Abstract
Aims:
Although extracorporeal cardiopulmonary resuscitation (ECPR) improves survival outcomes in refractory cardiac arrest, morbidity and mortality remain significantly high. Information on causes of death in ECPR is limited; however, some evidence suggests withdrawal of life sustaining therapy (WLST) is a major factor in ECPR-associated mortality. We sought to describe the patients experiencing WLST after ECPR.
Methods:
The international Extracorporeal Life Support Organization (ELSO) Registry was retrospectively queried for patients more than 18 years old supported with ECPR who underwent WLST due to family request from 2007–2017. These patients were split into groups for descriptive and multivariable analysis: early (WLST <72 hours from cannulation) and routine WLST.
Results:
Overall, 411 ECPR patients experienced WLST (median age 42 years IQR=28–51; 31.7% female) over the 10-year period. 55.5% (n=228) underwent early WLST with a median ECPR duration of 24 hours (IQR=7–48) versus routine WLST (median=147 hours; IQR=105–238). In multivariable regression analysis, lower arterial blood gas pH (aOR=−3.1; 95% CI=2.18–2.8; p=0.04), arterial oxygen saturation (aOR=1.12; 95% CI=1.01–1.23; p=0.02), and higher peak inspiratory pressure (aOR=0.84; 95% CI=0.71–1.00; p=0.05) were independently associated with early WLST. Early WLST patients experienced higher rates of all ECMO-related complications except for infections.
Conclusions:
More than half of ECPR patients experienced early WLST within 72 hours. The patients with early WLST had worse markers of severe critical illness at 24 hours and experienced higher rates of complications. Further research should include an appropriate control group to better adjust confounders for ECPR-associated death and focus on prognostication.
Keywords: Withdrawal of Life Sustaining Therapy, ECPR, ECMO, cardiac arrest
Introduction
Although the use of extracorporeal membrane oxygen therapy (ECMO) is increasing, survival for extracorporeal cardiopulmonary resuscitation (ECPR) is unchanging.1,2 ECPR provides cardiopulmonary support while patients receive treatment to reverse the underlying condition(s) that caused their refractory cardiac arrest. Recent evidence suggested that ECPR may improve mortality rates as well as neurological outcomes when compared to conventional cardiopulmonary resuscitation (CPR).3–5 As ECPR becomes a valuable tool for improving the outcomes for patients with refractory cardiac arrest, it is essential to understand how withdrawal of life sustaining therapy (WLST) influences the mortality in patients with ECPR.
By the nature of ECMO’s ability to provide adequate cardiopulmonary perfusion, WLST is one of few means patients on ECMO support can die. There are few studies on WLST in ECMO patients, which have previously only included small subsets of ECPR patients.6,7 WLST in ECMO patients have been associated with male sex, older age, increased baseline illness severity at time of cannulation, and being on at least one other form of life sustaining therapy including but not limited to mechanical ventilation, renal replacement therapy, and ventricular assist devices.6,7 However, these studies did not isolate ECPR patients who are at the highest risk of multi-organ failure including brain injury.8
Current guidelines for post-conventional cardiac arrest patients recommend avoiding early WLST within 72 hours from initial presentation or rewarming from targeted temperature management for comatose patients.9,10 Early WLST is not recommended due to confounders such as sedatives or paralytics as well as unstable hemodynamics and the effects of hypothermia. Therefore, early WLST within 72 hours after ECMO cannulation may result in patients experiencing WLST who would otherwise have had an acceptable long-term outcome. The most common reasons for WLST include perceived poor neurological prognosis, multi-system organ failure, and/or medical futility determined by the family or providers.11–13 A recent international consensus statement by the World Health Organization and The Transplantation Society recommends a delay in a WLST decision for patients with ECMO support to account for more accurate prognostication.14 Current prospective trials, including those focused on ECPR, limit WLST decisions for the first 72 hours as a way to mitigate early confounding bias.5
In our study, using the Extracorporeal Life Support Organization (ELSO) Registry, we aimed to describe the demographics and ECMO-specific factors of ECPR patients who underwent WLST. We hypothesized that early WLST is common in patients after ECPR and that greater illness severity was associated with the timing of WLST.
Materials and Methods
Study Design and Population
Data were extracted from the ELSO Registry, an international database. Records from the ELSO Registry are voluntarily reported and include patient demographics, clinical characteristics, complications, hemodynamic and laboratory values, and characteristics surrounding deaths occurring during ECMO. The registry uses current International Classification of Diseases (ICD) codes for past medical history.
Patients 18 years and older who received ECPR from 2007–2017 and underwent WLST were included in our study. Exclusion criteria included other indications for ECMO (non-ECPR), survivors at discharge, and patients who had another reason for death such as hemorrhage, diagnosis incompatible with life, or irreversible organ failure. Data were only included until 2017 as the ELSO Registry data collection form was updated at that time with regards to the section “reasons for death” in 2018.
