Abstract
Precision medicine (PM) informed by next-generation sequencing (NGS) poses challenges for health technology assessment (HTA). To date, there has been limited reimbursement of genomic testing with NGS in Canada, particularly for whole-genome and whole-exome sequencing (WGS/WES). Through a structured literature review, we examine Canadian economic evidence and evidentiary challenges for the adoption of genomic testing. We searched Medline (PubMed) for published Canadian studies generating economic evidence for PM informed by NGS. Our search focused on studies examining the costs and/or value of NGS. We reviewed included studies and summarized results according to evaluation type, clinical context, NGS technology, and test strategy. We then grouped HTA challenges encountered by authors when evaluating NGS. Our review included twenty-five studies. To determine the economic impacts of NGS-informed PM in Canada, studies applied cost-effectiveness analysis (52%, n = 13), stated preference analysis (20%, n = 5), cost-consequence analysis (16%, n = 4), and healthcare resource utilization or costing analysis (12%, n = 3). NGS panels were the most common technology evaluated (n = 13), followed by WGS and/or WES (n = 8). The included studies highlighted multiple challenges when generating economic evidence, many of which remain unaddressed. Challenges were broadly related to (1) accounting for all NGS outcomes; (2) addressing uncertainty; and (3) improving consistency of economic approaches. Canadian studies are beginning to produce estimates of the economic impacts of NGS-informed PM, yet challenges for HTA remain. While solutions and real-world evidence are generated, lifecycle health technology management methods can be designed to better support resource allocation decisions for genomic testing in Canada.
Electronic supplementary material
The online version of this article (10.1007/s12687-019-00428-5) contains supplementary material, which is available to authorized users.
Keywords: Precision medicine, Health technology assessment, Next-generation sequencing, Genomic testing, Canada
Introduction
Canada has a decentralized healthcare system, with health services primarily funded, regulated, and provided by the provinces and territories. Interventions that are being considered for adoption initially undergo a centralized review process (Cheung et al. 2016; Joly and Ramos-Paque 2010). Final reimbursement is the responsibility of each jurisdiction. For health technologies, including commercial testing, the review process begins with a safety and clinical effectiveness assessment by Health Canada. For laboratory services, the process begins with an assessment at the provincial or territorial level. If approved, the Canadian Agency for Drugs and Technologies in Health (CADTH) or the CADTH pan Canadian Oncology Drug Review (pCODR) formally reviews health technology assessment (HTA) evidence for clinical effectiveness, cost-effectiveness, and implementation. CADTH and CADTH pCODR then issue recommendations, which provinces and territories are not compelled to follow. Price negotiations and reimbursement decisions can vary widely across jurisdictions.
The HTA process aims to support evidence-informed resource allocation decisions for new and existing health technologies. Evaluations of the economic impacts of these technologies are a critical component of the HTA evidence base. In Canada, economic evaluation guidelines recommend conducting cost-effectiveness analysis (CEA) to evaluate health technologies, with outcomes measured in terms of quality-adjusted survival informed by a preference-based instrument (CADTH 2017). CEAs explore the trade-offs between incremental costs and incremental effects of competing technologies and express results in terms of a single metric, the incremental cost-effectiveness ratio (ICER). Additional forms of HTA evidence common in Canada include costing analyses, which focus only on cost and resource utilization impacts; stated preference studies, which enumerate stakeholders’ preference-based values for technology attributes; and cost-consequence analyses, which report a range of disaggregated costs and outcomes rather than emphasizing a single summary ratio (Drummond et al. 2015).
Precision medicine (PM) informed by next-generation sequencing (NGS) is challenging current HTA processes in Canada. There is increasing demand for genomic testing with costly NGS technologies from both clinicians and patients (Agyeman and Ofori-Asenso 2015). These technologies are capable of sequencing either panels of multiple genes, all protein coding regions of genes (exomes), or the whole genome. Health systems are pressured to adopt NGS-informed PM interventions for which there is often insufficient evidence available to support decision-making. There is concern among the HTA community that reimbursement decisions will move towards lower evidentiary standards because of this strong demand for genomic testing and the limited evidence base on effectiveness and cost-effectiveness. In response, parallel HTA processes are being developed in several provinces to manage NGS-informed PM on-boarding. For example, the Ontario Personalized Health Network and Quebec Network for Personalized Health Care are creating separate HTA processes for NGS interventions, which are not yet finalized (Génome Québec 2016; Ontario Personalized Medicine Network n.d.). Health Quality Ontario has developed unique recommendations for economic evaluations of medical genetic technologies and these evaluations must be reviewed by a specialized genetics committee prior to making funding recommendations (Health Quality Ontario 2018).
To date, there is limited reimbursement and uptake of genomic testing involving NGS technologies in Canada, particularly relating to the clinical implementation of whole-genome and whole-exome sequencing (WGS/WES). The use of WGS and WES to inform patient care is confined to research settings and few NGS panels have achieved public reimbursement. Current funding for NGS panels varies across jurisdictions, with approved clinical contexts ranging from disease screening, to hereditary cancer testing, to predicting risk of recurrence in breast cancer patients (AHS 2017; Centre for Clinical Genomics n.d.; Newborn Screening Ontario n.d.). British Columbia is the sole province to implement an NGS panel as a diagnostic tool for assessing tumor heterogeneity in newly diagnosed patients and informing treatment planning across multiple tumor sites (BC Cancer 2016).
In this study, we examine whether current uptake of genomic testing involving NGS in Canada aligns with available economic evidence and which evidentiary challenges for HTA remain. Through a structured literature review, we highlight key methods used when valuing NGS-informed PM approaches and summarize study findings and challenges. We then consider whether parallel HTA processes are needed to guide the appropriate implementation of genomic testing in Canada.
Methods
Search strategy
We conducted a literature search of full-text peer-reviewed articles in Medline (PubMed). We restricted our search to English-language articles published between January 1, 2005, and August 27, 2018. We identified additional records from citations in key articles and research team suggestions. Our detailed search strategy is provided in the Appendix. Two researchers (DW and DAR) independently evaluated the title and abstract of all publications to identify articles for inclusion. We focused on published Canadian studies generating economic evidence for PM informed by NGS. Reviewers compared their results and resolved differences through consensus. After ordering the publications, researchers read full texts thoroughly to assess relevance and excluded studies that did not evaluate costs or did not focus on NGS.
