Abstract
Recent success of mRNA-based covid vaccines have bolstered the strength of nucleic acids as a therapeutic platform. The number of new clinical trial candidates is skyrocketing with the potential to address many unmet clinical needs. Despite the advancements in other aspects, systemic delivery of nucleic acids to target site remains a major challenge. Thus, nucleic acid-based therapy is yet to reach its full potential. In this review, we shed light on a select few prospective technologies that exhibit substantial potential over traditional nanocarrier designs for nucleic acid delivery. We critically analyze these systems with specific attention to the possibilities for clinical translation.
Keywords: Nucleic acid, Lipid Nanoparticles, Gene Delivery, mRNA Delivery, RNAi
Introduction:
Nucleic acid therapies have emerged as a potential cure-to-all solution to many diseases owing to the possibility of specific alteration of misfiring genes (such as gene editing, inhibition, etc.).1,2 Recent advancements in the understanding of disease pathogenesis have created demands for gene therapy as a promising alternative to traditional medicine, especially in hard-to-cure diseases.3 The increased rate in clinical approval of nucleic acid therapeutics and innumerable candidates in different phases of clinical trials demonstrate the potential of nucleic acids as therapeutics. The clinical success of oligonucleotide-based drug Spinraza (Eondys 51) followed by FDA approval of first siRNA drug Onpattro (Patisiran, ALNTTR02)4 brought the spotlight onto the potentiality of gene therapies. The recent approvals of various nucleic acid based COVID-19 vaccines (Pfizer & BioNTech, Moderna, Johnson & Johnson) by the FDA and EMA have instigated a new wave of nucleic acid-based therapies.5–8
Although the therapeutic success of nucleic acid-based vaccines is recognized, there are a few delivery barriers which limit the recognition of nucleic acids as viable therapeutics in broader biomedical applications. First, nucleic acids with their high residual negatively charged backbone are impermeable to the plasma membrane.3,9 Second, unmodified nucleic acids are systemically unstable due to the presence of nucleases in bodily fluids.10 Both these factors have necessitated the development of smart strategies to efficiently deliver functional nucleic acids.9–11 Utilization of a delivery vector often provides protection against nucleases alongside efficient intracellular delivery across cell membrane.11–16 10,18 For intracellular delivery, viral vector platforms show promise, but these have some drawbacks such as immunogenicity, difficulty of assembly, cytotoxicity and inflammatory responses.17–19 Non-viral delivery platforms hold the potential to address some of these shortcomings.14–16,20–24 Among non-viral delivery systems, lipid nano assemblies are considered especially promising.11,23,25–28 The recent success of lipid nanoparticle (LNP) based mRNA vaccines brought the spotlight on self-assembled lipid nanostructures as “go-to” delivery vehicles for therapeutic nucleic acids.
Despite the clinical success of LNPs, there are still significant room of improvement. In general, major bottlenecks in lipid-based nano assemblies include poor long-term stability29,30 low cytosolic delivery31 and lack of active targeting for site-specific delivery. Explicit attention to address these pitfalls presents an enormous opportunity. In this review, we survey and critically analyze the ongoing research on nucleic acid drugs (Figure 1) with a specific focus on unmet needs, pitfalls, and potential scopes for improvement.
Figure 1:
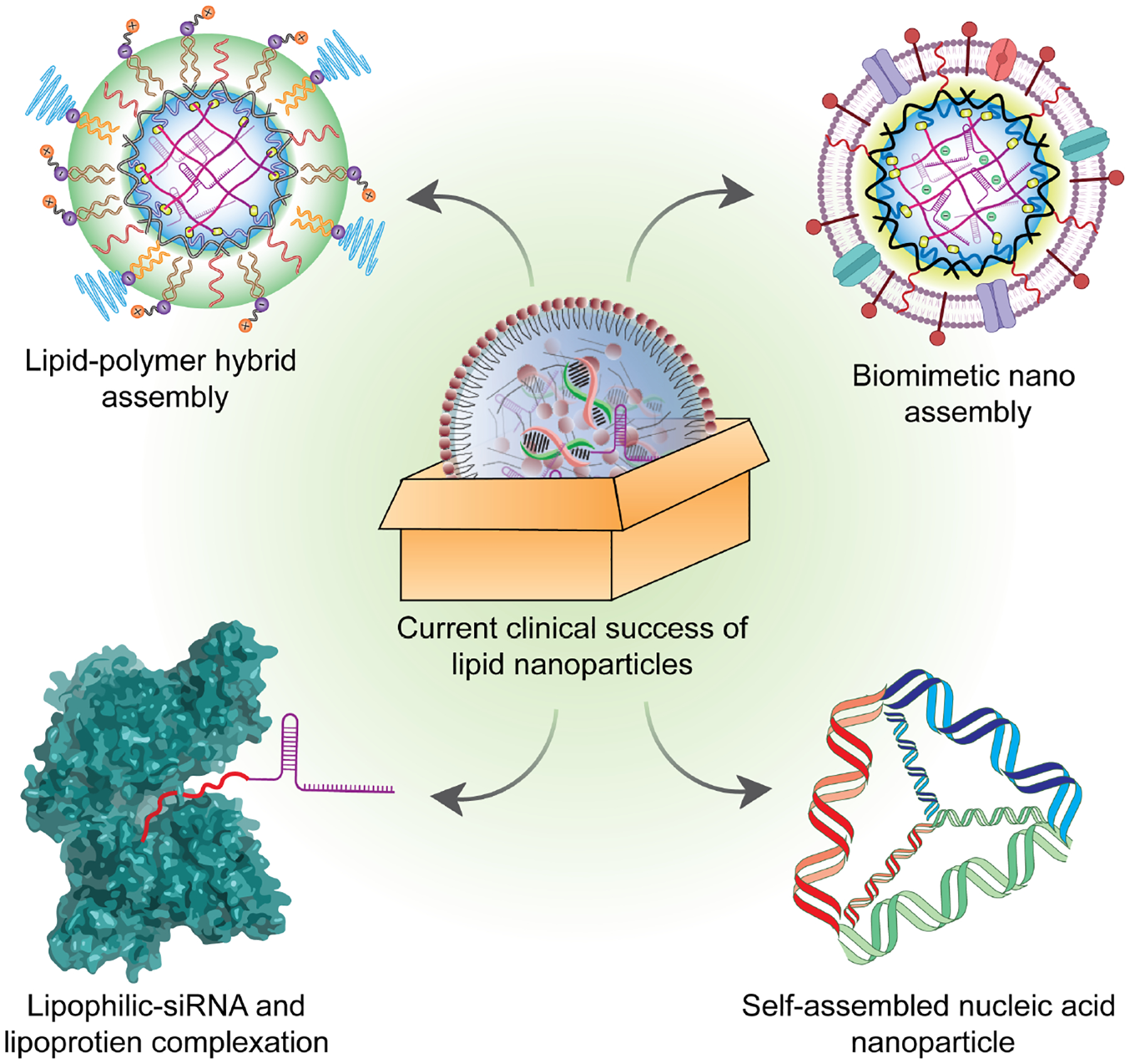
The figure highlights four different methodologies for nucleic acid delivery with potential clinical adaptability for future nucleic acid therapeutics.
The success journey of lipid nanoparticles for gene delivery:
Recent success with mRNA vaccines against COVID-19 highlights the prospect of LNPs as delivery vectors for gene therapies.32 Here, we list a few noteworthy candidates that are progressing in clinical trials for a variety of applications (Table 1). For example, Arcturus Therapeutics has presented an interesting nucleic acid approach towards COVID-19 vaccines. They use a self-replicating mRNA encapsulated in their LUNAR® LNP that is based on a proprietary ionizable lipid, DSPC, cholesterol, and PEG2000-DMG.33 Their self-replicating RNA is comprised of the replicase genes, nsP1-nsP4 of the Venezuelan equine encephalitis virus along with the pre-fusion spike protein of SARS-CoV-2. This candidate has shown exciting results in pre-clinical trials due its self-amplifying capability that is owed to the replicase genes, nsP1-nsP4, allowing for increased expression of the spike protein when compared to the pre-fusion spike protein alone. nsP4 encodes for an RNA polymerase which is primarily responsible for the spike protein to replicate, causing the increased expression, while nsP1 and nsP2 aid in the stability of the mRNA’s that are produced through capping and a polyA tail. This self-replicating RNA vaccine is double stranded and this double stranded nature can also cause an adaptive immune response through a type I IFN response. This mRNA based COVID-19 vaccine, CureVac, is in the clinical pipeline with a commercial name CVnCoV. The lipid-based formulation, which encapsulates the mRNA encoding the spike protein, is composed of an ionizable amino lipid, a phospholipid, cholesterol, and a PEGylated lipid.34 These two formulations suggest the overlapping themes that are seen in many successful LNP formulations, i.e., using a combination of ionizable lipids with a PEGylated lipid and a phospholipid, along with a certain percentage of cholesterol.
Table 1.
