Abstract
Purpose
Obesity is associated with a higher risk of mortality in women with ovarian cancer. Exercise has improved body composition among cancer survivors, yet no randomized controlled trial has explored the effect of exercise on body composition in women with ovarian cancer. In this analysis, we examined the effect of a six-month aerobic exercise intervention on body composition among ovarian cancer survivors in the Women’s Activity and Lifestyle Study in Connecticut (WALC).
Methods
Women with ovarian cancer (N = 144) were randomized in a 1:1 ratio to 6 months of an aerobic exercise intervention or attention-control, and body composition was measured as a secondary outcome at baseline and 6 months via dual-energy X-ray absorptiometry (DEXA). Women with at least one DEXA scan were included in the analysis (N = 103).
Results
On average, participants were 57.1 (± 8.7) years old and 1.6 (± 0.9) years since diagnosis. Women randomized to exercise maintained weight during the trial (− 0.11 kg, P = 0.82), while women in attention-control gained weight (+ 1.40 kg, P = 0.03); however, the between-group difference did not reach statistical significance (P = 0.09). We found no statistically significant differences by study arm for changes in body fat percentage, bone mineral density, or lean body mass.
Conclusions
Weight was maintained as a result of a 6-month aerobic exercise intervention among post-treatment ovarian cancer survivors. Future exercise and healthy eating interventions should consider additional measures (e.g., computer tomography scans, D3-creatinine) to more accurately assess changes in body composition.
Implications for Cancer Survivors
Moderate-intensity aerobic exercise may help ovarian cancer survivors maintain weight.
Keywords: Exercise, Body weight, Body composition, Ovarian cancer
Introduction
In 2021, there will be approximately 21,410 women diagnosed with ovarian cancer in the USA [1]. With an estimated 13,770 ovarian cancer deaths in the USA in 2021, ovarian cancer is the fifth leading cause of cancer-related deaths among women [1]. Due to the lack of effective screening tests, ovarian cancer remains the most lethal gynecological cancer. About 60% of cases are detected at late stages, with 5-year survival rates of 42% and 26% for stage III and stage IV disease, respectively [2]. Many surgical and pharmacological advances have improved the prognosis for ovarian cancer, but modifiable lifestyle factors should also be considered.
Obesity is relevant to both risk of ovarian cancer and outcomes in women diagnosed with ovarian cancer. A meta-analysis of 25,157 women found higher body mass index (BMI) was associated with an increased risk of ovarian cancer among never-users of hormonal therapy [3]. Data suggest 24 to 57% of women with ovarian cancer are overweight, and 10 to 35% are obese [4–10]. Importantly, meta-analyses and individual studies have also found that obesity pre-, post-, or at diagnosis is a predictor of higher occurrence of surgical complications and worse overall survival among women with ovarian cancer [4, 5, 11–13]. These data suggest the relevance of interventions for women diagnosed with ovarian cancer that address obesity. However, involuntary weight loss and being underweight (BMI < 18.5 kg/m2) after treatment are also associated with worse survival among women with advanced ovarian cancer [6, 14, 15]. Several studies of women undergoing ovarian cancer treatment indicated involuntary weight loss (from 1.2 to 2.2 kg) during chemotherapy treatment was common [14–16]. Based on current knowledge, achieving and maintaining a healthy weight (BMI 18.5–25.0 kg/m2) may improve the prognosis of women diagnosed and treated for ovarian cancer.
Body weight or BMI alone does not fully describe body composition or differentiate between fat mass and lean body mass (LBM). Therefore, having body composition measures from whole-body dual-energy X-ray absorptiometry (DEXA) scans is important to understand the changes in body composition more fully, with objective measures of total fat mass, LBM, and bone mineral density (BMD).
The American Cancer Society recommends 2.5 h of moderate-intensity exercise per week and twice-weekly resistance training for cancer survivors [17] because of its role in improving quality of life, physical function, arthralgia, mental health, and cancer-related fatigue [18–20]. However, most women diagnosed with ovarian cancer (64–81%) are not meeting physical activity guidelines [21]. Exercise trials have shown weight management and muscle preservation in breast and prostate cancer survivors [22–24], but thus far, to our knowledge, no study has examined the effect of exercise on body composition in women treated for ovarian cancer. Therefore, we examined the effect of a 6-month aerobic exercise intervention on body composition among women treated for ovarian cancer in the Women’s Activity and Lifestyle Study in Connecticut (WALC).
Methods
The WALC study
The study design, recruitment strategy, and intervention content of the WALC Study have been described previously [25]. Briefly, 144 women treated for ovarian cancer were enrolled and randomized in a 1:1 ratio to a 6-month exercise intervention (N = 74) or an attention-control (N = 70) arm between May 1, 2010, and March 20, 2014. Eligible participants were 18 to 75 years old, English-speaking, diagnosed with stage I–IV ovarian cancer within the past 4 years, completed chemotherapy at least 1 month prior to randomization, exercising less than 90 min per week, and received physician consent to participate.
Recruitment and study visits
Women from Connecticut were recruited using the Rapid Case Ascertainment Shared Resource of the Yale Cancer Center, a Connecticut Tumor Registry field arm that identified women from all hospitals in Connecticut. Women were also recruited at two additional study sites: Dana-Farber Cancer Institute (Boston, MA) and Geisinger Health Systems (Danville, PA). Some women living outside Connecticut learned about the study through national support groups, physicians, or brochures in clinic waiting rooms, and were screened via telephone. If eligible, women completed baseline questionnaires and were then randomized.
The study was approved by the Connecticut Department of Public Health and the Yale Human Investigation Committees, Dana-Farber/Harvard Cancer Center Institutional Review Board (IRB), Geisinger Health Systems IRB, and all 21 Connecticut hospital IRBs (ClinicalTrials.gov: NCT02107066, https://clinicaltrials.gov/ct2/show/NCT02107066). All participants gave written informed consent.
Measures
Self-reported sociodemographic variables were collected at baseline. Disease stage, time since diagnosis, chemotherapy, treatment status, and history of recurrence were obtained via self-report, with an additional questionnaire completed by the participants’ physicians to obtain physician-verified treatment information and review of medical records.
Physical activity levels were assessed using the Modifiable Physical Activity Questionnaire [26], which asked about the duration and frequency of 20 recreational activities during the previous 6 months. For women randomized to the exercise intervention arm, Daily Activity Logs completed by the women and Call Logs completed by the interventionists were used to monitor weekly progress towards the weekly exercise goal (150 min).
