Abstract
Neovascularization, the formation of new blood vessels, has received much research attention due to its implications in physiological processes and diseases. Most studies using traditional in vitro and in vivo platforms find challenges in recapitulating key cellular and mechanical cues of the neovascularization processes. Microfluidic in vitro models have been presented as an alternative to these limitations due to their capacity to leverage microscale physics to control cell organization and integrate biochemical and mechanical cues, such as shear stress, cell-cell interactions, or nutrient gradients, making them an ideal option for recapitulating organ physiology.
Much has been written about the use of microfluidics in vascular biology models from an engineering perspective. However, a review introducing the different models, components and progress for new potential adopters of these technologies was absent in the literature. Therefore, this paper aims to approach the use of microfluidic technologies in vascular biology from a perspective of biological hallmarks to be studied and written for a wide audience ranging from clinicians to engineers. Here we review applications of microfluidics in vascular biology research, starting with design considerations and fabrication techniques. After that, we review the state of the art in recapitulating angiogenesis and vasculogenesis, according to the hallmarks recapitulated and complexity of the models. Finally, we discuss emerging research areas in neovascularization, such as drug discovery, and potential future directions.
Keywords: Angiogenesis, in vitro models, microfluidics, microphysiological systems, anti-angiogenics, vasculogenesis
Introduction
The formation of new blood vessels, known as neovascularization, includes the physiological processes of angiogenesis and vasculogenesis, which have incredibly diverse human biology roles. The vasculogenesis process typically refers to the generation of novel blood vessels from scratch, especially during embryonic development, whereas angiogenesis is the development of new blood vessels from pre-existing ones. Under healthy conditions, the roles of these physiological processes are primarily supportive. For example, during development, angiogenesis can augment oxygen and nutrient delivery to developing organs, and following tissue injury, angiogenesis can repair damaged vascular networks and support tissue regeneration1. However, in the onset of diseases such as diabetes, asthma, cardiovascular disease, and cancer, angiogenesis can also play integral roles in tissue destruction and can be a vital factor in the morbidity and mortality of these conditions2,3. The incredible diversity of these roles and their importance in both normal biology and disease pathology have made angiogenesis an area of intense scientific research. The focus of this research is equally diverse, with topics that range from understanding the biology of angiogenesis to the development of therapies that target the pathways that drive the angiogenic process4,5. In this review, we focus most of our descriptions in angiogenesis, since its therapeutic possibilities are more explored in the literature, although we have dedicated a specific section to vasculogenesis, and have included novel vasculogenesis models in our sections, when appropriate.
Angiogenesis occurs as an orderly cascade of molecular events (Figure 1). In normal tissues, this cascade is tightly regulated by a balance of growth factors and angiogenic inhibitors. While angiogenic inhibitors (e.g., angiostatin, miRNA) circulate through the blood or are anchored in the ECM, angiogenic growth factors (e.g., vascular endothelial growth factor, fibroblast growth factor, and interleukin-8) are released by oxygen-deprived tissues or in response to hypoxia.
Figure 1. Angiogenesis occurs as an orderly cascade of molecular events:
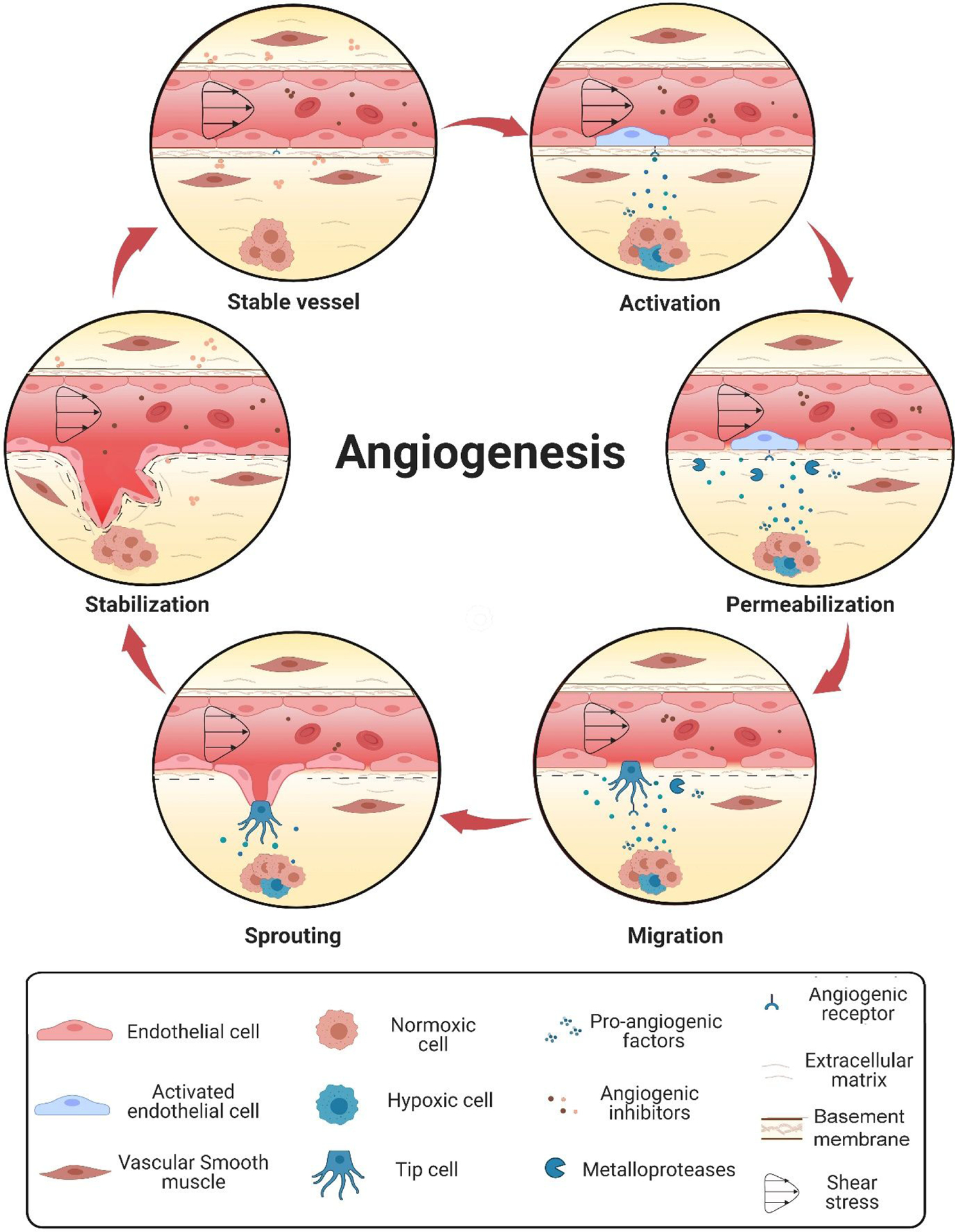
(1) activation of angiogenesis by angiogenic stimuli, (2) proteolytic enzyme production and subsequent degradation of the basement membrane and perivascular extracellular matrix (3) migration of endothelial cells and primary sprout formation (4) lumenation and formation of tube-like structures, and (5) novel basement membrane secretion and maturation into functional vessels capable of supporting blood flow.
Released angiogenic factors activate endothelial cells in mature capillaries to initiate the angiogenic cascade. Following activation, endothelial cells secrete proteases (e.g., MMPs and heparinase) that degrade adjacent basement membrane and extracellular matrix (ECM). This remodeling, in turn, releases additional anchored pro-angiogenic factors, which further activates endothelial cells and increases vessel permeability to substrate diffusion. Activated endothelial cells migrate outward from the capillary and proliferate to form new sprouts oriented toward the source of the stimulus. After that, endothelial cells organize into hollow tubes and create new basement membranes for vascular stability. Newly formed blood vessels also recruit mural cells, (e.g., smooth muscle cells and pericytes), to stabilize the vascular architecture into mature functioning vessels capable of supporting blood flow.
Research in angiogenesis has led to the development of an array of therapies that have improved patient outcomes6. There are several available angiogenesis-directed therapies in cancer alone that can reduce tumor burden, improve symptoms, and increase survival for patients with an array of tumor types7. However, despite these important advances, there continue to be significant gaps in our knowledge of the angiogenic process that limit continued progress in these fields. Therefore, further research is needed to improve our understanding of angiogenesis and identify new pathways for therapeutic targeting.
Most of the studies published in the field of vascular biology research employ mouse models and 2D cell cultures as the primary platforms to study the mechanisms of angiogenesis, such as cell migration (e.g., Teflon fence, Transwell or wound healing assays) or microvasculature assembly (i.e., tube formation assay on Matrigel). These techniques are currently considered the gold standard of in vitro angiogenesis assays since they are cost-effective, high-throughput, and user-friendly. However, 2D techniques struggle to recapitulate key cellular and mechanical cues in the angiogenic process, dramatically affecting cell phenotype (Figure 1)8,9. Therefore, there remains a pressing need to develop and standardize models capable of mimicking features of the in vivo tumor microenvironment (TME)(i.e., the niche in which a tumor develops), such as nutrient gradients, 3D cell migration, or ECM-cell interactions, without sacrificing throughput and discriminatory capacity.
Microfluidic devices (i.e., those handling volumes in the μl scale) offer a range of solutions to the challenges posed by conducting research using traditional 2D and 3D mediums. Microfluidic devices have been used to model blood vessels and angiogenesis for the last few decades due to their balance of the best features offered by traditional in vitro and in vivo models (i.e., simplicity, tractability, and cost-effectiveness) additional small-scale benefits10.
Furthermore, microscale physics confer additional advantages to these models for recapitulating organ physiology (e.g., fine control over distances, mechanical cues, tissue organization, and geometries)11. The device’s small size also reduces the quantity of expensive reagents (e.g., cytokines, drugs, scaffolding proteins) and precious biological samples (e.g., patient-derived or circulating tumor cells) required for each assay. These advantages over traditional in vitro models make microfluidic devices ideal platforms for angiogenesis focused research.
Most reviews discussing microfluidic modeling of angiogenesis focus only on a description of technologies and publish studies without previously establishing basic microfluidic principles necessary to understand the models and their limitations. Here we present a comprehensive and critical review that balances a biological and an engineering perspective, and summarizes recent progress in the field, including the numerous papers mimicking (brain and other) microvascular networks using microfluidic devices.
Design considerations for microfluidic modeling
Microfluidic devices can harness the unique properties of fluids at the submillimeter scale. The highly predictable microscale physics (e.g., capillarity and laminar flow) dominate over classical macroscale physics (e.g., gravity), resulting in a great degree of control in the design and operation of the devices for the user.
Device fabrication
There are a limited number of fabrication techniques capable of fabricating microfluidic devices since few strategies have enough resolution to create these small structures. The most commonly used are soft lithography, micromolding, micromachining or milling, and, more recently, 3D printing12. We will focus on the first two techniques, which are some of the most commonly used approaches to fabrication.