A list of major nucleic acid candidates currently in clinical trials
| Name | Company | Formulation | Nucleic acid | Disease | Target/encoded protein | Clinical trial phase | ID number |
|---|---|---|---|---|---|---|---|
| ARCT-154 | Arcturus Therapeutics, Inc. | LUNAR (LNP) | self-replicating mRNA | COVID-19 vaccine | Spike Protein | Phase 3 | NCT05012943 |
| ARCT-810 | Arcturus Therapeutics, Inc. | LUNAR (LNP) | mRNA | Ornithine Transcarbamylase Deficiency | Ornithine Transcarbamylase (OTC) | Phase 1,2 | NCT04442347 |
| ARCoV | Walvax Biotechnology, Abogen Biosciences | Ionizable lipid | mRNA | COVID-19 vaccine | receptor binding domain (amino acids 319–541) | Phase 3 | NCT04847102 |
| mRNA-1647 | Moderna | Ionizable LNP | mRNA | Cytomegalovirus Infection | CMV gH Pentamer complex and herpesvirus glycoprotein (gB) protein | Phase 3 | NCT05085366 |
| mRNA-1345 | Moderna | Ionizable LNP | mRNA | Respiratory Syncytial Virus | RSV Fusion Protein | Phase 2, 3 | NCT05127434 |
| mRNA-4157 | Moderna/Merck | Ionizable LNP | mRNA | Solid tumors/Melanoma | tumor-associated antigens | Phase 2 | NCT03313778 |
| mRNA-3705 | Moderna | Ionizable LNP | mRNA | Methylmalonic acidemia | human Methylmalonyl-Coenzyme A Mutase (hMUT) | Phase 1, 2 | NCT04899310 |
| mRNA-2416 | Moderna | Ionizable LNP | mRNA | Relapsed/Refractor y solid tumor malignancies lymphoma, Ovarian Cancer | OX40 Ligand (OX40L) | Phase 1, 2 | NCT03323398 |
| mRNA-1273.617 | Moderna | Ionizable LNP | mRNA | COVID-19 delta variant | N/A | Phase 2 | NCT04927065 |
| mRNA-1273 | Moderna | Ionizable LNP | mRNA | COVID-19 | Spike Protein | Phase 3 | NCT04860297 |
| mRNA-5671 | Moderna/Merck | LNP | mRNA | Neoplasms | KRAS | Phase 1 | NCT03948763 |
| BNT112 | BioNTech | RNA-Lipoplex | mRNA | Prostate Cancer | prostatic acid phosphatase (PAP), prostate-specific antigen (PSA), 3 antigens | Phase 1, 2 | NCT04382898 |
| BNT111 | BioNTech | RNA-Lipoplex | mRNA | Advanced Melanoma | NY-ESO-1, MAGE-A3, and TPTE | Phase 2 | NCT04526899 |
| BNT113 | BioNTech | RNA-Lipoplex | mRNA | human papillomavirus 16 (HPV16)+ head and neck cancer | HPV-16 E6 and E7 | Phase 2 | NCT04534205 |
| BNT122 | BioNTech/Genentech | RNA-Lipoplex (RNA-LPX) | mRNA | 1L Melanoma/Adjuvant Colorectal Cancer | N/A | Phase 2 | NCT04486378 |
| BNT151 | BioNTech | LNP | mRNA | Solid Tumors | IL-2 | Phase 1, 2 | NCT04455620 |
| BNT161 | BioNTech/Pfizer | LNP | mRNA | Seasonal Influenza | influenza antigens | Phase 1 | N/A |
| BNT162 | BioNTech/Pfizer | LNP | mRNA | COVID-19 | Spike protein | Phase 4 | NCT05168709 |
| CVnCoV | CureVac AG | Ionizable LNP | mRNA | COVID-19 vaccine | Spike protein | Phase 3 | NCT04652102 |
| CV7202 | CureVac AG | LNP | mRNA | Rabies | Rabies lyssavirus glycoprotein (RABV-G) | Phase 1 | NCT03713086 |
| INT-1B3 | InteRNA | LNP | miRNA | Solid Tumors | miR-193a-3p | Phase 1 | NCT04675996 |
| MiNA | MTL-CEBPA | NOV340 Smarticles | saRNA | Liver Cancer | CCAAT/enhancer-binding protein alpha (CEBPA) | Phase 1 | NCT02716012 |
| Onpattro (patisiran) | Alnylam | LNP | siRNA | ATTR Amyliodosis-Expansion | Transthyretin | Phase 3 | NCT03997383 |
In addition to LNPs, BioNTech also uses RNA-Lipoplexes (RNA-LPX) in their large number of mRNA-based formulations in the pipeline. In another successful candidate, BNT113, utilizes the RNA-LPX platform containing a cationic liposome consisting of N-[1-(2,3-dioleyloxy) propyl]-N, N, N-trimethylammonium chloride (DOTMA) and 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE).35 This BNT113 is a vaccine formulation against human papillomavirus (HPV), a virus that has been linked to causing cancer. Similarly, MiNA, a company focused on small activating RNAs (saRNA), has developed a formulation for liver cancer that is in Phase 1 clinical trial. The saRNA here is designed to upregulate CCAAT/enhancer-binding protein alpha (CEBPA) that is encapsulated in a proprietary liposomal nanoparticle, NOV340 SMARTICLES®.36 These ionizable liposomes are anionic or neutral that become slightly cationic in the tumor environment to enhance cellular internalizations.37 This charge-conversion strategy presents a new direction in designing delivery vectors for targeted therapeutics.38
Lipid nanoparticles, liposomes and other lipid conjugation strategies have become a popular choice for clinical gene therapy. Though a significant number of clinical candidates have been already approved or being evaluated, as exemplified in Table 1, there remains a significant room for improvement. In the next section, we discuss few next generation strategies that promise to overcome some of the stumbling blocks in the current generation of clinical candidates.
Next generation materials for efficient nucleic acid delivery:
An array of synthetic materials ranging from polymeric nanoparticles39,40 to lipid assemblies25,41,42 and cell penetrating peptides20,21 has been reported as alternatives of viral gene delivery vectors for efficient and non-toxic nucleic acid delivery. The success of LNPs in the clinic has generated more confidence in non-viral technologies than ever before. As a consequence, new technologies that can address pitfalls of lipid nano assemblies are emerging. In this review, we focus on a select few strategies with the potential for clinical translation of nucleic acids therapeutics.
Core/ shell lipid-polymer-hybrid assemblies:
Lipid based nanocarriers, such as liposomes, solid-lipid nanoparticles and other nanostructured lipid vectors offer advantages in features such as bioavailability, trapping efficiency, and low production cost.43,44 These advantages are also accompanied by some liabilities, often arising from poor encapsulation stability and high polydispersity.45,46 On the other hand, polymeric nanoassemblies are known to exhibit excellent encapsulation stability, low polydispersity, and convenient synthetic procedures.47,48 However, their poor bioavailability has been major stumbling block in their clinical translation.47,49 Lipid-polymer hybrids are being developed as the next generation formulation that strive to include the best of both lipid and polymeric systems.
Structurally, lipid-polymer hybrids consist of two functional components (i) a hydrophobic polymer/lipid core to facilitate stable encapsulation of the cargo (ii) a stealth lipid layer for enhanced biocompatibility of the carrier.50–52 This bi-component approach often offers significant tunability in carrier design. The independent structural tunability of each of these modules opens parametric optimizations that are critical for maximizing on-target delivery efficacy of functional biologics. Despite the success of this concept in efficient delivery of hydrophobic drugs,52,53encapsulation of highly charged and hydrophilic nucleic acids poses a significant barrier. To this end, a differentially charged hollow core-shell approach has been employed with a lipid-polymer-lipid hybrid design. GFP siRNA was encapsulated in a cationic lipid inner core surrounded by a hydrophobic PLGA shell and a neutral lipid encasing to form a stealth surface layer (Figure 2A).54,55 Following the success of initial concept, an improved version of lipid-polymer hybrid using lipidoid G0-C14 in the nanoparticle core, which showed a significant increase in stability with release half-life of ~9 days mark, compared to ~8 h lipofectamine2000.55,56 In vivo validation of the hybrid formulations in delivering PHB1 siRNA established the potential of the nano delivery platform with ~76% decrease in PHB1 expression in treated group.56 Inspired by such initial promise, several structural variations in polymer-lipid combinations have been explored.57,58 Among them, charge-reversing polymers appeared to be particularly interesting due their ability to switch surface charge from cationic to anionic or vice versa, on demand, which mitigates the cation-mediated toxicity observed with traditional polymeric transfection agents.38,58 To this end, our group envisaged a polymer system that uses a “bait-and-switch” strategy to incarcerate siRNA in a tightly packed polymeric core using a novel ad hoc electrostatic encapsulation process that eliminates cationic charges.59,60 To further improve the system by harnessing the advantages of lipid coating, the formulation was completed by encasing a zwitterionic lipid shell using hydrophobic alkyl chains on the polymer as handles to obtain “virus-inspired” symbiotic self-assemblies (Figure 2C).61 These nanoparticles show efficient silencing of three different genes GFP, PLK1 and MDR1 with negligible cytotoxicity compared to commercial transfection agents (Figure 2D, E). A prime utility of the tunability design is the scope it provides to modulate the nanoparticles with specific targeting abilities. For instance, role of surface coating in such lipid-polymer hybrids has been investigated with a specific focus on penetration across blood-brain-barrier (BBB).62 In one such study, a PLGA core was coated with DSPE-PEG alongside four discrete lipids, viz., polysorbate 80 (PS 80), poloxamer 188 (Pluronic F-68), DSPE-PEG-glutathione (GSH) and DSPE-PEG-transferrin (Tf). These formulations were then used to study the effect of each individual components in facilitating BBB penetration of nanoparticles. Among all the variations GSH-NP and PS 80-NPs showed the maximum amount of penetration through intact BBB. With further optimization PS 80-NPs showed an impressive 90% decrease in luciferase expression in Neuro-2a cells. The BBB penetration ability of PS 80-NPs were further evaluated in weight drop induced traumatic brain injury (TBI) model. Dy677-siRNA loaded PS 80-NPs showed five-fold and three-fold higher fluorescence intensity in brain tissue compared to free siRNA and PEG-NPs. Finally, Tau-siRNA loaded PS 80-NPs showed approximately 70% down regulation of Tau expression levels in primary neuron cells followed by 50% blocking of Tau expression in mice model, which explicitly demonstrates the potential of this tunable design. Overall, lipid-polymer hybrids present a robust delivery platform with enhanced stability and extensive structural tunability. This indeed opens new avenues for optimizing delivery efficiency and potential translation to the clinic.
