from the National Institute of Occupational Safety and Health (to ZJS, R01OH011528) and the Indiana Clinical and Translational Science Institute (to ZJS, UL1TR002529).
Footnotes
Disclosures
ZJS has received consultant fees from Otsuka Holdings Co., Ltd. Otherwise, no other potential conflicts of interest, financial or otherwise, are declared by the authors.
References
- al-Mashhadani SA, Gader AG, al Harthi SS, Kangav D, Shaheen FA & Bogus F (1994). The coagulopathy of heat stroke: alterations in coagulation and fibrinolysis in heat stroke patients during the pilgrimage (Haj) to Makkah. Blood Coagul Fibrinolysis 5, 731–736. [DOI] [PubMed] [Google Scholar]
- Alele FO, Malau-Aduli BS, Malau-Aduli AE & Crowe MJ (2021). Haematological, Biochemical and Hormonal Biomarkers of Heat Intolerance in Military Personnel. Biology 10, 1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzeer AH, Al-Arifi A, Warsy AS, Ansari Z, Zhang H & Vincent JL (1999). Nitric oxide production is enhanced in patients with heat stroke. Intensive Care Med 25, 58–62. [DOI] [PubMed] [Google Scholar]
- Argaud L, Ferry T, Le Q-H, Marfisi A, Ciorba D, Achache P, Ducluzeau R & Robert D (2007). Short-and long-term outcomes of heatstroke following the 2003 heat wave in Lyon, France. Archives of Internal Medicine 167, 2177–2183. [DOI] [PubMed] [Google Scholar]
- Armstrong LE, Casa DJ, Millard-Stafford M, Moran DS, Pyne SW & Roberts WO (2007). Exertional heat illness during training and competition. Medicine & Science in Sports & Exercise 39, 556–572. [DOI] [PubMed] [Google Scholar]
- Audet GN, Quinn CM & Leon LR (2015). Point-of-care cardiac troponin test accurately predicts heat stroke severity in rats. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 309, R1264–R1272. [DOI] [PubMed] [Google Scholar]
- Bajzar L (2000). Thrombin activatable fibrinolysis inhibitor and an antifibrinolytic pathway. Arterioscler Thromb Vasc Biol 20, 2511–2518. [DOI] [PubMed] [Google Scholar]
- Bouchama A, Abuyassin B, Lehe C, Laitano O, Jay O, O’Connor FG & Leon LR (2022a). Classic and exertional heatstroke. Nature Reviews Disease Primers 8, 1–23. [DOI] [PubMed] [Google Scholar]
- Bouchama A, Abuyassin B, Lehe C, Laitano O, Jay O, O’Connor FG & Leon LR (2022b). Classic and exertional heatstroke. Nature Reviews Disease Primers 8, 8. [DOI] [PubMed] [Google Scholar]
- Bouchama A, Bridey F, Hammami MM, Lacombe C, al-Shail E, al-Ohali Y, Combe F, al-Sedairy S & de Prost D (1996a). Activation of coagulation and fibrinolysis in heatstroke. Thromb Haemost 76, 909–915. [PubMed] [Google Scholar]
- Bouchama A, Hammami MM, Haq A, Jackson J & al-Sedairy S (1996b). Evidence for endothelial cell activation/injury in heatstroke. Crit Care Med 24, 1173–1178. [DOI] [PubMed] [Google Scholar]
- Bouchama A, Parhar RS, el-Yazigi A, Sheth K & al-Sedairy S (1991). Endotoxemia and release of tumor necrosis factor and interleukin 1 alpha in acute heatstroke. J Appl Physiol 70, 2640–2644. [DOI] [PubMed] [Google Scholar]
- Bouchama A, Roberts G, Al Mohanna F, El-Sayed R, Lach B, Chollet-Martin S, Ollivier V, Al Baradei R, Loualich A, Nakeeb S, Eldali A & de Prost D (2005). Inflammatory, hemostatic, and clinical changes in a baboon experimental model for heatstroke. J Appl Physiol 98, 697–705. [DOI] [PubMed] [Google Scholar]
