Abstract
Background
Viral infections are a proposed possible cause of inflammatory central nervous system (CNS) demyelinating diseases, including multiple sclerosis (MS), neuromyelitis optica spectrum disorder (NMOSD), and myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD). During the past 2 years, CNS demyelinating events associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection have been reported, but causality is unclear.
Objective
To investigate the relationship between CNS demyelinating disease development and exacerbation with antecedent and/or concurrent SARS-CoV-2 infection.
Methods
A systematic literature review of all publications describing either a new diagnosis or relapse of CNS demyelinating diseases (MS, NMOSD, MOGAD) in association with SARS-CoV-2 infection was performed utilizing PRISMA guidelines. Descriptive statistics were used for data analysis, using a case analysis approach.
Results
Sixty-seven articles met the inclusion criteria for the study. Most of the reported cases of NMOSD (n = 13, 72.2% of reported cases) and MOGAD (n = 27, 96.5% of reported cases) were of new disease onset, presenting with typical clinical and radiographic features of these conditions, respectively. In contrast, reported MS cases varied amongst newly diagnosed cases (n = 10, 10.5% of reported cases), relapses (n = 63, 66.4%) and pseudo-relapses (n = 22, 23.2%). The median duration between COVID-19 infection and demyelinating event onset was 11.5 days (range 0–90 days) in NMOSD, 6 days (range−7 to +45 days) in MOGAD, and 13.5 days (range−21 to +180 days) in MS. Most cases received high-dose corticosteroids with a good clinical outcome.
Conclusion
Based upon available literature, the rate of CNS demyelinating events occurring in the setting of preceding or concurrent SARS-CoV-2 infection is relatively low considering the prevalence of SARS-CoV-2 infection. The clinical outcomes of new onset or relapsing MS, NMOSD, or MOGAD associated with antecedent or concurrent infection were mostly favorable. Larger prospective epidemiological studies are needed to better delineate the impact of COVID-19 on CNS demyelinating diseases.
Keywords: COVID-19, multiple sclerosis (MS), neuromyelitis optica spectrum disorder (NMOSD), myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD), diagnosis, relapse, exacerbation
Introduction
Multiple sclerosis (MS), neuromyelitis optica spectrum disorder (NMOSD), and myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD) are immune-mediated inflammatory demyelinating diseases of the central nervous system (CNS). While the cause of these conditions is unknown, it is proposed that an interaction between genetic predisposition and behavioral, environmental, and personal factors contribute to disease development. Among the environmental factors involved, viral infections are considered a possible triggering factor.
Prior studies have shown a higher rate of multiple sclerosis (MS) exacerbation in temporal association with viral infections, especially upper respiratory tract infections caused by influenza A virus and Epstein Barr virus (EBV) (1). EBV has also been proposed as a causal agent in the onset of MS (2, 3). Likewise, preceding infections have been proposed as a possible trigger for the induction of pathogenic mechanisms leading to the development of NMOSD and MOGAD (4–10).
During the past 2 years, neurological complications associated with SARS-CoV-2 infection, the aetiologic agent of the coronavirus disease 2019 (COVID-19), have been reported. Some of these complications are thought to be caused by direct damage to the nervous system as a result of direct viral invasion (11). However, in most cases, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) CSF RNA test is negative, and an immune-mediated mechanism is postulated (12–15). In this latter category, reports of MS, NMOSD, and MOGAD cases presenting either as new diagnoses or disease relapses in temporal association with COVID-19 have been accumulating.
This systematic review aims to summarize the available data regarding the occurrence of new disease onset and disease exacerbation of MS, NMOSD, and MOGAD associated with SARS-CoV-2 infection.
Materials and methods
This systematic literature review was performed utilizing PRISMA guidelines. Electronic searches for published literature were conducted by a medical librarian using Ovid MEDLINE (1946 to present), Embase.com (1947 to present), and Web of Science (1900 to present). The searches were run in December 2021. A search update was run in May 2022.
The search strategy incorporated controlled vocabulary and free-text synonyms for the concepts of multiple sclerosis (MS), neuromyelitis optica spectrum disorder (NMOSD), myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD), relapse, new diagnosis, and COVID-19. The full database search strategies are documented in Appendix 1. No restrictions on language or any other search filters were applied. All identified studies were combined and de-duplicated in a single reference manager (EndNote). The citations were then uploaded into Covidence systematic review software.
The full reference list of all selected papers was screened for additional relevant sources. Publications meeting the purpose of the review that were not identified through the initial electronic search were added manually to the final review. The paper selection and data extraction process were carried out independently by two authors (IL and SN), with a third author available in case of disagreements.
To ensure maximal coverage of the currently available data pertinent for the topic of this review, we included all available case reports, case series, and cohort studies that met the pre-defined case selection criteria, presented either as manuscripts in peer-reviewed scientific journals or as posters or oral presentations in a scientific congress.
Descriptive statistics was used to present the data from reported cases, using a case analysis approach. Cases with missing data points were excluded from the analysis of the missing variable.
Case selection
We included patients of any age with confirmed COVID-19 and case description consistent with a new diagnosis or a relapse of MS, NMOSD, or MOGAD, in accordance with the 2017 revised McDonald criteria for MS (16), the 2015 international consensus diagnostic criteria for NMOSD (17), and the international recommendations on the diagnosis of MOGAD (18), respectively. Patients fulfilling a diagnosis of clinically isolated syndrome (CIS), considered as having a high likelihood of MS, were included as well. A relapse was defined as a clinical episode reflecting a focal or multifocal CNS demyelinating event lasting at least 24 h, in the absence of fever or active infection (16). When such an event was reported during an acute febrile state related to COVID-19, it was regarded as a pseudo-relapse, even when considered a relapse in the original publication.
COVID-19 cases were included if meeting one of the following criteria, as defined by the United States Centers for Disease Control and Prevention and the Infectious Diseases Society of America: (1) clinical symptoms consistent with COVID-19 without laboratory confirmation in the absence of an alternative explanation, (2) nasopharyngeal swab positive for COVID-19 PCR with or without symptoms, or (3) positive COVID-19 serologies with or without symptoms (19, 20).
No assumptions were made regarding the duration between COVID-19 and the onset of neurological manifestations. Missing data was noted as not available.
Exclusion criteria
Cases describing clinical manifestations consistent with demyelinating events of the CNS (i.e., optic neuritis, transverse myelitis, acute disseminated encephalomyelitis, etc.) not fulfilling the diagnostic criteria for MS, NMOSD, or MOGAD as described above, were excluded from this review. Papers reporting a suspected diagnosis of COVID-19 that do not fulfill the diagnostic criteria described above, and papers not available for full-text review were also excluded.
Results
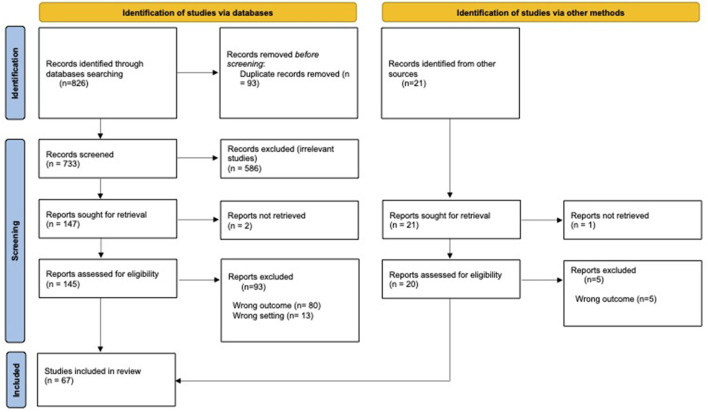
Sixty-seven articles were included in the final review. Twelve articles describe post-COVID-19 NMOSD (21–32), 25 describe post-COVID-19 MOGAD (33–56), and 29 describe post-COVID-19 MS (57–85). One paper describes three patients with post-COVID-19 demyelinating events, of which one is NMOSD, one- MOGAD, and one- clinically isolated syndrome (CIS) (86). Another paper describes various CNS inflammatory diseases, of which three were MOGAD and one—NMOSD (87). A PRISMA flow chart illustrating the article selection process is presented in Figure 1.
Figure 1.
PRISMA flow chart of the article selection process.
Post-COVID-19 NMOSD
Cases of post-COVID-19 NMOSD are summarized in Table 1.
Table 1.