Soft lithography is based on using a photoresist and selective UV-curing (i.e., photolithography) to produce a detailed master mold, which is then used to shape a thermally cured polymer (i.e., soft lithography) (Figure 2A). The shaped polymer is typically bonded to glass to create a fully enclosed device13. This three-step technique is highly popular and presents many advantages: low price, ease of prototyping, and higher versatility10. The most commonly used materials for this technique are the photoresist SU-8 and the polymer polydimethylsiloxane (PDMS), with the latter being responsible for most advantages and limitations of the technique14. PDMS is reasonably priced, highly biocompatible once cured, optically transparent to light, and relatively inert15. However, many authors report PDMS interactions with hydrophobic molecules and high permeability to gases and fluids, presenting challenges in disease modeling and drug testing16. While many modifications have been proposed to PDMS over the years, this material remains one of the most popular options for fabricating microfluidic devices17.
Figure 2. Schematic representation of the three main techniques described in this review to fabricate microfluidic devices.

(A) Soft lithography: A mask is designed for each layer required to generate the desired SU-8 master mold. Then, subsequent layers of photoresist material are spun on a silicon wafer, and the UV mask is used to expose certain regions of the photoresist for UV light to polymerize. The non-exposed areas are later removed using a solvent. A mixture of PDMS and curling material is poured on the SU-8 master mold and let polymerize under heat. Polymerized devices are then extracted from the master mold, assembled, and bonded to a glass substrate to produce the final device. (B) Micromilling: A pattern is designed in AutoCAD. A sheet of material (e.g., plastic) is placed onto the machine support. The programmed micromill removes bulk material that can then be bonded to a substrate via high pressure, ultrasound, heat, or chemical solvents. (C) Micromolding (injection molding): A steel mold is designed with AutoCAD and machined. In the molding injector, plastic granules are melted and loaded into the mold. When the piece is cooled, it is ejected using pins that push it out of the mold, which can then be used again.
In contrast to soft lithography, the micromilling (also called CNC milling) fabrication process uses rotary cutters to remove bulk material and create microscale features18. Once the device is finished, it is typically bonded to plastic by high pressure, ultrasound, heat, or chemical solvents to create a fully enclosed device (Figure 2B). The wide diversity of cutting tools (size, materials, and shapes) makes the milling amenable to fabricating devices with different materials, shapes, and precision19 Micromilling applications include the generation of molds20 and the design of new prototypes and open microfluidic devices21,22. Despite the versatility and low fabrication times, the need for large equipment and highly specialized training have reduced the usage of micromilling compared to other methods for microfluidics18.
Micromolding, on the other hand, is a set of techniques used to shape plastic polymers (e.g., cyclic olefin polymer or polystyrene) with the support of a mold (Figure 2C). Injection molding is one such technique, which involves the fabrication of a highly durable mold to shape the plastic polymers. This highly scalable technology is frequently used to manufacture everyday plastic objects, but the high durability required for the mold increases costs. Further, the technique presents limitations in size and the shape of the features that can be generated, since it is subject to the limitations of two engineering processes: the micromilling (also called micromachining) typically used to generate the mold; and the thermoplastic molding process, which results in the smoothing of certain angled interfaces (e.g., some squares become rounded after molding the thermoplastic). Therefore, micromolding is not chosen for small-scale and minute detail model development but rather for the commercial fabrication of robust and well-characterized devices23. Alternative strategies to solve some of these issues are being developed, especially in the generation of smaller and more accurate molds24–26. Since plastic polymers solve many of the challenges PDMS presents (e.g., molecule sequestration and high permeability), commercially available devices are on the rise. Currently, dozens of companies (e.g., Ibidi, MIMETAS) are flooding the market with micromolded, user-friendly products often developed from PDMS prototypes.27
Porous interfaces: leveraging hydrogels and membranes in microfluidics
Although cells can be seeded directly on the surface of a device, microfluidic devices often contain hydrogels and membranes, which can also be used as substrates for cell attachment. Often, microfluidic designs will present adaptations to facilitate hydrogel injection and control its confinement. Hydrogels of synthetic or natural origin provide cells with a 3D architecture and mechanical and biochemical cues, similar to those in vivo. While many natural ECM options have been reported (e.g., hyaluronic acid, Matrigel, alginate), collagen and fibrin remain some of the most popular due to their ubiquity in primary tissues, robustness, and ease of use9. However, synthetic PEG derivatives and silk scaffolds have also been used as cell substrates in microdevices28.
Microdevices with several channels or chambers typically leverage two different techniques to co-culture different cell types: microfluidic valves or porous membranes29. Microfluidic valves, a popular approach in microfluidics, leverage surface tension and geometry changes to pin a hydrogel at an interface. When the hydrogel polymerizes, a hydrogel-media interface is created and can be used as a substrate for cell seeding, often allowing cells to migrate into the hydrogel or remodel its architecture and composition30. A different approach relies on synthetic membranes, usually coated with ECM proteins, to enhance cell adhesion and separate the different compartments. Membranes vary according to their constituting material (e.g., polyesters, polycarbonates, PDMS), which in turn can influence their mechanical properties (e.g., flexibility) and pore size. Pore size (typically 5 – 0.1 μm) remains important for cell-based assays as membranes are often meant to retain most cells seeded on top of them while also allowing for the diffusion of molecules31. Conversely to the hydrogel pining approach, ECM remodeling studies are not often possible using membranes alone. Hybrid strategies of using a membrane for hydrogel pinning have also been reported32,33.
Cell sources for microfluidic modeling of vasculature
Vessels range in size and complexity (e.g., arteries, capillaries), and are different according to the tissue of origin. However, the cells composing, and stabilizing blood vessels can be sorted within a handful of categories: Endothelial cells (hematogenous or lymphatic), vascular smooth muscle cells, pericytes and fibroblasts Within these lineages, cells also vary according to the tissue of origin. Together, this amounts to a large variety of vascular cells that could be used within microfluidic models. However, availability of cells for modeling is limited to commercial sources, iPSC-derived cells and primary cells from mouse models or human tissue samples (see table 1). Cell availability continues to be a hurdle for microfluidic modeling, resulting in only a few cell types that are highly popular for microfluidic studies.
Table 1:
Summary of vascular cell types and fibroblasts used in the microfluidic studies reviewed in this article. N=no, Y=yes
| Cell type | Name | Tissue of origin | Organism of origin | Description | Commercial availability | Refs |
|---|---|---|---|---|---|---|
| Endothelial cells | hASC-EC | adipose | human | human adipose stem cell derived endothelial | N | 37 |
| hBTAECs | breast | human | human breast tumor associated endothelial | Y | 38 | |
| CF cell-derived EC | cord blood | human | colony forming cell-derived endothelial | N | 39–42 | |
| iPSC-EC | dermal (iPSC) | human | human iPSC derived endothelial | Y | 43 | |
| GENCs | kidney | unknown | glomerular endothelial | Y | 44 | |
| KMVEC | kidney | mouse | kidney microvascular endothelial | N | 45 | |
| TENC/NENC | kidney tumor/adjacent normal | human | human primary kidney patient derived/normal adjacent endothelial cells | N | 46,47 | |
| HLMVEC | lung | human | human lung microvascular | Y | 48,49 | |
| HMVEC | microvascular - | human | human microvascular endothelial | Y | 50–54 | |
| HUVEC | umbilical cord | human | Y | 40,50,51,54–79 | ||
| hTERT hMVEC |
- | human | hTERT immortalized microvascular endothelial | N | 8,80 | |
| HLEC | - | human | human lymphatic endothelial | Y | 81,82 | |
| Pericytes | pericytes | brain | rat | primary rat brain pericytes | Y | 43 |
| HPP | brain | human | human pericytes | Y | 57 | |
| hVP | - | human | human vascular pericytes | Y | 59 | |
| Smooth muscle cells | hVSMC | - | human | human primary vascular smooth muscle | Y | 59 |
| Fibroblasts | NDF | Skin | human | normal dermal fibroblasts | Y | 51,59,83 |
| BJ-5ta | human | immortalized human foreskin fibroblasts | Y | 38 | ||
| NHLF | Lung | human | Normal human lung fibroblasts | Y | 39–43,56,57,60,76 | |
| HNF | Head and neck cancer | human | Head and neck patient-derived fibroblasts | N | 82 |
Single endothelial tubes (constructed as endothelial cells lining a single channel) have served, as a starting point for studying vascular biology as previously discussed, and the cells of choice have traditionally been human umbilical cord endothelial cells. The popularity of HUVECs is likely due to their ease of availability, since they are both commercially available and easy to extract from donated umbilical cords, and their culture conditions and biology are well-known. Microvascular endothelial cells are also a popular alternative, and more recently the field has turned to induced pluripotent stem cells and commercial organotypic sources (Table 1). Next, fibroblasts have been a popular addition to microfluidic vascular models. The high variability of fibroblasts and additional sources have been nicely reviewed elsewhere34–36.
More recently, smooth muscle cells, and pericytes have started to be included in vascular biology models. Recently more companies have started to commercialize cells of these lineages, yet sources of smooth muscle cells and pericytes remain more limited. We expect that the availability of these cells will continue to increase and facilitate the generation of new organotypic vascular models.
Microfluidic Models in Vascular Biology Research
The wide range of fabrication techniques and design options available for microfluidic platforms have enabled the development of a highly diverse array of devices for vascular biology research. This section will review the main types of microfluidic devices that have been applied applied in the field of vasculogenesis and angiogenesis.
Model classification according to cell seeding substrate
The study of vascular biology requires building vascular interfaces84. Said interfaces have been created using the polymers constituting the devices, 2D membranes, or 3D porous scaffolding (e.g., collagen, PEG-DA). Since cell substrates condition the events of the angiogenic cascade that can be modeled, we classified the microfluidic modeling approaches for angiogenesis based on the nature of the substrate used (Figure 3):
Cells are seeded on the device material (2D only): The polymer constituting the device, sometimes including 2D membranes, is the substrate for cell seeding. This approach, sometimes (and hereafter) called 2D vascular interfaces, was most often used in early reports where cell confluency, alignment, and responses to shear stress were assessed. It also allows for higher throughput, and hence is chosen over other experimental layouts for prototyping more complex geometries or adding large scale mechanical stimuli.48 These setups excel at providing maximum throughput. However, the membranes used to create these platforms hinder endothelial cell migration and invasion, thereby limiting the relevance of some typical readouts of angiogenesis (e.g., sprouting).
Cells are seeded on a scaffolding material (3D only) and not the device building material: In this layout, a natural or synthetic matrix is used as a substrate for cell seeding, and the devices are only used to contain the scaffolding. The most common approaches to seeding the cells within 3D porous scaffolding are sacrificial molding of structures within the matrix (hereafter called 3D scaffolding models or devices)85,86 and self-assembled vessels (hereafter called microvascular assembly models or MVA models), which are discussed in detail in the following section. Other approaches have also been reported, such as the “viscous finger patterning” technique87 or 3D bioprinting. While shear stress studies are possible using these layouts, they are more challenging and remain less ubiquitous in the literature. Conversely, other readouts are uniquely enabled by these technologies due to their full 3D structure: cell migration, sprouting, ECM remodeling are all ideally performed in these platforms. Conversely, their throughput tends to be lower than 2D or 2D/3D approaches. Notably, this approach is becoming more popular in later years.