- Bruchim Y, Ginsburg I, Segev G, Mreisat A, Avital Y, Aroch I & Horowitz M (2017). Serum histones as biomarkers of the severity of heatstroke in dogs. Cell stress & chaperones 22, 903–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchim Y, Segev G, Kelmer E, Codner C, Marisat A & Horowitz M (2015). Hospitalized dogs recovery from naturally occurring heatstroke; does serum heat shock protein 72 can provide prognostic biomarker? Cell Stress and Chaperones 21, 123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capacchione JF & Muldoon SM (2009). The relationship between exertional heat illness, exertional rhabdomyolysis, and malignant hyperthermia. Anesthesia & Analgesia 109, 1065–1069. [DOI] [PubMed] [Google Scholar]
- Casa DJ, Armstrong LE, Kenny GP, O’Connor FG & Huggins RA (2012). Exertional heat stroke: new concepts regarding cause and care. Current sports medicine reports 11, 115–123. [DOI] [PubMed] [Google Scholar]
- Casa DJ, DeMartini JK, Bergeron MF, Csillan D, Eichner ER, Lopez RM, Ferrara MS, Miller KC, O’Connor F & Sawka MN (2015). National Athletic Trainers’ Association position statement: exertional heat illnesses. Journal of athletic training 50, 986–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantler S, Griffiths A, Matu J, Davison G, Jones B & Deighton K (2021). The effects of exercise on indirect markers of gut damage and permeability: A systematic review and meta-analysis. Sports Medicine 51, 113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao TC, Sinniah R & Pakiam JE (1981). Acute heat stroke deaths. Pathology 13, 145–156. [DOI] [PubMed] [Google Scholar]
- Chapman CL, Johnson BD, Parker MD, Hostler D, Pryor RR & Schlader ZJ (2020a). Kidney physiology and pathophysiology during heat stress and the modification by exercise, dehydration, heat acclimation and aging. Temperature In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman CL, Johnson BD, Vargas NT, Hostler D, Parker MD & Schlader ZJ (2020b). Both hyperthermia and dehydration during physical work in the heat contribute to the risk of acute kidney injury. Journal of Applied Physiology 128, 715–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GY & Nuñez G (2010). Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol 10, 826–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W-T, Lin C-H, Hsieh M-H, Huang C-Y & Yeh J-S (2012). Stress-induced cardiomyopathy caused by heat stroke. Annals of Emergency Medicine 60, 63–66. [DOI] [PubMed] [Google Scholar]
- Cheuvront SN, Chinevere TD, Ely BR, Kenefick RW, Goodman DA, McClung JP & Sawka MN (2008). Serum S-100 beta response to exercise-heat strain before and after acclimation. ARMY RESEARCH INST OF ENVIRONMENTAL MEDICINE NATICK MA THERMAL AND MOUNTAIN; …. [DOI] [PubMed] [Google Scholar]
- Chun J-K, Choi S, Kim H-H, Yang HW & Kim CS (2019). Predictors of poor prognosis in patients with heat stroke. Clinical and Experimental Emergency Medicine 6, 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I, Rider P, Carmi Y, Braiman A, Dotan S, White MR, Voronov E, Martin MU, Dinarello CA & Apte RN (2010). Differential release of chromatin-bound IL-1alpha discriminates between necrotic and apoptotic cell death by the ability to induce sterile inflammation. Proc Natl Acad Sci U S A 107, 2574–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall CG & Wilson TE (2014). Human Cardiovascular Responses to Passive Heat Stress. Comp Physiol 5, 17–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MS & Barrett MR (2021). Effect of conditioning and physiological hyperthermia on canine skeletal muscle mitochondrial oxygen consumption. J Appl Physiol (1985) 130, 1317–1325. [DOI] [PubMed] [Google Scholar]