Figure 2:
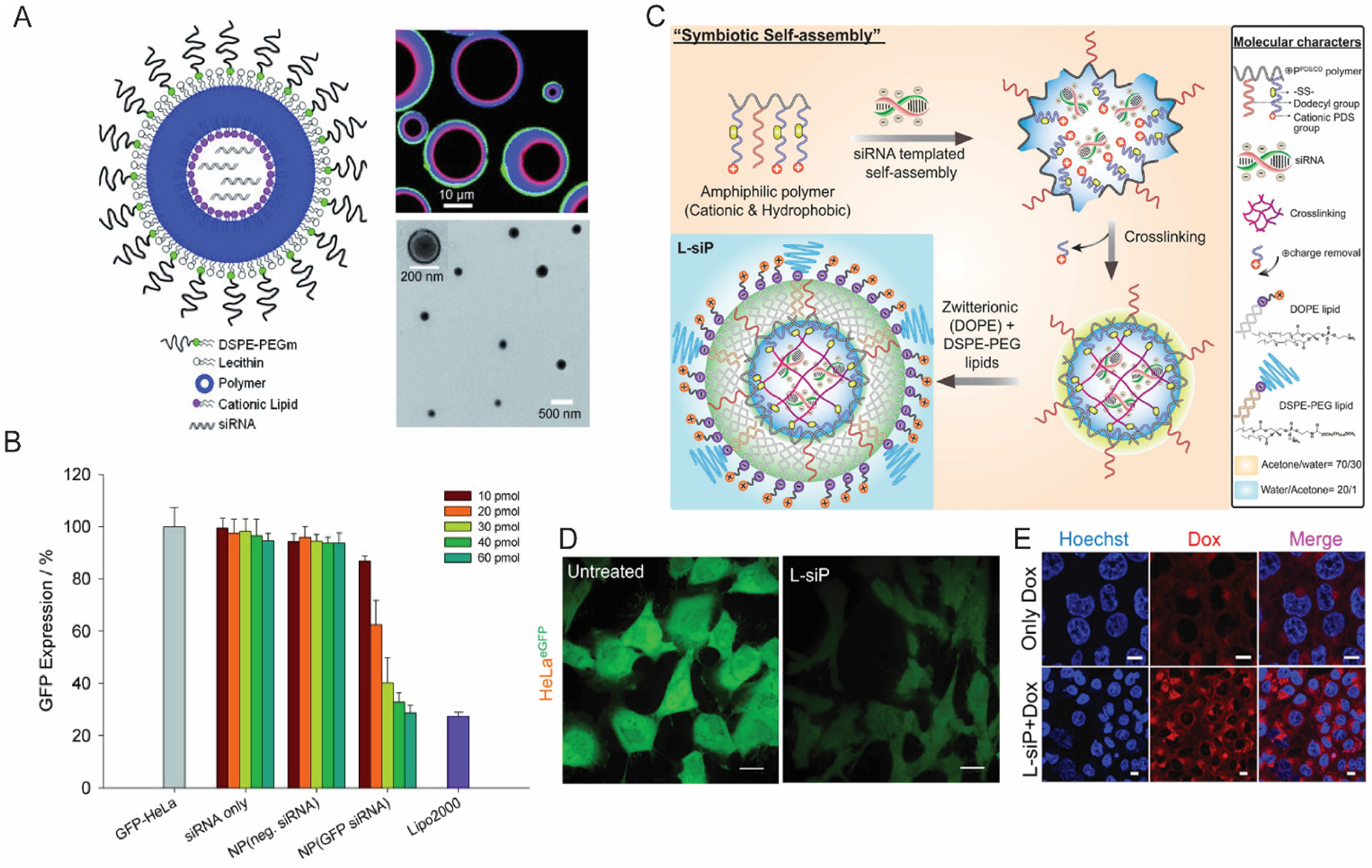
(A) A schematic illustration of core/shell lipid-polymer-lipid hybrid nanomaterial, with confocal microscopy image (right top) and TEM image (right bottom) confirming the core/shell morphology (B) Flow cytometry data showing dose dependent GFP knockdown in HeLa cells. (Reproduced with permission from reference 54, Copyright 2011 Wiley-VCH Verlag GmbH & Co) (C) A schematic illustration depicting the formulation procedure of “virus mimicking” lipid-polymer hybrid particles. (D) Confocal microscopy data showing efficient knockdown of GFP in HeLa cells; scale bar, 20 μm. (E) Effect of treating NCI-ADR/RES cells with MDR1 siRNA using lipid-polymer hybrid nano-assemblies; scale; 10 μm. (Reproduced with permission from reference 61, Copyright 2019 American Chemical Society)
Biomimetic nano-assemblies:
Bioinspired nanoparticles have gained popularity in the nucleic acid delivery. Although advancements in the field of nanotechnology have aided nanoparticle-mediated biomacromolecular delivery platforms, in vivo performance of nanoparticles remains underwhelming. Thus, there is significant interest in using biomimetic nanoparticles (BNP) to address critical shortcomings in purely synthetic nanomaterials.63 Owing to their similarity with the surface functionalities of the parent cell, BNPs provide a wide range of functions such as low immunogenic response, long circulation time and innate disease relevant targeting abilities (Figure 3A& B).63–65 BNPs can be divided into two broad categories (i) endogenous membrane debris such as cell membrane fragments, exosomes and microvesicles, with the latter two generally classified together as extracellular vesicles (EVs). (ii) cell-membrane coated nanoparticles, where a synthetically prepared nanoparticle is wrapped in cell membrane camouflage.
Figure 3:
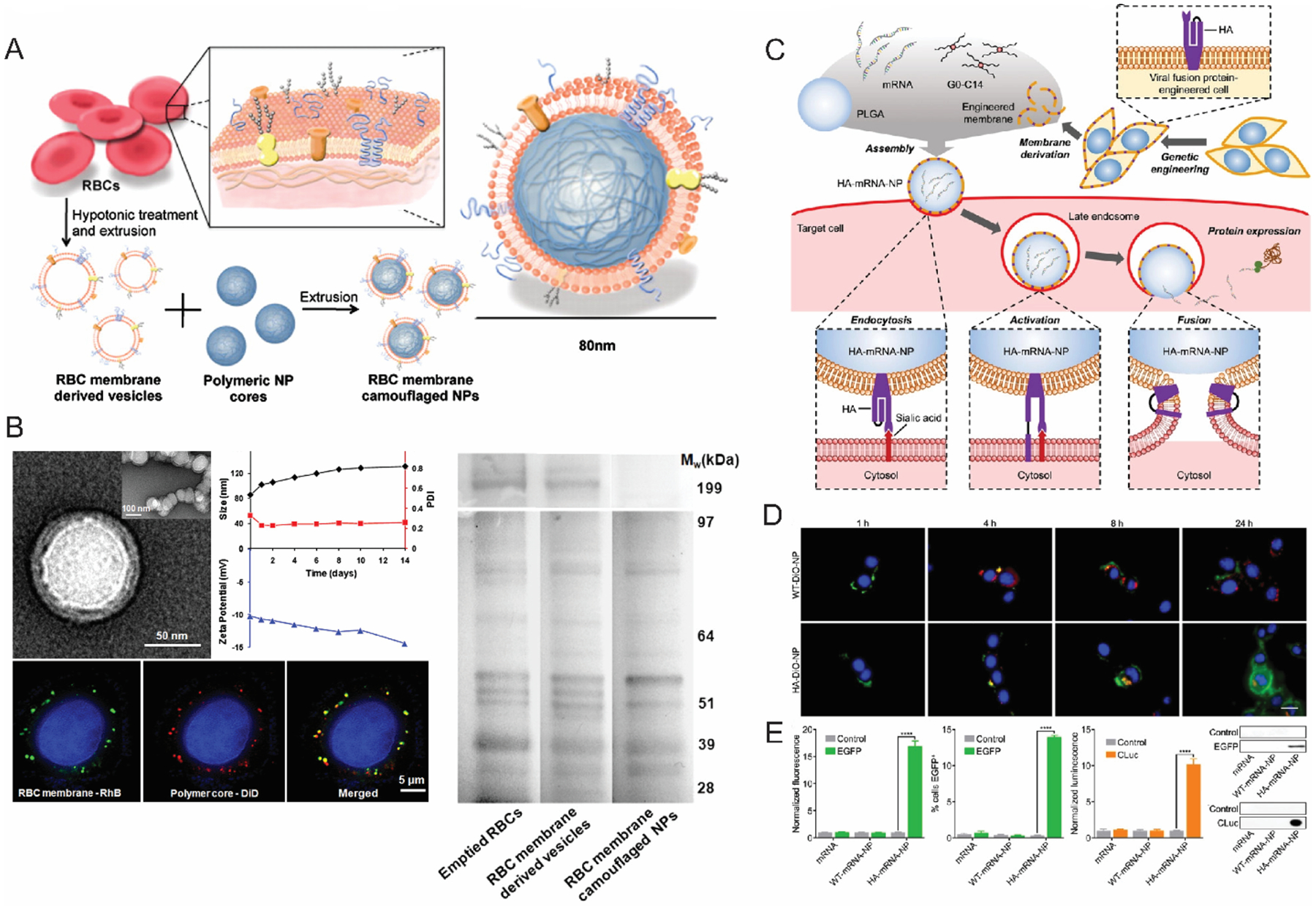
(A) A schematic illustration of RBC membrane coated PLGA nanoparticles (B) TEM and confocal microscopy characterization of RBC membrane coated nanoparticles (left); size and zeta potential data showing the stability of nanoparticles; SDS-PAGE data (right) shows the similarity of protein expression of the RBC derived membranes with native RBC membranes (Reproduced with permission from reference 65, Copyright 2011 The National Academy of Sciences of the USA) (C) mRNA delivery strategy utilizing genetically modified cells expressing viral fusion protein hemagglutinin (HA). (D) Endosomal escape and cytosolic localization of Dio-labeled nanoparticles in B16-WT cells; scale bar 20 μm (E) Expression of eGFP and cLUC in B16-WT cells, confirmed with flow cytometry and western blots. (Reproduced with permission from reference 83, Copyright 2022 Wiley-VCH Verlag GmbH & Co)
A variety of cell types like, red blood cells (RBC)66,67, immune cells68,69, cancer cells64,70 or stem cells71–73 have been exploited in the past for their unique targeting capabilities. For example, owing to the “self-markers” (e.g., CD47 proteins, various peptides and glycans) RBC-based nanoparticles can evade immune clearance effectively, which prolongs the circulation half-life.74,75 Vectors designed from red blood cells have shown promising results in delivering therapeutic cargo ranging from small drug molecules to much larger biomolecules such as proteins and nucleic acids.65,75 In one example, rapamycin-loaded PLGA particles camouflaged in a RBC membrane envelope show successful delivery to atherosclerotic plaques with minimal macrophage mediated phagocytosis.74 The potential of BNPs is not only limited to evading immune clearance and excellent targeting capabilities. In past few years, advancements in BNP engineering process have gained attention in many cumbersome targets, such as translocating therapeutics across blood brain barrier (BBB). For instance, GAPDH and BACE1 siRNA were delivered specifically to neurons, microglia, and oligodendrocytes across the BBB through endogenous EVs isolated from primary dendritic cells expressing LAMP2b which is specifically known to fuse with neuronal RVG peptides.76 In general, the modularity offered in BNP systems is unparalleled due to the compatibility with widespread selection of cell types and characteristics.77–80 For instance, blood cell membranes are usually used for prolonged circulation, whereas immune cells and tumor cells provide explicit targeting capabilities.63,66,67,71 Interestingly, engineered cell membranes and hybrid cell membranes were also reported as precursors for vector design process.81,82 Unlike vectors derived from a single cell type, these hybrids are not limited to the native characteristics of the source cells i.e., these vectors can be conferred with a wide range of functions. An example of that is the use of genetically engineered B16F10 cells that express a viral fusion protein hemagglutinin (HA) to mimic viral mechanism of payload delivery (Figure 3C).83 HA expression on the surface of influenza viruses has been reported to cause escape from endosomal entrapment.84 HA proteins consist of two subunits. While the HA1 subunit facilitates the attachment of virus on cell surface, HA2 subunit undergoes conformational change triggered by low pH at late endosomal stage causing fusion of viral envelope with endosomal membrane resulting in endosomal escape of the viral payload.85–87 These engineered nanoparticles were used to deliver EGFP and CLuc mRNA into cytosol (Figure 3D), which indeed exhibits a superior endosomal escape ability and significantly better transfection (Figure 3E). Overall, the robustness, modularity and structural similarities with cellular membranes make BNPs an attractive choice for therapeutic gene delivery with potential for clinical success.
