Abstract
Increasing evidence suggests that the intestinal microbiota composition could play a role in specific pathologies such as hypertension, obesity and diabetes. This study aims to demonstrate that the intestinal microbiota modulated by a diet creating dysbiosis increased the size of the myocardial infarction and that probiotics could attenuate this effect. To do this, microbiota transplants from rats fed a dysbiotic or non-dysbiotic diet in the presence or absence of probiotics were performed for 10 days on rats whose microbiota had been previously suppressed by antibiotic therapy. Then, the anterior coronary artery of the transplanted rats was occluded for 30 min. Infarct size was measured after 24 h of reperfusion, while signaling pathways were evaluated after 15 min of reperfusion. Intestinal resistance, plasma concentration of LPS (lipopolysaccharides), activation of NF-κB and Akt and composition of the microbiota were also measured. Our results demonstrate a larger infarct size in animals transplanted with the dysbiotic microbiota without probiotics compared to the other groups, including those that received the dysbiotic microbiota with probiotics. This increase in infarct size correlates with a higher firmicutes/bacteroidetes ratio, NF-kB phosphorylation and plasma LPS concentration, and a decrease in intestinal barrier resistance and Akt. These results indicate that dysbiotic microbiota promotes an increase in infarct size, an effect that probiotics can attenuate.
Subject terms: Cardiovascular biology, Myocardial infarction, Biochemistry, Physiology, Cardiology, Medical research
Introduction
Over the past decades, studies have shown a role for the intestinal microbiota in several physiological functions1. In addition to participating in the catabolism of certain substances, experimental results indicate that the microbiota may also contribute to the integrity of the intestinal barrier, the control of the immunological response, the prevention of the spread of pathogenic microorganisms, and the absorption and metabolism of certain nutrients. An imbalance in the composition of the microbiota can have significant consequences on the development or amplification of pathologies such as cardiovascular disease.
We have known for several decades that our diet can have an important effect on the development of cardiovascular diseases by increasing, among other things, triglycerides and cholesterol. However, despite this obvious link between diet and cardiovascular disease, the intestinal microbiota has been a neglected player. The intestinal microbiota can not only be altered by diet, but it may also contribute to cardiovascular disease by increasing the patient’s inflammatory state.
Diets may induce an “imbalance” in the gut microbial community, leading to dysbiosis. Dysbiosis has frequently been reported in patients with cardiovascular risks2. Certain types of high carbohydrate diets may increase Bifidobacteria3 while high-fat diets are associated with reduced Bacteroidetes, increased Firmicutes and increased Gram−/Gram+ ratio, leading to dysbiosis and ultimately to inflammation4,5.
Among other things, variation in the composition of the microbiota could alter the integrity of the intestinal barrier6. The layer of epithelial cells that constitutes the intestinal barrier makes it possible to form, with the layer of mucus and other types of cells, a physicochemical barrier that prevents the translocation of intestinal contents into the bloodstream. The presence of dysbiosis can induce the remodeling of this barrier, allowing the transfer of intestinal contents to the bloodstream, causing an increase in inflammation.
There is evidence, although limited, that the microbiota can influence the size of myocardial infarction. For example, drinking liquids containing the probiotic Lactobacillus plantarum 299v has been shown to reduce myocardial infarct size in rats7. Another study by Lam et al. has demonstrated an association between certain metabolites in the gut microbiota and the severity of myocardial infarction8. We have also reported that the integrity of the intestinal wall was altered after myocardial infarction and that this alteration was reduced in the presence of probiotics, which suggests a link between heart damage and the gut microbiota9. These results indicate that the probiotics may have beneficial effects on the integrity of the intestinal barrier in presence of dysbiosis.
Because inflammation influences the development of cardiovascular diseases, and because dysbiosis can contribute to inflammation, we hypothesised that microbiota from a dysbiotic (D) diet might increase infarct size compared to a non-dysbiotic (ND) diet. This effect could be attenuated in the presence of probiotics, in an attempt to restore balance.
To determine the effect of microbiota on myocardial infarct size, we proceeded to transplant microbiota isolated from D or ND donor rats (donor) ± probiotics in recipient rats, treated previously with antibiotics to remove commensal microbiota.
Materials and methods
Ethics statement
The experiments complied with the animal care guidelines published by the Canadian Council on Animal Care. The procedures performed (Grou57) were approved by the local Animal Care Committee of the Hôpital du Sacré-Coeur de Montréal. Our study experiments were performed in accordance with ARRIVE guidelines (Animal Research: Reporting of In Vivo Experiments)10.
Experimental design
One hundred twelve adult Sprague–Dawley albino male rats (approximately 300 g) (Charles River, Saint-Constant, QC, Canada) were housed in individual cages at 22 °C with a relative humidity of 40%–50% and subjected to a 12 h light/dark cycle, starting at 8:00 a.m. The rats had three days of acclimatisation to their environment with a standard diet (Charles River). The rats were then randomly separated into two groups: 36 donor rats and 76 recipient rats.
The donor group was divided into 4 subgroups, and for a minimum of 21 days before collecting their faeces for faecal transplants and throughout the rest of the protocol, they were fed with a ND or D diet (Table 1) combined or not with probiotics dissolved in water (Bifidobacterium longum R0175 and Lactobacillus helveticus R0052).
Table 1.
Composition of the diets used in the study.
| Non-dysbiotic diet | Dysbiotic diet | |
|---|---|---|
| % diet weight | ||
| Protein | 22.0 | 22.0 |
| Carbohydrate | 42.9 | 42.9 |
| Fat | 20.3 | 20.3 |
| Lipids (g/kg) | ||
| Saturated | 78.1 | 71.3 |
| Monounsaturated | 33.7 | 39.7 |
| Polyunsatured | 83.2 | 88.2 |
In parallel, the recipient group was fed ad libitum with a standard diet (Charles River) throughout the study. The recipients received a mixture of antibiotics in their drinking water for 15 days to suppress their gut microbiota.
