Abstract
Seed germination is one of the critical stages of plant life, and many quantitative trait loci (QTLs) control this complex trait. Meta-analysis of QTLs is a powerful computational technique for estimating the most stable QTLs regardless of the population's genetic background. Besides, this analysis effectively narrows down the confidence interval (CI) to identify candidate genes (CGs) and marker development. In the current study, a comprehensive genome-wide meta-analysis was performed on QTLs associated with germination in rice. This analysis was conducted based on the data reported over the last two decades. In this case, various analyses were performed, including seed germination rate, plumule length, radicle length, germination percentage, coleoptile length, coleorhiza length, radicle fresh weight, germination potential, and germination index. A total of 67 QTLs were projected onto a reference map for these traits and then integrated into 32 meta-QTLs (MQTLs) to provide a genetic framework for seed germination. The average CI of MQTLs was considerably reduced from 15.125 to 8.73 cM compared to the initial QTLs. This situation identified 728 well-known functionally characterized genes and novel putative CGs for investigated traits. The fold change calculation demonstrated that 155 CGs had significant changes in expression analysis. In this case, 112 and 43 CGs were up-regulated and down-regulated during germination, respectively. This study provides an overview and compares genetic loci controlling traits related to seed germination in rice. The findings can bridge the gap between QTLs and CGs for seed germination.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12298-022-01232-1.
Keywords: Expression analysis, Meta-QTL, Rice, Seed germination traits
Introduction
Rice (Oryza sativa L.) is one of the most important crops and staple food products. It is the principal food for the majority of the world's population. Also, it is ranked third in agricultural products (Rao et al. 2010; Islam et al. 2019). From the food security viewpoint, it is the second most major strategic product after wheat (Matzke et al. 2000). However, the population growth, climate change, and loss of agricultural land revealed the necessity of finding novel ways for plant breeders to meet the increasing demand for high-yielding rice (Wu et al. 2016).
Seed germination is the first step of rice seedling development. Besides, it is one of the most critical plant life stages, particularly while facing environmental stress (Wang et al. 2011; Guo et al. 2019). Also, it is a complex biological process that starts with water uptake by the dry seed and ends with the appearance of the embryonic axis, usually the radicle, through the structures surrounding it (Li et al. 2011; Bewley et al. 2013).
From a genetic viewpoint, seed germination is a polygenic and complex trait, which is controlled by QTLs. These traits are highly influenced by the environment conditions and cannot be detected by classical approaches of quantitative genetics (Lai et al. 2016). The QTL mapping is a powerful approach for identifying the action, interaction, numbers, and chromosomal locations of loci affecting such complex traits (Zhang et al. 2017). Previous studies demonstrated that QTL mapping as a detection tool dramatically increased the genetic control of traits (Zhang et al. 2005a; Dong et al. 2017).
Over the last two decades, many QTLs related to seed germination traits have been reported in rice using different genetic backgrounds (Redona and Mackill 1996; Cui et al. 2002; Zhang et al. 2005a, b; Wang et al. 2010, 2011; Mardani et al. 2013; Lai et al. 2016; Li et al. 2017; Sanchouli et al. 2021; Zeng et al. 2021). However, several factors (e.g., marker sets, statistical methods, parents, size and generation of populations, experimental design, and environment) can strongly influence detection, location, and QTLs significance level (Van and McHale 2017). On the other hand, undesirable epistatic interactions between different genetic backgrounds and the inconsistency and variability of QTLs due to the mentioned factors may limit their application in breeding programs (Islam et al. 2019).
The meta-analysis of QTLs is a suitable and powerful computational approach for resolving this issue (Islam et al. 2019; Khahani et al. 2021). It integrates independent QTL studies into a single dataset to estimate the number of "true" QTLs and obtain a more accurate estimate of QTL positions by reducing the confidence interval (CI) (Khowaja et al. 2009; Li et al. 2013b; Acuña-Galindo et al. 2015). In recent years, this method has frequently been used by various authors for some plants such as maize (Hao et al. 2010), barley (Li et al. 2013b), wheat (Acuña-Galindo et al. 2015), and Soybean (Van and McHale, 2017). Also, several reports are available regarding the meta-analysis of QTLs in rice for traits such as panicle-related traits (Wu et al. 2016), seedling-stage salt tolerance (Islam et al. 2019), disease resistance (Kumar and Nadarajah, 2020), yield and yield-related traits (Khahani et al. 2021), and drought response (Selamat and Nadarajah, 2021).
These studies defined a genome-wide landscape on the most stable loci associated with reliable genetic markers and candidate genes (CGs). The results can be used in future studies such as marker-assisted selection, identifying CGs, molecular breeding, and genetic engineering (Khahani et al. 2020; Selamat and Nadarajah 2021). Wu et al. (2016) employed a meta-analysis to identify 87 meta-QTLs (MQTLs) for panicle-related traits across 82 populations. In this case, 24 CGs (e.g., EP3, LP, MIP1, HTD1, DSH1, and OsPNH1) were recognized in these MQTL regions. Selamat and Nadarajah (2021) identified 70 MQTLs for 13 traits in rice that respond to drought. In this case, several genes were annotated in the MQTL areas through Blast2GO. These genes were associated with regulatory proteins to regulate signal transduction and gene expression that respond to drought stress.
The literature survey demonstrated that the previous studies paid less attention to the meta-analysis of QTLs for seed germination and related traits. Thus, the current study performed a comprehensive genome-wide meta-analysis on QTLs reported over the last two decades. This analysis was conducted on seed germination in rice. In this case, several analyses were performed, including seed germination rate (GR), plumule length (PL), radicle length (RL), germination percentage (GP), coleoptiles length (CL), coleorhiza length (COL), radicle fresh weight (RFW), germination potential (GPO), and germination index (GI). The objective of this study was to perform a meta-analysis to identify regions of the rice genome associated with seed germination traits. Also, this study identified molecular markers and potential CGs present in the meta-QTL regions so that the obtained results provide valuable information for applying in marker-assisted selection and genetic engineering of rice.
Materials and methods
Data collection
The literature survey was conducted by collecting published articles (up to the year 2021) regarding QTL mapping of seed germination and its component traits in rice from PubMed (http://www.ncbi.nlm.nih.gov/pubmed) and Google Scholar (https://scholar.google.com/). In this case, the search process was accomplished using appropriate keywords including “meta-QTL”, “seed germination”, “seed vigor”, “rice”, and “QTL mapping”. Besides, QTLs identified from rice associated with seed germination (available online: Gramene (https://archive.gramene.org/qtl/) were considered in the current study.
All QTL studies except those lacking proper genetic map information or QTL-related information were used in the meta-analysis of QTLs. This information consists of the mapping population’s parent, type, and size, the logarithm of odds (LOD score), the proportion of phenotypic variance (R2), molecular markers flanking, additive effects of favorable alleles, and QTL position. Two types of input data text files (i.e., map and QTL information files) were prepared for each study according to the instruction manual of BioMercator v3/v4 (Veyrieras et al. 2007). The position of genetically controlling regions of traits was determined by applying a meta-analysis of QTLs and merging different QTLs collected from independent studies. Indeed, this procedure was performed by constructing the consensus map regardless of their genetic backgrounds, population type, and evaluated locations and years (Arcade et al. 2004; Sosnowski et al. 2012).
In this study, the empirical formulas proposed by Darvasi and Soller (1997) and Guo et al. (2006) were employed for data homogenization and CI estimation, respectively. At the 95% level for BC and F2 populations, the QTL CI is formulated as Eq. (1). In addition, the RIL and DH populations were formulated as Eqs. (2) and (3), respectively.
| 1 |
| 2 |
| 3 |
where N is the population size, and is the proportion of the total variance explained by the individual QTL.
The construction of consensus map and QTLs projection
A rice reference map developed by Islam et al. (2019) was utilized for constructing the consensus map process. This reference genetic map was integrated by International Rice Microsatellite Initiative 2003 (McCouch et al. 2002) and SNP-based high-density linkage maps (De Leon et al. 2016; Gimhani et al. 2016). This situation generated 12,096 markers with an average distance of 0.14 cM between markers. Also, the average chromosome length was 139.47 cM for a total length of 1673.63 cM. In the first step, an integrated consensus map was developed using maps of all studies and the reference map. In the second step, the QTL detected in each study were projected onto the consensus map using BioMercator V4.2.3 (Veyrieras et al. 2007).
Meta-analysis of QTLs
MQTL analysis was performed on an integrated consensus map for each chromosome through Goffinet and Gerber algorithm (2000) and employing BioMercator V4.2.3 available at https://urgi.versailles.inra.fr/Tools/BioMercator-V4. According to Goffinet and Gerber algorithm (2000), if n individual QTLs are available, BioMercator examines the most likely assumption between 1, 2, 3, 4, and n underlying QTLs. Besides, the most likely QTL arrangement in every five models is determined using the maximum likelihood method. The calculation process was performed by assuming a Gaussian distribution for each model. Among the five MQTLs, the best fit was derived by analyzing the lowest Akaike information criterion (AIC) value. In each model, the consensus QTL positions were determined as the mean of QTL distribution by maximizing the likelihood. Besides, the consensus QTL was reported as a “real” QTL/MQTL. Also, the individual QTLs were analyzed for all traits related to seed germination as a meta-trait (Germination Traits (GT)) using the option “regrouping the traits into meta-traits” in the software. The GT-related traits included seed germination rate (GR), plumule length (PL), radicle length (RL), germination percentage (GP), coleoptiles length (CL), coleorhiza length (COL), radicle fresh weight (RFW), germination potential (GPO), and germination index (GI). Various studies reported the significant relationship between germination components and related traits (Borjas Artica, 2017; Zeng et al. 2021; Yang et al. 2019a; Dimaano et al. 2020).
A circular plot was drawn using an R/Shiny application (ShinyCircos) to visualize the position of each MQTL (Yu et al. 2018).
Detection of CGs within the MQTL genomic regions
The genes underlying individual MQTL regions were identified in the 0.1 Mb interval on either side (i.e., upstream and downstream) of the MQTL's peak position (total 0.2 Mb region). This procedure was accomplished using the BioMart tool in Ensembl Plants (https://plants.ensembl.org/biomart/martview). The RNA-seq data deposited in Genevestigator (https://genevestigator.com/gv/) was employed to expression profiling of candidate genes in the rice germination procedure. These data available as log2 transformed TPM (transcripts per million) values. Only candidate genes showing fold change (FC) ≥ 2 or FC ≤ - –2 relative to control were considered as differentially expressed genes. PlantTFDB (http://planttfdb.gao-lab.org/) and iTAK (http://itak.feilab.net/cgi-bin/itak/index.cgi) used to identify potential transcription factors (TF) and kinase coding gens.
Gene ontology (GO) analysis was carried out by establishing a framework and set of concepts to describe the functions of gene products and pre-digestion of elucidating the biological implications of unique genes (Zhou et al. 2020). In the current study, the significant enrichment analysis of gene ontology (GO) terms was carried out using TBtools software (P < 0.05). This procedure was performed on CGs with considerable changes in the expression patterns survey.