Lipophilic conjugates linked to siRNA:
Another well-explored approach involves lipoprotein particles, formed by the complexation between lipophilic-siRNA and lipophilic proteins. Hydrophobic conjugation of siRNA with cholesterol, lipids, fatty chain conjugates have shown to increase the stability, biodistribution, and gene silencing efficiency.88–90 These lipophilic siRNAs have affinity towards lipoproteins that are rich in phospholipids and cholesterol. The journey started with covalent conjugation of cholesterol to siRNA which facilitated the cellular internalizations and efficient gene silencing.91 In this example, a mechanistic aspect of mRNA degradation by cholesterol conjugated apoB-siRNA (Chol-apoB-siRNA) was studied. The modified siRNA could silence the apoliprotein B (apoB) gene in vivo in transgenic mouse model.71 In another example, cholesterol, bile acids and long chain fatty acids were conjugated to siRNA and the delivery mechanism of these lipophilic-siRNAs were studied in vivo.92 In this study, the preassembled formulation of cholesterol-siRNA with lipoprotein (high density lipoprotein) was ~8 to ~15 times more effective at silencing apoB protein expression than the same amount of unbound cholesterol-siRNA. The length of fatty acid chains and the binding affinity of these conjugates to lipoproteins were found to be crucial for efficient and selective internalizations. The longer chain siRNA conjugates (docosanyl and stearoyl) were more effective at apoB gene silencing in vivo than the shorter chain conjugates (lauroyl and myristoyl) because the former showed strong interaction with high density lipoproteins. In another study, the preferential association of lipophilic siRNAs with lipoproteins based on their hydrophobicity was evaluated in vivo. Higher hydrophobic siRNA (hsiRNA) conjugates spontaneously associated with low density lipoprotein whereas, the lesser hydrophobic conjugates preferred high-density lipoproteins (Figure 4a, b).90 Spontaneous binding with lipoprotein improved the plasma half-life of these hsiRNAs and showed slower clearance kinetics. The cell-specific distribution and gene-silencing efficacies of these lipoprotein-hsiRNA complexes were determined by PpibmRNA level quantification in different organs of mice.
Figure 4:
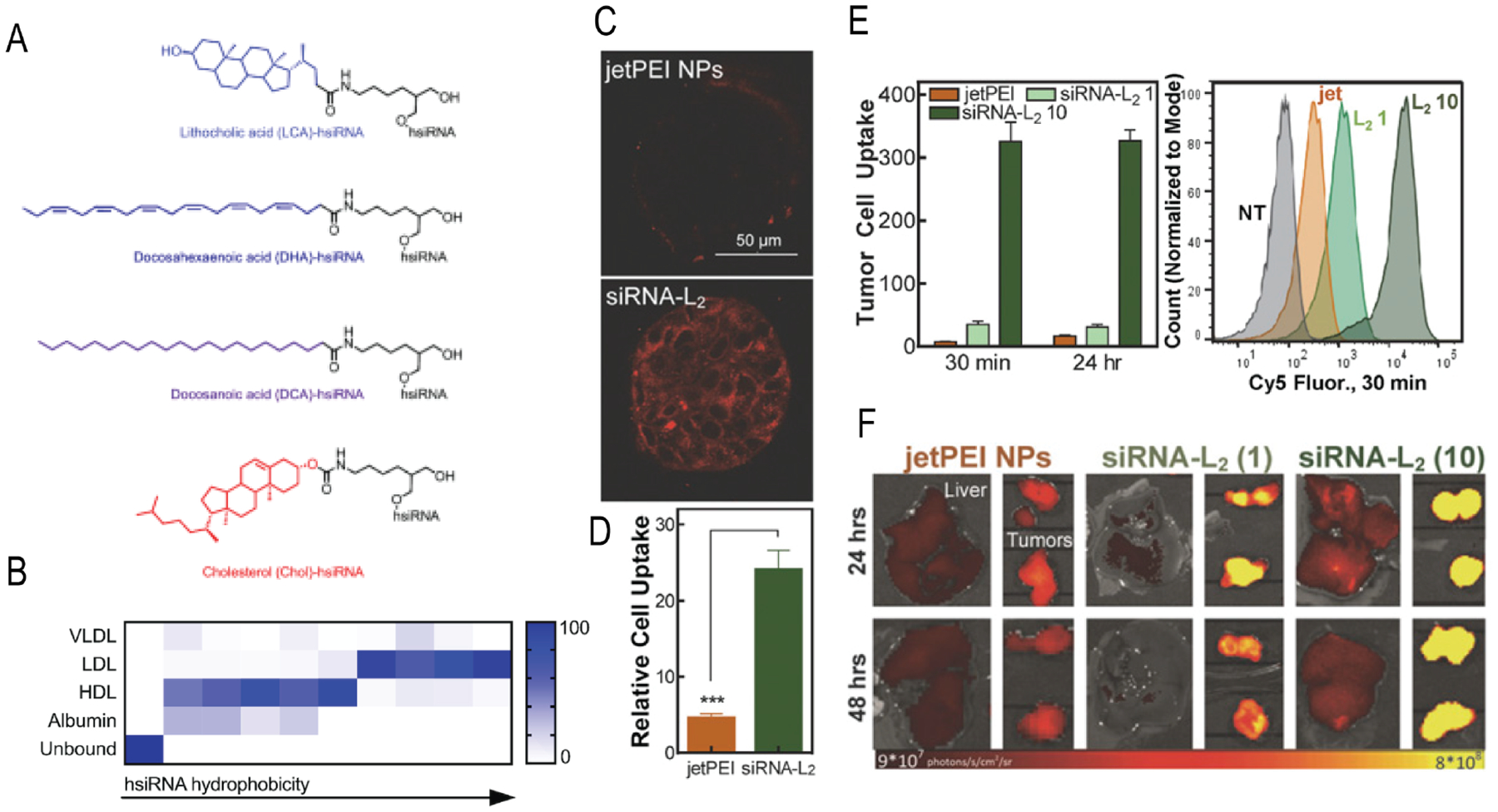
(A) Different chemical structures conjugated to hsiRNA to understand the effect of hydrophobicity towards lipoproteins. (B) The summary of the effect of hsiRNA hydrophobicity on protein binding. (Reproduced with permission from reference 90, Copyright 2018 Oxford University Press) (C) Confocal microscopy showing the tumor spheroid penetrations and internalizations of siRNA-L2. (D) Comparative cellular uptake of Cy-5 conjugated siRNA-L2 and jetPEI NPs with Cy5 siRNA in MCF-7 spheroids. (E) Internalization of jetPEI NPs (1 mg/kg) and siRNA-L2 (1 mg/kg and 10 mg/kg) in tumor cells isolated from xenograft mouse model post 1 h injection. (F) Accumulations of jetPEI NPs and siRNA-L2 in liver and tumor. (Reproduced with permission from reference 93, Copyright 2017 The National Academy of Sciences of the USA)
Recently, diacyl lipid conjugated siRNA was found to bind albumin which significantly increased the blood circulation time with increased tumor penetration and accumulation (Figure 4c, d).93 Serum has abundance of albumin (>40 mg/mL) with ~20-day circulation half-life.94 Albumin has been used extensively as carrier of therapeutics and a few formulations such as Abraxane, Levemir, and Optison already been approved for clinical applications.95,96 The diacyl lipid modified siRNA (siRNA-L2) showed 5.7-fold increased circulation half-life and 8.6-fold increased bioavailability compared to free siRNA. Also, in comparison to commercial siRNA nanocarrier jetPEI, siRNA-L2 showed 19-fold better tumor accumulations and 46-fold increase in per-tumor-cell uptake in a triple-negative breast cancer mouse model (Figure 4e, f). The albumin-hitchhiking of lipophilic siRNA conjugates is (i) a synthetically simple strategy, (ii) showed improved tumor cellular uptake with enhanced gene silencing efficacy, and (iii) better pharmacokinetics and safe at high dose. Therefore, this approach possesses the potential for clinal translation in RNAi-based cancer therapy.
