Abstract
Cannabis is the most commonly used federally illegal drug in the United States and world, especially among people of reproductive age. In addition, the potency of cannabis products has increased significantly in the past decade. This is concerning because the available evidence suggests an adverse effect from cannabis exposure on male and female reproductive health. Exposure to cannabinoids may have differential impacts on female reproductive health across a woman’s lifespan, from preconception to pregnancy, throughout lactation, and during menopause. Even more, cannabis use has been associated an adverse effect on fetal outcomes, and longer-term offspring health and developmental trajectories. Despite the prevalence of cannabis use, there is limited available evidence regarding its safety, especially in regard to reproductive health, pregnancy and lactation. The biological effects of cannabis are mediated by the endocannabinoid system and studies have reported the presence of cannabinoid receptors in the male and female reproductive tract, on sperm and the placenta, suggesting the endocannabinoid system plays a role in regulating reproduction. Cannabis use can impact male and female fertility and has been associated with altered reproductive hormones, menstrual cyclicity and semen parameters. Use of cannabis in males has also been associated with erectile dysfunction, abnormal spermatogenesis, and testicular atrophy. In females, cannabis use has been associated with infertility and abnormal embryo implantation and development. The main psychoactive component of cannabis, delta-9-tetrahydrocannabinol (THC), can also cross the placenta and has been detected in breastmilk. Maternal cannabis use during pregnancy and lactation has been associated with adverse effects including small for gestational age infants, preterm birth, fetal neurodevelopmental consequences, and impaired offspring sociobehavioral and cognitive development. The prevalence of cannabis use to alleviate menopausal symptoms has also increased despite the limited information on its benefits and safety. As cannabis use is on the rise, it is critical to understand its impact on reproductive health and offspring developmental outcomes. This is an understudied, but timely subject, with much needed information to guide healthcare providers and those interested in conceiving, or that are pregnant and lactating, as well as those at the end of their reproductive time span.
Keywords: cannabis, marijuana, menopause, maternal cannabis use, THC, cannabinoids, delta-9-tetrahydrocannabinol, reproductive health, substance use, fertility, cannabis use disorder, preterm birth, small for gestational age, low birth weight
CONDENSATION:
The use of cannabis and its impact on reproductive health and offspring outcomes.
INTRODUCTION
Cannabis is the most commonly used federally illegal drug in the United States (US) and world, in part due to widespread legalization and increasing social acceptability and accessibility.1 Prevalence of cannabis use is on the rise, especially among people of reproductive age, including during the COVID-19 pandemic in part due to heightened anxiety and stress (Figure 1).2,3 In addition, the potency of cannabis products has increased by almost two-fold in the past decade.4 This is concerning because the available evidence suggests an adverse effect from cannabis exposure on male and female reproductive health, pregnancy and fetal outcomes, and longer-term offspring health and developmental trajectories.
Figure 1. Past year cannabis use among females aged 12 or older: 2010–2020.48,185.
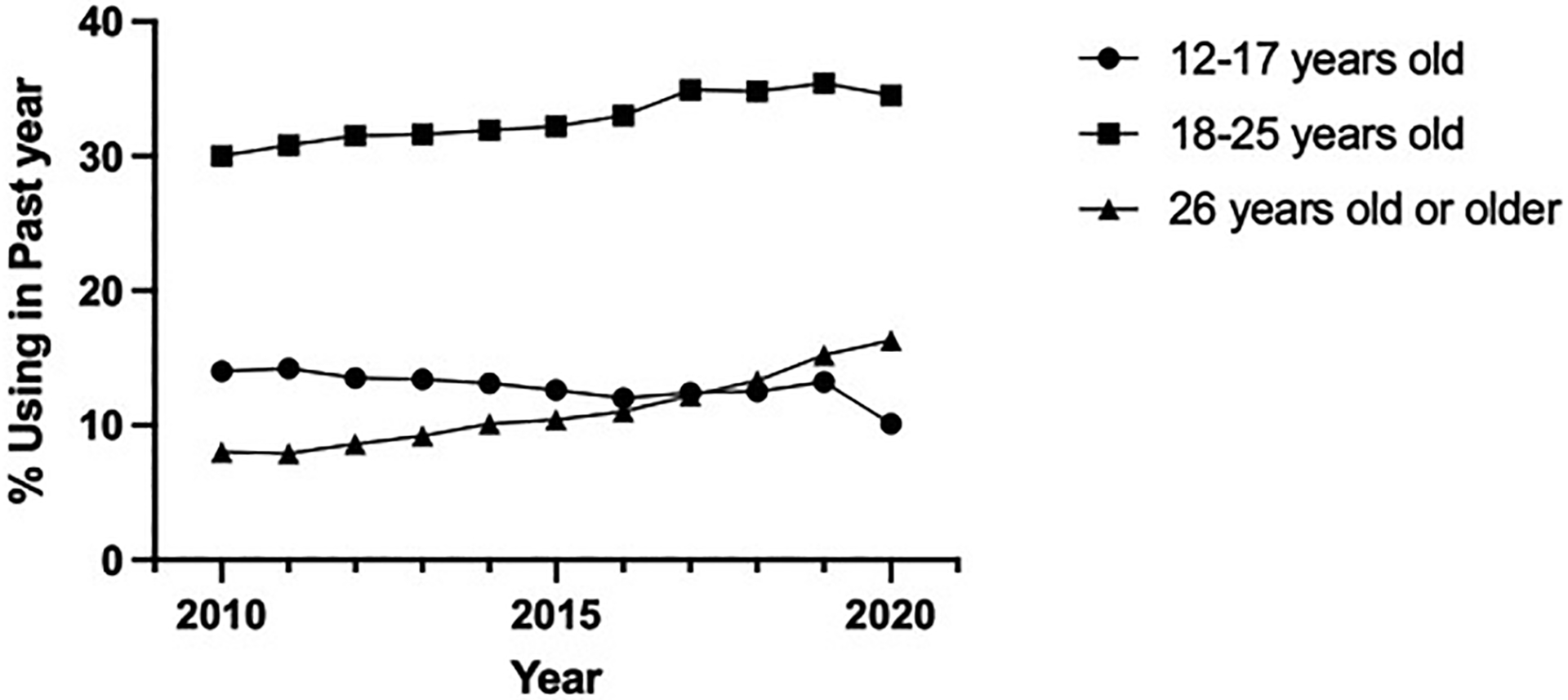
Prevalence of past year cannabis use has increased most rapidly in females aged 26 years or older.
The biological effects of cannabis are mediated by the endocannabinoid system. Expression of endocannabinoid receptors has been demonstrated in the fetus as early as 5 weeks gestation5 and the endocannabinoid system has been detected at early stages of development (Figure 2).6 Published studies have reported the presence of cannabinoid receptors in the male and female reproductive tract, on sperm and the placenta,7,8 suggesting the endocannabinoid system plays a role in regulating reproduction.9,10 Cannabis use can impact male and female fertility and has been associated with altered reproductive hormones, menstrual cyclicity and semen parameters.11–13 The main psychoactive component of cannabis, delta-9-tetrahydrocannabinol (THC), can also cross the placenta and has been detected in breastmilk. Maternal cannabis use during pregnancy and lactation has been associated with adverse effects including small for gestational age (SGA) infants, preterm birth (PTB), fetal neurodevelopmental consequences, and impaired offspring sociobehavioral and cognitive development.14–23
Figure 2. Human endocannabinoid system.
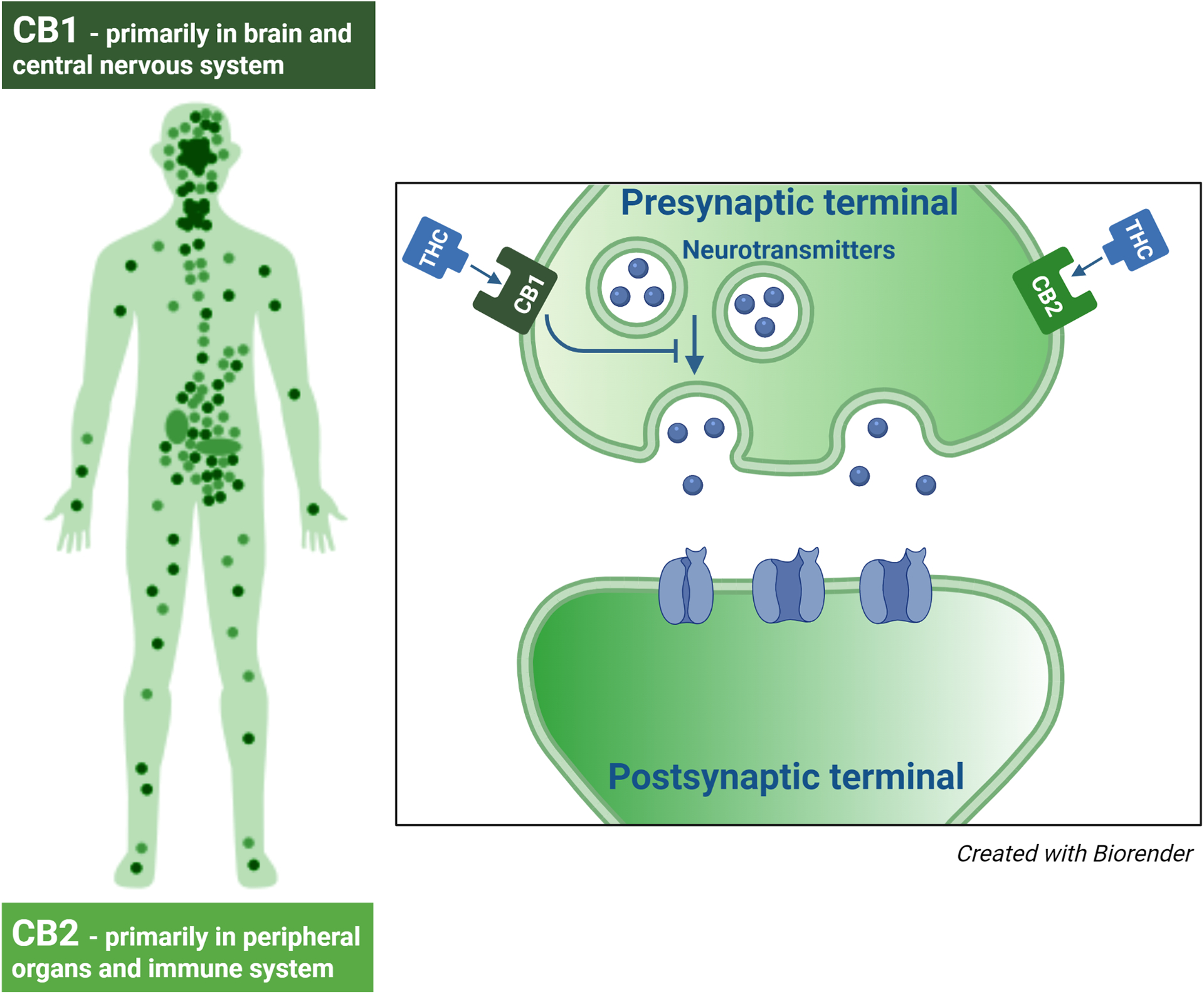
Consists of cannabinoid receptors and endocannbinoids. The two most common cannabinoid receptors are CB1 and CB2. CB1 receptors are predominantly located in the brain and central nervous system, but can also be found in other tissues. CB2 receptors are largely found in peripheral organs, especially cells associated with the immune system.
