Abstract
High-throughput next-generation sequencing technologies have seen an increase in use in most developed countries. The translation of genomic testing into clinical practice challenges the traditional model of medical care in France and raises numerous medical, legal, ethical, organizational, and financial issues. In order to allow the population to use this revolution to its advantage, France has conceived the French Plan for Genomic Medicine 2025. Its aim is to improve health and quality of life, to organize new pathways of care and counseling, and to make decisions about insurance coverage. It has also been designed to drive innovation and promote economic growth in France by incorporating genomic medicine into the French health care system. These issues can be addressed through evaluations developed to aid the decision-making process in the context of resource scarcity. Health economists can help to resolve these resource allocation challenges by measuring the impact of this technological revolution on patients, caregivers, providers, and the health care system. In this paper, we will review challenges associated with implementing genomic testing in France. One of the pilot studies of the French Plan for Genomic Medicine 2025 will be presented as an illustration of the role of health economists in overcoming some of the challenges of this technological revolution.
Keywords: Genomics, Health care pathway, Public health, Economics
Introduction
Genome research has been revolutionized by recently developed high-throughput next-generation sequencing (NGS) technologies that produce vast amounts of sequence data. NGS is novel in that it can sequence vast numbers of DNA base pairs in a single sequencing reaction. Medical genetics as a whole is experiencing a technological upheaval which is the result of the development of targeted gene panels, whole exome sequencing (WES), and whole genome sequencing (WGS).
Public access to genomic medicine raises significant medical, legal, ethical, organizational, and financial questions (Callier et al. 2016; Ojha and Thertulien 2005; Morgan et al. 2003): with the spread of ultra-high-throughput sequencing, a considerable number of patients, and not only those with rare diseases and cancer, will ultimately benefit from routine genomic investigation. Higher-resolution diagnoses are expected to reduce the number of expensive and futile diagnostic procedures (Stark et al. 2017; Monroe et al. 2016; Ontario Health (Quality) 2020), shorten time frames (Gainotti et al. 2018) and therefore modify the existing organization of care, and result in more effective therapeutic strategies and fewer adverse reactions. Non-medical outcomes associated with NGS for the patients and their families also have to be considered (Foster et al. 2009; Kohler et al. 2017; Turrini and Prainsack 2016).
In parallel, an innovative industrial framework is expected to emerge to support the system’s expanding scope and provide solutions to numerous technological challenges, in particular how to store the vast amounts of generated data. The expansion of this sector is therefore also expected to be a source of economic development and employment. Indeed, genomic medicine has also become a field of international competition supported by national policy. The USA, the UK, and China, in particular, have invested significant resources in this sector and have mobilized their biggest companies to find innovative technological solutions to the problems raised by large-scale ultra-high-throughput sequencing and the handling of big data (National Centre for Human Genomics Research 2018). France, with an annual capacity for just 20,000 exomes and 10,000 genomes, lags behind countries that can do several tens of thousands of runs per year. Genomic medicine is a fast growing industrial opportunity—an opportunity that has already attracted the interest of companies like Google, Apple, Facebook, Amazon, Microsoft, and Samsung (GAFAMS)—and a unique chance to develop a national industrial framework of high strategic, medical, scientific, and economic value. Countries that are not able to keep up will find themselves disadvantaged, the ultimate risk being technological dependence. Moreover, other European countries including Germany, Estonia, the Netherlands, and Slovenia have already started integrating genomic medicine into their health care systems, thereby increasing the risk of the French system losing ground and resulting in domestic patients going abroad for these services.
In this context, the French Plan for Genomic Medicine 2025 was drawn up in order to support France’s efforts to become internationally competitive and to respond to the various challenges presented by genomic medicine. The French initiative includes 14 measures, linking health care, research, and industry. Genome sequencing will be performed by 12 ultra-high-throughput platforms covering the whole country, two of which will start sequencing this year: SeqOIA and AURAGEN. A national data analysis facility (Central Analyser of Data, CAD) will interpret and store data, and interface with other national and international databases. A Reference Center for Innovation, Expertise and Transfer (CRefIX) is developing the procedures, tools, and technologies to be deployed at the sequencing centers and the CAD, and actively supports the implementation of the first clinical pilot projects including pilot cohorts for rare diseases, cancer, and common disease (diabetes). As a result, France will be capable of sequencing 235,000 genomes per year by 2025 (Lethimonnier and Levy 2018).