Qualitative synthesis
Two researchers (DW and DAR) independently reviewed included studies and extracted information on the following characteristics, as applicable: first author, publication year, title, journal of publication, province or territory, funding source, clinical context, NGS technology, test strategy, comparator, evaluation type, data and study design, attributes or cost components, study perspective, model type, study period/time horizon, reference year, discount rate, outcome measures, final estimates, and willingness-to-pay (WTP) threshold.
Given the differences across included studies, we summarized findings according to evaluation type, clinical context, NGS technology, and test strategy. We converted all monetary outcomes, including costs and ICERs, to 2018 Canadian dollars using the Canadian Health Care Consumer Price Index (Statistics Canada 2018). While this conversion helped to ensure that monetary outcomes were similarly represented across studies, the assumptions underlying these outcomes were heterogeneous and caution must be exercised when drawing direct comparisons. We identified challenges through authors’ statements on reasons for estimating economic impacts of NGS and discussions of study limitations. We restricted quality assessment of the studies to analyzing transparency through adherence to reporting standards (Bridges et al. 2011; Husereau et al. 2013).
Results
Search results
Figure 1 presents the acquisition and flow of included studies. Our literature search identified 86 records. We identified 5 additional records from citations in key articles. After screening titles and abstracts, we excluded 47 records and assessed 44 full-text articles for eligibility. Of these, 25 studies met our inclusion criteria. Detailed study characteristics and references for included studies are available in Supplemental Materials. Studies were excluded if they did not examine NGS (n = 13), use Canadian data (n = 3), or conduct economic analysis (n = 3).
Fig. 1.
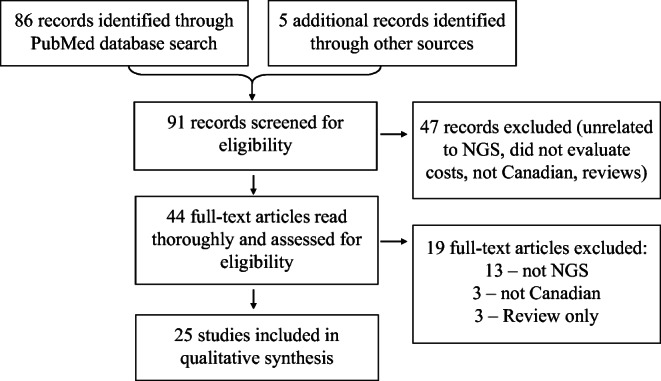
Study acquisition flow. NGS, next-generation sequencing. Other sources included citations in key articles and research team suggestions
Study characteristics
The first identified study examining economic impacts of NGS-informed PM within a Canadian context was published in 2010 (Tsoi et al. 2010) and the number of published studies increased over time. Table 1 summarizes the characteristics of the 25 included studies. Of these, the majority (64%) evaluated NGS-driven approaches within either Ontario (n = 8) or British Columbia (n = 8). Over half of included studies (n = 15) focused on oncology. Other clinical contexts included rare diseases (n = 5) and prenatal screening (n = 2). Three studies did not either specify a clinical context or evaluated NGS-informed PM approaches across multiple disease areas.
Table 1.
Characteristics of included studies
| Study characteristics | n | % |
|---|---|---|
| Province | ||
| Ontario | 8 | 32 |
| British Columbia | 8 | 32 |
| Prairies (Alberta and Manitoba) | 4 | 16 |
| Canada-Wide | 2 | 8 |
| Quebec (alone or with USA) | 2 | 8 |
| Maritimes (Nova Scotia) | 1 | 4 |
| Study funding | ||
| Public | 19 | 76 |
| Not stated | 3 | 12 |
| Private | 1 | 4 |
| Public and private | 1 | 4 |
| None | 1 | 4 |
| Year of publication | ||
| 2016–2018 | 12 | 48 |
| 2013–2015 | 9 | 36 |
| 2010–2012 | 4 | 16 |
| Clinical context | ||
| Oncology | 15 | 60 |
| Rare diseases | 5 | 20 |
| Multiple/not specific | 3 | 12 |
| Prenatal screening | 2 | 8 |
| NGS technology | ||
| NGS panels | 13 | 52 |
| Oncotype DX | 6 | 24 |
| WGS/WGTA | 5 | 20 |
| Not specific | 4 | 16 |
| WES and WGS | 2 | 8 |
| WES | 1 | 4 |
| Test strategy | ||
| Prognosis | 10 | 40 |
| Diagnosis | 9 | 36 |
| Guide treatment | 3 | 12 |
| Other/multiple | 3 | 12 |
WES, whole-exome sequencing; WGS/WGTA, whole-genome sequencing/whole-genome and transcriptome analysis, including cell-free DNA testing
NGS panels were the most common technology evaluated (n = 13), followed by WGS and/or WES (n = 8), including cell-free DNA testing. Four included studies did not specify the NGS technology being assessed. Primary strategies for genomic testing included prognosticating disease (n = 10), diagnosing patients (n = 9), and guiding treatment (n = 3). Two studies explored multiple testing strategies and one study focused on returning secondary genomic findings (SFs). SFs are clinically relevant genetic findings unrelated to the original indication for undergoing testing. Nearly half (46%, n = 6) of studies that evaluated NGS panels examined Oncotype DX (Genomic Health) for predicting breast cancer patients’ risk of recurrence.
Methods and outcomes of included studies
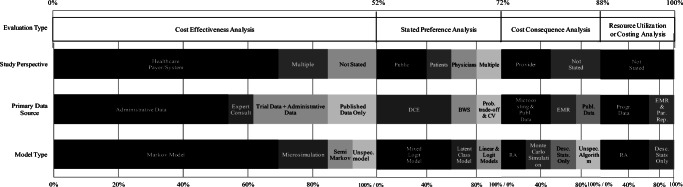
To determine the economic impacts of NGS-informed PM in Canada, 52% of studies applied cost-effectiveness analysis (n= 13), 20% conducted stated preference analysis (n = 5), 16% used cost-consequence analysis (n = 4), and 12% examined healthcare resource utilization or costs (n = 3). Figure 2 summarizes the study designs and methods used in the included studies.
Fig. 2.