A transplant of the intestinal microbiota from the donor group to the recipient group was performed 3 days after the end of the antibiotic treatment, and this was done for 10 consecutive days. The recipient group was then divided into subgroups corresponding to the subgroups of the donor group: one group received the microbiota from a ND diet with vehicle; a second group received the microbiota from a ND diet with probiotics; a third group received the microbiota from a D diet with vehicle; and fourth group received the microbiota from a D diet with probiotics. After 10 days of transplantation, the recipient rats underwent cardiac surgery where the left anterior descending artery was occluded for 30 min. Some rats were sacrificed after 15 min of reperfusion to allow evaluation of signaling pathways. The remaining rats were sacrificed after 24 h of reperfusion and were used to evaluate myocardial infarct size, intestinal permeability, as well as plasma concentration of LPS.
Diets
Animals from both donor groups received a specific diet rich in lipids (22% protein, 42.9% carbohydrates, 20.3% fat) from Envigo Teklab (Madison, WI, USA). The detail of the composition of the diets enriched in fat is presented in Table 1. All the recipient rats received a standard diet (Charles River).
Probiotics
The blend of Lactobacillus. helveticus R0052 and Bifidobacterium longum R0175 was administered by suspending the freeze-dried culture or the vehicle only (maltodextrin) in 200 mL of tap water. Each rat in the probiotics group received a daily dose of 109 colony-forming units. The drinking solution was freshly prepared each day for the duration of the experiments. Water intake was monitored throughout the investigation to ensure that a sufficient amount of bacteria was administered.
Antibiotic therapy
Before microbiota transplantation, recipient rats received a mixture of antibiotics in their drinking water for 10 days (ampicillin 1.25 g/L, Aurobindo; imipenem/cilastatin 350 mg/L, Sandoz; vancomycin 600 mg/mL, Sandoz; metronidazole 60 mg/L, Hospira; ciprofloxacin 40 mg/L, Sandoz) in order to suppress their intestinal microbiota as described in Kelly et al.11 Antibiotics in powder form (ampicillin, imipenem, and vancomycin) were dissolved in 5% dextrose beforehand. Water intake was monitored daily to ensure that a sufficient amount was consumed.
Preparation of the gut microbiota
The gut microbiota solutions were prepared according to an established technique12 and recommended by a European consensus13. Fresh faeces from the same animals (6 rats/diet) were pooled, homogenised in 10 ml of sterile phosphate-buffered saline, and centrifuged for 10 min at 4 °C. The supernatants’ optical density values were calculated and diluted to obtain 1 × 1010 bacteria/500 µl. This preparation was freshly prepared, added daily to gelatin (10 ml), and given to recipients.
Surgical procedures
Anesthesia was induced by ketamine xylazine (80 mg/kg and 10 mg/kg i.p., respectively) and maintained with isoflurane (1%). The animals were intubated and placed on a respirator. The left thoracotomy was undertaken, and the left anterior descending coronary artery was occluded with a 4.0 silk suture and plastic snare. Ischemia was assessed with ST-segment alterations and ventricular subepicardial cyanosis. The suture was removed after 30 min of ischemia for reperfusion. The thorax was closed, and the rats were injected with analgesics (0.05 mg/kg of butorphanol sc)14. After 15 min or 24 h of reperfusion, decapitation was used for sacrifice to prevent biochemical pathway modifications.
Infarct size measurement
The hearts were removed immediately after euthanasia and placed in dishes kept on crushed ice. They were washed with saline by retrograde perfusion via the aorta. The left anterior descending coronary artery was occluded at the same site as for MI induction (see above) to map the area at risk (AR) by Evans blue infusion (0.5%). The hearts were frozen (− 80° C for 5 min), sliced into 4 transverse 2 mm sections and placed in 2,3,5-triphenyltetrazolium chloride solution (1%, pH 7.4) at 37° C for 10 min to better distinguish the area of necrosis (I) from the AR. The different regions were carefully drawn on a glass plate, photocopied, and cut. Thereafter, the complete infarct region, AR and left ventricle (LV), were weighed separately to express MI as percentages of necrosis (I) of the AR (I/AR × 100), and AR as percentages of the LV area (AR/LV × 100).
Biochemical analysis
Plasma LPS concentration
Plasma samples were prepared in MAPK buffer (20% Mix 5X pH 7.5 (50 mM Tris–HCl, 20 mM β-glycerophosphate, 20 mM sodium fluoride, 5 mM EDTA, 10 mM EGTA, 1 mM Na3VO4), 1.6 mM benzamidine, 2.5 M PMSF, 1 M leupeptin, 5 M DTT, 1 M microcystin, 100 M Triton X-100), and a final concentration of 200 μg of protein was added in an ELISA plate. The measurements were taken in accordance with the instructions provided by the supplier (Rat LPS ELISA kit, MyBioSources [cat: MBS268498]).
Ex vivo intestinal resistance
Small intestine resistance was measured with a Ussing Chamber15. Using a 5 cm segment of jejunum, the mucosa was bluntly stripped from the seromuscular layer and a 1 cm2 of mucosa was placed in the cassette. Intestinal transepithelial resistance was measured continuously during 90 min. Chambers were continuously oxygenated to prevent cell death. Results at 90 min were reported for comparisons.