Cells can be seeded on a scaffolding material (3D) and on the device building material (2D): The (often pinning based) architecture of these devices can be leveraged to pattern cells on a combination of polymer and 3D scaffolding (i.e., 2D/3D). Notably, some of these devices are the same as those used for 3D only (e.g., MVA models), but the interfaces of the material are used for cell seeding as well. The use of device materials has implications on cell morphology and physiology, due to the stiffer nature of devices and 2D organization of cells. A square cross-section vessel model is produced by using both hydrogel interfaces (produced by microfluidic valve or membrane pinning) and device walls. Several variations of this design have been reported with 3 or 5 microchannels, typically pinning 1–3 hydrogels through microfluidic posts51. A similar layout results from perfusing a 2D vascular interface device with a hydrogel. Finally, other devices have used pinning principles with a different design, such as the unique Stacks design, a modular design that allows assembly and disassembly of different hydrogel layers88. These 2D/3D setups present a balance in throughput as compared to 3D or 2D systems and benefit from the mechanical stability of using building materials as part of their seeding substrate. Therefore, they have shown the highest compatibility with angiogenesis-related readouts (e.g., shear stress, mechanical forces, permeability, sprouting). Conversely, there has been discussions in the literature indicating that blood vessel models in these setups are not fully tubular nor fully established on a substrate of physiological stiffness, and therefore their functionality may be affected89.
Figure 3: Classification of microphysiological models vascular interfaces used for angiogenesis research according to the substrate supporting the endothelial cells.
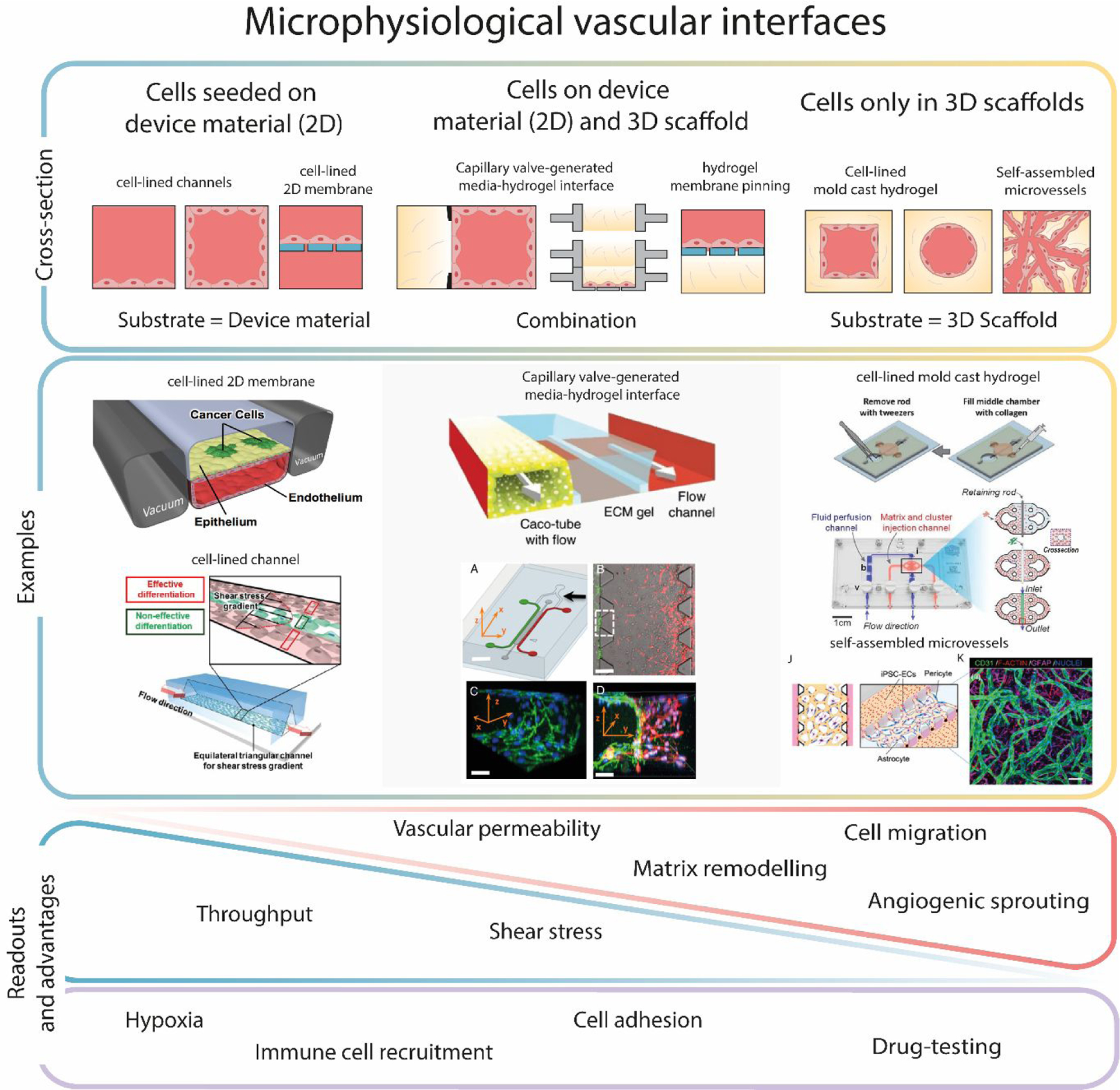
In these models, cells can be supported on the device material (2D), a 3D ECM-like scaffold (of natural or synthetic origin) (3D), or both 2D and 3D (2D/3D). Different layout configurations are shown, with device walls depicted as bold black lines, ECM-like scaffolds in yellow, membranes in blue, culture media in red. Literature examples and advantages of each model are shown below37,43,49,51,55,90,91. The triangles symbolize the applicability of those readouts to each configuration (apex = lower applicability), whereas the rectangle indicates that readouts are possible across the different configurations. Figures reproduced with permission.
Certain readouts are equally enabled by all three approaches, such as immune cell recruitment and drug testing (Figure 3), since those applications are not especially affected by model constraints.
Microfluidic models for Microvascular Assembly
Although most research to date has focused on angiogenesis as the main mechanism behind new vessel development, there is another important mechanism that has recently come into the spotlight in microfluidic models92. Specifically, vasculogenesis is an important biological process defined as the differentiation of precursor cells (e.g., angioblasts) into endothelial cells and the de novo formation of a primitive vascular network, whereas angiogenesis is defined as the growth of new capillaries from pre-existing blood vessels93. Vasculogenesis consists of three major steps: induction of vascular cell precursors like angioblasts (in a fibroblast growth factor (FGF)-dependent mechanism), assembly of primordial vessels (through vascular endothelial growth factor/vascular endothelial growth factor receptor signalling, VEGF/VEGFR) and transition from vasculogenesis to angiogenesis94.
Microfluidic microvascular assembly (MVA) models were first reported in 2013 simultaneously by two independent laboratories95, and leveraged designs with a central microchamber and flanking channels (Figure 3). In these models, the tubular network or MVA can be accessed via the flanking channels to add additional cell types, drugs, or antibodies for staining (Figure 3)56. Resulting blood vessel models present a smaller diameter that more closely resembles that of capillaries, and therefore their flow and transport rates of nutrients and oxygen to tissues are closer to those seen in vivo. Initial studies evaluated embedded culture of Human Umbilical Cord Endothelial Cells (HUVEC) and human lung fibroblasts in a fibrin and collagen 3D matrix in the central microchamber. Upon applying a fluid flow, HUVEC cells reorganized and self-assembled into a tubular network within 4–5 days, demonstrating that this model can be used to recapitulate tubular structures. Subsequent studies using alternative endothelial cell models (e.g., iPSC-ECs) and additional cell types (e.g., pericytes) have also been successful in these models43,57,58,96.
Several research groups have leveraged the MVA model to study vascular processes. Notably, to model anastomosis, Wang et al. created vessels on the flanking channels of the microvascular bed model and applied transendothelial flows and VEGF gradients to induce a vascular system model integrating an artery, a capillary network, and a vein39. The three vascular types were connected via two anastomosis-like junctions, as demonstrated by the barrier function exhibited by the conduit model. DiVito et al. created an MVA model via hydrodynamic focusing, a technique that leverages relative pressures of two or more fluids within a channel to align particles or cells in a specific area and simulated angiogenesis tubulogenesis, and anastomosis59. Later, other authors repurposed this model to mimic tumor angiogenesis40,60 and metastasis61, with an emphasis on anti-angiogenesis drug testing41,62, which is discussed in the dedicated section of this review.
Microfluidic applications in vascular biology research
One of the key advantages of microfluidics over traditional cell culture platforms is the high user control and customizability, which allows the user to incorporate many different environmental factors that play a role in the biological process of interest, including a variety of cell types and biophysical or biochemical cues11. Further, another advantage of microfluidics is the capability to evaluate functional readouts, which is typically defined as those that directly evaluate the physical response of cells to specific microenvironmental stimuli or treatments (e.g., cell migration, cell-cell junctions)97.
This section will review the various hallmarks of vasculogenesis for which microfluidic devices have been designed and discuss the roles of select devices in angiogenesis and vasculogenesis including the effect of biophysical and biochemical cues and functional readouts uniquely enabled by these technologies (Figure 4).
Figure 4:

Summary of the applications (larger portion of graph) and main functional readouts (extruded section) in microphysiological models used to investigate contributing factors to neovascularization. Main biophysical factors studied in microfluidics include the response to Shear Stress98, Interstitial Forces75, Cell-Cell interactions99, Extracellular matrix84. The principal biochemical factors studied are Soluble Factors85 and Hypoxia100. The main functional evaluated in microfluidic models are vessel permeability56 and angiogenic sprouting101. Figures reproduced with permissions.
Biophysical factors in vascular biology
Mechanical forces in blood vessels are tightly controlled due to their crucial role in maintaining vessel homeostasis. These forces are dependent on the flow pattern of fluid within the vessels, which in most of the circulatory system is laminar. Laminar flow is characterized by a parallel orientation to the vessel, with a series of molecular layers slipping past each other with increasing velocity towards the center of the vessel (a detailed description of the physics involved in fluid flow patterns can be found in102. This flow pattern results in two types of fluid-exerted forces: intravascular and transvascular.
Intravascular forces are those exerted within the vessel due to fluid flow. The most studied intravascular forces are shear stress, which is the stress exerted on the endothelium due to tangential blood flow. Endothelial shear stress depends on the flow rate, the blood’s viscosity, and the tube’s geometry. In general, mean shear stress is higher in small arterioles (60–80 dynes/cm2)103 and lower in large veins (<1 dyne/cm2)104. Blood behaves as non- Newtonian fluid in small capillaries and is, therefore, less predictable than other fluids (e.g., water). Shear stress induces several biological responses in endothelial cells mediated by the mechanosensing proteins integrins, including cytoskeletal reorganization, regulation of differentiation, proliferation, migration, and angiogenic sprouting. Conversely, dysregulation of shear stress is observed in pathologies, such as atherosclerosis105.
Transvascular forces, mainly interstitial pressure, are those exerted outward of the vessel by the fluid and primarily depend on the contained fluid volume. Fluid volume results from the equilibrium of two phenomena: hydrostatic pressure of blood, forcing fluid out of the capillaries, and the oncotic pressure driving fluid from the interstitial space back into the capillaries and lymphatics.106
The following section reviews some of the most important microfluidic devices reported to study fluidic forces and their effects on angiogenesis.