- Davis MS, Fulton MR & Popken AA (2020). Effect of hyperthermia and acidosis on equine skeletal muscle mitochondrial oxygen consumption. Comparative Exercise Physiology 17, 171–179. [Google Scholar]
- Dehbi M, Baturcam E, Eldali A, Ahmed M, Kwaasi A, Chishti MA & Bouchama A (2010). Hsp-72, a candidate prognostic indicator of heatstroke. Cell stress & chaperones. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehbi M, Uzzaman T, Baturcam E, Eldali A, Ventura W & Bouchama A (2012). Toll-like receptor 4 and high-mobility group box 1 are critical mediators of tissue injury and survival in a mouse model for heatstroke. PLoS One 7, e44100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demartini JK, Casa DJ, Stearns R, Belval L, Crago A, Davis R & Jardine J (2015). Effectiveness of cold water immersion in the treatment of exertional heat stroke at the Falmouth Road Race. Med Sci Sports Exerc 47, 240–245. [DOI] [PubMed] [Google Scholar]
- Dematte JE, O’Mara K, Buescher J, Whitney CG, Forsythe S, McNamee T, Adiga RB & Ndukwu IM (1998). Near-fatal heat stroke during the 1995 heat wave in Chicago. Annals of internal medicine 129, 173–181. [DOI] [PubMed] [Google Scholar]
- El-Kassimi FA, Al-Mashhadani S, Abdullah AK & Akhtar J (1986). Adult respiratory distress syndrome and disseminated intravascular coagulation complicating heat stroke. Chest 90, 571–574. [DOI] [PubMed] [Google Scholar]
- Esmon CT & Owen WG (1981). Identification of an endothelial cell cofactor for thrombin-catalyzed activation of protein C. Proc Natl Acad Sci U S A 78, 2249–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorenza M, Lemminger AK, Marker M, Eibye K, Iaia FM, Bangsbo J & Hostrup M (2019). High-intensity exercise training enhances mitochondrial oxidative phosphorylation efficiency in a temperature-dependent manner in human skeletal muscle: implications for exercise performance. FASEB J 33, 8976–8989. [DOI] [PubMed] [Google Scholar]
- Gallucci S & Matzinger P (2001). Danger signals: SOS to the immune system. Curr Opin Immunol 13, 114–119. [DOI] [PubMed] [Google Scholar]
- Gauss H & Meyer K (1917). Heat stroke: report of one hundred and fifty-eight cases from Cook County hospital, Chicago. The American Journal of the Medical Sciences (1827–1924) 154, 554. [Google Scholar]
- Gearing AJH & Newman W (1993). Circulating adhesion molecules in disease. Immunology Today 14, 506–512. [DOI] [PubMed] [Google Scholar]
- Geng Y, Ma Q, Liu Y-N, Peng N, Yuan F-F, Li X-G, Li M, Wu Y-S, Li B-l & Song W-b (2015). Heatstroke induces liver injury via IL-1β and HMGB1-induced pyroptosis. Journal of hepatology 63, 622–633. [DOI] [PubMed] [Google Scholar]
- Hadi HA, Carr CS & Al Suwaidi J (2005). Endothelial dysfunction: cardiovascular risk factors, therapy, and outcome. Vascular health and risk management 1, 183. [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S, Iwasaka H, Goto K, Ochi Y, Mizunaga S, Saikawa T & Noguchi T (2010). Recombinant thrombomodulin prevents heatstroke by inhibition of high-mobility group box 1 protein in sera of rats. Shock 34, 402–406. [DOI] [PubMed] [Google Scholar]
- Hall DM, Baumgardner KR, Oberley TD & Gisolfi CV (1999). Splanchnic tissues undergo hypoxic stress during whole body hyperthermia. Am J Physiol 276, G1195–1203. [DOI] [PubMed] [Google Scholar]
- Hall DM, Buettner GR, Matthes RD & Gisolfi CV (1994). Hyperthermia stimulates nitric oxide formation: electron paramagnetic resonance detection of .NO-heme in blood. J Appl Physiol (1985) 77, 548–553. [DOI] [PubMed] [Google Scholar]