Height and weight were measured by study staff using standardized procedures at in-person visits at baseline and 6 months. Participants were weighed in light clothing without shoes; measurements were rounded up to the nearest 0.1 kg. Height without shoes was measured using a stadiometer, with measurements rounded up to the nearest 0.5 cm. Body composition, including total fat mass, LBM and BMD were measured by whole-body DEXA scans (Hologic QDR 1500, Hologic Inc., Waltham, Mass) and participants followed standard guidelines for scans regarding drug and food intake. All DEXA scans were evaluated by a radiologist blinded to the study arm. For participants who did not complete in-person visits due to not being within driving distance of recruiting hospitals, no physical assessments were conducted, and DEXA scans were not obtained (N = 41).
Intervention
The exercise intervention consisted of home-based moderate-intensity aerobic exercise facilitated by weekly phone calls from an American College of Sports Medicine (ACSM)-Certified Cancer Exercise Trainer. Women were counseled on increasing their physical activity to 150 min/week of moderate-intensity aerobic exercise, mainly via brisk walking. Adherence was measured using self-report seven-day Daily Activity Logs on the type, duration, and intensity (based on heart rate monitors) of exercise. Achieving the exercise goal of 150 min/week was considered adherent a priori. Participants also reported their exercise levels during weekly telephone calls with the exercise trainer. Using a 26-chapter book developed for the study informed by the Social Cognitive Theory [27], the trainer provided weekly counseling including motivational interviewing technique via telephone during which they discussed educational topics on exercise and reviewed a weekly ovarian cancer health education topic to increase participant’s exercise levels.
The attention-control health education arm received weekly phone calls from a WALC staff member, on ovarian cancer health education topics along with a 26-chapter book that only contained ovarian cancer survivorship-related information.
Statistical analysis
This secondary analysis only includes women who completed in-person visits and had their body composition measured by DEXA, either only at baseline (N = 20) or at both baseline and 6 months (N = 83) (Fig. 1). At baseline, 53 women in the exercise arm and 50 women in the control arm completed DEXA scans at baseline, with 44 (83%) and 39 (78%) women respectively completing a DEXA scan at 6 months.
Fig. 1.
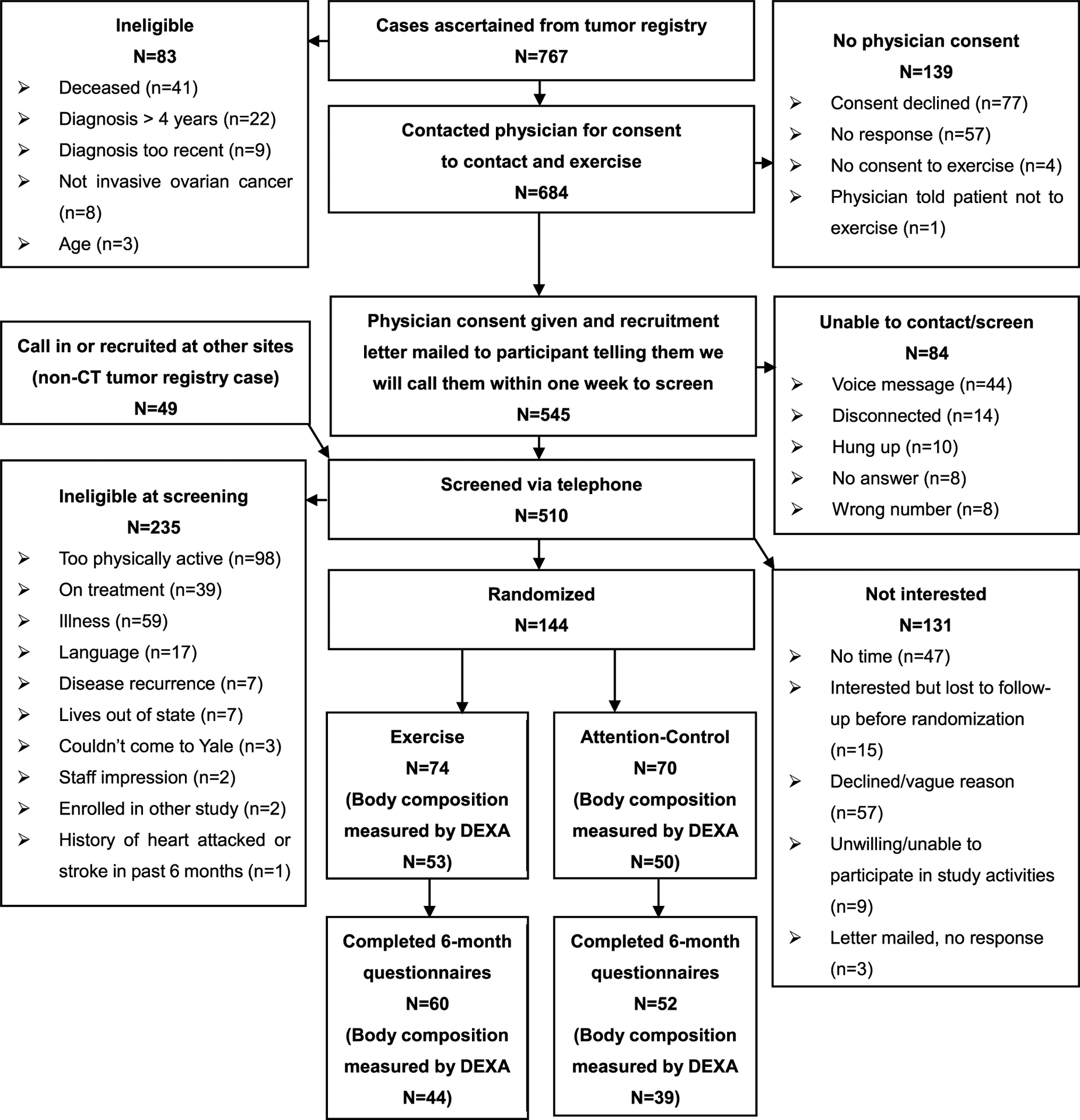
CONSORT diagram
Baseline demographic and clinical characteristics were compared using Student’s t-test, the chi-square test, or Fisher’s exact test. A mixed-model repeated measures analysis was used to evaluate a 6-month change in body composition between women randomized to exercise intervention versus attention-control according to the intention-to-treat principle [28] and baseline measures were retained as part of the response profile. Linear contrasts were used to obtain the change in body composition in each group and group differences, while baseline values of the two arms were constrained to be equal. This approach accounted for the baseline difference between the two arms, although the difference did not reach statistical significance (all P > 0.05). Least squares means and 95% confidence intervals (CI) estimated from the models were reported. The study site was included as a covariate in the models. We also evaluated recurrence before study enrollment and time since diagnosis as covariates, but these were excluded from the final models as they did not affect the parameter estimates.
