Abstract
Hematopoietic stem cells (HSCs) with age-associated somatic mutations that disproportionally contribute to hematopoiesis generate the condition known as clonal hematopoiesis (CH). While CH conveys increased risk of hematologic cancer, there is also strong association between CH and cardiovascular disease (CVD). Accumulating evidence suggests that inflammation mechanistically links CH to CVD, and we hypothesized that CH may be a predictive biomarker of CVD in conditions of chronic inflammation. One such patient population is people living with HIV (PLWH) who also have substantially increased incidence of CVD and CH [1]. We studied the association between CH and CVD in PLWH using samples from ACTG Study A5001 (or ALLRT), a prospective clinical trial of HIV-infected persons with long term follow-up. We observed a positive association between CH and CVD in PLWH independent of traditional CVD risk factors. Moreover, in CVD cases the CH clone was identifiable in the blood years prior to CVD diagnosis, unlike PLWH with CH who did not suffer CVD. With the lifespan of PLWH increasing due to advances in treatment, our results indicate that the presence of CH and its clonal dynamics could be used as a prognostic biomarker to risk of CVD in PLWH.
INTRODUCTION
Despite effective antiretroviral therapy (ART), people living with HIV (PLWH) continue to experience excess morbidity and mortality from both AIDS and non-AIDS co-morbidities [2, 3]. Most notable of the co-morbidities is cardiovascular disease (CVD), which occurs at significantly higher rates in PLWH [4] and is a leading cause of death in PLWH [5, 6]. Factors contributing to increased risk of CVD in PLWH are multiple, inter-related and complex, with some shared with the general population but others unique to HIV. Although traditional demographic and lifestyle / behavioral factors (age, gender, smoking, substance abuse and socioeconomic status) are clearly important, the underlying mechanisms behind increased CVD risk in PLWH are unclear.
Clonal hematopoiesis (CH), the age-related expansion of pre-malignant clones in the blood [7, 8], has emerged as a risk factor not only for development of haematological malignancies, but also other non-hematopoietic disorders including CVD and ischemic stroke [9, 10]. In various retrospective studies, CH mutations in DNMT3A, TET2, ASXL1, and JAK2 were each individually associated with CVD, with mechanistic validation supported by mouse models [11, 12]. CH is emerging as a significant risk factor for the development of CVD and efforts to mitigate this effect by targeting either the mutant clones or their downstream effects is the subject of intensifying research efforts.
Here we utilized samples from the ACTG Study A5001 (or ALLRT), a prospective observational study of PLWH aged >40 years who received randomized assignment of their initial ART regimen through an interventional trial and followed long term [13]. We examined the incidence of CH in PLWH to determine if the increased incidence of CVD could be linked to underlying CH driven by specific mutations that may be shaped by the chronic inflammation of the disease, or the specific types of therapy received. Results from this study show that CH is associated with CVD in PLWH. Interestingly, a trend towards a higher growth rate for CH mutations was observed among those developed CVD compared to those who did not, and the CH clone was detectable up to five years prior to CVD diagnosis. With the lifespan of PLWH increasing due to advances in treatment, we raise the potential for using CH as a biomarker for risk of CVD in these individuals.
METHODS
Patient Samples
Patient demographics and sample characteristics for ALLRT have previously been described [13]. PBMC samples were obtained from participants who suffered a CVD event (cases) close to the time of the event / diagnosis and a prior collected sample. ALLRT participants who had no evidence of CVD were used as control comparators, matched by age and gender to cases. We calculated the artherosclerotic cardiovascular disease (ASCVD) score as previously described [14]. Inputs for the ASCVD score calculations consisted of age at CVD event or time of second blood draw for controls, total cholesterol count, total high-density lipoprotein, treated or untreated systolic blood pressure, current smoker status, and age interaction terms amongst those listed.