Data Collection and Definitions
WLST in this study was defined as participants who met both of the following registry questions in the case report form: “Died on extracorporeal life support [ECLS] or ECLS withdrawn in anticipation of death” and the reason selected for death was “parental or family request.” The following data were then extracted for all patients meeting the inclusion criteria: pre-ECPR demographics, primary diagnoses, pre-ECPR support, ECPR clinical variables including hemodynamic data, and complications. Pre-ECPR support data were measured no more than six hours before cannulation and included arterial blood gas (ABG) values, fraction of inspired oxygenation (FiO2), systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), peak inspiratory pressure (PIP), positive end expiratory pressure (PEEP), and mean pulmonary arterial pressure (PAP). Twenty-four-hour data were obtained between 18 and 30 hours after ECPR initiation. Among complications, the “ECMO circuit mechanical failure” variable included oxygenator failure, cracks in pigtail connectors, clots in the hemofilter, clots in the circuit component, circuit change, cannula problems, air in the circuit, pump failure, and tubing rupture. Cardiac complications included CPR, arrythmia, hypertension requiring treatment, inotropes, tamponade, or myocardial stunning. Neurologic complications included brain death, hemorrhagic or ischemic stroke, or seizures. Metabolic complications included hyper- or hypoglycemia, hyperbilirubinemia, acidemia, or alkalemia. Pulmonary complications included pneumothorax or pulmonary hemorrhage. Hematologic complications included disseminated intravascular coagulopathy, gastrointestinal hemorrhage, cannulation site bleeding, surgical site bleeding, or hemolysis.
This study was approved by Johns Hopkins Hospital institutional review board. Informed consent is obtained by each of the participating ELSO registry sites.
Patients were stratified into two groups according to timing of WLST – “Early WLST” patients were defined by WLST ≤ 72 hours from ECPR cannulation. “Routine WLST” patients were defined by WLST > 72 hours from cannulation. A 72-hour cutoff was based on the prior guidelines for post cardiac arrest prognostication and WLST.9,10,14
Statistical Analysis
Baseline characteristics, pre-ECPR variables, on ECPR variables, and outcome variables were assessed for distribution normality and compared between the early and routine WLST groups. Results were expressed as median with interquartile range (IQR) for non-normally distributed continuous variables, means with standard deviation for normally distributed variables, and as proportions or percentages for categorical variables. Continuous variables were compared using Mann-Whitney U tests or unpaired t-tests as appropriate after assessing for normal distribution. Categorical variables were compared using Chi-square tests. A p-value of <0.05 was considered to be statistically significant.
Univariate logistic regression analysis was then performed to assess for unadjusted associations between demographics, clinical variables, and ECMO variables on WLST timing and were reported as odds ratios (ORs) with 95% confidence intervals (CI). Pre-specified variables included race, sex, baseline pH, and pH at 24 hours. Variables with a p-value < 0.20 on univariate analysis, as well as those > 0.20 but determined to be clinically significant, were included in the multivariable logistic regression analysis with WLST timing (early WLST vs routine WLST) as the primary outcome. The final multivariable regression model was evaluated using the Harrell C statistic and the Homer-Lemeshow goodness of fit test. All statistical analyses were performed using Stata version 15.1 (StataCorp LLC, College Station, TX).
Results
From 2007–2017, a total of 411 patients (median age 38.7 years; 31.7% female) were cannulated for ECPR and underwent WLST (Table 1). The total number of patients increased each year from 2007–2016 and stabilized from 2016–2017 (Figure 1). As a group, patients spent a median of 55 hours (IQR=21–134) on ECMO support prior to WLST. Of these patients, 55.5% (n=228) underwent early WLST in the first 72 hours (median age 44; IQR=28.5–51; 34.9% female) with a median time from ECPR cannulation to WLST of 24 hours (IQR=7–48]. The first 24-hour period after cannulation had the highest frequency of WLST events (n=133) (Figure 2). No significant differences between early WLST and routine WLST groups were observed for demographics or pre-ECPR support (Tables 1 and 2).
Table 1.