Methods overview for included studies. DCE, discrete choice experiment; BWS, best-worst scaling; CV, contingent valuation; electronic medical record; RA, regression adjustment; Publ., published; Desc. Stats, descriptive statistics; Unspec., unspecified; Par. Rep., parental reports. All cost-effectiveness studies used published data and all cost-consequence studies used program data, either alone or in combination with the data sources listed above.
Resource utilization or costing analysis
When exploring resource utilization or costs, study aims ranged from estimating the costs of each component of NGS-informed PM, to forecasting future costs, to analyzing resource utilization in the 6 months prior to genomic testing, to calculating cost savings from a centralized center for out-of-province test referrals. To explore these diverse outcomes, studies used program data (n = 2), parental reports (n = 1), and electronic medical records (n = 1). Data was analyzed using regression modeling (n = 2), non-parametric bootstrapping (n = 1), and descriptive statistics (n = 2).
After estimating costs of each component of NGS-informed PM, studies reported that major cost drivers included sequencing and bioinformatics. Total costs ranged from $1,724 to $5,748 per sample when applying single sample WES or WGS to identify pathogenic variants in patients with autism spectrum disorder (ASD), and reached $36,336 per patient when applying WGS and transcriptome analysis to guide treatment planning for patients with incurable cancers (Tsiplova et al. 2017; Weymann et al. 2017). Weymann et al. (2017) forecasted future costs of WGS and transcriptome analysis and found that total costs were unlikely to reach critical thresholds in the next 10 years owing to non-decreasing bioinformatics costs.
In the 6 months prior to genomic testing with NGS, Dragojlovic et al. (2018b) estimated that children with suspected genetic disorders experienced an average of 5 outpatient visits and 10 hospitalizations. When exploring the economic impacts of a centralized center for out-of-province test referrals, Lilley et al. (2013) estimated annual cost savings of $119,222.
Cost-consequence analysis
Studies estimated cost-consequences of NGS-informed PM using published data sources (n = 3), program data (n = 4), microcosting data (n = 2), and electronic medical records (n = 1). Data was analyzed using regression modeling (n = 1), Monte Carlo simulation (n = 1), descriptive statistics (n = 2), and unspecified algorithmic approaches (n = 1). While cost-consequence studies did not conduct full incremental analyses, authors often reported incremental costs divided by incremental yield if a comparator strategy was examined.
Studies found that more comprehensive NGS strategies involving WES or WGS increased both costs and diagnostic yield of testing. Dragojlovic et al. (2018a) reported that the costs of applying WES to diagnose children with suspected genetic disorders ranged from $11,000 per positive diagnosis for single sample WES to $19,340 per positive diagnosis for trio WES. Tsiplova et al. (2017) estimated that replacing chromosomal microarray (CMA) with WGS or WES to identify pathogenic variants in patients with ASD costs more than $25,000 per additional positive finding. Okun et al. (2014) estimated that replacing standard testing with primary cell-free DNA testing for trisomy disorders costs over $470,000 per additional prenatally diagnosed pregnancy.
When examining the downstream impacts of WGS for identifying a genetic diagnosis in children with developmental delay, Hayeems et al. (2017) concluded that the volume of healthcare resources used was similar after WGS or CMA, yet the nature of these resources differed, with CMA leading to further diagnostic investigations and WGS leading to care tailored according to genotypic variants.
Stated preference analysis determining benefits and acceptability
Stated preference analysis was used to value a wide range of outcomes resulting from genomic testing involving NGS. Methods included discrete choice experiments (DCE) (n = 3), best-worst scaling (BWS) (n = 1), or a combination of contingent valuation (CV) and probability trade-off testing (n = 1). Studies elicited preferences for NGS outcomes from the perspectives of the Canadian general public (n = 2), patients (n = 1), health-care providers (n = 1), or from both the public and patients (n = 1). All studies included attributes related to health and non-health outcomes. Non-health outcomes were captured in process (e.g., turnaround time) and informational (e.g., risk of future disease) attributes. To explore heterogeneity while modeling preference data, studies estimated latent class (n = 1) or mixed logit models (n = 3). Model findings were summarized in terms of relative importance of attributes (n = 4), predicted uptake (n = 2), and/or WTP (n = 2).
All studies concluded that respondents valued both health and non-health outcomes of NGS. There was also strong evidence of preference heterogeneity, both within and across respondents. Najafzadeh et al. (2013) found that patients valued NGS attributes differently from the general public. Patients placed higher importance on test sensitivity whereas the general public placed higher importance on health outcomes and testing procedures. Within the general public, Regier et al. (2015) found that preferences for receiving SFs depended on the information returned. Some respondents valued SF information on all genetic disorders, regardless of treatment availability, and others were only willing to pay for information on disorders with effective medical treatment available.
Cost-effectiveness analysis
All CEAs compared NGS-informed PM approaches with standard testing or management and one study directly compared two NGS technologies. To build cost-effectiveness models, all studies used published data. Additional data sources included administrative and/or site-specific data (n = 10), new analyses of clinical trial data (n = 3), and input from expert consultation (n = 1). Cost-effectiveness was estimated using the Markov modeling (n = 9), microsimulation (n = 2), semi-Markov decision analytic modeling (n = 1), or an unspecified decision model (n = 1), and summarized as ICERs. To determine cost-effectiveness, 84% of studies compared ICERs to threshold values representing decision-makers’ WTP for an additional one unit gain in effectiveness.
Oncology studies found that when NGS panels were used to prognosticate disease or diagnose patients (n = 11), mean ICERs were often cost-effective at WTP thresholds of either $50,000 or $100,000 per quality-adjusted life year (QALY) or life-year gained (LYG). This finding held true in all studies evaluating the use of Oncotype DX, a 21-gene panel, for predicting risk of recurrence in breast cancer patients and informing treatment de-escalation (n = 6). After applying probabilistic analysis, 75% of oncology studies reported fewer than 80% of sampled ICERs were cost effective at stated WTP thresholds and 38% reported fewer than 60% of ICERs were cost-effective. Similarly, high variability was observed in evaluations of Oncotype DX. This indicates that there exists substantial decision uncertainty regarding clinical adoption of NGS panels.