Results and discussion
Analysis of identified QTLs associated with germination components and their related traits
Seed germination is the first step of plant growth, and it is a critical process for seedling establishment and crop yield (Han and Yang 2015). Also, seed germination and related traits are redacted by QTLs. Although numerous QTLs have been reported for seed germination in rice (Miura et al. 2002; Cui et al. 2002; Zhang et al. 2005a, b; Wang et al. 2010; Li et al. 2013a; Mardani et al. 2013; Liu et al. 2014; Xie et al. 2014; Cheng et al. 2015; Mahender et al. 2015; Yang et al. 2019a; Dimaano et al. 2020; Zeng et al. 2021), the knowledge of the gene networks regarding the control of rice seed germination remains limited (Yang et al. 2019a).
A total of 90 individual QTLs related to GT were derived from 14 studies, and then they were combined to discover consensus genomic regions associated with seed germination traits in rice. These studies included ten populations: two F2, one backcross, one double haploid, and six recombinant inbred lines (RIL) populations. Table S1 represents these 14 studies, which have been reported since 1996. Among these populations, RIL is the most preferred and suitable mapping population. Indeed, this mapping population is immortal and consists of a series of homozygous lines that can be reproduced without genetic change occurring in different locations and years. This situation makes RILs highly suited for mapping QTLs (Collard et al. 2005). According to Table S1, these studies have different numbers, types of markers, parents, and population sizes. The population size in the current study ranges from 71 to 282 lines. Also, the markers' number is between 45 (Behrozbeh et al. 2019) and 236 (Mardani et al. 2013; Rabiei et al. 2014).
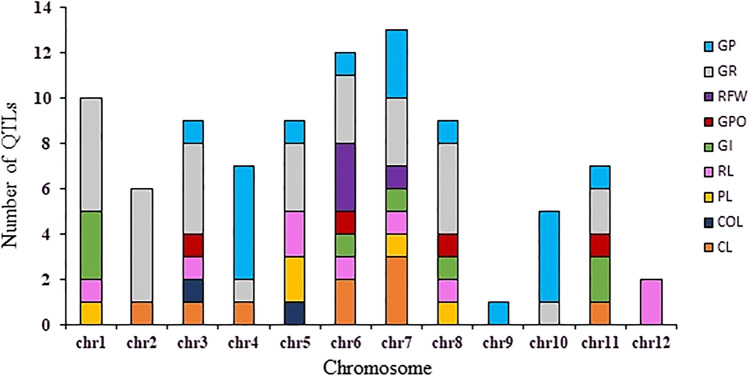
QTLs were unevenly scattered across the 12 rice chromosomes, ranging from one on chromosome 9 to 13 on chromosome 7. In this case, the average was 7.5 QTL per chromosome (Fig. 1). As shown in Fig. 1, the numbers of QTLs per trait were different on the chromosomes. Among these traits, the GR and GP had the highest numbers of QTLs of 31 and 18, respectively. The other traits were ranked as follows: RL, CL, GI, PL, GPO, RFW, and COL, respectively. Also, this figure depicts that chromosomes 6 and 7 have QTLs for seven traits; chromosomes 3, 5, and 8 have QTLs for six traits; chromosomes 1 and 11 have QTLs for four traits; chromosome 4 has QTLs for three traits; chromosomes 2 and 10 have QTLs for two traits; chromosomes 9 and 12 have QTLs for one trait. The phenotypic variance explained (PVE%) by the individual QTLs varied from 0.3 to 68.5%, and the CI of markers was different from 0.9 to 47.2 cM.
Fig. 1.
Number and distribution of initial quantitative trait loci (QTLs) for nine traits related to seed germination on 12 chromosomes in rice based on 14 QTL mapping studies. The germination-related traits included seed germination rate (GR), plumule length (PL), radicle length (RL), germination percentage (GP), coleoptiles length (CL), coleorhiza length (COL), radicle fresh weight (RFW), germination potential (GPO), germination index (GI). The length of the bars indicates the number of QTLs
Detected MQTLs for germination traits (GT)
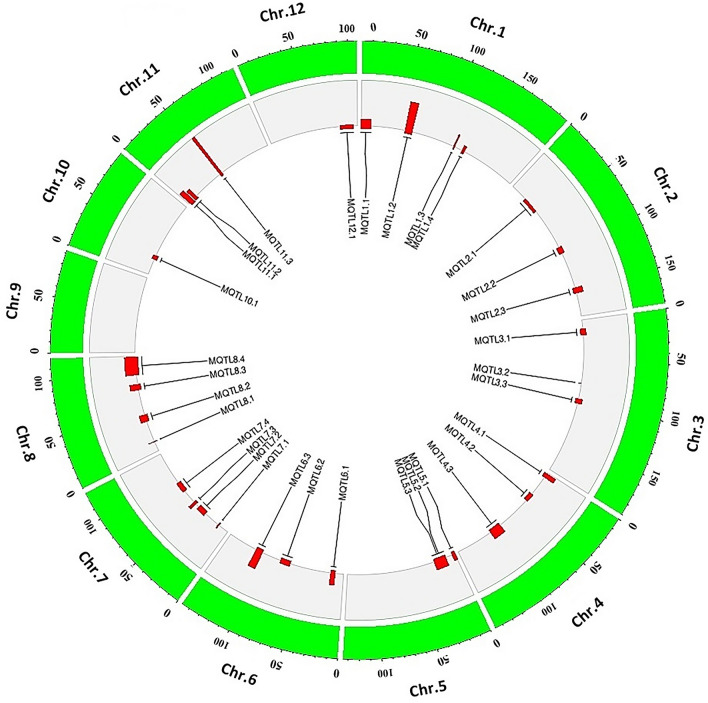
The meta-analysis does not include all the existing data in the studies. This issue is due to the unavailability of QTL data or missing principal information on some QTLs (Flather et al. 1997; Pogue and Yusuf 1998). However, it can enhance the knowledge of the genetic basis for complex traits using the integrated results of multiple QTL studies, robust identification of the genetic regions, and validation of QTL effects on environments/genetic backgrounds (Swamy and Sarla 2011a; Van and McHale 2017). In the present study, a meta-analysis of QTLs was performed to integrate the locations of 90 QTLs associated with seed germination. These data were collected from nine traits (i.e., GR, PL, RL, GP, CL, COL, RFW, GPO, and GI traits) based on the information of QTLs, such as population size, type and number of markers, and additional QTL information (Table S1). In the current study, 67 initial QTLs (74.4%) were successfully projected onto the consensus map and considered for the meta-analysis. Also, 32 MQTLs were identified on 11 chromosomes if at least one MQTL was observed on each chromosome (Figs. 2, 3, and 4). In this case, the MQTL was not detected on chromosome 9. Table 1 provides a comprehensive list of 32 MQTLs with information, including physical distance, genetical position, AIC value, CI (95%), and marker interval. According to Table 1, the MQTLs had different numbers of original QTLs and traits. Besides, chromosome 7 had the highest number of projected QTLs (10 QTLs) with four MQTLs. Also, the original QTLs for GR and GP traits were present in 16 and 11 regions out of the 32 MQTL regions, respectively. In addition, the original QTLs for other traits were GI (eight MQTL), RL and CL (six MQTL), PL, and GPO (four MQTL), COL (three MQTL), and RFW (one MQTL) traits, respectively. The mean PVE% for individual MQTLs ranged from 3.4 (MQTL 3.2) to 54.9% (MQTL 11.3) (Fig. 5). A comparison was made between the 95% CIs of initial QTLs and detected MQTLs. The results showed that the CI of most MQTLs was smaller than their respective initial QTLs. Besides, the average CIs reduced from 15.125 to 8.73 cM. This issue confirmed the meta-analysis power in increasing the QTL location precision. In this case, 9 MQTLs had a CI fewer than the value of 5 cM. Jan et al. (2021) introduced criteria for the low CI, high PVE mean (%), and the number of QTLs. This procedure was carried out by excluding the MQTL for choosing breeders’ MQTLs.
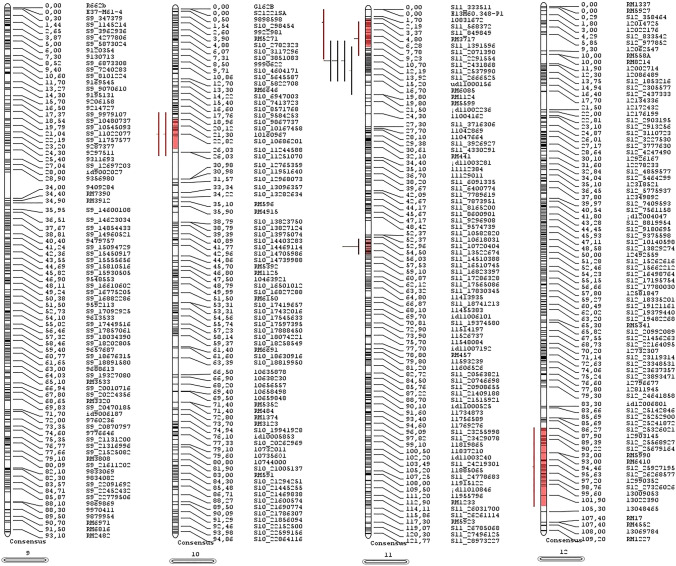
Fig. 2.
The identified MQTLs are displayed for seed germination traits on rice on chromosomes 1, 2, 3, and 4. Also, the filled colors on the chromosome arm represent 95% CI of each MQTL region. The brown color denotes the first MQTL, grey second MQTL, orange third MQTL, and fourth blue MQTL on each chromosome. The molecular markers' names and positions (cM) are shown on the right side. Table 1 gives the details of each MQTL
Fig. 3.
The identified MQTLs are displayed for seed germination traits on rice on chromosomes 5, 6, 7, and 8. Also, the filled colors on the chromosome arm represent 95% CI of each MQTL region. The brown color denotes the first MQTL, grey second MQTL, orange third MQTL, and fourth blue MQTL on each chromosome. The molecular markers' names and positions (cM) are shown on the right side. Table 1 gives the details of each MQTL
Fig. 4.
The identified MQTLs are displayed for seed germination traits on rice on chromosomes 9, 10, 11, and 12. Also, the filled colors on the chromosome arm represent 95% CI of each MQTL region. The brown color denotes the first MQTL, grey second MQTL, orange third MQTL, and fourth blue MQTL on each chromosome. The molecular markers' names and positions (cM) are shown on the right side. Table 1 gives the details of each MQTL
Table 1.