Western blotting: Akt, NF-kB
Western blot analysis was performed as described previously16. Briefly, tissues were homogenised by sonication in lysis buffer (1% Triton X-100, 0.32 mol/L sucrose, 10 mmol/L Tris (pH 8.0), 5 mmol/L EDTA, 2 mmol/L DTT, 1 mmol/L PMSF, 10 mg/mL leupeptin, 10 mg/mL Pepstatin A, 10 mg/mL aprotinin). The tissue homogenates were incubated for 30 min at 4 °C and centrifuged at 10 000 g for 15 min. Protein concentrations of the supernatant were quantified by the Lowry method. Aliquots of 100 µg protein were loaded in polyacrylamide gels (10%–15%) and migrated at 150 V for 75 min in a mini-gel apparatus (BioRad Laboratories, Hercules, CA, USA). Proteins were transferred to nitrocellulose membranes with a Trans-Blot semi-dry transfer cell (BioRad Laboratories). Using SNAP i.d. 2.0 system (Millipore, Etobicoke, ON, Canada), non-specific sites were blocked for 20 min incubation in Odyssey blocking buffer (Li-CoR, Lincoln, NE, USA) (1:1 with PBS). After PBS washing, membranes were incubated 10 min with primary antibody 1:1000 phospho-Akt (S473) (Rabbit Ab Cell signalling, Whitby, ON, Canada), total Akt (Rabbit Ab Cell signalling), NF-κB (Rabbit Ab Cell signalling) and phospho NF-kappaB p65 (Rabbit Ab Cell signalling). After washing, the membranes were incubated 10 min with secondary antibody 1: 15 000 (anti-rabbit IRDye 800CW Li-Cor). After washing, membranes were scanned with Odyssey LI-COR Clx, and band intensities were analysed with Image Studio. The same membranes were placed in stripping buffer (0.1 mol/l glycine, 1% SDS, pH 2.0, 1 h at room temperature) and reused with the same technique for ratio determination phosphoAkt/total Akt, and phosphoNF-κB/total NF-κB.
Microbiota composition
Stools from recipient rats were collected at the moment of the sacrifice and stored at − 80 °C. Total DNA was extracted from stool samples using the QIAmp Fast DNA Stool miniKit from Qiagen Sciences (Germantown, MD, USA). The gut microbiota composition was determined using 16S amplicon sequencing following Illumina 16S Metagenomic Sequencing Library Preparation. Briefly, template gDNA was amplified with 16S universal primers targeting the V3–V4 regions (forward: 5′-CCTACGGGNGGCWGCAG-3′; reverse 5′-GACTACHVGGGTATCTAATCC-3′). Following the described 25 cycles of amplification, amplicons were visualised on a 2% agarose 96-wells precast gel (Invitrogen, G72080). The PCR products were purified with Agencourt AMPure beads (Beckman Coulter, A63881). Five µL of the amplicon PCR reaction was used as template for 8 cycles Index PCR using Illumina barcode indexes Nextera XT V2 primer set (Illumina, FC-131-2001). Another round of Ampure Beads purification was done following Index PCR. The purified PCR reactions were quantified with Quant-iT PicoGreen dsDNA assay (Life Technologies, P7589) on a fluorescent plate reader. A 200 ng PCR reaction quantity was taken from each sample and pooled into a single tube that was quantified with QuBit Broad Range assay (ThermoScientific, Q32853) according to manufacturer’s instructions. The pool was diluted to 1 ng/µL and quality controlled by running a High Sensitivity D1000 TapeStation assay (Agilent, 5067-5584/5585). The library was denatured with 0.2 N NaOH and loaded at a 14 pM concentration. Sequencing was performed on a MiSeq platform using V3 chemistry kit with 2 × 250 bp reads.
Statistical analysis
The results are expressed as mean ± SEM. The number of animals was calculated based on a range of effect size of 30% from previous studies, alpha error of 0.05, and power of 0.8. Statistical analysis was performed using a 2-way ANOVA with Diet and Probiotics as independent variables. When the interaction was significant, a decomposition was performed to detect differences in the simple effect. Simple linear regression analysis was performed between the ratio of Firmicutes/Bacteroidetes and infarct size, expressed as a percent of the area at risk, or LPS to assess potential correlations between those factors. Analysis was performed using SPSS v. 26, and p < 0.05 values were considered to be significant.
Results
Hemodynamic data indicated that the heart rate was stable during the experiment for the different groups (Table 2). On the other side, the mean arterial pressure decreased significantly in the four groups during the experiment, as compared to before occlusion,. However, no significant difference was observed between groups throughout the experiment eliminating the cardiac work as a factor to explain the difference in other measurements.
Table 2.
Hemodynamic data.
| Non-dysbiotic | Non-dysbiotic + probiotics | Dysbiotic | Dysbiotic + probiotics | |
|---|---|---|---|---|
| Before occlusion | ||||
| HR | 218.6 ± 6.8 | 232.3 ± 10.4 | 222.1 ± 10.4 | 225.1 ± 13.4 |
| MAP | 73.7 ± 6.2 | 74.3 ± 6.9 | 91.5 ± 12.6 | 81.8 ± 10.9 |
| 20 min occlusion | ||||
| HR | 219.1 ± 10.7 | 221.6 ± 7.3 | 226.6 ± 6.4 | 219.9 ± 12.6 |
| MAP | 61.9 ± 7.4* | 49.7 ± 1.8* | 71.0 ± 7.1* | 54.8 ± 6.4* |
| Reperfusion | ||||
| HR | 230.4 ± 15.2 | 212.3 ± 6.9 | 220.4 ± 6.4 | 204.9 ± 7.0 |
| MAP | 63.0 ± 8.6* | 50.8 ± 2.3* | 55.2 ± 7.1* | 55.8 ± 8.2* |
HR heart rate (beats/min), MAP mean arterial pressure (mm Hg).
*p < 0.05 compared to before occlusion values.
Infarct size
Our results indicate that the dysbiosis-vehicle animals presented a 50% larger infarct size, expressed as a percent of the area at risk, as compared to the animal receiving the ND diet microbiota (p < 0.05). This increase in infarct size in the dysbiosis-vehicle group was prevented with probiotics indicating that microbiota participate to the progression of the infarct size in these conditions (Fig. 1).
Figure 1.