- Hall DM, Buettner GR, Oberley LW, Xu L, Matthes RD & Gisolfi CV (2001). Mechanisms of circulatory and intestinal barrier dysfunction during whole body hyperthermia. Am J Physiol Heart Circ Physiol 280, H509–521. [DOI] [PubMed] [Google Scholar]
- Hammami MM, Bouchama A & Al Sedairy S (1998a). Levels of soluble L-selectin and E-selectin in heatstroke and heatstress. Chest 114, 949–950. [DOI] [PubMed] [Google Scholar]
- Hammami MM, Bouchama A, Shail E, Aboul-Enein HY & Al-Sedairy S (1998b). Lymphocyte subsets and adhesion molecules expression in heatstroke and heat stress. J Appl Physiol 84, 1615–1621. [DOI] [PubMed] [Google Scholar]
- Hart L, Egier B, Shimizu A, Tandan P & Sutton J (1980). Exertional heat stroke: the runner’s nemesis. Canadian Medical Association Journal 122, 1144. [PMC free article] [PubMed] [Google Scholar]
- Hausfater P, Doumenc B, Chopin S, Le Manach Y, Santin A, Dautheville S, Patzak A, Hericord P, Mégarbane B & Andronikof M (2010). Elevation of cardiac troponin I during non-exertional heat-related illnesses in the context of a heatwave. Critical care 14, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsi-Hsing Y, Ching-Ping C, Juei-Tang C & Lin MT (2010). Inhibition of acute lung inflammation and injury is a target of brain cooling after heatstroke injury. J Trauma 69, 805–812. [DOI] [PubMed] [Google Scholar]
- Huisse MG, Pease S, Hurtado-Nedelec M, Arnaud B, Malaquin C, Wolff M, Gougerot-Pocidalo MA, Kermarrec N, Bezeaud A, Guillin MC, Paoletti X & Chollet-Martin S (2008). Leukocyte activation: the link between inflammation and coagulation during heatstroke. A study of patients during the 2003 heat wave in Paris. Crit Care Med 36, 2288–2295. [DOI] [PubMed] [Google Scholar]
- IPCC (2021). Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press. [Google Scholar]
- Jarmuszkiewicz W, Woyda-Ploszczyca A, Koziel A, Majerczak J & Zoladz JA (2015). Temperature controls oxidative phosphorylation and reactive oxygen species production through uncoupling in rat skeletal muscle mitochondria. Free Radic Biol Med 83, 12–20. [DOI] [PubMed] [Google Scholar]
- Ji J, Gao J, Wang C, Ouyang L, Liu Z & Liu Z (2021). Characteristics and outcome of exertional heatstroke patients complicated by acute hepatic injury: a cohort study. Journal of Clinical and Translational Hepatology 9, 655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser E, Kuzmits R, Pregant P, Burghuber O & Worofka W (1989). Clinical biochemistry of neuron specific enolase. Clinica chimica acta 183, 13–31. [DOI] [PubMed] [Google Scholar]
- Kew M, Abrahams C, Levin N, Seftel H, Rubenstein A & Bersohn I (1967). The effects of heatstroke on the function and structure of the kidney. QJM: An International Journal of Medicine 36, 277–300. [PubMed] [Google Scholar]
- King MA, Leon LR, Mustico DL, Haines JM & Clanton TL (2015). Biomarkers of multiorgan injury in a preclinical model of exertional heat stroke. Journal of Applied Physiology 118, 1207–1220. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Mimuro S, Sato T, Kobayashi A, Kawashima S, Makino H, Doi M, Katoh T & Nakajima Y (2018). Dexmedetomidine preserves the endothelial glycocalyx and improves survival in a rat heatstroke model. Journal of Anesthesia 32, 880–885. [DOI] [PubMed] [Google Scholar]
- Kultz D (2005). Molecular and evolutionary basis of the cellular stress response. Annu Rev Physiol 67, 225–257. [DOI] [PubMed] [Google Scholar]