In this context, the goal of this article is to describe the policy challenges associated with implementing genomic testing in France and show the need for multidisciplinary evaluation of NGS that considers the health and non-health effects associated with NGS required for its generalization. To this end, we will first describe how the public health decision-making process is supported in France. We will then illustrate how the French Plan for Genomic Medicine 2025, through its different axes and one of its four pilot studies, can contribute, with the help of health economists, to measuring the impact of NGS on the various stakeholders involved in this technological revolution and help the resource allocation decision.
Public health decision-making and resource allocation in France: the need for a multidisciplinary approach
To ensure the sustainability of a health system based largely on collective financial responsibility for health care, choices must be made in the allocation of resources (Commission évaluation économique et de santé publique (CEESP) Activity Report 2017). The evaluation of public health interventions is therefore essential. The goal of these evaluations is to evaluate the cost of an intervention to help determine the terms and conditions of its reimbursement, but also to make recommendations to public decision-makers in order guide implementation and modification. In this context, added value generated by the implementation of NGS can be measured in terms of resources saved and improved health benefits (such as higher quality of life, longer life expectancy) (Institute of Medicine; Board on Health Sciences Policy; Roundtable on Translating Genomic-Based Research for Health; Adam C. Berger and Steve Olson 2013).
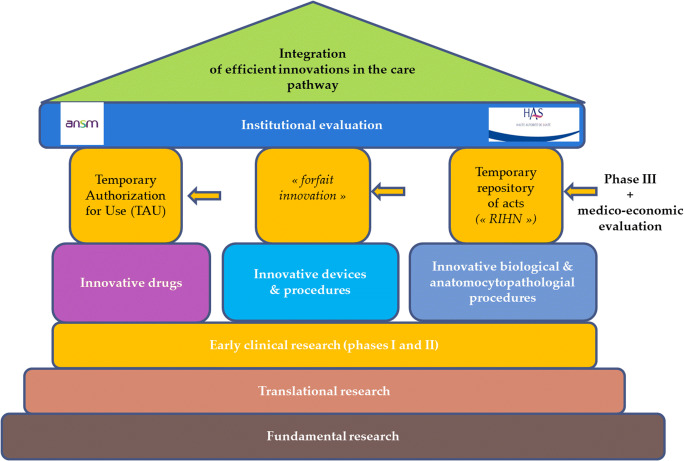
In France, public health authorities refer to the HAS (National Authority for Health). The HAS is an independent public administrative institute with a scientific vocation (https://www.has-sante.fr/portail/jcms/fc_1249588/fr/accueil) whose aim is to actively contribute to the decision-making process by producing public health recommendations based on a population-based approach (assessment of the benefit/risk ratio with the integration of economic and organizational aspects) (HAS 2018). The evaluation criteria are traditional clinical-related endpoints such as effectiveness, quality, and safety of care, with study designs that can differ according to the population, the scientific question, and ethical considerations. Efficiency is also explored—cost-utility analyses are recommended when quality of life is considered as a main criterion; otherwise, cost-effectiveness analyses can be conducted (as an illustration, Fig. 1 shows the French reimbursement process of innovative biological and anatomocytopathological procedures). But public decisions can also be based on “non-health” outcomes (Vass et al. 2017). The activities of the HAS are therefore becoming more cross-cutting, and in addition to traditional clinical and medico-economic endpoints, particular attention is given to the patient-centered approach that includes patients’ perceptions, expectations, preferences, and acceptance of innovations.
Fig. 1.
Reimbursement decision-making processa for innovations in France. Source: Adapted from the Instruction DGOS/PF4 no 2015–258 of the 31st July of the Ministry of Health (http://solidarites-sante.gouv.fr/fichiers/bo/2015/15-08/ste_20150008_0000_0113.pdf). aBefore considering a possible coverage by the insurance system, evidence of their validity in terms of reliability, accuracy, reproducibility, and performance should be demonstrated. After examination, these procedures can be listed in a temporary repository of innovative acts. This registration is only possible if a prospective and comparative collection of data to validate the clinical effectiveness, the efficiency of these procedures, is carried out. After 3 years, if data are considered robust, a reimbursement decision by the collectivity will be or not taken. A similar system exists for innovative drugs and medical devices
In general, it is important to evaluate health interventions with a multidimensional approach. The need for such approaches is reaffirmed in the field of genetics because the traditional model of medical care, traditionally based on the main questions of effectiveness, safety, and efficiency, is challenged (for example, with the controversial question of incidental findings, and variants of uncertain significance, but also with the question of the long-term consequences beyond the patient due to hereditary results, and more generally the psychological and social impact of having an uncertain diagnosis) (Gainotti et al. 2018). It appears essential that decisions relative to the implementation of NGS are based on the value of genetic interventions as measured narrowly by clinical utility, i.e., defined in terms of mortality, morbidity (Grosse and Khoury 2006), and efficiency, and more broadly by personal utility, in terms of “health awareness and behaviors, personal choice and interest as well as the psychological effects of disease risk perception” as defined by Foster et al. (2009; Turrini and Prainsack 2016). Health economists can provide a framework and various tools for measuring these utilities. The French Plan for Genomic Medicine 2025 gives health economists the opportunity to participate in the valuation of these health and non-health outcomes.