Information of identified Meta-QTL (MQTL) from Meta-analysis of QTLs for seed germination traits in rice
| Meta-QTL | Chr | AIC | QTL Model | Markers interval (QTL region) | Closest SSR marker | Meta-QTL peak position (cM) | Meta-QTL physical distance (bp) | No. of original QTLs | Mean Initial CI (cM) | Mean Phenotypic Variance of the QTL (%) | Meta-QTL CI (95%) cM | No. of gene |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MQTL1.1 | 1 | 110.93 | 4 | G8027˽115,511 | RM3148 | 5.59 | 16,078,082–2,800,122 | 1 | 14.9 | 11.17 | 13.05 | 41 |
| MQTL1.2 | S1_7778029˽S1_8924173 | RM1287 | 58.56 | 10,307,015–12,264,117 | 2 | 15.95 | 36.7 | 10.55 | 20 | |||
| MQTL1.3 | S1_25275937˽S1_25593034 | RM7419 | 117.76 | 27,626,912–28,333,800 | 3 | 14.35 | 18.52 | 2.17 | 23 | |||
| MQTL1.4 | S1_28101401˽S1_28947942 | RM6666 | 130.04 | 31,305,693–32,019,592 | 3 | 7.13 | 9.92 | 4 | 34 | |||
| MQTL2.1 | 2 | 41.39 | 3 | S2_3978527˽id2005033 | RM6233 | 32.57 | 4,414,124–9,032,782 | 1 | 20.58 | 4 | 20.58 | 26 |
| MQTL2.2 | S2_21852479˽S2_22880103 | RM5804 | 98.57 | 23,862,405–25,302,318 | 3 | 18.46 | 6.92 | 8.82 | 28 | |||
| MQTL2.3 | S2_31117728˽S2_32291905 | RM1251 | 150.98 | 34,081,397–35,160,225 | 2 | 12.5 | 11.77 | 7.37 | 19 | |||
| MQTL3.1 | 3 | 77.9 | 3 | RM1332˽S3_2715095 | RM6849 | 15.66 | 2,435,994–4,316,639 | 3 | 19.83 | 7.047 | 8.26 | 19 |
| MQTL3.2 | S3_12754378˽id3009515 | RM6881 | 78.53 | 16,685,678–16,685,710 | 1 | 0.9 | 3.4 | 0.9 | 3 | |||
| MQTL3.3 | S3_14738562˽S3_16599892 | RM5626 | 100.59 | 23,628,236–24,671,291 | 2 | 9.42 | 8.395 | 6.44 | 26 | |||
| MQTL4.1 | 4 | 25.21 | 3 | S4_598874˽RM2811 | RM335 | 4.98 | 176,849–2,074,339 | 2 | 22.79 | 15.725 | 6 | 24 |
| MQTL4.2 | id4002942˽id4003491 | RM6314 | 38.34 | 1,663,548–18,627,911 | 1 | 11.02 | 5.6 | 11.02 | 10 | |||
| MQTL4.3 | RM2636˽RM3276 | RM317 | 94.79 | 29,219,406–30,715,786 | 1 | 15.3 | 12.7 | 15.3 | 25 | |||
| MQTL5.1 | 5 | 64.56 | 3 | S5_103866˽S5_816467 | RM507 | 1.58 | 71,514–10,676,235 | 3 | 11.27 | 11.27 | 4.92 | 35 |
| MQTL5.2 | RM1024˽S5_3774907 | RM3345 | 17.79 | 1,174,375–3,187,129 | 4 | 22.49 | 13.43 | 9.61 | 20 | |||
| MQTL5.3 | S5_2096055˽S5_4565557 | RM4710 | 21.52 | 2,084,818–1,174,375 | 4 | 22.49 | 13.43 | 13.56 | 40 | |||
| MQTL6.1 | 6 | 44.38 | 3 | S6_2282606˽S6_3465712 | RM6536 | 13.97 | 2,364,986–4,439,584 | 2 | 8.82 | 17.19 | 6.24 | 10 |
| MQTL6.2 | S6_11989668˽RM3827 | RM7551 | 70.87 | 17,483,480–21,950,443 | 3 | 14.18 | 6.77 | 12.19 | 6 | |||
| MQTL6.3 | S6_24093510˽S6_25108804 | RM5509 | 108.14 | 26,873,633–28,149,934 | 1 | 9.3 | 24 | 9.3 | 27 | |||
| MQTL7.1 | 7 | 65.69 | 4 | S7_2565701˽S7_2691147 | RM5055 | 14.82 | 2,557,838–3,174,427 | 2 | 24.6 | 8.45 | 2 | 20 |
| MQTL7.2 | RM8263˽RM8022 | RM6081 | 41.62 | 4,688,095–8,165,670 | 1 | 12.11 | 6.8 | 12.11 | 26 | |||
| MQTL7.3 | S7_8556262˽S7_13750881 | RM2530 | 54.13 | 15,357,245–15,357,268 | 4 | 13.44 | 11.8 | 4.19 | 12 | |||
| MQTL7.4 | S7_19631949˽S7_22277522 | RM7564 | 79.82 | 20,733,917–23,958,017 | 2 | 25.63 | 6.13 | 13.54 | 28 | |||
| MQTL8.1 | 8 | 80.72 | 4 | S8_1128224˽S8_1537411 | RM3702 | 13.08 | 1,182,996–1,555,519 | 4 | 13.28 | 10.41 | 0.99 | 27 |
| MQTL8.2 | S8_5250169˽RM6429 | RM7057 | 43.72 | 5,102,982–8,379,130 | 2 | 28.18 | 10.16 | 9.07 | 22 | |||
| MQTL8.3 | S8_23316147˽RM556 | RM195 | 82.59 | 21,137,914–22,335,423 | 2 | 10.8 | 12.87 | 7.63 | 22 | |||
| MQTL8.4 | S8_25110888_RM281 | RM6765 | 108.47 | 24,065,708–3,174,380 | 1 | 22.48 | 15.93 | 22.48 | 22 | |||
| MQTL10.1 | 10 | 8.47 | 1 | S10_10102852˽S10_11244588 | RM4455 | 22.84 | 10,178,447–11,221,582 | 2 | 7.9 | 6.6 | 5.59 | 17 |
| MQTL11.1 | 11 | 40.93 | 3 | S11_680258˽RM7557 | RM3717 | 5.68 | 1,174,156–2,327,502 | 2 | 12.52 | 18.9 | 6.54 | 29 |
| MQTL11.2 | RM2459˽S11_2838776 | RM2459 | 12.51 | 2,391,067–3,025,484 | 4 | 9.92 | 14.74 | 4.92 | 33 | |||
| MQTL11.3 | S11_10847755˽S11_16282381 | RM7391 | 55.21 | 9,884,968–13,366,650 | 1 | 3.7 | 54.9 | 3.7 | 12 | |||
| MQTL12.1 | 12 | 6.7 | 1 | S12_25505958˽RM17 | RM5282 | 96 | 23,606,775–27,024,556 | 1 | 16.47 | 5 | 16.47 | 22 |
Fig. 5.
A circular plot represents the overall MQTL information, where the inner layer indicates the names and positions of MQTLs on the chromosomes. The width and height of the bars horizontally and vertically present CI and mean phenotypic variance (PVE) on each of the 12 rice chromosomes, respectively
The identified CGs in the MQTL regions
A total of 728 CGs were identified in the 32 MQTL regions (Table S2). In each MQTL, the average value was 22.75 CGs. Also, MQTL1.1 covers an area of 13.28 Mb in chromosome 1 through 41 genes containing the most CGs. But MQTL3.2 had three CGs and a physical length of 0.000032 Mb in chromosome 3. It was the smallest number of CGs (Table 1).
The molecular function of CGs was considered through the expression patterns obtained from the transcriptome datasets. Expression data were available for only 522 out of 728 CGs (Table S3), and only 155 CGs had significant changes (FC ≥ 2, or FC ≤ − 2).
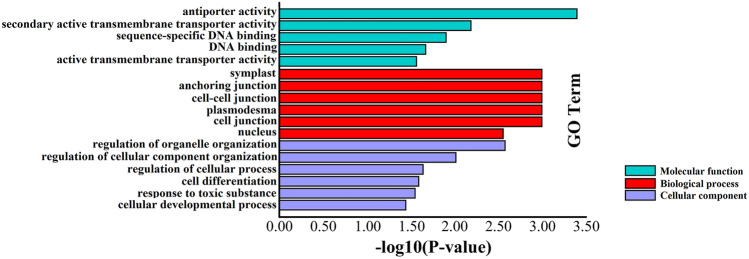
The significant enrichment analysis of gene ontology (GO) terms was carried out on the 155 CGs to understand the molecular function and identify common characteristics of CGs in biological functions. Thus, the GO terms were classified into the three main GO categories (i.e., biological process, molecular function, and cellular component) and 17 GO terms (Fig. 6, Table S4). In the biological process category, the CGs are involved in various processes such as the organelle organization regulation, cellular component organization regulation, cellular process regulation, cell differentiation, response to a toxic substance, and cellular developmental process. In the cellular component category, the CGs were active in different cell places such as symplast, anchoring cell, cell–cell junctions, plasmodesma, and nucleus. In the molecular function category, various biochemical activities of gene products were available such as antiporter activity, secondary active transmembrane transporter, sequence-specific DNA binding activity, DNA binding, and active transmembrane transporter activity.
Fig. 6.