Self-assembled nucleic acid nanoparticles:
Exploring the base pair complementarity driven self-assembly to create nucleic acid based nanosystems for delivery is another interesting direction in this area. DNA nanotechnology has been exploited to develop delivery vehicles where DNA self-assembles through the Watson-Crick base pairing.97,98 The potential for this platform arises from their (i) well defined shape and easily tunable size with monodispersity, (ii) biocompatibility, (iii) convenient functionalization with desired chemical conjugations, and (iv) programmability to introduce stimuli-responsive release of cargo.99–101 This technology has been explored for intracellular drug102,103 and protein delivery,104 and has the potential to be extended to nucleic acids. For example, a self-assembled DNA tetrahedral nanostructure was reported for siRNA delivery which showed effective gene silencing in vivo in a nude mouse bearing KB xenograft tumor.105 The tetrahedron was decorated with cancer-targeting folate ligands and it showed >50% firefly luciferase gene silencing in HeLa cells. The study showed the importance of number and spatial orientation of these ligands on the surface of the particle. The precise control in size of these nanoparticles (~28.6 nm) avoided renal filtrations and showed improved blood circulation time (half-life ~24.2 min) than the siRNA itself (half-life ~6 min). These nanostructures can be programmed to be responsive to external and internal stimuli. Many studies are shown to use endogenous stimuli to release the cargo. In one example, a DNA ‘nanosuitcase’ has been shown to stably encapsulate siRNA and release it only in the presence of the endogenous recognition sequence (oligonucleotide trigger) (Figure 5A).106 The flexibility with this system offers AND-gated responses for both targeting and synergistic therapies. The DNA cage showed controlled release of siRNA upon recognition of two trigger strands (Bcl-2 and Bcl-xL) related to antiapoptotic genes. The successful release of the cargo in fixed HeLa cells was monitored via FRET studies. The strategic placement of Cy3 and Cy5 dyes in the nanosuitcase exhibited FRET signal and the successful release of cargo was monitored by the disappearance of signal (Figure 5B). In another example, a spherical micellar particle was synthesized from monodispersed sequence specific DNA-polymer conjugates.107 This spherical nucleic acid (SNA) showed stable encapsulation of 18-mer antisense oligonucleotide with a triggered release of cargo via strand displacement. The hydrophobic core and DNA corona formed stable micellar particles in aqueous media. SNA could stably encapsulate phosphorothioated antisense oligonucleotide (ASO) and efficiently downregulate luciferase in HeLa cells. In essence, ease of synthesis, high specificity towards stimuli, and ability to introduce AND gated strategies108 makes nucleic acid based nanoassemblies potential candidates for delivery.
Figure 5:
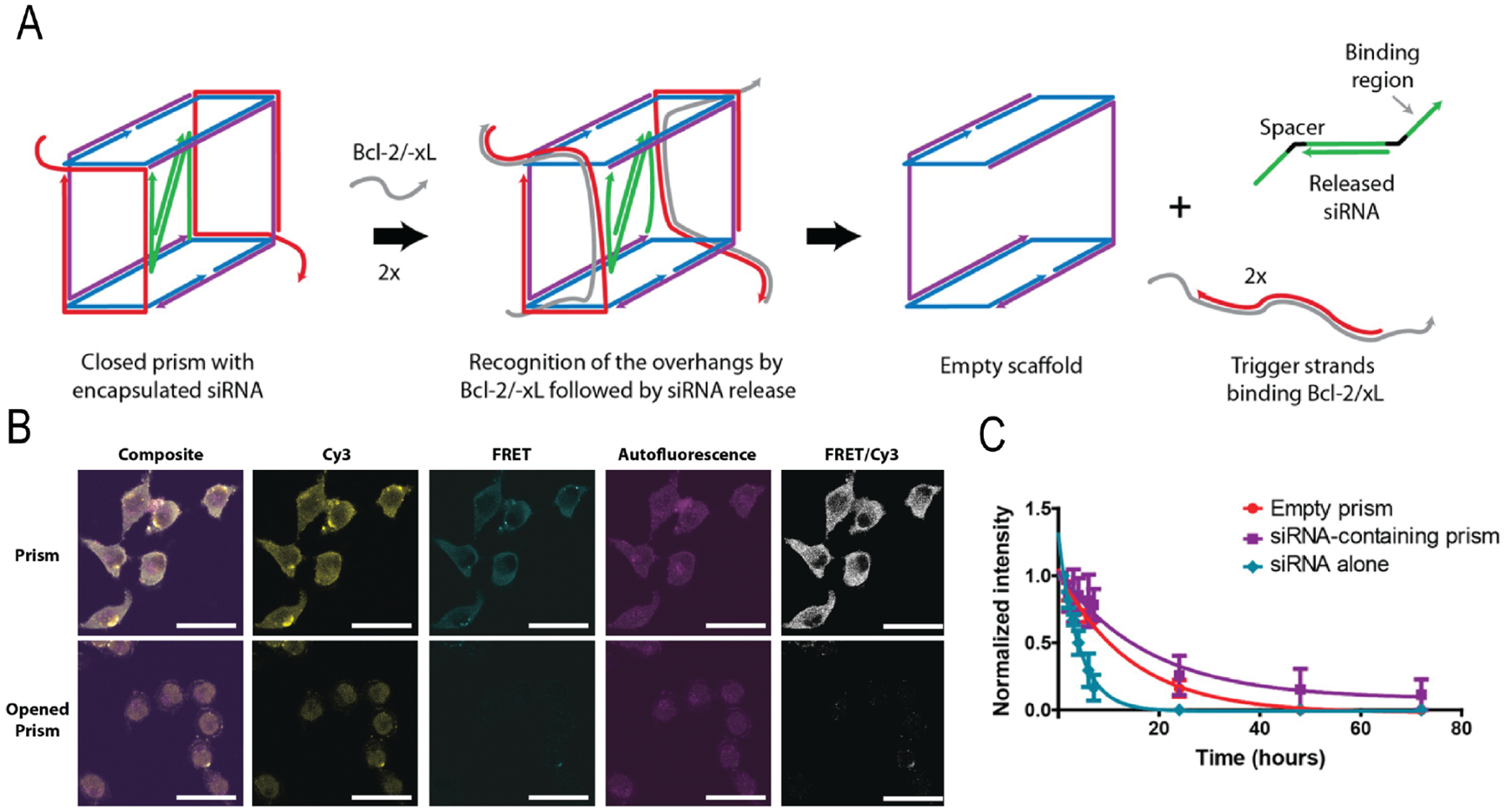
(A) Schematic representation of siRNA-encapsulating prism and siRNA release mechanism. (B) Spectral images of prism in fixed cells before and after the release of cargo. (C) Stability of siRNA-encapsulated prism in serum. (Reproduced with permission from reference 106, Copyright 2016 American Chemical Society)
Discussion and perspective:
In this review, we have discussed a few strategies for nucleic acid delivery that have shown exciting potential during the initial stages of investigations. These systems share conceptual similarities to address major drawbacks of traditionally utilized lipid-based nanoassemblies. Despite achieving initial success, these technologies are far from perfect. As mentioned previously, stability of nanocarriers has been one of the key hurdles faced by the traditional lipid-based systems. In this context lipid-polymer hybrids offer the potential to address this pitfall due the extent of tunability in both the polymer design and selection of lipid components. Careful design of the polymer core can ensure stable long-term encapsulation of nucleic acid for a long period, whereas systematic variation in the lipid shell can optimize delivery requirements.54,61 Both lipid-polymer hybrids hold the key benefit of modularity in their approach. Despite the ingenuity from a materials standpoint, significant hurdles remain before their adaptation in the clinic. For this system, scalability must be addressed by simplifying formulation procedures. Although microfluidic platforms have aided the production ability of these platforms,109 these technologies are at a relatively rudimentary stage and further understanding of manufacturing procedures is critical to ensure clinical success. In addition, biomimetic nanoparticles inherit intrinsic characteristic of the source cells, which in theory can mitigate targeting requirements to deliver genetic payloads. Interestingly, the clinical translation of BNPs is significantly hindered due to their heterogeneous properties depending on the source cells and preparation methods which can impact their performance.78–80,110 On the other hand, the innate characteristics of the source cells might also cause immunogenic responses. For instance, immune cell derived vectors have been shown to generate immune responses via pro inflammatory M1-macrophage polarization.77 Likewise, lipophilic RNAs showed excellent pharmacokinetics profile with promising gene silencing, but the modification process also affects its potency. Previously GalNAc-siRNA conjugate have shown targetability towards liver hepatocytes,111 however, lipophilic conjugates exhibit limitations towards preferential accumulation in other desired disease sites. Lesser hydrophobic conjugates preferentially bind to high density lipoproteins (HDL) which accumulates in kidney, whereas higher hydrophobic chains prefer low density lipoproteins (LDL) and accumulates in liver.112 Further studies are needed for a detailed understanding of the relationship between conjugate structures and preferential tissue accumulation in vivo for successful clinical adoption of RNA conjugates. Local injection can be adopted as solution to targetability in some isolated cases, but a mechanistic understanding of intracellular trafficking and their retention time is still lacking. More importantly, the controlled release chemistry of these conjugates is not yet realized. For example, a cleavable linker can be installed to enhance pharmacokinetics and systemic stability. Similarly, nucleic acid nanoassembly platforms are relatively new and the investigations are limited to proof-of-concept demonstrations. The structural integrity of nucleic acid nanostructure upon cellular internalization is well-studied, but the effect of physiochemical properties towards pharmacokinetic bioavailability needs exploration. Alongside, understanding the effect of nucleic acid engineering on biodegradability and biocompatibility is also essential. Success here will impinge on addressing potential immunogenicity and dose-dependent toxicity, as well as optimizing pharmacokinetic and clearance mechanisms. In summary, given the clinical success of COVID-19 vaccines, there is a lot of excitement around the use of nucleic acid-based therapeutics in areas other than vaccines. Developing strategies that mitigates the shortcomings of promising delivery platforms and innovating new ones to harness the potential of nucleic acid therapeutics are forthcoming.