Demographic and Baseline Characteristics for Patients with Early vs. Routine Withdrawal of Life Sustaining Therapy
| Total (N=411) | Early WLST <72 hours (N=228) | Routine WLST > 72 hours (N=183) | p | |
|---|---|---|---|---|
| Age (years) | 42 (28–51) | 44 (28.5–51) | 41 (27–50) | 0.39 |
| Weight (kg)a | 83.5 (70–103) | 83 (69.1–103) | 83.8 (70.5–105) | 0.28 |
| Female Sex, n (%)a | 120 (31.7) | 75 (34.9) | 45 (27.1) | 0.11 |
| Hours on ECMOa | 55 (21–134) | 24 (7–48) | 147 (105–238) | <0.001 |
| Race, n (%)a | 0.13 | |||
| Asian | 47 (12.84) | 25 (12.4) | 22 (13.4) | |
| Black | 38 (10.38) | 18 (8.9) | 20 (12.2) | |
| Hispanic | 22 (6.01) | 9 (4.5) | 13 (7.9) | |
| White | 232 (63.39) | 135 (66.8) | 97 (59.1) | |
| Other | 22 (6.01) | 10 (5.0) | 12 (7.3) | |
| Unknown | 5 (1.37) | 5 (2.5) | 0 (0) | |
| ECMO mode, n (%)a | 0.79 | |||
| VA | 388 (94.4) | 212 (96.0) | 176 (97.7) | |
| VV | 2 (0.49) | 1 (0.45) | 1 (0.56) | |
| VVA | 4 (0.97) | 4 (1.8) | 0 (0) | |
| Conversion | 6 (1.46) | 4 (1.8) | 2 (1.1) | |
| Other | 1 (0.24) | 0 (0) | 1 (0.56) | |
| Pre-ECPR Support | ||||
| Vasopressor/Inotrope | 183 (44.5) | 97 (53.0) | 86 (47.0) | 0.76 |
| Renal replacement therapy | 19 (4.6) | 9 (47.4) | 10 (52.3) | 0.56 |
| Therapeutic hypothermia | 10 (2.4) | 5 (50.0) | 5 (50.0) | 0.80 |
| Cardiac Support | 0.48 | |||
| Pacemaker | 38 (9.2) | 17 (44.7) | 21 (55.3) | |
| Cardiopulmonary bypass | 18 (4.4) | 12 (66.7) | 6 (33.3) | |
| Intra-aortic balloon pump | 62 (15.1) | 35 (56.5) | 27 (43.5) | |
| Right ventricular assist device | 1 (0.24) | 1 (100) | 0 (0) | |
| Left ventricular assist device | 15 (3.6) | 6 (40.0) | 9 (60.0) |
WLST = Withdrawal of Life Sustaining Therapy, ECPR = Extracorporeal Cardiopulmonary Resuscitation, ECMO = Extracorporeal Membrane Oxygen Therapy, VA = Venoarterial, VV = Venovenous, VVA = Veno-Venoarterial
Missing values for some variables resulted in different denominators for the following variables: Weight (359) Sex (381) Hours (407) Race (366) Mode (401)
All data are presented as n (%) for categorical variables and median (IQR) for continuous variables
Boldface values indicate statistical significance (p < 0.05)
Figure 1:
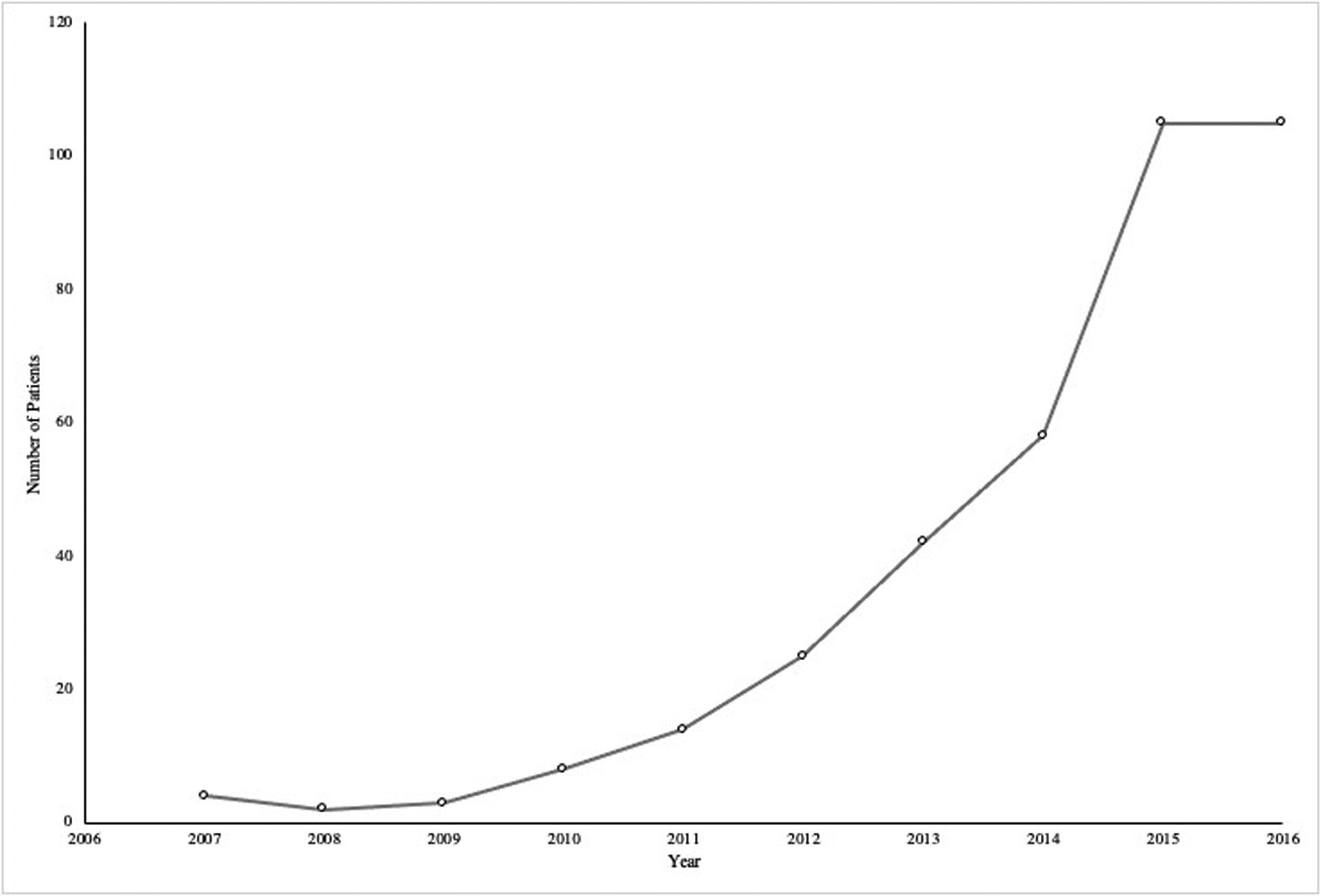
Patients Undergoing Withdrawal of Life Sustaining Therapy in Patients with Extracorporeal Cardiopulmonary Resuscitation by Year.