The contribution of the French Plan for Genomic Medicine 2025 to the public health decision-making
In 2015, the French Prime Minister commissioned Aviesan (French National Alliance for Life Sciences and Health) to develop and implement the Plan for Genomic Medicine 2025, which aims to position France as an international leader in personalized and precision medicine within the next 10 years, fully integrating genomic medicine into health care pathways and establishing a national genomic medicine industry. More precisely, the plan aims to establish by 2025 a generic care pathway with universal access to genomic medicine for all citizens affected by cancer, and to be capable of sequencing 235,000 genomes a year. Beyond 2025, the system’s capacity will be expanded to cover more common diseases. In order to meet the goals of the program, Aviesan has assembled a steering committee and several working groups. These groups are composed of institutional representatives, diverse experts from the fields of research, health care and business, and delegates from research and health care agencies, ministerial departments, and industrial enterprises. The challenges addressed by the French Plan for Genomic Medicine 2025 are synthesized in Table 1. (https://www.aviesan.fr/aviesan/accueil/toute-l-actualite/plan-france-medecine-genomique-2025): (Table 1).
Table 1.
Challenges addressed by the French Plan for Genomic Medicine 2025
| Challenges | Pursued goals |
|---|---|
| Public health challenge | By enabling patients to obtain equal access to personalized diagnosis, prognosis, and treatment |
| Scientific and clinical challenge | By reinforcing the links between the molecular analysis of the disease and the therapeutic benefit for the patient, which implies the simultaneous use of biological, clinical, and environmental databases |
| Technological challenge | By increasing the ability to acquire, store, distribute, interpret, and address these massive sets of patient data, which will facilitate emergence of computational sciences |
| Economic challenge | By providing opportunities to develop a new industrial sector and to boost innovation in health, growth, and jobs |
| By increasing efficiency and consequently reducing the cost for our health care system: less inadequate, imprecise, and costly tests; reduced analysis times; elimination or limitation of unnecessary drugs and certain disabling side effects, gain in life years |
Specific measures in the French Plan for Genomic Medicine 2025
In contrast to the initiatives launched elsewhere, notably the USA and the UK, the French Plan for Genomic Medicine exploits particularities of the health care system which covers patient care, training, and research with—especially in recent years—the development of broad-scope measures which strongly support this approach (governmental plans, establishing of spaces for dialogue between partners and companies, and definition of national health care and research strategies).
The Plan has been devised to meet the various needs identified at each of the steps along the care pathway and is designed around the patient/doctor partnership, from the ordering of genome analysis through the compilation of conclusions. It has three main targets and includes a series of measures to:
establish genomic medicine instruments for the care pathway:
by setting up a network of high-throughput sequencing platforms in order to cover the whole country, and meet the stated quantitative objectives—the first two sequencing platforms, AURAGEN in the Auvergne Rhône-Alpes region and SeqOIA in the Ile-de-France region, have been operational since 2019.
by establishing a National Center for Intensive Calculation (CAD, Collecteur analyseur de données) capable of processing and analyzing the huge volumes of data that will be generated and providing primary services for professional health care providers within the framework of their care pathways (in silico tests and aids to decision-making in diagnosis, establishing prognosis, and designing therapeutic strategies);
by generalizing standardized electronic patient medical records—a step that is indispensable when it comes to assimilating and exploiting genomic and clinical data;
by implementing pilot projects as of 2016, designed to overcome the technological, clinical, and regulatory obstacles along the pathway for cancer, rare diseases, and common diseases;
by appointing a National Consultative Ethics Committee (Comité consultatif national d’éthique) to examine the various steps of the care pathway with respect to ethical aspects of the collection, storage, and processing of clinical and genomic data as well as the guarantee of safe, high-quality care, and to prepare the evolution of current regulatory frameworks.