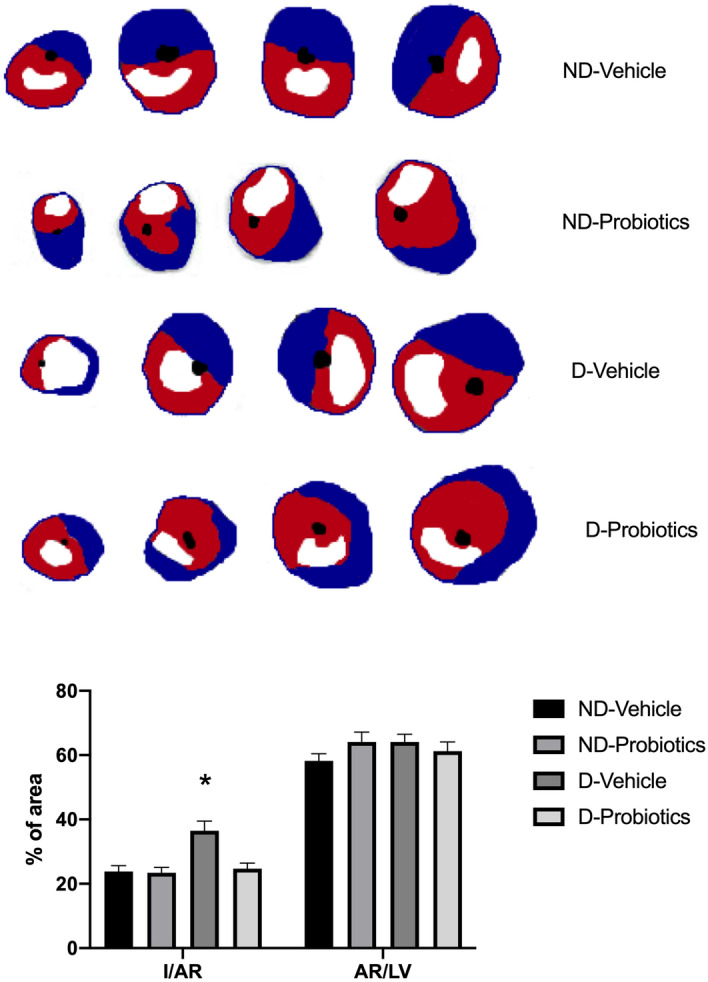
Upper panel: Representative images of myocardial infarction observed in the different groups (Blue: Evans Blue positive, normal region, White: TTC positive infarction, Red: TTC negative, Red and White represent AR). Lower panel: Infarct size (I) expressed as a percentage of the area at risk (AR), and AR as a percentage of the left ventricle (LV) after 24 h reperfusion. *p < 0.05 versus ND-vehicle and D-probiotics. ND none-dysbiotic, D dysbiotic.
Area at risk, expressed as percent of the left ventricle, are similar among groups (p > 0.05).
Firmicutes/bacteroidetes ratio
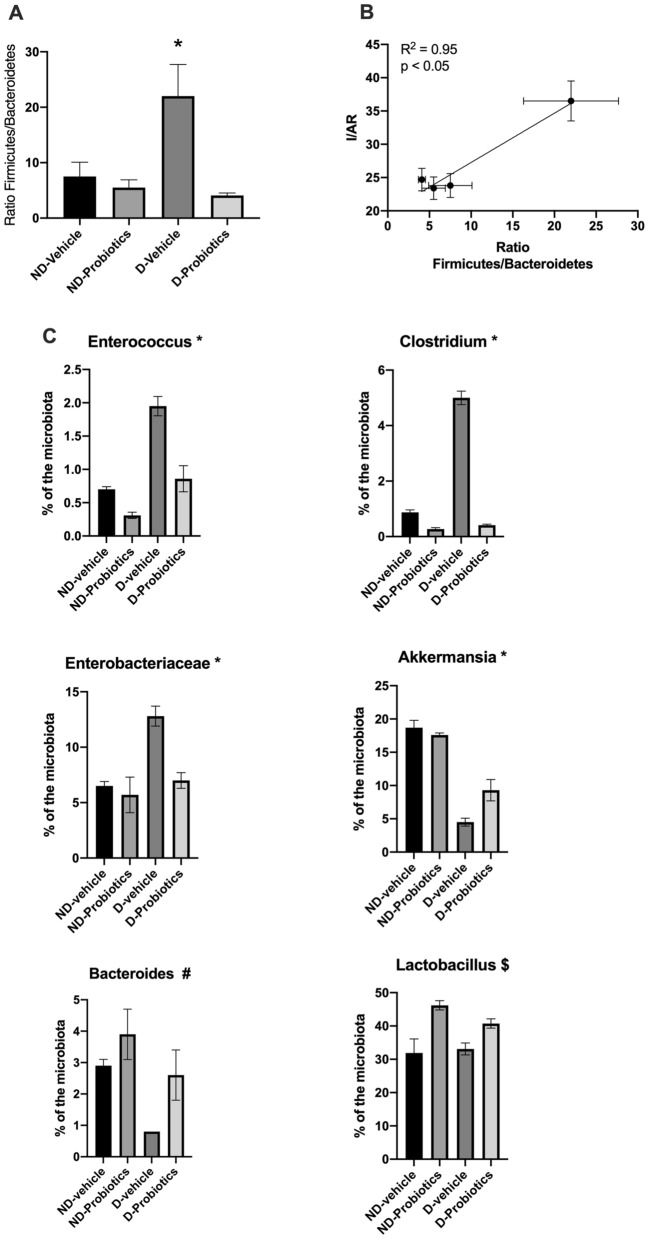
Firmicutes/Bacteroidetes ratio indicated a significant interaction between groups (F(1,19) 6.36; p < 0.05). Simple effects of this interaction indicated that the ratio in the D-vehicle group was significantly higher compared to the other groups. Probiotics alone reduced the ratio (F(1,19) 9.96; p < 0.05) as well as the ND diet compared to the D diet (F(1,19) 4.36; p = 0.051 (Fig. 2A).