Figure 2:
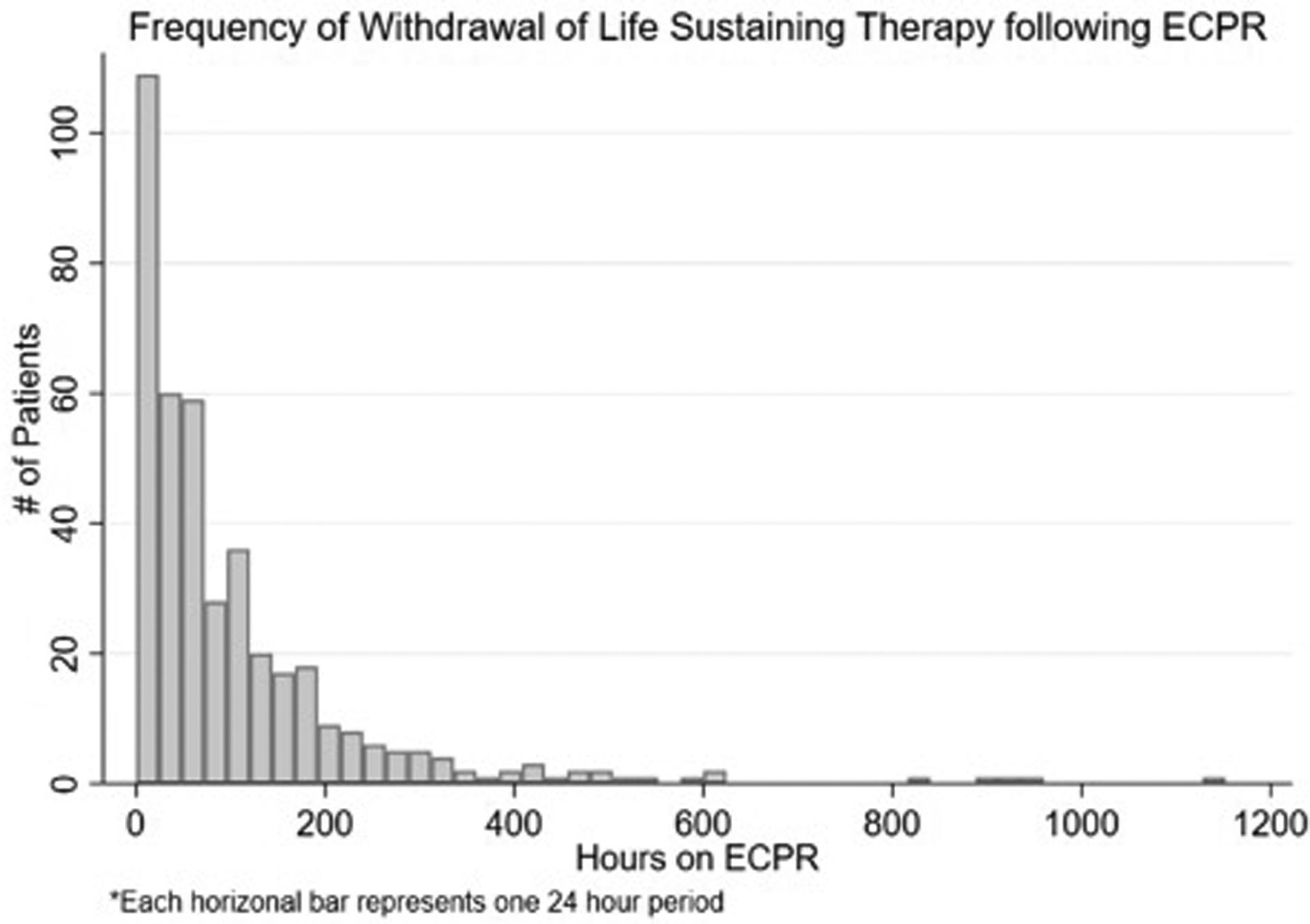
Frequency of Withdrawal of Life Sustaining Therapy following Extracorporeal Cardiopulmonary Resuscitation. Each bar is a 24-hour period. Over the first 72 hours, 228 patients have WLST. The first 24-hour period after initiation of ECPR has the highest frequency of withdrawal events (n=133).
Table 2.