-
(2)
respond to the increasing scope of the system with technological and regulatory changes integrated in the Plan’s dynamics:
by establishing a system for the assessment and validation of new indications for access to genomic diagnosis, ensuring development of the existing base with progressive integration into the care pathway;
by creating a national Reference Center for Technology, Innovation and Transfer (CRefIX, Centre de Référence, d’Innovation, d’eXpertise, et de transfert) turned towards the sequencing service, CAD, and companies;
by defining an economic model to ensure the sustainable integration of this new system into the health care system and health insurance, which should define costs and reimbursement conditions and promote the emergence of a “circuit of genomic medicine.” This circuit was designed to introduce genome sequencing into the health care pathway around the patient/physician partnership, from the prescription of a genome analysis to the medical report. This circuit offers companies not only an economic but also an operating framework for the future system guaranteeing its development in the long term;
by addressing industrial issues with support from a public/private sector task force;
by setting up special training programs in universities and schools in order to lay the groundwork of a multi- and inter-disciplinary genomic health system and to foster new skill sets to meet the specific challenges of data analysis and interpretation
-
(3)
implement necessary changes throughout development of the Plan to ensure public awareness and involvement (the general public, users, and patient support groups), with:
a system of governance that matches the Plan’s requirements, and the creation of special monitoring and steering mechanisms;
the creation of a registry to monitor international developments in the medical, technological, and regulatory aspects of genomic medicine;
the creation of an economic observatory to develop research programs on the medico-economic aspects related to the implementation of the Plan, and the organization of the transmission of information to, consultation with, and involvement of all those concerned.
The implementation of this plan is accompanied by an evaluation program whose specific aims are to validate the new clinical indications for access to genomic diagnostics and treatments, and to include these new genomic tests for these indications in a reimbursement process. The cost and efficiency of the new genomic technologies will be assessed, and the experiences of key stakeholders, patients, and their families, will be explored as well, with the involvement of the associations concerned. These types of evaluations will be essential for the development of best practices. One illustration of this evaluation process undertaken within the framework of the Plan is the DEFIDIAG (DEFIcience Intellectuelle DIAGnostic) study whose ambition is to consider the point of view of key stakeholders using an epidemiological approach but also social sand human sciences methodologies.
The value of a multidisciplinary approach: the case of the DEFIDIAG study
General medical context
DEFIDIAG is a prospective multicenter diagnostic study, and one of the four pilot studies of the French Genomic Plan. It illustrates the need to adopt a multidisciplinary methodology to approach the concept of value through a measure of clinical and personal utility (Foster et al. 2009). DEFIDIAG is a clinical trial sponsored by the French National Institute for Health and Medical Research (Inserm). It focuses on patients with intellectual disability (ID), a neurodevelopmental condition characterized before the age of 18 years by impaired intellectual performance and adaptive dysfunction. Similar to other countries, in France, ID affects around 1–3% of the general population, with around 15 per 1000 persons for mild ID and around 3 per 1000 for severe ID. ID is therefore a major public health concern, particularly because this population does not yet have access to optimal care in terms of diagnosis and therapy. Comorbidities are frequent in the form of recognized syndromic entities or co-manifesting with autism spectrum disorders, epilepsy encephalopathies, or a range of associated congenital malformations (Inserm 2018). Ante-perinatal environmental factors are responsible for about 15–20% of cases (i.e., prematurity, anoxia, infection, or fetal alcohol syndrome). The other main etiologies of ID are genetic, but because of the complexity of the genetics, the origin of the disorder remains unknown for more than 50% of patients. In France, diagnoses are globally based on dysmorphological clinical expertise, the search for fragile-X syndrome, the use of chromosomal analysis, and, if necessary, the study of targeted genes (Aviesan France Médecine Génomique 2018). A substantial number of families with children or relatives with ID are currently awaiting a molecular diagnosis in order to access specific rehabilitation (language or skills training, adapted psychosocial support), a better prognostic evaluation, specific treatments/diets, and improved family counseling (prenatal or preimplantation diagnosis).