Acknowledgements:
We are thankful for the support from the National Institutes of Health (GM-136395) and BTP training grant that partially supported JM (T32 GM135096).
References:
- 1.Opalinska JB & Gewirtz AM Nucleic-acid therapeutics: Basic principles and recent applications. Nature Reviews Drug Discovery 1, 503–514 (2002). [DOI] [PubMed] [Google Scholar]
- 2.Wang F, Zuroske T & Watts JK RNA therapeutics on the rise. Nature reviews. Drug discovery 19, 441–442 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Mitragotri S, Burke PA & Langer R Overcoming the challenges in administering biopharmaceuticals: Formulation and delivery strategies. Nature Reviews Drug Discovery 13, 655–672 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garber K Alnylam launches era of RNAi drugs. Nature Biotechnology 36, 777–778 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Krammer F SARS-CoV-2 vaccines in development. Nature 586, 516–527 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Richner JM, Himansu S, Dowd KA, Butler SL, Salazar V, Fox JM, Julander JG, Tang WW, Shresta S, Pierson TC, et al. Modified mRNA Vaccines Protect against Zika Virus Infection. Cell 168, 1114–1125.e10 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walsh EE, Frenck RW, Falsey AR, Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K, Mulligan MJ, Bailey R, et al. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. New England Journal of Medicine 383, 2439–2450 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. New England Journal of Medicine 383, 2603–2615 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samaridou E, Heyes J & Lutwyche P Lipid nanoparticles for nucleic acid delivery: Current perspectives. Advanced Drug Delivery Reviews 154–155, 37–63 (2020). [DOI] [PubMed] [Google Scholar]
- 10.Santa P, Garreau A, Serpas L, Ferriere A, Blanco P, Soni C & Sisirak V The Role of Nucleases and Nucleic Acid Editing Enzymes in the Regulation of Self-Nucleic Acid Sensing. Frontiers in Immunology 12, 1–25 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Semple SC, Akinc A, Chen J, Sandhu AP, Mui BL, Cho CK, Sah DWY, Stebbing D, Crosley EJ, Yaworski E, et al. Rational design of cationic lipids for siRNA delivery. Nature Biotechnology 28, 172–176 (2010). [DOI] [PubMed] [Google Scholar]
- 12.Tracy MA & Ph D Progress in the Development of Novel RNAi-Based Medicines : Research to Clinic Recent Accomplishments in Nucleic Acid-Based Medicines. (2016). [Google Scholar]
- 13.Rietwyk S & Peer D Next-Generation Lipids in RNA Interference Therapeutics. ACS Nano 11, 7572–7586 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Suzuki Y, Hyodo K, Suzuki T, Tanaka Y, Kikuchi H & Ishihara H Biodegradable lipid nanoparticles induce a prolonged RNA interference-mediated protein knockdown and show rapid hepatic clearance in mice and nonhuman primates. International Journal of Pharmaceutics 519, 34–43 (2017). [DOI] [PubMed] [Google Scholar]
- 15.Qiu N, Gao J, Liu Q, Wang J & Shen Y Enzyme-Responsive Charge-Reversal Polymer-Mediated Effective Gene Therapy for Intraperitoneal Tumors. Biomacromolecules 19, 2308–2319 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Yang HY, Li Y & Lee DS Recent Advances of pH-Induced Charge-Convertible Polymer-Mediated Inorganic Nanoparticles for Biomedical Applications. Macromolecular Rapid Communications 2000106, 1–15 (2020). [DOI] [PubMed] [Google Scholar]
- 17.Colella P, Ronzitti G & Mingozzi F Emerging Issues in AAV-Mediated In Vivo Gene Therapy. Molecular Therapy - Methods and Clinical Development 8, 87–104 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mingozzi F & High KA Immune responses to AAV vectors: Overcoming barriers to successful gene therapy. Blood 122, 23–36 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang D, Atochina-Vasserman EN, Maurya DS, Huang N, Xiao Q, Ona N, Liu M, Shahnawaz H, Ni H, Kim K, et al. One-Component Multifunctional Sequence-Defined Ionizable Amphiphilic Janus Dendrimer Delivery Systems for mRNA. Journal of the American Chemical Society 143, 12315–12327 (2021). [DOI] [PubMed] [Google Scholar]
- 20.Gooding M, Browne LP, Quinteiro FM & Selwood DL siRNA Delivery: From Lipids to Cell-penetrating Peptides and Their Mimics. Chemical Biology and Drug Design vol. 80 787–809 (2012). [DOI] [PubMed] [Google Scholar]
- 21.Kurrikoff K, Veiman KL, Künnapuu K, Peets EM, Lehto T, Pärnaste L, Arukuusk P & Langel Ü Effective in vivo gene delivery with reduced toxicity, achieved by charge and fatty acid -modified cell penetrating peptide. Scientific Reports 7, 1–11 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen C Chemical modifications of nucleic acid drugs and their delivery systems for gene-based therapy. 829–869 (2018) doi: 10.1002/med.21479. [DOI] [PubMed] [Google Scholar]
- 23.Hou X, Zaks T, Langer R & Dong Y Lipid nanoparticles for mRNA delivery. Nature Reviews Materials 6, 1078–1094 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dutta K, Das R, Medeiros J & Thayumanavan S Disulfide Bridging Strategies in Viral and Nonviral Platforms for Nucleic Acid Delivery. Biochemistry 60, 966–990 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samaridou E, Heyes J & Lutwyche P Lipid nanoparticles for nucleic acid delivery: Current perspectives. Advanced Drug Delivery Reviews 154–155, 37–63 (2020). [DOI] [PubMed] [Google Scholar]
- 26.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. New England Journal of Medicine 383, 2603–2615 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richner JM, Himansu S, Dowd KA, Butler SL, Salazar V, Fox JM, Julander JG, Tang WW, Shresta S, Pierson TC, et al. Modified mRNA Vaccines Protect against Zika Virus Infection. Cell 168, 1114–1125.e10 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maier MA, Jayaraman M, Matsuda S, Liu J, Barros S, Querbes W, Tam YK, Ansell SM, Kumar V, Qin J, et al. Biodegradable lipids enabling rapidly eliminated lipid nanoparticles for systemic delivery of RNAi therapeutics. Molecular Therapy 21, 1570–1578 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao P, Hou X, Yan J, Du S, Xue Y, Li W, Xiang G & Dong Y Long-term storage of lipid-like nanoparticles for mRNA delivery. Bioactive Materials 5, 358–363 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aso Y & Yoshioka S Effect of freezing rate on physical stability of lyophilized cationic liposomes. Chemical and Pharmaceutical Bulletin 53, 301–304 (2005). [DOI] [PubMed] [Google Scholar]
- 31.Gilleron J, Querbes W, Zeigerer A, Borodovsky A, Marsico G, Schubert U, Manygoats K, Seifert S, Andree C, Stöter M, et al. Image-based analysis of lipid nanoparticle-mediated siRNA delivery, intracellular trafficking and endosomal escape. Nature Biotechnology 31, 638–646 (2013). [DOI] [PubMed] [Google Scholar]
- 32.Corbett KS, Edwards DK, Leist SR, Abiona OM, Boyoglu-Barnum S, Gillespie RA, Himansu S, Schäfer A, Ziwawo CT, DiPiazza AT, et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature 586, 567–571 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Alwis R, Gan ES, Chen S, Leong YS, Tan HC, Zhang SL, Yau C, Low JGH, Kalimuddin S, Matsuda D, et al. A single dose of self-transcribing and replicating RNA-based SARS-CoV-2 vaccine produces protective adaptive immunity in mice. Molecular Therapy 29, 1970–1983 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gebre MS, Rauch S, Roth N, Yu J, Chandrashekar A, Mercado NB, He X, Liu J, McMahan K, Martinot A, et al. Optimization of non-coding regions for a non-modified mRNA COVID-19 vaccine. Nature 601, 410–414 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grunwitz C, Salomon N, Vascotto F, Selmi A, Bukur T, Diken M, Kreiter S, Türeci Ö & Sahin U HPV16 RNA-LPX vaccine mediates complete regression of aggressively growing HPV-positive mouse tumors and establishes protective T cell memory. OncoImmunology 8, 1–11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang KW, Tan CP, Reebye V, Chee CE, Zacharoulis D, Habib R, Blakey DC, Rossi JJ, Habib N & Sodergren MH MTL-CEBPA combined with immunotherapy or RFA enhances immunological anti-tumor response in preclinical models. International Journal of Molecular Sciences 22, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bader AG MiR-34 - a microRNA replacement therapy is headed to the clinic. Frontiers in Genetics 3, 1–9 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dutta K, Das R, Medeiros J, Kanjilal P & Thayumanavan S Charge-Conversion Strategies for Nucleic Acid Delivery. Advanced Functional Materials 31, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qiu N, Liu X, Zhong Y, Zhou Z, Piao Y, Miao L, Zhang Q, Tang J, Huang L & Shen Y Esterase-Activated Charge-Reversal Polymer for Fibroblast-Exempt Cancer Gene Therapy. Advanced Materials 28, 10613–10622 (2016). [DOI] [PubMed] [Google Scholar]