Pre- and 24 hours Post-Cannulation ECPR Variables in Patients with Early WLST vs. WLST
| Total (N=411) | Early WLST <72 Hours (N=228) | Routine WLST >72 Hours (N=183) | p | |
|---|---|---|---|---|
| Pre-ECPR ABGa | ||||
| pH | 7.18 (7.05–7.32) | 7.14 (7.0–7.32) | 7.2 (7.08–7.32) | 0.014 |
| PCO2 (mmHg) | 48.5 (33.9–66) | 49 (32–69) | 48 (36–64) | 0.36 |
| PO2 (mmHg) | 71.5 (50–150.5) | 74.5 (50–170) | 68 (48–137) | 0.48 |
| HCO3 (mmol/L) | 18.3 (14–23.05) | 18 (13–23) | 19 (14.2–24) | 0.12 |
| Pre-ECPR Hemodynamic Monitoringa | ||||
| SaO2 (%) | 91 (75–98) | 92 (74–98) | 88 (76–97) | 0.41 |
| SvO2 (%) | 53.5 (51–63.5) | 53.5 (50–76) | 53.5 (51–58.5) | 0.39 |
| SBP (mmHg) | 76 (56–97) | 72 (54–96) | 80 (58–100) | 0.33 |
| DBP (mmHg) | 44 (31–56.5) | 43.5 (29–57) | 44.5 (36–55) | 0.72 |
| MAP (mmHg) | 53 (38–68) | 50 (37–66) | 56 (43–71) | 0.14 |
| Cardiac Index (L/min/m2) | 2.26 (1.84–2.5) | 2.42 (2.18–2.9) | 1.9 (1.8–2.4) | 0.09 |
| Lactate (mmol/L) | 8.4 (4.3–14.5) | 7.5 (2.7–17.2) | 8.65 (6.9–12.5) | 0.82 |
| 4 Hour ECPR Circuit and Ventilator Settingsa | ||||
| Pump Flow (L/min) | 3.8 (3.0–4.5) | 3.52 (2.8–4.3) | 4.0 (3.1–4.6) | 0.01 |
| Rate (breaths/min) | 16 (14–23) | 16 (14–20) | 18 (14–24) | 0.79 |
| FiO2 (%) | 100 (100–100) | 100 (100–100) | 100 (100–100) | 0.89 |
| PIP (cmH2O) | 27 (22–32) | 26 (22–31) | 28 (21–33.8) | 0.5 |
| PEEP (cmH2O) | 6 (5–10) | 6 (5–10) | 6 (5–10) | 0.9 |
| Mean PAP (cmH2O) | 13 (10–17.5) | 13 (10–20) | 12 (10–16.7) | 0.41 |
| 24 Hour ABGa | ||||
| pH | 7.41 (7.34–7.46) | 7.37 (7.3–7.44) | 7.42 (7.37–7.47) | <0.001 |
| PCO2 (mmHg) | 38 (34–43) | 37.4 (33.5–42.1) | 38.1 (35–43) | 0.91 |
| PO2 (mmHg) | 249 (128–422) | 293 (129–435) | 236.5 (128–418) | 0.75 |
| HCO3 (mmol/L) | 24 (20.5–27) | 22 (18.3–25) | 25 (22–28) | <0.001 |
| 24 Hour Hemodynamic Monitoringa | ||||
| SaO2 (%) | 99 (97–100) | 99 (96–100) | 99 (97–100) | 0.01 |
| SBP (mmHg) | 90.5 (75.5–104) | 88 (72–103) | 91 (79–105) | 0.2 |
| DBP (mmHg) | 65 (55–73) | 62 (51–71) | 67 (58–74) | 0.02 |
| MAP (mmHg) | 72 (65–81) | 70 (64–80.5) | 73 (66–81) | 0.047 |
| Cardiac Index (L/min/m2) | 1.95 (1.2–2.3) | 2.1 (1.25–2.3) | 1.8 (1.1–2.58) | 0.9 |
| Lactate (mmol/L) | 3.6 (2.2–7.1) | 5.45 (2.8–20) | 3.3 (2.2–6.5) | 0.01 |
| 24 Hour ECPR Circuit and Ventilator Settingsa | ||||
| Pump Flow (L/min) | 4.0 (3.3–4.7) | 3.95 (3.1–4.5) | 4.1 (3.5–4.8) | 0.091 |
| Rate (breaths/min) | 12 (10–18) | 14 (12–18) | 12 (10–16) | 0.01 |
| FiO2 (%) | 50 (40–60) | 50 (40–100) | 40 (40–60) | <0.001 |
| PIP (cmH2O) | 26 (21–30) | 27 (23–32) | 25 (20–28) | <0.001 |
| PEEP (cmH2O) | 8 (5–10) | 8 (5–10) | 8 (5–10) | 0.16 |
| Mean PAP (cmH2O) | 13 (11–16) | 13 (11–17) | 12 (10–15) | 0.13 |
WLST = Withdrawal of Life Sustaining Therapy, ECPR = Extracorporeal Cardiopulmonary Resuscitation, ECMO = Extracorporeal Membrane Oxygen Therapy, ABG = Arterial Blood Gas, HCO3 = Bicarbonate, SBP = Systolic Blood Pressure, DBP = Diastolic Blood Pressure, MAP = Mean Arterial Pressure, PIP = Peak Inspiratory Pressure, PEEP = Positive End Expiratory Pressure, Mean PAP = Mean Pulmonary Arterial Pressure
Missing values for some variables resulted in different denominators for the following variables: Pre-ECMO ABG pH (286), Pre-ECMO PCO2 (284), Pre-ECMO PO2 (284), Pre-ECMO HCO3 (276), Pre-ECMO SaO2 (246), Pre-ECMO SvO2 (16), Pre-ECMO SBP (234), Pre-ECMO DBP (232), Pre-ECMO MAP (191), Pre-ECMO cardiac index (21), Pre-ECMO lactate (37), 4 hour pump flow (331), 4 hour rate (169), 4 hour FiO2 (230), 4 hour PIP (123), 4 hour PEEP (165), 4 hour mean PAP (112), 24 hour pH (267), 24 hour PCO2 (266), 24 hour PO2 (265), 24 hour HCO3 (265), 24 hour SaO2 (253), 24 hour SBP (228), 24 hour DBP (228), 24 hour MAP 241), 24 hour cardiac index (14), 24 hour lactate (39), 24 hour pump flow (260), 24 hour rate (251), 24 hour FiO2 (259), 24 hour PIP (214), 24 hour PEEP (251), 24 hour Mean PAP (185)
All data are presented as n (%) for categorical variables and median (IQR) for continuous variables.