Objectives and general methodology
The main objective of DEFIDIAG is to compare the percentage of genetic causal diagnoses identified in ID patients through GS analysis in trio (patient and both parents) and in solo (patient only) versus the use of the current French reference strategy (Fra-X + chromosomal microarray analysis + 44 genes panel strategy), as currently recommended by the ANPGM guidelines (Association nationale des praticiens de génétique moléculaire). In this study, each included patient is his/her own control and benefits from the strategies in parallel, with the results of these different strategies being interpreted blindly.
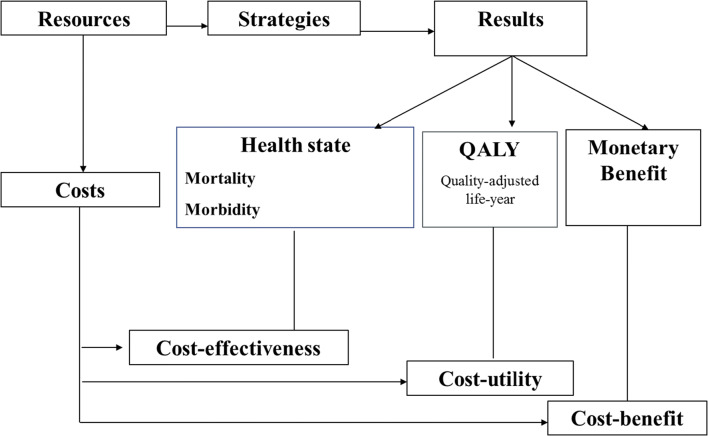
The secondary objectives of the study are to assess the diagnostic yields according to the various procedures and patient subgroups, and the overall feasibility of the proposed strategy. A medico-economic evaluation is also planned. The goal of medico-economic studies is to compare alternative strategies in an incremental way. Several types of medico-economic evaluations can be carried out: cost-effectiveness, cost-utility, and cost-benefit analyses. Though the three approaches consider the cost of the intervention and downstream costs, they measure health outcomes differently. In cost-effectiveness analyses, the outcome is a clinical indicator measured in terms of either morbidity or mortality; in cost-utility analyses, the outcome is expressed in terms of quality-adjusted life-years (QALY) gained; in cost-benefit analyses, the outcome is expressed in terms of the monetary value of health (Drummond et al. 2005) (Fig. 2). The two most commonly used approaches are cost-effectiveness and cost-utility analyses (HAS. 2011). The DEFIDIAG study includes a cost-effectiveness analysis because the main concern is the ability of genomic analysis to end the diagnostic odyssey of the patient. Finding the molecular cause can decrease the emotional and sometimes financial burden associated with the patient’s odyssey and help the parents focus on the symptoms of the disease and anticipate the future (Mollison et al. 2020).
Fig. 2.
Typology of medico-economic evaluations
The results will be therefore expressed in terms of cost per additional positive diagnosis. This design is commonly used in rare diseases (Ontario Health (Quality) 2020). Through the planned medico-economic evaluation, the DEFIDIAG study will also use the micro-costing method to provide precise information about the cost of genetic analyses. Estimating real costs is one way for health economists to measure the value of care strategies. The health authorities expect to receive this information, which may then be used to help determine the reimbursement for genetic tests within the health insurance system. But, besides the question of reimbursement, the estimation of cost is also important because the cost of the sequencing phase is expected to decrease in the near future. The cost evaluation of genetic testing could therefore play an incentive role in the generalization of the use of genetic tests in France (Institute of Medicine; Board on Health Sciences Policy; Roundtable on Translating Genomic-Based Research for Health; Adam C. Berger and Steve Olson 2013) and greatly impact the performance of the health care system if it is associated with a significant improvement in health outcomes.
Two impact studies have also been planned to explore the various aspects of such a complex decision. Again, the studies are based on a quantitative approach. The first aims to estimate, in patients investigated before their inclusion in the study, the cost of an extended search for a diagnosis that could have been avoided by first-line genomic analysis. The aim of the second impact study will be to estimate the frequency and nature of changes in medical and psychosocial follow-up induced by genomic analyses using a before-after study. The observed changes could be more intensive follow-up, the end of some medical procedures or changes in the nature of the follow-up. Data from the two impact studies are expected to reinforce efficiency results by demonstrating the importance of cost-savings, and they are also expected to have a clinical value in terms of medical decision-making and potential changes in the patients’ care pathways.