DNA Preparation, Exome Sequencing and ddPCR Analysis
Genomic DNA was extracted using the PureLink genomic DNA extraction kit (Invitrogen #K1820-02). Exome sequencing was performed at the McDonnell Genome Institute at Washington University to an average coverage depth of 250x. See Supplemental Methods for CH variant calling and annotation details. In brief, a consensus calling approach utilizing both Mutect2 and Vardict was used to identify mutations at a VAF of 2% or greater. After filtering for artifacts and germline mutations, we annotated CH mutations as putative drivers if they had been previously identified as a possible cancer driver mutations based on COSMIC, the Cancer Gene Census. OncoKB and previously reported CH literature [15, 16]. All procedural methods for droplet digital PCR (ddPCR) followed the manufacture protocol for the QX200 Droplet Digital PCR System (BioRad).
Data Sharing Statement
For original data including CH variant calls plus minimal clinical data and code needed to replicate findings, please contact bolton@wustl.edu or grantchallen@wustl.edu.
RESULTS AND DISCUSSION
Incidence and Spectrum of CH in PLWH and Correlation with CVD
With 27,105.2 total person years of follow-up for 4344 patients within the ALLRT cohort (those with history of hematologic malignancy were excluded), 86 participants suffered a CVD event (cases). 83 cases with available PBMCs were identified and matched to 78 controls (Supplemental Table 1). A variety of CVD events were reported in cases including myocardial infarction (MI; n=37), stroke / transient ischemic attack (TIA; n=39), coronary artery disease (CAD; n=4), congestive heart failure (n=1) and peripheral artery disease (PAD; n=2). 26 CH variants were identified in 23 individuals (three patients had two CH variants; Supplemental Table 2). The frequency of CH increased with age as expected (Figure 1A). Overall 18.1% of cases and 10.2% of controls were CH positive (Figure 1B) with the enrichment of CH in cases compared to controls being more appreciable with older (>50) age groups (Figure 1A). Even after accounting for race and traditional risk factors for CVD (as measured by 10-year risk of ASCVD score), we noted a significant association between CH and risk of CVD (OR=3.7, 95% CI 1.0–13.3, p=0.04). The enrichment of CH among PLWH who developed CVD was most notable for DNMT3A but also seen for other genes (Figure 1C). Among CH positive individuals however, there was no difference in VAF among CVD positive compared to CVD negative PLWH (Figure 1D).