Figure 2.
(A) Ratio of the Firmicutes/Bacteroidetes in the different recipient experimental groups *p < 0.05 versus ND-vehicle and D-probiotics. (B) Correlation between the ratio of Firmicutes/Bacteroidetes and infarct size expressed as a percent of the area at risk. To perform the regression, and since the measure were not performed in the same animals, we used the mean of the ratio and the infarct size for each group. (C) Relative abundance of different family/genus in the microbiota of the recipient animals at the time of sacrifice. *indicates a significant interaction in the analysis (p < 0.05), $ indicates a significant effect of the probiotics (p < 0.05) whereas # indicates a significant effect of the diet and probiotics (p < 0.05).
Interestingly, the relation between the infarct size and the Firmicutes/Bacteroidetes ratio indicated a strong relationship between both variables (r2 = 0.95, p < 0.05), giving another argument for a role of the microbiota on myocardial infarct size (Fig. 2B).
Composition of the microbiota
Figure 2C shows the percent of some family/genus observed in the microbiota isolated from the recipient rats at the time of the sacrifice. In four of them (Enteroccocus, Clostridium, Enterobacteriaceae, and Akkermensia), we observed a significant interaction (p < 0.05) between diet and probiotic. The percent of Enteroccocus, Clostridium, and Enterobacteriaceae was significantly more important (p < 0.05) in the D-vehicle group as compared to the other groups, while Akkermensia was significantly less abundant (p < 0.05). The relative abundance of the bacteroides was higher in the ND diet (p < 0.05) as compared to the D diet and more important in presence of probiotics, independently of the diet (p < 0.05).
We also observed that the relative abundance of the Lactobacillus was increased in the probiotics groups as compared to the vehicle groups.
LPS plasmatic concentrations and intestinal resistance
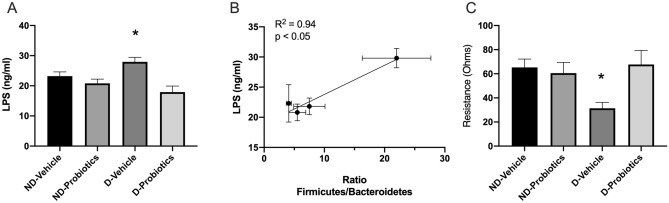
Because dysbiosis is associated with an increased in plasmatic concentration of LPS, we measured the concentrations of LPS in the different groups. The analysis indicated that the interaction between diet and probiotics was significant F(1,27) = 4.87, p < 0.05. The simple effect indicated that the concentration observed in the D-vehicle was significantly higher as compared to the concentrations observed in the ND-vehicle or D-probiotics (p < 0.05). We also observed that probiotics lowered the plasmatic concentrations (F(1,27) = 12.56, p < 0.05) (Fig. 3A). A correlation between the ratio of Firmicutes/Bacteroidetes found in the faeces and the plasmatic levels of LPS was found (Fig. 3B).
Figure 3.
(A) Plasmatic concentrations of LPS in the different groups. *indicates a significant interaction between the main factors (Diet and Probiotics; p < 0.05). (B) Correlation between the ratio of Firmicutes/Bacteroidetes and the plasmatic concentrations of LPS (ng/ml). To perform the regression, and since the measure were not performed in the same animals, we used the mean of the ratio and the infarct size for each group. (C) Gut barrier resistance (ohms) measured in the different groups with the Ussing Chambers. *p < 0.05 versus ND-vehicle and D-probiotics. ND none-dysbiotic D Dysbiotic.
Since LPS can be translocated from the gut to the circulation, we suspected that the gut permeability could have been altered. Measuring the colon resistance, we observed a significant interaction between diet and probiotics (F(1,25) = 5.22, p < 0.05). Simple effects analysis indicated that the resistance was lowered in the D-vehicle as compared to the other groups. Once again, the presence of probiotics in the D diet, results in a resistance that was similar to the one measured in the receiving ND diet, independently of the addition of probiotics (Fig. 3C).
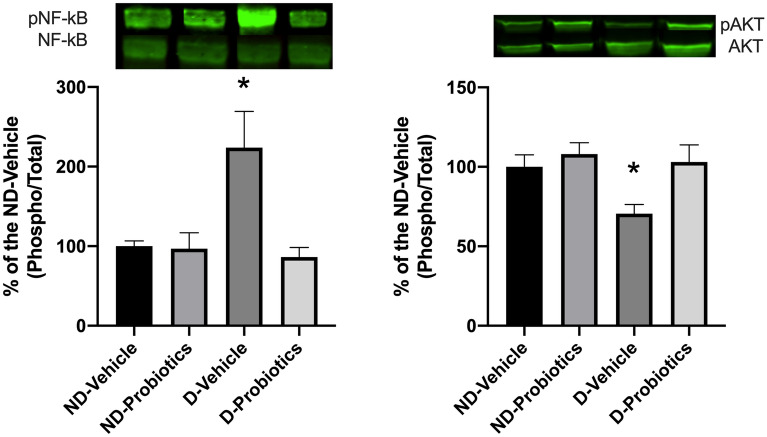
Akt and NF-κB
Two signaling pathways, Akt and NF-kB, known to influence the evolution of infarct size, were investigated to determine whether the modulation of the microbiota could alter their activation (Fig. 4). Significant interaction between diet and probiotics was observed (F(1,20) = 4.88, p < 0.05) and simple effects indicate that the phosphorylation of NF-kB is higher in the D-vehicle group as compared to the other one (Fig. 4; Left).
Figure 4.