- Laitano O, Garcia CK, Mattingly AJ, Robinson GP, Murray KO, King MA, Ingram B, Ramamoorthy S, Leon LR & Clanton TL (2020). Delayed metabolic dysfunction in myocardium following exertional heat stroke in mice. The Journal of Physiology 598, 967–985. [DOI] [PubMed] [Google Scholar]
- Laitano O, Oki K & Leon LR (2021). The Role of Skeletal Muscles in Exertional Heat Stroke Pathophysiology. Int J Sports Med 42, 673–681. [DOI] [PubMed] [Google Scholar]
- Lennon FE & Singleton PA (2011). Hyaluronan regulation of vascular integrity. Am J Cardiovasc Dis 1, 200–213. [PMC free article] [PubMed] [Google Scholar]
- Leon LR, Blaha MD & DuBose DA (2006a). Time course of cytokine, corticosterone, and tissue injury responses in mice during heat strain recovery. J Appl Physiol (1985) 100, 1400–1409. [DOI] [PubMed] [Google Scholar]
- Leon LR, Blaha MD & DuBose DA (2006b). Time course of cytokine, corticosterone, and tissue injury responses in mice during heat strain recovery. Journal of Applied Physiology 100, 1400–1409. [DOI] [PubMed] [Google Scholar]
- Leon LR & Bouchama A (2011). Heat stroke. Comprehensive Physiology 5, 611–647. [DOI] [PubMed] [Google Scholar]
- Li B, Jia Y-R, Gao W, Li H-P, Tao W-H & Zhang H-X (2020). The expression and clinical significance of neuron specific enolase and S100B protein in patients of severe heatstroke-induced brain injury. Medical Journal of Chinese People’s Liberation Army 45, 1282–1287. [Google Scholar]
- Li C, Su H-b, Li H, Li X, Wang H-m, Song Q & Hu J-h (2022). Severe acute liver injury in patients with exertional heat stroke associated with poor short-term prognosis. World Journal of Emergency Medicine 13, 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Liu Z, Shi X, Tong H & Su L (2021). Prognostic value of plasma exosomal levels of histone H3 protein in patients with heat stroke. Exp Ther Med 22, 922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y & Zhang Y (2019). Renoprotective effect of oral rehydration solution III in exertional heatstroke rats. Renal failure 41, 190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lip GY & Blann A (1997). von Willebrand factor: a marker of endothelial dysfunction in vascular disorders? Cardiovasc Res 34, 255–265. [DOI] [PubMed] [Google Scholar]
- Lippi GS F; Ceriotti F (2019). Diagnostic biomarkers of muscle injury and exertional rhabdomyolysis. Clin Chem Lab Med 57, 7. [DOI] [PubMed] [Google Scholar]
- Lowe G & Rumley A (2014). The relevance of coagulation in cardiovascular disease: what do the biomarkers tell us? Thrombosis and haemostasis 112, 860–867. [DOI] [PubMed] [Google Scholar]
- Lu K-C, Wang J-Y, Lin S-H, Chu P & Lin Y-F (2004a). Role of circulating cytokines and chemokines in exertional heatstroke. Critical care medicine 32, 399–403. [DOI] [PubMed] [Google Scholar]
- Lu KC, Lin SH, Chu P, Tsai WS & Lin YF (2004b). Correlation of neutrophil phagocytosis and lymphocyte adhesion molecules in exertional heat stroke. Am J Med Sci 327, 68–72. [DOI] [PubMed] [Google Scholar]
- Ma S, Evans RG, Iguchi N, Tare M, Parkington HC, Bellomo R, May CN & Lankadeva YR (2019). Sepsis‐induced acute kidney injury: a disease of the microcirculation. Microcirculation 26, e12483. [DOI] [PubMed] [Google Scholar]
- Malamud N, Haymaker W & Custer R (1946a). Heatstroke: a clinico-pathologic study of 125 fatal cases. Milit Surg 99, 397–449. [PubMed] [Google Scholar]
- Malamud N, Haymaker W & Custer RP (1946b). Heat Stroke. A Clinico-Pathologic Study of 125 Fatal Cases. Military surgeon 99, 397–449. [PubMed] [Google Scholar]