- 40.Insua I, Wilkinson A & Fernandez-Trillo F Polyion complex (PIC) particles: Preparation and biomedical applications. European Polymer Journal 81, 198–215 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hou X, Zaks T, Langer R & Dong Y Lipid nanoparticles for mRNA delivery. Nature Reviews Materials 6, 1078–1094 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nguyen J & Szoka FC Nucleic acid delivery: The missing pieces of the puzzle? Accounts of Chemical Research 45, 1153–1162 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davies N, Hovdal D, Edmunds N, Nordberg P, Dahlén A, Dabkowska A, Arteta MY, Radulescu A, Kjellman T, Höijer A, et al. Functionalized lipid nanoparticles for subcutaneous administration of mRNA to achieve systemic exposures of a therapeutic protein. Molecular Therapy - Nucleic Acids 24, 369–384 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arteta MY, Kjellman T, Bartesaghi S, Wallin S, Wu X, Kvist AJ, Dabkowska A, Székely N, Radulescu A, Bergenholtz J, et al. Successful reprogramming of cellular protein production through mRNA delivered by functionalized lipid nanoparticles. Proceedings of the National Academy of Sciences of the United States of America 115, E3351–E3360 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lball R, Bajaj P & Whitehead KA Achieving long-term stability of lipid nanoparticles: Examining the effect of pH, temperature, and lyophilization. International Journal of Nanomedicine 12, 305–315 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Danaei M, Dehghankhold M, Ataei S, Hasanzadeh Davarani F, Javanmard R, Dokhani A, Khorasani S & Mozafari MR Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 10, 1–17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Putnam D, Gentry CA, Pack DW & Langer R Polymer-based gene delivery with low cytotoxicity by a unique balance of side-chain termini. Proceedings of the National Academy of Sciences of the United States of America 98, 1200–1205 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schaffert D & Wagner E Gene therapy progress and prospects: Synthetic polymer-based systems. Gene Therapy 15, 1131–1138 (2008). [DOI] [PubMed] [Google Scholar]
- 49.Chen HT, Neerman MF, Parrish AR & Simanek EE Cytotoxicity, hemolysis, and acute in vivo toxicity of dendrimers based on melamine, candidate vehicles for drug delivery. Journal of the American Chemical Society 126, 10044–10048 (2004). [DOI] [PubMed] [Google Scholar]
- 50.Mieszawska AJ, Kim Y, Gianella A, van Rooy I, Priem B, Labarre MP, Ozcan C, Cormode DP, Petrov A, Langer R, et al. Synthesis of polymer-lipid nanoparticles for image-guided delivery of dual modality therapy. Bioconjugate Chemistry 24, 1429–1434 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mieszawska AJ, Gianella A, Cormode DP, Zhao Y, Meijerink A, Langer R, Farokhzad OC, Fayad ZA & Mulder WJM Engineering of lipid-coated PLGA nanoparticles with a tunable payload of diagnostically active nanocrystals for medical imaging. Chemical Communications 48, 5835–5837 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang L, Chan, J. M, Gu, F. X, Rhee J-W, Wang, A. Z, Radovic-Moreno, A. F, Alexis F, Langer R & Farokhzad, O. C Self-Assembled Lipid−Polymer Hybrid Nanoparticles: A Robust Drug Delivery Platform. ACS Nano 2, 1696–1702 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mieszawska AJ, Gianella A, Cormode DP, Zhao Y, Meijerink A, Langer R, Farokhzad OC, Fayad ZA & Mulder WJM Engineering of lipid-coated PLGA nanoparticles with a tunable payload of diagnostically active nanocrystals for medical imaging. Chemical Communications 48, 5835–5837 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shi J, Xiao Z, Votruba AR, Vilos C & Farokhzad OC Differentially charged hollow core/shell lipid-polymer-lipid hybrid nanoparticles for small interfering rna delivery. Angewandte Chemie - International Edition 50, 7027–7031 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shi J, Xu Y, Xu X, Zhu X, Pridgen E, Wu J, Votruba AR, Swami A, Zetter BR & Farokhzad OC Hybrid lipid-polymer nanoparticles for sustained siRNA delivery and gene silencing. Nanomedicine: Nanotechnology, Biology, and Medicine 10, e897–e900 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu X, Xu Y, Solis LM, Tao W, Wang L, Behrens C, Xu X, Zhao L, Liu D, Wu J, et al. Long-circulating siRNA nanoparticles for validating Prohibitin1-targeted non-small cell lung cancer treatment. Proceedings of the National Academy of Sciences of the United States of America 112, 7779–7784 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao W, Zhang C, Li B, Zhang X, Luo X, Zeng C, Li W, Gao M & Dong Y Lipid Polymer Hybrid Nanomaterials for mRNA Delivery. Cellular and Molecular Bioengineering 11, 397–406 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu X, Xiang J, Zhu D, Jiang L, Zhou Z, Tang J, Liu X, Huang Y & Shen Y Fusogenic Reactive Oxygen Species Triggered Charge-Reversal Vector for Effective Gene Delivery. Advanced Materials 28, 1743–1752 (2016). [DOI] [PubMed] [Google Scholar]
- 59.Jiang Z, Cui W, Prasad P, Touve, M. A, Gianneschi, N. C, Mager J & Thayumanavan S Bait-and-Switch Supramolecular Strategy To Generate Noncationic RNA–Polymer Complexes for RNA Delivery. Biomacromolecules 20, 435–442 (2018). [DOI] [PubMed] [Google Scholar]
- 60.Jiang Z, Cui W, Mager J & Thayumanavan S Postfunctionalization of Noncationic RNA–Polymer Complexes for RNA Delivery. Industrial & Engineering Chemistry Research 58, 6982–6991 (2019). [Google Scholar]
- 61.Dutta K, Bochicchio D, Ribbe AE, Alfandari D, Mager J, Pavan GM & Thayumanavan S Symbiotic Self-Assembly Strategy toward Lipid-Encased Cross-Linked Polymer Nanoparticles for Efficient Gene Silencing. ACS Applied Materials and Interfaces 11, 24971–24983 (2019). [DOI] [PubMed] [Google Scholar]
- 62.Li W, Qiu J, Li X-L, Aday S, Zhang J, Conley G, Xu J, Joseph J, Lan H, Langer R, et al. BBB pathophysiology–independent delivery of siRNA in traumatic brain injury. Science Advances 7, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen L, Hong W, Ren W, Xu T, Qian Z & He Z Recent progress in targeted delivery vectors based on biomimetic nanoparticles. Signal Transduction and Targeted Therapy 6, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fang RH, Hu CMJ, Luk BT, Gao W, Copp JA, Tai Y, O’Connor DE & Zhang L Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano Letters 14, 2181–2188 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hu CMJ, Zhang L, Aryal S, Cheung C, Fang RH & Zhang L Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proceedings of the National Academy of Sciences of the United States of America 108, 10980–10985 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li H, Jin K, Luo M, Wang X, Zhu X, Liu X, Jiang T, Zhang Q, Wang S & Pang Z Size Dependency of Circulation and Biodistribution of Biomimetic Nanoparticles: Red Blood Cell Membrane-Coated Nanoparticles. Cells 8, 881 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ben-Akiva E, Meyer RA, Yu H, Smith JT, Pardoll DM & Green JJ Biomimetic anisotropic polymeric nanoparticles coated with red blood cell membranes for enhanced circulation and toxin removal. Science Advances 6, 1–10 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Oroojalian F, Beygi M, Baradaran B, Mokhtarzadeh A & Shahbazi MA Immune Cell Membrane-Coated Biomimetic Nanoparticles for Targeted Cancer Therapy. Small vol. 17 (2021). [DOI] [PubMed] [Google Scholar]
- 69.Zhang Q, Dehaini D, Zhang Y, Zhou J, Chen X, Zhang L, Fang RH, Gao W & Zhang L Neutrophil membrane-coated nanoparticles inhibit synovial inflammation and alleviate joint damage in inflammatory arthritis. Nature Nanotechnology 13, 1182–1190 (2018). [DOI] [PubMed] [Google Scholar]
- 70.Liu L, Bai X, Martikainen MV, Kårlund A, Roponen M, Xu W, Hu G, Tasciotti E & Lehto VP Cell membrane coating integrity affects the internalization mechanism of biomimetic nanoparticles. Nature Communications 12, 1–12 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mu X, Li J, Yan S, Zhang H, Zhang W, Zhang F & Jiang J SiRNA Delivery with Stem Cell Membrane-Coated Magnetic Nanoparticles for Imaging-Guided Photothermal Therapy and Gene Therapy. ACS Biomaterials Science and Engineering 4, 3895–3905 (2018). [DOI] [PubMed] [Google Scholar]
- 72.Tian W, Lu J & Jiao D Stem cell membrane vesicle–coated nanoparticles for efficient tumor-targeted therapy of orthotopic breast cancer. Polymers for Advanced Technologies 30, 1051–1060 (2019). [Google Scholar]
- 73.Haraszti RA, Miller R, Stoppato M, Sere YY, Coles A, Didiot MC, Wollacott R, Sapp E, Dubuke ML, Li X, et al. Exosomes Produced from 3D Cultures of MSCs by Tangential Flow Filtration Show Higher Yield and Improved Activity. Molecular Therapy 26, 2838–2847 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang Y, Zhang K, Qin X, Li T, Qiu J, Yin T, Huang J, McGinty S, Pontrelli G, Ren J, et al. Biomimetic Nanotherapies: Red Blood Cell Based Core–Shell Structured Nanocomplexes for Atherosclerosis Management. Advanced Science 6, 1900172 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hu CMJ, Fang RH, Luk BT, Chen KNH, Carpenter C, Gao W, Zhang K & Zhang L “Marker-of-self” functionalization of nanoscale particles through a top-down cellular membrane coating approach. Nanoscale 5, 2664–2668 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S & Wood MJA Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nature Biotechnology 29, 341–345 (2011). [DOI] [PubMed] [Google Scholar]