Left panel: p-NF-kB/NF-kB ratio right panel: p-Akt/Akt ratio. Results are expressed as a percentage of the ND-vehicle group in the myocardial ischemic region assessed by in vitro western blotting after 15 min of reperfusion. * indicates a significant interaction between the main factors (p < 0.05; Diet and Probiotics). Bands are cropped from original western blots presented in supplementary information file: Fig. 1A,B.
No significant interaction was observed for Akt phosphorylation between the main factors (Diet and Probiotics), but we observed a higher level of phosphorylated Akt in the ND-diet group compared to the D-diet (F(1,21) = 4.0.65, p < 0.05) whereas the presence of probiotics induced a higher level of phosphorylation of AKT compared to the vehicle (F(1,21) = 6.42, p < 0.05; Fig. 4, Right).
Discussion
The present study indicates that the microbiota isolated from rats on a dysbiotic diet has a deleterious effect on the ischemic myocardium resulting in an increase of infarct size as compared to a non-dysbiotic diet. The addition of probiotics to this dysbiotic diet altered the presence of the different bacteria family/genus, reversed the loss of the integrity of the intestinal barrier and the plasmatic concentrations of LPS, attenuated the activation of the inflammatory pathway (NF-kB), increased the activation of the enzyme involved in a salvaged pathway (Akt), and finally resulted in a reduction of myocardial infarction size.
High-fat or high-carbohydrate diets in humans induce dysbiosis characterised by a decrease in Bacteroidetes and an increase in Firmicutes17, which is also reported in rodents4,5. Our dysbiotic diet was elevated in fats and also resulted in an increase in the Firmicutes/Bacteroidetes ratio. It is interesting to note that both diets used in the present study were elevated in fats, suggesting that it was not only the content of fats that was important in inducing dysbiosis but also its composition. The major difference between the diets used was the omega-3/omega-6 fatty acids ratio, which was lower in the dysbiotic diet. However, since the quantities of the different lipids vary between diets, it would be important to determine the impact of the different lipids in the development of dysbiosis.
It was also reported that a high-fat diet results in an increase in the Gram−/Gram+ bacteria ratio18. The increase in Gram− bacteria correlates with an increase in LPS, which promotes inflammation, as we also observed in the present study. We have also observed that the levels of different genera were increased in the dysbiotic diet without probiotics, such as Clostridium, Enterococcus, and Eubacterium, whereas Akkermensia was reduced. Some species of the first three families/genera are associated with an increase of inflammation although not in all of the species19–21, whereas some species associated with Akkermensia exhibit some anti-inflammatory properties22,23. However, we admit that our level of identification of the microbiotas was not sufficiently accurate to confirm this interesting hypothesis.
We also observed that the harmful effect of dysbiosis could be reversed by the addition of probiotics. The blend of probiotics that we used was previously shown to reduce apoptosis in the limbic system after myocardial infarction, while it was unable to reduce infarct size24. The rats used in this previous study were on a normal diet and without dysbiosis, as opposed to the present study9,25. Without dysbiosis, probiotics seem to have a minimal effect on the ischemic myocardium whereas, they attenuate the deleterious myocardial effect of dysbiosis in the presence of dysbiosis. Nevertheless, we must be aware that probiotics do not have the same effects, and it is important to target the mechanism we want to modify to choose the right probiotic or blend.
Although the design of the present study was not to determine the mechanism by which dysbiosis is deleterious for the ischemic myocardium, our convincing results of its impact might guide future research to identify the mechanisms involved.
Our findings point toward an increase in inflammation in the presence of dysbiosis, which can affect the size of the myocardial infarction. Many strategies have been elaborated to prevent or reduce inflammation during I/R, including the blockade of cytokines/chemokines, adhesion molecules, neutrophil depletion, and the administration of anti-inflammatory molecules26–33. Although not all of these have been successful, there are enough studies indicating that inflammation contributes negatively to myocardial infarction. Here we found that the activation of the myocardial NF-kB, a marker of inflammation, was greater in the dysbiotic group than in the other groups.
The inflammation observed in the dysbiotic group could be due to different factors, among which the measured elevation of plasmatic concentrations of LPS34. We have observed that the ratio of Firmicutes/Bacteroidetes in faeces correlated with the plasmatic concentrations of LPS. At this point, our results point toward a loss of the intestinal barrier integrity in the dysbiotic group, revealed by the reduced membrane resistance observed in the D-vehicle group. The increased permeability of the barrier could lead to bacterial product translocation and the initiation of an inflammatory response35,36, as pointed out by the increased circulatory LPS.
Regarding the protecting effect of probiotics on dysbiosis, some elements of the literature are worthwhile mentioning. For example, bacteria and their metabolites could also participate in the protective modulation of the infarct size by producing short chain fatty acids (SCFA), through their anti-inflammatory properties37,38. Non-digested polysaccharides are fermented by gut microbes, generating SCFAs, mostly acetate, propionate, and butyrate39,40, that could be beneficial for the myocardium, although this possibility is debated41. These metabolites present well-characterised anti-inflammatory properties and modulate cellular functions through G-protein coupled receptors (GPR41, GPR43, and GPR109A) or inhibiting histone deacetylases42–45.
Akt is among the different enzymes that are involved in the Reperfusion Injury Salvage Kinase (RISK) pathway, conferring cardioprotection and resulting in a reduction of infarct size when activated at the onset of reperfusion46,47. Lam et al. observed that the cardioprotection observed with vancomycin is lost in presence of the inhibition of Akt/PI3Kinase pathway and concluded that the gut microbiota metabolites are involved in the severity of the myocardial infarct size8 through the modulation of different signaling pathways.
Other mechanisms could also be involved. For example, it is suggested that the microbiota could contribute to cardiovascular disease through the production of phenylacetylglutamine (PAGIn)48. PAGln enhances platelet activation and thrombosis potential, leading to the development of myocardial infarction. However, MI was induced mechanically, and this aspect was not studied, but it remains that it could have contributed to our results.