Despite the prevalence of cannabis use, there is limited available evidence regarding its safety, especially in regard to reproductive health, pregnancy and lactation. This lack of information has resulted in approximately 70% of females in the US believing that consumption of cannabis once or twice per week is harmless24 and cannabis retailers promoting cannabinoids as safe, natural and effective ways to manage common daily ailments, including in pregnancy, such as insomnia, pain, and morning sickness.25 The heterogeneity in the existing human literature is due to methodologic issues, small sample sizes, lack of confirmatory testing, and difficulty controlling for confounders.26 The available animal literature focuses largely on the effects of acute cannabis exposure, and often studied modes of cannabis delivery not representative of human use (e.g. intravenous or oral gavage), thus limiting the translation of those findings to humans. Taken together, these factors contribute to the paucity of safety information.
As cannabis use is on the rise, especially among those of reproductive age, it is critical to understand its impact on reproductive health and offspring developmental outcomes. This is an understudied, but timely subject, with much needed information to guide healthcare providers and those interested in conceiving, or that are pregnant and lactating.
PHARMACOLOGY OF CANNABINOIDS
Cannabis, a plant of the Cannabaceae family, contains more than eighty biologically active chemical compounds. The most commonly known compounds are THC and cannabidiol. Cannabinoid receptors, CB1 and CB2, are distributed in the central nervous system and many peripheral tissues including reproductive, urinary and gastrointestinal tracts.8,27 THC is an agonist to CB1 and the CB2 subtype of cannabinoid receptors. Properties of cannabinoids that might be of therapeutic use include analgesia, muscle relaxation, immunosuppression, anti-inflammation, anti-allergic effects, sedation, improvement of mood, stimulation of appetite, anti-emesis, and antineoplastic effects.28 However, there is no Food and Drug Administration (FDA) approval for these therapeutic uses.
The most common mode of cannabis administration in both non-pregnant and pregnant populations is smoking followed by edibles.29,30 Smoking is the quickest method for THC to enter systematically and provides rapid onset and a short duration of symptoms (Table 1), which results in a lesser chance of overconsumption.31 Edibles are gaining popularity because they are palatable, discreet, and effects can last for hours (Table 1). Because edibles require gastrointestinal absorption, it takes longer before symptom onset and thus, can lend to a higher likelihood of overconsumption.31–35
Table 1.
Summary of different modes of cannabis delivery
| Method | Peak | Duration |
|---|---|---|
| Inhaled (vapor or smoke)181 | 9 minutes | 1–2 hours |
| Oral (drops, lozenge, spray)182 | 10–25 minutes | ≤10 hours |
| Ingested (capsules, edibles, powder, tablets)31–35 | 60 minutes – 5hours | ≤25hours |
| Transdermal (patch, gels)183 | 120 minutes | ≤48 hours |
| Rectal suppository184 | 60–120 minutes | ≤8 hours |
CANNABIS USE DISORDER
Cannabis use disorder (CUD) can develop in approximately 10 percent of regular cannabis users and 50 percent of chronic daily users.36 It is a problematic pattern of cannabis use associated with cognitive impairment and psychiatric comorbidity, with at least two manifestations in twelve months.36 Screening is generally by brief questionnaires at yearly preventative visits, or if prompted by signs or symptoms from the patient’s history and exam.36 In high-risk patient populations, drug testing can be considered. The diagnosis of CUD is guided by the patient’s cannabis use, signs and symptoms and functional impairment per the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) criteria.36
There are limited treatment options for CUD, but the goal is to achieve sustained abstinence from cannabis use, or reduced use that mitigates the patient’s cannabis-related symptoms. In half of patients with CUD, cessation of cannabis after heavy and prolonged use results in withdrawal symptoms.37 Options for treatment include brief intervention, psychosocial intervention, cognitive-behavioral therapy, and motivational enhancement therapy, or a combination. If these interventions fail, then adjunctive medications such as N-acetylcysteine, gabapentin, nabiximols, varenicline, and anti-depressants, can be considered, but are not FDA approved and some have limited safety information in pregnancy.38
LEGALIZATION OF CANNABIS AND DISPARITIES
Cannabis has been decriminalized in several regions of North America, Africa, Australia, Europe and South America. Use of cannabis has significantly increased due to widespread recreational cannabis legalization and increasing social acceptability and accessibility1. In 2021, cannabis was legalized for recreational use in eighteen US states.
Among adolescents, CUD has been associated with long-term adverse health, economic, and social implications. Individuals who have earlier initiation of cannabis are at an increased risk of lower levels of educational attainment, welfare dependence and unemployment, polysubstance use, including other illicit drugs, and psychotic symptomatology, suggesting that cannabis might contribute to racial and health inequity.39 Research suggests that early (12–14 years old) to late (15–17 years old) adolescence is a critical risk period for the initiation of substance use and may exacerbate racial and health inequities.40 While the legalization of cannabis may help reduce inequities in criminal justice, dysregulated cannabis use could widen gaps in health and social equity. Relative to the rates for whites, the odds of CUD are higher in American Natives and blacks but lower in Asians/Pacific Islanders and Hispanics.41 By CUD severity, the odds are also higher in blacks than whites at moderate and severe levels.41
A recent study showed that the prevalence of prenatal cannabis use increased after legalization in the state of Colorado.42 This was associated with an increase in fetal growth restriction (FGR) suggesting a population impact of legalization on obstetrical outcomes42 and the need to develop well-evaluated policies to mitigate the potential adverse maternal and fetal consequences of prenatal cannabis use.
CANNABIS USE AND IMPACT ON PUBERTY
The recent changes in cannabis legalization will also likely influence the prevalence of cannabis use by children and adolescents.43 This can result from increased availability, greater exposure to second-hand cannabis smoke, messaging that minimizes the health and behavioral risks, and the potential impact of role-modeling by adults who use cannabis on child and adolescent behavior. Studies have suggested that cannabis exposure can impact pediatric endocrine and metabolic health, and puberty.44 Prior animal studies have demonstrated that THC can delay puberty, interrupt sexual maturation, and impair growth and gonadal function.45,46 The limited human literature regarding the effect of cannabis use on puberty28 focuses largely on male subjects, consists of one abstract and a case report, and observed delayed puberty and reduced growth spurt in boys who smoked cannabis compared with non-smokers47.
CANNABIS USE AND MALE REPRODUCTIVE HEALTH
In 2020, the estimated prevalence of past year use was 34.5% amongst adult males ages 18 to 25 year-old and 16.3% in ages 26 years or older in the United States.48 Overall, the literature supports an adverse impact of paternal cannabis use on male reproductive health and offspring outcomes, but there is significant heterogeneity in the available published studies. It has been associated with erectile dysfunction, orgasmic dysfunction, and may cause premature or delayed ejaculation,49,50 but the contrary has also been reported.51 The effect of chronic cannabis use in men is inconsistent with some studies reporting minimal or no effect on follicle stimulating hormone (FSH) levels,52 an association with lower testosterone and luteinizing hormone (LH) levels,53,54 and poorer semen parameters,11,55–57 while other studies have not confirmed these findings.58–61 Previous animal studies suggest that acute exposure to THC can adversely impact spermatogenesis,62 including inhibition of Leydig cell function63, reduction in gonadotropins,64–66 testicular atrophy,67–72 and abnormal sperm morphology.73–76
IMPACT OF PATERNAL CANNABIS USE
In addition to affecting sperm function, cannabis may impact epigenetic regulators that can then influence the health and developmental trajectory of future offspring. A recent study reported that cannabis exposure in humans and rats is associated with altered widespread DNA methylation in sperm77. Affected genes identified are involved in early development, including neurodevelopment, while others are implicated in cancer. Additionally, paternal cannabis use during conception, pregnancy and the postnatal period has been significantly associated with sudden infant death syndrome, after adjusting for tobacco and alcohol co-use, but the underlying mechanism is unknown78.