Shear stress and interstitial forces in microfluidic vascular models
Microfluidic devices have inherent advantages for studying the effects of fluid flow on cell culture systems: a scale where laminar flow is dominant and highly customizable designs to recapitulate the vessel and cues of interest. Furthermore, these systems provide an easy interface with precision flow pumps, such as syringe or peristaltic, which deliver constant or pulsatile flow, respectively. The height of the microchannel and fluid flow pressure enabled by the pump determines the applied shear stress, thereby providing fine control over the fluid forces in these systems.
Most studies have evaluated the effects of shear stress on angiogenic hallmarks (Figure 1) other than the traditional angiogenic migration and sprouting. Early studies of shear stress in microfluidic cell cultures seeded a single cell type monolayer on a 2D vascular interface on PDMS, often coated with ECM proteins to enhance cell adhesion107. High-throughput versions with several channels running in parallel63 have been reported, and commercial versions of this device with different channel heights are available for modeling different shear stress ranges (e.g., IBIDI’s μ-Slide I Luer). 2D vascular interfaces have been used to investigate the effects of shear stress on multiple cell functions, such as cytoskeletal organization, immune cell recruitment, or drug uptake. Buchanan et al. were the first to modify this design to generate a spatiotemporal mapping of flow fields44,80. Other modifications in the geometry of the channels (e.g., diamond-shaped channels, corners, pillars) can be used to accommodate high and low-stress zones within the same chamber63. An example of these modifications was reported by Westein et al. to model stenotic lesions64 in vitro. Other authors have leveraged 2D membrane devices to combine the effects of stress with paracellular transport of small molecules65, cell-cell interactions, or cell migration across the membrane have been described66. Using these devices, Shirure et al. demonstrated that angiogenesis is directionally opposite to interstitial flow in stiff matrices at physiological interstitial flow (0.1–10 lm/s). 42
2D/3D microfluidic models have been used to analyze the effects of interstitial flow together with shear stress, angiogenic gradients, and cell-cell interactions within the TME. To this end, they used a 3-channel platform and used each of the channels as follows: 1) endothelialized channel, 2) 3D matrix, often including additional cell types of interest 3) optional channel for lymphatic drainage.52,68,108 These type devices have been helpful in the field of cancer research to mimic native tumor vascular flow dynamics and investigate cancer-ECM-endothelial interactions.38,53
3D scaffolding microdevices have been less popular to study flow-induced shear stress. Yet, they offer an independent modulation of flow-induced shear in a 3D microenvironment. Buchanan et al. created a microchannel lined with endothelial cells and surrounded by a collagen matrix containing embedded tumor cells109. Using this platform, the authors investigated the role of shear stress on vascular barrier function and tumor-endothelial crosstalk. However, the tubular geometry of Buchanan et al. device did not allow transmural shear since the flow was tangential to the apical surface of the endothelial-lined microchannel. Using a combination of 3D and 2D microfluidic devices, Galie et al. found that both transmural and luminal flow are required to induce and sustain flow-induced sprouting50. Recently, 3D scaffolding devices have been leveraged to generate curved vessels with a controlled diameter (120–400 μm), curvature, and torsion. This system reproduced the changes in wall shear stress induced by curvatures and generated a stress gradient in 3D. The authors found that, under high shear stress, spiral vessels increase the proliferation rate of endothelial cells. However, no clear relationship between fluid-induced forces and angiogenic sprouting has been established yet in microfluidic platforms.50,110
Cell-cell interactions in microfluidic vascular models
Several types of cells (e.g., erythrocytes, platelets, and immune cells) circulate in the blood and contact the endothelium. Although erythrocytes are the most abundant cells in human blood, platelets and immune cells circulate preferentially at the periphery of the bloodstream due to their small mass and therefore have higher chances of interaction with the endothelium111. Thus, vascularized microfluidic models represent ideal models to investigate the effects of blood cells on angiogenesis.
Early studies used 2D vascular interfaces (or commercial analogs) perfused with whole blood or a specific blood cell type to analyze blood- endothelium interactions. For instance, these devices have been used to study alterations in endothelial glycocalyx and cellular adherence and therapeutic strategies for sickle cell disease or malaria69,70,112. However, the impact of these alterations in the angiogenic process remains unknown.
Although 2D microfluidic devices can be useful to analyze the molecular cues involved in Endothelium-Blood cell interactions, they lack the 3D structure needed to recapitulate important processes such as matrix invasion during angiogenic sprouting, leading to the development of 3D scaffolding devices to model angiogenesis. The earliest 3D scaffolding devices leveraged PDMS to create microvascular-sized fluidic channels lined with endothelial cell monolayers in their inner 3D surface. This system reproduced quantifiable velocity profiles of red blood cells and cellular mechanisms of adhesion and aggregation; therefore, it has become a suitable device for studying microvascular occlusion and thrombosis72,113. However, solid polymeric materials present challenges to recapitulate relevant tissue stiffness, leading to endothelial dysfunction and compromised barrier function. To address this issue, Qiu et al. used an agarose-gelatin interpenetrating polymer network (IPN), which confers a more physiological relevant stiffness (from hundreds of Pa to 50 kPa) and maintain barrier function for over one month under continuous laminar flow condition (Figure 3)99. The introduction of porous membranes enabled the communication of different microfluidic chambers. One example is the Vessel-Chip reported by Barile et al., where a human endothelial model is perfused with whole human blood at physiologically relevant shear to model thrombosis114. Another 2D/3D microfluidic model was used to recreate the effect of immune cells and factors on endothelial cell migration. This device connected two endothelialized side channels through one migration channel. Endothelial cells and immune cells are placed in one channel, while VEGF and other cytokines were perfused in the other to investigate their effect on cell migration115.
Notably, these studies provided only a first approach of evaluating the effects of blood-endothelial interactions in regulating neovascularization. Therefore, there is still opportunity to evaluate the effects of blood-endothelial interactions on other angiogenic hallmarks (Figure 1) including traditional angiogenic migration and sprouting.
Extracellular matrix in microfluidic vascular models
The extracellular matrix (ECM) is a network of proteins, including glycosaminoglycans, proteoglycans, and collagen, which vary across different tissue provide biochemical, biomechanical, and structural cues for the development of organs116.
ECM composition and architecture are known to have important roles in the development of new vasculature and the stabilization of established networks117. While the role of ECM in angiogenesis remains understudied using microfluidic technologies, a few strategies have emerged for recapitulating the effects of native ECM in microfluidic devices. A first approach leveraged 2D microfluidic devices of PDMS or glass coated with a single or combination of purified ECM proteins (e.g., collagen, fibronectin, or laminin) to enhance cell adhesion113. However, PDMS has a higher stiffness than vascular ECMs (i.e., MPa instead of the physiological kPa range). To overcome this issue, three main approaches have been developed: (1) increasing the ratio of PDMS elastomer to curing agent118 (2) coating 2D PDMS devices with acrylamide/bisacrylamide gels119 (3) and embedding streptavidin-coated magnetic beads to modify the stiffness of the ECM upon the application of an external magnetic field120. Besides ECM stiffness, heterogeneity of pore diameter, number, and alignment results in differential effects on angiogenesis. To examine the relationship between pore size and migration, Keys et al. developed a PDMS microfluidic device containing a series of thin channels (1–3 μm in width) that mimic constraints in cell migration with different physiological pore sizes.121
3D hydrogels have also been applied to create endothelialized tubes typically encapsulated in collagen. These devices mimic the multiscale organization of vascular ECM and have shown to be effective models for investigating solute diffusion rates between the lumen and the ECM, cell-ECM interactions, and ECM remodeling (Figure 4)98,122. Similar endothelialized tubes have been leveraged to develop tumor organoid-vessel co-cultures to study vascular recruitment and tumor intravasation123. Early devices used type I collagen and fibrin hydrogels to reproduce the ECM and showed that higher concentrations of polymers resulted in decreased sprouting. However, the specific molecular cues responsible for this effect (e.g., matrix stiffness, porosity, degradability, viscoelasticity, and ligand engagement) remained unidentified124. Later, synthetic hydrogels (e.g., functionalized polyethylene glycol, hyaluronic acid, alginate, and dextran) were introduced and compared with their natural homologs, revealing that matrix permeability was a critical regulator of angiogenesis125–127. To reproduce a more physiological ECM, 3D hydrogels can be functionalized with integrin- or protease-binding sequences in studies of cell binding capacity and matrix degradability in angiogenesis128. Other authors leveraged 3D PDMS devices with microchannels to generate diffusion gradients of pro-angiogenic factors (e.g., VEGF) across the ECM and analyze its effect on endothelial sprouting129.
The ECM properties (stiffness, density, and microstructure) alter angiogenic behavior in microvessels, however, little experimental data are available and there is a gap in our knowledge about microvessel interaction with its matrix. We anticipate that recent advances in microfluidic devices, imaging and computational methods will enhance the control of ECM properties and enable further study of its implications on angiogenesis and vasculogenesis.
Biochemical factors in vascular biology
Biochemical factors contribute regulate the process blood vessel formation via spatial cues that direct vessel sprouting and maturation. Stimuli such as hypoxia or inflammation, provoke a localized release of cytokines and other soluble molecules, which effectively creates a gradient within the extracellular space. The establishment of this molecular gradient leads to the formation of a spatially controlled leading edge of cells, which induces localized angiogenesis and increased perfusion.
Soluble factors in microfluidic vascular models
At its core, blood is a connective tissue with vital roles in a variety of processes, including cellular nutrition, drug delivery, clot formation, immune and inflammatory response, and regulation of angiogenesis. These functions are tightly regulated through a complex communication between the blood, distant organs and the vascular endothelium mediated by adhesion mechanisms, fluid forces, and pro-angiogenic factors (e.g., VEGF, FGF). The alteration of this delicate balance results in pathological conditions affecting vascular systems and distant organs, including liver disease, cystic fibrosis, pulmonary arterial hypertension, and sickle cell disease.
Plasma is the acellular portion of blood, composed of an aqueous solution of small organic molecules, proteins, and salts. Plasma regulates angiogenesis through two main mechanisms: fluid forces and the presence of soluble proteins or factors. These soluble factors can be classified into 4 categories: angiogenic growth factors (e.g., VEGF, FGF, Ang-1), pro-inflammatory cytokines (e.g., TNF-alpha, IL-6), chemokines, and second messengers to angiogenic molecular pathways (e.g., S1P, cGAMP)130. While growth factors are the main drivers of angiogenesis, pro-inflammatory cytokines play a larger role in regulating vessel permeability. Finally, chemokines are responsible for recruiting immune cells, which, in turn, secrete angiogenic growth factors131. Most of the microfluidic designs described in this review have been leveraged to investigate the role of soluble factors in angiogenesis, with a particular interest in the role of angiogenic growth factors.
2D/3D devices were first used to study sprouting angiogenesis in response to VEGF and Angiopoietin-1 gradients68,73,132. Recently, an innovative capillarity-based design capable of stacking several hydrogel disks and re-assembling them studied the effects of cell-secreted chemokines (i.e., CXCL10, CXCL11, and IL-10) on HUVEC cells seeded in a hydrogel interface88. Less often, 2D/3D devices have been used to investigate the effects of pro-inflammatory cytokine TNF-α on endothelial integrity and cell junctions54. These studies revealed an alteration in endothelial cell morphology, followed by an increase in endothelial barrier permeability, and pointed to a fibrosis mechanism. However, 3D scaffolding models have proved more popular for these studies. For example, tubular mold casting devices in collagen hydrogels have investigated the effects and mechanism of pro-angiogenic growth factors, such as VEGF and sphingosine-1-phosphate (Figure 4)45,85, revealing increased angiogenic sprouting.