Among women randomized to the exercise intervention arm, we explored the effect of exercise on body composition by compliance with the exercise goal (< 150 min per week vs ≥ 150 min per week). Effect modification of exercise on body composition by adherence, disease stage, baseline obesity, educational level, employment status, and living alone was examined by including group by time by moderator interaction terms in the model as exploratory analyses in all women. Among women who were followed up at 6 months, we further explored variables associated with 5% or more change in body weight using a one-way analysis of variance, chi-square test, or Fisher’s exact test.
All analyses were conducted using SAS Version 9.4 (SAS, Cary, NC) and statistical tests were two-sided, with a 0.05 statistical significance level, except for exploratory effect moderation analyses where a 0.20 statistical significance level was used to detect a larger range of potential effect modifiers.
Results
Study population
The mean age of the 103 women was 57.1 ± 8.7 years, and the average time since ovarian cancer diagnosis was 1.6 ± 0.9 years (Table 1). A majority of the women had completed high school (96.1%), were non-Hispanic white (95.2%), employed (54.9%), married or living with a partner (74.8%), and post-menopausal at diagnosis (62.8%). Fifty-two percent of the women had stage III or stage IV ovarian cancer, 93.2% of the participants received chemotherapy before the study, and 17.5% had a cancer recurrence prior to enrollment. At baseline, women reported an average of 28.5 ± 43.9 min per week of physical activity, and their mean BMI was 29.4 ± 6.8 kg/m2. Thirty-two percent and fifty percent of women randomized to the exercise intervention (N = 17/53) and attention-control (N = 25/50) were obese at baseline, respectively (P = 0.06). The mean body fat percentage of the study sample was 40.6 ± 5.7%, mean LBM was 42.6 ± 7.5 kg, and mean BMD was 1.1 ± 0.1 g/cm2. All characteristics and baseline body composition were similar between the two study arms (all P > 0.05).
Table 1.
Baseline characteristics and body composition by study arm (N = 103)
| Characteristic | Total study population (N = 103)a,b | Study arm | P valuec | |
|---|---|---|---|---|
| Exercise (N = 53)a,b | Control (N = 50)a,b | |||
| Age | 57.1 ± 8.7 | 56.8 ± 9.4 | 57.4 ± 8.0 | 0.73 |
| Race/ethnicity | 0.20 | |||
| Non-Hispanic white | 98 (95.2) | 52 (98.1) | 46 (92.0) | |
| Other | 5 (4.9) | 1 (1.2) | 4 (8.0) | |
| Education level | 0.66 | |||
| No GED or equivalent | 4 (3.9) | 3 (5.7) | 1 (2.0) | |
| GED and some college/associates | 48 (46.6) | 23 (43.4) | 25 (50.0) | |
| College graduate or advanced degree | 51 (49.5) | 27 (50.9) | 24 (48.0) | |
| Employment status | 0.93 | |||
| Unemployed/retired | 46 (45.1) | 23 (43.4) | 23 (46.9) | |
| Employed part time (< 35 h/week) | 22 (21.6) | 12 (22.6) | 10 (20.4) | |
| Employed full time (≥ 35 h/week) | 34 (33.3) | 18 (34.0) | 16 (32.7) | |
| Marital status | 0.27 | |||
| Single | 10 (9.7) | 3 (5.7) | 7 (14.0) | |
| Divorced, separated, or widowed | 16 (15.5) | 10 (18.9) | 6 (12.0) | |
| Married or living with partner | 77 (74.8) | 40 (75.5) | 37 (74.0) | |
| Menopausal at baseline | 64 (62.8) | 29 (55.8) | 35 (70.0) | 0.14 |
| Cancer stage at diagnosis | 0.21 | |||
| Stage I | 24 (23.3) | 15 (28.3) | 9 (18.0) | |
| Stage II | 24 (23.3) | 8 (15.1) | 16 (32.0) | |
| Stage III | 37 (35.9) | 21 (39.6) | 16 (32.0) | |
| Stage IV | 17 (16.5) | 8 (15.1) | 9 (18.0) | |
| Unknown | 1 (1.0) | 1 (1.9) | 0 (0.0) | |
| Time since diagnosis (years) | 1.6 ± 0.9 | 1.7 ± 0.9 | 1.5 ± 0.9 | 0.39 |
| Chemotherapy prior to enrollment | 96 (93.2) | 50 (94.3) | 46 (92.0) | 0.71 |
| Cancer recurrence prior to enrollment | 18 (17.5) | 9 (17.0) | 9 (18.0) | 0.89 |
| Live alone | 14 (13.6) | 8 (16.0) | 6 (11.3) | 0.49 |
| Study site | 1.00 | |||
| Yale | 90 (87.4) | 46 (86.8) | 44 (88.0) | |
| Geisinger | 6 (5.8) | 3 (5.7) | 3 (6.0) | |
| DFCI | 7 (6.8) | 4 (7.6) | 3 (6.0) | |
| Physical activity (min/week) | 28.5 ± 43.9 | 21.7 ± 43.9 | 35.7 ± 43.2 | 0.11 |
| Weight (kg) | 77.2 ± 17.4 | 75.0 ± 17.0 | 79.6 ± 17.7 | 0.18 |
| BMI (kg/m2) | 29.4 ± 6.8 | 28.8 ± 6.9 | 29.9 ± 6.5 | 0.41 |
| Obesity (%) | 42 (40.8) | 17 (32.1) | 25 (50.0) | 0.06 |
| Body fat percentage (%) | 40.6 ± 5.7 | 40.4 ± 5.3 | 40.8 ± 6.1 | 0.71 |
| LBM (kg) | 42.6 ± 7.5 | 41.5 ± 7.8 | 43.8 ± 7.1 | 0.12 |
| BMD (g/cm2) | 1.1 ± 0.1 | 1.1 ± 0.1 | 1.1 ± 0.1 | 0.16 |
Abbreviations: BMD, bone mineral density; BMI, body mass index; DFCI, Dana-Farber Cancer Institute; LBM, lean body mass
Mean ± standard deviation for continuous variables and n (column %) for categorical variables
Numbers may not sum to the total due to missing data, and percentages may not sum to 100% due to rounding
P-value is for t-test (continuous variables), χ2 test (categorical variables), or Fisher’s exact test (cell counts < 5)
Women with DEXA (N = 103) included in this analysis were similar to women without DEXA (N = 41), except those without baseline DEXA were more likely to have an advanced degree and to have been self-referred (only 7 women were not self-referred), and further from date of diagnosis (data not shown).