CANNABIS USE AND FEMALE REPRODUCTIVE HEALTH
The concern for adverse effects from cannabis use on female reproductive health is because cannabis use is commonly used by reproductive age females and both CB1 and CB2 receptors are present in the hypothalamus, pituitary, ovary, and uterus.7,8 Currently, the existing literature suggests that cannabis can affect the processes involved with female reproductive health including the secretion of FSH and LH, ovulation and menstrual cyclicity. Although the exact underlying mechanism for these findings is unclear, the likely site of action is central79–85 but, can also be a direct THC effect at the gonadal level.86
Pre-clinical rat models have demonstrated that acute THC administration resulted in suppressed LH, FSH, and prolactin levels,85–87 and a 24-hour delay in ovulation.86 Non-human primates (NHP) have a similar THC plasma disposition, menstrual cycle length, and endocrine properties to humans.88 Prior NHP studies of chronic THC exposure have reported ovulatory dysfunction, increased menstrual cycle length, anovulation, and altered female reproductive hormone levels.12,82,89,90
The existing human literature is conflicting and has likely contributed to the increased perception of cannabis users seeking infertility treatment that cannabis is safe and will not adversely impact fertility61,91,92. Similar to prior NHP studies, Jukic et al. found that cannabis users had more anovulatory cycles compared with nonusers (43% vs. 15%).93 Some studies have found no significant association between cannabis use and spontaneous conception rate,94,95 while other studies have found that women that smoked cannabis within a year of trying to conceive were twice as likely to experience infertility secondary to ovulatory dysfunction.96 A recent study in women with a history of a prior first trimester pregnancy loss using cannabis preconception noted decreased fecundability despite increased intercourse frequency.97 In women that smoked cannabis 1 year prior to undergoing in vitro fertilization, after adjusting for cigarette smoking and other relevant confounders, one study noted 25% fewer oocytes retrieved and 28% fewer oocytes fertilized78 and another reported more than double the adjusted probability of pregnancy loss compared to non-smokers.98
CANNABIS USE IN PREGNANCY
Cannabis is now the most commonly used federally illegal drug in pregnancy,2,99–101 with prevalence of use nearly doubling in the past decade in the US.102 A recent report noted that the prevalence of last month cannabis use was over 4.9% among pregnant women aged 15–44 years old and rose to 8.5% in those aged 18–25 years-old.103 Characteristics associated with prenatal cannabis use include being single or unmarried, young, lower socioeconomic status, less education, or residing with a partner who also uses cannabis.104 Women that use cannabis in pregnancy often engage in polysubstance use with alcohol, tobacco, or other illicit drugs, that can result in an additive or synergistic effect.105 First trimester use of cannabis to treat nausea is common, and marks a developmental window when the fetus is most vulnerable to adversity; and half of female individuals who use cannabis continue to use throughout pregnancy.106,107 The most frequent methods of cannabis use during pregnancy are smoking, edibles, vaping, and topicals.108
The US Surgeon General, American College of Obstetricians and Gynecologists (ACOG), and the American Academy of Pediatrics (AAP) recommend that pregnant women should be counseled regarding the potential risks of prenatal cannabis use and encouraged to abstain from use in pregnancy and while breastfeeding.99,109,110 However, the continued high prevalence of use is partly because patients are unsure about the level of safety regarding prenatal cannabis use due to the heterogeneity in the available literature,111 healthcare providers are not appropriately counselling or educating patients,112–114 and cannabis retailers are promoting cannabis as a safe, natural and effective method for mitigating pregnancy symptoms.25,115 Patients most commonly report using cannabis during pregnancy to help with nausea, stress, sleep, and appetite changes.115,116
There is concern for adverse fetal and neonatal outcomes given THC can access and bind to cannabinoid receptors in the placenta and fetal brain (Figure 3).117–120 The limited, available evidence suggests that prenatal cannabis exposure is associated with a negative impact to the developing fetus and offspring.17,18,22,23,121 There is a possible increase in risk of miscarriage and stillbirth, but the results are inconsistent among studies, and many studies do not control for important confounders such as tobacco use.122,123 Some studies suggest an increased risk of neonatal intensive care unit (NICU) admission, SGA, placental abruption, 5-minute Apgar less than 4, and infant death.22,120,122,124,125 There is evidence prenatal cannabis exposure is associated with low birth weight (LBW)22 and PTB.120,126 The most recent systematic review and meta-analysis reported that cannabis use in pregnancy is associated with an increased risk of LBW (RR, 2.06 [95% CI, 1.25 to 3.42]; P = .005), SGA (RR, 1.61 [95% CI, 1.44 to 1.79]; P < .001), PTB (RR, 1.28 [95% CI, 1.16 to 1.42]; P < .001), and NICU admission (RR, 1.38 [95% CI, 1.18 to 1.62]; P < .001).120 The limited literature on teratogenicity is conflicting and inconsistent, but include reports of congenital anomalies with maternal cannabis use such as acrania, gastroschisis, esophageal atresia, and congenital diaphragmatic hernia.127,128
Figure 3. Adverse effects of cannabis consumption on the placenta, fetus and offspring.
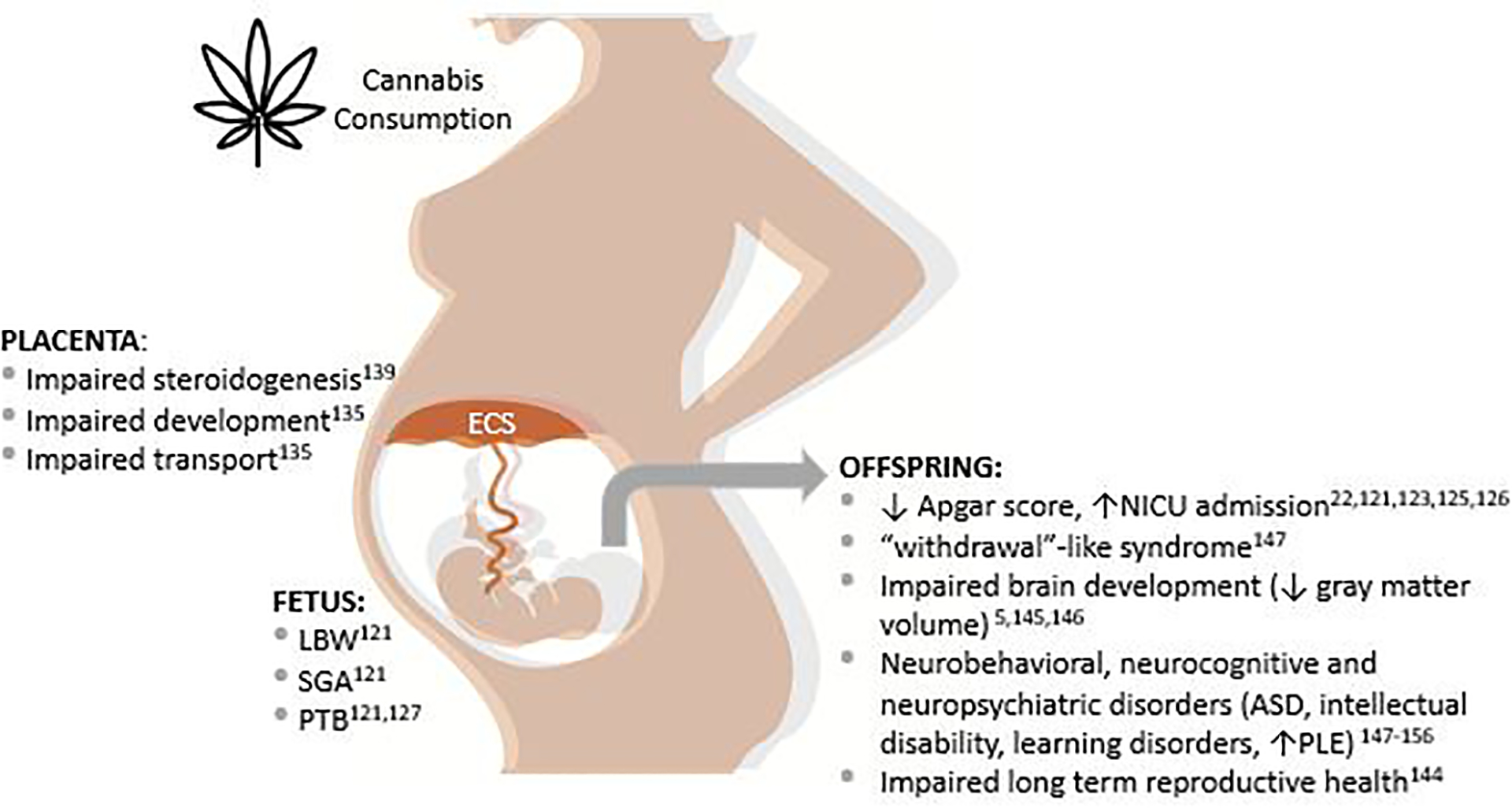
FGR: fetal growth restriction, SGA: small for gestational age, PTB: preterm birth, ASD: autism spectrum disorder, PLE: psychotic-like experience.
Amongst pregnant females that used cannabis in the past-year, it has been reported that 18.1% meet criteria for CUD.24 The rate of CUD increased from 1.8 to 9.4 per 1000 deliveries from 1993 to 2014 respectively.129 Recently, Shi et al. reported that infants born to mothers with CUD were more likely to experience adverse health outcomes, including SGA, PTB, low birth weight, and death within 1 year of birth, than those born to women without CUD.124,130 This adds to the growing body of evidence that prenatal cannabis exposure may be associated with poor birth outcomes.