Boldface values indicate statistical significance (p < 0.05).
Early WLST was associated with a lower pre-ECPR pH (7.14 vs 7.20; p=0.014) and lower pH at 24 hours after cannulation (7.37 vs 7.42; p<0.001). Lactate was persistently elevated at 24 hours in the early WLST group, whereas it had decreased from baseline in the routine WLST group (5.45 vs 3.3 mmol/L; p=0.009). The serum bicarbonate level was lower in the early WLST on 24-hour ABG (22 vs 25 mmoL/L; p<0.001). The early WLST group had higher ventilator settings 24 hours after cannulation, including higher respiratory rate (14 vs 12; p<0.008), higher FiO2 (50 vs 40%; p<0.001) and elevated peak inspiratory pressures (PIP) (27 vs 25 cmH2O; p<0.001). The early WLST group had a lower MAP 24 hours after cannulation (70 vs 73 mmHg; p=0.047) and DBP (62 vs 67 mmHg; p=0.02) (Table 2). Early WLST patients experienced higher rates of all ECMO-related complications except for infections (Table 3).
Table 3.
ECPR-associated Complication Rates in Early WLST vs. WLST
| Complications | Early WLST Frequency (n) | Early WLST Rate | Routine WLST Frequency (n) | Routine WLST Rate | p | 95% CI | OR |
|---|---|---|---|---|---|---|---|
| Mechanical | 37 | 14.1 | 55 | 3.6 | <0.0001 | 2.5–6.1 | 3.9 |
| Hemorrhagic | 84 | 31.9 | 94 | 6.1 | <0.0001 | 3.8–7.1 | 5.2 |
| Neurologic | 39 | 14.8 | 54 | 3.5 | <0.0001 | 2.7–6.5 | 4.2 |
| Renal | 116 | 44.1 | 175 | 11.4 | <0.0001 | 3.0–4.9 | 3.9 |
| Cardiovascular | 202 | 76.8 | 179 | 11.7 | <0.0001 | 5.4–8.1 | 6.6 |
| Pulmonary | 11 | 4.2 | 11 | 0.7 | 0.0001 | 2.3–14.8 | 6 |
| Infectious | 8 | 3.0 | 35 | 2.3 | 0.46 | 0.53–2.9 | 1.3 |
| Limb Ischemia | 15 | 5.7 | 29 | 1.9 | 0.0013 | 1.5–5.8 | 3 |
Each complication was defined in the methods section. Rate is defined as complications/day/100 patient to adjust for the duration of ECPR support. Odds Ratio (OR) of early WLST compared to WLST.
ECPR = Extracorporeal Cardiopulmonary Resuscitation, WLST = Withdrawal of Life Sustaining Therapy
Boldface values indicate statistical significance (p < 0.05)
In multivariable analysis, lower pH (aOR=−3.1, 95% CI 2.18–2.8, p=0.005), lower SaO2 (aOR=1.12, 95% CI 1.01–1.23, p= 0.02), lower pump flow (aOR=7.01, 95% CI 1.47–34.0, p=0.01), and higher PIPs (aOR=0.84, 95% CI 0.71–1.0, p=0.05) at 24 hours post-cannulation were independent risk factors for early WLST compared to routine WLST (Table 4). Notably, baseline pH and MAP was no longer significantly associated with early WLST when other covariates were adjusted. C statistic for multivariable regression model was 0.89.
Table 4.