Body composition
Women randomized to the exercise intervention maintained weight during the 6-month trial (− 0.11 kg, 95% CI: − 1.32 to 1.09 kg, P = 0.82), while women in the attention-control arm gained weight (+ 1.40 kg, 95% CI: 0.14 to 2.66 kg, P = 0.03) (Table 2). The between group difference in weight change was suggestive of the intervention preventing weight gain, but this did not reach statistical significance (− 1.51 kg, 95% CI: − 3.26 to 0.23 kg, P = 0.09). Women randomized to the exercise intervention had a similar change in body fat percentage at 6 months (+ 0.13%, 95% CI: − 0.79 to 1.05%, P = 0.78) compared to women randomized to attention-control (+ 0.38%, 95% CI: − 0.59 to 1.36%, P = 0.44; between group P = 0.71). Women in both groups had stable LBM during the study period (exercise: − 0.43 kg, 95% CI: − 1.07 to 0.20 kg vs. attention-control: + 0.31 kg, 95% CI: − 0.36 to 0.98 kg; between group P = 0.11). All participants had similar losses in BMD (between group P = 0.46); however, the reduction in BMD at 6 months was significant in the attention-control arm (− 0.01 g/cm2, 95% CI: − 0.02 to − 0.001, P = 0.03), but not the exercise arm (− 0.01 g/cm2, 95% CI: − 0.02 to 0.004, P = 0.22).
Table 2.
Effect of exercise intervention versus attention-control on body composition; baseline, and changes at 6 months (N = 103)
| Body composition | Exercise (N = 53)a | Control (N = 50)a | Between group difference (exercise-control), least square mean (95%CI)a | P value (between group effect)a | |
|---|---|---|---|---|---|
| Weight (kg) | Combined baseline | 76.69 (70.19, 83.19) | |||
| 6 months | 76.57 (69.96, 83.19) | 78.09 (71.46, 84.72) | |||
| 6-month change | −0.11 (−1.32, 1.09) | 1.40 (0.14, 2.66) | −1.51 (−3.26, 0.23) | 0.09 | |
| BMI (kg/m2) | Combined baseline | 29.11 (26.58, 31.64) | |||
| 6 months | 29.06 (26.48, 31.63) | 29.64 (27.06, 32.22) | |||
| 6-month change | −0.05 (−0.52, 0.41) | 0.53 (0.05, 1.01) | −0.58 (−1.25, 0.08) | 0.09 | |
| Body fat percentage (%) | Combined baseline | 42.83 (40.76, 44.89) | |||
| 6 months | 42.96 (40.71, 45.20) | 43.21 (40.94, 45.47) | |||
| 6-month change | 0.13 (−0.79, 1.05) | 0.38 (−0.59, 1.36) | −0.25 (−1.59, 1.09) | 0.71 | |
| LBM (kg) | Combined baseline | 39.83 (37.20, 42.47) | |||
| 6 months | 39.40 (36.74, 42.06) | 40.14 (37.48, 42.81) | |||
| 6-month change | −0.43 (−1.07, 0.20) | 0.31 (−0.36, 0.98) | −0.74 (−1.65, 0.17) | 0.11 | |
| BMD (g/cm2) | Combined baseline | 1.13 (1.09, 1.17) | |||
| 6 months | 1.12 (1.08, 1.16) | 1.12 (1.08, 1.16) | |||
| 6-month change | −0.01 (−0.02, 0.00) | −0.01 (−0.02, 0.00) | 0.01 (−0.01, 0.02) | 0.46 |
Abbreviations: BMD, bone mineral density; BMI, body mass index; LBM, lean body mass
Body composition values, exercise effect, and corresponding p-values are estimated using a mixed effect model adjusted for the study site
Moderation analyses
No significant differences in change in body composition were found for women who met or did not meet the a priori specified 150 min/week exercise goal in the intervention arm (all P > 0.20, data not shown).
Among all 103 women, there was some evidence of effect modification for various body composition measures by attendance to the 25 weekly exercise counseling phone calls, cancer stage, baseline BMI and if the participant lived alone (Table 3). For attendance, 27 (51%) women randomized to exercise and 10 (20%) women randomized to attention-control attended all 25 sessions. Exercisers attended 22.8 ± 3.6 sessions on average, which was significantly higher than the number of sessions women randomized to attention-control attended (20.9 ± 5.3 sessions, P = 0.04). Attendance to phone calls was positively correlated with average weekly exercise time during the study (Pearson correlation P = 0.05). Exercise had a more significant effect on weight loss, percentage body fat loss, and LBM preservation among women who attended all sessions (P for interaction = 0.07 (weight); 0.02 (body fat percentage); 0.04 (LBM)). In women who attended all sessions, exercisers experienced a greater loss in body weight and percentage body fat compared to attention-control women (weight: − 3.25, P = 0.02; percentage body fat: − 2.21%, P = 0.05) and had no significant change in LBM (0.55, P = 0.47). In contrast, no difference in change in weight or percentage of body fat between the exercise and attention-control groups was seen for women who were not 100% compliant with the phone sessions (P > 0.05), but exercise women lost more LBM compared to the control group (− 1.47 kg; P = 0.02). Compared to early-stage patients, exercise had a larger effect on body fat percentage loss among late-stage patients (P for interaction = 0.14), but less effect on LBM loss (P for interaction = 0.20). Exercise effectively reduced body weight and LBM in women with a baseline BMI ≥ 30 kg/m2 (exercise vs. control: weight: − 3.20 kg, P = 0.02; LBM: − 2.08 kg; P = 0.002), but not among women with a baseline BMI < 30 kg/m2. Women who lived alone during the study period and were randomized to exercise lost more weight (P = 0.003), body fat (P = 0.005), and LBM (P = 0.04), and preserved more BMD (P = 0.04) than attention-control women, but such effect was not seen in women who lived with other people. Education level and employment status did not modify these same associations (P for interaction > 0.20, data not shown).
Table 3.