Figure 1. Incidence and Spectrum of CH in PLWH and Correlation with CVD.
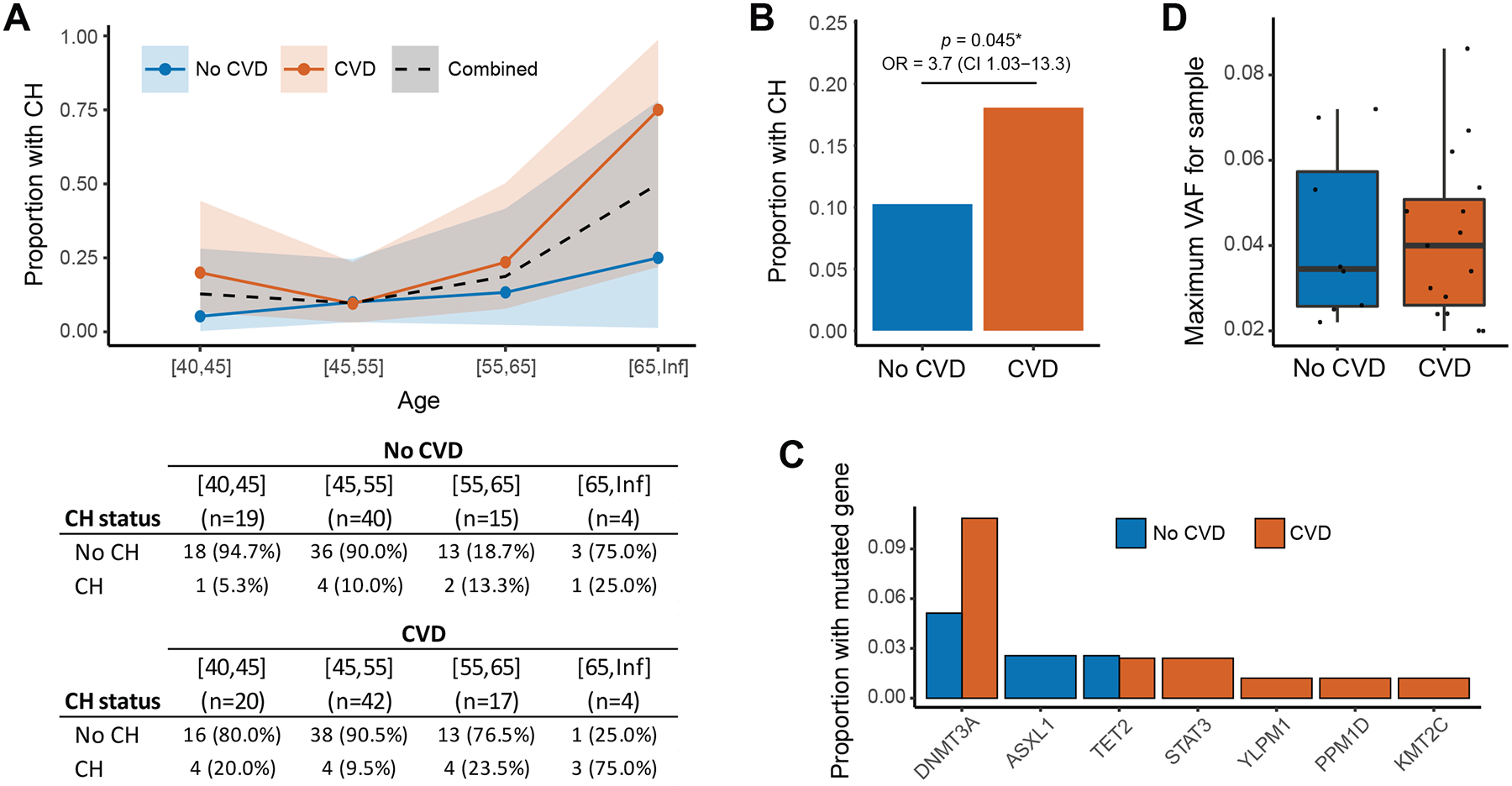
(A) Proportion of PLWH with CH by age stratified by those with and without a CVD event. Ribbons represent the 95% confidence interval for the proportion based on a binomial distribution. (B) Proportion of patients with CH among those with and without CVD. Odds ratio with 95% confidence interval generated from conditional logistic regression model adjusted for race and 10-Year risk of hard ASCVD score. (C) Proportion of PLWH with CH in specific genes among those with and without a CVD event. (D) Distribution of the variant allele fraction (VAF) of CH mutations comparing PLWH with and without CVD.
CH is a Biomarker for CVD in PLWH
To characterize whether the growth rate of CH mutations might influence the subsequent risk for CVD in PLWH, genomic DNA was prepared from PBMC samples banked 4–5 years prior to the date of CVD or last follow-up for cases and controls respectively (on average 1364 days prior, range 736–2242 days) where available. ddPCR probes were designed to determine if the CH clone could be identified in the prior banked sample at any level. Of the 15 individuals for whom long-term prior samples were available, ddPCR probes were able to be designed for 16 variants. For all 16, we were able to validate the presence of the mutation in the sample processed for exome sequencing, providing confidence in our variant calling pipeline. Of the seven control patients with CH, the CH clone was identified in 3/7 (42.9%) of the prior-banked samples (Figure 2A). However, for the PLWH who suffered CVD associated with CH, the CH clone was identified in 7/8 (87.5%) of the prior banked samples (Figure 2B). One control patient had two DNMT3A variants identified by exome sequencing, one of which was detected in the prior banked sample (DNMT3AE505Ter) while the other (DNMT3AC537W) was not. There was a non-significant trend towards a higher growth rate for CH mutations observed among those who went on develop CVD compared to those who did not (Figure 2C; Supplemental Figure 1).
Figure 2. CH is a Biomarker for CVD in PLWH.