GO analysis of candidate genes (CGs) of germination traits
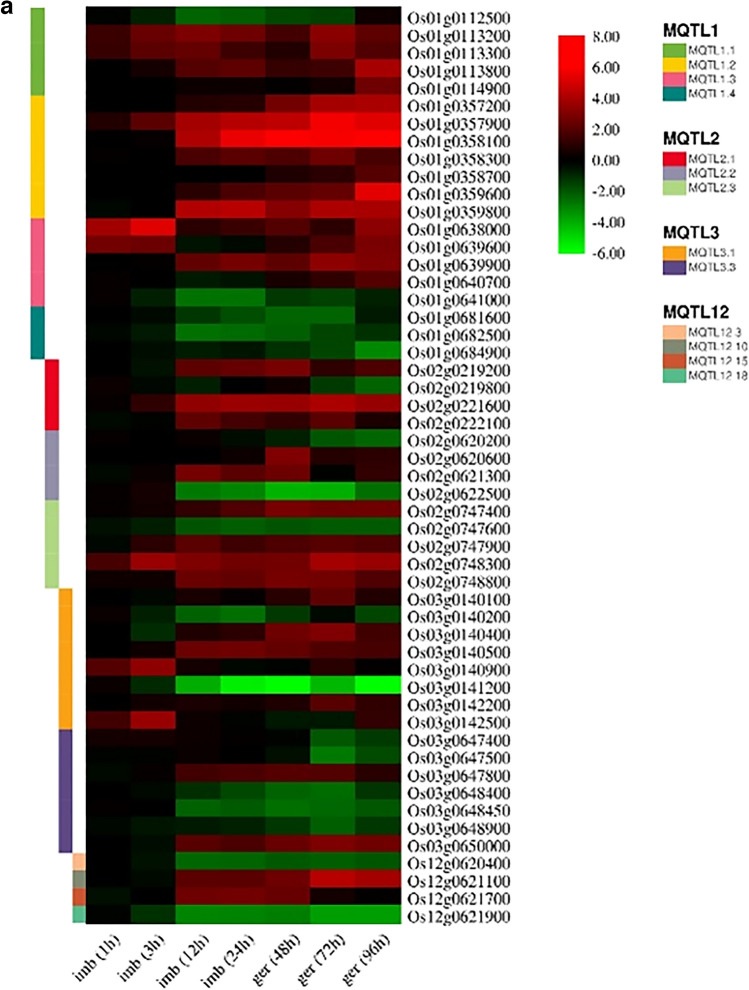
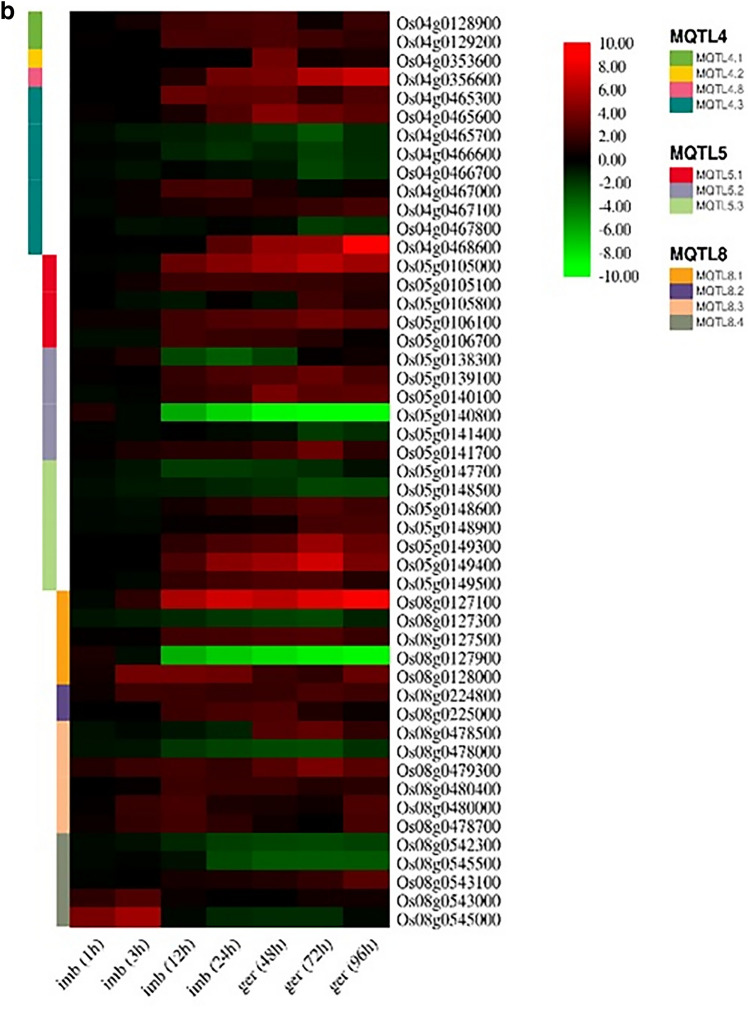
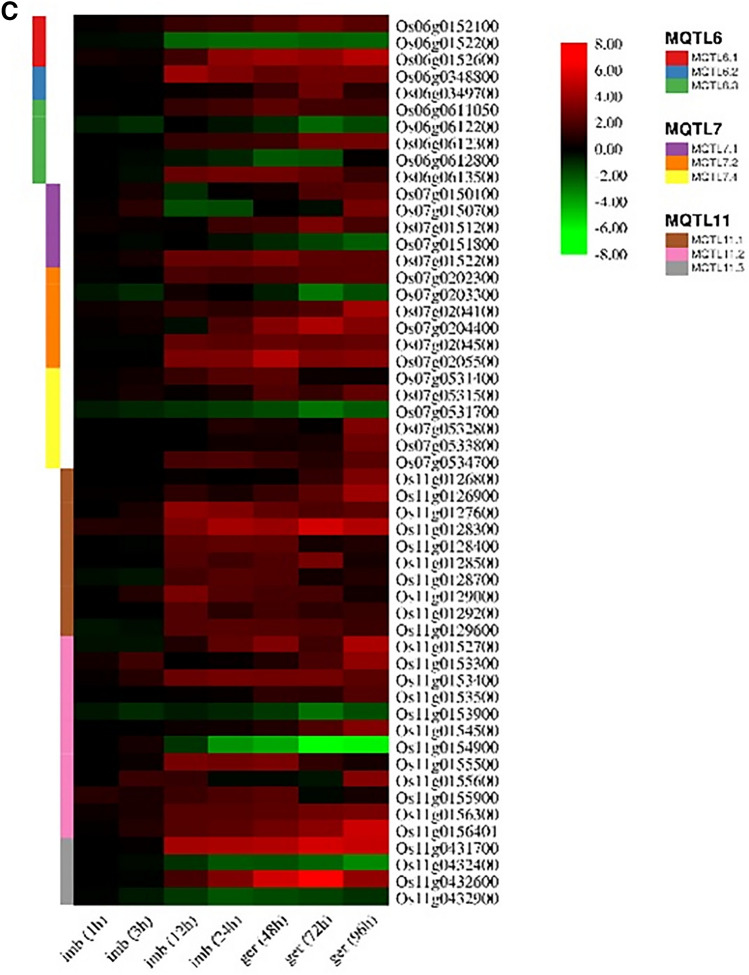
Among these 155 CGs, 112 and 43 CGs were up-regulated and down-regulated, respectively (Fig. 7a, b, and c; Table S5). These 155 CGs showing significant changes in expression belonged to only 29 out of 32 MQTLs. Although CGs and expression data were available for the remaining three MQTLs (i.e., MQTL3.2, MQTL7.3, and MQTL 10.1), these were insufficient for expression analysis. Expression profiling study revealed that most of the differentially expressed genes belong to chromosomes 11 (26 genes), 1 (20 genes), 5 (19 genes) and 8 (18 gens), respectively (Fig. 7a, b, and c; Table S5). Interestingly, expression pattern of promising genes showed that gradually increased the number of genes with FC ≥ 2 from the beginning of germination process to 96 h after germination. In 1 h after imbibition 2 genes, 3 h after imbibition 12 genes, 12 h after imbibition 51 genes, 24 h after imbibition 53 genes, 48 h after germination 65 genes, 72 h after germination 64 genes, and 96 h after germination 68 genes have expression level (FC) ≥ 2 (Fig. 7a, b, and c; Table S5). Similar results were observed for genes with FC ≤ 2. These results indicate an increase in seed metabolism during the germination process.
Fig. 7.
a Heat maps showing differential expression candidate genes (CGs) underlying the following 29 meta-QTLs (MQTL1, MQTL2, MQTL3, and MQTL12) are displayed. The existing scale on the right of each heatmap represents the fold changes ranging from + 8 to − 10, which differ in different heatmaps. b Heat maps showing differential expression candidate genes (CGs) underlying the following 29 meta-QTLs (MQTL4, MQTL5, and MQTL8) are displayed. The existing scale on the right of each heatmap represents the fold changes ranging from + 8 to − 10, which differ in different heatmaps. c Heat maps showing differential expression candidate genes (CGs) underlying the following 29 meta-QTLs (MQTL6, MQTL7, and MQTL11) are displayed. The existing scale on the right of each heatmap represents the fold changes ranging from + 8 to − 10, which differ in different heatmaps
Expression pattern of the identified 155 CGs was shown to encode various proteins such as protein kinases, conserved hypothetical protein, helix-loop-helix DNA domain-containing protein, cytochrome P450, the transcription factors, and beta-catenin-like protein. Some genes encode proteins of unknown function (Table S6). It is essential to investigate the influence of these genes on regulation of seed germination and its component traits in rice.
Transcription factors (TFs) are substantial regulatory proteins that play crucial roles in growth, development, and stress response in higher plants (Liu et al. 2001; Huo et al. 2019). In other words, TFs are mediators of stress responses and developmental programs (Licausi et al. 2013). Besides, protein kinases are dynamic regulatory proteins that act as principal regulators in diverse biological processes (Taylor and Kornev 2011; Naithani et al. 2021). Since the protein kinases and TFs were critical regulators, 27 CGs were evaluated in detail.
The Os01g0113200 (OsRLCK2), Os01g0113300 (OsRLCK3), Os01g0113800 (OsRLCK9), and Os01g0114900 were receptor kinase-related genes identified on MQTL1.1 and up-regulated during the seed germination stages. Besides, the OsRLCK2, OsRLCK3, and Os01g0114900 are receptor-like cytoplasmic kinases, which play substantial roles in diverse processes such as development and stress responses in rice (Vij et al. 2008). Zhao et al. (2020) described receptor kinase-related genes such as the OsRLCK2 and OsRLCK3. These genes might act as signal transducers in the stress response pathway and play pivotal roles in the initial imbibition stage during seed germination in rice. In addition, the OsRLCK3 was up-regulated as the salt stress-responsive gene in rice (Um et al. 2021). The OsRLCK9 is a representative of the receptor kinase LRK14. This issue was reported by Volante et al. (2017) during a search for CGs with a putative role in bakanae disease resistance. Also, Deng et al. (2018) stated that the OsRLCK9 was identified through the P-deficiency-stress and control samples in the leaf and root tissues of Dongxiang wild rice seedlings, and it was up-regulated during seed germination. This issue may be related to the receptor kinase LRK10 synthesis and a specific domain of tigr01615 family proteins. The OsRLCK9 gene (resistance-related receptor-like kinase) was previously reported in the regulatory networks for flavonoid and phytoalexin biosynthesis in rice leaves as one signal perceiving receptor kinases (Park et al. 2013). The analysis of the MQTL 2.3 candidate genes demonstrated three transcription factor coding genes, including Os02g0747400 (OsTCP9) and Os02g0747900 (ILI5) with FC ≥ 2 and Os02g0747600 (AP2/EREBP#022) with FC ≤ -2 during seed germination. Also, the OsTCP9 is a member of the TCP family genes identified in the rice genome. It is up-regulated in the P2 stage of panicle development (mainly involving differentiation of male meiocytes) and the S1 stage of seed development (Sharma et al. 2010).
Among these CGs, AP2/EREBP#022 belongs to the AP2/EREBP (ethylene-responsive element-binding protein) family. The AP2/EREBP gene family is a transcription factor family (Xiong et al. 2002) and plays a crucial role in developmental processes. Also, it provide resistance to biotic and abiotic stresses in plants (Liu and Zhang 2017; Dong et al. 2021). The ILI5 (OsPGL2) is a member of the bHLH (basic helix-loop-helix) gene family in rice (Carretero-Paulet et al. 2010; Mendes et al. 2014). In addition, it is associated with leaf angle changes in rice (Mantilla-Perez and Salas Fernandez 2017; Zhu et al. 2021). On the other hand, this gene controls grain length and rice weight through interaction with a typical bHLH protein APG (Heang and Sassa 2012; Qiu et al. 2015).