- Martin FA, Murphy RP & Cummins PM (2013). Thrombomodulin and the vascular endothelium: insights into functional, regulatory, and therapeutic aspects. Am J Physiol Heart Circ Physiol 304, H1585–H1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor PJ, Mellanby RJ, Baines EA, Villiers EJ, Archer J & Herrtage ME (2006). High serum troponin I concentration as a marker of severe myocardial damage in a case of suspected exertional heatstroke in a dog. Journal of Veterinary Cardiology 8, 55–62. [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Suzuki K, Ohtaki H, Nakamura M, Yamaga H, Yagi M, Honda K, Hayashi M & Dohi K (2021). A novel mouse model of heatstroke accounting for ambient temperature and relative humidity. Journal of Intensive Care 9, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora C, Dousset B, Caldwell IR, Powell FE, Geronimo RC, Bielecki CR, Counsell CW, Dietrich BS, Johnston ET & Louis LV (2017). Global risk of deadly heat. Nature Climate Change 7, 501–506. [Google Scholar]
- Murray KO, Brant JO, Iwaniec JD, Sheikh LH, de Carvalho L, Garcia CK, Robinson GP, Alzahrani JM, Riva A & Laitano O (2021). Exertional heat stroke leads to concurrent long‐term epigenetic memory, immunosuppression and altered heat shock response in female mice. The Journal of Physiology 599, 119–141. [DOI] [PubMed] [Google Scholar]
- Novosad VL, Richards JL, Phillips NA, King MA & Clanton TL (2013). Regional susceptibility to stress-induced intestinal injury in the mouse. American Journal of Physiology-Gastrointestinal and Liver Physiology 305, G418–G426. [DOI] [PubMed] [Google Scholar]
- Ohlstein EH, Vickery L, Sauermelch C & Willette RN (1990). Vasodilation induced by endothelin: role of EDRF and prostanoids in rat hindquarters. Am J Physiol 259, H1835–1841. [DOI] [PubMed] [Google Scholar]
- Pedersen BK & Febbraio MA (2008). Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev 88, 1379–1406. [DOI] [PubMed] [Google Scholar]
- Persson L, Hårdemark H, Gustafsson J, Rundström G, Mendel-Hartvig I, Esscher T & Påhlman S (1987). S-100 protein and neuron-specific enolase in cerebrospinal fluid and serum: markers of cell damage in human central nervous system. Stroke 18, 911–918. [DOI] [PubMed] [Google Scholar]
- Pires W, Veneroso CE, Wanner SP, Pacheco DA, Vaz GC, Amorim FT, Tonoli C, Soares DD & Coimbra CC (2017). Association between exercise-induced hyperthermia and intestinal permeability: a systematic review. Sports Medicine 47, 1389–1403. [DOI] [PubMed] [Google Scholar]
- Proctor EA, Dineen SM, Van Nostrand SC, Kuhn MK, Barrett CD, Brubaker DK, Yaffe MB, Lauffenburger DA & Leon LR (2020). Coagulopathy signature precedes and predicts severity of end-organ heat stroke pathology in a mouse model. J Thromb Haemost 18, 1900–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn CM, Duran RM, Audet GN, Charkoudian N & Leon LR (2014). Cardiovascular and thermoregulatory biomarkers of heat stroke severity in a conscious rat model. Journal of Applied Physiology 117, 971–978. [DOI] [PubMed] [Google Scholar]
- Rae DE, Knobel GJ, Mann T, Swart J, Tucker R & Noakes TD (2008). Heatstroke during endurance exercise: is there evidence for excessive endothermy? Med Sci Sports Exerc 40, 1193–1204. [DOI] [PubMed] [Google Scholar]
- Roberts GT, Ghebeh H, Chishti MA, Al-Mohanna F, El-Sayed R, Al-Mohanna F & Bouchama A (2008a). Microvascular injury, thrombosis, inflammation, and apoptosis in the pathogenesis of heatstroke: a study in baboon model. Arteriosclerosis, thrombosis, and vascular biology 28, 1130–1136. [DOI] [PubMed] [Google Scholar]