COVID-19 and NMOSD: Cases of para- and post-infectious disease development, relapse or pseudo-relapse.
| References | Number of patients | Diagnosis | Gender | Age | Ethnicity | Clinical presentation | AQP4-IgG status | Time from diagnosis of COVID-19 to clinical onset | Method of COVID-19 diagnosis | CSF SARS-CoV-2 PCR | Treatment of acute attack | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Barone et al. (22) | 1 | New onset | M | 35 | NA | ON + acute myositis | Positive (titer not reported) | 1 month | Clinical criteria + serology | NA | IVMP | Poor recovery of vision, full recovery of muscle symptoms |
| Batum et al. (23) | 1 | New onset | F | 50 | NA | LETM | Positive (titer not reported) | Concomitant | Clinical symptoms | NA | IVIG 0.4 g/kg for 5 days, then PLEX (10 courses every other day) + IVMP (750 mg every other day) | Some improvement in sensory function in the upper limbs, no motor improvement |
| Shaw et al. (29) | 1 | New onset | M | NA *Septuagenarian |
NA | ON + TM | Positive (titer not reported) | 9 days | SARS-CoV-2 PCR | NA | NA | Died due to sepsis and multiorgan failure |
| Chuang and Miskin (24) | 1 | New onset | NA | NA | NA | LETM + APS | Positive (titer not reported) | Neurological symptoms appeared shortly after COVID-19 diagnosis | Clinical symptoms + serology | NA | NA | NA |
| Corrêa et al. (25) | 1 | New onset | F | 51 | Caucasian | Encephalomyeloradiculitis | Positive (titer not reported) | 2 weeks | SARS-CoV-2 PCR | Negative | IVMP 1 gr X5 days followed by PLEX | Remarkable improvement |
| Nasreldein et al. (28) | 1 | New onset | F | 56 | NA | BON+ diencephalic syndrome (lethargy and disorientation) | NA | 2 weeks | SARS-CoV-2 PCR | NA | IVMP 1 gr/day (treatment duration not reported) | Deceased |
| Hooshmand et al. (27) | 1 | New diagnosis *Patient suffered from intractable emesis and visual loss 30 years prior |
M | 49 | NA | ON | Positive (1:10 by FACS assay) | 2 weeks | SARS-CoV-2 PCR | NA | NA | NA |
| Shukla et al. (30) | 1 | New onset | F | 13 | Asian | BON, APS, brainstem syndrome, cerebral syndrome | Negative | NA | Clinical criteria + serology | NA | CS, IVIG, Rituximab | Improved |
| Khair et al. (86) | 1 | New onset *Patient had an undiagnosed ADEM-like demyelinating episode 6 month prior |
F | 14 | NA | Left eye blurring of vision, neck pain, generalized fatigue, and right leg numbness | Positive (titer not reported) | Concomitant | SARS-CoV-2 PCR | NA | NA | NA |
| Ghosh et al. (26) | 1 | New onset | M | 20 | Asian-Indian | APS + LETM | Positive (titer not reported) | 5 days | SARS-CoV-2 PCR | NA | IVMP 1 gr/d for 5 days; RTX | Some improvement of the motor power in all limbs and resolution of the sensory symptoms |
| Jentzer et al. (31) | 1 | New onset | F | 71 | Caucasian | LETM | Positive (titer not reported) | 3 months | SARS-CoV-2 PCR | NA | NA | NA |
| Das et al. (32) | 1 | New onset | F | 16 | ON + LETM | Negative | 4 months | Clinical symptoms + serology | NA | IVMP + oral prednisone taper + RTX | Improvement of vision; outcome of myelopathic symptoms not reported | |
| Aubart et al. (87) | 1 *Also describes 3 MOGAD cases |
New onset | F | 14 | NA | ON | Positive (titer not reported) | NA *Inclusion criteria required positive testing for SARS-CoV-2 infection performed <6 weeks before onset of neurological symptoms or seroconversion following the symptoms with a prior history of SARS-CoV-2 exposure. |
SARS-CoV-2 PCR | NA | IVMP | Complete recovery |
| Apostolos-Pereira et al. (21) | 34 NMOSD patients who developed COVID-19 | Five patients (15%) presented neurologic manifestations (relapse or pseudo exacerbation) during or after SARS-CoV2 infection | NA | 48, 25, 16, 22, 32 | NA | 2- ON, 1-visual acuity worsening in previous ON, 1-TM, 1- not reported | 15 patients- positive; 7- negative; 7- not tested (all patients fulfilled the NMOSD diagnostic criteria). *The antibody status of the five patients who had relapse is not specified. |
In one patient neurological symptoms appeared 7 days after the viral infection, in one- concomitantly with the febrile illness, in the other 3- not reported | 18- SARS-CoV-2 PCR; 16- Clinical symptoms *The method of diagnosis of the five patients who had relapse is not specified. |
NA | 2- oral CS; 2- IVMP; 1- not reported | 3- Good recovery; 1- Poor recovery; 1- Worsening of EDSS from 4.0 to 5.0 |
APS, area postrema syndrome; BON, bilateral optic neuritis; CS, corticosteroids; IVIG, intravenous immunoglobulins; IVMP, intravenous methylprednisolone; LETM, longitudinally extensive transverse myelitis; ON, optic neuritis; PLEX, plasma exchange; RTX, rituximab; TM, transverse myelitis.
Collectively, 13 case reports and one case series describe the occurrence of 18 NMOSD-related clinical events in the context of COVID-19 (21–32, 86, 87). Eight patients were females, four were males, and in six cases the patients' sex was not reported. The mean age was 33.24 ± 18.5 years.
Ten case reports describe the onset of newly diagnosed NMOSD in people without previous neurological disease (22–26, 28–30, 32, 87). Two case reports describe people with previously undiagnosed neurological disease who then presented with a second clinical manifestation in temporal association to COVID-19, leading to an NMOSD diagnosis (27, 86). In one case, the aquaporin-4 antibodies (AQP4 Abs) were retrospectively found to be positive in a stored serum sample drawn 11 months before SARS-CoV-2 infection and more than a year before the clinical onset of NMOSD (31). Apostolos-Pereira et al. report a series of 34 NMOSD patients who developed COVID-19. Five of these patients (15%) developed neurological manifestations that were regarded as relapse or pseudo-relapse during or after SARS-CoV2 infection (21).
In 10 case reports, AQP4-IgG Abs were positive (22–27, 29, 31, 86, 87). In two case reports the AQP4 abs were negative (30, 32) and each fulfilled the diagnostic criteria for seronegative NMOSD. In one report, AQ4 serostatus was not reported (28). In the case series by Apostolos-Pereira et al., 15 patients tested positive for the AQP4-IgG Abs, 7 tested negative, and in 7 the antibody testing was not available (all patients fulfilled the NMOSD diagnostic criteria). The AQP4 antibody status of the five patients who had a relapse was not specified (21).
Neurological symptoms appeared after a median of 11.5 days (range 0–90 days) from COVID-19 diagnosis. In all cases, COVID-19 symptoms preceded the occurrence of neurological symptoms.
Treatment consisted of corticosteroid (CS) monotherapy in seven cases (21, 22, 28, 87), CS + rituximab in two cases (26, 32), intravenous methylprednisolone (IVMP)+ intravenous immunoglobulin (IVIG)+plasma exchange (PLEX) in one case (23), CS +IVIG+ rituximab in one case (30), and CS + PLEX in one case (25). In the remaining six cases, the treatment regimen was not reported (21, 24, 27, 29, 86). A favorable outcome (i.e., improvement of neurological symptoms) was reported in eight patients (21, 25, 26, 30, 32, 87), while poor neurological outcome (i.e., worsening of neurological disability) was reported in four patients (21–23). Two patients deceased due to systemic complications (28, 29). The clinical outcome was not reported for the remaining four patients (24, 27, 31, 86).
Post-COVID-19 MOGAD
Post-COVID-19 MOGAD cases are summarized in Table 2.
Table 2.
COVID-19 and MOGAD: Cases of para- and post-infectious disease development or exacerbation.