The Os03g0650000 (OsSh1) was located in MQTL 3.3 and up-regulated during seed germination. It was identified as a gene in seed shattering control (Htun et al. 2014; Ishikawa et al. 2021; Zhang et al. 2021). Besides, this gene may function downstream of qSH1, which accounts for a principal gene involved in abscission zone differentiation (Li et al. 2020) and controlling cell numbers at the abscission (Lin et al. 2012).
The Os04g0356600 on MQTL4.2 is another gene identified in this study. This gene encoded protein kinase and was up-regulated during seed germination stages. Also, it belonged to the rice SDRLK family genes (the S-domain subfamily of receptor-like kinases) (Naithani et al. 2021).
Two genes were identified on MQTL5.2 on chr5 (i.e., Os05g0139100 (APG) and Os05g0140100 (OsMYB2P-1)). These genes significantly changed expression patterns (FC ≥ 2) and encoded transcription factors. Besides, the APG/OsPIL16 gene was reported to function in leaf angle regulation (Heang and Sassa 2012; Wang et al. 2020). Since it is a member of the bHLH transcription factor family, it is known as a regulator of grain size (Heang and Sassa 2012; Yang et al. 2018; Li et al. 2018b). This situation is efficient for grain yield improvement in rice (Yang et al. 2018). The OsMYB2P-1 was another gene, and it was up-regulated during seed germination. This gene was initially identified in vigor-related QTL (Yan et al. 1998) and belonged to the MYB transcription factor family (one of the largest transcription factor families) (Zhang et al. 2012). Dai et al. (2012) reported that the OsMYB2P-1 was involved in phosphorus starvation signaling and root architecture of rice. Also, its overexpression in rice conferred greater tolerance to Pi starvation. Thus, they suggested that the OsMYB2P-1 could act as a Pi-dependent regulator in controlling the expression of Pi transporters.
The expression pattern of CGs showed that the Os06g0152200 (OsBBX16) was down-regulated during germination. This gene was identified on MQTL6.1 and encoded a BBX transcription factor (the B-box), which accounts for a kind of zinc finger transcription factor (Klug and Schwabe 1995; Huang et al. 2012; Khanna et al. 2009; Shalmani et al. 2019). Besides, it was reported that the OsBBX16 could participate in the light signaling pathway (Huang et al. 2012).
The Os06g0348800 (OsGLK1) gene on MQTL6.2 and the Os06g0612300 and Os06g0613500 (OsbHLH095) genes on MQTL6.3 (FC ≥ 2) are the CGs identified in chromosome 6 that encode transcription factors. Besides, the OsGLK1 encodes a transcription factor involved in the regulation of photosynthesis (Zhang et al. 2021). Also, it was reported that the overexpression of this gene along with OsGLK2 induced the expression of genes associated with chloroplast biogenesis (Nakamura et al. 2009; Wang et al. 2013). According to Li et al. (2007) and Li et al. (2006), the Os06g0612300 and OsbHLH095 genes belonged to the zinc finger protein gene and the bHLH gene family in rice, respectively.
The Os07g0150700 (CIPK23) and Os07g0152200 were two CGs identified on MQTL7.1 with significant expression changes that encode protein kinase. These genes were up-regulated during seed germination. Also, the CIPK23 gene was identified as the drought-responsive gene (Yang et al. 2008; Lopus et al. 2020) and salt-responsive gene (Liu et al. 2020a) in rice. In addition, Yang et al. (2008) reported that overexpression of this gene enhanced drought tolerance in rice plants. Zhao et al. (2020) detected the Os07g0152200 in a study of signaling-related differentially expressed genes involved in phase I of seed germination in rice. The authors found that in the same vein as the OsRLCK2 and OsRLCK3 on MQTL1.1, this gene (receptor-like protein kinase precursor) was associated with the gene responses involved in the initial imbibition of rice seed germination.
The Os07g0534700 on MQTL7.4 was identified as a gene that encoded protein kinase. It had significant expression changes and showed up-regulation during seed germination. Silveira et al. (2015) and Niño and Cho (2020) detected the Os07g0534700 in drought stress response and bacterial blight disease in two rice cultivars (Douradão and Jinbaek cultivars), respectively. Naithani et al. (2021) reported that the Os07g0534700 was regulated in response to biotic (bacterial panicle blight response and leaf-sheath blight response) and abiotic stresses (chilling response). Also, they found that this gene was a member of the SDRLK family and significantly affected plant development and responded to the biotic and abiotic stresses.
In the MQTL11.1 region, four CGs (i.e., Os11g0126900 (NAC122), Os11g0127600 (ONAC045), Os11g0128300, and Os11g0128500) encoded transcription factors. These genes showed significant changes in expression patterns and were up-regulated during seed germination. The NAC122 gene was one of the stress-related genes. It encoded the reported transcription factor related to stress resistance (Yang et al. 2019b). This gene belonged to NAC (nascent polypeptide-associated complex) TFs. Also, its expression was induced by abiotic stresses in rice leaves and roots (Li et al. 2018a; Lang et al. 2021; Liang et al. 2021) and identified as a stress-responsive NAC gene according to Zheng et al. (2009). The Os11g0128500 encoded the MYB-like DNA binding domain protein (Mohanty 2021), and its role was indicated in drought stress (Tan et al. 2020). In addition, it regulated the transcription of auxin-responsive genes (Deng et al. 2018).
In this case, 3 out of 33 CGs were identified in the MQTL11.2 region (i.e., Os11g0152700 (OsbZIP79), Os11g0154500 (NAC17), and Os11g0154900 (OsbZIP80)). These CGs had significant changes in expression patterns and encoded transcription factors. The NAC17 and OsbZIP79 were up-regulated, and the OsbZIP80 was down-regulated during seed germination. Su et al. (2020) indicated that the NAC17 gene was a member of the NAC gene family and involved in abiotic stress responses. Also, Chi et al. (2011) suggested that this gene was up-regulated under juglone stress in rice roots. On the other hand, Kan et al. (2017) showed that NAC17 was one of the early glutamate-responsive genes and encoded transcription factors in rice roots. Besides, it functions as a negative regulator of terpenoid phytoalexin biosynthesis in rice (Miyamoto et al. 2015; Guo et al. 2018). Some reports showed that the OsbZIP80 functioned as a dehydration stress-inducible gene in rice (Nijhawan et al. 2008; Hoang et al. 2019). It is worth noting that the expression of this gene is induced by ABA, suggesting that this gene is one of the OsbZIP09 target genes (involved in controlling seed germination in rice).
The candidate gene Os12g0621100 (OsYAB6) was identified on MQTL12.1 (FC ≥ 2) during seed germination. This gene encoded the YABBY transcription factor (Liu et al. 2020b). In the same vein as the OsSh1 gene, it is a member of the YABBY transcription factor family and plays a vital role in rice morphogenesis (Xia et al. 2017).
The findings showed that the discussed CGs were the main players in rice seed germination. Also, these CGs had multiple functions and played different roles in plants, ranging from stress response to rice plant growth and development. This information can be reviewed to elucidate the molecular basis of seed germination and its use in rice breeding programs.
It is worth noting, the present study is the first and most comprehensive meta-analysis QTL to identify stable loci controlling germination traits in rice. It is the first study to identify the positions of QTLs associated with seed germination and narrowed down the average CI of MQTLs from 15.125 to 8.73 compared to the initial QTLs; moreover, led to identifying a set of promising candidate genes (155 CGs with significant changes) associated with germination.
Nevertheless, it is essential to perform further analyses to understand genes' precise role in seed germination and the interactions among the expressed proteins. In addition, further work will be required to validate experiments for the identified MQTLs and biological functions of CGs in rice.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank Dr. Md. Shofiqul Islam, at Genetics Research Scientist at Indiana Crop Improvement Association, Lafayette, Indiana, and Qasim Raza, at University of Agriculture Faisalabad, Pakistan for their kind assistance. Also, we would like to acknowledge to Research Core of Seed Production and Processing of Agronomic, Horticultural and Medicinal Plants located in University of Guilan for providing the spiritual support.
Abbreviations
- AIC
Akaike information criterion
- BC
Backcross
- CG
Candidate gene
- CI
Confidence interval
- CL
Coleoptiles length
- COL
Coleorhiza length
- DH
Double Haploids
- GI
Germination index
- GO
Gene ontology
- GP
Germination percentage
- GPO
Germination potential
- GR
Germination rate
- GT
Germination traits
- MQTL
Meta-QTL
- PL
Plumule length
- QTL
Quantitative trait loci
- RFW
Radicle fresh weight
- RIL
Recombinant inbred lines
- RL
Radicle length
- TF
Transcription factor
Author contribution
SV collected all data, contributed to data analysis, and wrote the draft of the manuscript; AS conceived and designed the project, performed meta-QTL of analysis, and revised the manuscript, AA, performed the bioinformatics analyses and revised the manuscript. All authors have read and approved the final manuscript.
Funding
No funding was received for this work.