The present study focused on the acute phase of myocardial infarction, but there are also recent reports on the effect of the microbiota on heart failure progression. This is an interesting avenue because heart failure develops a few weeks after the acute phase of myocardial infarction. Given the present results, one could hypothesize that a dysbiotic diet contributes to the development of heart failure.
Some limitations in the present study deserve to be mentioned. Since we observed different effects of the transplanted microbiota on myocardial infarct size, we now believe that the donor microbiota could have differed according to the diet used, but this measure was unfortunately not planned from the start. It has been reported that the effect of high fat diets on the Firmicutes/Bacteroidetes ratio is similar to what was found here in recipient rats49,50, suggesting that the diets may have indeed altered the composition of the donor’s microbiota. Also, the effect of the antibiotic treatment itself was not assessed. However, since all animals were treated the same across groups, we believe that the impact of the antibiotic treatment was similar. Therefore future studies will aim to characterize the donors’ microbiota, and the potential impact of the antibiotic treatment on recipient rats.
Overall the present study shows that a dysbiotic diet may contribute to the extent of myocardial infarction, for which the addition of probiotics could be protective. Dysbiotic microbiota could be harmful by affecting the integrity of the gut barrier promoting an inflammatory state, and precipitating the development of cardiovascular diseases and infarct size.
Supplementary Information
Author contributions
M.A.G., C.B., G.F., K.G., O.M. and J.A. contribute to the acquisition and the analysis of the data. T.A.T., E.C., R.G. and G.R. contribute to the conception, design of the work, interpretation of the data and the writing of the manuscript. All authors reviewed the manuscript.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon a reasonable request as assessed by the Animal Research Ethics Committee of the corresponding author’s research center.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-20826-z.
References
- 1.Andoh A. Physiological role of gut microbiota for maintaining human health. Digestion. 2016;93:176–181. doi: 10.1159/000444066. [DOI] [PubMed] [Google Scholar]
- 2.Novakovic M, et al. Role of gut microbiota in cardiovascular diseases. World J. Cardiol. 2020;12:110–122. doi: 10.4330/wjc.v12.i4.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pokusaeva K, Fitzgerald GF, van Sinderen D. Carbohydrate metabolism in bifidobacteria. Genes Nutr. 2011;6:285–306. doi: 10.1007/s12263-010-0206-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de La Serre CB, et al. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;299:G440–448. doi: 10.1152/ajpgi.00098.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turnbaugh PJ, Backhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host. Microbe. 2008;3:213–223. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kinashi Y, Hase K. Partners in leaky gut syndrome: Intestinal dysbiosis and autoimmunity. Front. Immunol. 2021;12:673708. doi: 10.3389/fimmu.2021.673708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lam V, et al. Intestinal microbiota determine severity of myocardial infarction in rats. FASEB J. 2012;26:1727–1735. doi: 10.1096/fj.11-197921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lam V, et al. Intestinal microbial metabolites are linked to severity of myocardial infarction in rats. PLoS ONE. 2016;11:e0160840. doi: 10.1371/journal.pone.0160840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arseneault-Breard J, et al. Combination of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 reduces post-myocardial infarction depression symptoms and restores intestinal permeability in a rat model. Br. J. Nutr. 2012;107:1793–1799. doi: 10.1017/S0007114511005137. [DOI] [PubMed] [Google Scholar]
- 10.Percie du Sert, N. et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol.18, e3000410. 10.1371/journal.pbio.3000410 (2020). [DOI] [PMC free article] [PubMed]
- 11.Kelly JR, et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 2016;82:109–118. doi: 10.1016/j.jpsychires.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 12.Li M, et al. Fecal microbiota transplantation and bacterial consortium transplantation have comparable effects on the re-establishment of mucosal barrier function in mice with intestinal dysbiosis. Front. Microbiol. 2015;6:692. doi: 10.3389/fmicb.2015.00692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cammarota G, et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017;66:569–580. doi: 10.1136/gutjnl-2016-313017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boucher M, et al. Reduction of apoptosis in the amygdala by an A(2A) adenosine receptor agonist following myocardial infarction. Apoptosis. 2006;11:1067–1074. doi: 10.1007/s10495-006-6313-6. [DOI] [PubMed] [Google Scholar]
- 15.Khazoom F, et al. Impact of uric acid on liver injury and intestinal permeability following resuscitated hemorrhagic shock in rats. J. Trauma Acute Care Surg. 2020;89:1076–1084. doi: 10.1097/ta.0000000000002868. [DOI] [PubMed] [Google Scholar]
- 16.Tran Quang T, et al. Effect of Resolvin D1 on experimental myocardial infarction. Exp. Clin. Cardiol. 2014;20:6704–6712. [Google Scholar]
- 17.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: Human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 18.Nicholson JK, et al. Host–gut microbiota metabolic interactions. Science. 2012;336:1262–1267. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 19.Mukherjee A, Lordan C, Ross RP, Cotter PD. Gut microbes from the phylogenetically diverse genus Eubacterium and their various contributions to gut health. Gut Microbes. 2020;12:1802866. doi: 10.1080/19490976.2020.1802866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, et al. Eubacterium rectale contributes to colorectal cancer initiation via promoting colitis. Gut Pathogens. 2021;13:2. doi: 10.1186/s13099-020-00396-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van den Munckhof ICL, et al. Role of gut microbiota in chronic low-grade inflammation as potential driver for atherosclerotic cardiovascular disease: a systematic review of human studies. Obes. Rev. 2018;19:1719–1734. doi: 10.1111/obr.12750. [DOI] [PubMed] [Google Scholar]