In other disease areas, studies measured effectiveness for NGS-informed PM approaches using outcomes other than QALYs or LYG, such as number of additional cases identified. When WGS or WES was used to identify rare pathogenic variants within 18 months of patients’ diagnosis with ASD (n = 1), all sampled ICERs were below $20,000 per additional child identified (Yuen et al. 2018). Conclusions about cost-effectiveness were highly sensitive to the choice of WTP threshold and less than 25% of sampled ICERs were below $10,000 per child identified. When prenatal testing for trisomy disorders, replacing first trimester screening with universal cell-free DNA testing resulted in a mean ICER of $1.63 million per additional case detected (Nshimyumukiza et al. 2018). The probability that combined approaches to cell-free DNA testing were cost-effective varied greatly depending on the choice of WTP threshold.
Evidentiary challenges
The included studies highlighted multiple challenges when generating economic evidence to inform the adoption of genomic testing in Canada, summarized in Table 2. These challenges can be aggregated into three broad categories that relate to (1) accounting for all NGS outcomes; (2) addressing uncertainty; and (3) improving consistency of economic approaches.
Table 2.
Summary of evidentiary challenges when evaluating NGS technologies
| Challenges | Examples | Addressed | Gaps |
|---|---|---|---|
| 1) Accounting for all NGS outcomes |
- Health outcomes - Non-health outcomes: ○ Information ○ Process ○ Test characteristics |
- Preference studies include non-health attributes |
- Should economic evaluations incorporate non-health outcomes? - If so, how? |
| 2) Addressing uncertainty |
- Limited data available: ○ Accuracy ○ Clinical outcomes ○ Canadian context - Heterogeneity of NGS - Future changes in services and outcomes |
- Parameter uncertainty ○ Probabilistic and deterministic analysis - Decision uncertainty ○ Value of information - Model uncertainty ○ Scenario analysis |
- Accuracy in economic evaluations - Uncertainty in stated preference studies |
| 3) Improving consistency |
- Difficult to capture benefits of NGS: ○ Diagnostic odyssey ○ Comparison strategies ○ Downstream effects (e.g., cascade testing) - Study design and methods in the absence of RCT data |
- Transparency around ad hoc decisions |
- Guidance on: ○ Data collection ○ Study designs ○ Statistical methods |
NGS, next-generation sequencing; RCT, randomized controlled trial
Challenge 1—accounting for all NGS outcomes
NGS applications are heterogeneous and range from diagnosing patients, to identifying targeted treatment options, to prognosticating disease. Not all applications of NGS lead to immediate changes in clinical management, creating challenges for estimation of clinical and cost-effectiveness. Patients may value NGS information irrespective of its impact on health, termed personal utility, and the direction (and magnitude) of this utility is dependent on the information returned (Regier et al. 2018). Utility for NGS outcomes can also depend on the testing process. For example, NGS tests may vary in terms of costs, accuracy, necessary procedures (e.g., bone marrow biopsy versus blood sample), and wait time for results (Cuffe et al. 2014; Najafzadeh et al. 2013).
To date, Canadian studies have applied stated preference methods to estimate preference-based utility for NGS health and non-health outcomes. By including a patient-informed range of attributes describing non-health outcomes, studies found that respondents valued informational and process attributes alongside attributes describing health outcomes (Marshall et al. 2016; Regier et al. 2015). Despite this, we did not identify any studies that incorporated utility or WTP for non-health outcomes into economic evaluations of NGS technologies. If the Canadian healthcare system aims to maximize patients’ preference-based utility, ignoring values for non-health outcomes when estimating the economic impacts of NGS-informed PM could lead to over- or under-investment in these technologies (Regier et al. 2018).
Canadian economic evaluation guidelines currently recommend that all reference case analyses report cost-effectiveness in terms of costs per QALY (CADTH 2017). QALYs capture important aspects of clinical benefit, including length of life and health-related quality of life, and provide a common denominator for comparing health interventions, yet the instruments that support the generation of QALYs do not account for all benefits of NGS that patients value and may be inappropriate in some settings, particularly in context to genomic testing (Buchanan et al. 2013). Oncology studies included in this review consistently reported effectiveness gains in terms of LYG and QALY, despite evidence demonstrating that stakeholders’ value genomic information beyond its’ impact on these clinical outcomes. Outside of oncology, no studies included in this review reported QALYs when evaluating NGS technologies. When focusing on diagnosing genetic or chromosome disorders, studies often measured benefits using diagnostic yield because of insufficient data on long-term health outcomes for rare or untreatable disorders and ethical concerns for valuing prenatal testing outcomes involving pregnancy termination.
In the USA, recommendations for economic evaluation have recently emerged supporting a reference case that accounts for both health and non-health outcomes (Sanders et al. 2016). Despite these recommendations, no clear guidance exists on how best to incorporate non-health outcomes into traditional economic evaluation frameworks, nor on how decision-makers can best compare economic evaluations that incorporate non-health outcomes with evaluations that do not. Additional research is needed to determine whether these new forms of HTA evidence are acceptable to health system stakeholders, including decision-makers, patients, providers, and the general public. If so, information on how much decision-makers are willing to pay for effectiveness gains not measured in LYG or QALYs would provide context for these estimates.
Challenge 2—addressing uncertainty in economic analyses
All studies included in this review highlighted the challenges of managing uncertainty in their approaches for evaluating the economic impacts of NGS-informed PM. This uncertainty was largely driven by a lack of available evidence on the clinical effectiveness of NGS-informed care and the variable accuracy of NGS information for directing clinical management. Variability in accuracy stems from the probabilistic nature of the relationship between NGS results and individual response to a change in management (Laksman and Detsky 2011; Najafzadeh et al. 2013). To date, the primary focus of NGS has been on discovery and individualization. Few studies have assessed clinical and cost-effectiveness through randomized controlled trials (RCTs), largely because traditional trial designs are unable to control for genomic-level heterogeneity, which can shape individual outcomes from NGS-informed PM (Schork 2015). There has been an amplification of uncertainty in accuracy and outcomes estimates resulting from this lack of RCT evidence (Weymann et al. 2018).