Data availability
The data used in this study are available from supplementary materials (S1, S2, and S3 excel files) and the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Acuña-Galindo MA, Mason RE, Subramanian NK, Hays DB. Meta-analysis of wheat QTL regions associated with adaptation to drought and heat stress. Crop Sci. 2015;55(2):477–492. doi: 10.2135/cropsci2013.11.0793. [DOI] [Google Scholar]
- Arcade A, Labourdette A, Falque M, Mangin B, Chardon F, Charcosset A, Joets J. BioMercator: integrating genetic maps and QTL towards discovery of candidate genes. Bioinformatics. 2004;20(14):2324–2326. doi: 10.1093/bioinformatics/bth230. [DOI] [PubMed] [Google Scholar]
- Behrozbeh MJ, Sabouri H, Hossein Moghaddam H, Nakhzari Moghaddam A, Rahemi Karizaki A, Rezaei M. Mapping of QTLs affecting salinity tolerance in Iranian rice population at germination stage. Agric Biotechnol J. 2019;11(2):1–22. [Google Scholar]
- Bewley JD, Bradford KJ, Hilhorst HWM, Nonogaki H. Seeds (physiology of development, germination and dormancy) 3. New York Heidelberg Dordrecht London: Springer; 2013. [Google Scholar]
- Borjas Artica AH (2017) Molecular genetics of cold tolerance at germination and seedling stages in rice. Dissertation, University of Louisiana State
- Carretero-Paulet L, Galstyan A, Roig-Villanova I, Martínez-García JF, Bilbao-Castro JR, Robertson DL. Genome-wide classification and evolutionary analysis of the bHLH family of transcription factors in Arabidopsis, poplar, rice, moss, and algae. Plant Physiol. 2010;153(3):1398–1412. doi: 10.1104/pp.110.153593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, He Y, Yang B, Lai Y, Wang Z, Zhang H. Association mapping of seed germination and seedling growth at three conditions in indica rice (Oryza sativa L.) Euphytica. 2015;206:103–115. doi: 10.1007/s10681-015-1477-1. [DOI] [Google Scholar]
- Chi WC, Fu SF, Huang TL, Chen YA, Chen CC, Huang HJ. Identification of transcriptome profiles and signaling pathways for the allelochemical juglone in rice roots. Plant Mol Biol. 2011;77(6):591–607. doi: 10.1007/s11103-011-9841-6. [DOI] [PubMed] [Google Scholar]
- Collard BC, Jahufer MZ, Brouwer JB, Pang EC. An introduction to markers, quantitative trait loci (QTL) mapping and marker-assisted selection for crop improvement: the basic concepts. Euphytica. 2005;142(1):169–196. doi: 10.1007/s10681-005-1681-5. [DOI] [Google Scholar]
- Cui KH, Peng SB, Xing YZ, Xu CG, Yu SB, Zhang Q (2002) Molecular dissection of seedling-vigor and associated physiological traits in rice. Theor Appl Genet 105:745–753. https://link.springer.com/article/10.1007%2Fs00122-002-0908-2. [DOI] [PubMed]
- Dai X, Wang Y, Yang A, Zhang WH. OsMYB2P-1, an R2R3 MYB transcription factor, is involved in the regulation of phosphate-starvation responses and root architecture in rice. Plant Physiol. 2012;159(1):169–183. doi: 10.1104/pp.112.194217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darvasi A, Soller M. A simple method to calculate resolving power and confidence interval of QTL map location. Behav Genet. 1997;27(2):125–132. doi: 10.1023/A:1025685324830. [DOI] [PubMed] [Google Scholar]
- De Leon TB, Linscombe S, Subudhi PK. Molecular dissection of seedling salinity tolerance in rice (Oryza sativa L.) using a high-density GBS-based SNP linkage map. Rice. 2016;9:1–22. doi: 10.1186/s12284-016-0125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng QW, Luo XD, Chen YL, Zhou Y, Zhang FT, Hu BL, Xie JK. Transcriptome analysis of phosphorus stress responsiveness in the seedlings of Dongxiang wild rice (Oryza rufipogon Griff.) Biol Res. 2018;51(1):1–12. doi: 10.1186/s40659-018-0155-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimaano NG, Ali J, Mahender A, Baltazar AM, Diaz MG, Pang YL, Acero BL, Li Z. Identification of quantitative trait loci governing early germination and seedling vigor traits related to weed competitive ability in rice. Euphytica. 2020;216:159. doi: 10.1007/s10681-020-02694-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong XY, Fan SX, Jin LI, Qi WA, Li MR, Jiang X, Liu ZY, Yin YC, Wang JY. Identification of QTLs for seed storability in rice under natural aging conditions using two RILs with the same parent Shennong 265. J Integr Agric. 2017;16(5):1084–1092. doi: 10.1016/S2095-3119(16)61579-4. [DOI] [Google Scholar]
- Dong C, Xi Y, Chen X, Cheng ZM. Genome-wide identification of AP2/EREBP in Fragaria vesca and expression pattern analysis of the FvDREB subfamily under drought stress. BMC Plant Biol. 2021;21(1):1–14. doi: 10.1186/s12870-021-03095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flather MD, Farkouh ME, Pogue JM, Yusuf S. Strengths and limitations of meta-analysis: larger studies may be more reliable. Control Clin Trials. 1997;18(6):568–579. doi: 10.1016/S0197-2456(97)00024-X. [DOI] [PubMed] [Google Scholar]
- Gimhani DR, Gregorio GB, Kottearachchi NS, Samarasinghe WL. SNP-based discovery of salinity-tolerant QTLs in a bi-parental population of rice (Oryza sativa) Mol Genet Genom. 2016;291:2081–2099. doi: 10.1007/s00438-016-1241-9. [DOI] [PubMed] [Google Scholar]
- Goffinet B, Gerber S (2000) Quantitative trait loci: a meta-analysis, Genetics 155: 463–473. https://academic.oup.com/genetics/article-abstract/155/1/463/6048039. [DOI] [PMC free article] [PubMed]
- Guo B, Sleper DA, Lu P, Shannon JG, Nguyen HT, Arelli PR. QTLs associated with resistance to soybean cyst nematode in soybean meta-analysis of QTL locations—retraction. Crop Sci. 2006;46(1):595–602. doi: 10.2135/cropsci2005.04-0036-2. [DOI] [Google Scholar]
- Guo J, Xu C, Wu D, Zhao Y, Qiu Y, Wang X, Ouyang Y, Cai B, Liu X, Jing S, Shangguan X. Bph6 encodes an exocyst-localized protein and confers broad resistance to planthoppers in rice. Nat Genet. 2018;50(2):297–306. doi: 10.1038/s41588-018-0039-6. [DOI] [PubMed] [Google Scholar]
- Guo T, Yang J, Li D, Sun K, Luo L, Xiao W, Wang J, Liu Y, Wang S, Wang H, Chen Z. Integrating GWAS, QTL, mapping and RNA-seqto identify candidate genes for seed vigor in rice (Oryza sativa L.) Mol Breed. 2019;39:87. doi: 10.1007/s11032-019-0993-4. [DOI] [Google Scholar]
- Han C, Yang P. Studies on themolecular mechanismsof seedgermination. Proteomics. 2015;15:1671–1679. doi: 10.1002/pmic.201400375. [DOI] [PubMed] [Google Scholar]
- Hao Z, Li X, Liu X, Xie C, Li M, Zhang D, Zhang S. Meta-analysis of constitutive and adaptive QTL for drought tolerance in maize. Euphytica. 2010;174(2):165–177. doi: 10.1016/j.foodpol.2009.11.003. [DOI] [Google Scholar]
- Heang D, Sassa H. Antagonistic actions of HLH/bHLH proteins are involved in grain length and weight in rice. PLoS ONE. 2012;7(2):e31325. doi: 10.1371/journal.pone.0031325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang GT, Van Dinh L, Nguyen TT, Ta NK, Gathignol F, Mai CD, Jouannic S, Tran KD, Khuat TH, Do VN, Lebrun M. Genome-wide association study of a panel of Vietnamese rice landraces reveals new QTLs for tolerance to water deficit during the vegetative phase. Rice. 2019;12(1):1–20. doi: 10.1186/s12284-018-0258-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Htun TM, Inoue C, Chhourn O, Ishii T, Ishikawa R. Effect of quantitative trait loci for seed shattering on abscission layer formation in Asian wild rice Oryza rufipogon. Breed Sci. 2014;64(3):199–205. doi: 10.1270/jsbbs.64.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Zhao X, Weng X, Wang L, Xie W. The rice B-box zinc finger gene family: genomic identification, characterization, expression profiling and diurnal analysis. PLoS ONE. 2012;7(10):e48242. doi: 10.1371/journal.pone.0048242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo Y, Xiong W, Su K, Li Y, Yang Y, Fu C, Wu Z, Sun Z. Genome-wide analysis of the TCP gene family in switchgrass (Panicum virgatum L.) Int J Genom. 2019 doi: 10.1155/2019/8514928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa R, Castillo CC, Htun TM, Numaguchi K, Inoue K, Oka Y, Ogasawara M, Sugiyama S, Takama N, Orn C, Inoue C. A stepwise route to domesticate rice by controlling seed shattering and panicle shape. BioRxiv. 2021 doi: 10.1101/2021.12.02.470680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam M, Ontoy J, Subudhi PK. Meta-analysis of quantitative trait loci associated with seedling-stage salt tolerance in rice (Oryza sativa L.) Plants. 2019;8:33. doi: 10.3390/plants8020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan I, Saripalli G, Kumar K, Kumar A, Singh R, Batra R, Sharma PK, Balyan HS, Gupta PK (2021) Meta-QTLs and candidate genes for stripe rust resistance in wheat. Sci Rep 11(1):1–13. https://www.nature.com/articles/s41598-021-02049-w [DOI] [PMC free article] [PubMed]
- Kan CC, Chung TY, Wu HY, Juo YA, Hsieh MH. Exogenous glutamate rapidly induces the expression of genes involved in metabolism and defense responses in rice roots. BMC Genom. 2017;18(1):1–17. doi: 10.1186/s12864-017-3588-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khahani B, Tavakol E, Shariati V, Fornara F. Genome wide screening and comparative genome analysis for Meta-QTLs, ortho-MQTLs and candidate genes controlling yield and yield-related traits in rice. BMC Genom. 2020;21:1–24. doi: 10.1186/s12864-020-6702-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khahani B, Tavakol E, Shariati V, Rossini L. Meta-QTL and ortho-MQTL analyses identified genomic regions controlling rice yield, yield-related traits and root architecture under water deficit conditions. Sci Rep. 2021;11:1–18. doi: 10.1038/s41598-021-86259-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna R, Kronmiller B, Maszle DR, Coupland G, Holm M, Mizuno T, Wu SH. The Arabidopsis B-box zinc finger family. Plant Cell. 2009;21(11):3416–3420. doi: 10.1105/tpc.109.069088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khowaja FS, Norton GJ, Courtois B, Price AH. Improved resolution in the position of drought-related QTLs in a single mapping population of rice by meta-analysis. BMC Genom. 2009;10:276. doi: 10.1186/1471-2164-10-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug A, Schwabe JW. Zinc fingers. FASEB J. 1995;9(8):597–604. doi: 10.1096/fasebj.9.8.7768350. [DOI] [PubMed] [Google Scholar]