- 22.Dao MC, et al. Akkermansia muciniphila abundance is lower in severe obesity, but its increased level after bariatric surgery is not associated with metabolic health improvement. Am. J. Physiol. Endocrinol. Metab. 2019;317:E446–e459. doi: 10.1152/ajpendo.00140.2019. [DOI] [PubMed] [Google Scholar]
- 23.Schneeberger M, et al. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci. Rep. 2015;5:16643. doi: 10.1038/srep16643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Girard S-A, et al. Lactobacillus helveticus and Bifidobacterium longum taken in combination reduce the apoptosis propensity in the limbic system after myocardial infarction in a rat model. Br. J. Nutr. 2009;102:1420–1425. doi: 10.1017/S0007114509990766. [DOI] [PubMed] [Google Scholar]
- 25.Girard SA, et al. Lactobacillus helveticus and Bifidobacterium longum taken in combination reduce the apoptosis propensity in the limbic system after myocardial infarction in a rat model. Br. J. Nutr. 2009;102:1420–1425. doi: 10.1017/S0007114509990766. [DOI] [PubMed] [Google Scholar]
- 26.Airaghi L, Lettino M, Manfredi MG, Lipton JM, Catania A. Endogenous cytokine antagonists during myocardial ischemia and thrombolytic therapy. Am. Heart J. 1995;130:204–211. doi: 10.1016/0002-8703(95)90430-1. [DOI] [PubMed] [Google Scholar]
- 27.Blancke F, et al. Systemic inflammation and reperfusion injury in patients with acute myocardial infarction. Mediators Inflamm. 2005;2005:385–389. doi: 10.1155/MI.2005.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carnieto A, Jr, Dourado PM, Luz PL, Chagas AC. Selective cyclooxygenase-2 inhibition protects against myocardial damage in experimental acute ischemia. Clinics (Sao Paulo) 2009;64:245–252. doi: 10.1590/S1807-59322009000300016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Hoog VC, et al. Leucocyte expression of complement C5a receptors exacerbates infarct size after myocardial reperfusion injury. Cardiovasc. Res. 2014;103:521–529. doi: 10.1093/cvr/cvu153. [DOI] [PubMed] [Google Scholar]
- 30.Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc. Res. 2002;53:31–47. doi: 10.1016/S0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- 31.Gu Q, et al. Inhibition of TNF-alpha reduces myocardial injury and proinflammatory pathways following ischemia-reperfusion in the dog. J. Cardiovasc. Pharmacol. 2006;48:320–328. doi: 10.1097/01.fjc.0000250079.46526.38. [DOI] [PubMed] [Google Scholar]
- 32.Simpson PJ, Fantone JC, Mickelson JK, Gallagher KP, Lucchesi BR. Identification of a time window for therapy to reduce experimental canine myocardial injury: Suppression of neutrophil activation during 72 hours of reperfusion. Circ. Res. 1988;63:1070–1079. doi: 10.1161/01.RES.63.6.1070. [DOI] [PubMed] [Google Scholar]
- 33.Steffens S, Montecucco F, Mach F. The inflammatory response as a target to reduce myocardial ischaemia and reperfusion injury. Thromb. Haemost. 2009;102:240–247. doi: 10.1160/TH08-12-0837. [DOI] [PubMed] [Google Scholar]
- 34.Liu L, et al. Up-regulated TLR4 in cardiomyocytes exacerbates heart failure after long-term myocardial infarction. J. Cell Mol. Med. 2015;19:2728–2740. doi: 10.1111/jcmm.12659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der Hee B, Wells JM. Microbial regulation of host physiology by short-chain fatty acids. Trends Microbiol. 2021 doi: 10.1016/j.tim.2021.02.001. [DOI] [PubMed] [Google Scholar]
- 36.Wang L, et al. The effects of probiotics on total cholesterol: A meta-analysis of randomized controlled trials. Medicine. 2018;97:e9679. doi: 10.1097/MD.0000000000009679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohira H, Tsutsui W, Fujioka Y. Are short chain fatty acids in gut microbiota defensive players for inflammation and atherosclerosis? J. Atheroscler. Thromb. 2017;24:660–672. doi: 10.5551/jat.RV17006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vinolo MA, Rodrigues HG, Nachbar RT, Curi R. Regulation of inflammation by short chain fatty acids. Nutrients. 2011;3:858–876. doi: 10.3390/nu3100858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trompette A, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014;20:159–166. doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- 40.Tan J, et al. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014;121:91–119. doi: 10.1016/b978-0-12-800100-4.00003-9. [DOI] [PubMed] [Google Scholar]
- 41.Li M, et al. Pro- and anti-inflammatory effects of short chain fatty acids on immune and endothelial cells. Eur. J. Pharmacol. 2018;831:52–59. doi: 10.1016/j.ejphar.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 42.Macia L, et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat. Commun. 2015;6:6734. doi: 10.1038/ncomms7734. [DOI] [PubMed] [Google Scholar]
- 43.Maslowski KM, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith PM, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang PV, Hao L, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. USA. 2014;111:2247–2252. doi: 10.1073/pnas.1322269111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hausenloy DJ, Tsang A, Yellon DM. The reperfusion injury salvage kinase pathway: A common target for both ischemic preconditioning and postconditioning. Trends Cardiovasc. Med. 2005;15:69–75. doi: 10.1016/j.tcm.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 47.Hausenloy DJ, Yellon DM. Reperfusion injury salvage kinase signalling: Taking a RISK for cardioprotection. Heart Fail. Rev. 2007;12:217–234. doi: 10.1007/s10741-007-9026-1. [DOI] [PubMed] [Google Scholar]
- 48.Nemet I, et al. A cardiovascular disease-linked gut microbial metabolite acts via adrenergic receptors. Cell. 2020;180:862–877.e822. doi: 10.1016/j.cell.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cani PD, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 50.Mahowald MA, et al. Characterizing a model human gut microbiota composed of members of its two dominant bacterial phyla. Proc. Natl. Acad. Sci. USA. 2009;106:5859–5864. doi: 10.1073/pnas.0901529106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon a reasonable request as assessed by the Animal Research Ethics Committee of the corresponding author’s research center.