Placental development, function and pregnancy outcomes
Animal studies have demonstrated that the endocannabinoid system is present in midgestational placentas, where it is suggested to play a critical role in placentation, trophoblast differentiation, as well as fetal outcomes.131 In the human term placenta, CB1 receptor expression has been demonstrated in the amniotic epithelium, reticular and decidual cells suggesting that the placenta is a likely target for cannabinoid action.132 Studies demonstrate that THC inhibits the migration of the epithelial layer of human placental amnion tissue through the regulation of metalloproteinases, affecting the development of the amnion during the gestation and contributing to preterm labor and other adverse pregnancy outcomes.133 In vitro studies have shown that THC impairs cytotrophoblasts fusion and biochemical differentiation, inhibits trophoblast cell turnover and, consequently, can impair placental development134. This is consistent with histological studies demonstrating increased syncytiotrophoblastic knots and fibrin deposition in the villous stroma of human placentas from cannabis users.135 In rodents, prenatal THC exposure induces FGR and increased placental weight resulting in increased fetal/placental weight ratio.135–137 Abnormal placental steroidogenesis and estrogen signaling induced by THC might also explain placental dysfunction and adverse pregnancy outcomes in women that use cannabis during pregnancy.138
Pharmacodynamics of Prenatal Cannabis Use
Prior animal studies have shown that fetal blood and tissue THC concentrations are approximately 10% lower than maternal blood levels,139 but can be higher with chronic heavy exposure.140 In humans, THC levels in umbilical cord blood samples were 3 to 6 times lower than simultaneously collected maternal blood.141
OFFSPRING OUTCOMES WITH MATERNAL CANNABIS USE
The recent rise in prevalence of prenatal cannabis use has been associated with increasing evidence of associated adverse effects for fetal and neonatal developmental outcomes.142 In THC-exposed rat offspring, deleterious effects on fetal ovarian development and long-term reproductive health have been demonstrated such as accelerated folliculogenesis and follicular development arrest, transient effects on circulating steroid hormones level, and reduced ovarian vasculatures.143 It has also been demonstrated that exposure to THC in utero affects fetal brain development increasing the risk for neurocognitive and neuropsychiatric disorders, suggesting that maternal use has interfered with the proper maturation of the brain.5,144,145 In utero exposure to THC has also been linked to a “withdrawal”-like syndrome in newborns146 and increased aggressive behavior and attention deficits were observed in offspring as early as at 18 months of age.146,147 Abnormal verbal and visual reasoning, hyperactivity, attention deficit, and impulsivity have also been noted in preschool children born to mothers that used THC in pregnancy.21,146 A negative association of short-term memory with first and/or second trimester cannabis usage has also been described148. These changes in neurocognitive and behavioral function persisted throughout the school years and at age 10, depression and anxiety was observed in these children.21,149,150 Most recently, maternal cannabis use has been associated with an increased incidence of neurobehavioral changes, mental health issues, autism spectrum disorder, attention problems, attention scores, intellectual disability, and learning disorders.122,151–155 However, other studies have not demonstrated an association with childhood cognitive impairments.154,156
Maternal cannabis use has been associated with increased psychotic-like experiences in pre-adolescent offspring. Genetic susceptibilities in parents and their offspring, including epigenetic transgenerational changes of substance use and psychiatric disorders may play a role.157 A recent study also identified prenatal cannabis exposure as a risk factor for psychopathology during middle childhood.153 Prenatal cannabis exposure before and after maternal knowledge of pregnancy were associated with higher psychotic life experiences and internalizing, externalizing, attention, thought, social, and sleep problems, as well as reduced cognitive function and gray matter volume in children aged 9 to 11 years.153 Only the observed associations with cannabis exposure before maternal knowledge of pregnancy showed to be dependent on potential confounders such as socioeconomic status and familial history of psychopathology.153
Offspring Epigenetic Regulation
Altered epigenetic regulation can impact gene transcription in response to different environmental stimuli, including the intrauterine environment.158 Epigenetic mechanisms are increasingly recognized as a critical factor in the relationship between early life experience and risk of psychopathology.159 Pre-gestational and gestational cannabis exposure has been shown to alter epigenetic processes, such as DNA methylation and histone modifications, with functional consequences for gene expression. In utero exposure to cannabis has been associated with fetal epigenetic programming of genes and some molecular pathways in brain regions involved in the development of autism spectrum disorder, attention deficit hyperactivity disorder, schizophrenia, addiction, and other psychiatric diseases.160 A former study aimed to identifying the neurobiology underlying the risk of addiction vulnerability in humans detected diminished dopamine receptor D2 mRNA expression in fetal brain specimens of the nucleus accumbens, from mothers using cannabis who underwent elective abortions between 18 and 22 weeks of gestation compared to controls.161 The nucleus accumbens core is an important component of motor and reward circuits, respectively162 and disruptions in the dopamine signaling pathways could lead to adverse psychiatric outcomes.163
BREASTFEEDING AND CANNABIS USE
Overall, there is very little known about cannabis use and lactation. With recent legalization, the prevalence of cannabis use while breastfeeding has increased and is approximately 5%164 with up to 18% reported in certain populations.165 Lactating mothers tend to increase cannabis use within the first two months after childbirth.166 This is concerning because THC is lipid soluble and transferred through breastmilk where it is stored in lipid-filled tissue and slowly release over time in the offspring. This includes the offspring brain where it can impact sensitive neurodevelopmental processes. Thus, the Center for Disease Control and Prevention (CDC), ACOG and AAP all recommend against using cannabis in any form while lactating.99,109
The passage of THC into breastmilk has not been extensively studied, but the literature suggests that with chronic use, THC can concentrate in human breastmilk, up to 7.5 times, relative to plasma.166,167 When cannabis is smoked, it has been shown that THC levels peak in breastmilk 1 hour post inhalation and remain detectable for 6 days after use.164,168 Infants that are exclusively breastfed have been found to ingest a mean of 2.5% of the maternal THC dose used.164,168 A prior study found that offspring exposed to THC in breastmilk within the first month of life can have decreased motor development compared with those not exposed.169 However, the literature is limited on the effects of THC exposure through breastfeeding on long-term offspring neurodevelopment. Other studies have suggested that infants exposed to THC through breast milk experience more lethargy, less frequent feeding, growth delay, poor sucking, and shorter feeding times.169–173
CANNABIS AND MENOPAUSE
An increasing number of women are using cannabis to manage their menopausal symptoms174,175 with the frequency of cannabis use significantly correlating to the number and severity of menopausal symptoms.174 While cannabis use appears to mitigate musculoskeletal discomfort, irritability, insomnia, depression, anxiety, and hot flashes, other symptoms such as heart discomfort, exhaustion, vaginal dryness, and bladder problems were not alleviated by cannabis use.174 There is a significant gap between the marketed benefits of cannabis and the supporting evidence in the medical literature.176 Prior studies, published in the 1970s, have reported the pro- and anti-estrogenic properties of THC.177 178
However, the underlying mechanism for which THC provides symptom relief is unclear and the data on the efficacy and safety of cannabis use for menopausal symptom relief is limited and further evidence is needed to guide informed decision making.179
CONCLUSION
In summary, despite the increase in cannabis use, there is limited available evidence regarding its safety, especially in regard to reproductive health, pregnancy and lactation. Considering the existing literature suggests that cannabis use has health implications for women, men, and subsequent offspring, it is concerning almost 70% of females in the US believe that consumption of cannabis is safe in pregnancy and only approximately half of US healthcare providers discouraged the perinatal use of cannabis.180 While the literature regarding the safety of cannabis use is limited, women and their health care providers should be informed of the potential adverse effects of cannabis use, especially when planning to conceive, during pregnancy and the postpartum period.
Funding:
NIH P51-OD-011092
Footnotes
Conflict of Interest Statement: None of the authors have financial or other relationships that could result in a conflict of interest.
Disclaimer: The contents of this article represent the authors’ views and do not constitute an official position of the National Institutes of Health or the United States Government.
Bibliography
- 1.Merz F United Nations Office on Drugs and Crime: World Drug Report 2017. 2017. SIRIUS-Zeitschrift für Strategische Analysen. 2018;2(1):85–86. [Google Scholar]
- 2.Young-Wolff KC, Ray GT, Alexeeff SE, et al. Rates of Prenatal Cannabis Use Among Pregnant Women Before and During the COVID-19 Pandemic. JAMA. 2021;326(17):1745–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imtiaz S, Wells S, Rehm J, et al. Cannabis use during the COVID-19 pandemic in Canada: a repeated cross-sectional study. Journal of addiction medicine. 2021;15(6):484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ElSohly MA, Chandra S, Radwan M, Gon C, Church JC. A comprehensive review of cannabis potency in the USA in the last decade. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 2021. [DOI] [PubMed] [Google Scholar]