- Kumar IS, Nadarajah K. A Meta-analysis of quantitative trait loci associated with multiple disease resistance in rice (Oryza sativa L.) Plants. 2020;9:1491. doi: 10.3390/plants9111491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y, Cheng J, He Y, Yang B, Wang Z, Zhang H. Identification of QTLs with additive, epistatic, and QTL × seed maturity interaction effects for seed vigor in rice. Plant Mol Biol Rep. 2016 doi: 10.1007/s11105-015-0913-7. [DOI] [Google Scholar]
- Lang H, He Y, Zeng F, Xu F, Zhao M, Ma D. Comparative transcriptomic and physiological analyses of weedy rice and cultivated rice to identify vital differentially expressed genes and pathways regulating the ABA response. Sci Rep. 2021;11(1):1–16. doi: 10.1038/s41598-021-92504-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Duan X, Jiang H, Sun Y, Tang Y, Yuan Z, Guo J, Liang W, Chen L, Yin J, Ma H. Genome-wide analysis of basic/helix-loop-helix transcription factor family in rice and Arabidopsis. Plant Physiol. 2006;141(4):1167–1184. doi: 10.1104/pp.106.080580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Xu W, Yang W, Kong Z, Xue Y. Genome-wide gene expression profiling reveals conserved and novel molecular functions of the stigma in rice. Plant Physiol. 2007;144(4):1797–1812. doi: 10.1104/pp.107.101600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Sun P, Zhou H, Chen S, Yu S. Identification of quantitative trait loci associated with germination using chromosome segment substitution lines of rice (Oryza sativa L.) Theor Appl Genet. 2011;123:411–420. doi: 10.1007/s00122-011-1593-9. [DOI] [PubMed] [Google Scholar]
- Li L, Liu X, Xie K, Wang Y, Liu F, Lin Q, Wang W, Yang C, Lu B, Liu S, Chen L. qLTG-9, a stable quantitative trait locus for low-temperature germination in rice (Oryza sativa L.) Theor Appl Genet. 2013;126(9):2313–2322. doi: 10.1007/s00122-013-2137-2. [DOI] [PubMed] [Google Scholar]
- Li WT, Liu C, Liu YX, Pu ZE, Dai SF, Wang JR, Lan XJ, Zheng YL, Wei YM. Meta-analysis of QTL associated with tolerance to abiotic stresses in barley. Euphytica. 2013;189:31–49. doi: 10.1007/s10681-012-0683-3. [DOI] [Google Scholar]
- Li CS, Shao GS, Wang L, Wang ZF, Mao YJ, Wang XQ, Zhang XH, Liu ST, Zhang HS. QTL identification and fine mapping for seed storability in rice (Oryza sativa L.) Euphytica. 2017;213:127. doi: 10.1007/s10681-017-1913-5. [DOI] [Google Scholar]
- Li N, Liu H, Sun J, Zheng H, Wang J, Yang L, Zhao H, Zou D. Transcriptome analysis of two contrasting rice cultivars during alkaline stress. Sci Rep. 2018;8(1):1–16. doi: 10.1038/s41598-018-27940-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Xu R, Duan P, Li Y. Control of grain size in rice. Plant Reprod. 2018;31(3):237–251. doi: 10.1007/s00497-018-0333-6. [DOI] [PubMed] [Google Scholar]
- Li F, Komatsu A, Ohtake M, Eun H, Shimizu A, Kato H. Direct identification of a mutation in OsSh1 causing non-shattering in a rice (Oryza sativa L.) mutant cultivar using whole-genome resequencing. Sci Rep. 2020;10(1):1–13. doi: 10.1038/s41598-020-71972-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Tabien RE, Tarpley L, Mohammed AR, Septiningsih EM. Transcriptome profiling of two rice genotypes under mild field drought stress during grain-filling stage. AoB Plants. 2021;13(4):plab043. doi: 10.1093/aobpla/plab043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licausi F, Ohme-Takagi M, Perata P. APETALA 2/Ethylene responsive factor (AP 2/ERF) transcription factors: mediators of stress responses and developmental programs. New Phytol. 2013;199(3):639–649. doi: 10.1111/nph.12291. [DOI] [PubMed] [Google Scholar]
- Lin Z, Li X, Shannon L, Yeh CT, Wang ML, Bai G, Peng Z, Li J, Trick H, Clemente T, Doebley J. Parallel domestication of the Shattering1 genes in cereals. Nat Genet. 2012;44(6):720–724. doi: 10.1038/ng.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Zhang T. Expansion and stress responses of the AP2/EREBP superfamily in cotton. BMC Genom. 2017;18(1):1–16. doi: 10.1186/s12864-017-3517-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Zhang G, Chen S. Structure and regulatory function of plant transcription factors. Chin Sci Bull. 2001;46(4):271–278. doi: 10.1007/BF03187184. [DOI] [Google Scholar]
- Liu L, Lai Y, Cheng J, Wang L, Du W, Wang Z, Zhang H. Dynamic quantitative trait locus analysis of seed vigor at three maturity stages in rice. PLoS ONE. 2014;9(12):e115732. doi: 10.1371/journal.pone.0115732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Shabala S, Zhang J, Ma G, Chen D, Shabala L, Zeng F, Chen ZH, Zhou M, Venkataraman G, Zhao Q. Melatonin improves rice salinity stress tolerance by NADPH oxidase-dependent control of the plasma membrane K+transporters and K+homeostasis. Plant Cell Environ. 2020;43(11):2591–2605. doi: 10.1111/pce.13759. [DOI] [PubMed] [Google Scholar]
- Liu S, Li X, Yang H, Qian Q, Lin X. Ectopic expression of BoYAB1, a member of YABBY gene family in Bambusa oldhamii, causes leaf curling and late flowering in Arabidopsis thaliana. J Hortic Sci Biotechnol. 2020;95(2):169–174. doi: 10.1080/14620316.2019.1661289. [DOI] [Google Scholar]
- Lopus M, Tomy P, Binesh MK. Character-ization of drought responsive genes of CIPK families in rice, maize and sorghum. J Rice Res Dev. 2020;3(1):87–94. doi: 10.36959/973/425. [DOI] [Google Scholar]
- Mahender A, Anandan A, Pradhan SK. Early seedling vigour, an imperative trait for direct-seeded rice: an overview on physio-morphological parameters and molecular markers. Planta. 2015;241:1027–1050. doi: 10.1007/s00425-015-2273-9. [DOI] [PubMed] [Google Scholar]
- Mantilla-Perez MB, Salas Fernandez MG. Differential manipulation of leaf angle throughout the canopy: current status and prospects. J Exp Bot. 2017;68(21–22):5699–5717. doi: 10.1093/jxb/erx378. [DOI] [PubMed] [Google Scholar]
- Mardani Z, Rabiei B, Sabouri H, Sabouri A. Mapping of QTLs for germination characteristics under non-stress and drought stress in rice. Rice Sci. 2013;20(6):391–399. doi: 10.1016/S1672-6308(13)60150-X. [DOI] [Google Scholar]
- Matzke MA, Mette MF, Mateo AJ. Transgene silencing by the host nomedefense: implication for the evolution of epigenetic control mechanisms in plants and vertebrates. Plant Mol Biol. 2000;43(401–15):25. doi: 10.1023/a:1006484806925. [DOI] [PubMed] [Google Scholar]
- McCouch SR, Teytelman L, Xu Y, Lobos KB, Clare K, Walton M, Fu B, Maghirang R, Li Z, Xing Y, Zhang Q. Development and mapping of 2240 new SSR markers for rice (Oryza sativa L.) DNA Res. 2002;9:199–207. doi: 10.1093/dnares/9.6.257. [DOI] [PubMed] [Google Scholar]
- Mendes CD, Borba TC, Bueno LG, Cruzeiro GA, Mendonça JA, Pantalião GF, Vianello RP, Brondani C. Análise de associação quanto à produtividade e seus caracteres componentes em linhagens e cultivares de arroz de terras altas. Pesqui Agropecu Bras. 2014;49:771–782. doi: 10.1590/S0100-204X2014001000004. [DOI] [Google Scholar]
- Miura K, Lin S, Yano M, Nagamine T (2002) Mapping quantitative trait loci controlling seed longevity in rice (Oryza sativa L.). Theor. Appl. Genet. 104: 981–986. https://link.springer.com/article/10.1007%2Fs00122-002-0872-x. [DOI] [PubMed]
- Miyamoto K, Nishizawa Y, Minami E, Nojiri H, Yamane H, Okada K. Overexpression of the bZIP transcription factor OsbZIP79 suppresses the production of diterpenoid phytoalexin in rice cells. J Plant Physiol. 2015;173:19–27. doi: 10.1016/j.jplph.2014.09.001. [DOI] [PubMed] [Google Scholar]
- Mohanty B. Promoter architecture and transcriptional regulation of genes upregulated in germination and coleoptile elongation of diverse Rice genotypes tolerant to submergence. Front Genet. 2021;12:235. doi: 10.3389/fgene.2021.639654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naithani S, Dikeman D, Garg P, Al-Bader N, Jaiswal P. Beyond gene ontology (GO): using biocuration approach to improve the gene nomenclature and functional annotation of rice S-domain kinase subfamily. Peer J. 2021;15(9):e11052. doi: 10.7717/peerj.11052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H, Muramatsu M, Hakata M, Ueno O, Nagamura Y, Hirochika H, Takano M, Ichikawa H. Ectopic overexpression of the transcription factor OsGLK1 induces chloroplast development in non-green rice cells. Plant Cell Physiol. 2009;50(11):1933–1949. doi: 10.7717/peerj.11052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijhawan A, Jain M, Tyagi AK, Khurana JP. Genomic survey and gene expression analysis of the basic leucine zipper transcription factor family in rice. Plant Physiol. 2008;146(2):333. doi: 10.1104/pp.107.112821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niño MC, Cho YG. Transcriptional Modulation of Resistance against Xanthomonas oryzae pv.oryzae Korean Race K2 in japonica Rice. Agron. 2020;10(7):960. doi: 10.3390/agronomy10070960. [DOI] [Google Scholar]
- Park HL, Lee SW, Jung KH, Hahn TR, Cho MH. Transcriptomic analysis of UV-treated rice leaves reveals UV-induced phytoalexin biosynthetic pathways and their regulatory networks in rice. Phytochemistry. 2013;96:57–71. doi: 10.1016/j.phytochem.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Pogue J, Yusuf S. Overcoming the limitations of current meta-analysis of randomised controlled trials. Lancet. 1998;351:47–52. doi: 10.1016/S0140-6736(97)08461-4. [DOI] [PubMed] [Google Scholar]
- Qiu X, Pang Y, Yuan Z, Xing D, Xu J, Dingkuhn M, Li Z, Ye G. Genome-wide association study of grain appearance and milling quality in a worldwide collection of indica rice germplasm. PLoS ONE. 2015;10(12):e0145577. doi: 10.1371/journal.pone.0145577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabiei B, Mardani Z, Ghomi K, Sabouri H, Sabouri A (2014) The effect of rice chromosome 1 on traits associated with drought and salinity tolerance at germination and seedling stages. Seed Plant Improvement J, 30(1)
- Rao Y, Dong G, Zeng D, Hu J, Zeng L, Gao Z, Zhang G, Guo L, Qian Q. Genetic analysis of leaffolder resistance in rice. J Genet Genom. 2010;37:325–331. doi: 10.1016/S1673-8527(09)60050-3. [DOI] [PubMed] [Google Scholar]
- Redona ED, Mackill DJ. Mapping quantitative trait loci for seedling vigor in rice using RFLPs. Theor Appl Genet. 1996;92:395–402. doi: 10.1007/BF00223685. [DOI] [PubMed] [Google Scholar]
- Sanchouli S, Ghorbanzadeh Neghab M, Sabouri H, Zare Mehrjerdi M. Identification of gene locations affecting germination components in the Iranian recombinant inbred lines of rice (Oryza sativa L.) under different drought and salinity stresses. Environ Stresses Crop Sci (ESCS) 2021;13(4):1281–2122. doi: 10.22077/escs.2020.2470.1650. [DOI] [Google Scholar]