| References | Number of patients | Diagnosis | Gender | Age | Ethnicity | Clinical presentation | Method of COVID-19 diagnosis | CSF SARS-CoV-2 PCR | Time from diagnosis of COVID-19 to clinical onset | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Zhou et al. (44) | 1 | New onset | M | 26 | Hispanic | BON +TM | SARS-CoV-2 PCR | Negative | A few days | IVMP 1 gr X 5d followed by oral prednisone taper | Rapid improvement in vision, outcome of myelopathic symptoms not reported |
| Ide et al. (35) | 1 | New onset | F | 24 | NA | ON+ TM (diagnosed as ADEM d/t additional brain lesions) | SARS-CoV-2 PCR | Negative | 3 weeks | IVMP 1 gr X5 days followed by prednisolone taper | Visual symptoms Improved spontaneously; other symptoms improved after treatment |
| Khan et al. (36) | 1 | New onset | M | 11 | NA | BON | SARS-CoV-2 PCR | NA | 4 days | IVMP + prednisone taper | Improved vision |
| Kogure et al. (37) | 1 | New onset | M | 47 | Asian | ON (clinically unilateral, but bilateral optic nerve enhancement on MRI) | SARS-CoV-2 PCR | Negative | Concomitant | IVMP 1 gr X 3 days + prednisone taper | Rapid improvement in pain and vision |
| Pinto et al. (40) | 1 | New onset | F | 44 | NA | CNS inflammatory vasculopathy | SARS-CoV-2 PCR | Negative (repeated twice) | 7 days | IVMP 1 gr X5 days followed by oral prednisolone 60 mg/d, + PLEX | Rapid clinical improvement |
| Woodhall et al. (43) | 1 | Relapse | F | 39 | NA | ON | SARS-CoV-2 PCR | NA | 6 days | IVMP 1 g/day for 5 days followed by five cycles of PLEX | Partial improvement |
| Sawalha et al. (41) | 1 | New onset | M | 44 | Hispanic | BON | SARS-CoV-2 PCR | NA | One week | IVMP 1 g/day for 5 days followed by prednisone taper | Complete recovery in one eye, remarkable recovery but not complete in the other eye |
| Khair et al. (86) |
1 *Also describes one case of NMOSD and one CIS |
New onset *Concomitant NMDAR Abs |
F | 16 | NA | Headache, blurred vision, leg numbness, and weakness. | SARS-CoV-2 PCR | NA | Concomitant | NA | NA |
| Lindan et al. (38) | 1 | New onset | M | 4 | NA | Seizures, facial palsy, and four limb dysfunction | SARS-CoV-2 serology | NA | NA | IVMP | Marked improvement |
| Peters et al. (39) | 1 | New onset | M | 23 | NA | Headaches and dysesthesia followed by seizures, inattention and cognitive slowing | SARS-CoV-2 PCR | Negative | Initial neurological symptoms developed concomitantly with positive COVID-testing; further symptoms developed over the next 4 weeks | IVMP 1 gr X5 days followed by oral steroid taper | Gradual clinical and radiological resolution |
| Vraka et al. (42) | 1 *Describes another case of encephalopathy with negative MOG-IgG |
New onset | F | 13 months | NA | ADEM | SARS-CoV-2 PCR | Negative | Concomitant | Steroids | Gradual improvement |
| Ahsan et al. (33) | 1 | New onset | F | 7 | NA | ADEM | SARS-CoV-2 serology | NA | Neurological symptoms preceded COVID-19 by a week | IVIG 2 g/kg over 3 days | Gradual improvement (almost returned to her baseline with mild dysarthria) |
| de Ruijter et al. (34) | 1 | New onset | M | 15 | Caucasian | BON | Clinical criteria | NA | A few weeks | IVMP 1 gr/d for 3 days | Improved (almost full recovery) |
| Durovic et al. (47) | 1 | New onset | M | 22 | NA | Encephalitis | SARS-CoV-2 PCR | Negative | 3 days | IVMP 1 gr/d for 5 days | Complete clinical and radiological resolution |
| Jumah et al. (46) | 1 | New onset *Concomitant HHV6 infection |
M | 61 | NA | LETM | SARS-CoV-2 PCR + serology | Negative | 1 week | IVMP 1 gr/d for 5 days + prednisone taper + Gancyclovir, PLEX (7 sessions) | Marked improvement |
| Sinha et al. (45) | 1 | New onset | F | 11 | NA | BON | SARS-CoV-2 PCR | NA | 3 days | IVMP 1 gr/d + IVIG 2gr/kg for 5 days + prednisone taper | Improved |
| Yang et al. (55) | 1 | New onset | M | 57 | NA | LETM | SARS-CoV-2 PCR | NA | 3 weeks | IVIG × 4 days, then five sessions of PLEX, then IVMP 1 gr/d for 5 days + steroid taper | NA |
| Assavapongpaiboon et al. (50) | 1 | New onset | F | 35 | Thai | BON | SARS-CoV-2 PCR | Negative | 1 week | IVMP 1 gr/d for 5 days + steroid taper | Improved |
| Dias da Costa et al. (52) | 1 | New onset | M | 31 | NA | LETM | SARS-CoV-2 serology | Negative | 21 days | IVMP 1 gr/d for 5 days + steroid taper | Almost complete resolution of motor and sensory symptoms, mild urinary symptoms |
| Doukas et al. (53) | 1 | New onset | M | 40 | NA | TM | SARS-CoV-2 serology | NA | 12 days | IVMP 1 gr/d for 5 days + steroid taper | Gradual improvement |
| Jossy et al. (54) | 1 | New onset | M | 38 | NA | ON | SARS-CoV-2 serology | NA | 6 weeks | IVMP 1 gr/d for 3 days + steroid taper | Complete resolution |
| Rojas-Correa et al. (49) | 1 | New onset | M | 69 | NA | BON | Clinical criteria | Negative | 45 days | IVMP 1 gr/d for 5 days + steroid taper | Improved |
| Sardar et al. (48) | 1 | New onset | F | 38 | NA | BON *Diagnosed with concomitant idiopathic intracranial hypertension |
Clinical criteria | NA | 2 weeks | IVMP for 5 days, PLEX, IVIG for 5 days Acetazolamide |
Significant improvement |
| Žorić et al. (51) | 1 | New onset | M | 63 | NA | ON | SARS-CoV-2 serology | NA | 4 weeks | IVMP 1 gr/d for 5 days + steroid taper | Improved |
| Aubart et al. (87) | 3 *Also describes one case of NMOSD |
New onset | 2M, 1F | 1.5, 4, 10 | NA | ADEM | SARS-CoV-2 PCR or serology | NA | NA *Inclusion criteria required positive testing for SARS-CoV-2 infection performed <6 weeks before onset of neurological symptoms or seroconversion following the symptoms with a prior history of SARS-CoV-2 exposure. |
2- IVMP, 1- not treated | Complete recovery |
| Cay-Martínez et al. (56) | 1 | New onset | F | 7 | NA | ADEM | SARS-CoV-2 serology | Negative | 1 week | IVMP + PLEX + IVIG + oral prednisone taper | Resolution of facial and upper extremity weakness, mild improvement in leg weakness |
ON, optic neuritis; BON, bilateral optic neuritis; TM, transverse myelitis; LETM, longitudinally extensive transverse myelitis; ADEM, acute demyelinating encephalomyelitis; IVMP, intravenous methylprednisolone; IVIG, intravenous immunoglobulins; PLEX, plasma exchange.
A total of 28 cases of MOGAD occurring in temporal relation to COVID-19 have been described (33–47, 56, 86, 87). Seventeen were males, and 11 were females. The mean age was 28.1 ± 20.3 years (range 1–69 years; 10 patients <18 years old). The median time between COVID-19 and neurological symptoms was 6 days (range−7-+45 days). In one case, the neurological symptoms preceded the diagnosis of COVID-19 by 1 week (33). In four cases, neurological symptoms developed concomitantly with COVID-19 (37, 39, 42, 86), and in the remaining 23, COVID-19 diagnosis preceded the onset of neurological symptoms. In 27 (96.5%), a new diagnosis of MOGAD was made in people without prior neurological disease. In one case, a relapse occurred in a patient with known MOGAD (43). The MOG-IgG antibodies were positive in all cases. In one case, NMDAR antibodies and MOG-IgG antibodies were detected concomitantly (86). In another case, human herpes virus 6 (HHV6) PCR was also positive (46). Eighteen patients were treated with CS alone (intravenous methylprednisolone followed by oral prednisone taper, n = 15; IVMP alone, n = 2; details of steroid regimen were not described, n = 1) (42). One patient was treated with intravenous immunoglobulins (IVIG) alone (33). One patient received IVMP+ IVIG (45), three received IVMP + PLEX (40, 43, 46), and three received IVMP+ PLEX+ IVIG (48, 55, 56). One patient was not treated (87). The treatment regimen was not described for one patient (38). Clinical improvement was reported for 26 patients (93%).
Post COVID-19 MS
Table 3 illustrates MS cases occurring in the context of COVID-19.
Table 3.
COVID-19 and MS: Cases of para- and post-infectious disease development, relapse or pseudo-relapse.