- 5.Wu C-S, Jew CP, Lu H-C. Lasting impacts of prenatal cannabis exposure and the role of endogenous cannabinoids in the developing brain. Future neurology. 2011;6(4):459–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martínez-Peña AA, Perono GA, Gritis SA, et al. The impact of early life exposure to cannabis: The role of the endocannabinoid system. International Journal of Molecular Sciences. 2021;22(16):8576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunne C The effects of cannabis on female and male reproduction. BCMJ. 2019;61:282–285. [Google Scholar]
- 8.Uhlén M, Fagerberg L, Hallström BM, et al. Tissue-based map of the human proteome. Science. 2015;347(6220). [DOI] [PubMed] [Google Scholar]
- 9.Aquila S, Guido C, Santoro A, et al. Human sperm anatomy: ultrastructural localization of the cannabinoid1 receptor and a potential role of anandamide in sperm survival and acrosome reaction. The Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology. 2010;293(2):298–309. [DOI] [PubMed] [Google Scholar]
- 10.Jensen B, Chen J, Furnish T, Wallace M. Medical marijuana and chronic pain: a review of basic science and clinical evidence. Current pain and headache reports. 2015;19(10):1–9. [DOI] [PubMed] [Google Scholar]
- 11.Gundersen TD, Jørgensen N, Andersson A-M, et al. Association between use of marijuana and male reproductive hormones and semen quality: a study among 1,215 healthy young men. American journal of epidemiology. 2015;182(6):473–481. [DOI] [PubMed] [Google Scholar]
- 12.Ryan KS, Mahalingaiah S, Campbell LR, et al. The effects of delta-9-tetrahydrocannabinol exposure on female menstrual cyclicity and reproductive health in rhesus macaques. F&S Science. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryan KS, Bash JC, Hanna CB, Hedges JC, Lo JO. Effects of marijuana on reproductive health: preconception and gestational effects. Current opinion in endocrinology, diabetes, and obesity. 2021;28(6):558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alhusen JL, Lucea MB, Bullock L, Sharps P. Intimate partner violence, substance use, and adverse neonatal outcomes among urban women. The Journal of pediatrics. 2013;163(2):471–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Varner MW, Silver RM, Hogue CJR, et al. Association between stillbirth and illicit drug use and smoking during pregnancy. Obstetrics and gynecology. 2014;123(1):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayatbakhsh MR, Flenady VJ, Gibbons KS, et al. Birth outcomes associated with cannabis use before and during pregnancy. Pediatric research. 2012;71(2):215–219. [DOI] [PubMed] [Google Scholar]
- 17.El Marroun H, Tiemeier H, Steegers EA, et al. Intrauterine cannabis exposure affects fetal growth trajectories: the Generation R Study. Journal of the American Academy of Child & Adolescent Psychiatry. 2009;48(12):1173–1181. [DOI] [PubMed] [Google Scholar]
- 18.Hurd Y, Wang X, Anderson V, Beck O, Minkoff H, Dow-Edwards D. Marijuana impairs growth in mid-gestation fetuses. Neurotoxicology and teratology. 2005;27(2):221–229. [DOI] [PubMed] [Google Scholar]
- 19.Day NL, Goldschmidt L, Thomas CA. Prenatal marijuana exposure contributes to the prediction of marijuana use at age 14. Addiction. 2006;101(9):1313–1322. [DOI] [PubMed] [Google Scholar]
- 20.Fernández-Ruiz J, Berrendero F, Hernández ML, Romero J, Ramos J. Role of endocannabinoids in brain development. Life Sciences. 1999;65(6–7):725–736. [DOI] [PubMed] [Google Scholar]
- 21.Goldschmidt L, Richardson GA, Willford J, Day NL. Prenatal marijuana exposure and intelligence test performance at age 6. Journal of the American Academy of Child & Adolescent Psychiatry. 2008;47(3):254–263. [DOI] [PubMed] [Google Scholar]
- 22.Gunn J, Rosales C, Center K, et al. Prenatal exposure to cannabis and maternal and child health outcomes: a systematic review and meta-analysis. BMJ open. 2016;6(4):e009986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conner SN, Bedell V, Lipsey K, Macones GA, Cahill AG, Tuuli MG. Maternal marijuana use and adverse neonatal outcomes. Obstetrics & Gynecology. 2016;128(4):713–723. [DOI] [PubMed] [Google Scholar]
- 24.Ko JY, Farr SL, Tong VT, Creanga AA, Callaghan WM. Prevalence and patterns of marijuana use among pregnant and nonpregnant women of reproductive age. American journal of obstetrics and gynecology. 2015;213(2):201. e201–201. e210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dickson B, Mansfield C, Guiahi M, et al. Recommendations from cannabis dispensaries about first-trimester cannabis use. Obstetrics and gynecology. 2018;131(6):1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.ElSohly MA, Mehmedic Z, Foster S, Gon C, Chandra S, Church JC. Changes in cannabis potency over the last 2 decades (1995–2014): analysis of current data in the United States. Biological psychiatry. 2016;79(7):613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grotenhermen F Pharmacology of cannabinoids. Neuroendocrinology letters. 2004;25(1/2):14–23. [PubMed] [Google Scholar]
- 28.National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on the Health Effects of Marijuana: An Evidence Review and Research Agenda. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington (DC): National Academies Press (US); 2017. Jan 12. II, Therapeutic Effects. Available from: https://www.ncbi.nlm.nih.gov/books/NBK425767/. [Google Scholar]
- 29.Subbaraman MS, Kerr WC. Cannabis use frequency, route of administration, and co-use with alcohol among older adults in Washington state. J Cannabis Res. 2021;3(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Young-Wolff KC, Adams SR, Wi S, Weisner C, Conway A. Routes of cannabis administration among females in the year before and during pregnancy: Results from a pilot project. Addict Behav. 2020;100:106125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goodwin RS, Gustafson RA, Barnes A, Nebro W, Moolchan ET, Huestis MA. Δ9-tetrahydrocannabinol, 11-hydroxy-Δ9-tetrahydrocannabinol and 11-nor-9-carboxy-Δ9-tetrahydrocannabinol in human plasma after controlled oral administration of cannabinoids. Therapeutic drug monitoring. 2006;28(4):545–551. [DOI] [PubMed] [Google Scholar]
- 32.Wall ME, Sadler BM, Brine D, Taylor H, Perez‐Reyes M. Metabolism, disposition, and kinetics of delta‐9‐tetrahydrocannabinol in men and women. Clinical Pharmacology & Therapeutics. 1983;34(3):352–363. [DOI] [PubMed] [Google Scholar]
- 33.Gustafson RA, Moolchan ET, Barnes A, Levine B, Huestis MA. Validated method for the simultaneous determination of Δ9-tetrahydrocannabinol (THC), 11-hydroxy-THC and 11-nor-9-carboxy-THC in human plasma using solid phase extraction and gas chromatography–mass spectrometry with positive chemical ionization. Journal of Chromatography B. 2003;798(1):145–154. [DOI] [PubMed] [Google Scholar]
- 34.Reyes MP, Lipton MA, Timmons MC, Wall ME, Brine DR, Davis KH. Pharmacology of orally administered Δ9‐tetrahydrocannabinol. Clinical Pharmacology & Therapeutics. 1973;14(1):48–55. [DOI] [PubMed] [Google Scholar]
- 35.Ohlsson A, Lindgren JE, Wahlen A, Agurell S, Hollister L, Gillespie H. Plasma delta‐9‐tetrahydrocannabinol concentrations and clinical effects after oral and intravenous administration and smoking. Clinical Pharmacology & Therapeutics. 1980;28(3):409–416. [DOI] [PubMed] [Google Scholar]
- 36.American Psychiatric Association A. Diagnostic and statistical manual of mental disorders. Vol 3: American Psychiatric Association; Washington, DC; 1980. [Google Scholar]
- 37.Bahji A, Stephenson C, Tyo R, Hawken ER, Seitz DP. Prevalence of cannabis withdrawal symptoms among people with regular or dependent use of cannabinoids: a systematic review and meta-analysis. JAMA network open. 2020;3(4):e202370–e202370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bahji A, Meyyappan AC, Hawken ER, Tibbo PG. Pharmacotherapies for cannabis use disorder: A systematic review and network meta-analysis. International Journal of Drug Policy. 2021;97:103295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fergusson DM, Boden JM, Horwood LJ. Psychosocial sequelae of cannabis use and implications for policy: findings from the Christchurch Health and Development Study. Social psychiatry and psychiatric epidemiology. 2015;50(9):1317–1326. [DOI] [PubMed] [Google Scholar]
- 40.Drugs UNOo, Crime. World Drug Report 2018. United Nations publication, Sales No. E. 18. XI. 9 2018. [Google Scholar]
- 41.Hasin DS, Kerridge BT, Saha TD, et al. Prevalence and correlates of DSM-5 cannabis use disorder, 2012–2013: findings from the National Epidemiologic Survey on Alcohol and Related Conditions–III. American Journal of Psychiatry. 2016;173(6):588–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gnofam M, Allshouse AA, Stickrath EH, Metz TD. Impact of marijuana legalization on prevalence of maternal marijuana use and perinatal outcomes. American journal of perinatology. 2020;37(01):059–065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ammerman S, Ryan S, Adelman WP, et al. The impact of marijuana policies on youth: clinical, research, and legal update. Pediatrics. 2015;135(3):e769–e785. [DOI] [PubMed] [Google Scholar]
- 44.Kuhn C, Ignar D, Windh R. Endocrine function as a target of perinatal drug effects: Methodologic issues. Methodological issues in controlled studies on effects of prenatal exposure to drug abuse. 1991:206. [PubMed] [Google Scholar]
- 45.Field E, Tyrey L. Delayed sexual maturation in the female rat during chronic exposure to delta-9-tetrahydrocannabinol. Life sciences. 1984;35(17):1725–1730. [DOI] [PubMed] [Google Scholar]
- 46.Gupta D, Elbracht C. Effect of tetrahydrocannabinols on pubertal body weight spurt and sex hormones in developing male rats. Research in experimental medicine. 1983;182(2):95–104. [DOI] [PubMed] [Google Scholar]
- 47.Jabeen S, Raja GK, Akram M, Ahmad A, Qayyum M, Rizvi SR. Evidence of stimulation of pubertal development and suppression of growth rate in boys smoking marijuana in cigarettes. Paper presented at: Endocrine Abstracts2015. [Google Scholar]
- 48.Center for Behavioral Health Statistics and Quality. Key substance use and mental health indicators in the United States: Results from the 2020 National Survey on Drug Use and Health. Rockville, MD: Substance Abuse and Mental Health Services Administration;2021. [Google Scholar]
- 49.Smith AM, Ferris JA, Simpson JM, Shelley J, Pitts MK, Richters J. Cannabis use and sexual health. The journal of sexual medicine. 2010;7(2):787–793. [DOI] [PubMed] [Google Scholar]