Exercise effect on body composition stratified by attendance to intervention phone calls, cancer stage at diagnosis, baseline obesity (BMI ≥ 30 kg/m2), education, and living alone (N = 103)
| Six-month change | Weight (kg) | Body fat percentage | LBM (kg) | BMD (g/cm2) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exercise effecta | P | P interaction | Exercise effectb | P | P interaction | Exercise effectb | P | P interaction | Exercise effectb | P | P interaction | |
| Attendance | 0.07 | 0.02 | 0.04 | 0.43 | ||||||||
| 100% | −3.58 (−6.55,−0.63) | 0.02 | −2.21 (−4.44, 0.02) | 0.05 | 0.55 (−0.97, 2.08) | 0.47 | −0.00 (−0.03,0.02) | 0.81 | ||||
| Not 100% | −0.07 (−2.39,2.25) | 0.95 | 1.19 (−0.57, 2.94) | 0.18 | −1.47 (−2.67,−0.27) | 0.02 | 0.01 (−0.01,0.03) | 0.32 | ||||
| Cancer stage | 0.88 | 0.14 | 0.20 | 0.60 | ||||||||
| Stage I/II | −1.52 (−3.97,0.94) | 0.22 | 0.68 (−1.24, 2.59) | 0.48 | −1.26 (−2.55, 0.02) | 0.05 | 0.00 (−0.02, 0.03) | 0.85 | ||||
| Stage III/IV | −1.25 (−3.91, 1.41) | 0.35 | −1.45 (−3.50, 0.60) | 0.16 | −0.03 (−1.41, 1.35) | 0.97 | 0.01 (−0.01,0.04) | 0.37 | ||||
| Baseline obesity | 0.11 | 0.34 | 0.01 | 0.85 | ||||||||
| No | −0.33 (−2.64, 1.99) | 0.78 | −0.83 (−2.63,0.96) | 0.36 | 0.29 (−0.86, 1.44) | 0.62 | 0.01 (−0.01,0.03) | 0.56 | ||||
| Yes | −3.20 (−5.85,−0.55) | 0.02 | 0.49 (−1.59, 2.57) | 0.64 | −2.08 (−3.41,−0.75) | 0.002 | 0.00 (−0.02, 0.03) | 0.73 | ||||
| Live alone | 0.01 | 0.005 | 0.17 | 0.05 | ||||||||
| No | −0.61 (−2.44, 1.23) | 0.51 | 0.48 (−0.92, 1.88) | 0.50 | −1.02 (−2.01,−0.03) | 0.04 | −0.00 (−0.02, 0.01) | 0.83 | ||||
| Yes | −6.95 (−11.50,−2.41) | 0.003 | −4.93 (−8.36,−1.50) | 0.005 | 0.82 (−1.61,3.25) | 0.72 | 0.04 (0.00, 0.08) | 0.04 | ||||
Abbreviations: BMD, bone mineral density; LBM, lean body mass
Table values are estimated using mixed effect model adjusted for study site
Least square means (95% CI)
Percent weight change analysis
We further compared demographic and clinical characteristics of women by percentage weight change from baseline to 6 months, regardless of study arm, to understand variables associated with a clinically significant weight change (data not shown). Among 83 women who completed both baseline and 6-month DEXA scans, 6 women lost more than 5% baseline weight at the end of the study, 7 women gained more than 5% baseline body weight, while the majority (N = 70) maintained a relatively stable weight. Basic demographic characteristics, including age, race/ethnicity, education level, employment status, marital status, and living alone were similar between the three weight change groups, as well as clinical characteristics, baseline physical activity level, and baseline BMI and LBM. Women who maintained stable body weight had lower body fat percentage at baseline (39.87 ± 5.88%) compared to the two other groups (weight gain: 44.50 ± 3.98%, P = 0.046; weight loss: 45.15 ± 3.96%, P = 0.03). Change in body fat percentage (P < 0.001), but not change in LBM (P = 0.91), followed a similar pattern.
Discussion
We found a suggestive benefit of a 6-month randomized controlled trial of moderate-intensity exercise versus attention-control on maintenance of body weight and BMI among women who had been treated for ovarian cancer. We did not observe differences by study arm for percent body fat, LBM, and BMD. Among women randomized to attention-control, there was statistically significant weight gain and BMD loss.
Weight change following diagnosis among women with ovarian cancer is an important topic worth further examination. To our knowledge, there are no other exercise trials on weight and body composition changes in women treated for ovarian cancer, but previous observational studies have described weight change during and shortly after treatment. Gil and colleagues found the mean body weight of 33 ovarian cancer patients treated with adjuvant chemotherapy decreased after surgery and then returned to baseline levels by 1 year (P = 0.003) [16]. Among these women, early-stage patients had a 2.8 ± 2.0 kg weight gain during treatment, while patients with advanced-stage disease had a 1.5 ± 1.5 kg weight loss [16]. Weight loss among advanced-stage patients at the initiation of treatment was also seen by Hess et al. [14], 645 ovarian cancer patients on two different chemotherapy regimens experienced weight loss after the first chemotherapy cycle (− 2.2 kg for cisplatin/paclitaxel, − 1.2 kg for carboplatin/paclitaxel). Women either returned to pre-treatment weight status (carboplatin/paclitaxel regimen) or maintained a lower weight until the end of the treatment (cisplatin/paclitaxel regimen) [14]. Mardas et al. observed a similar reduction in body weight after the first chemotherapy cycle followed by a recovery (P < 0.001) among 165 ovarian cancer patients [15]. In contrast to existing research, women in our study were on average 1.6 years from diagnosis and had completed chemotherapy. Attention-control women gained weight during the trial. This mirrors observational studies of weight gain after the first cycle of chemotherapy, but to our knowledge studies of post-treatment ovarian cancer survivors are lacking and there are no other interventions to which we can compare our findings. Of note, participating in an intervention trial can impact the behaviors of control arm participants, so observational studies of a population similar to ours might observe greater weight gain. Therefore, the post-treatment weight trajectory of women treated for ovarian cancer remains largely unknown and warrants future research.
A meta-analysis of 8245 ovarian cancer patients found obesity pre-diagnosis, at diagnosis or at the beginning of chemotherapy, was a predictor of worse overall survival [11]. However, weight gain during chemotherapy has been associated with improved overall survival in women with ovarian cancer in observational studies [14, 15]. Post-treatment underweight status has also had a detrimental impact on ovarian cancer survival in observational data [6]. Weight loss in observational studies is more likely to be involuntary rather than intentional weight loss. Involuntary weight loss during cancer treatment could be a marker of poorer overall health status or cancer progression, with poor appetite, treatment-related nausea, chemotherapy-induced muscle loss, or physical inactivity during treatment implicated in the weight change [29]. While the role of weight gain on overall survival among ovarian cancer survivors requires further study, weight gain, or obesity may result in extraglandular estrogens from adipose tissue which could stimulate the proliferation of residual malignant cells, potentially leading to disease recurrence [30]. Overall, weight management, including prevention of involuntary weight loss due to chemotherapy side effects and an increase in fat mass, may improve ovarian cancer prognosis.