- Roberts GT, Ghebeh H, Chishti MA, Al-Mohanna F, El-Sayed R, Al-Mohanna F & Bouchama A (2008b). Microvascular injury, thrombosis, inflammation, and apoptosis in the pathogenesis of heatstroke: a study in baboon model. Arterioscler Thromb Vasc Biol 28, 1130–1136. [DOI] [PubMed] [Google Scholar]
- Ruell PA, Simar D, Périard JD, Best S, Caillaud C & Thompson MW (2014). Plasma and lymphocyte Hsp72 responses to exercise in athletes with prior exertional heat illness. Amino Acids 46, 1491–1499. [DOI] [PubMed] [Google Scholar]
- Schlader ZJ, Chapman CL, Sarkar S, Russo L, Rideout TC, Parker MD, Johnson BD & Hostler D (2017). Firefighter work duration influences the extent of acute kidney injury. Med Sci Sport Exer 49, 1745–1753. [DOI] [PubMed] [Google Scholar]
- Schrier RW, Hano J, Keller HI, Finkel RM, Gilliland PF, Cirksena WJ & Teschan PE (1970). Renal, metabolic, and circulatory responses to heat and exercise. Studies in military recruits during summer training, with implications for acute renal failure. Ann Intern Med 73, 213–223. [DOI] [PubMed] [Google Scholar]
- Segev G, Daminet S, Meyer E, De Loor J, Cohen A, Aroch I & Bruchim Y (2015). Characterization of kidney damage using several renal biomarkers in dogs with naturally occurring heatstroke. The Veterinary Journal 206, 231–235. [DOI] [PubMed] [Google Scholar]
- Sharma S, Chaudhary P, Sandhir R, Bharadwaj A, Gupta RK, Khatri R, Bajaj AC, Baburaj TP, Kumar S, Pal MS, Reddy PK & Kumar B (2021). Heat-induced endoplasmic reticulum stress in soleus and gastrocnemius muscles and differential response to UPR pathway in rats. Cell Stress Chaperones 26, 323–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shave R, George KP, Atkinson G, Hart E, Middleton N, Whyte G, Gaze D & Collinson PO (2007). Exercise-induced cardiac troponin T release: a meta-analysis. Medicine and science in sports and exercise 39, 2099–2106. [DOI] [PubMed] [Google Scholar]
- Shepley BR, Ainslie PN, Hoiland RL, Donnelly J, Sekhon MS, Zetterberg H, Blennow K & Bain AR (2021). Negligible influence of moderate to severe hyperthermia on blood-brain barrier permeability and neuronal parenchymal integrity in healthy men. Journal of Applied Physiology 130, 792–800. [DOI] [PubMed] [Google Scholar]
- Shieh SD, Shiang JC, Lin YF, Shiao WY & Wang JY (1995). Circulating angiotensin-converting enzyme, von Willebrand factor antigen and thrombomodulin in exertional heat stroke. Clin Sci (Lond) 89, 261–265. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Sun SC, Colcolough HL & Burch GE (1968). Heat stroke. An electron microscopic study of endothelial cell damage and disseminated intravascular coagulation. Arch Intern Med 122, 43–47. [DOI] [PubMed] [Google Scholar]
- Stacey MJ, Hill NE, Parsons IT, Wallace J, Taylor N, Grimaldi R, Shah N, Marshall A, House C & O’Hara JP (2022). Relative changes in brain and kidney biomarkers with Exertional Heat Illness during a cool weather marathon. PLOS ONE 17, e0263873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong H-S, Tang Y-Q, Chen Y, Qiu J-M, Wen Q & Su L (2011a). Early elevated HMGB1 level predicting the outcome in exertional heatstroke. Journal of Trauma and Acute Care Surgery 71, 808–814. [DOI] [PubMed] [Google Scholar]
- Tong H, Tang Y, Chen Y, Yuan F, Liu Z, Peng N, Tang L & Su L (2013). HMGB1 activity inhibition alleviating liver injury in heatstroke. Journal of Trauma and Acute Care Surgery 74, 801–807. [DOI] [PubMed] [Google Scholar]