Finally, several MVA models have been recently reported to investigate the effects of angiogenic growth factors, such as VEGF96 and PDGF-BB74, either adding them exogenously or using specific inhibitors57. While most reports have investigated the role of angiogenic growth factors, few have studied the role of pro-inflammatory cytokines (Figure 4),75 chemokines, and other important secondary messengers in angiogenesis. Of note, a recent report from Campisi et al. examined the role of tumor-secreted secondary messenger cGAMP in an MVA model. The authors noted how this molecule promoted an increase in oxidative stress in their model and activated the endothelial cells in the microvasculature model76.
Hypoxia in microfluidic vascular models
All tissues in the human body rely on oxygen to support their metabolic requirements. Decreases in local oxygen tension in tissues (i.e., hypoxia) therefore results in the activation of an array of compensatory intra- and extracellular pathways to increase oxygen supply. Angiogenesis is a key component of this compensatory response, restoring oxygen through increased blood flow to hypoxic areas. Studies evaluating hypoxia in vitro have encountered important challenges with replicating relevant oxygen levels: while atmospheric oxygen tension is 20%, normoxia within tissues varies between 3% to 7.4%, and tumor oxygenation is further reduced at <2%133. Conventional methods of replicating tissue oxygenation in vitro use chemical preconditioning (e.g., CoCl2 treatment) or incubators that modify oxygen tension by mixing in carbon dioxide or nitrogen gas, both of which present limitations. Chemical preconditioning often presents off-target effects, including cytotoxicity, and specialized incubators are expensive and cannot guarantee a loss of relevant oxygen tension as samples are manipulated for maintenance or imaging134.
Microfluidic technologies can overcome many of these limitations, supporting both fine-tuning of oxygen tension and analysis of the effects of hypoxia in cell cultures. However, this remains an active field of research since PDMS is not ideally suited to study the impact of hypoxia due to its high oxygen diffusivity135. Therefore, constant control of oxygen tension is required to maintain a hypoxic environment within PDMS devices. Alternatively, oxygen-impervious materials (e.g., COP)136 or PDMS modifications have been proposed to mitigate this issue137.
Notably, there is very little literature evaluating the effects of varying oxygen concentrations in traditional angiogenic hallmarks, such as sprouting (Figure 1). Therefore, most of the approaches described here are first uses of microfluidic technologies in vascular biology studies. 2D interfaces on porous membranes and 2D/3D models have been used to study the effects of hypoxia. These designs used one of the channels to perfuse oxygen scavengers (i.e., molecules capturing oxygen, such as sodium sulfite) or gasses capable of displacing oxygen in culture (e.g., CO2). In contrast, the other channel was used for cell culture134. This methodology allows for rapid equilibration times and supports the creation of oxygen gradients. An early example of this application was reported in 2012 (Figure 4)100 and demonstrated that hypoxia enhanced breast cancer cell migration.
One of the few reports studying effects of hypoxia in the endothelium was recently reported by Lam et al. The authors used a 5-channel pinning device to create a 2D/3D model, where an endothelial layer was cultured in the central channel, flanked by two stromal chambers. One of the outermost channels was used to perfuse media containing sodium sulfite (thereby depleting oxygen), whereas the other was used to perfuse normally oxygenated media138. This setup established a left-to-right oxygen gradient and enabled spatiotemporal control of oxygen tension around a vessel network, including cycling around two oxygen values to simulate intermittent hypoxia described in some pathologies.
To simulate hypoxia within a more advanced vessel architecture, Wu et al. created 2D PDMS channels with shapes resembling normal and tumor-associated capillary beds. The authors used a dialysis membrane to separate the cell culture from a fluid flow of sodium sulfite-supplemented media. The dialysis membrane allowed the exchange only of oxygen scavengers. Different oxygen tensions were achieved by varying the media flow rate or scavenger flow rate, validating this platform to simulate tumor hypoxia and vascular anomalies.
A recent report from Hsu et al. developed a microfluidic device fabricated in 4-layers alternating PDMS and laser-etched acrylic and used it to generate an MVA model139. A combinatorial screening of hyaluronic acid concentrations in the hydrogel and different oxygen tensions evaluated their effects on the MVA density. Their experiments revealed that oxygen gradients promoted the proliferation and assembly of the endothelial cell networks within the hydrogels, whereas the hyaluronic acid promoted networking but not proliferation. Furthermore, the combination of oxygen gradients and hyaluronic acid further promoted vascularization, reinforcing the importance of different biochemical cues and gradients in the angiogenic process.
Other authors have leveraged cell consumption to generate hypoxic gradients similar to those observed in tumor tissues as an alternative to oxygen scavengers. For example, we recently leveraged a 3D scaffolding setup with two tubular structures: one mimics a blood vessel, whereas the other had a densely packed mass of renal cell carcinoma cells. In this setup, we observed the generation of hypoxia, secretion of growth factors, and an increase in the angiogenic response of the nearby blood vessel model140. Overall, the effects of different levels of hypoxia in the different neovasculogenic mechanisms remain largely understudied and may be therapeutically targetable, therefore creating opportunities for future studies in this topic.
Functional readouts
Functional readouts are those that directly evaluate treatment response on live cells (e.g., cell migration, cell proliferation) and consider additional factors such as the tissue of origin, the tumor microenvironment, or the immune system. These readouts are capable of quantifying the performance or behavior of cells to directly evaluate response to an environmental stimulus or treatment and can help provide more robust indicators of biological effect, or in the case of drugs, predictors to identify the optimal treatment141. Two such readouts in vascular biology are vessel barrier function and permability; and angiogenic sprouting97. The following section reviews the use of microfluidic devices to the effect of blood and barrier function permeability in blood vessel formation and remodeling.
Barrier function and permeability
The structure of blood vessels, which comprises tightly joined endothelial cells and a basement membrane, creates a barrier that restricts the movement of water, solutes, proteins, and blood cells between the intravascular and interstitial compartments. The endothelial barrier contributes to the function and homeostasis of several organs (e.g., kidney, intestine, eye, brain). Therefore, diminished barrier function can lead to severe pathologies such as organ failure, sepsis, inflammatory, neurodegenerative diseases142. The evaluation of barrier function has been one of the most popular readouts in microfluidic models, including blood vessels. For example, 2D/3D microfluidic models have been helpful for reproducing biological barriers such as brain, intestinal, or lung epithelial barriers143–147. The most studied barrier functions include the blood-brain barrier (BBB), lymphatic system, kidney-barrier, and eye-barrier. In this review, we focused on the physiological systems where barrier function plays a role in angiogenesis, thereby excluding popular barrier function models, such as BBB, which have been reviewed elsewhere143,148
Other barrier studies have leveraged 3D microfluidic devices to analyze tumor-blood vessel crosstalk and drug response, either as organoids or disperse cells in a 3D collagen matrix. In particular, the study of drug transport and efficacy within TMEs has generated considerable research interest). Recent studies show that the presence of tumor cells and conventional chemotherapeutics increase vascular leakiness77. However, antiangiogenic drugs can rescue normal vessel permeability47. Microvascular endothelial barriers have also been modeled using 3D devices.
An example was reported by Kim et al., who used microneedles to create an array of microchannels embedded within the bulk of a collagen matrix. As well as enabling the transport of nutrients, chemical compounds, biomolecules, and cell suspensions, this device supported the application of flow-induced mechanical stimuli149. Recently, 3D microfluidic devices have been applied to model blood vessel barriers in other organs, such as the blood-retinal barrier, crucial for maintaining adequate vision150. This unique model enabled 3D co-cultures of monolayers of human retinal pigment epithelium cells with human endothelial cells lining a microvessel151. In addition, microvessels demonstrated a well-defined geometry and physiological permeability, which increased in a dose-dependent manner with oxidative stress exposure, making this a useful model for the study of age-related macular degeneration.
Angiogenic sprouting and remodeling
Sprouting angiogenesis is the main process by which new blood vessels grow from existing ones. It occurs in several well-characterized stages and is controlled by the presence of angiogenic and antiangiogenic molecules. Moreover, vessel sprouting undergoes extensive remodeling during angiogenesis until they form a mature vasculature. This process includes steps of vascular pruning and regression of selected branches. Traditional in vitro cell cultures can be used to investigate endothelial cell migration and proliferation. However, they fall short of recapitulating the 3D environment of endothelial cells and integrating mechanical and chemical stimuli precisely. Therefore, microfluidic devices have been presented as a solution to overcome most of these challenges.
Due to the limitations of most 2D devices to assess 3D sprouting, 3D devices have been preferably selected. The first studies used collagen hydrogels within PDMS devices where endothelial cells were cultured in 3D parallel channels68. Using this methodology, the authors measured endothelial sprout length into the ECM67. To evaluate the effects of chemical cues and fluidic forces, gradients of angiogenic factors have been established in the systems74,85, and fluidic forces have been applied (e.g., perfusion)78. Complex geometries enabled the generation of temporally or spatially defined pro-angiogenic factor gradients 129. A combination of VEGF-165 phorbol 12-myristate 13-acetate (PMA) and sphingosine-1-phosphate (S1P) promoted sprouting while perfusion acts as an essential survival and vascular stabilization factor. In addition, tumoral organoids, or other cell types have been embedded within the collagen matrix, revealing a pro-angiogenic effect measured as an increase in angiogenic sprouting55.
While these systems are highly informative, the parallel endothelial channels did not fully reproduce the physiological organization of vasculature. The use of 3D capillary beds set in multiple planes has been proposed to overcome this drawback and reproduced the “tree-like” capillary structure shown in vivo152. Another limitation of most microfluidic devices was the rectangular shape of their chamber, which did not reproduce the physiological circular tube-like structure29,89. Consequently, the traditional chambers were substituted by a series of interconnected open-top microwells filled with collagen. The authors found that the distribution of sprouts in this model was more regular and generated more tube-like structures153.
Therapies targeting angiogenesis
Upregulation of angiogenic pathways is a well-known hallmark of cancer154, as initially postulated by Judah Folkman in 1971155. He proposed that tumor growth would stagnate at 1–2 mm3 size without additional blood vessel recruitment. Therefore, targeting tumor vasculature would limit nutrient and oxygen supply to the tumor, thereby hindering growth and promoting regression156. Other authors alluded to restructuring tumor vasculature that would revert the disorganization and leakiness into a “normalized” phenotype157. As a positive side-effect to this mechanism of action, it was postulated that a normalization of the vasculature would lead to a more efficient cytotoxic drug delivery to the tumor and a decrease in the intratumoral fluid pressure158.
Since these hypotheses were presented, and especially in the last two decades, targeting angiogenic pathways has gained much interest and resulted in effective anti-cancer treatment strategies. These strategies include tyrosine kinase inhibitors (TKIs), which inhibit VEGF effects on effector cells, and monoclonal antibodies, which bind to and inhibit VEGF directly. While these agents have been found to be effective in a range of cancers (e.g., Kidney, lung, brain, colon), the contexts of use for these agents and optimal therapeutic combinations continue to be under investigation. A few studies have recently leveraged microfluidic models to study TKI effectiveness in vitro55, including both in MVA models40,62 and 3D scaffolding models79. Recent models have focused on renal cell carcinoma and, notably, evaluating personalized model responses to TKI agents46,47. Although these models are in their infancy, their use could be instrumental in determining the therapeutic effectiveness of combination therapies and optimizing therapeutic regimens.