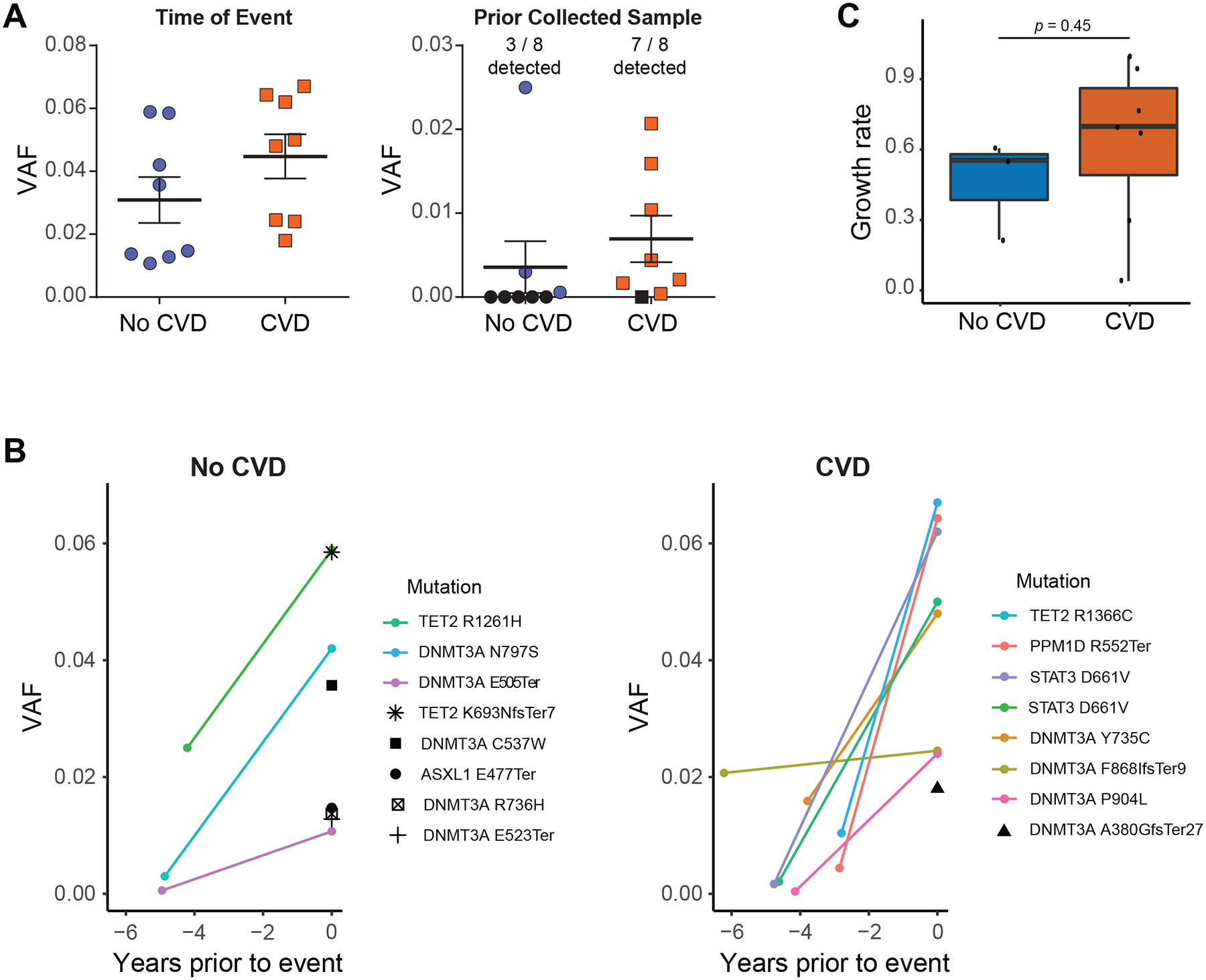
(A) VAF of CH mutations in PBMCs from PLWH quantified by ddPCR at the time of CVD event or a prior collected sample. The CH mutation identified in CVD cases at the time of event was observed in the prior collected sample for 7 out of 8 patients, whereas only 3 out of 8 CH clones were identified in the prior banked sample for PLWH without CVD. Black shapes denote a mutation was not detected. (B) Change in VAF of mutations between the initial blood collection timepoint and the collection closest to the CVD event or last follow-up for cases and controls respectively. Mutations that were detected at both times points are displayed using exponential growth curves while mutations that were only detectable at 2nd blood draw are shown with single black shapes. (C) Calculated clonal expansion rate for CH mutations among PLWH with and without CVD. Shaded bands represent intra-quartile ranges.