Additional sources of uncertainty include variability across NGS services, potential future changes in NGS outcomes, and informational uncertainty. The extent to which studies accounted for other forms of uncertainty in their economic analyses varied across evaluation frameworks. CEAs addressed high levels of uncertainty around clinical and cost outcomes through deterministic sensitivity analysis, probabilistic analysis, and/or value of information analysis. Few CEAs incorporated accuracy of NGS information for directing clinical management in their evaluations (n=3). To account for varying assumptions about NGS services and future outcomes, CEAs, costing studies, and stated preference studies often applied scenario analysis. While stated preference studies frequently included attributes related to accuracy and prediction error of genomic testing in choice tasks, none accounted for uncertainty in clinical effectiveness outcomes. Designing choice tasks that present complex information on uncertainty for NGS outcomes remains an important area for future research.
Informational uncertainty requires consideration when implementing genomic technologies into practice, although certain forms may not be relevant for economic evaluation. Variants of unknown significance (VUS) are one such genomic finding wherein the pathogenicity of a detected variant is entirely unknown and may never be discovered. To date, the majority of VUS are reclassified as benign, a process that can take several years to establish (Macklin et al. 2018). Current guidelines recommend that VUS should not be used to guide clinical decisions on the basis that their clinical utility is unknown and immeasurable (Richards et al. 2015). The potential downstream consequences of returning VUS to patients include increased patient anxiety and the potentially unnecessary uptake of prophylactic surgical procedures (Pollard et al. 2019). For these reasons, the uncertainty related to VUS is outside the scope of economic evaluations and, appropriately, was not considered in the included studies.
Challenge 3—improving consistency of economic approaches
NGS services vary widely across clinical contexts, as do the approaches used to evaluate them. In the absence of clear guidelines for economic evaluations in NGS-informed PM, studies assessing costs and benefits are often forced to make ad hoc decisions to address challenges. Variability in these decisions can reduce the comparability of final outcomes estimates across studies. To ensure decision-makers have access to reliable economic evidence that supports reimbursement decisions for NGS-informed PM in Canada, consistency of data collection, study design, and statistical methods must improve, as must the reporting of economic analyses (Faulkner et al. 2012; Husereau et al. 2014). Included studies often did not adhere to common reporting guidelines (Bridges et al. 2011; Husereau et al. 2013), with seven studies not stating their study’s perspective, three not disclosing their study funding, two not providing a reference year for their estimates, two not stating a time horizon for their analysis, and two not describing their modeling approach.
There is currently no guidance on the minimum amount of information genomic studies must record to facilitate health economic analyses. In diagnostic settings, standardization of which healthcare utilization data can be attributed to a patients’ diagnostic odyssey will be critical for accurate estimation of changes following genomic testing. For all NGS-informed PM strategies, studies require consistent recommendations on which downstream health and non-health consequences are relevant for economic evaluation and which comparison strategies are most appropriate.
Given that RCTs are uncommon in NGS-informed PM, robust study designs are needed to generate reliable counterfactuals for these approaches. N-of-1 trials are a proposed solution but their validity strongly depends on the assumption of clinical stability, which may be violated for certain clinical contexts (Bedard et al. 2013; Lillie et al. 2011). Quasi-experimental methods, such as matching, present an opportunity to evaluate NGS outside of controlled trial settings. Matching methods are increasingly used to mitigate selection bias and generate counterfactuals for NGS approaches (Barcenas et al. 2017; Presley et al. 2018). Matching involves selecting a group of controls most similar to individuals in a treatment group based on their observable characteristics (Stuart 2010). To date, no Canadian studies have incorporated matching into economic analyses of NGS-informed PM. There remains considerable scope for future research exploring the ability of quasi-experimental methods to adjust for confounding in observational studies of NGS-informed PM.
Discussion
We conducted a structured literature review of current Canadian evidence examining the economic impacts of NGS-informed PM. We found that the majority of available evidence focuses on NGS panels (52%, n = 13) and nearly half (46%, n = 6) of these studies evaluated Oncotype DX (Genomic Health) for predicting breast cancer patients’ risk of recurrence. This finding aligns with current implementation of NGS-informed PM in Canada, which has focused on NGS panels for disease screening, hereditary cancer testing, and prognosticating treatment response in breast cancer patients (AHS 2017; Centre for Clinical Genomics n.d.; Newborn Screening Ontario n.d.). Studies for adopted technologies reported that mean ICERs for NGS panels were cost-effective at WTP thresholds of $50,000 or $100,000 per LYG or QALY, but conclusions were subject to significant uncertainty. This suggests that decision-makers may have a high tolerance for decision uncertainty and further evidence of cost-effectiveness is crucial to ensure health system sustainability.
The majority of studies identified in this review (60%, n = 15) evaluated the economic impacts of genomic testing using NGS in oncology, which supports Canadian implementation of NGS to date, yet in international settings, genomic testing is also being implemented in disease areas outside of oncology (National Institutes of Health 2018). For example, Genomics England has implemented WGS for diagnosing rare diseases as part of the 100,000 Genomes Project, despite a lack of available economic evidence supporting this application (Turnbull et al. 2018). The current emphasis on evaluating NGS-informed PM in oncology has resulted in a relative lack of economic evidence to guide decision-making for NGS in other disease areas. Implementation of genomic testing is beginning to surpass the available evidence base outside of Canada. Research is needed to fill this evidentiary gap and inform adoption across a broader spectrum, both in Canada and abroad.
We identified eight studies that examined the economic impacts of more comprehensive NGS technologies in Canada. Of these, one study estimated the costs of applying WGS and transcriptome analysis to inform treatment planning for advanced cancers. The benefits of this approach remain unknown. To inform the adoption of WGS and WES in Canada, decision-makers require evidence of the trade-offs between costs and benefits of applying comprehensive NGS technologies to guide treatment planning. Seven studies evaluated WGS and/or WES for diagnosing genetic or chromosome disorders and reported that these approaches increased both costs and diagnostic yield of testing compared to existing testing strategies. Conclusions about cost-effectiveness of WGS and/or WES for diagnostics were highly sensitive to the choice of WTP threshold. To provide context for ICER estimates, Canadian decision-makers must clarify whether they are willing to pay for effectiveness gains not measured in LYG or QALYs and if so, how much.