Emerging topics and perspectives
The increased utilization of microfluidic technologies has led to great strides in angiogenesis research. These features have helped researchers better replicate in vivo environments while maintaining the experimental flexibility and analytical capability of in vitro models and independently evaluating factors driving angiogenesis. Recently, researchers in the field have increasingly included more physiologically relevant cues for the model at hand. These include patient-derived or iPSC cell sources, native extracellular matrices, and human serum159. Yet, the importance of each of these factors on angiogenesis remains to be elucidated on a model-to-model basis, such as the precise effects of shear stress in angiogenesis.
Novel and exciting angiogenesis models are recently emerging, such as lymphangiogenesis 81,82, retinal angiogenesis, and reproductive organ models99,160. The field is recognizing the great potential of microfluidic models in recapitulating these environments for basic biology or translational purposes. We anticipate that these and other applications will continue to emerge and gain interest in the field, leading to exciting research advances.
While the advances in microfluidic-enabled research are promising, widespread adoption of microfluidics remains a challenge. Specialized knowledge and training remain a necessity for aspiring microfluidic researchers. In response to this limitation, a great effort in the commercialization of microfluidic devices is underway, with dozens of companies filling the market need for easy-to-use microfluidic devices for in vitro modeling27. Furthermore, with a handful of PDMS-built models being validated through multiple different applications, it is reasonable for companies to invest in a transition to more large-scale fabrication methods capable of replacing the use of PDMS with more inert plastic-based alternatives.11 Some other technical challenges that remain are the degree of control over the geometry, diameter, and layers of the vessels recapitulated, and the limited throughput of devices. Regarding the lack of control over vessel size, smaller vessels are currently recapitulated using MVA methods, which provide very limited control over the resulting network. Conversely, mold casting strategies typically produce larger vessels. However, none of these strategies are currently able to finely control the positioning of supporting cell types (e.g., pericytes)29. Bioprinting has been proposed as a potential solution to some of these challenges, as its resolution may increase to provide that additional layer of control over model building161. Finally, model tissue (also called organ-on-a-chip) vascularization remains an unresolved issue of interest for many biological fields.
Going forward, the advantages of microfluidic devices for recapitulating tissue pathophysiology by integrating spatial, biochemical, and biomechanical cues will likely provide insight into the mechanisms of angiogenesis, both in health and vascular-impacting diseases. Several research areas may benefit, especially from microfluidics, such as cardiac diseases162 and regenerative therapy, where microfluidics can be used to study the disease pathology and generate new liquid biopsy diagnostic tools163. Likewise, there is an increasing interest in the field of neuroscience, where several microfluidic models of brain microvasculature have been developed. Future microfluidic applications will likely investigate blood endothelial interactions, brain-blood barrier function, tissue vascularization, and the effect of angiogenic/anti-angiogenics drugs to treat conditions such as stroke or Alzheimer’s. However, more work is needed to develop standardized devices that can be subject to clinical use approval.
The integration of relevant cues is also of importance in new drug discovery, where accurate cell behavior and response/resistance to therapies are fundamental. We anticipate that microfluidic models will serve as a complement to traditional in vitro testing. First the more accurate tissue mimicry may reveal new cell and tissue behaviors than those observed in traditional in vitro models. Further, microfluidic models are ideally suited to investigate therapeutic targets challenging to observe in traditional in vitro models, hence creating new research lines and therapeutic opportunities for vascular-related disorders.
Finally, we anticipate that the improved microenvironmental modeling of microfluidic systems will provide a more comprehensive risk-benefit assessment of newly developed therapies, both for drug development and from a precision medicine perspective. An example lies in TKI treatment of cancers. We anticipate TKI therapy will continue to be a relevant clinical treatment option. For example, combinations of TKI drugs164 or TKI and immunotherapy agents (e.g., anti-PD-1/PD-L1) are getting FDA approval to treat several cancers, following evidence that TKIs play a role in overcoming resistance to immunotherapies. Thus, we foresee that microfluidic angiogenesis models will continue to complement traditional in vitro and in vivo models in assessing the effectiveness and safety of therapeutic combinations with TKI agents in individual patients.
Acknowledgments
Authors would like to acknowledge Dr Duane Juang for his comments on the fabrication section. This work was supported by the NIH (R33CA225281) and the Assistant Secretary of Defense for Health Affairs through the Prostate Cancer Research Program, Award No. W81XWH-18-1-0273 to DK. The U.S. Army Medical Research Acquisition Activity, 820 Chandler Street, Fort Detrick MD 21702-5014 is the awarding and administering acquisition office. Opinions, interpretations, conclusions and recommendations are those of the author and are not necessarily endorsed by the Department of Defense.
Footnotes
Conflict of interest statement
David J. Beebe holds equity in Bellbrook Labs LLC, Tasso Inc., Salus Discovery LLC, Lynx Biosciences Inc., Stacks to the Future LLC, Turba LLC, Flambeau Diagnostics LLC, and Onexio Biosystems LLC. David J. Beebe is also a consultant for Abbott Laboratories.
References
- 1.Carmeliet P and Jain RK, Nature, 2011, 473, 298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fallah A, Sadeghinia A, Kahroba H, Samadi A, Heidari HR, Bradaran B, Zeinali S and Molavi O, Biomedicine & Pharmacotherapy, 2019, 110, 775–785. [DOI] [PubMed] [Google Scholar]
- 3.Eelen G, Treps L, Li X and Carmeliet P, Circulation Research, 2020, 127, 310–329. [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D, Perspectives on the Future of Angiogenesis Research, Springer, Boston, MA, 2008. [Google Scholar]
- 5.Potente M, Gerhardt H and Carmeliet P, Cell, 2011, 146, 873–887. [DOI] [PubMed] [Google Scholar]
- 6.Yoo SY and Kwon SM, Mediators of Inflammation, 2012, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vasudev NS and Reynolds AR, Angiogenesis, 2014, 17, 471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchanan CF, Voigt EE, Szot CS, Freeman J, Vlachos PP and Rylander MN, Tissue Engineering Part C: Methods. 2013, 20, 64–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker BM and Chen CS, J Cell Sci, 2012, 125, 3015–3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Folch A, Introduction to bioMEMS, CRC Press, 2016. [Google Scholar]
- 11.Ayuso JM, Park K-Y, Virumbrales-Muñoz M and Beebe DJ, APL Bioengineering, 2021, 5, 010902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sollier E, Murray C, Maoddi P, di Carlo D, Lab Chip, 2011, 11, 3752–3765. [DOI] [PubMed] [Google Scholar]
- 13.Weibel DB, DiLuzio WR and Whitesides GM, Nature Reviews Microbiology 2007, 5, 209–218. [DOI] [PubMed] [Google Scholar]
- 14.Walsh DI, Kong DS, Murthy SK and Carr PA, Trends in Biotechnology, 2017, 35, 383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berthier E, Young EWK and Beebe D, Lab on a Chip, 2012, 12, 1224–1237. [DOI] [PubMed] [Google Scholar]
- 16.Regehr KJ, Domenech M, Koepsel JT, Carver KC, Ellison-Zelski SJ, Murphy WL, Schuler LA, Alarid ET and Beebe DJ, Lab on a Chip, 2009, 9, 2132–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gokaltun A, Yarmush ML, Asatekin A and Usta OB, Technology, 2017, 05, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guckenberger DJ, de Groot TE, Wan AMD, Beebe DJ and Young EWK, Lab Chip, 2015, 15, 2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Câmara MA, Rubio JCC, Abrão AM and Davim JP, Journal of Materials Science & Technology, 2012, 28, 673–685. [Google Scholar]
- 20.Wilson M, Kota N, Kim Y, Wang Y, Stolz B, LeDuc P and Ozdoganlar O, Lab Chip, 2011, 11, 1550–1555. [DOI] [PubMed] [Google Scholar]
- 21.Berthier E, Guckenberger DJ, Cavnar P, Huttenlocher A, Keller N and Beebe DJ, Lab Chip, 2013, 13, 424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ando Y, Ta HP, Yen DP, Lee S-S, Raola S and Shen K, Scientific Reports, 2017, 7, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papautsky I and Peterson ETK, Encyclopedia of Microfluidics and Nanofluidics, 2015, 2102–2116. [Google Scholar]
- 24.Novak R, Ranu N and Mathies RA, Lab Chip, 2013, 13, 1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim SJ, Yang H, Kim K, Lim YT and Pyo HB, Electrophoresis, 2006, 27, 3284–3296. [DOI] [PubMed] [Google Scholar]
- 26.Tosello G, Bissacco G, Tang PT, Hansen HN and Nielsen PC, Microsystem Technologies, 2008, 14, 1757–1764. [Google Scholar]
- 27.Zhang B and Radisic M, Lab Chip, 2017, 17, 2395–2420. [DOI] [PubMed] [Google Scholar]
- 28.Raeber GP, Lutolf MP and Hubbell JA, Biophysical Journal, 2005, 89, 1374–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Virumbrales-Muñoz M, Ayuso JM, Gong MM, Humayun M, Livingston MK, Lugo-Cintrón KM, McMinn P, Álvarez-García YR and Beebe DJ, Chemical Society Reviews, 2020, 49, 6402–6442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tung C, Krupa O, Apaydin E, Liou J-J, Diaz-Santana A, Kim BJ and Wu M, Lab on a Chip, 2013, 13, 3876–3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Jong J, Lammertink RGH and Wessling M, Lab Chip, 2006, 6, 1125–1139. [DOI] [PubMed] [Google Scholar]