| References | Number of patients | Diagnosis | Gender | Age | Ethnicity | Clinical presentation | Method of COVID-19 diagnosis | DMT before COVID-19 infection | Time from diagnosis of COVID-19 to clinical onset | CSF SARS-CoV-2 qPCR | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case reports | ||||||||||||
| Moore et al. (77) | 1 | New onset | M | 28 | NA | Brainstem syndrome (vertigo, oscillopsia, diplopia, facial numbness) | Clinical criteria | None | Neurological symptoms appeared 10 days after COVID-19 symptoms | Negative | IVMP 1 g/day for 3 days followed by prednisone taper | Improved |
| Pignolo et al. (79) | 2 | 1 new onset, 1 relapse | M, F | 21,52 | NA | Hand paresthesia and facial nerve palsy; Right-sided weakness and clumsiness | Clinical criteria, serology | None (new onset) Cladribine (relapse) |
MS onset a few days after COVID-19; MS relapse 2 months after COVID-19 | Negative in one case (new onset), NA in the other | IVMP 1 g/day for 5 days | Relapse- fully resolved, new onset disease- partial recover |
| Fragoso et al. (66) | 1 | New onset | F | 27 | Caucasian | Left side dysesthesia | Clinical criteria | None | 6 months | Negative | NA | NA |
| Wildemann et al. (81) | 1 | MS relapse and Takotsubo cardiomyopathy | F | 39 | NA | Brainstem syndrome (dizziness, diplopia, dysarthria, dysphagia) | SARS-CoV-2 PCR | DMF | 10 days | Negative | IVMP 2 gr/day for 5 days + PLEX (seven courses) | Slow improvement |
| Yavari et al. (82) | 1 | New onset | F | 24 | NA | Diplopia, facial nerve palsy, fingertips paresthesia | SARS-CoV-2 PCR | None | 1 month | NA | IVMP 1 gr/day for 4 days | Improved |
| Palao et al. (78) | 1 | New onset | F | 29 | NA | ON | SARS-CoV-2 serology | None | 2–3 weeks | Negative | IVMP 1 gr/day (treatment duration not reported) followed by oral prednisolone taper | Improved |
| Florae et al. (65) | 1 | Relapse *3 weeks post-partum |
F | 40 | Caucasian | Right sided paresthesia and motor disability | SARS-CoV-2 PCR | None | No systemic symptoms, tested positive on swab PCR upon admission | NA | IVMP 1 gr/d for 3 days; hydroxychloroquine 4 g/day, lopinavir/ritonavir 4 tablets/day for 10 days, and azithromycin 1 g/day, for 3 days | Remission of neurological deficit after 2 weeks |
| Khair et al. (86) | 1 | CIS | M | 8 | NA | Double vision, worsening fine motor skills, and ataxic gait | Clinical criteria | None | 1 month | NA | NA | NA |
| Kataria et al. (69) | 3 | Pseudo-relapse | 2M, 1F | 65, 52, 69 | NA | Fatigue, general weakness | SARS-CoV-2 PCR | GA | Concomitant | NA | Only COVID-19 management | Improved to baseline status |
| Barzegar et al. (57) | 1 | Relapse | F | 42 | NA | Muscle aches, gait difficulty, sensory disturbances, and weakness on the right side | SARS-CoV-2 PCR | Fingolimod | Neurological symptoms preceded COVID-19 symptoms by 6 days | NA | Initially IVMP 1 gr/d for 3 days; then azithromycin, ceftriaxone, hydroxychloroquine, oseltamivir, and piperacillin/tazobactam | Gradual improvement |
| Domingues et al. (63) | 1 | CIS | F | 42 | NA | Left side paresthesia | Clinical criteria | None | Concomitant | Positive | No steroids, COVID-19 management not detailed | Full recovery |
| Jaisankar et al. (67) | 1 | Pseudo-relapse | M | 45 | Caucasian | Dysphagia, altered mental status, general deterioration | SARS-CoV-2 PCR | None | COVID-19 diagnosed 2 weeks prior to neurological deterioration. *Also diagnosed with acute renal failure, anemia, PE and sepsis. | NA | IVMP (dose and duration not reported). Received fluids, packed red blood cells and transfusions, anticoagulants, ciprofloxacin | Ongoing disability |
| Karsidag et al. (68) | 2 | Two patients with new-onset MS (*+1 ADEM) | 1F, 1M | 42, 32 | NA | Jaw and left facial pain and paresthesia; numbness in left jaw | Clinical criteria | None | 2–3 weeks; 4 months | 1 Negative, 1 Positive | 1-IVMP 1 gr/d for 7 days; 1- IVMP 1 gr/d for 10 days | Improved |
| Möhn et al. (76) | 1 | Relapse | M | 42 | NA | Gait and limb ataxia | SARS-CoV-2 PCR | Teriflunomide | Neurological symptoms preceded COVID-19 by 3 weeks | NA | IVMP 1 gr/d for 4 days | Initial improvement, then worsened concomitantly to COVID symptoms |
| Finsterer (84) | 1 | Relapse | F | 27 | NA | TM | Clinical criteria | IFNβ-1a | 2 weeks | NA | CS | Slow improvement |
| Observational case series and cohort studies of MS patients | ||||||||||||
| Khurana et al. (71) | 5 RRMS patients | 1 relapse | 3F, 2M | Mean (SD) age 35.60 (13.94) | NA | NA | SARS-CoV-2 PCR | Treated with DMT, type not specified | NA | NA | NA | NA |
| Maghzi et al. (73) | 3 RRMS, 1 SPMS, 1 RIS | No relapses | 3M, 2F | Mean 53.6 | NA | NA | SARS-CoV-2 PCR | Teriflunomide | NA | NA | NA | NA |
| Mantero et al. (74) | 7 RRMS patients | No relapses. 1 pseudo-relapse | 5F, 2M | Mean 35.9 ± 11.4 | NA | Left hand paresthesia | Clinical criteria | DMF | Concomitant | NA | NA | NA |
| Conway et al. (60) | 72 RRMS, 21 SPMS, 8 PPMS, 2 CIS, eight related disorders | 2/111 (1.8%) relapses, 19 (17.2%) pseudo-relapses and 27 (24.3%) with worsening of pre-existing MS symptoms. Five patients (4.5%) had new MRI lesions on T2 or T1Gd scans | 85 females (77%) | Mean age 49 (SD 12.2) years | NA | NA | Clinical criteria | NA | NA | NA | NA | |
| Chyzhyk et al. (59) | 17 relapsing MS patients | No clinical or radiological signs of MS disease activity During 6 months of observation |
4M, 13 F | Mean age 38 ± 7.6 years | NA | NA | Clinical criteria | Treated with DMT, type not specified | NA | NA | NA | |
| Czarnowska et al. (61) | 426 individuals with MS | 27 patients (6.34%) had a relapse at 3 months after the initial infection | 142M, 284F | Mean 40.27 ± 10.12 | NA | Symptoms during the relapse were as following: pyramidal track symptoms (16 people), cerebellar symptoms (eight people), sensory deficit (four people), brainstem symptoms (3 people), urinary incontinence (1 person) | SARS-CoV-2 PCR (n = 361), SARS-CoV-2 serology (n = 24) or combination of tests | Interferon beta (n = 77); GA (n = 43); DMF (n = 171); teriflunomide (n = 34); fingolimod (n = 16); natalizumab (n = 29); ocrelizumab (n = 29); cladribine (n = 7); alemtuzumab (n = 1); mitoxantrone (n = 1); ozanimod (n=12); other (n = 12); none (n = 4) *Type of DMT in patients who relapsed not specified |
The mean time for relapse occurrence after the SARS-CoV-2 infection was 43 days | All treated with IVMP 3–5 gr | NA | |
| Michelena et al. (75) | 41 MS patients with confirmed COVID-19 diagnosis | 25 patients (61%) reported neurological worsening, three patients (7.7%) met criteria for relapse | 24 F, 17M | Mean 42.9 years (SD 11.3) | NA | Motor (n = 12) Sensory (n = 10) Visual (n = 7) Balance disorders (n = 3) Memory (n = 6) Fatigue (n = 13) | SARS-CoV-2 PCR | 35 treated with DMTs (23-oral DMTs, 4-injectables, 8-monoclonal antibodies) | Concomitant (n = 16), within the 1st month (n = 5), beyond the 1st month (n = 4) | NA | CS (type, dose, and duration not reported) | NA |
| Luetic et al. (72) | 17 RRMS and 1 RIS patients | No MS relapses occurred during or after COVID-19 course. | 13 F, 5M | Mean 41.2 ± 12.6 | NA | NA | 11- SARS-CoV-2 PCR; 8- Clinical criteria | Teriflunomide | NA | NA | NA | NA |
| Etemadifar et al. (64) | A retrospective cohort study comparing the risk of relapse in RRMS patients with (n = 56) and without COVID-19 (n = 69) *Within 6 months from COVID |
4 patients in the MS-COVID-19 group (7.14%) had a relapse compared to 18 patients in the RRMS without COVID-19 group (26.09%). Incidence rate ratio: 0.275; p = 0.026 | COVID-19 group: 40 F/15 M; non COVID-19 group: 62 F/ 7 M | COVID-19 group: 36.89 (±9.06); non-COVID-19 group: 36.19 (±8.97) | NA | 2-limb paresthesia, 1-diplopia, 1-lower extremity weakness | SARS-CoV-2 PCR | Teriflunomide (n = 3); fingolimod (n = 9); DMF (n = 22); AZA (n = 5); Interferon ß 1b (n = 3; Interferon ß 1a (n = 6); GA(n = 3); RTX (n = 3); NTZ (n = 2) *Type of DMT in patients who relapsed not specified |
Only reported that the 4 relapses in COVID-19 confirmed patients occurred after COVID-19 diagnosis | NA | NA | NA |
| Etemadifar et al. (83) | A prospective-retrospective hybrid single center cohort study comparing the risk of relapse during 1 year pre- and post-COVID-19 period in 53 RRMS patients *Some patients may have been included in the previous study by the same first author |
11 patients (20.75%) in the post-COVID-19 period and 16 patients (30.19%) in the pre-COVID-19 period experienced a relapse (p = 0.30) | 45 F, 8M | Mean 38.42 (SD 8.77) | NA | NA | Clinical criteria or SARS-CoV-2 PCR *Number of patients in each group not specified |
IFN beta (n = 4); DMF (n = 21); teriflunomide (n = 1); GA (n = 1); fingolimod (n = 12); RTX (n = 9); AZA (n = 2); none (n = 3) | NA | NA | NA | NA |
| Barzegar et al. (58) | A retrospective observational study comparing the relapse rate among 41 MS patients with confirmed COVID-19 during a pre-defined at-risk period (from 2 weeks before to 5 weeks after COVID-19) and the previous 2 years | Five patients had a relapse during the defined at-risk period. Other two patients had neurological worsening that did not meet clinical relapse definition. Increased relapse rate during the at-risk period (RR: 2.566, 95% CI: 1.075–6.124, P = 0.034) | 31 females, 10 males | Mean 35.10 ± 9.20 | NA | NA | SARS-CoV-2 PCR | NA | All relapses occurred after the onset of COVID-19 (Mean 3.2 weeks, range 1–5 weeks) | NA | NA | |
| Paybast et al. (85) | 202 MS patients followed for 1 year | 25 patients developed COVID-19, of which 1 (4%) had a relapse | 164F, 37M | 38.09 ± 10.44 | NA | TM | SARS-CoV-2 PCR | NA | NA | NA | PLEX | NA |
| General observational studies | ||||||||||||
| Sandoval et al. (80) | 13 pediatric patients with confirmed COVID-19 and new-onset neurological manifestations | 1 patient with new-onset multifocal demyelination consistent with MS | M | 14 | ON, sixth nerve palsy, asymmetric paraparesis | SARS-CoV-2 PCR | None | No systemic symptoms, tested positive on swab PCR upon admission | NA | IVMP (dose and duration not reported) | Significant clinical improvement) | |
| Khedr et al. (70) | 439 patients with confirmed/probable COVID-19 | 2 MS relapse (among those with probable COVID-19, n = 62) | NA | NA | NA | NA | SARS-CoV-2 PCR | NA | NA | NA | NA | NA |
| Dhillon et al. (62) | Case series of 29 inpatients presented with COVID-19 and neurological disorders, 2 MS patients | 1 MS relapse | M | 56 | White | Worsening of limb weakness and dysarthria | SARS-CoV-2 PCR | NA | NA | NA | NA | Ongoing disability |
CIS, clinically isolated syndrome; DMT, disease modifying therapy; ON, optic neuritis; TM, transverse myelitis; IVMP, intravenous methylprednisolone; CS, corticosteroids; PLEX, plasma exchange.