In our study, women randomized to exercise who were 100% adherent to the counseling phone calls had significant weight loss, body fat loss, and stable LBM compared to women in the attention-control group who were fully adherent. No difference in change in body composition was seen for those who were not 100% compliant with the phone calls, except for LBM loss. We did not observe differences by study arm for the other DEXA body composition measures. While there are no other studies to which we could compare our results, the small sample size in sub-groups, type of exercise recommended, and the relatively short duration of our intervention could play a role in these null findings. Previous studies suggest resistance exercise effectively preserved lean body mass in cancer survivors [31–37] and could be more effective compared to aerobic exercise [38]. Future intervention studies should consider combined resistance and aerobic training, which is recommended by the American Cancer Society and the ACSM [17, 19].
We could not assess sarcopenia in our sample due to a lack of muscle strength measurements. As a syndrome characterized by loss of skeletal muscle mass and low muscle strength or physical performance [39], sarcopenia is associated with worse survival in ovarian cancer patients [7, 9, 10, 40–43]. The prevalence of sarcopenia ranges from 11 to 68% among women with ovarian cancer [44], highlighting that interventions to preserve muscle mass could be important for cancer-related outcomes in these women. Chemotherapy can increase muscle protein degeneration [45], and side effects of chemotherapy, such as fatigue, may lead to physical inactivity, thus exacerbating muscle loss. As more than 90% of women in our study were treated with chemotherapy, mirroring rates in ovarian cancer patients in general, assessing sarcopenia would be an important component for future work in this area. One limitation of our study is that we measured LBM via DEXA, which is only a surrogate for muscle mass. However, previous exercise trials among cancer survivors have measured body composition via DEXA [34, 38, 46] and found a significant change in LBM and/or fat mass after 12 to 17 weeks, supporting our approach of assessing body composition via DEXA over a 6-month period. Currently, computer tomography (CT) is considered the gold standard for the non-invasive assessment of muscle quantity. Although the agreement between DEXA and CT measured sarcopenia is high, to better understand sarcopenia in ovarian cancer patients and explore potential ways to preserve muscle mass, future studies should employ more accurate measurements of muscle mass, such as D3-creatinine or CT [47, 48].
Women who had a BMI ≥ 30 kg/m2 at baseline lost more body weight compared to women with BMI < 30 kg/m2 due to exercise, which could be driven by the loss in LBM we also observed in this group. Although weight loss during chemotherapy has been associated with worse survival [6, 14, 15], the effect of post-treatment intentional weight loss among obese ovarian cancer survivors has not been explored yet. We also observed that women who lived alone lost more weight compared to women who lived with others, which could be explained by that the intervention not addressing relatedness or could potentially be due to women living alone having different responsibilities and time for exercise. Our stratified results are exploratory and more data are needed to examine these potential differential effects to better guide future lifestyle interventions for ovarian cancer survivors.
To our knowledge, this is the first randomized controlled trial that examined the effect of exercise on body composition in women treated for ovarian cancer. Strengths of our study include the population-based recruitment, the relatively large sample size for this cancer type, and limited exclusion criteria making our population generalizable to other ovarian cancer survivors. Our results add to the evidence that exercise is not only feasible and safe, but potentially beneficial for weight maintenance among ovarian cancer survivors who have completed treatment. Since our intervention did not specifically target weight loss, we did not intervene on diet, but future studies could consider adding a dietary intervention along with exercise, which could lead to a more substantial effect on body weight change [49]. In addition, incorporating resistance training as mentioned above could also help to prevent potential muscle mass loss that often parallels weight loss.
In summary, there was a suggestion that weight and BMI were maintained as a result of a 6-month exercise intervention of 150 min/week of primary brisk walking in our population of ovarian cancer survivors who were on average 1.6 years from diagnosis. Several in-progress studies should provide more evidence on this important topic in the near future. The Physical Activity and Dietary intervention in women with OVArian cancer (PADOVA) study was recently initiated to study the effect of a combined exercise and dietary intervention on the body composition of 122 ovarian cancer patients undergoing chemotherapy [50]. The Lifestyle Intervention for Ovarian Cancer Enhanced Survival (LIVES) study, which enrolled 1200 women with ovarian cancer in a 2-year diet and exercise intervention on progression-free survival will also have measures of body composition [51]. Although accumulating evidence from observational studies has shown being physically active is associated with lower mortality in ovarian cancer patients [52, 53], our understanding of the effect of exercise and weight change on ovarian cancer outcomes, such as recurrence and survival, is still limited. Large observational studies with extended follow-up and additional randomized controlled trials are needed to examine such associations and understand if body composition is a mediating variable. Future studies should also explore effective ways to promote weight management and prevent muscle loss to help improve the prognosis of ovarian cancer survivors.
Acknowledgements
Certain data used in this study were obtained from the Connecticut Tumor Registry located in the Connecticut Department of Public Health. The authors assume full responsibility for analyses and interpretation of the data collected from the Connecticut Tumor Registry. We thank all the study participants and physicians; Rajni Mehta, Director of the Rapid Case Ascertainment Shared Resource of the Yale Cancer Center; and the following Connecticut hospitals: Charlotte Hungerford Hospital, Bridgeport Hospital, Danbury Hospital, Hartford Hospital, Middlesex Hospital, New Britain General Hospital, Bradley Memorial Hospital, Yale/New Haven Hospital, St. Francis Hospital and Medical Center, St. Mary’s Hospital, Hospital of St. Raphael, St. Vincent’s Medical Center, Stamford Hospital, William W. Backus Hospital, Windham Hospital, Eastern Connecticut Health Network, Griffin Hospital, Bristol Hospital, Johnson Memorial Hospital, Day Kimball Hospital, Greenwich Hospital, Lawrence and Memorial Hospital, Milford Hospital, New Milford Hospital, Norwalk Hospital, Sharon Hospital, and Waterbury Hospital.
Funding
This study was supported by the National Cancer Institute at the National Institutes of Health (NCI 5R01CA138556, P30 CA016359) and by the National Center for Advancing Translational Science at the National Institutes of Health (UL1TR000142). The funding agencies had no involvement in the study design, the collection, analysis and interpretation of data, in the writing of the report, or the decision to submit the paper for publication.
Footnotes
Ethics approval The study was approved by the Connecticut Department of Public Health and the Yale Human Investigation Committees, Dana-Farber/Harvard Cancer Center Institutional Review Board (IRB), Geisinger Health Systems IRB, and all 21 Connecticut hospital IRBs. The trial is registered at ClinicalTrials.gov: NCT02107066 (https://clinicaltrials.gov/ct2/show/NCT02107066).