- 50.Pizzol D, Demurtas J, Stubbs B, et al. Relationship between cannabis use and erectile dysfunction: a systematic review and meta-analysis. American journal of men’s health. 2019;13(6):1557988319892464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bhambhvani HP, Kasman AM, Wilson-King G, Eisenberg ML. A survey exploring the relationship between cannabis use characteristics and sexual function in men. Sexual medicine. 2020;8(3):436–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cone EJ, Johnson RE, Moore JD, Roache JD. Acute effects of smoking marijuana on hormones, subjective effects and performance in male human subjects. Pharmacology Biochemistry and Behavior. 1986;24(6):1749–1754. [DOI] [PubMed] [Google Scholar]
- 53.Dalterio S, Badr F, Bartke A, Mayfield D. Cannabinoids in male mice: effects on fertility and spermatogenesis. Science. 1982;216(4543):315–316. [DOI] [PubMed] [Google Scholar]
- 54.Kolodny RC, Masters WH, Kolodner RM, Toro G. Depression of plasma testosterone levels after chronic intensive marihuana use. New England Journal of Medicine. 1974;290(16):872–874. [DOI] [PubMed] [Google Scholar]
- 55.Hembree WC, Zeidenberg P, Nahas GG. Marihuana’s effects on human gonadal function. In: Marihuana. Springer; 1976:521–532. [Google Scholar]
- 56.HEMBREE W III, Nahas G, Zeidenberg P, Huang H. Changes in human spermatozoa associated with high dose marihuana smoking. In: Marihuana Biological Effects. Elsevier; 1979:429–439. [DOI] [PubMed] [Google Scholar]
- 57.Issidorides MR. Observations in chronic hashish users: nuclear aberrations in blood and sperm and abnormal acrosomes in spermatozoa. Advances in the Biosciences. 1978;22:377–388. [DOI] [PubMed] [Google Scholar]
- 58.Mendelson JH, Kuehnle J, Ellingboe J, Babor TF. Plasma testosterone levels before, during and after chronic marihuana smoking. N Engl J Med. 1974;291(20):1051–1055. [DOI] [PubMed] [Google Scholar]
- 59.Mendelson JH, Ellingboe J, Kuehnle JC, Mello NK. Effects of chronic marihuana use on integrated plasma testosterone and luteinizing hormone levels. Journal of Pharmacology and Experimental Therapeutics. 1978;207(2):611–617. [PubMed] [Google Scholar]
- 60.Schaefer CF, Gunn C, Dubowski K. Normal plasma testosterone concentrations after marihuana smoking. The New England journal of medicine. 1975;292(16):867–868. [DOI] [PubMed] [Google Scholar]
- 61.Block RI, Farinpour R, Schlechte JA. Effects of chronic marijuana use on testosterone, luteinizing hormone, follicle stimulating hormone, prolactin and cortisol in men and women. Drug and alcohol dependence. 1991;28(2):121–128. [DOI] [PubMed] [Google Scholar]
- 62.Grimaldi P, Orlando P, Di Siena S, et al. The endocannabinoid system and pivotal role of the CB2 receptor in mouse spermatogenesis. Proceedings of the National Academy of Sciences. 2009;106(27):11131–11136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dalterio S, Bartke A, Burstein S. Cannabinoids inhibit testosterone secretion by mouse testes in vitro. Science. 1977;196(4297):1472–1473. [DOI] [PubMed] [Google Scholar]
- 64.Symons A, Teale J, Marks V. Proceedings: Effect of delta9-tetrahydrocannabinol on the hypothalamic-pituitary-gonadal system in the maturing male rat. The Journal of endocrinology. 1976;68(3):43P–44P. [PubMed] [Google Scholar]
- 65.Smith C, Moore C, Besch N, Besch P. EFFECT OF DELTA-9 TETRAHYDROCANNABINOL (THC) ON SECRETION OF MALE SEX-HORMONE IN RHESUS-MONKEY. Paper presented at: Pharmacologist1976. [Google Scholar]
- 66.Murphy LL, Gher J, Steger RW, Bartke A. Effects of Δ9-tetrahydrocannabinol on copulatory behavior and neuroendocrine responses of male rats to female conspecifics. Pharmacology Biochemistry and Behavior. 1994;48(4):1011–1017. [DOI] [PubMed] [Google Scholar]
- 67.Dixit V, Gupta CL, Agrawal M. Testicular degeneration and necrosis induced by chronic administration of cannabis extract in dogs. Endokrinologie. 1977;69(3):299–305. [PubMed] [Google Scholar]
- 68.Dixit V, Sharma V, Lohiya N. The effect of chronically administered cannabis extract on the testicular function of mice. European journal of pharmacology. 1974;26(1):111–114. [DOI] [PubMed] [Google Scholar]
- 69.Thompson GR, Mason MM, Rosenkrantz H, Braude MC. Chronic oral toxicity of cannabinoids in rats. Toxicology and applied pharmacology. 1973;25(3):373–390. [DOI] [PubMed] [Google Scholar]
- 70.Banerjee A, Singh A, Srivastava P, Turner H, Krishna A. Effects of chronic bhang (cannabis) administration on the reproductive system of male mice. Birth Defects Research Part B: Developmental and Reproductive Toxicology. 2011;92(3):195–205. [DOI] [PubMed] [Google Scholar]
- 71.Harclerode J Endocrine effects of marijuana in the male: preclinical studies. NIDA Res Monogr. 1984;44:46–64. [PubMed] [Google Scholar]
- 72.Hedges JC, Hanna CB, Bash JC, et al. Chronic delta-9-tetrahydrocannabinol exposure impacts testicular volume and male reproductive health in rhesus macaques Fertility and sterility. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.HUANG HF, NAHAS GG, HEMBREE WC III. Effects of marihuana inhalation on spermatogenesis of the rat. In: Marihuana Biological Effects. Elsevier; 1979:419–427. [DOI] [PubMed] [Google Scholar]
- 74.ZIMMERMAN AM, ZIMMERMAN S, RAJ AY. Effects of cannabinoids on spermatogenesis in mice. In: Marihuana Biological Effects. Elsevier; 1979:407–418. [DOI] [PubMed] [Google Scholar]
- 75.Carroll K, Pottinger A, Wynter S, DaCosta V. Marijuana use and its influence on sperm morphology and motility: identified risk for fertility among Jamaican men. Andrology. 2020;8(1):136–142. [DOI] [PubMed] [Google Scholar]
- 76.Pacey A, Povey A, Clyma J-A, et al. Modifiable and non-modifiable risk factors for poor sperm morphology. Human Reproduction. 2014;29(8):1629–1636. [DOI] [PubMed] [Google Scholar]
- 77.Murphy SK, Itchon-Ramos N, Visco Z, et al. Cannabinoid exposure and altered DNA methylation in rat and human sperm. Epigenetics. 2018;13(12):1208–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Klonoff-Cohen HS, Natarajan L, Chen RV. A prospective study of the effects of female and male marijuana use on in vitro fertilization (IVF) and gamete intrafallopian transfer (GIFT) outcomes. American journal of obstetrics and gynecology. 2006;194(2):369–376. [DOI] [PubMed] [Google Scholar]
- 79.Smith CG, Besch NF, Smith RG, Besch PK. Effect of tetrahydrocannabinol on the hypothalamic-pituitary axis in the ovariectomized rhesus monkey. Fertility and Sterility. 1979;31(3):335–339. [DOI] [PubMed] [Google Scholar]
- 80.Smith C, Smith M, Besch N, Smith R, Asch R. Effect of delta-nine-tetrahydrocannabinol (THC) on female reproductive function. Marihuana: Chemistry, Biochemistry and Cellular Effects Pergamon Press, New York. 1980;449. [Google Scholar]
- 81.Asch RH, Fernandez EO, Smith CG, Pauerstein CJ. Precoital single doses of Δ9-tetrahydrocannabinol block ovulation in the rabbit. Fertility and sterility. 1979;31(3):331–334. [PubMed] [Google Scholar]
- 82.Asch RH, Smith CG. Effects of delta 9-THC, the principal psychoactive component of marijuana, during pregnancy in the rhesus monkey. J Reprod Med. 1986;31(12):1071–1081. [PubMed] [Google Scholar]
- 83.TYREY L δ−9-Tetrahydrocannabinol suppression of episodic luteinizing hormone secretion in the ovariectomized rat. Endocrinology. 1978;102(6):1808–1814. [DOI] [PubMed] [Google Scholar]
- 84.Dalterio S, Bartke A, Roberson C, Watson D, Burstein S. Direct and pituitary-mediated effects of Δ9-THC and cannabinol on the testis. Pharmacology Biochemistry and Behavior. 1978;8(6):673–678. [DOI] [PubMed] [Google Scholar]
- 85.Chakravarty I, Sheth AR, Ghosh JJ. Effect of acute delta 9-tetrahydrocannabinol treatment on serum luteinizing hormone and prolactin levels in adult female rats. Fertility and sterility. 1975;26(9):947–948. [DOI] [PubMed] [Google Scholar]
- 86.Ayalon D, Nir I, Cordova T, et al. Acute effect of delta1-tetrahydrocannabinol on the hypothalamo-pituitary-ovarian axis in the rat. Neuroendocrinology. 1977;23(1):31–42. [DOI] [PubMed] [Google Scholar]
- 87.Dalterio S, Mayfield D, Bartke A. Effects of delta 9-THC on plasma hormone levels in female mice. Substance and alcohol actions/misuse. 1983;4(5):339–345. [PubMed] [Google Scholar]
- 88.Stouffer RL, Woodruff TK. Nonhuman primates: a vital model for basic and applied research on female reproduction, prenatal development, and women’s health. ILAR journal. 2017;58(2):281–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Besch NF, Smith CG, Besch PK, Kaufman RH. The effect of marihuana (delta-9-tetrahydrocannabinol) on the secretion of luteinizing hormone in the ovariectomized rhesus monkey. American journal of obstetrics and gynecology. 1977;128(6):635–642. [DOI] [PubMed] [Google Scholar]
- 90.Asch RH, SMITH CG, SILER-KHODR TM, PAUERSTEIN CJ. Effects of Δ9-tetrahydrocannabinol during the follicular phase of the rhesus monkey (Macaca mulatto). The Journal of Clinical Endocrinology & Metabolism. 1981;52(1):50–55. [DOI] [PubMed] [Google Scholar]
- 91.Jordan T, Ngo B, Jones C. The use of cannabis and perceptions of its effect on fertility among infertility patients. Human reproduction open. 2020;2020(1):hoz041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.White AJ, Sandler DP, D’Aloisio AA, et al. Antimüllerian hormone in relation to tobacco and marijuana use and sources of indoor heating/cooking. Fertility and sterility. 2016;106(3):723–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jukic AMZ, Weinberg CR, Baird DD, Wilcox AJ. Lifestyle and reproductive factors associated with follicular phase length. Journal of women’s health. 2007;16(9):1340–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wise LA, Wesselink AK, Hatch EE, et al. Marijuana use and fecundability in a North American preconception cohort study. J Epidemiol Community Health. 2018;72(3):208–215. [DOI] [PubMed] [Google Scholar]
- 95.Kasman AM, Thoma ME, McLain AC, Eisenberg ML. Association between use of marijuana and time to pregnancy in men and women: findings from the National Survey of Family Growth. Fertility and sterility. 2018;109(5):866–871. [DOI] [PubMed] [Google Scholar]
- 96.Mueller BA, Daling JR, Weiss NS, Moore DE. Recreational drug use and the risk of primary infertility. Epidemiology. 1990:195–200. [DOI] [PubMed] [Google Scholar]
- 97.Mumford S, Flannagan K, Radoc J, et al. Cannabis use while trying to conceive: a prospective cohort study evaluating associations with fecundability, live birth and pregnancy loss. Human Reproduction. 2021;36(5):1405–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nassan FL, Arvizu M, Mínguez-Alarcón L, et al. Marijuana smoking and markers of testicular function among men from a fertility centre. Human Reproduction. 2019;34(4):715–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Obstetricians ACo, Gynecologists. Marijuana use during pregnancy and lactation. October), https://www.acog.org/Clinical-Guidance-and-Publications/Committee-Opinions/Committee-on-Obstetric-Practice/Marijuana-Use-During-Pregnancy-and-Lactation. 2017.