Multivariable Logistic Regression Analysis of Risk Factors of Early Withdrawal of Life Sustaining Therapy
| Variable | Adjusted Odds Ratio (95% CI) | p |
|---|---|---|
| Demographic | ||
| Race | 0.67 (0.43–1.04) | 0.07 |
| Female Sex | 0.13 (0.01–1.2) | 0.07 |
| Pre-ECPR | ||
| pH | 0.49 (0.008–28.5) | 0.73 |
| HCO3 | 1.14 (1.01–1.29) | 0.8 |
| MAP | 0.98 (0.95–1.02) | 0.29 |
| 4 Hour Post-Cannulation | ||
| Pump flow | 0.60 (0.19–1.91) | 0.39 |
| 24 Hour Post-Cannulation | ||
| pH | −3.1 (2.18–2.8) | 0.005 |
| HCO3 | 0.97 (0.84–1.12) | 0.71 |
| SaO2 | 1.12 (1.01–1.23) | 0.02 |
| SBP | 0.98 (0.95–1.02) | 0.6 |
| DBP | 0.973 (0.90–1.05) | 0.47 |
| Pump flow | 7.01 (1.47–34.0) | 0.01 |
| Rate | 1.01 (0.90–1.14) | 0.8 |
| FiO2 | 0.97 (0.94–1.01) | 0.12 |
| PIP | 0.84 (0.71–1.00) | 0.05 |
| PEEP | 0.98 (0.80–1.21) | 0.87 |
| Mean PAP | 0.92 (0.85–1.00) | 0.06 |
ECPR = Extracorporeal Cardiopulmonary Resuscitation, HCO3 = bicarbonate, MAP = mean arterial pressure, SBP = Systolic blood pressure, DBP = diastolic blood pressure, PIP = Peak Inspiratory Pressure, PEEP = Postive End Expiratory Pressure, Mean PAP = Pulmonary Arterial Pressure
Boldface values indicate statistical significance (p < 0.05)
Discussion
In this study, we described the demographics and ECMO-associated factors of the patients undergoing WLST in the ELSO registry. We primarily examined the patients who underwent early WLST within the first 72 hours compared to those with routine WLST. Early WLST was commonly observed within the first 72 hours, which represented over half of the patients in ECPR population. Interestingly, the patients experiencing early WLST had more complications and had laboratory, hemodynamic, and physiologic markers of being more critically ill compared to those with routine WLST.
Timing of WLST
It is notable that over half (55.5%) of the population experienced WSLT in the first 72 hours in our study. This is surprising given that the current guidelines, expert opinions, and recent ECPR trials recommended avoiding early WLST in patients after cardiac arrest, especially considering the resource utilization and cost associated with ECPR support.5,10,13,14 The results of our study are consistent with a prior study showing more than one third of non-survivors of ECPR were decannulated within 24 hours of ECMO initiation,15 although it is unclear how many of these were due to WLST. Early WLST precludes any chance of recovery during a time period with clinical and prognostic uncertainty, which may in fact lead to excess mortality.16 Currently, there are insufficient data on how to predict outcomes in ECMO patients, which is a major drawback in prognostication in this population. To date, no studies have attempted to determine an appropriate duration of ECMO prior to WLST.
Risk Factors for Early WLST
Previously, age was positively associated with early WLST in ECMO patients in a single cohort study.7 In this study, baseline demographic and pre-ECPR variables were comparable between early versus WLST groups, except for a lower pre-ECPR ABG pH was associated with early WLST. However, this association did not hold when adjusted with covariates in the multivariable regression model. We found, however, some markers of illness severity in the first 24 hours of ECMO support were significantly correlated with an early WLST decision. These factors include lower pH on 24-hour ABG, higher serum lactate levels, lower SaO2, lower MAP, and elevated PIPs in the patients with early WLST (vs. routine WLST). Most of these factors, other than lactate and MAP, were still independently associated with early WLST after adjusting for other covariates.
Most ECMO-related complications were more common in patients with early WLST (Table 3), which may indicate more severe critical illness in the early WLST group. Complications may have influenced timing of WLST decisions given that almost all complications were experienced at higher rates in the early WLST group. Neurologic complications, which were higher in the early WLST group, are known to have an important role in timing and occurrence of WLST.11–12 There are a lack of data if ECPR patients undergoing WLST have higher rates of neurologic complications compared to the broader ECPR population. Successful ECPR can provide sufficient perfusion and reduce duration of low flow state (reducing primary and secondary brain injury) in comparison to conventional CPR.5 ECPR can also rapidly sustain targeted temperature management, which has been associated with improved neurologic outcomes after cardiac arrest.17–18 Altogether, ECPR is expected to increase in use and may allow cardiac arrest patients to have improved survival with better neurologic outcomes.3 However, acute brain injury and long-term neurological outcomes will be key factors in driving the outcome research of ECPR.8 Therefore, further research is warranted to understand the impact of different risk factors such as neurological complications during ECMO support.
Limitations
Our study has several limitations. Our study cohort did not have a control group of non-WLST patients: we focused only on those who had the primary reason for death cited as being due to family request, as this was the only way to study these patients in the ELSO registry and was specifically recommended by the ELSO organization. We do not know if the other reasons listed may have also prompted families and care teams to initiate WLST. WLST is often a complex decision, it is possible other reasons listed as cause of death may have prompted families and care teams to initiate WLST. We did not differentiate between out-of-hospital arrests and other cardiac arrests prior to initiation of ECPR, which is an important within group difference. The data are sourced from a voluntary registry, so compliance and accuracy are unknown. The ELSO registry includes brain death as a complication; in clinical practice brain death is considered equivalent to cardiopulmonary death and therefore mortality. There is insufficient data to understand when these patients were declared brain dead or what stage of testing they were when with regards to the WLST decision. As it is considered a complication in the registry, it was included in this study as a neurologic complication and these patients were not excluded. Multiple comparisons in the univariable analysis may have found some associations by chance and the statistical significance may not correlate to clinical significance, rather an effect of the large sample size. We made a careful effort to address this limitation by pre-selecting covariates that have biological and mechanistical plausibility for inclusion in the final models, acknowledging there are sparse data to guide us on the variable selection. Finally, the retrospective nature of our review precludes evaluation of causality. In the future, prospective trials able to account for the complexity of factors that influence WLST decisions and have an adequate control group are needed to further understand this phenomenon in this group of patients.