- Selamat N, Nadarajah KK. Meta-Analysis of quantitative traits loci (QTL) Identified in Drought Response in Rice (Oryza sativa L.) Plants. 2021;10:716. doi: 10.3390/plants10040716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalmani A, Jing XQ, Shi Y, Muhammad I, Zhou MR, Wei XY, Chen QQ, Li WQ, Liu WT, Chen KM. Characterization of B-BOX gene family and their expression profiles under hormonal, abiotic and metal stresses in Poaceae plants. BMC Genom. 2019;20(1):1–22. doi: 10.1186/s12864-018-5336-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Kapoor M, Tyagi AK, Kapoor S. Comparative transcript profiling of TCP family genes provide insight into gene functions and diversification in rice and Arabidopsis. J Plant Mol Biol Biotechnol. 2010;1:24–38. [Google Scholar]
- Silveira RD, Abreu FR, Mamidi S, McClean PE, Vianello RP, Lanna AC, Carneiro NP. Expression of drought tolerance genes in tropical upland rice cultivars (Oryza sativa) Embrapa Milho e Sorgo-Artigo Em Periódico Indexado (ALICE) 2015 doi: 10.4238/2015.July.27.6. [DOI] [PubMed] [Google Scholar]
- Sosnowski O, Charcosset A, Joets J. BioMercator V3: an upgrade of genetic map compilation and quantitative trait loci meta-analysis algorithms. Bioinformatics. 2012;28(15):2082–2083. doi: 10.1093/bioinformatics/bts313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L, Fang L, Zhu Z, Zhang L, Sun X, Wang Y, Wang Q, Li S, Xin H. The transcription factor VaNAC17 from grapevine (Vitis amurensis) enhances drought tolerance by modulating jasmonic acid biosynthesis in transgenic Arabidopsis. Plant Cell Rep. 2020;39(5):621–634. doi: 10.1007/s00299-020-02519-x. [DOI] [PubMed] [Google Scholar]
- Swamy BP, Sarla N. Meta-analysis of yield QTLs derived from inter-specific crosses of rice reveals consensus regions and candidate genes. Plant Mol Biol Rep. 2011;29:663–680. doi: 10.1007/s11105-010-0274-1. [DOI] [Google Scholar]
- Tan L, Ijaz U, Salih H, Cheng Z, Ni Win Htet N, Ge Y, Azeem F. Genome-wide identification and comparative analysis of MYB transcription factor family in Musa acuminata and Musa balbisiana. Plants. 2020;9(4):413. doi: 10.3390/plants9040413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SS, Kornev AP. Protein kinases: evolution of dynamic regulatory proteins. Trends Biochem Sci. 2011;36(2):65–77. doi: 10.1016/j.tibs.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um T, Park T, Shim JS, Kim YS, Lee GS, Choi IY, Kim JK, Seo JS, Park SC. Application of upstream open reading frames (uORFs) editing for the development of stress-tolerant crops. Int J Mol Sci. 2021;22(7):3743. doi: 10.3390/ijms22073743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van K, McHale LK. Meta-analyses of QTLs associated with protein and oil contents and compositions in soybean [Glycine max (L.) Merr.] Seed. Int J Mol Sci. 2017;18(6):1180. doi: 10.3390/ijms18061180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veyrieras JB, Goffinet B, Charcosset A. MetaQTL: a package of new computational methods for the meta-analysis of QTL mapping experiments. BMC Bioinform. 2007;8:1–6. doi: 10.1186/1471-2105-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vij S, Giri J, Dansana PK, Kapoor S, Tyagi AK. The receptor-like cytoplasmic kinase (OsRLCK) gene family in rice: organization, phylogenetic relationship, and expression during development and stress. Mol Plant. 2008;1(5):732–750. doi: 10.1093/mp/ssn047. [DOI] [PubMed] [Google Scholar]
- Volante A, Tondelli A, Aragona M, Valente MT, Biselli C, Desiderio F, Bagnaresi P, Matic S, Gullino ML, Infantino A, Spadaro D. Identification of bakanae disease resistance loci in japonica rice through genome wide association study. Rice. 2017;10(1):1–16. doi: 10.1186/s12284-017-0168-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZF, Wang JF, Bao YM, Wang FH, Zhang HS. Quantitative trait loci analysis for rice seed vigor during the germination stage. J Zhejiang Univ Sci B. 2010;11(12):958–964. doi: 10.1631/jzus.B1000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Wang J, Bao Y. Quantitative trait loci controlling rice seed germination under salt stress. Euphytica. 2011;178:297–307. doi: 10.1007/s10681-010-0287-8. [DOI] [Google Scholar]
- Wang P, Fouracre J, Kelly S, Karki S, Gowik U, Aubry S, Shaw MK, Westhoff P, Slamet-Loedin IH, Quick WP, Hibberd JM. Evolution of GOLDEN2-LIKE gene function in C3 and C4 plants. Planta. 2013;237(2):481–495. doi: 10.1007/s00425-012-1754-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Liu C, Li Q, Chen Z, Sun S, Wang X. Spatiotemporal resolved leaf angle establishment improves rice grain yield via controlling population density. Iscience. 2020;23(9):101489. doi: 10.1016/j.isci.2020.101489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Huang M, Tao X, Guo T, Chen Z. Quantitative trait loci identification and meta-analysis for rice panicle-related traits. Mol Gen Genomics. 2016;291:1927–1940. doi: 10.1007/s00438-016-1227-7. [DOI] [PubMed] [Google Scholar]
- Xia ML, Tang DY, Yang YZ, Li YX, Wang WW, Lü H, Liu XM, Lin JZ. Preliminary study on the rice OsYABBY6 gene involving in the regulation of leaf development. J Life Sci Res. 2017;21:23–30. [Google Scholar]
- Xie L, Tan Z, Zhou Y, Xu R, Feng L, Xing Y, Qi X. Identification and fine mapping of quantitative trait loci for seed vigor in germination and seedling establishment in rice. J Integr Plant Biol. 2014;56:749–759. doi: 10.1111/jipb.12190. [DOI] [PubMed] [Google Scholar]
- Xiong L, Schumaker KS, Zhu JK. Cell signaling during cold, drought, and salt stress. Plant Cell. 2002;14(supp_l1):S165–S183. doi: 10.1105/tpc.000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Zhu J, He C, Benmoussa M, Wu P. Molecular dissection of developmental behavior of plant height in rice (Oryza sativa L.) Genetics. 1998;150(3):1257–1265. doi: 10.1093/genetics/150.3.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Kong Z, Omo-Ikerodah E, Xu W, Li Q, Xue Y. Calcineurin B-like interacting protein kinase OsCIPK23 functions in pollination and drought stress responses in rice (Oryza sativa L.) J Genet Genom. 2008;35(9):531–543. doi: 10.1016/S1673-8527(08)60073-9. [DOI] [PubMed] [Google Scholar]
- Yang X, Ren Y, Cai Y, Niu M, Feng Z, Jing R, Mou C, Liu X, Xiao L, Zhang X, Wu F. Overexpression of OsbHLH107, a member of the basic helix-loop-helix transcription factor family, enhances grain size in rice (Oryza sativa L.) Rice. 2018;11(1):1–12. doi: 10.1186/s12284-018-0237-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Yang G, Yang M, Su L, Xia L, Li D, Huang C, Zhou D, Liu Y, Wang H, Chen Z, Guo T. Quantitative trait locus analysis of seed germination and early seedling growth in rice. Front Plant Sci. 2019;10:1582. doi: 10.3389/fpls.2019a.01582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Xu K, Chen S, Li T, Xia H, Chen L, Liu H, Luo L. A stress-responsive bZIP transcription factor OsbZIP62 improves drought and oxidative tolerance in rice. BMC Plant Biol. 2019;19(1):1–15. doi: 10.1186/s12870-019-1872-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Ouyang Y, Yao W. ShinyCircos: an R/Shiny application for interactive creation of Circos plot. Bioinformatics. 2018;34(7):1229–1231. doi: 10.1093/bioinformatics/btx763. [DOI] [PubMed] [Google Scholar]
- Zeng P, Zhu P, Qian L, Mi Y, Lin Z, Dong S, Dong H, Zhang H, Cheng J. Identification and fine mapping of qGR6.2, a novel locus controlling rice seed germination under salt stress. BMC Plant Biol. 2021;21:36. doi: 10.1186/s12870-020-02820-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZH, Qu XS, Wan S, Chen LH, Zhu YG. Comparison of QTL controlling seedling vigour under different temperature conditions using recombinant inbred lines in rice (Oryza sativa) Ann Bot. 2005;95:423–429. doi: 10.1093/aob/mci039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZH, Yu SB, Yu T, Huang Z, Zhu YG. Mapping quantitative trait loci (QTLs) for seedling-vigor using recombinant inbred lines of rice (Oryza sativa L.) Field Crops Res. 2005;91:161–170. doi: 10.1016/j.fcr.2004.06.004. [DOI] [Google Scholar]
- Zhang T, Zhao X, Wang W, Pan Y, Huang L, Liu X, Zong Y, Zhu L, Yang D, Fu B. Comparative transcriptome profiling of chilling stress responsiveness in two contrasting rice genotypes. PLoS ONE. 2012;7:e43274. doi: 10.1371/journal.pone.0043274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A, Liu C, Chen G, Hong K, Gao Y, Tian P, Peng Y, Zhang B, Ruan B, Jiang H, Guo L. Genetic analysis for rice seedling vigor and fine mapping of a major QTL qSSL1b for seedling shoot length. Breed Sci. 2017;67:307–315. doi: 10.1270/jsbbs.16195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Wang Y, Deng C, Zhao S, Zhang P, Feng J, Huang W, Kang S, Qian Q, Xiong G, Chang Y. High-quality genome assembly of Huazhan and Tianfeng, the parents of an elite rice hybrid Tian-you-hua-zhan. Sci China Life Sci. 2021;65:1–14. doi: 10.1007/s11427-020-1940-9. [DOI] [PubMed] [Google Scholar]
- Zhao J, He Y, Li X, Weng X, Feng D, Ying J, Wang Z. An integrated RNA-Seq and physiological study reveals gene responses involving in the initial imbibition of seed germination in rice. Plant Growth Regul. 2020;90(2):249–263. doi: 10.1007/s10725-019-00567-2. [DOI] [Google Scholar]
- Zheng X, Chen B, Lu G, Han B. Overexpression of a NAC transcription factor enhances rice drought and salt tolerance. Biochem Biophys Res Commun. 2009;379(4):985–989. doi: 10.1016/j.bbrc.2008.12.163. [DOI] [PubMed] [Google Scholar]
- Zhou G, Wang J, Zhang X, Guo M, Yu G. Predicting functions of maize proteins using graph convolutional network. BMC Bioinform. 2020;21(16):1–6. doi: 10.1186/s12859-020-03745-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu CC, Wang CX, Lu CY, Wang JD, Zhou Y, Xiong M, Zhang CQ, Liu QQ, Li QF. Genome-wide identification and expression analysis of OsbZIP09 target genes in rice reveal its mechanism of controlling seed germination. Int J Mol Sci. 2021;22(4):1661. doi: 10.3390/ijms22041661. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this study are available from supplementary materials (S1, S2, and S3 excel files) and the corresponding author on reasonable request.