Stated preference analysis revealed that Canadian patients, the public, and physicians value information on both health and non-health outcomes from NGS, including those associated with SFs. Although the Canadian public values information on SFs (Regier et al. 2015), the Canadian College of Medical Geneticists (CCMG) does not currently endorse returning SFs after NGS testing (Boycott et al. 2015). Similarly, CADTH guidelines do not recommend including non-health outcomes in economic evaluations despite evidence of stakeholder value (CADTH 2017). Instead, these guidelines specify that effectiveness gains should be measured using QALYs. To ensure that the adoption of PM aligns with stakeholder preferences, future studies will benefit from guidance on how and whether non-health outcomes from NGS should be included in economic evaluations.
When assessing the economic impacts of NGS-informed PM in Canada, 52% of studies (n = 13) applied CEA, 20% conducted stated preference analysis (n = 5), 16% used cost-consequence analysis (n = 4), and 12% examined healthcare resource utilization or costs (n = 3). Regardless of the method applied, studies highlighted multiple challenges when generating economic evidence for genomic testing with NGS, many of which remain unaddressed. Challenges were broadly related to (1) accounting for all NGS outcomes; (2) addressing uncertainty in economic analyses; and (3) improving consistency of economic approaches. These challenges have been previously articulated and partially addressed when evaluating the economic impacts of microarray technologies (Regier et al. 2009; Regier et al. 2010; Wordsworth et al. 2007). For example, past research found evidence of personal utility when diagnosing genetic causes of idiopathic intellectual disability in children and incorporated preference-based values into an economic evaluation of CMA versus cytogenic testing, yet all evidentiary challenges identified, including valuing health and non-health outcomes, are exacerbated in context to NGS technologies, owing to the breadth and complexity of genomic results (Regier et al. 2018).
While challenges related to NGS do not necessitate new parallel HTA processes, comprehensive solutions may take time. Risk-sharing reimbursement and disinvestment policies present an opportunity to alleviate public pressure on health systems to invest in unproven NGS technologies while economic evidence is being generated (Carlson et al. 2010; Hollis 2016). By providing temporary access to NGS-informed PM, risk-sharing policies can balance the desire to fund good-value technologies with the need to provide patients with timely access to potentially beneficial care, yet transparent disinvestment processes are needed to ensure affordability if real-world evidence conflicts with initial reimbursement agreements.
This study should be interpreted in light of its limitations. First, we present a scoping review of published Canadian studies that generate economic evidence for PM informed by NGS and do not necessarily report on all investigations into this research area. Second, the overall quality of the included studies is not reported. Our quality appraisal is limited to assessing transparency of reporting and adherence to reporting guidelines within individual studies. Third, studies included in this review vary considerably in terms of the applications of genomic testing being evaluated, methodological approaches, and underlying assumptions. As a result, we are unable to conclude whether specific applications of NGS-informed PM in Canada provide value-for-money. Despite these limitations, the scoping nature of our review allows us to identify key evidentiary challenges researchers are facing when evaluating NGS technologies as well as highlight gaps in the Canadian evidence base.
Conclusions
Studies are beginning to produce reliable estimates of the economic impacts of NGS-informed PM in Canada but barriers for HTA remain. Researchers require direction on incorporating non-health outcomes into economic evaluation, managing amplified uncertainty around outcomes estimates, and standardizing data collection, study designs, and statistical methods. While these challenges are being addressed and the evidence base for NGS continues to grow, reimbursement and disinvestment policies that share risk between payers and manufacturers can support resource allocation decisions for genomic testing in Canada.
Electronic supplementary material
(DOC 199 kb)
Funding Information
This work was supported by BC Cancer Foundation’s Strategic Priority Fund Awards. The Canadian Centre for Applied Research in Cancer Control is funded by the Canadian Cancer Society Grant No. 2015-703549.
Compliance with ethical standards
Conflict of interest
Deirdre Weymann, Nick Dragojlovic, and Samantha Pollard report no conflicts of interest. Dean A. Regier has received travel support from Illumina to attend conferences in Boston, USA, and Barcelona, Spain.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Resource Allocation in Genetic and Genomic Medicine
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Agyeman AA, Ofori-Asenso R. Perspective: does personalized medicine hold the future for medicine? J Pharm Bioallied Sci. 2015;7:239–244. doi: 10.4103/0975-7406.160040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AHS (2017) Breast Cancer Molecular Testing – Oncologist Alberta Health Services. https://www.albertahealthservices.ca/assets/info/hp/cancer/if-hp-cancer-guide-moleculartesting-bulletin.pdf. Accessed 29/10/2018
- Barcenas CH, Raghavendra A, Sinha AK, Syed MP, Hsu L, Patangan MG, Jr, Chavez-MacGregor M, Shen Y, Hortobagyi GH, Valero V, Giordano SH, Ueno NT, Tripathy D. Outcomes in patients with early-stage breast cancer who underwent a 21-gene expression assay. Cancer. 2017;123:2422–2431. doi: 10.1002/cncr.30618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BC Cancer (2016) New genetic tests become standard of cancer care in BC. BC Cancer. http://www.bccancer.bc.ca/about/news-stories/news/2016/new-genetic-tests-become-standard-of-cancer-care-in-bc. Accessed 29 Oct 2018