However, alternative approaches can be used to estimate the utility associated with NGS. Indeed, the measure of a change may be small (such as a decrease in the number of medical procedures), but the value of this change can be significant for the patients. In addition to clinical endpoints, the non-health-related outcomes and the way patients and caregivers view the changes brought on by genomic testing are essential to decision-making (Vass et al. 2017). It is important for decision-makers to be adequately informed of the value of genetic testing for citizens. One way to explore non-health-related outcomes is to mobilize social science research methods that use qualitative and mixed studies (Pluye and Hong 2014). In the DEFIDIAG study, sociologists and clinical psychologists will conduct three series of interviews among parents and patients presenting with mild ID: after inclusion, immediately after the disclosure of results, and at 1 year after the disclosure of results. Our goal is to explore patient and parent perception of WGS. The concepts of burden, family, and patient expectations regarding WGS, emotional adjustment, and coping strategies will be explored. These non-medical personal benefits are essential for assessing how patients, their families, and providers are affected by genetic testing. In addition to effectiveness, avoided costs, and efficiency, these are key points for the success of a durable implementation of NGS in France.
Place of DEFIDIAG in a dynamic research context in France and internationally
There are already a number of published international studies regarding the implementation of NGS. In a 2016 study in the Netherlands, the combination of the relatively high costs of the traditional diagnostic trajectory and the high diagnostic potential of WES led researchers to conclude that WES was a relevant and cost-effective option in patient diagnostics (Monroe et al. 2016). In the USA, the costs and consequences of the use of WES and WGS for autism spectrum disorders was also evaluated positively (Tsiplova et al. 2017). The reimbursement of first-line WES for rare genetic diseases has very recently been shown to be cost-effective (tripling the diagnostic rate for one-third of the cost) in infants with suspected monogenic disorders (Stark et al. 2017). However, a systematic review of the literature published in 2018 concluded that few international studies had carefully evaluated the costs, effectiveness, and cost-effectiveness of WES and WGS tests, and the authors indicated that reliable estimates were urgently needed to support the translation of these tests into clinical practice (Schwarze et al. 2018). More recently, the Ontario Health Quality published a report on the use of genome-wide sequencing analyzed in terms of efficiency but also of budget impact (Ontario Health (Quality) 2020). In France, to our knowledge, no published study has assessed the overall performance and efficiency of first-line WGS for patients with ID while taking into account the standards and reimbursement procedures in place in France. DEFIDIAG is expected to demonstrate the efficiency of the NGS strategy suggested in the international literature. Indeed, this strategy makes it possible to obtain a higher diagnostic yield and thus a reduction of the time to diagnosis, which has traditionally been a significant source of expenses for the health system and of anxiety for parents. DEFIDIAG will also provide novel data on the real costs of NGS in order to help determine tariffs and the terms of reimbursement. But the challenge is also societal. For the patient, NGS is expected to optimize certain aspects of care, such as monitoring and perhaps treatment, and to provide great improvements in genetic counseling. For families, this technique will make it possible to identify the risks of recurrence in offspring and to limit the anxiety associated with causality. For providers, genetic testing could modify the shared decision-making process. At the macroeconomic level, reduced health expenditures will be expected as the result of earlier diagnoses. Finally, the implementation and development of NGS represents an opportunity for international companies to invest and develop in the field of digital health (such as the development of new skills in the analysis and the management of databases and algorithmic procedures or the development of new treatments based on genetic data).
The DEFIDIAG study is one among many currently underway in France. Its findings will complement the results of other ongoing French studies including:
the DISSEQ study (funded in 2015 and coordinated by Pr. C Thauvin, GAD team [Génétique des anomalies du développement], Dijon University Hospital), whose aim is to compare the cost and effectiveness of a reference strategy (including array-CGH, Fra-X, targeted genes including a 44-gene panel) and two alternatives strategies composed of (1) array-CGH, Fra-X syndrome, and a larger panel of 459 genes, and (2) Fra-X syndrome and ES;
the SEQUAPRE study (coordination: Pr. L. Faivre, GAD team, Dijon University Hospital), which targets parents of children with development disorders who are eligible for ES. The goal is to reveal preferences of parents of children with regard to the disclosure of WES results and to understand the expectations and the experience of families and geneticists towards ES after the results are delivered (Peyron et al. 2018);
the FIND study (coordination: Pr L Faivre, GAD team Dijon University Hospital), which focuses on unsolicited data produced by NGS in diagnosis. The study concerns adults or the parents of children/adults under tutorship or guardianship with developmental abnormalities who will have access to a diagnosis for the first time. It is based on questionnaires exploring anxiety, depression, quality of life and utility, and individual interviews. All of these studies are in line with the HAS’s multidisciplinary approach—they incorporate “non-health” value information that can be used to optimize the implementation of NGS in France in this medical indication.