Consent to participate All participants gave written informed consent.
Competing interests The authors declare no conflicts of interest.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Trabert B, DeSantis CE, Miller KD, Samimi G, Runowicz CD, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68:284–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collaborative Group on Epidemiological Studies of Ovarian Cancer. Ovarian cancer and body size: individual participant meta-analysis including 25,157 women with ovarian cancer from 47 epidemiological studies. PLoS Med. 2012;9:e1001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pavelka JC, Brown RS, Karlan BY, Cass I, Leuchter RS, Lagasse LD, et al. Effect of obesity on survival in epithelial ovarian cancer. Cancer. 2006;107:1520–4. [DOI] [PubMed] [Google Scholar]
- 5.Kumar A, Bakkum-Gamez JN, Weaver AL, McGree ME, Cliby WA. Impact of obesity on surgical and oncologic outcomes in ovarian cancer. Gynecol Oncol. 2014;135:19–24. [DOI] [PubMed] [Google Scholar]
- 6.Kim SI, Kim HS, Kim TH, Suh DH, Kim K, No JH, et al. Impact of underweight after treatment on prognosis of advanced-stage ovarian cancer. J Immunol Res. 2014;2014:349546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aust S, Knogler T, Pils D, Obermayr E, Reinthaller A, Zahn L, et al. Skeletal muscle depletion and markers for cancer cachexia are strong prognostic factors in epithelial ovarian cancer. PLoS One. 2015;10:e0140403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bandera EV, Lee VS, Rodriguez-Rodriguez L, Powell CB, Kushi LH. Impact of chemotherapy dosing on ovarian cancer survival according to body mass index. JAMA Oncol. 2015;1:737–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rutten IJ, van Dijk DP, Kruitwagen RF, Beets-Tan RG, Old-eDamink SW, van Gorp T. Loss of skeletal muscle during neoadjuvant chemotherapy is related to decreased survival in ovarian cancer patients. J Cachexia Sarcopenia Muscle. 2016;7:458–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar A, Moynagh MR, Multinu F, Cliby WA, McGree ME, Weaver AL, et al. Muscle composition measured by CT scan is a measurable predictor of overall survival in advanced ovarian cancer. Gynecol Oncol. 2016;142:311–6. [DOI] [PubMed] [Google Scholar]
- 11.Protani MM, Nagle CM, Webb PM. Obesity and ovarian cancer survival: a systematic review and meta-analysis. Cancer Prev Res (Phila). 2012;5:901–10. [DOI] [PubMed] [Google Scholar]
- 12.Nagle CM, Dixon SC, Jensen A, Kjaer SK, Modugno F, deFazio A, et al. Obesity and survival among women with ovarian cancer: results from the Ovarian Cancer Association Consortium. Br J Cancer. 2015;113:817–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heus C, Smorenburg A, Stoker J, Rutten MJ, Amant FCH, van Lonkhuijzen L. Visceral obesity and muscle mass determined by CT scan and surgical outcome in patients with advanced ovarian cancer. A retrospective cohort study. Gynecol Oncol. 2021;160:187–92. [DOI] [PubMed] [Google Scholar]
- 14.Hess LM, Barakat R, Tian C, Ozols RF, Alberts DS. Weight change during chemotherapy as a potential prognostic factor for stage III epithelial ovarian carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2007;107:260–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mardas M, Stelmach-Mardas M, Madry R. Body weight changes in patients undergoing chemotherapy for ovarian cancer influence progression-free and overall survival. Support Care Cancer. 2017;25:795–800. [DOI] [PubMed] [Google Scholar]
- 16.Gil KM, Frasure HE, Hopkins MP, Jenison EL, von Gruenigen VE. Body weight and composition changes in ovarian cancer patients during adjuvant chemotherapy. Gynecol Oncol. 2006;103:247–52. [DOI] [PubMed] [Google Scholar]
- 17.Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62:243–74. [DOI] [PubMed] [Google Scholar]
- 18.Speck RM, Courneya KS, Masse LC, et al. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010;4:87–100. [DOI] [PubMed] [Google Scholar]
- 19.Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvao DA, Pinto BM, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42:1409–26. [DOI] [PubMed] [Google Scholar]
- 20.Irwin ML, Cartmel B, Gross CP, Ercolano E, Li F, Yao X, et al. Randomized exercise trial of aromatase inhibitor-induced arthralgia in breast cancer survivors. J Clin Oncol. 2015;33:1104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones TL, Sandler CX, Spence RR, Hayes SC. Physical activity and exercise in women with ovarian cancer: a systematic review. Gynecol Oncol. 2020;158:803–11. [DOI] [PubMed] [Google Scholar]
- 22.Irwin ML, Alvarez-Reeves M, Cadmus L, Mierzejewski E, Mayne ST, Yu H, et al. Exercise improves body fat, lean mass, and bone mass in breast cancer survivors. Obesity (Silver Spring). 2009;17:1534–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopez P, Taaffe DR, Newton RU, GalvÃo DA. Resistance exercise dosage in men with prostate cancer: systematic review, meta-analysis, and meta-regression. Med Sci Sports Exerc. 2021;53:459–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez P, Galvão DA, Taaffe DR, Newton RU, Souza G, Trajano GS, et al. Resistance training in breast cancer patients undergoing primary treatment: a systematic review and meta-regression of exercise dosage. Breast Cancer. 2021;28:16–24. [DOI] [PubMed] [Google Scholar]
- 25.Zhou Y, Cartmel B, Gottlieb L, Ercolano EA, Li F, Harrigan M, et al. Randomized trial of exercise on quality of life in women with ovarian cancer: women’s activity and lifestyle study in Connecticut (WALC). J Natl Cancer Inst. 2017;109(12):djx072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kriska AM, Knowler WC, LaPorte RE, Drash AL, Wing RR, Blair SN, et al. Development of questionnaire to examine relationship of physical activity and diabetes in Pima Indians. Diabetes Care. 1990;13:401–11. [DOI] [PubMed] [Google Scholar]
- 27.Bandura A Social cognitive theory: an agentic perspective. Annu Rev Psychol. 2001;52:1–26. [DOI] [PubMed] [Google Scholar]
- 28.Fitzmaurice GM, Laird NM, Ware JH. Applied longitudinal analysis. New York: Wiley; 2012. [Google Scholar]
- 29.Mattox TW. Treatment of unintentional weight loss in patients with cancer. Nutr Clin Pract. 2005;20:400–10. [DOI] [PubMed] [Google Scholar]