- Tong H, Wan P, Zhang X, Duan P, Tang Y, Chen Y, Tang L & Su L (2014). Vascular endothelial cell injury partly induced by mesenteric lymph in heat stroke. Inflammation 37, 27–34. [DOI] [PubMed] [Google Scholar]
- Tong HS, Tang YQ, Chen Y, Qiu JM, Wen Q & Su L (2011b). Early elevated HMGB1 level predicting the outcome in exertional heatstroke. J Trauma 71, 808–814. [DOI] [PubMed] [Google Scholar]
- Umemura Y, Ogura H, Matsuura H, Ebihara T, Shimizu K & Shimazu T (2018). Bone marrow-derived mononuclear cell therapy can attenuate systemic inflammation in rat heatstroke. Scand J Trauma Resusc Emerg Med 26, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eldik L, Jensen R, Ehrenfried BA & Whetsell W Jr (1986). Immunohistochemical localization of S100 beta in human nervous system tumors by using monoclonal antibodies with specificity for the S100 beta polypeptide. Journal of Histochemistry & Cytochemistry 34, 977–982. [DOI] [PubMed] [Google Scholar]
- Wallace RF, Kriebel D, Punnett L, Wegman DH & Amoroso PJ (2007). Prior heat illness hospitalization and risk of early death. Environmental research 104, 290–295. [DOI] [PubMed] [Google Scholar]
- Wang J-C, Chien W-C, Chu P, Chung C-H, Lin C-Y & Tsai S-H (2019). The association between heat stroke and subsequent cardiovascular diseases. PLOS ONE 14, e0211386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward MD, King MA, Gabrial C, Kenefick RW & Leon LR (2020). Biochemical recovery from exertional heat stroke follows a 16-day time course. PLOS ONE 15, e0229616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson P, Shirreffs SM & Maughan RJ (2005). Blood-brain barrier integrity may be threatened by exercise in a warm environment. Am J Physiol Regul Integr Comp Physiol 288, R1689–1694. [DOI] [PubMed] [Google Scholar]
- Wells JM, Brummer RJ, Derrien M, MacDonald TT, Troost F, Cani PD, Theodorou V, Dekker J, Méheust A & De Vos WM (2017). Homeostasis of the gut barrier and potential biomarkers. American Journal of Physiology-Gastrointestinal and Liver Physiology 312, G171–G193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiticar R, Laba D & Smith S (2008). Exertional heat stroke in a young man with a documented rise in troponin I. Emergency Medicine Journal 25, 283–284. [DOI] [PubMed] [Google Scholar]
- Wu M, Wang C, Liu Z, Zhong L, Yu B, Cheng B & Liu Z (2021). Clinical characteristics and risk factors associated with acute kidney injury inpatient with exertional heatstroke: an over 10-year intensive care survey. Frontiers in medicine 8, 692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HH, Chang CP, Cheng RT & Lin MT (2009). Attenuation of acute lung inflammation and injury by whole body cooling in a rat heatstroke model. J Biomed Biotechnol 2009, 768086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Li Z, Zhao Y, Zhou F, Zhang Y, Gao J, Yin T, Hu X, Mao Z & Xiao J (2017). Outcome and risk factors associated with extent of central nervous system injury due to exertional heat stroke. Medicine 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Fan X, Zhong Z, Xu G & Shen J (2015). Association of plasma diamine oxidase and intestinal fatty acid–binding protein with severity of disease in patient with heat stroke. The American journal of emergency medicine 33, 867–871. [DOI] [PubMed] [Google Scholar]
- Zhang X, Chen Y, Tang L, Zhang Y, Duan P, Su L & Tong H (2018). The liver sinusoidal endothelial cell damage in rats caused by heatstroke. European Journal of Inflammation 16, 2058739218794328. [Google Scholar]