- 77.Deng G, Sun Z, Li S, Peng X, Li W, Zhou L, Ma Y, Gong P & Cai L Cell-Membrane Immunotherapy Based on Natural Killer Cell Membrane Coated Nanoparticles for the Effective Inhibition of Primary and Abscopal Tumor Growth. ACS Nano 12, 12096–12108 (2018). [DOI] [PubMed] [Google Scholar]
- 78.Mohsen MO, Gomes AC, Vogel M & Bachmann MF Interaction of viral capsid-derived virus-like particles (VLPs) with the innate immune system. Vaccines 6, 1–12 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang Z, Shi J, Xie J, Wang Y, Sun J, Liu T, Zhao Y, Zhao X, Wang X, Ma Y, et al. Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation. Nature Biomedical Engineering 4, 69–83 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gudbergsson JM, Jønsson K, Simonsen JB & Johnsen KB Systematic review of targeted extracellular vesicles for drug delivery – Considerations on methodological and biological heterogeneity. Journal of Controlled Release 306, 108–120 (2019). [DOI] [PubMed] [Google Scholar]
- 81.Rao L, Wu L, Liu Z, Tian R, Yu G, Zhou Z, Yang K, Xiong HG, Zhang A, Yu GT, et al. Hybrid cellular membrane nanovesicles amplify macrophage immune responses against cancer recurrence and metastasis. Nature Communications 11, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cai J-X, Liu J-H, Wu J-Y, Li Y-J, Qiu X-H, Xu W-J, Xu P & Xiang D-X Hybrid Cell Membrane-Functionalized Biomimetic Nanoparticles for Targeted Therapy of Osteosarcoma. International Journal of Nanomedicine 17, 837–854 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Park JH, Mohapatra A, Zhou J, Holay M, Krishnan N, Gao W, Fang RH & Zhang L Virus-Mimicking Cell Membrane-Coated Nanoparticles for Cytosolic Delivery of mRNA. Angewandte Chemie - International Edition 61, 1–5 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Russell CJ, Hu M & Okda FA Influenza Hemagglutinin Protein Stability, Activation, and Pandemic Risk. Trends in Microbiology 26, 841–853 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sup Kim C, Epand RF, Leikina E, Epand RM & Chernomordik L. v. The Final Conformation of the Complete Ectodomain of the HA2 Subunit of Influenza Hemagglutinin Can by Itself Drive Low pH-dependent Fusion. Journal of Biological Chemistry 286, 13226–13234 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wadia JS, Stan R. v. & Dowdy SF Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nature Medicine 10, 310–315 (2004). [DOI] [PubMed] [Google Scholar]
- 87.Thoennes S, Li ZN, Lee BJ, Langley WA, Skehel JJ, Russell RJ & Steinhauer DA Analysis of residues near the fusion peptide in the influenza hemagglutinin structure for roles in triggering membrane fusion. Virology 370, 403–414 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hong S, Choi DW, Kim HN, Park CG, Lee W & Park HH Protein-based nanoparticles as drug delivery systems. Pharmaceutics 12, 1–28 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xi J & Liu H Recent Advances in the Design of Self‐Delivery Amphiphilic Drugs and Vaccines. Advanced Therapeutics 3, 1900107 (2020). [Google Scholar]
- 90.Osborn MF, Coles AH, Biscans A, Haraszti RA, Roux L, Davis S, Ly S, Echeverria D, Hassler MR, Godinho BMDC, et al. Hydrophobicity drives the systemic distribution of lipid-conjugated siRNAs via lipid transport pathways. Nucleic Acids Research 47, 1070–1081 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Soutschek J, Akinc A, Bramlage B, Charisse K, Constien R, Donoghue M, Elbashir S, Gelck A, Hadwiger P, Harborth J, et al. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature 432, 173–178 (2004). [DOI] [PubMed] [Google Scholar]
- 92.Wolfrum C, Shi S, Jayaprakash KN, Jayaraman M, Wang G, Pandey RK, Rajeev KG, Nakayama T, Charrise K, Ndungo EM, et al. Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nature Biotechnology 25, 1149–1157 (2007). [DOI] [PubMed] [Google Scholar]
- 93.Sarett SM, Werfel TA, Lee L, Jackson MA, Kilchrist K. v., Brantley-Sieders D & Duvall CL Lipophilic siRNA targets albumin in situ and promotes bioavailability, tumor penetration, and carrier-free gene silencing. Proceedings of the National Academy of Sciences of the United States of America 114, E6490–E6497 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Commisso C, Davidson SM, Soydaner-Azeloglu RG, Parker SJ, Kamphorst JJ, Hackett S, Grabocka E, Nofal M, Drebin JA, Thompson CB, et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature 497, 633–637 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Larsen MT, Kuhlmann M, Hvam ML & Howard KA Albumin-based drug delivery: harnessing nature to cure disease. Molecular and Cellular Therapies 4, 1–12 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tao H. yu, Wang R. qi, Sheng W. jin & Zhen Y. su. The development of human serum albumin-based drugs and relevant fusion proteins for cancer therapy. International Journal of Biological Macromolecules 187, 24–34 (2021). [DOI] [PubMed] [Google Scholar]
- 97.Chi Q, Yang Z, Xu K, Wang C & Liang H DNA nanostructure as an efficient drug delivery platform for immunotherapy. Frontiers in Pharmacology 10, 1–17 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ma W, Zhan Y, Zhang Y, Mao C, Xie X & Lin Y The biological applications of DNA nanomaterials: current challenges and future directions. Signal Transduction and Targeted Therapy 6, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Krishnan Y & Seeman NC Introduction: Nucleic Acid Nanotechnology. Chemical Reviews 119, 6271–6272 (2019). [DOI] [PubMed] [Google Scholar]
- 100.Lacroix A & Sleiman HF DNA Nanostructures: Current Challenges and Opportunities for Cellular Delivery. ACS Nano 15, 3631–3645 (2021). [DOI] [PubMed] [Google Scholar]
- 101.Hu Q, Li H, Wang L, Gu H & Fan C DNA Nanotechnology-Enabled Drug Delivery Systems. Chemical reviews 119, 6459–6506 (2019). [DOI] [PubMed] [Google Scholar]
- 102.Sun P, Zhang N, Tang Y, Yang Y, Chu X & Zhao Y SL2B aptamer and folic acid dual-targeting DNA nanostructures for synergic biological effect with chemotherapy to combat colorectal cancer. International Journal of Nanomedicine 12, 2657–2672 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Prinzen AL, Saliba D, Hennecker C, Trinh T, Mittermaier A & Sleiman HF Amplified elf-Immolative Release of Small Molecules by Spatial Isolation of Reactive Groups on DNA-Minimal Architectures. Angewandte Chemie - International Edition 59, 12900–12908 (2020). [DOI] [PubMed] [Google Scholar]
- 104.Henry SJW & Stephanopoulos N Functionalizing DNA nanostructures for therapeutic applications. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology 13, 1–21 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lee H, Lytton-Jean AKR, Chen Y, Love KT, Park AI, Karagiannis ED, Sehgal A, Querbes W, Zurenko CS, Jayaraman M, et al. Molecularly self-assembled nucleic acid nanoparticles for targeted in vivo siRNA delivery. Nature Nanotechnology 7, 389–393 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bujold KE, Hsu JCC & Sleiman HF Optimized DNA “nanosuitcases” for Encapsulation and Conditional Release of siRNA. Journal of the American Chemical Society 138, 14030–14038 (2016). [DOI] [PubMed] [Google Scholar]
- 107.Fakih HH, Fakhoury JJ, Bousmail D & Sleiman HF Minimalist Design of a Stimuli-Responsive Spherical Nucleic Acid for Conditional Delivery of Oligonucleotide Therapeutics. ACS Applied Materials and Interfaces 11, 13912–13920 (2019). [DOI] [PubMed] [Google Scholar]
- 108.Cutler JI, Auyeung E & Mirkin CA Spherical Nucleic Acids. Journal of the American Chemical Society 134, 1376–1391 (2012). [DOI] [PubMed] [Google Scholar]
- 109.Kim Y, Lee Chung B, Ma M, Mulder WJM, Fayad ZA, Farokhzad OC & Langer R Mass production and size control of lipid-polymer hybrid nanoparticles through controlled microvortices. Nano Letters 12, 3587–3591 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ma Y, Nolte RJM & Cornelissen JJLM Virus-based nanocarriers for drug delivery. Advanced Drug Delivery Reviews 64, 811–825 (2012). [DOI] [PubMed] [Google Scholar]
- 111.Nair JK, Willoughby JLS, Chan A, Charisse K, Alam MR, Wang Q, Hoekstra M, Kandasamy P, Kelin A. v., Milstein S, et al. Multivalent N -acetylgalactosamine-conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing. Journal of the American Chemical Society 136, 16958–16961 (2014). [DOI] [PubMed] [Google Scholar]
- 112.Biscans A, Coles A, Echeverria D & Khvorova A The valency of fatty acid conjugates impacts siRNA pharmacokinetics, distribution, and efficacy in vivo. Journal of Controlled Release 302, 116–125 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]