- Bedard PL, Hansen AR, Ratain MJ, Siu LL. Tumour heterogeneity in the clinic. Nature. 2013;501:355–364. doi: 10.1038/nature12627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boycott K, Hartley T, Adam S, Bernier F, Chong K, Fernandez BA, Friedman JM, Geraghty MT, Hume S, Knoppers BM, Laberge AM, Majewski J, Mendoza-Londono R, Meyn MS, Michaud JL, Nelson TN, Richer J, Sadikovic B, Skidmore DL, Stockley T, Taylor S, van Karnebeek C, Zawati MH, Lauzon J, Armour CM, Canadian College of Medical Geneticists The clinical application of genome-wide sequencing for monogenic diseases in Canada: position statement of the Canadian College of Medical Geneticists. J Med Genet. 2015;52:431–437. doi: 10.1136/jmedgenet-2015-103144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges JF, et al. Conjoint analysis applications in health—a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value Health. 2011;14:403–413. doi: 10.1016/j.jval.2010.11.013. [DOI] [PubMed] [Google Scholar]
- Buchanan J, Wordsworth S, Schuh A. Issues surrounding the health economic evaluation of genomic technologies. Pharmacogenomics. 2013;14:1833–1847. doi: 10.2217/pgs.13.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CADTH (2017) Guidelines for the economic evaluation of health technologies. Canada. 4th ed. Ottawa:
- Carlson JJ, Sullivan SD, Garrison LP, Neumann PJ, Veenstra DL. Linking payment to health outcomes: a taxonomy and examination of performance-based reimbursement schemes between healthcare payers and manufacturers. Health policy. 2010;96:179–190. doi: 10.1016/j.healthpol.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Centre for Clinical Genomics (n.d.) CC Genomics Hereditary Cancer Panel. http://www.ccgenomics.ca/hcp-panel.html. Accessed 29 Oct 2018
- Cheung MC, Chan KK, Sabharwal M, Fields A, Chambers A, Evans WK. Comparing assessment frameworks for cancer drugs between Canada and Europe: what can we learn from the differences? ESMO open. 2016;1:e000124. doi: 10.1136/esmoopen-2016-000124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuffe S, Hon H, Qiu X, Tobros K, Wong CKA, de Souza B, McFarlane G, Masroor S, Azad AK, Hasani E, Rozanec N, Leighl N, Alibhai S, Xu W, Issa AM, Liu G. Cancer patients’ acceptance, understanding, and willingness-to-pay for pharmacogenomic testing. Pharmacogenet Genomics. 2014;24:348–355. doi: 10.1097/FPC.0000000000000061. [DOI] [PubMed] [Google Scholar]
- Dragojlovic N, Elliott AM, Adam S, van Karnebeek C, Lehman A, Mwenifumbo JC, Nelson TN, du Souich C, Friedman JM, Lynd LD. The cost and diagnostic yield of exome sequencing for children with suspected genetic disorders: a benchmarking study. Genet Med. 2018;20:1013–1021. doi: 10.1038/gim.2017.226. [DOI] [PubMed] [Google Scholar]
- Dragojlovic N, Kim E, Elliott AM, Study C, Friedman JM, Lynd LD. Evaluating the use of parental reports to estimate health care resource utilization in children with suspected genetic disorders. J Eval Clin Pract. 2018;24:416–422. doi: 10.1111/jep.12876. [DOI] [PubMed] [Google Scholar]
- Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW (2015) Methods for the economic evaluation of health care programmes. Oxford university press
- Faulkner E, Annemans L, Garrison L, Helfand M, Holtorf AP, Hornberger J, Hughes D, Li T, Malone D, Payne K, Siebert U, Towse A, Veenstra D, Watkins J. Challenges in the development and reimbursement of personalized medicine—payer and manufacturer perspectives and implications for health economics and outcomes research: a report of the ISPOR Personalized Medicine Special Interest Group. Value Health. 2012;15:1162–1171. doi: 10.1016/j.jval.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Génome Québec (2016) A new initiative to facilitate the implementation of personalized medicine in Canada. Génome Québec. http://www.genomequebec.com/267-en/news-a-new-initiative-to-facilitate-the-implementation-of-personalized-medicine-in-canada/. Accessed 29 Oct 2018
- Hayeems RZ, Bhawra J, Tsiplova K, Meyn MS, Monfared N, Bowdin S, Stavropoulos DJ, Marshall CR, Basran R, Shuman C, Ito S, Cohn I, Hum C, Girdea M, Brudno M, Cohn RD, Scherer SW, Ungar WJ. Care and cost consequences of pediatric whole genome sequencing compared to chromosome microarray. Eur J Hum Genet. 2017;25:1303–1312. doi: 10.1038/s41431-017-0020-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Quality Ontario (2018) Health technology assessments methods and process guide: version 2.0. https://www.hqontario.ca/Portals/0/documents/evidence/reports/hta-methods-and-process-guide-en.pdf. Accessed 15 Feb 2019
- Hollis A. Sustainable financing of innovative therapies: a review of approaches. PharmacoEconomics. 2016;34:971–980. doi: 10.1007/s40273-016-0416-x. [DOI] [PubMed] [Google Scholar]
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, Augustovski F, Briggs AH, Mauskopf J, Loder E, on behalf of the CHEERS Task Force Consolidated health economic evaluation reporting standards (CHEERS) statement. Cost Eff Resour Alloc. 2013;11:6. doi: 10.1186/1478-7547-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husereau D, Marshall DA, Levy AR, Peacock S, Hoch JS. Health technology assessment and personalized medicine: are economic evaluation guidelines sufficient to support decision making? Int J Technol Assess Health Care. 2014;30:179–187. doi: 10.1017/S0266462314000142. [DOI] [PubMed] [Google Scholar]
- Joly Y, Ramos–Paque E (2010) Approval of new pharmacogenomic tests: is the Canadian regulatory process adequate? CJLT 8:2
- Laksman Z, Detsky AS. Personalized medicine: understanding probabilities and managing expectations. J Gen Intern Med. 2011;26:204–206. doi: 10.1007/s11606-010-1515-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley M, Christian S, Blumenschein P, Chan S, Somerville M. A centralized approach to out–of–province genetic testing leads to cost savings: the Alberta experience. Clin Genet. 2013;84:373–377. doi: 10.1111/cge.12077. [DOI] [PubMed] [Google Scholar]
- Lillie EO, Patay B, Diamant J, Issell B, Topol EJ, Schork NJ. The n–of–1 clinical trial: the ultimate strategy for individualizing medicine? Per Med. 2011;8:161–173. doi: 10.2217/pme.11.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macklin S, Durand N, Atwal P, Hines S. Observed frequency and challenges of variant reclassification in a hereditary cancer clinic. Genet Med. 2018;20:346–350. doi: 10.1038/gim.2017.207. [DOI] [PubMed] [Google Scholar]