Conclusion
France, along with a number of other countries, is currently experiencing a revolution in the field of genomic medicine. The French Plan for Genomic Medicine 2025 responds to a very strong political impetus. Multidisciplinary research is urgently needed in order to prepare the field of genomic medicine as it becomes increasingly accessible to the French population. The findings of the current research will also be essential for establishing recommendations and supporting geneticists as they harmonize their practices. Finally, this research is needed to determine the precise terms of reimbursement for these new technologies, and to justify the use of resources within financial limitations. Health economists can contribute substantially to this research. They are well-placed to tackle the complex question of value, including non-health value such as the perception and the experience of the disease and of the care pathway, and to attempt to untangle the urgent societal issues that are raised by genetic testing, in coordination with geneticists, biologists, epidemiologists, and social and human sciences researchers.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
This article is part of the topical collection on Resource Allocation in Genomic Medicine (Slade).
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Catherine Lejeune, Email: catherine.lejeune@u-bourgogne.fr.
on behalf of the DEFIDIAG study group, FHU Translad and Aviesan:
Marie-Laure Humbert-Asensio, Christine Binquet, Jean-Francois Deleuze, Christelle Delmas, Hélène Dollfus, Hélène Esperou, Laurence Faivre, Thierry Frebourg, Bénédicte Gerard, Francis Guillemin, Delphine Heron, Franck Lethimonnier, Stanislas Lyonnet, Carine Malle, Sylvie Odent, Aurore Pélissier, Christine Peyron, Valerie Seror, Christel Thauvin, and Damien Salanville
References
- Aviesan France Médecine Génomique (2018) 2025 http://www.defiscience.fr/wp-content/uploads/2016/09/22.06.2016_rapport_france_medecine_genomique_2025.pdf Accessed 29 Oct 2018
- Callier SL, Abudu R, Mehlman MJ, Singer ME, Neuhauser D, Caga-Anan C, Wiesner GL. Ethical, legal, and social implications of personalized genomic medicine research: current literature and suggestions for the future. Bioethics. 2016;30(9):698–705. doi: 10.1111/bioe.12285. [DOI] [PubMed] [Google Scholar]
- Commission évaluation économique et de santé publique (CEESP) Activity Report (2017) https://www.has-sante.fr/portail/upload/docs/application/pdf/2018-07/rapport_activite_ceesp_2017.pdf Accessed 19 Mar 2019
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the eocnomic evaluation of health care programmes. Oxford: University Press; 2005. [Google Scholar]
- Foster MW, Mulvihill JJ, Sharp RR. Evaluating the utility of personal genomic information. Genet Med. 2009;11(8):570–574. doi: 10.1097/GIM.0b013e3181a2743e. [DOI] [PubMed] [Google Scholar]
- Gainotti S, Mascalzoni D, Bros-Facer V, Petrini C, Floridia G, Roos M, Salvatore M, Taruscio D. Meeting patients’ right to the correct diagnosis: ongoing international initiatives on undiagnosed rare diseases and ethical and social issues. Int J Environ Res Public Health. 2018;15(10):1–16. doi: 10.3390/ijerph15102072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse SD, Khoury MJ. What is the clinical utility of genetic testing? Genet Med. 2006;8(7):448–450. doi: 10.1097/01.gim.0000227935.26763.c6. [DOI] [PubMed] [Google Scholar]
- HAS (2011) Choix méthodologiques pour l’évaluation économique à la HAS. https://www.has-sante.fr/portail/upload/docs/application/pdf/2011-11/guide_methodo_vf.pdf. Accessed 29 Oct 2018
- HAS (2018) Projet stratégique 2013–2016. https://www.has-sante.fr/portail/upload/docs/application/pdf/2013-09/projet_strategique_synthese.pdf. Accessed 29 Oct 2018
- Inserm (2018) Déficiences intellectuelles. Expertise collective. Synthèse et recommandations. https://www.inserm.fr/information-en-sante/expertises-collectives/deficiences-intellectuelles Accessed 29 Oct 2018