Fifteen case reports and case series reported the occurrence of MS relapse/pseudo-relapse or the onset of a first demyelinating event consistent with MS or CIS in 19 patients (57, 63, 65–69, 76–79, 81, 82, 84, 86). Twelve observational case series and cohort studies documented the occurrence of relapses or pseudo-relapses among patients with a known diagnosis of MS and confirmed diagnosis of COVID-19 (58–61, 64, 71–75, 83, 85). Collectively, 54 relapses and 20 pseudo-relapses were reported in 804 patients (6.7 and 2.5%, respectively).
Three observational cohort studies of COVID-19 patients with various neurological manifestations reported MS cases (62, 70, 80). Overall, one case of multifocal demyelination consistent with MS and three MS relapses were reported among 481 patients (0.8%).
Considering all the reported cases, a total of 73 demyelinating events consistent with CIS/MS (10 new diagnoses and 63 relapses) and 22 events defined as pseudo-relapse were reported in 1,305 people (5.6 and 1.7%, respectively). Of these 73 events, 11 were in females, 10 in males, and sex was not reported in the remaining 64 cases. The mean age was 38.45 ± 15.93 years. Most relapses or first demyelinating events consistent with MS/CIS occurred after the onset of COVID-19. However, in two cases neurological symptoms preceded the diagnosis of COVID-19 by 6 and 21 days, respectively (57, 76). The median time from COVID-19 diagnosis to demyelinating event onset was 13.5 days (range−21-180 days).
Nine hundred eighty-six people with a known MS and COVID-19 diagnosis were reported. Of these, 624 (63.3%) were treated with various disease-modifying treatments (DMTs), 14 (1.5%) were not treated, and for 165 (16.7%), the information on DMTs was not reported. Twenty-one MS patients treated with DMTs (3.4%) had a relapse in temporal association with COVID-19. Five MS patients treated with DMTs (0.8%) had a pseudo relapse, and 144 (23.1%) did not have neurological worsening. The remaining 455 MS patients treated with DMTs (73%) were reported in larger cohorts in which some people were not treated. The information regarding relapses in these cohorts was not stratified between treated and untreated patients (61, 75).
Most MS/CIS cases received treatment with IVMP 1 gram for 3–5 days (57, 61, 65, 68, 76, 77, 79, 80, 82) and had a favorable outcome (57, 65, 68, 77–82). Treatment of pseudo-relapses was primarily focused on COVID-19 management, with return to baseline neurological status upon infection recovery (63, 69).
Discussion
This systematic review summarizes the currently available data on the occurrence of demyelinating CNS events in the context of COVID-19. As noted, the vast majority of NMOSD and MOGAD cases represent newly diagnosed cases presenting with the typical clinical, radiological, and laboratory findings associated with these two disorders. In contrast, the MS cases described vary between newly diagnosed cases, relapses, and pseudo-relapses. The patients' age of diagnosis in the three disease groups was relatively similar to the age of diagnosis reported in the literature for non-COVID-19 related cases. The clinical presentations and treatment approach were also similar to non-COVID-19 related cases (for further details, please see Tables 1–3).
Several mechanisms involved in the pathogenesis of demyelinating events in the context of SARS-CoV-2 infection have been proposed. These may be related to either direct viral neurotropism or induction of aberrant immune response. The neurotrophic features of the Coronavirus family have been previously reported for the Middle East respiratory syndrome coronavirus (MERS-COV) and SARS-COV-1, and similar evidence has been occasionally reported for SARS-CoV-2 (88–91). However, the fact that the SARS-CoV-2 PCR test in the CSF was negative in many of the reported cases (25, 35, 37, 39, 40, 42, 44, 66, 68, 77–79, 81) would argue against this mechanism of direct pathogenicity. Conversely, some evidence favors the theory of para-infectious or post-infectious immune-mediated etiology. In fact, SARS-CoV-2 infection leads to hyperactivation of pro-inflammatory T cells resulting in increased levels of inflammatory cytokines and chemokines (92) and decreased regulatory T cells to impair immune response (93). The resulting pro-inflammatory hyperimmune state may activate specific immune-mediated mechanisms resulting in CNS inflammation and damage. The favorable response to immunotherapy in the majority of the reported cases appears to support this theory.
The distinction between relapse and pseudo-relapse may not always be straightforward. According to the 2017 McDonald criteria, a relapse should be defined in the absence of fever or acute infection; hence, new or worsening neurological symptoms developed during a febrile illness or in the presence of acute infection in a patient with a known diagnosis of MS should not be defined as true relapse, but rather regarded as a pseudo-relapse. However, there may be situations where the diagnosis of true relapse should still be considered even in the context of acute infection. For example, a true relapse should be considered if the onset of new symptoms is associated with clinical signs that can be attributed to a specific anatomical localization that has not been previously described or correlated with the presence of a new symptomatic MRI lesion. Following this rationale, a few of the described clinical worsening in MS cases were felt to be better classified as pseudo-relapses (67, 69).
Prior studies propose that MS relapses in temporal association with viral infections occur between 1 and 2 weeks before infection to 3–5 weeks after (94–98). Andersen et al. and Correale et al. reported that the highest frequency of relapses and infection-related MS attacks occurred during the first 2 weeks after infection onset (94). In the series reported by Sibley et al., the median time between the onset of infection and occurrence of MS exacerbation was 8 days (95). Buljevac et al. reported a mean duration of 9.5 days between the onset of infection and clinical MS exacerbation. Both Buljevac et al. and Correale et al. also compared the relapse rate ratio during different time intervals and found that the highest rate ratio was observed from weeks 1 to 4, while the exacerbation rate ratio for weeks 3–5 was lower and not statistically significant compared to the non-at risk period (97, 98). Considering these data, MS relapses occurring more than 4–5 weeks from an infection are probably not related to the prior infectious insult. Therefore, MS cases that occurred >6 weeks from COVID-19 (64, 66, 68, 79, 83), although included in this review in order to provide a comprehensive review of available data, are thought to be more likely coincidental and not related to the preceding infectious insult. Likewise, the relation between SARS-CoV-2 infection and the NMOSD and MOGAD cases developing >6 weeks after the infection (31, 32, 49, 54) remains uncertain. The case of MOGAD occurring in temporal association to both HHV6 and COVID-19 infection (46) may also confound the association between COVID-19 and MOGAD.
The use of disease-modifying therapies (DMTs) may be associated with an increased risk of viral and bacterial infections. Early in the course of the COVID-19 pandemic, this notion led to significant concerns regarding COVID-19 outcomes for people with neuroimmunological diseases. While some reports described a less favorable COVID-19 course in people treated with B-cell depleting agents, the use of other DMTs does not seem to be associated with such an increased risk (99–101). Another aspect of interest is whether the efficacy of DMTs is maintained during the pandemic. However, the currently available data is not sufficient to answer this question. While relapses were reported in only 3.4% of MS patients treated with DMTs, information about DMTs use and relapses was available for a relatively small proportion of patients (169/624, 27.1%). The fact that the majority of NMOSD and MOGAD cases reported are of newly diagnoses rather than relapses of previously diagnosed disease, may suggest that the efficacy of immunotherapy during the pandemic is maintained. In the series reported by Apostolos-Pereira et al., 97% of NMOSD patients (33/34) continued their prescribed immunotherapy during the pandemic. The relatively low incidence of neurological exacerbation reported by the authors (5/34, 15%) may further support this theory (21). Still, prospective studies comparing the rate of relapse between COVID-19 patients treated with DMTs and untreated patients are required to answer this question.
The current literature pertaining to the occurrence of demyelinating events in temporal association with COVID-19 is primarily composed of case reports, case series, and relatively small cohort studies. Therefore, while the rate of such events appears low based upon this review, especially considering the high prevalence of SARS-CoV-2 infection, the available data does not permit the determination of whether the rate of CNS demyelinating events (either new onset or true relapse) differs among people with confirmed COVID-19 compared to those who do not contract the infection. Additional questions that remain unanswered at this point are whether there are differences in the severity of demyelinating attacks and the response to acute treatments between demyelinating events occurring in association with COVID-19 and those not associated with the infection.