In summary, our data suggest that CH is a strong and independent prognostic factor for CVD in PLWH beyond traditional CVD risk factors. Due to the prevalence of CVD in PLWH, the clinical utility of screening for CH should be further explored. Our data, in combination with epidemiologic and mechanistic evidence linking CH to inflammation and CVD, suggests that CH may be a particularly informative marker of CVD risk in individuals prone to inflammatory stress such as PLWH. Future investigation including functional studies will be important to clarify these mechanisms and to develop interventional strategies in high-risk populations such as PLWH.
Supplementary Material
HIGHLIGHTS.
A positive association between CH and CVD in people living with HIV (PLWH).
The CH clone was identifiable years prior to CVD diagnosis.
Higher growth rate of CH mutations among PLWH who developed CVD.
ACKNOWLEDGEMENTS
We thank all members of the Challen laboratory for ongoing contributions and critical discussion. We thank the McDonnell Genome Institute at Washington University for genomic analysis, partially supported by NCI Grant CA91842 and by ICTS/CTSA NIH Grant UL1TR000448. We would like to acknowledge all participating sites and thank the participants from the ALLRT cohort.
This work was supported by the National Institutes of Health (DK124883 to G.A.C). Samples collected from the ALLRT cohort that were used in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award numbers UM1 AI068634, UM1 AI068636 and UM1 AI106701 (R.M.P., K.M.E., K.K.T., K.W.).
G.A.C. is a scholar of the Leukemia and Lymphoma Society.
Footnotes
Disclosure of Conflicts of Interest
The authors have no financial interests relevant to this work to disclose.
REFERENCES
- [1].Dharan NJ, Yeh P, Bloch M, et al. HIV is associated with an increased risk of age-related clonal hematopoiesis among older adults. Nat Med. 2021;27:1006–1011. [DOI] [PubMed] [Google Scholar]
- [2].Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet. 2013;382:1525–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Martin A, Smith DE, Carr A, et al. Reversibility of lipoatrophy in HIV-infected patients 2 years after switching from a thymidine analogue to abacavir: the MITOX Extension Study. AIDS. 2004;18:1029–1036. [DOI] [PubMed] [Google Scholar]
- [4].Freiberg MS, Chang CC, Kuller LH, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173:614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rodger AJ, Lodwick R, Schechter M, et al. Mortality in well controlled HIV in the continuous antiretroviral therapy arms of the SMART and ESPRIT trials compared with the general population. AIDS. 2013;27:973–979. [DOI] [PubMed] [Google Scholar]
- [6].Adih WK, Selik RM, Hu X. Trends in Diseases Reported on US Death Certificates That Mentioned HIV Infection, 1996–2006. J Int Assoc Physicians AIDS Care (Chic). 2011;10:5–11. [DOI] [PubMed] [Google Scholar]
- [7].Xie M, Lu C, Wang J, et al. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat Med. 2014;20:1472–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Challen GA, Goodell MA. Clonal hematopoiesis: mechanisms driving dominance of stem cell clones. Blood. 2020;136:1590–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jaiswal S, Fontanillas P, Flannick J, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371:2488–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Genovese G, Kahler AK, Handsaker RE, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med. 2014;371:2477–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jaiswal S, Natarajan P, Silver AJ, et al. Clonal Hematopoiesis and Risk of Atherosclerotic Cardiovascular Disease. New England Journal of Medicine. 2017;377:111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fuster JJ, MacLauchlan S, Zuriaga MA, et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science. 2017;355:842–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Smurzynski M, Collier AC, Koletar SL, et al. AIDS clinical trials group longitudinal linked randomized trials (ALLRT): rationale, design, and baseline characteristics. HIV Clin Trials. 2008;9:269–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Goff DC Jr., Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–73. [DOI] [PubMed] [Google Scholar]
- [15].Bick AG, Weinstock JS, Nandakumar SK, et al. Inherited causes of clonal haematopoiesis in 97,691 whole genomes. Nature. 2020;586:763–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bolton KL, Ptashkin RN, Gao T, et al. Cancer therapy shapes the fitness landscape of clonal hematopoiesis. Nat Genet. 2020;52:1219–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