- 100.Martin CE, Longinaker N, Mark K, Chisolm MS, Terplan M. Recent trends in treatment admissions for marijuana use during pregnancy. Journal of addiction medicine. 2015;9(2):99–104. [DOI] [PubMed] [Google Scholar]
- 101.Volkow ND, Han B, Compton WM, McCance-Katz EF. Self-reported medical and nonmedical cannabis use among pregnant women in the United States. Jama. 2019;322(2):167–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brown QL, Sarvet AL, Shmulewitz D, Martins SS, Wall MM, Hasin DS. Trends in marijuana use among pregnant and nonpregnant reproductive-aged women, 2002–2014. Jama. 2017;317(2):207–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McCance-Katz EF. The national survey on drug use and health: 2017. Substance Abuse and Mental Health Services Administration. https://www.samhsa.gov/data/sites/default/files/nsduh-ppt-09-2018.pdf. Web site. Published 2019. Accessed December 20, 2021. [Google Scholar]
- 104.Fried P, Watkinson B, Grant A, Knights R. Changing patterns of soft drug use prior to and during pregnancy: A prospective study. Drug and alcohol dependence. 1980;6(5):323–343. [DOI] [PubMed] [Google Scholar]
- 105.Chabarria KC, Racusin DA, Antony KM, et al. Marijuana use and its effects in pregnancy. American journal of obstetrics and gynecology. 2016;215(4):506. e501–506. e507. [DOI] [PubMed] [Google Scholar]
- 106.Beatty JR, Svikis DS, Ondersma SJ. Prevalence and perceived financial costs of marijuana versus tobacco use among urban low-income pregnant women. Journal of addiction research & therapy. 2012;3(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Passey ME, Sanson-Fisher RW, D’Este CA, Stirling JM. Tobacco, alcohol and cannabis use during pregnancy: clustering of risks. Drug and Alcohol Dependence. 2014;134:44–50. [DOI] [PubMed] [Google Scholar]
- 108.Young-Wolff KC, Adams SR, Wi S, Weisner C, Conway A. Routes of cannabis administration among females in the year before and during pregnancy: Results from a pilot project. Addictive behaviors. 2020;100:106125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ryan SA, Ammerman SD, O’Connor ME, et al. Marijuana use during pregnancy and breastfeeding: implications for neonatal and childhood outcomes. Pediatrics. 2018;142(3). [DOI] [PubMed] [Google Scholar]
- 110.General OotS. US Surgeon General’s advisory: marijuana use and the developing brain. In: Washington, DC: Author; 2019. [Google Scholar]
- 111.Bayrampour H, Zahradnik M, Lisonkova S, Janssen P. Women’s perspectives about cannabis use during pregnancy and the postpartum period: An integrative review. Preventive medicine. 2019;119:17–23. [DOI] [PubMed] [Google Scholar]
- 112.Woodruff K, Scott KA, Roberts SC. Pregnant people’s experiences discussing their cannabis use with prenatal care providers in a state with legalized cannabis. Drug and Alcohol Dependence. 2021;227:108998. [DOI] [PubMed] [Google Scholar]
- 113.Barbosa-Leiker C, Brooks O, Smith CL, Burduli E, Gartstein MA. Healthcare professionals’ and budtenders’ perceptions of perinatal cannabis use. The American journal of drug and alcohol abuse. 2021:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Holland CL, Rubio D, Rodriguez KL, et al. Obstetric health care providers’ counseling responses to pregnant patient disclosures of marijuana use. Obstetrics and gynecology. 2016;127(4):681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chang JC, Tarr JA, Holland CL, et al. Beliefs and attitudes regarding prenatal marijuana use: Perspectives of pregnant women who report use. Drug and alcohol dependence. 2019;196:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vanstone M, Taneja S, Popoola A, et al. Reasons for cannabis use during pregnancy and lactation: a qualitative study. CMAJ. 2021;193(50):E1906–E1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cristino L, Di Marzo V. Fetal cannabinoid receptors and the “dis‐joint‐ed” brain. The EMBO journal. 2014;33(7):665–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kenney SP, Kekuda R, Prasad PD, Leibach FH, Devoe LD, Ganapathy V. Cannabinoid receptors and their role in the regulation of the serotonin transporter in human placenta. American Journal of Obstetrics and Gynecology. 1999;181(2):491–497. [DOI] [PubMed] [Google Scholar]
- 119.Lorenzetti V, Solowij N, Fornito A, Ian Lubman D, Yucel M. The association between regular cannabis exposure and alterations of human brain morphology: an updated review of the literature. Current pharmaceutical design. 2014;20(13):2138–2167. [DOI] [PubMed] [Google Scholar]
- 120.Marchand G, Masoud AT, Govindan M, et al. Birth Outcomes of Neonates Exposed to Marijuana in Utero: A Systematic Review and Meta-analysis. JAMA Network Open. 2022;5(1):e2145653–e2145653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fergusson D, Horwood L, Northstone K. Avon Longitudinal Study of Pregnancy and Childhood. Maternal use of cannabis and pregnancy outcome. BJOG. 2002;109(1):21–27. [DOI] [PubMed] [Google Scholar]
- 122.National Academies of Sciences Engineering and Medicine. The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. Washington, DC: The National Academies Press; 2017. [PubMed] [Google Scholar]
- 123.Varner MW, Silver RM, Hogue CJR, et al. Association between stillbirth and illicit drug use and smoking during pregnancy. Obstet Gynecol. 2014;123(1):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shi Y, Zhu B, Liang D. The associations between prenatal cannabis use disorder and neonatal outcomes. Addiction. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Corsi DJ, Walsh L, Weiss D, et al. Association between self-reported prenatal cannabis use and maternal, perinatal, and neonatal outcomes. Jama. 2019;322(2):145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Conner SN, Bedell V, Lipsey K, Macones GA, Cahill AG, Tuuli MG. Maternal marijuana use and adverse neonatal outcomes. Obstet Gynecol. 2016;128(4):713–723. [DOI] [PubMed] [Google Scholar]
- 127.Reece AS, Hulse GK. Epidemiological overview of multidimensional chromosomal and genome toxicity of cannabis exposure in congenital anomalies and cancer development. Scientific reports. 2021;11(1):1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.van Gelder MM, Donders ART, Devine O, Roeleveld N, Reefhuis J, Study NBDP. Using bayesian models to assess the effects of under‐reporting of cannabis use on the association with birth defects, National Birth Defects Prevention Study, 1997–2005. Paediatr Perinat Epidemiol. 2014;28(5):424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shi Y, Zhong S. Trends in cannabis use disorder among pregnant women in the US, 1993–2014. Journal of general internal medicine. 2018;33(3):245–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gabrhelík R, Mahic M, Lund IO, et al. Cannabis use during pregnancy and risk of adverse birth outcomes: a longitudinal cohort study. European addiction research. 2021;27(2):131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sun X, Dey SK. Endocannabinoid signaling in female reproduction. ACS Chemical Neuroscience. 2012;3(5):349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Park B, Gibbons H, Mitchell M, Glassa M. Identification of the CB1 cannabinoid receptor and fatty acid amide hydrolase (FAAH) in the human placenta. Placenta. 2003;24(5):473–478. [DOI] [PubMed] [Google Scholar]
- 133.Yao J, He Q, Liu M, et al. Effects of Δ (9)-tetrahydrocannabinol (THC) on human amniotic epithelial cell proliferation and migration. Toxicology. 2018;394:19–26. [DOI] [PubMed] [Google Scholar]
- 134.Costa M, Fonseca B, Marques F, Teixeira N, Correia-da-Silva G. The psychoactive compound of Cannabis sativa, Δ9-tetrahydrocannabinol (THC) inhibits the human trophoblast cell turnover. Toxicology. 2015;334:94–103. [DOI] [PubMed] [Google Scholar]
- 135.Chang X, Bian Y, He Q, et al. Suppression of STAT3 signaling by Δ9-tetrahydrocannabinol (THC) induces trophoblast dysfunction. Cellular Physiology and Biochemistry. 2017;42(2):537–550. [DOI] [PubMed] [Google Scholar]
- 136.Benevenuto SG, Domenico MD, Martins MAG, et al. Recreational use of marijuana during pregnancy and negative gestational and fetal outcomes: an experimental study in mice. Toxicology. 2017;376:94–101. [DOI] [PubMed] [Google Scholar]
- 137.Natale BV, Gustin KN, Lee K, et al. Δ9-tetrahydrocannabinol exposure during rat pregnancy leads to symmetrical fetal growth restriction and labyrinth-specific vascular defects in the placenta. Scientific reports. 2020;10(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Maia J, Almada M, Midão L, et al. The Cannabinoid Delta-9-tetrahydrocannabinol Disrupts Estrogen Signaling in Human Placenta. Toxicological Sciences. 2020;177(2):420–430. [DOI] [PubMed] [Google Scholar]
- 139.Lee C, Chiang C. Maternal-fetal transfer of abused substances: pharmacokinetic and pharmacodynamic data. NIDA Research monograph. 1985;60:110–147. [PubMed] [Google Scholar]
- 140.Hutchings DE, Martin BR, Gamagaris Z, Miller N, Fico T. Plasma concentrations of delta-9-tetrahydrocannabinol in dams and fetuses following acute or multiple prenatal dosing in rats. Life sciences. 1989;44(11):697–701. [DOI] [PubMed] [Google Scholar]
- 141.Blackard C, Tennes K. Human placental transfer of cannabinoids. The New England journal of medicine. 1984;311(12):797. [DOI] [PubMed] [Google Scholar]
- 142.Grzeskowiak LE, Grieger JA, Andraweera P, et al. The deleterious effects of cannabis during pregnancy on neonatal outcomes. Medical Journal of Australia. 2020;212(11):519–524. [DOI] [PubMed] [Google Scholar]