In conclusion, the rate of CNS demyelinating events occurring in the context of SARS-CoV-2 infection is relatively low given the global prevalence of infection. The clinical outcomes of new-onset or relapsing MS, NMOSD, or MOGAD associated with antecedent or concurrent SARS-CoV-2 infection is mostly favorable. Larger prospective epidemiological studies are needed to better characterize the impact of COVID-19 on CNS demyelinating diseases.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
IL, SN, GM, and MiL contributed to the conception and design of the study. IL and MiL organized the database. IL and SN performed the data collection. IL wrote the first draft of the manuscript. MeL conducted the literature search. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.970383/full#supplementary-material
References
- 1.Oikonen M, Laaksonen M, Aalto V, Ilonen J, Salonen R, Erälinna JP, et al. Temporal relationship between environmental influenza A and Epstein-Barr viral infections and high multiple sclerosis relapse occurrence. Mult Scler. (2011) 17:672–80. 10.1177/1352458510394397 [DOI] [PubMed] [Google Scholar]
- 2.Ramagopalan SV, Valdar W, Dyment DA, DeLuca GC, Yee IM, Giovannoni G, et al. Association of infectious mononucleosis with multiple sclerosis. A population-based study. Neuroepidemiology. (2009) 32:257–62. 10.1159/000201564 [DOI] [PubMed] [Google Scholar]
- 3.Marrodan M, Alessandro L, Farez MF, Correale J. The role of infections in multiple sclerosis. Mult Scler. (2019) 25:891–901. 10.1177/1352458518823940 [DOI] [PubMed] [Google Scholar]
- 4.Graber DJ, Levy M, Kerr D, Wade WF. Neuromyelitis optica pathogenesis and aquaporin 4. J Neuroinflammation. (2008) 5:22. 10.1186/1742-2094-5-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhong X, Zhou Y, Lu T, Wang Z, Fang L, Peng L, et al. Infections in neuromyelitis optica spectrum disorder. J Clin Neurosci. (2018) 47:14–9. 10.1016/j.jocn.2017.10.005 [DOI] [PubMed] [Google Scholar]
- 6.Jarius S, Ruprecht K, Kleiter I, Borisow N, Asgari N, Pitarokoili K, et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 2: epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflammation. (2016) 13:280. 10.1186/s12974-016-0718-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakajima H, Motomura M, Tanaka K, Fujikawa A, Nakata R, Maeda Y, et al. Antibodies to myelin oligodendrocyte glycoprotein in idiopathic optic neuritis. BMJ Open. (2015) 5:7766. 10.1136/bmjopen-2015-007766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramanathan S, Reddel SW, Henderson A, Parratt JD, Barnett M, Gatt PN, et al. Antibodies to myelin oligodendrocyte glycoprotein in bilateral and recurrent optic neuritis. Neurol Neuroimmunol Neuroinflamm. (2014) 1:e40. 10.1212/NXI.0000000000000040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cobo-Calvo A, Ruiz A, Maillart E, Audoin B, Zephir H, Bourre B, et al. Clinical spectrum and prognostic value of CNS MOG autoimmunity in adults: The MOGADOR study. Neurology. (2018) 90:e1858–e69. 10.1212/WNL.0000000000005560 [DOI] [PubMed] [Google Scholar]
- 10.Koga M, Takahashi T, Kawai M, Fujihara K, Kanda T. A serological analysis of viral and bacterial infections associated with neuromyelitis optica. J Neurol Sci. (2011) 300:19–22. 10.1016/j.jns.2010.10.013 [DOI] [PubMed] [Google Scholar]
- 11.Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J, et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. (2020) 94:55–8. 10.1016/j.ijid.2020.03.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gutiérrez-Ortiz C, Méndez-Guerrero A, Rodrigo-Rey S, San Pedro-Murillo E, Bermejo-Guerrero L, Gordo-Mañas R, et al. Miller Fisher syndrome and polyneuritis cranialis in COVID-19. Neurology. (2020) 95:e601–e5. 10.1212/WNL.0000000000009619 [DOI] [PubMed] [Google Scholar]
- 13.Goel K, Kumar A, Diwan S, Kohli S, Sachdeva HC, Ganapathy U, et al. Neurological manifestations of COVID-19: a series of seven cases. Indian J Crit Care Med. (2021) 25:219–23. 10.5005/jp-journals-10071-23723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rahimi K. Guillain-Barre syndrome during COVID-19 pandemic: an overview of the reports. Neurol Sci. (2020) 41:3149–56. 10.1007/s10072-020-04693-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paterson RW, Brown RL, Benjamin L, Nortley R, Wiethoff S, Bharucha T, et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. (2020) 143:3104–20. 10.1093/brain/awaa240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. (2018) 17:162–73. 10.1016/S1474-4422(17)30470-2 [DOI] [PubMed] [Google Scholar]
- 17.Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. (2015) 85:177–89. 10.1212/WNL.0000000000001729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jarius S, Paul F, Aktas O, Asgari N, Dale RC, de Seze J, et al. MOG encephalomyelitis: international recommendations on diagnosis and antibody testing. J Neuroinflammation. (2018) 15:134. 10.1186/s12974-018-1144-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanson KE, Caliendo AM, Arias CA, Hayden MK, Englund JA, Lee MJ, et al. The infectious diseases society of America Guidelines on the Diagnosis of COVID-19: molecular diagnostic testing. Clin Infect Dis. (2021) 2021:ciab048. 10.1093/cid/ciab048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prevention CfDCa . Coronavirus Disease 2019 (COVID-19) 2020 Interim Case Definition. (2020). Available online at: https://ndc.services.cdc.gov/case-definitions/coronavirus-disease-2019-2020-08-05/ (accessed August 5, 2020).
- 21.Apostolos-Pereira SL, Campos Ferreira L, Boaventura M, de Carvalho Sousa NA, Joca Martins G, d'Almeida JA, et al. Clinical features of COVID-19 on patients with neuromyelitis optica spectrum disorders. Neurol Neuroimmunol Neuroinflamm. (2021) 8:1060. 10.1212/NXI.0000000000001060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barone S, Rapisarda L, Manzo L, Mechelli A, Pascarella A, Bruno P, et al. A case of neuromyelitis optica spectrum disorder (NMOSD) and acute myositis following SARS-CoV-2 infection. J Neurol Sci. (2021) 429:119862. 10.1016/j.jns.2021.119862 [DOI] [Google Scholar]
- 23.Batum M, Kisabay Ak A, Mavioglu H. Covid-19 infection-induced neuromyelitis optica: a case report. Int J Neurosci. (2020) 2020:1–7. 10.1080/00207454.2020.1860036 [DOI] [PubMed] [Google Scholar]
- 24.Chuang T-Y, Miskin D. SARS-CoV-2 Associated Neuromyelitis optica (2590). Philadelphia, PA: AAN Enterprises; (2021). [Google Scholar]
- 25.Corrêa DG, de Souza Lima FC, da Cruz Bezerra D, Coutinho AC, Hygino da Cruz LC. COVID-19 associated with encephalomyeloradiculitis and positive anti-aquaporin-4 antibodies: cause or coincidence? Multiple Scler J. (2021) 27:973–6. 10.1177/1352458520949988 [DOI] [PubMed] [Google Scholar]
- 26.Ghosh R, De K, Roy D, Mandal A, Biswas S, Biswas S, et al. A case of area postrema variant of neuromyelitis optica spectrum disorder following SARS-CoV-2 infection. J Neuroimmunol. (2020) 350:577439. 10.1016/j.jneuroim.2020.577439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hooshmand S, Obeidat A, Brod S. Reactivation of Latent Neuromyelitis Optic Secondary to COVID-19 Infection: A Case Report (4562). Philadelphia, PA: AAN Enterprises; (2021). [Google Scholar]
- 28.Nasreldein A, Ibrahem H, Bahie A. Neuromyelitis optica spectrum disorders (NMOSD) attack triggered by COVID-19 infection (a case report). Multiple Scler J. (2020) 2020:77–8. [Google Scholar]
- 29.Shaw VC, Chander G, Puttanna A. Neuromyelitis optica spectrum disorder secondary to COVID-19. Br J Hosp Med. (2020) 81:1–3. 10.12968/hmed.2020.0401 [DOI] [PubMed] [Google Scholar]
- 30.Shukla V, Singh VA, Singh VR, Maharaj P, Fernandes M, Cadan S, et al. 542 Seronegative NMOSD–A Post SARS-CoV-2 Neurological Complication Associated With Paediatric Multisystem Inflammatory Syndrome (PIMS)? London: BMJ Publishing Group Ltd; (2021). 10.1136/archdischild-2021-rcpch.59 [DOI] [Google Scholar]