- 30.Cramer DW, Welch WR. Determinants of ovarian cancer risk. II. Inferences regarding pathogenesis. J Natl Cancer Inst. 1983;71:717–21. [PubMed] [Google Scholar]
- 31.Lønbro S, Dalgas U, Primdahl H, Johansen J, Nielsen JL, Aagaard P, et al. Progressive resistance training rebuilds lean body mass in head and neck cancer patients after radiotherapy–results from the randomized DAHANCA 25B trial. Radiother Oncol. 2013;108:314–9. [DOI] [PubMed] [Google Scholar]
- 32.Cormie P, Newton RU, Spry N, Joseph D, Taaffe DR, Galvão DA. Safety and efficacy of resistance exercise in prostate cancer patients with bone metastases. Prostate Cancer Prostatic Dis. 2013;16:328–35. [DOI] [PubMed] [Google Scholar]
- 33.Christensen JF, Jones LW, Tolver A, Jørgensen LW, Andersen JL, Adamsen L, et al. Safety and efficacy of resistance training in germ cell cancer patients undergoing chemotherapy: a randomized controlled trial. Br J Cancer. 2014;111:8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dawson JK, Dorff TB, Todd Schroeder E, Lane CJ, Gross ME, Dieli-Conwright CM. Impact of resistance training on body composition and metabolic syndrome variables during androgen deprivation therapy for prostate cancer: a pilot randomized controlled trial. BMC Cancer. 2018;18:368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.CeŠeiko R, Thomsen SN, Tomsone S, EglĪtis J, Vētra A, Srebnijs A, et al. Heavy resistance training in breast cancer patients undergoing adjuvant therapy. Med Sci Sports Exerc. 2020;52:1239–47. [DOI] [PubMed] [Google Scholar]
- 36.Kamel FH, Basha MA, Alsharidah AS, Salama AB. Resistance training impact on mobility, muscle strength and lean mass in pancreatic cancer cachexia: a randomized controlled trial. Clin Rehabil. 2020;34:1391–9. [DOI] [PubMed] [Google Scholar]
- 37.Wochner R, Clauss D, Nattenmüller J, Tjaden C, Bruckner T, Kauczor HU, et al. Impact of progressive resistance training on CT quantified muscle and adipose tissue compartments in pancreatic cancer patients. PLoS One. 2020;15:e0242785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adams SC, Segal RJ, McKenzie DC, Vallerand JR, Morielli AR, Mackey JR, et al. Impact of resistance and aerobic exercise on sarcopenia and dynapenia in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. Breast Cancer Res Treat. 2016;158:497–507. [DOI] [PubMed] [Google Scholar]
- 39.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ataseven B, Luengo TG, du Bois A, Waltering KU, Traut A, Heitz F, et al. Skeletal muscle attenuation (Sarcopenia) predicts reduced overall survival in patients with advanced epithelial ovarian cancer undergoing primary debulking surgery. Ann Surg Oncol. 2018;25:3372–9. [DOI] [PubMed] [Google Scholar]
- 41.Ubachs J, Ziemons J, Minis-Rutten IJG, Kruitwagen R, Kleijnen J, Lambrechts S, et al. Sarcopenia and ovarian cancer survival: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2019;10:1165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang CY, Sun FJ, Lee J. Prognostic value of muscle measurement using the standardized phase of computed tomography in patients with advanced ovarian cancer. Nutrition. 2020;72:110642. [DOI] [PubMed] [Google Scholar]
- 43.Chae SH, Lee C, Yoon SH, Shim SH, Lee SJ, Kim SN, et al. Sarcopenia as a predictor of prognosis in early stage ovarian cancer. J Korean Med Sci. 2021;36:e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McSharry V, Glennon K, Mullee A, Brennan D. The impact of body composition on treatment in ovarian cancer: a current insight. Expert Rev Clin Pharmacol. 2021:1–10; 1065–1074 [DOI] [PubMed] [Google Scholar]
- 45.Bozzetti F Chemotherapy-induced sarcopenia. Curr Treat Options Oncol. 2020;21:7. [DOI] [PubMed] [Google Scholar]
- 46.Dieli-Conwright CM, Courneya KS, Demark-Wahnefried W, Sami N, Lee K, Buchanan TA, et al. Effects of aerobic and resistance exercise on metabolic syndrome, sarcopenic obesity, and circulating biomarkers in overweight or obese survivors of breast cancer: a randomized controlled trial. J Clin Oncol. 2018;36:875–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33:997–1006. [DOI] [PubMed] [Google Scholar]
- 48.Evans WJ, Hellerstein M, Orwoll E, Cummings S, Cawthon PM. D(3) -Creatine dilution and the importance of accuracy in the assessment of skeletal muscle mass. J Cachexia Sarcopenia Muscle. 2019;10:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harrigan M, Cartmel B, Loftfield E, Sanft T, Chagpar AB, Zhou Y, et al. Randomized trial comparing telephone versus in-person weight loss counseling on body composition and circulating biomarkers in women treated for breast cancer: the lifestyle, exercise, and nutrition (LEAN) study. J Clin Oncol. 2016;34:669–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stelten S, Hoedjes M, Kenter GG, Kampman E, Huijsmans RJ, van Lonkhuijzen LR, et al. Rationale and study protocol of the Physical Activity and Dietary intervention in women with OVArian cancer (PADOVA) study: a randomised controlled trial to evaluate effectiveness of a tailored exercise and dietary intervention on body composition, physical function and fatigue in women with ovarian cancer undergoing chemotherapy. BMJ Open. 2020;10:e036854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thomson CA, Crane TE, Miller A, Garcia DO, Basen-Engquist K, Alberts DS. A randomized trial of diet and physical activity in women treated for stage II-IV ovarian cancer: rationale and design of the Lifestyle Intervention for Ovarian Cancer Enhanced Survival (LIVES): An NRG Oncology/Gynecologic Oncology Group (GOG-225) Study. Contemp Clin Trials. 2016;49:181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou Y, Chlebowski R, LaMonte MJ, Bea JW, Qi L, Wallace R, et al. Body mass index, physical activity, and mortality in women diagnosed with ovarian cancer: results from the Women’s Health Initiative. Gynecol Oncol. 2014;133:4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Minlikeeva AN, Cannioto R, Jensen A, Kjaer SK, Jordan SJ, Diergaarde B, et al. Joint exposure to smoking, excessive weight, and physical inactivity and survival of ovarian cancer patients, evidence from the Ovarian Cancer Association Consortium. Cancer Causes Control. 2019;30:537–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