- 143.Martínez-Peña AA, Lee K, Petrik JJ, Hardy DB, Holloway AC. Gestational exposure to Δ9-THC impacts ovarian follicular dynamics and angiogenesis in adulthood in Wistar rats. Journal of Developmental Origins of Health and Disease. 2021:1–5. [DOI] [PubMed] [Google Scholar]
- 144.Nashed MG, Hardy DB, Laviolette SR. Prenatal Cannabinoid Exposure: Emerging Evidence of Physiological and Neuropsychiatric Abnormalities. Frontiers in psychiatry. 2021;11:1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Brummelte S, Mc Glanaghy E, Bonnin A, Oberlander T. Developmental changes in serotonin signaling: Implications for early brain function, behavior and adaptation. Neuroscience. 2017;342:212–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Huizink A Prenatal cannabis exposure and infant outcomes: overview of studies. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2014;52:45–52. [DOI] [PubMed] [Google Scholar]
- 147.El Marroun H, Hudziak JJ, Tiemeier H, et al. Intrauterine cannabis exposure leads to more aggressive behavior and attention problems in 18-month-old girls. Drug and alcohol dependence. 2011;118(2–3):470–474. [DOI] [PubMed] [Google Scholar]
- 148.Day NL, Richardson GA, Goldschmidt L, et al. Effect of prenatal marijuana exposure on the cognitive development of offspring at age three. Neurotoxicology and teratology. 1994;16(2):169–175. [DOI] [PubMed] [Google Scholar]
- 149.El Marroun H, Bolhuis K, Franken IH, et al. Preconception and prenatal cannabis use and the risk of behavioural and emotional problems in the offspring; a multi-informant prospective longitudinal study. International journal of epidemiology. 2019;48(1):287–296. [DOI] [PubMed] [Google Scholar]
- 150.Rompala G, Nomura Y, Hurd YL. Maternal cannabis use is associated with suppression of immune gene networks in placenta and increased anxiety phenotypes in offspring. Proceedings of the National Academy of Sciences. 2021;118(47). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Corsi DJ, Donelle J, Sucha E, et al. Maternal cannabis use in pregnancy and child neurodevelopmental outcomes. Nat Med. 2020;26(10):1536–1540. [DOI] [PubMed] [Google Scholar]
- 152.Daha SK, Sharma P, Sah PK, Karn A, Poudel A, Pokhrel B. Effects of prenatal cannabis use on fetal and neonatal development and its association with neuropsychiatric disorders: A systematic review. Neurol Psychiatry Brain Res. 2020;38:20–26. [Google Scholar]
- 153.Paul SE, Hatoum AS, Fine JD, et al. Associations between prenatal cannabis exposure and childhood outcomes: results from the ABCD study. JAMA psychiatry. 2021;78(1):64–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Smid MC, Metz TD, McMillin GA, et al. Prenatal nicotine or cannabis exposure and offspring neurobehavioral outcomes. Obstet Gynecol. 2021:10.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Corsi DJ, Donelle J, Sucha E, et al. Maternal cannabis use in pregnancy and child neurodevelopmental outcomes. Nature medicine. 2020;26(10):1536–1540. [DOI] [PubMed] [Google Scholar]
- 156.Torres CA, Medina-Kirchner C, O’malley KY, Hart CL. Totality of the evidence suggests prenatal cannabis exposure does not lead to cognitive impairments: a systematic and critical review. Front Psychol. 2020;11:816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bolhuis K, Kushner SA, Yalniz S, et al. Maternal and paternal cannabis use during pregnancy and the risk of psychotic-like experiences in the offspring. Schizophrenia research. 2018;202:322–327. [DOI] [PubMed] [Google Scholar]
- 158.Lester BM, Conradt E, Marsit CJ. Are epigenetic changes in the intrauterine environment related to newborn neurobehavior? Epigenomics. 2014;6(2):175–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Roth TL, David Sweatt J. Annual Research Review: Epigenetic mechanisms and environmental shaping of the brain during sensitive periods of development. Journal of Child Psychology and Psychiatry. 2011;52(4):398–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Smith A, Kaufman F, Sandy MS, Cardenas A. Cannabis exposure during critical windows of development: Epigenetic and molecular pathways implicated in neuropsychiatric disease. Current environmental health reports. 2020;7:325–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.DiNieri JA, Wang X, Szutorisz H, et al. Maternal cannabis use alters ventral striatal dopamine D2 gene regulation in the offspring. Biological psychiatry. 2011;70(8):763–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zahm DS. An integrative neuroanatomical perspective on some subcortical substrates of adaptive responding with emphasis on the nucleus accumbens. Neuroscience & Biobehavioral Reviews. 2000;24(1):85–105. [DOI] [PubMed] [Google Scholar]
- 163.Zhu Z, Wang G, Ma K, Cui S, Wang J-H. GABAergic neurons in nucleus accumbens are correlated to resilience and vulnerability to chronic stress for major depression. Oncotarget. 2017;8(22):35933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bertrand KA, Hanan NJ, Honerkamp-Smith G, Best BM, Chambers CD. Marijuana use by breastfeeding mothers and cannabinoid concentrations in breast milk. Pediatrics. 2018;142(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wang GS. Pediatric concerns due to expanded cannabis use: unintended consequences of legalization. Journal of Medical Toxicology. 2017;13(1):99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Moss MJ, Bushlin I, Kazmierczak S, et al. Cannabis use and measurement of cannabinoids in plasma and breast milk of breastfeeding mothers. Pediatric Research. 2021:1–8. [DOI] [PubMed] [Google Scholar]
- 167.Perez-Reyes M, Wall ME. Presence of delta9-tetrahydrocannabinol in human milk. N Engl J Med. 1982;307(13):819–820. [DOI] [PubMed] [Google Scholar]
- 168.Baker T, Datta P, Rewers-Felkins K, Thompson H, Kallem RR, Hale TW. Transfer of inhaled cannabis into human breast milk. Obstetrics & Gynecology. 2018;131(5):783–788. [DOI] [PubMed] [Google Scholar]
- 169.Astley SJ, Little RE. Maternal marijuana use during lactation and infant development at one year. Neurotoxicology and teratology. 1990;12(2):161–168. [DOI] [PubMed] [Google Scholar]
- 170.Djulus J, Moretti M, Koren G. Marijuana use and breastfeeding. Canadian family physician. 2005;51(3):349–350. [PMC free article] [PubMed] [Google Scholar]
- 171.Medicine Io, Lactation NRCSoND, Lactation IoMSoNd, et al. Nutrition during lactation. https://www.ncbi.nlm.nih.gov/books/NBK235593/. Published 1991. Accessed December 20, 2021.
- 172.Tennes K, Avitable N, Blackard C, et al. Marijuana: prenatal and postnatal exposure in the human. NIDA Res Monogr. 1985;59:48–60. [PubMed] [Google Scholar]
- 173.Liston J. Breastfeeding and the use of recreational drugs-alcohol, caffeine, nicotine and marijuana. Breastfeeding review. 1998;6(2):27–30. [PubMed] [Google Scholar]
- 174.Lowry DE, Corsi DJ. Trends and correlates of cannabis use in Canada: a repeated cross-sectional analysis of national surveys from 2004 to 2017. CMAJ open. 2020;8(3):E487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Slavin MN, Farmer S, Earleywine M. Expectancy mediated effects of marijuana on menopause symptoms. Addiction Research & Theory. 2016;24(4):322–329. [Google Scholar]
- 176.Bar‐Lev Schleider L, Abuhasira R, Novack V. Medical cannabis: aligning use to evidence‐based medicine approach. British journal of clinical pharmacology. 2018;84(11):2458–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Rawitch AB, Schultz GS, Ebner KE, Vardaris RM. Competition of Δ9-tetrahydrocannabinol with estrogen in rat uterine estrogen receptor binding. Science. 1977;197(4309):1189–1191. [DOI] [PubMed] [Google Scholar]
- 178.Chakrabarty I, Sengupta D, Bhattacharya P, Ghosh J. Effect of cannabis extract on the uterine monoamine oxidase activity of normal and estradiol treated rats. Biochemical pharmacology. 1976;25(4):377–378. [DOI] [PubMed] [Google Scholar]
- 179.Mejia-Gomez J, Phung N, Philippopoulos E, Murphy K, Wolfman W. The impact of cannabis use on vasomotor symptoms, mood, insomnia and sexuality in perimenopausal and postmenopausal women: a systematic review. Climacteric. 2021;24(6):572–576. [DOI] [PubMed] [Google Scholar]
- 180.Young-Wolff KC, Gali K, Sarovar V, Rutledge GW, Prochaska JJ. Women’s questions about perinatal cannabis use and health care providers’ responses. Journal of Women’s Health. 2020;29(7):919–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Huestis MA, Sampson AH, Holicky BJ, Henningfield JE, Cone EJ. Characterization of the absorption phase of marijuana smoking. Clinical Pharmacology & Therapeutics. 1992;52(1):31–41. [DOI] [PubMed] [Google Scholar]
- 182.Monte AA, Zane RD, Heard KJ. The implications of marijuana legalization in Colorado. Jama. 2015;313(3):241–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Huestis MA. Human cannabinoid pharmacokinetics. Chemistry & biodiversity. 2007;4(8):1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Elsohly MA, Little TL Jr, Hikal A, Harland E, Stanford DF, Walker L. Rectal bioavailability of delta-9-tetrahydrocannabinol from various esters. Pharmacology Biochemistry and Behavior. 1991;40(3):497–502. [DOI] [PubMed] [Google Scholar]
- 185.Abuse S. Key substance use and mental health indicators in the United States: results from the 2019 National Survey on Drug Use and Health. 2020.