- 31.Jentzer A, Carra-Dallière C, Lozano C, Riviere S, Darmon O, Ayrignac X, et al. Neuromyelitis optica spectrum disorder following COVID-19 infection with increase in pre-existing anti-aquaporin-4 antibodies. J Neurol. (2022) 269:2850–3. 10.1007/s00415-022-10972-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Das D, Bhattacharjee H, Rehman O, Deori N, Magdalene D, Bharali G, et al. Neuromyelitis optica spectrum disorder post-COVID-19 infection: a rare case report from Northeast India. Indian J Ophthalmol. (2022) 70:1833–6. 10.4103/ijo.IJO_61_22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahsan N, Jafarpour S, Santoro JD. Myelin oligodendrocyte glycoprotein antibody encephalitis following severe acute respiratory syndrome coronavirus 2 in a pediatric patient. Clin Exp Pediatr. (2021) 64:310–2. 10.3345/cep.2020.01963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Ruijter NS, Kramer G, Gons RAR, Hengstman GJD. Neuromyelitis optica spectrum disorder after presumed coronavirus (COVID-19) infection: a case report. Mult Scler Relat Disord. (2020) 46:102474. 10.1016/j.msard.2020.102474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ide T, Kawanami T, Eriguchi M, Hara H. SARS-CoV-2-related myelin oligodendrocyte glycoprotein antibody-associated disease: a case report and literature review. Intern Med. (2022) 2022:21. 10.2169/internalmedicine.8709-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khan A, Panwala H, Ramadoss D, Khubchandani R. Myelin Oligodendrocyte Glycoprotein (MOG) antibody disease in a 11 year old with COVID-19 infection. Indian J Pediatr. (2021) 88:488–9. 10.1007/s12098-020-03656-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kogure C, Kikushima W, Fukuda Y, Hasebe Y, Takahashi T, Shibuya T, et al. Myelin oligodendrocyte glycoprotein antibody-associated optic neuritis in a COVID-19 patient: a case report. Medicine. (2021) 100:e25865. 10.1097/MD.0000000000025865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lindan CE, Mankad K, Ram D, Kociolek LK, Silvera VM, Boddaert N, et al. Neuroimaging manifestations in children with SARS-CoV-2 infection: a multinational, multicentre collaborative study. Lancet Child Adolesc Health. (2021) 5:167–77. 10.1016/S2352-4642(20)30362-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peters J, Alhasan S, Vogels CBF, Grubaugh ND, Farhadian S, Longbrake EE. MOG-associated encephalitis following SARS-CoV-2 infection. Mult Scler Relat Disord. (2021) 50:102857. 10.1016/j.msard.2021.102857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pinto AA, Carroll LS, Nar V, Varatharaj A, Galea I. CNS inflammatory vasculopathy with antimyelin oligodendrocyte glycoprotein antibodies in COVID-19. Neurol Neuroimmunol Neuroinflamm. (2020) 7:813. 10.1212/NXI.0000000000000813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sawalha K, Adeodokun S, Kamoga GR. COVID-19-induced acute bilateral optic neuritis. J Investig Med High Impact Case Rep. (2020) 8:2324709620976018. 10.1177/2324709620976018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vraka K, Ram D, West S, Chia WYE, Kurup P, Subramanian G, et al. Two paediatric patients with encephalopathy and concurrent COVID-19 infection: two sides of the same coin? Case Rep Neurol Med. (2021) 2021:6658000. 10.1155/2021/6658000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woodhall M, Mitchell JW, Gibbons E, Healy S, Waters P, Huda S. Case report: myelin oligodendrocyte glycoprotein antibody-associated relapse with COVID-19. Front Neurol. (2020) 11:598531. 10.3389/fneur.2020.598531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou S, Jones-Lopez EC, Soneji DJ, Azevedo CJ, Patel VR. Myelin oligodendrocyte glycoprotein antibody-associated optic neuritis and myelitis in COVID-19. J Neuroophthalmol. (2020) 40:398–402. 10.1097/WNO.0000000000001049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sinha R, Wander A, Kapoor A, Yadav R, Kumar A, Gulati S. Acute Demyelinating Syndrome (MOG Antibody Positive) associated with COVID-19 infection: a widening spectrum. Clin Pediatr. (2021) 60:501–3. 10.1177/00099228211037210 [DOI] [PubMed] [Google Scholar]
- 46.Jumah M, Rahman F, Figgie M, Prasad A, Zampino A, Fadhil A, et al. COVID-19, HHV6 and MOG antibody: a perfect storm. J Neuroimmunol. (2021) 353:577521. 10.1016/j.jneuroim.2021.577521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Durovic E, Bien C, Bien CG, Isenmann S. MOG antibody-associated encephalitis secondary to Covid-19: case report. BMC Neurol. (2021) 21:414. 10.1186/s12883-021-02449-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sardar S, Safan A, Okar L, Sadik N, Adeli G. The diagnostic dilemma of bilateral optic neuritis and idiopathic intracranial hypertension coexistence in a patient with recent COVID-19 infection. Clin Case Rep. (2021) 9:e04347. 10.1002/ccr3.4347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rojas-Correa DX, Reche-Sainz JA, Insausti-García A, Calleja-García C, Ferro-Osuna M. Post COVID-19 myelin oligodendrocyte glycoprotein antibody-associated optic neuritis. Neuroophthalmology. (2022) 46:115–21. 10.1080/01658107.2021.1916044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Assavapongpaiboon B, Apinyawasisuk S, Jariyakosol S. Myelin oligodendrocyte glycoprotein antibody-associated optic neuritis with COVID-19 infection: a case report and literature review. Am J Ophthalmol Case Rep. (2022) 26:101491. 10.1016/j.ajoc.2022.101491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Žorić L, Rajović-Mrkić I, Colak E, Mirić D, Kisić B. Optic neuritis in a patient with seropositive myelin oligodendrocyte glycoprotein antibody during the post-COVID-19 period. Int Med Case Rep J. (2021) 14:349–55. 10.2147/IMCRJ.S315103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dias da Costa M, Leal Rato M, Cruz D, Valadas A, Antunes AP, Albuquerque L. Longitudinally extensive transverse myelitis with anti-myelin oligodendrocyte glycoprotein antibodies following SARS-CoV-2 infection. J Neuroimmunol. (2021) 361:577739. 10.1016/j.jneuroim.2021.577739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Doukas SG, Santos AP, Mir W, Daud S, Zivin-Tutela TH. A rare case of myelin oligodendrocyte glycoprotein antibody-associated transverse myelitis in a 40-year-old patient with COVID-19. Cureus. (2022) 14:e23877. 10.7759/cureus.23877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jossy A, Jacob N, Sarkar S, Gokhale T, Kaliaperumal S, Deb AK. COVID-19-associated optic neuritis - a case series and review of literature. Indian J Ophthalmol. (2022) 70:310–6. 10.4103/ijo.IJO_2235_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang E, Husein A, Martinez-Perez J, Li T. Post-COVID-19 longitudinally extensive transverse myelitis with myelin oligodendrocyte glycoprotein antibodies. Case Rep Neurol Med. (2022) 2022:1068227. 10.1155/2022/1068227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cay-Martínez KC, Shen MY, Silver WG, Vargas WS. Postinfectious encephalomyelitis associated with myelin oligodendrocyte glycoprotein antibody in a pediatric patient with COVID-19. Pediatr Neurol. (2021) 124:40–1. 10.1016/j.pediatrneurol.2021.08.001 [DOI] [PubMed] [Google Scholar]
- 57.Barzegar M, Mirmosayyeb O, Nehzat N, Sarrafi R, Khorvash F, Maghzi AH, et al. COVID-19 infection in a patient with multiple sclerosis treated with fingolimod. Neurol Neuroimmunol Neuroinflamm. (2020) 7:753. 10.1212/NXI.0000000000000753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barzegar M, Vaheb S, Mirmosayyeb O, Afshari-Safavi A, Nehzat N, Shaygannejad V. Can coronavirus disease 2019 (COVID-19) trigger exacerbation of multiple sclerosis? A retrospective study. Mult Scler Relat Disord. (2021) 52:102947. 10.1016/j.msard.2021.102947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chyzhyk V, Sialitski M, Boika A, Bahamaz V, Mazurenka K, Ponomarev V. Is SARS-CoV-2 important for multiple sclerosis? Eur J Neurol. (2021) 2021:492. [Google Scholar]
- 60.Conway S, Healy B, Zurawski J, Severson C, Kaplan T, Stazzone L, et al. COVID-19 and neurologic outcomes in multiple sclerosis and related disorders. Multiple Scler J. (2021) 2021:765.35709663 [Google Scholar]
- 61.Czarnowska A, Kapica-Topczewska K, Zajkowska O, Adamczyk-Sowa M, Kubicka-Baczyk K, Niedziela N, et al. Symptoms after COVID-19 infection in individuals with multiple sclerosis in Poland. J Clin Med. (2021) 10:225225. 10.3390/jcm10225225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dhillon PS, Dineen RA, Morris H, Tanasescu R, Nikfekr E, Evans J, et al. Neurological disorders associated with COVID-19 hospital admissions: experience of a single tertiary healthcare center. Front Neurol. (2021) 12:640017. 10.3389/fneur.2021.640017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Domingues RB, Mendes-Correa MC, de Moura Leite FBV, Sabino EC, Salarini DZ, Claro I, et al. First case of SARS-CoV-2 sequencing in cerebrospinal fluid of a patient with suspected demyelinating disease. J Neurol. (2020) 267:3154–6. 10.1007/s00415-020-09996-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Etemadifar M, Sedaghat N, Aghababaee A, Kargaran PK, Maracy MR, Ganjalikhani-Hakemi M, et al. COVID-19 and the risk of relapse in multiple sclerosis patients: a fight with no bystander effect? Mult Scler Relat Disord. (2021) 51:102915. 10.1016/j.msard.2021.102915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Florea AA, Sirbu CA, Ghinescu MC, Plesa CF, Sirbu AM, Mitrica M, et al. SARS-CoV-2, multiple sclerosis, and focal deficit in a postpartum woman: a case report. Exp Ther Med. (2021) 21:92. 