- Institute of Medicine; Board on Health Sciences Policy; Roundtable on Translating Genomic-Based Research for Health; Adam C. Berger and Steve Olson (2013) The economics of genomic medicine: workshop summary health. Washington (DC): National Academies Press (US) [PubMed]
- Kohler JN, Turbitt E, Biesecker BB. Personal utility in genomic testing: a systematic literature review. Eur J Hum Genet. 2017;25(6):662–668. doi: 10.1038/ejhg.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lethimonnier F, Levy Y. Genomic medicine France 2025. Ann Oncol. 2018;29(4):784–785. doi: 10.1093/annonc/mdy027. [DOI] [PubMed] [Google Scholar]
- Mollison L, O’Daniel JM, Henderson GE, Berg JS, Skinner D. Parents’ perceptions of personal utility of exome sequencing results. Genet Med. 2020;22(4):752–757. doi: 10.1038/s41436-019-0730-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe GR, Frederix GW, Savelberg SM, de Vries TI, Duran KJ, van der Smagt JJ, Terhal PA, van Hasselt PM, Kroes HY, Verhoeven-Duif NM, Nijman IJ, Carbo EC, van Gassen KL, Knoers NV, Hövels AM, van Haelst MM, Visser G, van Haaften G. Effectiveness of whole-exome sequencing and costs of the traditional diagnostic trajectory in children with intellectual disability. Genet Med. 2016;18(9):949–956. doi: 10.1038/gim.2015.200. [DOI] [PubMed] [Google Scholar]
- Morgan S, Hurley J, Miller F, Giacomini M. Predictive genetic tests and health system costs. CMAJ. 2003;168(8):989–991. [PMC free article] [PubMed] [Google Scholar]
- National Centre for Human Genomics Research (2018) Technologies de la génomique pour la médecine personnalisée http://www.cea.fr/presse/Pages/dossiers/2018/genomique-medecine-personnalisee.aspx. Accessed 19 Mar 2019
- Ojha RP, Thertulien R. Health care policy issues as a result of the genetic revolution: implications for public health. Am J Public Health. 2005;95(3):385–388. doi: 10.2105/AJPH.2003.026708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ontario Health (Quality) Genome-wide sequencing for unexplained developmental disabilities or multiple congenital anomalies: a health technology assessment. Ont Health Technol Assess Ser. 2020;20(11):1–178. [PMC free article] [PubMed] [Google Scholar]
- Peyron C, Pélissier A, Béjean S. Preference heterogeneity with respect to whole genome sequencing. A discrete choice experiment among parents of children with rare genetic diseases. Soc Sci Med. 2018;214:125–132. doi: 10.1016/j.socscimed.2018.08.015. [DOI] [PubMed] [Google Scholar]
- Pluye P, Hong QN. Combining the power of stories and the power of numbers: mixed methods research and mixed studies reviews. Annu Rev Public Health. 2014;35:29–45. doi: 10.1146/annurev-publhealth-032013-182440. [DOI] [PubMed] [Google Scholar]
- Schwarze K, Buchanan J, Taylor JC, Wordsworth S. Are whole-exome and whole-genome sequencing approaches cost-effective? A systematic review of the literature. Genet Med. 2018;20(10):1122–1130. doi: 10.1038/gim.2017.24. [DOI] [PubMed] [Google Scholar]
- Stark Z, Schofield D, Alam K, Wilson W, Mupfeki N, Macciocca I, Shrestha R, White SM, Gaff C. Prospective comparison of the cost-effectiveness of clinical whole-exome sequencing with that of usual care overwhelmingly supports early use and reimbursement. Genet Med. 2017;19(8):867–874. doi: 10.1038/gim.2016.221. [DOI] [PubMed] [Google Scholar]
- Tsiplova K, Zur RM, Marshall CR, Stavropoulos DJ, Pereira SL, Merico D, Young EJ, Sung WWL, Scherer SW, Ungar WJ. A microcosting and cost-consequence analysis of clinical genomic testing strategies in autism spectrum disorder. Genet Med. 2017;19(11):1268–1275. doi: 10.1038/gim.2017.47. [DOI] [PubMed] [Google Scholar]
- Turrini M, Prainsack B. Beyond clinical utility: the multiple values of DTC genetics. Appl Transl Genom. 2016;8:4–8. doi: 10.1016/j.atg.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vass C, Rigby D, Payne K. The role of qualitative research methods in discrete choice experiments. Med Decis Mak. 2017;37(3):298–313. doi: 10.1177/0272989X16683934. [DOI] [PMC free article] [PubMed] [Google Scholar]