Conclusions
In this study of early WLST in ECPR, more than half of ECPR patients experienced WLST within 72 hours. The patients with early WLST had worse markers of severe critical illness at 24 hours compared to those with routine WLST. Early WLST was associated with higher rates of all complications other than infections. In light of recent guidelines and trials of cardiac arrest and ECPR, it would be prudent to limit WLST in ECPR patients within the first 72 hours. Further research should include an appropriate control group to better adjust confounders for ECPR-associated death and focus on prognostication.
Footnotes
Conflict of Interest: None
References
- 1.Richardson ACS, Mattheu S, Bailey M, et al. : ECMO cardio-pulmonary resuscitation (ECPR), trends in survival from an international multicentre cohort study over 12-years. Resuscitation 2017; 112:34–40. [DOI] [PubMed] [Google Scholar]
- 2.Thiagarajan RR, Barbaro RP, Rycus PT, et al. : Extracorporeal life support organization registry international report 2016. ASAIO J 2017; 63:60–7. [DOI] [PubMed] [Google Scholar]
- 3.Beyea MM, Tillmann BW, Iansavichene AE, et al. : Neurologic outcomes after extracorporeal membrane oxygenation assisted CPR for resuscitation. Resuscitation 2018;146–158. [DOI] [PubMed] [Google Scholar]
- 4.Inoue A, Hifumi T, Sakamoto T, et al. : Extracorporeal cardiopulmonary resuscitation for out-of-hospital cardiac arrest in adult patients. J Am Heart Assoc 2020;1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yannopoulos D, Bartos J, Raveendran G, et al. : Advanced reperfusion strategies for patients with out-of-hospital cardiac arrest and refractory ventricular fibrillation (ARREST): a phase 2, single centre, open-label, randomised controlled trial. Lancet 2020; 396:1807–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeMartino ES, Braus NA, Sulmasy DP, et al. : Decisions to withdraw extracorporeal membrane oxygenation support: Patient characteristics and ethical considerations. Mayo Clin Proc 2019; 96:620–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlson JM, Etchill EW, Enriquez CAG, et al. : Population characteristics and markers for withdrawal of life sustaining therapy in patients on extracorporeal membrane oxygenation (ECMO): Withdrawal of ECMO therapy. J Cardiothorac Vascular Anesth 2022; 36:833–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Migdady I, Rice C, Deshpande A, et al. : Brain injury and neurologic outcome in patients undergoing extracorporeal cardiopulmonary resuscitation: A systematic review and meta-analysis. Crit Care Med 2020;48:e611–19. [DOI] [PubMed] [Google Scholar]
- 9.Callaway CW, Donnino MW, Fink EL, et al. : Part 8: Post-cardiac arrest care: 2015 American heart association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2015;132:S465–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wijdicks EFM, Hijdra A, Young GB, et al. : Practice parameter: Prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review) report of the quality standards subcommittee of the American academy of neurology. Neurology 2006;67:203–10. [DOI] [PubMed] [Google Scholar]
- 11.Elmer J, Torres C, Aufderheide TP, et al. : Association of early withdrawal of life-sustaining therapy for perceived neurological prognosis with mortality after cardiac arrest. Resuscitation 2016;102:127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geocadin RG, Buitrago MM, Torbey MT, et al. : Neurologic prognosis and withdrawal of life support after resuscitation from cardiac arrest. Neurology 2006;67:105–8. [DOI] [PubMed] [Google Scholar]
- 13.Matthews EA, Magid-Bernstein J, Presciutti A, et al. : Categorization of survival and death after cardiac arrest. Resuscitation 2017;114:79–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dominguez-Gil B, Ascher N, Capron AM, et al. : Expanding controlled donation after the circulatory determination of death: statement from an international collaborative. Intensive Care Med 2021;47:265–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haas NL, Coute RA, Hsu CH, et al. : Descriptive analysis of extracorporeal cardiopulmonary resuscitation following out-of-hospital cardiac arrest- An ELSO registry study. Resuscitation 2017;119:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.May TL, Ruthazer R, Riker RR, et al. : Early withdrawal of life support after resuscitation from cardiac arrest is common and may result in additional deaths. Resuscitation 2019; 139:308–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernard SA, Smith K, Cameron P, et al. : Rapid Infusion of Cold Hartmanns (RICH) Investigators. Induction of therapeutic hypothermia by paramedics after resuscitation from out-of-hospital ventricular fibrillation cardiac arrest: a randomized controlled trial. Circulation 2010;122:737–42. [DOI] [PubMed] [Google Scholar]
- 18.Nielson N, Wetterslev J, Cronbert T, et al. : Targeted temperature management at 33°C versus 36°C after cardiac arrest. N Engl J Med 2013;369:2197–206. [DOI] [PubMed] [Google Scholar]