10.3892/etm.2020.9524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fragoso YD, Pacheco FAS, Silveira GL, Oliveira RA, Carvalho VM, Martimbianco ALC. COVID-19 in a temporal relation to the onset of multiple sclerosis. Mult Scler Relat Disord. (2021) 50:102863. 10.1016/j.msard.2021.102863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jaisankar PJ, Kucera A, Lomiguen CM, Chin J. Complications of COVID-19 pneumonia and multiple sclerosis exacerbation. Cureus. (2021) 13:e17506. 10.7759/cureus.17506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Karsidag S, Sahin S, Ates MF, Cinar N, Kendirli S. Demyelinating disease of the central nervous system concurrent with COVID-19. Cureus. (2021) 13:e17297. 10.7759/cureus.17297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kataria S, Tandon M, Melnic V, Sriwastava S. A case series and literature review of multiple sclerosis and COVID-19: clinical characteristics, outcomes and a brief review of immunotherapies. eNeurologicalSci. (2020) 21:100287. 10.1016/j.ensci.2020.100287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Khedr EM, Abo-Elfetoh N, Deaf E, Hassan HM, Amin MT, Soliman RK, et al. Surveillance study of acute neurological manifestations among 439 Egyptian patients with COVID-19 in Assiut and Aswan University Hospitals. Neuroepidemiology. (2021) 55:109–18. 10.1159/000513647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Khurana D, Kaur G, Chellapa R. COVID-19 in multiple sclerosis: experience from North India. J Neurol Sci. (2021) 429:118164. 10.1016/j.jns.2021.118164 [DOI] [Google Scholar]
- 72.Luetic G, Menichini ML, Burgos M, Alonso R, Carnero Contentti E, Carrá A, et al. COVID-19 in Argentine teriflunomide-treated multiple sclerosis patients: first national case series. Mult Scler Relat Disord. (2021) 53:103049. 10.1016/j.msard.2021.103049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maghzi AH, Houtchens MK, Preziosa P, Ionete C, Beretich BD, Stankiewicz JM, et al. COVID-19 in teriflunomide-treated patients with multiple sclerosis. J Neurol. (2020) 267:2790–6. 10.1007/s00415-020-09944-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mantero V, Abate L, Basilico P, Balgera R, Salmaggi A, Nourbakhsh B, et al. COVID-19 in dimethyl fumarate-treated patients with multiple sclerosis. J Neurol. (2021) 268:2023–5. 10.1007/s00415-020-10015-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Michelena G, Casas M, Eizaguirre MB, Pita MC, Cohen L, Alonso R, et al. Can COVID-19 exacerbate multiple sclerosis symptoms? A case series analysis. Mult Scler Relat Disord. (2022) 57:103368. 10.1016/j.msard.2021.103368 [DOI] [PubMed] [Google Scholar]
- 76.Möhn N, Saker F, Bonda V, Respondek G, Bachmann M, Stoll M, et al. Mild COVID-19 symptoms despite treatment with teriflunomide and high-dose methylprednisolone due to multiple sclerosis relapse. J Neurol. (2020) 267:2803–5. 10.1007/s00415-020-09921-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moore L, Ghannam M, Manousakis G. A first presentation of multiple sclerosis with concurrent COVID-19 infection. eNeurologicalSci. (2021) 22:100299. 10.1016/j.ensci.2020.100299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Palao M, Fernández-Díaz E, Gracia-Gil J, Romero-Sánchez CM, Díaz-Maroto I, Segura T. Multiple sclerosis following SARS-CoV-2 infection. Mult Scler Relat Disord. (2020) 45:102377. 10.1016/j.msard.2020.102377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pignolo A, Aprile M, Gagliardo C, Giammanco GM, D'Amelio M, Aridon P, et al. Clinical onset and multiple sclerosis relapse after SARS-CoV-2 infection. Neurol Int. (2021) 13:695–700. 10.3390/neurolint13040066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sandoval F, Julio K, Méndez G, Valderas C, Echeverría AC, Perinetti MJ, et al. Neurologic features associated with SARS-CoV-2 infection in children: a case series report. J Child Neurol. (2021) 36:853–66. 10.1177/0883073821989164 [DOI] [PubMed] [Google Scholar]
- 81.Wildemann B, Jarius S, Lehmann LH, André F, Frey N, Schnitzler P, et al. COVID-19-related severe MS exacerbation with life-threatening Takotsubo cardiomyopathy in a previously stable patient and interference of MS therapy with long-term immunity against SARS-CoV-2. J Neurol. (2022) 269:1138–41. 10.1007/s00415-021-10779-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yavari F, Raji S, Moradi F, Saeidi M. Demyelinating changes alike to multiple sclerosis: a case report of rare manifestations of COVID-19. Case Rep Neurol Med. (2020) 2020:6682251. 10.1155/2020/6682251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Etemadifar M, Abhari AP, Nouri H, Salari M, Maleki S, Amin A, et al. Does COVID-19 increase the long-term relapsing-remitting multiple sclerosis clinical activity? A cohort study. BMC Neurol. (2022) 22:64. 10.1186/s12883-022-02590-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Finsterer J. SARS-CoV-2 triggered relapse of multiple sclerosis. Clin Neurol Neurosurg. (2022) 215:107210. 10.1016/j.clineuro.2022.107210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Paybast S, Hejazi SA, Molavi P, Habibi MA, Naser Moghadasi A. A one year follow of patients with multiple sclerosis during COVID-19 pandemic: a cross-sectional study in Qom province, Iran. Mult Scler Relat Disord. (2022) 60:103712. 10.1016/j.msard.2022.103712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Khair A, Husain S, Ortiz M, Kaur G, Bean S. Para and post-COVID-19 acute demyelinating disorders in children, expanding the spectrum of clinical and radiological characteristics. Ann Neurol. (2021) 2021:S113–S4. 10.7759/cureus.23405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Aubart M, Roux CJ, Durrleman C, Gins C, Hully M, Kossorotoff M, et al. Neuro-inflammatory disease following SARS-CoV-2 infection in children. J Pediatr. (2022) 247:22–8.e2. 10.1016/j.jpeds.2022.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhou Z, Kang H, Li S, Zhao X. Understanding the neurotropic characteristics of SARS-CoV-2: from neurological manifestations of COVID-19 to potential neurotropic mechanisms. J Neurol. (2020) 267:2179–84. 10.1007/s00415-020-09929-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Verstrepen K, Baisier L, De Cauwer H. Neurological manifestations of COVID-19, SARS and MERS. Acta Neurol Belg. (2020) 120:1051–60. 10.1007/s13760-020-01412-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Desforges M, Le Coupanec A, Dubeau P, Bourgouin A, Lajoie L, Dubé M, et al. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. (2019) 12:14. 10.3390/v12010014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wu Y, Xu X, Chen Z, Duan J, Hashimoto K, Yang L, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. (2020) 87:18–22. 10.1016/j.bbi.2020.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. (2020) 71:762–8. 10.1093/cid/ciaa248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Andersen O, Lygner PE, Bergström T, Andersson M, Vahlne A. Viral infections trigger multiple sclerosis relapses: a prospective seroepidemiological study. J Neurol. (1993) 240:417–22. 10.1007/BF00867354 [DOI] [PubMed] [Google Scholar]
- 95.Sibley WA, Bamford CR, Clark K. Clinical viral infections and multiple sclerosis. Lancet. (1985) 1:1313–5. 10.1016/S0140-6736(85)92801-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Panitch HS. Influence of infection on exacerbations of multiple sclerosis. Ann Neurol. (1994) 36(Suppl.):S25–8. 10.1002/ana.410360709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Buljevac D, Flach HZ, Hop WC, Hijdra D, Laman JD, Savelkoul HF, et al. Prospective study on the relationship between infections and multiple sclerosis exacerbations. Brain. (2002) 125:952–60. 10.1093/brain/awf098 [DOI] [PubMed] [Google Scholar]
- 98.Correale J, Fiol M, Gilmore W. The risk of relapses in multiple sclerosis during systemic infections. Neurology. (2006) 67:652–9. 10.1212/01.wnl.0000233834.09743.3b [DOI] [PubMed] [Google Scholar]
- 99.Simpson-Yap S, De Brouwer E, Kalincik T, Rijke N, Hillert JA, Walton C, et al. Associations of disease-modifying therapies with COVID-19 severity in multiple sclerosis. Neurology. (2021) 97:e1870–e85. 10.1212/WNL.0000000000012753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sormani MP, De Rossi N, Schiavetti I, Carmisciano L, Cordioli C, Moiola L, et al. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann Neurol. (2021) 89:780–9. 10.1002/ana.26028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Reder AT, Centonze D, Naylor ML, Nagpal A, Rajbhandari R, Altincatal A, et al. COVID-19 in patients with multiple sclerosis: associations with disease-modifying therapies. CNS Drugs. (2021) 35:317–30. 10.1007/s40263-021-00804-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.