- Marshall DA, Deal K, Bombard Y, Leighl N, MacDonald KV, Trudeau M. How do women trade-off benefits and risks in chemotherapy treatment decisions based on gene expression profiling for early-stage breast cancer? A discrete choice experiment. BMJ Open. 2016;6:e010981. doi: 10.1136/bmjopen-2015-010981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najafzadeh M, Johnston KM, Peacock SJ, Connors JM, Marra MA, Lynd LD, Marra CA. Genomic testing to determine drug response: measuring preferences of the public and patients using Discrete Choice Experiment (DCE) BMC Health Serv Res. 2013;13:454. doi: 10.1186/1472-6963-13-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health (2018) All of Us Research Program Operation Protocol. https://allofus.nih.gov/sites/default/files/aou operational protocol v1.7 mar 2018.pdf. Accessed 15/02/2019
- Newborn Screening Ontario (n.d.) Newborn Screening Ontario Molecular Diagnostics. https://www.newbornscreening.on.ca/en/diagnostic–testing/molecular–diagnostics. Accessed 29/10/2018
- Nshimyumukiza L, Beaumont JA, Duplantie J, Langlois S, Little J, Audibert F, McCabe C, Gekas J, Giguère Y, Gagné C, Reinharz D, Rousseau F. Cell-free DNA–based non-invasive prenatal screening for common aneuploidies in a Canadian Province: a cost-effectiveness analysis. J Obstet Gynaecol Can. 2018;40:48–60. doi: 10.1016/j.jogc.2017.05.015. [DOI] [PubMed] [Google Scholar]
- Okun N, Teitelbaum M, Huang T, Dewa CS, Hoch JS. The price of performance: a cost and performance analysis of the implementation of cell-free fetal DNA testing for down syndrome in Ontario, Canada. Prenat Diagn. 2014;34:350–356. doi: 10.1002/pd.4311. [DOI] [PubMed] [Google Scholar]
- Ontario Personalized Medicine Network (n.d.) Personalized Medicine Reports - Subcommittee 2: Evaluating Our Current Health Technology Assessment Capabilities in Light of Personalized Medicine Technologies. Ontario Genomics. http://www.ontariogenomics.ca/provincial-strategies/opmn/personalized-medicine-resources/. Accessed 29 Oct 2018
- Pollard S, Sun S, Regier DA (2019) Balancing uncertainty with patient autonomy in precision medicine. Nat Rev Genet. 20:251–252 [DOI] [PubMed]
- Presley CJ, Tang D, Soulos PR, Chiang AC, Longtine JA, Adelson KB, Herbst RS, Zhu W, Nussbaum NC, Sorg RA, Agarwala V, Abernethy AP, Gross CP. Association of broad-based genomic sequencing with survival among patients with advanced non–small cell lung cancer in the community oncology setting. Jama. 2018;320:469–477. doi: 10.1001/jama.2018.9824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regier D, Friedman J, Makela N, Ryan M, Marra C. Valuing the benefit of diagnostic testing for genetic causes of idiopathic developmental disability: willingness to pay from families of affected children. Clin Genet. 2009;75:514–521. doi: 10.1111/j.1399-0004.2009.01193.x. [DOI] [PubMed] [Google Scholar]
- Regier DA, Friedman JM, Marra CA. Value for money? Array genomic hybridization for diagnostic testing for genetic causes of intellectual disability. Am J Hum Genet. 2010;86:765–772. doi: 10.1016/j.ajhg.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regier DA, Peacock SJ, Pataky R, van der Hoek K, Jarvik GP, Hoch J, Veenstra D. Societal preferences for the return of incidental findings from clinical genomic sequencing: a discrete–choice experiment. Can Med Assoc J. 2015;187:E190–E197. doi: 10.1503/cmaj.140697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regier DA, Weymann D, Buchanan J, Marshall DA, Wordsworth S. Valuation of health and nonhealth outcomes from next-generation sequencing: approaches, challenges, and solutions. Value Health. 2018;21:1043–1047. doi: 10.1016/j.jval.2018.06.010. [DOI] [PubMed] [Google Scholar]
- Richards S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–423. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, Kuntz KM, Meltzer DO, Owens DK, Prosser LA, Salomon JA, Sculpher MJ, Trikalinos TA, Russell LB, Siegel JE, Ganiats TG. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. Jama. 2016;316:1093–1103. doi: 10.1001/jama.2016.12195. [DOI] [PubMed] [Google Scholar]
- Schork NJ. Personalized medicine: time for one-person trials. Nature. 2015;520:609–611. doi: 10.1038/520609a. [DOI] [PubMed] [Google Scholar]
- Statistics Canada (2018) Table 18–10–0004–01 Consumer Price index, monthly, not seasonally adjusted. https://www150.statcan.gc.ca/t1/tbl1/en/cv.action?pid=1810000401. Accessed 01 Oct 2018
- Stuart EA. Matching methods for causal inference: a review and a look forward statistical science: a review. J Inst Math Stat. 2010;25:1. doi: 10.1214/09-STS313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiplova K, Zur RM, Marshall CR, Stavropoulos DJ, Pereira SL, Merico D, Young EJ, Sung WWL, Scherer SW, Ungar WJ. A microcosting and cost–consequence analysis of clinical genomic testing strategies in autism spectrum disorder. Genet Med. 2017;19:1268–1275. doi: 10.1038/gim.2017.47. [DOI] [PubMed] [Google Scholar]
- Tsoi DT, Inoue M, Kelly CM, Verma S, Pritchard KI. Cost–effectiveness analysis of recurrence score–guided treatment using a 21–gene assay in early breast cancer. Oncologist. 2010;15:457–465. doi: 10.1634/theoncologist.2009-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull C et al (2018) The 100 000 genomes project: bringing whole genome sequencing to the NHS. BMJ 361:k1687 [DOI] [PubMed]
- Weymann D, Laskin J, Roscoe R, Schrader KA, Chia S, Yip S, Cheung WY, Gelmon KA, Karsan A, Renouf DJ, Marra M, Regier DA. The cost and cost trajectory of whole-genome analysis guiding treatment of patients with advanced cancers. Mol Genet Genom Med. 2017;5:251–260. doi: 10.1002/mgg3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weymann D, Pataky R, Regier DA (2018) Economic evaluations of next–generation precision oncology: a critical review. JCO Precision Oncology 2:1–23 [DOI] [PubMed]
- Wordsworth S, Buchanan J, Regan R, Davison V, Smith K, Dyer S, Campbell C, Blair E, Maher E, Taylor J, Knight SJL. Diagnosing idiopathic learning disability: a cost-effectiveness analysis of microarray technology in the National Health Service of the United Kingdom. Genomic Medicine. 2007;1:35–45. doi: 10.1007/s11568-007-9005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen T, Carter MT, Szatmari P, Ungar WJ (2018) Cost–effectiveness of genome and exome sequencing in children diagnosed with autism spectrum disorder Applied health economics and health policy 16:481–493 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 199 kb)