- 32.Raimondi I, Izzo L, Tunesi M, Comar M, Albani D and Giordano C, Frontiers in Bioengineering and Biotechnology, , DOI: 10.3389/fbioe.2019.00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi Y, Hyun E, Seo J, Blundell C, Kim HC, Lee E, Lee SH, Moon A, Moon WK and Huh D, Lab Chip, 2015, 15, 3350–3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Biffi G and Tuveson DA, Physiol Rev, 2021, 101, 147–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sahai E, Astsaturov I, Cukierman E, DeNardo DG, Egeblad M, Evans RM, Fearon D, Greten FR, Hingorani SR, Hunter T, Hynes RO, Jain RK, Janowitz T, Jorgensen C, Kimmelman AC, Kolonin MG, Maki RG, Powers RS, Puré E, Ramirez DC, Scherz-Shouval R, Sherman MH, Stewart S, Tlsty TD, Tuveson DA, Watt FM, Weaver V, Weeraratna AT and Werb Z, Nature Reviews Cancer, 2020, 20, 174–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Öhlund D, Handly-Santana A, Biffi G, Elyada E, Almeida AS, Ponz-Sarvise M, Corbo V, Oni TE, Hearn SA, Lee EJ, Chio IIC, il Hwang C, Tiriac H, Baker LA, Engle DD, Feig C, Kultti A, Egeblad M, Fearon DT, Crawford JM, Clevers H, Park Y and Tuveson DA, J Exp Med, 2017, 214, 579–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim HW, Lim J, Rhie JW and Kim DS, Microelectronic Engineering, 2017, 174, 24–27. [Google Scholar]
- 38.Pradhan S, Smith AM, Garson CJ, Hassani I, Seeto WJ, Pant K, Arnold RD, Prabhakarpandian B and Lipke EA, Scientific Reports, 2018, 8, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang X, Phan DTT, Sobrino A, George SC, Hughes CCW and Lee AP, Lab Chip, 2016, 16, 282–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sobrino A, Phan DTT, Datta R, Wang X, Hachey SJ, Romero-López M, Gratton E, Lee AP, George SC and Hughes CCW, Scientific Reports, 2016, 6, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Phan DTT, Wang X, Craver BM, Sobrino A, Zhao D, Chen JC, Lee LYN, George SC, Lee AP and Hughes CCW, Lab Chip, 2017, 17, 511–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shirure VS, Lezia A, Tao A, Alonzo LF and George SC, Angiogenesis, 2017, 20, 493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Campisi M, Shin Y, Osaki T, Hajal C, Chiono V and Kamm RD, Biomaterials, 2018, 180, 117–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lobo DP, Wemyss AM, Smith DJ, Straube A, Betteridge KB, Salmon AHJ, Foster RR, Elhegni HE, Satchell SC, Little HA, Pacheco-Gómez R, Simmons MJ, Hicks MR, Bates DO, Rodger A, Dafforn TR and Arkill KP, Nano Res, 2015, 8, 3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dang LTH, Aburatani T, Marsh GA, Johnson BG, Alimperti S, Yoon CJ, Huang A, Szak S, Nakagawa N, Gomez I, Ren S, Read SK, Sparages C, Aplin AC, Nicosia RF, Chen C, Ligresti G and Duffield JS, Biomaterials, 2017, 141, 314–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiménez-Torres JA, Virumbrales-Muñoz M, Sung KE, Lee MH, Abel EJ and Beebe DJ, EBioMedicine, 2019, 42, 408–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Virumbrales-Muñoz María, Chen Jiong, Ayuso Jose, Lee Moonhee, Jason Abel E and Beebe DJ, Lab Chip, 2020, 20, 4420–4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.(Dan) Huh D, Ann Am Thorac Soc, 2015, 12, S42–S44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hassell BA, Goyal G, Lee E, Sontheimer-Phelps A, Levy O, Chen CS and Ingber DE, Cell Rep, 2017, 21, 508–516. [DOI] [PubMed] [Google Scholar]
- 50.Galie PA, Nguyen D-HT, Choi CK, Cohen DM, Janmey PA and Chen CS, Proceedings of the National Academy of Sciences, 2014, 111, 7968–7973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zervantonakis IK, Hughes-Alford SK, Charest JL, Condeelis JS, Gertler FB and Kamm RD, Proceedings of the National Academy of Sciences, 2012, 109, 13515–13520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kwak B, Ozcelikkale A, Shin CS, Park K and Han B, Journal of Controlled Release, 2014, 194, 157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hockemeyer K, Janetopoulos C, Terekhov A, Hofmeister W, Vilgelm A, Costa L, Wikswo J and Richmond A, Biomicrofluidics, 2018, 12, 034102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Virumbrales-Muñoz M, Ayuso JM, Olave M, Monge R, de Miguel D, Martínez-Lostao L, le Gac S, Doblare M, Ochoa I and Fernandez LJ, Scientific Reports, 2017, 7, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miller CP, Tsuchida C, Zheng Y, Himmelfarb J, Akilesh S, CP M, C T, Y Z, J H and S A, Neoplasia, 2018, 20, 610–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Offeddu GS, Possenti L, Loessberg-Zahl JT, Zunino P, Roberts J, Han X, Hickman D, Knutson CG and Kamm RD, Small, 2019, 15, 1902393. [DOI] [PubMed] [Google Scholar]
- 57.Haase K, Gillrie MR, Hajal C and Kamm RD, Advanced Science, 2019, 6, 1900878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jeon JS, Bersini S, Whisler JA, Chen MB, Dubini G, Charest JL, Moretti M and Kamm RD, Integrative Biology, 2014, 6, 555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.DiVito KA, Daniele MA, Roberts SA, Ligler FS and Adams AA, Data in Brief, 2017, 14, 156–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nashimoto Y, Hayashi T, Kunita I, Nakamasu A, Torisawa J, Nakayama M, Takigawa-Imamura H, Kotera H, Nishiyama K, Miura T and Yokokawa R, Integr Biol (Camb), 2017, 9, 506–518. [DOI] [PubMed] [Google Scholar]
- 61.Gilardi M, Bersini S, Valtorta S, Proietto M, Crippa M, Boussommier-Calleja A, Labelle M, Moresco RM, Vanoni M, Kamm RD and Moretti M, Biomaterials, 2021, 276, 120975. [DOI] [PubMed] [Google Scholar]
- 62.Zeinali S, Bichsel CA, Hobi N, Funke M, Marti TM, Schmid RA, Guenat OT and Geiser T, Angiogenesis, 2018, 21, 861–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang X, Huk D, Wang Q, Lincoln J, Zhao Y, Zhang X, Huk DJ, Wang Q, Lincoln J and Zhao Y, Biomicrofluidics, 2014, 8, 054106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Westein E, van der Meer AD, Kuijpers MJE, Frimat J-P, van den Berg A and Heemskerk JWM, Proceedings of the National Academy of Sciences, 2013, 110, 1357–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Frost TS, Jiang L, Lynch RM and Zohar Y, Micromachines (Basel), 2019, 10, 533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin YH and Ingber DE, Science, 2010, 328, 1662–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Song JW, Bazou D and Munn LL, Methods in Molecular Biology, 2015, 1214, 243–254. [DOI] [PubMed] [Google Scholar]
- 68.Song JW and Munn LL, Proceedings of the National Academy of Sciences, 2011, 108, 15342–15347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carden M, Fay M, Lu X, Mannino RG, Sakurai Y, Ciciliano JC, Hansen CE, Chonat S, CH J, DK W and WA L, Blood, 2017, 130, 2654–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Introini V, Carciati A, Tomaiuolo G, Cicuta P and Guido S, Journal of the Royal Society Interface, 2018, 15, 20180773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen HY, der Tsai H, Chen WC, Wu JY, Tsai FJ and Tsai CH, Journal of Clinical Laboratory Analysis, 2001, 15, 251–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tsvirkun D, Grichine A, Duperray A, Misbah C and Bureau L, Scientific Reports, 2017, 7, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jeong GS, Han S, Shin Y, Kwon GH, Kamm RD, Lee S-H and Chung S, Analytical Chemistry, 2011, 83, 8454–8459. [DOI] [PubMed] [Google Scholar]
- 74.del Amo C, Borau C, Gutiérrez R, Asín J and García-Aznar JM, J Biomech, 2016, 49, 1340–1346. [DOI] [PubMed] [Google Scholar]
- 75.Kim S, Chung M, Ahn J, Lee S and Jeon NL, Lab Chip, 2016, 16, 4189–4199. [DOI] [PubMed] [Google Scholar]
- 76.Campisi M, Sundararaman S, Shelton SE, Knelson EH, Mahadevan NR, Yoshida R, Tani T, Ivanova E, Cañadas I, Osaki T, Lee SWL, Thai T, Han S, Piel BP, Gilhooley S, Paweletz CP, Chiono V, Kamm RD, Kitajima S and Barbie DA, Front Immunol, 2020, 11, 2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Haase K, Offeddu GS, Gillrie MR and Kamm RD, Adv Funct Mater, 2020, 30, 2002444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.van Duinen V, Zhu D, Ramakers C, van Zonneveld AJ, Vulto P and Hankemeier T, Angiogenesis, 2019, 22, 157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee S, Kim S, Koo D-J, Yu J, Cho H, Lee H, Song JM, Kim S-Y, Min D-H and Jeon NL, ACS Nano, 2020, 15, 338–350. [DOI] [PubMed] [Google Scholar]
- 80.Buchanan CF, Verbridge SS, Vlachos PP and Rylander MN, Cell Adh Migr, 2015, 8, 517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lugo-Cintrón KM, Ayuso JM, White BR, Harari PM, Ponik SM, Beebe DJ, Gong MM and Virumbrales-Muñoz M, Lab Chip, 2020, 20, 1586–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lugo-Cintrón KM, Ayuso JM, Humayun M, Gong MM, Kerr SC, Ponik SM, Harari PM, Virumbrales-Muñoz M and Beebe DJ, EBioMedicine, 2021, 73, 103634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bischel LL, Lee S-H and Beebe DJ, J Lab Autom, 2012, 17, 96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lugo-Cintrón KM, Gong MM, Ayuso JM, Tomko L, Beebe DJ, Virumbrales-Muñoz M and Ponik SM, Cancers (Basel), 2020, 12, 1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nguyen D-HT, Stapleton SC, Yang MT, Cha SS, Choi CK, Galie PA and Chen CS, Proceedings of the National Academy of Sciences, 2013, 110, 6712–6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jiménez-Torres JA, Peery SL, Sung KE and Beebe DJ, Advanced Healthcare Materials, 2016, 5, 198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bischel LL, Lee S-H and Beebe DJ, Journal of Laboratory Automation, 2012, 17, 96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yu J, Berthier E, Craig A, de Groot T, Sparks S, Ingram P, Jarrard D, Huang W, Beebe DJ and Theberge A, Nat Biomed Eng, 2019, 3, 830–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bischel LL, Sung KE, Jiménez-Torres JA, Mader B, Keely PJ and Beebe DJ, The FASEB Journal, 2014, 28, 4583–4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Trietsch SJ, Naumovska E, Kurek D, Setyawati M, Vormann MK, Wilschut KJ, Lanz HL, Nicolas A, Ng CP, Joore J, Kustermann S, Roth A, Hankemeier T, Moisan A and Vulto P, Nat Commun, 2017, 8, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Morgan MM, Livingston MK, Warrick JW, Stanek EM, Alarid ET, Beebe DJ and Johnson BP, Molecules, 2018, 8, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Poole TJ and Coffin JD, J Exp Zool, 1989, 251, 224–231. [DOI] [PubMed] [Google Scholar]
- 93.Risau W, Nature, 1997, 386, 671–674. [DOI] [PubMed] [Google Scholar]
- 94.Flamme I, Frölich T and Risau W, J Cell Physiol, 1997, 173, 206–10. [DOI] [PubMed] [Google Scholar]
- 95.Kim S, Lee H, Chung M and Jeon NL, Lab Chip, 2013, 13, 1489–1500. [DOI] [PubMed] [Google Scholar]
- 96.Kim JJH, Chung M, Kim S, Jo DH, Kim JJH and Jeon NL, PLOS ONE, 2015, 10, e0133880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ayuso JM, Virumbrales-Muñoz M, Lang JM and Beebe DJ, Nature Communications, 2022, 13, 3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Polacheck WJ, Kutys ML, Yang J, Eyckmans J, Wu Y, Vasavada H, Hirschi KK and Chen CS, Nature, 2017, 552, 258–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Qiu Y, Ahn B, Sakurai Y, Hansen CE, Tran R, Mimche PN, Mannino RG, Ciciliano JC, Lamb TJ, Joiner CH, Ofori-Acquah SF and Lam WA, Nature Biomedical Engineering, 2018, 2, 453–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Funamoto K, Zervantonakis IK, Liu Y, Ochs CJ, Kim C and Kamm RD, Lab Chip, 2012, 12, 4855–4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jusoh N, Ko J and Jeon NL, APL Bioengineering, 2019, 3, 36101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Beebe David J., and Mensing Glennys A. and Walker GM, Annu Rev Biomed Eng, 2003, 4, 261–286. [DOI] [PubMed] [Google Scholar]
- 103.Ballermann BJ, Dardik A, Eng E and Liu A, Kidney International, 1998, 54, S100–S108. [DOI] [PubMed] [Google Scholar]
- 104.Chatterjee S, Frontiers in Physiology, 2018, 0, 524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cunningham KS and Gotlieb AI, Lab Invest, 2005, 85, 9–23. [DOI] [PubMed] [Google Scholar]
- 106.Scallan J, Huxley VH and Korthuis RJ, Fluid Movement Across the Endothelial Barrier, Morgan & Claypool Life Sciences, 2010. [PubMed] [Google Scholar]
- 107.Siddique A, Meckel T, Stark RW and Narayan S, Colloids and Surfaces B: Biointerfaces, 2017, 150, 456–464. [DOI] [PubMed] [Google Scholar]
- 108.Song JW, Bazou D and Munn LL, Methods in Molecular Biology, 2015, 1214, 243–254. [DOI] [PubMed] [Google Scholar]
- 109.Buchanan CF, Voigt EE, Szot CS, Freeman JW, Vlachos PP and Rylander MN, Tissue Engineering Part C: methods, 2014, 20, 64–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wu SH, Zhang F, Yao S, Tang L, Zeng HT, Zhu LP and Yang Z, International Journal of Stem Cells, 2020, 13, 312–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hesh CA, Qiu Y and Lam WA, Micromachines (Basel), 2019, 1, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.White J, Krishnamoorthy S, Gupta D, Lancelot M, Moore N, Sarnaik S, Hobbs WE, Light DR and Hines P, British Journal of Haematology, 2016, 174, 970–982. [DOI] [PubMed] [Google Scholar]
- 113.Tsai M, Kita A, Leach J, Rounsevell R, Huang JN, Moake J, Ware RE, Fletcher DA and Lam WA, The Journal of Clinical Investigation, 2012, 122, 408–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Barrile R, van der Meer A, Park H, Fraser J, Simic D, Teng F, Conegliano D, Nguyen J, Jain A, Zhou M, Karalis J, Ingber DE, Hamilton G and Otieno M, Clin Pharmacol Ther, 2018, 104, 1240–1248. [DOI] [PubMed] [Google Scholar]
- 115.Wu X, Newbold M, Gao Z and Haynes C, Biochim Biophys Acta Gen Subj, 2017, 1861, 1122–1130. [DOI] [PubMed] [Google Scholar]
- 116.Yue B, J Glaucoma, 2014, 23, S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mongiat M, Andreuzzi E, Tarticchio G and Paulitti A, International Journal of Molecular Sciences, 2016, 17, 1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Polacheck WJ and Zhang CS, Nat Methods, 2016, 13, 415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Syed S, Karadaghy A and Zustiak S, Journal of Visualized Experiments : JoVE, 2015, 2015, 52643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.See HH, Herath SCB, Arayanarakool R, Du Y, Tan E, Ge R, Asadam H and Chen PCY, SLAS Technol, 2018, 23, 70–82. [DOI] [PubMed] [Google Scholar]
- 121.Keys J, Windsor A and Lammerding J, Methods Mol Biol, 2018, 1840, 101–118. [DOI] [PubMed] [Google Scholar]
- 122.Chrobak KM, Potter DR and Tien J, Microvasc Res, 2006, 71, 185–196. [DOI] [PubMed] [Google Scholar]
- 123.Silvestri V, Henriet E, Linville R, Wong A, Searson P and Ewald A, Cancer Res, 2020, 80, 4288–4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ghajar CM, Chen X, Harris JW, Suresh V, Hughes CCW, Jeon NL, Putnam AJ and George SC, Biophysical Journal, 2008, 94, 1930–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Martino MM, Brkic S, Bovo E, Burger M, Schaefer DJ, Wolff T, Gürke L, Briquez PS, Larsson HM, Gianni-Barrera R, Hubbell JA and Banfi A, Frontiers in Bioengineering and Biotechnology, 2015, 0, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sokic S and Papavasiliou G, Tissue Engineering Part A, 2012, 18, 2477–2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang WY, Kent RN, Huang SA, Jarman EH, Shikanov EH, Davidson CD, Hiraki HL, Lin D, Wall MA, Matera DL, Shin J-W, Polacheck WJ, Shikanov A and Baker BM, Acta Biomaterialia, 2021, 135, 260–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Trappmann B, Baker BM, Polacheck WJ, Choi CK, Burdick JA and Chen CS, Nature Communications 2017 8:1, 2017, 8, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Baker BM, Trappmann B, Stapleton SC, Toro E and Chen CS, Lab Chip, 2013, 13, 3246–3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Neve A, Cantatore FP, Maruotti N, Corrado A and Ribatti D, BioMed Research International, 2014, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sprague AH and Khalil RA, Biochem Pharmacol, 2009, 78, 539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shin Y, Jeon JS, Han S, Jung G-S, Shin S, Lee S-H, Sudo R, Kamm RD and Chung S, Lab Chip, 2011, 11, 2175–2181. [DOI] [PubMed] [Google Scholar]
- 133.Swartz HM and Dunn JF, Advances in Experimental Medicine and Biology, 2003, 530, 1–12. [DOI] [PubMed] [Google Scholar]
- 134.Shirure VS, Lam SF, Shergill B, Chu YE, Ng NR and George SC, Lab on a Chip, 2020, 20, 3036–3050. [DOI] [PubMed] [Google Scholar]
- 135.Rivera KR, Yokus MA, Erb PD, Pozdin VA and Daniele M, Analyst, 2019, 144, 3190–3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ayuso JM, Monge R, Llamazares GA, Moreno M, Agirregabiria M, Berganzo J, Doblaré M, Ochoa I and Fernández LJ, Frontiers in Materials, 2015, 0, 37. [Google Scholar]
- 137.Markov DA, Lillie EM, Garbett SP and McCawley LJ, Biomedical Microdevices 2013 16:1, 2013, 16, 91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lam SF, Shirure VS, Chu YE, Soetikno AG and George SC, PLOS ONE, 2018, 13, e0209574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hsu H-H, Ko P-L, Wu H-M, Lin H-C, Wang C-K and Tung Y-C, Small, 2021, 17, 2006091. [DOI] [PubMed] [Google Scholar]
- 140.Virumbrales-Muñoz M, Ayuso JM, Loken JR, Denecke KM, Rehman S, Skala MC, Abel EJ and Beebe DJ, Biomaterials, 2022, 283, 121454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Morgan MM, Johnson BP, Livingston MK, Schuler LA, Alarid ET, Sung KE and Beebe DJ, Pharmacology & Therapeutics, 2016, 165, 79–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rodrigues SF and Granger DN, Tissue Barriers, 2015, 3, e978720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Walter FR, Valkai S, Kincses A, Petneházi A, Czeller T, Veszelka S, Ormos P, Deli MA and Dér A, Sensors and Actuators B: Chemical, 2016, 222, 1209–1219. [Google Scholar]
- 144.Sellgren K, Hawkins B and Grego S, Biomicrofluidics, 2015, 9, 061102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Terrell-Hall TB, Ammer AG, Griffith JIG and Lockman PR, Fluids and Barriers of the CNS, 2017, 14, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Martínez-Redondo V, Pettersson AT and Ruas JL, Diabetologia, 2015, 58, 1969–1977. [DOI] [PubMed] [Google Scholar]
- 147.Brown JA, Pensabene V, Markov DA, Allwardt V, Neely MD, Shi M, Britt CM, Hoilett OS, Yang Q, Brewer BM, Samson PC, McCawley LJ, May JM, Webb DJ, Li DL, Bowman AB, Reiserer RS and Wikswo JP, Biomicrofluidics, 2015, 9, 054124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Augustine R, Aqel AH, Kalva SN, Joshy KS, Nayeem A and Hasan A, Translational Oncology, 2021, 14, 101087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kim JA, Kim HN, Im S-K, Chung S, Kang JY and Choi N, Biomicrofluidics, 2015, 9, 024115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Runkle EA and Antonetti DA, Methods Mol Biol, 2011, 686, 133–148. [DOI] [PubMed] [Google Scholar]
- 151.Arık YB, Buijsman W, Loessberg-Zahl J, Cuartas-Vélez C, Veenstra C, Logtenberg S, Grobbink AM, Bergveld P, Gagliardi G, den Hollander AI, Bosschaart N, van den Berg A, Passier R and van der Meer AD, Lab Chip, 2021, 21, 272–283. [DOI] [PubMed] [Google Scholar]
- 152.Chan JM, Zervantonakis IK, Rimchala T, Polacheck WJ, Whisler J and Kamm RD, PLOS ONE, 2012, 7, e50582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chen S, Zhang L, Zhao Y, Ke M, Li B, Chen L and Cai S, Biomicrofluidics, 2017, 11, 054111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hanahan D and Weinberg RA, Cell, 2000, 100, 57–70. [DOI] [PubMed] [Google Scholar]
- 155.Folkman J, The New England Journal of Medicine, 2010, 285, 1182–1186. [DOI] [PubMed] [Google Scholar]
- 156.He Y, Halford M, Achen M and Stacker SA, Biochem Soc Trans, 2014, 42, 1569–1575. [DOI] [PubMed] [Google Scholar]
- 157.Carmeliet P and Jain RK, Nat Rev Drug Discov, 2011, 10, 417–427. [DOI] [PubMed] [Google Scholar]
- 158.Jain RK, Nature Medicine, 2001, 7, 987–989. [DOI] [PubMed] [Google Scholar]
- 159.Chou DB, Frismantas V, Milton Y, David R, Pop-Damkov P, Ferguson D, MacDonald A, Vargel Bölükbaşı Ö, Joyce CE, Moreira Teixeira LS, Rech A, Jiang A, Calamari E, Jalili-Firoozinezhad S, Furlong BA, O’Sullivan LR, Ng CF, Choe Y, Marquez S, Myers KC, Weinberg OK, Hasserjian RP, Novak R, Levy O, Prantil-Baun R, Novina CD, Shimamura A, Ewart L and Ingber DE, Nature Biomedical Engineering, 2020, 4, 394–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gargus ES, Rogers HB, McKinnon KE, Edmonds ME and Woodruff TK, Nature Biomedical Engineering, 2020, 4, 381–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chen EP, Toksoy Z, Davis BA and Geibel JP, Frontiers in Bioengineering and Biotechnology, 2021, 9, 664188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ma Q, Ma H, Xu F, Wang X and Sun W, Microsystems & Nanoengineering, 2021, 7, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bluecher A, dos Santos SM, Ferreirós N, Labocha S, Meyer Dos Santos IMR, Picard-Willems B, Harder S and Singer OC, Thromb Haemost, 2017, 117, 519–528. [DOI] [PubMed] [Google Scholar]
- 164.Garcia VM, Basu B, Molife LR and Kaye SB, Clinical Cancer Research, 2012, 18, 3750–3761. [DOI] [PubMed] [Google Scholar]


